Abstract
This study evaluated the biomechanical microenvironmental stimulating effect of pulsed electromagnetic field (PEMF) on the regeneration of crush-injured rat sciatic nerve, when combined with bone marrow mesenchymal stem cells (BMSCs) and recombinant human nerve growth factor (rhNGF-β), in the form of an adenoviral vector-mediated NGF. Sprague–Dawley rats were equally distributed into six groups; PBS, BMSC, NGF-Ad + BMSC, PEMF + PBS, PEMF + BMSC and PEMF + NGF-Ad + BMSC. The PBS group received PBS (volume: 10μL/rat), the BMSC group with BMSCs (1 × 106 cell/10 μL/rat) and NGF-Ad group with the rhNGF-β Ad infected BMSCs (1 × 106 cell/10 μL/rat) immediate after right sciatic nerve crush injury. The PEMF groups were exposed to PEMF of 1mT, 50 Hz, 1 h/day. The rats were observed for 3 weeks. PEMF alone did not showed the positive effect compared with negative control group. The groups transplanted with BMSCs showed higher axonal regeneration compared with the groups without transplantation of the cells whether BMSC was infected with NGF-Ad or not and whether the animals received PEMF. PEMF + NGF-Ad + BMSC group showed the significantly highest number of axons than the other groups. Functionally, all groups showed marked improvement at 3 weeks postoperatively although the difference was not statistically significant among the groups. PEMF showed the positive effect when combined with BMSC and NGF-ad in aspect of number of axons. Therefore, combining the microenvironment stimulation methods of PEMF and conventional methods such as transplantation of stem cells and growth factor could be considered for the regeneration methods in the nerve damage.
Keywords: Peripheral nerve injury, PEMF, NGF, BMSC
Introduction
Despite the recent progress in repairing peripheral nerve injury, complete recovery does not always occur [1]. Several strategies have been developed to enhance the functional recovery of nerves, including cell therapies with mesenchymal stem cells, which create the favorable environment in peripheral nervous system due to properties such as high proliferation rates and multipotency [2]. Specifically, bone marrow mesenchymal stem cell (BMSC) transplants can promote nerve regeneration by differentiating into Schwann cell-like cells, releasing neurotrophic factors that enhance angiogenesis and nerve regeneration, and decreasing oxidative stress and inflammatory reactions [3].
Another strategy is the use of pulsed electromagnetic field (PEMF) stimulation, which has the advantage of non-invasiveness in its application. It had been reported that PEMF application can regenerate axonal damage of the sciatic nerve and form the myelin sheath [4]. PEMF was known to enhance nerve regeneration by increasing ionic permeability and triggering intracellular signal cascades, which facilitate various biological processes [5]. Other studies showed that PEMF stimulation promoted the release of NGF and BDNF by Schwann cells and neurite outgrowth [6].
Nerve Growth Factor (NGF) has been known to promote survival, proliferation and neurite outgrowth in sensory and sympathetic neurons [1]. However, due to its short half-life (2.3 h), an appropriate dose and repeated supply were necessary, and adenovirus serves as a vector, providing a platform for nutrition support and controlled expression. Our previous study showed enhanced peripheral nerve regeneration with recombinant human nerve growth factor (rhNGF-β) gene transfer using adenovirus in a crush-injured sensory nerve. The rhNGF- β adenovirus group showed increased expression of the NGF, p75 neurotrophin receptor, and trkA mRNA [7]. Other previous studies showed higher regeneration ability in PEMF and tacrolimus (FK506) groups compared to the control group and synergistic effect was found in PEMF + FK506 and PEMF + hDPSCs + FK506 groups in rat sciatic nerve crush injury [8].
Based on these previous studies, we aimed to evaluate the biomechanical microenvironmental stimulating effect of pulsed electromagnetic field (PEMF) on the regeneration of crush-injured rat sciatic nerve, when combined with bone marrow mesenchymal stem cells (BMSCs) and recombinant human nerve growth factor (rhNGF-β), in the form of adenoviral vector-mediated NGF.
Materials and methods
Isolation, culture and characterization of BMSCs
The methods of harvesting, culture and characterization of BMSCs were described previously [9]. Briefly, BMSCs were isolated from 5- day-old Spraque-Dawley male rats. After injection of Dulbecco's Modified Eagle Medium (DMEM) solution to cancellous bone of hind limbs, the leakage was collected and centrifuged to isolate the MSCs. The collected MSCs were cultivated until passage 5 and analyzed for its stem cell characteristics with immunocytochemical methods using CD29 (Biolegend, San Diego, CA, USA) and CD105 (Abcam Inc., Cambridge, UK) primary antibodies.
