Abstract
Poly(ethylene terephthalate) (PET) is a synthetic polymer widely used globally. The high PET resistance to biotic degradation and its improper destination result in the accumulation of this plastic in the environment, largely affecting terrestrial and aquatic animals. This work investigated post-consumer PET (PC-PET) degradation using five commercial hydrolase enzymes (Novozym 51032, CalB, Palatase, Eversa, Lipozyme TL). Humicola insolens cutinase (HiC, Novozym 51032) was the most active among the enzymes studied. Several important reaction parameters (enzyme type, dual enzyme system, enzyme concentration, temperature, ultrasound treatment) were evaluated in PC-PET hydrolysis using HiC. The concentration and the proportion (molar ratio) of hydrolysis products, terephthalic acid (TPA), mono(2-hydroxyethyl) terephthalate (MHET), and bis(2-hydroxyethyl) terephthalate (BHET), were significantly changed depending on the reaction temperature. The TPA released at 70 °C was 3.65-fold higher than at 50 °C. At higher temperatures, the conversion of MHET into TPA was favored. The enzymatic PET hydrolysis by HiC was very sensitive to the enzyme concentration, indicating that it strongly adsorbs on the polymer surface. The concentration of TPA, MHET, and BHET increased as the enzyme concentration increased, and a maximum was achieved using 40–50 vol % of HiC. The presented results add relevant data to optimizing enzyme-based PET recycling technologies.
Keywords: PET hydrolysis, Enzymatic depolymerization, Lipases, Terephthalic acid, Ultrasound, Poly(ethylene terephthalate)
Introduction
Poly(ethylene terephthalate) (PET), a synthetic aromatic polyester composed of ethylene glycol and terephthalic acid, is one of the most consumed plastics in the world (Crawford and Quinn 2017). PET shows several uses, such as bottles for beverages, medical materials, films, clothing, and technical textiles. The outstanding properties of PET, such as high strength, low weight, low permeability of gases, and chemical resistance, make PET an excellent packaging material (Crawford and Quinn 2017).
The increasing production of PET and its durability cause negative environmental and economic impacts due to the accumulation of this highly persistent material in aquatic and terrestrial ecosystems (Taniguchi et al. 2019). Therefore, there is a growing effort to find polymer degradation and recycling technologies.
Plastic recycling can be performed using different approaches, including thermal, mechanical, and chemical (Abdelaal et al. 2008; Geyer et al. 2016; Paszun and Spychaj 1997; Sinha et al. 2008). Only the chemical route converts PET to its original raw materials, which can then be reused, addressing a circular economy philosophy. However, high energy-consuming processes are carried out under severe temperature and pH conditions due to the polymer's high resistance to many chemicals.
Alternative environmentally friendly strategies for PET recycling have been evaluated, including microbial enzymes and microorganisms. The enzymes involved in PET degradation are mainly hydrolases like cutinases (EC 3.1.1.74), lipases (EC 3.1.1.3), carboxylesterases (EC 3.1.1.1), and PET hydrolase (PETase—EC 3.1.1.101) (Kawai et al. 2019; Danso et al. 2018; Castro et al. 2017). Compared to conventional chemical hydrolysis of polyester, enzymatic depolymerization with hydrolases leads to fewer side products and higher purity of the monomers for following re-polymerization processes (Castro et al. 2017).
Therefore, hydrolases are an environmentally friendly alternative due to the high specificity and efficiency of enzymes, which work under mild conditions with low energy input. These enzymes catalyze the hydrolysis of PET, releasing terephthalic acid (TPA), ethylene glycol (EG), and products of higher molecular weight, mainly mono(2-hydroxyethyl) terephthalate (MHET) and bis(2-hydroxyethyl) terephthalate (BHET) (Acero et al. 2011).
However, PET is a non-natural substrate for enzymatic reactions. The biodepolymerization decreases with the increase of the content of aromatic constituents on the polymer, as occurs in PET (Müller 2006). The performance of hydrolytic enzymes over the PET surface increases as the reaction temperatures are closer to the polymer's glass transition temperature (Tg) and decreases with the increase of its crystallinity. Most enzymes preferentially attack amorphous rather than crystalline regions of a polymer, where its chain mobility is less restricted than in highly crystallized areas. The mobility of the polymer chain increases above the Tg. At temperatures above the Tg, the amorphous parts of the polymer become flexible and more accessible to an enzymatic attack, which supports the search for thermostable enzymes (Kawai et al. 2019). Enzymatic PET hydrolysis increases with temperature, because there is a higher probability of surface chains leaving the polymer structure and temporarily forming a kind of loop that can penetrate the enzyme's active site near its glass transition temperature (Biundo et al. 2018; Fecker et al. 2018; Muller 2006).
Enzymatic depolymerization is influenced not only by the polymer's chemical structure and physical properties (Müller 2006; Urbanek et al. 2020) but also by an adequate interaction of the enzyme with the substrate at the solid–liquid interface (Biundo et al. 2018; Wei et al. 2016). The accessibility of the active site is a crucial parameter that enables the biodegradability of PET (Biundo et al. 2018; Fecker et al. 2018). Cutinases display a shallow, wide-open active site on the enzyme surface, allowing hydrolysis of water-insoluble hydrophobic polyesters. On the other hand, lipases and esterases contain the active site in a tunnel-forming structure, leading to vertical-type hydrolysis (Biundo et al. 2018). Only cutinases have been reported to degrade the inner block of PET films, although surface modification of PET is possible with lipases, esterases, and cutinases (Kawai et al. 2019).
