Abstract
Dysfunction of glucagon-secreting α-cells participates in the progression of diabetes, and glucagon receptor (GCGR) antagonism is regarded as a novel strategy for diabetes therapy. GCGR antagonism upregulates glucagon and glucagon-like peptide 1 (GLP-1) secretion and, notably, promotes β-cell regeneration in diabetic mice. Here, we aimed to clarify the role of GLP-1 receptor (GLP-1R) activated by glucagon and/or GLP-1 in the GCGR antagonism–induced β-cell regeneration. We showed that in db/db mice and type 1 diabetic wild-type or Flox/cre mice, GCGR monoclonal antibody (mAb) improved glucose control, upregulated plasma insulin level, and increased β-cell area. Notably, blockage of systemic or pancreatic GLP-1R signaling by exendin 9-39 (Ex9) or Glp1r knockout diminished the above effects of GCGR mAb. Furthermore, glucagon-neutralizing antibody (nAb), which prevents activation of GLP-1R by glucagon, also attenuated the GCGR mAb–induced insulinotropic effect and β-cell regeneration. In cultured primary mouse islets isolated from normal mice and db/db mice, GCGR mAb action to increase insulin release and to upregulate β-cell–specific marker expression was reduced by a glucagon nAb, by the GLP-1R antagonist Ex9, or by a pancreas-specific Glp1r knockout. These findings suggest that activation of GLP-1R by glucagon participates in β-cell regeneration induced by GCGR antagonism in diabetic mice.
Article Highlights
Glucagon receptor (GCGR) antagonism promotes β-cell regeneration in type 1 and type 2 diabetic mice and in euglycemic nonhuman primates. Glucagon and glucagon-like peptide 1 (GLP-1) can activate the GLP-1 receptor (GLP-1R), and their levels are upregulated following GCGR antagonism.
We investigated whether GLP-1R activated by glucagon and/or GLP-1 contributed to β-cell regeneration induced by GCGR antagonism.
We found that blockage of glucagon–GLP-1R signaling attenuated the GCGR monoclonal antibody–induced insulinotropic effect and β-cell regeneration in diabetic mice.
Our study reveals a novel mechanism of β-cell regeneration and uncovers the communication between α-cells and β-cells in regulating β-cell mass.
Introduction
Loss of functional β-cell mass contributes to the development of diabetes (1). Glucagon, which is secreted from α-cells and mainly acts on the glucagon receptor (GCGR), has gained increasing attention owing to its counterregulatory function on glucose metabolism to insulin (2). Excessive glucagon secretion is common in patients with type 1 and type 2 diabetes (3). Hence, GCGR has been selected as a potential target for diabetes therapy. GCGR antagonism by distinct methods, including gene deletion (4), antisense oligonucleotides (5), small molecule antagonists (6), and antibody (7,8), improves glucose control in animals and humans with type 1 diabetes (T1D) or type 2 diabetes (T2D).
Although liver is the main target of glucagon action, glucagon also acts on β-cells and is responsible for insulin secretion (9–13). GCGR antagonism promotes β-cell regeneration (characterized by the increased β-cell mass through β-cell proliferation, β-cell redifferentiation, conversion of α- to β-cells, and β-cell neogenesis from progenitors) in T1D and T2D mice (7,14–16) and in euglycemic nonhuman primates (17). The beneficial effect might be mediated via glucagon-like peptide 1 (GLP-1), because plasma GLP-1 level and intestinal GLP-1 content were greatly elevated by GCGR antagonism (18). However, GLP-1 is immediately inactivated by local gut and systemic ubiquitous expression of dipeptidyl peptidase 4 and is rapidly cleared from circulation by the kidney (19,20). Therefore, it is somewhat difficult for intestinal L-cell–derived GLP-1 to exert effects on β-cells. α-Cells also own the ability to secrete GLP-1 (21,22). Whether α-cell–derived GLP-1 secretion is enhanced by GCGR antagonism and whether it participates in β-cell regeneration remains to be clarified.
GLP-1 exerts its protective effects on β-cells via activation of the GLP-1 receptor (GLP-1R) (20). Notably, glucagon also activates GLP-1R (9,13,23,24). Hyperglucagonemia and an elevated GLP-1 level are common when the glucagon–GCGR signaling pathway is blocked (25,26), making it complicated to clarify which hormone activates GLP-1R and whether the activated GLP-1R is responsible for β-cell regeneration.
In this study, we used REMD 2.59, a human GCGR monoclonal antibody (mAb), to specifically antagonize GCGR and detected its effect on β-cell regeneration in db/db mice (a T2D model) and streptozotocin (STZ)-induced T1D mice. First, we verified that glucagon and GLP-1 levels in the circulation and pancreas were elevated by GCGR mAb. Then, we determined whether GLP-1R signaling participated in GCGR mAb–induced β-cell regeneration by using the GLP-1R antagonist exendin 9-39 (Ex9) or global and pancreas-specific Glp1r-knockout mice. Next, whether glucagon–GLP-1R signaling took part in the process was investigated by application of the glucagon-neutralizing antibody (nAb) GLU-001, which could bind to glucagon and prevent it binding with GLP-1R under the GCGR mAb treatment condition. In addition, we clarified whether islet-derived GLP-1 was enhanced by GCGR mAb and determined the involvement of glucagon–GLP-1R signaling in GCGR mAb–mediated regulation of β-cell function and phenotype in primary normal and diabetic mouse islets. Our study provides a novel insight into understanding of glucagon–GLP-1R signaling and reveals a new mechanism of GCGR antagonism–induced β-cell regeneration.
Research Design and Methods
Animal Experiments
All animal experiments were approved by the Peking University Animal Care and Use Committee. Male db/db mice (8 weeks old) were treated for 4 weeks with IgG, GCGR mAb, Ex9, or GCGR mAb combined with Ex9. GCGR mAb REMD 2.59 (5 mg/kg; REMD Biotherapeutics, Camarillo, CA) and IgG (5 mg/kg, as control) were intraperitoneally injected weekly. Ex9 (50 nmol/kg/day; Bachem, Bubendorf, Switzerland) or saline was administered by micro-osmotic pumps (ALZET, Cupertino, CA) (27).
To induce the T1D model, male and female global Glp1r-knockout (Glp1r−/−) mice (Supplementary Fig. 1A and B) and wild-type (WT) Glp1r+/+ littermates, and male pancreas-specific Glp1r-knockout (Glp1rpan−/−) mice and Glp1r-flox or Pdx1-Cre (Flox/cre) littermates (Supplementary Fig. 1C and D) were injected with STZ (125 mg/kg; MilliporeSigma, St. Louis, MO) at the age of 8–12 weeks. Diabetic mice were treated with 5 mg/kg GCGR mAb or IgG for 4 weeks.
STZ-induced T1D male C57BL/6J mice (12 weeks old) were treated with 5 mg/kg IgG or GCGR mAb with 4 mg/kg A-TNP (as control of glucagon nAb) or glucagon nAb GLU-001 (Novo Nordisk A/S, Bagsvaerd, Denmark) for 4 weeks. GLU-001 and A-TNP were injected intraperitoneally daily (28).
Glucose Monitoring and Glucose Tolerance Test
Blood glucose level in samples from a tail vein was monitored by OneTouch Ultra glucometer (LifeScan, Milpitas, CA). Glucose tolerance tests were performed after overnight fasting. Blood glucose levels were measured at baseline, 30, 60, and 120 min after intraperitoneal injection of 2 g/kg glucose.
Immunofluorescent Staining and Analysis
Pancreatic tissues were fixed with 10% (v/v) neutral-buffered formalin and embedded in paraffin. Sections (5-μm-thick) were dewaxed, hydrated, blocked with goat serum, and incubated with primary antibodies at 4°C overnight and with secondary antibodies for 1 h at room temperature, followed by nuclear staining with DAPI. Fluorescence was imaged using a Leica TCS SP8 confocal fluorescence microscope (Leica Microsystems, Wetzlar, Germany) or automatic digital slide scanner (Pannoramic MIDI, 3DHISTECH, Budapest, Hungary). The antibodies are summarized in Supplementary Table 1.