Multiplication and purification of rhNGF-β adenovirus
The rhNGF-β adenovirus was multiplied and purified based on a previously reported protocol [7]. The NGF-Adenovirus (NGF-Ad) shuttle vector and pJM17, a plasmid containing the entire adenoviral genome, were co-transfected into 293 cells to generate homologous recombination between the adenovirus genome sequences (Fig. 1a). The constructed replication-incompetent NGF-Ad was isolated as a single plaque to remove wild adenovirus.
Fig. 1.
Single treatment of mesenchymal stem cells. a Multiplication and purification of rhNGF-β adenovirus. NGF-Ad was co-transfected into 293 cells. b Mesenchymal stem cell culture. c rhNGH- β gene transfection into BMSCs. BMSCs infected with rhNGF- β adenovirus were monitored for GFP expression under a fluorescence microscope 24 h after infection (original magnification × 20)
The isolated NGF-Ad vector was infected into 293 cells that were expanded by subculture. The cells were harvested 2 days later when they all showed cytotoxicity. These cells were frozen and thawed 5 times and an rhNGF-β-Ad suspension was obtained by vigorous shaking at every stage. The suspension was purified by double cesium chloride-gradient ultracentrifugation. The viral stock was then dialyzed against phosphate-buffered saline (PBS) and stored at − 80 °C until further use [7].
rhNGH- β gene transfection into BMSCs
Passage zero BMSCs were seeded at a density of 1 × 104 cells in a 100 mm dish for 24 h. Then, 2 ml of serum-free α-minimum essential medium (α-MEM) was added and the cells were incubated at 37 °C for 60 min. The BMSCs were then infected with rhNGF-β Ad at a multiplicity of infection (MOI) of 70. After removing the media, the cells were washed once with α-MEM and then re-cultured in normal medium for 24 h for green fluorescent protein (GFP) expression. The BMSCs infected with rhNGF- β adenovirus were monitored for GFP expression under a fluorescence microscope 24 h after infection (Fig. 1b, c). When the number of GFP-positive cells became more than 80% of the total number of cells, the cells were used in the experiment.
Experimental design and surgical procedures
Male Sprague–Dawley rats (200–250 g, 6-week-old) were randomly allocated into 6 groups (n = 18 each); PBS, BMSC, NGF-Ad + BMSC, PEMF + PBS, PEMF + BMSC and PEMF + NGF-Ad + BMSC. All animal surgical and experimental procedures were approved by Institutional Animal Care and Use Committee of Seoul National University (SNU-130201–2) and conducted in accordance with the care guidelines of the Laboratory of Animal Resources of Seoul National University, Republic Korea.
The surgical procedures were performed as described previously [10]. A crush injury was made in the middle of the right sciatic nerve by compressing a 4 mm-wide needle holder to the second ratchet for 1 min. The injury site was marked with a single 9–0 ethilon (Ethicon, LLC., USA) epineural stitch at the distal end of the injury for later identification.
The PBS group was administered with PBS (volume: 10 μL/rat), the BMSC and PEMF + BMSC groups received BMSCs (1 × 106 cell/10 μL/rat), and the NGF-Ad + BMSC and PEMF + NGF-Ad + BMSC groups were transplanted with rhNGF-β Ad-infected BMSCs (1 × 106 cell/10 μL/rat) immediately after sciatic nerve injury using a Hamilton syringe with a 33-gauge needle (Fig. 2a). Nerve regeneration was analyzed for 3 weeks.
Fig. 2.
Experimental operation & electromagnetic fields equipment. a PBS, BMSCs or NGF-Ad infected BMSCs were injected to SD rat sciatic nerve after crush injury. b PEMF was carried out with pulse trains with 50 Hz frequency and intensity of 1 mT. c Overview of PEMF device. PEMF was generated in a pair of Helmholtz coil of 30 cm diameter and 15 cm distance. The rats of PEMF groups were put into a plastic cage between the two coils 1 h/day
Pulsed electromagnetic fields (PEMFs) exposure
PEMF was generated with a pair of Helmholtz coils of 30 cm diameter, and the distance of the two coils was kept at 15 cm (Fig. 2b, c). The rats of PEMF groups were put into a plastic cage between the two coils, where they could freely wander around. PEMF was delivered with 50 Hz frequency and 1 mT for 3 weeks (1 h /day) in a silent room at a temperature of 21 °C–25 °C [8].
