Abstract
Background
Over-crowded surgical trays result in perioperative inefficiency and unnecessary costs. While methodologies to reduce the size of surgical trays have been described in the literature, they each have their own drawbacks. In this study, we compared three methods: (1) clinician review (CR), (2) mathematical programming (MP), and (3) a novel hybrid model (HM) based on surveys and cost analysis. While CR and MP are well documented, CR can yield suboptimal reductions and MP can be laborious and technically challenging. We hypothesized our easy-to-implement HM would result in a reduction of surgical instruments in both the laminectomy tray (LT) and basic neurosurgery tray (BNT) that is comparable to CR and MP.
Methods
Three approaches were tested: CR, MP, and HM. We interviewed 5 neurosurgeons and 3 orthopedic surgeons, at our institution, who performed a total of 5437 spine cases, requiring the use of the LT and BNT over a 4-year (2017–2021) period. In CR, surgeons suggested which surgical instruments should be removed. MP was performed via the mathematical analysis of 25 observations of the use of a LT and BNT tray. The HM was performed via a structured survey of the surgeons’ estimated instrument usage, followed by a cost-based inflection point analysis.
Results
The CR, MP, and HM approaches resulted in a total instrument reduction of 41%, 35%, and 38%, respectively, corresponding to total cost savings per annum of $50,211.20, $46,348.80, and $44,417.60, respectively.
Conclusions
While hospitals continue to examine perioperative services for potential inefficiencies, surgical inventory will be increasingly scrutinized. Despite MP being the most accurate methodology to do so, our results suggest that savings were similar across all three methods. CR and HM are significantly less laborious and thus are practical alternatives.
Keywords: Surgical instruments, Surgical trays, Spine surgery, Optimization, Standardization, Cost savings, Mathematical model, Newsvendor model, Clinician review
Background
Currently, there is a growing interest in the implementation of industrial and business management concepts within the healthcare sector to improve operations [1], [2], [3]. Standardization is one such method leading to reduced variability, increased order, increased productivity, and improved safety [4].
Curiously, standardization of perioperative surgical operations is not quite yet modus operandi [5], [6], [7], [8], despite surgical care being one of the major contributors to hospital expenses [9], [10], [11], with inefficiency and variability being among the main culprits [10], [11], [12], [13], [14]. Because surgical instruments comprise a significant portion of perioperative budgets [15], [16], [17], [18], surgical ‘trays’ make ideal standardization candidates [19,20].
Commonly, surgical institutions conduct ad hoc addition of surgical instruments to a surgical tray without a formal analysis or review process, and often without any regular process for the audit and removal of less used instruments [21]. This results in increasingly crowded surgical trays, which, in addition to being economically unsustainable [22], can increase error rate and decrease the percentage of instruments used, regardless of operative procedure [15]. An example of this inefficiency is highlighted in the literature whereby a study of 38 spine cases performed by orthopedic surgeons and neurosurgeons found that only 58% of instruments were used at least once [23].
Different approaches have been used to standardize and optimize tray configuration, all resulting in reduced instruments per surgical tray [20]. Approaches relying on clinician review (CR), through the use of surgical experts’ analyses or Lean practices, employ interviews and checklists, among other methods to reduce the number of instruments on the surgical tray. They have been shown to result in a decrease in the weight of the tray [24,25], a decrease in time to sterilize and package the instruments [22,24,25], a decrease in operation room (OR) set-up time [21,26], and a decrease in costs [21,23,25,27,28], without compromising quality, safety, and efficiency [21,25,[29], [30], [31], [32]. However, CR, though pragmatic, is often procedure-specific and not generalizable, relying heavily on clinician input and recall, which are particularly vulnerable to recency bias [33]. Approaches relying on mathematical programming (MP) focus on minimizing costs, and have demonstrated similar favorable results [34], [35], [36], [37]. Of particular interest is the Newsvendor Model, a customized single-period inventory model, which has been shown to outperform CR [38]. The model can simultaneously consider multiple surgical procedures, the surgeon's needs, the patient's safety, ease of tray set-up, instrument wear-and-tear, and associated costs [38]. A major barrier to the implementation of mathematical models in surgical tray optimization is its reliance on labor-intensive observation data collection for each surgical procedure.