For cell quantification, three to four sections (which covered the whole pancreas) per pancreas from three mice per group were imaged. The area of positive-staining cells was analyzed by Fiji software (National Institutes of Health, Bethesda, MD) (29). Islet clusters with ≤10 cells are defined as small islets, indicating islet neogenesis (30).
Primary Mouse Islet Intervention
Primary mouse islets were isolated as previously reported (31). Islets from 8-week-old male normal C57BL/6J and db/db mice were treated with 1,000 nmol/L GCGR mAb or IgG in the absence or presence of Ex9 (200 nmol/L) or glucagon nAb (10 mg/L) for 24 h in a high glucose (30 mmol/L) condition. Islets from 8-week-old male Glp1rpan−/− mice were treated with 1,000 nmol/L GCGR mAb or IgG for 24 h.
Hormone Measurements
ELISA kits specific for insulin, C-peptide, glucagon, and active GLP-1 are summarized in Supplementary Table 1. The hormone levels in pancreatic lysates and islet culture supernatants were normalized to the protein content of pancreatic lysates or cultured islets, respectively.
RNA Extraction, Reverse Transcription, and Quantitative PCR
Total RNA was isolated using Trizol reagent (Thermo Fisher Scientific, Waltham, MA), and cDNA was synthesized with a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific). Quantitative PCR was performed using THUNDERBIRD SYBR qPCR Mix (Toyobo Co., Ltd., Osaka, Japan) on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific). Relative gene expression was normalized to Actb and calculated using the 2−ΔΔCt method. Primer sequences are summarized in Supplementary Table 2.
Statistical Analysis
If data were Gaussian distributed, they are presented as mean ± SEM and were analyzed by one-way or two-way ANOVA, followed by the Dunnett T3 multiple comparisons test, Tukey multiple comparisons test, or Bonferroni multiple comparisons test among three or more groups, or unpaired Student t test (two-tailed) between two groups, as appropriate. If data were not Gaussian distributed, they are presented as median (interquartile range) and were analyzed by Kruskal-Wallis test, followed by the Dunn multiple comparisons test among three or more groups, or the Mann-Whitney test (two-tailed) between two groups, as appropriate. P < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA).
Data and Resource Availability
The data sets and resources generated and analyzed during the current study are available from the corresponding authors upon reasonable request.
Results
GCGR Antagonism Promotes β-Cell Regeneration and Upregulates Plasma and Pancreatic Glucagon and GLP-1 Levels in T2D and T1D Mice
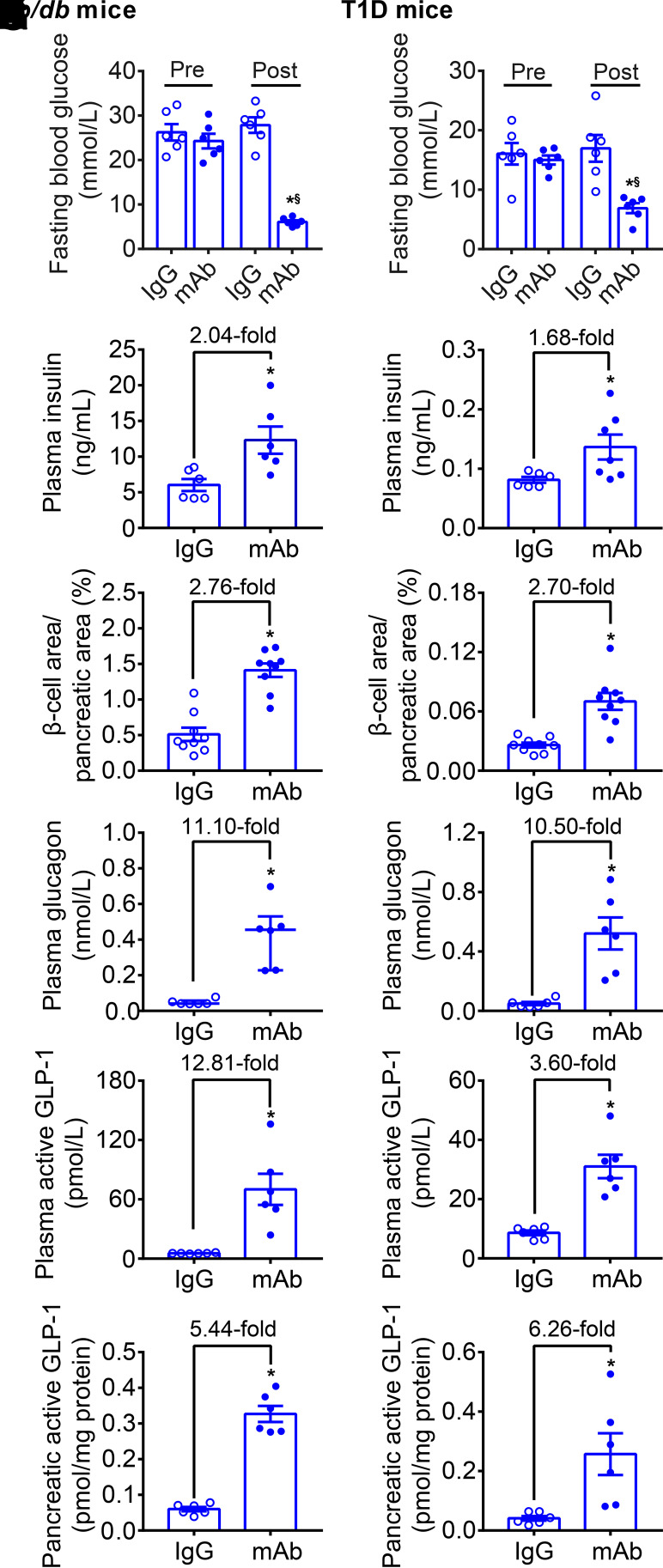
In db/db mice and T1D mice, no significant difference was found between GCGR mAb and IgG groups in body weight during the 4-week treatment (Supplementary Fig. 2A and B). Compared with baseline or IgG control, GCGR mAb significantly lowered the fasting and random blood glucose levels in two diabetic mice (Fig. 1A and B and Supplementary Fig. 2C and D). Plasma insulin level was increased 2.04- and 1.68-fold by GCGR mAb in db/db mice and T1D mice, respectively (Fig. 1C and D). An approximately threefold increase of β-cell area was observed in the GCGR mAb group in two diabetic mice (Fig. 1E and F and Supplementary Fig. 3), suggestive of β-cell regeneration. GCGR mAb expanded α-cell area (Supplementary Figs. 2E and F and 3), and increased plasma glucagon (∼11-fold), plasma GLP-1 (db/db: 12.81-fold; T1D: 3.60-fold), and pancreatic GLP-1 (∼5- to 6-fold) levels in two diabetic models (Fig. 1G–L), indicative of changes of α-cells in number and function. These results suggested that elevated glucagon and GLP-1 levels might be involved in GCGR mAb–induced β-cell regeneration.
Figure 1.
GCGR mAb promotes β-cell regeneration and upregulates plasma and pancreatic glucagon and GLP-1 levels in T2D and T1D mice. Male db/db mice (8 weeks) were used as a T2D model. Male C57BL/6J mice (12 weeks) were injected with STZ to induce a T1D model. Mice were treated weekly with IgG (5 mg/kg, as control) or GCGR mAb (5 mg/kg) for 4 weeks. A, C, E, G, I, and K: Parameters in db/db mice. B, D, F, H, J, and L: Parameters in T1D mice. A and B: Fasting blood glucose. C and D: Plasma insulin (n = 6 mice/group). E and F: Quantification of the β-cell area per pancreatic section (n = 3 sections/mouse × 3 mice/group). G and H: Plasma glucagon. I and J: Plasma active GLP-1. K and L: Pancreatic active GLP-1 (n = 6 mice/group). Data are expressed as the mean ± SEM or median (interquartile range). Statistical analysis was performed by two-way ANOVA, followed by the Bonferroni multiple comparisons test in A and B, by unpaired Student t test in C−F and H−L, or by Mann-Whitney test in G. *P < 0.05 vs. IgG control; §P < 0.05 vs. pretreatment in the same group.
