Abstract
This study investigated the differential effects of Bacillus subtilis–fermented products (SFPs) and Bacillus licheniformis–fermented products (LFPs) on the growth performance, intestinal morphology, intestinal gene expression, cecal microbiota community, and microbial carbohydrate-active enzyme composition of broilers. In total, 160 one-day-old unsexed Arbor Acres broiler chicks were randomly allocated to 4 treatment groups (with 8 replicates per group and 5 chicks per replicate): control (CON), enramycin (ENM), SFP, and LFP groups, which were fed a basal diet, basal diet supplemented with 10 mg/kg ENM, basal diet supplemented with 108 colony-forming units (CFU) of B. subtilis spores per gram of feed, and basal diet supplemented with 108 CFU of B. licheniformis spores per gram of feed, respectively. LFP treatment resulted in a higher (P < 0.01) body weight at the age of 35 d and higher average daily gain over 15 to 35 (P < 0.05) and 1 to 35 (P < 0.05) d of age than did the CON and SFP treatments. The average villus heights in the jejunum were longer (P < 0.05) in the LFP group than those in the SFP group. Moreover, the LFP group exhibited a higher jejunal barrier function gene expression (mucin 2, zonula occludens-1, and occludin) and cecal antioxidant gene expression (superoxide dismutase) than did the CON group (P < 0.05). A principal coordinate analysis of cecal microbiota and carbohydrate-active enzyme composition demonstrated distinct clustering among the groups. Lactobacillus crispatus abundance in the cecal digesta was higher (P < 0.01) in the SFP group than in the CON and LFP groups. Finally, microbial glycosyltransferase 2 gene expression in the cecal digesta was higher in the LFP group than in the CON and ENM groups. In conclusion, LFPs can improve the growth performance, increase intestinal barrier function and antioxidant gene expression, and modulate cecal microflora composition and carbohydrate-active enzyme composition of broilers. The overall effect of LFPs on growth promotion in broilers was superior to that of SFPs.
Key words: Bacillus licheniformis, Bacillus subtilis, broiler, carbohydrate-active enzyme, microbiota
INTRODUCTION
Antibiotic growth promoters (AGPs) are commonly used to prevent infection and improve growth performance in poultry. However, in poultry, antibiotic overuse and misuse have contributed to an increased risk of antibiotic resistance (Marshall and Levy, 2011). Many countries have restricted the use of AGPs as feed additives for enhancing growth in poultry production. Therefore, determining the effective alternatives to AGPs that can maintain the health status and improving the growth performance of the broilers are imperative.
Probiotics have gained considerable attention as an alternative to AGPs in poultry feeds. Among all probiotics, Bacillus species are the most commonly used probiotics in poultry production because of their ability to form endospores, which allows them to survive in harsh environments (with low pH, high bile salt levels, and high heat) and germinate in the gastrointestinal tract of poultry (Ramlucken et al., 2020; Bahaddad et al., 2022). Bacillus species can produce antimicrobial substances against enteric pathogens and provide extracellular enzymes, which then reduce infection risk and enhance nutrient utilization (Ramlucken et al., 2020). Bacillus subtilis and Bacillus licheniformis strains are widely used as probiotics for broilers. Studies have demonstrated that B. subtilis or B. licheniformis supplementation can prevent enteric diseases and increase growth performance in broilers (Grant et al., 2018; Aljumaah et al., 2020; Zhao et al., 2020).
The effects of B. subtilis and B. licheniformis on broiler performance have been studied (Gong et al., 2018; Musa et al., 2019; Xu et al., 2021). B. subtilis and B. licheniformis supplementation at similar concentrations are similar in their effects on growth and necrotic enteritis prevention (Gong et al., 2018; Musa et al., 2019). By contrast, B. licheniformis supplementation produces results superior to those after B. subtilis supplementation in terms of growth performance, immunity, and cecal short-chain fatty acid levels in broilers (Xu et al., 2021). Because these findings in the literature have been inconsistent, the differences in the effects of B. subtilis and B. licheniformis supplementation on broiler performance warrants clarification.
Commercial probiotics, including B. subtilis and B. licheniformis, are mainly produced by submerged fermentation. Submerged fermentation is relatively expensive due to high cost of required media and instruments compared with solid-state fermentation. Solid-state fermentation only requires simple feed ingredients (bran or soybean meal) as the substrate and very less instrument, capital investment, and energy expenditure (Yafetto, 2022). Due to the environmentally friendly and low-cost process, solid-state fermentation has the potential of being a possible alternative to submerged fermentation for probiotic production. However, the production of probiotics as feed additives using solid-state fermentation is still not widely used in animal production.
Our previous studies have demonstrated that supplementation with Bacillus sp.-solid-state fermentation products (B. subtilis–fermented products [SFPs] and/or B. licheniformis–fermented products [LFPs]) can improve growth performance, alleviate inflammation, and modulate gut microbiota in broilers (Cheng et al., 2018, Cheng et al., 2021a, Cheng et al., 2021b; Chen and Yu, 2020, 2021; Yu et al., 2021; Chang and Yu, 2022). However, the differential effects of SFPs and LFPs in broilers from multiple perspectives are required. An integrated analysis of the gut microbiota and microbial carbohydrate-active enzyme gene composition, as affected by the 2 fermented products, is required to investigate the underlying mechanism. B. subtilis and B. licheniformis produce extracellular enzymes and secondary metabolites to differing extents (Sumi et al., 2015; Elshaghabee et al., 2017); thus, the mechanisms underlying their antimicrobial effects may differ (Tran et al., 2022). Therefore, we hypothesized that SFPs and LFPs differentially regulate growth performance, intestinal morphology, intestinal gene expression, cecal microbiota community, and microbial carbohydrate-active enzyme gene composition in broilers.
In the current study, we investigated the differential effects of a SFP and LFP on the performance, intestinal morphology, and intestinal gene expression in broilers. We also performed an integrated analysis of changes in gut microbiota and microbial carbohydrate-active enzyme gene composition in response to SFP and LFP treatment through metagenomic sequencing in broilers.
MATERIALS AND METHODS
Ethics Statement
The protocols used in the present study were reviewed and approved by the Institutional Animal Care and Use Committee of National Ilan University (109-26).
Birds and Diets
B. subtilis (BCRC 14718) and B. licheniformis (ATCC 12713) were obtained from the Food Industry Research and Development Institute (Hsinchu, Taiwan). The SFP and LFP preparation methods used in this study have been detailed elsewhere (Cheng et al., 2021a ; Chen and Yu, 2021). The SFPs and LFPs were produced by solid-state fermentation. The detailed composition of SFPs and LFPs is listed in Table 1. In the fermented products used in this study, the B. subtilis and B. licheniformis spore concentrations were 3.8 × 109 and 4.4 × 109 colony-forming units (CFU)/g, respectively. In total, 160 one-day-old unsexed Arbor Acres broiler chicks, with an average body weight of 41.9 ± 0.13 g, were obtained from a local commercial hatchery and randomly allocated to 4 treatment groups (40 chicks per group): 1) control (CON) group (basal diet alone), 2) enramycin (ENM) group (basal diet supplemented with 10 mg/kg ENM), 3) SFP (basal diet supplemented with 108 CFU B. subtilis spore/g of feed), and 4) LFP (basal diet supplemented with 108 CFU B. licheniformis spore/g of feed). Each group had 8 replicates with 5 birds per replicate. The birds were reared in stainless-steel cages and given ad libitum access to feed and water during the experiment. Nutrient levels in basal diets were formulated to meet the National Research Council (1994) nutrient requirements of broiler chickens (Table 2). The experimental diets were given for 35 d, including starter (d 1–14) and growth (d 15–35) phases. A 20:4 h light–dark photoperiod was applied during the experimental period. Ambient temperature was maintained at 33°C at the age of 1 to 3 d and then gradually reduced by 2°C per wk to a final temperature of approximately 24°C. The broilers were vaccinated with Newcastle disease and infectious bronchitis vaccines through nose drops on experiment d 4 and 14, respectively. Body weight was measured weekly, and feed intake was measured daily. Average daily gain, average daily feed intake, and feed conversion ratio were calculated for d 1 to 14, 15 to 35, and 1 to 35 intervals. Mortality was recorded through visual observations daily.
