Graphical abstract
Keywords: Ultrasound, Caffeoylquinic acids, Transformation, Degradation kinetics, Isomerization
Highlights
-
•
The increase of pH accelerated the degradation of 3,5-diCQA, 4,5-diCQA and 3,4-diCQA.
-
•
The isomerization of diCQAs was more likely to occur under neutral and alkaline conditions.
-
•
Ultrasound boosted the degradation and isomerization of three diCQAs.
-
•
EGCG and VC slowed down the degradation of diCQAs treated with and without ultrasound.
Abstract
Dicaffeoylquinic acids (diCQAs) are found in a variety of edible and medicinal plants with various biological activities. An important issue is the low stability of diCQAs during extraction and food processing, resulting in the degradation and transformation. This work used 3,5-diCQA as a representative to study the influence of different parameters in ultrasonic treatment on the stability of diCQAs, including solvent, temperature, treatment time, ultrasonic power, duty cycle, and probe immersion depth. The generation of free radicals and its influence were investigated during the treatment. The stability of three diCQAs (3,5-diCQA, 4,5-diCQA and 3,4-diCQA) under the certain ultrasonic condition at different pH conditions was evaluated and found to decrease with the increase of pH, further weakened by ultrasonic treatment. Ultrasound was found to accelerate the degradation and isomerization of diCQAs. Different diCQAs showed different pattern of degradation and isomerization. The stability of diCQAs could be improved by adding epigallocatechin gallate and vitamin C.
1. Introduction
Caffeoylquinic acid derivatives (CQAs) are widely distributed in nature, which have been claimed to have various biological effects. The most common CQAs is 5-CQA (chlorogenic acid), but there are isomers of other structures in plants. Dicaffeoylquinic acids (diCQAs), such as 3,4-diCQA, 3,5-diCQA and 4,5-diCQA, can also be found [1], [2]. However, the degradation and transformation of CQAs in the processing are great challenges in their application [3]. The presence of light and the change of temperature and pH cause the degradation and isomerization of CQAs. DiCQAs are much more unstable than mono-CQAs [4]. Dawidowicz & Typek [5] studied the transformation of CQAs during coffee bean roasting process. A decrease of the amount of mono-CQAs and diCQAs was found with the increase of roasting time and temperature. Such chemical transformation processes of CQAs may actually occur during their extraction from plant sources and subsequent product processing, which may change or destroy the bioactivity and quality of final products [6].
Ultrasound, as a non-thermal technology, is well-known to achieve the objective of sustainable ‘‘green” chemistry. The physical and chemical effects induced by ultrasonic cavitation have been used to improve the efficiency of various processes in food industry, such as extraction, freezing/thawing, homogenization/emulsification, and filtration [7]. It has been widely used in innovative food processing of fruits, vegetables, juices, and other products for cooking, cutting, foaming, separation, and degassing [8]. Ultrasound was successfully used to extract CQAs from sweet potato peels. Increasing ultrasonic power intensified the sonochemical hydrolysis of 3,4-diCQA [9].
In our previous study, the effect of ultrasound on the stability of mono-CQAs has been studied [10], [11]. However, the transformation pattern of diCQAs in ultrasonic system has not been studied yet. The present work therefore studied the degradation and isomerization pattern of there diCQAs (3,5-diCQA, 4,5-diCQA and 3,4-diCQA) under ultrasound. The effects of different treatment conditions including solvents, time, temperature, ultrasonic power density, probe immersion depth and duty cycle on the stability of diCQAs were also evaluated. Epigallocatechin gallate (EGCG) and vitamin C (VC) as two common antioxidants were added to help stabilize three diCQAs. The degradation kinetics of three diCQAs were conducted at different pH conditions.
2. Materials and methods
2.1. Materials
The standard of three diCQA isomers (3,5-, 4,5-, and 3,4-diCQA) (purity ≥ 99%), puerarin (purity ≥ 99.71%) and EGCG (purity ≥ 99.78%) were purchased from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China). Ascorbic acid (VC) was purchases from Shanghai Aladdin Co., Ltd. (Shanghai, China). Terephthalic acid (TPA) and 2-hydroxyterephthalic acid (TPA-OH) were obtained from Sigma-Aldrich Chemical Co., Ltd. (Shanghai, China). Chromatographic grade acetonitrile and methanol were purchased from Merck KGaA company (Darmstadt, Germany). All other reagents used were of analytical grade or purer.
2.2. Ultrasonic treatment
The solutions of 3,5-, 3,4- and 4,5-diCQA with a concentration of 0.1 mg/mL (30 mL) were taken into brown cylindrical glass bottles (diameter: 2.6 cm, height: 9.5 cm), and then treated by a probe ultrasonic processor at 22 kHz (JY92-IIDN, Ningbo Scientz Biotechnology Co., Ltd., Ningbo, China). During the ultrasonic treatment, the bottle was put in a thermostatic water bath to control the temperature.
Different solvent (absolute methanol, absolute ethanol, water, 50% (v/v) methanol in water, and 50% (v/v) ethanol in water), temperature (10, 20, 30, 40, 50, and 60 °C), treatment time (10, 20, 30, 40, 50, and 60 min), ultrasonic power density (3, 6, 9, 12, 15, and 18 W/mL), duty cycle (0, 16.7, 33.3, 50, 66.7, 83.3, and 100%) and probe immersion depth (1, 2, 3, 4, 5, and 6 cm) were studied during ultrasonic treatment. The probe immersion depth was defined as the distance from the liquid level to the bottom of the ultrasonic probe. The duty cycle is the ratio of the ultrasonic on time to the total time. Each variable was carried out while maintaining the other variables fixed. The experiments were conducted in triplicate. After ultrasonic treatment, samples were filtrated through 0.22 μm-filter and detected using HPLC.
2.3. Quantification of three diCQAs
The analysis of diCQAs was performed on an Agilent 1260 series HPLC system equipped with a UV detector and a Phenomenon Luna-C18 column (250 mm × 4.6 mm, 5 μm). The filtrated sample (10 µL) was injected into the HPLC system and eluted by acetonitrile (A) and 0.1% phosphoric acid (B) at a flow rate of 0.8 mL/min with the gradient program as follows: 0–8 min, 15% A; 8–12 min, 15%–30% A; 12–16 min, 30% A; 16–20 min, 30%–15% A; 20–25 min, 15% A. The detection wavelength was 330 nm. The column temperature was kept at 35 °C. Calibration curves for three diCQA isomers in the concentration range of 0.03–0.2 mg/mL were established and used to calculate the isomers’ contents in samples.
2.4. Measurement of hydroxyl radicals (•OH)
The production of •OH was measured by monitoring the formation of TPA-OH from the reaction of TPA and •OH as described by Deng et al. [12]. TPA solution (10 μmol/L) was prepared using 50% methanol in water (v/v). Then, 0.2000 mg 3,5-diCQA was dissolved in 30 mL TPA solution. The mixture was then treated by ultrasound with different time (10, 20, 30, 40, 50, and 60 min). Afterwards the fluorescence intensity of generated TA-OH was measured at 326 nm as an excitation wavelength and at 432 nm as an emission wavelength. TPA solution without 3,5-diCQA was used as reference.
2.5. Degradation kinetics of diCQA at different pH
The solution of diCQA (3.88 mmol/L, 2 mL), in 50% (v/v) methanol, was mixed with 25 mL buffer of different pH (0.1 M acetate buffer, pH 4.69; 0.1 M phosphate buffer, pH 7.06; 0.1 M phosphate buffer, pH 7.96; 0.1 M Tris-HCl buffer, pH 9.22). The mixture was then treated by ultrasound as the following conditions: ultrasonic power density 15 W/mL, temperature 40 °C, duty cycle 66.67%, and probe immersion depth 2 cm. During the treatment, 100 μL reaction solution was taken out at 0, 10, 30, 60, 90, 120, 150, 180, and 210 min, then mixed with 100 μL puerarin (internal standard, 0.2 mg/mL in 50% (v/v) methanol) and 800 μL mobile phase (0.1% phosphoric acid-acetonitrile, 4:1). After completely mixing, samples were analyzed as previously described in 2.3. The sample treated at the same condition without ultrasound was analyzed in the same way as control.
