Abstract
The rapid evolution of wearable technology in healthcare sectors has created the opportunity for people to measure their blood pressure (BP) using a smartwatch at any time during their daily activities. Several commercially-available wearable devices have recently been equipped with a BP monitoring feature. However, concerns about recalibration remain. Pulse transit time (PTT)-based estimation is required for initial calibration, followed by periodic recalibration. Recalibration using arm-cuff BP monitors is not practical during everyday activities. In this study, we investigated recalibration using PTT-based BP monitoring aided by a deep neural network (DNN) and validated the performance achieved with more practical wrist-cuff BP monitors. The PTT-based prediction produced a mean absolute error (MAE) of 4.746 ± 1.529 mmHg for systolic blood pressure (SBP) and 3.448 ± 0.608 mmHg for diastolic blood pressure (DBP) when tested with an arm-cuff monitor employing recalibration. Recalibration clearly improved the performance of both DNN and conventional linear regression approaches. We established that the periodic recalibration performed by a wrist-worn BP monitor could be as accurate as that obtained with an arm-worn monitor, confirming the suitability of wrist-worn devices for everyday use. This is the first study to establish the potential of wrist-cuff BP monitors as a means to calibrate BP monitoring devices that can reliably substitute for arm-cuff BP monitors. With the use of wrist-cuff BP monitoring devices, continuous BP estimation, as well as frequent calibrations to ensure accurate BP monitoring, are now feasible.
Keywords: Blood pressure, Recalibration, Attention mechanism, Electrocardiogram, Photoplethysmogram, MAE, DNN, Signal processing
Introduction
Blood pressure (BP) is critically important for the diagnosis and prevention of cardiovascular diseases [1]. BP is affected by the physical and environmental conditions that are able to cause fluctuations in BP levels throughout the day. Therefore, it is essential that BP is tracked through continuous measurement [2]. The most accurate and direct method for assessing BP is through the measurement of arterial blood pressure (ABP) using arterial catheters [3]. However, this is an invasive method that increases the risk of infections; therefore, it is not suitable for daily monitoring. A more convenient and non-invasive BP monitoring method that has been suggested employs an oscillometric cuff-based approach using a sphygmomanometer to indirectly measure the BP via the Korotkoff sounds of the arteries as the cuff wrapped around the arm is deflated [4]. A disadvantage of this method is that users could feel discomfort due to the pressures exerted from the cuff while measuring the BP [5]; furthermore, the substantial bulk of the cuff could be viewed as inappropriate for daily monitoring. As a consequence, numerous studies have suggested a cuffless approach that indirectly estimates BP on a continuous basis using the pulse transit time (PTT), which is defined as the time delay between the pulse from an electrocardiograph (ECG) and the pulse from a photoplethysmogram (PPG) [5]. The PTT is usually calculated as the time interval between the ECG’s R wave and the peak of the PPG. The PTT has been shown to have a strong correlation with BP [6, 7]. Therefore, it has played a prominent role in the development of cuffless techniques to measure BP on a continuous basis [8–11]
Although the PTT-based estimation of BP has contributed substantially to cuffless wearable technology for monitoring BP, the majority of studies have indicated the necessity for calibrating this method [12–16]. Subsequent studies have demonstrated the superior performance of BP estimation methodologies that are based on deep neural networks (DNNs) using different biomedical signals. Schlesinger et al. [17] proposed a Siamese convolutional neural network (CNN) method in which recalibration was performed by retraining the activation layer with the calibrated feature vectors that are repeatedly updated by subtracting the output feature vector of the pre-trained model from the calibrated feature vectors. Their calibration-free model exhibited a mean absolute difference (MAD) of mmHg for systolic BP (SBP) and for diastolic BP (DBP), whereas the calibrated model exhibited an MAD of mmHg for SBP and mmHg for DBP. Song et al. [18] also proposed a recalibration system called “stacked DNN”, that relearns the model. In their method, the BP estimated from the model is concatenated with the subsequent features in order to recalibrate the current BP value. Kauchee et al. [19] proposed various machine learning models such as linear regression, decision trees, and support vector machines (SVM) for BP estimation and validated the improvement of accuracy using the calibration approach. However, all of these calibration approaches were executed with invasive arterial BP or from a sphygmomanometer, rendering them impractical in an outdoors environment. Following the development of wearable devices, smartwatch manufacturers are adopting wearable blood pressure monitoring systems using ECG and PPG sensors embedded in the smartwatches. Examples include the Samsung Galaxy Watch, the Apple Watch, and the Google Fitbit [20, 21]. However, it remains mandatory to recalibrate these smartwatches on a regular basis, using traditional arm-cuff BP monitors [22]. Moreover, there is no consensus on how often calibration needs to be performed to ensure accurate BP monitoring. McCarthy et al. [23] assessed the duration for which blood pressure estimation remained accurate, using the PTT-based approach proposed by Chen et al. [24] and Poon et al. [9]. They concluded that Chen’s algorithm produced an accurate BP estimation for 5 min, whereas Poon’s algorithm sustained accurate measurements for only 2 min before recalibration was required.
In the present study, we propose a more practical recalibration approach using wearable wrist-cuff BP monitors. These wrist-cuff BP monitors have been proven to measure relatively accurate BPs by comparing their output with BPs measured by arm-cuff sphygmomanometers [25, 26]. The method proposed in this study consists of two steps: (1) BP estimation through the attention mechanism of DNNs presented by Eom et al. [27]; (2) a recalibration step that updates the model parameters based on the mean absolute error (MAE) between the estimation and the ground truth. The error trend associated with prediction pre- and post-recalibration serves as a prerequisite for investigating the time interval of recalibration. The main contribution of this study is the demonstration that the methodology is substantially more accurate and accessible in terms of practicality. The study validates that regular recalibration by a wrist-mounted BP monitor is accurately performed and can serve as a substitute for the conventional arm-cuff BP measurement and also establishes an appropriate calibration interval. The purpose of the study was fourfold: (1) to demonstrate the performance of the BP prediction model throughout the testing sessions; (2) to confirm that the recalibration could improve the accuracy of the prediction; (3) to verify how long the estimation remained accurate before recalibration was required; (4) to verify whether the wrist-cuff BP monitor could replace the conventional arm-cuff BP monitor for the recalibration.
Related work
The recalibration of PTT-based BP estimation has been the subject of several studies [12–16]. After the initial calibration of the device, a BP estimation only remains reliable for a certain period, and periodic recalibration is required to sustain reliability in the long term [28, 29].
