Abstract
Introduction
The clinical benefits of advanced therapies (i.e., biologics and small-molecule drugs) in the treatment of moderate-to-severe ulcerative colitis (UC) have been demonstrated; however, there is less clarity regarding the economic and health-related quality of life (HRQoL) impact of these treatments. We conducted a systematic literature review to synthesize data on cost, healthcare resource utilization (HCRU), and HRQoL for patients who received approved advanced therapies for moderate-to-severe UC in the United States and Europe.
Methods
Databases including MEDLINE, Embase, the Database of Abstracts of Reviews of Effects (DARE), the National Health Service Economic Evaluation Database (NHS EED), and EconLit were searched systematically to identify observational studies published between January 1, 2010 and October 14, 2021 that assessed the impact of advanced therapies on cost, HCRU, and/or HRQoL in adults with moderate-to-severe UC. Supplementary gray literature searches of conference proceedings from the past 4 years (January 2018 to October 2021) were also performed.
Results
47 publications of 40 unique cost/HCRU studies and 13 publications of nine unique HRQoL studies were included. Findings demonstrated that biologics have a positive impact on indirect costs (i.e., productivity, presenteeism, and absenteeism) and HRQoL. High costs of biologics were not always fully offset by reductions in cost and HCRU associated with disease management. For many patients, treatment switching and dose escalations were required, thus increasing drug costs, particularly when switching across treatment classes.
Conclusion
These findings highlight a high unmet need for therapies for moderate-to-severe UC that can reduce the healthcare burden and impact on society. Further research is warranted, as the reported evidence was limited by the small sample sizes of some treatment groups within a study.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02488-z.
Keywords: Ulcerative colitis, Biologic therapy, Systematic literature review, Direct costs, Indirect costs, Healthcare resource utilization, Health-related quality of life
Plain Language Summary
Although advanced therapies, such as biologics and small-molecule drugs, have shown clinical benefit in treating moderate-to-severe ulcerative colitis, their economic impact and effect on patients’ quality of life is less clear. This study comprehensively reviewed the cost and use of healthcare resources associated with starting treatment with advanced therapies for ulcerative colitis, as well as the impact of these treatments on quality of life. We found that while biologics have a benefit on work productivity, work attendance, work absence, and quality of life, the high costs of biologics were not always fully met by reductions in disease management costs and healthcare resources. Many patients needed to switch treatments or required dose increases, which were expensive. There is a high unmet need for therapies for moderate-to-severe ulcerative colitis that can reduce healthcare costs, use of healthcare resources, and effect on society.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02488-z.
Key Summary Points
| Why carry out this study? |
| Advanced therapies (i.e., biologics and small-molecule drugs) have demonstrated clear clinical benefits for the treatment of moderate-to-severe ulcerative colitis; however, the economic and health-related quality of life impact of these treatments are less well understood. |
| We conducted a systematic literature review to synthesize data on cost and healthcare resource utilization and assess health-related quality of life for patients treated with approved advanced therapies for moderate-to-severe ulcerative colitis. |
| What was learned from the study? |
| This study demonstrates the positive impact that biologic therapies have had on indirect costs (i.e., productivity, presenteeism, and absenteeism) as well as health-related quality of life, but highlights that the high costs of biologics are not always fully offset by reductions in cost and resource use associated with disease. |
| For many patients, treatment switching and dose escalation were required, albeit at considerable expense, especially when switching between treatment classes and to less convenient routes of administration (e.g., subcutaneous to intravenous). |
| This systematic review highlights a high unmet need for therapies for moderate-to-severe ulcerative colitis that reduce the healthcare burden and impact on society. |
Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease that primarily affects the colon and rectum [1] and is most often diagnosed between the ages of 30 and 40 years [2]. The prevalence of UC varies worldwide and is most often diagnosed in developed regions, such as Europe and the USA, where approximately one in every 200–747 and 350 people, respectively, are affected [3]. Although most patients with UC experience mild disease activity, 20% of patients with UC experience at least one severe exacerbation over the course of their disease [4]. On the basis of the Truelove and Witts criteria and Mayo Clinic score, patients with moderate-to-severe disease experience frequent daily (four or more) bloody stools [5], and have severe endoscopic disease activity (presence of ulcers), are dependent on or refractory to corticosteroids, and/or are at high risk of colectomy [6–8]. Patients typically experience periods of remission between symptomatic flares, which can lead to abdominal pain, vomiting, diarrhea, fever, rectal bleeding, weight loss, and even gastrointestinal bleeding [1, 9].
The goal of treatment is to induce and maintain remission, as well as to prevent and manage complications, improve health-related quality of life (HRQoL), and achieve mucosal healing [8, 10–12]. Patients living with UC can have a variable disease course; therefore, treatment choice largely depends on disease activity (active or remission), severity, and steroid dependence [8, 10, 11]. Most patients with moderate-to-severe disease in the USA and Europe typically receive conventional therapies, including aminosalicylates (5-ASAs), corticosteroids, and/or immunomodulators (i.e., azathioprine and 6-mercaptopurine) as first-line therapy, but these therapies are often insufficient to induce or maintain adequate response [4, 13].
Over the past 20 years, the development and approval of biologic agents has provided additional treatment options for patients with moderate-to-severe UC. The first biologics to receive regulatory approval in the USA and Europe were the tumor necrosis factor-α antagonists (anti-TNFs) infliximab, adalimumab, and golimumab, followed by the anti-integrin agent vedolizumab, and the interleukin-12/23 antagonist ustekinumab. For many patients, biologic agents successfully induce and maintain remission, in turn reducing the need for hospitalization and colectomy [12]. However, the effectiveness of biologics often diminishes over time, leading to the need for dose escalation or treatment switching [14]. As such, new biologics as well as small-molecule drugs that target intracellular transduction pathways are being developed for the treatment of moderate-to-severe UC [15–21]. The US Food and Drug Administration and European Medicines Agency recently approved two Janus kinase (JAK) inhibitors, tofacitinib and upadacitinib, and a sphingosine 1-phosphate receptor modulator, ozanimod, for the treatment of moderate-to-severe UC [15, 22–27].
With the emergence of additional treatments for patients with moderate-to-severe UC, an up-to-date indirect treatment comparison has established that these treatments vary with respect to comparative efficacy and safety [12]. Although the clinical benefits of biologics and small-molecule drugs in the treatment of moderate-to-severe UC are well understood, there is less clarity regarding the economic and humanistic impact of these treatments. Some studies have reported the high cost of biologic therapies, and some have suggested costs may be reduced from decreased hospitalizations, emergency department visits, surgeries, and improvements in indirect costs and HRQoL [28–32]. However, there is an important need to collect and comprehensively synthesize these data.
These considerations invite questions about economic and HRQoL outcomes related to treatment with biologics in patients with moderate-to-severe UC. Given that anti-TNFs have been available for more than 20 years [33], real-world evidence (including long-term follow-up data and data on treatment switching) is now available for assessment. Therefore, we conducted a systematic literature review to quantify the cost and healthcare resource utilization (HCRU) and assess HRQoL for patients treated with approved biologic therapy and small-molecule drugs for moderate-to-severe UC.
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [34–36], International Society for Pharmacoepidemiology guidelines for good pharmacoepidemiology practice [37], methodology outlined by the Cochrane Collaboration, and applicable regulatory requirements [38]. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The study protocol was not registered but is available upon request.
Systematic Literature Review
Systematic searches were conducted via OvidSP in MEDLINE, Embase, the Database of Abstracts of Reviews of Effects (DARE), National Health Service Economic Evaluation Database (NHS EED), and EconLit to identify relevant observational studies (including prospective and retrospective cohort studies and cross-sectional analyses) published between January 1, 2010 and October 14, 2021, involving adult patients (18 years or older) with moderate-to-severe UC treated with approved biologic and small-molecule therapies. Two search strategies (one on economic outcomes and another on HRQoL outcomes) were developed in accordance with the pre-specified population, intervention, comparison, outcome, and study design (PICOS) framework (Supplementary Material Tables 1 and 2). Supplementary gray literature searches of conference proceedings from the past 4 years (January 1, 2018 to October 14, 2021) were also performed.
Study inclusion criteria are described in Table 1. The overall objective was to summarize evidence on cost and HCRU and assess HRQoL for patients treated with approved advanced therapies (biologics and small-molecule drugs) for moderate-to-severe UC. Geography was limited to the USA and Europe because of the high prevalence of UC in those regions [3].
Table 1.
PICOS framework
| Domain | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Adults (≥ 18 years) with moderate-to-severe UC |
Pediatric patients (< 18 years) Patients with mild UC Mixed populations without results reported separately for adults with moderate-to-severe UC Patients with UC who were entirely comorbid |
| Interventions/comparators | Approved biologic and small-molecule agents including, but not limited to, adalimumab, golimumab, infliximab, tofacitinib, ustekinumab, or vedolizumab, or biosimilar versions of these therapies | No interventions or comparators of interest |
| Outcomes |
Economic outcomes Total costs (direct + indirect) Direct costs and individual cost components (i.e., healthcare visits, ED visits, hospitalizations) Indirect costs (i.e., productivity losses; absenteeism, presenteeism, WPAI score) HCRU (i.e., healthcare visits, ED visits, hospitalizations, LOS) Patient-reported outcomes (HRQoL and utilities) Change from baseline in the following disease-specific and generic HRQoL measures: SF-36 EQ-5D IBDQ SIBDQ Utilities/disutilities |
Economic outcomes Hospitalizations reported as a risk factor for another outcome such as colectomies or postoperative complications Studies examining/reporting: Only readmission rates following surgical procedures for UC Primarily on clinical outcomes Only on postoperative resource use (e.g., hospitalizations, readmission rates) Patient-reported outcomes (HRQoL and utilities) Outcomes based on different surgical approaches Outcomes of interest for only a select group of patients with specific surgical-related adverse events |
| Study design | Observational studies, including prospective and retrospective cohort studies and cross-sectional analyses |
Case reports or case series, letters to the editor, editorials, comments, notes, narrative reviews, clinical trials, systematic literature reviews, or meta-analyses Studies focusing solely on comparisons between UC and Crohn’s disease |
| Timeframe |
Full-text articles: January 1, 2010 to October 14, 2021 Conference abstracts: January 1, 2018 to October 14, 2021 |
Full-text articles published prior to January 1, 2010 Conference abstracts presented prior to January 1, 2018 |
CUCQ Crohn’s and Ulcerative Colitis Questionnaire, ED emergency department, EQ-5D EuroQol-5D, HCRU healthcare resource utilization, HRQoL health-related quality of life, IBDQ Inflammatory Bowel Disease Questionnaire, LOS length of stay, RFIPC Rating Form of Inflammatory Bowel Disease Patient Concerns, SF-36 36-item Short Form questionnaire, SIBDQ Short Inflammatory Bowel Disease Questionnaire, UC ulcerative colitis, WPAI Work Productivity and Activity Impairment
All abstracts and subsequent full texts were screened by two independent investigators, with disagreements resolved through discussion and consensus or by a third investigator. Study and patients’ characteristics, methodology, results, and conclusions from the accepted studies were extracted into a prespecified data extraction form by a single investigator and validated by a second investigator. Study findings were summarized in a descriptive synthesis.