Table 1.
Composition of Bacillus species–fermented products.
| Bacillus subtilis–fermented products | Bacillus licheniformis–fermented products | |
|---|---|---|
| Analyzed value, g kg−1 | ||
| Crude protein | 276 | 290 |
| Crude fat | 40 | 42 |
| Carbohydrate | 545 | 527 |
| Ash | 64 | 63 |
| Water | 75 | 78 |
| Calorie, kcal/kg | 3644 | 3646 |
| Spore, CFU/g | 3.8 × 109 | 4.4 × 109 |
Table 2.
Basal diet compositions.
| Item | d 1–14 | d 15–35 |
|---|---|---|
| Ingredient, g kg−1, as fed basis | ||
| Corn, yellow | 554.2 | 607.3 |
| Soybean meal, 36.7% CP | 355.2 | 315.3 |
| Fish meal, 60% CP | 39.9 | 36.3 |
| Vegetable oil | 35.2 | 30.2 |
| Limestone | 15.2 | 12.7 |
| Salt | 3.0 | 3.0 |
| Monocalcium phosphate | 9.2 | 7.8 |
| Mineral premix1 | 2.0 | 2.0 |
| Vitamin premix2 | 2.0 | 2.0 |
| DL-methionine, 98% | 2.0 | 2.0 |
| L-lysine, 98% | 1.0 | 0.6 |
| Choline chloride | 0.5 | 0.5 |
| Calculated value3, g kg−1 | ||
| Dry matter | 889.0 | 887.0 |
| Crude protein | 221.6 | 206.3 |
| Analyzed calcium | 10.2 | 8.7 |
| Analyzed total phosphorus | 6.9 | 6.3 |
| Lysine | 11.2 | 9.5 |
| Methionine + Cystine | 8.5 | 7.6 |
| ME, kcal/kg | 3081.1 | 3057.2 |
Supplied per kilogram of diet: 32 mg of Mn (MnSO4·H2O), 16 mg of Fe (FeSO4·7H2O), 24 mg of Zn (ZnO), 2 mg of Cu (CuSO4·5H2O), 800 μg of I (KI), 200 μg of Co (CoSO4), and 60 μg of Se.
Supplied per kilogram of diet: 5232 IU of vitamin A, 800 IU of vitamin D, 8.3 IU of vitamin E, 2.2 mg of menadione, 2 mg of pyridoxine HCl, 8 mg of cyanocobalamin, 10 mg of nicotine amid, 0.3 mg of folic acid, 20 mg of D-biotin, and 160 mg of choline chloride.
Calculated according to tables of feed composition and nutritive values in Taiwan.
Sample Collection
Birds were euthanized through carbon dioxide inhalation on d 35 and immediately dissected. Two broilers per replicate were chosen randomly, and their intestinal segments (i.e., duodenum, jejunum, ileum, and cecum) were collected for intestinal morphology and gene expression analysis, and cecal digesta collected from each bird were pooled for metagenomic sequencing. The details of intestinal segment sampling locations were provided in our previous study (Cheng et al., 2021a).
Intestinal Morphology
The collected intestinal segments were fixed in 4% buffered formaldehyde for 24 h, dehydrated in ethanol, equilibrated in xylene, embedded in paraffin wax, and cut into 5-μm-thick sections. The sections were then mounted on glass slides, stained with hematoxylin and eosin, and examined under a microscope coupled with a digital camera (Olympus, Tokyo, Japan). Ten well-oriented, distinct villi were identified on each slide and measured for villus height, crypt depth, and their ratio.
Intestinal Gene Expression
Total RNA was extracted from the small intestine and cecum by using the acid guanidinium thiocyanate–phenol–chloroform extraction method. cDNA synthesis was performed using the iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA). The expression of genes (catalase [CAT], superoxide dismutase [SOD], mucin 2 [MUC2], zonula occludens-1 [ZO-1], occludin [OCLN], and claudin [CLDN]) was measured on a CFX connect PCR detection system (Bio-Rad, Hercules, CA). The cDNA was amplified using the iQ SYBR Green Supermix kit (Bio-Rad, Hercules, CA), with initial denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for 15 s and 60°C for 30 s. The sequence of specific oligonucleotide primers for quantitative reverse transcription-PCR is listed in Table 3. The mRNA expression of each genes using the 2−ΔΔCt method were calculated after normalizing to it to the 18S rRNA gene.
Table 3.
Primer sequences for quantitative reverse transcription-PCR.
| Gene1 | Primer sequences (5′-3′) |
|---|---|
| CAT | F: ACCAAGTACTGCAAGGCGAA R: TGAGGGTTCCTCTTCTGGCT |
| SOD | F: AGGGGGTCATCCACTTCC R: CCCATTTGTGTTGTCTCCAA |
| MUC2 | F: GCCTGCCCAGGAAATCAAG R: CGACAAGTTTGCTGGCACAT |
| ZO-1 | F: CCGCAGTCGTTCACGATCT R: GGAGAATGTCTGGAATGGTCTGA |
| OCLN | F: GTAGTCGGGTTCGTTTCC R: GACCTGATTGCCTAGAGTGT |
| CLDN | F: GATTTACTCCTACGCTGGTGAC R: CACAAAGATGGCTATTAGTCCC |
| 18S rRNA | F: ATAACGAACGAGACTCTGGCA R: CGGACATCTAAGGGCATCACA |
CAT = catalase; SOD = superoxide dismutase; MUC2 = mucin 2; ZO-1 = zonula occludens-1; OCLN = occludin; CLDN = claudin.
Metagenomic Sequencing
Procedures for metagenomic sequencing and bioinformatics analysis have been detailed in our previous study (Chen and Yu, 2022a). In brief, total genomic DNA from the cecal digesta was extracted using the ZymoBIOMICS DNA Miniprep Kit (Zymo Research, Irvine, CA). Metagenomic DNA paired-end libraries were prepared using the Illumina Nextera XTLibrary Preparation Kit (Illumina, San Diego, CA). Sequencing was performed on the Illumina NovaSeq 6000 platform (Illumina). After sequencing, the raw reads were cleaned by removing adaptor sequences and low-quality reads using Trimmomatic (version 0.38). The reads that mapped to chicken, human, maize, soybean, wheat, and fish genomes were filtered out using Bowtie2 (version 2.3.4.1). For each sample, the clean reads were assembled using MEGAHIT (version 1.1.3), and gene prediction was performed on contigs >500 bp by using Prodigal (version 2.6.3). The unaligned sequences from each assembly were compared for removing redundancy using CD-HIT (version 4.6.6). The taxonomic assignment of sequences was conducted using DIAMOND (version 0.9.22.123). Protein sequences of open reading frames predicted in the contigs that carried carbohydrate-active enzyme-like open reading frames were annotated using the DIAMOND against the Carbohydrate-Active enZYmes Database. Bioinformatics analysis of microbiota and carbohydrate-active enzyme genes was implemented in MicrobiomeAnalyst (Dhariwal et al., 2017), followed by a comprehensive analysis in R (version 0.84). Fisher's alpha index and the Shannon index were used to evaluate the richness and evenness of the microbial and carbohydrate-active enzyme gene compositions, respectively. Heatmap analysis and principal coordinate analysis (PCoA) were used to evaluate the overall differences in bacterial community and carbohydrate-active enzyme gene composition. The results of correlation analyses were visualized using R.
Statistical Analysis
Individual cages were considered replicates, each replicate was defined as an experimental unit. The data were tested for normality using the Shapiro–Wilk test. One-way analysis of variance with Tukey's honestly significant difference test was used for analyzing differences. Significance was indicated at P ≤ 0.05. PCoA based on Bray–Curtis dissimilarity metrics was used to determine similarity in microbial community and carbohydrate-active enzyme gene composition. Spearman's correlation coefficient was calculated for correlations between growth performance and microbial carbohydrate-active enzyme gene expression. All statistical analyses were performed using SAS software (version 9.4, 2012; SAS Institute, Cary, NC).