A longer calibration interval reduces the accuracy of an estimation [30]. There is no consensus on how often a PTT-based BP monitor should be recalibrated. Mukkamala et al. [31] proposed a maximum calibration period for PTT-based BP monitoring, suggesting different calibration intervals for different age groups and genders. As an example, they recommended an interval of one year for persons of age 30 and six months for persons of age 70. However, their predictions were specifically applicable to a PTT-based estimation of the BP in the aorta based on measurements on the feet; therefore, the suggested intervals might not be suitable for wearable devices. Choi et al. [28] suggested predicting a PTT-based BP by using the Hilbert-Huang transform and verified that the initial calibration should be based on forty measurements of DBP and SBP. They obtained this result by testing their algorithm on carefully selected samples from the MIMIC database [32] which is approximately 0.32 seconds of BP data that experimenting with time intervals of 30, 60, and 90 minutes for recalibrating a cuff-type BP monitor. Yoon et al. [33] explored how often calibration is required for accurate BP monitoring using the pulse arrival time (PAT) method and suggested that at least four recalibrations of 60 s duration each are required to maintain monitoring for a period of 12 h, implying a recalibration once every 3 h on average. El-Hajj et al. [30] suggested that the use of PTT parameters to predict the BP would only be valid for one day following a calibration. However, these calibration intervals were proposed on the basis of measuring the ambulatory blood pressure (ABP) either by catheters or by using an arm-cuff BP monitor. Both of these methods could be detrimental to the adoption of wearable BP monitoring devices owing to their inconvenience while performing daily life tasks. In addition, feature extraction could be costly, and the methods require manual adjustment in order to generate accurate predictions [34]. Recently, several studies have suggested calibration-free BP estimation based on the recurrent neural networks (RNN). Maher et al. [35] proposed an RNN-based approach for predicting continuous BP using the ECG and PPG signals. However, like the other PPG-based algorithms, the RNN-based method is also subject to decaying accuracy over time. Predictions of time-series data using RNNs have indicated a decrease in accuracy with an increase in the elapsed time from the start of the prediction [36–39]. Therefore, model updates require new data to maintain their accuracy as the time-step increases. The validity of measurements produced by wrist-worn BP monitors has been questioned. Certain studies have claimed that such monitors overestimate the actual BP values because of incorrect positioning [40, 41]. Other studies were conducted to validate the reliability of wrist-worn sphygmomanometers. Ali et al. [42] observed the performance of wrist-type sphygmomanometers compared with standard mercury sphygmomanometers and concluded that wrist-type BP monitors produced highly valid measurements in obese people [42]. Kario et al. [43] found that wrist-type BP devices were reliable compared to ABP monitoring [43]. Melvile et al. [44] examined the accuracy of BP measurements obtained by wrist-cuff monitors compared with those obtained with central intra-arterial catheters and demonstrated that the mean errors of the wrist-cuff BPs post-calibration were mmHg for systolic BP and mmHg for diastolic BP. These values constituted improvements of 33% and 73% in the precision for SBP and DBP, respectively. The present study observed the prediction accuracy of PTT-based BP under periodic recalibration conditions and verified the accuracy of a wrist-based BP monitor compared with the conventional arm-cuff BP monitor.
Material and methods
Data acquisition
Experiment setup
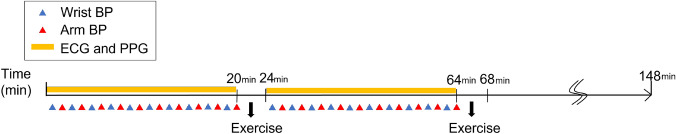
The experimental setup is illustrated in Fig. 1. Twelve subjects were recruited for the study at Kwangwoon University (eleven males and one female with ages years). All the participating subjects were in good health. Consent was obtained from all participants. They were informed of the purpose of the study, the methodology, the experimental equipment, and the possible side effects associated with the experiment. Subjects with any medical conditions were excluded from the experiment. This experiment was approved by the Institutional Review Board of Kwangwoon University (IRB No.7001546-20200823-HR(SB)-008-07).
Fig. 1.
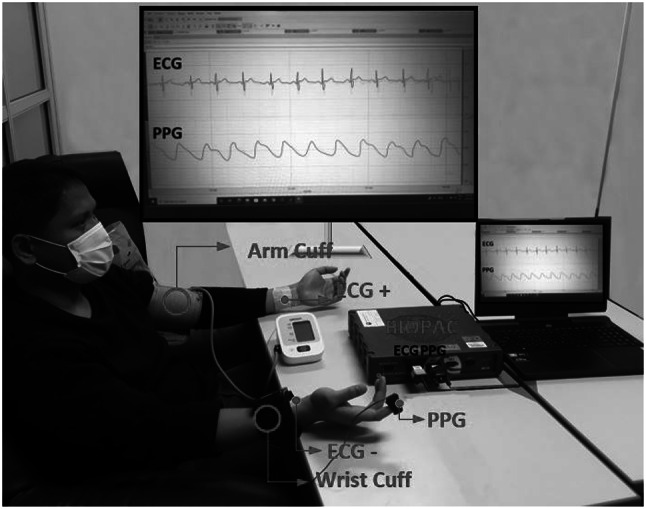
Experiment Setup. ECG and PPG signals were measured simultaneously. At the same time, the blood pressure was measured at the interval of 2 minute, alternating between the wrist cuff monitor and the arm cuff monitor
Experiment systems
ECG and PPG signals were measured simultaneously. Concomitantly, the BP was measured at intervals of 2 min, alternating between the wrist-cuff monitor and the arm-cuff monitor. A bio-potential acquisition system was utilized to measure the ECG and PPG signals. The system consisted of a bio-signal amplifier for data acquisition; ground, reference, and recording electrodes for the ECG; and wrist-cuff and arm-cuff BP monitors. For the acquisition of lead I ECG signals, Ag/AgCl gold-plated cups (EL 160 Gold Cup, Biopac Systems inc., Goleta, CA, USA) were attached to the inner sides of the subject’s left and right wrists and to the left limb of the subject. A PPG sensor (SS4LA, Biopac Systems inc., Goleta, CA, USA) was worn on the subject’s right index finger. The ECG and PPG signal data were measured simultaneously using a BIOPAC MP36 (Biopac Systems inc., Goleta, CA, USA). The sampling frequency of the ECG and PPG signals was set to 250 Hz. Two automatic digital BP monitors were used to measure the blood pressure, one as an arm-cuff monitor and the other as a wrist-cuff monitor. The arm-cuff BP monitor (HEM7121, OMRON Healthcare, Inc. Japan) was worn on the left arm [45–47], and the wrist-cuff BP monitor (HEM6232T, OMRON, Japan) was worn on the subject’s right wrist, measuring the SBP and DBP values. When measuring the wrist BP, the wrist-cuff monitor was positioned at the same level as the heart for correct measurement. The blood pressure was measured at intervals of two minutes, alternating between the wrist- and arm-cuff monitors. The duration of the experiment was set to 3 h to ensure that a sufficient number of data were acquired to train and test the model. Additionally, seven sessions of physical exercise were included during the experiment to increase the variability of the BP [48–50]. All subjects were asked to avoid the intake of alcohol, smoking, and taking food for 30 min prior to the experiment [51]. The experimental protocol is illustrated in Fig. 2. The experimental design included two sessions.
Fig. 2.
Experiment protocol
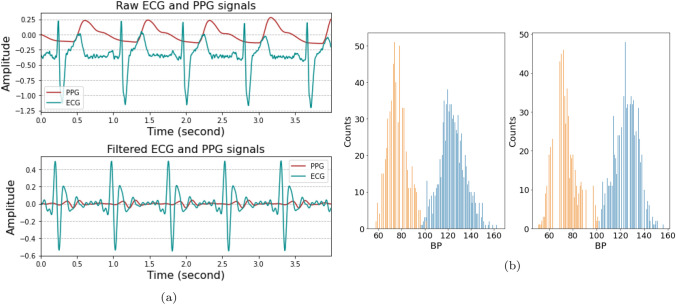
In the first session, the subject was in a relaxed state and measurements by the ECG and PPG were conducted while the subject sat in an armchair for twenty minutes. Arm and wrist BPs were measured simultaneously at intervals of 2 min. To ensure that the two different BP monitors did not affect each other, and taking into consideration that a single BP measurement took approximately 45 s, the first measurement taken by the wrist BP monitor was made 30 s after the onset. This was followed by the second measurement taken by the arm BP monitor once the wrist BP monitor was completely deflated. In the second session, subjects moved to the ergometer cycle with the sensors unplugged and started cycling for 4 min to elevate their BP [52]. After completion of the exercise, the subject returned to the chair to resume the measurements. The histograms of the measured wrist and arm BPs are depicted in Fig. 3b.