Systemic or Pancreatic GLP-1R Signaling Participates in Glucose-Lowering and Insulinotropic Effects Induced by GCGR Antagonism in T2D and T1D Mice
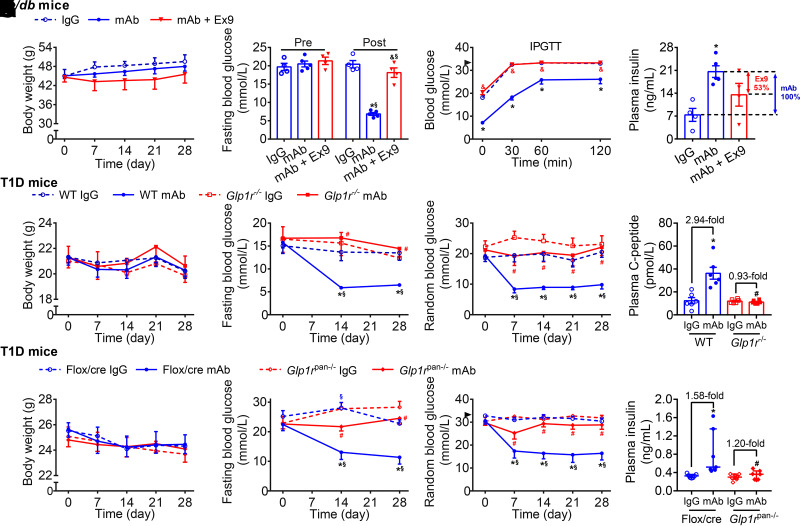
We used Ex9, a specific GLP-1R antagonist without affecting GCGR (9), to investigate the involvement of GLP-1R in GCGR mAb–mediated metabolic effects. Since db/db mice treated with Ex9 alone died rapidly owing to uncontrollable hyperglycemia, this group was excluded. Body weight was comparable among IgG, GCGR mAb, and GCGR mAb + Ex9 groups (Fig. 2A). Four-week treatment of GCGR mAb significantly lowered fasting glucose and improved glucose tolerance compared with IgG treatment, and Ex9 addition abolished these effects (Fig. 2B and C). Plasma insulin level was higher in the GCGR mAb group than in the IgG group and decreased by 53% in the GCGR mAb + Ex9 group versus the GCGR mAb group (Fig. 2D).
Figure 2.
Systemic or pancreatic GLP-1R signaling participates in glucose-lowering and insulinotropic effects induced by GCGR antagonism in T2D and T1D mice. A–D: Parameters in db/db mice. Male db/db mice (8 weeks) were treated with IgG (5 mg/kg/week, n = 4), GCGR mAb (5 mg/kg/week, n = 5), or GCGR mAb combined with Ex9 (50 nmol/kg/day, n = 4) for 4 weeks. E–H: Parameters in T1D Glp1r−/− mice and WT Glp1r+/+ littermates. Male and female Glp1r−/− mice and WT littermates were injected with STZ to induce T1D models at the age of 8–12 weeks and treated weekly with IgG (5 mg/kg) or GCGR mAb (5 mg/kg) for 4 weeks (n = 6 mice/group). I–L: Parameters in T1D Glp1rpan−/− mice and Flox/cre littermates. Male Glp1rpan−/− mice and Flox/cre littermates were injected with STZ to induce T1D models at the age of 8−12 weeks and treated weekly with IgG (5 mg/kg, as control) or GCGR mAb (5 mg/kg) for 4 weeks (n = 7 mice/group). A, E, and I: Body weight. B, F, and J: Fasting blood glucose. Blood glucose during intraperitoneal glucose tolerance test (IPGTT) (C) or random blood glucose (G and K). The arrowheads in C and K indicate the upper detection limit (33.3 mmol/L) of the glucometer. Plasma insulin (D and L) or C-peptide (H). Data are expressed as the mean ± SEM or median (interquartile range). Statistical analysis was performed by two-way ANOVA, followed by the Bonferroni multiple comparisons test in A−C, the Tukey multiple comparisons test in E–G and I–K, or by one-way ANOVA, followed by the Bonferroni multiple comparisons test in D, the Tukey multiple comparisons test in H, or by the Kruskal-Wallis test, followed by the Dunn multiple comparisons test in L. *P < 0.05 vs. IgG control in the same genotype of mice; §P < 0.05 vs. pretreatment in the same group; &P < 0.05 vs. GCGR mAb in db/db mice; #P < 0.05 vs. WT or Flox/cre littermates on the same treatment.
STZ-induced T1D models of Glp1r−/− mice and WT mice were treated with GCGR mAb or IgG. Mice in the four groups had similar body weight (Fig. 2E). Compared with IgG control, GCGR mAb significantly decreased the fasting and random blood glucose levels in WT mice but not in Glp1r−/− mice (Fig. 2F and G). GCGR mAb remarkably increased the plasma C-peptide level in WT mice, but this effect disappeared in Glp1r−/− mice (Fig. 2H).
GLP-1R is expressed not only in pancreata but also in other tissues, such as brain, where GLP-1R signaling is involved in control of food intake and thus influences glucose metabolism (20). Therefore, we constructed Glp1rpan−/− mice to detect the effect of pancreatic GLP-1R. STZ-induced T1D models of Glp1rpan−/− mice and Flox/cre littermates were treated with GCGR mAb or IgG. Results showed that there was no difference in body weight among the four groups (Fig. 2I). Compared with IgG control, GCGR mAb remarkably lowered the fasting and random blood glucose levels in Flox/cre mice but not in Glp1rpan−/− mice (Fig. 2J and K). GCGR mAb significantly increased plasma insulin level in Flox/cre mice, and this effect disappeared in Glp1rpan−/− mice (Fig. 2L).
Collectively, these results suggested that systemic and pancreas-specific GLP-1R blockage diminished the GCGR antagonism–induced glucose-lowering and insulinotropic effects.
Systemic or Pancreatic GLP-1R Signaling Contributes to β-Cell Mass Expansion Induced by GCGR Antagonism in T2D and T1D Mice
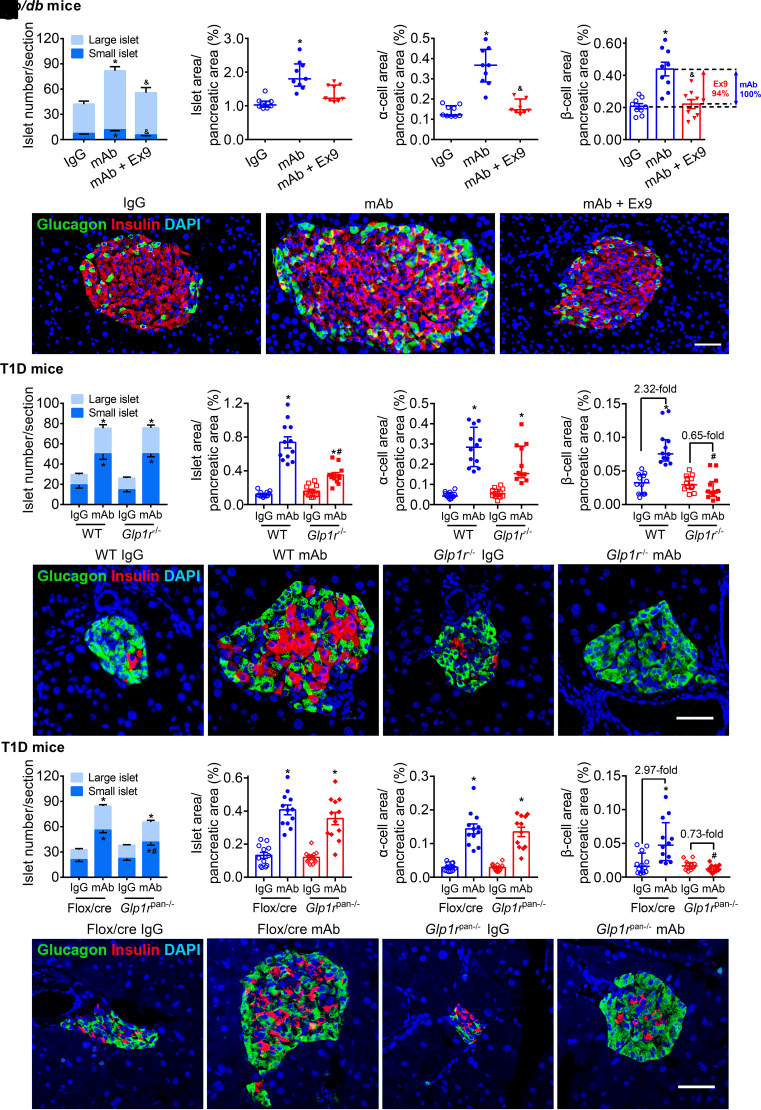
In db/db mice, GCGR mAb remarkably increased the number of islets, including small islets (≤10 cells) and large islets, islet area, α-cell area, and β-cell area, and Ex9 addition attenuated these effects, with 94% decrease in β-cell area (Fig. 3A–E and Supplementary Fig. 4), indicating that pharmacological blockage of systemic GLP-1R signaling diminished GCGR antagonism−induced islet regeneration.
