Summary
Background
Vitamin D insufficiency is associated with risk of multiple sclerosis (MS) relapse; whether supplementation influences prognosis is unknown. The Vitamin D to Ameliorate MS (VIDAMS) trial aimed to determine if high dose (5000 International Units (IU)/day) versus low dose (600 IU/day) vitamin D3, added to daily glatiramer acetate (GA), reduced the risk of clinical relapse in people with established relapsing remitting MS (RRMS) over 96 weeks.
Methods
VIDAMS is a randomised, phase 3, double-blind, multi-centre, controlled trial conducted at sixteen neurology clinics in the United States. Participants with MAGNIMS 2010 RRMS, aged 18–50 years, with recent disease activity were eligible to enroll if they had an Expanded Disability Status Scale score ≤4.0; minimum serum 25-hydroxyvitamin D level of 15 ng/ml within 30 days of screening; and average ≤ 1000 IU supplemental vitamin D3 daily in the 90 days prior to screening. Of 203 screened, 183 were eligible for the 30-day run-in to assess GA adherence, after which 172 were randomised 1:1 to low dose vitamin D3 (LDVD) or high dose vitamin D3 (HDVD), and were followed every 12 weeks for 96 weeks. The primary outcome was the proportion that experienced a confirmed relapse and analyses used Kaplan Meier and Cox proportional hazards models. 165 participants returned for ≥1 follow-up visit and were included in the primary and safety analyses; 140 completed a week 96 visit. This study was registered with ClinicalTrials.gov, NCT01490502.
Findings
Between March 22, 2012 and March 8, 2019, 172 participants were enrolled and randomised (83 LDVD, 89 HDVD) and differed at baseline only in gender and race: more males received HDVD (31%) than LDVD (16%), and fewer Black participants received HDVD (12%) than LDVD (22%). Among 165 participants with at least one follow-up visit, the proportion experiencing confirmed relapse did not differ between LDVD and HDVD [at 96 weeks: 32% vs. 34%, p = 0.60; hazard ratio (HR): 1.17 (0.67, 2.05), p = 0.57]. There was no hypercalcaemia. Three participants developed nephrolithiasis or ureterolithiasis (1 in the LDVD and 2 in the HDVD group). Two were possibly related to study drug; and one was presumed related to concomitant treatment with topiramate for migraine.
Interpretation
VIDAMS provides evidence that HDVD supplementation, added to GA, does not reduce the risk of clinical relapse in people with RRMS. Taken together with the null findings of previous trials, these results suggest that prescribing higher doses of vitamin D for purposes of modifying the RRMS course may not be beneficial.
Funding
This investigation was supported by a grant from the National Multiple Sclerosis Society (RG 4407A2/1). Teva Neuroscience, Inc. provided Copaxone (GA) for the duration of the trial.
Keywords: Relapsing-remitting multiple sclerosis, Vitamin D supplementation, Disease activity
Research in context.
Evidence before this study
We searched PubMed for the terms (“vitamin D”) AND (“supplementation”) AND (“multiple sclerosis”), published before February 19, 2022. Vitamin D is a known immunomodulator, and low serum 25(OH)D is associated with higher risk of developing MS and with increased clinical and radiological activity in RRMS. However, most evidence suggesting a role for vitamin D in MS activity arises from observational studies with inherent possible limitations, including confounding and reverse causality, so it remains unclear if vitamin D supplementation impacts the course of established RRMS. Three randomised clinical trials of vitamin D supplementation did not demonstrate an apparent benefit but also were limited in duration or size.
Added value of this study
The VIDAMS trial provides evidence that HDVD, as add-on to GA, does not reduce disease activity in established RRMS compared to LDVD. Taken together with the null findings of previous trials, these results suggest that prescribing higher doses of vitamin D for purposes of modifying the RRMS course may not be beneficial.
Implications of all the available evidence
Although common practice, based on these findings, prescribing HDVD for the purpose of reducing clinical and imaging disease activity in established RRMS does not appear to be helpful. We cannot exclude that a different level of serum 25(OH)D is needed to see a benefit, or that only subgroups of people with MS respond to HDVD supplementation. Future research includes a planned meta-analysis using individual patient-level data from CHOLINE, SOLAR and VIDAMS trials to increase sample size and statistical power and further explore these questions.
Introduction
Multiple sclerosis (MS) is a demyelinating and neurodegenerative autoimmune disease of the central nervous system with known environmental and genetic risk factors.1 Vitamin D is a known immunomodulator, and low serum 25-hydroxyvitamin D [25(OH)D] is associated with higher risk of developing MS and with increased clinical and radiological activity in relapsing-remitting MS (RRMS).2, 3, 4, 5 However, most evidence suggesting a role for vitamin D in MS activity arises from observational studies with inherent possible limitations, including confounding and reverse causality.3, 4, 5 Two early pilot trials demonstrated potential promise but had limitations and were not designed as definitive trials, so it remained unclear after their completion if vitamin D supplementation impacts the course of established RRMS.6,7 Three randomised clinical trials of vitamin D supplementation did not demonstrate an apparent benefit with respect to the primary outcome but also were limited in duration or size and had some other limitations that reduce confidence in the results.8, 9, 10 These trials also tested the effect of vitamin D supplementation as add-on to interferon beta as the MS disease-modifying therapy, where there is a possible interaction between vitamin D and interferon beta, underscoring the importance of evaluating the effect of vitamin D supplementation in the context of other MS therapies.11 We sought to test whether vitamin D supplementation as add-on to a first-line disease-modifying therapy (DMT) reduces disease activity among people with active MS.