Fig. 3.
The data used for estimation of the BP. a the pre-processing of the input data. The top one denotes the raw signals and the bottom one denotes after band-pass filtering to remove baseline drift and noise. b the blood pressure distribution. The left one is the distribution of the arm BP and the right one is wrist BP
Data pre-processing
The total duration of the experiment was 3 h, including exercise. Consequently, the datasets were 2 h 20 min long. Since the BP values were measured once every 2 min, providing 70 data labels in a dataset, a large number of datasets were required to train and test the DNN model effectively.
In order to augment the data, a window of 10 s duration with 1 s overlap was applied to segment the dataset. The BP labels were assigned as follows: for the first half of the 2 min interval, the data were labeled with the previous BP, whereas for the last half of the interval, the data were labeled with the subsequent BP. The input segment to the BP estimation included 10 s ECG and PPG recordings in a dimension of (). Once the raw data had been collected, a second-order Butterworth band-pass filter was applied to remove the baseline drift and the noise of the data. The cut-off frequency of the ECG was between 0.5 and 35 Hz, and that of the PPG was between 0.5 and 15 Hz [27]. The input data before and after the Butterworth band-pass filtering are presented in Fig. 3a.
Proposed method
Deep learning algorithm
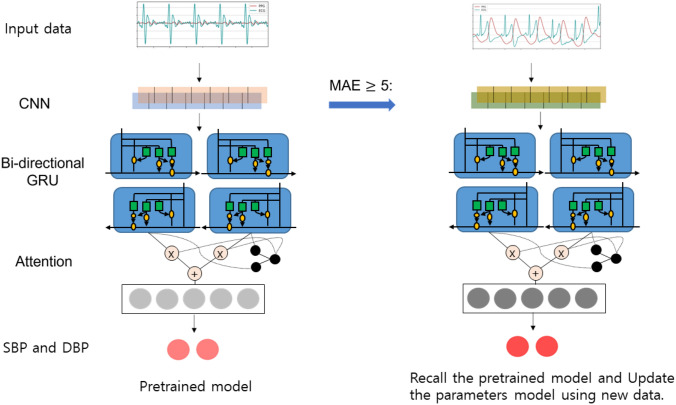
Recently, there have been several studies suggesting the use of a CNN-based long short-term memory (LSTM) model for various tasks [53]. One such task is estimation, where the attention mechanism has been shown to produce good results [54]. In this study used a deep neural network (DNN) inspired by Eom et al. [27], which comprises a series of CNN-based bi-directional gated recurrent unit (GRU) attention steps to predict the SBP and DBP. The DNN architecture was implemented in Python 3.7.1 and Tensorflow 2.3.1 environments. First, two-channel PPG and ECG data were fed into the CNN model as input, and spatial features were extracted. The CNN model consisted of ten 1D - CNN models in which batch normalization was added after each layer; the max pooling layer was placed after every two or three CNN layers to reduce the size of the data. The output size of the data became smaller as the number of feature maps was increased up to 512, with () features at the end of the CNN. Second, the spatial features of the data were fed into the bi-directional GRU layers to extract the temporal information of the data. The bidirectional GRU model used in this study considered a sequential relationship within the given time steps that learned both past information and the information going forward. In this study, the data contained thirty-one time steps, each consisting of a 512-dimensional vector input and 64 hidden units that output () features. Finally, an attention mechanism was applied to establish which vector input had been used most frequently to predict the output and to assign a weighted attention to that vector. Input vectors with a sequence of thirty-one time steps were trained to calculate attention score vectors, subsequently calculating the attention vector by multiplying it with the original hidden state vectors.
| 1 |
Recalibration process
As stated earlier, periodic calibrations were necessary to prevent the model’s accuracy from degrading. Recalibration was performed by calibrating the training model with new data. The recalibration proceeded as follows: (1) the model predicted the BP using the test signals, and monitored the MAE expressed in Eq. (1); (2) if the error of the predicted BP was larger than a predetermined threshold, the previously-trained model was retrained with the new dataset to predict the subsequent BP.
In this process, the initially-trained model was continually reused and updated with new information, allowing an accurate prediction over the long term. For recalibration, 100 min datasets were used to estimate the BP at an interval of 2 min; if the MAE was higher than 5 mmHg, the model was subjected to the training process to learn the current data and retain the estimation. The threshold for model retraining was set at 5 mmHg of absolute difference, in accordance with the Association for the Advancement of the Medical Instrumentation (AAMI) standard 81060-2:2013 [55]. The overflow of the recalibration process is displayed in Fig. 5.
Fig. 5.
Overview of the process
Results
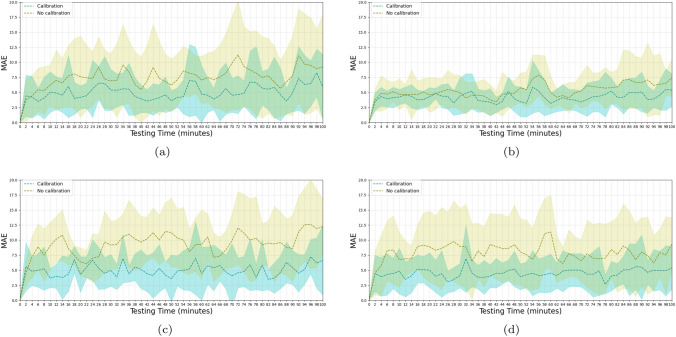
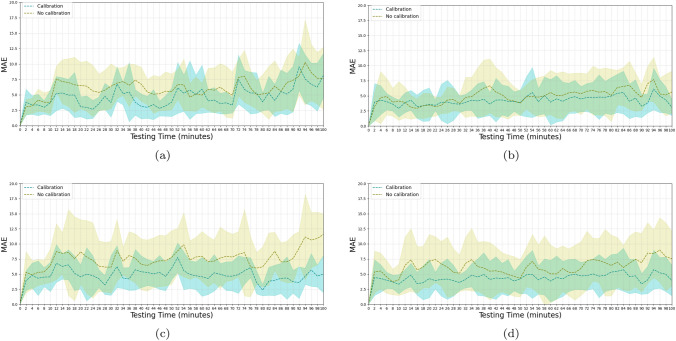
After 40 min of model training using wrist-cuff BP, a test was conducted with the model architecture illustrated in Fig. 4 to predict the BP every 2 min for a total period of 100 min. The accuracy was evaluated in terms of the MAE and SD, compared with the reference BP. The performance was evaluated under either calibrated or uncalibrated conditions, using wrist-cuff BP. The test was also conducted using conventional arm-cuff BP to compare the accuracy with that of wrist-cuff BP. Finally, the test was conducted using the linear regression method to compare the performance with that of the DNN approach. The overall MAE values associated with the BP predictions for the two cuff-based methods, with and without recalibration, are listed in Tables 1 and 2. For all conditions except the arm-cuff DBP with the calibrated linear regression model which had an MAE of mmHg, the DNN model with the attention mechanism outperformed the PTT-based linear regression model. For DBP, the calibrated models for arm-cuff BP had MAE values of mmHg and mmHg, which exhibited superior performance in comparison with the calibrated models of the wrist-cuff BP (the associated MAE values for the wrist-cuff BP were mmHg and mmHg). The continuous variations as functions of time of the MAE and SD, for calibrated and uncalibrated conditions, are illustrated in Figs. 6 and 7. The blue dotted line and the shaded area represent the average MAE and standard deviation, respectively across the twelve experimental subjects obtained with the recalibration