The degradation kinetics of diCQAs at different pH conditions were fitted by Weibull model [13]:
where Ci is the concentration of diCQA at t min, C0 is the initial concentration, k is the reaction rate constant, n is a shape constant (n > 1, the function is an increasing function (the degradation rate increases with time); n < 1, the function is a decreasing function (the degradation rate decreases with time); n = 1, the function satisfies the exponential distribution (the equation is equivalent to a simple first-order kinetic model).
According to above equation, the degradation parameters k and n can be calculated by nonlinear least square fitting with origin2019 software (OriginLab, MA, USA). Afterwards, the degradation half-life (t1/2) was calculated as follow:
2.6. Protective effect of EGCG and VC
The solution of diCQAs (3.88 mmol/L, 5 mL) in 50% (v/v) methanol, was mixed with 20 mL buffer of different pH as mentioned above. Then 2 mL antioxidant (EGCG or VC) was added to reach the final molar concentration ratio of the antioxidant and diCQAs at 2:1, 1:1 and 1:2. After that, the mixed sample was treated by ultrasound.
2.7. Statistical analysis
Experiments for each treatment were conducted in triplicate. The experimental data was processed by SPSS 22.0 (SPSS Inc., Chicago, IL, USA) and represented as mean value ± standard deviation. Duncan analysis of variance was used to assess the significance of differences between treatments. Significance levels were tested at p < 0.05.
3. Results and discussion
3.1. Effect of different treatment conditions on the stability of 3,5-diCQA
3.1.1. Solvent
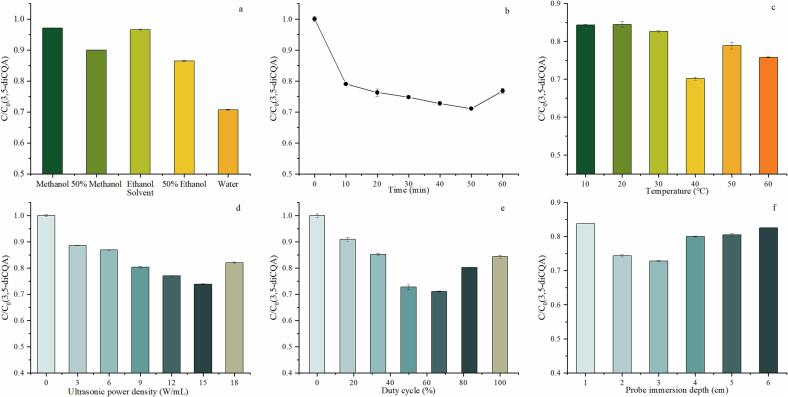
The selection of solvent is an important factor affecting the extraction efficiency, which is related to the solubility of targeted compounds and the penetration power of solvent into the matrix [14]. In addition to the influence on the extraction efficiency, the solvent also affects the intensity of ultrasonic cavitation [15]. Water, water-based organic-aqueous solvents, and organic solvents are commonly used in the extraction of bioactive compounds. Therefore, as a representative, 3,5-diCQA dissolved in absolute methanol, 50% (v/v) methanol, absolute ethanol, 50% (v/v) ethanol, and water was treated by ultrasound (15 W/mL) at 40 ℃ for 50 min with a duty cycle of 66.7% and a probe immersion depth of 3 cm. The result is shown in Fig. 1a.
Fig. 1.
Effect of different conditions on the degradation of 3,5-diCQA.
As shown in Fig. 1a, 3,5-diCQA was degraded most severely in water, followed by 50% ethanol. The ultrasonic stability of 3,5-diCQA decreased with the increase of water content in the solvent. It has been reported that CQAs are more unstable in methanol than in 50% methanol, which is inconsistent with the results of this work 4. This is because the stability of CQAs with ultrasonic treatment may be different from that without ultrasonic treatment because of the effect of acoustic cavitation. Acoustic cavitation, also described as sonochemical effect, is generated with the formation, growth and collapse of acoustic bubbles. Different solvent carries the combined effect of viscosity, vapor pressure and surface tension that may influence sonochemical effect. The solvent with low vapor pressure, high viscosity, and high surface tension generally has high threshold for cavitation, because more energy is needed to overcome the intermolecular force in the liquid to form bubbles. However, once cavitation begins, the solvent with lower vapor pressure, higher viscosity, and higher surface tension forms stronger cavitation [16]. Water shows the lowest vapor pressure, highest viscosity, and highest surface tension in the five solvents, so the most intense cavitation was generated in water system, which led to the most degradation of 3,5-diCQA. Besides the formation of highly reactive radical species during cavitation also attack 3,5-diCQA. Water in the system can participated the generation of reactive radicals, thus causing more damage to 3,5-diCQA. Consequently, in order to clarify the degradation mechanism of diCQAs by ultrasound, water was selected as the solvent for subsequent experimental research.
3.1.2. Treatment time
To investigate the influence of ultrasonic time, 3,5-diCQA, dissolved in water, was treated by ultrasound (15 W/mL) at 40℃ with a duty cycle of 66.7% and a probe immersion depth of 3 cm for different time. As shown in Fig. 1b, with the increasing time, the 3,5-diCQA content decreased sharply within 10 min at first, then slowly until 50 min. The content of 3,5-diCQA increased slightly at 60 min, which may result from the transformation of diCQAs isomers. It indicates that the prolonged ultrasonic attack will continuously increase the degradation degree of 3,5-diCQA until reach the limitation. Similar results have been reported that the degradation induced by ultrasound was limited because of the energy attenuation under high-intensity or long-time ultrasound irradiation [17].
3.1.3. Temperature
The effect of temperature on the stability of 3,5-diCQA (in water) was studied under 15 W/mL ultrasonic treatment for 50 min using a duty cycle of 66.7% and a probe immersion depth of 3 cm. As shown in Fig. 1c, different temperature affected the ultrasonic degradation of 3,5-diCQA. When treating samples below 40 °C, the degradation degree of 3,5-diCQA caused by ultrasound was small. The most severe degradation occurred at 40 °C. The degradation of 3,5-diCQA was alleviated when the processing temperature exceeding 40 °C. The change of the degradation of 3,5-diCQA under ultrasound caused by different temperatures may be resulted from three aspects: the influence of temperature on the stability of 3,5-diCQA; the influence of temperature on ultrasonic effect; both of the former two. The thermal stability of CQAs (mono-CQAs and diCQAs) aqueous solution in boiling water has been studied. During heating, diCQAs could isomerize to each other and degrade to mono-CQAs, caffeic acid and the compounds with the formula of C15H14O6 [18]. Nevertheless, the stability of diCQAs below 100 °C has been hardly studied. Li et al. [19] reported that the higher the heating temperature (45–105 °C), the more the content of chlorogenic acids decreased. With the increase of temperature, the degradation of diCQA could be more intense. In contrast, however, the intensity of ultrasonic cavitation decreases with increasing temperature. This is because a cushioning effect on collapse, attributing to the vapor pressure increases due to the temperature rise [20]. Therefore, due to the dual effects of temperature and ultrasound, the most serious degradation of substances in the experiment occurred at 40 °C.
