Abstract
The present study investigated whether neonatal exposure to the proinflammatory endotoxin lipopolysaccharide (LPS) followed by an antibiotic (ATB)-induced dysbiosis in early adulthood could induce neurodevelopmental disorders-like behavioral changes in adult male rats. Combining these two stressors resulted in decreased weight gain, but no significant behavioral abnormalities were observed. LPS treatment resulted in adult rats' hypoactivity and induced anxiety-like behavior in the social recognition paradigm, but these behavioral changes were not exacerbated by ATB-induced gut dysbiosis. ATB treatment seriously disrupted the gut bacterial community, but dysbiosis did not affect locomotor activity, social recognition, and acoustic reactivity in adult rats. Fecal bacterial community analyses showed no differences between the LPS challenge exposed/unexposed rats, while the effect of ATB administration was decisive regardless of prior LPS exposure. ATB treatment resulted in significantly decreased bacterial diversity, suppression of Clostridiales and Bacteroidales, and increases in Lactobacillales, Enterobacteriales, and Burkholderiales. The persistent effect of LPS on some aspects of behavior suggests a long-term effect of early toxin exposure that was not observed in ATB-treated animals. However, an anti-inflammatory protective effect of ATB cannot be assumed because of the increased abundance of pro-inflammatory, potentially pathogenic bacteria (Proteus, Suttrella) and the elimination of the bacterial families Ruminococcaceae and Lachnospiraceae, which are generally considered beneficial for gut health.
Keywords: Rats, Lipopolysaccharide, Antibiotics, Behavior, Microbiome
1. Introduction
The current state of the art suggests an important communication between the residing intestinal microorganisms, brain development, function, and behavior associated with psychiatric disorders [[1], [2], [3], [4], [5], [6]]. The concept known as the microbiota-gut-brain axis is now widely accepted [[7], [8], [9], [10], [11], [12], [13], [14]]. In neuroscience, the number of studies on the interactions between the brain, gastrointestinal tract, gut bacteria, and the bidirectional relationship between these systems is steadily increasing, including manipulation of gut microbial diversity by novel prebiotics as potential alternative therapeutic possibilities for the treatment of neurodevelopmental disorders [15]. The rodent model of psychiatric disorders is a valuable source of information that can significantly expand our understanding of the role of the gut microbiota in the etiopathology of mental diseases and elucidate the effects of the microbiome on behavior and cognitive function [16,17]. Exposure of rodents to inflammatory agents, such as the proinflammatory endotoxin lipopolysaccharide (LPS), during early development, is one of the animal models used to study neurodevelopmental disorders [[18], [19], [20], [21], [22], [23]]. LPS is part of the outer membrane of Gram-negative bacteria and is known to induce a strong immune response, resulting in increased levels of proinflammatory mediators (e.g., IL-1β, TNF-α) that affect the brain [24] and induce sickness behavior [25].
Increased anxiety-like and depressive-like behavior after LPS administration have been reported in mice [[26], [27], [28], [29], [30]] and rats [[31], [32], [33], [34], [35], [36]]. In these studies, a decrease in locomotor activity, food intake, social interaction, and/or exploration of novel objects was observed in rodents several hours after the endotoxin insult. Studies on the long-term effects of LPS treatment are not as consistent, showing increased anxiety-like behavior [37,38], compulsive responses [39], and impaired social interaction [40], while no impact on locomotor activity was observed in rats in rats [21,39] and no significant changes in anxiety- and depression-like behaviors were observed in adult mice [41]. On the other hand, studies mostly agree that LPS leads to disruption of sensorimotor gating [21,[42], [43], [44], [45]], expressed as the prepulse inhibition (PPI) deficit, a proposed biomarker of schizophrenia-like behavior [[46], [47], [48]].
Although neonatal LPS immune activation is considered a crucial factor in vulnerability to neuropsychopathological disorders, Bilbo et al. [49] observed in a rat model that some impairment effects may only occur once a secondary stressor is introduced. In particular, complex neurodevelopmental disorders such as schizophrenia or autism may require both exposures, i.e., a specific infection at a specific time in early development combined later with other environmental hits [50]. The type of stressor is essential information to consider and significantly affects the outcome [51,52]. The second hit can include a secondary immune challenge [49], different kinds of stress [37,53,54], or gut dysbiosis induced by antibiotics [11,55].
Antimicrobial treatment can be applied to induce microbiome perturbation under controlled conditions. It can therefore be used in animals as a methodological tool to evaluate the effects of the gut microbiota on behavior [16,56]. In mice and rats, antibiotic administration in early life [[57], [58], [59]], adolescence [60], and adulthood [[61], [62], [63]] has been well studied and shown to alter many factors, including hormone levels, gene expression, anxiety-related responses, exploratory and social behaviors, and cognitive functions [22]. On the other hand, the effect of LPS on the gut microbiome is less studied. This bacterial endotoxin is a potent activator of innate immune responses [64]. It has been well studied and was shown to be able to change many factors, including hormonal levels, gene expression, anxiety-related reactions, exploratory and social behavior, and cognitive functions. LPS-induced cytokines are not derived only from innate or adaptive immune cells but also from intestinal epithelial cells and thus may be essential factors in intestinal inflammation [65]. Gut microorganisms actively participate in the host inflammatory response through cytokine-microbiota or microbiota-cytokine modulation interactions [66]. This bidirectional relationship shapes the gut microbiota's structure, and microbial composition changes can inhibit or stimulate inflammatory pathways [67]. However, the number of studies investigating the impact of LPS on the microbiome in laboratory animals is still limited, and results vary between rodent species. Significant effects on gut bacteria have been reported in mice [[68], [69], [70]]. Still, no effect of toxin treatment on fecal bacterial community composition has been found in rats [71] or hamsters [55]. It is clear that the different rodent models are not comparable and that further research is needed to evaluate the consequences of an early inflammatory event on the gut microbiome.
Animal models are undoubtedly valuable tools for studying the onset of neurodevelopmental disorders, explaining the origin of psychiatric abnormalities, creating model examples of pathological symptoms, or testing the effects of drugs that are not feasible in humans [72]. Animal models are also critical for establishing cause-and-effect relationships between disease, behavior, and other factors, such as the microbiome [73]. The rodent model of neurodevelopmental disorders is often based on the “two hit hypothesis"' formulated as early as 1999 by Bayer et al. [74]. This concept proposed that the development of the disease process requires a genetic predisposition (first hit) combined with the second hit(s) early or later in life, which may include multiple environmental factors such as viral infection(s), birth complications, or social stressors. This idea was reflected in animal models and led to a spate of rodent models of neurodevelopmental disorders using several different first/second insults [50]. Prenatal or early life LPS challenge is often used as the first hit, but the types of the second hit vary from study to study. Intestinal dysbiosis induced by antibiotic treatment is not commonly used. Still, this type of second insult is worth investigating because there is growing evidence that the mammalian digestive tract microbiome affects host physiology, modulates immunity, development, and nutrient uptake [75,76], and that its disruption affects not only physical but also mental health [8] due to the dysregulation of the gut-microbiome-brain axis [3,5,11,[77], [78], [79], [80], [81]].
The present study aimed to evaluate whether two stressors, the first applied in early life and the second in early adulthood, could induce neurodevelopmental changes in adulthood in a rodent model. LPS treatment as a first stressor mimics a bacterial infection, and antibiotic treatment as a second stressor influences intestinal bacteria. The connections known as the gut-microbiome-brain axis can consequently influence animals' exploratory, social behaviors and cognitive functions. Male rats were exposed to a combination of neonatal LPS challenge and antibiotic treatment in early adulthood to examine effects on locomotor activity, juvenile conspecific rat recognition, and sensorimotor gating. Behavioral tests were selected because they are known to be both relevant to symptoms of neurodevelopmental disorders [82] and affected by changes in gut microbiota composition induced by antibiotic treatment [83]. As a secondary outcome, we determined the impact of both insults on the gut microbiome assessed by high-throughput sequencing (HTS) of 16 S rRNA fragments of fecal bacteria. We hypothesize that the neonatal subchronic LPS insult has long-term effects on behavior in early adult rats and gut bacterial composition. Still, it is unclear whether antibiotic treatment in early adulthood can amplify or attenuate this presumed effect and induce behavioral deviations in rats, including gut microbiota profile.
2. Materials and methods
Ethical statement
The animal experiment was conducted at the National Institute of Mental Health (Klecany, Czech Republic). Animal handling, treatment, and clinical examinations were performed in accordance with instructions of the National Committee for the Care and Use of Laboratory Animals and were approved by the Local Animal Care Committee (MHCR No. 35/2017, 17,668/2017-3OVZ, April 13, 2017). All procedures were performed in concordance with the European legislation Directive 2010/63/EU on protecting animals for scientific purposes and the Czech legislation Act on the Protection of Animals from Cruelty no. 246/1992.
2.1. Animals, treatment, and study design
One hundred fifty-three male Wistar/Hann rats (Velaz Ltd., Czech Republic) used in the experiments were housed in standard 48 × 21 × 21-cm polysulfone cages (Tecniplast, Buguggiate, Italy) in an air-conditioned room (22 ± 2 °C) with a standard 12 h light/12 h dark cycle (lights on from 06:00–18:00). Rat litters with their mothers were obtained from the supplier on postnatal day 2 (PD 2). On PD 5, rats were permanently identified using finger marks, weighed, and the animals of each litter were divided into two cohorts. The first group was treated with lipopolysaccharide (LPS), an endotoxin from E. coli, serotype O26:B6 (L-8274, Sigma-Aldrich, St. Louis, MO, USA). LPS was dissolved in 0.9% NaCl and administered intraperitoneally (i.p.) at a dose of 2 mg/day/kg body weight (b.w.) to neonatal rats for 5 consecutive days (PD 5–9). A vehicle control containing 0.9% NaCl was injected on the same days in the second group of animals that formed the control cohort (CTL). All pups were weaned on PD 28 and kept in smaller groups (3–4 animals) according to litter and treatment. Rats were fed a normal chow diet (feed mixture ST-1, Velaz, Prague, Czech Republic) and were given tap water to drink ad libitum. From PD 50 to the end of the experiment, male rats were kept in pairs or a maximum of three animals per cage, depending on neonatal pretreatment and adult treatments.
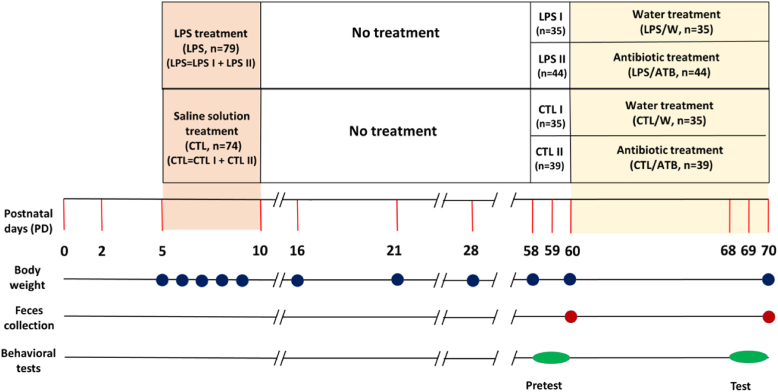
The endotoxin-treated group (LPS, n = 79) and the saline-treated control group (CTL, n = 74) were further randomly divided into LPS I (n = 35) and CTL I (n = 35) subgroups, which were transformed into two water-treated control groups LPS/W (n = 35) and CTL/W (n = 35). The other two subgroups LPS II (n = 44) and CTL II (n = 39), were transformed into antibiotic-treated (ATB) groups LPS/ATB (n = 44) and CTL/ATB (n = 39). The early adult rats in the antibiotic groups received the ATB mixture for 10 consecutive days in sterile drinking water (PD 60–69). In contrast, the rats in the control groups received the same amount of sterilized tap water (W). The antibiotic cocktail (50 ml/animal/day) consisted of metronidazole (500 mg/L; B. Braun, Melsungen, Germany), vancomycin (250 mg/L; Mylan S.A.S, Saint Priest), and colistine (15,000 U/mg; Sigma-Aldrich) to target a broad spectrum of both Gram-positive and Gram-negative bacteria [[84], [85], [86]]. Drinking water in all cages was lightly sweetened (1% sucrose solution, Sigma-Aldrich) to suppress the bitter taste of the antibiotics. Sweetened water was provided to all animals in all groups from day 60, i.e., rats in the antibiotic groups LPS/ATB and CTL/ATB and non-antibiotic groups of rats LPS/W and CTL/W to ensure the same conditions. Drinking water was replaced daily, and water consumption in each cage was recorded. Animal body weights were recorded daily at PD 5–9 and subsequently at PD 16, PD 21, PD 28, PD 58, PD 60, and PD 70. Behavioral tests were performed as pretests before ATB or water treatment (PD 58–60) and as tests at the end of ATB or water treatment (PD 68–70). Feces were collected at the beginning (PD 60) and at the end of ATB or water treatment (PD 70). The schematic of the study design is shown in Fig. 1.
