Abstract
The canonical view of PI3Kα signaling describes phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) generation and activation of downstream effectors at the plasma membrane or at microtubule-bound endosomes. Here, we show that colorectal cancer (CRC) cell lines exhibit a diverse plasma membrane–nuclear distribution of PI3Kα, controlling corresponding levels of subcellular PtdIns(3,4,5)P3 pools. PI3Kα nuclear translocation was mediated by the importin β-dependent nuclear import pathway. By PtdIns(3,4,5)P3 affinity capture mass spectrometry done in the presence of SDS on CRC cell lines with PI3Kα nuclear localization, we identified 867 potential nuclear PtdIns(3,4,5)P3 effector proteins. Nuclear PtdIns(3,4,5)P3 interactome proteins were characterized by noncanonical PtdIns(3,4,5)P3-binding domains and showed overrepresentation for nuclear membrane, nucleolus, and nuclear speckles. The nuclear PtdIns(3,4,5)P3 interactome was enriched for proteins related to RNA metabolism, with splicing reporter assays and SC-35 foci staining suggesting a role of epidermal growth factor–stimulated nuclear PI3Kα signaling in modulating pre-mRNA splicing. In patient tumors, nuclear p110α staining was associated with lower T stage and mucinous histology. These results indicate that PI3Kα translocation mediates nuclear PtdIns(3,4,5)P3 effector signaling in human CRC, modulating signaling responses.
Keywords: PI3Kα; nuclear import pathway; PtdIns(3,4,5)P3 interactome; pre-mRNA splicing; colorectal cancer
Graphical Abstract
Highlights
-
•
PI3Kα translocation regulates nuclear PtdIns(3,4,5)P3 levels in colorectal cancer.
-
•
PI3Kα shuttling is mediated by the importin β-dependent nuclear import pathway.
-
•
PtdIns(3,4,5)P3 interactome profiling implicates roles in mRNA metabolism.
-
•
PI3Kα localization relates to clinical subtypes of colorectal cancer.
In Brief
The canonical view of PI3Kα signaling describes PtdIns(3,4,5)P3 generation and activation of downstream effectors at the plasma membrane. Here, we show that colorectal cancer cell lines exhibit a diverse plasma membrane–nuclear distribution of PI3Kα, controlling corresponding subcellular PtdIns(3,4,5)P3 pools, and characterize the nuclear PtdIns(3,4,5)P3 interactome. Our findings support a model in which nuclear translocation of PI3Kα defines a mechanism for spatial organization of PtdIns(3,4,5)P3 effector signaling, associated with colorectal cancer subtypes, and modulating signaling responses.
Compartmentalization of second messenger signaling pathways is a principal mechanism for determining the specificity in cellular responses. PI3Kα signaling is critical for normal growth and development and is frequently hyperactivated in human malignancies including colorectal cancer (CRC) (1). Canonical agonist-stimulated PI3Kα signaling is triggered by recruitment of the p110α catalytic subunit via p85 regulatory subunits to activated receptor tyrosine kinases (RTKs) at the plasma membrane or at microtubule-bound endosomes in the cytoplasm, resulting in the phosphorylation of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2) to generate phosphatidylinositol-3,4,5-trisphosphate (PtdIns(3,4,5)P3) (1, 2). PtdIns(3,4,5)P3 binds to effector proteins including the serine/threonine kinases AKT (AKT serine/threonine kinase), PDK1 (pyruvate dehydrogenase kinase 1), and SIN1 (stress-activated protein kinase–interacting 1) via binding motifs including pleckstrin homology (PH), phox homology (PX), plant homeodomain (PHD), and C1 and C2 domains (3). AKT is activated by phosphorylation mediated by PDK1 and the mammalian target of rapamycin complex 2 and can act at various intracellular sites to phosphorylate downstream substrates that regulate cell survival, proliferation, growth, and metabolism (1). The generation of PtdIns(3,4,5)P3 is negatively regulated by phosphatase and tensin homolog (PTEN) and SHIP1 (SH2 domain-containing inositol 5′-phosphatase 1), two lipid phosphatases that dephosphorylate PtdIns(3,4,5)P3 at the 3 or 5′ positions of the inositol ring, respectively (3).
Activating mutations in PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha), the gene encoding p110α, and loss of PTEN protein expression are found in ∼15% and ∼30% of human CRCs, respectively (4). Several studies of anti–epidermal growth factor receptor (EGFR) antibody treatment in patients with metastatic CRC have reported resistance for tumors with PIK3CA mutation or PTEN loss (5, 6). Conversely, PIK3CA mutation has been associated with CRC response to aspirin treatment with an increased survival rate after surgery (7, 8).
While PI3Kα signaling is classically considered to be initiated at the plasma membrane/cytosolic compartment, other class I PI3Ks, including PI3Kβ and PI3Kγ, have also been detected in the nucleus (9), forming a functionally distinct nuclear PI3K signaling axis that can be stimulated in response to agonists, such as nerve growth factor, platelet-derived growth factor, and insulin (10, 11, 12, 13). Nuclear PI3K signaling may be initiated by nuclear import of activated PI3Kβ and PI3Kγ (13, 14) or via activation of nuclear RTKs or GTPases (15, 16, 17). A single study expressing GFP-tagged p110α has noted nuclear localization in CRC (HCT116) and breast cancer (MCF7) cells, although endogenous protein was not analyzed (18). To date, only a few nuclear PI3K signaling–associated PtdIns(3,4,5)P3-effector proteins have been described including nucleophosmin (NPM)/B23 and ErbB3-binding protein 1 involved in cell survival and cell growth (19, 20), the ribonuclear protein ALY (Aly/REF export factor; THO complex subunit 4) involved in mRNA export (21), the nuclear upstream binding factor UBF-1 involved in RNA I polymerase activity (22) and PtdIns(3,4,5)P3-binding protein (PIP3BP) involved in the regulation of actin cytoskeleton (23). Similar to the plasma membrane pool, dephosphorylation of nuclear PtdIns(3,4,5)P3 can occur by nuclear PTEN and SHIP1 phosphatases (24, 25).
Here, we demonstrate that nuclear translocation of PI3Kα, mediated by the importin β-dependent nuclear import pathway, is a principal regulator of nuclear PtdIns(3,4,5)P3 levels in CRC cells. Potential roles of nuclear PI3Kα signaling were explored by comprehensive interactome profiling of the nuclear PtdIns(3,4,5)P3 effector network, highlighting functions related to RNA metabolism including pre-mRNA splicing. Our biochemical, cell biology, and patient data indicate that PI3Kα translocation mediates nuclear PtdIns(3,4,5)P3 effector signaling in human CRC, modulating signaling responses.
Experimental Procedures
CRC Cell Lines
A total of 58 CRC cell lines were studied as detailed in supplemental Table S1. Cells were cultured with Dulbecco's modified Eagle’s medium and 10% fetal bovine serum at 37 °C and 10% CO2. PIK3CA (exons 9 and 20) and KRAS (codons 12, 13, and 61) mutation status, detected by direct DNA sequencing, DNA mismatch repair (MMR) status, assessed by the Bethesda consensus panel of microsatellite markers, and PTEN protein expression, assessed by Western blot, for these cell lines have been reported previously (26, 27, 28).
Patients
Tissue microarrays (TMAs) from 406 patients with stages I–IV CRC recruited at the Royal Melbourne Hospital, Melbourne Private Hospital, and Western Hospital Footscray in Melbourne in Australia were examined (4, 29). Patients with familial polyposis syndromes, ulcerative colitis, or Crohn disease–associated CRC were excluded. All patients gave informed consent, and the study was approved by the relevant hospital and institutional ethics committees (WEHI HREC 12/19). Human studies were conducted in accordance with the Declaration of Helsinki.
TMAs comprised of 1 mm diameter tissue cores derived from archival primary tumor specimens, with up to four tumor tissue cores per patient harvested from the areas of highest tumor cell percentage. PIK3CA (exons 9 and 20) and KRAS (codons 12, 13, and 61) mutation status, detected by direct DNA sequencing, and MMR status, assessed by either the Bethesda consensus panel of microsatellite markers or immunohistochemistry, for these tumors have been reported previously (4, 29).
Immunofluorescence and Confocal Microscopy
For immunofluorescence (IF) studies, cells were grown on glass coverslips in 6-well plates to ∼80% confluency. Cells were fixed with 4% paraformaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 10 min, and blocked in 5% bovine serum albumin/Tris-buffered saline for 30 min. Cells were incubated with primary antibodies against p110α (catalog no.: 4249; Cell Signaling Technology, 1:250 dilution), lamin B1 (catalog no.: ab8982; Abcam, 1:100 dilution), nucleolin (catalog no.: 39-6400; Invitrogen, 1:100 dilution), SC-35 (catalog no.: S4045; Sigma, 1:2000 dilution), PML (PML nuclear body scaffold; catalog no.: ab96051; Abcam, 1:100 dilution), and coilin (catalog no.: ab87913; Abcam, 1:1000 dilution) for 1 h at room temperature, followed by incubation with fluorescent-conjugated Alexa Fluor 488 and Alexa Fluor 546 secondary antibodies (A27034 and A11003; Life Technologies, 1:500 dilution) for 1 h at room temperature. Cells were stained with HCS CellMask Red Stain (catalog no.: H32712; Life Technologies, 1:5000 dilution) and/or 4′,6-diamidino-2-phenylindole for 5 min and mounted in DPX Mountant medium (Sigma).
For PtdIns(3,4,5)P3 IF staining, glutathione-S-transferase (GST)-GRP1PH domain reporter was produced as previously described (30). Cells were incubated with GST-tagged GRP1PH domain reporter for 1 h at room temperature, followed by incubation with primary antibody against GST (catalog no.: ab19256; Abcam, 1:500 dilution) and fluorescent-conjugated secondary antibody as described previously. For the soluble PtdIns(3,4,5)P3 competition assay with GST-GRP1PH domain reporter, cells were preincubated with 10 μg PtdIns(3,4,5)P3 for 1 h prior to staining.
For siRNA-based knockdown assays of p110α, IF staining was performed 72 and 96 h post siRNA transfection. Cells were incubated with primary antibodies against p110α or GST-tagged GRP1PH domain reporter as described previously.
For EGF stimulation assays, cell lines were stimulated with EGF (200 ng/ml) with or without pretreatment with 25 μM BYL719 for 1 h or p110α knockdown for 96 h over a time course of 5, 10, and 20 min. Cells were incubated with GST-tagged GRP1PH domain reporter as described previously.
For importazole assays, cell lines were treated with 40 μM importazole over a time course of 1, 3, 6, and 24 h. Cells were incubated with primary antibodies against p110α or GST-tagged GRP1PH domain reporter as described previously.
For SC-35 foci assays, cell lines were stimulated with EGF (200 ng/ml) for 10 min with or without pretreatment with 25 μM BYL719 for 1 h. Cells were incubated with primary antibodies against SC-35 as described previously.
