Highlights
-
•
The ongoing increment of diabetes mellitus (DM) could affect previous achievement on tuberculosis (TB) treatment progress in high-burden countries like Ethiopia.
-
•
In contrast with most previous studies, a very high prevalence of DM was observed among people diagnosed with drug susceptible TB.
-
•
Although the overall TB treatment success rate seems consistent with previous trends in Ethiopia, people diagnosed with TB and DM are at higher the risk for of poor TB treatment outcome was ten times higher among patients diagnosed DM.
-
•
That suggests the need for an enhanced bi-directional screening and integrated managements of TB and DM as part of the routine healthcare program.
Keywords: Tuberculosis, Diabetes mellitus, Treatment outcomes
Abstract
Background
The impact of diabetes mellitus on tuberculosis (TB) treatment outcomes has not been well investigated in most sub-Saharan countries including Ethiopia. The current study aimed to determine the association between diabetes mellitus and unsuccessful TB treatment outcomes among drug-susceptible TB patients treated at selected health facilities in Addis Ababa, Ethiopia.
Methods
This health facility-based prospective cohort study was conducted at six randomly selected public health centers in Addis Ababa, from August 2020 until November 2021. Clinically diagnosed adult pulmonary and extra pulmonary TB patients were recruited at the time of treatment initiation. A multivariable logistic regression analysis was used to estimate the association between diabetes and unsuccessful TB treatment outcomes.
Results
Among the total 267 enrolled participants, 9.7% of patients with TB were identified to have diabetes comorbidity. Of patients with diabetes and TB, 9 (34.6%) were newly diagnosed based on glucose test results. Despite an overall high TB treatment success rate (94.0%), more than one-fourth (26.9%) of patients with diabetes had a poor TB treatment outcome (26.9%), which was remarkably higher compared to patients without diabetes (3.7%). In multivariable regression, the adjusted odds of poor TB treatment outcome among those with diabetes was 14.8 (95% CI 3.5 – 62.7) times the odds of poor outcome patients without diabetes.
Conclusion
Diabetes was significantly associated with increased odds of poor TB treatment outcomes among patients in Addis Ababa, Ethiopia.
1. Introduction
According to the World Health Organization [1], diabetes mellitus (DM) is a key risk factor for TB disease and is associated with poor TB treatment outcomes. Chronic concomitant diseases, including diabetes, are associated with impaired host immunity that increases susceptibility to TB infection, TB disease, and related with poor treatment outcomes [2]. Patients with diabetes and uncontrolled hyperglycaemia appear to be at higher risk for TB than those with controlled blood glucose levels, which is an important determinant for the interaction effect [3], [4]. The most common clinical characteristics of TB, such as decrease in appetite, weight loss, and reduced physical activity may acerbate hyperglycaemia among patients with diabetes [5]. Although the dual burden and bidirectional relationship between TB-diabetes diseases is a well-recognized problem for global TB control, the area has not been well-studied in low- and middle-income countries in which TB is a major public health problem [6], [7]. The rapid emergence of non-communicable diseases (NCDs) [3], [8] and the increasing incidence of diabetes in TB high-burden countries may affect the global reduction in the number of TB deaths and negatively impact recent progress in TB diagnosis and treatment [1].
Adult cases of diabetes mellitus are estimated to increase by 69% from 2010 to 2030 in most low- and middle-income countries [9]. Despite rapidly rising diabetes rates and an expected doubling of burden in the next two decades, there is limited data regarding diabetes and TB from sub-Saharan countries like Ethiopia [3], [5]. Previously, a low prevalence of diabetes (less than 2%) was reported in Ethiopia [10]. However, a recent systematic review and meta‑analysis estimated a relatively higher pooled diabetes prevalence [6.5% (95% CI (5.8, 7.3)] [11]. Furthermore, prevalence estimates of undiagnosed diabetes (5.8%) and impaired fasting glucose (8.9%) suggest that the true diabetes disease burden may be much higher [12]. Although there is a lack of precise evidence, the average prevalence of diabetes among patients with TB in Ethiopia is assumed to be substantially higher than the general population [13], [14], [15].
While the impact of diabetes on TB treatment outcomes is not well investigated in sub-Saharan regions, it has been estimated that diabetes triples the risk of acquiring TB disease and increases the probability of adverse TB treatment outcomes such as failure, death, and recurrent TB [2], [8]. Existing data suggest that the relationship between diabetes and TB outcomes varies dramatically by region and setting [1], [3], [14], [16], [17]. In Ethiopia, a recent prospective cohort study reported diabetes to be associated with a 4-fold increased rate of death among patients with TB from the South-Eastern Amhara Region [14]. The current prospective study aimed to estimate the association between diabetes mellitus and unsuccessful TB treatment outcomes among drug-susceptible TB patients treated at selected health facilities in Addis Ababa, Ethiopia.