Fig. 1.
The study design diagram shows the experimental rat groups and time schedule of treatments (PD), body weight determinations (blue circles), fecal sampling (red circles), and behavioral pretests and tests (green ovals). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Behavioral testing
All animals were consequently subjected to the three behavioral tests—open field test (OFT), social recognition test, and prepulse inhibition (PPI) evaluation. OFT is commonly used as a marker of basal animal activity, including the animals' responsiveness to a stressogenic and novel environment. Experimentally it is suggested that locomotor activation (i.e., hyperlocomotion) is similar to the positive clinical signs in neurodevelopmental disorders. The social recognition test, based on short-term working memory, reflects the ability of social animals to recognize and remember familiar individuals of the same species. In neurodevelopmental disorders, frequent negative symptoms (social withdrawal) and cognitive symptoms (memory impairment) are described. Supported experimental procedures can be observed a similar spectrum of symptoms. The last behavioral test, prepulse inhibition of startle (PPI), is a frequently used method in evaluating the effect of drugs in various models of neuropsychiatric disorders because this reaction is believed to be a marker of sensorimotor gating. Deficient PPI is described in several neuropsychiatric disorders [87]. The advantage of PPI is based on the fact that it can be measured across species using very similar experimental parameters.
Rats were tested in two blocks: Pretests (PD 58–60) and Tests (PD 68–70). The pretests were performed before the second stressor treatment to examine the effects of LPS toxin on the behavior of the early adult rats (CTL I vs. LPS I, CTL II vs. LPS II). The tests were performed at the end of ATB (or water) treatment to examine the long-term consequences of neonatal LPS insult alone (CTL/W vs. LPS/W), of early adult ATB treatment alone (CTL/W vs. CTL/ATB), and in combination with neonatal LPS hit (LPS/W vs. LPS/ATB).
2.2.1. Open field test
The Open Field Test (OFT) was used to assess locomotor and spontaneous exploratory activity. Locomotor activity was measured with a video camera connected to an Ethovision video tracking system (EthoVision Color Pro-Version 3.1, Noldus Information Technology, Wageningen, The Netherlands). Rats were placed individually in the center of the dimly lit arena (80 × 80 cm with black walls 30 cm high), which was located in a soundproof room, and locomotor activity was assessed as the length of trajectory in cm over the 30-min series of trials (pretest and test). The arenas were cleaned between each trial.
2.2.2. Social recognition
The social recognition abilities of rats were assessed using the modified juvenile recognition procedure described by Richter et al. [88]. Briefly, male rats tested were individually caged for 24 h prior to the confrontation with a juvenile conspecific rat (22–26 days old) that represented a social stimulus. Immature rats were housed in small cages for 3 h before being transferred to the home cage of the tested adult. Social behavior was assessed during a 5-min interaction period. The assessment was based on the adult's exploration of the young rat's head, body, anogenital area, forepaw manipulation, and touching. After 30 min, the exposure of a young animal was repeated to determine whether the second exposure reflected juvenile-related memory. Testing was performed as a pretest before ATB administration and as a test at the end of ATB administration. The exploratory behavior of the adult towards the young animal was recorded and analyzed using the software ACTIVITIES [89]. The program generated pulses (at intervals of 0.1 s) and calculated the number of occurrences, total duration (i.e., total time spent performing the activity), and latency (i.e., time from onset of observation to first occurrence) of selected behavioral elements (recorded by key codes). In addition, rearing, a nonsocial parameter indicating anxiety-like behavior, was also documented in these circumstances. Experiments were scored by an experimenter who was blinded to the treatment groups.
2.2.3. Acoustic Startle Response and prepulse inhibition
Acoustic Startle Response (ASR) and Prepulse Inhibition (PPI) tests were performed using the SR-LAB startle response system (San Diego Instruments, USA), as described previously [90]. Briefly, rats underwent a 5-min adaptation period in a startle chamber with continuous white noise background (70 dB). Then, 50 trials were presented in a pseudorandom order: 10 without stimulus for 30 m s, 30 prepulse + pulse inhibition with prepulse intensities of 3 (PP3), 5 (PP5), and 10 (PP10) dB [A] above background for 30 m s, and 10 startle pulse alone with intensity 120 dB (P120) for 30 m s. The interval between the prepulse and the startle pulse was fixed at 70 m s. The inter-trial period ranged from 10 to 20 s, and the entire session lasted 17 min. All animal groups were tested in mixed order, always with litter-matched controls. ASR was assessed as the amplitude of the motor reaction expressed in mV. Prepulse inhibition was expressed as % PPI and calculated using formula [100 - (100 × startle amplitude at PP)/P120], where PP is the average response to specific prepulse + pulse intensity trials and P120 is the startle alone. The total PP (PPx) was expressed as the mean of all different prepulses + pulses (PP3 + PP5 + PP10). Data from animals whose average response to the startle trials was below 10 mV (i.e., nonresponders) were excluded from the statistical calculations.
2.2.4. Statistical analysis
Behavioral tests and body weights were analyzed using BMDP and SigmaStat statistical software. All data, with some exceptions, were analyzed using a two-way between-subjects ANOVA with neonatal pretreatment as one factor and antibiotic treatment as the second factor. The exceptions are as follows. The pretest and test ASR and PPI values were compared using a three-way repeated-measures ANOVA model with two between-subjects factors of pretreatment (LPS vs. CTL), treatment (ATB vs. W), and one within-subjects factor of the experimental phase (pretest vs. test). The habituation curves resulting from the open field test were analyzed using a four-way repeated-measures ANOVA with two between-subjects factors of pretreatment and treatment and two within-subjects factors of experimental phase and time (6 levels, i.e., 5-min segments). Finally, body weight analyses up to PD 60 were performed using a one-way between-subjects ANOVA with pretreatment as the only factor. Bonferroni-corrected Student t-tests were used as post-hoc tests. Test results were considered significant when p < 0.05. Significant ANOVA F-test and Student t-test results are accompanied by eta-squared (eta-sq) and Cohen's d (d) effect size coefficients, resp. All data were expressed as means ± S.E.M.
2.3. Microbiome analysis
2.3.1. Sample collection
Fresh fecal pellets were obtained aseptically by direct defecation of rats into a sterile 120-ml container (VWR, Radnor, PA, USA). Samples were collected from the 153 rats at PD 60, the pretest phase (baseline), and PD 70, the test phase (end of ATB or water treatment) as described above. A total of 306 samples were collected to track individual changes in the fecal microbiome as a result of drug treatment. Samples were immediately frozen, transferred on dry ice to the Laboratory of Anaerobic Microbiology of the Institute of Animal Physiology and Genetics of the Czech Academy of Sciences (Czech Republic), and stored at −25 °C until use.
2.3.2. DNA extraction and PCR amplification
According to the manufacturer's protocol, genomic DNA was isolated from fecal samples using the DNeasy PowerSoil Kit (Qiagen, Hilden, Germany). The concentration and purity of the extracted nucleic acids were checked using a NanoDrop 2000c UV–Vis spectrophotometer (Thermo Scientific, Waltham, MA, USA). DNA extracts were stored at −25 °C until use. The hypervariable regions V4–V5 of bacterial 16 S rRNA were amplified with primers BactB-F: GGATTAGATACCCTGGTAGT and BactB-R: CACGACACGAGCTGACG according to Fliegerova et al. [91] using EliZyme HS Robust MIX Red (Elisabeth Pharmacon, Croydon, UK). Thermal cycling conditions were as follows: 5 min initial denaturation at 95 °C; 30 cycles of denaturation at 95 °C for 30 s, annealing at 57 °C for 30 s and elongation at 72 °C for 30 s; final elongation at 72 °C for 5 min.
2.3.3. High-throughput sequencing
PCR amplicons (∼300 bp) were purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany). Libraries were prepared using the NEBNext Fast DNA Library Prep Set for Ion Torrent (New England BioLabs, UK) and the Ion Xpress Barcode Adapters 1–96 Kit (Thermo Fisher Scientific, Waltham, MA, USA). The amplicons were then pooled in equimolar ratios based on the concentration determined using a KAPA Library Quantification Kit (KAPA Biosystems). The sequencing template was prepared by emulsion PCR in an OneTouch 2 instrument using an Ion PGM OT2 HiQ View kit (ThermoFisher Scientific, Waltham, MA, USA). HTS was performed in an Ion Torrent PGM platform with an Ion 316 Chip Kit v2 BC (ThermoFisher Scientific) using an Ion PGM Hi-Q View Sequencing Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's protocols.
2.3.4. Bioinformatics and statistical analysis
Sequence data retrieved from the Ion Torrent Software Suite in fastq format were analyzed following the procedure of Bolyen et al. [92]. Reads were processed as described previously [93]. Briefly, sequences were trimmed, quality filtered, and chimeras were removed. The Amplicon Sequence Variants (ASVs) were generated using DADA2 software. The dataset was subsampled to a minimum of 6000 reads per sample to enable equal sampling depth. Sequence processing resulted in the elimination of 10 samples. The following 296 samples of respective groups of animals were included in the bacterial community structure analysis: the baseline LPS (n = 76) and CTL (n = 72) groups, test phase antibiotic-treated groups CTL/ATB (n = 39) and LPS/ATB (n = 43), and the water-treated groups CTL/W (n = 33) and LPS/W (n = 33). The VSEARCH-based consensus classifier was used to assign taxonomy against Greengenes database version 13_8 [94]. Bacterial community alpha diversity was assessed using Chao1, evenness, Faith's phylogenetic diversity, and the Shannon index. Beta diversity was assessed using Jaccard's distance metric with Qiime2 version 2020.2 [92]. Sequence data and information were deposited in the Sequence Read Archive under accession number PRJNA765490.
The bacterial community was assessed using the parameters of alpha and beta diversity. Alpha diversities among groups of differently treated animals were compared using the Kruskal-Wallis H test. Statistical p-values and q-values with Benjamini-Hochberg false discovery rate are reported. Beta diversities among the studied groups were assessed with a nonparametric permutational multivariate ANOVA (PERMANOVA) test. The PERMDISP test was performed to support the PERMANOVA results. Linear discriminant analysis (LDA) effect size with standard parameters [95] was used to determine bacterial taxa with significantly different abundance levels.
3. Results
3.1. Body weight
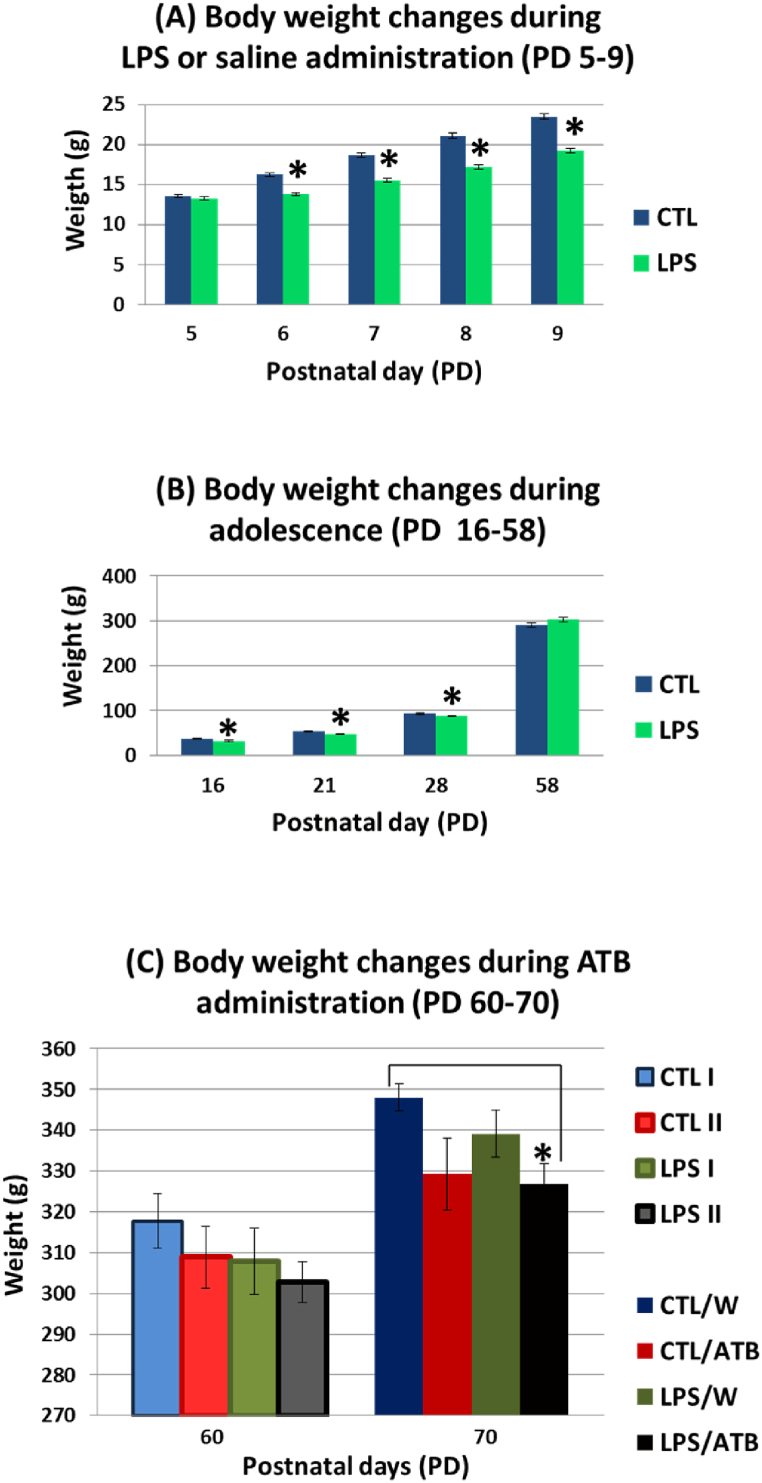
Body weight gain was significantly suppressed during neonatal LPS treatment (since PD 6) as shown by the results of ANOVA (PD 6: F (1,151) = 63.57, p < 0.0001, η2 = 0.2963; PD 7: F (1,151) = 77.19, p < 0.0001, η2 = 0.3383; PD 8: F (1,151) = 94.37, p < 0.0001, η2 = 0.3846, PD 9: F (1,151) = 94.01, p < 0.0001, η2 = 0.3837) (Fig. 2(A)). The significant negative effect of endotoxin persisted until weaning (PD 16–28) (PD 16: F (1,24) = 10.56, p = 0.0034, η2 = 0.3056; PD 21: F (1,24) = 8.67, p = 0.0071, η2 = 0.2654; PD 28: F (1,86) = 4.74, p = 0.0315, η2 = 0.0522), but was diminished during further development of the animals (Fig. 2(B)). The significant changes in body weight were induced by the 10-day treatment with the ATB cocktail (PD 70: F (1,149) = 6.19, p = 0.0140, η2 = 0.0399) (Fig. 2(C)). A lower weight gain in both groups (CTL/ATB, LPS/ATB) compared to the respective controls (CTL/W, LPS/W) was clearly documented. However, a post-hoc analysis showed only a difference between the LPS/ATB and CTL/W groups (t = −3.28 df = 75, p = 0.0016, d = −0.7112). The body weight development in adulthood was not affected by early LPS administration (Fig. 2(C)), as the lower weight gain from weaning (PD 28) to adulthood (PD 70) of animals in the LPS/W group was not significantly different from the control group (CTL/W).
