Abstract
Sulfur is a vital element that all living things require, being a component of proteins and other bio-organic substances. The various kinds and varieties of microbes in nature allow for the transformation of this element. It also should be emphasized that volatile sulfur compounds are typically present in food in trace amounts. Life cannot exist without sulfur, yet it also poses a potential health risk. The colon’s sulfur metabolism, which is managed by eukaryotic cells, is much better understood than the S metabolism in gastrointestinal bacteria. Numerous additional microbial processes are anticipated to have an impact on the content and availability of sulfated compounds, as well as intestinal S metabolism. Hydrogen sulfide is the sulfur derivative that has attracted the most attention in relation to colonic health, but it is still unclear whether it is beneficial or harmful. Several lines of evidence suggest that sulfate-reducing bacteria or exogenous hydrogen sulfide may be the root cause of intestinal ailments, including inflammatory bowel diseases and colon cancer. Taurine serves a variety of biological and physiological purposes, including roles in inflammation and protection, additionally, low levels of taurine can be found in bodily fluids, and taurine is the primary sulfur component present in muscle tissue (serum and urine). The aim of this scoping review was to compile data from the most pertinent scientific works about S compounds' existence in food and their metabolic processes. The importance of S compounds in various food products and how these compounds can impact metabolic processes are both stressed in this paper.
Keywords: Sulfur metabolism, Sulfur assimilation, Gut microbiome, Sulfate-reducing bacteria, Hydrogen sulfide, Toxicity
1. Introduction
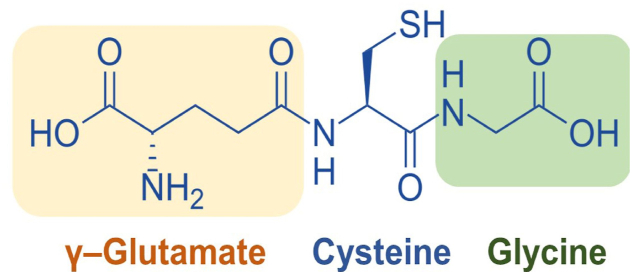
Sulfur (S) is an important element and necessary for all living organisms. It is included in bio-organic compounds, such as proteins (in the form of incorporated amino acids, e.g., cysteine and methionine) [1,2], antibiotics and antimicrobial compounds (e.g., penicillins, sulfonamides, allicin) [[3], [4], [5]], thioethers, thiols or mercaptans, sulfonium ions, sulfoxides and sulfones, dimethylsulfoniopropionate, dimethyl sulfoxide (a common solvent), sulfolane (a common sulfone), and glutathione (preventing damage to important cellular components [6,7], protection against free radicals, peroxides and heavy metals) (Fig. 1) [8]. S is involved in the metabolism of living organisms in oxidized forms (sulfate, sulfite, thiosulfate, etc.) and reduced forms (S2−, HS−, and hydrogen-sulfide [H2S]). S may exist in the following oxidation states: −2, −1, 0, +1, +2, +3, +4, +5, +6 [9].
Fig. 1.
The chemical structure glutathione (GSH).
S may be transformed in nature by different types and groups of microorganisms [[10], [11], [12], [13], [14], [15]]. This process may include the following steps: a) organic S compounds may be oxidized/transformed to sulfite and consequently to sulfate, which can be immobilized or returned into organic S compounds; b) organic S compounds may be decomposed by the special group of microorganisms which can produce H2S (which may be oxidized to SO, SO32− and SO42−); c) H2S may be also formed in the process of dissimilatory sulfate reduction [12,[16], [17], [18], [19], [20], [21], [22], [23]]. Elemental S may also be reduced to H2S by sulfur reducing microorganisms (i.e., the genera Desulfuromonas and Desulforella). The same final product, H2S, may be produced by sulfate-reducing bacteria (SRB), including the genera Desulfovibrio, Desulfotomaculum, Desulfomicrobium and many other genera of this physiological group [[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]].
Many food commodities and foodstuffs contain S compounds that can further influence the gut microbiota, as these compounds are degraded in the stomach. The important physical factor in the release of S from complex organic compounds is the pH levels of the stomach (pH = 1–3) environment, and its poor microbiome (101–103 CFU/mL) due to its extreme conditions. In this microenvironment, S compounds from food may be degraded by acidophilic species of Lactobacillus, Streptococcus, Staphylococcus and some members of the Enterobacterales order [[33], [34], [35]].
Food generally contains trace levels of volatile sulfur compounds (VSCs); these substances contribute to both good and bad flavors in food, and they have a potent odor [36]. To emphaize its role, S is the third most prevalent mineral in the human body and the sixth most abundant macromineral in breast milk [37]. On the other hand, the food industry, especially the beverage sector, uses sulfating agents or sulfur (IV) oxoion compounds as preservatives on a large scale. Additionally, sulfite, bisulfite, and metabisulfite serve as enzyme inhibitors, oxygen scavengers, bleaching agents, antibacterial agents, and more [38].
S is both necessary for life and is a potential health risk. The metabolism of S by gastrointestinal microorganisms is less understood than colonic S metabolism, which is handled by eukaryotic cells. Sulfated substances in the colon can either be organic (such as host mucins and dietary amino acids) or inorganic (such as sulfates and sulfites) in origin. Sulfate-reducing bacteria (SRB), which are widespread colonic inhabitants, have been the most thoroughly investigated of the microorganisms involved in colonic S metabolism [32,[39], [40], [41], [42]]. The content and availability of sulfated substances, as well as intestinal sulfur metabolism, are expected to be influenced by numerous additional microbial processes. The S derivative that has garnered the most interest in relation to colonic health is H2S, but it is still unclear whether it is harmful or advantageous. SRBs or exogenous H2S may be an important factor in developing intestinal illnesses, in particular inflammatory bowel diseases (IBDs) and colon cancer, according to different levels of evidence [12,14,16,17,22,24,35,43]. Due to this, it is important to obtain and collect new information about the biological processes related to the transformation of S.
The aim of the comprehensive review was to collect information evidencing the most relevant previous publications about the presence of S compounds in food and their metabolic pathways. The work aims to emphasize the importance of S compounds in different food commodities and how these compounds may affect metabolic processes.
2. Search strategy and methodology
The scientific literature published in the English language was used to compile the present scoping review. Sources that were used to collect the target articles were the Web of Science (WoS), Scopus, PubMed and Google Scholar databases. Keywords used in WoS, Scopus and Google Scholar consisted of sulfur compounds in food commodities and the metabolism of found sulfur compounds in food; i.e. the following keywords used: “sulfate in food”, “sulfur dioxide”, “sulfate-reducing bacteria”, “sulfur metabolism”, “sulfur sugars”, “carrageenans metabolism”; the names of the articles that were discovered served as the basis for manually preselecting the search results. Articles used in this review were published between 1943.01.01. and 2022.12.31.
3. Sulfur-containing compounds
S belongs to the same group (IV) in the periodic table of elements together with oxygen (O2), selenium (Se), tellurium (Te), which results in a similar behavior of their organic derivatives [9]. For example, water, H2S, hydrogen selenide (H2Se), and hydrogen tellurium (H2Te) are structurally related compounds. Water has no smell, while hydrogen sulfide has an unpleasant smell, this property is also intense in the case of H2Se and H2Te. Another interesting group of compounds is methanol, methanethiol and methaneselenol. Methanol has virtually no odor, while methanethiol has a strong and unpleasant odor. Methaneselenol has the most pungent foul odor [36]. Many VSCs are produced during food processing and storage. Processing may include heating, smoking, cooking, frying, roasting, irradiation and enzymatic processes involved in flavor formation. Exposure to light and ionizing radiation may also cause flavor changes during processing and storage. The following reactions may occur during heat processing: oxidation, degradation, hydrolysis, dehydration, condensation, decarboxylation, among others. In this context, it is worth noting the important role of the Maillard reaction and Strecker degradation in the formation of aromas of many processed foods [36]. A survey of sulfur-containing volatiles in food shows that they contain one of the functional groups such as thiols, sulfides, sulfoxides, sulfones, thiocyanases, thiosulfinates, thiazoles and thiolanes [44].
Thiolates (HS–C group) may act as metal ligands, as bases in enzymatic reactions (protease) and in group transfer reactions (coenzyme A [CoA] transfer); they also forms the basis of disulfide bridges and control redox potential (glutathione). The sulfide (CS2–C group), on the other hand, acts as a redox group (lipoic acid) and a metal ligand. A thioester (H3C–SC group), such as methionine, acts as a methyl donor, a ligand in enzymes and the formation of various vitamins, e.g., thiamin and biotin. Sulfate-group (CO–SO3–group) and sulfonate group (C–SO3–) change in the feeding behavior of nature, for example lipid solubility [45]. Table 1 [45], summarizes the sulfur-containing compounds present in biological matrices, in addition to the ubiquitous sulfate ions.
Table 1.
Basic structure of sulfur-containing groups and their occurrence (data from Raab and Feldmann, 2019) [45].
| Sulfur-containing compounds | Present in |
|---|---|
| HS–CH2–R | cystine |
| H3C–S–CH2–R | methionine |
| R–S–S–R | disulfide (e.g. cystine, a relative of allin) |
| HOS–R | sulfenic acid (post-translational modification of cysteine in proteins) |
| HO2S–R | sulfinic acid (the modification of cysteine in proteins) |
| HO3–S–CH2–R | in fat |
| HO3–S–O–CH2–R | in fat |
The major S-containing amino acids are methionine and cysteine, which are present in most proteins [46]. Methionine itself is essential for humans, while cysteine can be synthesized in a five-step process from methionine. Cysteine exhibits redox activity and often binds thiophilic metal ions in the catalytic center of proteins. It is also often involved in stabilizing the three-dimensional (3D) structure of proteins by forming disulfide bridges [47]. There are very few carbohydrates that contain S. Among the most common ones are carrageenans, which are linear sulfated polysaccharides in red seaweed, which is an important raw material and additive in the food and pharmaceutical industries [48]. The addition of S compounds has the effect of directly forming polyfunctional mercaptans from their precursors. Additives of S compounds therefore not only affect the metabolism of polyfunctional mercaptan precursors, but also create aroma. Regarding the effect of S compounds on the consumption of polyfunctional mercaptan precursors, yeast could absorb glutathione-3-mercaptohexanol (GLUMH) to obtain GSH, cysteine and/or S present in this precursor. In addition, according to these results, glutathione-4-mercapto-4-methyl-2-pentanone (GLUMP) is transformed into CYSM [49].
4. Sulfur compounds in food
VSCs are generally present in food in trace amounts; these compounds have a strong odor and contribute to both pleasant and unpleasant flavors in foods [36]. Amino acids containing S are methionine, cysteine, cystine, homocysteine, homocysteine and taurine [37]. For a long time, scientists have been studying S compounds present in various types of food. Cooked foods usually contain numerous S compounds, especially heterocyclic compounds such as thiazoles, thiophenes and thiazolines. Other cooked foods such as bread, potato products, nuts, popcorn, cheese and coffee also contain many S compounds. Aliphatic thiols have been found in fruits, vegetables and dairy products. It has been reported that more than half of the volatile compounds in garlic, onions, leeks and chives contain S [50]. The use of sulfating agents or sulfur (IV) oxoion compounds as preservatives is extensive in the food industry, including the beverage industry. Sulfite, bisulfite, and metabisulfite have many functions such as bleaching agents, antimicrobial agents, oxygen scavengers, reducing agents, and enzyme inhibitors [38]. VSCs are important components of food flavors. Sulfides represent a major chemical class with more than 440 compounds, of which the disulfide group alone represents about 100 different molecules [38].