2. Methods
2.1. Study design and participants
A prospective cohort study was conducted in Addis Ababa, Ethiopia, from August 2020 until November 2021. Using two-stage sampling techniques, six public health centers were randomly selected, and then eligible participants were consecutively enrolled at the time of anti-TB treatment initiation. Diagnosis of TB disease was done according to routine healthcare practices using clinical workup including rapid and sensitive diagnostic tests such as Xpert MTB/RIF Ultra assay, sputum microscopy, and/or chest x-ray [18]. Clinically diagnosed adult pulmonary TB and extrapulmonary TB patients who were eligible for first line anti-TB treatment were included. Exclusion criteria included clinically confirmed MDR-TB cases, patients with age less than 18 years, and pregnant patients were excluded. All patients in the study received a standard six-month TB treatment regimen with a combination of four first line anti-TB drugs and were followed by trained health professionals working at the public health centers until the end of TB treatment.
2.2. Study measures and variables
The primary study exposure of interest was diabetes status. Previously known diabetes status was recoded based on self-reported history and clinical assessment. In addition, all participants were screened using either random blood glucose or oral glucose tolerance tests at the time of TB treatment initiation and at the end of each phase of the treatment period. Thus, newly diagnosed diabetes status was defined based on thresholds and cut-off points of blood glucose levels as per the guidelines developed by the International Union against Tuberculosis and Lung Disease [3], [8]. Patients with random blood glucose (RBG) or oral glucose tolerance (OGTT) of ≥ 200 mg/dl and/or fasting plasma (blood) glucose test (FBS/FPGT) of ≥ 126 were considered as diabetic while those with RBG levels ranging from 140 −199 or FBS of 110–125 were defined as pre-diabetic [8].
Other covariates included socio-demographic, behavioural and clinical history of participants and were collected using a structured questionnaire. HIV status of participants was recorded based on the routine provider-initiated counselling and testing. History of chronic non-communicable diseases (NCD) were self-reported including hypertension, high cholesterol, heart disease, stroke, kidney disease and hepatitis or chronic liver disease. Follow up measurements such as blood glucose measures and routine diagnostic test results used to evaluate TB treatment outcomes were recorded at the end of each phase of the treatment period.
The primary study outcome of interest was evaluated and classified according to national guidelines as successful or unfavourable TB treatment outcomes [18]. Briefly, patients with a successful TB outcome included 1) smear positive cases which were “cured”, i.e., becoming bacteriologically negative by the end of therapy or 2) smear negative or smear unknown cases diagnosed on clinical grounds which “complete” the standard course of therapy with clinical improvement. Unfavourable outcomes represented those patients who failed therapy, died, were lost to follow up, or who transferred out with unknown outcomes [18].
2.3. Statistical analyses
Participant characteristics associated with prevalent diabetes mellitus at TB treatment initiation were assessed with chi-square analysis. A binary logistic regression was used to determine the association between diabetes and poor TB treatment outcome. We used the lowest akaki information criteria (AIC) and maximum Likelihood Ratio-test value while performing stepwise backward elimination procedures. We also considered clinical relevance of variables associated with the exposure and outcome while developing the final multivariable model. The observed associations were reported as adjusted odds ratios (aOR) and 95% confidence intervals. Data entry and management was performed with Epi-data and analyzed with STATA (Version-14 StataCorp LP, USA) and R-package (Studio).
2.4. Ethical considerations
The study protocol was reviewed and approved by AHRI/ALERT Ethics Review Committee (#PO/26/19) and the Addis Ababa city administration public health research and emergency management directorates. Written informed consent was taken from all eligible participants before enrolment.
3. Results
3.1. Baseline characteristics of the study participants
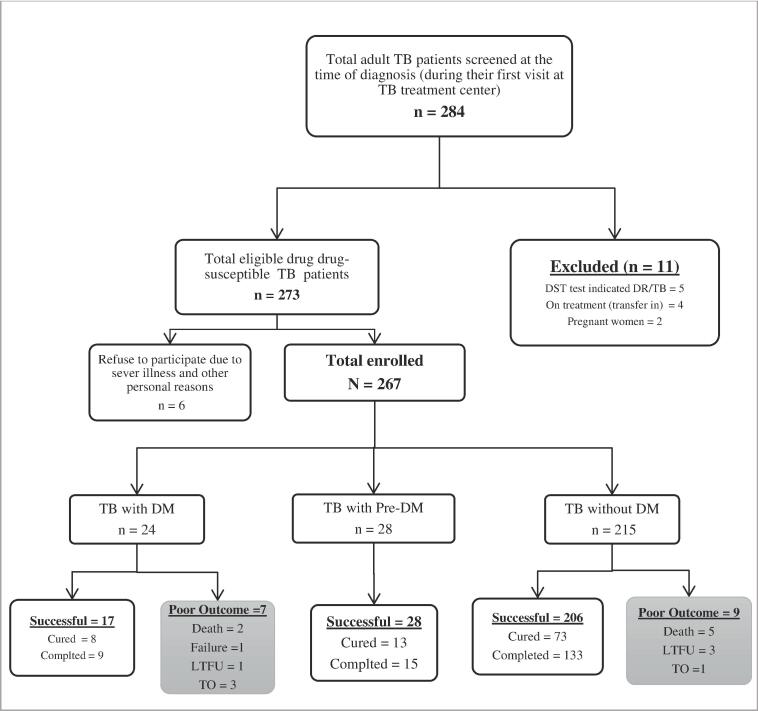
A total of 267 drug susceptible TB patients met the inclusion criteria and were enrolled at the time TB treatment initiation (Fig. 1). At the time of TB treatment initiation DM comorbidity was observed in 8.9% (24/267) of participants and 10.5% (28/267) had pre-diabetes. The majority (58.1%) of the participants were less than 35 years old, with a mean age of 34 years (standard deviation 13) and mean BMI of 20 Kg/m2 (standard deviation 3.8). Most participants were male (53.6%) and 66.3% had either primary or secondary school education.