Images for five fields per cell line were taken with a Zeiss LSM 780 confocal microscope controlled by ZEN software (Zeiss). All images were acquired using a 63× oil-immersion objective lens (numerical aperture 1.4) and processed using Metamorph software (Molecular Devices). For quantification, the mean fluorescent intensity of channels of interest in each cell was measured using Fiji software (ImageJ) (31). Colocalization was quantified using Fiji software for thresholded Mander's split colocalization coefficient using Costes Auto threshold method, which ranges from 0 to 1, expressing the fraction of intensity in a channel that is located in pixels where there is above threshold intensity in the other color channel; 0 means no colocalization and 1 represents perfect colocalization.
Cell Line siRNA Transfections
CRC cell line transfections with siRNAs were conducted using Lipofectamine 2000 (Invitrogen) as per the manufacturer’s instructions for the following siRNAs: siPIK3CA #1 (Dharmacon siGENOME siRNA D-003018-07), siPIK3CA#2 (Dharmacon siGENOME siRNA D-003018-08).
Western Blot Analysis
Protein isolation was performed using radioimmmunoprecipitation assay lysis buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10 mM EDTA), protease inhibitor cocktail Roche cOmplete (Roche), and phosphatase inhibitor PhosSTOP (Roche). A total of 30 μg of protein was resolved on a NuPAGE Novex 4 to 12% Bis–Tris gel (Life Technologies). Proteins were transferred onto nitrocellulose using the iBlot Dry Blotting System (Life Technologies) and blocked in 5% bovine serum albumin/Tris-buffered saline for 1 h. Following overnight incubation with primary antibody against p110α (catalog no.: 4249; Cell Signaling Technology, 1:250 dilution), SRSF1 (catalog no.: 32-4500; Invitrogen, 1:250 dilution), ALY (catalog no.: 12655; Cell Signaling Technology, 1:1000 dilution), and EIF4A3 (eukaryotic translation initiation factor 4A3; catalog no.: ab180573; Abcam, 1:1000 dilution), lamin B1 (catalog no.: ab8982; Abcam, 1:100 dilution), and tubulin (catalog no.: 2128; Cell Signaling Technology, 1:1000 dilution) at 4 °C, the blot was incubated with fluorescent-conjugated goat anti-rabbit 800 and antimouse 800 secondary antibodies (catalog nos.: 926-32211 and 926-32210; LiCor, 1:10,000 dilution) at room temperature for 1 h. Fluorescence was visualized using the Odyssey Infrared Imaging System (LI-COR).
Nuclear Lysate Extraction
DLD1 and SW480 cells were grown in 10 cm dishes. Cells were dissociated by scraping and pelleted at 1500 rpm for 5 min. Cells were washed twice with PBS. Cytoplasm–membrane fraction was isolated using a hypotonic buffer (20 mM Tris–HCl [pH 7.4], 10 mM NaCl, 3 mM MgCl2), protease inhibitor cocktail Roche Complete, and phosphatase inhibitor PhosSTOP. Cells were lysed on ice for 10 min, followed by an additional 5 min with the addition of 1% Triton X-100. Following centrifugation and removal of the cytoplasmic fraction, the remaining nuclear pellet was washed twice with 1% Triton X-100 and lysed using on ice for 30 min in radioimmmunoprecipitation assay buffer (50 mM Tris–HCl [Ph 7.5], 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, and 10 mM EDTA), protease inhibitor cocktail Roche cOmplete, and phosphatase inhibitor PhosSTOP.
Anion/cation-Exchange Chromatography
DLD1 and SW480 nuclear extracts were prefractionated using anion (Q-Fast Flow) and cation (S-Fast Flow) equilibrated in 10 mM Tris–HCl (pH 9), 0.005% Tween-20, and 10 mM sodium acetate (pH 6), 0.005% Tween-20, respectively. Bound proteins were eluted in 1 M NaCl, and eluted fractions were pooled and underwent buffer exchange in 0.005% Tween-20/PBS for subsequent affinity assays.
PtdIns(3,4,5)P3 Affinity Capture
Sodium salts of dipalmitoyl analogs of phosphatidylinositol-(3,4,5)-triphosphate were synthesized as described previously (32, 33) and prepared for attachment to Affi-10 beads (Bio-Rad) by conjugating an ω-amino glycerol side chain to the inositol moiety. NH2-PtdIns(3,4,5)P3 was attached to Affi-10 beads following the protocol previously described for NH2-PtdIns(3,5)P2 and NH2-PtdIns(4,5)P2 (34). The efficiency of PtdIns(3,4,5)P3 conjugation was verified by Biacore analysis (Biacore 3000; GE Healthcare Bio-Sciences AB) using a calibration curve generated by titrating PtdIns(3,4,5)P3 (twofold dilution series from 200 to 3.1 μg/ml) over neomycin immobilized onto a CM5 carboxymethylated biosensor surface (Biacore) using amine coupling chemistry. The PtdIns(3,4,5)P3 Affi-Gel-10 beads were incubated with purified anion (Q Fast Flow) and cation (S Fast Flow) chromatographic nuclear fractions (DLD1 and SW480). Briefly, incubation was performed initially with ethanolamine-derivatized Affi-Gel-10 blank beads (200 μl beads for 500 μl sample) for 2 h at 4 °C to remove proteins that bind nonspecifically. After removal of the blank derivatized beads by centrifugation (5 min at 480g), the precleared fraction was incubated overnight at 4 °C with PtdIns(3,4,5)P3 Affi-Gel-10 or Affi-Gel-10 blank derivatized beads (100 μl beads for 500 μl of extract). The beads were then washed five times with 1.5 ml PBS containing 0.005% (v/v) Tween-20 before desorbing the bound proteins using 100 μl of SDS-PAGE buffer (LDS NuPAGE sample buffer; Invitrogen) at 95 °C for 5 min and detected using SDS-PAGE and sensitive Coomassie staining. Following overnight destaining in water and imaging using the Odyssey Infrared Imaging System, gel lanes were cut into 10 to 15 sections per lane and prepared for protein digestion.
LC/MS–MS Analysis
Reduction, alkylation, tryptic digestion, and peptide extraction from protein bands were performed as described previously (35). Generated tryptic peptides were concentrated to ∼20 μl by centrifugal lyophilization for LC/MS–MS analysis. LC/MS–MS was carried out on an LTQ Orbitrap Elite mass spectrometer (Thermo Scientific) with a nanoESI interface in conjunction with an Ultimate 3000 RSLC nanoHPLC (Dionex Ultimate 3000). The LC system was equipped with an Acclaim PepMap nanotrap column (Dinoex-C18, 100 Å, 75 μm × 2 cm) and an Acclaim PepMap RSLC analytical column (Dinoex-C18, 100 Å, 75 μm × 15 cm). The tryptic peptides were injected into the enrichment column at an isocratic flow of 5 μl/min of 3% v/v CH3CN containing 0.1% v/v formic acid for 5 min applied before the enrichment column was switched in-line with the analytical column. The eluents were 0.1% v/v formic acid (solvent A) and 100% v/v CH3CN in 0.l% v/v formic acid (solvent B). The flow gradient was (i) 0 to 5 min at 3% B, (ii) 5 to 6 min, 3 to 6% B, (iii) 6 to 18 min, 6 to 10% B, (iv) 18 to 38 min, 10 to 30% B, (v) 38 to 40 min, 30 to 45% B, (vi) 40 to 42 min, 45 to 80% B, (vii) 42 to 45 min at 80% B, (viii) 45 to 46 min, 80 to 3% B, and (ix) 46 to 53 min at 3% B. The LTQ Orbitrap Elite spectrometer was operated in the data-dependent mode with nanoESI spray voltage of 2.0 kV, capillary temperature of 250 °C, and S-lens RF value of 55%. All spectra were acquired in positive mode with full scan mass spectrometry (MS) spectra scanning from m/z 300 to 1650 in the FT mode at 240,000 resolution after accumulating to a target value of 1.0 × 106. Lock mass of 445.120025 was used. The top 20 most intense precursors were subjected to collision-induced dissociation with normalized collision energy of 30 and activation q of 0.25. Dynamic exclusion of 45 s was applied for repeated precursors.
PtdIns(3,4,5)P3 Interactome Identification
All MS raw files were analyzed in a single run with MaxQuant (http://maxquant.org), version 1.8.3, against the human UniProt database. The database used was https://www.uniprot.org/proteomes/UP000005640 (Homo sapiens accessed on November 14, 2019). Protein search was against UniProtKB (Swiss-Prot) reviewed proteins (20,386 proteins). Parameters used were cysteine carbamidomethylation as a fixed modification; methionine oxidation, acetylation (protein N termini), N-terminal glutamate to pyro glutamate (Gln-pyro_glu), and phosphorylation (STY) as variable modifications; enzyme specificity as strict trypsin with a maximum of two missed cleavages; minimum peptide length of seven amino acids; main search peptide tolerance of 4.5 ppm; IT MS/MS tolerance of 0.5 Da, peptide-spectrum match, protein and site false discovery rate (FDR) of 1%; match between runs as match from and to; decoy as revert and contaminants included.
Four independent biological replicates were analyzed for each PtdIns(3,4,5)P3 nuclear localization (PIP3nuc) DLD1 and SW480 cells. Proteins identified in at least two experiments (with two peptides, FDR of 1%) in both cell lines were considered a member of the PtdIns(3,4,5)P3 nuclear protein interactome.
Enrichment Analyses
Functional domain enrichment was performed for InterPro motifs using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) (36,37). Investigator-defined PtdIns(3,4,5)P3-binding motifs were translated into “regular expression vectors” for searching against protein sequences using Python (https://www.python.org/). To test for compartmentalization of nuclear PtdIns(3,4,5)P3 interactome proteins, enrichment analysis was performed against genes with experimentally validated subcellular localization data by the Human Protein Atlas using a hypergeometric test (38). Enrichment analysis for Gene Ontology (GO) biological processes was performed using DAVID. Protein–protein interaction network was achieved using the Search Tool for the Retrieval of Interacting Genes/Proteins database (STRING, http://string-db.org). A confidence interaction network was visualized with a highest confidence (0.900) using experiments as active interaction source (39). Biological process enrichment analyses were performed on the protein–protein interaction network.
Production of Recombinant Proteins
PH domain (Bruton tyrosine kinase [BTK], Q06187: 3–133), PH-like domain (SSRP1, Q08945: 1–107), PHD-type domain (tripartite motif containing 28 [TRIM28], Q13263: 627–672), RNA recognition motif 1 (RRM1) domains ± PtdIns(3,4,5)P3-binding domain (non-POU domain containing octamer binding [NONO], Q15233: 74–141; paraspeckle component 1 [PSPC1], Q8WXF1: 82–154), RRM1 domain (HNRNPA3, P51991: 35–118), and serine/arginine (S/R)-rich domain (SRSF1, Q07955: 179–248) were produced as GST-fusion proteins. Domains were generated in the pGEX4T1 vector as previously described, verified by DNA sequencing (32, 34, 40) and transformations were carried out using BL21-competent cells. GST fusion proteins were purified using glutathione agarose beads. Protein purity was analyzed using Coomassie-stained SDS-PAGE, and protein identity was confirmed using LC/MS–MS analysis.