Methods
The Vitamin D to Ameliorate Multiple Sclerosis (VIDAMS) trial (NCT01490502 Vitamin D Supplementation in Multiple Sclerosis), which enrolled participants between March 22, 2012 and March 8, 2019 and completed data collection on May 15, 2021, was a phase 3, multi-centre, randomised controlled, double-blind trial to evaluate if high dose vitamin D3 (HDVD) supplementation as add-on to daily glatiramer acetate (GA) treatment, reduces MS disease activity as compared to low dose vitamin D3 supplementation (LDVD). The study was conducted at 16 neurology clinics in the United States (US); local institutional review board/ethics committee approval was secured before each site began enrollment and all participants provided written informed consent. An independent data and safety monitoring board (DSMB), consisting of two MS specialists and a statistician, met twice yearly to review enrollment progress, protocol deviations and safety data. The University of Southern California Advanced Imaging in MS (AIMS) Laboratory served as the independent, blinded magnetic resonance imaging (MRI) reading centre.12
Study population
The VIDAMS trial study design has been described previously.13 Briefly, people with McDonald 2005 relapsing-remitting MS (RRMS)14 less than 10 years since diagnosis, between 18 and 50 years old, were invited to participate if they had recent disease activity (≥1 clinical attack in the past year AND at least one new silent T2 or gadolinium-enhancing (Gd+) lesion on brain MRI within the past year, OR ≥ 2 clinical attacks in the past 2 years, one of which was in the past year). Those with McDonald 2005 clinically isolated syndrome (CIS)14 but Magnetic Resonance Imaging in MS (MAGNIMS) RRMS15 were also eligible to participate if at screening, disease onset was within the past year. Additional eligibility requirements included: Kurtzke Expanded Disability Status Scale (EDSS)16 score ≤4.0; minimum serum 25(OH)D of 15 ng/ml within 30 days of screening; and average of ≤1000 IU supplemental vitamin D3 daily (in addition to multivitamin amount) in the 90 days before screening. Women of child-bearing potential were asked to use contraception/avoid pregnancy during the trial. All participants were required to refrain from taking cod liver oil or additional vitamin D, except as part of a multivitamin (with maximum 600 IU vitamin D3). Other exclusions are detailed in Bhargava et al., 2014.13
Randomisation and masking
After successful screening, participants began a one-month run-in of self-administered daily subcutaneous injections of 20 mg GA (Copaxone, provided by Teva Neuroscience, Inc., Parsippany, NJ), after which medication adherence was assessed. Participants who missed ≤3 GA doses were randomised 1:1 to receive blinded study vitamin D (VD): 600 IU/day (LDVD) or 5000 IU/day (HDVD) tablets. The 600 IU/day dose was the recommended daily allowance at the trial's start which was expected to produce little change in serum 25(OH)D levels. The 5000 IU/day dose was chosen to produce a serum 25(OH)D level of approximately 60 ng/ml.17 The randomisation scheme developed by the study statistician was stratified by study site and based on randomly-permuted blocks of varied size to ensure balance and masking of treatment assignments within each site. The two doses of vitamin D3 study drug were identical in appearance; quality assurance testing was completed for each manufactured batch during the trial (Continental Vitamin Company, Los Angeles, CA). Participants were encouraged to take the GA and study drug together daily, to minimise missed doses, and within an hour of a meal, since vitamin D3 is fat-absorbed. Treating clinicians, study coordinators, study participants, outcomes assessors, and MRI readers were all blinded to study drug dose.
Outcomes
The primary endpoint was clinical relapse, and the primary objective was to determine if HDVD compared to LDVD supplementation, as an add-on to GA for RRMS, was associated with a decrease in the proportion of participants who experienced a confirmed clinical relapse at 96 weeks. Clinical relapse was defined as new or worsening symptoms referable to the central nervous system, lasting at least 24 hours, occurring at least 30 days since the prior attack. To be confirmed, a relapse needed to be accompanied by worsening in the EDSS score (>0.5 points) or in the functional systems (FS) scales (2 points on ≥1 FS scale or 1 point on ≥2 FS scales) since the prior exam, as determined by the examining neurologist, who was blinded to the clinical course and group assignment. Probable relapses did not require accompanying change on the EDSS or FS scales. Pseudo-exacerbations (worsening symptoms in the context of an infection or fever) were not considered relapses.
Secondary objectives included examining if HDVD supplementation was associated with differences in new T2 lesions on MRI, brain volume loss (whole brain and normalised grey matter), annualised relapse rate, number of relapses requiring intravenous steroid treatment, time to first confirmed relapse, proportion experiencing sustained EDSS progression (increase in EDSS score of at least 1.0 point at week 48, compared to baseline, sustained at week 96), or changes in the Multiple Sclerosis Functional Composite (MSFC), binocular low-contrast letter acuity (LCLA using 2.5% Sloan chart), or health-related quality of life (HRQoL; using Functional Assessment in MS [FAMS, version 4.0] questionnaire), compared to LDVD supplementation.18, 19, 20
In-person study visits were held every 12 weeks and included blood samples for calcium levels. Serum was frozen at baseline and five follow-up visits to measure serum 25(OH)D levels as a batch at the trial's end (liquid chromatography/mass spectrometry; Heartland Laboratories, Ames, IA). Per protocol, safety outcomes that required temporary or permanent discontinuation of study drug were hypercalcaemia/severe hypercalcaemia, self-reported development of kidney stones, or unintended pregnancy.
Every 24 weeks, participants were examined by the treating clinician and completed research assessments administered by a blinded examiner, specifically the MSFC [timed 25-foot walk test (T25FWT), nine-hole peg test (9HPT) and 3-second paced auditory serial addition test (PASAT-3)], LCLA and FAMS. At baseline and weeks 48 and 96, participants underwent 3 Tesla (3T) clinical brain MRI with and without gadolinium contrast and had a blinded EDSS. Between visits, participants were contacted by phone or email to check on adherence to GA and study VD and to assess for symptoms concerning for MS relapse. Suspected relapse at any time during the trial prompted an unscheduled visit to assess for clinical relapse (with EDSS). Participants who experienced excessive disease activity [≥2 relapses or excessive MRI activity (>3 new T2 lesions on the year 1 brain MRI) or one relapse plus >2 new T2 lesions on the year 1 MRI] were permitted to change from GA to another MS DMT at the discretion of the treating physician but remained on study VD and continued trial follow-up.