Fig. 1.
Flow chart shows study progress and TB treatment outcome among drug-susceptible tuberculosis patients treated at selected health facilities in Addis Ababa; Ethiopia.
The prevalence of diabetes was higher among participants with age of above 45 years old (19.3%) compared to those 18–35 years (7.1%). Diabetes was also associated with history smoking (18.6% among smokers vs. 7.1% among never smokers) and alcohol consumption (16.9% among alcohol users vs. 5.4% among no alcohol users) (Table 1).
Table 1.
Baseline characteristics of participants with tuberculosis by diabetes status, Addis Ababa, Ethiopia 2020–2021.
| Characteristics of participants | Diabetes n = 24 (8.9 %) | Prediabetes n = 28 (10.5%) | No diabetes n = 215 (79.6%) | Total N = 267 | P-value (X2-test) |
|---|---|---|---|---|---|
| Age Groups | |||||
| 18–35 years | 11 (7.1) | 14 (9.0) | 130 (83.9) | 155 (58.1) | |
| 35–44 years | 2 (3.6) | 8 (14.6) | 45 (81.8) | 55 (20.6) | 0.02 |
| Above 45 years | 11(19.3) | 6 (10.5) | 40 (70.2) | 57 (21.4) | |
| BMI in Kg/m2 | |||||
| Median (IQR) | 20 (18,24) | 19 (17,21) | 19 (17,21) | 19 (17,21) | 0.09 |
| Gender | |||||
| Male | 18 (12.6) | 13 (9.1) | 112 (78.3) | 143 (53.6) | 0.07 |
| Female | 6 (4.8) | 15(12.1) | 103 (83.1) | 124 (46.4) | |
| Education | |||||
| <Grade 1 | 5 (9.3) | 9(16.7) | 40 (74.1) | 54 (20.2) | |
| Grade 1–12 | 15 (8.5) | 18(10.2) | 144 (81.4) | 177 (66.3) | 0.32 |
| >Grade 12 | 4 (11.1) | 1 (2.8) | 31 (86.1) | 36 (13.5) | |
| Occupation | |||||
| Non-Employed | 10 (9.1) | 15 (13.6) | 85 (77.3) | 110 (41.2) | 0.36 |
| Employed | 14(8.9) | 13 (8.3) | 130(82.8) | 157 (58.8) | |
| Marital status | |||||
| Single | 9 (7.6) | 11 (9.2) | 99(83.2) | 119 (44.6) | |
| Married | 13(10.6) | 13(10.6) | 97(78.9) | 123 (46.1) | 0.78 |
| Others | 2(8.0) | 4 (16.0) | 19(76.0) | 25 (9.4) | |
| TB disease category | |||||
| Smear negative PTB | 13 (10.9) | 12(10.1) | 94 (79.0) | 119 (44.6) | |
| Smear positive PTB | 8 (12.5) | 7 (10.9) | 49 (76.6) | 64 (24.0) | 0.33 |
| EPTB | 3 (3.6) | 9(10.7) | 72 (85.7) | 84 (31.5) | |
| HIV | |||||
| Negative | 23 (10.6) | 23 (10.6) | 171 (78.8) | 217 (81.3) | 0.15 |
| Positive | 1 (2.0) | 5 (10.0) | 44 (88.0) | 50 (18.7) | |
| Other NCDs | |||||
| No | 21 (8.5) | 25(10.2) | 200 (81.3) | 246 (92.1) | 0.53 |
| Yes | 3 (14.3) | 3(14.3) | 15 (71.4) | 21 (7.9) | |
| Smoking | |||||
| Never | 16 (7.1) | 24(10.7) | 184 (82.1) | 224 (83.9) | 0.05 |
| Ever | 8 (18.6) | 4 (9.3) | 31 (72.1) | 43 (16.1) | |
| Alcohol | |||||
| Never | 10 (5.4) | 23(12.5) | 151 (82.1) | 184 (68.9) | 0.01 |
| Ever | 14 (16.9) | 5 (6.0) | 64(77.1) | 83 (31.1) |
We used Kruskal rank test for continuous and Pearson chi-square test for categorical variable.
Other NCDs include high blood pressure, cholesterol, heart disease, renal disease and liver disease (Hepatitis).