Biosensor Analysis
Experiments were performed using a Biacore 3000 biosensor (Biacore Life Sciences, GE Healthcare). NH2-PtdIns(3,4,5)P3 analog (1 mg, 2 mg/ml in PBS) was conjugated to biotin using Sulfo-NHS-biotin (Thermo Scientific) (1/2 ratio, 2 h incubation at room temperature), purified using a Superdex peptide connected to an 1100Agilent HPLC, and immobilized onto neutravidin-derivatized sensor surface as previously described (32). Various concentration of GST-PH domain (BTK: 1.8, 0.9, 0.45, 0.22, and 0.11 μM), GST-PH like (SSRP1: 49.5, 24.7, 12.3, 6.15, 3.07, and 1.5 μM), GST-PHD-type (TRIM28: 22.8, 11.4, 5.7, 2.8, 1.4, and 0.7 μM), putative PIP-interacting domain (GST-PIP-RRM domains of NONO: 2.15, 1.07, 0.54, 0.27, 0.13, 0.067, and 0.033 μM; PSPC1: 5.25, 2.62, 1.31, 0.66, and 0.33 μM), and GST-RRM domains (NONO: 2.15, 1.07, 0.54, 0.27, 0.13, 0.067, and 0.033 μM; PSPC1: 5.25, 2.62, 1.31, 0.66, and 0.33 μM) were run across the PtdIns(3,4,5)P3 biosensor surface. Kinetic constants were derived from the resulting sensorgrams with BIAEVALUATION 4.1 software (Biacore Life Sciences) using (i) global analysis with a 1:1 model that includes terms for mass transfer of analyte to the surface and (ii) a 1:1 Langmuir model.
PtdIns(3,4,5)P3 Immunoprecipitation
Nuclear extracts of DLD1 and SW480 were prepared as per the nuclear lysate extraction protocol described previously. Immunoprecipitation was conducted according to the PtdIns(3,4,5)P3 affinity capture protocol, bound proteins were eluted in 0.2 M glycine (pH 2.0), and separated on 4 to 12% SDS-PAGE gels prior to Western blotting.
Cell-Based RG6 Splicing Reporter Assays
The RG6 fluorescent splicing reporter plasmid was kindly provided by Thomas Cooper (Addgene plasmid #80167; http://n2t.net/addgene:80167; Research Resource Identifier: Addgene_80167) (41). For in vivo splicing assays, 1 × 105 cells were plated in 12-well plates and transiently transfected with 1 μg RG6 DNA. Forty-eight hours post transfection, cells were trypsinized, pelleted at 1500 rpm for 5 min, and resuspended in 300 μl of PBS for flow cytometry. Each condition had six replicates, and two separate experiments were conducted for each cell line. Flow cytometry was performed on the LSR IIW flow cytometer (BD Biosciences) and was analyzed using FlowJo software (Becton Dickinson). Each sample was gated for 10,000 live cell events. Splicing was assessed as the dsRED:enhanced GFP ratio of transfected cells.
In vitro RG6 Splicing Reporter Assays
SKCO1 cells were serum starved for 1 h, and nuclear extracts were prepared as previously described (42). RG6 RNA transcripts were produced with T7 RNA polymerase using the Riboprobe in vitro transcription system (Promega). Splicing reactions were performed at 30 °C for 3 h in a final volume of 20 μl. Reactions contained 30% nuclear lysate, 2500 U/ml RNasin, 0.4 mM ATP, 20 mM creatine phosphate, 3 mM MgCl2, 0.6% polyvinyl alcohol, and 3 ng RNA probe. To test for the role of PtdIns(3,4,5)P3 in splicing, 250 μM soluble PtdIns(3,4,5)P3 was added to the reaction (43). Splicing reactions were subjected to proteinase K digestion and phenol/chloroform extraction before reverse transcription and PCR amplification using RG6-specific primers and subsequent analysis by 1% agarose gel electrophoresis.
Immunohistochemistry
p110α protein was detected by immunohistochemistry using rabbit anti-p110α monoclonal antibody (catalog no.: 4249; Cell Signaling Technology, 1:50 dilution). All assays were performed on a Discovery ULTRA IHC/ISH research platform (Ventana). Sections from TMAs were freshly cut to 4 μm and baked at 60 °C for 10 min followed by deparaffinization at 69 °C for 10 min. Antigen retrieval was conducted at 60 °C for 3 h using the EnVision FLEX Target Retrieval Solution (Dako). Primary antibody was added to each slide for 2 h at room temperature, followed by incubation with an anti-rabbit HQ linker (catalog no.: 760-4815; Ventana) for 30 min at room temperature and anti-HQ-horseradish peroxidase secondary antibody (catalog no.: 760-4820; Ventana) for a further 30 min at room temperature. TMAs were counterstained with hematoxylin. Stained slides were imaged on an Aperio ScanScope AT Slide Scanner (Leica Biosystems) with a 20× objective. The immunostained slides were evaluated for the presence of nuclear p110α protein staining by an anatomical pathologist (M.C.) blinded to other clinical and molecular data. Tumors exhibiting nuclear p110α protein staining in >5% of cells were scored as nuclear p110α positive.
PTEN was detected by immunohistochemistry using mouse anti-PTEN monoclonal antibody (clone 6H2.1; Cascade Bioscience, 1:100 dilution). Sections from TMAs were freshly cut to 4 μm and deparaffinized in xylene. Antigen retrieval was performed by microwaving in 10 mmol/l sodium citrate buffer pH 6 for 15 min. Blocking was achieved with 3% hydrogen peroxidase in methanol and 2.5% horse serum (S-2012-50; Vector Laboratories). Primary antibody was added to each slide for 90 min at room temperature, followed by incubation with an antimouse secondary antibody (catalog no.: MP-7402; Vector Laboratories) according to the manufacturer’s instructions. Visualization was performed using DAB chromogen for 10 min. The immmunostained slides were evaluated for loss of PTEN expression, defined as the absence of staining, by an anatomical pathologist (M.C.) blinded to other clinical and molecular data.
Experimental Design and Statistical Rationale
For the PtdIns(3,4,5)P3 interactome profiling, four independent biological replicates were analyzed for each PIP3nuc DLD1 and SW480 cells. Proteins identified in at least in two experiments for each cell line, with a minimum of two unique peptides (1% FDR at peptide and protein levels) were selected. Peptide and protein identifications are provided in supplemental Table S2. The MS proteomics data have been deposited in the ProteomeXchange Consortium Database (http://www.proteomexchange.org/) via the PRIDE (44) partner repository with the dataset identifier PXD021936. Statistical tests used for all other experiments, numbers of replicates, and definitions of statistical significance are described in the relevant figure legends. All bar charts show mean ± standard error of the mean. Statistical analyses were performed using the statistical computing software R (R Development Core Team, 2011). For univariate analyses, differences between groups were assessed using the Fisher’s exact test for categorical variables and the Student's t test for continuous variables as indicated. Multivariate analysis for the association of nuclear p110α staining in primary tumors with clinicopathologic and molecular features was performed using a generalized linear model. Colocalization was assessed using Mander's split colocalization coefficient. Associations between PtdIns(3,4,5)P3 and cell line mutation status were assessed using Wilcoxon rank-sum test. Outcome analyses were conducted for 5-year disease-free survival and overall survival. Cox proportional hazard models were used to assess the associations of tumor mucinous component with disease-free survival in the context of patient clinicomolecular features and adjuvant treatment. Hazard ratios and 95% confidence intervals were calculated. All comparisons were two sided, and p values of <0.05 were considered statistically significant.
Results
Variable Plasma Membrane–Nuclear Distribution of PtdIns(3,4,5)P3 Across CRC Cell Lines
Aberrant PtdIns(3,4,5)P3 signaling is a principal driver of CRC cell proliferation, survival, and tumor growth (1). Although classically viewed in the context of PI3Kα signaling at the plasma membrane or at microtubule-bound endosomes in the cytoplasm, it is recognized that PtdIns(3,4,5)P3 generation also occurs in the nucleus to regulate diverse biological functions (9). To elucidate the role of nuclear PtdIns(3,4,5)P3 signaling in CRC cells, we measured the plasma membrane–nuclear distribution of PtdIns(3,4,5)P3 in a panel of 58 human CRC cell lines using IF staining with a previously validated PtdIns(3,4,5)P3-specific GST-GRP1PH domain reporter (30, 45, 46) (supplemental Table S1). PtdIns(3,4,5)P3 reporter staining was detected in both the plasma membrane–cytoplasmic and the nuclear compartments in all CRC cell lines. However, there was an unexpected variation in PtdIns(3,4,5)P3 reporter staining intensities across the cell line panel, ranging from predominant plasma membrane–cytoplasmic (PIP3mem) localization in 74% (43 of 58) of cell lines to predominant PIP3nuc localization in 26% (15 of 58) of cell lines (Fig. 1, A and B). GST-GRP1PH reporter specificity for detection of PtdIns(3,4,5)P3 was confirmed for both plasma membrane and nuclear pools by competition assays with soluble PtdIns(3,4,5)P3 (Fig. 1C). The plasma membrane–nuclear distribution of PtdIns(3,4,5)P3 was not associated with PIK3CA or KRAS mutation status or with PTEN loss of protein expression (Fig. 1B).
Fig. 1.
PtdIns(3,4,5)P3subcellular localization in a panel of 58 CRC cell lines.A, representative images of immunofluorescence staining for PtdIns(3,4,5)P3 using a GST-GRP1PH domain reporter showing CRC cell lines with predominant nuclear (DLD1, SW480, CACO2) or membrane (SKCO1, LIM1215, LOVO) PtdIns(3,4,5)P3 localization. Scale bar represents 10 μm. B, quantification of the PtdIns(3,4,5)P3 plasma membrane–nuclear distribution across 58 CRC cell lines. Red boxes indicate the presence of a PIK3CA or KRAS mutation. Blue boxes indicate loss of PTEN protein expression. Data show mean ± SEM for five images per cell line with n > 20 cells per image. Statistical significance was determined using the Student’s t test. C, representative images of immunofluorescence staining for PtdIns(3,4,5)P3 GST-GRP1PH domain reporter in PIP3mem SKCO1 and PIP3nuc DLD1 cells for competition assays with soluble PtdIns(3,4,5)P3 (8.8 μM). Scale bar represents 10 μm. CRC, colorectal cancer; GST, glutathione-S-transferase; PIP3mem, PtdIns(3,4,5)P3 plasma membrane–cytoplasmic localization; PIP3nuc, PtdIns(3,4,5)P3 nuclear localization; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate.