It is still challenging to understand the hydrolysis of PET catalyzed by enzymes. Research into enzyme type and reaction conditions is crucial to improving the competitiveness of this route. Some pretreatments, like the application of ultrasound (Pellis et al. 2016) or the pre-incubation of PET film with anionic surfactants (Furukawa et al. 2018), have been investigated with the aim of improving PET depolymerization. Ultrasound is an emerging sustainable technology that meets the principles of green chemistry and enhances the rate of several processes (Chatel 2018; Córdova et al. 2022; Hoo et al. 2022). Ultrasound has been shown to improve the efficiency of the biocatalysts by modifying the structure of the enzyme and the substrate or by increasing the substrate mass transfer rate in the reaction system (Córdova et al. 2022). The intense physical forces such as shear forces, shock waves, turbulence, and microjets caused by the mechanical effects of ultrasound intensify the mass transfer, decrease the diffusion barrier, and contribute to modifying substrate structure (Hoo et al. 2022; Vartolomei et al. 2022). Acoustic cavitation may also produce enzyme structure modifications with a higher exposure of the active site, which becomes more accessible to the substrate, facilitating enzyme–substrate interaction, decreasing the activation energy, and increasing the rate of hydrolysis (Córdova et al. 2022). Increasing the monomer yield while reducing the reaction time is necessary to improve the enzyme recycling process. In the present work, several PET enzymatic hydrolysis reaction parameters were investigated to contribute to the enhancement of the post-consumer PET (PC-PET) depolymerization processes. Previously, our group investigated the effect of some variables during enzymatic hydrolysis of a different post-consumer PET sample assorted from a supplier (Castro et al. 2019; Eugenio et al. 2021, 2022a). Here, the investigation of the comparative performance of varying enzyme sources (among lipases and cutinase), the simultaneous use of different hydrolases (dual enzyme system), the use of ultrasound energy, the reaction temperature, the enzyme concentration, and the reaction time on depolymerization of PET sample obtained from post-consumer non-carbonated mineral water bottles were studied, thus adding knowledge to the results, we have previously reported (Carniel et al. 2017; Castro et al. 2017, 2018, 2019; Malafatti-Picca et al. 2019; Eugenio et al. 2021, 2022a).
Materials and methods
Chemicals
Terephthalic acid (TPA, 98% purity) and bis-(2-hydroxyethyl) terephthalate (BHET, 99.8% purity) were purchased from Sigma-Aldrich. Mono-(2-hydroxyethyl) terephthalate (MHET) was synthesized through the enzymatic hydrolysis of bis-(2-hydroxyethyl) terephthalate by our group (Eugenio et al. 2022b).
PET samples were obtained from post-consumer non-carbonated mineral water bottles (Crystal® brand). The post-consumer PET (PC-PET) presents an intrinsic viscosity of 0.7453 ± 0.0032 dl g−1, molar mass equal to 42,737 ± 288 g mol−1, a polymerization degree of 222.4 ± 1.5, and crystallinity of 36.6 ± 0.5% (Castro et al. 2017).
Enzymes
Novozymes kindly provided liquid preparations of Candida antarctica lipase B (CalB, Lipozyme® CALB L), Rhizomucor miehei lipase (RmL, Palatase® 20000L), Candida antarctica lipase (CaL, Eversa® transform), Thermomyces lanuginosus lipase (TlL, Lipozyme® TL) and Humicola insolens cutinase (HiC, product Novozym® 51032, listed as a lipase by the supplier). The protein concentrations of CalB, RmL, CaL, TlL, and HiC were determined according to the Bradford method (Bradford 1976), and the obtained results were 11.2, 3.9, 25.6, 21.4, and 11.2 mg mL−1, respectively.
PET hydrolysis
Before the hydrolysis reaction, PET squares of approximately 0.5 cm with 0.1 mm thickness were washed with detergent, subsequently with distilled water, and finally with a mixture of water and ethanol (1:1 volume ratio) for 10 min using orbital stirring (200 rpm) and then dried using the Moisture analyzer (Mettler HB43-S) at 105 °C. The reactions were carried out in two different reactors: a 50-mL batch reactor stirred in an orbital shaker-incubator (Tecnal TE-420) and a 10-mL reactor with magnetic stirring (EasyMax 102 Reactor, Mettler Toledo). The effects of reaction temperature (50–70 °C) and enzyme concentration (3.2–80% v/v) on enzymatic PET hydrolysis were investigated using the EasyMax reaction system with magnetic stirring (800 rpm) since the maximum temperature of the orbital shaker incubator is 55 °C. The reaction medium (5 mL) consisted of a mixture of the commercial enzyme diluted in phosphate buffer 0.1 mol L−1 (0.086 M Na2HPO4 and 0.014 M KH2PO4 at pH = 8.0), PET (5 g L−1 PET) and sodium azide (0.2% wt/v). Control experiments (without biocatalyst) were performed at 70 °C (blank test) at the same reaction conditions. A standard deviation of 15% was found when triplicates were analyzed, considered acceptable, given the heterogeneity of the reaction medium.
The ultrasound energy was also used to treat the reaction medium using an ultrasound water bath (Branson 2510, heating power 245 W, 230 V, 42 kHz, Branson Ultrasonics Corporation). Sonication treatment of the reaction medium (3.2 vol% HiC; 5 g L−1 PET; phosphate buffer 0.1 mol L−1 pH = 8.0) was performed at different times (0, 10, 20, and 30 min). The reaction further proceeded in an orbital shaker-incubator, at 50 °C, for 7 days. In another experiment, the reaction medium in the absence of biocatalyst (5 g L−1 PET; phosphate buffer 0.1 mol L−1 pH = 8.0) underwent ultrasound treatment for 30 min. After, the biocatalyst was added to the medium, and the reaction was carried out at 50 °C for 7 days.
Quantification of the enzymatic hydrolysis products
The concentrations of TPA, MHET, and BHET formed during PET hydrolysis were determined by high-performance liquid chromatography (HPLC). Aliquots of the reaction medium were mixed with ice-cold methanol for protein precipitation. Then, the suspension was filtered through a 0.45 μm PTFE filter membrane. TPA, MHET, and BHET were quantified by HPLC using a chromatograph (Waters) equipped with a binary HPLC Pump (1525 model, Waters), a UV/Visible detector (2489 model, Waters), and an Eclipse Plus C18 column (Agilent Technologies) of 4.6 mm × 250 mm and 5 μm particle diameter. The column temperature was maintained at 30 °C, and an acetonitrile/0.05% formic acid gradient was used as a mobile phase (0.5 mL min−1). Detection was done at 254 nm. The TPA, MHET, and BHET concentrations were determined based on standard curves in a concentration range from 1 up to 100 mg L−1 using methanol as solvent.