Probiotic strains (L. acidophilus NCFM, L. plantarum 299v, L. rhamnosus GG, L. reuteri ATCC55730 and L. reuteri BR11), L. delbrueckii ATCC4797, L. plantarum ATCC14917 and Lactococcus lactis MG1363 were found to produce the following VSCs: H2S, methanethiol, dimethyldisulfide and dimethyltrisulfide. L. reuteri ATCC55730 and L. lactis MG1363 were discovered to produce the lowest amount of VSCs. Numerous probiotic strains have the capacity to produce high quantities of VSCs, and various strains of the same species have significantly varying capacities for VSC production [51]. To regulate oxidation processes and limit the growth of native yeast and bacteria, primarily acetic acid bacteria, SO2 must be introduced to the must during the wine production [51,52].
5. Determination of sulfur compounds in food
The small amount of S compounds in many foods makes their analysis and quantification challenging. Extraction without further fractionation will not result in a sufficiently high concentration in many cases, thus gas chromatography with mass spectrometry (GC/MS) is mainly used in practice. The most commonly used detectors are flame photometric detectors (FPD) and chemiluminescence detectors (SCD) [50]. Sulfate determination may also be performed by ion chromatography with conductivity detection [53]. A study by Restaino et al. [54] used high-performance capillary electrophoresis (HPCE) to determine keratan sulfate and hyaluronic acid. They used an elemental analyzer (EA) attached to a 34S/32S isotope ratio mass spectrometer (IRMS) to determine sulfites in food as a sulfur isotope ratio [55]. An internationally recognized method for determining SO2 in wine is the Monier-Williams method, which consists of the extraction of SO2 in the gas phase from wine samples and subsequent acid-base titration [56]. Another established method is the iodometric Ripper method [57].
5.1. Occurrence of sulfur in selected food commodities
5.1.1. Herbs, spices and seasonings
The Allium genus includes garlic, onions, leeks and chives, which contain a variety of S compounds. More than 50% of VSCs have been isolated from these foodstuffs [58]. These compounds include diallyl disulfide and the corresponding thiosulfinate derivative (allicin), which are enzymatically released from the sulfoxide flavoring agent (alliin) when garlic cloves are crushed [58]. Block et al. [59] found that Allium plants, when cut or crushed, release alliinase enzymes that convert sulfoxide precursors to sulfenic acid intermediates, which then condense to form aromatic thiosulfinates [59].
5.1.2. Fruits
Global production and demand for tropical fruits is increasing every year as consumers are drawn to the exotic flavors and potential health benefits of these fruits. VSCs are often responsible for the juicy, fresh aroma of tropical fruits [60]. Dead fruits such as watermelon, durian, strawberries, pineapple, kiwi fruit, yellow passion fruit, grapefruit and pears contain VSCs. Up to 16 VSCs have been reported in strawberries. Volatile strawberry sulfides include H2S, methanethiol, dimethyl sulfide, dimethyl disulfide, and dimethyl trisulfide. Strawberry thioesters include methyl thioacetate, methyl thiopropionate, methyl thiobutyrate, ethyl thiobutanoate, methyl thiohexanoate, methyl (methylthio) acetate, and others. VSCs, such as methanethiol, dimethyldisulfide, dimethylsulfide, methylthioacetate and methylthiobutanoate are important components for distinguishing between “older'” European strawberry cultivars [61,62].
5.1.3. Vegetables
In plants, such as garlic and onions, organic sulfur compounds are produced to protect against bacterial, fungal and animal attacks. Microorganisms are usually killed by these compounds and animals that eat these plants avoid them in the future. These S compounds are formed enzymatically from S-containing amino acids and from alk (en)yl-l-cysteine sulfoxides. Enzyminase is contained in a clove of garlic in large quantities [63,64]. The genus Allium includes not only onions and garlic but many other vegetables such as shallots, leeks and chives. The characteristic smell and taste of this vegetable is (at least partly) attributed to S-containing compounds. S actually makes up about 1% of the dry weight of garlic and up to 0.5% of the dry weight of onions. These S-containing compounds in garlic and onion are largely derived from the precursors gamma-glutamyl-S-alk (en)yl-l-cysteines and S-alk (en)yl-l-cysteine sulfoxides (ASCO) [65].
5.1.4. Pastry
Both volatile and non-volatile compounds in bread contribute to its flavor. These compounds include acids, alcohols, aldehydes, esters, ethers, furans, hydrocarbons, ketones, lactones, pyrazines, pyrroles and last but not least, S compounds [[66], [67], [68]]. There were 44 identified sulfur-containing compounds involved in bread aroma. Methionine and cysteine are precursors of various aliphatic S compounds. Heterocyclic S compounds, such as thiophenes are formed during reactions of aldehydes with H2S or with mercaptic aldehyde (the degradation product of cysteine by the Strecker reaction). Examples of these compounds identified in bread aroma are 5-methyl-4,5-dihydro-3-[2H]-thiophenone and thiophenones. Thiazoles represent another chemical class whose precursors appear to be cysteinamine. Strecker degradation plays a dominant role in the formation of thiazoles. Of particular interest are furans, which are easily substituted with S groups. A number of these types of compounds may be formed by reactions of furfural or pentose with H2S [69,70].
5.1.5. Meat and seafood
The characteristic flavor of cooked meat comes from the thermal reactions occurring during heating, especially the Maillard reaction, lipid oxidation, the interaction between the products of the Maillard reaction and the products of lipid oxidation, as well as the degradation of thiamine. These reactions include processes leading to the formation of a wide variety of products that represent a large number of volatile compounds. Heterocyclic compounds, especially those containing S, are important aromatic compounds produced by the Maillard reaction, which are responsible for the meaty, roasted, cooked flavor of meat [71,72].
5.1.6. Dairy products
VSCs have been found to be responsible for S off-flavors produced during heat treatment of milk. When examining raw, pasteurized and ultra high temperature treated (UHT) milk, significantly higher concentrations of hydrogen sulfide (H2S), methanethiol, carbon disulfide, dimethyltrisulfide, and dimethylsulfoxide was detected in UHT milk [73]. Sulfuric compounds are also present in yogurts and mature cheeses. In the case of ripening cheeses, structurally diverse classes of molecules have been shown to be responsible for the S flavor, providing a variety of characteristic aromatic notes. This includes cheeses such as Limburger, Camembert, but also Cheddar. Many S flavors in cheese are associated with the formation of methanethiol, for which not only bacteria but also yeast are responsible. These bacteria include the genera Lactobacillus, Lactococcus, and Brevibacterium linens. Of the yeasts, Geotrichum candidum stands out. Methyl sulfoxide is generally believed to be the result from degradation of l-methionine catalyzed by intracellular l-methionine gamma-lyase [74].
5.1.7. Wine
Wine is a hydroalcoholic solution containing hundreds of compounds that originate from grapes or are formed during winemaking and storage. Some of these compounds affect the aroma of the wine. These compounds determine the quality of the wine and are characteristic of each wine variety. S compounds contained in wines are divided into five different groups according to their structure: thiols, sulfides, polysulfides, thioesters, and heterocyclic compounds. However, in the literature, another type of division may be encountered, which is according to their volatility: with a boiling point below 90 °C (volatile compounds) and above 90 °C (less volatile compounds) [75]. Sulfites play an integral role in the winemaking process. These additives are used to sterilize barrels or tanks prior to fermentation and are added to grape must and wine at various stages of production, to control the growth of undesirable types of yeast and bacteria that cause spoilage. Sulfites are also present in the final bottled wine and prevent oxidation and prevent oxidation and help maintain the desirable sensory characteristics of the wine during its production, transport and marketing [76].
Health Canada cites a maximum amount of sulfites that should be present in specific foods as being at 5000 ppm [77]. The Joint Food and Agriculture Organization/World Health Organization (FAO/WHO) Expert Committee on Food Additives (JECFA) developed an acceptable daily intake (ADI) for sulfites in 1974 (set at 0.7 mg/kg body weight, expressed as sulfur dioxide, or 42 mg for a 60 kg adult), which was also endorsed by the Scientific Committee for Food (SCF) in 1994. Foods must be labeled appropriately – according to the Food and Drug Administration (FDA) – if they contain sulfites at levels higher than 10 ppm [78]. Sulfite levels in red and white wine are usually at the mean concentrations 70 mg/L to 122 mg/L. Accordingly, consuming two glasses (450 mL) of wine per day for a person weighing 60 kg is equivalent to consuming 75–130% of the ADI for sulfites. An increased sulfite intake, that is typical for the western diet, may result in an average total dietary exposure to sulfites for adults and children that is 294% of the ADI [79].
6. Sulfur dioxide as an allergen
Sulfur dioxide, sodium and potassium bisulfite, and metabisulfites are widely used in food preparation, processing, and storage. Sodium and potassium bisulfites and metabisulphites are converted into sulfuric acid and sulfur dioxide itself in solutions [80]. Regulation (EU) No. 1169/2011 of the European Parliament and of the Council on the provision of food information to consumers lists substances causing allergies or intolerances in Annex II. Point 12. mentions sulfur dioxide and sulphites in amounts higher than 10 mg/kg or 10 mg/L, expressed as total SO2.
The physiological role of SO2 is obscure due to the lack of efficient tools for monitoring and imaging. A fluorescent probe MS-Bindol may be used for the detection of SO2 derivatives (the detection limit is 0.2 nM) [81]. The excessive intake of SO2 may cause diarrhea, hypotension, allergic reactions and asthma in susceptible individuals, in addition to contributing to cardiovascular and cerebrovascular diseases, lung cancer, and other diseases [[82], [83], [84]]. SO2 and its derivatives also play an important biological role in redox equilibrium balance [[85], [86], [87]].
Treatment with 2,4,6-trinitrobenzenesulfonic acid (TNBS) in experimental conditions resulted in increased oxidative stress in studies done on rats. All of these oxidative changes were undone by SO2, which also helped the rats with colitis. Combining TNBS with an inhibitor of the endogenous SO2 synthesis worsened colitis. The results of this study pointed to a role for IBD in the down-regulation of the SO2/glutamate oxaloacetate transaminase pathway. Due to its anti-inflammatory and antioxidant properties, SO2 may have a preventive role in colitis. IBD is accompanied with down-regulation of the SO2/glutamate oxaloacetate transaminase pathway. SO2 may be utilized (following extensive investigation) with prescribed medications for a synergistic effect, because it is endogenously produced at low concentrations and is not hazardous [88]. A recent study demonstrated that SO2 caused alterations in antioxidative status and lipid peroxidation in the stomach and intestines of mice. The levels of TBARS (thiobarbituric acid) in the stomach and intestine were considerably increased after exposure to SO2 at all concentration levels. Increased SO2, however, resulted in much higher catalase (affected in a dose-dependent manner) activity and significantly lower glutathione levels (both in female and male mice) as well as superoxide dismutase and glutathione peroxidase activity. The studies support the notion that SO2 is hazardous to the lining of mammals' stomachs and intestines, same as for their respiratory systems, as it can induce oxidative damage. Certainly, toxicological effects of SO2 on all organs of humans and animals require additional research and higher evidence rigor [88].