PtdIns(3,4,5)P3 Plasma Membrane–Nuclear Distribution is Associated With PI3Kα Subcellular Localization
Canonical PtdIns(3,4,5)P3 generation by PI3Kα at the plasma membrane or microtubule-bound endosomes is initiated by agonist stimulation of RTKs (1, 2). To examine whether the differential subcellular localization of PtdIns(3,4,5)P3 in CRC cells was reflected by a change in PI3Kα localization, the subcellular distribution of PI3K catalytic subunit p110α was assessed in seven PIP3mem CRC cell lines (SKCO1, LIM1215, COLO320, SW948, LS513, HCC2998, and LOVO) and seven PIP3nuc cell lines (DLD1, SW480, HT55, CACO2, HCT15, HCT116, and HCA7). The localization of the PI3K-p110α subunit correlated with the differential plasma membrane–nuclear distribution of PtdIns(3,4,5)P3, with predominant plasma membrane–cytoplasmic staining in the PIP3mem cells and nuclear staining in the PIP3nuc cells (Fig. 2A). Specificity of the p110α antibody staining was confirmed by siRNA knockdown of p110α (PIK3CA) in both a PIP3mem (SKCO1) and PIP3nuc (DLD1) cell line (Figs. 2, B and C and S1).
Fig. 2.
Subcellular distribution of p110ɑ in PIP3nucversus PIP3memCRC cell lines.A, representative images of immunofluorescence staining for class I PI3K catalytic subunit p110α in seven PIP3nuc (DLD1, SW480, HT55, CACO2, HCT15, HCT116, HCA7) and seven PIP3mem (SKCO1, LIM1215, COLO320, SW948, LS513, HCC2998, LOVO) cell lines. Scale bar represents 10 μm. B and C, Western blot and representative images of immunofluorescence staining for siRNA-based knockdown of p110α (PIK3CA) and siRNA negative control (siNEG) in PIP3nuc DLD1 and PIP3mem SKCO1 cells. Scale bars represents 10 μm. CRC, colorectal cancer; PIP3mem, PtdIns(3,4,5)P3 plasma membrane–cytoplasmic localization; PIP3nuc, PtdIns(3,4,5)P3 nuclear localization.
PtdIns(3,4,5)P3 Levels in the Nucleus are Determined by PI3Kα Activity
Canonical PtdIns(3,4,5)P3 generation by PI3Kα at the plasma membrane or microtubule-bound endosomes is initiated by agonist stimulation of RTKs (1,2). To investigate whether agonist-activated PI3K signaling modulated nuclear PtdIns(3,4,5)P3 generation in CRC cells, PIP3nuc DLD1 and SW480 cells were stimulated with EGF (200 ng/ml) over a 20 min time course. EGF stimulation resulted in a significant increase in the ratio of nuclear to cytoplasmic PtdIns(3,4,5)P3 reporter staining after 5 to 10 min, reaching a plateau, and returning to baseline by 20 min (Fig. 3A). This response pattern was similar to that observed for the ratio of cytoplasmic to nuclear PtdIns(3,4,5)P3 staining in PIP3mem SKCO1 and LIM1215 cells after EGF stimulation (Fig. 3A). In PIP3nuc DLD1 and SW480 cells, EGF-induced changes in PtdIns(3,4,5)P3 reporter staining were further mirrored by a transient increase in the ratio of nuclear to cytoplasmic p110α staining with the latter further confirmed for PIP3nuc CACO2 cells (supplemental Fig. S2).
Fig. 3.
EGF-stimulated PI3Kα signaling controls nuclear PtdIns(3,4,5)P3levels in CRC cells.A, representative images and quantification of PtdIns(3,4,5)P3 responses to EGF (200 ng/ml) stimulation in PIP3nuc (DLD1, SW480) and PIP3mem (SKCO1, LIM1215) CRC cell lines over a 20 min time course using immunofluorescence microscopy with a GST-GRP1PH domain reporter. Scale bar represents 20 μm. B, representative images and quantification of PtdIns(3,4,5)P3 in PIP3nuc DLD1 and PIP3mem SKCO1 cells serum-starved for 1 h and treated with EGF (200 ng/ml) for 10 min ± pretreatment with the PI3Kα-specific inhibitor BYL719 (25 μM) for 1 h using immunofluorescence microscopy with a GST-GRP1PH domain reporter. Scale bar represents 10 μm. C, representative images and quantification of PtdIns(3,4,5)P3 responses to EGF (200 ng/ml) stimulation ± siRNA knockdown of p110α (PIK3CA) in PIP3nuc (DLD1) CRC cells using immunofluorescence microscopy with a GST-GRP1PH domain reporter. Scale bar represents 10 μm. Data show mean ± SEM from five images per condition with n > 20 cells per image. Statistical significance was determined compared with baseline (A) or between all groups (B and C) using Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗∗p < 0.0001. CRC, colorectal cancer; EGF, epidermal growth factor; GST, glutathione-S-transferase; PIP3nuc, PtdIns(3,4,5)P3 nuclear localization; PIP3mem, PtdIns(3,4,5)P3 plasma membrane–cytoplasmic localization; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate.
To confirm that generation of PtdIns(3,4,5)P3 in the nucleus was due to the enzymatic activity of PI3Kα, EGF-induced PtdIns(3,4,5)P3 response in PIP3nuc DLD1 and SW480 cells was assessed in the presence of the PI3Kα-specific inhibitor BYL719 (47). BYL719 treatment reduced PtdIns(3,4,5)P3 generation in the nucleus of both PIP3nuc CRC cell lines to below residual baseline levels at 1 h postserum starvation (Figs. 3B and S3). Similar results were obtained for the PtdIns(3,4,5)P3 levels at the plasma membrane in PIP3mem SKCO1 cells (Fig. 3B). The relationship between PI3Kα catalytic activity and PtdIns(3,4,5)P3 generation in the nuclear compartment was confirmed using siRNA knockdown of p110α (PIK3CA) in PIP3nuc DLD1 cells (Fig. 3C).
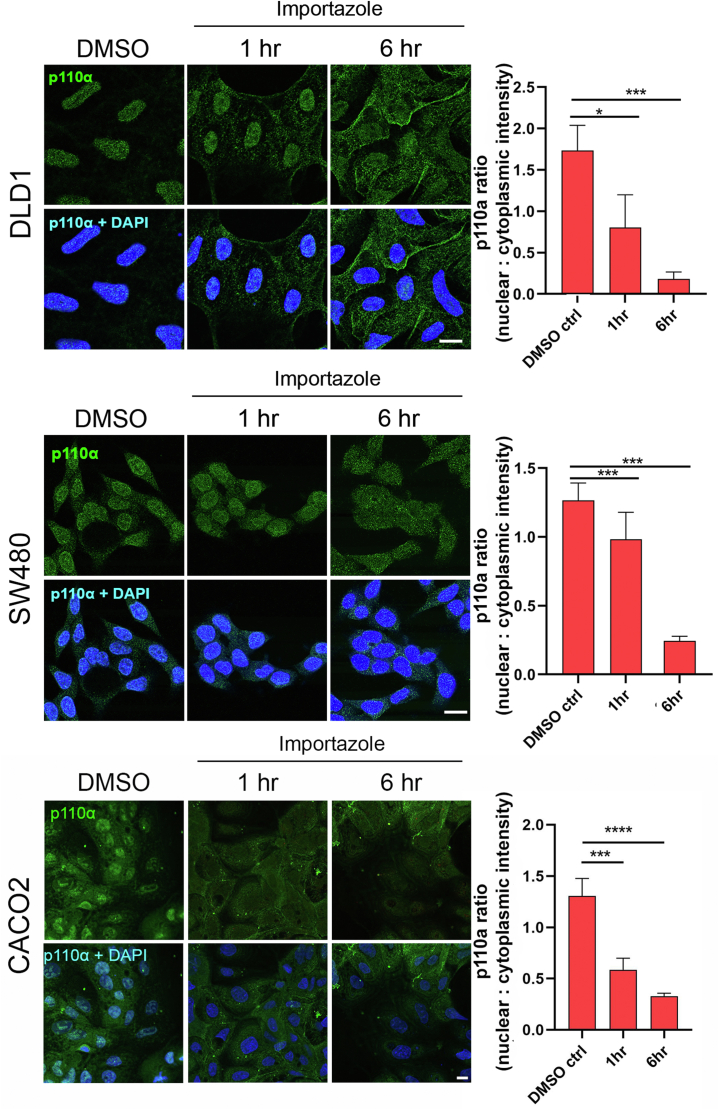
PI3Kα Nuclear Translocation is Mediated by the Importin β-Dependent Nuclear Import Pathway
The importin β-dependent nuclear import pathway is a major pathway of protein translocation into the nucleus (48). Although the p110α catalytic and associated regulatory subunits (p85α, p55α, p50α, p85β, and p55γ) do not contain classical nuclear localization sequence, nuclear localization of regulatory subunits has been described in various cellular systems with shuttling linked to complex formation with proteins that contain a nuclear localization sequence (13, 49, 50, 51). To probe for the involvement of the importin-β-dependent nuclear import pathway in the trafficking of PI3Kα and the impact on nuclear PtdIns(3,4,5)P3 levels, PIP3nuc DLD1, SW480, and CACO2 cells grown in standard medium with 10% fetal bovine serum were treated with importazole, a selective importin-β inhibitor, and assayed for p110α localization as nuclear to cytoplasmic ratio by IF microscopy (Fig. 4). Consistent with a block of the importin β-dependent nuclear import pathway, a significant increase in cytoplasmic and reduction in nuclear p110α levels was observed by 6 h post-treatment in both cell lines.
Figure 4.
PI3Kα nuclear translocation is mediated by the importin β-dependent nuclear import pathway. Representative images and quantification of p110α subcellular localization in PIP3nuc DLD1, SW480, and CACO2 cells treated with importazole (40 μM), a selective importin-β inhibitor, over a 6 h time course. Scale bar represents 10 μm. Data show mean ± SEM from five images per condition with n > 20 cells per image. Statistical significance was determined using Student’s t test. ∗p < 0.05, ∗∗∗p < 0.001. PIP3nuc, PtdIns(3,4,5)P3 nuclear localization.
Affinity Capture and MS-Based Interactome Profiling of the Nuclear PtdIns(3,4,5)P3 Effector Network in the Presence of SDS
PtdIns(3,4,5)P3 regulates cellular functions through recruitment of effector proteins via PtdIns(3,4,5)P3-binding domains (32). To gain insights into the potential biological functions of nuclear PtdIns(3,4,5)P3 in CRC cells, we established a workflow to map the nuclear protein interactome in PIP3nuc DLD1 and SW480 cells using affinity capture combined with high-resolution and unbiased proteomic analysis (supplemental Fig. S4, A and B). Nuclear protein extracts were prepared using Mono Q anion or Mono S cation exchange chromatography, and proteins were injected over an immobilized ω-amino PtdIns(3,4,5)P3 derivative conjugated to Affi-10 beads; preincubation with blank ethanolamine-derivatized Affi-gel10 beads was used to remove nonspecific binders (supplemental Fig. S4C). To increase the resolution of MS, a gel fractionation step was incorporated, and samples were subjected to LC/MS–MS analysis on an LTQ Orbitrap Elite mass spectrometer. Four independent biological replicates were analyzed for each cell line. In total, our experiments identified 1237 unique proteins present in at least two experiments in either cell line (1104 genes for DLD1 and 998 genes for SW480) indicating high reproducibility of protein identifications (supplemental Fig. S4D). Proteins identified in at least two experiments (with two peptides, FDR of 1%) in both cell lines were considered a member of the PtdIns(3,4,5)P3 nuclear protein interactome, yielding 867 candidate proteins (supplemental Fig. S4E and supplemental Table S3).