RESULTS
Growth Performance, Intestinal Morphology, and Intestinal Gene Expression
The effects of SFP and LFP treatments on broiler growth performance is summarized in Table 4. Compared with CON and SFP treatments, LFP treatment resulted in increased (P ≤ 0.01) body weight at 35 d of age. The LFP group had higher average daily gain at 15 to 35 (P ≤ 0.05) and 1 to 35 (P ≤ 0.05) d of age than did the CON and SFP groups. No significant between-group differences were identified in the ADFI or feed conversion ratio during the experimental period. Compared with CON treatment, LFP treatment resulted in increased (P ≤ 0.05) mortality during the experimental period. The effects of SFP and LFP treatments on broiler intestinal morphology are summarized in Table 5. The average villus heights in the duodenum were higher (P ≤ 0.05) in the LFP group than in the ENM group. The LFP group had higher (P ≤ 0.05) average villus heights in the jejunum compared with the SFP group. No significant between-group differences in ileal morphology were identified. The effects of SFP and LFP treatments on broiler intestinal gene expression are summarized in Table 6. CAT and SOD mRNA expression was lower in the duodenum in the LFP group than in the SFP group (P ≤ 0.01 and P ≤ 0.001, respectively). MUC2 expression in the duodenum was lower (P ≤ 0.05) in the CON group than in the ENM group. Moreover, OCLN mRNA expression in the duodenum was lower (P ≤ 0.001) in the LFP group than in the other groups, and CAT mRNA expression in the jejunum was lower (P ≤ 0.01) in the LFP group than in the CON and LFP groups. By contrast, SOD expression in the jejunum was higher (P ≤ 0.05) in the LFP and SFP groups than in the ENM group. Moreover, MUC2 and ZO-1 mRNA expression in the jejunum was higher in the LFP group than in the CON group (P ≤ 0.001 and P ≤ 0.05), and OCLN expression in the jejunum was higher (P ≤ 0.05) in the LFP and SFP groups than in the CON group. Compared with the other treatments, SFP treatment induced (P ≤ 0.001) CLDN mRNA expression in the jejunum and CAT mRNA expression in the ileum. ZO-1 expression in the ileum was higher (P ≤ 0.01) in the ENM group than in the CON and SFP groups. Compared with SFP treatment, LFP treatment induced (P ≤ 0.05) OCLN mRNA expression in the ileum. CAT expression in the cecum was higher (P ≤ 0.01) in the ENM group than in the CON group. Compared with CON treatment, LFP treatment induced (P ≤ 0.05) SOD mRNA expression in the cecum. MUC2 expression in the cecum was higher (P ≤ 0.05) in the LFP group than in the SFP group. Compared with CON treatment, ENM treatment induced (P ≤ 0.05) ZO-1 mRNA expression in the cecum. OCLN expression in the cecum was higher (P ≤ 0.001) in the ENM and SFP groups than those in the other groups. Compared with CON treatment, SFP treatment induced (P ≤ 0.01) CLDN mRNA expression in the cecum.
Table 4.
Effects of fermented products on broiler growth performance.
| Item | Treatment1 |
|||||
|---|---|---|---|---|---|---|
| CON | ENM | SFP | LFP | SEM2 | P-value3 | |
| Body weight (g/bird) | ||||||
| 1 d | 41.9 | 41.9 | 41.9 | 42.0 | 0.02 | 0.336 |
| 35 d | 1712.4b | 1782.1ab | 1731.0b | 1908.0a | 22.02 | 0.003 |
| Average daily gain (g/d/bird) | ||||||
| 1–14 d | 24.5 | 25.7 | 25.0 | 25.0 | 0.36 | 0.640 |
| 15–35 d | 64.0b | 66.2ab | 64.0b | 70.6a | 0.86 | 0.011 |
| 1–35 d | 48.2b | 50.0ab | 48.3b | 52.4a | 0.55 | 0.019 |
| Average daily feed intake (g/d/bird) | ||||||
| 1–14 d | 28.5 | 29.5 | 28.2 | 28.3 | 0.34 | 0.564 |
| 15–35 d | 105.6 | 104.2 | 102.7 | 110.7 | 1.57 | 0.313 |
| 1–35 d | 74.8 | 74.4 | 72.9 | 77.7 | 0.99 | 0.382 |
| Feed conversion ratio | ||||||
| 1–14 d | 1.16 | 1.15 | 1.13 | 1.16 | 0.01 | 0.677 |
| 15–35 d | 1.66 | 1.58 | 1.61 | 1.57 | 0.03 | 0.648 |
| 1–35 d | 1.55 | 1.49 | 1.51 | 1.48 | 0.02 | 0.621 |
| Mortality rate (%) | ||||||
| 1–35 d | 2.5b | 12.5ab | 5.0ab | 15.0a | 1.78 | 0.030 |
CON = Basal diet; ENM = Basal diet supplemented with enramycin; SFP = Basal diet supplemented with B. subtilis–fermented products; LFP = Basal diet supplemented with B. licheniformis–fermented products.
SEM = standard error of means.
P-value was obtained by ANOVA.
Means in the row without common superscripts are significantly different (P ≤ 0.05). Mean value representing results from 8 replicate cages per treatment (n = 8).
Table 5.
Effects of fermented products on broiler intestinal morphology.
| Item | Treatment1 |
||||||
|---|---|---|---|---|---|---|---|
| CON | ENM | SFP | LFP | SEM2 | P-value3 | ||
| Duodenum | Villus height (μm) | 1833.9ab | 1698.8b | 1860.9ab | 1981.5a | 35.50 | 0.029 |
| Crypt depth (μm) | 145.6 | 148.6 | 129.1 | 169.4 | 8.95 | 0.730 | |
| Villus height: crypt depth | 12.7 | 12.3 | 14.8 | 14.4 | 0.70 | 0.541 | |
| Jejunum | Villus height (μm) | 907.3ab | 870.6ab | 809.1b | 946.2a | 18.61 | 0.048 |
| Crypt depth (μm) | 128.1 | 123.6 | 104.1 | 121.1 | 5.17 | 0.398 | |
| Villus height: crypt depth | 7.3 | 7.6 | 7.9 | 8.0 | 0.33 | 0.840 | |
| Ileum | Villus height (μm) | 711.0 | 677.5 | 718.5 | 718.0 | 14.67 | 0.750 |
| Crypt depth (μm) | 107.6 | 115.1 | 105.6 | 124.1 | 4.46 | 0.471 | |
| Villus height: crypt depth | 6.7 | 6.1 | 7.0 | 5.9 | 0.26 | 0.410 | |
CON = Basal diet; ENM = Basal diet supplemented with enramycin; SFP = Basal diet supplemented with B. subtilis–fermented products; LFP = Basal diet supplemented with B. licheniformis–fermented products.
SEM = standard error of means.
P-value was obtained by ANOVA.
Mean values in the row without identical superscripted letters are significantly different (P ≤ 0.05). Mean value representing results from 4 replicate cages per treatment (n = 4).
Table 6.
