Abstract
In this study, the influence of γ-irradiation with different dose (0, 4, 8, and 16 kGy) on chemical composition, physicochemical features and bioactivities of polysaccharides extracted from Lentinula edodes (LEP) were investigated. The carbohydrate content (from 59.47 to 70.96%), the solubility, the ⋅OH and DPPH scavenging ability of LEP increased with the increased γ-irradiation dose, while the protein content, the weight-average and number-average molecular weight of LEP were significantly decreased with the increased γ-irradiation dose. Moreover, γ-irradiation treatment caused LEP color changes and surface topography destroyed. γ-Irradiated LEP showed higher hypoglycemic activities in vitro than that of non-irradiated LEP. Moreover, γ-irradiated LEP had better proliferation promoting effects on Lactobacillus rhamnosus and L. plantarum. These results showed that γ-irradiation treatment changes the physicochemical features of LEP, thus affects its antioxidant, hypoglycemic and prebiotic properties, which suggests that γ-irradiated LEP has potential application in the pharmaceutical industries and functional foods.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-022-01234-5.
Keywords: γ-Irradiation, Polysaccharides, Antioxidant, Hypoglycemic activity, Prebiotic proliferation
Introduction
Lentinula edodes (formerly Lentinus edodes) is the second most artificially cultivated edible fungus in the world (Li et al., 2019). Because of its unique flavour and high nutritional value, L. edodes has become a favourite food in East Asia. In the past few centuries, L. edodes has been used as traditional medicine diet in folk of China (Li et al., 2019). Currently, numerous bioactive compounds including polysaccharides, ergosterol, polyphenols and dietary fibres have been isolated from L. edodes (Liu et al., 2020). Among which, L. edodes polysaccharides receive a lot of attention due to its outstanding immunomodulatory, antibacterial, antioxidant and antitumor activities (Sheng et al., 2021). β-(1-3)-d-glucan, a typical polysaccharide from L. edodes named lentinan, has been used as a drug for cancer therapy based on pharmacology and clinical trial research (Rao et al., 2021).
Although L. edodes is easy to obtain, there are still a series of problems in acquisition of high qualified polysaccharides, such as low bioactivity, poor solubility and high viscosity (Rao et al., 2021). Nowadays, modification methods including γ-irradiation, carboxymethylation, and phosphorylation have been used to improve the activity of mushroom polysaccharides (Xie et al., 2020). In addition, polysaccharides combined with microelement such as selenium have been proved to be a new method for enhancing their bioactivity (Liu et al., 2022). It had been reported that poor solubility and high viscosity of polysaccharides generally relate to its high molecular weights (Mw), and polysaccharides with high Mw are hard to penetrate the cell membrane to exert pharmacological effects (Akram et al., 2017). Therefore, the decrease of Mw may contribute to enhance solubility and reduce viscosity of polysaccharides. Recently, γ-irradiation has received extensive attention for its ability to decrease the Mw and modify the structure of polysaccharides in a green and environmentally friendly manner (Akram et al., 2017). Ren et al. (2018) found that the Mw of γ-irradiated Astragalus polysaccharides was significantly decreased, and the solubility was significantly increased. Xiong et al. (2020) certified that γ-irradiation treatment effectively reduced the Mw of Morchella sextelata polysaccharides and enhanced its antioxidant activity. Xu et al. (2021) demonstrated that γ-irradiation treatment decreased the viscosity of Undaria pinnatifida root extracts and improved its anti-inflammatory activity. However, the influence of γ-irradiation on physicochemical and bioactivity of L. edodes polysaccharides (LEP) has not been fully studied. In this study, the chemical composition, physicochemical properties as well as the antioxidant, hypoglycemic and prebiotic activity of γ-irradiated LEP were determined to characterize the γ-irradiation influence.
Materials and methods
Materials and reagents
The dried L. edodes (Berk.) were obtained from Hubei Haowei Technology Co., Ltd. (Zhongxiang, China) with a proper traceability record. 4-Nitrophenyl-β-d-glucopyranoside (PNPG), 3-methyl-1-phenyl-2-pyrazoline-5-one (PMP), α-amylase and α-glucosidase were purchased from Sigma-Aldrich Chemical Co., Ltd. (St. Louis, MO, USA). Monosaccharides standards and bovine serum albumin were provided by Yuanye Biological Technology Co., Ltd. (Shanghai, China). Lactobacillus plantarum CGMCC-15801 and L. rhamnosus CGMCC-16103 were purchased from Zhongke Jiayi Biological Engineering Co., Ltd. (Shandong, China). All other analytical grade chemicals used in this study were purchased from Sinopharm Co., Ltd. (Beijing, China).
Polysaccharides extraction and γ-irradiation treatment
The L. edodes fruiting bodies were grinded to powder (80 mesh). Oligosaccharides and small molecules were removed from the powder with reported method (Yin et al., 2018). L. edodes polysaccharides (LEP) were extracted according to our previous method (Chen et al., 2020). Then, LEP was irradiated with a cobalt-60 source irradiator at Hubei Irradiation Experiment Center (Wuhan, China). The γ-irradiation dose was set as 16, 8, 4 and 0 kGy, the corresponding samples were named as LEP16, LEP8, LEP4 and LEP0 (non-irradiated), respectively.
Chemical composition and color characteristics analysis
Total carbohydrates content, uronic acid content, protein content, sulfate radical content and the pH value of different samples were determined according to our previous methods (Yin et al., 2022).
The color parameters including yellowness to blueness (b), redness to greenness (a) and lightness (L) were measured by a colorimeter (CM-5, MINOLTA, Osaka, Japan). The total color difference (△E) was calculated as follows:
Molecular weight determination
An Agilent 1100 series HPLC system with RID detector was used to determine the Mw of different samples. According to our previous method (Chen et al., 2020), the parameters were set as follows: column, PL aquagel-OH MIXED (8 μm, 7.5 × 300 mm); column temperature, 35 °C; mobile phase, 0.05 M Na2SO4 solution; flow rate, 0.9 mL/min; injection volume, 20 μL. The average MW of different samples was calculated from the calibration curve of dextran standards (Dextran T-series, T-180, T-3K, T-7K, T-10K, T-40K, T-70K, T-500K and T-2000K).