3.2. Follow-up measurement and poor TB outcomes
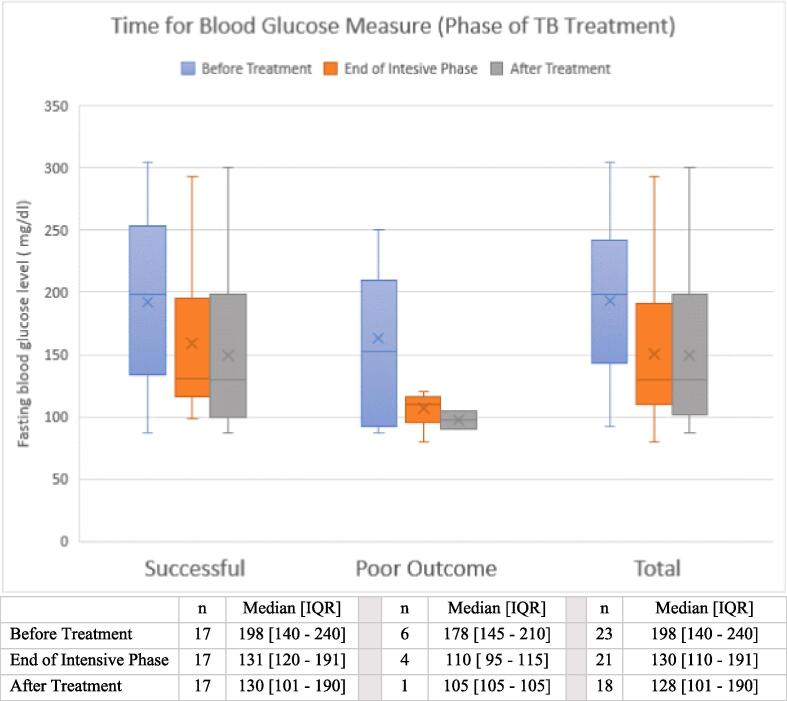
During TB treatment, two additional cases of diabetes were diagnosed. One of the incident diabetes diagnoses during treatment had pre-diabetes at the time of TB treatment initiation. While the follow up blood glucose measure for all other prediabetes cases found to be within the normal range; and hance they were diagnosed as TB patients without diabetes during treatment period. Among a subset (N = 23) of participants with repeated fasting glucose measures during treatment, the median fasting glucose level reduced from 198 mg/dl (IQR 140–240 mg/dl) at baseline to 128 mg/dl (IQR 101–190 mg/dl) at the end of TB treatment (Fig. 2).
Fig. 2.
Summary score of fasting blood glucose measured during TB treatment among patients with diabetes mellitus, which classified based on TB treatment outcome.
Overall, 251 of 267 (94.0%) participants had a successful TB treatment outcome, including 94/251 (35.2%) who were cured and 157/251 (58.8%) who completed TB treatment (Table 2 & Fig. 1). Among the 16 study participants with poor TB outcomes seven of them died, four were loss to follow up, four transferred out and one patient with treatment failure.
Table 2.
Diabetes mellitus and clinical characteristics associated with poor TB treatment outcomes among participants with drug susceptible TB from Ethiopia.
| Patients Characteristics | TB treatment outcome |
Crude estimates |
Adjusted estimatesa |
|
|---|---|---|---|---|
| Successful n = 251 (94.0%) | Poor n = 16 (6.0%) | OR (95% CI) | aOR (95% CI) | |
| DM status | ||||
| TB only | 232 (96.3) | 9 (3.7) | Ref | Ref |
| TBDMb | 19 (73.1) | 7 (26.9) | 9.5 (3.2 – 28.3) *** | 14.8 (3.5 – 62.7) *** |
| Age Group | ||||
| 18–35 years | 149 (96.1) | 6 (3.9) | Ref | Ref |
| 35–44 years | 52 (94.5) | 3 (5.5) | 1.4 (0.6 – 5.9) | 1.0 (0.2 – 5.7) |
| Above 45 years | 50 (87.7) | 7 (12.3) | 3.5 (1.1 – 10.8)* | 2.0 (0.5 – 8.3) |
| BMI (n = 250) | ||||
| Less than 18.5 | 115 (95.8) | 5 (4.2) | 0.6 (0.2 – 2.0) | 1.0 (0.2 – 4.2) |
| 18.5–24.9 | 116 (93.5) | 8 (6.5) | Ref | Ref |
| Above 25 | 19 (86.4) | 3 (13.6) | 2.3 (0.6 – 9.4) | 1.8 (0.3 – 10.6) |
| Gender | ||||
| Male | 136 (95.1) | 7 (4.9) | Ref | Ref |
| Female | 115 (92.7) | 9 (7.3) | 1.5 (0.5 – 4.2) | 2.0 (0.4 – 10.3) |
| TB disease category | ||||
| Smear negative PTB | 117 (98.3) | 2 (1.7) | Ref | Ref |
| Smear positive PTB | 56 (87.5) | 8 (12.5) | 8.4 (1.7 – 40.6) ** | 16.4 (2.3 –119.3)** |
| EPTB | 78 (92.9) | 6 (7.1) | 4.5 (0.9 – 22.9) | 9.7 (1.3 – 73.1) * |
| HIV | ||||
| Negative | 205 (94.5) | 12 (5.5) | Ref | Ref |
| Positive | 48 (92.0) | 4 (8.0) | 1.5 (0.5 – 4.8) | 3.0 (0.7 – 13.4) |
| Other NCDs | ||||
| No | 234 (95.1) | 12 (4.9) | Ref | Ref |
| Yes | 17 (81.0) | 4 (19.0) | 4.6 (1.3 – 15.8) * | 5.3 (1.0 – 28.0) * |
| Smoking | ||||
| Never | 210 (93.8) | 14 (6.2) | Ref | Ref |
| Ever | 41 (95.3) | 2 (4.7) | 0.7 (0.2 – 3.3) | 0.3 (0.03 – 2.5) |
| Alcohol | ||||
| Never | 174 (94.6) | 10 (5.4) | Ref | Ref |
| Ever | 77 (92.8) | 6 (7.2) | 1.4 (0.5 – 3.9) | 3.0 (0.5 –18.6) |
a Adjusted logistic regression model included all variables listed in rows in Table 2.