In this study, we attempt to overcome these respective barriers of CR and MP by using a hybrid CR-MP approach (HM), in which CR interview data is fed into a detailed cost inflection point model, to optimize two trays required for laminectomy procedures, the laminectomy tray (LT) and basic neurosurgery tray (BNT). We compare the percent reduction in instruments obtained by our hybrid model (HM) to a CR-only approach and an MP-only approach (ie, the Newsvendor model). We hypothesize that the HM will result in a reduction of surgical instruments in both LT and BNT that is comparable to a CR-only and MP-only approach, creating an efficient and practical method of large-scale surgical tray optimization in busy surgical centers.
Methods
This is a quality improvement study conducted at a large academic, level 1 trauma center which performs over 1400 spine and neurosurgery procedures annually, involving the cervical, thoracic, and lumbar spine, such as, but not limited to, decompressions, fusions, and laminectomies. These procedures all require LT and BNT, which we seek to optimize using three methods of surgical tray optimization: (1) a clinician-review-only approach (CR), (2) a mathematical Newsvendor model (MP) reliant on archetypal surgical procedure observation, and (3) a hybrid clinician-review-and-mathematical-modeling approach (HM) based on detailed cost inflection point analysis. We compare the reduction in tray instruments resulting from each approach. Ethical approval to perform the study was obtained from the institution's Research Ethics Board.
Clinician review (CR)
We interviewed 5 neurosurgeons and 3 orthopedic surgeons who performed a total of 83 different types of surgical procedures, for a total of 5437 surgical cases, requiring the use of the LT and/or BNT, from April 1, 2017, to February 15, 2021. Surgeons were to identify, globally speaking, the surgical instruments which must remain on and those that can be removed from the LT and BNT. Then, we used the majority method to determine the instruments that would remain and those that would be removed from the trays: we conservatively defined the majority as 50%, such that instruments which were deemed necessary by 4 out of the 8 surgeons would remain on the tray.
Newsvendor model (MP)
A trained observer attended 25 randomly selected laminectomy procedures requiring both LT and BNT and noted the use of the instruments from each tray. Processes in the Medical Department Reprocessing Department (MDRD) and the operating room (OR) were also observed to quantify the costs of underage and overage for each instrument. Observations were implemented in the Newsvendor model to solve for the instruments to be removed from each tray, as described in detail by Toor et al. [38].
Hybrid approach: cost-based inflection point model (HM)
During the CR interviews, we also asked each surgeon to identify which instruments to remain on the trays for each type of surgical procedure performed by the respective surgeon, a total of 83 different procedures, including cervical, thoracic, lumbar, and thoracolumbar procedures. We calculated a weighted average for the necessity of each instrument based on the frequency that each procedure was performed by each surgeon. Using the model, as described by Mhlaba et al. [39] and Toor et al., [38] we calculate a cost-based inflection point for each instrument. Instruments for which the weighted average of necessity on the tray exceeded the cost-based point of inflection were deemed globally necessary for all procedures and were retained on the tray. Conversely, instruments for which the cost-based point of inflection exceeded the weighted average of necessity on the tray were removed from the tray and peel-packed.
In brief, the cost-based point of inflection is the ratio of the cost of overage (Coverage) and the cost of underage (Cunderage), such that inflection point equals Coverage ÷ Cunderage. The cost of overage addresses the cost incurred by having the instrument in excess on the tray when it is not needed. It includes the cost of reprocessing the instrument, the cost associated with the contamination of the instrument, and the cost of depreciation of the instrument due to wear-and-tear. The cost of underage, on the other hand, is the cost associated with not having an instrument on the tray when it is needed. It comprises the cost of peel-packing the instrument, the cost of picking the peel packed instrument, and the cost of retrieving the instrument. The costs associated with the point of inflection model were calculated as described in Toor et al. 2021 [38]. To facilitate calculations, each instrument was estimated to cost $100, with a lifetime of 10 years, taking 1.3 min to reprocess, and costing $0.50 per minute of reprocessing.
