Abstract
This study was designed to depict prevalence and antimicrobial resistance characteristics of Proteus mirabilis (P. mirabilis) strains in 4 chicken farms and to probe the transfer mechanism of resistance genes. A total of 187 P. mirabilis isolates were isolated from 4 chicken farms. The susceptibility testing of these isolates to 14 antimicrobials showed that the multidrug resistance (MDR) rate was as high as 100%. The β-lactamase resistance genes blaOXA-1, blaCTX-M-1G, blaCTX-M-9G and colistin resistance gene mcr-1 were highly carried in the P. mirabilis isolates. An MDR strain W47 was selected for whole genome sequencing (WGS) and conjugation experiment. The results showed that W47 carried 23 resistance genes and 64 virulence genes, and an SXT/R391 integrated conjugative elements (ICEs) named ICEPmiChn5 carrying 17 genes was identified in chromosome. ICEPmiChn5 was able to be excised from the chromosome of W47 forming a circular intermediate, but repeated conjugation experiments were unsuccessful. Among 187 P. mirabilis isolates, 144 (77.01%, 144/187) isolates carried ICEPmiChn5-like ICEs, suggesting that ICEs may be the major vector for the transmission of resistance genes among MDR chicken P. mirabilis strains in this study. The findings were conducive to insight into the resistance mechanism of chicken P. mirabilis strains and provide a theoretical basis for the use of antibiotics for the treatment of MDR P. mirabilis infections in veterinary clinic.
Key words: chicken, P. mirabilis, multidrug resistance, transmission mechanism, ICEPmiChn5
INTRODUCTION
Proteus mirabilis (P. mirabilis) belonging to the family of Enterobacteriaceae without spores, pili, or flagella has the ability to migrate and grow, and are widespread in the intestines of humans and animals and in the environment soil and water (O'Hara et al., 2000). As a common clinical zoonotic pathogen in the intestinal tract of humans and animals, P. mirabilis can cause gastroenteritis in the host and is associated with extraintestinal infections such as tympanitis, meningitis, pneumococcal urethritis (Drzewiecka, 2016; Gong et al., 2019). In recent years, food poisoning cases associated with P. mirabilis have been frequently reported in worldwide. In Datong from 2016 to 2017 (Shanxi Province, China), the proportion of food poisoning incidents related to P. mirabilis was 3.61% (Gong et al., 2019). Moreover, P. mirabilis isolated from broiler chickens and human feces were highly homologous, suggesting that poultry products might be one of the sources of human infections (Girlich et al., 2020; Yu et al., 2021). P. mirabilis is naturally resistant to several antibiotics, including colistin, nitrofuran, tigecycline, and tetracycline (Lu et al., 2021). Worryingly, the irrational use of clinical antibacterial agents has led to the emergence and spread of multidrug resistance (MDR) P. mirabilis, which have been increasingly reported (Girlich et al., 2020). The MDR rate of P. mirabilis reported in Northeast China was 76.7% (Sun et al., 2020), in Brazil was 78.13% (Sanches et al., 2019), and in Shandong Province, China was 100% (Li et al., 2022). The widespread spread of MDR P. mirabilis might pose a threat to the clinical treatment of infections caused by P. mirabilis in humans and animals.
Integrative and conjugative elements (ICEs), played an important role in the dissemination and accumulation of genes relevant to antibiotic and heavy metal resistance, have been detected in P. mirabilis strains from humans and animals (Harada et al., 2010; Mata et al., 2011). ICEs, mobile chromosomal genetic elements, cannot replicate autonomously and must be integrated into the host chromosome to function. In the ICE berg database (https://db-mml.sjtu.edu.cn/ICEbreg), SXT/R391 ICEs were the largest family of the ICEs, and widely present in Enterobacteriaceae bacteria. The basic structure of ICEs is conserved with 52 core genes involved in integration/excision, conjugation, and regulation of ICEs (Bie et al., 2017). Five hot spots (HS1–HS5) and 5 variable regions (VRI to VRV) are insertion points of new DNA fragments related to the acquisition of resistance genes (Rodríguez-Blanco et al., 2012; Lei et al., 2018). Int, a tyrosine recombinase with a full length of 1,242 bp, is responsible for the integration of ICE into the 5′ end of prfc gene in host chromosome. setC/D gene, located at the 3′ end of SXT/R391 ICEs, is a transcriptional activator of ICEs and can activate the conjugated function of ICEs. Therefore, the existence of ICEs was often detected by int and setC/D genes (Harada et al., 2010; Bioteau et al., 2018). SXT/R391 ICEs have been identified in P. mirabilis isolates from human, gull, chicken, and swine (Aberkane et al., 2016; Siebor et al., 2018; Lu et al., 2021). Many SXT/R391 ICEs carrying drug resistance genes significantly associated with the MDR phenotype complicated the treatment of infections caused by P. mirabilis with MDR ICEs, which has brought about the widespread attention. However, the report regarding the epidemiological survey of SXT/R391 ICEs in chicken P. mirabilis is rare to date.