Effect of fermented products on broiler intestinal gene expression.
| Item | Treatment1 |
||||||
|---|---|---|---|---|---|---|---|
| Gene2 | CON | ENM | SFP | LFP | SEM3 | P-value4 | |
| Duodenum | CAT | 1.02ab | 1.08ab | 1.53a | 0.43b | 0.13 | 0.009 |
| SOD | 1.08b | 0.63c | 1.38a | 0.95b | 0.07 | <0.001 | |
| MUC2 | 1.17a | 0.76b | 0.81ab | 0.79ab | 0.06 | 0.033 | |
| ZO-1 | 1.00 | 1.08 | 0.99 | 1.08 | 0.03 | 0.583 | |
| OCLN | 0.98a | 1.13a | 0.77b | 0.98a | 0.04 | <0.001 | |
| CLDN | 1.05 | 0.59 | 0.93 | 0.83 | 0.07 | 0.088 | |
| Jejunum | CAT | 0.73b | 1.78ab | 2.78a | 0.35b | 0.11 | 0.002 |
| SOD | 1.00ab | 0.40b | 1.17a | 1.14a | 0.11 | 0.018 | |
| MUC2 | 1.01b | 1.07b | 0.91b | 2.45a | 0.17 | <0.001 | |
| ZO-1 | 0.90b | 1.98ab | 1.38ab | 2.23a | 0.17 | 0.016 | |
| OCLN | 0.92b | 2.09ab | 2.38a | 2.29a | 0.20 | 0.015 | |
| CLDN | 1.19b | 1.80b | 2.86a | 1.45b | 0.17 | <0.001 | |
| Ileum | CAT | 1.11b | 0.68b | 1.90a | 0.57b | 0.13 | <0.001 |
| SOD | 1.05 | 2.04 | 3.06 | 2.35 | 0.33 | 0.198 | |
| MUC2 | 1.23 | 1.10 | 1.40 | 1.00 | 0.07 | 0.164 | |
| ZO-1 | 1.00b | 1.97a | 1.21b | 1.53ab | 0.12 | 0.006 | |
| OCLN | 1.04ab | 1.34ab | 0.92b | 1.50a | 0.08 | 0.015 | |
| CLDN | 0.84 | 1.70 | 1.55 | 1.61 | 0.18 | 0.320 | |
| Cecum | CAT | 0.98b | 2.11a | 1.53ab | 1.75ab | 0.14 | 0.009 |
| SOD | 1.00b | 1.59ab | 2.26ab | 2.89a | 0.26 | 0.049 | |
| MUC2 | 0.90ab | 0.96ab | 0.71b | 1.22a | 0.06 | 0.016 | |
| ZO-1 | 0.89b | 1.47a | 0.91ab | 1.10ab | 0.08 | 0.035 | |
| OCLN | 0.80b | 1.46a | 1.34a | 0.82b | 0.083 | <0.001 | |
| CLDN | 1.15b | 1.43ab | 2.01a | 0.97ab | 0.12 | 0.007 | |
CON = Basal diet; ENM = Basal diet supplemented with enramycin; SFP = Basal diet supplemented with B. subtilis–fermented products; LFP = Basal diet supplemented with B. licheniformis–fermented products.
CAT = catalase; SOD = superoxide dismutase; MUC2 = mucin 2; ZO-1 = zonula occludens-1; OCLN = occludin; CLDN = claudin.
SEM = standard error of means.
P-value was obtained by ANOVA.
Mean values in the row without identical superscripted letters are significantly different (P ≤ 0.05). Mean value representing results from 5 replicate cages per treatment (n = 5) except SOD in duodenum and CAT in cecum (n = 4)
Cecal Microbiota Composition
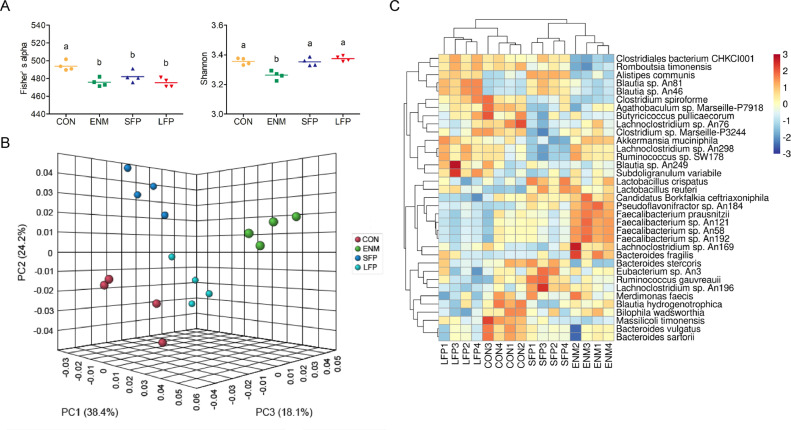
The microbial species richness (i.e., Fisher's alpha) in the cecal digesta was lower (P ≤ 0.05) in the ENM, SFP, and LFP groups than in the CON group (Figure 1A). The microbial species evenness (Shannon) in the cecal digesta was lower (P ≤ 0.05) in the ENM group than in the other groups (Figure 1A). The PCoA results indicated a clear difference in bacterial community composition between the groups (Figure 1B). Figure 1C presents the heatmap indicating the 35 most abundant bacteria in the cecal digesta of broilers at species level. Some bacterial clusters, such as those including Massilicoli timonensis, Bacteroides vulgatus, and Bacteroides sartorii were specifically identified in the CON group. Specific bacterial clusters including Faecalibacterium prausnitzii, Faecalibacterium sp. An121, Faecalibacterium sp. An58, and Faecalibacterium sp. An192, were identified only in the ENM group. Some bacterial clusters, such as those including Alistipes communis, Blautia sp. An81, and Blautia sp. An46, partially overlapped between the LFP and SFP groups. Specific bacterial clusters, such as those including Lactobacillus crispatus, Ruminococcus gauvreauii, and Lachnoclostridium sp. An196, were identified only in the SFP group. Similar bacterial clusters, such as those including Clostridiales bacterium CHKCI001 and Romboutsia timonensis, were identified in the CON, LFP, and SFP groups. The effects of SFP and LFP treatments on the bacterial taxonomy in the cecal digesta of broilers are summarized in Table 7. At the phylum level, the abundance of the phylum Firmicutes was higher (P ≤ 0.05) in the ENM group than in the CON group, whereas that of the phylum Bacteroidetes was lower (P ≤ 0.01) in the ENM group than those in the CON and SFP groups. At the genus level, the abundance of the genus Bacteroides was lower (P ≤ 0.05) in ENM or LFPs than those in the CON group, whereas that of the genera Faecalibacterium and Lachnoclostridium was higher (P ≤ 0.01) in the ENM group than in the SFP and LFP groups. Compared with the other groups, the SFP group demonstrated higher (P ≤ 0.001) abundance of the genus Lactobacillus but lower (P ≤ 0.001) abundance of the genus Clostridium. Compared with the other groups, the SFP and LFP groups demonstrated higher (P ≤ 0.001) abundance of the genus Blautia and the CON and LFP groups demonstrated higher (P ≤ 0.05) abundance of the genus Erysipelatoclostridium. At the species level, the SFP group had the highest abundance of Bacteroides stercoris and R. gauvreauii, whereas the ENM group demonstrated the highest (P ≤ 0.05) abundance of Candidatus Borkfalkia ceftriaxoniphila, Faecalibacterium sp. An58, and F. prausnitzii. The abundance of Lachnoclostridium sp. An76 and Blautia hydrogenotrophica was lower (P ≤ 0.05) in the SFP and LFP groups than in the CON group. The abundance of C. bacterium CHKCI001 was lower (P ≤ 0.001) in the ENM group than in the other groups. The abundance of L. crispatus was higher (P ≤ 0.01) in the SFP group than in the CON and LFP groups. The abundance of B. vulgatus was higher (P ≤ 0.01) in the CON group than in the ENM and LFP groups. The SFP demonstrated a lower (P ≤ 0.01) abundance of Clostridium sp. Marseille-P3244 than did the CON group. The abundance of Clostridium spiroforme was higher (P ≤ 0.01) in the CON and LFP groups than in the ENM group. The abundance of Lactobacillus reuteri was higher (P ≤ 0.01) in the ENM and SFP groups than in the CON group. The abundance of R. timonensis was higher (P ≤ 0.001) in the LFP group than in the ENM and SFP groups, and that of Akkermansia muciniphila was higher (P ≤ 0.01) in the ENM and LFP groups than in the SFP group. SFP and LFP treatments led to the highest (P ≤ 0.001) abundance of Blautia sp. An81.
Figure 1.
Microbial community structures in cecal digesta for comparison. (A) Scatter plot of bacterial alpha diversity in the CON, ENM, SFP, and LFP groups. Each bar represents mean ± standard deviation (n = 4). Different superscripts indicate significant differences between groups. (B) PCoA of the cecal bacterial communities in the CON, ENM, SFP, and LFP groups (n = 4). (C) Heatmap of microbial species abundance in cecal digesta. Abundance distribution of 35 dominant species (Y axis) across all samples (X axis) is displayed (n = 4). The values are normalized using the Z-score. Abbreviations: CON, basal diet; ENM, basal diet supplemented with enramycin; LFP, Basal diet supplemented with B. licheniformis–fermented products; PCoA, principal coordinate analysis; SFP, Basal diet supplemented with B. subtilis–fermented products.