bParticipants with diabetes at baseline (n = 24) or diagnosed during TB treatment (n = 2). TB only included those with prediabetes at baseline and those with no diabetes.
Star (*) referred level of statistical significance (P-value); *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
Other NCDs include high blood pressure– cholesterol– heart disease– renal disease and liver disease (Hepatitis).
TBDM referred for patients diagnosed for both Tuberculosis and diabetes.
TB disease category was based on basline sputum smear result performed before TB treatment initiation.
3.3. Relationship between diabetes and poor TB treatment outcome
Among TB patients with diabetes (including the two cases diagnosed during treatment), 26.9% (7/26) had poor TB treatment outcomes. From the total cohorts with poor treatment outcome, TBDM comorbidity accounted for two of the seven (2/7) deaths, three of the four (3/4) transferred out, one of the four (1/4) lost to follow up and the only one failed treatment cases (Fig. 1). In unadjusted analyses, the odds of poor TB outcome were 9.5 (95 %CI 3.2–28.3) times the odds of poor outcome among participants without diabetes (Table 2).
The odds of poor treatment outcome remained significantly higher among TB patients diagnosed with diabetes mellites (Table 2) after multivariable adjustment. The adjusted odds of poor TB outcome among those with diabetes was 14.8 (95 %CI 3.5–62.7) times the odds of poor TB outcome observed among those without diabetes. Other factors associated with poor TB outcome in multivariable analysis included smear positive pulmonary TB (adjusted odds ratio [aOR] = 16.4; 95 %CI 2.3 –119.3), extrapulmonary TB (aOR = 9.7; 95 %CI 1.3 – 73.1), and history of other NCDs (aOR = 5.3; 95 %CI 1.0 – 28.0).
4. Discussion
The dual burden of tuberculosis and diabetes gained substantial attention in the past decade as diabetes prevalence increased dramatically in countries already afflicted with a high burden of tuberculosis [4]. The current study primarily aimed to estimate the relationship between diabetes mellitus and with poor TB treatment outcomes among patients with TB from Ethiopia. The study’s main finding indicated that diabetes was associated with a significant increased odds of poor TB outcome. More than one-quarter of patients with diabetes had a poor TB treatment outcome compared to less than one in twenty among patients without diabetes.
Diabetes was common among patients with TB in our cohort, with nearly 10% diabetes prevalence at the time of TB treatment initiation. Our findings are consistent with one of the most recent studies conducted at selected health facilities in Addis Ababa [19] and nearly comparable with the pooled prevalence (12.8%), estimated from previous studies which reported comorbidity of DM among TB patients in Ethiopia [20]. Our results are also comparable with the pooled estimate of diabetes prevalence among patients with TB (9.0%) generated from published articles reported in Sub-Saharan Africa ranging from 6.0 to 12.0%; and subgroup analysis indicated that Ethiopia (10%) was the third countries with highest prevalence next to Nigeria (15%) and Tanzania (11%) [21]. However, the estimated prevalence of diabetes in patients with TB from our study was substantially lower than findings reported in several European and Asian countries where in some settings ∼ 50% diabetes prevalence was observed among patients with TB [5], [7], [22].
Diabetes mellitus is an established risk factor for poor TB treatment outcomes [1], [23]. Although there is remarkable heterogeneity between studies, a comprehensive global systematic review and meta-analysis [23] estimated approximately two times increased average risk of death (aOR = 1.88 (1.59 – 2.21) and treatment failure (aOR = 2.19 (1.45 – 3.31) among patients with TB and diabetes. Our study is one of the few to confirm that the negative impact of diabetes on TB outcomes is also present in Ethiopia. Even after adjusting for other key patient characteristics, the odds of unsuccessful treatment outcome among patients with diabetes in our study was more than tenfold higher compared to patients without diabetes. The observed risk difference in our study further confirms the first prospective cohort study conducted in Ethiopia [14].which found an increased death rate related to TB-diabetes comorbidity among newly diagnosed adult TB patients treated with six month standard treatment at selected health facilities in South-Eastern part of Amhara; with hazard ratio of 4 compared to TB only patients. It might be due to the study period, sociodemographic and geographical differences, the relative risk of poor TB treatment outcome estimated from the first prospective cohort was still lower than out estimate.