The MRI protocol and post-processing and analysis plan were previously described in detail.13 Briefly, brain acquisition was performed on a 3T system (GE, Philips or Siemens) using a quadrature or phased-array head coil and included a 3D isotropic volumetric T1-weighted gradient echo (MPRAGE) sequence, proton density and T2-weighted dual echo sequences, and pre- and post-contrast T1-weighted spin echo sequences. MRI readers were blinded to the treatment group and clinical course. Normalised brain parenchymal (nBPV), grey matter (nGMV) and white matter volumes (nWMV) were calculated from MPRAGE images using SIENAX (Oxford, UK) and whole brain volume change at 48 and 96 weeks compared to baseline was computed and converted to percent brain volume change using SIENA.21, 22, 23 T2 lesion volume and the number of gadolinium-enhancing lesions were measured at each time point, and the number of new and enlarged T2 lesions was computed at weeks 48 and 96 compared to baseline.
Statistical analysis
As previously described,13 sample size estimates were calculated from a published study, in which 16% of those randomised to high-dose vitamin D versus 37% of those in the control arm had a relapse.6 With a two-sided alpha of 0.05, a total of 156 patients would provide 80% power to detect a difference of 16% vs 37%. The sample size was increased to 172 to allow for a 10% drop-out rate. Baseline demographic, clinical and MRI measures were summarised for each treatment group, and demographic differences of ≥10% were identified for inclusion in multivariable models based on pre-specified criteria. All analyses were completed as modified intention-to-treat (mITT) in which the eligible analysis population are those who were randomised and attended at least one follow-up study visit. The primary endpoint was the cumulative probability of a clinical relapse (confirmed; confirmed or probable) at 96 weeks. The original protocol initially specified a logistic regression model to assess differences in the proportion of participants with a relapse between treatment arms. However, we noted incomplete follow-up across participants and determined, prior to the initiation of any data analysis, that methods that more appropriately address this type of data should be applied. Therefore, we assessed the primary endpoint with an analysis of the time until clinical relapse using a Cox proportional hazards model to account for incomplete follow-up for all participants, adjusting for unbalanced covariates as described above. At 96-weeks, the unadjusted proportion of participants with a clinical relapse (confirmed; confirmed or probable) by study VD dose group was compared using Kaplan Meier curves and the log-rank test. Annualised relapse rate was analysed using an Andersen-Gill model for recurrent events. Negative binomial models compared the number of relapses requiring treatment between arms. Change over time in MSFC Z scores (and component raw scores) was analysed using a linear mixed effects model with an unstructured covariance matrix and random subject-specific intercepts. We tested for differences in change in MSFC (or components) over time using a cross-product term of treatment status and time. Time was considered as a linear term, and sensitivity analyses modeled time categorically. For analyses of raw MSFC score components, a log transformation was applied to those with skewed distributions; results for these variables are presented as the geometric mean. We also pre-specified clinically significant worsening in MSFC as a confirmed (at the subsequent visit) 20% change from baseline. For change in LCLA, we considered a confirmed change of at least 7 letters, which is considered clinically meaningful.24 For HRQoL, change over time in FAMS total score and FAMS components (emotional well-being, social well-being, general contentment, mobility, symptoms, and thinking/fatigue) were assessed using linear mixed effects models with unstructured covariance matrices and subject-specific random intercepts. Safety outcomes, development of hypercalcaemia or nephrolithiasis, were compared descriptively with a plan to formally test for differences between treatment arms if the number of events for either adverse event exceeded 10. Common injection-related adverse effects of GA were documented during the trial but not analysed since they were not part of the intervention.
Change over time in grey matter, whole brain, and T2 lesion volume were analysed using linear mixed effects models with unstructured covariance structures and random intercepts. The number of new or enlarged T2 lesions, number of Gd + lesions, and the composite number of new lesions (sum of new/enlarged T2 and Gd + lesions) were analysed using negative binomial models incorporating follow-up time as an offset term. We considered all secondary outcomes as such; therefore, no adjustments were made for multiple comparisons. We also completed a set of planned per protocol analyses in which we: (1) accounted for change in DMT over follow-up, considering DMT change as a time-varying covariate; (2) adjusted for time-varying adherence to GA (non-adherent defined as ≥4 missed doses in the preceding 3 month period); (3) compared individuals who completed the study with those who withdrew early; (4) assessed potential mediation by change in 25(OH)D levels from baseline using instrumental variables and 2-stage residual inclusion with study-group assignment as the instrument and change in 25(OH)D level from baseline as the exposure of interest.25
Role of the funding source
The funding sources of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Results
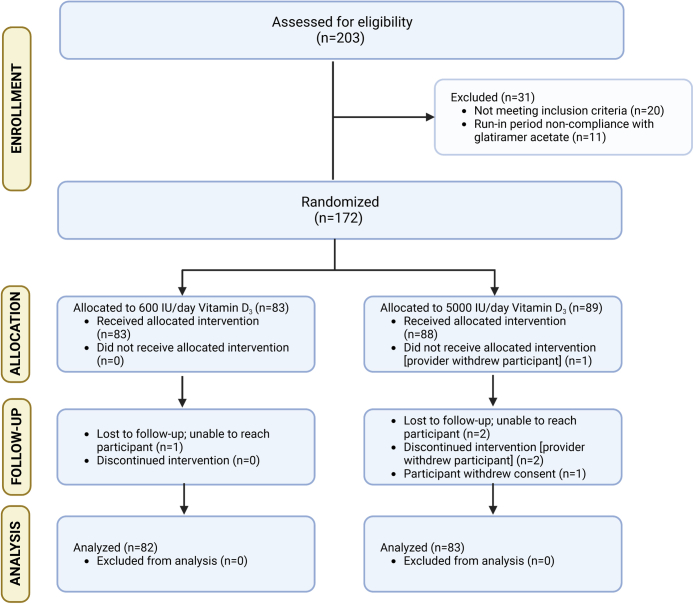
We screened 203 potentially eligible candidates who provided consent; of 183 who entered the run-in period, 172 successfully completed it and were eligible for randomisation (Fig. 1). Baseline characteristics are displayed in Table 1. A larger proportion of males received HDVD (16% LDVD vs. 31% HDVD) and a smaller proportion of Black participants received HDVD (22% LDVD vs. 12% HDVD); these covariates were, as pre-specified, included in multivariable models.
Fig. 1.
CONSORT diagram.
Table 1.