Results and discussion
Effects of enzyme type on PET depolymerization
Enzymes involved in PET degradation are mainly hydrolases. Various hydrolases, such as cutinases, lipases, carboxylesterases, and esterases, have been shown to degrade PET to different extents (Joo et al. 2018). Some of them are PET surface-modifying enzymes and do not degrade the building blocks of PET; they only hydrolyze the surface polymer chain. PET depolymerization requires substantial degradation of the building blocks of PET, and only a limited number of cutinases have been recognized as PET hydrolases (Kawai et al. 2019). This work tested five widely used commercial hydrolases, four lipases (CalB, RmL, CaL, TlL), and one cutinase (HiC) in PET depolymerization. These enzymes are active against ester bonds. The PET hydrolysis reactions were carried out for 7 days at 50 ºC. The reaction time was chosen considering the previous results of the group (Castro et al. 2019; Eugenio et al. 2021, 2022a) and reported works (Ronkvist et al. 2009; Müller et al. 2005). According to the results in Fig. 1, the cutinase HiC and the lipases CaL and RmL could hydrolyze PET into TPA, MHET, or BHET. HiC enzyme was the most active at 50 °C, and TPA was the predominant product. CalB and TlL were not active under the experimental conditions tested.
Fig. 1.
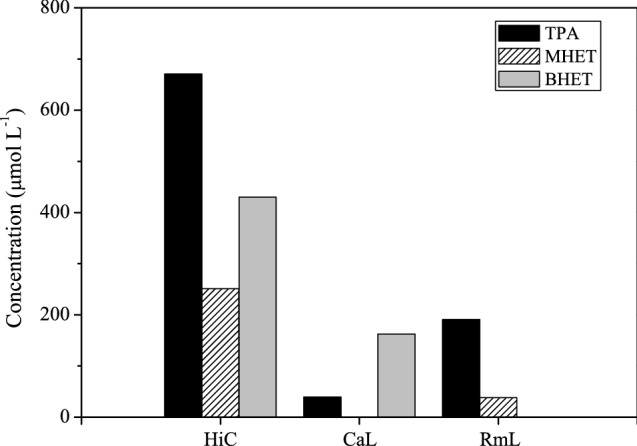
Depolymerization of post-consumer PET using different enzymes: Humicola insolens cutinase (HiC), Candida antarctica lipase (CaL), and Rhizomucor miehei lipase (RmL). Experimental conditions: 5 g L−1 PET; 50 vol% biocatalyst; phosphate buffer (pH = 8.0, 0.1 mol L−1); 200 rpm; 50 °C; 7 days
Previously, our group used a dual enzyme system, which consisted of HiC and CalB as biocatalysts in depolymerizing post-consumer PET in a one-pot approach (using the mixture of two enzymes simultaneously) or in a process in which the enzymes were employed sequentially (Castro et al. 2017; Carniel et al. 2017). It was observed that this dual enzyme system could favor the degradation of PET, because CalB presented a good activity for BHET and MHET hydrolysis to TPA, favoring the PET hydrolyzing activity of HiC. However, only some proportions of these enzymes were evaluated, and a more detailed investigation was needed to optimize this process.
Therefore, the PC-PET hydrolysis using this approach was further investigated using a mixture of HiC and CalB in different proportions at 50 °C (Fig. 2). As can be observed in Fig. 2, the use of a mix of HiC (87.5%) and CalB (12.5%) enhanced TPA concentration by 1.13-fold, which can be attributed to a higher specificity of CalB to catalyze the hydrolysis of MHET, as also seen in the previous investigation (Castro et al. 2017). However, CalB proportions higher than 12.5% resulted in a more evident decrease in TPA concentration, while for MHET concentration, a slighter effect was noted. The reduction of TPA concentration is related to the lower amount of HiC present in the reaction medium. TPA/MHET molar ratio, when only HiC was used, was equal to 1.9. On the other hand, when only CalB was used, the TPA/MHET molar ratio was equivalent to 6.7, confirming the ability of CalB to hydrolyze MHET. When different amounts of CalB and HiC were used, the TPA/MHET molar ratio was around 1.9, indicating that the presence of the CalB enzyme did not improve MHET hydrolysis in this reaction system at 50 °C.
Fig. 2.
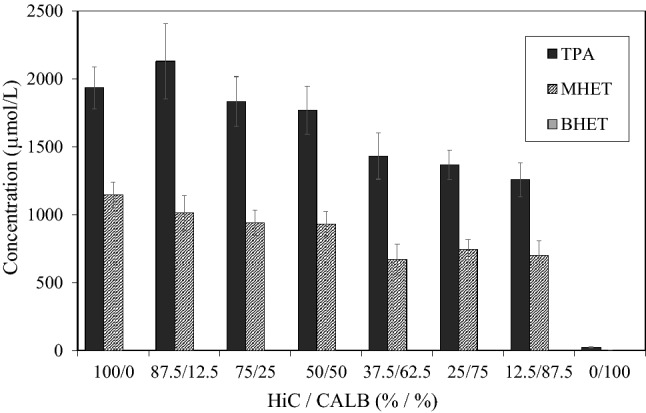
Influence of the use of a dual enzyme system on post-consumer PET depolymerization. Experimental conditions: Humicola insolens cutinase (HiC) and Candida antarctica lipase B (CalB); 3.2 vol% biocatalyst (total amount); 5 g L−1 PET; phosphate buffer (pH = 8.0 and 0.1 mol L−1); 200 rpm; 50 °C; 7 days. Error bars indicate the standard deviations of triplicate measurements
The improvement of the presence of CalB in the PET depolymerization using HiC cutinase was reported in the literature for other PET sources and under distinct experimental conditions (Castro et al. 2017) to a different extent from what was found in this work. The best result reported by the authors was obtained using a PET sample that was pretreated with ethylene glycol for 22 h at 37 °C before the hydrolysis reaction. Castro et al. (2017) studied the synergy between these enzymes over five industrial PET resins and two post-consumer PET samples. The authors observed that the combined use of the two enzymes increased TPA and MHET concentrations substantially only when the PET bottle was used after 14 days of reaction, and at the end of the test with amorphous PET, at 60 °C.