Nuclear PtdIns(3,4,5)P3 Interactome Proteins are Characterized by Noncanonical PtdIns(3,4,5)P3-Binding Domains
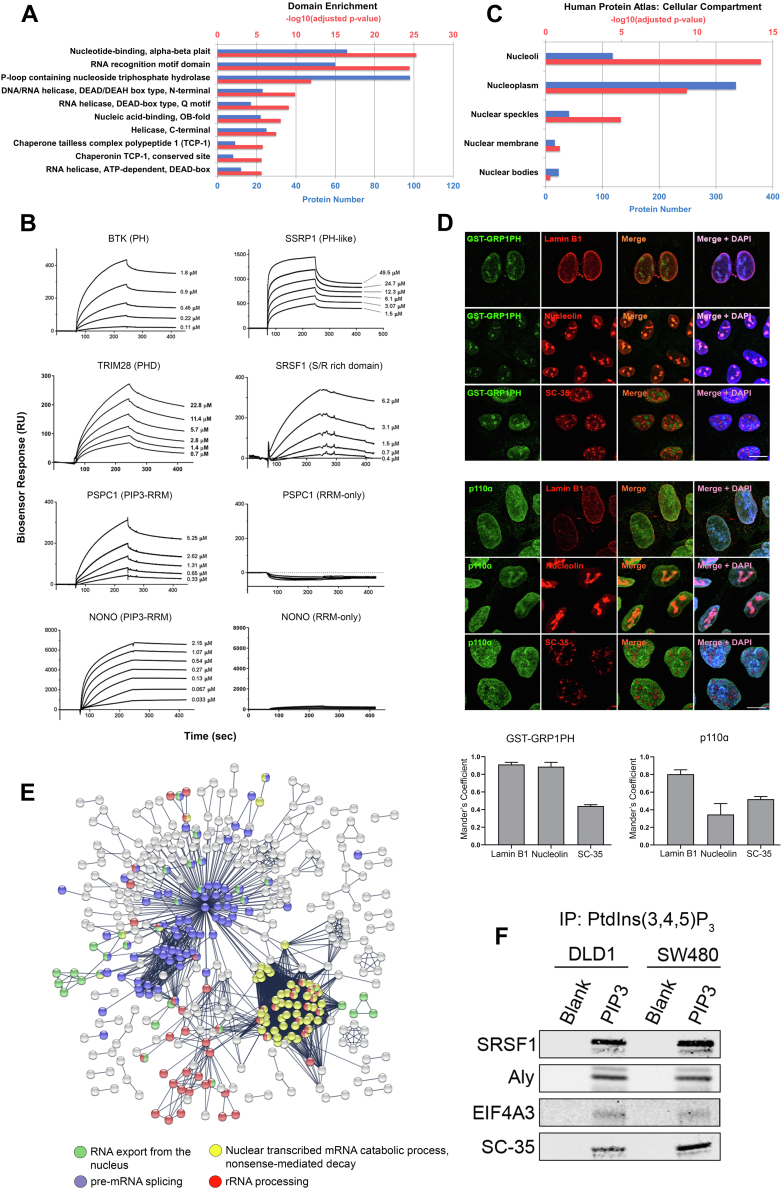
Protein interactions with PtdIns(3,4,5)P3 are mediated by distinctive PtdIns(3,4,5)P3-binding domains (52). To identify putative direct PtdIns(3,4,5)P3-binding proteins among our interactome candidates, functional domain enrichment was performed for InterPro motifs (36, 37). In contrast to our previous PtdIns(3,4,5)P3 protein interactome studies for plasma membrane–cytoplasmic protein extracts from CRC cells (32), domain enrichment analysis for nuclear PtdIns(3,4,5)P3 interactome proteins did not identify an over-representation of canonical PtdIns(3,4,5)P3-binding domains such as PH (two proteins), PH-like (three proteins), and PHD-type (four proteins) domains (supplemental Table S5). Instead, nuclear PtdIns(3,4,5)P3 interactome proteins were significantly enriched for RNA- and DNA-binding domains, including nucleotide-binding domains with an alpha–beta plait structure, RRM domains, P-loop containing nucleoside triphosphate hydrolase, DNA/RNA helicase, DEAD/DEAH box type, N-terminal and RNA helicase, DEAD-box type, Q motif (Fig. 5A and supplemental Table S5).
Fig. 5.
Nuclear PtdIns(3,4,5)P3interactome characterization for protein domains, nuclear compartmentalization and biological process enrichment.A, bar graph showing nuclear PtdIns(3,4,5)P3 interactome enrichment for Interpro protein domains. B, PtdIns(3,4,5)P3 binding affinities of the PH domain of BTK, PH-like domain of SSRP1, PHD-type domain of TRIM28, S/R-rich domain of SRSF1, PIP3-RRM, and RRM-only domains of PSPC1, and PIP3-RRM and RRM-only domains of NONO as determined by biosensor analysis. C, bar graph showing nuclear PtdIns(3,4,5)P3 interactome enrichment for experimentally validated subcellular protein localization data from the Human Protein Atlas. D, representative images and quantification of colocalization of PtdIns(3,4,5)P3 GST-GRP1PH domain reporter or p110α with lamin B1, nucleolin, and SC-35 in PIP3nuc DLD1 cells using immunofluorescence staining. Scale bar represents 10 μm. Data show mean ± SEM from five images per condition with n > 20 cells per image. Colocalization was determined using Mander’s coefficient. E, STRING network showing nuclear PtdIns(3,4,5)P3 interactome enrichment for putative protein–protein complexes based on high-quality and experimentally validated interactions (confidence score >0.9). F, Western blot of nuclear PtdIns(3,4,5)P3 interactome candidates SRSF1, Aly, EIF4A3, and SC-35 pulled down with PtdIns(3,4,5)P3 beads in nuclear fractions of PIP3nuc DLD1 and SW480 CRC cells. BTK, Bruton tyrosine kinase; CRC, colorectal cancer; GST, glutathione-S-transferase; NONO, non-POU domain containing octamer binding; PH, pleckstrin homology; PHD, plant homeodomain; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate; RRM, RNA recognition motif; S/R, serine/arginine; TRIM28, tripartite motif containing 28.
To reconcile this observation, we expanded our search to other investigator-defined PtdIns(3,4,5)P3-binding motifs that are not included in standard search algorithms. A basic consensus motif described by Lemmon et al. (53), [Ф]-X-K-X-[GASP]-X∗-[KR]-X∗-[RK]-X-R-X[FL], was detected within or overlapped with the RRM domain of eight candidate proteins (supplemental Table S6), whereas an extended PH domain motif described by Jungmichel et al. (54), G-X[3]-K-X[7,13]-[FW]-X[2]-R-X-F-X[30,80]-[KR]-X[12,14]W, was not found among our interactome members. We further examined an unstructured positively charged lysine/arginine-rich motif (K/R-X(n3–7)-K-X-K/R-K/R) previously reported as the dominant interaction site of nuclear proteins with PtdIns(4,5)P2 by Lewis et al. (55), which may also form an interaction site for PtdIns(3,4,5)P3. Consistent with this suggestion, we identified this motif in 336 proteins (p = 4.67 × 10−35) including 108 proteins with RNA- and DNA-binding domains (supplemental Table S7). A further six RRM proteins contained a polybasic S/R-rich domain. Taken together, these data suggest that the enrichment of RNA- and DNA-binding proteins among the nuclear PtdIns(3,4,5)P3 protein interactome is at least partly explained by the presence of noncanonical PtdIns(3,4,5)P3-binding sites including polybasic K/R- or S/R-rich motifs.
To validate the identified binding motifs, selected GST-tagged recombinant protein domains were assessed for their ability to bind immobilized PtdIns(3,4,5)P3 using biosensor assays. Canonical PtdIns(3,4,5)P3-binding domains detected in our nuclear protein interactome, including the PH domain of BTK, the PH-like domain of SSRP1, and the PHD-type domain of TRIM28, exhibited high PtdIns(3,4,5)P3-binding affinities (KD) of 40, 819, and 2.17 μM, respectively (Fig. 5B). We next examined PtdIns(3,4,5)P3 binding for the [Ф]-X-K-X-[GASP]-X∗-[KR]-X∗-[RK]-X-R-X[FL] motifs overlapping the RRM domains (referred to as PIP3-RRM) of NONO and PSPC1; constructs with the [Ф]-X-K-X-[GASP]-X∗-[KR]-X∗-[RK]-X-R-X[FL] motif omitted and leaving only the RRM domain (referred to as RRM only) were also produced to test for loss of PtdIns(3,4,5)P3 binding. The PIP3–RRM domains of PSPC1 and NONO bound PtdIns(3,4,5)P3 with high affinity (9 and 250 nM, respectively), whereas RRM-only domains showed complete loss or a 70-fold reduction in PtdIns(3,4,5)P3 binding for PSPC1 and NONO, respectively (Fig. 5B). As a representative of the over-represented polybasic interaction sites for PtdIns(3,4,5)P3, we examined the S/R-rich domain of SRSF1, obtaining a PtdIns(3,4,5)P3-binding affinity of 1.04 μM (Fig. 5B).
Nuclear PtdIns(3,4,5)P3 Interactome Proteins are Localized in the Nuclear Membrane, Nucleolus, and Nuclear Speckles
Proteins with related functions tend to be compartmentalized in particular regions of the cell. To test for compartmentalization of nuclear PtdIns(3,4,5)P3 interactome proteins, enrichment analysis was performed using experimentally validated subcellular localization data from the Human Protein Atlas (38) (Fig. 5C). Overall, 49.3% (427 of 867) of interactome proteins had experimentally confirmed nuclear localization (p = 5.88 × 10−16). Significant compartment enrichment was observed for the nucleoplasm (335 proteins, p = 9.60 × 10−10), nucleoli (118 proteins, p = 1.80 × 10−14), and nuclear speckles (41 proteins, p = 1.65 × 10−05), but not for the nuclear membrane, although classic nuclear membrane markers including lamin B1 and lamin B2 were represented among our interactome proteins. No enrichment was observed for nuclear bodies.