procedure, whereas the yellow line and area indicate the results obtained without the recalibration procedure. The results obtained for the SBP using the arm-cuff-based predictions of the DNN model are displayed in Fig. 7a. First, the MAE was 3.82 mmHg at 2 min. However, at 12 min, it reached 5.20 mmHg, requiring the model to be recalibrated. The calibration was performed for 4 min from 16 to 20 min, during which the MAE gradually dropped and declined to 3.06 mmHg at 20 min. The uncalibrated model displayed a similar pattern, generating an MAE of 3.06 mmHg at 2 min, rising to 7.56 mmHg at 12 min before gradually declining to a level not much below 5 mmHg. This pattern of a rise and subsequent gradual decline in the error continued throughout the test, clarifying the effect of recalibration. Under the recalibration of wrist-cuff measurements, the accuracy improved to better than 5 mmHg, which is the validation standard. An MAE threshold of 5 mmHg was set as the maximum allowed error during the BP prediction; if the MAE exceeded that threshold, the recalibration procedure would be conducted, using either the arm-cuff or the wrist-cuff BP monitor. The process of consecutive recalibrations was restricted to five iterations. The iteration size was determined experimentally. The prediction performance of the DNN model was mmHg for SBP and mmHg for DBP when tested with the arm-cuff BP labels under the recalibration condition. When the model was tested with the wrist-cuff BP labels under the recalibration condition, the MAE was mmHg for SBP and mmHg for DBP. These results demonstrated the high level of performance of the wrist-cuff BP monitor for the recalibration of the BP estimation model, whose accuracy was already reported in a previous study [56]. The results demonstrated that the wrist-cuff BP monitor could be as accurate as the arm-cuff BP monitor. Accordingly, the wrist-cuff BP monitor has the potential to be used to calibrate the cuffless continuous BP monitoring device, as a substitute for an arm-cuff BP monitor. Such a substitution could be convenient for BP monitoring because of its greater ease of wearing, compared with the arm-cuff BP monitor. Under the uncalibrated condition, the performance achieved with the arm-cuff and wrist-cuff BP monitors deteriorated over time. We inferred that the error might have accumulated as testing progressed, causing the observed deterioration. As a benchmarking test, we conducted a PTT-based BP estimation using linear regression and compared the result with that of the DNN method.
Fig. 4.
Model architecture. The left figure shows the overall architecture in training step. After training is finished, the model estimates SBP and DBP and monitors the MAE. If the MAE is 5 or above, the pre-trained model is recalled and new data set are fed in to the model to relearn and calibrate
Table 1.
BP prediction results in average MAE depending on two different cuff-based without the recalibration
| Arm BP without recalibration | Wrist BP without recalibration | |||
|---|---|---|---|---|
| DNN | PTT | DNN | PTT | |
| SBP | 6.200 | 7.606 | 7.657 | 9.480 |
| DBP | 4.964 | 5.381 | 6.219 | 7.956 |
Table 2.
BP prediction results in average MAE depending on two different cuff-based with the recalibration
| Arm BP with recalibration | Wrist BP with recalibration | |||
|---|---|---|---|---|
| DNN | PTT | DNN | PTT | |
| SBP | 4.745 | 5.102 | 4.852 | 5.044 |
| DBP | 4.233 | 4.216 | 4.413 | 4.516 |
Fig. 6.
Testing results of Linear regression BP prediction with and without the recalibration. a tested with arm-cuff SBP b tested with arm-cuff DBP c tested with wrist-cuff SBP d tested with wrist-cuff DBP
Fig. 7.
Testing results of DNN BP prediction with and without the recalibration. a tested with arm-cuff SBP b tested with arm-cuff DBP c tested with wrist-cuff SBP d tested with wrist-cuff DBP
The performance of the BP prediction models is illustrated in Figs. 6 and 7, where the MAEs associated with the PTT-based linear regression and the DNN are presented. The models were trained and calibrated with the upper arm-cuff-based BP monitors, as these are known to generate accurate BP readings [57]. Overall, the model in the recalibration condition exhibited a lower MAE than the model in the uncalibrated condition. The trend clearly shows the effect of the recalibration, as the error improved four times within a 100 min period. In Fig. 7, we indicate the error trends of SBP and DBP, respectively, estimated using the DNN model. In Fig. 7a, the MAE keeps increasing up to a level of mmHg at 12 min, at which point calibration is introduced to lower the error. After the recalibration, the error gradually decreases to mmHg at 24 min before increasing again, displaying a fluctuating pattern. In Fig. 7b, we display the variation in the MAE of the DBP, which is more erratic than that of the SBP throughout the measuring period. In Fig. 6a and b, we illustrate the MAEs of the SBP and DBP, estimated by the linear regression model using the PTT calculated from the ECG and PPG signals. We display the negative correlation between PTT and BP [7] in Fig. 8. The PTT was calculated from the interval between the R peak of the ECG and the peak of the PPG detected by the Pan-Tompkins algorithm [58]. We considered the trend despite a few automated detection errors. Due to the possibility of the errors, the proposed method does not depend on the peak detection, but the raw data itself using the data-driven neural network architecture. However, A linear regression model that requires the extraction of features from ECG and PPG signals is more difficult to implement in order to calculate PTT. Furthermore, the linear regression yields higher values of the MAE and STD compared with the DNN, for both SBP and DBP, with and without recalibration. The performance was inferior to the DNN. Overall, the recalibration procedure improved the performance under all conditions. The DNN model performed better than linear regression except for one condition, shown in Fig. 6b and Fig. 7b, where the linear regression model achieved a value of mmHg, compared with for the DNN model.
Fig. 8.