Although there is a scarcity of evidence related to diabetes and TB treatment outcomes in Ethiopia and other African countries [23], our data have important implications for national TB control efforts in Ethiopia. Limited evidence from Ethiopia and nearby regions suggests the adverse effect of diabetes on TB outcomes may be greater than in Asia or other settings where the TB-diabetes relationship has been evaluated [20], [23]. For example, two studies—one from Nigeria [24] and one from Tanzanian [25] did not report a significant increased risk of unfavourable outcomes in patients with TB and diabetes. Another study from Tanzania, reported that patients with TB and hyperglycaemia had three times (aOR, 3.3; 95% CI, 1.2–9.3) higher odds of poor treatment outcome (failure or death) than patients without diabetes [26]. In another study from Tanzania, diabetes was associated with a fivefold increased risk of mortality (RR = 5.0, 95% CI 2.4–11.0) during the intensive phase of TB treatment [27]. A cross-sectional study from Somalia reported that the odds of poor TB outcomes among patients diagnosed with diabetes was eight-times higher that TB patients without diabetes (OR: 8.02 (1.79–35.86) [28]. Similarly, a case–control study from Egypt reported more than a nine-fold increased risk of treatment failure (RR = 9.32 (2.7–31.69) among patients with TB and diabetes compared to those without diabetes [29]. However, as mentioned earlier, the above two studies used different approaches and research methodologies; and the estimates may not truly reflect the relationship between DM and TB treatment outcome. Overall, the magnitude of the association between diabetes and poor TB treatment outcome reported from this Ethiopian study is greater than estimates from several studies in Africa and substantially greater than the pooled estimate from larger meta-analyses.
The current study also reported that patients with TB and a known history of other chronic NCDs are at increased risk of poor TB treatment outcomes. Despite the need for further investigation, key NCDs that include chronic obstructive pulmonary disease, cardiovascular disease, and liver disease likely interfere with patients’ response to anti-tuberculosis treatment [1], [30]. In contrast to other studies, the current study did not observe significant differences in TB outcomes by HIV status [31], [32], [33], [34], [35], [36]. This might be due to methodological and participant selection differences; wherein previous reports did not adjust for other factors such as diabetes and relied on retrospective research designs. Recent improvements in Ethiopian healthcare services for patients with TB-co-infection might also explain the lack of effect of HIV in this study.
5. Limitations
This study was subject to limitations. First, the current study was confined by resources and the COVID-19 pandemic, and recruitment was restricted to specific geographical areas and population groups. Recruitment occurred in selected health facilities located only in the capital city and we did not include patients with MDR TB. Additional work is needed to expand enrolment to a representative sample of patients with TB from across Ethiopia and with diverse clinical presentations of TB. Nonetheless, to our knowledge, this is only the second prospective cohort study which investigated the impact of diabetes on TB treatment outcomes in Ethiopia. Thus, the findings are likely considerably stronger as compared to self-reported diabetes or retrospective analysis of medical records which may be prone to substantial misclassification of exposure and outcome variables. Second, the relatively short follow up period and lack of knowledge regarding the exact time of diabetes onset likely limited the study in terms of exposure ascertainment and outcome evaluation. Hence, the results should be interpreted in the light of these mentioned limitations and other sources of bias such as misclassification of diabetes and glycemic status.
6. Conclusion
In this prospective cohort study of patients with TB from Ethiopia, we observed a high overall treatment success rate. However, patients with diabetes comorbidity were at a increased risk of poor treatment outcomes, with more than one-quarter experiencing unsuccessful TB outcomes. The current study further supports existing evidence of an adverse effect of diabetes on TB treatment outcomes, and extends this observation to the understudied region of Ethiopia. Additional prospective longitudinal studies that incorporate more diverse populations are necessary to better understand how TB treatment can be modified to reduce risk of poor outcomes among individuals with diabetes.
Funding
This research project was supported by an NIH Fogarty International Center Global Infectious Diseases grant (D43TW009127) through the Ethiopia-Emory Tuberculosis-Research Training Program (EETB-RTP). The EETB-RTP represents collaboration between Emory University, Addis Ababa University, the Armauer Hansen Research Institute (AHRI), and the Ethiopian Public Health Institute (EPHI).
CRediT authorship contribution statement
Hawult T. Adane: Conceptualization, Methodology, Project administration, Investigation, Data curation, Formal analysis, Validation, Visualization, Writing – original draft. Rawleigh C. Howe: Data curation, Methodology, Supervision, Validation, Visualization, Writing – review & editing. Liya Wassie: Validation, Visualization, Writing – review & editing. Matthew J. Magee: Data curation, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank all study participants, collaborating health facilities and research nurses, who assisted in recruiting and enrolling study participants. We also would like to thank the EETB-RTP team and the AHRI staff in facilitating the conduct of this study.