To examine whether PtdIns(3,4,5)P3 pools exhibited subnuclear compartmentalization reflecting the enrichment of PtdIns(3,4,5)P3 interactome proteins, PIP3nuc DLD1 and SW480 cells were costained with the PtdIns(3,4,5)P3 reporter and antibodies against lamin B1 (nuclear membrane), nucleolin (nucleoli), and SC-35 (nuclear speckles) (Figs. 5D and S5). PIP3nuc DLD1 cells were also examined for markers for PML bodies (PML) and Cajal bodies (coilin) (supplemental Fig. S6). Subnuclear PtdIns(3,4,5)P3 pools as detected by GST-GRP1PH reporter staining were colocalized with the nuclear membrane (Mander’s coefficient, 0.91 in DLD1, 0.78 in SW480), the nucleoli (Mander’s coefficient, 0.89 in DLD1, 0.73 in SW480) and, to a lesser extent, with nuclear speckles (Mander’s coefficient, 0.44 in DLD1, 0.66 in SW480). Diffuse GST-GRP1PH reporter staining was further evident throughout the nucleoplasm, but staining did not colocalize with PML bodies or Cajal bodies (supplemental Fig. S6).
Consistent with PtdIns(3,4,5)P3 subnuclear localization, p110α was colocalized with the nuclear membrane (Mander’s coefficient, 0.81 in DLD1, 0.80 in SW480), and, to a lesser extent, with the nucleoli (Mander’s coefficient, 0.74 in DLD1, 0.35 in SW480) and nuclear speckles (Mander’s coefficient, 0.44 in DLD1, 0.52 in SW480) (Figs. 5D and S5).
The Nuclear PtdIns(3,4,5)P3 Interactome is Enriched for Proteins Related to RNA Metabolism
To identify putative functions of nuclear PtdIns(3,4,5)P3 interactome proteins, GO enrichment analysis was performed, identifying significant enrichment for nuclear processes related to RNA metabolism, including rRNA processing, pre-mRNA splicing, mRNA surveillance, and RNA export from the nucleus (supplemental Table S8). Furthermore, STRING analysis for putative protein–protein complexes based on high-quality, experimentally validated interactions (confidence score >0.9, (39)) identified a protein–protein interaction network with 854 nodes and 2385 edges (p value <1.0 × 10−16), again highlighting subnetworks associated with rRNA processing, pre-mRNA splicing, mRNA surveillance, and mRNA transport (Fig. 5E).
To validate interactions between PtdIns(3,4,5)P3 and putative effector proteins of the pre-mRNA splicing and RNA transport pathways, PtdIns(3,4,5)P3 binding of SRSF1, SRSF2/SC-35, EIF4A3, and ALY was confirmed using immunoprecipitation studies on PIP3nuc DLD1 and SW480 nuclear extracts (Fig. 5F).
Nuclear PI3Kα Signaling is Associated With Modulation of pre-mRNA Splicing
Among members of the pre-mRNA splicing pathway identified in the nuclear PtdIns(3,4,5)P3 interactome were multiple SR proteins and heterogeneous nuclear ribonucleoproteins, components of the U1, U2, U4, U5, and U6 spliceosomal RNA complexes, and regulatory complexes such as the PRP19 and exon junction complexes (56) (Fig. 6A). To determine whether nuclear PtdIns(3,4,5)P3 generation was associated with changes in pre-mRNA splicing, live-cell RG6 fluorescent splicing reporter assays were performed on PIP3nuc DLD1, SW480, and CACO2 cells. This assay enables the quantification of two alternative splicing events from a single construct resulting in the mutually exclusive expression of a dsRED protein (exon skipping) or a GFP protein (exon inclusion) (41). EGF stimulation resulted in significant alterations of ratios of dsRED:GFP-expressing cells in all three PIP3nuc cell lines, with a decrease in GFP-expressing cells and an increase in dsRED-expressing cells, indicating enhanced exon skipping (Fig. 6B). In contrast, the PIP3mem cells showed no significant splicing reporter responses to EGF stimulation or BYL719 inhibition (Fig. 6B).
Fig. 6.
Nuclear PI3Kα signaling is associated with modulation of pre-mRNA splicing.A, schematic highlighting identified nuclear PtdIns(3,4,5)P3 interactome proteins and their role in the pre-mRNA splicing process. Proteins identified in the interactome are listed in red boxes. B, representative flow cytometry plots for RG6 splicing reporter assays on PIP3nuc DLD1 and PIP3mem SKCO1 cells, serum-starved for 1 h, and stimulated with EGF (200 ng/ml) for 24 h ± pretreatment with BYL719 (25 μM) for 1 h. Quantification of dsRed:GFP ratios for flow cytometry–based RG6 splicing reporter assays on PIP3nuc DLD1, SW480, and CACO2 cells and PIP3mem SKCO1 and LIM1215 cells. Data show the mean ± SEM for six biological replicates per condition. Statistical significance was determined using Student’s t test. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. C, representative images of immunofluorescence staining for SC-35 foci in PIP3nuc DLD1 and PIP3mem SKCO1 cells, serum starved for 1 h, and stimulated with EGF (200 ng/ml) for 10 min ± pretreatment with BYL719 (25 μM) for 1 h. Scale bar represents 10 μm. Data show the mean ± SEM from five images per condition with n > 20 cells per image. Statistical significance was determined using Student’s t test. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001. D, Agarose gel image of PCR products from in vitro splicing assays with the RG6 probe using nuclear extract from PIP3mem SKCO1 CRC cells in the absence or presence of soluble PtdIns(3,4,5)P3 (250 μM) or PtdIns(4,5)P2 (250 μM). PIP3mem, PtdIns(3,4,5)P3 plasma membrane–cytoplasmic localization; PIP3nuc, PtdIns(3,4,5)P3 nuclear localization; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate; PtdIns(3,4,5)P3, phosphatidylinositol-3,4,5-trisphosphate.
Nuclear speckles (also known as SC-35 domains) are known to regulate the availability of splicing factors at transcription sites, and changes in the size of nuclear speckles have been associated with signals that influence active pre-mRNA splicing (57). The PIP3nuc cells exhibited an increase in SC-35 foci area 10 min after EGF stimulation; this effect was blocked with BYL719 treatment (Fig. 6C). In contrast, PIP3mem cells showed a minor decrease in SC-35 foci area when stimulated with EGF, but this was not affected by BYL719 inhibition (Fig. 6C).
To probe for a direct role of the PtdIns(3,4,5)P3 levels in pre-mRNA splicing processes, cell-free splicing assays were performed on nuclear lysates from PIP3mem SKCO1 cells with and without addition of soluble PtdIns(3,4,5)P3 or PtdIns(4,5)P2 as a control (Fig. 6D). For these in vitro conditions, efficient exon skipping was observed for the RG6 splicing reporter at baseline and with addition of PtdIns(4,5)P2, whereas exon retention was increased with addition of soluble PtdIns(3,4,5)P3, consistent with a direct role of nuclear PtdIns(3,4,5)P3 levels in modulating pre-mRNA splicing outcomes. Nonetheless, further detailed analyses at the transcriptome level will be required to conclusively demonstrate a direct role of nuclear PtdIns(3,4,5)P3 levels in modulating pre-mRNA splicing outcomes.
Nuclear PI3Kα Localization is Prevalent in Primary Colorectal Tumors and Associated With Clinicopathologic Features
To determine the prevalence of nuclear PI3Kα localization in primary colorectal tumors and investigate associations with clinicomolecular features and disease outcomes, we performed immunohistochemistry analysis on TMAs for 406 stage I–IV CRC patients. Among tumors, 13% (51 of 403) had mutations in PIK3CA and 37% (149 of 406) in KRAS; 30% (120 of 406) showed loss of PTEN protein expression and 20% (81 of 401) had deficient DNA MMR. As observed for CRC cell lines, primary cancers exhibited a highly variable subcellular distribution of p110α staining, with 64% (259 of 406) of cases exhibiting plasma membrane–cytoplasmic p110α localization and 36% (147 of 406) of cases exhibiting nuclear p110α staining ranging from ∼5% to 60% of tumor cells (Fig. 7).
Fig. 7.
Immunohistochemistry staining for p110α localization in patient tumors. Representative images of p110α immunohistochemistry staining in CRC tissue cores, exhibiting plasma membrane–cytoplasmic (#437, #450, #446) or nuclear (#527, #432, #423) expression. Scale bar represents 20 μm. CRC, colorectal cancer.
Prevalence of nuclear p110α staining was similar across tumor stages I–IV, between well/moderately and poorly differentiated cases and between tumor locations, and there was no association with patient gender or age at diagnosis (p > 0.05 for all comparisons; supplemental Table S9A). Consistent with our findings in the CRC cell lines, nuclear p110α staining was not associated with tumor PIK3CA or KRAS mutation status, or PTEN protein expression, and there was no association with tumor MMR status (p > 0.05 for all comparisons; supplemental Table S9B). Nuclear p110α localization was not associated with overall survival for stage I–IV patients or disease-free survival for stage II/III patients (p > 0.05 for all comparisons; supplemental Table S10).
However, nuclear p110α staining was significantly associated with lower T stage and mucinous histology (p < 0.05 for both comparisons; supplemental Table S9). These latter associations were maintained in multivariate logistic regression analysis including other clinicopathologic features (p < 0.01 for both comparisons).
Discussion
Aberrant PI3Kα signaling caused by mutation or epigenetic alterations is a key driver of CRC development and a clinical marker of tumor sensitivity to aspirin and anti-EGFR antibody therapy (5, 6, 7, 8). The canonical view of agonist-activated PI3Kα signaling depicts PtdIns(3,4,5)P3 generation and recruitment of effector proteins at the plasma membrane or on microtubule-bound endosomes in the cytoplasm (1, 2). Here, we demonstrate that PI3Kα can also translocate into the nucleus mediated by the importin β-dependent nuclear import pathway, consistent with one previous study reporting nuclear localization of overexpressed GFP-tagged p110α in HCT116 cells (18). Nonetheless, the detailed mechanism regulating PI3Kα shuttling into the nucleus remains to be determined. We show that differential PI3Kα localization and activity is a principal determinant of the plasma membrane–cytoplasmic-nuclear distribution of PtdIns(3,4,5)P3 in CRC cells, generating a wide spectrum of subcellular distribution profiles.
Similar to the plasma membrane–cytoplasmic PtdIns(3,4,5)P3 pool, the nuclear PtdIns(3,4,5)P3 pool in CRC cells was responsive to EGF agonist stimulation in a PI3Kα-dependent manner. Previously, nuclear PtdIns(3,4,5)P3 signaling had only been attributed to PI3Kβ and PI3Kγ (13, 14), triggered by EGF (15), platelet-derived growth factor (58), nerve growth factor (59) or insulin stimulation (10, 11, 17, 59). The mechanism of agonist signal transduction activating nuclear PI3Kα signaling remains to be elucidated with nuclear shuttling of activated PI3Kα, a prime candidate analogous to findings for PI3Kβ and PI3Kγ (13, 14). Accordingly, we noted a transient increase in nuclear p110α staining in PIP3nuc CRC cell lines in response to EGF stimulation. However, p110α was already observed in the nucleus of serum-starved CRC cells, indicating that EGF is not required for nuclear localization.