Here, the drug resistance of 187 strains of P. mirabilis isolated from chicken farms in Henan and Jiangxi Provinces was analyzed. Whole genome sequencing (WGS) analysis of a typical multidrug-resistant P. mirabilis W47 was performed to insight into the connection between genotype and phenotypes. Characteristics of an SXT/R391 ICE named ICEPmiChn5 in chromosome of W47 were analyzed to understand the transmission mechanism of resistance genes in chicken P. mirabilis isolates. To determine the existence of SXT/R391 ICEs in P. mirabilis isolated from 4 chicken farms, the int and setC/D genes were detected. This study is the first investigation on prevalence of SXT/R391 ICEs in P. mirabilis of chicken origin.
MATERIALS AND METHODS
Sample Collection and Identification of P. Mirabilis
Autoclaved cotton swabs and 10 mL centrifuge tubes were used to collect chicken feces samples. In this study, a total of 473 fecal samples were collected from 4 chicken farms in Henan and Jiangxi Province between August and November 2020. A total of 298 samples were collected from Kaifeng City, 62 from Nanyang City, 17 from Zhumadian City and 96 from Nankang City of Jiangxi Province. A total of 187 suspected P. mirabilis strains were isolated and purified on SS agar plates incubating at 37°C for 12 to 16 h. Then suspected P. mirabilis isolates were confirmed using the VITEK-2 Compact (Biomérieux, Marcy l'Etoile, France). Briefly, pure colonies were selected from 18 to 24 h cultures, and suspended with physiological saline. Insert the bacterial identification card into the bacterial suspension and put it into the filling bin for bacterial identification.
Antimicrobial Susceptibility Testing
The agar dilution method was used to detect the minimal inhibitory concentrations (MICs) of 14 antimicrobial agents for P. mirabilis strains. The 14 tested antimicrobials contained ampicillin (AMP), cefotaxime (CTX), ceftazidime (CAZ), cefquinome (CEQ), meropenem (MEM), tigecycline (TIG), tetracyclines (TET), doxycycline (DOX), amikacin (AN), streptomycin (STR), ciprofloxacin (CIP), fosfomycin (FOS), colistin (COL), and florfenicol (FLR). The results were interpreted based on the Clinical and Laboratory Standards Institute (CLSI, 2022). Breakpoints of tigecycline (>2 mg/L) and florfenicol (>16 mg/L) were interpreted according to EUCAST (http://mic.eucast.org/Eucast2/). E. coli strain ATCC 25922 was used as the quality control. MDR was defined as acquired nonsusceptibility to at least one agent in 3 or more antimicrobial drug classes (Cerceo et al., 2016).
Detection of Drug Resistance Genes
DNA templates were prepared by the boiling method of DNA extraction, and resistance genes including the β-lactamase resistance genes blaCTX-M-1G, blaCTX-M-9G, and blaOXA-1, carbapenem resistance genes blaKPC and blaNDM, colistin resistance genes mcr-1 and mcr-9, tigecycline resistance genes tet(X1), tet(X3), and tet(X4), and fosfomycin resistance gene fosA3 were screened by PCR (Table S1). The PCR reactions were performed using Takara Taq DNA polymerase under the following conditions: denaturation at 95°C for 5 min; followed by 30 cycles at 95°C for 30 s, annealing for 30 s and 72°C for 45 s; and a final extension step at 72°C for 10 min. The annealing temperatures were listed in Table S1.
WGS and Identification of ICEPmiChn5
To probe the genetic basis of antibiotic resistance and transmission in chicken P. mirabilis strains, a typical MDR P. mirabilis strain W47 was selected for the WGS. The genomic DNA of the strain W47 was extracted using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany). WGS was performed using Illumina Hiseq 2000 and Oxford Nanopore Technologies MinION platforms. Sequencing reads including short-read and long-read data were assembled with Unicycler 0.4.4 with the hybrid assembly strategy as described previously (Wick et al., 2017). The sequence was initially annotated using RAST server (http://rast.nmpdr.org), and corrected manually based on NCBI (https://www.ncbi.nlm.nih.gov/). The CGE server (https://cge.cbs.dtu.dk/services/) and IS finder (https://isfinder.biotoul.fr/) were used to identify resistance genes and IS elements. The presence of an SXT/R391 ICE located at positions 636,501 to 752,736 in chromosome was further confirmed by PCR and sequencing targeting the left and right junction regions with primers W47cycle and W47drop (Table S1). A comparative alignment was conducted using Easyfig software (Sullivan et al., 2011).