Fig. 2.
Body weight gain of rats during neonatal LPS treatment (A), during adolescence (B), and before (PD 60) and after (PD 70) 10-day ATB cocktail treatment (C). Results are expressed as mean ± SEM for the respective animal groups, *p < 0.05.
3.2. Behavioral tests
All animals were subjected to behavioral tests in the following order: open field test, social recognition, and prepulse inhibition concerning the ethological approach (i.e., all animal groups were tested in mixed order, always with litter-matched controls). The tests were chosen to reflect the expected changes in behavior. All animal groups were tested randomly and during the same daytime period from 6:30 a.m. to 2:00 p.m., with one test per day.
3.2.1. Open field test
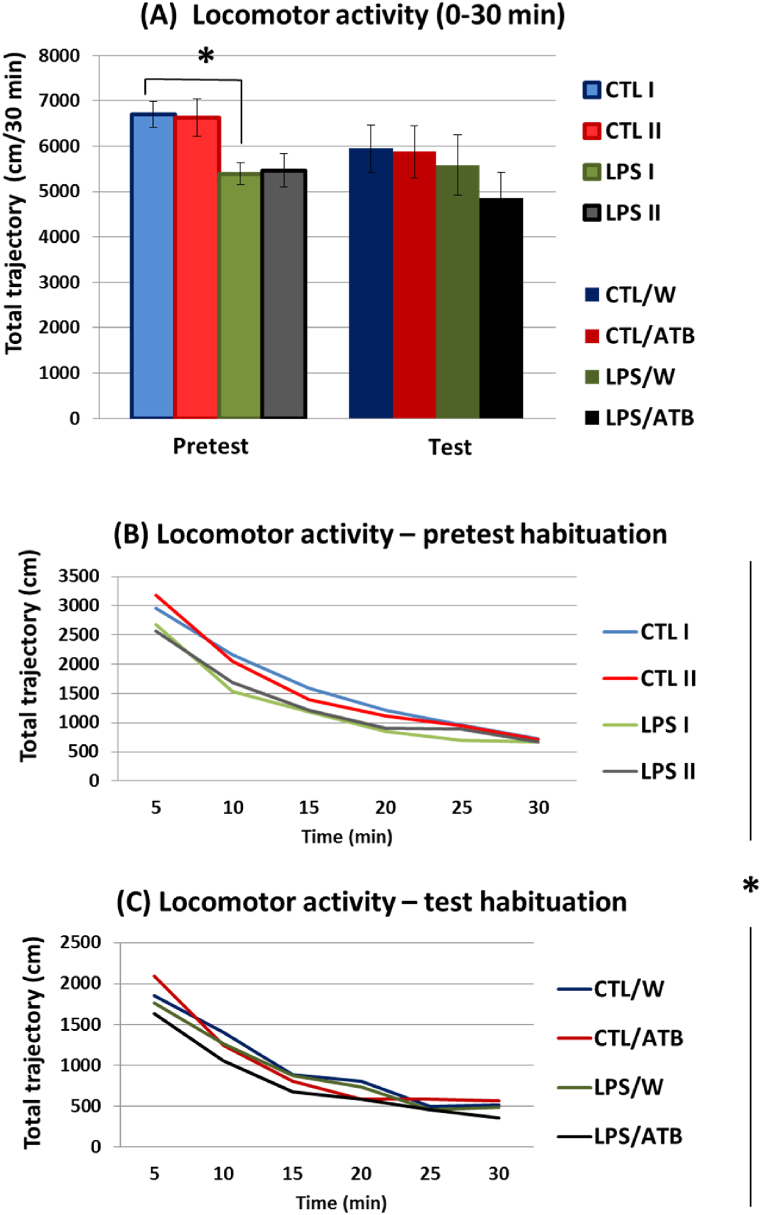
In the open field pretest (i.e., before administration of the antibiotics), rats neonatally treated with the toxin showed significantly reduced locomotor activity (F (1,44) = 9.09; p = 0.0043, η2 = 0.1712). The rats in the LPS I group traveled a shorter distance than the untreated CTL I group during 30 min, as shown in Fig. 3(A) (pretest) and supported by post-hoc analysis (t = −3.48, df = 21, p = 0.0021, d = −1.4199). In the test session (i.e., at the end of antibiotic administration), no significant differences in distance traveled were observed between the 4 groups (CTL/W, CTL/ATB, LPS/W, and LPS/ATB) (Fig. 3(A), test), indicating that neither LPS (F (1,44) = 1.38, p = 0.2466) nor the ATB cocktail (F (1,44) = 0.45, p = 0.5056) affected the locomotor activities of the rats.
Fig. 3.
The spontaneous exploratory activity of differently treated groups of adult rats was studied in the open field pretest (PD58/60) and test (PD68/70). The locomotor activity of the rats during the 30 min of the two sessions was expressed as a total trajectory (A). Results are given as mean ± SEM for each animal group, *p < 0.05. The habituation curves of the rats in the six 5-min blocks of the pretest (B) and test (C) sessions. Values are expressed as the mean distance traveled within each 5-min block.
On the other hand, the comparison of the habituation curves between the groups of animals showed a different locomotor activity of the rats treated with LPS (F (1,44) = 6.84, p = 0.0121, η2 = 0.1345) in the pretest and test sessions (i.e., mean values from 6 to 6 blocks of measurements taken during the pretest and test, respectively). The toxin-treated groups displayed a lower score of total distance traveled. Statistically significant differences in six 5-min measurement blocks (F (5,220) = 2.53, p = 0.0297, η2 = 0.0544) documented different dynamics of the habituation curve in the LPS-treated rats. Panels B and C in Fig. 3 show that the curves of the LPS groups (LPS I and LPS II in the pretest, LPS/W, LPS/ATB in the test) are not parallel to the corresponding curves of the CTL groups (CTL I and CTL II in pretest, CTL/W and CTL/ATB in test), indicating faster habituation of the control groups. Thus, neonatal LPS treatment induced total hypoactivity in adult rats, especially in the open field pretest (Fig. 3(B)), whereas ATB administration had no significant effects on spontaneous exploratory activity and habituation parameters (Fig. 3(C)).
3.2.2. Social recognition
Two expositions examined the total exploration and social investigation of the juveniles during the pretest and test sessions (in total, four juvenile object expositions). Both sessions resulted in similar social contact with the conspecific juvenile of all tested rat groups. Thus, social memory performance did not appear to be affected by both LPS administration in the early neonatal period and the 10-day ATB treatment in adulthood (data not shown).
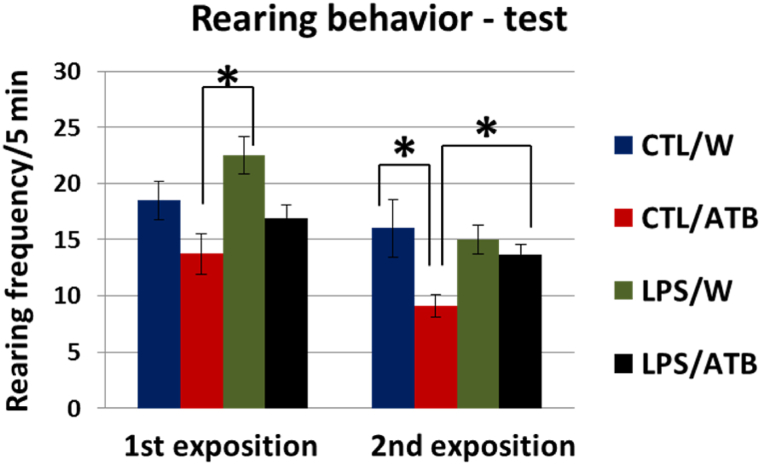
However, a different behavior was observed in relation to rearing, which was used in this test as a parameter related to emotional–anxiety–provoking activity. While no significant differences were observed in the pretest, the differences were evident in both expositions of the test (Fig. 4). Two-way ANOVA showed the influence of both treatments, i.e., LPS and ATB, indicating that LPS induced an increase in rearing. In contrast, the effect of ATB was the opposite. This is already evident at the first exposure of the juvenile, where the LPS/W group had a significantly higher rearing frequency, as shown by the post hoc analysis of the difference between the CTL/ATB and LPS/W groups (t = −3.35, df = 26, p = 0.0025, d = −1.8892). At the re-exposition, after 30 min, no significant differences were found between the LPS/W and CTL/W groups. The ATB treatment alone (CTL/ATB) significantly decreased rearing frequency (F (1,26) = 7.23, p = 0.0123, η2 = 0.2176), while LPS insult in combination with ATB administration (LPS/ATB) significantly increased this behavior. This influence on rearing frequency was confirmed by post-hoc analysis, which showed significant differences between the CTL/ATB and CTL/W groups (t = 3.30, df = 26, p = 0.0028, d = 1.5036) and the LPS/ATB and CTL/ATB groups (t = −2.58, df = 26, p = 0.0160, d = −1.4712).
Fig. 4.
Rearing behavior of adult rats in social recognition test consisting of two expositions of the same juvenile subject. Results are expressed as mean ± SEM for each group of animals, *p < 0.05.
3.2.3. ASR and PPI
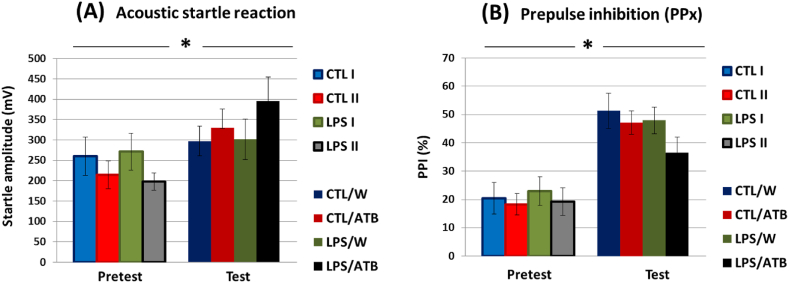
Acoustic startle responses did not differ significantly between animal groups in either session, indicating that neither early life LPS insult of rats by LPS nor antibiotic treatment of adults affected acoustic reactivity (Fig. 5(A)). Drug treatment resulted in only mild nonsignificant hyper-responsiveness to the startle pulse in animals in the LPS/ATB group (Fig. 5(A), test). However, a comparison of pretest and test values revealed a significant difference between these two datasets showing higher acoustic sensitivity of all animal groups in the test session (pretest vs. test: F (1,57) = 16.98, p < 0.0001, η2 = 0.2295) and higher sensitivity to strong acoustic stimuli elicited by the ATB treatment (F (1,57) = 6.93, p = 0.0109, η2 = 0.1084) (CTL/ATB, LPS/ATB).
