Highlights
-
•
We presented the first transcriptome-wide profile of m6A modifications in prostate cancer.
-
•
What's more, we developed the MMM score, which utilizes m6A-modified differentially expressed genes to predict the biochemical recurrence of prostate cancer patients. This score was verified in nine external multi-center cohorts.
-
•
Additionally, our findings indicate that patients with a high MMM score have a poorer response to ARSI therapy and immunotherapy.
-
•
Two hub genes in the MMM score, TLE1 and PFKL, were found to be directly modified by m6A and promote invasion of prostate cancer cells.
Keywords: Prostate cancer, N6-methyladenosine modified mRNA, Prognostic signature, Biochemical recurrence, Androgen receptor signaling inhibitor, Immunotherapy
Abstract
Novel biomarkers are urgently needed to improve the prediction of clinical outcomes and guide personalized treatment for prostate cancer (PCa) patients. However, the role of N6-methyladenosine (m6A) modifications in PCa initiation and progression remains largely elusive. In our study, we collected benign Prostate Hyperplasia (BPH), localized PCa, and metastatic PCa samples from patients and performed methylated RNA immunoprecipitation sequencing (MeRIP-Seq) to map m6A-methylated mRNAs. Furthermore, we developed a prognostic signature based on 239 differentially methylated RNAs and the TCGA-PRAD dataset, which can be used to calculate an m6A-modified mRNA (MMM) score for a PCa patient, validated by independent multi-center cohorts. Our findings revealed that differential m6A modifications were positively correlated with altered expressions of mapped m6A-modified mRNAs. Higher MMM scores were associated with shorter times to biochemical recurrence (BCR) in PCa patients, and the MMM scoring system outperformed three well-established signatures in nine independent validation cohorts, as demonstrated by Kaplan-Meier survival analysis, C-index and ROC. Patients who did not respond to androgen receptor signaling inhibitor (ARSI) therapy and immunotherapy were found to have high MMM scores. Two hub genes, TLE1 and PFKL, were confirmed to have m6A sites through MeRIP-qPCR, and their knockdown promoted PCa cell invasion. Bioinformatics analysis of single-cell databases identified cell types with high transcript abundance levels of these two genes. In summary, our study is the first to perform transcriptome-wide m6A mapping in prostate tissues. The translational potential of a prognostic signature, comprising m6A-methylated mRNAs, in predicting clinical outcomes and therapy responses for PCa patients, is demonstrated.
Graphical abstract

Background
In 2021, the estimated number of new cases of prostate cancer (PCa) in the United States was 248,530, with an estimated number of deaths of up to 34,130, and this cancer was the most frequently diagnosed cancer among males in the United States [1]. Metastasis is mainly responsible for the poor prognoses of patients with PCa; nearly 8% of newly diagnosed PCa patients develop metastasis [2]. The 5-year relative survival rate of localized PCa is >99%, while the 5-year relative survival rate for distant/metastatic PCa is approximately 30.6% [3]. Clinically, the prevention and treatment of metastatic PCa remain significantly challenging since multiple molecular mechanisms are involved in PCa metastasis [4]. Thus, uncovering the cellular and molecular mechanisms underlying PCa metastasis, such as epithelial−mesenchymal transition (EMT), autophagy, and epigenetic modification, will likely offer potential therapeutic strategies.
As an increasing number of advanced technologies for analyzing epigenetics have emerged, the impact of epigenetics on cancer has attracted the attention of scientists. Traditionally, epigenetic regulation is applied via various reversible chemical modifications of DNA and histones that tune gene expression without changing original genome sequences [5]. However, recently, RNA modification, a new branch of epigenetics, has gained attention given that these modifications have been identified as significant posttranscriptional regulators of gene expression [6]. To date, the number of RNA modifications that have been identified with advanced detection methods, including N6-methyladenosine (m6A), 5-methylcytosine (m5C) and N1-methyladenosine (m1A), exceed 150 [6,7]. Among these chemical modifications, methylation of adenosine at position 6, known as m6A, which is widespread in messenger RNAs (mRNAs) and noncoding RNAs, is the best characterized.
Emerging evidence has suggested that dysregulation of both the global abundance of m6A and the expression levels of its regulators is associated with various types of cancers and is essential for cancer initiation, progression, drug resistance and relapse [8]. However, given the limited evidence of previous studies, the relationship between m6A-modified mRNA and PCa remains to be elucidated.