Confirmation of the Circular Extrachromosomal Form and Conjugal Transfer of ICEPmiChn5
Primers W47cycle-F and W47cycle-R were designed downstream of attL and upstream of attR to verify the circular intermediate of ICEPmiChn5, and primers W47drop-F and W47drop-R were designed upstream of attL and downstream of attR to verify whether ICEPmiChn5 could be excised from the chromosome (Table S1). To identify the mobility of ICEPmiChn5, conjugation experiments were performed by using W47 as the donor and the azide-resistant E. coli J53 as the recipient. Colonies of W47 and E. coli J53 were respectively cultured in LB broth for 4 to 5 h at 37°C. They were mixed at a 1:4 ratio in fresh LB broth and incubated for 4 h at 37°C. Transconjugants were selected on MacConkey agar plates supplemented with 150 mg/L sodium azide and 32 mg/L florfenicol. PCR was performed with the primers W47cycle and W47drop for the identification of transconjugants.
PCR Screening for ICEPmiChn5-like ICEs
To investigate the prevalence of ICEPmiChn5-like ICEs in P. mirabilis isolated from 4 chicken farms, primers were designed to amplify conserved regions (int and setC/D genes) of the SXT/R391 family ICEs backbone as previous studied (Harada et al., 2010; Bioteau et al., 2018). The presence of SXT/R391 ICEs in 187 P. mirabilis strains was determined by PCR and sequencing with primers int and setC/D listed in Table S1. The strain W47 was used as a positive control to verify the presence of int and setC/D genes in ICEPmiChn5-like ICEs.
Nucleotide Sequence Accession Numbers
The complete sequences of the chromosome and ICEPmiChn5 in P. mirabilis W47 have been submitted to GenBank under the accession numbers CP104986 and OP490725, respectively.
RESULTS AND DISCUSSION
Isolation and Identification of P. Mirabilis Strains
A total of 187 P. mirabilis strains were initially identified from 473 fecal samples in this study, and the isolation rate was 39.53% (187/473). 40.05% (151/377) isolates were isolated from 3 chicken farms in Henan Province, including 39.26% (117/298) isolates in Kaifeng City, 45.16% (28/62) isolates in Nanyang City, 35.29% (6/17) isolates in Zhumadian City, 37.50% (36/96) isolates were isolated from Nankang City in Jiangxi Province (Figure S1). Li et al. reported that the isolation rate of P. mirabilis was 0.81 to 17.19% in 6 chicken farms in Shandong Province from May to July 2019 (Li et al., 2022). Comparatively, the isolation rate of P. mirabilis was high in 4 chicken farms in this study. Pathogens in chicken feces might contaminate chicken products during slaughter, and can then be transmitted to humans through the food chain, and cause human foodborne illness (Guo et al., 2019; Sanches et al., 2019; Yu et al., 2021). Therefore, in modern broiler breeding, proper disposal of poultry excreta should be paid attention to.
Antimicrobial Resistant Phenotype and MDR Profiles
Antimicrobial susceptibility testing of 187 isolates was performed using 14 antimicrobials in 8 categories in this study (Figure 1A). A total of 187 P. mirabilis isolates with natural resistance to tigecycline, doxycycline, tetracycline and colistin exhibited MDR phenotypes with the multiple resistance rate of 100% (187/187). 93.58, 86.10, 86.10, and 78.07% of strains were respectively resistant to streptomycin, ciprofloxacin, florfenicol, and ampicillin. The resistance to amikacin, cephalosporins, and fosfomycin was relatively less serious, and the resistance rates varied from 4.28 to 22.99%. The lowest resistance rate to meropenem was 3.21% (6/187). The proportion of isolates resistant to 6 to 11 drugs was 90.37% (169/187), and the rate of drug resistance to 7 antibiotics was the highest (32.09%, 60/187). Two isolates (1.07%) with the highest drug resistance to 13 drugs were identified from the chicken farm in Nankang City (Figure 1B). The results of AGAR drug sensitivity test were expressed by heat map and bar chart of drug resistance map (Figure 1C). The drug resistance rate of isolates in chicken farms in Nanyang City and Zhumadian City was higher than that of isolates in the other 2 regions, which might be related to the using rate of antibacterials in farms.
Figure 1.
The drug resistance of 187 P. mirabilis strains. (A) The susceptibility results of 14 kinds of antibacterial drugs are presented in the resistance rate (%). (B) The MDR of 187 strains of P. mirabilis. (C) P. mirabilis heat resistance spectrum.
The MDR P. mirabilis W47 was resistant to 12 tested antibacterials, but susceptible to meropenem and amikacin. WGS analysis indicated that the strain W47 contained one 3,912,942 kb chromosome with a G/C content of 39%, and 2 small plasmids (8,955 bp and 2,742 bp in size, respectively) without any resistant determinant. The chromosome possessed 64 virulence genes and 23 resistance genes including tetracycline resistance genes tet(C) and tet(R), fluoroquinolone resistance gene aac(6′)-Ib-cr, bleomycin resistance gene blmS, trimethoprim resistance genes dfrA14 and dfrA32, streptomycin resistance genes aph(3″)-Ib, aph(6)-Id, and strB/A, benzalkonium chloride resistance gene qacEdeltal, chloramphenicol resistance genes cat, catB3, and floR, streptomycin and spectinomycin resistance gene aadA2, β-lactamase resistance gene blaOXA-1, sulfonamide resistance genes sul1 and sul2, rifampicin resistance gene arr3, erythromycin resistance gene ereA, kanamycin and neomycin resistance gene aphA1, which was consistent with the resistance phenotype. At present, the MDR of P. mirabilis isolated from chicken has been very serious. We must closely monitor the use of antimicrobial drugs in chicken farms and put forward prevention and control measures to avoid the threat of MDR bacteria to public health.