Results
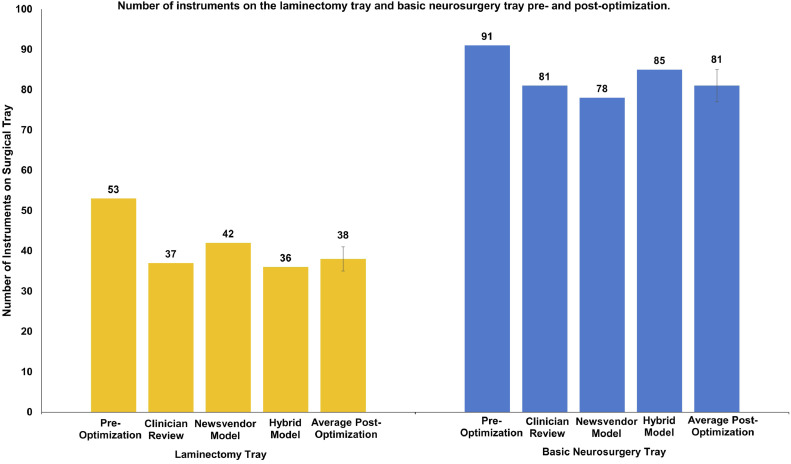
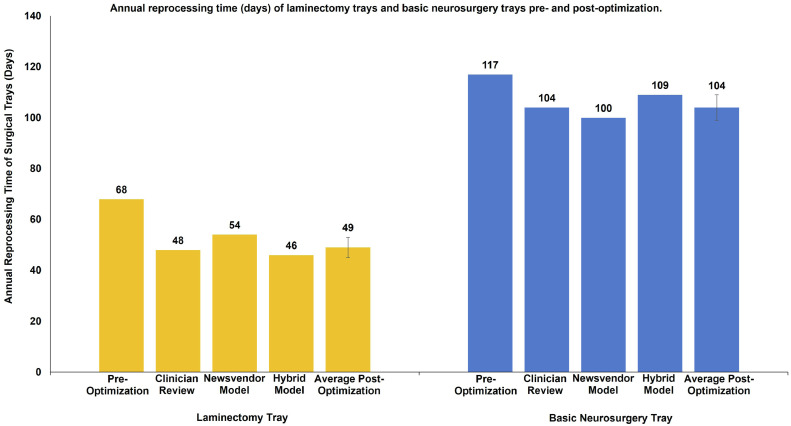
Table 1 summarizes the reduction of the number of surgical instruments on the LT and BNT using the clinician review approach (CR), the mathematical Newsvendor model (MP), and the hybrid cost-based point of inflection model (HM). Overall, as Fig. 1 illustrates, the three approaches yielded similar reductions in instrument numbers of 30%, 21%, and 32%, for the LT, and 11%, 14%, and 7%, for the BNT, using CR, the MP, and the HM respectively. This translates into a reduction of time spent reprocessing the instrument of 21, 14, and 22 days on the LT (Fig. 2), and 13, 17, and 8 days on the BNT (Fig. 2), using CR, MP, and HM, respectively.
Table 1.
Reduction of the number of surgical instruments on the laminectomy tray (LT) and basic neurosurgery tray (BNT) using the clinician review approach, the mathematical Newsvendor model, and the hybrid cost-based point of inflection model.
| Laminectomy tray | Basic neurosurgery tray | |
|---|---|---|
| N° instruments (% Size reduction) | N° instruments (% Size reduction) | |
| Preoptimization | 53 | 91 |
| Clinician Review | 37 (30.19%) | 81 (10.99%) |
| Newsvendor Model | 42 (20.75%) | 78 (14.29%) |
| Hybrid Model | 36 (32.08%) | 85 (6.59%) |
| Average% Size Reduction (± Standard Deviation) | 27.67 (± 6.068) | 10.62 (± 3.86) |
Fig. 1.
Number of instruments on the laminectomy tray and basic neurosurgery tray pre- and postoptimization.
Fig. 2.
Annual reprocessing time (days) of laminectomy trays and basic neurosurgery trays pre- and postoptimization.