Functional assessment, SFI (sciatic function index)
The footprints were recorded 1, 2 and 3 weeks postoperatively for all groups. The footprints were obtained by the method described by Jolicoeur et al. [11]. The sciatic function index (SFI) was calculated by the formula of Bain et al.[12]. Measurements were taken for each footprint, including the print length (PL), the distance between the tip of the longest toe and the heel, the toe spread (TS), the distance between the first and fifth toes, and the intermediate toe spread (IT), the distance between the second and fourth toes, for both the normal (N) and experimental (E) paws. Then, SFI was calculated:
SFI = -38.3 x (EPL—NPL) / NPL + 109.5 x (ETS—NTS) / NTS + 13.3 x (EIT—NIT) / NIT—8.8. As an indicator of nerve function, SFI values around -100 indicate total loss of function whereas values around 0 indicate normal function [8].
Retrograde labeling
Retrograde labeling and counting of back-labeled sensory neurons were performed at the end of the 3-week follow-up period. The sciatic nerves of sixth rats in each group were labeled with 4% Fluorogold (FG) (Fluorochrome, LLC, Denver, CO, USA), while using distilled water as a negative control. The sciatic nerves were sharply cut at a distance of 10 mm distal to the injury site,soaked in 4% FG for 1 h in vaseline well, washed thoroughly with saline and then the wound was closed.
Five days after retrograde labeling, L4 and L5 dorsal root ganglia (DRG) were harvested and serially sectioned into fresh-frozen 20 μm thick sections using a Cryo-Cut microtome (Leica CM3050S Cryostat, Leica Microsystems, Wetzlar, Germany) at − 18 °C. A confocal microscope (Olympus, FV- 300, Tokyo, Japan) was used to capture images of the DRG sections at 20 × magnification [8].
Histomorphometric analysis
At the end of the 3-week follow-up period, six rats per group were used for histomorphometric analysis. The specimens were harvested at a distance of 5 mm distal to the injury site. Serial transverse semi-thin sections of 2 μm thickness were cut using a microtome (Leica, Ultracut, UCT, Vienna, Austria) and stained with 1% Toluidine Blue for light microscopy examination, including axon counts and densities at 40 × magnification (Olympus, BX41, TF, Japan) [8].
Statistical analysis
Statistical analysis was carried out using SPSS ver 25 (SPSS, Chicago, IL). Mean differences and standard deviations were calculated for the variables measured. One-way analysis of variance followed by Fisher's protected least significant difference test was used to determine statistical significance. A P value < 0.05 was considered statistically significant.
Results
rhNGF-β gene transfection into BMSCs
After 24 h, most of the BMSCs were transfected with rhNGF-β adenovirus, and GFP expression was observed under a fluorescence microscope without any changes in cell morphology (Fig. 1c).
Functional assessment, SFI (Sciatic functional index)
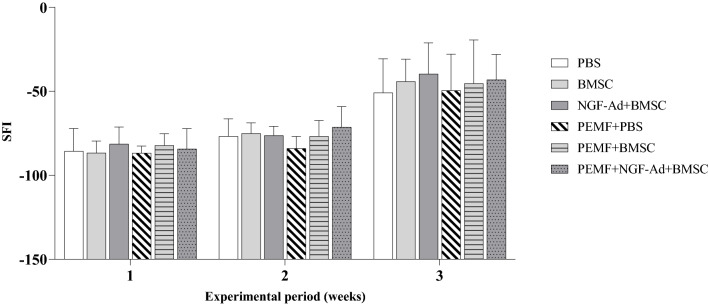
Figure 3 shows the overall changes of SFI. One week after the crush injury, SFI was around -90 due to the loss of function induced by the nerve damage. A gradual increase in SFI was observed during the following 2 weeks. However, there were no statistical differences between the groups, except for the PEMF and PEMF + NGF-Ad + BMSC group (− 82.94 ± 7.24 and -71.34 ± 12.27, respectively, p = 0.0173) at 2 weeks post-operatively.
Fig. 3.