The Prevalence of Antimicrobial Resistance Genes
Various antibiotic resistance genes were identified among the 187 P. mirabilis strains isolated from chicken farms in 4 different regions (Figure 2). β-Lactamase resistance genes were the most common, including blaOXA-1 for the highest proportion of 77.54% (145/187), 6.42% (12/187) for blaCTX-M-1 group, and 17.65% (33/187) for blaCTX-M-9 group. For CTX-M extended-spectrum β-lactamases (ESBLs), the rate of blaCTX-M-9 group was high in Nanyang, Kaifeng, and Nankang, while the proportion of blaCTX-M-1 group was high in Zhumadian and Nankang. Among 187 P. mirabilis strains, 34.76% (65/187) carried mcr-1, 12.30% (23/187) carried fosA3, 0.53% (1/187) carried tet(X1), 0.53% (1/187) carried tet(X4), 0.53% (1/187) carried blaNDM, and the blaKPC detection rate was 0. The detection rate of drug resistance genes in P. mirabilis isolates from Nankang chicken farm was the lowest, while the highest for Zhumadian chicken farm (Figure 2), which was corresponded to the resistance phenotype. The mcr-1 was detected only in Kaifeng and Nanyang, and fosA3 was detected only in Kaifeng and Zhumadian. Among the 19 fosA3-positive strains in Kaifeng, 8 strains carried blaCTX-M and mcr-1 genes, 2 strains carried mcr-1 gene, and 8 strains carried blaCTX-M gene. Four P. mirabilis strains coharboring blaCTX-M and fosA3 genes were also detected in Zhumadian chicken farm. Fosfomycin is only approved for the clinical infections in China, so the high prevalence of fosA3 detected in this study might be was resulted from the coselection by other antimicrobials, such as cephalosporins and colistin. Other studies also observed the cotransfer of fosA3 with blaCTX-M or mcr-1 (Yang et al., 2014; Cao et al., 2020).
Figure 2.
Detection of drug resistance genes in 187 strains of P. mirabilis. Orange represents positive, blue represents negative.
The β-lactamase resistance genes blaOXA-1, blaCTX-M-1G, blaCTX-M-9G, and colistin resistance gene mcr-1 were highly carried in P. mirabilis isolates in this study. However, the positive rates of blaNDM and blaKPC were low, with 0.53% (1/187) and 0, respectively. New Delhi metal β-lactamase (NDM-1) can hydrolyze almost all β-lactams antibiotics including cephalosporins and carbapenems (Wang et al., 2021), so the disease infected by NDM-1-produced pathogens can only be treated with a few antibiotics, such as tigecycline and colistin. Unfortunately, P. mirabilis is naturally resistant to colistin and tigecycline, and poses huge challenges to clinical treatments. Meanwhile, the clinical potential of tigecycline and colistin has been significantly confined by the emergence and prevalence of tet(X4) and mcr-1. In recent years, blaNDM-1 has been continuously reported to be located on ICEs of P. mirabilis chromosomes (Kong et al., 2020; He et al., 2021). ICEs, as the reservoir of drug resistance genes, could accelerate the transmission of resistance genes between the same or different bacterial genera. Carbapenems and tigecycline were not approved for the treatment of veterinary clinical infections, it was likely that the coselection by other antimicrobials such as cephalosporins, first- and second-generation tetracyclines, was the main drivers for the emergence and diffusion of the blaNDM and tet(X4) genes (Ma et al., 2022). Continuous monitoring of antimicrobial resistance in animal original bacteria contributes to promote the rational use of antimicrobial agents, and delay the development of drug resistance may be an effective method to reduce the rate of antimicrobial resistance.