It was shown that SO2 – at a concentration of 500 mg/L – had little effect on any of the microorganisms (L. acidophilus KI, L. rhamnosus R11, L. plantarum, Bifidobacterium animalis Bo, and B. animalis Bb12) and pathogenic bacterial strains that could affect gastrointestinal the system (Listeria monocytogenes, Escherichia coli, Salmonella enteritidis, and Bacillus cereus), a higher concentration of SO2 (1000 mg/L) had an inhibiting effect on B. animalis. Only L. monocytogenes, and to a lesser extent E. coli were suppressed by SO2 (at 1000 mg/L) treatment among the pathogenic strains. The other probiotic strains were not significantly inactivated, and it appears that the SO2 was protecting them. Additionally, the metabolism of B. animalis Bo was considerably impacted by SO2 (at 1000 mg/L), particularly the microorganism’s glucose metabolism. There were no changes in the production of organic acids, like acetic, propionic and butyric acid, although lactic and citric acids were impacted, and succinic acid production was apparently reduced. Generally, SO2 is a safe molecule to employ for food preservation, which seemingly does not affect the gut microbiome when consumed with food. SO2 has no antibacterial activity when consumed by contaminating bacteria or when these bacteria are found in the gut [89].
S-based agents are added to many foods and beverages, such as wine, dried fruit, crackers, dried vegetables, potatoes, fish, mustard because they enhance the antimicrobial and the antioxidant activity, and are useful in enzymatic and non-enzymatic browning reactions [90]. S compounds that cause allergies include sulfites (sulfur dioxide, bisulfites, and metabisulfites), antibiotic sulfonamides such as sulfamethoxazole, and nonantibiotic sulfonamides such as furosemide, and thiazides. Even some medicines contain S, such as omeprazole or disulfiram, which contain a thiuram group [91]. The adverse effect of ingesting sulfur dioxide is more often an intolerance than an allergic reaction. Although the most common symptom is asthma (bronchospasm and wheezing), foods and food additives are not common asthma triggers. In addition, adverse reactions to sulfite compounds are rare in non-asthmatic and non-sensitive individuals [92]. Asthmatic symptoms occurring 1–2 min after ingestion of soft drinks may be caused by the inhalation of gas or rapid absorption through the buccal mucosa. Inhalation of 1 ppm sulfur dioxide may cause bronchoconstriction in asthmatics [80].
Individual reactions to sulfur dioxide vary widely. While many individuals may tolerate up to 4 g of sulfite per day without adverse effects (i.e., approximately 50 mg/kg body weight), while others will complain of headaches, nausea, diarrhea, or a feeling of fullness after ingesting very small amounts [93]. The possible adverse effects of long-term sodium bisulfite administration in the diet of experimental animals (laboratory rats) are summarized in Table 2 [94,94].
Table 2.
Amount of dietary sodium bisulfite administered to rats over one year and its adverse effects (data from Fitzhugh et al., 1946) [94].
| Amount of sodium bisulfite | Negative effect |
|---|---|
| 0.5–2% | Damage to the nervous system, reproductive organs, bone tissue, kidneys and other internal organs |
| Doses lesser than 0.25% | They do not cause any pathological symptoms except diarrhea at levels above 0.1% |
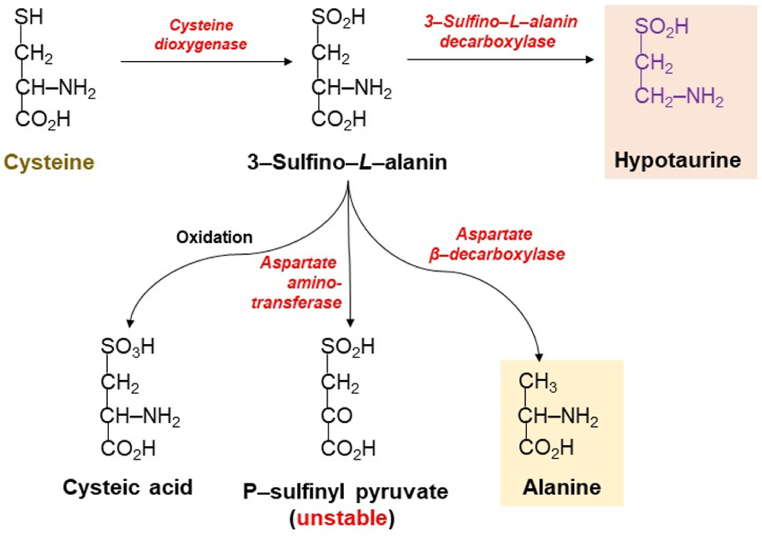
Intestinal microorganisms have a high capacity in biotransformation of S compounds [95]. H2S is described as a significant signaling molecule, with important roles in the protection of bacteria antibiotic and oxidative stress [96,97]. The source of H2S can be S amino acids and it is the product of metabolism [98]. Methionine and cysteine (S-containing amino acids) are metabolized in the way where the transfer of methyl groups is involved, and it is with the formation of S excretory forms. Methionine S is transferred to serine and synthesized to cysteine [98]. Cysteine undergoes catabolism by several pathways: i) thiol is oxidized to a sulfinic acid (the initial step of Cys catabolism), ii) S released from Cys before the oxidation. It should be emphasized that the available knowledge of Cys catabolism is mainly from studies done in rodents. Cys represents the substrate for other pathways as well: synthesis of cysteinyl-tRNAs for protein synthesis, glutathione synthesis, coenzyme A synthesis, cofactors of molybdenum, and thiol-modified tRNAs. Free cysteine is regenerated by protein and glutathione, coenzyme A degradation generates cysteamine (decarboxylated cysteine) and the end products are hypotaurine and taurine (Fig. 2) [98].
Fig. 2.
The biotransformation of cysteine sulfinic acid.
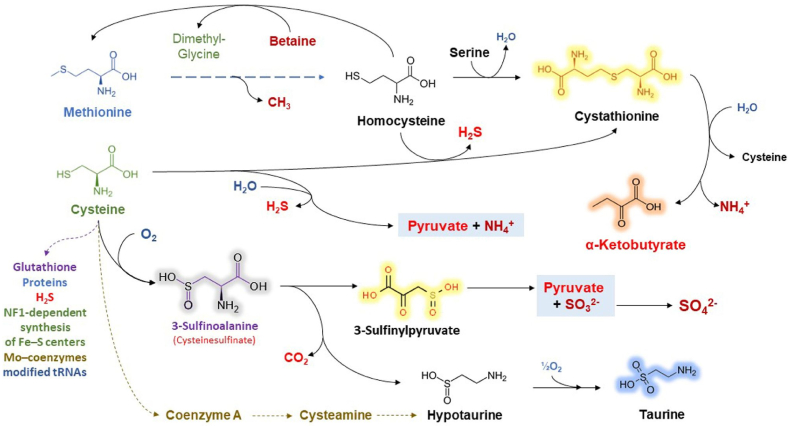
Methionine and cysteine are amino acids containing S, and play an important role in the metabolism of humans and animals, but they also play a significant role in the metabolism of different microorganisms as a growth factor [98]. Methionine adenosyltransferase (MAT) I/III and glycine N-methyltransferase (GNMT), found in the liver, are capable of transmethylation of methionine and homocysteine. The lack of MAT and GNMT results in hypermethioninemia. Cystathionine β-synthase (CBS) is activated by S-adenosylmethionine (SAM) and further metabolism of homocysteine (Hcy) is continued at the transsulfuration pathway. The catabolism of methionine (Met) molecules is affected by transmethylation plus transsulfuration. The S atom of Met is transferred to serine due to the synthesis of cysteine. Cys catabolism and S oxidation pathways are influencing the oxidation and excretion of Met sulfur. Cysteine dioxygenase 1 oxidizes the excessive amount of cysteine that is further metabolized to taurine of sulfate. H2S is produced by desulfhydration pathways and it is further oxidized to sulfate [18]. The excessive amount of cysteine and homocysteine in the desulfhydration process may be elevated in the case of higher concentrations of cysteine and homocysteine. The scheme of the metabolism of amino acids containing S is presented in Fig. 3.
Fig. 3.
The metabolism of amino acids containing sulfur.
About 20% of the dietary methionine intake – which is mostly transmethylated to homocysteine and trans-sulfurated to cysteine – is metabolized in the gastrointestinal tract (GIT), which is a physiologically significant location of S amino acid (SAA) metabolism in the body. About 25% of the body's total transmethylation and transsulfuration occurs in the GIT. Additionally, 25% of the diet's cysteine intake is used by the gut, which also absorbs 65% of the splanchnic first-pass amount of cysteine. According to recent research, intestinal metabolism of dietary methionine and cysteine is essential for the development of the intestinal mucosa. Methionine and cysteine are precursors of significant compounds, in addition to being necessary for the synthesis of proteins. The main physiologic methyl donor in mammalian cells and a precursor for polyamine production is S-adenosylmethionine, a metabolite of methionine. The rate-limiting amino acid for the production of glutathione, the main cellular antioxidant in mammals, is cysteine. It is necessary to conduct more research to determine how SAA metabolism controls intestinal function and growth, as well as how it plays a role in the emergence of gastrointestinal disorders [99].
Another important compound that is absorbed from the diet is sulfinic acid; the structure of sulfinic acid is the following: R–SO(OH) [100]. The sulfinate anion is a conjugate base of sulfinic acid. Cysteine is converted by the enzyme cysteine dioxygenase into the corresponding sulfinate. The sulfinic acid hypotaurine represents the product of that catabolic reaction. Esters of sulfinic acid are sulfinite. Sultines are cyclic sulfinite esters. The derivates of sulfinic acid may be found in all living systems [100,101]. A sulfinate anion is the conjugated base of a sulfinic acid. Cysteine is converted to the sulfinate by the enzyme cysteine dioxygenase. Hypotaurine is one of the product of this catabolic reaction. Esters of sulfinic acid are sulfinite (sultines are cyclic sulfinite esters) [102].
Aminoalkylsulfonic acids, and particularly 3-sulfino-alanine (also better known as cysteinesulfinic acid) and 2-aminoethylsulfinic acid or hypotaurine, are the most important compounds in this metabolism. All these derivates are linked with taurine and cysteine, possessing different physiological activities [103]. Cysteinesulfinic acid is formed from cysteine by the action of the сysteine dioxygenase 1 enzyme [104]. It should be emphasized that the metabolic rate of cysteinesulfinic acid is different for various species: cysteinesulfinic acid is transferred to hypotaurine. Cysteinesulfinic acid is a substrate for tryptophanase [105], and glutamic acid (Glu) decarboxylase from E . coli. Cysteinesulfinic acid, Asp and Glu are acting in the same way in the central nervous system (CNS). Cysteinesulfinic acid is accelerating gamma-aminobutyric acid (GABA) liberation from the hippocampus of the brain (Zn2+ or insulin are stopping this effect) [102].