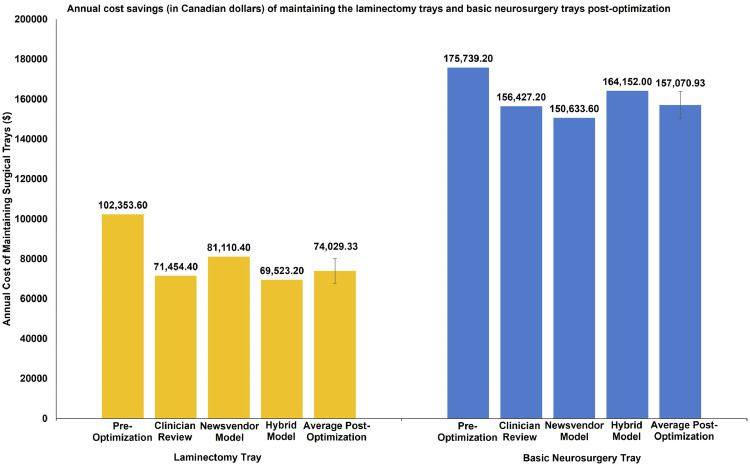
We calculated the cost savings resulting from a decreased tray size, due to savings in the cost of reprocessing the instruments, the cost of the time spent reprocessing the instrument, and depreciation of the value of the instrument due to wear-and-tear. The annual cost savings resulting from optimization of the LT are $30,899.20, $21,243.20, and $32,830.40 (Fig. 3), while those for the BNT are $19,312.00, $25,105.60, $11,587.20 (Fig. 3) using CR, MP, and the HM, respectively. Table 2 summarizes the cost savings following the optimization of the LT and BNT.
Fig. 3.
Annual cost savings (in Canadian dollars) of maintaining the laminectomy trays and basic neurosurgery trays postoptimization. The annual cost of maintaining laminectomy trays and basic neurosurgery trays comprises the cost of reprocessing the instruments, the cost of the time spent reprocessing the instruments, and the depreciation cost of each instrument.
Table 2.
Reprocessing time saved (days) and total annual cost savings resulting from the reduction of instruments on the laminectomy tray (LT) and basic neurosurgery tray (BNT) following surgical tray optimization using clinician review approach, the mathematical Newsvendor model, and the hybrid cost-based point of inflection model.
| Time saved in reprocessing tray (days) | Total annual cost savings ($)* | ||
|---|---|---|---|
| Laminectomy Tray | Clinician Review | 20.57 | 30,899.20 |
| Newsvendor Model | 14.14 | 21,243.20 | |
| Hybrid Model | 21.85 | 32,830.40 | |
| Average | 18.85 | 28,324.27 | |
| STDEV | 4.13 | 6207.94 | |
| Basic Neurosurgery Tray | Clinician Review | 12.86 | 19,312.00 |
| Newsvendor Model | 16.71 | 25,105.60 | |
| Hybrid Model | 7.713 | 11,587.20 | |
| Average | 12.43 | 18,668.27 | |
| STDEV | 4.51 | 6782.15 |
In Canadian dollars.
Discussion
Over-crowded surgical trays containing rarely-used surgical instruments are economically unsustainable and reduce operational efficiency [15,[21], [22], [23]. Clinician review and Mathematical programming are common approaches to reduce the number of instruments on surgical trays, which have demonstrated potential to reduce the weight of trays, the time to reprocess instruments, operation room set-up time, and associated costs [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32],[34], [35], [36],38]. Whereas the former is vulnerable to inaccurate clinician recall, the latter relies on cumbersome and impractical observational data collection. In this study, we took advantage of the practicality of the former approach and the robustness of the latter approach by developing a hybrid model (HM) based on inflection point cost analysis to reduce the number of instruments on the LT and the BNT used in over 5000 cases at a large academic trauma center. We compared the percent reduction in instruments, costs, and reprocessing time, from our hybrid model to a CR-only approach and an MP-only approach, specifically the Newsvendor model (MP).
In the CR approach, we interviewed surgeons who identified the surgical instruments to retain, in general, on the LT and BNT, such that only instruments that were deemed necessary by 50% of surgeons were retained. For the MP approach, a trained observer attended 25 randomly selected procedures and noted the use of the instruments from each tray. Observations were implemented in the Newsvendor model [38] to optimize both trays. Through our hybrid model, each surgeon identified the necessary instruments for each procedure type performed. We then calculated the weighted average of necessity of each instrument based on the frequency that each procedure was performed by each surgeon. Instruments for which the weighted average of necessity on the tray exceeded the cost-based point of inflection were deemed globally necessary for all procedures and were retained on the tray.