Monosaccharide composition analysis
The monosaccharide composition of different samples was analyzed according to the method describe by Yin et al. (2022). Briefly, 1.0 mL of trifluoroacetic acid solution (0.5 mol/L) and 2.0 mg of sample were mixed and hydrolyzed at 120 °C for 6 h in a sealed tube. After dried and washed with methanol, the hydrolyzed samples were obtained. Then, 200.0 μL of hydrolyzed samples mixed with 200.0 μL of PMP solution (0.5 mol/L) and 400.0 μL of NaOH solution (0.3 mol/L) and reacted at 70 °C for 2 h. Finally, terminate the reaction by adding 400.0 μL of HCl solution (0.3 mol/L). Then, 20.0 μL of filtered sample was injected into the Shimadzu LC-20A HPLC system with the mobile phase of acetonitrile and phosphate buffer (0.05 mol/L, pH 6.7) (83:17, v/v) at the flow rate of 1.0 mL/min at 35 °C. The column was Shimadzu InertSustain AQ-C18 (5 μm, 4.6 × 250 mm) and the detective wavelength was 245 nm.
UV–Vis spectrum, FT-IR spectrum and SEM analysis
The UV–Vis spectra of different samples were determined with the UV–Vis spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan). The FT-IR spectra of different samples were analyzed with the Nexus 470 spectrometer (Nicolet, Madison, USA). Briefly, KBr and dried sample were mixed and ground into powder, then a disk of the mixture was prepared and measured according to the instruction manual. The surface topography of different samples was captured by a Gemini SEM300 system (Carl Zeiss, Oberkochen, Germany), the dried sample were sputtered with gold and the acceleration voltage was 5.0 kV.
Solubility and Congo red test
The solubility of different samples was measured according to the method described by Shang et al. (2018). Briefly, a beaker with 125.0 mL distilled water was placed in a thermostatic magnetic stirrer. The stirred speed was set at 60 r/min and the temperatures was set as 20, 40, 60, 80, 100 °C, respectively. 0.25 g sample was added to the baker and the completely dissolved time at each temperature was recorded.
The Congo red experiment was conducted with the reported method of Chen et al. (2020). 1.5 mL of Congo red solution (2.0 mmol/L) mixed with 0.5 mL of sample solution (2.0 mg/mL), then NaOH solution with different concentrations was added to ensure the final concentration of NaOH ranged from 0.4 to 0.0 M. After stand at room temperature for 10 min, the maximum absorption wavelength of the mixture was recorded with the UV–Vis spectrophotometer at 400–600 nm.
In vitro antioxidant activities analysis
The DPPH and ⋅OH radical scavenging ability and the reducing power of different samples were determined with the method described by Chen et al. (2020). The absorbance of samples with different concentrations (0.5 to 3.0 mg/mL) were measured by the UV–vis spectrophotometer, and ascorbic acid (Vc) was used as the positive control.
In vitro hypoglycemic activities analysis
The inhibitory ability towards α-amylase and α-glucosidase are important indicators to evaluate the in vitro hypoglycemic activities of active compounds. According to the method of Wang et al. (2018), α-amylase inhibitory experiment was conducted as follows: 20.0 μL of sample solution with different concentration (2.0–10.0 mg/mL) and 20.0 μL of α-amylase solution (1.0 U/mL) were mixed. After incubated at 37 °C for 10 min, 40.0 μL of starch solution (0.5%, w/v) was added and incubated at 37 °C for another 10 min. Then, 80.0 μL of DNS reagent was added and mixed. Finally, terminate the reaction by incubating at boiling water for 5 min. The absorbance of mixture was recorded with Multiskan SkyHigh microplate reader (Thermo Scientific, Waltham, USA) at 540 nm.
The α-glucosidase inhibitory ability was determined with the method of Chen et al. (2020). Briefly, 20.0 μL of α-glucosidase solution (1000 U/mL), 40.0 μL of sample with different concentration (2.0–10.0 mg/mL) and 120.0 μL of phosphate buffer (pH 7.0, 0.25 M) were mixed. After incubated at 37 °C for 10 min, 20.0 μL of PNPG solution (5 mM) was added and incubated for another 10 min. Finally, the reaction was terminated by adding 80.0 μL of 0.2 M sodium carbonate. The absorbance of mixture was recorded with Multiskan SkyHigh microplate reader at 405 nm.
Probiotics activity
Lactobacillus rhamnosus and Lactobacillus plantarum were used to evaluate the probiotics activity of different samples with the method described by Hu et al. (2021). L. rhamnosus and L. plantarum were respectively inoculated on MRS solid medium. After incubated at 37 °C for 24 h under anaerobic conditions, single colony was inoculated into MRS liquid medium and cultivated at 37 °C for another 24 h. Finally, 5% (v/v) bacterial liquid was added to the MRS liquid medium in which glucose was replaced by different LEP and control (fructo-oligosaccharides, FOS) (10 mg/mL) as the carbon source. Under anaerobic conditions, the cultures were incubated at 37 °C for 48 h. Then, the pH values and the absorbance at 600 nm were recorded at every 8 h.
Statistical analysis
Each experiment was repeated at least 3 times and data was demonstrated as mean ± standard deviation (SD). Data obtained by one-way ANOVA and Duncan's multiple range tests using SPSS software. All statistics were based on the 95% confidence level, p < 0.05 was considered statistically significant.