The main S compound found in the muscle tissue is taurine, although low concentrations of taurine may be found in the bodily fluids (serum and urine). Taurine has important biological and physiological functions, including both protective and inflammatory roles [[106], [107], [108]]. Higher level of taurine may be found in the small intestine [109]. The taurine treatment showed a protective effect on intestinal injuries due to ischemia–reperfusion and lipopolysaccharides [110,111]. The study conducted by Shimizu et al. showed that taurine supplementation reduced significantly diarrhea severity in C57BL/6 mice [112]. Taurine supplementation also possessed an ameliorative effect, as it showed the improved antioxidant defense and duodenum tissue integrity [113]. Otherwise, there are also studies indicating taurine supplementation to be without a positive effect [114,115]. The amount of taurine supplementation is also important, as it was found that 3% of taurine supplementation had adverse effects on villus height, diarrhea index, and crypt depth [10]. The plant source of sulfur compounds are partially represented with glutathione, the major intracellular thiol, providing the protection from the oxidative injury (with in vitro studies showing the same trend) [116,117].
6.1. Sulfosugars from green plants and algae
Sulfoquinovose (sulfosugar), a sulfonic acid derivative of glucose, was found as a chemical building block in green vegetables such as spinach, lettuce, and in algae. Sulfosugars may be used by microorganisms as a potential nutrient. According to David Schleheck, sulfoquinovose stimulates the growth of very specific key organisms in the gut microbiome. Among these enzymes include the dihydropteroate synthase (DHPS) of the key organism Eubacterium rectale. E. rectale decomposes sulfoquinovose to a S compound, dihydroxypropane sulfonate, which serves as an energy source for other intestinal bacteria, such as Bilophila wadsworthia; B. wadsworthia produces hydrogen sulfide from DHPS. Glutathione is produced by the human body, but it can also be ingested through food. Okra, spinach, avocados, and asparagus are some of the foods that are the richest in glutathione [118]. The thiol redox status is increasing the level of oxidized glutathione (GSSG) (6 to 7-fold) and protein-bound reduced glutathione (GSH) (2-fold), while non-protein GSH level remains at the same level. The whole process may be conducted due to increased mucosal GSH synthesis or transport from the circulation. Cellular non-protein thiols, cysteine and cystine are non changed after luminal exposure to oxidants. Glutathione is the most abundant low molecular mass cellular thiol that is included in the removal of electrophiles and oxidants [119]. Glutathione is also defined as non-protein thiol able to remove oxidants and xenobiotics [120].
Cellular metabolism may be affected by protein GSH mixed disulfide formation. Activities of the adenylate cyclase (AC), phosphofructokinase (PFK) and phosphorylase phosphatase enzymes are altered with the S-thiolation of the protein [121]. Other low molecular mass thiols, such cysteine and cystine, are not affected by oxidant exposure in the cells, suggesting that they may not be directly engaged in oxidant defense, albeit they may be used for GSH synthesis. After oxidant exposure, no change in protein-bound cysteine was seen. After oxidant exposure, the GSSG level increased by almost 7-times, although mucosal GSH did not drop at the same time. The thiol redox state in the mucosa was changed as a result. It's possible that enhanced cellular synthesis or transfer from circulation is to blame for the observed rise in mucosal GSH. The amount of mucosal GSH decreased after buthionine sulfoximine (BSO) inhibited the synthesis of GSH. After BSO treatment, oxidant exposure nevertheless led to an increase in mucosal GSH. This rise is most likely the result of GSH being taken up from the bloodstream. It is known that the transport system found in the brush border membranes allows the intestinal epithelium to absorb GSH from the diet as well as biliary secretions [122]. According to previous studies, the liver is the primary organ responsible for GSH synthesis, and this GSH is then transferred to extrahepatic tissues [123]. It has been demonstrated in isolated kidney cells that extracellular GSH is transported to promote detoxification by the Na+-dependent uptake pathway found in the basolateral membranes [124].
Sulfur dioxide gas, hydrogen sulfite, metabisulfite, and sulfur salts including potassium, calcium, or sodium are all referred to as “sulfites” in applications for food and pharmaceuticals. Beer, wine, juices, dried fruit, meats, processed fish, processed seafood, and some canned items all include these compounds as additives. As metabolites of yeast, they also exist naturally in several fermented foods [125]. Sulfite additives are introduced into foodstuffs to limit microbial development, to avoid browning, and to preserve food [119]. According to the regulation on sulfites, they are declared as “Generally Regarded as Safe” (GRAS) (per the FDA). Otherwise, sulfites have been the subject of numerous reviews, and research that have demonstrated they can be harmful to people at levels as low as 1 ppm [126]. Many food commodities contain high levels of sulfites and they represent health concerns for some individuals. It has been challenging to pinpoint the precise threshold at which these preservatives become toxic, as there is a lack of statistical data regarding individual sensitivities and consumer intake levels [127]. During food preparation and digestion, as well as interactions between these additives and naturally occurring ingredients in food, uncertain reactions might take place. Government regulatory authorities typically deem sulfites and other preservatives acceptable for ingestion at concentrations up to 5000 ppm. Sulfites and other preservatives are considered food additives used for the reduction of bacterial contamination. It has been demonstrated that bactericidal and bacteriostatic drug consumption harms the beneficial bacteria in the human gut, and this harm has been linked to the development of a number of disorders. A study utilizing serial dilution and drop plates revealed bactericidal effects on all Lactobacillus species at concentrations between 1000 and 3780 ppm after 4 h of exposure, and effects on S. thermophilus at 2000 ppm NaHSO3 after 6 h of exposure [79].
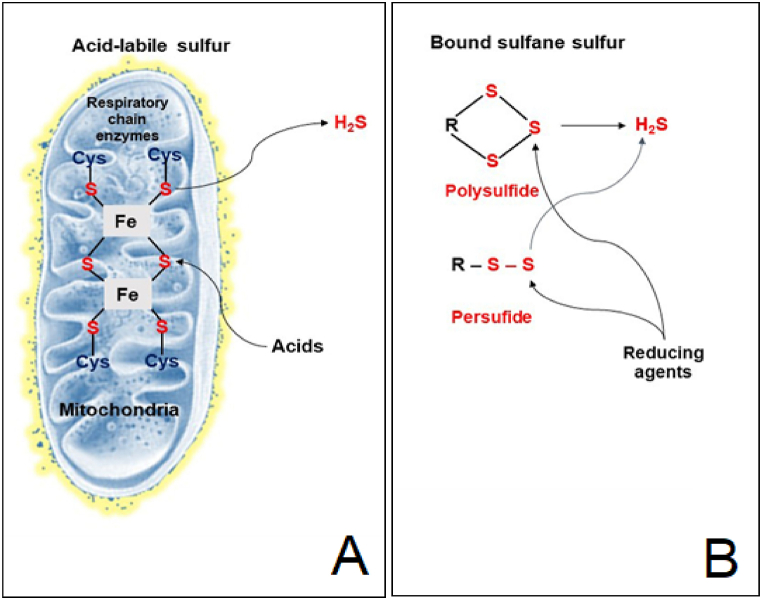
The hazardous gas hydrogen sulfide (H2S), which has a rotten-egg odor, was well described in antecedent studies. Previous reports related to this molecule have shown physiological involvement in processes like neuromodulation, vascular tone regulation, cytoprotection against oxidative stress, angiogenesis, anti-inflammation, and oxygen sensing since the discovery of endogenous H2S in the mammalian brain two decades ago. Several H2S-producing enzymes, including cystathionine–synthase, cystathionine–lyase, and 3-mercaptopyruvate sulfurtransferase, have undergone extensive research and are well understood. It was discovered that polysulfides – which have more interior S atoms than H2S – are potential signaling molecules, with greater efficacy than that of H2S, for activating ion channels, transcription factors, and tumor suppressors [128]. H2S liberation is made possible by enzymes and released from cellular reserves [129]. Acid-labile S and bound sulfane S are the two types of H2S that can be stored. Iron-sulfur (Fe–S) complexes coupled to respiratory chain enzymes are the principal source of acid-labile S [128]. Under acidic circumstances, this complex releases H2S. On the other hand, when reduced, bound sulfane S, which is present in proteins as sulfurated cysteine residues, also releases H2S (Fig. 4).
Fig. 4.
The release of H2S from acid-labile sulfur (A) and bound sulfane sulfur (B).
Proteins contain bound sulfane S as persulfide or polysulfide, which, in reducing environments, releases H2S. H2S that has been absorbed can be retrieved using a reducing agent like dithiothreitol [129,130]. To bind to proteins as a persulfide or polysulfide, H2S must be oxidized [131]. When dihydrolipoic acid (DHLA) is available at pH 8.0, or when endogenous amounts of cysteine and glutathione are present at pH 8.4, the lysates of neurons and astrocytes produce H2S [132]. Na+/HCO3− transporters are active during membrane repolarization, which causes the intracellular pH to change to alkaline, and causes the release of H2S from bound sulfane S [133]. Because H2S is created and destroyed by enzymes, the S of H2S is integrated into cysteine residues after being oxidized to dihydridotin (H2Sn), and the reaction between thiol and H2Sn releases H2S, therefore the concentration of free H2S in cells may be altered considerably in a short period of time. It was found that the H2S concentration in the aorta is 1 μM and 7 nM in the blood. However, no sulfide was discovered in the blood after the testing of various species [134].
7. The metabolism of carrageenans by intestinal bacteria
One of the phycocolloids, carrageenan, is a sulfated galactan composed of linear chains of galactose and 3,6-anhydrogalactose with alternating -(1,3) and -(1,4) linkages. Carrageenan is further divided into three types based on the quantity and location of sulfated ester(s). κ-, ι-, and λ-carrageenases are the main enzymes that break down carrageenans. All of them are endohydrolases that produce oligo-carrageenans by cleaving the internal -(1,4) links of carrageenans. Only bacteria, specifically Gram-negative bacteria, can produce these enzymes. The majority of marine bacteria produce these extracellular enzymes, and they are active throughout a wide temperature range [135,136]. Carrageenans have a significant potential to influence prostaglandin E2 (PGE2) synthesis and activate IL-1 and IL-6 synthesis at high concentrations. Carrageenans must have a low sulfate content and a high molecular weight in order to influence PGE2 production. Although less effectively than cholestyramine, the least sulfated/-carrageenan could retain bile salt penetration the most. The activity of pancreatic lipase was unaffected by the polysaccharides [137].
Carrageenans are hydrophilic colloids (water soluble gums) of commercial importance that are found as matrix material in many species of red seaweeds (comprising up to 50% dry weight; Rhodophyta), where they perform a structural function similar to that of cellulose in land plants. They are a class of biomolecules made up of sugar units connected to sulfate half-esters in linear polysaccharide chains. Depending on the amount of sulfate substituents per disaccharide unit, it may be found in three different forms: one in -carrageenan, two in -carrageenan, and three in –carrageenan units. Due to these characteristics, carrageenans may create extremely viscous solutions, dissolve in water, and maintain their stability across a wide pH range [138,139]. Substances that mix with water to create colloid systems are known as hydrocolloids. Agar, alginates, and carrageenans are hydrocolloid polysaccharides that are employed in the many biotechnological industries and have substantial economic and technological significance because of their unique physico-chemical characteristics [111,140,141]. As microbial enzymes are extremely selective for their substrates and produce oligo-derivatives that are homogenous in molecular weight, this method of producing oligo-carrageenan may be more favorable than acid hydrolysis [139]. The anticancer and antiviral properties of the oligosaccharides produced from carrageenan suggested that oligo-carrageenan might have great promise for future biomedical and physiological applications [142].