Fig. 5.
Acoustic startle response (A) and prepulse inhibition (B) of differentially treated groups of adult rats assessed in pretest (PD58/59) and test (PD68/69) sessions. PPx represents the mean of all different prepulses (PP3, PP5, and PP10). Results are given as mean ± SEM for each group of animals, *p < 0.05.
Prepulse inhibition was not significantly different between groups at both the pretest and test, suggesting that neonatal LPS treatment and early adult ATB administration had no effect in adulthood. However, a comparison of the pretest and test values revealed again a significant difference between these two datasets showing the increased reactivity of all groups in the test session (Fig. 5(B)) (pretest vs. test: F (1,57) = 93.67, p < 0.0001, η2 = 0.6217). Both tests indicate that the animals' previous experience with the experimental setup resulted in a better response of the rats to the stimuli, regardless of the type of treatment. However, it appears that rats treated with LPS/ATB scored worse on PPI than animals of other groups.
3.3. Microbiome analysis
The present study analyzed two hundred and ninety-six fecal samples for bacterial diversity. Baseline samples were collected from 148 animals at PD 60 (CTL=CTL I + CTL II, LPS = LPS I + LPS II), and test samples were collected from 148 rats at PD 70 (CTL/W, LPS/W, CTL/ATB, and LPS/ATB) were analyzed for bacterial community composition to evaluate the effects of neonatal LPS insult and early adult ATB insult on the fecal microbiome in adulthood.
3.3.1. Alpha and beta diversity
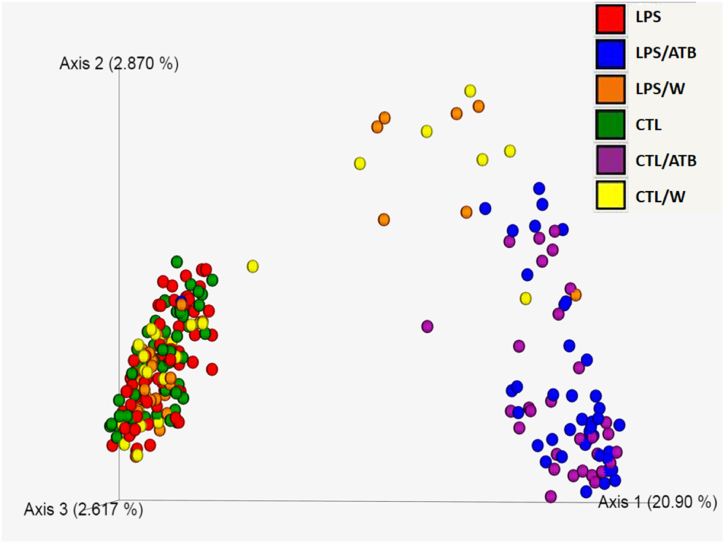
Bacterial community structure in feces from differentially treated rats was qualitatively and quantitatively examined for species richness and evenness, Faith's phylogenetic diversity, and Shannon entropy. Alpha diversity metrics indicated a significant effect of treatment with the antibiotic cocktail, which resulted in a significant decrease in diversity in the CTL/ATB and LPS/ATB groups of animals, as shown in the alpha diversity boxplots (Supplementary Figure S1). Kruskal-Wallis pairwise tests for all calculated metrics showed significant differences (0.001 ≤ p 0.05) between the two ATB-treated animal groups (CTL/ATB and LPS/ATB) and all other non-antibiotic groups (CTL, CTL/W, LPS, LPS/W). The decrease in diversity in the antibiotic-treated samples was similar in both ATB groups (CTL/ATB and LPS/ATB). No significant difference was observed between them, indicating the crucial influence of the antibiotic cocktail regardless of the previous LPS treatment. No significant differences were found between the CTL and LPS groups (PD 60) and between the CTL/W and LPS/W groups (PD 70), indicating that the animals recovered from the toxin-induced insult at an early age (PD 5–9). Shannon entropy values determined by the pairwise Kruskal-Wallis H test are summarized for all rat groups in Table S1. Beta diversity, which assesses the similarity of bacterial communities between rat groups, was determined using Jaccard's nonphylogenetic distance matrix. The main separation on axis 1 was driven by ATB treatment (Fig. 6). The CTL/ATB (purple) and LPS/ATB (blue) groups clustered together (non-significant differences PERMANOVA p = 0.081) and were significantly separated from four other rat groups (PERMANOVA p = 0.001). Even if non-antibiotic groups clustered together, the community structures of samples collected at PD 60 were significantly different from those collected at PD 70, i.e., the CTL group (green) was different from the CTL/W group (yellow) (PERMANOVA p = 0.001), and the LPS group (red) was different from the LPS/W group (orange) (PERMANOVA p = 0.001). This difference can most likely be attributed to the sweetening of the water used to suppress the bitter taste of the antibiotics, which was applied to all animals, including the control groups. On the other hand, samples collected on the same day did not differ significantly, i.e., at PD 60, the control CTL group could not be distinguished from the LPS group (PERMANOVA p = 0.596) and at PD 70, the control CTL/W group could not be distinguished from the LPS/W groups (PERMANOVA p = 0.314). Pairwise PERMANOVA and PERMDISP results for all groups of rats are summarized in Table S1.
Fig. 6.
Principal coordinate analysis (PCoA) showing the Jaccard's distance matrix of bacterial 16 S rRNA amplicons from fecal samples from six groups of differently treated rats. Each dot represents one sample, and a different color indicates each rat group. The percentage of variation explained by the plotted principal coordinates is indicated on the axes. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Taxonomical composition
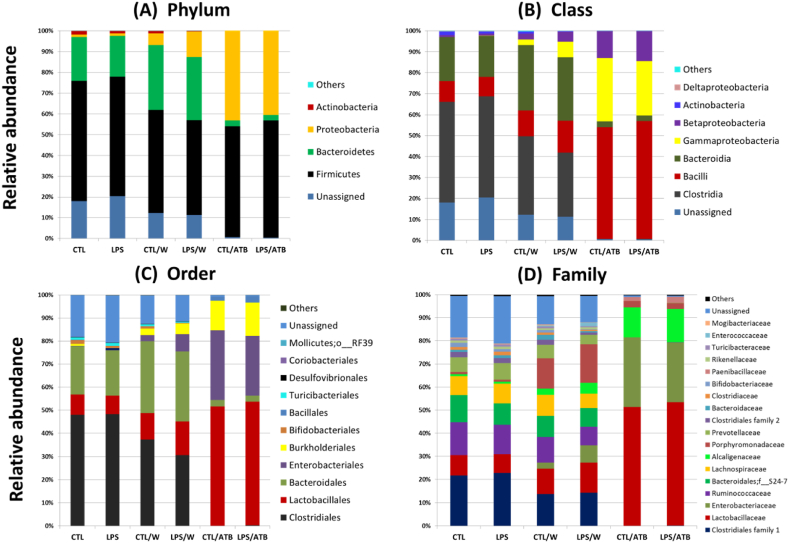
A total of 9 phyla (including 110 bacterial phylotypes) were detected in the rat groups studied, but only 4 of them, including Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, had meaningful relative abundance (Fig. 7(A)). The abundances of the phyla Verrucomicrobia, Cyanobacteria, Deferribacteres, Tenericutes, and TM7 were considerably low (< 0.03%) and are summarized as “others” in Fig. 7(A). Regardless of treatment, Firmicutes were detected as the dominant phylum in all groups (45.6–57.9%). Bacteroidetes was the second dominant phylum in the non-antibiotic-treated groups (19.7–31.2%), followed by a low abundance of Actinobacteria (0.3–1.8%), as shown in Fig. 7(A). In both ATB-treated groups (CTL/ATB, LPS/ATB), Proteobacteria (52%), dominated by order Enterobacteriales (25.1–30.1%), and Burkholderiales (13.0–14.4%), were the second most abundant phylum (40.4–43.2%), while Actinobacteria were depleted (Fig. 7(A, B, C)). In the non-antibiotic groups, Firmicutes and Bacteroidetes were mainly represented by the order Clostridiales (30.6–48.3%) and Bacteroidales (19.7–31.2%), respectively, and a high relative abundance of unassigned bacteria (11.4–20.4%) was observed in all four groups (CTL, LPS, CTL/W, LPS/W). The ATB treatment completely suppressed Clostridiales, while Lactobacillales were induced and formed the most abundant order (51.4–53.2%), as shown in Fig. 7(C). The reduced bacterial diversity due to antibiotic treatment of animals (CTL/ATB, LPS/ATB) is well seen at the family level (Fig. 7(D)). Both ATB-treated animal groups were dominated by Lactobacillaceae (51.4–53.2%), represented by the genus Lactobacillus, Enterobacteriaceae (26–31.1%), represented by Proteus and unassigned genus, and Alcaligenaceae (13–14.4%) represented by the genus Sutterella. ATB treatment considerably suppressed the abundance of unassigned bacteria (0.5–0.7%).
Fig. 7.
Relative abundance of fecal bacteria at the phylum (A), class (B), order (C), and family (D) levels in six groups of differently treated rats. Only taxa with a relative abundance of ≥0.1% in at least one group are reported.
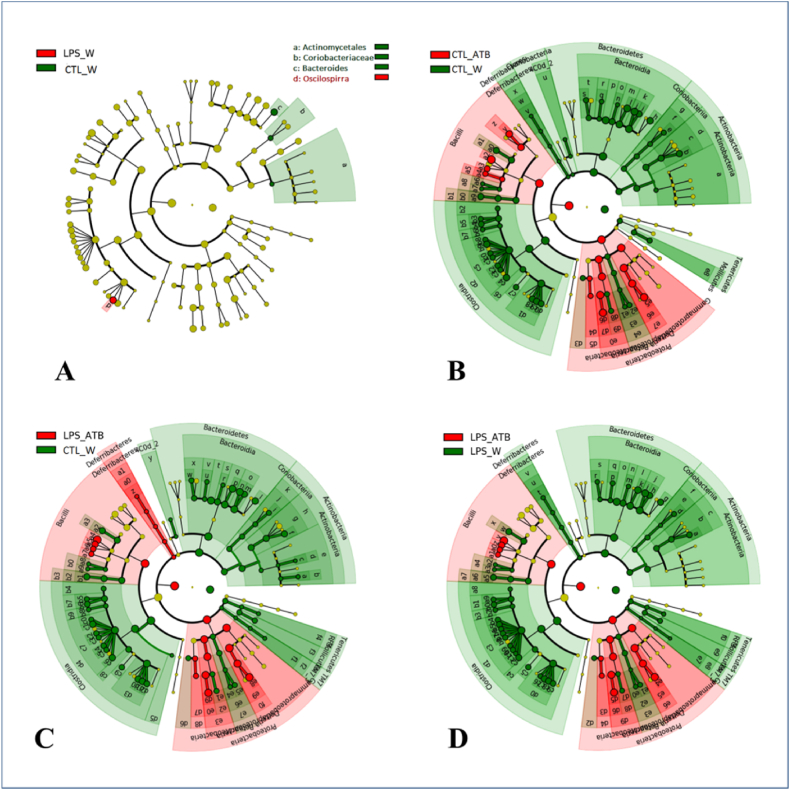
To elucidate specific phylotypes that respond to LPS and/or ATB treatment, the linear discriminant analysis (LDA) effect size (LEfSe) algorithm was applied to identify taxa that are more abundant in one group than in the other corresponding group of animals. To identify specific phylotypes that respond to LPS and/or ATB treatment, the linear discriminant analysis (LDA) algorithm was applied to determine the effect size (LEfSe) to identify more abundant taxa in one group than in the other group of animals. Regarding the influence of LPS, the four bacterial taxa were differentially abundant in the CTL/W and LPS/W groups (LDA score >3.0). Oscilospirra was overrepresented in the LPS/W group, whereas Bacteroides, Actinomycetales, and Coriobacteriaceae were overrepresented in control CTL/W group, as shown by a cladogram (Fig. 8(A)) and a histogram (Fig. S2). However, no significant differences were found between the LPS and CTL groups in PD 60 (data not shown). Regarding the influence of the antibiotic cocktail, the 95 bacterial taxa differed significantly in abundance between the CTL/ATB and CTL/W groups (LDA score >2.4), as shown in Fig. 8(B) and Fig. S3. ATB treatment (CTL/ATB) enriched 19 phylotypes of the phylum Proteobacteria and the class Bacilli. Among the 76 taxa enriched in the control group (CTL/W), the phylotypes of Bacteroidetes, Clostridia, and Actinobacteria are meaningful. In contrast, the taxa of Tenericutes, Defferibacteria, and Cyanobacteria are negligible due to their low relative abundance (together 0.03%). The combined treatment with LPS and ATB resulted in 103, and 98 differentially expressed phylotypes, respectively (LDA score >2.4), based on the comparison of the LPS/ATB group with the CTL/W (Fig. 8(C) and Fig. S4(A)) and LPS/W (Fig. 8(D) and Fig. S4(B)) groups, respectively. The overrepresented phylotypes are similar to those of the antibiotic-only treated group (CTL/ATB). Thus, the observed effect can be mainly attributed to the application of the antibiotic, which is confirmed by the limited changes in the LPS/W group (Fig. 8(A)) and the nonsignificant changes in LPS-induced taxa abundance determined by LEfSe analysis of the LPS and CTL groups (PD 60) and the CTL/ATB and LPS/ATB groups (PD 70).