Result and discussion
Chemical composition of γ-irradiated LEP
The chemical composition of LEP γ-irradiated at 0, 4, 8, and 16 kGy (LEP0, LEP4, LEP8, and LEP16) was showed in Table 1. LEP16 exhibited the highest carbohydrate content (70.96%), followed by LEP8 (64.01%), LEP4 (60.92%), and LEP0 (59.47%). The results showed the carbohydrate content increased with the increased irradiation dose. Similar results had reported by Zhou et al. (2021), who found γ-irradiation treatment significantly increased the carbohydrate content in Angelica dahurica crude polysaccharides in a dose-dependent manner. Moreover, Yin et al. (2022) and Akram et al. (2017) respectively reported the γ-irradiation treatment on the fruiting body of Schizophyllum commune and L. edodes can significantly increase the purity of polysaccharides. The increased carbohydrate content might attribute to larger polysaccharide molecules degradation, which increased the water soluble of polysaccharide (Akram et al., 2017). LEP8 (4.82%) and LEP16 (4.81%) showed similar protein content, but lower than that in LEP4 (5.63%) and LEP0 (6.64%). Obviously, γ-irradiation treatment decreased the protein content of LEP in a dose-dependent manner, and Yin et al. (2022) had also reported the similar results in γ-irradiated S. commune. The highest uronic acid and sulfate radical content were appeared in LEP0 (3.28%) and LEP8 (3.45%), respectively. However, it seemed that there was no specific trend in uronic acid and sulfate radical content. In addition, there was no significant difference observed in pH value between the different LEP.
Table 1.
The chemical composition, monosaccharide molar ratio and color characteristics of different LEP
| Index | Polysaccharides | |||
|---|---|---|---|---|
| LEP0 | LEP4 | LEP8 | LEP16 | |
| Chemical composition | ||||
| Carbohydrate content (%) | 59.47 ± 0.008c | 60.92 ± 0.007c | 64.01 ± 0.015b | 70.96 ± 0.012a |
| Protein content (%) | 6.64 ± 0.002a | 5.63 ± 0.003b | 4.82 ± 0.0016c | 4.81 ± 0.0003c |
| Uronic acid content (%) | 3.28 ± 0.0006a | 3.07 ± 0.0008a | 2.11 ± 0.0009b | 3.16 ± 0.0026a |
| Sulfate radical content (%) | 2.39 ± 0.0030b | 1.86 ± 0.0020b | 3.45 ± 0.0012a | 1.76 ± 0.0062b |
| pH | 6.89 ± 0.028a | 6.90 ± 0.042a | 6.48 ± 0.014a | 6.60 ± 0.014a |
| Monosaccharide molar ratio (molar %) | ||||
| Mannose | 1.00 | 1.00 | 1.00 | 1.00 |
| Ribose | 0.04 | 0.03 | 0.03 | 0.03 |
| Rhamnose | 0.03 | 0.03 | 0.07 | 0.05 |
| Glucuronic acid | 0.25 | 0.21 | 0.44 | 0.42 |
| Galacturonic acid | ND | ND | ND | ND |
| Glucose | 0.29 | 0.55 | 1.15 | 0.2 |
| Galactose | ND | ND | ND | ND |
| Xylose | 0.03 | 0.01 | 0.02 | 0.02 |
| Arabinose | 0.26 | 0.18 | 0.14 | 0.15 |
| Fucose | 0.17 | 0.08 | 0.29 | 0.43 |
| Color characteristics | ||||
| L* | 48.66 ± 0.67b | 53.33 ± 0.37a | 48.34 ± 0.44b | 54.31 ± 0.12a |
| a* | 2.29 ± 0.08a | 1.22 ± 0.07c | 1.45 ± 0.11b | 1.12 ± 0.18c |
| b* | 6.48 ± 0.34a | 4.00 ± 0.13d | 4.94 ± 0.23b | 4.38 ± 0.42bc |
| ∆E | 0.00 ± 0.00c | 5.46 ± 0.34a | 2.45 ± 0.53b | 6.19 ± 0.46a |
Mean values (n = 3) within the same row with different superscript letters (a to d) are significantly different at ρ < 0.05. ND, not detected. L* represents lightness, a* represents redness to greenness, b* represents yellowness to blueness, and ∆E represents total difference with respect to a control group
Monosaccharide composition and molecular weight
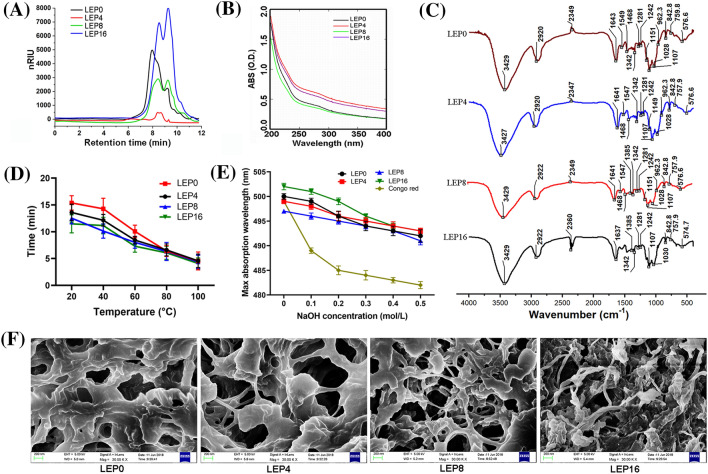
The monosaccharide molar ratios of γ-irradiated LEP were presented in Table 1. The main monosaccharide in different LEP were mannose, glucose, glucuronic acid, arabinose and fucose. Among them, mannose and glucose showed the highest molar ratio in LEP0, LEP4 and LEP8 when mannose was used as a reference, while mannose, glucuronic acid and fucose showed highest molar ratio in LEP16. The different molar ratio may relate to the large polysaccharide molecules degradation caused by γ-irradiation. Interestingly, the highest glucose content appeared in LEP8 but not in LEP16, this could be attribute to γ-irradiation treatment which resulted in the fracture of polysaccharides chain, the different monosaccharide composition of main or side chain might affect the monosaccharide molar ratios of different LEP. Moreover, it had been reported that the glucose γ-irradiated in high dose would be oxidated and cleaved to form gluconic acid, glucuronic acid, xylose etc. (Wang, 2015), therefore, the low content of glucose in LEP16 might be due to the oxidation of glucose. The HPLC spectra of Mw for different LEP was showed in Fig. 1a. Two peaks were observed in different samples, peak in front represented for high molecular component and the peak in the back represented for low molecular component. The Mw, Mn and polydispersity index information of different LEP was presented in Table 2. The Mw, Mn and polydispersity index of LEP were significantly decreased with the increased γ-irradiation dose, which showed the polysaccharides Mw decreased and the molecular weight distribution narrowed. The average Mw of LEP0, LEP4, LEP8 and LEP16 were 691.2, 492.4, 472.1 and 301.0 kDa, respectively. Interestingly, the Mn and polydispersity index of high molecular component in different LEP were decreased with the increased γ-irradiation dose in our study, which indicated the molecular chain length of γ-irradiated LEP was shorter and the molecular weight distribution was narrower than that of LEP0. Similarly, the Mw of polysaccharides from L. edodes (Akram et al., 2017), Astragalus membranaceus (Ren et al., 2018), Agaricus bisporus (Khan et al., 2015), Saccharomyces cerevisiae (Khan et al., 2016), Avena sativa (Shah et al., 2015) and S. commune (Yin et al., 2022) were decreased by γ-irradiation. In addition, the Mw, Mn and polydispersity index of low molecular component in different LEP were similar and no significant difference was observed.