3.1.4. Ultrasonic power density
Ultrasonic power density plays a very important role on ultrasonic cavitation. To clarify its effect on the stability of 3,5-diCQA, the aqueous solution of 3,5-diCQA was treated by ultrasound with different power density at 40℃ for 50 min using a duty cycle of 66.7% and a probe immersion depth of 3 cm. As shown in Fig. 1d, with the increase of ultrasonic power density, the retention of 3,5-diCQA gradually decreased until reaching 15 W/mL and then increased. Generally, the increase of ultrasonic power density will increase the intensity of ultrasonic cavitation. When the ultrasonic power density exceeded 15 W/mL, the cavitation bubbles may grow too big to collapse or collapse weakly, resulting in a reduction in the cavitation effect [21]. In addition, too many bubbles adsorbed on the surface of the ultrasonic probe may hinder the transmission of ultrasonic waves [22].
3.1.5. Duty cycle
Duty cycle of ultrasonic treatment decides the exposure time to the irradiation in one cycle, varied based on the on/off time. The aqueous solution of 3,5-diCQA was treated by ultrasound (15 W/mL) at 40℃ for 50 min with a duty cycle of 66.7% and a probe immersion depth of 3 cm using different duty cycle. The degradation of 3,5-diCQA first increased and then decreased with the increase of duty cycle (Fig. 1e). The content of 3,5-diCQA reached the minimum at the duty cycle of 66.7%. It is known that periodic on–off switching of ultrasound, named as pulse ultrasound, is a way to intense acoustic cavitation. Pulsed ultrasound can prevent the formation of excessive cavitation bubbles and bubble aggregation, and reduce the acoustic field attenuation caused thereby. Owing to the limited pulse time, the partial growth and dissolution of cavitation bubbles may provide bases for the bubbles in the next cycle, generating stronger and more stable cavitation [23]. Under the same cycle time, lower duty cycle means shorter effective ultrasonic time and longer pulse interval. On the one hand, it reduces the total energy input of ultrasound, on the other hand, it causes bubble dissolution in a long pulse interval, which is not conducive to cavitation effects. Furthermore, the excessive duty cycle leads to the excessive bubble formation, creating a barrier to dissipative energy transfer and resulting in lower cavitation effects [24]. From the foregoing, the effect of duty cycles on the ultrasonic degradation of 3,5-diCQA can be explained.
3.1.6. Probe immersion depth
The immersion depth of the ultrasonic probe is tightly related to the propagation, attenuation and reverberation of ultrasound [25]. Changing the probe immersion depth from 1 cm to 6 cm, the 3,5-diCQA solution (in water) was treated by ultrasound (15 W/mL) at 40℃ for 50 min with a duty cycle of 66.7% and a probe immersion depth of 3 cm. The effect of probe immersion depth was studied and the results are shown in Fig. 1f. As the probe immersion depth increasing from 1 cm to 3 cm, the degradation of 3,5-diCQA increased. When the probe immersion depth is shallow, the emitting surface of the ultrasonic wave is far from the bottom of the vessel. The cavitation intensity is known to decrease exponentially on moving away from the probe and vanish at a certain distance [26]. Therefore, the ultrasonic energy cannot reach the liquid at the bottom and cannot be evenly distributed to the whole system, attributed to the attenuation of ultrasonic wave. Flow pattern of the liquid caused by acoustic streaming and reflection, so as the extent of mixing in the reactor is dependent on the distance of probe immersed in the solution. Better mixing occurs at a medium depth [27]. As the probe immersion depth increased to 3 cm, better mixing happened with some reflection of ultrasonic wave from the bottom of the reactor, which also improve the cavitational activity [28]. Consequently, the highest degradation of 3,5-diCQA was found. With the continuous increase of probe immersion depth (from 4 cm to 6 cm), the probe is getting closer to the bottom of the container, the ultrasonic wave hits the container during transmission, causing severe attenuation and forming standing wave because of the complex characteristics of wave irradiation and reflection [29]. Therefore, the degradation effect was weakened at the high probe immersion depth. Cao et al. [30] conducted the physical field simulation of ultrasound with different probe height (80–10 mm). They found that the solution affected by the acoustic wave has the largest range when the distance between probe and glass bottom was 40 mm, indicating the best effect of ultrasonic radiation. An optimum immersion depth needs to be selected so as to get maximum ultrasonic effects.
3.2. Evaluation of radicals
To clarify the mechanism of ultrasonic degradation of diCQAs, the free radical content in the ultrasonic system was monitored and showed in Fig. 2. The concentration of TPA-OH generated in ultrasonic system can be used to represent the free radical yield. The TPA-OH concentration increased along with the ultrasonic time, indicating an accumulation of free radicals during sonication (solid column in Fig. 2). However, less TPA-OH was generated in the ultrasonic system with 3,5-diCQA, and its concentration showed no obvious variation law with time (patterned column in Fig. 2). It can be inferred from the above result that the existence of 3,5-diCQA consumed the generated free radicals in the ultrasonic system and made it unable to react with TPA. CQAs have been reported to have the ability to scavenge peroxyl radical, hydroxyl radical, and singlet oxygen [31]. The process of scavenging free radicals is also the process of oxidative degradation of CQAs. Krimmel et al. [32] studied the OH-radical induced degradation of chlorogenic acid and found that •OH cleaved the phenolic glycoside bond and released caffeic acid from chlorogenic acid. Therefore, it can be concluded that one cause of ultrasonic degradation of diCQA is the free radical formed by cavitation.
Fig. 2.
The concentration of TPA-OH generated in ultrasonic system with and without 3,5-diCQA.
3.3. Degradation and transformation of three diCQAs at different pH
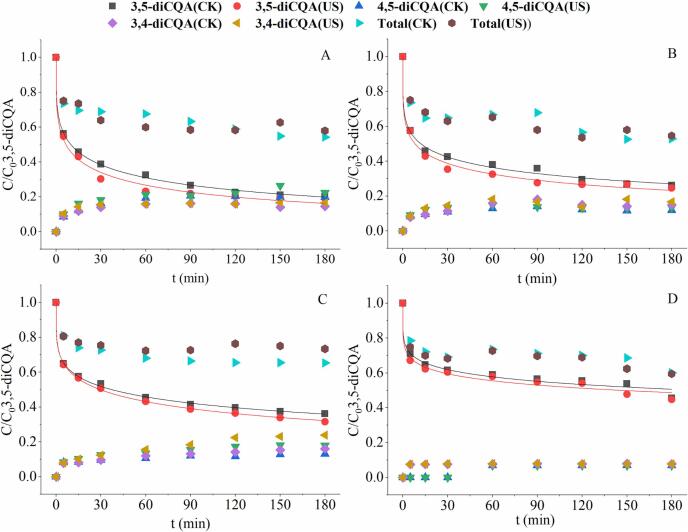
The degradation and transformation of 3,5-diCQA with and without ultrasonic treatment were evaluated at pH 9.22, 7.96, 7.06 and 4.69, as shown in Fig. 3. The ultrasonic conditions were as follow: ultrasonic power density 15 W/mL, temperature 40 °C, duty cycle 66.67%, and probe immersion depth 2 cm. The change of 3,5-diCQA content was described according to Weibull model and the kinetic parameters were accordingly calculated and listed in Table 1.
Fig. 3.
The transformation and degradation kinetics of 3,5-diCQA at pH 9.22 (A), 7.96 (B), 7.06 (C), and 4.69 (D).
Table 1.