Outcomes are presented as reported in each respective study with costs (total, direct, and indirect) reported as US dollars, euros, or British pound sterling at the time of each study. Findings were qualitatively synthesized.
Results
Summary of Included Studies
The literature searches identified 3006 unique publications, of which 56 reporting on 47 unique studies met the inclusion criteria. Of the 47 unique studies, 40 reported on cost/HCRU and nine reported on HRQoL including two cost/HCRU studies that also reported HRQoL. The PRISMA diagram in Fig. 1 [39–42] illustrates the flow of references through the review.
Fig. 1.
PRISMA diagram of study attrition. aTwo studies reported across four publications on both the economic burden of UC and associated HRQoL [39–42]. HRQoL health-related quality of life, PRISMA Preferred Reporting Items for Systematic reviews and Meta-Analyses, PRO patient-reported outcome, SLR systematic literature review, UC ulcerative colitis
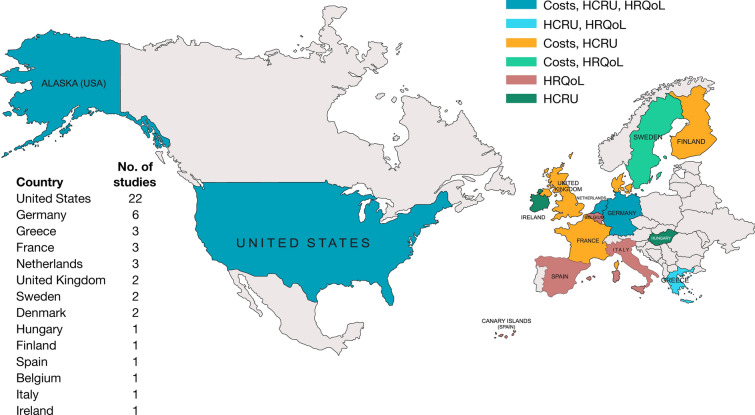
Of the 56 publications that met the inclusion criteria, the evidence base was reported primarily as full-text articles (33 [58.9%]), with 23 (41.1%) conference abstracts. Across the 47 unique studies identified, 21 (44.7%) focused on the USA, 25 (53.2%) on Europe, and one (2.1%) reported data for both regions. Figure 2 presents the distribution of outcomes by country [43, 44]. Most studies were retrospective cohorts (34 [72.3%]), followed by prospective cohorts (10 [21.3%]) and cross-sectional (3 [6.4%]). For the cohort studies, follow-up duration ranged from 2 months to 12 years. Data collection ranged from January 1991 to September 2019, with most studies (40 [85.1%]) within the 2010–2017 timeframe when only adalimumab, golimumab, infliximab, and vedolizumab were approved for UC (Supplementary Material, Fig. 1). Table 2 provides additional details on the included studies [28–32, 39–41, 43–89].
Fig. 2.
Geographic distribution of reported outcomes. Two studies reported data from multiple regions: one study from the Netherlands and Belgium [43] and one from the USA and France [44]. HCRU healthcare resource utilization, HRQoL health-related quality of life
Table 2.
Characteristics of included studies
| Study; publication type | Study design | Country | Data source | Study years | Study population | Sample size | Advanced therapy | Follow-up duration, months | Outcomes reported |
|---|---|---|---|---|---|---|---|---|---|
| USA | |||||||||
| Bornheimer 2019 [45]; abstract | Retrospective cohort | USA | IQVIA RWD Adjudicated Claims Database | 01/2011 to 12/2017 | Patients with UC receiving ≥ 1 IBD-related biologic | 4832 | Not specified | 60 | Costs |
| Borren 2020 [46]; article | Prospective cohort | USA | Prospective Registry for IBD Study | 12/2014 to 06/2018 | Patients with moderate-to-severe UC initiating biologic therapy | 308 | ADA, IFX, UST, or VDZ | 12.4 | HRQoL |
| Cai 2020 [47]; abstract | Retrospective cohort | USA | IBM MarketScan Commercial Claims Database | 01/2017 to 12/2017 | Adults with UC and ≥ 1 medical or pharmacy claim for a biologic | 6414 | Not specified | 12 | Costs, HCRU |
| Carter 2011 [30]; article | Retrospective cohort | USA | IMS LifeLink database | 09/2004 to 03/2009 | Patients with UC newly initiating IFX | 420 | IFX | 12–14 | Costs, HCRU |
| Chapman 2019 [48]; abstract | Retrospective cohort | USA | PharMetrics Plus claims data | 01/2013 to 10/2017 | Patients with UC newly initiating biologic therapy | 3595 | ADA, GOL, IFX, or VDZ | 12 | HCRU |
| Chen 2021 [49]; abstract | Retrospective cohort | USA | Optum Research Database | 2016 to 2019 | Patients with UC treated with biologic therapy | NR | Anti-TNF or VDZ | NR | HCRU |
| Chiorean 2020 [50]; article | Retrospective cohort | USA | IBM MarketScan databases | 01/2000 to 09/2017 | Patients with UC who switched from an initial anti-TNF to another anti-TNF or VDZ | 1348 | ADA, GOL, IFX, or VDZ | 6 | Costs, HCRU |
| Cross 2020 [51]; abstract | Retrospective cohort | USA | Corrona IBD registry | 5/2017 to 9/2019 | Patients with UC on biologics/JAK inhibitors | 315 | Not specified | NR | Costs |
| Hunter 2019 [52]; abstract | Cross-sectional | USA | Truven Health MarketScan Commercial and Medicare Supplemental Databases | 01/2007 to 12/2017 | Patients with UC currently receiving biologics | NR | Not specified | NA | HCRU |
| Kirchgesner 2021 [44]; article | Retrospective cohort | USA | IBM MarketScan and Optum’s Clinformatics Data Mart Database |
MarketScan (2004 to 2018); Optum (2005 to 2019) |
Anti-TNF-naïve patients with moderate-to-severe UC initiating biologic therapy | 878 | IFX | 9.3 | HCRU |
| Kochar 2018 [53]; abstract | Retrospective cohort | USA | Truven Health MarketScan database | 05/2014 to 12/2015 | Patients with UC who are new users of VDZ | 249 | VDZ | 6 | HCRU |
| Long 2019 [28]; article | Retrospective cohort | USA | IQVIA RWD Adjudicated Claims Database | 07/2011 to 07/2014 | Patients with UC who were new and chronic users (≥ 60 days) of anti-TNFs | 2851 | ADA, CZP, GOL, or IFX | 2 | Costs, HCRU |
| Long 2020 [29]; article | Retrospective cohort | USA | IBM MarketScan Research Databases | 01/2012 to 03/2017 | Patients with UC initiating biologic therapy | 2331 | ADA, GOL, IFX, or VDZ | 12 | Costs, HCRU |
| Naegeli 2019 [54]; abstract | Cross-sectional | USA | IBM MarketScan Commercial, Medicaid, and Medicare Supplemental Claims database | 01/2017 to 12/2017 | Patients with UC receiving biologics | 7705 | Not specified | NA | HCRU |
| Nguyen 2020 [55]; abstract | Cross-sectional | USA | IBM Watson Health MarketScan database | 2010 to 2017 | Patients with UC initiating biologic therapy | 7331 | Not specified | NA | HCRU |
| Null 2017 [56]; article | Retrospective cohort | USA | Humana Research Database | 01/2007 to 12/2014 | Patients with UC initiating biologic therapy | 295 | ADA or IFX | 12 | Costs, HCRU |
| Patel 2018 [57]; abstract | Retrospective cohort | USA | Explorys Universe database | 05/2014 to 10/2017 | Biologic-naïve patients with UC initiating treatment with IFX or VDZ | 150 | IFX or VDZ | 12 | HCRU |
| Perera 2018 [58]; article | Retrospective cohort | USA | Truven Health MarketScan Commercial and Medicare Supplemental Databases | 04/2010 to 03/2015 | Patients with UC initiating biologics | 2195 | ADA, CZP, GOL, IFX, NAT, UST, or VDZ | 12 | Costs, HCRU |
| Pilon 2020 [59]; article | Retrospective cohort | USA | Optum Healthcare Solutions, Inc employer claims database | 01/1999 to 03/2017 | Patients with moderate-to-severe UC receiving biologics | 889 | ADA, CZP, GOL, IFX, NAT, or VDZ | 58.8 | Costs |
| Rubin 2020 [60]; article | Retrospective cohort | USA | IBM MarketScan Commercial Claims & Encounters and Medicare Supplemental & Coordination of Benefits databases | 01/2001 to 12/2014 | Patients with UC and ≥ 1 anti-TNF drug claim | 4451 | Anti-TNF (not specified) | 12 | Costs |
| Stewart 2021 [61]; article | Retrospective cohort | USA | Optimum Clinformatics Data Mart | 01/2013 to 12/2018 | Patients with UC initiating an anti-TNF agent | 492 | ADA or GOL | 12 | HCRU |
| Wolf 2021 [62]; abstract | Retrospective cohort | USA | MarketScan Commercial Claims data | 01/2012 to 12/2016 | Patients with UC initiating biologic therapy | 2972 | ADA, IFX, GOL, or VDZ | 24 | Costs, HCRU |
| Europe | |||||||||
|
Armuzzi 2021 [63]; abstract Armuzzi 2018 [32]; article |