Table 7.
Effects of fermented products on bacterial taxonomy in broiler cecal digesta.
| Item | Treatment1 |
|||||
|---|---|---|---|---|---|---|
| CON | ENM | SFP | LFP | SEM2 | P-value3 | |
| Phylum (%) | ||||||
| Firmicutes | 74.39b | 78.90a | 75.95ab | 77.83ab | 0.62 | 0.025 |
| Bacteroidetes | 20.27a | 15.80b | 19.44a | 17.04ab | 0.60 | 0.010 |
| Genus (%) | ||||||
| Bacteroides | 18.14a | 14.25b | 15.84ab | 14.46b | 0.52 | 0.011 |
| Faecalibacterium | 6.83b | 10.71a | 6.72b | 5.76b | 0.52 | <0.001 |
| Lachnoclostridium | 5.75ab | 5.93a | 5.18c | 5.29bc | 0.10 | 0.003 |
| Lactobacillus | 4.75c | 6.86ab | 8.72a | 5.51bc | 0.45 | <0.001 |
| Blautia | 4.80b | 5.19b | 6.58a | 6.79a | 0.24 | <0.001 |
| Clostridium | 2.14a | 2.04a | 1.88b | 2.08a | 0.03 | <0.001 |
| Ruminococcus | 1.74 | 1.69 | 1.90 | 1.76 | 0.04 | 0.246 |
| Erysipelatoclostridium | 2.01a | 1.19b | 1.42ab | 1.85a | 0.11 | 0.011 |
| Species (%) | ||||||
| Bacteroides stercoris | 2.19ab | 0.94b | 2.69a | 1.45ab | 0.25 | 0.039 |
| Lachnoclostridium sp. An76 | 2.41a | 1.93ab | 1.63b | 1.81b | 0.09 | 0.003 |
| Clostridiales bacterium CHKCI001 | 2.50a | 1.29b | 2.59a | 2.98a | 0.18 | <0.001 |
| Lactobacillus crispatus | 0.69b | 0.88ab | 1.11a | 0.64b | 0.06 | 0.005 |
| Bacteroides vulgatus | 0.72a | 0.53b | 0.56ab | 0.51b | 0.03 | 0.008 |
| Merdimonas faecis | 0.73 | 0.73 | 0.71 | 0.65 | 0.01 | 0.156 |
| Ruminococcus gauvreauii | 0.63ab | 0.60b | 0.84a | 0.54b | 0.04 | 0.010 |
| Lachnoclostridium sp. An169 | 0.73 | 0.92 | 0.68 | 0.70 | 0.04 | 0.047 |
| Candidatus Borkfalkia ceftriaxoniphila | 0.64b | 0.87a | 0.72ab | 0.41c | 0.05 | <0.001 |
| Blautia hydrogenotrophica | 0.60a | 0.50ab | 0.42b | 0.43b | 0.02 | 0.003 |
| Clostridium sp. Marseille-P3244 | 0.59a | 0.54ab | 0.47b | 0.55ab | 0.02 | 0.009 |
| Clostridium spiroforme | 0.70a | 0.30b | 0.42ab | 0.70a | 0.06 | 0.009 |
| Lactobacillus reuteri | 0.50b | 0.90a | 1.01a | 0.79ab | 0.06 | 0.003 |
| Romboutsia timonensis | 0.53ab | 0.18c | 0.36bc | 0.58a | 0.05 | <0.001 |
| Faecalibacterium sp. An58 | 0.43b | 0.67a | 0.40b | 0.35b | 0.03 | <0.001 |
| Akkermansia muciniphila | 0.31ab | 0.54a | 0.15b | 0.55a | 0.05 | 0.004 |
| Faecalibacterium prausnitzii | 0.43b | 0.59a | 0.42b | 0.38b | 0.02 | <0.001 |
| Blautia sp. An81 | 0.41b | 0.43b | 0.56a | 0.65a | 0.03 | <0.001 |
CON = Basal diet; ENM = Basal diet supplemented with enramycin; SFP = Basal diet supplemented with B. subtilis–fermented products; LFP = Basal diet supplemented with B. licheniformis–fermented products.
SEM = standard error of means.
P-value was obtained by ANOVA.
Mean values in the row without identical superscripted letters are significantly different (P ≤ 0.05). Mean value representing results from 4 replicate cages per treatment (n = 4).
Microbial Carbohydrate-Active Enzyme Gene Composition in the Cecal Digesta
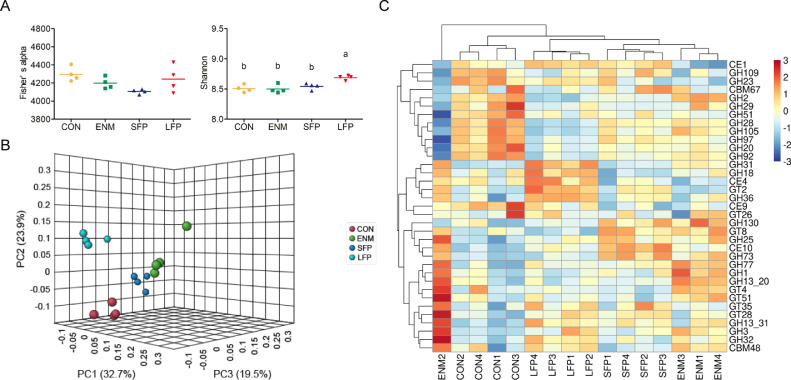
The between-group differences in the richness of microbial carbohydrate-active enzyme gene were nonsignificant (Figure 2A). By contrast, the evenness of microbial carbohydrate-active enzyme genes in the cecal digesta was higher (P ≤ 0.05) in LFP group than in the other groups (Figure 2A). The PCoA results exhibited a clear separation of microbial carbohydrate-active enzyme gene composition among the groups (Figure 2B). Figure 2C presents a heatmap showing the 35 most abundant microbial carbohydrate-active enzyme genes in the cecal digesta. The glycoside hydrolase genes GH51, GH97, GH20, and GH92 were identified only in the CON group, whereas GH77, GH1, and GH13_20 were identified only in the ENM group. The carbohydrate esterase 10 gene (CE10) was identified only in the SFP group. GH73 and the glycosyltransferase (GT) 8 gene (GT8) were identified in the ENM and SFP groups. The carbohydrate-active enzyme genes GH31, GH18, GT2, and GH36 were identified in the LFP group. The effects of SFP and LFP treatments on the microbial carbohydrate-active enzyme genes in the cecal digesta of broilers are summarized in Table 8. GT2 abundance was higher (P ≤ 0.01) in the LFP group than in the CON and ENM groups. GT4 and GH1 abundance were the highest (P ≤ 0.01 and P ≤ 0.001, respectively) in the ENM group, and CE10 abundance was the highest (P < 0.001) in the SFP group. GH2 abundance was higher (P ≤ 0.01) in the LFP group than in the CON and ENM groups, that of GH3 was higher (P ≤ 0.05) in the ENM group than in the CON group, that of CE4 was higher (P ≤ 0.05) in the LFP group than in the ENM and SFP groups, that of GH25 was higher (P ≤ 0.01) in the ENM and SFP groups than in the CON group, and that of GT51 was higher (P ≤ 0.01) in the ENM group than in the CON and SFP groups. By contrast, CE1 abundance was lower (P ≤ 0.001) in the ENM group than in the other groups. GH20 and GH28 abundance was higher (P ≤ 0.05 and P ≤ 0.01) in the CON group than in the LFP group, and that of GH31 was higher (P ≤ 0.001) in the ENM and LFP groups than in the CON and SFP groups. The between-group differences in the total microbial carbohydrate-active enzyme gene numbers in the cecal digesta were nonsignificant.
Figure 2.