Degradation kinetic parameters of 3,5-diCQA, 4,5-diCQA and 3,4-diCQA at different pH.
| Parameters | pH 9.22 |
pH 7.96 |
pH 7.06 |
pH 4.69 |
|||||
|---|---|---|---|---|---|---|---|---|---|
| CK | US | CK | US | CK | US | CK | US | ||
| 3,5-diCQA | k | 2.9E−2 | 4.3E−2 | 1.8E−2 | 2.7E−2 | 6.0E−3 | 9.0E−3 | 7.8E−4 | 9.3E−4 |
| n | 0.293 | 0.292 | 0.236 | 0.241 | 0.247 | 0.272 | 0.195 | 0.183 | |
| t1/2/h | 7.50 | 4.97 | 8.56 | 5.79 | 26.7 | 21.7 | 130 | 92.9 | |
| R2 | 0.999 | 0.991 | 0.994 | 0.991 | 0.999 | 0.999 | 0.977 | 0.977 | |
| 4,5-diCQA | k | 2.2E−2 | 3.6E−2 | 1.0E−2 | 1.5E−2 | 3.0E−3 | 6.0E−3 | 4.6E−7 | 4.9E−7 |
| n | 0.295 | 0.264 | 0.324 | 0.329 | 0.284 | 0.334 | 0.097 | 0.091 | |
| t1/2/h | 9.83 | 5.12 | 26.4 | 17.6 | 64.6 | 41.5 | 2.14E+5 | 1.50E+5 | |
| R2 | 0.998 | 0.991 | 0.994 | 0.998 | 0.992 | 0.995 | 0.960 | 0.982 | |
| 3,4-diCQA | k | 2.9E−2 | 4.7E−2 | 2.3E−2 | 3.4E−2 | 1.2E−2 | 1.7E−2 | 1.6E−7 | 3.6E−7 |
| n | 0.252 | 0.242 | 0.239 | 0.251 | 0.245 | 0.269 | 0.076 | 0.066 | |
| t1/2/h | 5.93 | 3.33 | 6.80 | 4.99 | 13.1 | 10.9 | 1.69E+5 | 3.22E+4 | |
| R2 | 0.997 | 0.996 | 0.984 | 0.997 | 0.989 | 0.989 | 0.998 | 0.999 | |
As shown in Fig. 3, the content of 3,5-diCQA with and without ultrasonic treatment showed the similar decrease pattern. The content of 3,5-diCQA descended sharply in the first 30 min and then the descent rate slowed down. As the pH increased from 4.69 to 9.22, the degradation constant k of 3,5-diCQA without ultrasonic treatment increased from 7.8E−4 to 2.9E−2, the degradation half-life decreased from 130 h to 7.50 h. confirming the intensification of 3,5-diCQA degradation. Within three hours of treatment time, the degradation ratio of 3,5-diCQA reached about 55% at pH 4.69, while about 85% at pH 9.22. At the same pH condition, ultrasound accelerated the degradation of 3,5-diCQA with higher k value and lower t1/2 value (Table 1). The degradation constant k of 3,5-diCQA increased about 48%, 50%, 50%, and 19% at pH 9.22, 7.96, 7.06 and 4.69 after ultrasonic treatment.
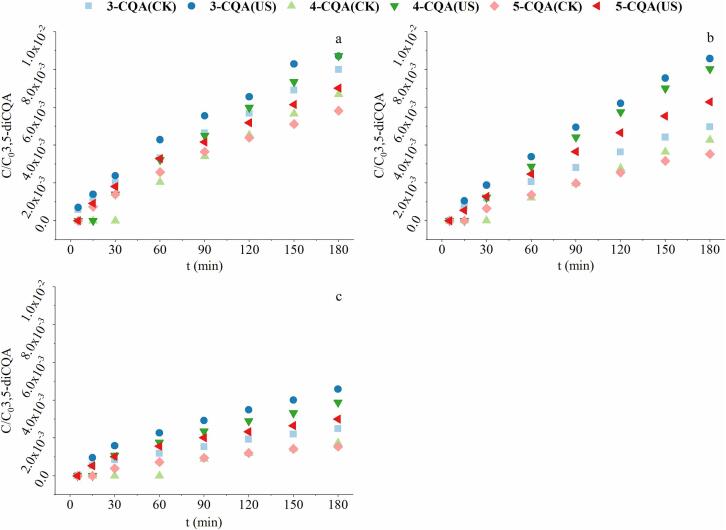
Furthermore, the contents of total CQAs (Fig. 3), two isomers of diCQAs (Fig. 3) and three mono-CQAs (Fig. 4) were also measured at different pH conditions. The degradation ratio of total CQAs at pH 9.22 and 7.96 (around 55%) was higher than that at pH 7.06 (35%) and 4.69 (40%). Moreover, the total CQAs content were higher than those of 3,5-diCQA in the same system, which indicating the formation of other CQAs during the treatment. As indicated in Fig. 4, the amount of mono-CQAs produced increased with the increasing alkalinity of the system, which was consistent with the decrease of 3,5-diCQA content. There was no mono-CQAs detected at pH 4.69. Among the three mono-CQAs detected during treatment, 3-CQA was the most abundant, followed by 4-CQA and 5-CQA. Meanwhile, 3-CQA was the first detected, then 5-CQA and 4-CQA. In the sample without ultrasonic treatment, 5-CQA can’t be detected until the time reached 30 min at pH 7.96 and 7.06. At pH 9.22, the time when 5-CQA can be monitored was advanced to 15 min. 4-CQA started to be detected much later (90 min at pH 7.06, 60 min at pH 7.96 and 9.22). It can be inferred from the above results that, diCQAs could degrade to mono-CQAs. The main degradation product of 3,5-diCQA was 3-CQA. But the 3-CQA will also be converted to 4- and 5-CQA in the process. With ultrasonic treatment, the time at which 4-CQA began to be detectable was advanced from 30 min (pH 9.22 & 7.96) and 60 min (pH 7.06) to 15 min. The initial detectable time for 5-CQA also advanced from 15 min to 5 min (pH 7.96 & 7.06) by ultrasound. Thus, in general, ultrasonic treatment increased the content of mono-CQAs and advanced their detection time, illustrating that ultrasound could accelerate the degradation of 3,5-diCQA.
Fig. 4.
The generation of mono-CQAs during the treatment of 3,5-diCQA at pH 9.22 (a), 7.96 (b), and 7.06 (c).
Besides degradation, diCQAs has been reported to undergo isomerization with each other [33]. At pH 4.69 (Fig. 3D), two isomers, 4,5- and 3,4-diCQA were found in relatively small amounts (lower than 10%). In the first 30 min, almost no 4,5-diCQA were detected in both of the sample with and without ultrasonic treatment. By inference, 3,5-diCQA is more likely to isomerize to 3,4-diCQA. The effect of ultrasound on the formation of isomers was not obvious. The possible reason is that 3,5-diCQA showed a good stability at pH 4.69. With increasing pH, the contents of isomers increased obviously. At pH 7.06 and 7.96, the concentration of 3,4-diCQA was slightly higher than that of 4,5-diCQA, but at pH 9.22, the concentration of 4,5-diCQA was higher. It is due to the easier isomerization of 3,5-diCQA to 3,4-diCQA. However, under strong alkaline conditions (pH 9.22), 3,4-diCQA was less stable than 4,5-diCQA, and degraded more seriously, resulting in a lower content. Higher isomer contents were detected in samples with ultrasonic treatment, indicating that ultrasound promoted the isomerization of 3,5-diCQA.
The degradation and transformation of 4,5-diCQA and 3,4-diCQA are displayed in Fig. S1 & S3. The generation of mono-CQAs are shown in Fig. S2 & S4. The kinetic parameters are listed in Table 1. The stability of 4,5-diCQA and 3,4-diCQA was decreased with increasing pH value. Under the same condition, samples with ultrasonic treatment showed higher k value and lower t1/2 value, indicating the ultrasonic-enhanced degradation of 4,5- and 3,4-diCQA. In general, similar to 3,5-diCQA, 4,5- and 3,4-diCQA were degraded into mono-CQAs and isomerized to other diCQA isomers during the treatment.