Baseline characteristics of participants.
| Characteristic | Vitamin D3 600 IU/day (n = 83) |
Vitamin D3 5000 IU/day (n = 89) |
|---|---|---|
| Age, mean ± SD | 34.2 (7.7) | 34.5 (7.1) |
| Female gender, n (%) | 70 (84) | 61 (69) |
| Race, n (%) | ||
| White/Caucasian | 61 (73) | 71 (80) |
| Black/African American | 18 (22) | 11 (12) |
| Other | 3 (3.6) | 5 (5.6) |
| Race not reported | 1 (1.2) | 2 (2.2) |
| Hispanic or Latino ethnicity, n (%) | 12 (15) | 18 (20) |
| Ethnicity not reported | 1 (1.2) | 0 (0) |
| Body Mass Index (kg/m2),a mean ± SD | 30.6 (9.4) | 28.7 (6.3) |
| EDSS, median (interquartile range) | 2.0 (1.0–2.5) | 2.0 (1.5–2.5) |
| T2-weighted lesion volume (ml),b mean ± SD | 3.7 (8.9) | 2.7 (3.5) |
| Brain volume (ml),c mean ± SD | 1488.3 (107.0) | 1496.1 (88.0) |
| Grey matter volume (ml),c median (interquartile range) | 772.7 (735.4–809.2) | 781.5 (737.8–808.0) |
| 25-hydroxyvitamin D level, ng/ml,d mean ± SD | 28.3 (9.9) | 29.6 (10.3) |
| MSFC z-score,e mean ± SD | 0.5 (0.4) | 0.6 (0.5) |
| Timed 25-foot walk test, seconds,f mean ± SD | 4.8 (1.4) | 4.7 (1.6) |
| Nine-hole peg test, seconds,e mean ± SD | 20.1 (3.8) | 19.6 (3.3) |
| PASAT-3 second, total correct,g mean ± SD | 48.7 (10.2) | 50.2 (9.7) |
| Binocular 2.5% low-contrast letter acuity,e mean ± SD | 36 (10) | 34 (10) |
| Overall FAMS score, mean ± SD | 126 (24) | 126 (27) |
EDSS (Expanded Disability Status Scale); MSFC (Multiple Sclerosis Functional Composite); PASAT (Paced Auditory Serial Addition Test); FAMS (Functional Assessment in Multiple Sclerosis).
Study sites that enrolled at least one participant included: Johns Hopkins University (coordinating center and enrolling site), University of California, San Francisco, Swedish Medical Services, University of Rochester, University of Virginia, Oregon Health and Science University, Columbia University, University of Massachusetts in Worcester, Stanford University, Washington University at St. Louis, Anne Arundel Health System Research Institute, Cleveland Clinic, Yale University, University of Pennsylvania, Dignity Health Medical Foundation and Icahn School of Medicine at Mount Sinai.
Missing for 2 in 600 IU dose group and 3 in 5000 IU dose group.
Missing for 8 in 600 IU dose group and 8 in 5000 IU dose group.
Missing for 9 in 600 IU dose group and 7 in 5000 IU dose group.
Missing for 6 in 600 IU dose group and 7 in 5000 IU dose group.
Missing for 3 in 5000 IU dose group.
Missing for 1 in 600 IU dose group and 3 in 5000 IU dose group.
Missing for 1 in 600 IU dose group and 5 in 5000 IU dose group.
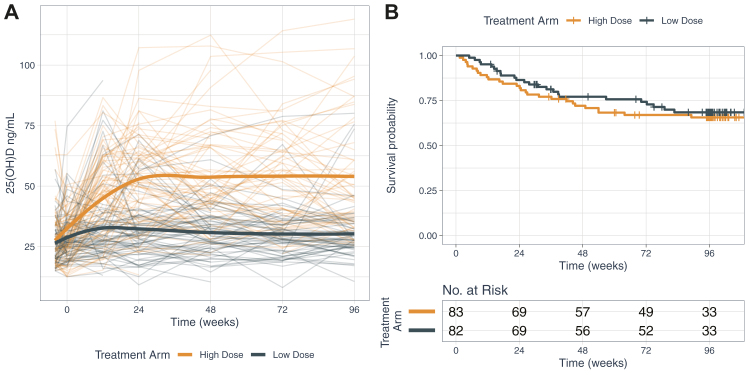
Participants randomised to LDVD achieved mean 25(OH)D serum levels of 31.9 ng/ml after 24 weeks of supplementation, and 30.3 ng/ml by 96 weeks (Fig. 2 (panel A)). Those receiving HDVD achieved mean serum 25(OH)D levels of 52.5 ng/ml after 24 weeks and 54.0 ng/ml by 96 weeks (both p for difference <0.001). Plots by season showed similar differences between dose groups.
Fig. 2.
Serum 25-hydroxyvitamin D levels by treatment arm (panel A); Kaplan Meier plot of proportion with confirmed relapse by treatment arm (panel B). Panel A depicts a spaghetti plot of individual participant serum 25(OH)D levels at each time point from baseline to week 96 for low dose (dark blue lines) compared to high dose (orange lines) vitamin D treatment arms with overlaid loess curves of mean levels over time. Panel B shows the results of the Kaplan Meier survival analysis (risk of confirmed relapse at 96 weeks) for low dose (dark blue line) compared to high dose (orange line) vitamin D treatment arms (p = 0.60 by log rank test); the hazard ratio (95% confidence interval) from the Cox proportional hazard model is 1.17 (0.67–2.05); p = 0.57.
Primary outcome
The primary analyses included 165 participants who were analysed following a mITT principle. There were no differences in confirmed clinical relapse risk between HDVD versus LDVD (Hazard Ratio [HR]: 1.17; 95% CI: 0.67 to 2.05; p = 0.57). The proportional hazards assumption was checked and the Cox proportional hazards model met the assumption. At 96 weeks, the cumulative proportion of participants with confirmed relapse did not differ between LDVD and HDVD groups (24 relapses vs. 28 relapses; 32% vs. 34%; p = 0.60; Fig. 2 (panel B)).