As a dual enzyme system only yielded a marginal increase of post-consumer PET hydrolysis in a narrow range of CALB content at 50 °C, further experiments in this work were carried out using HiC solely as a biocatalyst.
Effects of PET pretreatment in an ultrasound reactor
Ultrasound technology has been used to improve several enzymatic processes. The main outcome of these processes is the enhancement of mass transfer, which facilitates reagents' movement to the active site of the enzyme and the reaction products to the medium, increasing catalytic efficiency (Córdova et al. 2022; Gonçalves et al. 2015; Vartolomei et al. 2022; Wang et al. 2011). Ultrasound energy is also known to reduce the activation energy and enthalpy of enzymatic reactions. On the other hand, enzymes can be activated or inactivated by ultrasound irradiation. Shock waves released from cavitation bubbles can alter the native structure of the enzyme caused by breaking hydrogen bonds and van der Waals interactions (Pellis et al. 2016). High-power ultrasound may result in enzyme denaturation. A low-intensity, short-duration ultrasonic treatment is more likely to enhance enzyme activity, while prolonged exposure may lead to a loss of stability and a decrease in enzyme activity (Vartolomei et al. 2022).
The influence of pretreatment in an ultrasound reactor on the PET's depolymerization using HiC was studied using two experiments. Firstly, one investigation was carried out to investigate if sonication in the absence of the enzyme could improve the polymer segment's mobility in the crystalline phase of the PET. The mixture of PET and buffer was submitted to ultrasound for 30 min, and after that, the enzyme was added. However, no improvement in results was observed. In another experiment, the reaction medium (PET + enzyme + buffer) underwent treatment during 0, 10, 20, and 30 min. According to the results shown in Fig. 3, after 7 days, no significant differences were observed for the concentration of TPA, MHET, and BHET, which indicates that cavitation benefits were not noted. On the other hand, there was no evidence of enzyme deactivation, which suggests that HiC was not affected by the collapse of bubbles. The molar fractions of TPA (0.66), MHET (0.32), and BHET (0.02) did not change after sonication in any experiment.
Fig. 3.
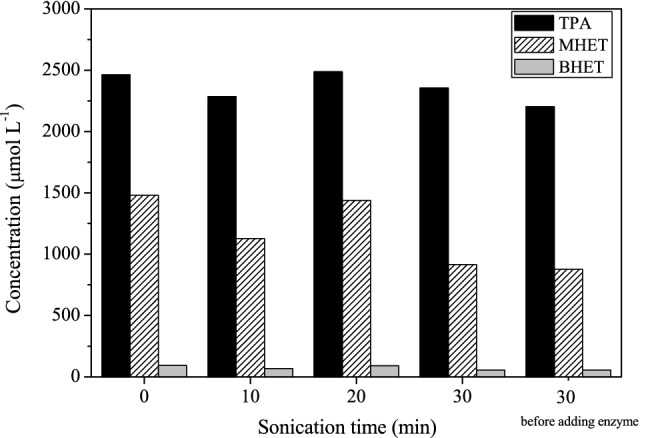
Influence of sonication on post-consumer PET depolymerization in the presence of Humicola insolens cutinase (HiC). Experimental conditions: 3.2 vol% biocatalyst; 5 g L−1 PET; phosphate buffer (pH = 8.0 and 0.1 mol L−1); 200 rpm; 50 °C; 7 days. Sonication was applied for 10, 20, and 30 min in the enzyme's presence and 30 min before adding the biocatalyst
Pellis et al. (2016) studied the enzymatic hydrolysis of PET in the presence of recombinant Thermobifida cellulosilytica cutinase 1 (Thc_cut1) using ultrasound. The application of ultrasound for a short time (10 min) enhanced the enzymatic hydrolysis by 8% of crystalline PET powder. The sonication led to a 5.2-fold increase in TPA, and a 6.6-fold increase of MHET released compared to the enzyme treatment alone after 24 h. When a 28% crystalline PET powder was used, the positive effect (2.9-fold increase in the released TPA) of ultrasound energy (30 min) on the enzymatic activity was lower than that observed with 8% crystalline PET powder. The effect of sonication using PET film was also investigated. They noticed that the ultrasound treatment (10 min) enhanced by 1.2-fold the TPA released in the hydrolysis of PET films with a crystallinity of 7% after 72 h of reaction. Longer sonication times (30 and 60 min) led to partial inactivation of the biocatalyst due to cavitation's mechanical and chemical effects during ultrasound treatment. The authors concluded that ultrasound energy affects the biocatalyst stability and activity, and its impact is reduced when using substrates with a less available surface (film vs. powder). Therefore, the results observed in this work with 36.6% crystallinity PET film agree with those followed by Pellis et al. (2016).
Influence of reaction temperature on PET depolymerization
The temperature of the enzyme-catalyzed hydrolysis of polyesters is critical because both enzymatic activity and polymer chain mobility are directly affected by temperature.
The Tg value of PET is approximately 80 °C, but it is lowered in the water, resulting in increased chain mobility (Kawai et al. 2019). Water molecules enter between the polymer chains in a swelling effect, weakening hydrogen bonds, and randomizing polymer chains, thus increasing polymer chain flexibility and their accessibility to enzymes (Kawai et al. 2019). Therefore, higher reaction temperatures are expected to result in faster rates of PET enzymatic depolymerization in an aqueous solution. However, the thermal denaturation of the enzyme must also be considered. Thus, the enzyme must be thermostable for efficient enzymatic depolymerization of PET.
Our group (Eugenio et al. 2021) has already tested the enzymatic hydrolysis of PET at temperatures higher than 70 °C. However, the best observed temperature was 70 °C. For this reason, in the PC-PET experiments, we only evaluated temperatures of 50, 60, and 70 °C. As shown in Fig. 4, the PET hydrolysis increased as the reaction temperature increased. The TPA released at 70 °C is 3.65-fold higher than at 50 °C. These results indicate the high thermal stability of HiC, as our group had already observed (Eugenio et al. 2021). The HiC cutinase is a thermostable enzyme, showing significant activity even at 85 °C (Eugenio et al. 2021; Baker et al. 2012).
Fig. 4.