At pH 4.69, 4,5- and 3,4-diCQA were relatively stable, so the degradation products mono-CQAs were not detected (Fig. S2 & S4). Generally, the contents of mono-CQAs increased with the increasing pH, demonstrating the steadily serious degradation of 4,5- and 3,4-diCQA. The main degradation products of 4,5-diCQA and 3,4-diCQA were 5-CQA and 3-CQA, respectively. Compared with samples without ultrasonic treatment, the contents of mono-CQAs detected in samples with ultrasonic treatment were significantly higher, confirming that ultrasound caused more degradation products to be generated and promoted the degradation of diCQAs.
As shown in Fig. S1D & Fig. S3D, the quantities of isomers were relatively low at pH 4.69 (lower than 15%), which means the weak isomerization of 4,5- and 3,4-diCQA at this condition. During the treatment of both 4,5- and 3,4-diCQA, 3,5-diCQA accounted for a larger fraction of the two generated isomers. This suggests that both 4,5-diCQA and 3,4-diCQA are inclined to isomerize to 3,5-diCQA. Additionally, ultrasound slightly enhanced the isomerization of 4,5- and 3,4-diCQA under neutral and alkaline conditions.
As described in Fig. 3, S1, and S3, three diCQAs have different degradation rates. Comparing the degradation kinetics of the three diCQAs (Table 1), it is found that the degradation constant k of 3,4-diCQA was the largest, followed by 3,5-diCQA and 4,5-diCQA under the same pH condition. In other words, the order of stability of the three diCQAs is 4,5-diCQA > 3,5-diCQA > 3,4-diCQA.
3.4. Effect of EGCG and VC
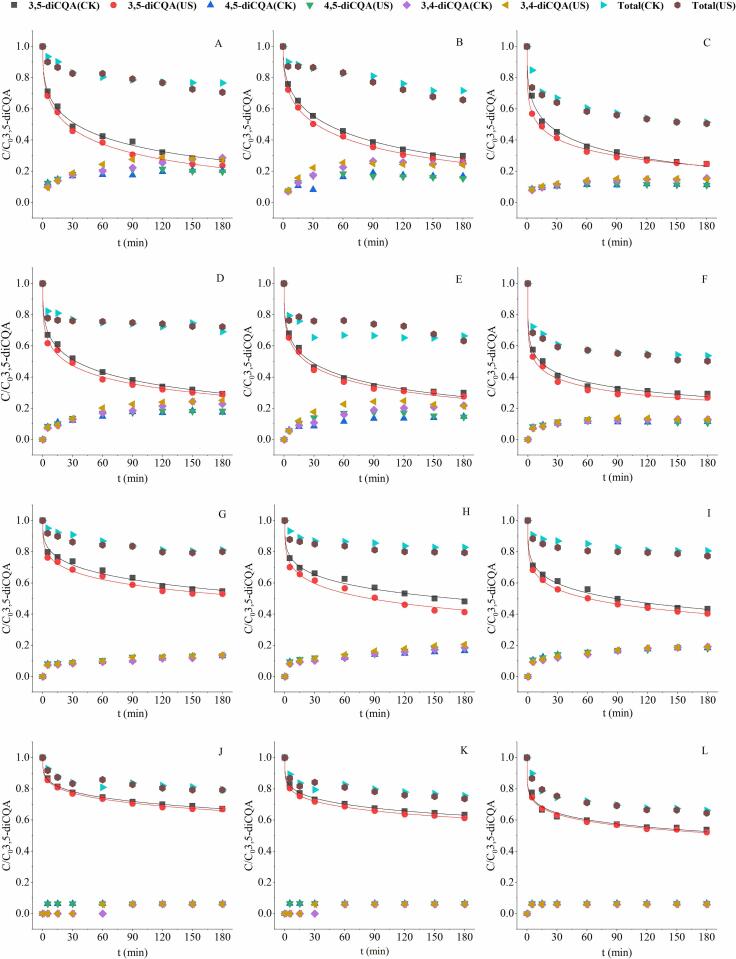
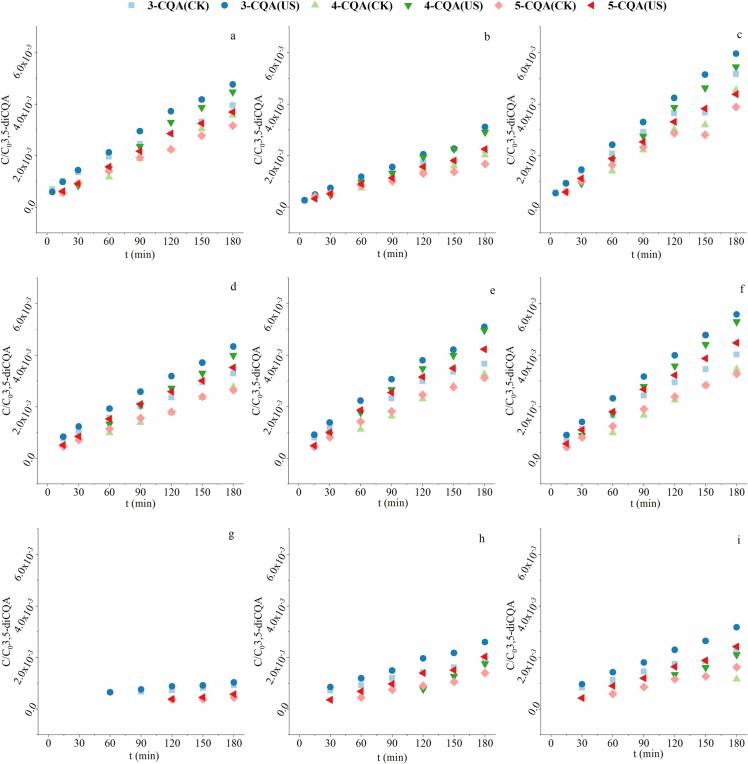
In order to reduce the degradation of diCQAs, EGCG and VC were added into the system. Afterwards, the degradation and transformation of three diCQAs after adding EGCG and VC were analyzed. Fig. 5 shows the content change of 3,5-diCQA and total CQAs during treatment, as well as the formation of two isomers (3,4- and 4,5-diCQA) when adding different amount of EGCG. Fig. 6 shows the generation of corresponding mono-CQAs degraded from 3,5-diCQA. Based on Weibull model, the kinetic parameters are listed in Table 2.
Fig. 5.
Effect of different dose of EGCG (EGCG:3,5-diCQA = 2:1 (A, D, G, J), 1:1 (B, E, H, K), 1:2 (C, F, I, L)) on the stability of 3,5-diCQA with and without ultrasound at pH 9.22 (A, B, C), 7.96 (D, E, F), 7.06 (G, H, I) and 4.69 (J, K, L).
Fig. 6.
Effect of different dose of EGCG (EGCG:3,5-diCQA = 2:1 (a, d, g), 1:1 (b, e, h), 1:2 (c, f, i)) on the formation of mono-CQAs with and without ultrasound at pH 9.22 (a, b, c), 7.96 (d, e, f), and 7.06 (g, h, i).
Table 2.