Secondary outcomes
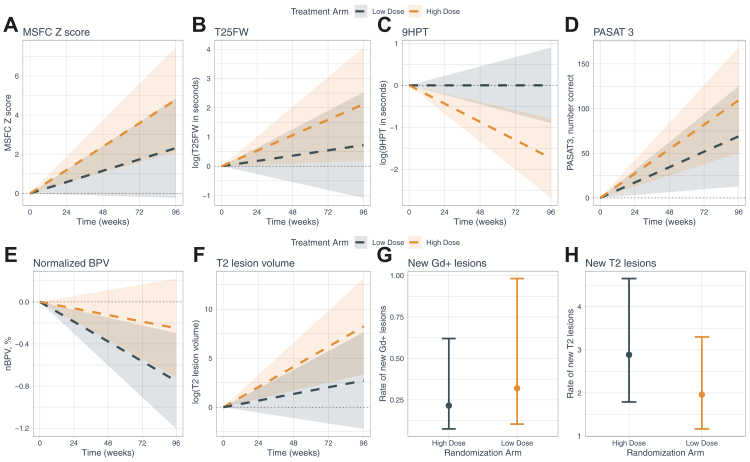
When varying how the relapse outcome was defined as per the pre-specified analysis plan, there remained no apparent differences between LDVD and HDVD groups (Supplementary eTable 1). Only 10 participants total (4 (5%) in the LDVD group and 6 (8%) in the HDVD group; p = 0.60) of the 140 participants who completed the week 96 visit experienced sustained disability progression on the EDSS. No differences were found between groups in the average change over time in LCLA, FAMS, MSFC or the components of MSFC with one exception (Supplementary eTable 2, Fig. 3). The LDVD group had better performance on the 9HPT versus the HDVD group, but the difference was not clinically meaningful (i.e., change between groups was less than the accepted clinically significant 20% change).
Fig. 3.
Secondary clinical and MRI outcomes (panels A–H). Plotted values for panels A–F denote change in a given outcome for each treatment arm as derived from a linear mixed model. Change was measured according to the mean of a given outcome between baseline and end of study using all available data. The shaded regions denote the 95% confidence intervals of the estimated slope for a given outcome for each treatment arm. For panels G–H, the plotted values denote the estimated rate of new lesions by treatment arm and associated 95% CI. Abbreviations: MSFC (Multiple Sclerosis Functional Composite); T25FW (Timed 25-Foot Walk Test); 9HPT (Nine-hole Peg Test); nBPV (normalised Brain Parenchymal Volume); PASAT 3 (Paced Auditory Serial Addition Test – 3 second); Gd+ (Gadolinium-positive).
There were no differences in MRI outcomes between the two groups either in terms of change from baseline to 96 weeks (nBPV, nGMV, T2 lesion volume) or the rate of developing new or enlarged T2-bright lesions, Gd + enhancing lesions, or in the composite measure (Fig. 3, Supplementary eTables 3 and 4).
Per protocol analyses
Per protocol analyses were conducted, taking account of change in MS therapy, GA adherence, early withdrawal and change in serum 25(OH)D levels. Findings were similar to the original analysis cohort; no significant effect of HDVD supplementation was found on relapse risk.
Safety outcomes
Safety outcomes, serious adverse events (SAEs) and adverse events (AEs) occurring in ≥5% of either study group are displayed in Table 2. There was no hypercalcaemia. Three participants developed nephrolithiasis or ureterolithiasis (1 in the LDVD and 2 in the HDVD group). Two were possibly related to study drug; and one was presumed related to concomitant treatment with topiramate for migraine. Per the treatment protocol, two of the three discontinued study VD but continued in the study. The third discontinued topiramate and then withdrew from follow-up one month later for an unrelated reason. There were no other withdrawals from follow-up due to adverse events except for unplanned pregnancies. No congenital abnormalities were reported, and none of the pregnancy outcomes was deemed related to study VD. One participant attempted suicide during the trial, was hospitalised, and was treated successfully thereafter.
Table 2.
Safety outcomes, serious adverse events and other adverse events.
| Vitamin D3 600 IU/day (n = 82) | Vitamin D3 5000 IU/day (n = 83) | |
|---|---|---|
| Safety outcome | ||
| Hypercalcaemia | 0 (0) | 0 (0) |
| Nephrolithiasis/ureterolithiasis | 1 (1.2) | 2 (2.4) |
| Serious adverse events, n (%) | ||
| Suicide attempt resulting in hospitalisation | 1 (1.2) | 0 (0) |
| Other unplanned hospitalisation | 4 (4.9) | 15a(18.1) |
| Planned hospitalisation | 1 (1.2) | 3 (3.6) |
| Pregnancy | 6b(7.3) | 3 (3.6) |
| Other adverse events, n (%) | ||
| Upper respiratory infection | 44 (53.7) | 40 (48.2) |
| Numbness or tingling | 22 (26.8) | 21 (25.3) |
| Headache/worsened headache/migraine | 14 (17.1) | 22 (26.5) |
| Fatigue/increased fatigue | 14 (17.1) | 11 (13.3) |
| Joint pain or swelling | 10 (12.2) | 12 (14.5) |
| Myalgia | 11 (13.4) | 7 (8.4) |
| Sinusitis | 12 (14.6) | 5 (6.0) |
| Sleep disorder | 5 (6.1) | 11 (13.3) |
| Depression/increased depression/severe depression | 6 (7.3) | 10 (12.0) |
| Influenza | 7 (8.5) | 6 (7.2) |
| Abdominal pain/cramp/discomfort | 8 (9.8) | 4 (4.8) |
| Nausea | 7 (8.5) | 4 (4.8) |
| Limb pain | 6 (7.3) | 4 (4.8) |
| Cough | 5 (6.1) | 5 (6.0) |
| Urinary tract infection | 7 (8.5) | 3 (3.6) |
| Urinary dysfunction | 3 (3.7) | 6 (7.2) |
| Dizziness/increased dizziness | 4 (4.9) | 5 (6.0) |
| Spasm/cramp (non-abdominal) | 3 (3.7) | 6 (7.2) |
| Bronchitis | 5 (6.1) | 4 (4.8) |
| Anxiety/worsening anxiety | 3 (3.7) | 6 (7.2) |
| Sore throat ( ± streptococcal infection) | 4 (4.9) | 2 (2.4) |
Two participants accounted for 11 unplanned hospitalisations; one was admitted 4 times for MS relapse; the other had 7 unplanned hospitalisations, 4 of which were re-admissions following issues after elective surgery. 7 unplanned hospitalisations were for MS relapse and were deemed possibly related to study drug (this was the hypothesis of the trial). MS relapses themselves were not considered adverse events for purposes of this trial and most were managed in the outpatient setting.