Fig. 8.
Cladograms generated with LEfSe software show taxa with different abundance in fecal samples from rats treated with LPS and/or an antibiotic cocktail compared with their respective controls. Red regions indicate taxa enriched in LPS- and/or antibiotic-treated animals, whereas regions in green indicate taxa enriched in the respective control groups. (A) Four differentially abundant bacterial phylotypes between the groups of LPS-treated rats and the control group (LPS/W vs. CTL/W). (B) Ninety-five differentially abundant bacterial phylotypes between the group of antibiotic-treated rats and the control group (CTL/ATB vs. CTL/W). (C) One hundred and three differentially abundant bacterial phylotypes between the group of rats treated with LPS and antibiotics and the control group without any treatment (LPS/ATB vs. CTL/W). (D) Ninety-eight differentially abundant bacterial phylotypes between the group of rats treated with LPS and antibiotics and the control group treated with LPS (LPS/ATB vs. LPS/W). The names of the significantly enriched phylotypes are indicated in the LEfSe histograms of the LDA scores (Supplementary Figures S2–S4). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Here, we use an animal model to determine whether the early-life LPS challenge, followed by an ATB insult in early adulthood, can induce the behavioral changes relevant to neurodevelopmental disorders. However, the general results of our study suggest that this combination of two stressors did not have significant effects on the behavior of the rats. The strong tendency toward higher vulnerability to acoustic reflex and lower levels of PPI in the LPS/ATB-treated group was evident but not statistically proven. This finding is inconsistent with the neurodevelopmental disorder-like models of several authors [21,40,[43], [44], [45],96], all of whom described an LPS-induced deficit in prepulse inhibition in adult animals. However, in contrast to our work, these studies used either neonatal intrahippocampal [40] or prenatal intraperitoneal LPS injections [21,[43], [44], [45]], suggesting that the injection site and timing of LPS application appear to be critical for subsequent behavioral changes. Surprisingly, there is no information on the effect of antibiotic treatment and the resulting disruption of the microbiome on sensorimotor gating, as animals in the relevant (antibiotic-using) studies [60,62,97,98] have not been tested for prepulse inhibition and/or responses to intense acoustic stimuli. The usefulness of including such tests is illustrated by Giovanoli et al. [53], who show that antibiotic treatment can prevent the development of sensorimotor gating deficiency induced by combined prenatal immune activation and peripubertal stress in a mouse model of neurodevelopmental disorders. This finding may suggest that the antibiotics used as a second stressor in our study may have acted in the opposite direction and protected the animals from the consequences of LPS-induced inflammation. However, the results of the LPS-treated group (without ATB treatment) suggest that the effects of the early-life immune insult were transient. Adult rats generally overcame the endotoxin-induced discomfort documented in young rats by significantly reduced weight gain by weaning. No downstream effects of LPS on social behavior and sensorimotor gating in rats were detected in their adulthood.
On the other hand, LPS-challenged rats exhibited locomotor hypoactivity, but only in the pretest, lower habituation ability in OFT, and increased frequency of rearing as an indicator probably stress-related anxiety-like behavior in the presence of conspecific pups. Rearing behavior appears very sensitive to context situations [99]. It seems that experimental arrangement induced this response in LPS-pretreated rats. These results suggest some long-lasting consequences of LPS treatment, but the effect of endotoxin insult was less complex than in other studies. While the suppressive effect of LPS on weight gain [31,90,[100], [101], [102], [103], [104]] and locomotor activity [31,34,101,[105], [106], [107], [108], [109], [110], [111]] is well known, the depressive effect of LPS on social exploration of rats described by many authors [31,100,101,111] was not observed in our study.
No negative changes were observed in the fecal bacterial population of LPS-treated animals, again suggesting that adult rats overcame the early-age endotoxin insult. Increased occurrence of Oscillospira (LPS/W, PD 70) may even be considered beneficial because this bacterium is known to produce butyrate [112], which is a major source of energy for colon epithelial cells, plays an important role in maintaining the stability of the gut microbiota, alleviates mucosal inflammation, and strengthens the epithelial defense barrier [113,114]. This bacterium, commonly found in 60% of humans [115], is significantly decreased in patients with inflammatory bowel disease (IBD) [116,117] and was significantly reduced in SI rats (social isolation of rat pups from weaning as an animal model of schizophrenia) [118]. On the other hand, Xia et al. [68] described a higher abundance of this genus in adult LPS-treated mice immediately after 5 days of LPS challenge, which is inconsistent with IBD studies in humans and seems to be in contradiction with the proinflammatory nature of LPS [119]. However, due to its recent discovery and difficult cultivation, little is known about Oscillospira metabolism and/or physiology [112].
On the other hand, the effect of ATB on the microbiome was immense. A 10-day antibiotic treatment drastically reduced microbial diversity and altered the bacterial composition of adult rat feces. Both groups of ATB-treated animals were clearly separated from the non-antibiotic groups in PCoA analysis, regardless of LPS treatment. A significant decrease in bacterial diversity due to ATB treatment is consistent with the results of several studies in rats [62,[120], [121], [122]], mice [60,61,123], and hamsters [55]. However, the disturbances of the microbial community in the studies mentioned above were highly dependent on the type of antibiotics administered and the study design. In our work, the most apparent changes were a significant increase in Proteobacteria, a decrease in Bacteroidetes, and a depletion of Actinobacteria, phyla with low abundance, and unassigned species. The relative abundance of Firmicutes was not altered, but the community composition within this phylum was notably affected. Proteobacteria is a common phylum of mammal GIT, ranging from 2.5 to 10% in the healthy rat intestine [[124], [125], [126]]. Its elevated abundance is, however, increasingly recognized as a possible microbial signature of the disease state [127]. In our study, Proteobacteria were represented at the lowest taxonomically identifiable level by the family Enterobacteriaceae and the genera Proteus and Sutterella.
The family Enterobacteriaceae includes proinflammatory, potentially pathogenic bacteria [128], Proteus species possess many virulence factors potentially relevant to gastrointestinal pathogenicity [129,130], and Sutterella was found to be associated with GI infections [131] and inflammatory bowel disease [[132], [133], [134]]. The increased abundance of Sutterella spp. has also been detected in the feces of children with autism spectrum disorders (ASD) [[135], [136], [137]], indicating the possible role of this bacterium in neurodevelopmental diseases. The effect of antibiotics on the Firmicutes phylum resulted in extensive suppression of the diverse bacterial diversity (found in the groups without antibiotics) and monoculture of the genus, which accounted for 94% of the Firmicutes in the LPS/ATB group. Antibiotics also depleted the majority of Clostridia and eliminated the Ruminococcaceae and Lachnospiraceae families, which are generally considered beneficial for gut health [138]. These effects and diminishing Bacteroidetedes strongly suggest gastrointestinal disturbances induced by ATB treatment. Therefore, it was surprising that the observed suppression of bacterial diversity had no effect on rat behavior. These results are in contradiction with data from the rodent literature showing the negative effect of ATB on spatial recognition memory in rats [62,120], ATB-induced cognitive deficits [123], disruption of novel object recognition memory [61], increased exploratory behavior [139], increased immobility and reduced social recognition [140] in mice, and a reduction in the duration of nose-to-nose investigation after antibiotic treatment in hamsters [55].
On the other hand, Hoban et al. [62] observed that antibiotic treatment of adult rats had no effect on anxiety-related behavior assessed using Elevated Plus Maze (EPM) and Open Field (OF) tests. Cross-comparison of experimental results is complex and sometimes problematic because results are strongly influenced by many variable factors, such as type and duration of drug treatment, type of drug administration and dose, age of animals at the time of insult/test, type of behavioral tests, breed of rodents, number of animals in an experiment, source of animals, and even factors related to rodent husbandry. Our study design is similar to the hamster study by Sylvia et al. [55]. Antibiotic treatment of adult hamsters strongly affected gut microbial communities, but this effect was not influenced by LPS insult at an early age, similar to our study. On the other hand, LPS-treated male hamsters exhibited anxiety-like behaviors after antibiotic treatment in adulthood, a phenomenon that was not observed in our experiments.
4.1. The limitations of this study
This study's primary purpose was to evaluate late behavioral consequences of early infection-like insult together with the effect of gut microbiota manipulation. Systematic early postnatal LPS administration was used as a neurodevelopmental model of immune dysregulation that is relevant as one aspect of etiopathology of aberrant behavior and psychopathological changes. Inflectional agent initiated during the neonatal period is associated with the manifestation of changes over time. These changes are documented in behavioral, biochemical (neurotransmission), and histopathological processes. Considering microbiota's effect on brain function, our model has evaluated the effect of ATB on the status of the gut microbiome. The limitations of the supposed model were a requirement of precisely defined days of LPS/ATB administration and the timing of behavioral testing.
Due to the ethological approach of behavioral (testing with litter-mates, the same daytime periods, postnatal days) rules, we could not use females, herewith gender deviations in behavioral features it can be supposed. This approach was chosen based on knowledge of sex differences in responding to the experimental conditions and behavioral tests [30,[141], [142], [143], [144]]. Female sex hormones influence brain function and behavior, introducing additional variables into the study. Females of rats, thus, have not been included in our study due to concerns about the impact of variations in the female estrous cycle on behavioral responses.
Moreover, the presented study does not reflect biochemical analyses of neurotransmitters and cytokines in the brain and serum of experimental animals. However, neurotransmitter changes are frequently in the neuropsychiatric disorders described.
The sweetening of drinking water from day 60 is another limitation of this study, as sucrose may affect intestinal bacteria. Water was prepared in the same way for both experimental and control groups, being compared to ensure equal conditions for all animals. A gavage will be used to administer antibiotics to eliminate this deficiency in future experiments.
In a future study, we would consider the evaluation of behavioral profiles while observing gender differences in rats and biochemical analyses of neurotransmitters and pro-inflammatory cytokines to obtain more complex information about interactions between neonatal infection-like risk vs. antibiotic dysbiosis.
In conclusion, the two-hit model tested in this study did not induce neurodevelopmental disorders-like behavior in adult male rats. Apart from reduced weight gain, no behavioral abnormalities were caused by neonatal LPS immune challenge combined with an early adult antibiotic insult. A sensorimotor gating deficit was observed, considered a behavioral phenomenon relevant to neurodevelopmental disorders. Still, the difference between the two-hit-treated and the control groups was insignificant. Our results are quite surprising given the well-documented negative influence of early-age endotoxin treatment and the reported adverse effects of gut microbial disruption on rodent behavior. LPS-only treated animals showed in adulthood hypoactivity and induced anxiety-like behavior in the social recognition paradigm, but these behavioral changes were not exacerbated by ATB-induced gut dysbiosis. The gut bacterial community was seriously disrupted in animals treated with ATB alone, but the dysbiosis did not affect behavioral responses in the tests. The protective, anti-inflammatory effect of ATB administration cannot be supposed due to the significant increase in potentially harmful bacteria and elimination of beneficial microbes. The lack of interaction between LPS and ATB treatment suggests that independent mechanisms of action may exist for both insults. Comparison with data from the literature suggests that targeting and timing of hits significantly impact outcomes. Further work is needed to understand better how an immune challenge and microbiome dysbiosis may influence animal health status and behavior.
Author contribution statement
Hana Tejkalová: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kateřina Olša Fliegerová: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Simona Kvasnová:Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Hana Sechovcová: Performed the experiments; Wrote the paper.
Jakub Mrázek:Performed the experiments; Wrote the paper.
Jan Klaschka: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Lea Jakob: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Tomáš Páleníček: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data associated with this study has been deposited at the Sequence Read Archive under the accession number PRJNA765490.
Declaration of interest's statement
The authors declare the following conflict of interests: Tomaš Paleniček declares to have shares in „Psyon s. r.o”., has founded „PSYRES - Psychedelic Research Foundation” and has shares in “Společnost pro podporu neurovědního výzkumu s. r.o”. He reports consulting fees from GH Research and CB21-Pharma outside the submitted work. He is involved in Compass Pathways trials with psilocybin and MAPS clinical trial with MDMA outside the submitted work.
Additional information
Supplementary content related to this article has been published online at [URL].
Funding
This research was funded by Czech Health Research Council, grant number 17-31852A.
Declaration of competing interest
TP declare to have shares in „Psyon s. r.o”., has founded „PSYRES - Psychedelic Research Foundation” and has shares in “Společnost pro podporu neurovědního výzkumu s. r.o”. TP reports consulting fees from GH Research and CB21-Pharma outside the submitted work. TP is involved in Compass Pathways trials with psilocybin and MAPS clinical trial with MDMA outside the submitted work.