Effects of EGCG and VC on the degradation kinetic parameters of 3,5-diCQA.
| Additive Content | Parameters | pH 9.22 |
pH 7.96 |
pH 7.06 |
pH 4.69 |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| CK | US | CK | US | CK | US | CK | US | |||
| None | k | 2.9E−2 | 4.3E−2 | 1.8E−2 | 2.7E−2 | 6.0E−3 | 9.0E−3 | 7.8E−4 | 9.3E−4 | |
| n | 0.293 | 0.292 | 0.236 | 0.241 | 0.247 | 0.272 | 0.195 | 0.183 | ||
| t1/2/h | 7.50 | 4.97 | 8.56 | 5.79 | 26.7 | 21.7 | 130 | 92.9 | ||
| R2 | 0.999 | 0.991 | 0.994 | 0.991 | 0.999 | 0.999 | 0.977 | 0.977 | ||
| EGCG | 1:2 | k | 1.7E−2 | 2.2E−2 | 1.7E−2 | 2.5E−2 | 3.0E−3 | 4.0E−3 | 7.0E−4 | 7.7E−4 |
| n | 0.349 | 0.269 | 0.232 | 0.216 | 0.273 | 0.249 | 0.214 | 0.211 | ||
| t1/2/h | 16.8 | 8.47 | 8.51 | 7.35 | 65.1 | 43.2 | 175 | 157 | ||
| R2 | 0.996 | 0.998 | 0.995 | 0.995 | 0.995 | 0.995 | 0.990 | 0.993 | ||
| 1:1 | k | 1.0E−2 | 1.2E−2 | 1.2E−2 | 1.4E−2 | 2.0E−3 | 3.0E−3 | 2.2E−4 | 2.2E−4 | |
| n | 0.427 | 0.400 | 0.329 | 0.313 | 0.281 | 0.280 | 0.238 | 0.222 | ||
| t1/2/h | 36.4 | 26.9 | 21.0 | 17.0 | 127 | 63.4 | 701 | 599 | ||
| R2 | 0.996 | 0.973 | 0.993 | 0.994 | 0.998 | 0.991 | 0.981 | 0.978 | ||
| 2:1 | k | 1.1E−2 | 1.6E−2 | 1.0E−2 | 1.2E−2 | 1.0E−3 | 1.0E−3 | 1.9E−4 | 2.1E−4 | |
| n | 0.375 | 0.388 | 0.334 | 0.293 | 0.320 | 0.275 | 0.271 | 0.268 | ||
| t1/2/h | 26.8 | 19.7 | 26.1 | 17. 6 | 226 | 177 | 1.03E+3 | 887 | ||
| R2 | 0.995 | 0.998 | 0.996 | 0.994 | 0.992 | 0.989 | 0.991 | 0.988 | ||
| VC | 1:2 | k | 2.5E−2 | 3.2E−2 | 1.9E−2 | 3.0E−2 | 3.0E−3 | 3.0E−3 | 6.3E−4 | 7.5E−4 |
| n | 0.255 | 0.247 | 0.260 | 0.250 | 0.272 | 0.246 | 0.260 | 0.250 | ||
| t1/2/h | 6.93 | 5.02 | 9.26 | 5.52 | 70.9 | 46.6 | 283 | 221 | ||
| R2 | 0.997 | 0.994 | 0.995 | 0.994 | 0.984 | 0.995 | 0.996 | 0.998 | ||
| 1: 1 | k | 1.8E−2 | 2.7E−2 | 1.5E−2 | 2.2E−2 | 2.0E−3 | 4.0E−3 | 1.7E−4 | 1.7E−4 | |
| n | 0.267 | 0.262 | 0.316 | 0.287 | 0.295 | 0.287 | 0.272 | 0.262 | ||
| t1/2/h | 10.6 | 6.70 | 16.6 | 9.58 | 90.1 | 53.5 | 1.12E+3 | 1.04E+3 | ||
| R2 | 0.998 | 0.997 | 0.997 | 0.996 | 0.994 | 0.993 | 0.994 | 0.996 | ||
| 2: 1 | k | 1.6E−2 | 2.4E−2 | 1.0E−2 | 1.7E−2 | 2.0E−3 | 5.0E−3 | 2.5E−4 | 2.5E−4 | |
| n | 0.261 | 0.258 | 0.276 | 0.302 | 0.293 | 0.301 | 0.233 | 0.220 | ||
| t1/2/h | 11.5 | 7.21 | 19.3 | 13.7 | 98.8 | 44.0 | 580 | 514 | ||
| R2 | 0.990 | 0.992 | 0.998 | 0.998 | 0.995 | 0.993 | 0.999 | 0.998 | ||
According to the data shown in Table 2, the degradation constant (k) decreased and the half-life time (t1/2) increased after adding EGCG during both ultrasonic and non-ultrasonic treatment, which indicates that EGCG can effectively protect 3,5-diCQA. The protective effect was influenced by the dose of EGCG and pH. At pH 9.22, the addition amount of EGCG at 1:1 (the molar ratio of EGCG and 3,5-diCQA) reduced the degradation constant k by 66% for the control and 75% for the ultrasonic sample, extended t1/2 by 3.9 times for the control and 4.4 times for the ultrasonic sample, achieving the optimal protective effect on 3,5-diCQA under this condition. At the rest pH conditions, the protective effect of EGCG on 3,5-diCQA increased with the increase of EGCG dose. EGCG is a strong antioxidant due to due to the existence of the hydroxyl groups, which has been found to protect gardenia yellow pigment [34] and β-carotene [35]. EGCG can be prone to oxidative decomposition to protect the target substance. The protective effect of EGCG was much more obvious at pH 4.69 and 7.06, compared to that at pH 7.96 and 9.22. It is due to the decreased stability of EGCG in alkaline solution [36]. Furthermore, with the addition of EGCG, the difference between degradation constant (k) under ultrasonic and non-ultrasonic conditions was reduced, showing a better protective effect of EGCG under ultrasound. It is because the potential ability of EGCG to scavenge free radicals, including hydroxyl radicals and peroxyl radicals [37]. The existence of EGCG counteracted part of the ultrasonic attack induced by free radical effect against 3,5-diCQA.
To further explore the mechanism of the protective effect of EGCG, the formation of two isomers and mono-CQAs were evaluated and shown in Fig. 5, Fig. 6. Combined with the results displayed in Fig. 3, Fig. 5, it can be seen that the addition of EGCG affected the isomerization of 3,5-diCQA differently at different pH and dose. At alkaline pH (pH 9.22 & 7.96), high dose of EGCG (2:1 & 1:1) increased the amount of two isomers (3,4-diCQA and 4,5-diCQA), while low dose of EGCG inhibited the formation of isomer. At pH 7.06 & 4.69, the addition of EGCG showed repression of the isomerization. Besides, the inhibition of degradation of 3,5-diCQA to mono-CQAs was much stronger at alkaline conditions (pH 9.22 & 7.96), obtained by comparing Fig. 4, Fig. 6. That’s why EGCG still played a protective role on 3,5-diCQA even if the isomerization was promoted at certain conditions. It suggests that EGCG can prevent the decrease of 3,5-diCQA concentration by inhibiting its decomposition and isomerization, depending on pH environment.
The effect of VC on the stability of 3,5-diCQA were also measured. The corresponding results are shown in Figs. S5 & S6. The kinetic parameters obtained by Weibull model are listed in Table 2.
As shown in Table 2, the degradation constant (k) decreased and the half-life time (t1/2) increased after adding VC in samples with and without ultrasonic treatment, revealing the improved stability of 3,5-diCQA by adding VC. At pH 4.69, the optimum addition amount of VC was 1:1 (the molar ratio of VC and 3,5-diCQA), which reduced the degradation constant k by 78% for the control and 81% for the ultrasonic sample, extended t1/2 by 3.5 times for the control and 4.5 times for the ultrasonic sample. At the rest pH conditions, the optimal protective effect of VC occurred at the dose of 2:1 (the molar ratio of VC and 3,5-diCQA). VC has been reported to prevent phenolic acids from degradation. It serves as reducing agent that can stabilize the substances and recycle their free radical form [13], [38]. Similar mechanism may involve in the protective effect of VC on diCQAs.