Figure 3.
Systemic or pancreatic GLP-1R signaling contributes to β-cell mass expansion induced by GCGR antagonism in T2D and T1D mice. A–E: Parameters in db/db mice. Male db/db mice (8 weeks) were treated with IgG (5 mg/kg/week), GCGR mAb (5 mg/kg/week), or GCGR mAb combined with Ex9 (50 nmol/kg/day) for 4 weeks. F−J: Parameters in T1D Glp1r−/− mice and WT Glp1r+/+ littermates. Male and female Glp1r−/− mice and WT littermates were injected with STZ to induce T1D models at the age of 8–12 weeks and treated weekly with IgG (5 mg/kg) or GCGR mAb (5 mg/kg) for 4 weeks. K–O: Parameters in T1D Glp1rpan−/− mice and Flox/cre littermates. Male Glp1rpan−/− mice and Flox/cre littermates were injected with STZ to induce T1D models at the age of 8−12 weeks and treated weekly with IgG (5 mg/kg, as control) or GCGR mAb (5 mg/kg) for 4 weeks. A, F, and K: Quantification of the islet number per pancreatic section. An islet with a cell number ≤10 is defined as a small islet and the others as a large islet. B, G, and L: Quantification of the islet area per pancreatic section. C, H, and M: Quantification of the α-cell area per pancreatic section. D, I, and N: Quantification of the β-cell area per pancreatic section (n = 3–4 sections/mouse × 3 mice/group). E, J, and O: Representative images of an islet immunostained for glucagon, insulin, and DAPI. Scale bar = 50 μm. Data are expressed as the mean ± SEM or median (interquartile range). Statistical analysis was performed by one-way ANOVA, followed by the Tukey multiple comparisons test in A and K, the Dunnett T3 multiple comparisons test in D, F, G, L, and M, or by the Kruskal-Wallis test, followed by the Dunn multiple comparisons test in B, C, H, I, and N. *P < 0.05 vs. IgG control in the same genotype of mice; &P < 0.05 vs. GCGR mAb in db/db mice; #P < 0.05 vs. WT or Flox/cre littermates on the same treatment.
Similarly, GCGR mAb significantly increased the number of small islets and large islets, islet area, α-cell area, and β-cell area in T1D WT mice (Fig. 3F–J and Supplementary Fig. 5). In T1D Glp1r−/− mice, GCGR mAb still increased islet number, islet area, and α-cell area (Fig. 3F–H). However, islet area was smaller in Glp1r−/− mice than in WT mice after GCGR mAb treatment (Fig. 3G). Notably, GCGR mAb no longer increased β-cell area in T1D Glp1r−/− mice (Fig. 3I). These results suggested that genetic elimination of systemic GLP-1R signaling abolished GCGR antagonism−induced β-cell regeneration but had little effect on α-cell hyperplasia.
In T1D Flox/cre mice and Glp1rpan−/− mice, GCGR mAb remarkably increased the number of small islets and large islets, islet area, and α-cell area (Fig. 3K–M and O and Supplementary Fig. 6). However, the small islet number was lower in Glp1rpan−/− mice than in Flox/cre mice after GCGR mAb treatment (Fig. 3K). Again, GCGR mAb could not enlarge β-cell area in T1D Glp1rpan−/− mice (Fig. 3N). These results indicated that genetic elimination of pancreatic GLP-1R signaling abolished GCGR antagonism−induced β-cell regeneration, while it did not affect α-cell hyperplasia.
Systemic or Pancreatic GLP-1R Signaling Is Involved in β-Cell Self-Replication, α- to β-Cell Transdifferentiation, and β-Cell Neogenesis Triggered by GCGR Antagonism in T1D Mice
GCGR mAb promoted β-cell regeneration through β-cell self-replication, α- to β-cell transdifferentiation, and β-cell neogenesis in diabetic mice, as indicated by our previous lineage-tracing studies (14,15,32). Here, we detected the effect of GLP-1R signaling on these regeneration paths. In T1D WT or Flox/cre littermates, GCGR mAb significantly increased the ratio of BrdU+insulin+ cells (the proliferating β-cells) and glucagon+insulin+ cells (indicating β-cells transdifferentiated from α-cells), and β-cell number in small islets (representing β-cell neogenesis [30]) compared with IgG control. In T1D Glp1r−/− or Glp1rpan−/− mice, GCGR mAb had no such effects (Supplementary Figs. 7 and 8). These results suggested that GCGR antagonism boosted β-cell regeneration through β-cell self-replication, α- to β-cell transdifferentiation, and β-cell neogenesis in T1D mice, which was mediated via GLP-1R signaling.
Activation of GLP-1R by Glucagon Is Involved in β-Cell Regeneration Induced by GCGR Antagonism in T1D Mice
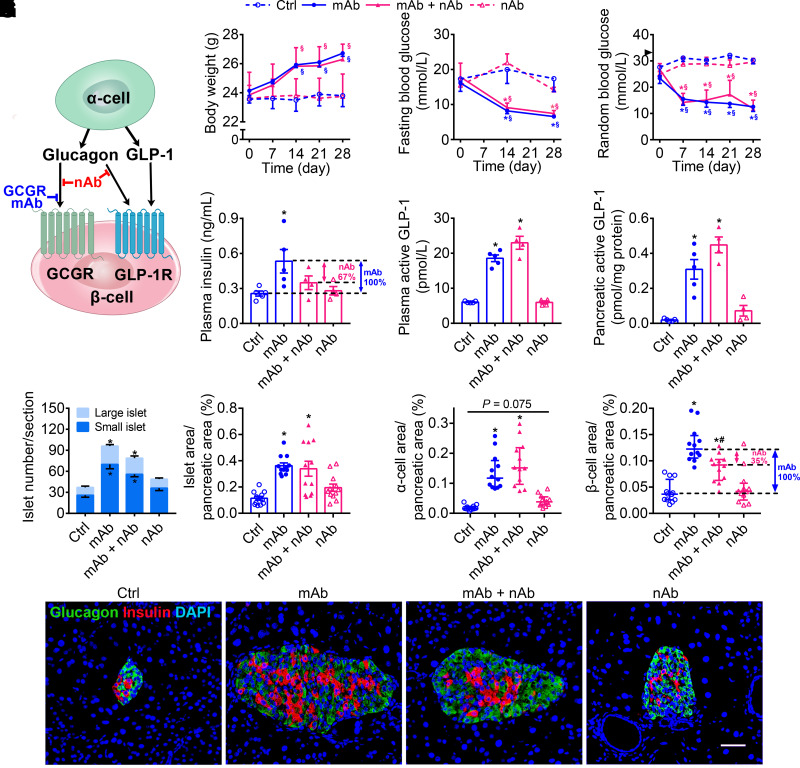
In addition to GLP-1, glucagon has been recognized as another endogenous ligand of GLP-1R (9,23,24). Since GCGR mAb upregulated the levels of glucagon and GLP-1 in the circulation and pancreas (Fig. 1G–L), we tried to clarify the relative contribution of glucagon and GLP-1 to GLP-1R activation. A specific glucagon nAb was used to prevent glucagon binding to GCGR and GLP-1R (33,34). In the context of GCGR mAb treatment, glucagon nAb could only block the binding of glucagon with GLP-1R since GCGR was already occupied by GCGR mAb, a more potent competitor than glucagon (35) (Fig. 4A).