Graph of weekly sciatic function index (SFI). Gradual increase of SFI was observed from 1 week postoperatively. The PEMF + NGF-Ad + BMSC group showed higher SFI than PEMF group at 2 weeks post-operatively (p = 0.0173, Fisher’s protected least significant difference test). Otherwise, no statistical differences were found among groups during 3 weeks
Retrograde tracing
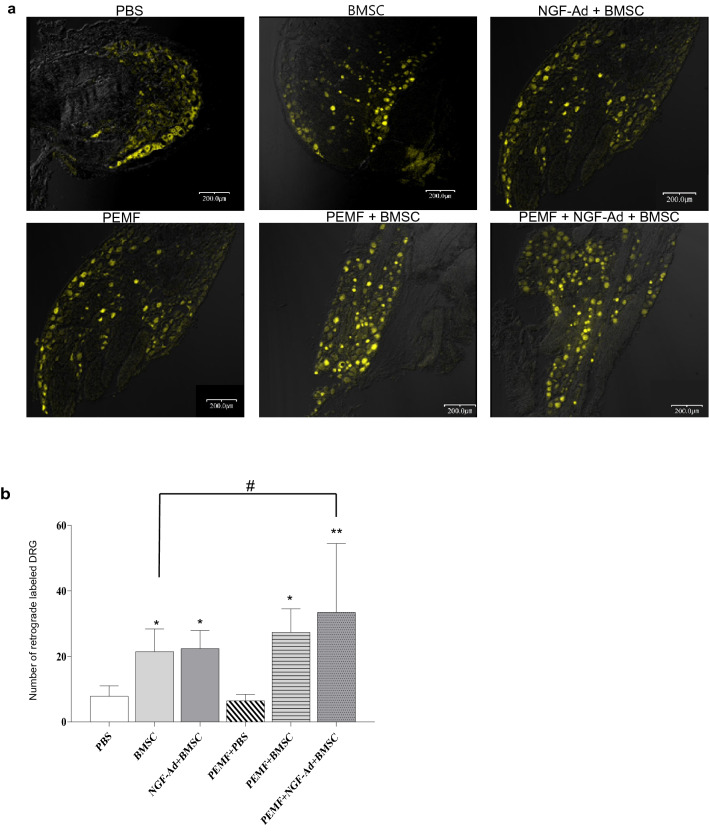
The number of retrogradely labeled neurons was significantly higher in the BMSC, NGF-Ad + BMSC, PEMF + BMSC and PEMF + NGF-Ad + BMSC groups (BMSC = 21.46 ± 6.91, NGF-Ad + BMSC = 22.40 ± 5.49, PEMF + BMSC = 27.38 ± 7.12 and PEMF + NGF-Ad + BMSC = 33.44 ± 21.02) compared to the other two groups (PBS = 7.83 ± 3.14, PEMF + PBS = 6.43 ± 1.97). Transplantation of BMSCs was assumed to have accelerated neuronal regeneration. Among the four groups that contained cells, the PEMF + NGF-Ad + BMSC group showed a significantly higher number of retrogradely labeled neurons compared to the BMSC group (Fig. 4a, b).
Fig. 4.
Retrograde labeling of dorsal root ganglion (DRG). a Representative fluorescence photomicrographs of DRG showed fluorescent (yellow color) cell bodies of retrograde labeled with Fluorogold, 3 weeks following the crush injury. Scale bar 200 μm. b Mean count of retrograde labeled DRG. All groups BMSCs exhibited a significantly higher number of back-labeled DRG than both the control and PEMF group. Furthermore, among the groups that were injected with BMSCs, the co-treatment of NGF-Ad-infected BMSCs and PEMF demonstrated a significantly higher number of back-labeled DRG as compared to BMSCs alone. All values are presented as mean ± SD (∗ : p < 0.05 vs. Control and PEMF, ∗ ∗ : p < 0.0001 vs. Control and PEMF, #: p < 0.05 between two selected groups)
Histomorphometric evaluation
The photographs of histologic features distal to the crush injury site were shown in Fig. 5a and the histomorphometric results were shown in Fig. 5b, c. Regenerated nerves showed the myelinated fibers with smaller diameter and a thinner myelin sheath. In axon count, the PEMF + NGF-Ad + BMSC group showed the highest number of axons, followed by the PEMF + BMSC and BMSC group, then the NGF-Ad + BMSC group, and finally the PBS and PEMF group. All groups showed statistically significant differences between each other except those between the PEMF + BMSC and BMSC groups and between the PBS and PEMF groups. The PEMF + NGF-Ad + BMSC, NGF-Ad + BMSC and BMSC groups showed higher axon density than the PBS and PEMF groups, and there were no statistical differences between the PEMF + NGF-Ad + BMSC, NGF-Ad + BMSC, and BMSC groups. The PEMF + BMSC group showed no differences compared to the other groups in axon density.
Fig. 5.