Fig. 1.
The physico-chemical properties of different LEP. (A) molecular weight distribution; (B) UV–visible spectrophotometer; (C) infrared spectral; (D) solubility; (E) Congo red test; (F) SEM photographs (×30,000)
Table 2.
Molecular weight characteristics of LEP treated with different γ-irradiation dose
| Dose (kGy) | RT (min) | Molecular weight parameters | |||
|---|---|---|---|---|---|
| Mw (Da)a | Mn (Da)b | Polydispersity c | Area / % | ||
| 0 | 7.967 | 691,200 | 382,200 | 1.81 | 89.13 |
| 9.194 | 22,360 | 19,510 | 1.15 | 10.87 | |
| 4 | 8.162 | 492,400 | 302,100 | 1.63 | 88.09 |
| 9.29 | 20,350 | 17,010 | 1.20 | 11.91 | |
| 8 | 8.44 | 472,100 | 298,800 | 1.58 | 62.51 |
| 9.243 | 23,010 | 19,530 | 1.18 | 37.49 | |
| 16 | 8.534 | 301,000 | 220,700 | 1.36 | 50.03 |
| 9.271 | 22,320 | 18,000 | 1.24 | 49.97 | |
aWeight-average molecular weight
bNumber-average molecular weight
cPolydispersity index (Mw/Mn)
Color characteristics
As shown in Table 1, the lightness (L*) of LEP4 and LEP16 were significantly increased when compared to LEP0, while the redness (a*) and yellowness (b*) values of LEP4, LEP8 and LEP16 were significantly decreased. Yin et al. (2022) and Akram et al. (2017) respectively reported the L* value of S. commune and L. edodes polysaccharides increased after the fruiting body were γ-irradiated. While Ren et al. (2018) and Alijani et al. (2011) reported that the b* value of γ-irradiated polysaccharides isolated from A. membranaceus and A. fluccosus were significantly increased. These results showed that different irradiation style and irradiation dose might affect the color values of polysaccharides. Moreover, the total color difference (ΔE) was significantly increased when compared to the LEP0. The irradiation-induced color change may relate to main chain scission or the double bonds formed during hydrogen abstraction reaction (Ren et al., 2018).
Observations by UV–Vis, FT-IR and SEM
As shown in Fig. 1b, four different LEP exhibited weak absorption at 280 nm and 260 nm, indicating both of them contained low level of protein and nucleic acids, which was consistent with the protein content and monosaccharide composition results in Table 1.
The results of FT-IR spectroscopy were showed in Fig. 1c. Generally, the weak absorption peak at 2920 cm−1 and the strong absorption peak at 3429 cm−1 were characteristic absorption peak for polysaccharides, which were the stretching of C–H group and O–H group, respectively. (Bi et al., 2022). The strong absorption peak at 1640 cm−1 belonged to the C=O asymmetric and symmetric stretching vibrations (Ren et al., 2018). The absorption peak at 1107 cm−1 and 1028 cm−1 might attribute to C–O stretching (Chen et al., 2020). The weak absorption peak at 960 cm−1 and 840 cm−1 might represent C–H bending vibrations. Obviously, all the different LEP showed the similar FT-IR spectroscopy, indicating γ-irradiation treatment had not change the main structures of LEP.
The surface topography of different LEP were presented in Fig. 1f. LEP0 showed a porous structure with irregularly shaped. However, γ-irradiation treatment resulted in the polysaccharides structure destruction and the collapse of holes, and the extent of structure destruction increased with the increased γ-irradiation dose. The destructive effects of γ-irradiation on polysaccharides microstructure had been reported in L. edodes (Akram et al., 2017), S. commune (Yin et al., 2022) and A. membranaceus (Ren et al., 2018).
Solubility and Congo red analysis
The solubility of different LEP showed in Fig. 1d. Obviously, higher temperature resulted in shorter dissolution time of LEP. At the same temperature, LEP16 had best solubility and the shortest dissolution time. The increased solubility may relate to the degradation of high Mw polysaccharides. After γ-irradiation, the portion of low Mw fraction increased from 10.87 to 49.97% (Table 2). Ratcliffe et al. (2005) deemed that the solubility may relate to the hydrogen bonding associated with inter-molecular. γ-Irradiation treatment broke the glycosidic bonds and caused chain scission, and decreased the inter-chain hydrogen bonds (Ren et al., 2018), which might contribute to the increased solubility. Similar results about γ-irradiated polysaccharides from bean (Hussain et al., 2014), A. membranaceus (Ren et al., 2018) and sterculia gum (Singh and Sharma, 2013) had been reported.
The biological activity of polysaccharides is closely linked to their triple helix conformation. At low concentrations of NaOH, Congo red can combine with triple helix conformation polysaccharides to shift the maximum absorption wavelength (λmax) to a longer wavelength (Meng et al., 2020). The results of Congo red experiment were showed in Fig. 1e. λmax of LEP was decreased with the increased NaOH concentration, indicating that all LEP might have not the triple helix conformation. Shang et al. (2018) reported that the heteropolysaccharide may not to form a triple-helix conformation. Therefore, LEP may belong to the group of heteropolysaccharide.