Genetic Structure and Conjugative Transfer of ICEPmiChn5
Sequence analysis showed that a 116,235 bp SXT/R391 ICE with 48% GC content containing a conserved integrase, inserted into the prfc gene in the chromosome of strain W47, and was named ICEPmiChn5 according to the nomology recommended by Burrus et al. (2006). ICEPmiChn5 contained 17 different resistance genes, including ereA, aadA2, aphA1, arr3, catB3, dfrA32, blmS, blaOXA-1, aac(6′)-Ib-cr, strB/A, tet(C), tet(R), floR, sul1, sul2, qacEdeltal (Figure 3). It consisted of a highly conserved genetic backbone involved in essential functions of SXT/R391 ICEs and variable insertion DNA at HS1, HS2, HS3, HS4, HS5, VRII, and VRIII (Figure 3). BLAST analysis showed that ICEPmiChn5 exhibited high homology to ICEPmiBCP11 and ICEPmiFra1 in P. mirabilis, with 99.97% identity at 99 and 93% coverage, as well as ICEPreChnRF-14-2, with 98.94% identity at 76% coverage, and ICESupCHN110003, with 99.69% identity at 55% coverage, suggesting these ICEs evolved from a common ancestor. Notably, among which, sequence analysis implied that ICESupCHN110003 was more likely to be an archetypal of other 4 ICEs during evolution. The VRⅢ region in these 5 ICEs showed high homology and all carried strB/A and sul2, and the other 4 ICEs carried floR except ICEPreChnRF-14-2 carrying tet(X6). Interestingly, the sequences of ICEPmiChn5, ICEPmiChnBCP11, and ICEPmiFra1 were essentially identical, with the largest difference being in the variable insertion region HS4 (Figure 3).
Figure 3.
Structural comparison between ICEPmiChn5 and 4 reported ICEs including ICESupChn110003, ICEPreChnRF14-2, ICEPmiFra1, and ICEPmiChnBCP11. The backbone of ICEs with conserved core genes is represented in the shadowed box. Among the backbone genes, excision and integration, transfer, regulation, unknown and known function, error-prone DNA repair genes are in blue, pink, purple, gray, yellow, respectively. ICESupChn110003 (MG014393.1), ICEPreChnRF14-2 (MT219827.1), ICEPmiFra1 (MF490434.1), ICEPmiChn5, and ICEPmiChnBCP11 (MG773277.1) are respectively in brown, cyan, light purple, light blue, and purple. Antibiotic resistance genes and mobile elements are in red and yellow. Regions of >99% homology in VRIII and HS4 are marked by dark gray shading.
In HS4 region, a 31,493 bp MDR region harboring 13 resistance genes, bracketed by 2 copies ISPpu12 in the same direction, were identified in ICEPmiChn5. ISPpu12-mediated composite transposons with different resistance genes were common in SXT/R391 type ICEs, and also existed in ICEPmiChnBCP11, ICEPreChnRF-14-2, and ICEPmiFra1. However, the left-hand ISPpu12 in ICEPmiChn5, ICEPreChnRF-14-2, and ICEPmiFra1 had a 2-nucleotide (nt) deletion in tnpA leading to a shortened transposes, but intact in ICEPmiChnBCP11. The MDR region of ICEPmiChn5 contained 5 IS26-flanked transposon segments. The first 2 segments ISPpu12-tet(C)-tet(R)-IS26 and IS26-aphA1-IS26, and the fourth segment IS26-dfrA32-ereA-aadA2-ISPpu12, were the same as that of 2 above-mentioned ICEs except ICESupCHN110003 and ICEPreChnRF14-2. The third segment IS26-aac(6′)-Ib-cr-blaOXA-1-catB3-arr3-qacEdeltal-sul1-IS26, was present in ICEPmiChn5, ICEPmiChnBCP11, and a E. coli plasmid pECXH3 (accession no. KY865324), suggesting the important role of IS26 in the transmission of resistance genes between different bacterial genera. Based on the above analysis, the MDR region in ICEPmiChn5 differed from that of ICEPmiFra1 by the insertion of 3 IS26-flanked transposons, and from that of ICEPmiChnBCP11 by the deletion of 3 IS26-flanked composite transposons carrying resistance genes. It was proposed that ICEPmiChn5 and ICEPmiFra1 may be 2 intermediate forms of ICEPmiChnBCP11 during evolution, involving the deletion of the fragment in VRII region and the insertion of IS26-mediated fragments in HS4 region, providing the evidence for the role of IS26 in formation of MDR regions.
A 17 bp direct repeat was detected at both the attL (5′-ATTATTTCCCACCCTGA-3′) and attR (5′-ATCATCTCCCACCCCGA-3′) ends of ICEPmiChn5, being used to determine whether ICEPmiChn5 could form a ring form. PCR and sequencing with primers W47 cycle and W47 drop also proved that ICEPmiChn5 could form a circular intermediate and excised from the chromosome of W47, and the junction kept the 17 bp sequence (Figure S1). WGS sequencing showed that ICEPmiChn5 had the necessary genes for conjugative transfer as ICEPmiChnBCP11 (Lei et al., 2018). However, despite repeated attempts, ICEPmiChn5 was not successfully transferred to the E. coli J53. Coincidentally, for ICEAplChn1, ICEApl2, and ICEPgs6Chn1 possessing all the essential genes for the ICEs mobility, no transconjugant was obtain by conjugation experiments (Xu et al., 2018; He et al., 2020). It was speculated that the nontransferability of ICEAplChn1 may be caused by the VRVI insertion between the mobI and rumB genes, responsible for the mobilization of SXT/R391 ICEs and error prone DNA repair, respectively (Wozniak et al., 2009 ; Xu et al., 2018). However, the nontransferable ICEPmiChn5, ICEPgs6Chn1, and ICEApl2 lacked VRVI insertion, implying that the nontransferability may be caused by other factors. The xis and setC/D genes in the genetic backbone were relevant to the transfer of ICEs (Burrus et al., 2003). So, the deletion of xis gene in ICEPmiChn5 may result in the horizontal transfer failure. Furthermore, the mutation of genes related to conjugative transfer or regulating, may impact the self-transferability of ICEPmiChn5, which should be further investigated.