Ethical considerations
The study protocol was reviewed and approved by AHRI/ALERT Ethics Review Committee and the Addis Ababa city administration public health research and emergency management directorates. Written informed consent was taken from all eligible participants before enrolment.
References
- 1.WHO, Global tuberculosis report 2021. Geneva: World Health Organization; 2021. Licence: CC!BY-NC-SA!3.0!IGO. 2021.
- 2.Dunachie S., Chamnan P. The double burden of diabetes and global infection in low and middle-income countries. Trans R Soc Trop Med Hyg. 2019;113(2):56–64. doi: 10.1093/trstmh/try124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harries A.D., Satyanarayana S., Kumar A.M., Nagaraja S.B., Isaakidis P., Malhotra S., et al. Epidemiology and interaction of diabetes mellitus and tuberculosis and challenges for care: a review. Public Health Action. 2013;3(Suppl 1):S3–S9. doi: 10.5588/pha.13.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez N., Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44(3):617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riza A.L., Pearson F., Ugarte-Gil C., Alisjahbana B., van de Vijver S., Panduru N.M., et al. Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol. 2014;2(9):740–753. doi: 10.1016/S2213-8587(14)70110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon C.Y., Murray M.B. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5(7):e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sane Schepisi M., Navarra A., Altet Gomez M.N., Dudnyk A., Dyrhol-Riise A.M., Esteban J., et al. Burden and Characteristics of the Comorbidity Tuberculosis-Diabetes in Europe: TBnet Prevalence Survey and Case-Control Study. Open Forum Infect Dis. 2019;6(1):p. ofy337. doi: 10.1093/ofid/ofy337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harries A.D., Kumar A.M., Satyanarayana S., Lin Y., Zachariah R., Lonnroth K., et al. Addressing diabetes mellitus as part of the strategy for ending TB. Trans R Soc Trop Med Hyg. 2016;110(3):173–179. doi: 10.1093/trstmh/trv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Misganaw A., Mariam D.H., Ali A., Araya T. Epidemiology of major non-communicable diseases in Ethiopia: a systematic review. J Health Popul Nutr. 2014;32(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 11.Zeru M.A., Tesfa E., Mitiku A.A., Seyoum A., Bokoro T.A. Prevalence and risk factors of type-2 diabetes mellitus in Ethiopia: systematic review and meta-analysis. Sci Rep. 2021;11(1):21733. doi: 10.1038/s41598-021-01256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yitbarek G.Y., Ayehu G.W., Asnakew S., Chanie E.S., Bayih W.A., Feleke D.G., et al. Undiagnosed diabetes mellitus and associated factors among adults in Ethiopia: a systematic review and meta-analysis. Sci Rep. 2021;11(1):24231. doi: 10.1038/s41598-021-03669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abera A., Ameya G. Pulmonary Tuberculosis and Associated Factors Among Diabetic Patients Attending Hawassa Adare Hospital, Southern Ethiopia. Open Microbiol J. 2018;12:333–342. doi: 10.2174/1874285801812010333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Workneh M.H., Bjune G.A., Yimer S.A. Diabetes mellitus is associated with increased mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients in South-Eastern Amahra Region, Ethiopia. Infect Dis Poverty. 2016;5:22. doi: 10.1186/s40249-016-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bishu K.G., Jenkins C., Yebyo H.G., Atsbha M., Wubayehu T., Gebregziabher M. Diabetes in Ethiopia: A systematic review of prevalence, risk factors, complications, and cost. J Obesity Medicine. 2019 [Google Scholar]
- 16.Alene K.A., Viney K., Gray D.J., McBryde E.S., Wagnew M., Clements A.C.A. Mapping tuberculosis treatment outcomes in Ethiopia. BMC Infect Dis. 2019;19(1):474. doi: 10.1186/s12879-019-4099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eshetie S., Gizachew M., Alebel A., van Soolingen D. Tuberculosis treatment outcomes in Ethiopia from 2003 to 2016, and impact of HIV co-infection and prior drug exposure: A systematic review and meta-analysis. PLoS One. 2018;13(3):e0194675. doi: 10.1371/journal.pone.0194675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fmoh . Sixth Edition. National Guidline; 2018. Guidelines for management of TB, DR-TB and leprosy in Ethiopia. [Google Scholar]