Comparison of microbial carbohydrate-active enzyme gene structure in the cecal digesta. (A) Scatter plot of alpha diversity of microbial carbohydrate-active enzyme gene composition in the CON, ENM, SFP, and LFP groups. Each bar represents mean ± standard deviation (n = 4). Different superscripts indicate significant differences between groups. (B) PCoA of the microbial carbohydrate-active enzyme gene composition in the CON, ENM, SFP, and LFP groups (n = 4). (C) Heatmap of microbial carbohydrate-active enzyme gene abundance in the cecal digesta. Abundance distribution of 35 dominant microbial carbohydrate-active enzyme genes (Y-axis) across all samples (X-axis) is displayed (n = 4). The values are normalized using the Z-score. Abbreviations: CON, basal diet; ENM, basal diet supplemented with enramycin; LFP, Basal diet supplemented with B. licheniformis–fermented products; PCoA, principal coordinate analysis; SFP, Basal diet supplemented with B. subtilis–fermented products.
Table 8.
Effects of fermented products on dominant carbohydrate-active enzyme gene in broiler cecal digesta.
| Treatment1 |
||||||
|---|---|---|---|---|---|---|
| Gene2 (%) | CON | ENM | SFP | LFP | SEM3 | P-value4 |
| GT2 | 13.64b | 13.75b | 14.07ab | 14.61a | 0.12 | 0.006 |
| GT4 | 6.80b | 7.00a | 6.72b | 6.78b | 0.03 | 0.006 |
| GH2 | 4.54a | 4.51a | 4.37ab | 4.27b | 0.04 | 0.008 |
| GH3 | 3.10b | 3.40a | 3.19ab | 3.28ab | 0.04 | 0.014 |
| CE10 | 2.63c | 2.85b | 3.04a | 2.79bc | 0.04 | <0.001 |
| CE4 | 2.69ab | 2.59b | 2.60b | 2.86a | 0.04 | 0.018 |
| GH1 | 1.91b | 2.30a | 1.99b | 1.92b | 0.05 | <0.001 |
| GH25 | 1.91b | 2.12a | 2.09a | 2.01ab | 0.02 | 0.009 |
| GT51 | 1.83b | 1.98a | 1.80b | 1.85ab | 0.02 | 0.009 |
| CE1 | 1.69a | 1.54b | 1.74a | 1.77a | 0.02 | <0.001 |
| GH20 | 1.78a | 1.57b | 1.62ab | 1.57b | 0.03 | 0.014 |
| GH28 | 1.71a | 1.50ab | 1.57ab | 1.39b | 0.03 | 0.008 |
| GH31 | 1.38b | 1.50a | 1.40b | 1.57a | 0.02 | <0.001 |
| GT28 | 1.37 | 1.44 | 1.44 | 1.43 | 0.01 | 0.234 |
| GH29 | 1.46 | 1.36 | 1.33 | 1.34 | 0.02 | 0.067 |
| CON | ENM | SFP | LFP | SEM | P value | |
| Total abundance5 | 164299 | 158394 | 150967 | 154980 | 4638.6 | 0.811 |
CON = Basal diet; ENM = Basal diet supplemented with enramycin; SFP = Basal diet supplemented with B. subtilis–fermented products; LFP = Basal diet supplemented with B. licheniformis–fermented products.
GT = glycosyltransferase; GH = glycoside hydrolase; CE = carbohydrate esterase.
SEM = standard error of means.
P-value was obtained by ANOVA.
Total carbohydrate-active enzyme gene abundance.
Mean values in the row without identical superscripted letters are significantly different (P ≤ 0.05). Mean value representing results from 4 replicate cages per treatment (n = 4).
Taxonomic Assignment of Microbial GT2
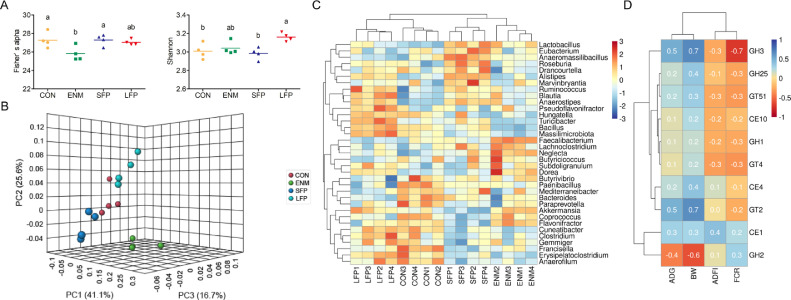
At the genus level, the richness of the taxonomic assignment of microbial GT2 was lower (P ≤ 0.05) in the ENM group than in the CON and SFP groups (Figure 3A). By contrast, the evenness of the taxonomic assignment of microbial GT2 was higher (P ≤ 0.05) in the LFP group than in the CON and SFP groups (Figure 3A). The PCoA results demonstrated a clear separation of taxonomic assignment of microbial GT2 composition at the genus level among the groups (Figure 3B). Figure 3C presents a heatmap demonstrating the dominant taxonomic assignment of microbial GT2 at the genus level. The genera Paenibacillus, Mediterraneibacter, and Bacteroides exhibited the highest proportions of GT2 in the CON group; the genera Faecalibacterium, and Neglecta had the highest proportions of GT2 in the ENM group; the genera Lactobacillus, Anaeromassilibacillus, and Roseburia exhibited the highest proportions of GT2 in the SFP group; and the genera Pseudoflavonifractor, Turicibacter, and Bacillus demonstrated the highest proportions of GT2 in the LFP group. Figure 3D presents the results of analysis of the correlation between the dominant carbohydrate-active enzyme genes in the cecal digesta and broiler growth performance. All detected microbial carbohydrate-active enzyme genes, except CE1 and GH2, were positively correlated with average daily gain and body weight and negatively correlated with feed conversion ratio. The average GT2 abundance was positively correlated with average daily gain and body weight and negatively correlated with feed conversion ratio. By contrast, the average GH2 abundance was negatively correlated with average daily gain and body weight.
Figure 3.
Taxonomic assignment of microbial GT2 at the genus level in the cecal digesta for comparison. (A) Scatter plot of alpha diversity of taxonomic assignment of microbial GT2 in the CON, ENM, SFP, and LFP groups. Each bar represents mean ± standard deviation (n = 4). Different superscripts indicate significant differences between groups. (B) PCoA of the taxonomic assignment of microbial GT2 gene in the CON, ENM, SFP, and LFP groups (n = 4). (C) Heatmap of abundance of taxonomic assignment of microbial GT2 gene in the cecal digesta. Abundance distribution of 35 dominant genera (Y-axis) across all samples (X-axis) is displayed (n = 4). The values are normalized using the Z-score. (D) Results of Spearman's correlation analysis between dominant carbohydrate-active enzyme genes and growth performance. Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; CON, basal diet; ENM, basal diet supplemented with enramycin; FCR: feed conversion ratio; LFP, Basal diet supplemented with B. licheniformis–fermented products; PCoA, principal coordinate analysis; SFP, Basal diet supplemented with B. subtilis–fermented products.