Acknowledgments
We would like to thank Dagmar Schierová for helpful advice during the HTS data analyses.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15417.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morais L.H., Schreiber 4th H.L., Mazmanian S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021;19:241–255. doi: 10.1038/s41579-020-00460-0. [DOI] [PubMed] [Google Scholar]
- 3.Cryan J.F., O'Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 4.Dinan T.G., Cryan J.F. Gut microbiota: a missing link in psychiatry. World Psychiatr. 2020;19:111–112. doi: 10.1002/wps.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maiuolo J., Gliozzi M., Musolino V., Carresi C., Scarano F., Nucera S., Scicchitano M., Oppedisano F., Bosco F., Ruga S., Zito M.C., Macri R., Palma E., Muscoli C., Mollace V. The contribution of gut microbiota-brain Axis in the development of brain disorders. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.616883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schächtle M.A., Rosshart S.P. The microbiota-gut-brain Axis in health and disease and its implications for translational research. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.698172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin C.R., Osadchiy V., Kalani A., Mayer E.A. The brain-gut-microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruce-Keller A.J., Salbaum J.M., Berthoud H.-R. Harnessing gut microbes for mental health: getting from here to there. Biol. Psychiatr. 2018;83:214–223. doi: 10.1016/j.biopsych.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherwin E., Dinan T.G., Cryan J.F. Recent developments in understanding the role of the gut microbiota in brain health and disease. Ann. N. Y. Acad. Sci. 2018;1420:5–25. doi: 10.1111/nyas.13416. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y.-K., Shin C. The microbiota-gut-brain Axis in neuropsychiatric disorders: pathophysiological mechanisms and novel treatments. Curr. Neuropharmacol. 2018;16:559–573. doi: 10.2174/1570159X15666170915141036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cryan J.F., O'Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., Guzzetta K.E., Jaggar M., Long-Smith C.M., Lyte J.M., Martin J.A., Molinero-Perez A., Moloney G., Morelli E., Morillas E., O'Connor R., Cruz-Pereira J.S., Peterson V.L., Rea K., Ritz N.L., Sherwin E., Spichak S., Teichman E.M., van de Wouw M., Ventura-Silva A.P., Wallace-Fitzsimons S.E., Hyland N., Clarke G., Dinan T.G. The microbiota-gut-brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 12.Rutsch A., Kantsjö J.B., Ronchi F. The gut-brain Axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spichak S., Bastiaanssen T.F.S., Berding K., Vlckova K., Clarke G., Dinan T.G., Cryan J.F. Mining microbes for mental health: determining the role of microbial metabolic pathways in human brain health and disease. Neurosci. Biobehav. Rev. 2021;125:698–761. doi: 10.1016/j.neubiorev.2021.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Ratsika A., Cruz Pereira J.S., Lynch C.M.K., Clarke G., Cryan J.F. Microbiota-immune-brain interactions: a lifespan perspective. Curr. Opin. Neurobiol. 2023;78 doi: 10.1016/j.conb.2022.102652. [DOI] [PubMed] [Google Scholar]
- 15.Munawar N., Ahsan K., Muhammad K., Ahmad A., Anwar M.A., Shah I., Al Ameri A.K., Al Mughairbi F. Hidden role of gut microbiome dysbiosis in schizophrenia: antipsychotics or psychobiotics as therapeutics? Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22147671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cryan J.F., Dinan T.G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 17.Gass P., Wotjak C. Rodent models of psychiatric disorders--practical considerations. Cell Tissue Res. 2013;354:1–7. doi: 10.1007/s00441-013-1706-7. [DOI] [PubMed] [Google Scholar]
- 18.Mohr A.E., ’sa Crawford M., Jasbi P., Fessler S., Sweazea K.L. Lipopolysaccharide and the gut microbiota: considering structural variation. FEBS Lett. 2022;596:849–875. doi: 10.1002/1873-3468.14328. [DOI] [PubMed] [Google Scholar]
- 19.Chamera K., Kotarska K., Szuster-Głuszczak M., Trojan E., Skórkowska A., Pomierny B., Krzyżanowska W., Bryniarska N., Basta-Kaim A. The prenatal challenge with lipopolysaccharide and polyinosinic:polycytidylic acid disrupts CX3CL1-CX3CR1 and CD200-CD200R signalling in the brains of male rat offspring: a link to schizophrenia-like behaviours. J. Neuroinflammation. 2020;17:247. doi: 10.1186/s12974-020-01923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey L., Boksa P. Prenatal and postnatal animal models of immune activation: relevance to a range of neurodevelopmental disorders. Dev. Neurobiol. 2012;72:1335–1348. doi: 10.1002/dneu.22043. [DOI] [PubMed] [Google Scholar]
- 21.Wischhof L., Irrsack E., Osorio C., Koch M. Prenatal LPS-exposure--a neurodevelopmental rat model of schizophrenia--differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;57:17–30. doi: 10.1016/j.pnpbp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Nikitina V.A., Zakharova M.V., Trofimov A.N., Schwarz A.P., Beznin G.V., Tsikunov S.G., Zubareva O.E. Neonatal exposure to bacterial lipopolysaccharide affects behavior and expression of ionotropic glutamate receptors in the Hippocampus of adult rats after psychogenic trauma. Biochemistry. 2021;86:761–772. doi: 10.1134/S0006297921060134. [DOI] [PubMed] [Google Scholar]
- 23.Skrzypczak-Wiercioch A., Sałat K. Lipopolysaccharide-induced model of neuroinflammation: mechanisms of action, research application and future directions for its use. Molecules. 2022;27 doi: 10.3390/molecules27175481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skelly D.T., Hennessy E., Dansereau M.-A., Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, [corrected] TNF-α and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dantzer R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Connor J.C., Lawson M.A., André C., Moreau M., Lestage J., Castanon N., Kelley K.W., Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatr. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haba R., Shintani N., Onaka Y., Wang H., Takenaga R., Hayata A., Baba A., Hashimoto H. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: possible role of activation of the central amygdala. Behav. Brain Res. 2012;228:423–431. doi: 10.1016/j.bbr.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 28.Sulakhiya K., Keshavlal G.P., Bezbaruah B.B., Dwivedi S., Gurjar S.S., Munde N., Jangra A., Lahkar M., Gogoi R. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci. Lett. 2016;611:106–111. doi: 10.1016/j.neulet.2015.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Fields C.T., Chassaing B., Castillo-Ruiz A., Osan R., Gewirtz A.T., de Vries G.J. Effects of gut-derived endotoxin on anxiety-like and repetitive behaviors in male and female mice. Biol. Sex Differ. 2018;9:7. doi: 10.1186/s13293-018-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cristino L.M.F., Chaves Filho A.J.M., Custódio C.S., Vasconcelos S.M.M., de Sousa F.C.F., Sanders L.L.O., de Lucena D.F., Macedo D.S. Animal model of neonatal immune challenge by lipopolysaccharide: a study of sex influence in behavioral and immune/neurotrophic alterations in juvenile mice. Neuroimmunomodulation. 2022;29:391–401. doi: 10.1159/000522055. [DOI] [PubMed] [Google Scholar]
- 31.Avitsur R., Pollak Y., Yirmiya R. Different receptor mechanisms mediate the effects of endotoxin and interleukin-1 on female sexual behavior. Brain Res. 1997;773:149–161. doi: 10.1016/s0006-8993(97)00927-x. [DOI] [PubMed] [Google Scholar]
- 32.Bluthé R.M., Bristow A., Lestage J., Imbs C., Dantzer R. Central injection of interleukin-13 potentiates LPS-induced sickness behavior in rats. Neuroreport. 2001;12:3979–3983. doi: 10.1097/00001756-200112210-00025. [DOI] [PubMed] [Google Scholar]
- 33.Taksande B.G., Chopde C.T., Umekar M.J., Kotagale N.R. Agmatine attenuates lipopolysaccharide induced anorexia and sickness behavior in rats. Pharmacol. Biochem. Behav. 2015;132:108–114. doi: 10.1016/j.pbb.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Moraes M.M.T., Galvão M.C., Cabral D., Coelho C.P., Queiroz-Hazarbassanov N., Martins M.F.M., Bondan E.F., Bernardi M.M., Kirsten T.B. Propentofylline prevents sickness behavior and depressive-like behavior induced by lipopolysaccharide in rats via neuroinflammatory pathway. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mendes-Lima T., Kirsten T.B., Rodrigues P.S., Sampaio A.C.S., Felício L.F., Rocha P.R.D.A., Reis-Silva T.M., Bondan E.F., Martins M.F.M., Queiroz-Hazarbassanov N., Bernardi M.M. Prenatal LPS induces sickness behaviour and decreases maternal and predatory behaviours after an LPS challenge. Int. J. Neurosci. 2020;130:804–816. doi: 10.1080/00207454.2019.1706505. [DOI] [PubMed] [Google Scholar]
- 36.Mansouri M., Sotoudeh M.M., Shamshirian A., Beheshti F., Hosseini M., Sadeghnia H.R. Beneficial effects of selenium against the behavioral consequences of lipopolysaccharide administration in rats. Learn. Motiv. 2021;74 doi: 10.1016/j.lmot.2021.101713. [DOI] [Google Scholar]
- 37.Saavedra L.M., Hernández-Velázquez M.G., Madrigal S., Ochoa-Zarzosa A., Torner L. Long-term activation of hippocampal glial cells and altered emotional behavior in male and female adult rats after different neonatal stressors. Psychoneuroendocrinology. 2021;126 doi: 10.1016/j.psyneuen.2021.105164. [DOI] [PubMed] [Google Scholar]
- 38.Babaei F., Mirzababaei M., Mohammadi G., Dargahi L., Nassiri-Asl M. Saccharomyces boulardii attenuates lipopolysaccharide-induced anxiety-like behaviors in rats. Neurosci. Lett. 2022;778 doi: 10.1016/j.neulet.2022.136600. [DOI] [PubMed] [Google Scholar]
- 39.Mora S., Martín-González E., Prados-Pardo Á., Flores P., Moreno M. Increased compulsivity in adulthood after early adolescence immune activation: preclinical evidence. Int. J. Environ. Res. Publ. Health. 2021;18 doi: 10.3390/ijerph18094684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu F., Zhang L., Ding Y.-Q., Zhao J., Zheng Y. Neonatal intrahippocampal injection of lipopolysaccharide induces deficits in social behavior and prepulse inhibition and microglial activation in rats: implication for a new schizophrenia animal model. Brain Behav. Immun. 2014;38:166–174. doi: 10.1016/j.bbi.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Babri S., Doosti M.-H., Salari A.-A. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain Behav. Immun. 2014;37:164–176. doi: 10.1016/j.bbi.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Foley K.A., MacFabe D.F., Kavaliers M., Ossenkopp K.-P. Sexually dimorphic effects of prenatal exposure to lipopolysaccharide, and prenatal and postnatal exposure to propionic acid, on acoustic startle response and prepulse inhibition in adolescent rats: relevance to autism spectrum disorders. Behav. Brain Res. 2015;278:244–256. doi: 10.1016/j.bbr.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 43.Basta-Kaim A., Budziszewska B., Leśkiewicz M., Fijał K., Regulska M., Kubera M., Wędzony K., Lasoń W. Hyperactivity of the hypothalamus-pituitary-adrenal axis in lipopolysaccharide-induced neurodevelopmental model of schizophrenia in rats: effects of antipsychotic drugs. Eur. J. Pharmacol. 2011;650:586–595. doi: 10.1016/j.ejphar.2010.09.083. [DOI] [PubMed] [Google Scholar]
- 44.Romero E., Guaza C., Castellano B., Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol. Psychiatr. 2010;15:372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- 45.Borrell J., Vela J.M., Arévalo-Martin A., Molina-Holgado E., Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 46.Kumari V., Soni W., Sharma T. Schizophrenia Research, Elsevier Science Bv Po Box 211, 1000 Ae Amsterdam, Netherlands. 1999. Clozapine normalises information processing deficits in schizophrenia; p. 175. [Google Scholar]
- 47.Weike A.I., Bauer U., Hamm A.O. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol. Psychiatr. 2000;47:61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow N.R., Light G.A., Thomas M.L., Sprock J., Calkins M.E., Green M.F., Greenwood T.A., Gur R.E., Gur R.C., Lazzeroni L.C., Nuechterlein K.H., Radant A.D., Seidman L.J., Siever L.J., Silverman J.M., Stone W.S., Sugar C.A., Tsuang D.W., Tsuang M.T., Turetsky B.I., Braff D.L. Deficient prepulse inhibition in schizophrenia in a multi-site cohort: internal replication and extension. Schizophr. Res. 2018;198:6–15. doi: 10.1016/j.schres.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bilbo S.D., Levkoff L.H., Mahoney J.H., Watkins L.R., Rudy J.W., Maier S.F. Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci. 2005;119:293–301. doi: 10.1037/0735-7044.119.1.293. [DOI] [PubMed] [Google Scholar]