Prospective cohort | Italy | GO-CARE study | NR | Patients with UC treated with GOL | 83 | GOL | 12.4 | HRQoL |
|
Bamias 2021 [64]; article Bamias 2019 [65]; abstract |
Prospective cohort | Greece | Greek tertiary GI-IBD centers | 11/2015 to 05/2019 | Patients with UC initiating therapy with VDZ | 96 | VDZ | 12.4 | HRQoL |
| Black 2016 [66]; article | Retrospective cohort | UK | Hospital Treatment Insights database (IMS Health Ltd, UK) | 01/2010 to 03/2014 | Patients with UC treated with ADA | 191 | ADA | 12 | Costs |
| Campbell-Hill 2018 [31]; abstract | Retrospective cohort | Germany | Patient charts from 15 German centers | 07/2014 to 10/2015 | Patients with UC initiating VDZ or an anti-TNF agent | 140 | ADA, GOL, IFX, or IFX biosimilar | 12 | Costs |
| Casellas 2012 [67]; article | Retrospective cohort | Spain | Crohn-Colitis Care Unit (UACC) at Hospital Universitario Valle de Hebrón | NR | Patients with UC treated with anti-TNFs | 11 | ADA or IFX | 12 | HRQoL |
| Desmond 2012 [68]; article | Prospective cohort | Ireland | IBD database of a tertiary referral center, Cork University Hospital | 01/1991 to 01/2009 | Patients with UC admitted to the Cork University Hospital | 25 | ADA or IFX | Median: 14.2 | HCRU |
|
Dignass 2020 [69]; article |
Retrospective cohort | Germany | German statutory health insurance database | 01/2013 to 12/2015 | Patients with UC newly initiating biologic therapy | 304 | ADA, GOL, IFX, or VDZ | 24 | Costs, HCRU |
| Eriksson 2021 [72]; article | Prospective cohort | Sweden | Swedish National Quality Register for IBD (SWIBREG); SVEAH Study | 06/2015 to 11/2018 | Patients with active UC at the onset of biologic treatment | 117 | VDZ | 12 | HRQoL |
| Gatopoulou 2021 [73]; article | Prospective cohort | Greece | GO-LIFE study | 2015 to 2018 | Anti-TNF-naïve patients with moderately to severely active UC and inadequate response, intolerability, or contraindication to conventional therapies | 81 | GOL | 12 | HRQoL |
| Khalili 2020 [74]; article | Retrospective cohort | Sweden | Swedish National Patient Register | 2014 | Patients with UC on anti-TNF therapy | 862 | Anti-TNF (not specified) | 12 | Costs |
| Kirchgesner 2021 [44]; article | Retrospective cohort | France | SNDS | 2009 to 2018 | Anti-TNF-naïve patients with moderate-to-severe UC initiating biologic therapy | 620 | IFX | 9.3 | HCRU |
| Lawton 2019 [75]; article | Retrospective cohort | France | Outpatient clinic at the Nancy University hospital | 11/2016 to 02/2017 | Patients with UC treated with ADA or IFX | 25 | ADA, IFX, or IFX biosimilar | 12 | Costs |
| Lo 2020 [76]; article | Prospective cohort | Denmark | Danish national registries | 01/2003 to 12/2004 | Patients with UC receiving biologics | 28 | Not specified | 144 | Costs |
| Lowenberg 2014 [77]; article | Retrospective cohort | Netherlands | Local hospital database | 11/2003 to 08/2012 | Patients hospitalized for severe corticosteroid-refractory UC and treated with IFX | 16 | IFX | Median: 34.5 | Costs, HCRU |
| Mandel 2014 [78]; article | Retrospective cohort | Hungary | Patients’ medical charts | 01/2008 to NR | Patients with steroid-refractory UC receiving at least one maintenance anti-TNF therapy | 42 | ADA or IFX | Median: 102 | HCRU |
| Mantzaris 2019 [79]; abstract | Prospective cohort | Greece | GO-LIFE study | NR | Anti-TNF-naïve patients with moderate-to-severe UC and inadequate response to conventional therapies | 50 | GOL | 6 | HCRU |
| Oussalah 2010 [80]; article | Retrospective cohort | France | Medical records of patients from five academic centers in France | 12/2007 to 12/2014 | Patients with UC initiating first-line anti-TNF therapy | 191 | ADA or IFX | Median: 18 | HCRU |
|
Picker 2021 [81] |
Retrospective cohort | Germany | AOK PLUS | 01/2015 to 06/2019 | Patients with UC undergoing advanced therapies | 574 | Anti-TNFs (not specified), TOF, or VDZ | 48 | Costs, HCRU |
| Pöllinger 2019 [84]; article | Retrospective cohort | Germany | Arvato Health Analytics GmbH database in cooperation with Gesundheitsforen Leipzig GmbH | 2007 to 2015 | Patients with moderate-to-severe UC treated with biologics | 154 | ADA | 12 | Costs |
| Sebastian 2019 [85]; article | Retrospective cohort | UK | 11 acute hospitals | 05/2016 to 05/2018 | Hospitalized, steroid-refractory patients with ASUC | 131 | IFX | 12 | HCRU |
| Singh 2017 [86]; article | Retrospective cohort | Denmark | Danish National Patient Registry | 2005 to 2014 | Biologic-naïve patients with UC | 275 | ADA or IFX |
Median: ADA 15.6 Median: IFX 27.6 |
HCRU |
|
Teich 2021 [39]; article Teich 2020 [40]; article Teich 2019 [42]; abstract |
Prospective cohort | Germany | GO CUTE study | 03/2014 to 08/2019 | Patients with UC who were suitable for GOL therapy | 282 | GOL | 24 | Costs, HCRU, HRQoL |
| van der Valk 2015 [41]; article | Prospective cohort | Netherlands | COIN study | 10/2010 to 10/2011 | Patients with UC on anti-TNF therapy | 34 | ADA or IFX | 24 | Costs, HRQoL |
| van Gennep 2017 [43]; article | Retrospective cohort | Netherlands, Belgium | Academic Medical Center in Amsterdam, the Netherlands, and the University Hospitals in Leuven, Belgium | 2010 to 01/2015 | Patients with moderate-to-severe UC starting treatment with an anti-TNF agent | 59 | ADA, GOL, or IFX | Median: 29 | HRQoL |
| Wilke 2020 [87]; article | Retrospective cohort | Germany | Regional German Sickness Fund | 01/2011 to 12/2015 | Patients newly diagnosed with UC initiating anti-TNF therapies | 131 | ADA, CZP, IFX, or GOL | Median: 51 | Costs |
|
Ylisaukko-Oja 2019 [88]; article Torvinen 2018 [89]; abstract |
Retrospective cohort | Finland | The Hospital District of Southwest Finland | 2014 to 2016 | Anti-TNF-naïve patients with UC initiating treatment with IFX | 110 | IFX | 12 | Costs, HCRU |
ADA adalimumab, CZP certolizumab pegol, GI gastrointestinal, GOL golimumab, HCRU healthcare resource utilization, HRQoL health-related quality of life, IBD inflammatory bowel disease, IFX infliximab, JAK Janus kinase, NA not applicable, NAT natalizumab, NR not reported, RWD real-world data, SNDS Système National des Données de Santé (French nationwide health insurance database), TNF tumor necrosis factor, UC ulcerative colitis, UST ustekinumab, VDZ vedolizumab
Details of patient characteristics were limited, mainly because the biologic-treated populations were reported as subgroups of broader inflammatory bowel disease or UC study populations. Approximately half of the included studies reported separate outcomes of interest for subsets of patients with UC treated with biologics. Sample sizes for biologic-treated patients ranged from 11 (the subset of patients with UC in a small study from Spain) to 7705 (a US claims study). European studies generally had smaller patient populations, particularly across treatment groups.
Regarding specific treatments, 22 (46.8%) of the 47 studies assessed a mix of advanced therapies, while others assessed specific drugs or reported outcomes separately by biologic agent (Fig. 3). Infliximab was the most frequently referenced treatment in 15 (31.9%) studies, followed by adalimumab in 11 (23.4%), golimumab in 10 (21.3%), and vedolizumab in 10 (21.3%) studies. One study assessed ustekinumab, but alongside other biologics and for a very limited sample of patients (n = 3). The off-label data on certolizumab pegol and natalizumab are not summarized in this review because of the lack of availability of data and small sample sizes when data were reported.
Fig. 3.