In addition, VC was found to inhibit the isomerization of phenotypic catechins, and the higher the concentration of VC, the stronger inhibition of isomerization [39]. Comparing Fig. 3 & S5, it can be seen that with the increasing addition amount of VC, the concentration of two isomerization products decreased slightly. Comparing Fig. 4 & S6, the presence of VC effectively reduced the formation of mono-CQAs, indicating a reduced degradation of 3,5-diCQA.
In terms of the contents of generated diCQAs (products of isomerization) and mono-CQAs (degradation products), the inhibition of VC on degradation and isomerization of 3,5-diCQA was weaker than that of EGCG except at pH 4.69. This is consistent with the results of degradation kinetics shown in Table 2 that the addition of EGCG led to a smaller rate constant k and a longer half-life time t1/2. Moreover, unlike EGCG, the addition of VC did not narrow the degradation difference of 3,5-diCQA with and without ultrasonic treatment. It reveals that VC has little protective effect on 3,5-diCQA degradation caused by ultrasonic attack.
The effects of EGCG and VC on 4,5- and 3,4-diCQA were also analyzed and shown in Figs. S7–S14. The kinetic parameters are listed in Table S1 & S2. The results were similar with those of 3,5-diCQA. Both EGCG and VC can enhance the stability of 4,5- and 3,4-diCQA. The effect of VC strengthened by increasing its concentration at all pH conditions, while the effect of EGCG increased by the increasing concentration at pH 9.22, 7.96 and 7.06. EGCG showed better protective effect on 4,5- and 3,4-diCQA at pH 4.69 and 7.06, while VC showed better protective effect on 4,5- and 3,4-diCQA at alkaline pH (pH 9.22 & 7.96), which is different with that on 3,5-diCQA. At neutral and acidic condition, the presence of VC caused significantly less degraded products of 4,5- and 3,4-diCQA compared with EGCG.
4. Conclusion
In general, changes of the solvent, treatment time, temperature, ultrasonic power density, ultrasonic duty cycle and probe immersion depth would affect the degradation of 3,5-diCQA. The stability of three diCQAs were decreased with the increase of pH at order of 4,5-diCQA > 3,5-diCQA > 3,4-diCQA. During the treatment, diCQAs would isomerize to each other and degrade to mono-CQAs, which can be accelerated by ultrasonic treatment. Generally, 3,5-diCQA is more likely to isomerize to 3,4-diCQA, while 4,5-diCQA and 3,4-diCQA are more preferentially isomerized to 3,5-diCQA. The dominant degradation product of 3,5- and 3,4-diCQA was 3-CQA, while the main degradation product of 4,5-diCQA was 5-CQA. EGCG and VC effectively prevented the loss of diCQAs during processing. Our study provided the fundamental research on the degradation pattern of three diCQAs with and without ultrasound. The parameters optimization is suggested before the processing of diCQAs. The acidic system environment is more conducive to the stability of diCQAs. Some protective agents can be used to enhance the stability of diCQAs, and reduce ultrasonic damage. The study is beneficial to the application of ultrasound in diCQAs extraction and processing.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31871763); Zhejiang Province Key Science and Technology Projects (No. 2023C02042 and 2023C02045); Agriculture and Social Development Projects of Hangzhou (202203A11).
CRediT authorship contribution statement
Danli Wang: Data curation, Writing – original draft, Funding acquisition. Yushi Wang: Validation, Data curation. Zhenlei Zhang: Investigation. Shaoping Qiu: Investigation, Methodology. Yawen Yuan: Validation. Gongshuai Song: Methodology. Ling Li: Visualization. Tinglan Yuan: Formal analysis. Jinyan Gong: Conceptualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2023.106401.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Meinhart A.D., Damin F.M., Caldeirão L., Filho M.J., Silva L.C., Constant L.S., Filho J.T., Wagner R., Godoy H.T. Study of new sources of six chlorogenic acids and caffeic acid. J. Food Compos. Anal. 2019;82:103244. doi: 10.1016/j.jfca.2019.103244. [DOI] [Google Scholar]
- 2.Raheem K.S., Botting N.P., Williamson G., Barron D. Total synthesis of 3,5-O-dicaffeoylquinic acid and its derivatives. Tetrahedron Lett. 2011;52:7175–7177.. doi: 10.1016/j.tetlet.2011.10.127. [DOI] [Google Scholar]
- 3.Magaña A.A., Kamimura N., Soumyanath A., Stevens J.F., Maier C.S. Caffeoylquinic acids: chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021;107(5):1299–1319. doi: 10.1111/TPJ.15390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue M., Shi H., Zhang J., Liu Q., Guan J., Zhang J., Ma Q. Stability and degradation of caffeoylquinic acids under different storage conditions studied by high-performance liquid chromatography with photo diode array detection and high-performance liquid chromatography with electrospray ionization collision-induced dissociation tandem mass spectrometry. Molecules. 2016;21:948. doi: 10.3390/molecules21070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawidowicz A.L., Typek R. Transformation of chlorogenic acids during the coffee beans roasting process. Eur. Food Res. Technol. 2017;243:379–390. doi: 10.1007/s00217-016-2751-8. [DOI] [Google Scholar]
- 6.Ianni F., Barola C., Blasi F., Moretti S., Galarini R., Cossignani L. Investigation on chlorogenic acid stability in aqueous solution after microwave treatment. Food Chem. 2022;374:131820. doi: 10.1016/j.foodchem.2021.131820. [DOI] [PubMed] [Google Scholar]
- 7.P. Chavan, P. Sharma, S.R. Sharma, T.C. Mittal, A.K. Jaiswal, Application of high-intensity ultrasound to improve food processing efficiency: A review, Foods. 11 (2022) 122, doi:10.3390/foods11010122. [DOI] [PMC free article] [PubMed]
- 8.Taha A., Mehany T., Pandiselvam R., Siddiqui S.A., Mir N.A., Malik M.A., Sujayasree O.J., Alamuru K.C., Khanashyam A.C., Casanova F., Xu X., Pan S., Hu H. Sonoprocessing: mechanisms and recent applications of power ultrasound in food. Crit. Rev. in Food Sci. Nutr. 2023 doi: 10.1080/10408398.2022.2161464. [DOI] [PubMed] [Google Scholar]
- 9.Elenilson G., Filho A., Sousa V.M., Rodrigues S., Brito E.S., Fernandes F.A.N. Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives. Ultrason. Sonochem. 2020;63:104911. doi: 10.1016/j.ultsonch.2019.104911. [DOI] [PubMed] [Google Scholar]
- 10.Wang D., Liu J., Qiu S., Wang J., Song G., Chu B., Li L., Xiao G., Gong J., Zheng F. Ultrasonic degradation kinetics and isomerization of 3- and 4-O-caffeoylquinic acid at various pH: The protective effects of ascorbic acid and epigallocatechin gallate on their stability. Ultrason. Sonochem. 2021;80:105812. doi: 10.1016/j.ultsonch.2021.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D., Wang J., Sun J., Qiu S., Chu B., Fang R., Li L., Gong J., Zheng F. Degradation kinetics and isomerization of 5-O-caffeoylquinic acid under ultrasound: Influence of epigallocatechin gallate and vitamin C. Food Chemistry: X. 2021;12:100147. doi: 10.1016/j.fochx.2021.100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng J., Cheng J., Liao X., Zhang T., Leng X., Zhao G. Comparative Study on Iron Release from Soybean (Glycine max) Seed Ferritin Induced by Anthocyanins and Ascorbate. J. Agric. Food Chem. 2021;58:635–641. doi: 10.1021/jf903046u. [DOI] [PubMed] [Google Scholar]
- 13.Narita Y., Inouye K. Degradation kinetics of chlorogenic acid at various pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions. J. Agric. Food Chem. 2013;61(4):966–972. doi: 10.1021/jf304105w. [DOI] [PubMed] [Google Scholar]
- 14.Zia S., Khan M.R., Shabbir M.A., Maan A.A., Khan M.K.I., Nadeem M., Khalil A.A., Din A., Aadil R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Intl. 2022;38(6):1166–1196. doi: 10.1080/87559129.2020.1772283. [DOI] [Google Scholar]
- 15.Desai V., Shenoy M.A., Gogate P.R. Degradation of polypropylene using ultrasound-induced acoustic cavitation. Chem. Eng. J. 2008;140:483–487. doi: 10.1016/j.cej.2007.11.030. [DOI] [Google Scholar]
- 16.Y.G. Adewuyi, Sonochemistry: Environmental science and engineering applications, Ind. Eng. Chem. Res. 40 (2001) 4681–4715, doi:10.1021/ie010096l.X.