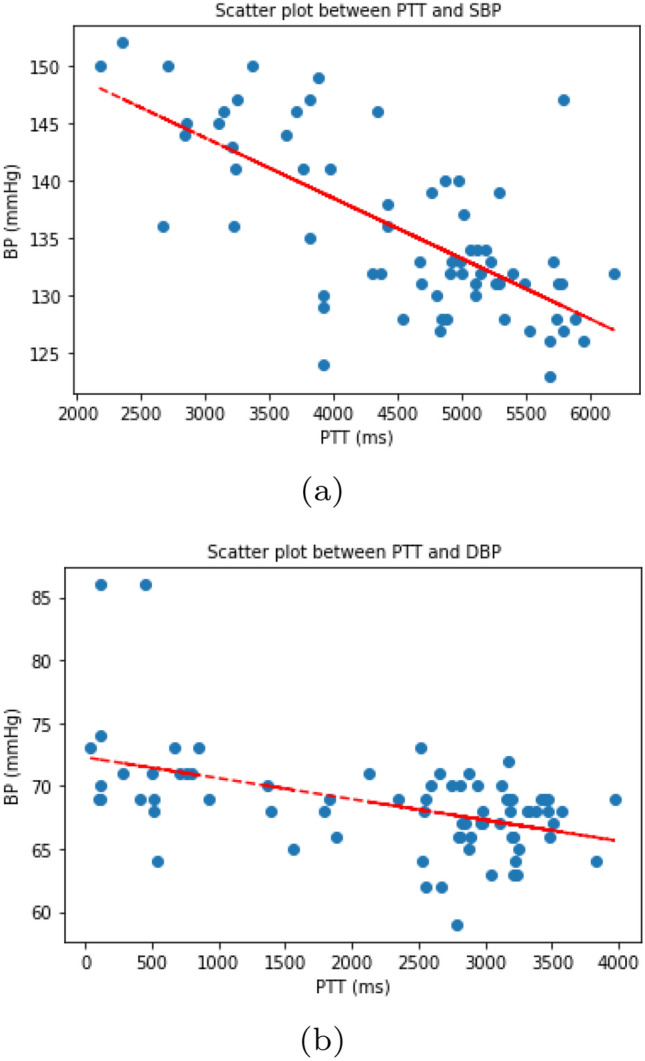
Scatter plots between PTT and BP (a SBP, b DBP). note the negative correlation between PTT and BP
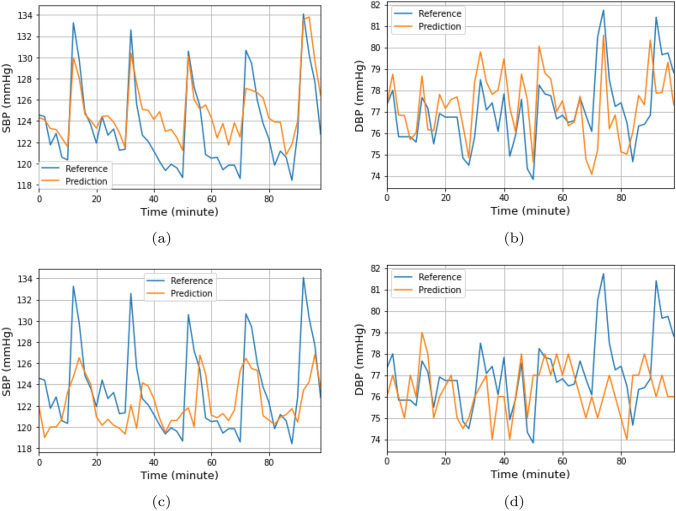
Generally there is a significant difference between the results achieved by the linear regression model with and without recalibration, compared with the DNN model. We present a comparison of wrist-cuff-based BP estimation under calibrated and uncalibrated conditions, respectively, throughout the 100 min test, in Fig. 9. The reference BP was accurately predicted under the calibrated condition, whereas an accurate prediction reflecting the trend change of the target BP was not achieved under the uncalibrated condition. The prediction under the calibrated condition corresponded to the reference value, especially in the variation of the elevation and decline of the BP. Specifically, the SBP presented a pattern consisting of a sharp rise followed by a gradual decline. This behavior resulted from the multiple exercise sessions included in the experiment with the aim of increasing the participant’s BP. The estimated DBP also accurately tracked the reference BP. The DBP displayed a tendency to remain at constant levels regardless of physical activity, showing no clear elevation after exercise. The DBP did not display the same degree of periodic behavior as the SBP [59, 60]. In Table 3, The T-test was performed to assess the statistical significance of MAE changes with or without recalibration. The p-values of the DNN-based approach were under 0.05 on both arm and wrist cuff monitors, giving a significant difference in recalibration. The p-values of the linear regression-based approach were also significant, though wrist DBP was shown to exceed its significance level. However, studies have shown that PTT-based prediction could be unreliable [61, 62], and not a good reference for blood pressure measurement.
Fig. 9.
Comparison of the wrist-cuff-based BP estimation in the recalibrated and uncalibrated conditions for 100 minutes. a tested using the wrist-cuff SBP with the recalibration b tested using the wrist-cuff DBP with the recalibration c tested using the wrist-cuff SBP without the recalibration d tested using the wrist-cuff DBP without the recalibration
Table 3.
T test was conducted to verify if the MAE of the recalibration is statistically different from that of uncalibrated condition, and to argue that the recalibrated wrist monitor can effectively predict the BP. It was tested for both arm and wrist BP
| Deep Neural Networks | Linear regression | |||||||
|---|---|---|---|---|---|---|---|---|
| t | p value | lower CI | upper CI | t | p value | lower CI | upper CI | |
| Arm calibration(Systolic) | 2.817 | 0.024 | 6.657 | −0.073 | 2.912 | 0.016 | 9.179 | 7.647 |
| Arm calibration(Diastolic) | 2.332 | 0.031 | 5.657 | −0.577 | 1.985 | 0.06 | 5.299 | 0.889 |
| Wrist calibration(Systolic) | 2.682 | 0.02 | 6.553 | 2.593 | 2.256 | 0.037 | 8.928 | 8.035 |
| Wrist calibration(Diastolic) | 2.359 | 0.043 | 5.451 | 0.771 | 2.17 | 0.054 | 7.95 | 1.25 |
Benchmarking results compared with the related works
The results obtained with the models used in related studies are listed in Table 4. Whereas [63–66] utilized datasets recorded during the resting states of the subjects, the subjects in [67] and [68] exercised during the experiment to cause a variation of BP. Simjanoska et al.[63] and Kachuee et al.[64] used features with conventional machine learning classifiers. Notwithstanding the sophisticated feature-engineering procedures for the BP estimation, the proposed approach based on raw data to reduce the effort demanded for the feature extraction yielded improved results. The deep learning architectures proposed by Aguirre et al.[65] and Slapničar et al.[66] using the raw data did not generate results superior to those of the proposed model. Dastjerdi et al.[67] and José M et al.[68] demonstrated that BP estimation using the linear regression model could generate results superior to those of our linear regression model without recalibration; however, with the inclusion of recalibration, our linear regression generated superior results.
Table 4.
Benchmarking results compared with the related works
| Method | Input | SBP | DBP | |
|---|---|---|---|---|
| Inputs | Signal | Error | Error | |
| Machine Learning [63] | Feature | ECG | MAE : 7.72 | MAE : 9.45 |
| AdaBoost [64] | Feature | ECG PPG | MAE : 8.21 STD : 5.43 | MAE : 4.31 STD : 3.52 |
| Seq2seq+Attention [65] | raw | PPG | MAE : 12.08 STD : 15.67 | MAE : 5.56 STD : 7.32 |
| ResNet-GRU [66] | raw | PPG | MAE : 9.43 | MAE : 6.88 |
| Linear regression [67] | Feature (PTT) | PCG PPG | MAE : 7.47 STD : 11.08 | MAE : 3.56 STD : 4.53 |
| Linear regression [68] | Feature (PAT) | ECG PPG | MAE : 7.78 | MAE : 4.21 |
| Linear regression with the recalibration (Proposed) | Feature (PTT) | ECG PPG | MAE : 5.10 STD : 3.45 | MAE : 4.22 STD : 2.19 |
| CNN+Bi-GRU+Attention with the recalibration (Proposed) | raw | MAE : 4.75 STD : 2.61 | MAE : 4.23 STD : 2.51 | |
Discussion
The purpose of this study was to demonstrate the performance of a cuffless BP monitoring device using wrist-cuff calibration for continuous BP monitoring during daily life tasks, taking advantage of the convenience of the wrist-cuff BP monitor. The recalibration was confirmed as a necessary procedure to ensure accurate BP readings by the cuffless BP monitoring system. In particular, regular calibration with an arm-cuff BP monitor is highly recommended for the continuous cuffless BP monitoring functions embedded in recent off-the-shelf smart devices. However, there is no universal standard for the recalibration interval. A continuous recalibration using a wrist-cuff BP could be feasible due to the recent development of wearable wrist-cuff-based BP monitors (for example, the Omron wearable blood pressure monitor [69]) [70]. However, these wrist-cuff BP monitors only generate results intermittently, leading to a time delay between BP estimates. The approach proposed in this study could overcome this drawback and provide more reliable continuous BP estimation compared with previous methods.