In vitro antioxidant activity of γ-irradiated LEP
As shown in Fig. 2a, the DPPH scavenging ability of four LEP showed a dose-dependent relationship. At the concentration of 3.0 mg/mL, LEP16 showed the highest DPPH scavenging ability with the value of 98.47%, which was comparable to that of Vc (92.65%), followed by LEP8 (87.55%), LEP4 (67.50%) and LEP0 (50.45%). Obviously, γ-irradiation treatment significantly increased the DPPH scavenging ability of LEP. Similar results were reported in γ-irradiated polysaccharides from S. cerevisiae (Khan et al., 2016), bean (Hussain et al., 2014), A. sativa (Shah et al., 2015) and A. bisporus (Khan et al., 2015). Hussain et al. (2014) speculated that the increased DPPH scavenging ability of γ-irradiated polysaccharides may attribute to new double bonds formation during irradiation degradation. The hydroxyl radicals scavenging ability of different LEP was determined and the results showed in Fig. 2b. All the LEP exhibited a dose–response relationship to hydroxyl radicals scavenging activity. At the concentration of 3.0 mg/mL, LEP4, LEP8 and LEP16 showed hydroxyl radicals scavenging rate of 24.72%, 32.88% and 37.61%, respectively, which were higher than that of LEP0 (13.0%). Obviously, γ-irradiation treatment increased the scavenging ability of LEP towards hydroxyl radicals. It had been reported that Mw of polysaccharides is associated with its antioxidant activity (Yarley et al., 2021). Zha et al. (2009) and Liu et al. (2010) had reported that low Mw of polysaccharides fractions exhibited higher antioxidant activity. In this study, the increased hydroxyl radicals scavenging rate of LEP may attribute to the decreased Mw caused by γ-irradiation, as low Mw means more hydroxyl groups exposure and less intramolecular hydrogen bonding (Mukhtar et al., 2017; Shah et al., 2015).
Fig. 2.
The antioxidant activities of different LEP in vitro. (A) DPPH radical scavenging activity; (B) hydroxyl radical scavenging activity; (C) reducing power
Antioxidants can donate electron and their electron-donating ability can be evaluate by reducing power assay, and higher absorbance at 700 nm indicates greater reducing power (Khan et al., 2016). As shown in Fig. 2c, four different LEP showed a concentrated-dependent reducing power with the increased concentrations. LEP4 exhibited greater reducing power, while LEP0, LEP8 and LEP16 showed the similar reducing power at the same concentration. Several studies had reported the increased reducing power of γ-irradiated polysaccharides from bean (Hussain et al., 2014), A. sativa (Shah et al., 2015), S. cerevisiae (Khan et al., 2016) and A. bisporus (Khan et al., 2015). Shah et al. (2015) deemed that the reducing power of polysaccharides increased after γ-irradiation treatment maybe relate to its increased hydrogen extraction ability. Although γ-irradiated LEP showed good antioxidant activity, but it could not exclude the contribution of bioactive impurities which was wrapped up by polysaccharides but exposed after γ-irradiation treatment. Therefore, purification of polysaccharides is need in follow-up studies.
In vitro hypoglycemic activity of γ-irradiated LEP
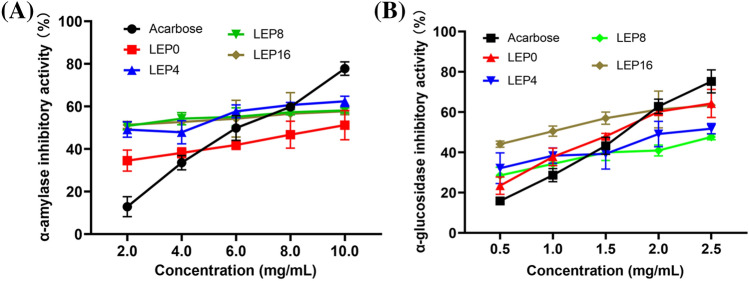
α-Amylase and α-glycosidase are important hydrolytic enzymes, and they can digest dietary carbohydrate to oligosaccharides (Wang et al., 2018). Inhibit the activity of these two enzymes can significantly suppress blood sugar index (Zeng et al., 2020). As shown in Fig. 3a, dose-dependent inhibitory effect on α-amylase by four LEP were observed at the concentrations from 2.0 to 10.0 mg/mL. Totally, the γ-irradiated LEP showed better α-amylase inhibitory effect than non-irradiated LEP, and the IC50 value of LEP16, LEP8, LEP4 and LEP0 were 1.22, 1.64, 1.25 and 2.40 mg/mL, respectively, although IC50 value of acarbose was 0.15 mg/mL. Moreover, four LEP exhibited a certain α-glycosidase inhibition ability in a dose-dependent manner (Fig. 3b). At 2.5 mg/mL, the inhibition rate of α-glycosidase in LEP16, LEP8, LEP4 and LEP0 were 68.81%, 52.38%, 55.35% and 65.30%, respectively. LEP16 showed the lowest IC50 value of 1.20 mg/mL, followed by LEP0 (1.90 mg/mL), LEP4 (2.91 mg/mL) and LEP8 (3.93 mg/mL). Interestingly, IC50 value of LEP16 was lower than that of acarbose (1.554 mg/mL). These results certified that the γ-irradiated LEP may have potential to be a hypoglycemic drug. It had been reported that uronic acid content, monosaccharide composition, glycosyl linkage type, Mw and molecular conformation can influence the inhibitory effects of polysaccharides on α-amylase and α-glycosidase (Mirzadeh et al., 2021). Mirzadeh et al. (2021) reported that carboxylic acid and hydroxyl groups on polysaccharides branched chains can easily interact with digestive enzymes residues via hydrogen bonds to form a polysaccharide-enzyme complex, which can successfully inhibit the activities of α-amylase and α-glucosidase. Cao et al. (2018) and Chen et al. (2018) had reported that polysaccharides with low Mw and high carboxylic acid content have higher inhibitory effect on α-amylase and α-glucosidase. LEP16 showed higher α-amylase and α-glucosidase inhibition rate maybe relate to its higher glucuronic acid and lower Mw which has more potential to exposure its active sites (Mirzadeh et al., 2021).
Fig. 3.