Figure 4.
Activation of GLP-1R by glucagon is involved in β-cell regeneration induced by GCGR antagonism in T1D mice. Male C57BL/6J mice (12 weeks) were injected with STZ to induce a T1D model. The diabetic mice were divided into four groups and given the 4-week treatments as follows: 1) control (Ctrl) group (n = 5), injected with IgG (5 mg/kg/week, as control of GCGR mAb) and A-TNP (4 mg/kg/day, as control of glucagon nAb); 2) GCGR mAb group (n = 5), received injection of GCGR mAb (5 mg/kg/week) and A-TNP; 3) GCGR mAb + glucagon nAb group (n = 4), injected with GCGR mAb and glucagon nAb (4 mg/kg/day); 4) glucagon nAb group (n = 4), received injection of IgG and glucagon nAb. A: Schematic diagram about the action of GCGR mAb and glucagon nAb. GCGR mAb could bind to GCGR and act as a competitive antagonist against glucagon, while glucagon nAb could prevent glucagon binding to GCGR and GLP-1R. In the context of GCGR mAb treatment, glucagon nAb could only block the binding of glucagon with GLP-1R since GCGR was already occupied by GCGR mAb, a more potent competitor than glucagon. B: Body weight. C: Fasting blood glucose. D: Random blood glucose. The arrowhead indicates the upper detection limit (33.3 mmol/L) of the glucometer. E: Plasma insulin. F: Plasma active GLP-1. G: Pancreatic active GLP-1. H: Quantification of the islet number per pancreatic section. An islet containing a cell number ≤10 is defined as a small islet and the others as a large islet. Quantification of the islet area (I), α-cell area (J), and β-cell area (K) per pancreatic section (n = 4 section/mouse × 3 mice/group). L: Representative images of an islet immunostained for glucagon, insulin, and DAPI. Scale bar = 50 μm. Data are expressed as the mean ± SEM or median (interquartile range). Statistical analysis was performed by two-way ANOVA, followed by the Bonferroni multiple comparisons test in B–D, by one-way ANOVA, followed by the Bonferroni multiple comparisons test in E, the Dunnett T3 multiple comparisons test in F–I, or by the Kruskal-Wallis test, followed by the Dunn multiple comparisons test in J and K. *P < 0.05 vs. Ctrl group; §P < 0.05 vs. pretreatment in the same group; #P < 0.05 vs. GCGR mAb group.
In T1D C57BL/6J mice, there was no significant difference in body weight among the four groups (Fig. 4B). The fasting and random blood glucose levels in the GCGR mAb group and the GCGR mAb + glucagon nAb group significantly decreased from baseline and were lower than those in the control group and glucagon nAb group after treatment (Fig. 4C and D). The glucose levels were comparable between GCGR mAb and GCGR mAb + glucagon nAb groups (Fig. 4C and D), suggesting that glucagon–GLP-1R signaling did not affect the glucose-lowing effect of GCGR mAb.
Plasma insulin level in the GCGR mAb group was higher than that in the control group, and this effect was attenuated by 67% when adding glucagon nAb on GCGR mAb treatment (Fig. 4E). Plasma and pancreatic GLP-1 levels were higher in the GCGR mAb group than in the control group, while they were comparable between the GCGR mAb and GCGR mAb + glucagon nAb groups (Fig. 4F and G), thereby excluding the possibility of compensation of GLP-1 production.
Compared with the control group, islet number, islet area, and α-cell area were significantly increased in the GCGR mAb group, and these parameters showed no difference between the GCGR mAb and GCGR mAb + glucagon nAb groups (Fig. 4H–J and L and Supplementary Fig. 9). Notably, β-cell area was larger in the GCGR mAb group than in the control group, and this effect was diminished by 35% after adding glucagon nAb (Fig. 4K and L). Moreover, glucagon nAb alone appeared to increase α-cell area (P = 0.075), but showed little effect on islet number, islet area, and β-cell area (Fig. 4H–L). These results suggested that glucagon–GLP-1R signaling at least partly contributed to β-cell regeneration but not α-cell hyperplasia induced by GCGR antagonism.
Glucagon–GLP-1R Signaling Takes Part in Promotion of Insulin Secretion Evoked by GCGR Antagonism In Vitro
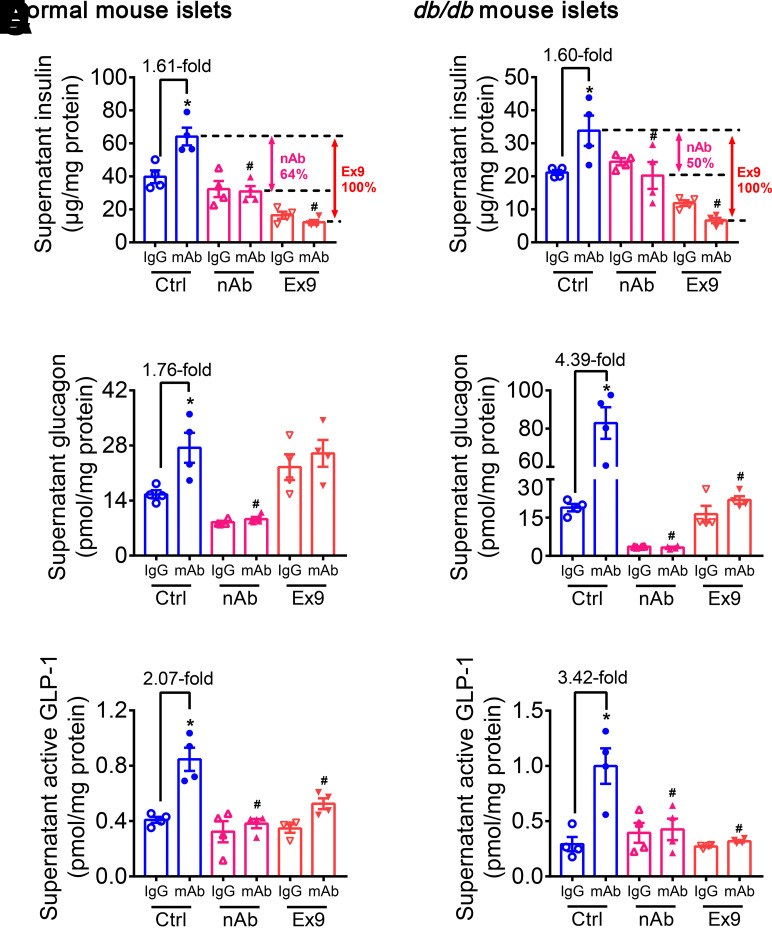
GLP-1 is mainly synthesized in and secreted from intestinal L cells (20). Recently, it has been demonstrated that α-cells–derived GLP-1 also plays an important role in glucose homeostasis, especially under metabolic stress (24,36–38). To determine the role of islet-derived GLP-1 in GCGR mAb–mediated regulation of β-cell identity, islets isolated from normal mice, db/db mice, and Glp1rpan−/− mice were used. Results showed that compared with IgG control, GCGR mAb significantly increased insulin, glucagon, and GLP-1 release in normal and diabetic mouse islets (Fig. 5). The above effects of GCGR mAb disappeared after blockage of GLP-1R by Ex9 or Glp1r knockout and after elimination of glucagon–GLP-1R signaling by glucagon nAb (Fig. 5 and Supplementary Fig. 10A–C). These results indicated that glucagon–GLP-1R signaling participated in the insulinotropic effect of GCGR mAb in vitro. By comparing insulin release levels among GCGR mAb groups, with or without other cotreatments, we inferred that glucagon and GLP-1 contributed 64% and 36% to the GCGR mAb–induced insulinotropic effect in normal mouse islets, respectively (Fig. 5A). In diabetic mouse islets, the relative contributions changed to 50% and 50%, respectively (Fig. 5B). These results suggested that both glucagon and GLP-1 activated GLP-1R, which contributed to the GCGR antagonism–induced insulinotropic effect in vitro.