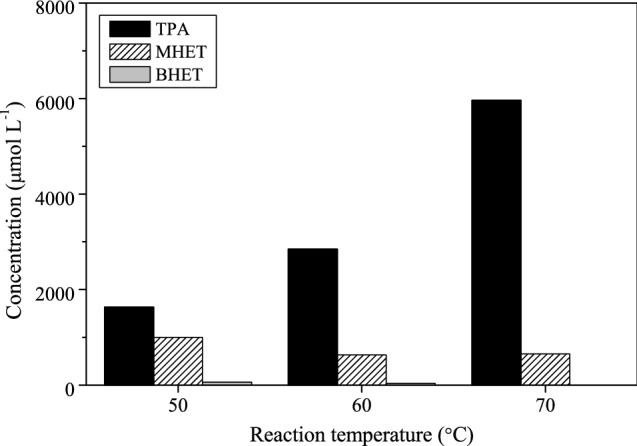
Influence of reaction temperature (50, 60, 70 °C) on post-consumer PET depolymerization in the presence of Humicola insolens cutinase (HiC). Experimental conditions: 3.2 vol% biocatalyst; 5 g L−1 PET; phosphate buffer (pH = 8.0 and 0.1 mol L−1); 800 rpm; 7 days
The degradation temperature must be high enough to increase the chain mobility of the polymer. This behavior was observed in this work as the Tg of the PET sample is 78 °C, and PET depolymerization was more effective at 70 °C. Ronkvist et al. (2009) also studied the hydrolysis of a low crystallinity (7.0 ± 0.5%) PET using HiC. They reported that the hydrolysis activity sharply increased at temperatures above 65 °C. Weinberger et al. (2017) also observed a significant increase in the hydrolysis of amorphous poly(ethylene 2,5-furanoate) (PEF) films using HiC when the reaction temperature increased from 50 to 65 °C.
It is interesting to highlight that the reaction temperature affects the concentration of degradation products and their molar ratios. The conversion of MHET into TPA is favored by reaction temperature increase (Fig. 5). A blank test (without biocatalyst) was also carried out at 70 °C, and the TPA, MHET, and BHET concentrations were negligible for 7 days.
Fig. 5.
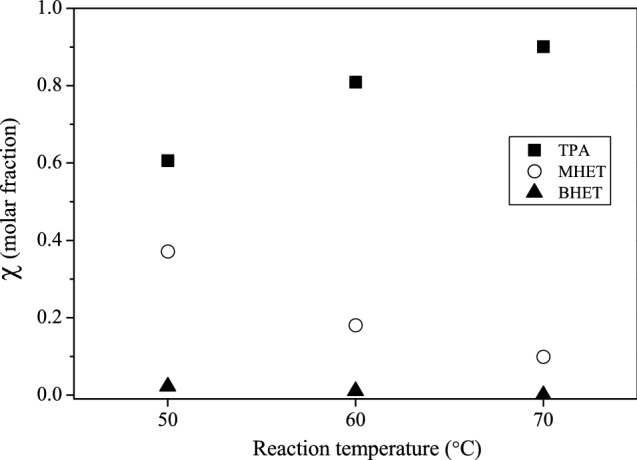
Influence of reaction temperature (50, 60, 70 °C) on post-consumer PET depolymerization in the presence of Humicola insolens cutinase (HiC) using the molar fraction of the degradation products. Experimental conditions: 3.2 vol% biocatalyst; 5 g L−1 PET; phosphate buffer (pH = 8.0 and 0.1 mol L−1); 800 rpm; 7 days
Influence of enzyme concentration and reaction time on PET depolymerization
The effects of HiC concentration on the depolymerization of PET were studied at 70 °C, and the results are illustrated in Figs. 6 and 7. The concentration of degradation products increased as the enzyme concentration increased, and a maximum was achieved at around 40–50 vol %. After that, the hydrolysis decreased. The effect of the biocatalyst (HiC) concentration on the hydrolysis of the PC-PET was also studied by Eugenio et al. (2021). The authors observed that the initial rate of enzymatic PC-PET depolymerization increased to a maximum value with the concentration of HiC, followed by a gradual decrease, indicating that the adsorption with hydrophobic domains present on the enzyme surface impacts PC-PET hydrolysis.
Fig. 6.
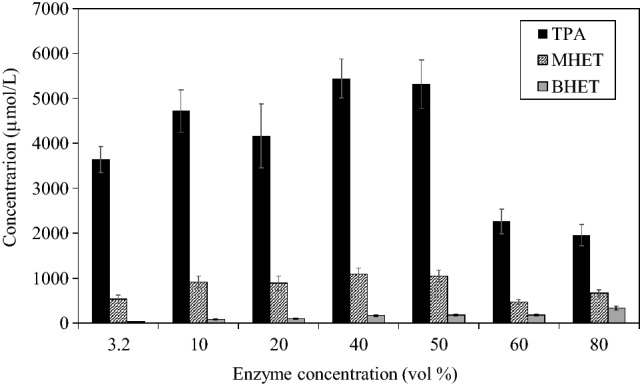
Influence of the enzyme concentration (3.2, 10, 20, 40, 50, 60, 80 vol %) on post-consumer PET depolymerization in the presence of Humicola insolens cutinase (HiC). Experimental conditions: 35 g L−1 PET; phosphate buffer (pH = 8.0 and 0.1 mol L−1); 800 rpm; 70 °C; 7 days. Error bars indicate the standard deviations of triplicate measurements
Fig. 7.