Hypoglycemic activity of different LEP in vitro. (A) α-amylase; (B) α-glucosidase
Prebiotic activity analysis
Lactobacillus rhamnosus and Lactobacillus plantarum are important beneficial bacterium in human intestinal tract, they can produce several kinds of carbohydrate-active enzymes, such as glycoside hydrolases, polysaccharide lyases, to catalyze the degradation of non-digested polysaccharides (Furtado et al., 2019). In this study, L. rhamnosus and L. plantarum have been used to evaluate the prebiotic activity of different LEP. As shown in Fig. 4a and 4c, L. rhamnosus and L. plantarum can growth rapidly by using the different LEP and FOS, as evidenced by the increased absorbance at 600 nm. Totally, LEP showed better effects of proliferation promoting on L. rhamnosus than FOS, in which the OD600 value of γ-irradiated LEP (LEP8 and LEP16) were higher than that of non-irradiated LEP (LEP0). Conversely, FOS had better proliferation promoting effects on L. plantarum than different LEP, and LEP4 showed better proliferation promoting effects than LEP0, LEP8 and LEP16. This may be due to different polysaccharide degradation strategies in different bacterium (Yin et al., 2020). In general, low Mw polysaccharides have better solubility and thus are easily contacted with the carbohydrate-active enzymes. Hu et al. (2021) reported that the enzymatic hydrolyzed mulberry leaf polysaccharide showed better proliferation promoting effects on Bifidobacterium adolescentis, B.bifidum, L. acidophilus and L. rhamnosus than unhydrolyzed mulberry leaf polysaccharide, which is consistent with our results. Moreover, Li et al. (2016) also considered that the prebiotic activity of polysaccharides may influence by their glycosidic bonds and Mw. Decreasing the pH value of the culture medium was also observed in Fig. 4b and 4d, which may be attribute to lactic acid and SCFAs production by L. rhamnosus and L. plantarum during the fermentation (Yin et al., 2020). FOS and different LEP show similar pH value decreased trend in L. rhamnosus while FOS shows lower pH value than other different LEP in L. plantarum. The results showed the L. plantarum may favor small molecular FOS rather than LEP.
Fig. 4.

Prebiotic activities of different LEP. (A) growth curves of Lactobacillus plantarum CGMCC-15801; (B) acid production curves of Lactobacillus plantarum CGMCC-15801; (C) growth curves of Lactobacillus rhamnosus CGMCC-16103; (D) acid production curves of Lactobacillus rhamnosus CGMCC-16103
In this study, L. edodes polysaccharides were extracted and γ-irradiated with different dose. γ-Irradiation treatment significantly increased the carbohydrate content, the total color difference value and the solubility, decreased the protein content and molecular weight, and destroyed the surface topography of polysaccharides. γ-Irradiated LEP showed higher reducing power, free radical scavenging activities against DPPH and HO· than that of non-irradiated LEP. Moreover, γ-irradiated LEP exhibited better inhibition rate on α-amylase and α-glycosidase in vitro when compared to LEP0. The γ-irradiated LEP had better proliferation promoting effects on L. rhamnosus and L. plantarum, therefore, they showed better prebiotic activity than LEP0. However, the bioactivity including hypoglycemic activity and prebiotic activity of γ-irradiated LEP were not increased in an γ-irradiation dose dependent manner. It’s well known that the γ-irradiation treatment will cause polysaccharides main chain or branching chain scission in disorder style. This disorder scission will significantly influence the polysaccharides bioactivity. It had been reported that conformation, molecular weight, functional groups, branching degree, and native types of polysaccharides can significantly influence its bioactivity (Guo et al. 2022). Therefore, purification and in vivo bioactivity determination are needed in further studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Fig. S1 HPLC spectra of monosaccharide composition. a: standard monosaccharide; b: LEP0; C: LEP4; d: LEP8; e: LEP16. (1-mannose, 2-ribose, 3-rhamnose, 4-glucuronic acid, 5-glucose, 6-xylose, 7-galacturonic acid, 8-galactose, 9-arabinose and 10-fucose). (TIF 5458 kb)
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 31801921 & 32072229). Young-aged Top-notch Talent Training Program of Hubei Academy of Agricultural Sciences (2022). Special Project for Science and Technology Innovation of Wuhan (2022020801010343). Project of Edible Fungus Industrial Technology System of Hubei Province (HBHZDZB-2021-023).
Declarations
Competing interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chaomin Yin, Email: yinchaomin@163.com.
Chen Li, Email: 840078399@qq.com.
Kun Ma, Email: 502152061@qq.com.
Xiuzhi Fan, Email: xzhfan@163.com.
Fen Yao, Email: 732900717@qq.com.
Defang Shi, Email: 68052302@qq.com.
Wenjing Wu, Email: 272081603@qq.com.
Jianhui Qiu, Email: 81316908@qq.com.
Guoyuan Hu, Email: hgy701@163.com.
Hong Gao, Email: highong@163.com.