The majority of seaweeds that utilize carrageenan as their primary component of the cell wall are red algae, or Rhodophyta. Carrageenans, which can make up as much as 50% of the dry weight of the carrageenophyte red seaweed genera Eucheuma, Gigartina, Hypnea, and Kappaphycus, are harvested from these plants [141]. While ι-carrageenan is mostly synthesized from E. denticulatum (also known as E. spinosum), κ-carrageenan is extracted primarily from K. alvarezii (sometimes known as E. cottonii in the commerce). The Gigartina and Chondrus genera of seaweeds, which produce λ-carrageenan as sporophytic plants, while creating a hybrid as gametophytic plants, are the source of carrageenan [143]. These "κ-2″ or “weak-gelling κ-carrageenans,” also known as “carrageenans of the/hybrid type,” are composed of mixed polysaccharide chains that contain both κ- and ι-units and vary from virtually pure ι-carrageenan to practically pure κ-carrageenan [135].
To avoid deterioration, the seaweed is swiftly dried and then bagged for transport to processing facilities. After the seaweed has been repeatedly washed to eliminate coarse contaminants, such as sand, salt, and marine life, carrageenan is extracted from the cell using hot alkali. When carrageenan is placed in a hot solution, it goes through purification and is then turned into powder. Alkaline pre-treatment duration and extraction parameters, such as temperature, pH, and duration, have a significant impact on the chemical structure and gelling qualities [144]. Carrageenan has a high degree of polydispersity and a high molecular mass (>100 kDa). Other carbohydrate residues and substituents, such as methyl ethers and pyruvate groups, are present in carrageenans in addition to the galactose and sulfate [140,145,146].
The three primary carrageenans used commercially all share the same basic structure. While one sulfate ester is present in κ-carrageenan, two and three sulfates are present per dimer in ι- and λ-carrageenan, respectively. Different carrageenans have different properties, depending on the quantity and positioning of ester sulfate groups. Lower solubility temperature and weaker gel strength are correlated with higher ester sulfate concentrations. κ-carrageenan contains about 25–30% of ester sulfate, ι-type carrageenan about 28–30% and λ-type carrageenan about 32–39%, respectively [147].
7.1. Microbial carrageenanolytic system
7.1.1. Enzymatic hydrolysis of carrageenan
κ-Carrageenases (EC 3.2.1.83), ι-carrageenases (EC 3.2.1.157), and λ-carrageenases (EC 3.2.1.162) are enzymes that are able to break down carrageenans. They are all endohydrolases that cleave the internal -(1–4) links of carrageenans, to produce oligogalactans of either the neocarrabiose or neoagarobiose series [148]. It has been speculated that κ-carrageenases and ι-carrageenases break internal connections rather than hydrolyzing units from the ends to degrade carrageenan, resulting in a string of homologous, even-numbered oligosaccharides. Both enzymes are processive, meaning they slide along the polysaccharide instead of dissociating from the substrate to cleave every potential link (DP4s and DP2s). Carrageenases break down by digestion to produce oligo-galactans of different sizes, which are most likely 2, 4, and 6° polymerized (DP) carbohydrates [149,150]. Carrageenases must bind to a chain length of four sugar residues in order to guarantee hydrolysis [139]. Different carrageenases display various hydrolysis polymer mechanisms [135,136]. Carrageenases hydrolyze their substrate through an inversion mechanism, which involves the starting compound‘s anomeric structure being reversed [145,149]. As S-containing carrageenans are widely used in the preparation of different food commodities, their potential beneficial or detrimental health effects should be studied more extensively.
8. Conclusion
The present scoping review dealt with compounds in different food commodities containing sulfur (S) and its compounds. Based on the currently available literature and evidence, it is crucial to emphasize the understanding of which food commodities are containing S compounds as on one hand, these compounds may considerably influence food sensory properties, but on the other hand may also influence the intestinal microbiota, even in a detrimental fashion. Based on the contents of this review, it should also be noted that the present findings and published works are insufficient to comprehensively explain the full scale of issues concerning the metabolism of sulfur compounds, and their beneficial or negative influences on mammalian health.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
9. Data availability statement
The paper is a review.
Funding statement
The research was supported by the project of FVHE/Tremlová/ITA2022from University of Veterinary Sciences, Brno, Czech Republic, grant number 2022ITA23
References
- 1.Brosnan J.T., Brosnan M.E. The sulfur-containing amino acids: an overview. J. Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 2.Kushkevych I., Cejnar J., Treml J., Dordević D., Kollar P., Vítězová M. Recent advances in metabolic pathways of sulfate reduction in intestinal bacteria. Cells. 2020;9:698. doi: 10.3390/cells9030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes R., Amador P., Prudêncio C. β-Lactams: chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013;24:7–17. doi: 10.1097/MRM.0b013e3283587727. [DOI] [Google Scholar]
- 4.Henry R.J. The mode of action of sulfonamides. Bacteriol. Rev. 1943;7:175–262. doi: 10.1128/br.7.4.175-262.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borlinghaus J., Albrecht F., Gruhlke M., Nwachukwu I., Slusarenko A. Allicin: chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alanazi A.M., Mostafa G.A.E., Al-Badr A.A. Elsevier; 2015. Glutathione; pp. 43–158. (Profiles of Drug Substances, Excipients and Related Methodology). [DOI] [PubMed] [Google Scholar]
- 7.Thomas R., Shoemaker C.B., Eriks K. The molecular and crystal structure of dimethyl sulfoxide, (H 3 C) 2 SO. Acta Crystallogr. 1966;21:12–20. doi: 10.1107/S0365110X66002263. [DOI] [Google Scholar]
- 8.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Phcog. Rev. 2010;4:118. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapter 1. Introduction to sulfur chemical biology. Royal Society of Chemistry; Cambridge: 2020. pp. 5–22. (Chemical Biology,). [DOI] [Google Scholar]
- 10.Liu S., Li L., Guo L., Jin H., Kou J., Li G. Sulfur transformation characteristics and mechanisms during hydrogen production by coal gasification in supercritical water. Energy Fuels. 2017;31:12046–12053. doi: 10.1021/acs.energyfuels.7b02505. [DOI] [Google Scholar]
- 11.Kushkevych I., Vítězová M., Vítěz T., Bartoš M. Production of biogas: relationship between methanogenic and sulfate-reducing microorganisms. Open Life Sci. 2017;12:82–91. doi: 10.1515/biol-2017-0009. [DOI] [Google Scholar]
- 12.Kushkevych I., Dordević D., Vítězová M. Possible synergy effect of hydrogen sulfide and acetate produced by sulfate-reducing bacteria on inflammatory bowel disease development. J. Adv. Res. 2021;27:71–78. doi: 10.1016/j.jare.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotrsova V., Kushkevych I. Possible methods for evaluation of hydrogen sulfide toxicity against lactic acid bacteria. Biointerface Res. Appl. Chem. 2019;9:4066–4069. doi: 10.33263/BRIAC94.066069. [DOI] [Google Scholar]
- 14.Kushkevych I., Leščanová O., Dordević D., Jančíková S., Hošek J., Vítězová M., Buňková L., Drago L. The sulfate-reducing microbial communities and meta-analysis of their occurrence during diseases of small–large intestine Axis. JCM. 2019;8:1656. doi: 10.3390/jcm8101656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Černý M., Vítězová M., Vítěz T., Bartoš M., Kushkevych I. Variation in the distribution of hydrogen producers from the clostridiales order in biogas reactors depending on different input substrates. Energies. 2018;11:3270. doi: 10.3390/en11123270. [DOI] [Google Scholar]
- 16.Kushkevych I., Dordević D., Vítězová M. Toxicity of hydrogen sulfide toward sulfate-reducing bacteria Desulfovibrio piger Vib-7. Arch. Microbiol. 2019;201:389–397. doi: 10.1007/s00203-019-01625-z. [DOI] [PubMed] [Google Scholar]
- 17.Kushkevych I., Coufalová M., Vítězová M., Rittmann S.K.-M.R. Sulfate-reducing bacteria of the oral cavity and their relation with periodontitis—recent advances. JCM. 2020;9:2347. doi: 10.3390/jcm9082347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Struk M., Kushkevych I. Perspectives of application of phototrophic sulfur bacteria in hydrogen sulfide utilization. MendelNet. 2018:537–541. [Google Scholar]
- 19.Kováč J., Vítězová M., Kushkevych I. Metabolic activity of sulfate-reducing bacteria from rodents with colitis. Open Med. 2018;13:344–349. doi: 10.1515/med-2018-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kushkevych I.V. Kinetic properties of pyruvate ferredoxin oxidoreductase of intestinal sulfate-reducing bacteria Desulfovibrio piger vib-7 and Desulfomicrobium sp. rod-9. Pol. J. Microbiol. 2015;64:107–114. [PubMed] [Google Scholar]
- 21.Kushkevych I., Vítězová M., Fedrová P., Vochyanová Z., Paráková L., Hošek J. Kinetic properties of growth of intestinal sulphate-reducing bacteria isolated from healthy mice and mice with ulcerative colitis. Acta Vet. Brno. 2017;86:405–411. doi: 10.2754/avb201786040405. [DOI] [Google Scholar]
- 22.Kushkevych I. In: Microorganisms. Blumenberg M., Shaaban M., Elgaml A., editors. IntechOpen; 2020. Isolation and purification of sulfate-reducing bacteria. [DOI] [Google Scholar]
- 23.Kushkevych I. Identification of sulfate-reducing bacteria strains of human large intestine. Biol. Stud. 2013;7:115–132. doi: 10.30970/sbi.0703.312. [DOI] [Google Scholar]