DISCUSSION
Few studies have studied the effects of B. subtilis and B. licheniformis on the growth performance of broilers (Gong et al., 2018; Musa et al., 2019; Xu et al., 2021). Supplementation with B. subtilis (1 × 105 CFU/g) or B. licheniformis (1 × 105 CFU/g) exhibit similar daily body weight gain in broilers (Gong et al., 2018). B. subtilis (2 × 109 CFU/g) and B. licheniformis (2 × 109 CFU/g) have been noted to demonstrate necrotic enteritis preventive (or partially preventive) effects similar to those of antibiotics in broilers (Musa et al., 2019). In the present study, compared with SFP treatment (1 × 108 CFU/g), LFP treatment (1 × 108 CFU/g) improved body weight at 35 d of age and led to average daily gain at 15 to 35 and 1 to 35 d of age in broilers. This observation agrees with the results of Xu et al. (2021); the authors observed that at identical concentration of supplementation (1.5 × 106 CFU/g), B. licheniformis more effectively promoted growth in broilers than did B. subtilis. Furthermore, dietary inclusion of B. licheniformis (3 × 106 CFU/g) improved body weight gain and reduced feed conversion ratio compared with B. subtilis (6.6 × 106 CFU/g) in broilers (Arif et al., 2021). It has been demonstrated that probiotic-mediated mucus production and secretion by intestinal goblet cells are crucial for the maintenance of intestinal epithelial barrier function (Ohland and Macnaughton, 2010). An intact intestinal epithelial barrier can protect the host from pathogens, prevent gut microbiota dysbiosis, and improve subsequent growth. This study also demonstrated that LFP supplementation promoted MUC2 expression in the jejunum and cecum in broilers. PCoA on cecal microbiota and microbial carbohydrate-active enzyme gene composition demonstrated a clear separation of the 4 main groups, indicating gut microbiota composition was modulated in response to LFP treatment. Previous study has shown that B. licheniformis is more effective in degrading the protein of soybean meal than is B. subtilis (Dai et al., 2022). This result indicates that B. licheniformis exhibits higher proteolytic enzyme activity, which may increase the nutrient utilization in the gastrointestinal tract and subsequent growth performance in broilers. Taken together, these results indicated that at identical concentration of supplementation, LFPs lead to superior growth performance than do SFPs. The growth promoting effects of LFPs in broilers may be mediated through the formation of an effective physical barrier in the gut through increased mucin production, competitive exclusion of enteric pathogens via cecal microbiota alteration, and promotion of nutrient utilization by highly efficient microbial proteolytic enzyme activity. LFP supplementation unexpectedly increased the mortality of broilers in the present study. There is no clear explanation for this results and more work will be needed in future to further investigate the effects of different concentration of LFP on the mortality of broilers.
On ingestion, spores from several Bacillus species can germinate and grow in the small intestinal tract of broilers (Cartman et al., 2008). Therefore, many hypotheses have been proposed for the mechanism through which Bacillus sp. regulate gut microbiota in broilers, such as the competitive exclusion of pathogens, production of antimicrobial peptides, and secretion of extracellular enzymes or secondary metabolites (Ramlucken et al., 2020). It has been reported that dietary supplementation with Bacillus sp.-based probiotics has positive effects on the modulation of intestinal microbiota in broilers (Luise et al., 2022). B. subtilis supplementation increases the abundance of beneficial bacteria and decreases the abundance of pathogens in broiler intestines (Bilal et al., 2021; Šimunović et al., 2022). Dietary supplementation with B. licheniformis promotes Lactobacillus sp. growth and prevents pathogen colonization in broiler intestines (Lin et al., 2017; Xu et al., 2018). Our previous studies, we have demonstrated that dietary supplementation with SFPs reverses dysbiosis of cecal microbial community in broilers by using inflammatory models (Chen and Yu, 2021, 2022a). Dietary LFP inclusion leads to the modulation of microbial composition in broiler intestines through an increase in Lactobacillus sp. abundance (Chen and Yu, 2020; Cheng et al., 2021b ). In the current study, we found that LFP supplementation reduced species richness in the cecal digesta of broilers. However, our previous study demonstrated that LFP supplementation increased species richness and evenness in the cecal digesta of broilers (Chen and Yu, 2022a). Furthermore, cecal bacterial abundance at the genus level differed between the results of the 2 studies. For instance, the abundance of Lactobacillus sp. in the cecal digesta of broilers increased in our previous study, but in the present study, the abundance of Lactobacillus sp. remained unchanged. This inconsistency may be due to the differences in dietary B. licheniformis spore concentrations used in the 2 studies. Our previous studies have demonstrated that SFP supplementation can modulate the cecal microbiota of broilers in lipopolysaccharide or dextran sulfate sodium-induced inflammatory models (Chen and Yu, 2021, 2022b). In the present study, similar to LFP supplementation, SFP supplementation reduced species richness in the cecal digesta of broilers. LFP and SFP supplementation consistently reduced the abundance of Lachnoclostridium sp. and increased the abundance of Blautia sp. By contrast, compared with LFP supplementation, SFP supplementation specifically increased Lactobacillus sp. abundance but reduced that of Clostridium sp. It has been demonstrated that B. subtilis and B. licheniformis differentially produce extracellular enzymes, antimicrobial peptides, and secondary metabolites (Velmurugan et al., 2009; Sumi et al., 2015; Elshaghabee et al., 2017). These microbe-derived substances also play a critical role in modulating gut microbiota composition (Liu et al., 2022). Thus, the differences in the effects of SFP and LFP supplementation on the cecal microbiota may be due to the differences in the production of extracellular enzymes, antimicrobial peptides, and secondary metabolites (Velmurugan et al., 2009; Sumi et al., 2015; Elshaghabee et al., 2017). These results indicated that at identical supplementation concentrations, SFPs and LFPs have a distinct effect on the regulation of cecal microbiota composition in broilers.
In the present study, the functional annotation of bacterial genome revealed that GT2 was the most dominant microbial carbohydrate-active enzyme gene in the cecal digesta of broilers and differentially regulated among groups. GTs catalyze the transfer of a glycosyl group from activated sugar donors to a diverse range of acceptors, such as lipids, proteins, antibiotics, xenobiotics, and phytotoxins (Bowles et al., 2005). GT-mediated glycosylation can alter the stability, chemical properties, and bioactivity of its acceptors (Bowles et al., 2005). Glycosylation also can occur on microbe-derived substances, such as mycotoxins. Mycotoxins can be glycosylated, generating modified mycotoxins with decreased toxicity (Li et al., 2020). Mycotoxins, which are toxic fungal secondary metabolites, constitute a serious problem for the gut health and growth of poultry. Mycotoxin contamination commonly occurs in the field as well as during animal feed processing and storage (Yang et al., 2019). LFP supplementation increased microbial GT2 expression in the cecal digesta, and broiler growth performance was positively correlated with microbial GT2 abundance, implying that microbial GT2 might regulate growth-related factors or their activity in the intestines of broilers through glycosylation. Whether LFP-mediated increase in microbial GT2 expression contributes to detoxification of mycotoxins through glycosylation remain to be confirmed. Differential carbohydrate-active enzyme gene distribution was also observed between the effects of SFP and LFP treatments because of the dominance of different bacterial taxa. These findings thus demonstrated that SFPs and LFPs not only differentially modulate cecal microbiota composition but also alter microbial carbohydrate-active enzyme gene levels in the cecal digesta of broilers. Whether alterations in the composition of microbial carbohydrate-active enzyme genes, particularly GT2, or their expression has a beneficial effect on gut health and growth of broilers warrant further investigation.
In conclusion, LFP supplementation improves the body weight and average daily gain, promotes jejunal and cecal intestinal barrier function and antioxidant gene expression, modulates cecal microbiota composition, and increases microbial glycosyltransferase 2 gene expression in broilers. Specifically, LFP promoted broiler growth more effectively than did SFP. This finding provides an improved understanding of the benefits of using LFPs as an antibiotic alternative in poultry feeds
ACKNOWLEDGMENTS
This work was supported by the Ministry of Science and Technology [MOST 110-2313-B-197-005-MY3] in Taiwan.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102670.