Biologic treatments evaluated by country. ADA adalimumab, BEL Belgium, DNK Denmark, GOL golimumab, UK United Kingdom, UST ustekinumab, VDZ vedolizumab
Cost Outcomes
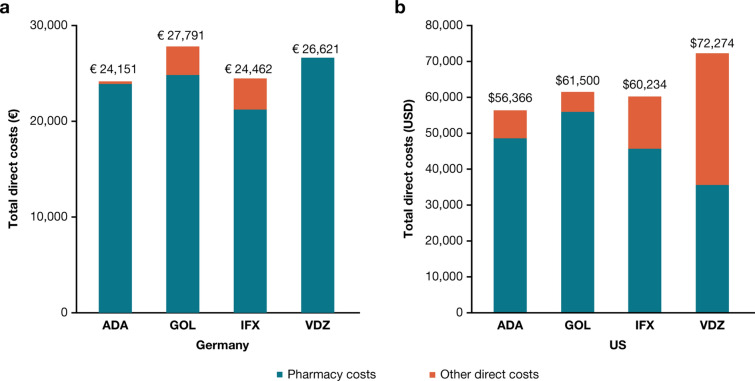
Of the 40 studies, 25 (62.5%) reported cost outcomes (12, USA; 13, Europe); details are provided in Supplementary Material Tables 3 and 4. Twenty-two (55.0%) of the 40 studies reported direct costs (11, USA; 11, Europe) either as total direct costs (medical and pharmacy) and/or individual components of medical costs (e.g., outpatient visits, hospitalization, surgical procedures, etc.). Irrespective of geography, total direct costs were driven by pharmacy expenses, given the high costs associated with biologic therapy, with similar trends observed in Germany (adalimumab, €24,151; golimumab, €27,791; infliximab, €24,462; vedolizumab, €26,621) and the USA (adalimumab, $56,366; golimumab, $61,500; infliximab, $60,234; vedolizumab, $72,274) for individual biologic agents (Fig. 4). Hospitalizations were associated with the highest medical costs, followed by outpatient visits and emergency services. This trend was evident in all studies except for Perera et al., which reported higher outpatient costs than hospital-related expenses for all biologics except adalimumab [58].
Fig. 4.
Direct costs in a euros and b US dollars associated with the first year of biologic therapy for patients with moderate-to-severe UC in the USA and Germany. Data on direct costs of moderate-to-severe UC were reported by two studies; one in the USA [58] and one in Germany [69]. ADA adalimumab, GOL golimumab, IFX infliximab, UC ulcerative colitis, USD United States dollar, VDZ vedolizumab
Direct Costs Associated with Initiation of and Switching Between Biologics
Of the 40 studies, four (10.0% [two, USA; two, Europe]) assessed the direct costs associated with initiating biologic therapy (Fig. 5; Supplementary Material Table 3). Trends were consistent across studies in biologic-naïve patients and those who had previously received anti-TNFs and were starting a new biologic.
Fig. 5.
Changes in direct healthcare costs (a)a and HCRU (b)b associated with initiation of biologic therapy (pre vs. post biologic use) in patients with moderate-to-severe UC. Green arrows indicate a positive outcome after initiation of biologic therapy (pre vs. post biologic use). Red arrows indicate a negative outcome after initiation of biologic therapy (pre vs. post biologic use). Gray horizontal arrows indicate conflicting data regarding changes in the outcome after initiation of biologic therapy (pre vs. post biologic use). aData on direct healthcare costs were reported by four publications [31, 50, 58, 69]. bData on HCRU were reported by 10 publications [39, 44, 50, 52, 55, 58, 61, 69, 78, 79]. ADA adalimumab, ED emergency department, GOL golimumab, HCRU healthcare resource utilization, IFX infliximab, UST ustekinumab, VDZ vedolizumab, UC ulcerative colitis, US United States
Among two USA studies, initiation of biologics was associated with reductions in mean costs of outpatient visits, hospitalizations, and emergency department visits over both 6 months and 1 year except for the initiation of vedolizumab, which was associated with increased outpatient visits [50, 58]. There were no data reported for the USA with regard to changes in medication use or surgical procedures after biologic initiation.
In contrast to the USA studies, two German studies demonstrated increased mean annual costs of outpatient visits, hospitalizations, emergency services, in addition to prescriptions and surgical procedures over the first year of biologic treatment [31, 69], with Dignass et al. reporting subsequent reductions in medical and pharmacy costs over the second year of treatment below those observed before biologic initiation. These findings suggest that the highest burden associated with biologic use is observed in the first year of treatment [69].
Of the 40 studies, two USA studies (5.0%) assessed cost outcomes associated with biologic switching and found that changing therapies can be expensive, particularly if a different drug class is involved. Chiorean et al. compared costs (2017 US$) associated with switching from one anti-TNF to another anti-TNF or vedolizumab, and found that switching to vedolizumab incurred significantly higher healthcare costs within the first 6 months after the switch versus switching to adalimumab or infliximab ($54,528 vs. $43,118–47,861, P < 0.05), but not when switching to golimumab ($54,528 vs. $49,677) [50]. These differences were driven by higher medical expenses (outpatient costs; $7768 vs. $5216, P < 0.05) and drug costs ($36,689 vs. $29,573, P < 0.05) for vedolizumab compared with adalimumab and by higher non-index drug-related pharmacy costs ($3825 vs. $2914, P < 0.05) compared with infliximab [50]. Among patients switching anti-TNFs, Null et al. found that patients treated with infliximab or adalimumab who remained on their initial anti-TNF (irrespective of dose stability) had lower mean quarterly total healthcare costs (2014 US$) versus patients who switched to the other anti-TNF ($9632–10,113 vs. $15,004) [56]. The biggest driver of higher costs related to treatment switching aside from costs related to anti-TNFs was inpatient medical costs ($3559 vs. $540–749) [56].
Indirect Costs
Six (15.0%) of the 40 studies (two, USA; four, Europe) reported indirect costs (Supplementary Material Table 4). Pilon et al. reported total indirect costs as $11,898 per person per year, representing 13.0% of the total cost burden of patients with UC who received biologics in the USA, which was primarily attributable to costs associated with work absences due to disability costs (Supplementary Material Table 4) [59]. In Germany, Teich et al. found that initiation of golimumab was associated with significant improvements (P < 0.0001) in work productivity and the capacity for daily activities starting as early as month 3, with these improvements maintained up to 24 months (end of study follow-up) [39]. Picker et al. also found that patients receiving anti-TNFs, vedolizumab, or tofacitinib who were previously treated with advanced therapies had fewer sick days from work than treatment-naïve patients (12.2 vs. 13.2) per year and lower costs associated with UC-related sick leave (€2909 vs. €3404 per patient-year) [81, 82]. Cross et al. found that patients in the USA who were treated with biologics or JAK inhibitors were burdened most by impaired daily activities due to UC, followed by loss of work productivity, work impairments, and work time missed. In comparison with patients treated with 5-ASAs, patients treated with biologics or JAK inhibitors were more likely to be employed, and experienced more frequent work and activity impairments across all four WPAI domains [51].
HCRU Outcomes
Thirty-one (77.5%) of the 40 studies reported HCRU outcomes (18, USA; 13, Europe; Supplementary Material Table 5). HCRU was driven primarily by use of outpatient services in the USA and Europe. After outpatient visits, the emergency department was the next most commonly used resource in the USA [50, 54, 56], while hospital services were used more frequently in Europe [39, 69, 88]. Reporting of surgical procedures alongside other services was limited to six studies, but consistently was the least-used service among patients treated with advanced therapies.
HCRU Outcomes Based on Initiation of and Switching Between Biologics
Ten (25.0%) of the 40 studies (five, USA; four, Europe; one from both the USA and France) assessed the impact of biologic initiation on healthcare services utilization (Fig. 3). Trends were generally consistent across studies whether patients were biologic therapy-naïve and initiating biologic treatment or had previously received an anti-TNF and were starting a new biologic therapy. However, three USA studies assessing golimumab (two on patients naïve to biologic therapy [58, 61] and one on patients switching biologics [50]) reported increased inpatient admissions and emergency department visits after initiation of a second biologic compared with the decreases observed upon initiation of first biologic agent.
Among the USA studies, initiation of biologic therapy was generally associated with reduced hospitalization rates and emergency department visits, but more frequent outpatient visits (consistent with our findings on costs). Patients initiating adalimumab had fewer outpatient visits after 6 and 12 months of treatment compared with baseline [50, 58, 61]. HCRU associated with initiation of golimumab was reported by the same three studies reporting data for adalimumab [50, 58, 61], but results were inconsistent across and within these studies regarding increased or decreased use of individual services.
The European (n = 4) and USA studies (n = 5) were largely consistent in reporting increased outpatient visits and decreased emergency services use over the first year of biologic initiation, while mixed results were observed across two studies that reported hospitalization rates. Similar to findings on costs, Dignass et al. noted overall increased HCRU associated with the first year of biologic initiation, with subsequent reductions below those observed before biologic initiation over the second year of treatment [69].
Consistent with findings on costs, USA patients switching from one anti-TNF to another anti-TNF were more likely to utilize healthcare services (emergency department, hospital, and outpatient) [56]. Chen et al. also suggested that the timing of anti-TNF switching may affect utilization of healthcare services wherein patients treated with an anti-TNF agent before switching to vedolizumab (irrespective of receipt of immunomodulators) experienced more hospitalizations, colectomies, and UC-related laboratory tests (Supplementary Material Table 5) [49]. Also consistent with findings on costs, Chiorean et al. demonstrated higher HCRU burden, driven by more frequent outpatient visits when patients switched from anti-TNF therapy to vedolizumab versus patients switching to adalimumab or golimumab [50]. However, use of outpatient services was similar to patients switching to infliximab, possibly because both drugs are administered intravenously rather than subcutaneously, as with adalimumab and golimumab.
HCRU Outcomes Associated with Dose Changes and Treatment Combinations
Four studies (two, USA; two, Europe) assessed the HCRU outcomes associated with dose changes. In a USA study by Null et al., escalation or reduction of adalimumab or infliximab doses was associated with slightly more frequent use of outpatient services (57.2% vs. 55.3%) and increased medication claims (average 3.83 vs. 3.75 claims) over 1 year compared with patients on a stable dose [56]. Meanwhile, a German study by Picker et al. suggested that dose escalation of infliximab in the first year of treatment was associated with slightly fewer hospitalizations (average per patient 0.3 vs. 0.4) or gastroenterologist visits (average per patient 2.1 vs. 2.3) versus patients who did not undergo dose escalation [82]. Other findings related to infliximab that were consistent across USA and European studies showed that the timing and dose of induction and maintenance therapy have an impact on HCRU. Sebastian et al. found that an accelerated regimen (two 5-mg/kg doses at week 0 and one 5-mg/kg dose on or before week 1 and/or before week 2) was associated with longer hospital stays and higher rates of colectomy than the standard regimen (5 mg/kg at weeks 0 and 2), although the between-group differences were not significant (P > 0.05) [85]. Similarly, patients who received the maintenance regimen of infliximab exhibited fewer hospitalizations in the first year of treatment versus those who received only induction therapy (10.6% vs. 16.4%). Some of these findings suggest that deviations from the approved labeling in a real-world setting may counteract some of the benefit associated with biologic therapy [30].