- 19.Jerene D., Muleta C., Ahmed A., Tarekegn G., Haile T., Bedru A., et al. High rates of undiagnosed diabetes mellitus among patients with active tuberculosis in Addis Ababa, Ethiopia. J ClinTuberc Other Mycobact Dis. 2022;27 doi: 10.1016/j.jctube.2022.100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alemu A., Bitew Z.W., Diriba G., Gumi B. Co-occurrence of tuberculosis and diabetes mellitus, and associated risk factors, in Ethiopia: a systematic review and meta-analysis. IJID Reg. 2021;1:82–91. doi: 10.1016/j.ijregi.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alebel A., Wondemagegn A.T., Tesema C., Kibret G.D., Wagnew F., Petrucka P., et al. Prevalence of diabetes mellitus among tuberculosis patients in Sub-Saharan Africa: a systematic review and meta-analysis of observational studies. BMC Infect Dis. 2019;19(1):254. doi: 10.1186/s12879-019-3892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gautam S., Shrestha N., Mahato S., Nguyen T.P.A., Mishra S.R., Berg-Beckhoff G. Diabetes among tuberculosis patients and its impact on tuberculosis treatment in South Asia: a systematic review and meta-analysis. Sci Rep. 2021;11(1):2113. doi: 10.1038/s41598-021-81057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huangfu P., Ugarte-Gil C., Golub J., Pearson F., Critchley J. The effects of diabetes on tuberculosis treatment outcomes: an updated systematic review and meta-analysis. Int J Tuberc Lung Dis. 2019;23(7):783–796. doi: 10.5588/ijtld.18.0433. [DOI] [PubMed] [Google Scholar]
- 24.Ayeni F.A., Oyetunde O.O., Aina B.A. The effect of collaborative care on treatment outcomes of newly diagnosed tuberculosis patients with Type-2 diabetes mellitus and adverse drug reaction presentations: A prospective study. Int J Mycobacteriol. 2021;10(3):285–292. doi: 10.4103/ijmy.ijmy_124_21. [DOI] [PubMed] [Google Scholar]
- 25.Nagu T., Ray R., Munseri P., Moshiro C., Shayo G., Kazema R., et al. Tuberculosis among the elderly in Tanzania: disease presentation and initial response to treatment. Int J Tuberc Lung Dis. 2017;21(12):1251–1257. doi: 10.5588/ijtld.17.0161. [DOI] [PubMed] [Google Scholar]
- 26.Boillat-Blanco N., Ramaiya K.L., Mganga M., Minja L.T., Bovet P., Schindler C., et al. Transient Hyperglycemia in Patients With Tuberculosis in Tanzania: Implications for Diabetes Screening Algorithms. J Infect Dis. 2016;213(7):1163–1172. doi: 10.1093/infdis/jiv568. [DOI] [PubMed] [Google Scholar]
- 27.Faurholt-Jepsen D., Range N., PrayGod G., Jeremiah K., Faurholt-Jepsen M., Aabye M.G., et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health. 2013;18(7):822–829. doi: 10.1111/tmi.12120. [DOI] [PubMed] [Google Scholar]
- 28.Kassim S.A., Cote A., Kassim S.M., Abbas M., Baig M., Ahmed A.M., et al. Factors influencing treatment outcomes of tuberculosis patients attending health facilities in Galkayo Puntland, Somalia. J Public Health (Oxf) 2021;43(4):887–895. doi: 10.1093/pubmed/fdaa146. [DOI] [PubMed] [Google Scholar]
- 29.Morsy A.M., Zaher H.H., Hassan M.H., Shouman A. Predictors of treatment failure among tuberculosis patients under DOTS strategy in Egypt. East Mediterr Health J. 2003;9(4):689–701. [PubMed] [Google Scholar]
- 30.Magee M.J., Salindri A.D., Gujral U.P., Auld S.C., Bao J., Haw J.S., et al. Convergence of non-communicable diseases and tuberculosis: a two-way street? Int J Tuberc Lung Dis. 2018;22(11):1258–1268. doi: 10.5588/ijtld.18.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tesema T., Seyoum D., Ejeta E., Tsegaye R. Determinants of tuberculosis treatment outcome under directly observed treatment short courses in Adama City, Ethiopia. PLoS One. 2020;15(4):e0232468. doi: 10.1371/journal.pone.0232468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Getie A., Alemnew B. Tuberculosis Treatment Outcomes and Associated Factors Among Patients Treated at Woldia General Hospital in Northeast Ethiopia: An Institution-Based Cross-Sectional Study. Infect Drug Resist. 2020;13:3423–3429. doi: 10.2147/IDR.S275568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hailemeskel S., Mohammed O.Y., Ahmed A.M. Retrospective assessment of the status and determinants of tuberculosis treatment outcome among patients treated in government hospitals in North Shoa Administrative Zone, Amhara Regional State, Ethiopia. Res Rep Trop Med. 2017;8:65–71. doi: 10.2147/RRTM.S129337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teshome Kefale A., Anagaw Y.K. Outcome of tuberculosis treatment and its predictors among HIV infected patients in southwest Ethiopia. Int J Gen Med. 2017;10:161–169. doi: 10.2147/IJGM.S135305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad S.R., Yaacob N.A., Jaeb M.Z., Hussin Z., Wan Mohammad W.M.Z. Effect of Diabetes Mellitus on Tuberculosis Treatment Outcomes among Tuberculosis Patients in Kelantan, Malaysia. Iran J Public Health. 2020;49(8):1485–1493. doi: 10.18502/ijph.v49i8.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali S.A., Mavundla T.R., Fantu R., Awoke T. Outcomes of TB treatment in HIV co-infected TB patients in Ethiopia: a cross-sectional analytic study. BMC Infect Dis. 2016;16(1):640. doi: 10.1186/s12879-016-1967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]