Conclusion
We proposed a novel deep learning architecture based on the CNN-BiGRU-Attention mechanism to estimate SBP and DBP accurately using a wrist-worn BP monitor. The model was first trained with ECG and PPG signals as input and arm BPs as labels. During the testing, the model was recalibrated when the difference between the prediction and the ground truth rose above 5 mmHg, in accordance with AAMI standards. We tested the linear regression and DNN models with and without recalibration. Both models performed better with recalibration. The DNN model outperformed the linear regression model. We found that periodic recalibration with the wrist-cuff BP produced BP measurements as accurate as those obtained with arm-cuff BP monitors.
Funding
This work was supported in part by the National Research Foundation of Korea (NRF) funded by the Korean Government (MSIT) under Grant NRF-2017R1A5A1015596; and in part by the Commercializations Promotion Agency for R&D Outcomes (COMPA) grant funded by the Korea government(MSIT) (No. 1711179046).
Declaration
Conflict of interest
All authors have no conficts of interest relevant to this study to disclose
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Youjung Seo, Saehim Kwon and Unang Sunarya have contributed equally to this work.
Contributor Information
Youjung Seo, Email: youjungseo0317@gmail.com.
Saehim Kwon, Email: ikeessa@naver.com.
Youngho Cho, Email: yhcho@daelim.ac.kr.
Cheolsoo Park, Email: parkcheolsoo@kw.ac.kr.
References
- 1.Shimada K, Fujita T, Ito S, Naritomi H, Ogihara T, Shimamoto K, Tanaka H, Yoshiike N. The importance of home blood pressure measurement for preventing stroke and cardiovascular disease in hypertensive patients: a sub-analysis of the japan hypertension evaluation with angiotensin ii antagonist losartan therapy (j-health) study, a prospective nationwide observational study. Hypertens Res. 2008;31(10):1903–1911. doi: 10.1291/hypres.31.1903. [DOI] [PubMed] [Google Scholar]
- 2.Puke S, Suzuki T, Nakayama K, Tanaka H, Minami S. Blood pressure estimation from pulse wave velocity measured on the chest. In: 2013 35th annual international conference of the IEEE engineering in medicine and biology society (EMBC), pp 6107–6110. IEEE; 2013. [DOI] [PubMed]
- 3.Meyer S, Sander J, Gräber S, Gottschling S, Gortner L. Agreement of invasive versus non-invasive blood pressure in preterm neonates is not dependent on birth weight or gestational age. J Paediatr Child Health. 2010;46(5):249–254. doi: 10.1111/j.1440-1754.2009.01679.x. [DOI] [PubMed] [Google Scholar]
- 4.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American heart association council on high blood pressure research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 5.Nyvad J, Christensen KL, Buus NH, Reinhard M. The cuffless SOMNOtouch NIBP device shows poor agreement with a validated oscillometric device during 24-h ambulatory blood pressure monitoring. J Clin Hypertens. 2021;23(1):61–70. doi: 10.1111/jch.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He X, Goubran RA, Liu XP. Evaluation of the correlation between blood pressure and pulse transit time. In: 2013 IEEE international symposium on medical measurements and applications (MeMeA), pp 17–20. IEEE; 2013.
- 7.Wong MY-M, Poon CC-Y, Zhang Y-T. An evaluation of the cuffless blood pressure estimation based on pulse transit time technique: a half year study on normotensive subjects. Cardiovasc Eng. 2009;9(1):32–38. doi: 10.1007/s10558-009-9070-7. [DOI] [PubMed] [Google Scholar]
- 8.Gesche H, Grosskurth D, Küchler G, Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112(1):309–315. doi: 10.1007/s00421-011-1983-3. [DOI] [PubMed] [Google Scholar]
- 9.Poon CCY, Zhang YT. Cuff-less and noninvasive measurements of arterial blood pressure by pulse transit time. In: 2005 IEEE engineering in medicine and biology 27th annual conference, p. 5877–5880. IEEE; 2006. [DOI] [PubMed]
- 10.McCarthy BM, O’Flynn B, Mathewson A. An investigation of pulse transit time as a non-invasive blood pressure measurement method. In: Journal of physics: conference series, vol 307, p. 012060. IOP Publishing; 2011.
- 11.Wang R, Jia W, Mao Z-H, Sclabassi RJ, Sun M. Cuff-free blood pressure estimation using pulse transit time and heart rate. In: 2014 12th international conference on signal processing (ICSP). IEEE; 2014. p. 115–118. [DOI] [PMC free article] [PubMed]
- 12.Shao J, Shi P, Sijung H, Hongliu Yu. A revised point-to-point calibration approach with adaptive errors correction to weaken initial sensitivity of cuff-less blood pressure estimation. Sensors. 2020;20(8):2205. doi: 10.3390/s20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiang Z, Xianxiang C, Zhen F, Yongjiao X, Qingyuan Z, Ting Y, Shanhong X. Cuff-less blood pressure measurement using pulse arrival time and a Kalman filter. J Micromech Microeng. 2017;27(2):024002. doi: 10.1088/1361-6439/27/2/024002. [DOI] [Google Scholar]
- 14.Griggs D, Sharma M, Naghibi A, Wallin C, Ho V, Barbosa K, Ghirmai T, Cao H, Krishnan SK. Design and development of continuous cuff-less blood pressure monitoring devices. In: 2016 IEEE sensors. IEEE; 2016. p. 1–3.
- 15.Barvik D, Cerny M, Penhaker M, Noury N. Noninvasive continuous blood pressure estimation from pulse transit time: a review of the calibration models. IEEE Rev Biomed Eng. 2021;15:138–151. doi: 10.1109/RBME.2021.3109643. [DOI] [PubMed] [Google Scholar]
- 16.Shao J, Shi P, Hu S. A unified calibration paradigm for a better cuffless blood pressure estimation with modes of elastic tube and vascular elasticity. J Sens 2021:2021.
- 17.Schlesinger O, Vigderhouse N, Eytan D, Moshe Y. Blood pressure estimation from ppg signals using convolutional neural networks and siamese network. In: ICASSP 2020–2020 IEEE international conference on acoustics, speech and signal processing (ICASSP). IEEE; 2020. p. 1135–1139.