Long et al. demonstrated that receipt of biologics in combination with immunosuppressants and/or corticosteroids was associated with higher annual rates of all-cause and UC-related hospitalizations and emergency department visits in the USA (Supplementary Material Table 5) [28]. By contrast, infliximab plus thiopurines was associated with lower rates of UC- or colectomy-related hospitalizations within 16 weeks of treatment initiation in the USA (6.3–7.9% vs. 11.1–11.3%) and in France (10.5% vs. 6.1%) compared with patients receiving infliximab monotherapy [44].
HRQoL
Evidence of the impact of biologic therapy on HRQoL in the USA and Europe was limited. Only one USA study and eight European studies from Germany, Greece, Italy, the Netherlands, and Sweden assessed HRQoL in biologic-treated patients (Supplementary Material Table 6). All studies, irrespective of biologic agent or geographic region, demonstrated improved Inflammatory Bowel Disease Questionnaire (IBDQ), Short IBDQ (SIBDQ), EuroQol-5D (EQ-5D), and Short Form-12 (SF-12) scores after initiation of biologic treatment. Significant improvements in HRQoL were observed as early as 3 months in three studies [42, 46, 65] and were maintained up to 1 year [46, 65, 67, 72, 73] and 2 years [39, 42], respectively.
Discussion
To our knowledge, this is the first systematic literature review focused on the impact of advanced therapies on economic and HRQoL outcomes in patients with moderate-to-severe UC. Forty studies included across the USA and Europe demonstrated that the economic burden in patients with moderate-to-severe UC starting treatment with biologics or small-molecule drugs was high in these regions, driven primarily by the cost of these treatments and outpatient visits associated with administration and monitoring. However, biologic initiation was shown to reduce the indirect cost burden to these patients and improve quality of life.
Aside from prescription costs increasing the total healthcare costs associated with biologic treatments, hospitalization-related expenses were the main drivers of medical costs, followed by outpatient visit and emergency services costs. Among healthcare services, outpatient visits were the most common, followed by emergency department visits within the USA studies, while hospital services were used more frequently than the emergency services in Europe. Initiation of biologic therapy was generally associated with reduced medical costs (outpatient, hospital, emergency) and visits (hospital and emergency), increased outpatient visits, and significant improvements in work and activity impairments and quality of life over a range of follow-up from 3 months to 2 years. Despite some reductions in costs and HCRU, the high cost of advanced therapies may not be fully offset. Dignass et al. demonstrated that first year of biologic treatment (1–12 months) was often associated with increased medical and pharmacy costs and HCRU, with subsequent cost reductions below those observed before biologic initiation over the second year of treatment (13–24 months) [69]. These findings suggest that the highest burden associated with biologic use is observed in the first year of treatment.
In general, our findings are consistent with a recently published systematic literature review that assessed cost-of-illness of inflammatory bowel disease, and concluded that since the introduction of biologic treatments, the cost of medications has increased, but the costs associated with inpatient visits, hospitalizations, and surgery have not declined enough to offset increases in treatment costs [90]. Similarly, a recent systematic review on the economic burden of Crohn’s disease in Europe found the cost of biologics to be the main driver, over and above the cost of surgery and hospitalizations. Similar trends have also been observed in moderate-to-severe psoriasis, another chronic inflammatory condition that is commonly treated with similar biologic therapies, such as adalimumab and infliximab [91, 92].
Although the use of biologics adds a substantial cost to patient care, further consideration is required regarding how treatment patterns and switching impact overall cost and HCRU trends in real-world settings. A recent publication by Huynh et al. noted that physicians in Europe reported lack of treatment effectiveness as the primary reason for treatment switching in patients with UC [93]. Bokemeyer et al. found that loss of response can occur within 5 months of treatment (median time to inadequate response 4.8 months), with as many as 75% of patients exhibiting signs of inadequate response, defined as augmentation, corticosteroid dependence, discontinuation, escalation, hospitalization, surgery, or switching after the first year on biologics and increasing to 85% over 2 years [94]. Patients who switched treatments were more likely to incur higher healthcare costs, use more healthcare services, and require hospitalization, often due to adverse events.
Findings from our review suggest that switching to more convenient routes of administration (e.g., intravenous to subcutaneous) is associated with lower healthcare costs and HCRU, specifically intravenous administration of infliximab compared with subcutaneous administration of adalimumab [56]. This finding is further substantiated by a recent study by Bergqvist et al., which examined patients switching from intravenously administered vedolizumab to subcutaneous administration, and found a 15% reduction in costs associated with the subcutaneous autoinjector, along with increased patient satisfaction and comparable efficacy and safety [95]. On the contrary, a study by Causey et al. found that self-administered subcutaneous injections were associated with reduced compliance, and in turn increased use of emergency or hospital services [96]. For most patients and physicians, ease of administration is an important factor in managing UC; nearly half of patients treated with biologic therapies (47%) prefer oral treatment over injectable [97].
Given the current unmet need in UC for more cost-effective treatments resulting in greater medication adherence with better long-term efficacy without dose escalation or concomitant therapies, several oral treatments have been recently approved. These therapies include the small-molecule drugs tofacitinib (2018), ozanimod (2021), and upadacitinib (2022). On the basis of indirect comparisons, these new treatments have demonstrated significant superiority over adalimumab in terms of endoscopic improvements and similar efficacy compared with the other biologics in their ability to induce remission [12, 98–101]. The approval of oral treatments options shows great promise with their ability to mitigate the increased costs and HCRU associated with other routes of administration. However, assessing the economic impact of these treatments within the context of their clinical efficacy is beyond the framework of this review. Additionally, the emergence of biosimilars also offers additional, less expensive treatment options to help manage the cost of treating UC. Biosimilars are also preferred by 30% of physicians in Europe as first-line therapy because they are more affordable to patients [93].
Furthermore, costs and HCRU associated with administration of treatment are important to consider in the context of the evolving treatment landscape with the emergence of oral therapies. However, administration and monitoring costs associated with biologics were rarely reported, representing a substantial gap in the literature, with only one USA study reporting costs for infliximab and adalimumab [56]. As adalimumab can be self-administered subcutaneously, administration costs were not reported. However, infliximab, which is administered intravenously—most often at outpatient clinics [30]—had a reported annual administration cost of $1634 per patient, on average [56]. Costs associated with patient monitoring after biologic administration (i.e., through blood panels and assays) were similar between adalimumab and infliximab ($21 vs. $42, respectively) [56].
Study Limitations
While one of the primary benefits of conducting a systematic literature review is to capture all available evidence, the searches performed for this review relied on accurate database indexing and clear descriptions of the study populations in the titles and abstracts of manuscripts. The searches were conducted in October 2021, and therefore did not capture recent, relevant studies, including the full manuscript of Bokemeyer et al. [94], which was published after completion of our review. Despite this limitation, more recently published evidence on the economic burden and HRQoL for patients with moderate-to-severe UC in the USA and Europe has been sparse, and is not anticipated to impact the overall conclusions of this review.
Overall, the outcomes reported were quite heterogeneous across studies, with a small subset of studies providing the most recent, robust, and comprehensive data on the cost and HCRU impact of biologics for the USA and Germany [50, 58, 69]. These studies were the main contributors to the descriptive trends, depicting the impact of initiation of biologic therapy. In general, data from other studies reporting a smaller set of outcomes were consistent with the findings of these studies. Whether the changes observed in medical and pharmacy costs and services after initiation of advanced therapies are statistically significant remains to be seen as no statistical comparisons were performed by the study authors.
Substantial variability was also observed in how outcomes were reported with respect to units, costing years, and/or follow-up duration, thus limiting our ability to synthesize the evidence in its entirety. Most studies reported annual costs or annualized rates of HCRU while others captured 3-month or 6-month data or costs and/or HCRU over the entire study period (i.e., up to 4 years). Furthermore, HCRU was often captured as a proportion of patients or a mean number of visits, but studies rarely reported both.
Most studies collected data from large claims databases, which do not fully characterize whether patients are truly naïve to biologics or if previous biologics of a different class have been received. Therefore, an assumption based on the enrollment criteria reported by the study was required, which typically was defined on the basis of the absence or presence of prescriptions for biologic agents within a particular timeframe (i.e., 6 to 12 months) before the index date. Moreover, the studies included here focused on the USA and Europe, which have advanced healthcare systems; hence, these findings may not be representative of other countries.
The reported evidence was also limited by small sample sizes for some studies or particular treatment groups within a study in which findings should be interpreted carefully. One-third of the studies reported results for fewer than 50 patients (range 3–48). More than half of these studies reported on biologic-treated patients as a subset of a larger study and one included groups of patients receiving more newly approved treatments (e.g., ustekinumab) or unapproved treatments (e.g., certolizumab and natalizumab). Thus, data specifically for certolizumab and natalizumab were omitted because of concerns around interpretability of findings based on their off-label use. Additionally, many (41.1%) studies were captured as conference abstracts with limited or inadequate information, so the results should be interpreted with caution.
Other limitations include the retrospective and observational nature of the studies included. Given the inconsistencies across studies, additional data on analyses by age, sex, cultural background, socioeconomic status, treatment access, detailed profiling of different therapies, and in-depth analyses on initiation, switching, and dose escalation of therapies are needed.