Histomorphometric analysis. a Photomicrographs of histologic features at distal to the crush injury site, 3 weeks post-operatively. Representative microphotographs show the regenerating nerves with small myelinated fibers. Toluidine blue stain. Scale bar 100 μm. b Histograms of axon counting in each group. The PEMF + NGF-Ad + BMSC group showed significantly higher numbers of axons compared to the other groups. The BMSC and PEMF + BMSC groups also showed higher numbers of axons compared to the NGF-Ad and BMSC group, as well as the PBS and PEMF groups, which had the least number of axons. c Histogram of axon density in each group. The axon density was significantly higher in the BMSC, NGF-Ad + BMSC, and PEMF + NGF-Ad + BMSC groups compared to the PBS and PEMF groups. All values are presented as mean ± SD. (∗ : p < 0.05, ∗ ∗ : p < 0.0001 vs. Control and PEMF, #: p < 0.05, ##: p < 0.01; ###: p < 0.0001 between two selected groups)
Discussion
This study evaluated the biomechanical microenvironmental stimulating effect of pulsed electromagnetic field (PEMF) on the regeneration of crush-injured rat sciatic nerve, when combined with bone marrow mesenchymal stem cells (BMSCs) and recombinant human nerve growth factor(rhNGF-β) in the form of adenoviral vector-mediated NGF. The results showed that PEMF alone did not demonstrate a positive effect compared to the negative control group. The synergistic effect of BMSC, NGF-Ad and PEMF was observed based on the superiority of the number of axons. However, significant differences were not observed in retrograde labeled DRG and axon density from NGF-Ad + BMSC and PEMF + BMSC groups.
In sciatic nerve crush injury, the SFI was reported to increase until 4 weeks due to the rapid spontaneous repair process. Thereafter, no further improvement was observed, although nerve regeneration was progressing because SFI only measures the toe extension [13]. Also, the SFI showed limitation at 1 and 2 weeks postoperatively due to the foot drop caused by paralysis and clawing of the toes [14]. Correlations between SFI and histomorphometric data have been controversial, as Munro et al. found no correlation [15], while Wang et al. found a correlation between functional indexes such as SFI, ankle angle and toe angle and histomorphometric analysis [13]. There was no correlation between SFI and histomorphometric results in our study. These limitation of SFI measurements could explain the lack of correlation.
The BMSCs showed positive effect on the number of back labeled DRG, axon count and axon density. Although some studies reported no effect on bone marrow-derived mesenchymal stem cells over peripheral nerve regeneration [16], it was difficult to compare with our results due to the different size of the nerve defect in the previous study. Recently, it has been reported that BMSCs exert their regenerative effect by releasing various extracellular vesicles that transfer genetic information and exercise their biological effect [17]. Also, BMSC transplantation was reported to enhance nerve regeneration in a rat model of hindlimb replantation [3], and it seemed that BMSCs certainly have an effect on peripheral nerve regeneration.
The animals treated with PEMF without transplantation of BMSCs didn’t show better results than the animals of negative control with neither PEMF nor BMSCs. This result was different from our previous study by Kim et al. [8], which showed a significantly higher number of retrograde labeled DRG, axon number and axon density in the PEMF group compared to the control group, although the conditions for giving electromagnetic fields were different. PEMF was delivered with 60 Hz frequency and 1.5 mT for 3 weeks (1 h/day) in Kim et al.’s study, while PEMF with 50 Hz frequency and 1mT were used in this study. The conditions of this study were based on our previous study, which evaluated the influence of frequency and exposure time of PEMF and found the highest Schwann cell proliferation and mRNA expression of BDNF and S100 under 50 Hz, 1mT PEMF exposure for 1 h/day [18]. These PEMF conditions may help the differentiation of MSCs into Schwann cells, and there would be no significant effect in the conditions without cell transplantation.
Due to the inherent characteristic of neurons to transmit electrochemical signals throughout the nervous system, electrical stimulation was considered to affect neural differentiation of stem cells or neuron maturation [19]. EMFs was reported to influence the influx of calcium ions, a crucial regulator of various cellular functions, resulting in changes to the concentration of ions across the cell membrane and the transmembrane potential. EMF was also known to induce the formation of reactive oxygen species (ROS), which influence ATP production and to affect spindle microtubules related to stem cell differentiation [20]. Therefore, EMFs can alter stem cell fate and determine their differentiation by influencing charges in cell components and thus cell communication by regulating the signals delivered to cells [20]. Beck-Broichsitter et al. used PEMF of 35mT, 33 Hz, 12 min/day for 12 weeks in a rat median nerve model and found that PEMF increased the regeneration of nerve functionally. Similarly, De Pedro et al. [21] with PEMF (0.9–1 mT, 15 Hz, 4 week), Mohammadi et al. [22] with PEMF (0.2mT, 2 Hz, 4 h/day, 1–5 days/week) reported positive effect of PEMF in rat peripheral nerve defect models.