Includes 2 pregnancies for 1 participant; one additional participant became pregnant during run-in period and was withdrawn prior to randomisation.
Discussion
Although they produced expected differential changes in serum 25(OH)D levels, HDVD compared to LDVD supplementation, as an add-on to GA, was not associated with a reduced proportion of participants with clinical relapse in established RRMS over 96 weeks of treatment in VIDAMS. Secondary clinical and MRI outcomes in VIDAMS also did not demonstrate a benefit from taking HDVD. Differences in 9HPT outcomes between the groups were likely due to chance in the context of multiple comparisons and were not clinically meaningful. Vitamin D supplementation appeared to be safe, with no occurrences of hypercalcaemia and only 3 cases of nephrolithiasis or ureterolithiasis, two in the HDVD (one attributed to topiramate) and one in the LDVD group.
These findings corroborate those from placebo-controlled trials that tested HDVD as add-on to interferon β-1a, which also did not show significant benefit.8,9 The CHOLINE trial's primary outcome was change in the ARR at 96 weeks; no differences were found between very high dose vitamin D3 (100,000 IU every other week) and placebo groups (rate ratio 0.799, 95% CI 0.481, 1.32, p = 0.38), although notably there was substantial drop-out.8 For SOLAR (vitamin D3 14,007 IU/day or placebo as add-on to interferon beta), the primary outcome was revised to no evidence of disease activity-3 (NEDA-3) at 48 weeks after experiencing difficulty recruiting, and only approximately 2/3 of the original planned target was enrolled.9 The proportion with NEDA-3 was similar between the VD and placebo groups (odds ratio 0.93, 95% CI 0.53, 1.63, p = 0.80). While some exploratory or subgroup analyses within these trials were reported as favorable in the vitamin D arms, such analyses must be interpreted with caution in the context of negative overall results and since those findings were noted only in subsets of the enrolled participants (CHOLINE) or appear to have been driven by outliers (SOLAR). Another high versus low dose cholecalciferol supplementation trial with interferon β-1b also had null findings and was halted early, though the final sample size was likely too small to yield definitive results.10
Vitamin D supplementation trials, many quite small, in other autoimmune diseases in which insufficiency thereof has been proposed as a risk or prognostic factor have had mixed results.26,27 It is difficult to reconcile the results herein with the strong associations in MS observational studies of lower vitamin D levels with greater risk of disease incidence or disease activity. Some studies have suggested that sunlight or ultraviolet radiation exerts a possible effect on risk of MS disease activity, independent of vitamin D pathways; if true, vitamin D supplementation would not likely alter outcomes.28,29 It is also plausible that low sunlight exposure or low vitamin D serum levels could be reflective of an MS prodromal phase or of greater accumulated subclinical disease activity.30 Were these conditions true, lower vitamin D levels might appear to be a risk factor for MS or a prognostic factor for worse MS outcomes, when in reality, the MS caused lower vitamin D levels due to related behavioral change (reverse causality). These are interesting areas for future research.
Like all studies, our trial has limitations. First, its size may have limited the ability to detect smaller effects of HDVD supplementation. Its size also likely explains the randomisation imbalances, which we attempted to mitigate by adjusting for variables with large imbalances. The infrequent capture of EDSS may have limited the ability to detect sustained progression of disability. However, it is likely that the short study duration was more of a contributor, since disability accumulates slowly. Further, brain volume changes, particularly nGMV, are strongly associated with subsequent disability change and herein, did not differ by vitamin D dose.31 It is possible that the dosages chosen for both arms may have obscured a treatment effect, where perhaps a greater absolute serum 25(OH)D level for the higher-dose arm or a greater difference between groups was needed, although we point out that in observational studies, the relationship of 25(OH)D levels and MS outcomes appeared to be linear and that different dosing regimens, with use of placebo groups, did not produce different conclusions in the SOLAR and CHOLINE trials. While the longer than expected recruitment could have introduced variability related to study team turnover, we had a manual of procedures to guide study teams and a trial manager who held monthly calls with site coordinators and was available for questions; further, site principal investigators were responsible for continuity and training of new team members. Finally, while slow recruitment was likely in part due to the evolving landscape of available MS therapeutics, it is unlikely that a trial in which vitamin D was added to higher-efficacy therapies would demonstrate a therapeutic benefit for HDVD that was not detectable with GA, which is moderate in efficacy.
HDVD, as add-on to GA, did not reduce disease activity in established RRMS compared to LDVD in the VIDAMS trial. Taken together with the null findings of previous trials, these results suggest that prescribing higher doses of vitamin D for purposes of modifying the RRMS course may not be beneficial. We cannot exclude that a different level of serum 25(OH)D is needed to see a treatment effect, or that only subgroups of people with MS respond to HDVD supplementation. Future research includes a planned individual participant data meta-analysis from CHOLINE, SOLAR and VIDAMS trials to increase statistical power and further explore these questions.
Contributors
Sandra D. Cassard was responsible for data curation, project administration, supervision, writing the original draft of the manuscript, and directly accessed and verified the underlying data.