Appendix. Supplementary materials
REFERENCE
- Aljumaah M.R., Alkhulaifi M.M., Abudabos A.M., Aljumaah R.S., Alsaleh A.N., Stanley D. Bacillus subtilis PB6 based probiotic supplementation plays a role in the recovery after the necrotic enteritis challenge. PLoS One. 2020;15 doi: 10.1371/journal.pone.0232781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif M., Akteruzzaman M., Tuhin-Al-Ferdous S.S.Islam, Das B.C., Siddique M.P., Kabir S.M.L. Dietary supplementation of Bacillus-based probiotics on the growth performance, gut morphology, intestinal microbiota and immune response in low biosecurity broiler chickens. Vet. Anim. Sci. 2021;14 doi: 10.1016/j.vas.2021.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahaddad S.A., Almalki M.H.K., Alghamdi O.A., Sohrab S.S., Yasir M., Azhar E.I., Chouayekh H. Bacillus species as direct-fed microbial antibiotic alternatives for monogastric production. Probiotics Antimicrob. Proteins. 2022;29:1–16. doi: 10.1007/s12602-022-09909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilal M., Si W., Barbe F., Chevaux E., Sienkiewicz O., Zhao X. Effects of novel probiotic strains of Bacillus pumilus and Bacillus subtilis on production, gut health, and immunity of broiler chickens raised under suboptimal conditions. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D., Isayenkova J., Lim E.K., Poppenberger B. Glycosyltransferases: managers of small molecules. Curr. Opin. Plant Biol. 2005;8:254–263. doi: 10.1016/j.pbi.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Cartman S.T., La Ragione R.M., Woodward M.J. Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl. Environ. Microbiol. 2008;74:5254–5258. doi: 10.1128/AEM.00580-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W.Y., Yu Y.H. Effect of Bacillus species-fermented products and essential oils on growth performance, gut morphology, cecal short-chain fatty acid levels, and microbiota community in broilers. Poult. Sci. 2022;101:101970. doi: 10.1016/j.psj.2022.101970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis–fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Y., Yu Y.H. Bacillus subtilis–fermented products ameliorate the growth performance and alter cecal microbiota community in broilers under lipopolysaccharide challenge. Poult. Sci. 2021;100:875–886. doi: 10.1016/j.psj.2020.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis–fermented products and enramycin differentially modulate microbiota and antibiotic resistome in the cecal digesta of broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.Y., Yu Y.H. Bacillus subtilis–fermented products ameliorate the growth performance, alleviate intestinal inflammatory gene expression, and modulate cecal microbiota community in broilers during the starter phase under dextran sulfate sodium challenge. J. Poult. Sci. 2022;59:261–272. doi: 10.2141/jpsa.0210139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.H., Horng Y.B., Dybus A., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. J. Poult. Sci. 2021;58:30–39. doi: 10.2141/jpsa.0200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.H., Horng Y.B., Chen W.J., Hua K.F., Dybus A., Yu Y.H. Effect of fermented products produced by Bacillus licheniformis on the growth performance and cecal microbial community of broilers under coccidial challenge. Animals. 2021;11:1245. doi: 10.3390/ani11051245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.H., Zhang N., Han J.C., Chang C.W., Hsiao F.S.H., Yu Y.H. Optimization of surfactin production from Bacillus subtilis in fermentation and its effects on Clostridium perfringens-induced necrotic enteritis and growth performance in broilers. J. Anim. Physiol. Anim. Nutr. 2018;102:1232–1244. doi: 10.1111/jpn.12937. [DOI] [PubMed] [Google Scholar]
- Dai C., Hou Y., Xu H., Huang L., Dabbour M., Mintah B.K., He R., Ma H. Effect of solid-state fermentation by three different Bacillus species on composition and protein structure of soybean meal. J. Sci. Food Agric. 2022;102:557–566. doi: 10.1002/jsfa.11384. [DOI] [PubMed] [Google Scholar]
- Dhariwal A., Chong J., Habib S., King I.L., Agellon L.B., Xia J. MicrobiomeAnalyst: a web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45(W1):W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshaghabee F.M.F., Rokana N., Gulhane R.D., Sharma C., Panwar H. Bacillus as potential probiotics: status, concerns, and future perspectives. Front. Microbiol. 2017;8:1490. doi: 10.3389/fmicb.2017.01490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L., Wang B., Mei X., Xu H., Qin Y., Li W., Zhou Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci J. 2018;89:1561–1571. doi: 10.1111/asj.13089. [DOI] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Li P., Su R., Yin R., Lai D., Wang M., Liu Y., Zhou L. Detoxification of mycotoxins through biotransformation. Toxins. 2020;12:121. doi: 10.3390/toxins12020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Xu S., Zeng D., Ni X., Zhou M., Zeng Y., Wang H., Zhou Y., Zhu H., Pan K., Li G. Disruption in the cecal microbiota of chickens challenged with Clostridium perfringens and other factors was alleviated by Bacillus licheniformis supplementation. PLoS One. 2017;12 doi: 10.1371/journal.pone.0182426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Tan Y., Cheng H., Zhang D., Feng W., Peng C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. 2022;13:1106–1126. doi: 10.14336/AD.2022.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luise D., Bosi P., Raff L., Amatucci L., Virdis S., Trevisi P. Bacillus spp. probiotic strains as a potential tool for limiting the use of antibiotics, and improving the growth and health of pigs and chickens. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.801827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa B.B., Duan Y., Khawar H., Sun Q., Ren Z., Elsiddig Mohamed M.A., Abbasi I.H.R., Yang X. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Physiol. Anim. Nutr. 2019;103:1039–1049. doi: 10.1111/jpn.13082. [DOI] [PubMed] [Google Scholar]
- Ohland C.L., Macnaughton W.K. Probiotic bacteria and intestinal epithelial barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;298:G807–G819. doi: 10.1152/ajpgi.00243.2009. [DOI] [PubMed] [Google Scholar]
- Ramlucken U., Lalloo R., Roets Y., Moonsamy G., Jansenvan van Rensburg C., Thantsha M.S. Advantages of Bacillus-based probiotics in poultry production. Livest. Sci. 2020;241 [Google Scholar]
- Šimunović K., Sahin O., Erega A., Štefanič P., Zhang Q., Mandic Mulec I., Smole Možina S., Klančnik A. Bacillus subtilis PS-216 spores supplemented in broiler chicken drinking water reduce Campylobacter jejuni colonization and increases weight gain. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.910616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi C.D., Yang B.W., Yeo I.C., Hahm Y.T. Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can. J. Microbiol. 2015;61:93–103. doi: 10.1139/cjm-2014-0613. [DOI] [PubMed] [Google Scholar]
- Tran C., Cock I.E., Chen X., Feng Y. Antimicrobial Bacillus: metabolites and their mode of action. Antibiotics. 2022;11:88. doi: 10.3390/antibiotics11010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velmurugan N., Choi M.S., Han S.S., Lee Y.S. Evaluation of antagonistic activities of Bacillus subtilis and Bacillus licheniformis against wood-staining fungi: in vitro and in vivo experiments. J. Microbiol. 2009;47:385–392. doi: 10.1007/s12275-009-0018-9. [DOI] [PubMed] [Google Scholar]
- Xu S., Lin Y., Zeng D., Zhou M., Zeng Y., Wang H., Zhou Y., Zhu H., Pan K., Jing B., Ni X. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018;8:1744. doi: 10.1038/s41598-018-20059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Yu Y., Shen Y., Li Q., Lan J., Wu Y., Zhang R., Cao G., Yang C. Effects of Bacillus subtilis and Bacillus licheniformis on growth performance, immunity, short chain fatty acid production, antioxidant capacity, and cecal microflora in broilers. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yafetto L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: a review and bibliometric analysis. Heliyon. 2022;8:e09173. doi: 10.1016/j.heliyon.2022.e09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.K., Cheng Y.H., Tsai W.T., Liao R.M., Chang C.S., Chien W.C., Jhang J.C., Yu Y.H. Prevalence of mycotoxins in feed and feed ingredient between 2015 and 2017 in Taiwan. Environ. Sci. Pollut. R. 2019;26:23798–23806. doi: 10.1007/s11356-019-05659-0. [DOI] [PubMed] [Google Scholar]
- Yu Y.H., Wu C.M., Chen W.J., Hua K.F., Liu J.R., Cheng Y.H. Effectiveness of Bacillus licheniformis-fermented products and their derived antimicrobial lipopeptides in controlling coccidiosis in broilers. Animals. 2021;11:3576. doi: 10.3390/ani11123576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zeng D., Wang H., Qing X., Sun N., Xin J., Luo M., Khalique A., Pan K., Shu G., Jing B., Ni X. Dietary probiotic Bacillus licheniformis H2 enhanced growth performance, morphology of small intestine and liver, and antioxidant capacity of broiler chickens against Clostridium perfringens-induced subclinical necrotic enteritis. Probiotics Antimicrob. Proteins. 2020;12:883–895. doi: 10.1007/s12602-019-09597-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.