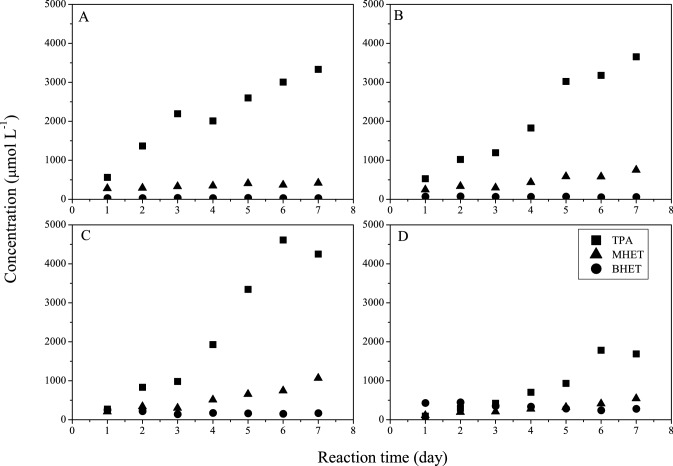
Influence of enzyme concentration and reaction time on post-consumer PET depolymerization in the presence of Humicola insolens cutinase (HiC). Experimental conditions: 5 g L−1 PET; phosphate buffer (pH = 8.0 and 0.1 mol L−1); 800 rpm; 70 °C; 7 days. A 3.2 vol% of enzyme; B 10 vol% of enzyme; C 40 vol% of enzyme; D 80 vol% of the enzyme
Mukai et al. (1993) studied the kinetics and mechanism of enzymatic degradation on the surface of a polyhydroxyalkanoate (PHA) film using three types of extracellular PHA depolymerase from Alcaligenes faecalis, Pseudomonas pickettii, and Comamonas testosteroni. They also verified that the enzymatic degradation increased to a maximum value as the concentration of PHA depolymerase grew and then gradually decreased. The authors presented a schematic model for the influence of enzyme concentration. There is enough space for adsorption and hydrolysis at low enzyme concentrations until the optimum concentration. However, at high enzyme concentrations, the enzyme only adsorbs through the binding domain, and hydrolysis does not occur.
This mechanism can explain the increase in the concentration of hydrolysis products as the concentration of enzyme increases, and hydrolysis decreases when higher enzyme concentrations are used. Figure 6 shows that the product concentrations increased with enzyme concentration up to 40% v/v. At higher enzyme concentrations (> 50% v/v), the product concentration decreased, indicating that too many protein molecules are adsorbed, and the appropriate orientation of the active site to the surface area may be hindered. The results show the strong dependence of the surface area of PET on enzymatic depolymerization. However, a specific substrate-binding domain is absent in the cutinases reported for PET hydrolysis, unlike polyhydroxyalkanoate depolymerase. The enzyme adsorption to the PET surface is presumably mediated by hydrophobic regions surrounding the catalytic site (Kawai et al. 2019; Urbanek et al. 2020).
The heterogeneous enzymatic hydrolysis of PET nanoparticles in the presence of a polyester hydrolase (TfCut2) from Thermobifida fusca KW3 was studied using turbidimetric analysis (Wei et al. 2014). The kinetics of the enzymatic hydrolysis of different PET nanoparticles was investigated with a constant PET concentration (0.25 mg mL−1) and varying enzyme concentrations of up to 80 μg mL−1. The reaction rate for nanoparticles from PET film reached a maximum at TfCut2 concentration of 60 μg mL−1 and then decreased fast.
To understand the PET depolymerization in the presence of HiC, the formation of the degradation products, TPA, MHET, and BHET, was evaluated using four concentrations of the enzyme (3.2, 10, 40, 80 vol%) over 7 days (Fig. 7). The profiles of these products are similar in the presence of 3.2, 10, and 40 vol% of the enzyme. After 2 days, TPA was the main product, while BHET remained stable at low concentrations, and MHET concentration slightly increased in the presence of 40 vol% of the enzyme. On the other hand, for the higher concentration (80 vol%), TPA became the main product after 4 days. For all concentrations of enzyme used, TPA concentration increased throughout reaction time. A steep increase was observed after 3 days when 40 vol% of HiC cutinase was used.
TPA was the dominant hydrolysis product from PET for all conditions tested in this work, agreeing with what is disclosed in the literature (Ronkvist et al. 2009). The same behavior was also reported for different cutinases from Thermobifida cellulosilytica and Thermobifida fusca (Acero et al. 2011). On the other hand, MHET was the predominant product after PET surface modification with a cutinase from Fusarium solani pisi (Vertommen et al. 2005). Different ratios of TPA and MHET were reported for PET hydrolysis depending on incubation time and material (fabrics or films) using cutinases from Thermobifida fusca and Fusarium solani (Eberl et al. 2009).
Conclusions
This work used commercial hydrolases for PET depolymerization for the purpose of enzymatic PET recycling. Several variables were studied to contribute to an optimization process for enzyme-based PET degradation. The enzymatic PET hydrolysis route allows for the use of monomers in re-polymerization processes, thus addressing a circular economy approach needed on the planet. Among the enzymes tested, Humicola insolens cutinase (HiC) showed the highest activity for PET hydrolysis. HiC was active for PET depolymerization into terephthalic acid, ethylene glycol, and intermediates like mono-(2-hydroxyethyl) terephthalate (MHET) and bis(2-hydroxyethyl) terephthalate (BHET), that can be reused for synthesis. The hydrolysis activity of HiC and the selectivity to TPA were favored as the reaction temperature increased until 70 °C, which evidenced the high thermal stability of HiC. The concentration of the released products (TPA, MHET, BHET) showed a different molar ratio with the temperature increase. Although Candida antarctica lipase B (CalB) is effective in catalyzing the hydrolysis of MHET to TPA, when mixtures of different amounts of CalB and HiC (a dual enzyme system) were used, an improvement in TPA concentration was only observed when 12.5% of CalB was added to the total enzyme stream. An ultrasound-assisted reaction system was evaluated to enhance the enzymatic hydrolysis of PET, but no PET depolymerization improvement was observed. Other pretreatments, such as the addition of surfactants and additives, should be investigated in order to increase PET depolymerization. The increase of enzyme concentration beyond 50 vol % hampers the hydrolysis of intermediate products, which can be attributed to the high coverage of the PET film surface by the enzyme. The biocatalytic depolymerization of PET arrives as an environmentally friendly process for the recycling of post-consumer plastic wastes. Enzyme technology can be an alternative solution for the treatment of plastic wastes that disintegrate forming micro-plastics and nano-plastics over time. The presented results contribute to important knowledge about the promising enzymatic route for PET depolymerization.
Acknowledgements
The authors thank UFRJ, UERJ, IFRJ, and Petrobras for supporting the research.
Author contribution
All authors contributed to the study conception and design. All authors read and approved the final manuscript. RB: conceptualization, methodology, investigation, writing—original draft. CdOV: conceptualization, methodology, investigation, writing—original draft. AMdC: supervision, writing—review and editing. MAPL: conceptualization, methodology, supervision, writing—review and editing.