- 24.Kushkevych I., Dordević D., Kollar P., Vítězová M., Drago L. Hydrogen sulfide as a toxic product in the small–large intestine Axis and its role in IBD development. JCM. 2019;8:1054. doi: 10.3390/jcm8071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushkevych I., Castro Sangrador J., Dordević D., Rozehnalová M., Černý M., Fafula R., Vítězová M., Rittmann S.K.-M.R. Evaluation of physiological parameters of intestinal sulfate-reducing bacteria isolated from patients suffering from IBD and healthy people. JCM. 2020;9:1920. doi: 10.3390/jcm9061920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushkevych I., Dordević D., Vítězová M., Rittmann S.K.-M.R. Environmental impact of sulfate-reducing bacteria, their role in intestinal bowel diseases, and possible control by bacteriophages. Appl. Sci. 2021;11:735. doi: 10.3390/app11020735. [DOI] [Google Scholar]
- 27.Kushkevych I., Vítězová M., Kos J., Kollár P., Jampílek J. Effect of selected 8-hydroxyquinoline-2-carboxanilides on viability and sulfate metabolism of Desulfovibrio piger. J. Appl. Biomed. 2018;16:241–246. doi: 10.1016/j.jab.2018.01.004. [DOI] [Google Scholar]
- 28.Kushkevych I., Dordević D., Vítězová M., Kollár P. Cross-correlation analysis of the Desulfovibrio growth parameters of intestinal species isolated from people with colitis. Biologia. 2018;73:1137–1143. doi: 10.2478/s11756-018-0118-2. [DOI] [Google Scholar]
- 29.Kushkevych I., Abdulina D., Dordević D., Rozehnalová M., Vítězová M., Černý M., Svoboda P., Rittmann S.K.-M.R. Basic bioelement contents in anaerobic intestinal sulfate-reducing bacteria. Appl. Sci. 2021;11:1152. doi: 10.3390/app11031152. [DOI] [Google Scholar]
- 30.Abdulina D., Kováč J., Iutynska G., Kushkevych I. ATP sulfurylase activity of sulfate-reducing bacteria from various ecotopes. 3 Biotech. 2020;10:55. doi: 10.1007/s13205-019-2041-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kushkevych I., Kollar P., Ferreira A.L., Palma D., Duarte A., Lopes M.M., Bartos M., Pauk K., Imramovsky A., Jampilek J. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing bacteria. J. Appl. Biomed. 2016;14:125–130. doi: 10.1016/j.jab.2016.01.005. [DOI] [Google Scholar]
- 32.Kushkevych I., Dordević D., Kollár P. Analysis of physiological parameters of Desulfovibrio strains from individuals with colitis. Open Life Sci. 2019;13:481–488. doi: 10.1515/biol-2018-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brenner D.J., Krieg N.R., Staley J.T., Garrity G.M. Bergey's Manual of Systematic Bacteriology. second ed. Springer; Boston, MA, USA: 2005. The proteobacteria, Part C: the alpha-, beta-, delta-, and epsilonproteobacteria; p. 1388. [Google Scholar]
- 34.Růžičková M., Vítězová M., Kushkevych I. The characterization of Enterococcus genus: resistance mechanisms and inflammatory bowel disease. Open Med. 2020;15:211–224. doi: 10.1515/med-2020-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kushkevych I., Kotrsová V., Dordević D., Buňková L., Vítězová M., Amedei A. Hydrogen sulfide effects on the survival of lactobacilli with emphasis on the development of inflammatory bowel diseases. Biomolecules. 2019;9:752. doi: 10.3390/biom9120752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shankaranarayana M.L., Raghavan B., Abraham K.O., Natarajan C.P., Brodnitz H.H. Volatile sulfur compounds in food flavors. CRC Crit. Rev. Food Technol. 1974;4:395–435. doi: 10.1080/10408397409527163. [DOI] [Google Scholar]
- 37.Parcell S. Sulfur in human nutrition and applications in medicine. Alternative Med. Rev. 2002;7:22–44. [PubMed] [Google Scholar]
- 38.Roberts A.C., McWEENY D.J. The uses of sulphur dioxide in the food industry: a review. Int. J. Food Sci. Technol. 2007;7:221–238. doi: 10.1111/j.1365-2621.1972.tb01658.x. [DOI] [Google Scholar]
- 39.Kushkevych I., Fafula R., Parák T., Bartoš M. Activity of Na+/K+-activated Mg2+-dependent ATP-hydrolase in the cell-free extracts of the sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9. Acta Vet. Brno. 2015;84:3–12. doi: 10.2754/avb201585010003. [DOI] [Google Scholar]
- 40.Kushkevych I., Kos J., Kollar P., Kralova K., Jampilek J. Activity of ring-substituted 8-hydroxyquinoline-2-carboxanilides against intestinal sulfate-reducing bacteria Desulfovibrio piger. Med. Chem. Res. 2018;27:278–284. doi: 10.1007/s00044-017-2067-7. [DOI] [Google Scholar]
- 41.Kushkevych I., Kollar P., Suchy P., Parak T., Pauk K., Imramovsky A. Activity of selected salicylamides against intestinal sulfate-reducing bacteria. Neuroendocrinol. Lett. 2015;36(Suppl 1):106–113. [PubMed] [Google Scholar]
- 42.Kushkevych I., Abdulina D., Kováč J., Dordević D., Vítězová M., Iutynska G., Rittmann S.K.-M.R. Adenosine-5′-phosphosulfate- and sulfite reductases activities of sulfate-reducing bacteria from various environments. Biomolecules. 2020;10:921. doi: 10.3390/biom10060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kushkevych I., Dordević D., Vítězová M. Analysis of pH dose-dependent growth of sulfate-reducing bacteria. Open Med. 2019;14:66–74. doi: 10.1515/med-2019-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwimmer S., Friedman M. Genesis of volatile sulfur-containing food flavors. Flavour Ind. 1972;1972 [Google Scholar]
- 45.Raab A., Feldmann J. Biological sulphur-containing compounds – analytical challenges. Anal. Chim. Acta. 2019;1079:20–29. doi: 10.1016/j.aca.2019.05.064. [DOI] [PubMed] [Google Scholar]
- 46.Rappel C., Schaumlöffel D. The role of sulfur and sulfur isotope dilution analysis in quantitative protein analysis. Anal. Bioanal. Chem. 2008;390:605–615. doi: 10.1007/s00216-007-1607-2. [DOI] [PubMed] [Google Scholar]
- 47.Rampler E., Dalik T., Stingeder G., Hann S., Koellensperger G. Sulfur containing amino acids – challenge of accurate quantification. J. Anal. At. Spectrom. 2012;27:1018. doi: 10.1039/c2ja10377j. [DOI] [Google Scholar]
- 48.Necas J., Bartosikova L. Carrageenan: a review. Vet. Med. 2013;2013:187–205. [Google Scholar]
- 49.Gorman J.J., Wallis T.P., Pitt J.J. Protein disulfide bond determination by mass spectrometry. Mass Spectrom. Rev. 2002;21:183–216. doi: 10.1002/mas.10025. [DOI] [PubMed] [Google Scholar]
- 50.Mussinan C.J., Keelan M.E., editors. Sulfur Compounds in Foods. American Chemical Society; Washington, DC: 1994. [DOI] [Google Scholar]
- 51.Sreekumar R., Al-Attabi Z., Deeth H.C., Turner M.S. Volatile sulfur compounds produced by probiotic bacteria in the presence of cysteine or methionine. Lett. Appl. Microbiol. 2009 doi: 10.1111/j.1472-765X.2009.02610.x. [DOI] [PubMed] [Google Scholar]
- 52.Carrete R., Vidal M.T., Bordons A., Constanti M. Inhibitory effect of sulfur dioxide and other stress compounds in wine on the ATPase activity of Oenococcus oeni. FEMS (Fed. Eur. Microbiol. Soc.) Microbiol. Lett. 2002;211:155–159. doi: 10.1111/j.1574-6968.2002.tb11218.x. [DOI] [PubMed] [Google Scholar]
- 53.Yamada I., Shibuya H., Matsubara O., Umehara I., Makino T., Numano F., Suzuki S. Pulmonary artery disease in Takayasu's arteritis: angiographic findings. Am. J. Roentgenol. 1992;159:263–269. doi: 10.2214/ajr.159.2.1352939. [DOI] [PubMed] [Google Scholar]
- 54.Restaino S., Mereu L., Finelli A., Spina M.R., Marini G., Catena U., Turco L.C., Moroni R., Milani M., Cela V., Scambia G., Fanfani F. Robotic surgery vs laparoscopic surgery in patients with diagnosis of endometriosis: a systematic review and meta-analysis. J. Robotic Surg. 2020;14:687–694. doi: 10.1007/s11701-020-01061-y. [DOI] [PubMed] [Google Scholar]
- 55.Kelly K.K., Meadows S.M., Cripps R.M. Drosophila MEF2 is a direct regulator of Actin57B transcription in cardiac, skeletal, and visceral muscle lineages. Mech. Dev. 2002;110:39–50. doi: 10.1016/S0925-4773(01)00586-X. [DOI] [PubMed] [Google Scholar]
- 56.Esparza I., Martínez-Inda B., Cimminelli M.J., Jimeno-Mendoza M.C., Moler J.A., Jiménez-Moreno N., Ancín-Azpilicueta C. Reducing SO2 doses in red wines by using grape stem extracts as antioxidants. Biomolecules. 2020;10:1369. doi: 10.3390/biom10101369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mandrile L., Cagnasso I., Berta L., Giovannozzi A.M., Petrozziello M., Pellegrino F., Asproudi A., Durbiano F., Rossi A.M. Direct quantification of sulfur dioxide in wine by Surface Enhanced Raman Spectroscopy. Food Chem. 2020;326 doi: 10.1016/j.foodchem.2020.127009. [DOI] [PubMed] [Google Scholar]
- 58.Boelens M.H., Van Gemert L.J. Volatile character-impact sulfur compounds and their sensory properties. Perfum. Flavor. 1993;1993:29–39. [Google Scholar]
- 59.Block E. Biological activity of Allium compounds: recent results. Acta Hortic. 2005:41–58. doi: 10.17660/ActaHortic.2005.688.4. [DOI] [Google Scholar]
- 60.Cannon R.J., Ho C.-T. Volatile sulfur compounds in tropical fruits. J. Food Drug Anal. 2018;26:445–468. doi: 10.1016/j.jfda.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du X., Whitaker V., Rouseff R. Changes in strawberry volatile sulfur compounds due to genotype, fruit maturity and sample preparation: Florida strawberry sulfur volatiles. Flavour Fragrance J. 2012;27:398–404. doi: 10.1002/ffj.3107. [DOI] [Google Scholar]
- 62.Cecatto A.P., Calvete E.O., Nienow A.A., da Costa R.C., Mendonça H.F.C., Pazzinato A.C. Culture systems in the production and quality of strawberry cultivars. Acta Sci. Agron. 2013;35:471–478. doi: 10.4025/actasciagron.v35i4.16552. [DOI] [Google Scholar]
- 63.Fei M.L., Tong L., Wei L., De Yang L. Changes in antioxidant capacity, levels of soluble sugar, total polyphenol, organosulfur compound and constituents in garlic clove during storage. Ind. Crop. Prod. 2015;69:137–142. doi: 10.1016/j.indcrop.2015.02.021. [DOI] [Google Scholar]
- 64.Ariga T., Seki T. Antithrombotic and anticancer effects of garlic-derived sulfur compounds: a review. Biofactors. 2006;26:93–103. doi: 10.1002/biof.5520260201. [DOI] [PubMed] [Google Scholar]
- 65.Nicastro H.L., Ross S.A., Milner J.A. Garlic and onions: their cancer prevention properties. Cancer Prev. Res. 2015;8:181–189. doi: 10.1158/1940-6207.CAPR-14-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pétel C., Onno B., Prost C. Sourdough volatile compounds and their contribution to bread: a review. Trends Food Sci. Technol. 2017;59:105–123. doi: 10.1016/j.tifs.2016.10.015. [DOI] [Google Scholar]