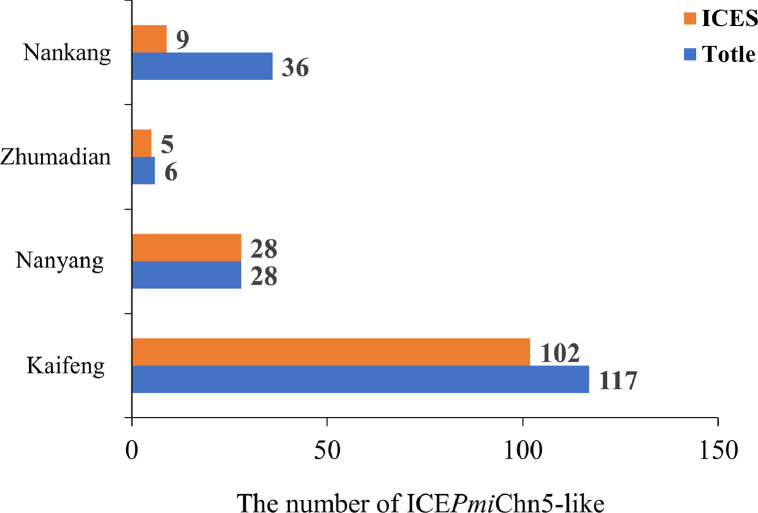
Prevalence of ICEPmiChn5-Like in 187 Isolates of P. Mirabilis
The 187 isolates of P. mirabilis involved in this study were detected by setC/D and int genes to determine the existence of SXT/R391 ICEs, with the results that 144 (77.01%, 144/187) isolates of P. mirabilis carried ICEPmiChn5-like ICEs (Figure 4). Results analysis found that the highest detection rate (100%, 28/28) of SXT/R391 ICEs was in Nanyang chicken farm, and the lowest detection rate (25%, 9/36) was in Nankang chicken farm, which was positively related to the resistance phenotype and genotype, further indicating the significant role of ICEs in accumulation and transmission of resistance genes. The identification rates of ICEs in Kaifeng and Zhumadian chicken farm were 87% (102/117) and 83% (5/6), respectively.
Figure 4.
Prevalence of ICEPmiChn5-like ICEs in 187 P. mirabilis isolates.
To our knowledge it is the first time that so many SXT/R391 ICEs have been identified in P. mirabilis from chickens on such a large scale. The SXT/R391 ICEs were frequently found in MDR P. mirabilis isolates from clinical specimens and food-producing animals (Bontron et al., 2019). There was a growing concern over the possibility of transmission of MDR SXT/R391 ICEs through the food chain. The positive ratio of blaOXA-1 in the 144 ICEs-harboring isolates in this study was 97% (139/144), and the blaOXA-1 was located in the HS4 region of ICEPmiChn5 and ICEPmiChnBCP11, inferring that blaOXA-1 might be transmitted by ICEs in P. mirabilis of chicken origin. More studies are required to investigate the prevalence of ICEs in other hosts and environments.
CONCLUSIONS
In this study, we investigated the drug resistance of P. mirabilis strains from 4 chicken farms, and explored the transmission mechanism of resistance genes. The MDR of P. mirabilis strains was serious, as high as 100%. The detection rate of β-lactamase resistance gene blaOXA-1 in 187 strains of P. mirabilis was the highest (77.54%), and the antibiotic resistance of P. mirabilis strains in Zhumadian was most serious. An ICE named ICEPmiChn5 carrying 17 resistance genes was identified in the MDR strain W47. The epidemiological survey of ICEPmiChn5-like ICEs was performed with the results that 144 strains carried ICEs with the proportion of 77.01%, suggesting the role of ICEs in evolution of MDR strains. Further studies should evaluate the transfer risk SXT/R391 ICEs in P. mirabilis from chicken origin into human clinical pathogens.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 32072923), the Program for Science and Technology Innovation Talents in Universities of Henan Province (No. 23HASTIT039).
DISCLOSURES
The authors hereby declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102640.