In another aspect, there were controversies related to the effects of PEMF. Groot et al., reported no increase in intracellular calcium or production of ROS after exposure to extremely low frequency EMFs in naive and chemically stressed pheochromocytoma cells [23]. Walker et al. [5] studied the effects of PEMF (2 Hz, 0.03, 0.3, 3 mT) 4 h/day, for 5 days and found no difference in functional recovery in rat peripheral nerve defect models. Baptista et al. also reported functional and histological results with PEMF (72 Hz, 2G) 30 min/ day, 5 days a week for 3 weeks, and they found decreased regeneration in morphometric assessment and increased free radicals in the PEMF nerves [24]. However, the experimental setups often differ in many key parameters, such as the time, intensity, and type of exposure, making it difficult to compare results and reach a consensus [25]. Similarly, Kang et al., demonstrated that specific electromagnetic field conditions (frequency and magnetic flux density) significantly regulate osteogenic differentiation of adipose-derived stem cells in vitro [26].
In the NGF-Ad + BMSC group and PEMF + NGF-Ad + BMSC group of our study, we did not observe a clear additive effect of NGF-Ad compared to the BMSCs groups. NGF was known to involve differentiation of MSC after crosstalk between energy homeostasis and mitochondrial remodeling. After binding to TrkA, NGF can activate the phosphatidylinositol 3-hydroxy kinase (PI3K)-protein kinase B (Akt) and Ras-mitogen-activated protein kinase (MAPK)- extracellular signal-regulated kinase (Erk) signaling pathways in MSCs and stimulate the neural differentiation of MSCs [27]. Although NGF has indirect and positive effects on angiogenesis, neuronal survival, Schwann cell proliferation and viability during peripheral nerve development and regeneration [2], its clinical use remains controversial. Initial clinical studies exploring its use in patients with peripheral neuropathies induced by diabetes showed that phase I and phase II clinical trials confirmed the protective action of NGF. However, the phase III trial did not confirm the results of the previous trials, and the clinical studies were halted due to undesired side-effects such as peripheral pain [28]. In other clinical studies on the central nervous system, intracerebral injections of adeno-associated viral vector – nerve growth factor (AAV2-NGF) into each hemisphere of the nucleus basalis of Meynert in mild to moderate Alzheimer-associated dementia patients were well-tolerated, but there was no evidence of efficacy [29]. On the contrary, recent animal studies on spinal injury models showed that rats transfected with neural stem cells with adeno-associated virus carrying NGF and five hypoxia-responsive elements (5HRE), which controlled the NGF expression, and promoted tissue repair and functional recovery [30]. According to Wu et al.’s studies, accurate control of NGF gene expression is essential in nerve regeneration, and our study results and the clinical studies mentioned above could be related to the lack of sensitive control of NGF gene expression, possibly under-expression.
Our effort to increase the effect of NGF-Adenovirus by using PEMF showed additive results in the number of axons. There were few studies that evaluated the combined effect of NGF and PEMF. Zhang et al. reported that neurite outgrowth was observed in PC12 cells in 50 and 70 Hz PEMF in the presence of NGF [31]. However, some studies reported that both NGF-like activity and NGF protein levels were decreased when exposed to PEMF following rat sciatic nerve injury [32]. Although our NGF expression may have been influenced by PEMF, the results in the PEMF + NGF-Ad + BMSC group showed higher mean values than the other groups, which was not consistent with the findings of Longo et al. This may be related with the different setups as discussed above. Our study was limited to in vivo study and molecular and genetic changes were not evaluated. Therefore, further studies with more careful analysis of NGF in molecular or genetic levels would be necessary.
Conclusion
PEMF showed the better effect when combined with BMSC and NGF-ad in aspect of the number of axons. However, PEMF alone did not show a positive effect compared to the negative control group. Therefore, a combination of microenvironmental stimulation by PEMF and conventional methods such as tramsplantation of stem cells and growth factors could be considered as a treatment method for the regeneration of nerve damage.
Acknowledgements
Sang-Yoon Lee and Bongju Kim contributed equally as a first authors. This research was supported by a grant from the Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HI20C2114).
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by S-L, BK and S-HL. The first draft of the manuscript was written by KP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Korea Health Technology R&D Project through Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HI20C2114).