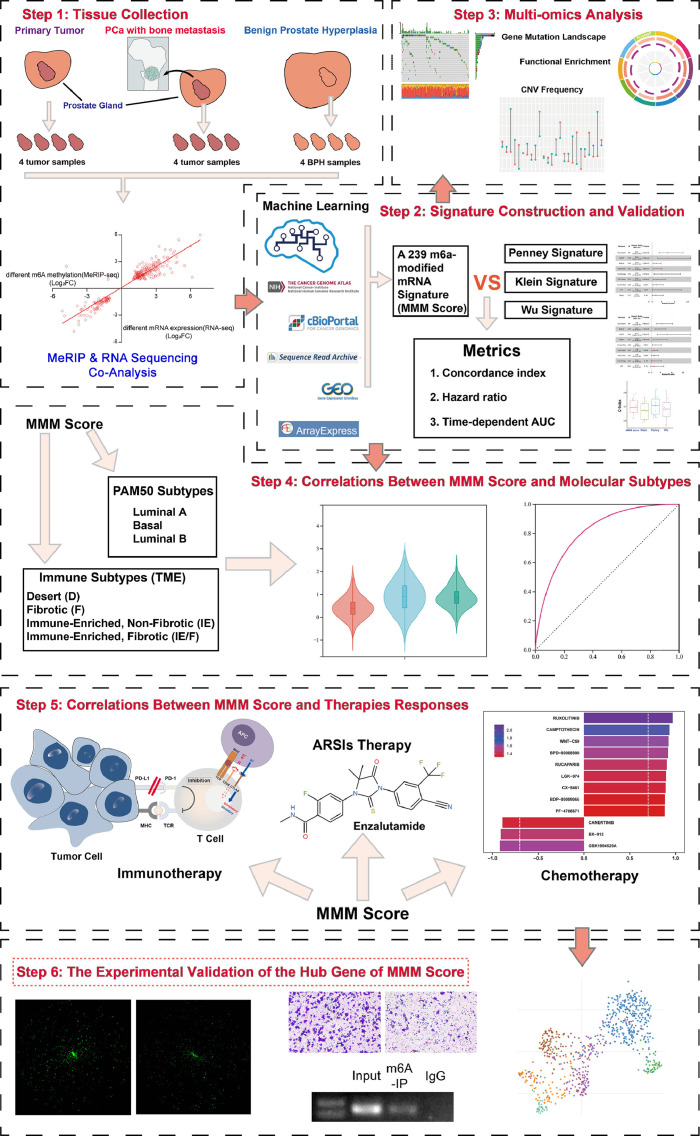
In this study, we presented the first m6A transcriptome-wide profile in benign prostatic hyperplasia (BPH) and primary PCa. We also used both bioinformatic and experimental studies to establish an m6A-modified mRNA-based scoring system (the MMM score) for classifying prostate tumors and for predicting clinical outcomes or response to various therapies with multicenter cohort data. Finally, we used a single-cell dataset, cell assays and MeRIP-qPCR analysis to verify the hub genes considered by the MMM score. Given its variety of research approaches and reasonably substantial clinical translational value, this study adds to the body of knowledge on RNA modifications in PCa. The workflow is shown in Fig. 1.
Fig. 1.
The workflow of this study.
Materials and methods
Sample collection
Four benign human prostate tissue samples (benign group) were taken from Benign Prostate Hyperplasia by enucleation of the prostate. Four primary tumor site samples taken from primary prostate carcinoma without metastasis at diagnosis (primary group) and four metastatic site samples taken from prostate carcinomas with metastasis at diagnosis (metastasis group) by biopsies. Half of the biopsies tissues were stained with Hematoxylin and Eosin (HE) and evaluated by a trained pathologist. Samples’ clinical information were shown in Table S1.
High throughout methylated RNA immunoprecipitation and mRNA sequencing
About 300 ng RNA from each specimen were using for RNA-sequencing and about 9ug RNA from each specimen were using for MeRIP-sequencing. The detail of MeRIP-Seq, RNA-Seq could be found in the supplemental methods.
Public dataset preprocessing
In this study, eleven PCa public datasets and three immunotherapy cohorts were included (Table S2). The detailed processing workflows were shown in our previous study [9]. In briefly, TCGA-PRAD were downloaded and preprocessed using the R package GDCRNATools 1.18.0. Affymetrix microarray datasets were downloaded from GEO/ArrayExpress and normalized with the R package oligo 1.58.0 [9], the processed intensity data were downloaded directly from GEO. Gene/probe names in each dataset were converted to the Ensembl IDs. Besides, the source of processed data for three immunotherapy cohorts, including melanoma and advanced renal carcinoma, can be found in Table S2. These cohorts were utilized for the preliminary validation of the relationship between the MMM score and immunotherapy response.
The copy number variation data were collected from the UCSC (http://xena.ucsc.edu/). The maftools R package 2.13.0 was used to plot the somatic mutation data. Sigle cell analysis of a metastatic castration resistant prostate cancer sample was performed by single cell portal (singlecell.broadinstitute.org).