Figure 5.
Glucagon–GLP-1R signaling takes part in promotion of insulin secretion evoked by GCGR antagonism in vitro. Primary mouse islets were isolated from 8-week-old male normal mice and db/db mice and cultured with 1,000 nmol/L GCGR mAb or IgG in the absence or presence of Ex9 (200 nmol/L) or glucagon nAb (10 mg/L) for 24 h in a high glucose (30 mmol/L) condition. A, C, and E: Parameters in normal mouse islets. B, D, and F: Parameters in db/db mouse islets. Ctrl, PBS control. A and B: Supernatant insulin level. C and D: Supernatant glucagon level. E and F: Supernatant active GLP-1 level (n = 4). Data are expressed as the mean ± SEM. Statistical analysis was performed by one-way ANOVA, followed by the Tukey multiple comparisons test. *P < 0.05 vs. IgG control on the same cotreatment; #P < 0.05 vs. GCGR mAb on the control cotreatment.
Activation of GLP-1R by Glucagon Participates in Regulation of β-Cell Identity by GCGR Antagonism In Vitro
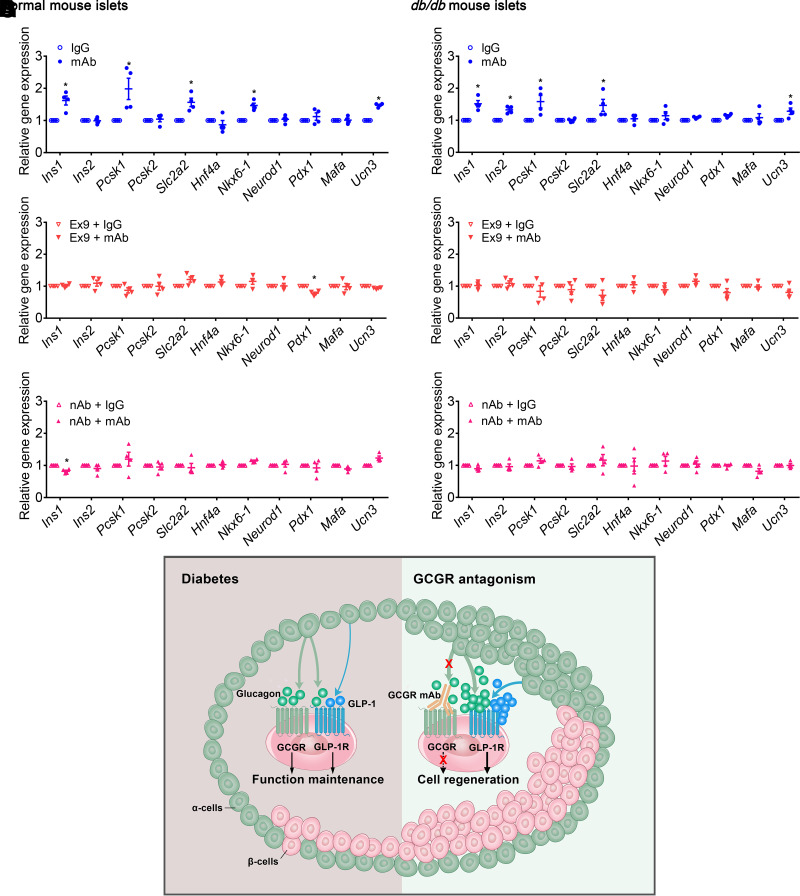
β-Cell identity was further evaluated by detecting the expression of genes related to insulin synthesis and processing (Ins1, Ins2, Pcsk1, and Pcsk2), glucose transporter (Slc2a2), key transcription factors (Hnf4a, Nkx6-1, Neurod1, and Pdx1) and mature β-cell markers (Mafa and Ucn3). Results showed that GCGR mAb upregulated the mRNA levels of Ins1, Pcsk1, Slc2a2, Nkx6-1, and Ucn3 in normal mouse islets, and Ins1, Ins2, Pcsk1, Slc2a2, and Ucn3 in diabetic mouse islets (Fig. 6A and B). Blockage of GLP-1R by Ex9 or Glp1r knockout and elimination of glucagon–GLP-1R signaling by glucagon nAb attenuated the effects of GCGR mAb (Fig. 6C–F and Supplementary Fig. 10D). These results suggested that GCGR mAb influenced β-cell identity in primary mouse islets, which was mediated via glucagon–GLP-1R signaling.
Figure 6.
Activation of GLP-1R by glucagon participates in regulation of β-cell identity by GCGR antagonism in vitro and the working model for this study. Primary mouse islets were isolated from 8-week-old male normal mice and db/db mice and cultured with 1,000 nmol/L GCGR mAb or IgG in the absence or presence of Ex9 (200 nmol/L) or glucagon nAb (10 mg/L) for 24 h in a high glucose (30 mmol/L) condition. A, C, and E: Relative mRNA levels in normal mouse islets. B, D, and F: Relative mRNA levels in db/db mouse islets (n = 4). Data are expressed as the mean ± SEM. Statistical analysis was performed by unpaired Student t test. *P < 0.05 vs. IgG control. G: Working model for the involvement of glucagon–GLP-1R signaling in β-cell regeneration induced by GCGR antagonism. In diabetic mice, α-cell–derived glucagon and GLP-1 act on β-cell GCGR and GLP-1R to maintain β-cell function. GCGR antagonism upregulates α-cell–derived glucagon and GLP-1 production. High concentration of glucagon, together with elevated GLP-1 level, can activate GLP-1R on islet cells (especially β-cells) and thus promote β-cell regeneration.
Discussion
In this study, we showed that antagonistic GCGR mAb increased β-cell area through β-cell proliferation, α- to β-cell transdifferentiation, and β-cell neogenesis in diabetic mice, and upregulated insulin secretion and the expression of β-cell specific markers in cultured mouse islets, suggesting that glucagon could influence β-cell mass and identity. Besides, the levels of glucagon and GLP-1 in circulation and the pancreas were greatly elevated by GCGR mAb. The GLP-1R antagonist Ex9 and global and pancreatic Glp1r knockout attenuated the effects of GCGR mAb on glucose metabolism and β-cell area and identity in T2D and T1D mice and in primary mouse islets. By using glucagon nAb to block the binding of glucagon with GLP-1R, we found that glucagon–GLP-1R signaling was responsible for the increased β-cell area induced by the GCGR mAb in T1D mice and involved in regulation of β-cell identity by GCGR antagonism in cultured mouse islets.
GCGR antagonism displays an efficacious glucose control in T1D or T2D animals and humans (4–8). Our series of studies showed that GCGR antagonism promoted β-cell regeneration in T1D and T2D mice (14–16,32), with GLP-1R activation as one of underlying mechanisms identified in this study. Thirteen-week treatment of GCGR mAb increased β-cell mass and the number of Nkx6.1+/Pdx1+ α-cells in cynomolgus monkeys (17), suggestive of β-cell regeneration in nonhuman primates. Although its effect on β-cell mass in humans is absent, GCGR antagonism was reported to increase plasma insulin and C-peptide levels in T2D patients and reduce daily insulin use in T1D patients (5,8). In T1D mice with transplanted human islets, GCGR mAb increased human insulin level even after a 2-week washout (7), indicating that GCGR antagonism might promote β-cell regeneration in humans. Importantly, compared with rodent islets, human islets contain more α-cells (especially GLP-1–secreting α-cells), which are adjoining to β-cells instead of surrounding β-cells, making communication of α-cells with β-cells more convenient (22,39). Moreover, high-dose GGGR antibody upregulated serum glucagon and GLP-1 levels in healthy people (40). These observations suggest that α-cells may play more important roles in regulation of β-cell mass and function by GCGR antagonism in humans.