- 67.Ruiz J.A., Quilez J., Mestres M., Guasch J. Solid-phase microextraction method for headspace analysis of volatile compounds in bread crumb. Cereal Chem. J. 2003;80:255–259. doi: 10.1094/CCHEM.2003.80.3.255. [DOI] [Google Scholar]
- 68.Ruiz M.A., Pincus A.L., Dickinson K.A. NEO PI-R predictors of alcohol use and alcohol-related problems. J. Pers. Assess. 2003;81:226–236. doi: 10.1207/S15327752JPA8103_05. [DOI] [PubMed] [Google Scholar]
- 69.Pu D., Zhang H., Zhang Y., Sun B., Ren F., Chen H. Characterization of the key aroma compounds in white bread by aroma extract dilution analysis, quantitation, and sensory evaluation experiments. J. Food Process. Preserv. 2019;43 doi: 10.1111/jfpp.13933. [DOI] [Google Scholar]
- 70.Parliment T.H., Morello M.J., McGorrin R.J., editors. Thermally Generated Flavors: Maillard, Microwave, and Extrusion Processes. American Chemical Society; Washington, DC: 1993. [DOI] [Google Scholar]
- 71.Mottram D.S. Flavour formation in meat and meat products: a review. Food Chem. 1998;62:415–424. doi: 10.1016/S0308-8146(98)00076-4. [DOI] [Google Scholar]
- 72.Kosowska M., Majcher M.A., Fortuna T. Volatile compounds in meat and meat products. Food Sci. Technol. 2017;37:1–7. doi: 10.1590/1678-457x.08416. [DOI] [Google Scholar]
- 73.Vazquez-Landaverde P.A., Torres J.A., Qian M.C. Quantification of trace volatile sulfur compounds in milk by solid-phase microextraction and gas chromatography–pulsed flame photometric detection. J. Dairy Sci. 2006;89:2919–2927. doi: 10.3168/jds.S0022-0302(06)72564-4. [DOI] [PubMed] [Google Scholar]
- 74.Spinnler H.E., Berger C., Lapadatescu C., Bonnarme P. Production of sulfur compounds by several yeasts of technological interest for cheese ripening. Int. Dairy J. 2001;11:245–252. doi: 10.1016/S0958-6946(01)00054-1. [DOI] [Google Scholar]
- 75.Mestres M., Busto O., Guasch J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A. 2000;881:569–581. doi: 10.1016/S0021-9673(00)00220-X. [DOI] [PubMed] [Google Scholar]
- 76.Vally H., Thompson P. Allergic and asthmatic reactions to alcoholic drinks. Addiction Biol. 2003;8:3–11. doi: 10.1080/1355621031000069828. [DOI] [PubMed] [Google Scholar]
- 77.Health Canada Legislation and guidelines—food and nutrition. 2017. http://www.hc-sc.gc.ca/fn-an/legislation/index-eng.php
- 78.U.S. Food and Drug Administration . vol. 182. 2014. Sulfur Dioxide; p. 3862.http://www.gpo.gov/fdsys/granule/CFR-2012-title21-vol3/CFR-2012-title21-vol3-sec182-3862 (Food and Drug Title 21: Code of Federal Regulations). [Accessed 15 August 15] [Google Scholar]
- 79.Irwin S.V., Fisher P., Graham E., Malek A., Robidoux A. Sulfites inhibit the growth of four species of beneficial gut bacteria at concentrations regarded as safe for food. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hannuksela M., Haahtela T. Hypersensitivity reactions to food additives. Allergy. 1987;42:561–575. doi: 10.1111/j.1398-9995.1987.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhang X., Zhang L., Liu S., Zhu X., Zhou P., Cheng X., Zhang R., Zhang L., Chen L. Insight into sulfur dioxide and its derivatives metabolism in living system with visualized evidences via ultra-sensitive fluorescent probe. J. Hazard Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127179. [DOI] [PubMed] [Google Scholar]
- 82.Zhao Y., Ma Y., Lin W. A near-infrared and two-photon ratiometric fluorescent probe with a large Stokes shift for sulfur dioxide derivatives detection and its applications in vitro and in vivo. Sensor. Actuator. B Chem. 2019;288:519–526. doi: 10.1016/j.snb.2019.01.170. [DOI] [Google Scholar]
- 83.Pundir C.S., Rawal R. Determination of sulfite with emphasis on biosensing methods: a review. Anal. Bioanal. Chem. 2013;405:3049–3062. doi: 10.1007/s00216-013-6753-0. [DOI] [PubMed] [Google Scholar]
- 84.Silva K.R.B., Raimundo I.M., Gimenez I.F., Alves O.L. Optical sensor for sulfur dioxide determination in wines. J. Agric. Food Chem. 2006;54:8697–8701. doi: 10.1021/jf061553h. [DOI] [PubMed] [Google Scholar]
- 85.Olson K.R. H2S and polysulfide metabolism: conventional and unconventional pathways. Biochem. Pharmacol. 2018;149:77–90. doi: 10.1016/j.bcp.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Yang B., Xu J., Zhu H.-L. Recent progress in the small-molecule fluorescent probes for the detection of sulfur dioxide derivatives (HSO3−/SO32−) Free Radic. Biol. Med. 2019;145:42–60. doi: 10.1016/j.freeradbiomed.2019.09.007. [DOI] [PubMed] [Google Scholar]
- 87.Yang Q., Lan T., He W. Recent progress in reaction-based fluorescent probes for active sulfur small molecules. Dyes Pigments. 2021;186 doi: 10.1016/j.dyepig.2020.108997. [DOI] [Google Scholar]
- 88.Banerjee S., Ghosh S., Sinha K., Chowdhury S., Sil P.C. Sulphur dioxide ameliorates colitis related pathophysiology and inflammation. Toxicology. 2019;412:63–78. doi: 10.1016/j.tox.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 89.Shah U.A., Pintado M.M. The effect of sulphur dioxide on probiotic and pathogenic bacteria of the human gastrointestinal tract. 2019. https://repositorio.ucp.pt/bitstream/10400.14/31510/1/Tese%20Completo%20Usman%20final%20version%20%20%28Oct%202019%29%20%281%29.pdf
- 90.Iammarino M., Di Taranto A., Muscarella M. Investigation on the presence of sulphites in fresh meat preparations: estimation of an allowable maximum limit. Meat Sci. 2012;90:304–308. doi: 10.1016/j.meatsci.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 91.Stohs S.J., Miller M.J.S. A case study involving allergic reactions to sulfur-containing compounds including, sulfite, taurine, acesulfame potassium and sulfonamides. Food Chem. Toxicol. 2014;63:240–243. doi: 10.1016/j.fct.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 92.Stockley C.S., Johnson D.L. Adverse food reactions from consuming wine: adverse food reactions from consuming wine. Aust. J. Grape Wine Res. 2015;21:568–581. doi: 10.1111/ajgw.12171. [DOI] [Google Scholar]
- 93.Lück E., Jager M. Springer Berlin Heidelberg; Berlin, Heidelberg: 1997. Antimicrobial Food Additives. [DOI] [Google Scholar]
- 94.Fitzhugh O.G., Woodard G. The toxicities of compounds related to 2,3-dimercaptopropanol (BAL) with a note on their relative therapeutic efficiency. J. Pharmacol. Exp. Therapeut. 1946;87:23–27. [PubMed] [Google Scholar]
- 95.Barić I., Ćuk M., Fumić K., Vugrek O., Allen R.H., Glenn B., Maradin M., Pažanin L., Pogribny I., Radoš M., Sarnavka V., Schulze A., Stabler S., Wagner C., Zeisel S.H., Mudd S.H. S ‐Adenosylhomocysteine hydrolase deficiency: a second patient, the younger brother of the index patient, and outcomes during therapy. J. Inherit. Metab. Dis. 2005;28:885–902. doi: 10.1007/s10545-005-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Paul R., Borah A. L-DOPA-induced hyperhomocysteinemia in Parkinson's disease: elephant in the room. Biochim. Biophys. Acta Gen. Subj. 2016;1860:1989–1997. doi: 10.1016/j.bbagen.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 97.Miller J.W., Selhub J., Nadeau M.R., Thomas C.A., Feldman R.G., Wolf P.A. Effect of L-dopa on plasma homocysteine in PD patients: relationship to B-vitamin status. Neurology. 2003;60:1125–1129. doi: 10.1212/01.WNL.0000055899.24594.8E. [DOI] [PubMed] [Google Scholar]
- 98.Stipanuk M.H. Metabolism of sulfur-containing amino acids: how the body copes with excess methionine, cysteine, and sulfide. J. Nutr. 2020;150:S2494–S2505. doi: 10.1093/jn/nxaa094. [DOI] [PubMed] [Google Scholar]
- 99.Bauchart-Thevret C., Stoll B., Burrin D.G. Intestinal metabolism of sulfur amino acids. Nutr. Res. Rev. 2009;22:175–187. doi: 10.1017/S0954422409990138. [DOI] [PubMed] [Google Scholar]
- 100.Patai S., editor. Sulphinic Acids, Esters and Derivatives. John Wiley & Sons, Inc.; Chichester, UK: 1990. [DOI] [Google Scholar]
- 101.Schubart R. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, editor. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2000. Sulfinic acids and derivatives. a25_461. [DOI] [Google Scholar]
- 102.Kalir A., Kalir H.H. In: Sulphinic Acids, Esters and Derivatives (1990) Patai S., editor. John Wiley & Sons, Inc.; Chichester, UK: 1990. Biological activity of sulfinic acid derivatives; pp. 665–676. [DOI] [Google Scholar]
- 103.Jacobsen J.G., Smith L.H. Biochemistry and physiology of taurine and taurine derivatives. Physiol. Rev. 1968;48:424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- 104.Wollmann H., Raether G. Efficacy testing of stabilizing agents in epinephrine model solutions. 19: stability of drugs and preparations. Pharmazie. 1983;38:37–42. [PubMed] [Google Scholar]
- 105.Watanabe T., Snell E.E. The interaction of Escherichia coli tryptophanase with various amino acids and their analogs. J. Biochem. 1977;82:733–745. doi: 10.1093/oxfordjournals.jbchem.a131750. [DOI] [PubMed] [Google Scholar]
- 106.Huxtable R.J. Physiological actions of taurine. Physiol. Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 107.Wen C., Li F., Zhang L., Duan Y., Guo Q., Wang W., He S., Li J., Yin Y. Taurine is involved in energy metabolism in muscles, adipose tissue, and the liver. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201800536. [DOI] [PubMed] [Google Scholar]
- 108.Marcinkiewicz J., Kontny E. Taurine and inflammatory diseases. Amino Acids. 2014;46:7–20. doi: 10.1007/s00726-012-1361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huxtable R.J., Lippincott S.E. Relative contribution of diet and biosynthesis to the taurine content of the adult rat. Drug-Nutr Interact. 1982;1:153–168. [PubMed] [Google Scholar]
- 110.Sukhotnik I., Aranovich I., Ben Shahar Y., Bitterman N., Pollak Y., Berkowitz D., Chepurov D., Coran A.G., Bitterman A. Effect of taurine on intestinal recovery following intestinal ischemia-reperfusion injury in a rat. Pediatr. Surg. Int. 2016;32:161–168. doi: 10.1007/s00383-015-3828-3. [DOI] [PubMed] [Google Scholar]