M6a modified mRNA signature development and verification
239 newly identified m6A-methylated differentially expressed genes were used to develop an m6A-modified mRNA signature score (MMM score) by ridge penalty (Cox-Ridge) in PCaDB platform [9]. Three well established signatures (Klein, Penney and Wu) were used for comparison [[10], [11], [12]]. In these signatures, TCGA-PRAD acted as a training set and the other nine PCa datasets acted as validation sets. Their coefficients were shown in Table S3. The MMM score was calculated by expression profile data and coefficient of the corresponding gene with the formula as follows:
The n is the number of 239 differentially methylated genes in the prognostic prediction model, Coef(i) represents the coefficient, and X(i) means the relative expression level of 239 differentially methylated genes identified by ridge regression in PCaDB platform.
Three metrics were included in signature comparison, hazard ratio (HR) estimated by the Kaplan Meier (KM) analysis, concordance index (C-index) and time-dependent receiver operating characteristics (ROC) curve. R package survival 3.2 was used to KM analyses [9]. R package survivalROC 1.0.3.1 was used to estimate the area under the ROC curves (AUC) [9]. R package survcomp 1.42.0 was used to calculate C-index [9].
Genomics of drug sensitivity in cancer analysis
To further investigate the drug sensitivity correlation of MMM score in PCa, the effects of the MMM score on drug response from the Genomics of Drug Sensitivity in Cancer (GDSC, https://www.cancerrxgene.org/) database. The Spearman correlation between the MMM score and drugs’ IC50 based on the GDSC database in PCa cell lines.
Cell culture, transfection and transwell assays
22RV1 was used for transwell assays. The details of cell culture, transfection and transwell assays could be found in supplemental methods.
Statistical analysis
R (version 4.1.2) and related packages were applied to all statistical analyses. We used the t-test to compare continuous variables between two groups if they fit the normal distribution, and the Wilcoxon test was used to compare two independent non-parametric samples. The Pearson coefficients were used to analyze the correlation between continuous variables. P < 0.05 was regarded as statistically significant.
Results
Identification of differentially expressed genes in PCa by RNA-seq
In our study, four benign group, four primary group and four metastasis group samples were selected for RNA-seq and MeRIP-seq. In brief, through the analysis of mRNA expression profiles, we identified significant differences in gene expression and biological functions among the three groups. The details of these description could be found in the supplemental results (Fig. S1-S2 and Table S4-S7).
The general features of the m6A modification pattern in human benign prostate tissues and prostate carcinoma tissues
Next, we detected the m6A modification pattern in prostate tissues. The benign group, primary group and metastasis group were involved for analysis. Briefly, we found the significant different methylated peaks and biological function among the three group. The details of these description could be found in the supplemental results (Fig. S3-S5 and Table S8-S10).
The different m6A modification patterns between PCa primary and metastatic samples
Specifically, we analyzed the differences in the m6A modification pattern between the primary group and the metastasis group. When compared to the primary group, the metastasis group showed 3165 hypermethylated m6A modification peaks and 1433 hypomethylated m6A modification peaks (|fold change|>2, P<0.05) (Fig. 2A, TableS11). The aberrant m6A peaks representing mRNAs were enriched in hallmarks such as early estrogen response, EMT, TNFa signaling via NF-κB and angiogenesis, which are related to cancer metastasis (Fig. 2B) [[13], [14], [15]]. In addition, the 3164 hypermethylated m6A modification peaks representing mRNAs in the metastasis group were found to be enriched in KEGG pathway terms such as ECM-receptor interaction, protein digestion and absorption, cell adhesion molecules and proteoglycans in cancer (Fig. 2C) and enriched in GO function terms such as cell adhesion and cell–cell adhesion via plasma membrane adhesion molecules (Fig. 2E). The 1433 hypomethylated m6A modification peaks representing mRNAs were enriched in KEGG pathway terms such as Hippo signaling pathway, HIF-1 signaling pathway, and Rap1 signaling pathway (Fig. 2D) and enriched in GO function terms such as cytosolic ribosome, nuclear-transcribed mRNA catabolic process nonsense-mediated decay, and SRP-dependent cotranslational protein targeting to membrane (Fig. 2F). All enrichment analysis results are in Table S12.
Fig. 2.
The global m6A modification pattern in the prostate carcinoma metastasis group compared to the primary group.
(A) The volcano plot of different m6A peaks between the primary group and the metastasis group (|fold change|>2, P<0.05).
(B) The graph shows the hallmarks that the aberrant m6A modification peaks in the metastasis group compared to the primary group are enriched in.