The three approaches, the CR, MP, and HM successfully decreased the number of instruments per tray (Table 1, Fig. 1). This resulted in a proportional reduction in annual tray reprocessing time amongst the three approaches (Fig. 2). We also considered savings in the cost of reprocessing the instruments, in the cost of the time spent reprocessing the instruments, and in depreciation of the value of the instrument due to wear-and-tear. Annual savings were again similar amongst the three optimization approaches, averaging $(28,324.27 ± 6207.94) and $(18,668.27 ± 6782.15) for LT and BNT, respectively (Table 2).
The CR approach allows for the expedient appraisal of each instruments’ usage. However, this approach offers a rather crude estimate of the instruments’ use as it does not consider variations in use based on procedure-type and the incidence of each procedure type. In addition, the CR approach is dichotomous as it can only classify instruments as “retain” or “remove”, without allowing for the adjustment of multiple counts of the same instrument within the tray. The MP approach, on the other hand, can solve for the optimal number of each instrument if there are multiple counts of the same instrument within the same tray. MP can, in theory, also account for variations in the use of each instrument based on procedure-type and the incidence of each procedure type [38]. However, MP relies on direct, cumbersome, observations of surgical procedures, and is thus limited to a small sample of observed procedures. The HM, though also reliant on a dichotomous “retain/remove” decision system, does not rely on direct observation of procedures; rather, it takes advantage of the surgeon's accounts of their use of each instrument for each procedure, whilst also considering the frequency of each procedure, to arrive at a “retain/remove” decision in a time-efficient manner. Therefore, the HM is endowed with the practicality of CR, yet has improved accuracy as it incorporates an attenuated version of MP. As such, we recommend the use of our hybrid model for the time-efficient optimization of trays.
Conclusions
While hospitals continue to examine perioperative services for potential inefficiencies, surgical inventory will be increasingly scrutinized. Despite MP being the most accurate methodology to do so, our results suggest that savings were similar across all three methods. CR and our HM are significantly less laborious and thus are viable alternatives.
Summary sentence
Despite mathematical programming being the most accurate way to analyze surgical inventory for potential inefficiencies, our results suggest that clinician review and a novel hybrid model are less laborious, demonstrate similar savings, and are thus practical alternatives.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
FDA device/drug status: Not applicable.
Author disclosures: SB: Nothing to disclose. JT: Nothing to disclose. AA: Nothing to disclose. JRL: Nothing to disclose. MRM: Nothing to disclose. JL: Nothing to disclose.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.xnsj.2023.100208.
Appendix. Supplementary materials
References
- 1.Young T., Brailsford S., Connell C., Davies R., Harper P., Klein J.H. Using industrial processes to improve patient care. BMJ. 2004;328:162–164. doi: 10.1136/BMJ.328.7432.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaissi A. Learning from other industries: lessons and challenges for health care organizations. Health Care Manag (Frederick) 2012;31:65–74. doi: 10.1097/HCM.0B013E318242D399. [DOI] [PubMed] [Google Scholar]
- 3.Daultani Y., Chaudhuri A., Kumar S. A decade of lean in healthcare: current state and future directions. Glob Bus Rev. 2015;16:1082–1099. doi: 10.1177/0972150915604520. [DOI] [Google Scholar]
- 4.Timmermans S., Epstein S. A world of standards but not a standard world: toward a sociology of standards and standardization. Annu Rev Sociol. 2010;36:69–89. doi: 10.1146/ANNUREV.SOC.012809.102629. [DOI] [Google Scholar]
- 5.De Regge M., Gemmel P., Meijboom B. How operations matters in healthcare standardization. Int J Oper Prod Manag. 2019;39:1144–1165. doi: 10.1108/IJOPM-03-2019-0227. [DOI] [Google Scholar]