Glucagon secreted from α-cells might have a direct effect on β-cell mass and identity. Multiple studies have shown that exogenous glucagon input increases insulin secretion from rodent and human β-cells (9,41). Similarly, intraislet glucagon is needed for adequate insulin secretion in vivo (9,10,42). However, whether glucagon can affect β-cell regeneration remains unclear. Coculture of α-TC1.9 cells (an α-cell line) with Min6 cells (a β-cell line) promoted β-cell proliferation (43). Impaired β-cell formation induced by Gcg knockdown could be restored by infusion of glucagon or GLP-1 analog in zebrafish, suggesting that the peptides encoded by Gcg were required for β-cell regeneration (44). In this study, we found that glucagon and GLP-1 levels in plasma and pancreas were greatly elevated after GCGR mAb treatment. Therefore, we supposed that elevated levels of glucagon and GLP-1 participated in GCGR antagonism–induced β-cell regeneration.
Multiple lines of evidence have proved that GLP-1R activation enhances insulin secretion and improves glucose control (20). Notably, GLP-1R activation promotes β-cell regeneration in diabetic mice and in cultured human islets (45,46). GCGR antagonist– or Gcgr knockout–mediated improvement in glucose control is dependent on functional pancreatic GLP-1R (26,47). However, the effect of GLP-1R on β-cell regeneration has not yet been investigated. Our recent report showed that the combination of GCGR mAb and liraglutide (a GLP-1R agonist) could not further increase β-cell area compared with GCGR mAb alone (48), suggesting that GCGR mAb itself already activated pancreatic GLP-1R sufficiently. By using the GLP-1R antagonist Ex9 and global and pancreatic Glp1r-knockout mice, our studies demonstrated that GLP-1R signaling contributed to GCGR antagonism–induced β-cell regeneration.
Classically, GLP-1R is thought to be activated by GLP-1. Recently, glucagon has been identified as a GCGR and GLP-1R dual agonist (9,23,24). In isolated human islets or perfused rodent pancreata, blockage of either GCGR or GLP-1R impaired exogenous glucagon-stimulated insulin secretion, and simultaneous antagonism of these two receptors abolished insulin output (9,24), albeit Ex9 only had a tendency to reduce glucagon-stimulated insulin secretion in some reports (49). Similarly, exogenous glucagon given under fed conditions robustly stimulated insulin secretion. Glucagon failed to induce meaningful insulin secretion in β-cell–specific Glp1r-knockout mice, while it promoted insulin secretion in β-cell–specific Gcgr-knockout mice, demonstrating that β-cell GLP-1R and GCGR are indispensable and dispensable for glucagon-induced insulin secretion, respectively (10). Consistently, we found that plasma insulin level and β-cell area were increased under the hyperglucagonemic state induced by GCGR mAb and that the insulinotropic effect of GCGR mAb was attenuated or even disappeared when glucagon–GLP-1R signaling was blocked in diabetic mice and in cultured mouse islets.
Since GLP-1R can be activated by glucagon and GLP-1 (9,23,24) and these two hormones were upregulated by GCGR antagonism (25,26), figuring out which one is responsible for GLP-1R activation is difficult. Traditional hormone gene deletion is not suitable owing to the same encoding gene Gcg. GLP-1R antagonist Ex9 is unavailable because it antagonizes activation of GLP-1R by both glucagon and GLP-1 (9). Fortunately, we got a specific glucagon nAb that prevented glucagon binding with GLP-1R and thus distinguished glucagon–GLP-1R signaling and GLP-1–GLP-1R signaling. We found that glucagon–GLP-1R signaling at least partly participated in the insulinotropic effect and β-cell regeneration induced by GCGR mAb. Of course, involvement of GLP-1–GLP-1R signaling could not be excluded because the GCGR mAb–mediated increment of the β-cell area was only partially reversed by glucagon nAb. Therefore, both glucagon and GLP-1 are involved in GCGR mAb–induced β-cell regeneration. To our knowledge, this is the first study to explore the contribution of glucagon–GLP-1R signaling to β-cell regeneration.
Our study has some limitations. First, involvement of GLP-1–GLP-1R signaling was not studied directly due to absence of available tools. Maybe using the clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein 9 (Cas9) system to accurately target the exon encoding GLP-1 instead of deleting full-length Gcg gene is feasible (50).
Second, expression of Glp1r was eliminated not only in β-cells but also in other pancreatic cells in Glp1rpan−/− mice, and therefore, β-cell–specific Glp1r-knockout mice may be needed to clarify the direct effect of GLP-1R on β-cells. However, GCGR antagonism or GLP-1R activation promotes β-cell regeneration from several sources, including β-cell proliferation, α- to β- transdifferentiation, and β-cell neogenesis from progenitors (7,14,15,17,32). In this regard, pancreas-specific knockout (Pdx1-Cre) was more suitable than β-cell–specific knockout (Ins1/Ins2-Cre); the latter could only block the regenerated β-cells from themselves.
Third, inducible knockout mice are more suitable for gene function research in adults. However, we used GLP-1R antagonist Ex9 as a pharmacological blockage to determine the function of GLP-1R in adult mice.
Finally, supraphysiological concentration of glucagon or glucagon analog to activate GLP-1R may be a more direct way to study glucagon function on β-cell regeneration.
In summary, our study reveals the mechanism of β-cell regeneration induced by GCGR antagonism, uncovers the communication between α-cells and β-cells in regulating β-cell mass and identity, and provides a new insight for GCGR antagonism–induced β-cell regeneration through activation of glucagon–GLP-1R signaling (Fig. 6G).
Article Information
Acknowledgments. The authors thank Prof. Yingmei Feng (Beijing Youan Hospital, Beijing, China) for the kindly gift of Glp1r−/− mice and Prof. Jingjing Zhang (The Second Xiangya Hospital of Central South University, Changsha, China) for the kindly gift of Pdx1-Cre mice. The authors are grateful to Dr. Hai Yan (REMD Biotherapeutics, Camarillo, CA) for providing GCGR mAb and Dr. Christian L. Brand (Diabetes Research Unit, Novo Nordisk A/S, Bagsvaerd, Denmark) for providing glucagon nAb GLU-001 and its control antibody A-TNP.
Funding. This work was supported by National Natural Science Foundation of China research grants 81830022 (T.H.), 81970671 (R.W.), 82270843 (R.W.), 82170875 (T.H.), and 82271611 (K.Y.). This work was also supported by Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation and Talent Project of Clinical Key Project of Peking University Third Hospital (R.W.).
The funding agencies were not involved in the design of the study, the collection, analysis, and interpretation of data, writing the report, and did not impose any restrictions regarding the publication of the report.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.W., X.C., and Y.J. did the statistical analyses. T.W., X.C., Y.J., K.W., D.W., F.L., X.L., and L.G. performed the experiments. T.W., X.C., K.Y., J.Y., T.H., and R.W. interpreted the results. T.W., T.H., and R.W. prepared the manuscript. J.Y., T.H., and R.W. designed the study. T.H. and R.W. contributed to the conception. T.H. and R.W. controlled the decision to publish. All authors reviewed and edited the manuscript. T.H. and R.W. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.22141916.
T.W. and X.C. contributed equally.