References
- Akram K, Shahbaz HM, Kim GR, Farooq U, Kwon JH. Improved extraction and quality characterization of water-soluble polysaccharide from gamma-irradiated Lentinus edodes. Journal of Food Science. 2017;82(2):296–303. doi: 10.1111/1750-3841.13590. [DOI] [PubMed] [Google Scholar]
- Alijani S, Balaghi S, Mohammadifar MA. Effect of gamma irradiation on rheological properties of polysaccharides exuded by A. Fluccosus and A. Gossypinus. International Journal of Biological Macromolecules. 2011;49(4):471–479. doi: 10.1016/j.ijbiomac.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Bi ZH, Zhao Y, Hu J-H, Ding J, Yang P, Liu Y, Lu Y, Jin Y, Tang HM, Liu YH, Zhang YQ. A novel polysaccharide from Lonicerae Japonicae Caulis: Characterization and effects on the function of fibroblast-like synoviocytes. Carbohydrate Polymers. 2022;292:119674. doi: 10.1016/j.carbpol.2022.119674. [DOI] [PubMed] [Google Scholar]
- Cao CL, Huang Q, Zhang B, Li C, Fu X. Physicochemical characterization and in vitro hypoglycemic activities of polysaccharides from Sargassum pallidum by microwave-assisted aqueous two-phase extraction. International Journal of Biological Macromolecules. 2018;109:357–368. doi: 10.1016/j.ijbiomac.2017.12.096. [DOI] [PubMed] [Google Scholar]
- Chen SL, Shang HM, Yang JY, Li R, Wu HX. Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Industrial Crops and Products. 2018;121:18–25. doi: 10.1016/j.indcrop.2018.04.063. [DOI] [Google Scholar]
- Chen ZY, Yin CM, Fan XZ, Ma K, Yao F, Zhou RR, Shi DF, Cheng W, Gao H. Characterization of physicochemical and biological properties of Schizophyllum commune polysaccharide extracted with different methods. International Journal of Biological Macromolecules. 2020;156:1425–1434. doi: 10.1016/j.ijbiomac.2019.11.183. [DOI] [PubMed] [Google Scholar]
- Furtado LL, Martins ML, Ramos AM, da Silva RR, Leite BRD, Martins EMF. Viability of probiotic bacteria in tropical mango juice and the resistance of the strains to gastrointestinal conditions simulated in vitro. Semina-Ciencias Agrarias. 2019;40(1):149–162. doi: 10.5433/1679-0359.2019v40n1p149. [DOI] [Google Scholar]
- Guo QB, Huang XJ, Kang J, Ding HH, Liu Y, Wang NF, Cui SW. Immunomodulatory and antivirus activities of bioactive polysaccharides and structure-function relationship. Bioactive Carbohydrates and Dietary Fibre. 2022;27:100301. doi: 10.1016/j.bcdf.2021.100301. [DOI] [Google Scholar]
- Hu TG, Zou YX, Li EN, Liao ST, Wu H, Wen P. Effects of enzymatic hydrolysis on the structural, rheological, and functional properties of mulberry leaf polysaccharide. Food Chemistry. 2021;355:129608. doi: 10.1016/j.foodchem.2021.129608. [DOI] [PubMed] [Google Scholar]
- Hussain PR, Wani IA, Suradkar PP, Dar MA. Gamma irradiation induced modification of bean polysaccharides: Impact on physicochemical, morphological and antioxidant properties. Carbohydrate Polymers. 2014;110:183–194. doi: 10.1016/j.carbpol.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Khan AA, Gani A, Shah A, Masoodi FA, Hussain PR, Wani IA, Khanday FA. Effect of γ-irradiation on structural, functional and antioxidant properties of β-glucan extracted from button mushroom (Agaricus bisporus) Innovative Food Science & Emerging Technologies. 2015;31:123–130. doi: 10.1016/j.ifset.2015.05.006. [DOI] [Google Scholar]
- Khan AA, Gani A, Masoodi FA, Amin F, Wani IA, Khanday FA, Gani A. Structural, thermal, functional, antioxidant & antimicrobial properties of β-D-glucan extracted from baker's yeast (Saccharomyces cereviseae)-Effect of γ-irradiation. Carbohydrate Polymers. 2016;140:442–450. doi: 10.1016/j.carbpol.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Li PJ, Xia JL, Nie ZY, Shan Y. Pectic oligosaccharides hydrolyzed from orange peel by fungal multi-enzyme complexes and their prebiotic and antibacterial potentials. LWT-Food Science and Technology. 2016;69:203–210. doi: 10.1016/j.lwt.2016.01.042. [DOI] [Google Scholar]
- Li SF, Wang AJ, Liu LN, Tian GR, Xu FF. Extraction of polysaccharides under vacuum condition from Lentinus edodes stipe and their antioxidant activities in vitro. Food Science and Biotechnology. 2019;28(3):759–767. doi: 10.1007/s10068-018-0513-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Wang HY, Pang XB, Yao WB, Gao XD. Characterization and antioxidant activity of two low-molecular-weight polysaccharides purified from the fruiting bodies of Ganoderma lucidum. International Journal of Biological Macromolecules. 2010;46(4):451–457. doi: 10.1016/j.ijbiomac.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu J, Liu C, Zhang X, Zhao Z, Xu J, Zhang X, Zhou K, Gao P, Li D. Selenium-containing polysaccharides isolated from Rosa laevigata Michx fruits exhibit excellent antioxidant and neuroprotective activity in vitro. International Journal of Biological Macromolecules. 2022;209(Pt A):1222–1233. doi: 10.1016/j.ijbiomac.2022.04.146. [DOI] [PubMed] [Google Scholar]
- Liu Y, Luo ML, Liu F, Feng X, Ibrahim SA, Cheng L, Huang W. Effects of freeze drying and hot-air drying on the physicochemical properties and bioactivities of polysaccharides from Lentinula edodes. International Journal of Biological Macromolecules. 2020;145:476–483. doi: 10.1016/j.ijbiomac.2019.12.222. [DOI] [PubMed] [Google Scholar]
- Meng Y, Lyu FZ, Xu XJ, Zhang LN. Recent advances in chain conformation and bioactivities of triple-helix polysaccharides. Biomacromolecules. 2020;21(5):1653–1677. doi: 10.1021/acs.biomac.9b01644. [DOI] [PubMed] [Google Scholar]
- Mirzadeh M, Lelekami AK, Khedmat L. Plant/algal polysaccharides extracted by microwave: A review on hypoglycemic, hypolipidemic, prebiotic, and immune-stimulatory effect. Carbohydrate Polymers. 2021;266:118134. doi: 10.1016/j.carbpol.2021.118134. [DOI] [PubMed] [Google Scholar]