Our subnuclear localization data identifying PI3Kα and PtdIns(3,4,5)P3 at the nuclear envelope, nucleoli, and nuclear speckles in PIP3nuc cells raises the question of the availability of the PtdIns(4,5)P2 precursor at these sites. Consistent with our data for PtdIns(3–5)P3, the presence of PtdIns(4,5)P2 in the nucleus has been reported for nuclear membrane, nucleoli, and nuclear speckle locations (60, 61). Nonmembrane-bound nuclear PtdIns(4,5)P2 has further been shown to interact with nucleic acids and proteins in the nucleoplasm (62), and the diffuse nuclear staining of p110α and PtdIns(3,4,5)P3 detected in our study may similarly indicate a nucleoplasm function.
Limited data are available regarding the effectors of nuclear PtdIns(3,4,5)P3 signaling (19, 20, 21, 22). Our interactome study identified a consensus interactome of 867 PtdIns(3,4,5)P3-binding proteins across two PIP3nuc CRC cell lines. In contrast to our previous plasma membrane–cytoplasmic PtdIns(3,4,5)P3 interactome studies in PIP3mem CRC cells (LIM1215), which discerned 58 proteins (20.5%) with canonical PtdIns(3,4,5)P3-binding sites among 282 interactome proteins (32), only nine (1.0%) such proteins were detected among the nuclear interactome proteins. Instead, the nuclear PtdIns(3,4,5)P3 interactome exhibited enrichment for polybasic K/R- or S/R-rich domains. Our data are consistent with a recent study that utilized the electrostatic interaction between neomycin and the phosphate group of PtdIns(3,4,5)P3, coupled with MS, to characterize PtdIns(3,4,5)P3-binding proteins for nuclear extracts from HELA cells, which similarly found an enrichment for polybasic motifs (63). One potential limitation of our PtdIns(3,4,5)P3 interactome study is nonspecific capture of proteins by Affi-Gel-10 beads, although we performed preincubation with blank beads to remove nonspecific binders with minimal residual protein detected in respective controls. A limitation of our PtdIns(3,4,5)P3-binding motif validation using biosensor analysis is that the ligand may not maintain its native configuration upon immobilization on the sensor chip surface, or that the ligand orientation may sterically hinder analyte binding.
GO enrichment as well as STRING analyses for nuclear PtdIns(3,4,5)P3 interactome proteins suggest potential roles of nuclear PI3Kα signaling in processes related to RNA metabolism, including rRNA processing, pre-mRNA splicing, mRNA surveillance, and RNA export from the nucleus. A role of EGF-activated nuclear PI3Kα signaling in pre-mRNA splicing was further indicated by our subnuclear localization, biochemical, and splicing reporter studies, although transcriptome level studies will be required to further substantiate these findings. Notably, PtdIns(4,5)P2 precursor has previously been implicated as a component of the pre-mRNA processing machinery through its interaction with splicing factors such as SC-35 and RNA pol II (43).
Our survey of p110α localization in tumor specimens from 406 stage I–VI CRC patients identified the presence of nuclear staining in 36% of cases. To our knowledge, this is the first report specifically considering p110α nuclear localization in CRC, with previous studies more broadly reporting on tumor p110α expression levels (64, 65). Our data suggest that nuclear p110α localization is related to clinical subtypes of CRC, with higher prevalence in tumors with lower T stage and mucinous histology. Whether this affects CRC sensitivity to anti-EGFR therapy or aspirin treatment remains to be explored.
In conclusion, our findings support a model in which nuclear translocation of PI3Kα defines a mechanism for spatial organization of PtdIns(3,4,5)P3 effector signaling across plasma membrane–cytoplasmic and nuclear compartments in human CRC, thereby modulating signaling responses.
Data Availability
The MS proteomics data generated during this study have been deposited in the ProteomeXchange Consortium Database (http://www.proteomexchange.org/) via the PRIDE (44) partner repository with the dataset identifier PXD021936.
Supplemental data
This article contains supplemental data.
Conflict of interest
The authors declare no competing interests.
Acknowledgments
We thank Professor Andrew Holmes and Dr Mark Gregory for technical support as well as Prof M. Schwab at the DKFZ for access to CRC cell lines.
Funding and additional information
This work was supported by the Victorian Government’s Operational Infrastructure Support Program, an Australian Rotary Health Scholarship (to M. P.), an NHMRC Senior Research Fellowship (grant no.: GNT1136119; to O. M. S.), and an NHMRC Project Grant (grant no.: GNT1050177, to O. M. S.).
Author contributions
M. P., B. C., and O. M. S. conceptualization; M. P., B. C., D. M., A. S., E. K., M. C., and O. M. S. formal analysis; M. P., B. C., C.-S. A., N. A. W., C. J. N., J. D., P. G., A. W. B., and O. M. S. investigation; M. P., B. C., and O. M. S. writing–original draft; M. P., B. C., D. M., A. S., E. K., C.-S. A., N. A. W., C. J. N., M. C., J. D., P. G., A. W. B., and O. M. S. writing–review & editing.
Supplemental Data
References
- 1.Hoxhaj G., Manning B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thapa N., Chen M., Horn H.T., Choi S., Wen T., Anderson R.A. Phosphatidylinositol-3-OH kinase signalling is spatially organized at endosomal compartments by microtubule-associated protein 4. Nat. Cell Biol. 2020;22:1357–1370. doi: 10.1038/s41556-020-00596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hennessy B.T., Smith D.L., Ram P.T., Lu Y., Mills G.B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 4.Day F.L., Jorissen R.N., Lipton L., Mouradov D., Sakthianandeswaren A., Christie M., et al. PIK3CA and PTEN gene and exon mutation-specific clinicopathologic and molecular associations in colorectal cancer. Clin. Cancer Res. 2013;19:3285–3296. doi: 10.1158/1078-0432.CCR-12-3614. [DOI] [PubMed] [Google Scholar]
- 5.De Roock W., Claes B., Bernasconi D., De Schutter J., Biesmans B., Fountzilas G., et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 6.Jhawer M., Goel S., Wilson A.J., Montagna C., Ling Y.H., Byun D.S., et al. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953–1961. doi: 10.1158/0008-5472.CAN-07-5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao X., Lochhead P., Nishihara R., Morikawa T., Kuchiba A., Yamauchi M., et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N. Engl. J. Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domingo E., Church D.N., Sieber O., Ramamoorthy R., Yanagisawa Y., Johnstone E., et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J. Clin. Oncol. 2013;31:4297–4305. doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- 9.Davis W.J., Lehmann P.Z., Li W. Nuclear PI3K signaling in cell growth and tumorigenesis. Front. Cell Dev. Biol. 2015;3:24. doi: 10.3389/fcell.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S.J. Insulin rapidly induces nuclear translocation of PI3-kinase in HepG2 cells. Biochem. Mol. Biol. Int. 1998;46:187–196. doi: 10.1080/15216549800203692. [DOI] [PubMed] [Google Scholar]
- 11.Martelli A.M., Borgatti P., Bortul R., Manfredini M., Massari L., Capitani S., et al. Phosphatidylinositol 3-kinase translocates to the nucleus of osteoblast-like MC3T3-E1 cells in response to insulin-like growth factor I and platelet-derived growth factor but not to the proapoptotic cytokine tumor necrosis factor alpha. J. Bone Mineral Res. 2000;15:1716–1730. doi: 10.1359/jbmr.2000.15.9.1716. [DOI] [PubMed] [Google Scholar]
- 12.Neri L.M., Milani D., Bertolaso L., Stroscio M., Bertagnolo V., Capitani S. Nuclear translocation of phosphatidylinositol 3-kinase in rat pheochromocytoma PC 12 cells after treatment with nerve growth factor. Cell Mol. Biol. (Noisy-le-grand) 1994;40:619–626. [PubMed] [Google Scholar]
- 13.Kumar A., Redondo-Munoz J., Perez-Garcia V., Cortes I., Chagoyen M., Carrera A.C. Nuclear but not cytosolic phosphoinositide 3-kinase beta has an essential function in cell survival. Mol. Cell Biol. 2011;31:2122–2133. doi: 10.1128/MCB.01313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metjian A., Roll R.L., Ma A.D., Abrams C.S. Agonists cause nuclear translocation of phosphatidylinositol 3-kinase gamma. A Gbetagamma-dependent pathway that requires the p110gamma amino terminus. J. Biol. Chem. 1999;274:27943–27947. doi: 10.1074/jbc.274.39.27943. [DOI] [PubMed] [Google Scholar]
- 15.Klein C., Gensburger C., Freyermuth S., Nair B.C., Labourdette G., Malviya A.N. A 120 kDa nuclear phospholipase Cgamma1 protein fragment is stimulated in vivo by EGF signal phosphorylating nuclear membrane EGFR. Biochemistry. 2004;43:15873–15883. doi: 10.1021/bi048604t. [DOI] [PubMed] [Google Scholar]
- 16.Ye K. PIKE/nuclear PI 3-kinase signaling in preventing programmed cell death. J. Cell. Biochem. 2005;96:463–472. doi: 10.1002/jcb.20549. [DOI] [PubMed] [Google Scholar]
- 17.Ye K., Hurt K.J., Wu F.Y., Fang M., Luo H.R., Hong J.J., et al. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell. 2000;103:919–930. doi: 10.1016/s0092-8674(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 18.Singh P., Dar M.S., Singh G., Jamwal G., Sharma P.R., Ahmad M., et al. Dynamics of GFP-fusion p110alpha and p110beta Isoforms of PI3K signaling pathway in normal and cancer cells. J. Cell Biochem. 2016;117:2864–2874. doi: 10.1002/jcb.25598. [DOI] [PubMed] [Google Scholar]
- 19.Karlsson T., Altankhuyag A., Dobrovolska O., Turcu D.C., Lewis A.E. A polybasic motif in ErbB3-binding protein 1 (EBP1) has key functions in nucleolar localization and polyphosphoinositide interaction. Biochem. J. 2016;473:2033–2047. doi: 10.1042/BCJ20160274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J.Y., Liu X., Cheng D., Peng J., Chan P.K., Wade P.A., et al. Nucleophosmin/B23, a nuclear PI(3,4,5)P(3) receptor, mediates the antiapoptotic actions of NGF by inhibiting CAD. Mol. Cell. 2005;18:435–445. doi: 10.1016/j.molcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Okada M., Jang S.W., Ye K. Akt phosphorylation and nuclear phosphoinositide association mediate mRNA export and cell proliferation activities by ALY. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8649–8654. doi: 10.1073/pnas.0802533105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drakas R., Tu X., Baserga R. Control of cell size through phosphorylation of upstream binding factor 1 by nuclear phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9272–9276. doi: 10.1073/pnas.0403328101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thacker E., Kearns B., Chapman C., Hammond J., Howell A., Theibert A. The arf6 GAP centaurin alpha-1 is a neuronal actin-binding protein which also functions via GAP-independent activity to regulate the actin cytoskeleton. Eur. J. Cell Biol. 2004;83:541–554. doi: 10.1078/0171-9335-00416. [DOI] [PubMed] [Google Scholar]