Appendix. Supplementary materials
REFERENCES
- Aberkane S., Compain F., Decré D., Dupont C., Laurens C., Vittecoq M., Pantel A., Solassol J., Carrière C., Renaud F., Brieu N., Lavigne J.P., Bouzinbi N., Ouédraogo A.S., Jean-Pierre H., Godreuil S. High prevalence of SXT/R391-related integrative and conjugative elements carrying blaCMY-2 in Proteus mirabilis isolates from gulls in the south of France. Antimicrob. Agents Chemother. 2016;60:1148–1152. doi: 10.1128/AAC.01654-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie L., Wu H., Wang X.H., Wang M., Xu H. Identification and characterization of new members of the SXT/R391 family of integrative and conjugative elements (ICEs) in Proteus mirabilis. Int. J. Antimicrob. Agents. 2017;50:242–246. doi: 10.1016/j.ijantimicag.2017.01.045. [DOI] [PubMed] [Google Scholar]
- Bioteau A., Durand R., Burrus V. Redefinition and unification of the SXT/R391 family of integrative and conjugative elements. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.00485-18. e00485-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontron S., Poirel L., Kieffer N., Savov E., Trifonova A., Todorova I., Kueffer G., Nordmann P. Increased resistance to carbapenems in Proteus mirabilis mediated by amplification of the bla(VIM-1)-carrying and IS26-associated class 1 integron. Microb. Drug Resist. 2019;25:663–667. doi: 10.1089/mdr.2018.0365. [DOI] [PubMed] [Google Scholar]
- Burrus V., Marrero J., Waldor M.K. The current ICE age: biology and evolution of SXT-related integrating conjugative elements. Plasmid. 2006;55:173–183. doi: 10.1016/j.plasmid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Burrus V., Waldor M.K. Control of SXT integration and excision. J. Bacteriol. 2003;185:5045–5054. doi: 10.1128/JB.185.17.5045-5054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y.P., Lin Q.Q., He W.Y., Wang J., Yi M.Y., Lv L.C., Yang J., Liu J.H., Guo J.Y. Co-selection may explain the unexpectedly high prevalence of plasmid-mediated colistin resistance gene mcr-1 in a Chinese broiler farm. Zool. Res. 2020;41:569–575. doi: 10.24272/j.issn.2095-8137.2020.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerceo E., Deitelzweig S.B., Sherman B.M., Amin A.N. Multidrug-resistant gram-negative bacterial infections in the hospital setting: overview, implications for clinical practice, and emerging treatment options. Microb. Drug Resist. 2016;22:412–431. doi: 10.1089/mdr.2015.0220. [DOI] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) Performance Standards for Antimicrobial Susceptibility Testing; Thirty-second Informational Supplement. CLSI Document M100-S32. CLSI; Wayne, PA: 2022. [Google Scholar]
- Drzewiecka D. Significance and roles of Proteus spp. bacteria in natural environments. Microb. Ecol. 2016;72:741–758. doi: 10.1007/s00248-015-0720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girlich D., Bonnin R.A., Dortet L., Naas T. Genetics of acquired antibiotic resistance genes in Proteus spp. Front. Microbiol. 2020;11:256. doi: 10.3389/fmicb.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z., Shi X., Bai F., He X., Zhang H., Li Y., Wan Y., Lin Y., Qiu Y., Chen Q., Hu Q., Cao H. Characterization of a novel diarrheagenic strain of Proteus mirabilis associated with food poisoning in China. Front. Microbiol. 2019;10:2810. doi: 10.3389/fmicb.2019.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S., Aung K.T., Tay M.Y.F., Seow K.L.G., Ng L.C., Schlundt J. Extended-spectrum β-lactamase-producing Proteus mirabilis with MDR isolated from raw chicken in Singapore: genotypic and phenotypic analysis. J. Glob. Antimicrob. Resist. 2019;19:252–254. doi: 10.1016/j.jgar.2019.10.013. [DOI] [PubMed] [Google Scholar]
- Harada S., Ishii Y., Saga T., Tateda K., Yamaguchi K. Chromosomally encoded blaCMY-2 located on a novel SXT/R391-related integrating conjugative element in a Proteus mirabilis clinical isolate. Antimicrob. Agents Chemother. 2010;54:3545–3550. doi: 10.1128/AAC.00111-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J., Sun L., Zhang L., Leptihn S., Yu Y., Hua X. A novel SXT/R391 integrative and conjugative element carries two copies of the bla(NDM-1) gene in Proteus mirabilis. mSphere. 2021;6 doi: 10.1128/mSphere.00588-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D., Wang L., Zhao S., Liu L., Liu J., Hu G., Pan Y. A novel tigecycline resistance gene, tet(X6), on an SXT/R391 integrative and conjugative element in a Proteus genomospecies 6 isolate of retail meat origin. J. Antimicrob. Chemother. 2020;75:1159–1164. doi: 10.1093/jac/dkaa012. [DOI] [PubMed] [Google Scholar]
- Kong L.H., Xiang R., Wang Y.L., Wu S.K., Lei C.W., Kang Z.Z., Chen Y.P., Ye X.L., Lai Y., Wang H.N. Integration of the blaNDM-1 carbapenemase gene into a novel SXT/R391 integrative and conjugative element in Proteus vulgaris. J. Antimicrob. Chemother. 2020;75:1439–1442. doi: 10.1093/jac/dkaa068. [DOI] [PubMed] [Google Scholar]