Kathryn C. Fitzgerald was responsible for formal analysis, software, visualisation, writing the original draft of the manuscript, and directly accessed and verified the underlying data. Peiqing Qian was responsible for conducting the investigation at her site, resources, supervision, reviewing and editing the manuscript. Susan A. Emrich was responsible for data curation, project administration, supervision, and directly accessed and verified the underlying data. Christina J. Azevedo was responsible for formal analysis, investigation, resources, supervision, reviewing and editing the manuscript. Andrew D. Goodman was responsible for conducting the investigation at his site, resources, supervision, reviewing and editing the manuscript. Elizabeth A. Sugar was responsible for methodology, software, reviewing and editing the manuscript. Daniel Pelletier was responsible for project administration, supervision, reviewing and editing the manuscript. Emmanuelle Waubant was responsible for conceptualisation, funding acquisition, conducting the investigation at her site, resources, supervision, reviewing and editing the manuscript. Ellen M. Mowry was responsible for conceptualisation, funding acquisition, conducting the overall investigation and at her site, methodology, project administration, resources, supervision, writing the original draft of the manuscript. All authors had full access to all the data in the study and accept responsibility for the decision to submit for publication.
Data sharing statement
Data collected for the study, including deidentified individual participant data and a data dictionary defining each field in the set, will be made available to others with publication. Proposed uses of the data should be sent to scassar1@jhmi.edu and data will be available with investigator support, after approval of a proposal, and with a signed data access agreement.
Declaration of interests
SD Cassard, SE Emrich, and D Pelletier have nothing to disclose. KC Fitzgerald reports research funding from NIH, NMSS, and the DoD. She has served on Data and Safety Monitoring Boards for trials funded by the NIH and NMSS, and has received honorarium for serving as an external thesis committee reviewer.
P Qian reports research funding for this clinical trial. She has served on a Data and Safety Monitoring Board for a trial funded by Janssen and has received speaking honorariums from Biogen and Bristol Myers Squibb. EA Sugar reports receiving salary support from NIH funds for statistical assistance with the design, execution and analysis of this project. CJ Azevedo has received personal compensation for participation on advisory boards or data and safety monitoring boards for Horizon Therapeutics, Genentech, Sanofi Genzyme, and TG Therapeutics; she has received honoraria for participation in CME activities from Catamount Medical Education, American Academy of Neurology, Spire Learning, and Efficient LLC,; she has received payment for serving on a grant review committee from the Department of Defense and for serving on a data and safety monitoring board from Genentech; she receives grant support from the National Multiple Sclerosis Society and the National Institutes of Health.
AD Goodman has received personal compensation for consulting from Genentech-Roche, Janssen, TG Therapeutics, Novartis, payment for expert testimony from EMD Serono, support for attending meetings from Biogen, payment for participation on a data and safety monitoring board from IMCYSE, and research support from Atara, Biogen, EMD Serono, and Sanofi Genzyme. E Waubant has participated in multicentre clinical trials funded by Genentech, Alexion and Biogen; she has current support from the NIH, NMSS, PCORI, CMSC and Race to Erase MS. She has received consulting fees from Emerald Pharmaceuticals, payments from Neurology Live and Yoga Moves MS, had support for attending the ACTRIMS 2022 and ECTRIMS 2022 conferences, volunteered on a data and safety monitoring board for a Novartis trial, chaired the International Women in Multiple Sclerosis (iWiMS) network (unpaid), and served as President-elect of ACTRIMS forum (unpaid). EM Mowry has received grant or research support from the National MS Society, Biogen, Genentech and Teva Neuroscience, has served on a data and safety monitoring board for an NIH trial and receives honoraria from UpToDate (editorial duties) and consulting fees from BeCare Link LLC.
Acknowledgements
This investigation was supported by a grant from the National Multiple Sclerosis Society (RG 4407A2/1). Teva Neuroscience, Inc. provided Copaxone (GA) for the duration of the trial. In addition, EMM received support from NIH K23 NS067055. We would like to acknowledge the study participants as well as the site principal investigators (PI) and lead study coordinators from each participating site:
Johns Hopkins University School of Medicine, Ellen M. Mowry, MD, MCR, site PI; Sonya U. Steele, Mary E. Frey, Madiha Qutab, MS, Ornusa Chalayon, Ikechukwu Chidobem, lead coordinators
University of California, San Francisco, Emmanuelle Waubant, MD, PhD, site PI; Nisha Raj Revirajan and Uk Sok Shin, lead coordinators
Swedish Medical Services, Peiqing Qian, MD site PI; Yuriko Courtney and Caryl Tongco, lead coordinators
University of Rochester, Andrew D. Goodman, MD, site PI
University of Virginia, David E. Jones, MD, site PI; Margaret F. Keller, RN, MS, lead coordinator
Oregon Health and Science University, Edward Kim, MD, site PI; Debbie Guess, RN, lead coordinator
Columbia University, Claire S. Riley, MD, site PI; Gabriella Tosto-D’Antonio, DNP and Kaho B. Onomichi, lead coordinators
University of Massachusetts in Worcester, Peter Riskind, MD, PhD, site PI; Carolyn F. Griffin, lead coordinator
Stanford University, Alexandra Goodyear, MD, site PI
Washington University at St. Louis, Anne H. Cross, MD, site PI; Susan Sommer-Mammenga, lead coordinator
Anne Arundel Health System Research Institute, Arash Farhadi, MD, site PI; Kathleen W. Gray, BSN, RN, CCRC lead coordinator
Cleveland Clinic, Daniel Ontaneda, MD, PhD, site PI; Susan L. Sharp and Dee Ivancic, lead coordinators
Yale University, Daniel Pelletier, MD, site PI
University of Pennsylvania, Clyde Markowitz, MD, site PI
Dignity Health Medical Foundation, Sabeen Lulu, MD, MCR, site PI; Lucy Ng-Price, MA, CCRC, lead coordinator
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101957.