- 18.Song K, Chung K, Chang J-H. Cuffless deep learning-based blood pressure estimation for smart wristwatches. IEEE Trans Instrum Meas. 2019;69(7):4292–4302. doi: 10.1109/TIM.2019.2947103. [DOI] [Google Scholar]
- 19.Kachuee M, Kiani MM, Mohammadzade H, Shabany M. Cuffless blood pressure estimation algorithms for continuous health-care monitoring. IEEE Trans Biomed Eng. 2016;64(4):859–869. doi: 10.1109/TBME.2016.2580904. [DOI] [PubMed] [Google Scholar]
- 20.Thomson EA, Nuss K, Comstock A, Reinwald S, Blake S, Pimentel RE, Tracy BL, Li K. Heart rate measures from the Apple Watch, Fitbit Charge HR 2, and electrocardiogram across different exercise intensities. J Sports Sci. 2019;37(12):1411–1419. doi: 10.1080/02640414.2018.1560644. [DOI] [PubMed] [Google Scholar]
- 21.Dörr M, Weber S, Birkemeyer R, Leonardi L, Winterhalder C, Raichle CJ, Brasier N, Burkard T, Eckstein J. iPhone App compared with standard blood pressure measurement—the iPARR trial. Am Heart J. 2021;233:102–108. doi: 10.1016/j.ahj.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Lee HY, Lee D-J, Seo J, Ihm S-H, Kim K-I, Cho EJ, Kim HC, Shin J, Park S, Sohn I-S, et al. Smartphone/smartwatch-based cuffless blood pressure measurement: a position paper from the Korean society of hypertension. Clin Hypertens. 2021;27(1):1–8. doi: 10.1186/s40885-020-00158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy BM, Vaughan CJ, O’flynn B, Mathewson A, Mathúna CÓ. An examination of calibration intervals required for accurately tracking blood pressure using pulse transit time algorithms. J Hum Hypertens. 2013;27(12):744–750. doi: 10.1038/jhh.2013.41. [DOI] [PubMed] [Google Scholar]
- 24.Chen W, Kobayashi T, Ichikawa S, Takeuchi Y, Togawa T. Continuous estimation of systolic blood pressure using the pulse arrival time and intermittent calibration. Med Biol Eng Comput. 2000;38(5):569–574. doi: 10.1007/BF02345755. [DOI] [PubMed] [Google Scholar]
- 25.Omboni S, Riva I, Giglio A, Caldara G, Groppelli A, Parati G. Validation of the Omron M5-I, R5-I and HEM-907 automated blood pressure monitors in elderly individuals according to the International Protocol of the European Society of Hypertension. Blood Pressure Monitor. 2007;12(4):233–242. doi: 10.1097/MBP.0b013e32813fa386. [DOI] [PubMed] [Google Scholar]
- 26.Ilman N, Altunkan S, Kayatürk N, Altunkan E. Validation of the Braun BP 3550 wrist blood pressure measuring device with a position sensor and an EasyClick cuff according to the International Protocol in adults. Blood Pressure Monitor. 2007;12(1):45–49. doi: 10.1097/01.mbp.0000218006.80827.dc. [DOI] [PubMed] [Google Scholar]
- 27.Eom H, Lee D, Han S, Hariyani YS, Lim Y, Sohn I, Park K, Park C. End-to-end deep learning architecture for continuous blood pressure estimation using attention mechanism. Sensors. 2020;20(8):2338. doi: 10.3390/s20082338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi Y, Zhang Q, Ko S. Noninvasive cuffless blood pressure estimation using pulse transit time and Hilbert-Huang transform. Comput Electr Eng. 2013;39(1):103–111. doi: 10.1016/j.compeleceng.2012.09.005. [DOI] [Google Scholar]
- 29.Kumar R, Dubey PK, Zafer A, Kumar A, Yadav S. Past, present and future of blood pressure measuring instruments and their calibration. Measurement. 2021;172:108845. doi: 10.1016/j.measurement.2020.108845. [DOI] [Google Scholar]
- 30.Ding X, Zhang Y, Tsang HK. Impact of heart disease and calibration interval on accuracy of pulse transit time-based blood pressure estimation. Physiol Meas. 2016;37(2):227. doi: 10.1088/0967-3334/37/2/227. [DOI] [PubMed] [Google Scholar]
- 31.Mukkamala R, Hahn J-O. Toward ubiquitous blood pressure monitoring via pulse transit time: predictions on maximum calibration period and acceptable error limits. IEEE Trans Biomed Eng. 2017;65(6):1410–1420. doi: 10.1109/TBME.2017.2756018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moody GB, Mark RG. A database to support development and evaluation of intelligent intensive care monitoring. In: Computers in cardiology 1996. IEEE; 1996. p. 657–660.
- 33.Yoon Y-Z, Kang JM, Kwon Y, Park S, Noh S, Kim Y, Park J, Hwang SW. Cuff-less blood pressure estimation using pulse waveform analysis and pulse arrival time. IEEE J Biomed Health Inform. 2017;22(4):1068–1074. doi: 10.1109/JBHI.2017.2714674. [DOI] [PubMed] [Google Scholar]
- 34.Zhou X, Peng R, Ding H, Zhang N, Li P. Validation of new and existing decision rules for the estimation of beat-to-beat pulse transit time. BioMed Res Int, 2015;2015. [DOI] [PMC free article] [PubMed]
- 35.Maher N. Non-invasive calibration-free blood pressure estimation based on artificial neural network 04 2019.
- 36.El-Hajj C, Kyriacou PA. A review of machine learning techniques in photoplethysmography for the non-invasive cuff-less measurement of blood pressure. Biomed Signal Process Control. 2020;58:101870. doi: 10.1016/j.bspc.2020.101870. [DOI] [Google Scholar]
- 37.Biancofiore F, Busilacchio M, Verdecchia M, Tomassetti B, Aruffo E, Bianco S, Di Tommaso S, Colangeli C, Rosatelli G, Di Carlo P. Recursive neural network model for analysis and forecast of pm10 and pm2.5. Atmos Pollut Res. 2017;8(4):652–659. doi: 10.1016/j.apr.2016.12.014. [DOI] [Google Scholar]
- 38.Cheng H, Xie Z, Shi Y, Xiong N. Multi-step data prediction in wireless sensor networks based on one-dimensional CNN and bidirectional LSTM. IEEE Access. 2019;7:117883–117896. doi: 10.1109/ACCESS.2019.2937098. [DOI] [Google Scholar]
- 39.Lyu P, Chen N, Mao S, Li M. LSTM based encoder-decoder for short-term predictions of gas concentration using multi-sensor fusion. Process Saf Environ Prot. 2020;137:93–105. doi: 10.1016/j.psep.2020.02.021. [DOI] [Google Scholar]
- 40.Rutschmann OT, Sarasin FP, Simon J, Vermeulen B, Riberdy L, Pechere-Bertschi A. Can wrist blood pressure oscillometer be used for triage in an adult emergency department? Ann Emerg Med. 2005;46(2):172–176. doi: 10.1016/j.annemergmed.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Casiglia E, Tikhonoff V, Albertini F, Palatini P. Poor reliability of wrist blood pressure self-measurement at home: a population-based study. Hypertension. 2016;68(4):896–903. doi: 10.1161/HYPERTENSIONAHA.116.07961. [DOI] [PubMed] [Google Scholar]