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experimental procedures were conducted in compliance with the the care guidelines of the laboratory of animal resources of Seoul National University and approved by the Institutional Animal Care and Use Committee of Seoul National University (SNU-130201-2).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li R, Li DH, Zhang HY, Wang J, Li XK, Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol Sin. 2020;41:1289–1300. doi: 10.1038/s41401-019-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moattari M, Kouchesfehani HM, Kaka G, Sadraie SH, Naghdi M. Evaluation of nerve growth factor (NGF) treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. J Craniomaxillofac Surg. 2018;46:898–904. doi: 10.1016/j.jcms.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Abbas OL, Ozatik O, Gonen ZB, Kocman AE, Dag I, Ozatik FY, Bahar D, Musmul A. Bone marrow mesenchymal stem cell transplantation enhances nerve regeneration in a rat model of Hindlimb replantation. Plast Reconstr Surg. 2019;143:758e–768e. doi: 10.1097/PRS.0000000000005412. [DOI] [PubMed] [Google Scholar]
- 4.Mert T, Gunay I, Gocmen C, Kaya M, Polat S. Regenerative effects of pulsed magnetic field on injured peripheral nerves. Altern Ther Health Med. 2006;12:42–49. [PubMed] [Google Scholar]
- 5.Walker JL, Kryscio R, Smith J, Pilla A, Sisken BF. Electromagnetic field treatment of nerve crush injury in a rat model: effect of signal configuration on functional recovery. Bioelectromagnetics. 2007;28:256–263. doi: 10.1002/bem.20302. [DOI] [PubMed] [Google Scholar]
- 6.Min Q, Parkinson DB, Dun XP. Migrating Schwann cells direct axon regeneration within the peripheral nerve bridge. Glia. 2021;69:235–254. doi: 10.1002/glia.23892. [DOI] [PubMed] [Google Scholar]
- 7.Li BH, Kim SM, Yoo SB, Kim MJ, Jahng JW, Lee JH. Recombinant human nerve growth factor (rhNGF-beta) gene transfer promotes regeneration of crush-injured mental nerve in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e26–34. doi: 10.1016/j.tripleo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim YT, Hei WH, Kim S, Seo YK, Kim SM, Jahng JW, Lee JH. Co-treatment effect of pulsed electromagnetic field (PEMF) with human dental pulp stromal cells and FK506 on the regeneration of crush injured rat sciatic nerve. Int J Neurosci. 2015;125:774–783. doi: 10.3109/00207454.2014.971121. [DOI] [PubMed] [Google Scholar]
- 9.Seo N, Lee SH, Ju KW, Woo J, Kim B, Kim S, Jahng JW, Lee JH. Low-frequency pulsed electromagnetic field pretreated bone marrow-derived mesenchymal stem cells promote the regeneration of crush-injured rat mental nerve. Neural Regen Res. 2018;13:145–153. doi: 10.4103/1673-5374.224383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sung MA, Jung HJ, Lee JW, Lee JY, Pang KM, Yoo SB, Alrashdan MS, Kim SM, Jahng JW, Lee JH. Human umbilical cord blood-derived mesenchymal stem cells promote regeneration of crush-injured rat sciatic nerves. Neural Regen Res. 2012;7:2018–2027. doi: 10.3969/j.issn.1673-5374.2012.26.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolicoeur FB, Rondeau DB, Hamel E, Butterworth RF, Barbeau A. Measurement of ataxia and related neurological signs in the laboratory rat. Can J Neurol Sci. 1979;6:209–215. doi: 10.1017/S0317167100119663. [DOI] [PubMed] [Google Scholar]
- 12.Bain J, Mackinnon S, Hunter D. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Wang T, Ito A, Aoyama T, Nakahara R, Nakahata A, Ji X, Zhang J, Kawai H, Kuroki H. Functional evaluation outcomes correlate with histomorphometric changes in the rat sciatic nerve crush injury model: a comparison between sciatic functional index and kinematic analysis. PLoS ONE. 2018;13:e0208985. doi: 10.1371/journal.pone.0208985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monte-Raso VV, Barbieri CH, Mazzer N, Yamasita AC, Barbieri G. Is the Sciatic Functional Index always reliable and reproducible? J Neurosci Methods. 2008;170:255–261. doi: 10.1016/j.jneumeth.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Munro CA, Szalai JP, Mackinnon SE, Midha R. Lack of association between outcome measures of nerve regeneration. Muscle Nerve. 1998;21:1095–1097. doi: 10.1002/(SICI)1097-4598(199808)21:8<1095::AID-MUS20>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Fernandes M, Valente SG, Sabongi RG, Gomes Dos Santos JB, Leite VM, Ulrich H, Nery AA, da Silva Fernandes MJ. Bone marrow-derived mesenchymal stem cells versus adipose-derived mesenchymal stem cells for peripheral nerve regeneration. Neural Regen Res. 2018;13:100–104. doi: 10.4103/1673-5374.224378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh A, Raghav A, Shiekh PA, Kumar A. Transplantation of engineered exosomes derived from bone marrow mesenchymal stromal cells ameliorate diabetic peripheral neuropathy under electrical stimulation. Bioact Mater. 2021;6:2231–2249. doi: 10.1016/j.bioactmat.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hei WH, Byun SH, Kim JS, Kim S, Seo YK, Park JC, Kim SM, Jahng JW, Lee JH. Effects of electromagnetic field (PEMF) exposure at different frequency and duration on the peripheral nerve regeneration: in vitro and in vivo study. Int J Neurosci. 2016;126:739–748. doi: 10.3109/00207454.2015.1054032. [DOI] [PubMed] [Google Scholar]