- 50.Guerrin C.G.J., Doorduin J., Sommer I.E., de Vries E.F.J. The dual hit hypothesis of schizophrenia: evidence from animal models. Neurosci. Biobehav. Rev. 2021;131:1150–1168. doi: 10.1016/j.neubiorev.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Bilbo S.D., Yirmiya R., Amat J., Paul E.D., Watkins L.R., Maier S.F. Bacterial infection early in life protects against stressor-induced depressive-like symptoms in adult rats. Psychoneuroendocrinology. 2008;33:261–269. doi: 10.1016/j.psyneuen.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarkar T., Patro N., Patro I.K. Perinatal exposure to synergistic multiple stressors leads to cellular and behavioral deficits mimicking Schizophrenia-like pathology. Biol. Open. 2022;11 doi: 10.1242/bio.058870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Giovanoli S., Engler H., Engler A., Richetto J., Feldon J., Riva M.A., Schedlowski M., Meyer U. Preventive effects of minocycline in a neurodevelopmental two-hit model with relevance to schizophrenia. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker A.K., Nakamura T., Byrne R.J., Naicker S., Tynan R.J., Hunter M., Hodgson D.M. Neonatal lipopolysaccharide and adult stress exposure predisposes rats to anxiety-like behaviour and blunted corticosterone responses: implications for the double-hit hypothesis. Psychoneuroendocrinology. 2009;34:1515–1525. doi: 10.1016/j.psyneuen.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Sylvia K.E., Deyoe J.E., Demas G.E. Early-life sickness may predispose Siberian hamsters to behavioral changes following alterations of the gut microbiome in adulthood. Brain Behav. Immun. 2018;73:571–583. doi: 10.1016/j.bbi.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cryan J.F., O'Mahony S.M. The microbiome-gut-brain axis: from bowel to behavior. Neuro Gastroenterol. Motil. 2011;23:187–192. doi: 10.1111/j.1365-2982.2010.01664.x. [DOI] [PubMed] [Google Scholar]
- 57.Ruiz V.E., Battaglia T., Kurtz Z.D., Bijnens L., Ou A., Engstrand I., Zheng X., Iizumi T., Mullins B.J., Müller C.L., Cadwell K., Bonneau R., Perez-Perez G.I., Blaser M.J. A single early-in-life macrolide course has lasting effects on murine microbial network topology and immunity. Nat. Commun. 2017;8:518. doi: 10.1038/s41467-017-00531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leclercq S., Mian F.M., Stanisz A.M., Bindels L.B., Cambier E., Ben-Amram H., Koren O., Forsythe P., Bienenstock J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017;8 doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cox L.M., Yamanishi S., Sohn J., Alekseyenko A.V., Leung J.M., Cho I., Kim S.G., Li H., Gao Z., Mahana D., Zárate Rodriguez J.G., Rogers A.B., Robine N., ’ng Loke P., Blaser M.J. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lach G., Fülling C., Bastiaanssen T.F.S., Fouhy F., Donovan A.N.O., Ventura-Silva A.P., Stanton C., Dinan T.G., Cryan J.F. Enduring neurobehavioral effects induced by microbiota depletion during the adolescent period. Transl. Psychiatry. 2020;10:382. doi: 10.1038/s41398-020-01073-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fröhlich E.E., Farzi A., Mayerhofer R., Reichmann F., Jačan A., Wagner B., Zinser E., Bordag N., Magnes C., Fröhlich E., Kashofer K., Gorkiewicz G., Holzer P. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav. Immun. 2016;56:140–155. doi: 10.1016/j.bbi.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoban A.E., Moloney R.D., Golubeva A.V., McVey Neufeld K.A., O'Sullivan O., Patterson E., Stanton C., Dinan T.G., Clarke G., Cryan J.F. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience. 2016;339:463–477. doi: 10.1016/j.neuroscience.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Reikvam D.H., Erofeev A., Sandvik A., Grcic V., Jahnsen F.L., Gaustad P., McCoy K.D., Macpherson A.J., Meza-Zepeda L.A., Johansen F.-E. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bryant C.E., Spring D.R., Gangloff M., Gay N.J. The molecular basis of the host response to lipopolysaccharide. Nat. Rev. Microbiol. 2010;8:8–14. doi: 10.1038/nrmicro2266. [DOI] [PubMed] [Google Scholar]
- 65.Andrews C., McLean M.H., Durum S.K. Cytokine tuning of intestinal epithelial function. Front. Immunol. 2018;9:1270. doi: 10.3389/fimmu.2018.01270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendes V., Galvão I., Vieira A.T. Mechanisms by which the gut microbiota influences cytokine production and modulates host inflammatory responses. J. Interferon Cytokine Res. 2019;39:393–409. doi: 10.1089/jir.2019.0011. [DOI] [PubMed] [Google Scholar]
- 67.Al Bander Z., Nitert M.D., Mousa A., Naderpoor N. The gut microbiota and inflammation: an overview. Int. J. Environ. Res. Publ. Health. 2020;17 doi: 10.3390/ijerph17207618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xia J., Gu L., Guo Y., Feng H., Chen S., Jurat J., Fu W., Zhang D. Gut microbiota mediates the preventive effects of dietary capsaicin against depression-like behavior induced by lipopolysaccharide in mice. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.627608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang N., Hua D., Zhan G., Li S., Zhu B., Jiang R., Yang L., Bi J., Xu H., Hashimoto K., Luo A., Yang C. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol. Biochem. Behav. 2019;176:93–100. doi: 10.1016/j.pbb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Song S., Liu J., Zhang F., Hong J.-S. Norepinephrine depleting toxin DSP-4 and LPS alter gut microbiota and induce neurotoxicity in α-synuclein mutant mice. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-72202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kentner A.C., Khan U., MacRae M., Dowd S.E., Yan S. The effect of antibiotics on social aversion following early life inflammation. Physiol. Behav. 2018;194:311–318. doi: 10.1016/j.physbeh.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Białoń M., Wąsik A. Advantages and limitations of animal schizophrenia models. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms23115968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franklin C.L., Ericsson A.C. Microbiota and reproducibility of rodent models. Lab. Anim. 2017;46:114–122. doi: 10.1038/laban.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bayer T.A., Falkai P., Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis. J. Psychiatr. Res. 1999;33:543–548. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- 75.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelly J.R., Minuto C., Cryan J.F., Clarke G., Dinan T.G. Cross talk: the microbiota and neurodevelopmental disorders. Front. Neurosci. 2017;11:490. doi: 10.3389/fnins.2017.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dinan T.G., Cryan J.F. Microbes, immunity, and behavior: psychoneuroimmunology meets the microbiome. Neuropsychopharmacology. 2017;42:178–192. doi: 10.1038/npp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dickerson F., Severance E., Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav. Immun. 2017;62:46–52. doi: 10.1016/j.bbi.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanji S., Fonseka T.M., Marshe V.S., Sriretnakumar V., Hahn M.K., Müller D.J. The microbiome-gut-brain axis: implications for schizophrenia and antipsychotic induced weight gain. Eur. Arch. Psychiatr. Clin. Neurosci. 2018;268:3–15. doi: 10.1007/s00406-017-0820-z. [DOI] [PubMed] [Google Scholar]
- 81.Deng H., He L., Wang C., Zhang T., Guo H., Zhang H., Song Y., Chen B. Altered gut microbiota and its metabolites correlate with plasma cytokines in schizophrenia inpatients with aggression. BMC Psychiatr. 2022;22:629. doi: 10.1186/s12888-022-04255-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Powell C.M., Miyakawa T. Schizophrenia-relevant behavioral testing in rodent models: a uniquely human disorder? Biol. Psychiatr. 2006;59:1198–1207. doi: 10.1016/j.biopsych.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang P., Tu K., Cao P., Yang Y., Zhang H., Qiu X.-T., Zhang M.-M., Wu X.-J., Yang H., Chen T. Antibiotics-induced intestinal dysbacteriosis caused behavioral alternations and neuronal activation in different brain regions in mice. Mol. Brain. 2021;14:49. doi: 10.1186/s13041-021-00759-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freeman C.D., Klutman N.E., Lamp K.C. Metronidazole. A therapeutic review and update. Drugs. 1997;54:679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 85.Wang F., Zhou H., Olademehin O.P., Kim S.J., Tao P. Insights into key interactions between vancomycin and bacterial cell wall structures. ACS Omega. 2018;3:37–45. doi: 10.1021/acsomega.7b01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El-Sayed Ahmed M.A.E.-G., Zhong L.-L., Shen C., Yang Y., Doi Y., Tian G.-B. Colistin and its role in the Era of antibiotic resistance: an extended review (2000-2019) Emerg. Microb. Infect. 2020;9:868–885. doi: 10.1080/22221751.2020.1754133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan A., Powell S.B. Sensorimotor gating deficits in “two-hit” models of schizophrenia risk factors. Schizophr. Res. 2018;198:68–83. doi: 10.1016/j.schres.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Richter K., Wolf G., Engelmann M. Social recognition memory requires two stages of protein synthesis in mice, Learn. MEM (Miner. Electrolyte Metab.) 2005;12:407–413. doi: 10.1101/lm.97505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Donát P. Measuring behaviour: the tools and the strategies. Neurosci. Biobehav. Rev. 1991;15:447–454. doi: 10.1016/s0149-7634(05)80130-7. [DOI] [PubMed] [Google Scholar]
- 90.Tejkalová H., Kaiser M., Klaschka J., Stastný F. Does neonatal brain ischemia induce schizophrenia-like behavior in young adult rats? Physiol. Res. 2007;56:815–823. doi: 10.33549/physiolres.931056. [DOI] [PubMed] [Google Scholar]
- 91.Fliegerova K., Tapio I., Bonin A., Mrazek J., Callegari M.L., Bani P., Bayat A., Vilkki J., Kopečný J., Shingfield K.J., Boyer F., Coissac E., Taberlet P., Wallace R.J. Effect of DNA extraction and sample preservation method on rumen bacterial population. Anaerobe. 2014;29:80–84. doi: 10.1016/j.anaerobe.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 92.Bolyen E., Rideout J.R., Dillon M.R., Bokulich N.A., Abnet C.C., Al-Ghalith G.A., Alexander H., Alm E.J., Arumugam M., Asnicar F., Bai Y., Bisanz J.E., Bittinger K., Brejnrod A., Brislawn C.J., Brown C.T., Callahan B.J., Caraballo-Rodríguez A.M., Chase J., Cope E.K., Da Silva R., Diener C., Dorrestein P.C., Douglas G.M., Durall D.M., Duvallet C., Edwardson C.F., Ernst M., Estaki M., Fouquier J., Gauglitz J.M., Gibbons S.M., Gibson D.L., Gonzalez A., Gorlick K., Guo J., Hillmann B., Holmes S., Holste H., Huttenhower C., Huttley G.A., Janssen S., Jarmusch A.K., Jiang L., Kaehler B.D., Kang K.B., Keefe C.R., Keim P., Kelley S.T., Knights D., Koester I., Kosciolek T., Kreps J., Langille M.G.I., Lee J., Ley R., Liu Y.-X., Loftfield E., Lozupone C., Maher M., Marotz C., Martin B.D., McDonald D., McIver L.J., Melnik A.V., Metcalf J.L., Morgan S.C., Morton J.T., Naimey A.T., Navas-Molina J.A., Nothias L.F., Orchanian S.B., Pearson T., Peoples S.L., Petras D., Preuss M.L., Pruesse E., Rasmussen L.B., Rivers A., Robeson M.S., 2nd, Rosenthal P., Segata N., Shaffer M., Shiffer A., Sinha R., Song S.J., Spear J.R., Swafford A.D., Thompson L.R., Torres P.J., Trinh P., Tripathi A., Turnbaugh P.J., Ul-Hasan S., van der Hooft J.J.J., Vargas F., Vázquez-Baeza Y., Vogtmann E., von Hippel M., Walters W., Wan Y., Wang M., Warren J., Weber K.C., Williamson C.H.D., Willis A.D., Xu Z.Z., Zaneveld J.R., Zhang Y., Zhu Q., Knight R., Caporaso J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37:852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schierová D., Březina J., Mrázek J., Fliegerová K.O., Kvasnová S., Bajer L., Drastich P. Gut microbiome changes in patients with active left-sided ulcerative colitis after fecal microbiome transplantation and topical 5-aminosalicylic acid therapy. Cells. 2020;9 doi: 10.3390/cells9102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rognes T., Flouri T., Nichols B., Quince C., Mahé F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4 doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Segata N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W.S., Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fortier M.-E., Luheshi G.N., Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav. Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 97.Sylvia K.E., Demas G.E. Acute intraperitoneal lipopolysaccharide influences the immune system in the absence of gut dysbiosis. Phys. Rep. 2018;6 doi: 10.14814/phy2.13639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pyndt Jørgensen B., Krych L., Pedersen T.B., Plath N., Redrobe J.P., Hansen A.K., Nielsen D.S., Pedersen C.S., Larsen C., Sørensen D.B. Investigating the long-term effect of subchronic phencyclidine-treatment on novel object recognition and the association between the gut microbiota and behavior in the animal model of schizophrenia. Physiol. Behav. 2015;141:32–39. doi: 10.1016/j.physbeh.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 99.Sturman O., Germain P.-L., Bohacek J. Exploratory rearing: a context- and stress-sensitive behavior recorded in the open-field test. Stress. 2018;21:443–452. doi: 10.1080/10253890.2018.1438405. [DOI] [PubMed] [Google Scholar]