- 42.Ali EA, Omar SM, Ibrahim Y, Al-Wutayd O, Adam I. Validation of the wrist blood pressure measuring device Omron RS6 (HEM-6221-E) among obese Sudanese patients according to the European Society of Hypertension International Protocol Revision 2010. F1000Research. 2020;9:1284. doi: 10.12688/f1000research.26442.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kario K, Shimbo D, Tomitani N, Kanegae H, Schwartz JE, Williams B. The first study comparing a wearable watch-type blood pressure monitor with a conventional ambulatory blood pressure monitor on in-office and out-of-office settings. J Clin Hypertens. 2020;22(2):135–141. doi: 10.1111/jch.13799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melville S, Teskey R, Philip S, Simpson JA, Lutchmedial S, Brunt KR. A comparison and calibration of a wrist-worn blood pressure monitor for patient management: assessing the reliability of innovative blood pressure devices. J Med Internet Res. 2018;20(4):e8009. doi: 10.2196/jmir.8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn JW, Ku Y, Kim HC. A novel wearable EEG and ECG recording system for stress assessment. Sensors. 2019;19(9):1991. doi: 10.3390/s19091991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang S, Zhang Y, Cho S-Y, Correia R, Morgan SP. Non-invasive cuff-less blood pressure estimation using a hybrid deep learning model. Opt Quantum Electron. 2021;53(2):1–20. doi: 10.1007/s11082-020-02667-0. [DOI] [Google Scholar]
- 47.Frese EM, Fick A, Sadowsky HS. Blood pressure measurement guidelines for physical therapists. Cardiopulm Phys Therapy J. 2011;22(2):5. doi: 10.1097/01823246-201122020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Zhang X, Cui P, Li S, Tang J. Key feature selection and model analysis for blood pressure estimation from electrocardiogram, ballistocardiogram and photoplethysmogram. IEEE Access. 2021;9:54350–54359. doi: 10.1109/ACCESS.2021.3070636. [DOI] [Google Scholar]
- 49.Nabeel PM, Jayaraj J, Mohanasankar S. Single-source PPG-based local pulse wave velocity measurement: a potential cuffless blood pressure estimation technique. Physiol Meas. 2017;38(12):2122. doi: 10.1088/1361-6579/aa9550. [DOI] [PubMed] [Google Scholar]
- 50.Huynh TH, Jafari R, Chung W-Y. Noninvasive cuffless blood pressure estimation using pulse transit time and impedance plethysmography. IEEE Trans Biomed Eng. 2018;66(4):967–976. doi: 10.1109/TBME.2018.2865751. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q, Zhou D, Zeng X. Highly wearable cuff-less blood pressure and heart rate monitoring with single-arm electrocardiogram and photoplethysmogram signals. Biomed Eng Online. 2017;16(1):1–20. doi: 10.1186/s12938-017-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang Z, Tamura T, Sekine M, Huang M, Chen W, Yoshida M, Sakatani K, Kobayashi H, Kanaya S. A chair-based unobtrusive cuffless blood pressure monitoring system based on pulse arrival time. IEEE J Biomed Health Inform. 2016;21(5):1194–1205. doi: 10.1109/JBHI.2016.2614962. [DOI] [PubMed] [Google Scholar]
- 53.Bak H, Lee S. A 1d cnn-lstm using wav2vec 2.0 for violent scene discrimination. IEIE Transactions on Smart Processing & Computing. 2022;11(2):92–6.
- 54.Lee Y, Kang B. Where to look: Visual attention estimation in road scene video for safe driving. IEIE Trans-actions on Smart Processing & Computing. 2022;11(2):105–11.
- 55.Landry C, Hedge ET, Hughson RL, Peterson SD, Arami A. Accurate blood pressure estimation during activities of daily living: a wearable cuffless solution. IEEE J Biomed Health Inform. 2021;25(7):2510–2520. doi: 10.1109/JBHI.2021.3054597. [DOI] [PubMed] [Google Scholar]
- 56.Mostafa MMA, Hasanin AM, Alhamade F, Abdelhamid B, Safina AG, Kasem SM, Hosny O, Mahmoud M, Fouad E, Rady A, et al. Accuracy and trending of non-invasive oscillometric blood pressure monitoring at the wrist in obese patients. Anaesth Crit Care Pain Med. 2020;39(2):221–227. doi: 10.1016/j.accpm.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Campbell NRC, Berbari AE, Cloutier L, Gelfer M, Kenerson JG, Khalsa TK, Lackland DT, Lemogoum D, Mangat BK, Mohan S, et al. Policy statement of the world hypertension league on noninvasive blood pressure measurement devices and blood pressure measurement in the clinical or community setting. J Clin Hypertens. 2014;16(5):320. doi: 10.1111/jch.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan J, Tompkins WJ. A real-time QRS detection algorithm. IEEE Trans Biomed Eng. 1985;3:230–236. doi: 10.1109/TBME.1985.325532. [DOI] [PubMed] [Google Scholar]
- 59.Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exercise. 2001;33(6):S484–S492. doi: 10.1097/00005768-200106001-00018. [DOI] [PubMed] [Google Scholar]
- 60.Brett SE, Ritter JM, Chowienczyk PJ. Diastolic blood pressure changes during exercise positively correlate with serum cholesterol and insulin resistance. Circulation. 2000;101(6):611–615. doi: 10.1161/01.CIR.101.6.611. [DOI] [PubMed] [Google Scholar]
- 61.Ding X, Yan BP, Zhang Y-T, Liu J, Zhao N, Tsang HK. Pulse transit time based continuous cuffless blood pressure estimation: a new extension and a comprehensive evaluation. Sci Rep. 2017;7(1):1–11. doi: 10.1038/s41598-017-11507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hahnen C, van Helmond N. Blood pressure measurement using pulse transit time. Nederlands Tijdschrift Voor Geneeskunde. 2019;163:D3408. [PubMed] [Google Scholar]
- 63.Simjanoska M, Gjoreski M, Gams M, Madevska Bogdanova A. Non-invasive blood pressure estimation from ECG using machine learning techniques. Sensors. 2018;18(4):1160. doi: 10.3390/s18041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kachuee M, Kiani MM, Mohammadzade H, Shabany M. Cuffless blood pressure estimation algorithms for continuous health-care monitoring. IEEE Trans Biomed Eng. 2017;64(4):859–869. doi: 10.1109/TBME.2016.2580904. [DOI] [PubMed] [Google Scholar]
- 65.Aguirre N, Grall-Maës E, Cymberknop LJ, Armentano RL. Blood pressure morphology assessment from photoplethysmogram and demographic information using deep learning with attention mechanism. Sensors. 2021;21(6):2167. doi: 10.3390/s21062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slapničar G, Mlakar N, Luštrek M. Blood pressure estimation from photoplethysmogram using a spectro-temporal deep neural network. Sensors. 2019;19(15):3420. doi: 10.3390/s19153420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dastjerdi AE, Kachuee M, Shabany M. Non-invasive blood pressure estimation using phonocardiogram. In: 2017 IEEE international symposium on circuits and systems (ISCAS); 2017. p. 1–4.
- 68.Bote JM, Recas J, Hermida R. Evaluation of blood pressure estimation models based on pulse arrival time. Comput Electr Eng. 2020;84:106616. doi: 10.1016/j.compeleceng.2020.106616. [DOI] [Google Scholar]
- 69.Blood pressure watch: Omron heartguide wrist bp monitor.
- 70.Watanabe N, Bando YK, Kawachi T, Yamakita H, Futatsuyama K, Honda Y, Yasui H, Nishimura K, Kamihara T, Okumura T, et al. Development and validation of a novel cuff-less blood pressure monitoring device. Basic Transl Sci. 2017;2(6):631–642. doi: 10.1016/j.jacbts.2017.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]