Data availability
Data available on request from the authors.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Contributor Information
Rodrigo Brackmann, Email: rodrigobrackmann@utfpr.edu.br.
Cláudia de Oliveira Veloso, Email: co.veloso@hotmail.com.
Aline Machado de Castro, Email: alinebio@petrobras.com.br.
Marta Antunes Pereira Langone, Email: marta.langone@gmail.com, Email: marta.langone@ifrj.edu.br, Email: langone@uerj.br, Email: marta.langone@pppeq.uerj.br.
References
- Abdelaal MY, Sobahi TR, Makki MSI. Chemical degradation of poly(ethylene terephthalate) Int J Polym Mater Polym. 2008;57(1):73–80. doi: 10.1080/00914030701329080. [DOI] [Google Scholar]
- Acero EH, Ribitsch D, Steinkellner G, Gruber K, Greimel K, Eiteljoerg I, Trotscha E, Wei R, Zimmermann W, Zinn M, Cavaco-Paulo A, Freddi G, Schwab H, Guebitz GM. Enzymatic surface hydrolysis of PET: effect of structural diversity on kinetic properties of cutinases from Thermobifida. Macromolecules. 2011;44:4632–4640. doi: 10.1021/ma200949p. [DOI] [Google Scholar]
- Baker PJ, Poultney C, Liu Z, Gross R, Montclare JK. Identification and comparison of cutinases for synthetic polyester degradation. Appl Microbiol Biotechnol. 2012;93:229–240. doi: 10.1007/s00253-011-3402-4. [DOI] [PubMed] [Google Scholar]
- Biundo A, Ribitsch D, Guebitz GM. Surface engineering of polyester-degrading enzymes to improve efficiency and tune specificity. Appl Microbiol Biotechnol. 2018;102:3551–3559. doi: 10.1007/s00253-018-8850-7. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carniel A, Valoni E, Nicomedes Junior J, Gomes AC, Castro AM. Lipase from Candida antarctica (CALB) and cutinase from Humicola insolens act synergistically for PET hydrolysis to terephthalic acid. Process Biochem. 2017;59:84–90. doi: 10.1016/j.procbio.2016.07.023. [DOI] [Google Scholar]
- Castro AM, Carniel A, Nicodemos Junior J, Gomes AC, Valoni E. Screening of commercial enzymes for poly(ethylene terephthalate) (PET) hydrolysis and synergy studies on different substrate sources. J Ind Microbiol Biotechnol. 2017;44:835–844. doi: 10.1007/s10295-017-1942-z. [DOI] [PubMed] [Google Scholar]
- Castro AM, Carniel A, Sirelli L, Dias ML, Menezes SMC, Chinelatto Junior LS, Honorato HA. Enzyme-catalyzed simultaneous hydrolysis-glycolysis reactions reveals tunability on PET depolymerization products Biochem. Eng J. 2018;137:239–246. [Google Scholar]
- Castro AM, Carniel A, Stahelin D, Chinelatto Junio LS, Honorato HA, Menezes SMC. High-fold improvement of assorted post-consumer poly(ethyleneterephthalate) (PET) packages hydrolysis using Humicola insolens cutinase as a single biocatalyst. Process Biochem. 2019;81:85–91. doi: 10.1016/j.procbio.2019.03.006. [DOI] [Google Scholar]
- Chatel G. How sonochemistry contributes to green chemistry? Ultrason Sonochem. 2018;40:117–122. doi: 10.1016/j.ultsonch.2017.03.029. [DOI] [PubMed] [Google Scholar]
- Córdova A, Henríquez P, Nuñez H, Rico-Rodriguez F, Guerrero C, Astudillo-Castro C, Illanes A. Recent advances in the application of enzyme processing assisted by ultrasound in agri-foods: a review. Catalysts. 2022;12:107. doi: 10.3390/catal12010107. [DOI] [Google Scholar]
- Crawford CB, Quinn B. Microplastic pollutants. Amsterdam: Elsevier Science; 2017. Physiochemical properties and degradation; pp. 57–100. [Google Scholar]
- Danso D, Schmeisser C, Chow J, Zimmermann W, Wei LC, Li X, Hazen T, Streit WR. New insights into the function and global distribution of polyethylene terephthalate (PET)—degrading bacteria and enzymes in marine and terrestrial metagenomes. Appl Environ Microbiol. 2018;84:1–13. doi: 10.1128/AEM.02773-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl A, Heumann S, Bruckner T, Araujo R, Cavaco-Paulo A, Kaufmann F, Kroutil W, Guebitz GM. Enzymatic surface hydrolysis of poly(ethylene terephthalate) and bis(benzoyloxyethyl) terephthalate by lipase and cutinase in the presence of surface active molecules. J Biotechnol. 2009;143:207–212. doi: 10.1016/j.jbiotec.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Eugenio EQ, Campisano ISP, Castro AM, Coelho MAZ, Langone MAP. Experimental and mathematical modeling approaches for biocatalytic post-consumer poly(ethylene terephthalate) hydrolysis. J Biotechnol. 2021;341:76–85. doi: 10.1016/j.jbiotec.2021.09.007. [DOI] [PubMed] [Google Scholar]
- Eugenio EQ, Campisano ISP, Castro AM, Coelho MAZ, Langone MAP. Kinetic Modeling of the post-consumer poly(Ethylene Terephthalate) hydrolysis catalyzed by cutinase from Humicola insolens. J Polym Environ. 2022;30:1627–1637. doi: 10.1007/s10924-021-02301-4. [DOI] [Google Scholar]
- Eugenio EQ, Campisano ISP, Dias AM, Castro AM, Coelho MAZ, Langone MAP. Novel efficient enzymatic synthesis of the key-reaction intermediate of PET depolymerization, mono(2-hydroxyethyl terephthalate)—MHET. J Biotechnol. 2022;358:102–110. doi: 10.1016/j.jbiotec.2022.08.019. [DOI] [PubMed] [Google Scholar]