Conclusions
The introduction of advanced therapies has provided new and effective treatment options for patients with moderate-to-severe UC, but the impact of these treatments on economic burden and HRQoL is less understood. Findings from this systematic review suggest that the economic burden in patients with moderate-to-severe UC initiating treatment with biologics or small-molecule drugs was high and primarily driven by treatment costs and costs associated with outpatient visits. It also demonstrates the positive impact that biologic therapies have had on indirect costs (i.e., productivity, presenteeism, and absenteeism) as well as quality of life. However, this review also highlights that the high costs of biologics are not always fully offset by reductions in cost and HCRU associated with disease. Many patients require treatment switching and dose escalations, which are costly, in particular when switching across treatment classes or for patients initiating biologics. It is unclear whether reduced indirect costs and improved HRQoL would offset the high costs of biologics, especially in the long term. Moreover, the advantages of small-molecule drugs over biologics need to be further substantiated in terms of economic, social, and personal impact. Thus, there remains a high unmet need for therapies for moderate-to-severe UC that reduce healthcare burden and impact on society. As use of newer biologic therapies expands globally, high-quality, prospective real-world studies that evaluate short- and long-term economic burden and HRQoL are required.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
Development of this study, manuscript, and all associated publication costs, including the journal’s Rapid Service Fee, were funded by Bristol Myers Squibb. The sponsor was involved in design and conduct of the study; analysis and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. Funding for writing assistance was provided by Bristol Myers Squibb.
Medical Writing and/or Editorial Assistance
Writing assistance was provided by Russell Craddock, PhD, of Parexel International, and was funded by Bristol Myers Squibb.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Allie Cichewicz, Tom Tencer, Heather Burnett, Jinender Kumar. Data curation: Allie Cichewicz, Sonya Egodage. Formal analysis: Allie Cichewicz. Funding acquisition: Heather Burnett, Jinender Kumar. Investigation: Allie Cichewicz, Sonya Egodage, Heather Burnett, Jinender Kumar. Methodology: Allie Cichewicz, Sonya Egodage, Heather Burnett, Jinender Kumar. Project administration: Allie Cichewicz, Sonya Egodage, Jinender Kumar. Resources: Allie Cichewicz, Sonya Egodage, Jinender Kumar. Supervision: Allie Cichewicz, Heather Burnett, Jinender Kumar. Validation: Allie Cichewicz, Sonya Egodage, Heather Burnett, Jinender Kumar. Visualization: Allie Cichewicz, Jinender Kumar. Writing original draft: Allie Cichewicz, Sonya Egodage, Heather Burnett. Writing—review and editing: Allie Cichewicz, Tom Tencer, Komal Gupte-Singh, Sonya Egodage, Heather Burnett, Jinender Kumar.
Disclosures
Allie Cichewicz, Sonya Egodage, and Heather Burnett are employed by Evidera, a business of PPD, part of Thermo Fisher Scientific, which provides consulting and other research services to pharmaceutical, medical device, and related organizations. In their salaried positions, they work with a variety of companies and organizations, and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Komal Gupte-Singh and Jinender Kumar are employees of Bristol Myers Squibb. Tom Tencer was an employee of Bristol Myers Squibb at the time the study was conducted; currently an employee of Alnylam Pharmaceuticals. Evidera received funding from Bristol Myers Squibb to participate in the study and the development of this manuscript.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
References
- 1.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365(18):1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 2.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140(6):1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 3.Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 4.Burri E, Maillard MH, Schoepfer AM, et al. Treatment algorithm for mild and moderate-to-severe ulcerative colitis: an update. Digestion. 2020;101(suppl 1):2–15. doi: 10.1159/000504092. [DOI] [PubMed] [Google Scholar]
- 5.Truelove SC, Witts LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br Med J. 1954;2(4884):375–378. doi: 10.1136/bmj.2.4884.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13(3):273–284. doi: 10.1093/ecco-jcc/jjy114. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625–1629. doi: 10.1056/NEJM198712243172603. [DOI] [PubMed] [Google Scholar]
- 8.Feuerstein JD, Isaacs KL, Schneider Y, et al. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450–1461. doi: 10.1053/j.gastro.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubinsky MC. Reviewing treatments and outcomes in the evolving landscape of ulcerative colitis. Postgrad Med. 2017;129(5):538–553. doi: 10.1080/00325481.2017.1319730. [DOI] [PubMed] [Google Scholar]
- 10.Raine T, Bonovas S, Burisch J, et al. ECCO guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohns Colitis. 2022;16(1):2–17. doi: 10.1093/ecco-jcc/jjab178. [DOI] [PubMed] [Google Scholar]
- 11.Ooi CJ, Fock KM, Makharia GK, et al. The Asia-Pacific consensus on ulcerative colitis. J Gastroenterol Hepatol. 2010;25(3):453–468. doi: 10.1111/j.1440-1746.2010.06241.x. [DOI] [PubMed] [Google Scholar]
- 12.Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–170. doi: 10.1016/S2468-1253(21)00377-0. [DOI] [PubMed] [Google Scholar]
- 13.Armuzzi A, DiBonaventura MD, Tarallo M, et al. Treatment patterns among patients with moderate-to-severe ulcerative colitis in the United States and Europe. PLoS ONE. 2020;15(1):e0227914. doi: 10.1371/journal.pone.0227914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gemayel NC, Rizzello E, Atanasov P, Wirth D, Borsi A. Dose escalation and switching of biologics in ulcerative colitis: a systematic literature review in real-world evidence. Curr Med Res Opin. 2019;35(11):1911–1923. doi: 10.1080/03007995.2019.1631058. [DOI] [PubMed] [Google Scholar]
- 15.Crohn’s & Colitis Foundation. Fact sheet: News from the IBD Help Center - recently approved treatments. https://www.crohnscolitisfoundation.org/sites/default/files/legacy/assets/pdfs/recently-approved-treatments.pdf. Accessed April 10, 2022.
- 16.Segal JP, LeBlanc JF, Hart AL. Ulcerative colitis: an update. Clin Med (Lond) 2021;21(2):135–139. doi: 10.7861/clinmed.2021-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tazi J, Begon-Pescia C, Campos N, Apolit C, Garcel A, Scherrer D. Specific and selective induction of miR-124 in immune cells by the quinoline ABX464: a transformative therapy for inflammatory diseases. Drug Discov Today. 2021;26(4):1030–1039. doi: 10.1016/j.drudis.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Sandborn WJ, Peyrin-Biroulet L, Zhang J, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):550–561. doi: 10.1053/j.gastro.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 19.Vermeire S, Hebuterne X, Tilg H, et al. Induction and long-term follow-up with ABX464 for moderate-to-severe ulcerative colitis: results of phase IIa trial. Gastroenterology. 2021;160(7):2595–2598. doi: 10.1053/j.gastro.2021.02.054. [DOI] [PubMed] [Google Scholar]
- 20.Leber A, Hontecillas R, Zoccoli-Rodriguez V, et al. The safety, tolerability, and pharmacokinetics profile of BT-11, an oral, gut-restricted lanthionine synthetase C-like 2 agonist investigational new drug for inflammatory bowel disease: a randomized, double-blind, placebo-controlled phase I clinical trial. Inflamm Bowel Dis. 2020;26(4):643–652. doi: 10.1093/ibd/izz094. [DOI] [PubMed] [Google Scholar]
- 21.Danese S, Klopocka M, Scherl EJ, et al. Anti-TL1A antibody PF-06480605 safety and efficacy for ulcerative colitis: a phase 2a single-arm study. Clin Gastroenterol Hepatol. 2021;19(11):2324–2332. doi: 10.1016/j.cgh.2021.06.011. [DOI] [PubMed] [Google Scholar]
- 22.RINVOQ (upadacitinib) [summary of product characteristics]. AbbVie Deutschland GmbH & Co. KG: Ludwigshafen, Germany; December 2019.
- 23.RINVOQ (upadacitinib) extended-release tablets, for oral use. AbbVie Biotechnology, Ltd.: North Chicago, IL; April 2022.
- 24.XELJANZ (tofacitinib) [summary of product characteristics]. Pfizer Europe MA EEIG: Brussels, Belgium; March 2022.
- 25.XELJANZ (tofacitinib) oral solution. Pfizer, Inc.: New York, NY; December 2021.
- 26.ZEPOSIA (ozanimod) [summary of product characteristics]. Bristol Myers Squibb Pharma EEIG: Dublin, Ireland; December 2021.
- 27.ZEPOSIA (ozanimod) capsules, for oral use. Celgene Corporation, a member of the Bristol Myers Squibb group: Summit, NJ; April 2022.
- 28.Long GH, Tatro AR, Oh YS, Reddy SR, Ananthakrishnan AN. Analysis of safety, medical resource utilization, and treatment costs by drug class for management of inflammatory bowel disease in the United States based on insurance claims data. Adv Ther. 2019;36(11):3079–3095. doi: 10.1007/s12325-019-01095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long MD, Smith TW, Dibonaventura M, et al. Real-world effectiveness of advanced therapies among patients with moderate to severe ulcerative colitis in the United States. Inflamm Bowel Dis. 2020;26(6):941–948. doi: 10.1093/ibd/izz204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter CT, Leher H, Smith P, Smith DB, Waters HC. Impact of persistence with infliximab on hospitalizations in ulcerative colitis. Am J Manag Care. 2011;17(6):385–392. [PubMed] [Google Scholar]
- 31.Campbell-Hill S, Stein D, Soni M, et al. Real-world drug treatment costs for ulcerative colitis and Crohn’s disease patients treated with vedolizumab vs anti-TNFalpha: results from a German retrospective chart review study. J Crohns Colitis. 2018;12(suppl 1)(1):S504. [Google Scholar]
- 32.Armuzzi A, Marchi S, Gasbarrini A, et al. GO-CARE: a prospective multi-centre observational study of golimumab effectiveness and quality of life in a real-life UC patient population in Italy. J Crohns Colitis. 2018;12(suppl 1):S497. [Google Scholar]
- 33.Melsheimer R, Geldhof A, Apaolaza I, Schaible T. Remicade® (infliximab): 20 years of contributions to science and medicine. Biologics. 2019;13:139–178. doi: 10.2147/BTT.S207246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 35.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Public Policy Committee, International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf. 2016;25(1):2–10. [DOI] [PubMed]
- 38.Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed: The Cochrane Collaboration; 2019.