- Lei C.W., Chen Y.P., Kang Z.Z., Kong L.H., Wang H.N. Characterization of a novel SXT/R391 integrative and conjugative element carrying cfr, bla(CTX-M-65), fosA3, and aac(6′)-Ib-cr in Proteus mirabilis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00849-18. e00849-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Peng C., Zhang G., Shen Y., Zhang Y., Liu C., Liu M., Wang F. Prevalence and characteristics of multidrug-resistant Proteus mirabilis from broiler farms in Shandong Province, China. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Zhao K., Xie H., Li R., Zhou M. Identification and characterization of a novel SXT/R391 integrative and conjugative element in a Proteus mirabilis food isolate. Foodborne Pathog. Dis. 2021;18:727–732. doi: 10.1089/fpd.2020.2886. [DOI] [PubMed] [Google Scholar]
- Ma J., Zhou W., Wu J., Liu X., Lin J., Ji X., Lin H., Wang J., Jiang H., Zhou Q., Zhao G., Yang H., Tang B. Large-scale studies on antimicrobial resistance and molecular characterization of Escherichia coli from food animals in developed areas of Eastern China. Microbiol. Spectr. 2022;10 doi: 10.1128/spectrum.02015-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata C., Navarro F., Miró E., Walsh T.R., Mirelis B., Toleman M. Prevalence of SXT/R391-like integrative and conjugative elements carrying blaCMY-2 in Proteus mirabilis. J. Antimicrob. Chemother. 2011;66:2266–2270. doi: 10.1093/jac/dkr286. [DOI] [PubMed] [Google Scholar]
- O'Hara C.M., Brenner F.W., Miller J.M. Classification, identification, and clinical significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000;13:534–546. doi: 10.1128/cmr.13.4.534-546.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Blanco A., Lemos M.L., Osorio C.R. Integrating conjugative elements as vectors of antibiotic, mercury, and quaternary ammonium compound resistance in marine aquaculture environments. Antimicrob. Agents Chemother. 2012;56:2619–2626. doi: 10.1128/AAC.05997-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches M.S., Baptista A.A.S., de Souza M., Menck-Costa M.F., Koga V.L., Kobayashi R.K.T., Rocha S.P.D. Genotypic and phenotypic profiles of virulence factors and antimicrobial resistance of Proteus mirabilis isolated from chicken carcasses: potential zoonotic risk. Braz. J. Microbiol. 2019;50:685–694. doi: 10.1007/s42770-019-00086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebor E., de Curraize C., Neuwirth C. Genomic context of resistance genes within a French clinical MDR Proteus mirabilis: identification of the novel genomic resistance island GIPmi1. J. Antimicrob. Chemother. 2018;73:1808–1811. doi: 10.1093/jac/dky126. [DOI] [PubMed] [Google Scholar]
- Sullivan M.J., Petty N.K., Beatson S.A. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wen S., Zhao L., Xia Q., Pan Y., Liu H., Wei C., Chen H., Ge J., Wang H. Association among biofilm formation, virulence gene expression, and antibiotic resistance in Proteus mirabilis isolates from diarrhetic animals in Northeast China. BMC Vet. Res. 2020;16:176. doi: 10.1186/s12917-020-02372-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Xu K., Zhao L., Tong R., Xiong L., Shi J. Recent research and development of NDM-1 inhibitors. Eur. J. Med. Chem. 2021;223 doi: 10.1016/j.ejmech.2021.113667. [DOI] [PubMed] [Google Scholar]
- Wick R.R., Judd L.M., Gorrie C.L., Holt K.E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak R.A., Fouts D.E., Spagnoletti M., Colombo M.M., Ceccarelli D., Garriss G., Déry C., Burrus V. Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS genetics. 2009;5 doi: 10.1371/journal.pgen.1000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Jia H., Cui G., Tong H., Wei J., Shao D., Liu K., Qiu Y., Li B., Ma Z. ICEAplChn1, a novel SXT/R391 integrative conjugative element (ICE), carrying multiple antibiotic resistance genes in Actinobacillus pleuropneumoniae. Vet. Microbiol. 2018;220:18–23. doi: 10.1016/j.vetmic.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Yang X., Liu W., Liu Y., Wang J., Lv L., Chen X., He D., Yang T., Hou J., Tan Y., Xing L., Zeng Z., Liu J.H. F33: A-: B-, IncHI2/ST3, and IncI1/ST71 plasmids drive the dissemination of fosA3 and blaCTX-M-55/-14/-65 in Escherichia coli from chickens in China. Front. Microbiol. 2014;5:688. doi: 10.3389/fmicb.2014.00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Joossens M., Van den Abeele A.M., Kerkhof P.J., Houf K. Isolation, characterization and antibiotic resistance of Proteus mirabilis from Belgian broiler carcasses at retail and human stool. Food Microbiol. 2021;96 doi: 10.1016/j.fm.2020.103724. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.