- Fecker T, Galaz-Davison P, Engelberger F, Narui Y, Sotomayor M, Parra LP, Ramírez-Sarmiento CA. Active site flexibility as a hallmark for efficient PET degradation by I. sakaiensis PETase. Biophys J. 2018;114:1302–1312. doi: 10.1016/j.bpj.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa M, Kawakami N, Oda K, Miyamoto K. Acceleration of enzymatic degradation of poly(ethylene terephthalate) by surface coating with anionic surfactants. Chemsuschem. 2018;11:4018–4025. doi: 10.1002/cssc.201802096. [DOI] [PubMed] [Google Scholar]
- Geyer B, Lorenz G, Kandelbauer A. Recycling of poly(ethylene terephthalate)—a review focusing on chemical methods. Express Polym Lett. 2016;7:559–586. doi: 10.3144/expresspolymlett.2016.53. [DOI] [Google Scholar]
- Gonçalves I, Silva C, Cavaco-Paulo A. Ultrasound enhanced laccase applications. Green Chem. 2015;17:1362–1374. doi: 10.1039/C4GC02221A. [DOI] [Google Scholar]
- Hoo DY, Low ZL, LOw DYS, Tang SY, Manickam S, Tan KW, Ban ZH. Ultrasonic cavitation: An effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason Sonochem. 2022;90:106176. doi: 10.1016/j.ultsonch.2022.106176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S, Cho IJ, Seo H, Son HF, Sagong H-Y, Shin TJ, Choi SY, Lee SY, Kim K-J. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nature Commun. 2018;9:382–394. doi: 10.1038/s41467-018-02881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F, Kawabata T, Oda M. Current knowledge on enzymatic PET degradation and its possible application to waste stream management and other fields. Appl Microbiol Biotechnol. 2019;103:4253–4268. doi: 10.1007/s00253-019-09717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafatti-Picca L, Chaves MRB, Castro AM, Valoni E, Oliveira VM, Marsaioli AJ, Angelis DF. Hydrocarbon-associated substrates reveal promising fungi for poly(ethylene terephthalate) (PET) depolymerization. Braz J Microbiol. 2019;50:633–648. doi: 10.1007/s42770-019-00093-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K, Yamada K, Doi Y. Kinetics and mechanism of heterogeneous hydrolysis of poly[(R)-3-hydroxybutyrate] film by PHA depolymerases. Int J Biol Macromol. 1993;15:361–366. doi: 10.1016/0141-8130(93)90054-P. [DOI] [PubMed] [Google Scholar]
- Müller RJ. Biological degradation of synthetic polyesters—enzymes as potential catalysts for polyester recycling. Process Biochem. 2006;41:2124–2128. doi: 10.1016/j.procbio.2006.05.018. [DOI] [Google Scholar]
- Müller R-J, Schrader H, Profe J, Dresler K, Deckwer W-D. Enzymatic degradation of poly(ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol Rapid Commun. 2005;26:1400–1405. doi: 10.1002/marc.200500410. [DOI] [Google Scholar]
- Paszun D, Spychaj T. Chemical recycling of poly(ethylene terephtalate) Ind Eng Chem Res. 1997;4:1373–1383. doi: 10.1021/ie960563c. [DOI] [Google Scholar]
- Pellis A, Gamerith C, Ghazaryan G, Ortner A, Acero EH, Guebitz GM. Ultrasound-enhanced enzymatic hydrolysis of poly(ethylene terephthalate) Bioresour Technol. 2016;218:1298–1302. doi: 10.1016/j.biortech.2016.07.106. [DOI] [PubMed] [Google Scholar]
- Ronkvist A, Xie W, Lu W, Gross RA. Cutinase-catalyzed hydrolysis of poly(ethylene terephthalate) Macromolecules. 2009;42:5128–5138. doi: 10.1021/ma9005318. [DOI] [Google Scholar]
- Sinha V, Patel MR, Patel JV. Pet waste management by chemical recycling: a review. J Polym Environ. 2008;1:8–25. [Google Scholar]
- Taniguchi I, Yoshida S, Hiraga K, Miyamoto K, Kimura Y, Oda K. Biodegradation of PET: current status and application aspects. ACS Catal. 2019;9:4089–4105. doi: 10.1021/acscatal.8b05171. [DOI] [Google Scholar]
- Urbanek AK, Mirończuk AM, García-Martín A, Saborido A, Mata I, Arroyo M. Biochemical properties and biotechnological applications of microbial enzymes involved in the degradation of polyester-type plastics. Biochim Biophys Acta Proteins Proteom. 2020;1868:140315. doi: 10.1016/j.bbapap.2019.140315. [DOI] [PubMed] [Google Scholar]
- Vartolomei A, Calinescu I, Vinatoru M, Gavrila MV. A parameter study of ultrasound assisted enzymatic esterification. Sci Rep. 2022;12:1421. doi: 10.1038/s41598-022-05551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertommen MAME, Nierstrasz VA, van der Veer M, Warmoeskerken MMCG. Enzymatic surface modification of poly(ethylene terephthalate) J Biotechnol. 2005;120:376–386. doi: 10.1016/j.jbiotec.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Wang J, Cao Y, Sun B, Wang C, Mo Y. Effect of ultrasound on the activity of alliinase from fresh garlic. Ultrason Sonochem. 2011;18:534–540. doi: 10.1016/j.ultsonch.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Wei R, Oeser T, Barth M, Weigl N, Lübs A, Schulz-Siegmund M, Hacker MC, Zimmermann W. Turbidimetric analysis of the enzymatic hydrolysis of polyethyleneterephthalate nanoparticles. J Mol Catal B Enzym. 2014;103:72–78. doi: 10.1016/j.molcatb.2013.08.010. [DOI] [Google Scholar]
- Wei R, Oeser T, Schmidt J, Meier R, Barth M, Then J, Zimmermann W. Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol Bioeng. 2016;113:1658–1665. doi: 10.1002/bit.25941. [DOI] [PubMed] [Google Scholar]
- Weinberger S, Canadell J, Quartinello F, Yeniad B, Arias B, Pellis A, Guebitz GM. Enzymatic degradation of poly(ethylene 2,5-furanoate) powders and amorphous films. Catalysts. 2017;7(318):1–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available on request from the authors.