- 17.Ma X., Wang W., Wang D., Ding T., Ye X., Liu D. Degradation kinetics and structural characteristics of pectin under simultaneous sonochemical-enzymatic functions. Carbohydr. Polym. 2016;154:176–185. doi: 10.1016/j.carbpol.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Zhang C., Ding G., Huang W., Wang Z., Bi Y., Xiao W. Investigating the thermal stability of six caffeoylquinic acids employing rapid-resolution liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. Eur. Food Res. Technol. 2015;240:1225–1234. doi: 10.1007/s00217-015-2425-y. [DOI] [Google Scholar]
- 19.Li Y., Wang Y., Li J., Gao W., Niu Z. Study on the stability of chlorogenic acids in stevia. China Food Additives. 2021;9:73–78. doi: 10.19804/j.issn1006-2513.2021.09.011. [DOI] [Google Scholar]
- 20.Patist A., Bates D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008;9(2):147–154. doi: 10.1016/j.ifset.2007.07.004. [DOI] [Google Scholar]
- 21.Wang J., Ma H., Pan Z., Qu W. Sonochemical effect of flat sweep frequency and pulsed ultrasound (FSFP) treatment on stability of phenolic acids in a model system. Ultrason. Sonochem. 2017;39:707–715. doi: 10.1016/j.ultsonch.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 22.Kentish S., Wooster T.J., Ashokkumar M., Balachandran S., Mawson R., Simons L. The use of ultrasonics for nanoemulsion preparation. Innov. Food Sci. Emerg. Technol. 2008;9:170–175. doi: 10.1016/j.ifset.2007.07.005. [DOI] [Google Scholar]
- 23.Xu S., Zong Y., Feng Y., Liu R., Liu X., Hu Y., Han S., Wan M. Dependence of pulsed focused ultrasound induced thrombolysis on duty cycle and cavitation bubble size distribution. Ultrason. Sonochem. 2015;22:160–166. doi: 10.1016/j.ultsonch.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Sancheti S.V., Gogate P.R. A review of engineering aspects of intensification of chemical synthesis using ultrasound. Ultrason. Sonochem. 2017;36:527–543. doi: 10.1016/j.ultsonch.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y., Ma G., Ye X., Kakuda Y., Meng R. Stability of all-trans-beta-carotene under ultrasound treatment in a model system: Effects of different factors, kinetics and newly formed compounds. Ultrason. Sonochem. 2010;17:654–661. doi: 10.1016/j.ultsonch.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Gogate P.R., Kabadi A.M. A review of applications of cavitation in biochemical engineering/biotechnology. Biochem. Eng. J. 2009;44:60–72. doi: 10.1016/j.bej.2008.10.006. [DOI] [Google Scholar]
- 27.Mohod A.V., Gogate P.R. Ultrasonic degradation of polymers: Effect of operating parameters and intensification using additives for carboxymethyl cellulose (CMC) and polyvinyl alcohol (PVA) Ultrason. Sonochem. 2011;18(3):727–734. doi: 10.1016/j.ultsonch.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y., No Y., Kim J. Geometric and operational optimization of 20-kHz probe-type sonoreactor for enhancing sonochemical activity. Ultrason. Sonochem. 2020;65:105065. doi: 10.1016/j.ultsonch.2020.105065. [DOI] [PubMed] [Google Scholar]
- 29.Son Y., Lim M., Song J.H., Khim J. Liquid height effect on sonochemical reactions in a 35 kHz sonoreactor. Jpn. J. Appl. Phys. 2017;48:1–4. doi: 10.1143/JJAP.48.07GM16. [DOI] [Google Scholar]
- 30.Cao P., Hao C., Ma C., Yang H., Sun R. Physical field simulation of the ultrasonic radiation method: an investigation of the vessel, probe position and power. Ultrason. Sonochem. 2020;76:105626. doi: 10.1016/j.ultsonch.2021.105626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mullen W., Nemzer B., Ou B., Stalmach A., Hunter J., Clifford M.N., Combet E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food Chem. 2011;59(8):3754–3762. doi: 10.1021/jf200122m. [DOI] [PubMed] [Google Scholar]
- 32.Krimmel B., Swoboda F., Solar S., Reznicek G. OH-radical induced degradation of hydroxybenzoic- and hydroxycinnamic acids and formation of aromatic products—A gamma radiolysis study. Radiat. Phys. Chem. 2010;79:1247–1254. doi: 10.1016/j.radphyschem.2010.07.001. [DOI] [Google Scholar]
- 33.Tian F., Ruan Q.J., Zhang Y., Cao H., Ma Z.G., Zhou G.L., Wu M.H. Quantitative Analysis of six phenolic acids in Artemisia capillaris (Yinchen) by HPLC-DAD and their transformation pathways in decoction preparation process. J. Anal. Methods Chem. 2020;2020:8950324. doi: 10.1155/2020/8950324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu J., Wang X., He Y., Li J., Ma K., Zhang Y., Li H., Yin C., Zhang Y. Stability evaluation of gardenia yellow pigment in presence of different phenolic compounds. Food Chem. 2022;373:131441. doi: 10.1016/j.foodchem.2021.131441. [DOI] [PubMed] [Google Scholar]
- 35.L. Liu, Y. Gao, D.J. McClements, E. Decker, A. Role of continuous phase protein, (–)-epigallocatechin-3-gallate and carrier oil on β-carotene degradation in oil-in-water emulsions, Food Chem. 210 (2016) 242–248, doi:10.1016/j.foodchem.2016.04.075. [DOI] [PubMed]
- 36.Sang S., Lambert J.D., Ho C.T., Yang C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011;64(2):87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Zhu N., Huang T.C., Yu Y., LaVoie E.J., Yang C.S., Ho C.T. Identification of oxidation products of (–)-epigallocatechin gallate and (–)-epigallocatechin with H2O2. J. Agric. Food Chem. 2000;48:979–981. doi: 10.1021/jf991188c. [DOI] [PubMed] [Google Scholar]
- 38.Nardini M., Cirillo E., Natella F., Mencarelli D., Comisso A., Scaccini C. Detection of bound phenolic acids: Prevention by ascorbic acid and ethylenediaminetetraacetic acid of degradation of phenolic acids during alkaline hydrolysis. Food Chem. 2002;79:119–124. doi: 10.1016/S0308-8146(02)00213-3. [DOI] [Google Scholar]
- 39.Chen L., Wang W., Zhang J., Cui H., Ni D., Jiang H. Dual effects of ascorbic acid on the stability of EGCG by the oxidation product dehydroascorbic acid promoting the oxidation and inhibiting the hydrolysis pathway. Food Chem. 2021;337:127639. doi: 10.1016/j.foodchem.2020.127639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.