- 100.Bluthé R.M., Dantzer R., Kelley K.W. Effects of interleukin-1 receptor antagonist on the behavioral effects of lipopolysaccharide in rat. Brain Res. 1992;573:318–320. doi: 10.1016/0006-8993(92)90779-9. [DOI] [PubMed] [Google Scholar]
- 101.Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–174. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- 102.Zhong Y., Zhang X., Hu X., Li Y. Effects of repeated lipopolysaccharide treatment on growth performance, immune organ index, and blood parameters of sprague-dawley rats. J Vet Res. 2018;62:341–346. doi: 10.2478/jvetres-2018-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang X., Zhu L., Hu J., Guo R., Ye S., Liu F., Wang D., Zhao Y., Hu A., Wang X., Guo K., Lin L. FGF21 attenuated LPS-induced depressive-like behavior via inhibiting the inflammatory pathway. Front. Pharmacol. 2020;11:154. doi: 10.3389/fphar.2020.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pires J.M., Foresti M.L., Silva C.S., Rêgo D.B., Calió M.L., Mosini A.C., Nakamura T.K.E., Leslie A.T.F., Mello L.E. Lipopolysaccharide-induced systemic inflammation in the neonatal period increases microglial density and oxidative stress in the cerebellum of adult rats. Front. Cell. Neurosci. 2020;14:142. doi: 10.3389/fncel.2020.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Foster C.G., Landowski L.M., Sutherland B.A., Howells D.W. Differences in fatigue-like behavior in the lipopolysaccharide and poly I:C inflammatory animal models. Physiol. Behav. 2021;232 doi: 10.1016/j.physbeh.2021.113347. [DOI] [PubMed] [Google Scholar]
- 106.On Wah D.T., Kavaliers M., Bishnoi I.R., Ossenkopp K.-P. Lipopolysaccharide (LPS) induced sickness in early adolescence alters the behavioral effects of the short-chain fatty acid, propionic acid, in late adolescence and adulthood: examining anxiety and startle reactivity. Behav. Brain Res. 2019;360:312–322. doi: 10.1016/j.bbr.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 107.Tishkina A., Stepanichev M., Kudryashova I., Freiman S., Onufriev M., Lazareva N., Gulyaeva N. Neonatal proinflammatory challenge in male Wistar rats: effects on behavior, synaptic plasticity, and adrenocortical stress response. Behav. Brain Res. 2016;304:1–10. doi: 10.1016/j.bbr.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 108.Batinić B., Santrač A., Divović B., Timić T., Stanković T., Obradović A.L., Joksimović S., Savić M.M. Lipopolysaccharide exposure during late embryogenesis results in diminished locomotor activity and amphetamine response in females and spatial cognition impairment in males in adult, but not adolescent rat offspring. Behav. Brain Res. 2016;299:72–80. doi: 10.1016/j.bbr.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 109.Harvey L., Boksa P. Do prenatal immune activation and maternal iron deficiency interact to affect neurodevelopment and early behavior in rat offspring? Brain Behav. Immun. 2014;35:144–154. doi: 10.1016/j.bbi.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 110.Kirsten T.B., Taricano M., Flório J.C., Palermo-Neto J., Bernardi M.M. Prenatal lipopolysaccharide reduces motor activity after an immune challenge in adult male offspring. Behav. Brain Res. 2010;211:77–82. doi: 10.1016/j.bbr.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 111.Yirmiya R., Rosen H., Donchin O., Ovadia H. Behavioral effects of lipopolysaccharide in rats: involvement of endogenous opioids. Brain Res. 1994;648:80–86. doi: 10.1016/0006-8993(94)91908-9. [DOI] [PubMed] [Google Scholar]
- 112.Gophna U., Konikoff T., Nielsen H.B. Oscillospira and related bacteria - from metagenomic species to metabolic features. Environ. Microbiol. 2017;19:835–841. doi: 10.1111/1462-2920.13658. [DOI] [PubMed] [Google Scholar]
- 113.Bourassa M.W., Alim I., Bultman S.J., Ratan R.R. Butyrate, neuroepigenetics and the gut microbiome: can a high fiber diet improve brain health? Neurosci. Lett. 2016;625:56–63. doi: 10.1016/j.neulet.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Canani R.B., Costanzo M.D., Leone L., Pedata M., Meli R., Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Konikoff T., Gophna U. Oscillospira: a central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016;24:523–524. doi: 10.1016/j.tim.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 116.Walters W.A., Xu Z., Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588:4223–4233. doi: 10.1016/j.febslet.2014.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pérez-Brocal V., García-López R., Nos P., Beltrán B., Moret I., Moya A. Metagenomic analysis of crohn's disease patients identifies changes in the virome and microbiome related to disease status and therapy, and detects potential interactions and biomarkers. Inflamm. Bowel Dis. 2015;21:2515–2532. doi: 10.1097/MIB.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 118.Dunphy-Doherty F., O'Mahony S.M., Peterson V.L., O'Sullivan O., Crispie F., Cotter P.D., Wigmore P., King M.V., Cryan J.F., Fone K.C.F. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav. Immun. 2018;68:261–273. doi: 10.1016/j.bbi.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 119.Gnauck A., Lentle R.G., Kruger M.C. The characteristics and function of bacterial lipopolysaccharides and their endotoxic potential in humans. Int. Rev. Immunol. 2016;35:189–218. doi: 10.3109/08830185.2015.1087518. [DOI] [PubMed] [Google Scholar]
- 120.Leigh S.-J., Kaakoush N.O., Westbrook R.F., Morris M.J. Minocycline-induced microbiome alterations predict cafeteria diet-induced spatial recognition memory impairments in rats. Transl. Psychiatry. 2020;10:92. doi: 10.1038/s41398-020-0774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu X., Zheng H., Lu R., Huang H., Zhu H., Yin C., Mo Y., Wu J., Liu X., Deng M., Li D., Cheng B., Wu F., Liang Y., Guo H., Song H., Su Z. Intervening effects of total alkaloids of corydalis saxicola bunting on rats with antibiotic-induced gut microbiota dysbiosis based on 16S rRNA gene sequencing and untargeted metabolomics analyses. Front. Microbiol. 2019;10:1151. doi: 10.3389/fmicb.2019.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Behr C., Ramírez-Hincapié S., Cameron H.J., Strauss V., Walk T., Herold M., Beekmann K., Rietjens I.M.C.M., van Ravenzwaay B. Impact of lincosamides antibiotics on the composition of the rat gut microbiota and the metabolite profile of plasma and feces. Toxicol. Lett. 2018;296:139–151. doi: 10.1016/j.toxlet.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 123.Desbonnet L., Clarke G., Traplin A., O'Sullivan O., Crispie F., Moloney R.D., Cotter P.D., Dinan T.G., Cryan J.F. Gut microbiota depletion from early adolescence in mice: implications for brain and behaviour. Brain Behav. Immun. 2015;48:165–173. doi: 10.1016/j.bbi.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 124.Čoklo M., Maslov D.R., Kraljević Pavelić S. Modulation of gut microbiota in healthy rats after exposure to nutritional supplements. Gut Microb. 2020;12:1–28. doi: 10.1080/19490976.2020.1779002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nagpal R., Wang S., Solberg Woods L.C., Seshie O., Chung S.T., Shively C.A., Register T.C., Craft S., McClain D.A., Yadav H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 2018;9:2897. doi: 10.3389/fmicb.2018.02897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li D., Chen H., Mao B., Yang Q., Zhao J., Gu Z., Zhang H., Chen Y.Q., Chen W. Microbial biogeography and core microbiota of the rat digestive tract. Sci. Rep. 2017;8 doi: 10.1038/srep45840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rizzatti G., Lopetuso L.R., Gibiino G., Binda C., Gasbarrini A. Proteobacteria: a common factor in human diseases. BioMed Res. Int. 2017;2017 doi: 10.1155/2017/9351507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maes M., Coucke F., Leunis J.-C. Normalization of the increased translocation of endotoxin from gram negative enterobacteria (leaky gut) is accompanied by a remission of chronic fatigue syndrome. Neuroendocrinol. Lett. 2007;28:739–744. http://nel.altamira.sk/userfiles/articlesnew/NEL280607A13.pdf accessed February 22, 2023. [PubMed] [Google Scholar]
- 129.Hamilton A.L., Kamm M.A., Ng S.C., Morrison M. Proteus spp. as putative gastrointestinal pathogens. Clin. Microbiol. Rev. 2018;31 doi: 10.1128/CMR.00085-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang M., Lv Y., Hou S., Liu Y., Wang Y., Wan X. Differential mucosal microbiome profiles across stages of human colorectal cancer. Life. 2021;11 doi: 10.3390/life11080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Molitoris E., Wexler H.M., Finegold S.M. Sources and antimicrobial susceptibilities of Campylobacter gracilis and Sutterella wadsworthensis. Clin. Infect. Dis. 1997;25(Suppl 2):S264. doi: 10.1086/516234. [DOI] [PubMed] [Google Scholar]
- 132.Gophna U., Sommerfeld K., Gophna S., Doolittle W.F., Veldhuyzen van Zanten S.J.O. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J. Clin. Microbiol. 2006;44:4136–4141. doi: 10.1128/JCM.01004-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lavelle A., Lennon G., O'Sullivan O., Docherty N., Balfe A., Maguire A., Mulcahy H.E., Doherty G., O'Donoghue D., Hyland J., Ross R.P., Coffey J.C., Sheahan K., Cotter P.D., Shanahan F., Winter D.C., O'Connell P.R. Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut. 2015;64:1553–1561. doi: 10.1136/gutjnl-2014-307873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaakoush N.O. Sutterella species, IgA-degrading bacteria in ulcerative colitis. Trends Microbiol. 2020;28:519–522. doi: 10.1016/j.tim.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 135.Williams B.L., Hornig M., Parekh T., Lipkin W.I. Application of novel PCR-based methods for detection, quantitation, and phylogenetic characterization of Sutterella species in intestinal biopsy samples from children with autism and gastrointestinal disturbances. mBio. 2012;3 doi: 10.1128/mBio.00261-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang L., Christophersen C.T., Sorich M.J., Gerber J.P., Angley M.T., Conlon M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism. 2013;4:42. doi: 10.1186/2040-2392-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang M., Ma W., Zhang J., He Y., Wang J. Analysis of gut microbiota profiles and microbe-disease associations in children with autism spectrum disorders in China. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Biddle A., Stewart L., Blanchard J., Leschine S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity. 2013;5:627–640. doi: 10.3390/d5030627. [DOI] [Google Scholar]
- 139.Bercik P., Denou E., Collins J., Jackson W., Lu J., Jury J., Deng Y., Blennerhassett P., Macri J., McCoy K.D., Verdu E.F., Collins S.M. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141:599–609. doi: 10.1053/j.gastro.2011.04.052. 609.e1–3. [DOI] [PubMed] [Google Scholar]
- 140.Guida F., Turco F., Iannotta M., De Gregorio D., Palumbo I., Sarnelli G., Furiano A., Napolitano F., Boccella S., Luongo L., Mazzitelli M., Usiello A., De Filippis F., Iannotti F.A., Piscitelli F., Ercolini D., de Novellis V., Di Marzo V., Cuomo R., Maione S. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav. Immun. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 141.Simpson J., Ryan C., Curley A., Mulcaire J., Kelly J.P. Sex differences in baseline and drug-induced behavioural responses in classical behavioural tests. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2012;37:227–236. doi: 10.1016/j.pnpbp.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 142.Scholl J.L., Afzal A., Fox L.C., Watt M.J., Forster G.L. Sex differences in anxiety-like behaviors in rats. Physiol. Behav. 2019;211 doi: 10.1016/j.physbeh.2019.112670. [DOI] [PubMed] [Google Scholar]
- 143.Blanchard D.C., Shepherd J.K., De Padua Carobrez A., Blanchard R.J. Sex effects in defensive behavior: baseline differences and drug interactions. Neurosci. Biobehav. Rev. 1991;15:461–468. doi: 10.1016/s0149-7634(05)80132-0. [DOI] [PubMed] [Google Scholar]
- 144.Cuskelly A., Hoedt E.C., Harms L., Talley N.J., Tadros M.A., Keely S., Hodgson D.M. Neonatal immune challenge influences the microbiota and behaviour in a sexually dimorphic manner. Brain Behav. Immun. 2022;103:232–242. doi: 10.1016/j.bbi.2022.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has been deposited at the Sequence Read Archive under the accession number PRJNA765490.