Appendix A. Supplementary data
References
- 1.Reich D.S., Lucchinetti C.F., Calabresi P.A. Multiple sclerosis. NEJM. 2018;378:169–180. doi: 10.1056/NEJMra1401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munger K.L., Levin L.I., Hollis B.W., Howard N.S., Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 3.Mowry E.M., Krupp L.B., Milazzo M., et al. Vitamin D status is associated with relapse rate in pediatric-onset multiple sclerosis. Ann Neurol. 2010;67:618–624. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 4.Mowry E.M., Waubant E., McCulloch C.E., et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72:234–240. doi: 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald K.C., Munger K.L., Köchert K., et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol. 2015;72(12):1458–1465. doi: 10.1001/jamaneurol.2015.2742. PMID: 26458124. [DOI] [PubMed] [Google Scholar]
- 6.Burton J.M., Kimball S., Vieth R., et al. A phase I/II dose-escalation trial of vitamin D3 and calcium in multiple sclerosis. Neurology. 2010;74:1852–1859. doi: 10.1212/WNL.0b013e3181e1cec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soilu-Hänninen M., Åivo J., Lindström B., et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatr. 2012;83:565–571. doi: 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 8.Camu W., Lehert P., Pierrot-Deseilligny C., et al. Cholecalciferol in relapsing-remitting MS: a randomized clinical trial (CHOLINE) Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e597. doi: 10.1212/NXI.0000000000000597. Print 2019 Sep. PMID: 31454777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hupperts R., Smolders J., Vieth R., et al. SOLAR Study Group. Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology. 2019;93(20):e1906–e1916. doi: 10.1212/WNL.0000000000008445. Epub 2019 Oct 8. PMID: 31594857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dörr J., Bäcker-Koduah P., Wernecke K.D., et al. High-dose vitamin D supplementation in multiple sclerosis - results from the randomized EVIDIMS (efficacy of vitamin D supplementation in multiple sclerosis) trial. Mult Scler J Exp Transl Clin. 2020;6(1) doi: 10.1177/2055217320903474. eCollection 2020 Jan–Mar. PMID: 32047645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng X., Wang Z., Howlett-Prieto Q., Einhorn N., Causevic S., Reder A.T. Vitamin D enhances responses to interferon-β in MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e622. doi: 10.1212/NXI.0000000000000622. PMID: 31582399; PMCID: PMC6807660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mowry E.M., Beheshtian A., Waubant E., et al. Quality of life in multiple sclerosis is associated with lesion burden and brain volume measures. Neurology. 2009;72:1760–1765. doi: 10.1212/WNL.0b013e3181a609f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhargava P., Cassard S., Steele S.U., et al. The vitamin D to ameliorate multiple sclerosis (VIDAMS) trial: study design for a multicenter, randomized, double-blind controlled trial of vitamin D in multiple sclerosis. Contemp Clin Trials. 2014 Nov;39(2):288–293. doi: 10.1016/j.cct.2014.10.004. Epub 2014 Oct 12. PMID: 25311447. [DOI] [PubMed] [Google Scholar]
- 14.Polman C.H., Reingold S.C., Edan G., et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the ‘McDonald Criteria'. Ann Neurol. 2005;58(6):840–846. doi: 10.1002/ana.20703. http://www.ncbi.nlm.nih.gov/pubmed/16283615 Available at: [DOI] [PubMed] [Google Scholar]
- 15.Montalban X., Tintoré M., Swanton J., et al. MRI criteria for MS in patients with clinically isolated syndromes. Neurology. 2010;74(5):427–434. doi: 10.1212/WNL.0b013e3181cec45c. Epub 2010 Jan 6. PMID: 20054006. [DOI] [PubMed] [Google Scholar]
- 16.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. http://www.ncbi.nlm.nih.gov/pubmed/6685237 Available at: [DOI] [PubMed] [Google Scholar]
- 17.Heaney R.P., Davies K.M., Chen T.C., Holick M.F., Barger-Lux M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 18.Cutter G.R., Baier M.S., Rudick R.A., et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122:101–112. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 19.Balcer L.J., Frohman E.M. Evaluating loss of visual function in multiple sclerosis as measured by low-contrast letter acuity. Neurology. 2010 Apr 27;74(Suppl 3):S16–S23. doi: 10.1212/WNL.0b013e3181dbb664. PMID: 20421569. [DOI] [PubMed] [Google Scholar]
- 20.Cella D.F., Dineen K., Arnason B., et al. Validation of the functional assessment of multiple sclerosis quality of life instrument. Neurology. 1996;47:129–139. doi: 10.1212/wnl.47.1.129. [DOI] [PubMed] [Google Scholar]
- 21.Smith S.M., Zhang Y., Jenkinson M., et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 22.Smith S.M., Jenkinson M., Woolrich M.W., et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier D., Garrison K., Henry R. Measurement of whole-brain atrophy in multiple sclerosis. J Neuroimaging. 2004 Jul;14(3 Suppl):11S–19S. doi: 10.1177/1051228404266264. PMID: 15228755. [DOI] [PubMed] [Google Scholar]
- 24.Balcer L.J., Raynowska J., Nolan R., et al. Multiple Sclerosis Outcome Assessments Consortium. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017 Apr;23(5):734–747. doi: 10.1177/1352458517690822. Epub 2017 Feb 16. PMID: 28206829; PMCID: PMC5407511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terza J.V., Basu A., Rathouz P.J. Two-stage residual inclusion estimation: addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531–543. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn J., Cook N.R., Alexander E.K., et al. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ. 2022;376 doi: 10.1136/bmj-2021-066452. PMID: 35082139; PMCID: PMC8791065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison S.R., Li D., Jeffery L.E., et al. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif Tissue Int. 2020;106:58–75. doi: 10.1007/s00223-019-00577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langer-Gould A., Lucas R., Xiang A.H., et al. MS sunshine study: sun exposure but not vitamin D is associated with multiple sclerosis risk in Blacks and Hispanics. Nutrients. 2018;10:268. doi: 10.3390/nu10030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostkamp P., Salmen A., Pignolet B., et al. German Competence Network Multiple Sclerosis (KKNMS) and the BIONAT Network. Sunlight exposure exerts immunomodulatory effects to reduce multiple sclerosis severity. Proc Natl Acad Sci U S A. 2021;118(1) doi: 10.1073/pnas.2018457118. Erratum in: Proc Natl Acad Sci U S A. 2021 Jul 20;118(29): PMID: 33376202; PMCID: PMC7817192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makhani N., Tremlett H. The multiple sclerosis prodrome. Nat Rev Neurol. 2021;17(8):515–521. doi: 10.1038/s41582-021-00519-3. Epub 2021 Jun 21. PMID: 34155379; PMCID: PMC8324569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher E., Lee J.C., Nakamura K., Rudick R.A. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64(3):255–265. doi: 10.1002/ana.21436. PMID: 18661561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.