- 19.Liang L, Liu C, Cai P, Han S, Zhang R, Ren N, Wang J, Yu J, Shang S, Zhou W, et al. Highly specific differentiation of MSCs into neurons directed by local electrical stimuli triggered wirelessly by electromagnetic induction nanogenerator. Nano Energy. 2022;100:107483. doi: 10.1016/j.nanoen.2022.107483. [DOI] [Google Scholar]
- 20.Safavi AS, Sendera A, Haghighipour N, Banas-Zabczyk A. The role of low-frequency electromagnetic fields on mesenchymal stem cells differentiation: a systematic review. Tissue Eng Regen Med. 2022;19:1147–1160. doi: 10.1007/s13770-022-00473-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Pedro J, Pérez-Caballer A, Dominguez J, Collia F, Blanco J, Salvado M. Pulsed electromagnetic fields induce peripheral nerve regeneration and endplate enzymatic changes. Bioelectromagn: J Bioelectromagn Soc Soc Phys Regulat Biol Med Eur Bioelectromagn Associat. 2005;26:20–27. doi: 10.1002/bem.20049. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi R, Faraji D, Alemi H, Mokarizadeh A. Pulsed electromagnetic fields accelerate functional recovery of transected sciatic nerve bridged by chitosan conduit: an animal model study. Int J Surg. 2014;12:1278–1285. doi: 10.1016/j.ijsu.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 23.de Groot MW, Kock MD, Westerink RH. Assessment of the neurotoxic potential of exposure to 50Hz extremely low frequency electromagnetic fields (ELF-EMF) in naive and chemically stressed PC12 cells. Neurotoxicology. 2014;44:358–364. doi: 10.1016/j.neuro.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Baptista AF, Goes BT, Menezes D, Gomes FCA, Zugaib J, Stipursky J, Gomes JR, Oliveira JT, Vannier-Santos MA, Martinez AMB. PEMF fails to enhance nerve regeneration after sciatic nerve crush lesion. J Peripher Nerv Syst. 2009;14:285–293. doi: 10.1111/j.1529-8027.2009.00240.x. [DOI] [PubMed] [Google Scholar]
- 25.Bertagna F, Lewis R, Silva SRP, McFadden J, Jeevaratnam K. Effects of electromagnetic fields on neuronal ion channels: a systematic review. Ann N Y Acad Sci. 2021;1499:82–103. doi: 10.1111/nyas.14597. [DOI] [PubMed] [Google Scholar]
- 26.Kang KS, Hong JM, Kang JA, Rhie JW, Jeong YH, Cho DW. Regulation of osteogenic differentiation of human adipose-derived stem cells by controlling electromagnetic field conditions. Exp Mol Med. 2013;45:e6. doi: 10.1038/emm.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zha K, Yang Y, Tian G, Sun Z, Yang Z, Li X, Sui X, Liu S, Zhao J, Guo Q. Nerve growth factor (NGF) and NGF receptors in mesenchymal stem/stromal cells: Impact on potential therapies. Stem Cells Transl Med. 2021;10:1008–1020. doi: 10.1002/sctm.20-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413. doi: 10.1016/S0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- 29.Rafii MS, Tuszynski MH, Thomas RG, Barba D, Brewer JB, Rissman RA, Siffert J, Aisen PS, Team ANS Adeno-associated viral vector (Serotype 2)-nerve growth factor for patients with alzheimer disease: a randomized clinical trial. JAMA Neurol. 2018;75:834–841. doi: 10.1001/jamaneurol.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Q, Xiang Z, Ying Y, Huang Z, Tu Y, Chen M, Ye J, Dou H, Sheng S, Li X, et al. Nerve growth factor (NGF) with hypoxia response elements loaded by adeno-associated virus (AAV) combined with neural stem cells improve the spinal cord injury recovery. Cell Death Discov. 2021;7:301. doi: 10.1038/s41420-021-00701-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Ding J, Duan W. A study of the effects of flux density and frequency of pulsed electromagnetic field on neurite outgrowth in PC12 cells. J Biol Phys. 2006;32:1–9. doi: 10.1007/s10867-006-6901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longo FM, Yang T, Hamilton S, Hyde JF, Walker J, Jennes L, Stach R, Sisken BF. Electromagnetic fields influence NGF activity and levels following sciatic nerve transection. J Neurosci Res. 1999;55:230–237. doi: 10.1002/(SICI)1097-4547(19990115)55:2<230::AID-JNR10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]