- 24.Bassi C., Ho J., Srikumar T., Dowling R.J., Gorrini C., Miller S.J., et al. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science. 2013;341:395–399. doi: 10.1126/science.1236188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nalaskowski M.M., Metzner A., Brehm M.A., Labiadh S., Brauer H., Grabinski N., et al. The inositol 5-phosphatase SHIP1 is a nucleo-cytoplasmic shuttling protein and enzymatically active in cell nuclei. Cell Signal. 2012;24:621–628. doi: 10.1016/j.cellsig.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 26.Mouradov D., Sloggett C., Jorissen R.N., Love C.G., Li S., Burgess A.W., et al. Colorectal cancer cell lines are representative models of the main molecular subtypes of primary cancer. Cancer Res. 2014;74:3238–3247. doi: 10.1158/0008-5472.CAN-14-0013. [DOI] [PubMed] [Google Scholar]
- 27.Ashraf S.Q., Nicholls A.M., Wilding J.L., Ntouroupi T.G., Mortensen N.J., Bodmer W.F. Direct and immune mediated antibody targeting of ERBB receptors in a colorectal cancer cell-line panel. Proc. Natl. Acad. Sci. U. S. A. 2012;109:21046–21051. doi: 10.1073/pnas.1218750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medico E., Russo M., Picco G., Cancelliere C., Valtorta E., Corti G., et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat. Commun. 2015;6:7002. doi: 10.1038/ncomms8002. [DOI] [PubMed] [Google Scholar]
- 29.Day F., Muranyi A., Singh S., Shanmugam K., Williams D., Byrne D., et al. A mutant BRAF V600E-specific immunohistochemical assay: correlation with molecular mutation status and clinical outcome in colorectal cancer. Target Oncol. 2015;10:99–109. doi: 10.1007/s11523-014-0319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmieri M., Nowell C.J., Condron M., Gardiner J., Holmes A.B., Desai J., et al. Analysis of cellular phosphatidylinositol (3,4,5)-trisphosphate levels and distribution using confocal fluorescent microscopy. Anal. Biochem. 2010;406:41–50. doi: 10.1016/j.ab.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 31.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. Fiji: an open-source platform for biological-image analysis. Nat. Met. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catimel B., Yin M.X., Schieber C., Condron M., Patsiouras H., Catimel J., et al. PI(3,4,5)P3 interactome. J. Proteome Res. 2009;8:3712–3726. doi: 10.1021/pr900320a. [DOI] [PubMed] [Google Scholar]
- 33.Conway S.J., Gardiner J., Grove S.J., Johns M.K., Lim Z.Y., Painter G.F., et al. Synthesis and biological evaluation of phosphatidylinositol phosphate affinity probes. Org. Biomol. Chem. 2010;8:66–76. doi: 10.1039/b913399b. [DOI] [PubMed] [Google Scholar]
- 34.Catimel B., Schieber C., Condron M., Patsiouras H., Connolly L., Catimel J., et al. The PI(3,5)P2 and PI(4,5)P2 interactomes. J. Proteome Res. 2008;7:5295–5313. doi: 10.1021/pr800540h. [DOI] [PubMed] [Google Scholar]
- 35.Gundry R.L., White M.Y., Murray C.I., Kane L.A., Fu Q., Stanley B.A., et al. Preparation of proteins and peptides for mass spectrometry analysis in a bottom-up proteomics workflow. Curr. Protoc. Mol. Biol. 2009 doi: 10.1002/0471142727.mb1025s88. Chapter 10:Unit10 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang da W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 37.Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucl. Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thul P.J., Akesson L., Wiking M., Mahdessian D., Geladaki A., Ait Blal H., et al. A subcellular map of the human proteome. Science. 2017;356:eaal3321. doi: 10.1126/science.aal3321. [DOI] [PubMed] [Google Scholar]
- 39.Snel B., Lehmann G., Bork P., Huynen M.A. String: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucl. Acids Res. 2000;28:3442–3444. doi: 10.1093/nar/28.18.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catimel B., Kapp E., Yin M.X., Gregory M., Wong L.S., Condron M., et al. The PI(3)P interactome from a colon cancer cell. J. Proteomics. 2013;82:35–51. doi: 10.1016/j.jprot.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Orengo J.P., Bundman D., Cooper T.A. A bichromatic fluorescent reporter for cell-based screens of alternative splicing. Nucl. Acids Res. 2006;34:e148. doi: 10.1093/nar/gkl967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayeda A., Krainer A.R. Preparation of HeLa cell nuclear and cytosolic S100 extracts for in vitro splicing. Met. Mol. Biol. 1999;118:309–314. doi: 10.1385/1-59259-676-2:309. [DOI] [PubMed] [Google Scholar]
- 43.Osborne S.L., Thomas C.L., Gschmeissner S., Schiavo G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J. Cell Sci. 2001;114:2501–2511. doi: 10.1242/jcs.114.13.2501. [DOI] [PubMed] [Google Scholar]
- 44.Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., et al. The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucl. Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gray A., Van Der Kaay J., Downes C.P. The pleckstrin homology domains of protein kinase B and GRP1 (general receptor for phosphoinositides-1) are sensitive and selective probes for the cellular detection of phosphatidylinositol 3,4-bisphosphate and/or phosphatidylinositol 3,4,5-trisphosphate in vivo. Biochem. J. 1999;344:929–936. [PMC free article] [PubMed] [Google Scholar]
- 46.Thiem S., Pierce T.P., Palmieri M., Putoczki T.L., Buchert M., Preaudet A., et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J. Clin. Invest. 2013;123:767–781. doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fritsch C., Huang A., Chatenay-Rivauday C., Schnell C., Reddy A., Liu M., et al. Characterization of the novel and specific PI3Kalpha inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials. Mol. Cancer Ther. 2014;13:1117–1129. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 48.Strom A.C., Weis K. Importin-beta-like nuclear transport receptors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-6-reviews3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia X., Cheng A., Akinmade D., Hamburger A.W. The N-terminal 24 amino acids of the p55 gamma regulatory subunit of phosphoinositide 3-kinase binds Rb and induces cell cycle arrest. Mol. Cell Biol. 2003;23:1717–1725. doi: 10.1128/MCB.23.5.1717-1725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pensa S., Neoh K., Resemann H.K., Kreuzaler P.A., Abell K., Clarke N.J., et al. The PI3K regulatory subunits p55alpha and p50alpha regulate cell death in vivo. Cell Death Differ. 2014;21:1442–1450. doi: 10.1038/cdd.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiu Y.H., Lee J.Y., Cantley L.C. BRD7, a tumor suppressor, interacts with p85alpha and regulates PI3K activity. Mol. Cell. 2014;54:193–202. doi: 10.1016/j.molcel.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Balla T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 2005;118:2093–2104. doi: 10.1242/jcs.02387. [DOI] [PubMed] [Google Scholar]
- 53.Lemmon M.A., Ferguson K.M. Molecular determinants in pleckstrin homology domains that allow specific recognition of phosphoinositides. Biochem. Soc. Trans. 2001;29:377–384. doi: 10.1042/bst0290377. [DOI] [PubMed] [Google Scholar]
- 54.Jungmichel S., Sylvestersen K.B., Choudhary C., Nguyen S., Mann M., Nielsen M.L. Specificity and commonality of the phosphoinositide-binding proteome analyzed by quantitative mass spectrometry. Cell Rep. 2014;6:578–591. doi: 10.1016/j.celrep.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 55.Lewis A.E., Sommer L., Arntzen M.O., Strahm Y., Morrice N.A., Divecha N., et al. Identification of nuclear phosphatidylinositol 4,5-bisphosphate-interacting proteins by neomycin extraction. Mol. Cell. Proteomics. 2011;10 doi: 10.1074/mcp.M110.003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoskins A.A., Moore M.J. The spliceosome: a flexible, reversible macromolecular machine. Trends Biochem. Sci. 2012;37:179–188. doi: 10.1016/j.tibs.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spector D.L., Lamond A.I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindsay Y., McCoull D., Davidson L., Leslie N.R., Fairservice A., Gray A., et al. Localization of agonist-sensitive PtdIns(3,4,5)P3 reveals a nuclear pool that is insensitive to PTEN expression. J. Cell Sci. 2006;119:5160–5168. doi: 10.1242/jcs.000133. [DOI] [PubMed] [Google Scholar]
- 59.Neri L.M., Martelli A.M., Borgatti P., Colamussi M.L., Marchisio M., Capitani S. Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3,4,5) trisphosphate synthesis precede PKC-zeta translocation to the nucleus of NGF-treated PC12 cells. FASEB J. 1999;13:2299–2310. [PubMed] [Google Scholar]
- 60.Boronenkov I.V., Loijens J.C., Umeda M., Anderson R.A. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol. Biol. Cell. 1998;9:3547–3560. doi: 10.1091/mbc.9.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fiume R., Faenza I., Sheth B., Poli A., Vidalle M.C., Mazzetti C., et al. Nuclear phosphoinositides: their regulation and roles in nuclear functions. Int. J. Mol. Sci. 2019;20:2991. doi: 10.3390/ijms20122991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sobol M., Krausova A., Yildirim S., Kalasova I., Faberova V., Vrkoslav V., et al. Nuclear phosphatidylinositol 4,5-bisphosphate islets contribute to efficient RNA polymerase II-dependent transcription. J. Cell Sci. 2018;131:jcs211094. doi: 10.1242/jcs.211094. [DOI] [PubMed] [Google Scholar]
- 63.Mazloumi Gavgani F., Slinning M.S., Morovicz A.P., Arnesen V.S., Turcu D.C., Ninzima S., et al. Nuclear phosphatidylinositol 3,4,5-trisphosphate interactome uncovers an enrichment in nucleolar proteins. Mol. Cell. Proteomics. 2021;20 doi: 10.1016/j.mcpro.2021.100102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui B., Tao J., Yang Y. Studies on the expression patterns of class I PI3K catalytic subunits and its prognostic significance in colorectal cancer. Cell Biochem. Biophys. 2012;62:47–54. doi: 10.1007/s12013-011-9257-6. [DOI] [PubMed] [Google Scholar]
- 65.Zhu Y.F., Yu B.H., Li D.L., Ke H.L., Guo X.Z., Xiao X.Y. PI3K expression and PIK3CA mutations are related to colorectal cancer metastases. World J. Gastroenterol. 2012;18:3745–3751. doi: 10.3748/wjg.v18.i28.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MS proteomics data generated during this study have been deposited in the ProteomeXchange Consortium Database (http://www.proteomexchange.org/) via the PRIDE (44) partner repository with the dataset identifier PXD021936.