- 39.Teich N, Grummer H, Jorgensen E, et al. Golimumab improves work productivity in patients suffering from moderate to severe ulcerative colitis: results of a prospective study over 24 months. BMC Gastroenterol. 2021;21(1):161. doi: 10.1186/s12876-021-01747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Teich N, Grummer H, Jorgensen E, et al. Golimumab in real-world practice in patients with ulcerative colitis: twelve-month results. World J Gastroenterol. 2020;26(21):2852–2863. doi: 10.3748/wjg.v26.i21.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Valk ME, Mangen MJ, Severs M, et al. Comparison of costs and quality of life in ulcerative colitis patients with an ileal pouch-anal anastomosis, ileostomy and anti-TNFα therapy. J Crohns Colitis. 2015;9(11):1016–1023. doi: 10.1093/ecco-jcc/jjv134. [DOI] [PubMed] [Google Scholar]
- 42.Teich N, Gruemmer H, Joergensen E, et al. Golimumab in real-world practice in patients with ulcerative colitis: 2-year interim results from a non-interventional trial in Germany. J Crohns Colitis. 2019;13(suppl 1):S337–S338. doi: 10.1093/ecco-jcc/jjy222.577. [DOI] [Google Scholar]
- 43.van Gennep S, Sahami S, Buskens CJ, et al. Comparison of health-related quality of life and disability in ulcerative colitis patients following restorative proctocolectomy with ileal pouch-anal anastomosis versus anti-tumor necrosis factor therapy. Eur J Gastroenterol Hepatol. 2017;29(3):338–344. doi: 10.1097/MEG.0000000000000798. [DOI] [PubMed] [Google Scholar]
- 44.Kirchgesner J, Desai RJ, Schneeweiss MC, Beaugerie L, Kim SC, Schneeweiss S. Emulation of a randomized controlled trial in ulcerative colitis with US and French claims data: infliximab with thiopurines compared to infliximab monotherapy. Pharmacoepidemiol Drug Saf. 2021;08:08. doi: 10.1002/pds.5356. [DOI] [PubMed] [Google Scholar]
- 45.Bornheimer R, Hass S, Nag A, Oster G. Economic burden of biologic treatment in patients with inflammatory bowel disease in the US. Am J Gastroenterol. 2019;114(suppl):S433–S444. doi: 10.14309/01.ajg.0000592484.27433.a0. [DOI] [Google Scholar]
- 46.Borren NZ, Tan W, Colizzo FP, et al. Longitudinal trajectory of fatigue with initiation of biologic therapy in inflammatory bowel diseases: a prospective cohort study. J Crohns Colitis. 2020;14(3):309–315. doi: 10.1093/ecco-jcc/jjz148. [DOI] [PubMed] [Google Scholar]
- 47.Cai Q, Ding Z, Teeple A, Gowda P, Muser E. Costs of inflammatory bowel disease-related hospitalizations and surgeries: a retrospective analysis in a commercially insured population S0871. Am J Gastroenterol. 2020;115(suppl):S449. doi: 10.14309/01.ajg.0000705532.18543.be. [DOI] [Google Scholar]
- 48.Chapman JC, DiBonaventura MD, Comerford E, et al. Extraintestinal manifestations and their impact on real-world treatment patterns and outcomes among patients with ulcerative colitis. Gastroenterology. 2019;156(suppl 3):S47–S48. doi: 10.1053/j.gastro.2019.01.127. [DOI] [Google Scholar]
- 49.Chen J, Zhao Y, Liu T, Candela N, Lasch K. P477 Assessment of treatment sequence and real-world outcomes in patients with ulcerative colitis. J Crohns Colitis. 2021;15(suppl 1)(1):S466–S467. doi: 10.1093/ecco-jcc/jjab076.600. [DOI] [Google Scholar]
- 50.Chiorean M, Afzali A, Cross RK, et al. Economic outcomes of inflammatory bowel disease patients switching to a second anti-tumor necrosis factor or vedolizumab. Crohns Colitis. 2020;2(2):otaa031. doi: 10.1093/crocol/otaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cross RK, Naegeli AN, Harrison RW, et al. Disease burden and patient-reported outcome measures among ulcerative colitis patients according to therapy at enrollment into the Corrona IBD Registry. Gastroenterology. 2020;158(6 suppl 1):S725–S726. doi: 10.1016/S0016-5085(20)32493-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunter T, Farrar M, Dong Y, Choong C, Naegeli AN. Medication use and healthcare resource utilization trends among adult ulcerative colitis patients in the United States: 2007–2017. Value Health. 2019;22(suppl 2):S180. doi: 10.1016/j.jval.2019.04.784. [DOI] [Google Scholar]
- 53.Kochar B, Winn A, Barnes EL, Long MD, Kappelman M. The national experience with vedolizumab. Gastroenterology. 2018;154(6 suppl 1):S67–S68. doi: 10.1016/S0016-5085(18)30682-6. [DOI] [Google Scholar]
- 54.Naegeli A, Hunter T, Dong Y, Choong C, Crandall W. Impact of biologic treatment of ulcerative colitis on healthcare resource utilization in US patients. United Eur Gastroenterol J. 2019;7(suppl 8):61. [Google Scholar]
- 55.Nguyen J, Xia Q, Sharma N, Kapoor G, Ahmad H, Zhuo J. Trends in inflammatory bowel disease (IBD) related hospitalizations, emergency department visits, and intestinal resection among patients with IBD in the era of biologics. Gastroenterology. 2020;158(6 suppl 1):S731. doi: 10.1016/S0016-5085(20)32507-5. [DOI] [Google Scholar]
- 56.Null KD, Xu Y, Pasquale MK, et al. Ulcerative colitis treatment patterns and cost of care. Value Health. 2017;20(6):752–761. doi: 10.1016/j.jval.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Patel H, Khalid JM, Shah S, Shah R, Berger A. Early use of vedolizumab vs infliximab in biologic-naive patients with ulcerative colitis: a real-world analysis of healthcare utilisation. J Crohns Colitis. 2018;12(suppl 1)(1):S385–S386. doi: 10.1093/ecco-jcc/jjx180.679. [DOI] [Google Scholar]
- 58.Perera S, Yang S, Stott-Miller M, Brady J. Analysis of healthcare resource utilization and costs after the initiation of biologic treatment in patients with ulcerative colitis and Crohn's disease. J Health Econ Outcomes Res. 2018;6(1):96–112. doi: 10.36469/9791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pilon D, Ding Z, Muser E, et al. Long-term direct and indirect costs of ulcerative colitis in a privately-insured United States population. Curr Med Res Opin. 2020;36(8):1285–1294. doi: 10.1080/03007995.2020.1771293. [DOI] [PubMed] [Google Scholar]
- 60.Rubin DT, Griffith J, Zhang Q, Hepp Z, Keshishian A. The impact of intestinal complications on health care costs among patients with inflammatory bowel disease treated with anti-tumor necrosis factor therapies. Inflamm Bowel Dis. 2020;27:2. doi: 10.1093/ibd/izaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stewart MJ, Bessissow T, Gregor J, et al. Subcutaneously administered anti-TNFs for the treatment of ulcerative colitis: a retrospective, propensity score-matched, US health claims analysis. Adv Ther. 2021;38(7):4115–4129. doi: 10.1007/s12325-021-01818-3. [DOI] [PubMed] [Google Scholar]
- 62.Wolf DC, Puckett J, Setyawan J, Rabbat C, Sloan S, Kamal-Bahl S. Real-world treatment patterns and healthcare costs among US commercially insured adults initiating biologics for ulcerative colitis. United Eur Gastroenterol J. 2021;9(suppl 8):519–520. [Google Scholar]
- 63.Soriano A, Marchi S, Benedetti A, et al. Real-life evaluation of golimumab effectiveness and quality of life in patients with moderate-to-severe ulcerative colitis: the prospective Go-Care study. United Eur Gastroenterol J. 2021;9(suppl 8):504–505. [Google Scholar]
- 64.Bamias G, Kokkotis G, Gizis M, et al. Predictors of response to vedolizumab in patients with ulcerative colitis: results from the Greek VEDO-IBD cohort. Dig Dis Sci. 2021;67(3):1007–1017. doi: 10.1007/s10620-021-06907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bamias G, Xourafas V, Tsoukali E, et al. Treatment outcomes following administration of vedolizumab in patients with moderate to severe ulcerative colitis. United Eur Gastroenterol J. 2019;7(suppl 8):642. [Google Scholar]
- 66.Black CM, Yu E, McCann E, Kachroo S. Dose escalation and healthcare resource use among ulcerative colitis patients treated with adalimumab in English hospitals: an analysis of real-world data. PLoS ONE. 2016;11(2):e0149692. doi: 10.1371/journal.pone.0149692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casellas F, Robles V, Borruel N, et al. Restoration of quality of life of patients with inflammatory bowel disease after one year with antiTNFα treatment. J Crohns Colitis. 2012;6(9):881–886. doi: 10.1016/j.crohns.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 68.Desmond AN, Shanahan F. Managing chronic disease in Ireland: hospital admission rates and clinical outcomes in a large ulcerative colitis population. Ir J Med Sci. 2012;181(1):65–71. doi: 10.1007/s11845-011-0760-y. [DOI] [PubMed] [Google Scholar]
- 69.Dignass A, Waller J, Cappelleri JC, et al. Living with ulcerative colitis in Germany: a retrospective analysis of dose escalation, concomitant treatment use and healthcare costs. J Med Econ. 2020;23(4):415–427. doi: 10.1080/13696998.2019.1707210. [DOI] [PubMed] [Google Scholar]
- 70.Dignass A, Waller J, Cappelleri JC, et al. P746 Living with ulcerative colitis in Germany: quantifying the socioeconomic impact of moderate to severe ulcerative colitis. J Crohns Colitis. 2019;13(suppl 1):S495. doi: 10.1093/ecco-jcc/jjy222.870. [DOI] [Google Scholar]