- 111.Xiao A., Xu C., Lin Y., Ni H., Zhu Y., Cai H. Preparation and characterization of κ-carrageenase immobilized onto magnetic iron oxide nanoparticles. Electron. J. Biotechnol. 2016;19:1–7. doi: 10.1016/j.ejbt.2015.10.001. [DOI] [Google Scholar]
- 112.Shimizu M., Zhao Z., Ishimoto Y., Satsu H. In: Azuma J., Schaffer S.W., Ito T., editors. Springer New York; New York, NY: 2009. Dietary taurine attenuates dextran sulfate sodium (DSS)-induced experimental colitis in mice; pp. 265–271. (Taurine 7). [DOI] [PubMed] [Google Scholar]
- 113.Ahmad M.K., Khan A.A., Ali S.N., Mahmood R. Chemoprotective effect of taurine on potassium bromate-induced DNA damage, DNA-protein cross-linking and oxidative stress in rat intestine. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuan J.M., Wang Zh. Effect of taurine on intestinal morphology and utilisation of soy oil in chickens. Br. Poultry Sci. 2010;51:540–545. doi: 10.1080/00071668.2010.506984. [DOI] [PubMed] [Google Scholar]
- 115.Huang C., Guo Y., Yuan J. Dietary taurine impairs intestinal growth and mucosal structure of broiler chickens by increasing toxic bile acid concentrations in the intestine. Poultry Sci. 2014;93:1475–1483. doi: 10.3382/ps.2013-03533. [DOI] [PubMed] [Google Scholar]
- 116.Linden D.R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxidants Redox Signal. 2014;20:818–830. doi: 10.1089/ars.2013.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dordević D., Jančíková S., Vítězová M., Kushkevych I. Hydrogen sulfide toxicity in the gut environment: meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021;27:55–69. doi: 10.1016/j.jare.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jones D.P., Coates R.J., Flagg E.W., Eley J.W., Block G., Greenberg R.S., Gunter E.W., Jackson B. Glutathione in foods listed in the national cancer institute's health habits and history food frequency questionnaire. Nutr. Cancer. 1992;17:57–75. doi: 10.1080/01635589209514173. [DOI] [PubMed] [Google Scholar]
- 119.Chang I.S., Kim B.H., Shin P.K. Use of sulfite and hydrogen peroxide to control bacterial contamination in ethanol fermentation. Appl. Environ. Microbiol. 1997;63:1–6. doi: 10.1128/aem.63.1.1-6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.LeBlanc J.G., Milani C., de Giori G.S., Sesma F., van Sinderen D., Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 121.Neta P., Huie R.E. Free-radical chemistry of sulfite. Environ. Health Perspect. 1985;64:209–217. doi: 10.1289/ehp.8564209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kerns J.C., Arundel C., Chawla L.S. Thiamin deficiency in people with obesity. Adv. Nutr. 2015;6:147–153. doi: 10.3945/an.114.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jiang X., Du B., Zheng J. Glutathione-mediated biotransformation in the liver modulates nanoparticle transport. Nat. Nanotechnol. 2019;14:874–882. doi: 10.1038/s41565-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sugio K., Inoda D., Masuda M., Azumaya I., Sasaki S., Shimono K., Ganapathy V., Miyauchi S. Transport of 2,4-dichloro phenoxyacetic acid by human Na+-coupled monocarboxylate transporter 1 (hSMCT1, SLC5A8) Drug Metabol. Pharmacokinet. 2019;34:95–103. doi: 10.1016/j.dmpk.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 125.Bemrah N., Vin K., Sirot V., Aguilar F., Ladrat A.-C., Ducasse C., Gey J.-L., Rétho C., Nougadere A., Leblanc J.-C. Assessment of dietary exposure to annatto (E160b), nitrites (E249-250), sulphites (E220-228) and tartaric acid (E334) in the French population: the second French total diet study. Food Addit. Contam. 2012;29:875–885. doi: 10.1080/19440049.2012.658525. [DOI] [PubMed] [Google Scholar]
- 126.Walker R. Sulphiting agents in foods: some risk/benefit considerations. Food Addit. Contam. 1985;2:5–24. doi: 10.1080/02652038509373522. [DOI] [PubMed] [Google Scholar]
- 127.Leclercq C., Molinaro M.G., Piccinelli R., Baldini M., Arcella D., Stacchini P. Dietary intake exposure to sulphites in Italy - analytical determination of sulphite-containing foods and their combination into standard meals for adults and children. Food Addit. Contam. 2000;17:979–989. doi: 10.1080/02652030010014402. [DOI] [PubMed] [Google Scholar]
- 128.Kimura H. Hydrogen sulfide and polysulfides as signaling molecules. Proc. Jpn. Acad. B Phys. Biol. Sci. 2015;91:131–159. doi: 10.2183/pjab.91.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishigami M., Hiraki K., Umemura K., Ogasawara Y., Ishii K., Kimura H. A source of hydrogen sulfide and a mechanism of its release in the brain. Antioxidants Redox Signal. 2009;11:205–214. doi: 10.1089/ars.2008.2132. [DOI] [PubMed] [Google Scholar]
- 130.Ogasawara Y., Isoda S., Tanabe S. Tissue and subcellular distribution of bound and acid-labile sulfur, and the enzymic capacity for sulfide production in the rat. Biol. Pharm. Bull. 1994;17:1535–1542. doi: 10.1248/bpb.17.1535. [DOI] [PubMed] [Google Scholar]
- 131.Toohey J.I. Sulfur signaling: is the agent sulfide or sulfane? Anal. Biochem. 2011;413:1–7. doi: 10.1016/j.ab.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 132.Mikami Y., Shibuya N., Kimura Y., Nagahara N., Ogasawara Y., Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem. J. 2011;439:479–485. doi: 10.1042/BJ20110841. [DOI] [PubMed] [Google Scholar]
- 133.Brookes N., Turner R.J. K(+)-induced alkalinization in mouse cerebral astrocytes mediated by reversal of electrogenic Na(+)-HCO3- cotransport. Am. J. Physiol. Cell Physiol. 1994;267:C1633–C1640. doi: 10.1152/ajpcell.1994.267.6.C1633. [DOI] [PubMed] [Google Scholar]
- 134.Whitfield N.L., Kreimier E.L., Verdial F.C., Skovgaard N., Olson K.R. Reappraisal of H2S/sulfide concentration in vertebrate blood and its potential significance in ischemic preconditioning and vascular signaling. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1930–R1937. doi: 10.1152/ajpregu.00025.2008. [DOI] [PubMed] [Google Scholar]
- 135.Chauhan P.S., Saxena A. Bacterial carrageenases: an overview of production and biotechnological applications. 3 Biotech. 2016;6:146. doi: 10.1007/s13205-016-0461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chauhan P.S., Gupta N. Insight into microbial mannosidases: a review. Crit. Rev. Biotechnol. 2017;37:190–201. doi: 10.3109/07388551.2015.1128878. [DOI] [PubMed] [Google Scholar]
- 137.Sokolova E.V., Kravchenko A.O., Sergeeva N.V., Davydova V.N., Bogdanovich L.N., Yermak I.M. Effect of carrageenans on some lipid metabolism components in vitro. Carbohydr. Polym. 2020;230 doi: 10.1016/j.carbpol.2019.115629. [DOI] [PubMed] [Google Scholar]
- 138.De Ruiter G.A., Rudolph B. Carrageenan biotechnology. Trends Food Sci. Technol. 1997;8:389–395. doi: 10.1016/S0924-2244(97)01091-1. [DOI] [Google Scholar]
- 139.Yao Z., Wu H., Zhang S., Du Y. Enzymatic preparation of κ-carrageenan oligosaccharides and their anti-angiogenic activity. Carbohydr. Polym. 2014;101:359–367. doi: 10.1016/j.carbpol.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 140.Rhein-Knudsen N., Ale M., Meyer A. Seaweed hydrocolloid production: an update on enzyme assisted extraction and modification technologies. Mar. Drugs. 2015;13:3340–3359. doi: 10.3390/md13063340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rhein-Knudsen N., Ale M.T., Ajalloueian F., Yu L., Meyer A.S. Rheological properties of agar and carrageenan from Ghanaian red seaweeds. Food Hydrocolloids. 2017;63:50–58. doi: 10.1016/j.foodhyd.2016.08.023. [DOI] [Google Scholar]
- 142.Li J., Hu Q., Seswita-Zilda D. Purification and characterization of a thermostable λ-carrageenase from a hot spring bacterium, Bacillus sp. Biotechnol. Lett. 2014;36:1669–1674. doi: 10.1007/s10529-014-1520-7. [DOI] [PubMed] [Google Scholar]
- 143.van de Velde F., Lourenço N.D., Pinheiro H.M., Bakker M. Carrageenan: a food-grade and biocompatible support for immobilisation techniques. Adv. Synth. Catal. 2002;344:815–835. doi: 10.1002/1615-4169(200209)344:8<815::AID-ADSC815>3.0.CO;2-H. [DOI] [Google Scholar]
- 144.Hilliou L., Larotonda F.D.S., Abreu P., Ramos A.M., Sereno A.M., Gonçalves M.P. Effect of extraction parameters on the chemical structure and gel properties of κ/ι-hybrid carrageenans obtained from Mastocarpus stellatus. Biomol. Eng. 2006;23:201–208. doi: 10.1016/j.bioeng.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 145.Michel G., Helbert W., Kahn R., Dideberg O., Kloareg B. The structural bases of the processive degradation of ι-carrageenan, a main cell wall polysaccharide of red algae. J. Mol. Biol. 2003;334:421–433. doi: 10.1016/j.jmb.2003.09.056. [DOI] [PubMed] [Google Scholar]
- 146.Michel A.-S., Mestdagh M.M., Axelos M.A.V. Physico-chemical properties of carrageenan gels in presence of various cations. Int. J. Biol. Macromol. 1997;21:195–200. doi: 10.1016/S0141-8130(97)00061-5. [DOI] [PubMed] [Google Scholar]
- 147.Campo V.L., Kawano D.F., da Silva D.B., Carvalho I. Carrageenans: Biological properties, chemical modifications and structural analysis – a review. Carbohydr. Polym. 2009;77:167–180. doi: 10.1016/j.carbpol.2009.01.020. [DOI] [Google Scholar]
- 148.Barbeyron T., Michel G., Potin P., Henrissat B., Kloareg B. ι-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of κ-carrageenases. J. Biol. Chem. 2000;275:35499–35505. doi: 10.1074/jbc.M003404200. [DOI] [PubMed] [Google Scholar]
- 149.Michel G., Chantalat L., Fanchon E., Henrissat B., Kloareg B., Dideberg O. The ι-Carrageenase of Alteromonas fortis. J. Biol. Chem. 2001;276:40202–40209. doi: 10.1074/jbc.M100670200. [DOI] [PubMed] [Google Scholar]
- 150.Lemoine M., Nyvall Collén P., Helbert W. Physical state of κ-carrageenan modulates the mode of action of κ-carrageenase from Pseudoalteromonas carrageenovora. Biochem. J. 2009;419:545–553. doi: 10.1042/BJ20080619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The paper is a review.