- 6.Cendán J.C., Good M. Interdisciplinary work flow assessment and redesign decreases operating room turnover time and allows for additional caseload. Arch Surg. 2006;141:65–69. doi: 10.1001/ARCHSURG.141.1.65. [DOI] [PubMed] [Google Scholar]
- 7.Cima R.R., Brown M.J., Hebl J.R., Moore R., Rogers J.C., Kollengode A., et al. Use of lean and six sigma methodology to improve operating room efficiency in a high-volume tertiary-care academic medical center. J Am Coll Surg. 2011;213:83–92. doi: 10.1016/J.JAMCOLLSURG.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Leslie M., Hagood C., Royer A., Reece C.P., Maloney S. Using lean methods to improve OR turnover times. AORN J. 2006;84:849–855. doi: 10.1016/S0001-2092(06)63971-9. [DOI] [PubMed] [Google Scholar]
- 9.Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22:233–236. doi: 10.1016/j.jclinane.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Devakos T., Gordon M., Gurnham M., et al. 2021. 2021 Ministry of Health spending plan review, Toronto. [Google Scholar]
- 11.Marchildon G., DiMatteo L. The Facts; Ottawa: 2011. Health Care Cost Drivers. [Google Scholar]
- 12.Overdyk F.J., Harvey S.C., Fishman R.L., Shippey F. Successful strategies for improving operating room efficiency at academic institutions. Anesth Analg. 1998;86:896–906. doi: 10.1213/00000539-199804000-00039. [DOI] [PubMed] [Google Scholar]
- 13.Marjamaa R., Vakkuri A., Kirvela O. Operating room management: why, how and by whom? Acta Anaesthesiol Scand. 2008;52:596–600. doi: 10.1111/J.1399-6576.2008.01618.X. [DOI] [PubMed] [Google Scholar]
- 14.Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22:233–236. doi: 10.1016/J.JCLINANE.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Stockert E.W., Langerman A. Assessing the magnitude and costs of intraoperative inefficiencies attributable to surgical instrument trays. J Am Coll Surg. 2014;219:646–655. doi: 10.1016/J.JAMCOLLSURG.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Prat F., Spieler J.F., Paci S., et al. Reliability, cost-effectiveness, and safety of reuse of ancillary devices for ERCP. Gastrointest Endosc. 2004;60:246–252. doi: 10.1016/S0016-5107(04)01685-2. [DOI] [PubMed] [Google Scholar]
- 17.Demoulin L., Kesteloot K., Penninckx F. A cost comparison of disposable vs reusable instruments in laparoscopic cholecystectomy. Surg Endosc. 1996;10:520–525. doi: 10.1007/BF00188399. [DOI] [PubMed] [Google Scholar]
- 18.Adler S., Scherrer M., Rückauer K.D., Daschner F.D. Comparison of economic and environmental impacts between disposable and reusable instruments used for laparoscopic cholecystectomy. Surg Endosc Other Interv Tech. 2005;19:268–272. doi: 10.1007/S00464-003-9232-4. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadi E., Masel D.T., Metcalf A.Y., Schuller K. Inventory management of surgical supplies and sterile instruments in hospitals: a literature review. Heal Syst. 2019;8:151. doi: 10.1080/20476965.2018.1496875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.dos Santos B.M., Fogliatto F.S., Zani C.M., Peres F.A.P. Approaches to the rationalization of surgical instrument trays: scoping review and research agenda. BMC Health Serv Res. 2021;21:1–15. doi: 10.1186/S12913-021-06142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farrokhi F.R., Gunther M., Williams B., Blackmore C.C. Application of lean methodology for improved quality and efficiency in operating room instrument availability. J Healthc Qual. 2015;37:277–286. doi: 10.1111/JHQ.12053. [DOI] [PubMed] [Google Scholar]