- 71.Dignass A, Waller J, Cappelleri JC, et al. P782 Living with ulcerative colitis in Germany: quantifying the healthcare resource utilisation and direct healthcare costs associated with the treatment of moderate to severe ulcerative colitis in Germany. J Crohns Colitis. 2019;13(suppl 1):S512. doi: 10.1093/ecco-jcc/jjy222.906. [DOI] [Google Scholar]
- 72.Eriksson C, Rundquist S, Lykiardopoulos V, et al. Real-world effectiveness of vedolizumab in inflammatory bowel disease: week 52 results from the Swedish prospective multicentre SVEAH study. Therap Adv Gastroenterol. 2021;14:17562848211023386. doi: 10.1177/17562848211023386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gatopoulou A, Christodoulou DK, Katsanos KH, et al. Effect of golimumab on health-related quality of life, other patient-reported outcomes and healthcare resource utilization in patients with moderate-to-severe ulcerative colitis: a real-world multicenter, noninterventional, observational study in Greece. Eur J Gastroenterol Hepatol. 2021;21:21. doi: 10.1097/MEG.0000000000002182. [DOI] [PubMed] [Google Scholar]
- 74.Khalili H, Everhov AH, Halfvarson J, et al. Healthcare use, work loss and total costs in incident and prevalent Crohn's disease and ulcerative colitis: results from a nationwide study in Sweden. Aliment Pharmacol Ther. 2020;52(4):655–668. doi: 10.1111/apt.15889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lawton J, Achit H, Pouillon L, et al. Cost-of-illness of inflammatory bowel disease patients treated with anti-tumour necrosis factor: a French large single-centre experience. United Eur Gastroenterol J. 2019;7(7):908–913. doi: 10.1177/2050640619853448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lo B, Vind I, Vester-Andersen MK, Bendtsen F, Burisch J. Direct and indirect costs of inflammatory bowel disease: ten years of follow-up in a Danish population-based inception cohort. J Crohns Colitis. 2020;14(1):53–63. doi: 10.1093/ecco-jcc/jjz096. [DOI] [PubMed] [Google Scholar]
- 77.Lowenberg M, Duijvis NW, Ponsioen C, van den Brink GR, Fockens P, D'Haens GR. Length of hospital stay and associated hospital costs with infliximab versus cyclosporine in severe ulcerative colitis. Eur J Gastroenterol Hepatol. 2014;26(11):1240–1246. doi: 10.1097/MEG.0000000000000187. [DOI] [PubMed] [Google Scholar]
- 78.Mandel MD, Balint A, Golovics PA, et al. Decreasing trends in hospitalizations during anti-TNF therapy are associated with time to anti-TNF therapy: results from two referral centres. Dig Liver Dis. 2014;46(11):985–990. doi: 10.1016/j.dld.2014.07.168. [DOI] [PubMed] [Google Scholar]
- 79.Mantzaris G, Gatopoulou A, Christodoulou D, et al. A real-world assessment of golimumab effect on quality of life, healthcare resource utilisation and work productivity in patients with ulcerative colitis in Greece: interim results from the GOLIFE study. J Crohns Colitis. 2019;13(suppl 1):S422. doi: 10.1093/ecco-jcc/jjy222.735. [DOI] [Google Scholar]
- 80.Oussalah A, Evesque L, Laharie D, et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol. 2010;105(12):2617–2625. doi: 10.1038/ajg.2010.345. [DOI] [PubMed] [Google Scholar]
- 81.Picker N, Bokemeyer B, Wilke T, Rosin L, Patel H. P404 Healthcare utilization and expenditures for patients with ulcerative colitis on advanced therapies in Germany. J Crohns Colitis. 2021;15(suppl 1):S411–S412. doi: 10.1093/ecco-jcc/jjab076.528. [DOI] [Google Scholar]
- 82.Picker N, Patel H, Wilke T, Rosin L, Bokemeyer B. P423 Treatment escalation and associated cost in German ulcerative colitis patients treated with advanced therapies P423. J Crohns Colitis. 2021;15(suppl 1):S427. doi: 10.1093/ecco-jcc/jjab076.547. [DOI] [Google Scholar]
- 83.Bokemeyer B, Picker N, Wilke T, Rosin L, Patel H. P464 Steroid dependency in patients with ulcerative colitis treated with advanced therapies in a German real-world-setting. J Crohns Colitis. 2021;15(1):S455–S456. doi: 10.1093/ecco-jcc/jjab076.587. [DOI] [Google Scholar]
- 84.Pöllinger B, Schmidt W, Seiffert A, Imhoff H, Emmert M. Costs of dose escalation among ulcerative colitis patients treated with adalimumab in Germany. Eur J Health Econ. 2019;20(2):195–203. doi: 10.1007/s10198-017-0953-z. [DOI] [PubMed] [Google Scholar]
- 85.Sebastian S, Myers S, Argyriou K, et al. Infliximab induction regimens in steroid-refractory acute severe colitis: a multicentre retrospective cohort study with propensity score analysis. Aliment Pharmacol Ther. 2019;50(6):675–683. doi: 10.1111/apt.15456. [DOI] [PubMed] [Google Scholar]
- 86.Singh S, Andersen NN, Andersson M, Loftus EV, Jr, Jess T. comparison of infliximab and adalimumab in biologic-naive patients with ulcerative colitis: a nationwide Danish cohort study. Clin Gastroenterol Hepatol. 2017;15(8):1218–1225. doi: 10.1016/j.cgh.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilke T, Groth A, Long GH, Tatro AR, Sun D. Rate of adverse events and associated health care costs for the management of inflammatory bowel disease in Germany. Clin Ther. 2020;42(1):130–143. doi: 10.1016/j.clinthera.2019.11.012. [DOI] [PubMed] [Google Scholar]
- 88.Ylisaukko-Oja T, Torvinen S, Ventola H, et al. Healthcare resource utilization and treatment costs of Finnish chronic inflammatory bowel disease patients treated with infliximab. Scand J Gastroenterol. 2019;54(6):726–732. doi: 10.1080/00365521.2019.1627579. [DOI] [PubMed] [Google Scholar]
- 89.Torvinen S, Ventola H, Herrala S, Schmidt S, Ylisaukko-oja T, Voutilainen M. The economic burden of chronic inflammatory bowel disease patients in Finland. Value Health. 2018;21(suppl 3):S144. doi: 10.1016/j.jval.2018.09.858. [DOI] [Google Scholar]
- 90.van Linschoten RCA, Visser E, Niehot CD, et al. Systematic review: societal cost of illness of inflammatory bowel disease is increasing due to biologics and varies between continents. Aliment Pharmacol Ther. 2021;54(3):234–248. doi: 10.1111/apt.16445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fonia A, Jackson K, Lereun C, Grant DM, Barker JN, Smith CH. A retrospective cohort study of the impact of biologic therapy initiation on medical resource use and costs in patients with moderate to severe psoriasis. Br J Dermatol. 2010;163(4):807–816. doi: 10.1111/j.1365-2133.2010.09944.x. [DOI] [PubMed] [Google Scholar]
- 92.Svedbom A, Dahlen J, Mamolo C, et al. Economic burden of psoriasis and potential cost offsets with biologic treatment: a Swedish register analysis. Acta Derm Venereol. 2016;96(5):651–657. doi: 10.2340/00015555-2329. [DOI] [PubMed] [Google Scholar]
- 93.Huynh L, Hass S, Peyrin-Biroulet L, et al. Real-world treatment patterns and physician preferences for biologics in moderate-to-severe inflammatory bowel disease: retrospective chart review in Europe. Crohns Colitis. 2022;4(1):otac001. doi: 10.1093/crocol/otac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bokemeyer B, Picker N, Wilke T, Rosin L, Patel H. Inadequate response, treatment patterns, health care utilization, and associated costs in patients with ulcerative colitis: retrospective cohort study based on German claims data. Inflamm Bowel Dis. 2022;28(11):1647–57. [DOI] [PMC free article] [PubMed]
- 95.Bergqvist V, Holmgren J, Klintman D, Marsal J. Real-world data on switching from intravenous to subcutaneous vedolizumab treatment in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2022;55(11):1389–1401. doi: 10.1111/apt.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Causey E, Shah N, Slaughter C, et al. Non-adherence to self-injectable biologic therapy in IBD increases ED visits and hospitalizations S834. Am J Gastroenterol. 2021;116:386–7.
- 97.Choi J, Lukanova R, Ahmad H, et al. S3307 Unmet need of an oral treatment in moderate to severe ulcerative colitis: findings from international patient and physician survey. Am J Gastroenterol. 2021;116:S1363.
- 98.Burr NE, Gracie DJ, Black CJ, Ford AC. Efficacy of biological therapies and small molecules in moderate to severe ulcerative colitis: systematic review and network meta-analysis. Gut. 2021 doi: 10.1136/gutjnl-2021-326390. [DOI] [PubMed] [Google Scholar]
- 99.Dubinsky MC, Betts KA, LaPensee K, et al. S694 Comparative efficacy and safety of ozanimod vs adalimumab and vedolizumab in patients with moderately to severely active ulcerative colitis. Am J Gastroenterol. 2021;116(suppl):S314. doi: 10.14309/01.ajg.0000776308.71765.85. [DOI] [Google Scholar]
- 100.Eaton K, Duperrouzel C, Bhandari P, et al. POSB17 Ozanimod for the treatment of biologic-experienced patients with moderate-to-severe ulcerative colitis: results from a systematic literature review and network meta-analyses. Value Health. 2022;25(1):S27–S28. doi: 10.1016/j.jval.2021.11.127. [DOI] [Google Scholar]
- 101.Eaton K, Duperrouzel C, Bhandari P, et al. Ozanimod for induction treatment of moderate-to-severe ulcerative colitis: results from a systematic literature review and network meta-analyses. Presented at 16th Congress of the European Crohn’s and Colitis Organisation, July 2–3 and 8–10, 2021:Abstract DOP69.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.