- Mukhtar R, Shah A, Noor N, Gani A, Wani IA, Ashwar BA, Masoodi FA. γ-irradiation of oat grain-Effect on physico-chemical, structural, thermal, and antioxidant properties of extracted starch. International Journal of Biological Macromolecules. 2017;104:1313–1320. doi: 10.1016/j.ijbiomac.2017.05.092. [DOI] [PubMed] [Google Scholar]
- Rao Z, Dong Y, Zheng X, Tang K, Liu J. Extraction, purification, bioactivities and prospect of lentinan: A review. Biocatalysis and Agricultural Biotechnology. 2021;37:102163. doi: 10.1016/j.bcab.2021.102163. [DOI] [Google Scholar]
- Ratcliffe I, Williams PA, Viebke C, Meadows J. Physicochemical characterization of konjac glucomannan. Biomacromolecules. 2005;6(4):1977–1986. doi: 10.1021/bm0492226. [DOI] [PubMed] [Google Scholar]
- Ren LN, Wang XF, Li S, Li JL, Zhu XD, Zhang L, Gao F, Zhou GH. Effect of gamma irradiation on structure, physicochemical and immunomodulatory properties of Astragalus polysaccharides. International Journal of Biological Macromolecules. 2018;120:641–649. doi: 10.1016/j.ijbiomac.2018.08.138. [DOI] [PubMed] [Google Scholar]
- Shah A, Masoodi FA, Gani A, Ashwar BA. Effect of γ-irradiation on antioxidant and antiproliferative properties of oat β-glucan. Radiation Physics and Chemistry. 2015;117:120–127. doi: 10.1016/j.radphyschem.2015.06.022. [DOI] [Google Scholar]
- Shang HM, Zhou HZ, Duan MY, Li R, Wu HX, Lou YJ. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from comfrey (Symphytum officinale L.) root. International Journal of Biological Macromolecules. 2018;112:889–899. doi: 10.1016/j.ijbiomac.2018.01.198. [DOI] [PubMed] [Google Scholar]
- Sheng KJ, Wang CL, Chen BT, Kang MJ, Wang MC, Liu K, Wang M. Recent advances in polysaccharides from Lentinus edodes (Berk.): Isolation, structures and bioactivities. Food Chemistry. 2021;358:129883. doi: 10.1016/j.foodchem.2021.129883. [DOI] [PubMed] [Google Scholar]
- Singh B, Sharma V. Influence of gamma radiation on the physicochemical and rheological properties of sterculia gum polysaccharides. Radiation Physics and Chemistry. 2013;92:112–120. doi: 10.1016/j.radphyschem.2013.06.006. [DOI] [Google Scholar]
- Wang F. Principles of food irradiation technology. In: Ha YM, editor. Modern Food Irradiation Processing Technology. Beijing: Science Press; 2015. p. 57. [Google Scholar]
- Wang L, Zhang B, Xiao J, Huang Q, Li C, Fu X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chemistry. 2018;249:127–135. doi: 10.1016/j.foodchem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Xie LM, Shen MY, Hong YZ, Ye HD, Huang LX, Xie JH. Chemical modifications of polysaccharides and their anti-tumor activities. Carbohydrate Polymers. 2020;229:115436. doi: 10.1016/j.carbpol.2019.115436. [DOI] [PubMed] [Google Scholar]
- Xiong C, Li P, Luo Q, Yan JY, Zhang J, Jin X, Huang WL. Effect of gamma-irradiation on the structure and antioxidant activity of polysaccharide isolated from the fruiting bodies of Morchella sextelata. Bioscience Reports. 40: Bsr20194522 (2020) [DOI] [PMC free article] [PubMed]
- Xu XT, Jeong SM, Lee JE, Kang WS, Ryu SH, Byun EB, Kim K, Byun EH, Ahn DH. Characterization and anti-inflammatory effects of gamma-irradiation on Undaria pinnatifida root water extracts. Biotechnology and Bioprocess Engineering. 2021;26(4):606–611. doi: 10.1007/s12257-020-0325-x. [DOI] [Google Scholar]
- Yarley OPN, Kojo AB, Zhou CS, Yu XJ, Gideon A, Kwadwo HH, Richard O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. International Journal of Biological Macromolecules. 2021;183:2262–2271. doi: 10.1016/j.ijbiomac.2021.05.181. [DOI] [PubMed] [Google Scholar]
- Yin CM, Fan XZ, Fan Z, Shi DF, Gao H. Optimization of enzymes-microwave-ultrasound assisted extraction of Lentinus edodes polysaccharides and determination of its antioxidant activity. International Journal of Biological Macromolecules. 2018;111:446–454. doi: 10.1016/j.ijbiomac.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Yin CM, Noratto GD, Fan XZ, Chen ZY, Yao F, Shi DF, Gao H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydrate Polymers. 2020;250:116942. doi: 10.1016/j.carbpol.2020.116942. [DOI] [PubMed] [Google Scholar]
- Yin CM, Shi DF, Chen ZY, Fan XZ, Yao F, Lu Q, Gao H. Comparative analysis of physicochemical characteristics and in vitro biological activities of polysaccharides from γ-irradiated and nonirradiated Schizophyllum commune. Radiation Physics and Chemistry. 2022;197:110177. doi: 10.1016/j.radphyschem.2022.110177. [DOI] [Google Scholar]
- Zeng AQ, Yang RJ, Yu SH, Zhao W. A novel hypoglycemic agent: Polysaccharides from laver (Porphyraspp.) Food & Function. 2020;11(10):9048–9056. doi: 10.1039/D0FO01195A. [DOI] [PubMed] [Google Scholar]
- Zha XQ, Wang JH, Yang XF, Liang H, Zhao LL, Bao SH, Luo JP, Xu YY, Zhou BB. Antioxidant properties of polysaccharide fractions with different molecular mass extracted with hot-water from rice bran. Carbohydrate Polymers. 2009;78(3):570–575. doi: 10.1016/j.carbpol.2009.05.020. [DOI] [Google Scholar]
- Zhou X, Shu XY, Li XK, Wan Y, Zhou YT, Hou DB. Optimization of extraction technology of crude polysaccharides from Angelica dahurica and effects of irradiation on its content and activity. Journal of Food Safety & Quality. 12(21):8508–8516 (2021) (in Chinese)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 HPLC spectra of monosaccharide composition. a: standard monosaccharide; b: LEP0; C: LEP4; d: LEP8; e: LEP16. (1-mannose, 2-ribose, 3-rhamnose, 4-glucuronic acid, 5-glucose, 6-xylose, 7-galacturonic acid, 8-galactose, 9-arabinose and 10-fucose). (TIF 5458 kb)