References
- 1. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 2020;16:349–362 [DOI] [PubMed] [Google Scholar]
- 2. Finan B, Capozzi ME, Campbell JE. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes 2020;69:532–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Unger RH, Orci L. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1975;1:14–16 [DOI] [PubMed] [Google Scholar]
- 4. Gelling RW, Du XQ, Dichmann DS, et al. Lower blood glucose, hyperglucagonemia, and pancreatic α cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A 2003;100:1438–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morgan ES, Tai LJ, Pham NC, et al. Antisense inhibition of glucagon receptor by IONIS-GCGRRx improves type 2 diabetes without increase in hepatic glycogen content in patients with type 2 diabetes on stable metformin therapy. Diabetes Care 2019;42:585–593 [DOI] [PubMed] [Google Scholar]
- 6. Pettus JH, D’Alessio D, Frias JP, et al. Efficacy and safety of the glucagon receptor antagonist RVT-1502 in type 2 diabetes uncontrolled on metformin monotherapy: a 12-week dose-ranging study. Diabetes Care 2020;43:161–168 [DOI] [PubMed] [Google Scholar]
- 7. Wang MY, Dean ED, Quittner-Strom E, et al. Glucagon blockade restores functional β-cell mass in type 1 diabetic mice and enhances function of human islets. Proc Natl Acad Sci U S A 2021;118:e2022142118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pettus J, Boeder SC, Christiansen MP, et al. Glucagon receptor antagonist volagidemab in type 1 diabetes: a 12-week, randomized, double-blind, phase 2 trial. Nat Med 2022;28:2092–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Svendsen B, Larsen O, Gabe MBN, et al. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 2018;25:1127–1134.e2 [DOI] [PubMed] [Google Scholar]
- 10. Capozzi ME, Wait JB, Koech J, et al. Glucagon lowers glycemia when β-cells are active. JCI Insight 2019;5:e129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ali S, Lamont BJ, Charron MJ, Drucker DJ. Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest 2011;121:1917–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, Schuit F. Dual glucagon recognition by pancreatic β-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes 1998;47:66–72 [DOI] [PubMed] [Google Scholar]
- 13. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 2000;43:1012–1019 [DOI] [PubMed] [Google Scholar]
- 14. Wei R, Gu L, Yang J, et al. Antagonistic glucagon receptor antibody promotes α-cell proliferation and increases β-cell mass in diabetic mice. iScience 2019;16:326–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui X, Feng J, Wei T, et al. Pro-α-cell-derived β-cells contribute to β-cell neogenesis induced by antagonistic glucagon receptor antibody in type 2 diabetic mice. iScience 2022;25:104567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang K, Cui X, Li F, et al. Glucagon receptor blockage inhibits β-cell dedifferentiation through FoxO1. Am J Physiol Endocrinol Metab 2023;324:E97–E113 [DOI] [PubMed] [Google Scholar]
- 17. Xi Y, Song B, Ngan I, et al. Glucagon-receptor-antagonism-mediated β-cell regeneration as an effective anti-diabetic therapy. Cell Rep 2022;39:110872. [DOI] [PubMed] [Google Scholar]
- 18. Lang S, Yang J, Yang K, et al. Glucagon receptor antagonist upregulates circulating GLP-1 level by promoting intestinal L-cell proliferation and GLP-1 production in type 2 diabetes. BMJ Open Diabetes Res Care 2020;8:e001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen L, Deacon CF, Ørskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999;140:5356–5363 [DOI] [PubMed] [Google Scholar]
- 20. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Intraislet production of GLP-1 by activation of prohormone convertase 1/3 in pancreatic α-cells in mouse models of β-cell regeneration. Islets 2010;2:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Campbell SA, Golec DP, Hubert M, et al. Human islets contain a subpopulation of glucagon-like peptide-1 secreting α cells that is increased in type 2 diabetes. Mol Metab 2020;39:101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cabrera O, Ficorilli J, Shaw J, et al. Intra-islet glucagon confers β-cell glucose competence for first-phase insulin secretion and favors GLP-1R stimulation by exogenous glucagon. J Biol Chem 2022;298:101484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Capozzi ME, Svendsen B, Encisco SE, et al. β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang S, Wei R, Wei T, et al. Glucagon receptor antagonism promotes the production of gut proglucagon-derived peptides in diabetic mice. Peptides 2020;131:170349. [DOI] [PubMed] [Google Scholar]
- 26. Jun LS, Millican RL, Hawkins ED, et al. Absence of glucagon and insulin action reveals a role for the GLP-1 receptor in endogenous glucose production. Diabetes 2015;64:819–827 [DOI] [PubMed] [Google Scholar]
- 27. Grigoryan M, Kedees MH, Charron MJ, Guz Y, Teitelman G. Regulation of mouse intestinal L cell progenitors proliferation by the glucagon family of peptides. Endocrinology 2012;153:3076–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brand CL, Jørgensen PN, Knigge U, et al. Role of glucagon in maintenance of euglycemia in fed and fasted rats. Am J Physiol 1995;269:E469–E477 [DOI] [PubMed] [Google Scholar]
- 29. Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee H, Lee YS, Harenda Q, et al. Beta cell dedifferentiation induced by IRE1α deletion prevents type 1 diabetes. Cell Metab 2020;31:822–836.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zmuda EJ, Powell CA, Hai T. A method for murine islet isolation and subcapsular kidney transplantation. J Vis Exp 2011;(50):2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cui X, Feng J, Wei T, et al. Pancreatic alpha cell glucagon-liver FGF21 axis regulates beta cell regeneration in a mouse model of type 2 diabetes. Diabetologia 2023;66:535–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holst JJ, Galbo H, Richter EA. Neutralization of glucagon by antiserum as a tool in glucagon physiology: lack of depression of basal blood glucose after antiserum treatment in rats. J Clin Invest 1978;62:182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sørensen H, Brand CL, Neschen S, et al. Immunoneutralization of endogenous glucagon reduces hepatic glucose output and improves long-term glycemic control in diabetic ob/ob mice. Diabetes 2006;55:2843–2848 [DOI] [PubMed] [Google Scholar]
- 35. Yan H, Gu W, Yang J, et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther 2009;329:102–111 [DOI] [PubMed] [Google Scholar]
- 36. Sancho V, Daniele G, Lucchesi D, et al. Metabolic regulation of GLP-1 and PC1/3 in pancreatic α-cell line. PLoS One 2017;12:e0187836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Traub S, Meier DT, Schulze F, et al. Pancreatic α cell-derived glucagon-related peptides are required for β cell adaptation and glucose homeostasis. Cell Rep 2017;18:3192–3203 [DOI] [PubMed] [Google Scholar]
- 38. Chambers AP, Sorrell JE, Haller A, et al. The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab 2017;25:927–934.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell JE, Newgard CB. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat Rev Mol Cell Biol 2021;22:142–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kostic A, King TA, Yang F, et al. A first-in-human pharmacodynamic and pharmacokinetic study of a fully human anti-glucagon receptor monoclonal antibody in normal healthy volunteers. Diabetes Obes Metab 2018;20:283–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Han C, Zhu W, et al. Glucagon potentiates insulin secretion via β-cell GCGR at physiological concentrations of glucose. Cells 2021;10:2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhu L, Dattaroy D, Pham J, et al. Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019;5:e127994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Green AD, Vasu S, Moffett RC, Flatt PR. Co-culture of clonal beta cells with GLP-1 and glucagon-secreting cell line impacts on beta cell insulin secretion, proliferation and susceptibility to cytotoxins. Biochimie 2016;125:119–125 [DOI] [PubMed] [Google Scholar]
- 44. Ye L, Robertson MA, Hesselson D, Stainier DYR, Anderson RM. Glucagon is essential for alpha cell transdifferentiation and beta cell neogenesis. Development 2015;142:1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism 2014;63:9–19 [DOI] [PubMed] [Google Scholar]
- 46. Saikia M, Holter MM, Donahue LR, et al. GLP-1 receptor signaling increases PCSK1 and β cell features in human α cells. JCI Insight 2021;6:e141851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gu W, Winters KA, Motani AS, et al. Glucagon receptor antagonist-mediated improvements in glycemic control are dependent on functional pancreatic GLP-1 receptor. Am J Physiol Endocrinol Metab 2010;299:E624–E632 [DOI] [PubMed] [Google Scholar]
- 48. Gu L, Wang D, Cui X, et al. Combination of GLP-1 receptor activation and glucagon blockage promotes pancreatic β-cell regeneration in situ in type 1 diabetic mice. J Diabetes Res 2021;2021:7765623 [Google Scholar]
- 49. Moens K, Berger V, Ahn JM, et al. Assessment of the role of interstitial glucagon in the acute glucose secretory responsiveness of in situ pancreatic β-cells. Diabetes 2002;51:669–675 [DOI] [PubMed] [Google Scholar]
- 50. Tellez K, Hang Y, Gu X, Chang CA, Stein RW, Kim SK. In vivo studies of glucagon secretion by human islets transplanted in mice. Nat Metab 2020;2:547–557 [DOI] [PMC free article] [PubMed] [Google Scholar]