- 22.Van Meter M.M., Adam R.A. Costs associated with instrument sterilization in gynecologic surgery. Am J Obstet Gynecol. 2016;215:652.e1–652.e5. doi: 10.1016/J.AJOG.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Lunardini D., Arington R., Canacari E.G., Gamboa K., Wagner K., McGuire K.J. Lean principles to optimize instrument utilization for spine surgery in an academic medical center: an opportunity to standardize, cut costs, and build a culture of improvement. Spine (Phila Pa 1976) 2014;39:1714–1717. doi: 10.1097/BRS.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 24.Capra R., Bini S.A., Bowden D.E., et al. Implementing a perioperative efficiency initiative for orthopedic surgery instrumentation at an academic center: a comparative before-and-after study. Med (United States) 2019;98:e14338. doi: 10.1097/MD.0000000000014338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koyle M., AlQarni N., Odeh R., et al. Reduction and standardization of surgical instruments in pediatric inguinal hernia repair - ScienceDirect. J Pediatr Urol. 2018;14:20–24. doi: 10.1016/j.jpurol.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 26.DeHaan A.M., Adams J.R., DeHart M.L., Huff T.W. Patient-specific versus conventional instrumentation for total knee arthroplasty: peri-operative and cost differences. J Arthroplasty. 2014;29:2065–2069. doi: 10.1016/J.ARTH.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 27.Hsu A., Gross C., Bhatia S., Levine B. Template-directed instrumentation in total knee arthroplasty: cost savings analysis. Orthopedics. 2012;35:e1596–e1600. doi: 10.3928/01477447-20121023-15. [DOI] [PubMed] [Google Scholar]
- 28.McLawhorn A.S., Carroll K.M., Blevins J.L., DeNegre S.T., Mayman D.J., Jerabek S.A. Template-directed instrumentation reduces cost and improves efficiency for total knee arthroplasty: an economic decision analysis and pilot study. J Arthroplasty. 2015;30:1699–1704. doi: 10.1016/J.ARTH.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 29.Siegel G.W., Patel N.N., Milshteyn M.A., Buzas D., Lombardo D.J., Morawa L.G. Cost analysis and surgical site infection rates in total knee arthroplasty comparing traditional vs. single-use instrumentation. J Arthroplasty. 2015;30:2271–2274. doi: 10.1016/J.ARTH.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 30.Kwon O.-.R., Kang K.-.T., Son J., Suh D-S, Heo D.B., Koh Y.-.G. Patient-specific instrumentation development in TKA: 1st and 2nd generation designs in comparison with conventional instrumentation. Arch Orthop Trauma Surg. 2017;137:111–118. doi: 10.1007/S00402-016-2618-2. [DOI] [PubMed] [Google Scholar]
- 31.Renson L., Poilvache P., Van den Wyngaert H. Improved alignment and operating room efficiency with patient-specific instrumentation for TKA. Knee. 2014;21:1216–1220. doi: 10.1016/J.KNEE.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Avansino J.R., Goldin A.B., Risley R., Waldhausen J.H.T., Sawin R.S. Standardization of operative equipment reduces cost. J Pediatr Surg. 2013;48:1843–1849. doi: 10.1016/J.JPEDSURG.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 33.Assmann C., Amarov B., Auspurg K., et al. Routledge; New York: 2015. Improving survey methods. [DOI] [Google Scholar]
- 34.Dobson G., Seidmann A., Tilson V., Froix A. Configuring surgical instrument trays to reduce costs. IIE Trans Healthc Syst Eng. 2015;5:225–237. doi: 10.1080/19488300.2015.1094759. [DOI] [Google Scholar]
- 35.Dollevoet T., van Essen J.T., Glorie K.M. Solution methods for the tray optimization problem. Eur J Oper Res. 2018;271:1070–1084. doi: 10.1016/J.EJOR.2018.05.051. [DOI] [Google Scholar]
- 36.Cardoen B., Beliën J., Vanhoucke M. On the design of custom packs: grouping of medical disposable items for surgeries. Int J Prod Res. 2015;53:7343–7359. doi: 10.1080/00207543.2015.1061221. [DOI] [Google Scholar]
- 37.Toor J., Du J.T., Koyle M., Abbas A., Shah A., Bassi G., et al. Inventory Optimization in the Perioperative Care Department Using Kotter's Change Model. Jt Comm J Qual Patient Saf. 2022;48:5–11. doi: 10.1016/J.JCJQ.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Toor J., Bhangu A., Wolfstadt J., et al. Optimizing the surgical instrument tray to immediately increase efficiency and lower costs in the operating room. Can J Surg. 2022;65:E275–E281. doi: 10.1503/cjs.022720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mhlaba J., Stockert E., Coronel M., Langerman A. Surgical instrumentation: the true cost of instrument trays and a potential strategy for optimization. J Hosp Adm. 2015;4:82–88. doi: 10.5430/jha.v4n6p82. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.