(C) KEGG pathway enrichment analysis of the genes represented by the hyper-methylated and (D) the hypo-methylated peaks in the metastasis group versus the primary group. (P<0.05).
(E) GO analysis of both the hyper-methylated peaks represented genes and (F) the hypo-methylated peaks represented ones, in the metastasis group compared to the primary group (P<0.05).
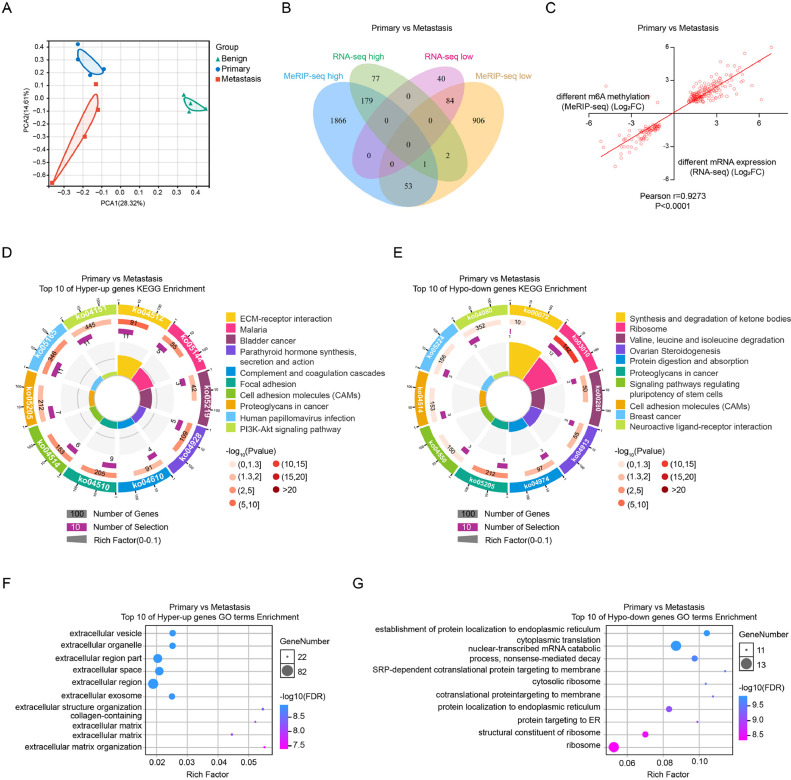
We then combined the MeRIP-seq data and RNA-seq data of the primary group and the metastasis group. First, we performed principal component analysis (PCA) among the benign group, primary group and metastasis group. As shown in Fig. 3A, the distribution among these three groups was significantly different. Similar to the benign group and primary group, the primary group and metastasis group shared 180 genes in the MeRIP-seq high category (fold change>2, P<0.05) and the RNA-seq high category (fold change>2, P<0.05); these genes were included in the ‘hyper up’ category, while 84 genes were downregulated and in the MeRIP-seq low category (fold change<−2, P<0.05) and the RNA-seq low category (fold change<−2, P<0.05); these genes were included in the ‘hypo down’ category (Fig. 3B). Strangely, only three genes were identified as belonging in the ‘hypo up’ category, and none of the genes fit in the ‘hyper down’ category. These results indicate a positive correlation between m6A modification and mRNA transcript levels in the primary group and the metastasis group (Pearson r = 0.9273, P<0.0001) (Fig. 3C).
Fig. 3.
The conjoint analysis of MeRIP-seq and RNA-seq between the primary group and the metastasis group.
(A) The graph of PCA analysis between the benign, primary, and metastasis groups.
(B) The Venn diagram shows the overlap of the differentially methylated genes in MeRIP-seq and the differentially transcribed genes in RNA-seq in the metastatic group, compared to the primary group.
(C) The correlation analysis between the differentially methylated genes in MeRIP-seq and the differentially transcribed genes in RNA-seq in the metastasis group versus the primary group.
(D) KEGG pathway enrichment analysis of the ‘hyper up’ genes and (E) the ‘hypo down’ genes in the metastasis group compared to the primary group (P<0.05).
(F) GO analysis of the ‘hyper up’ genes and (G) the ‘hypo down’ genes in the metastasis group compared to the primary group (P<0.05).
Subsequently, the ‘hyper up’ and ‘hypo down’ genes were subjected to KEGG pathway analysis and GO functional analysis (Table S13). As shown in Fig. 3D, the ‘hyper up’ genes were enriched in cancer pathway terms such as ECM-receptor interaction, proteoglycans in cancer and the PI3K-Akt signaling pathway, while the ‘hypo down’ genes were enriched in terms such as ribosome, protein digestion and absorption (Fig. 3E). For GO functional analysis, the ‘hyper up’ genes were significantly enriched in the terms extracellular vesicle and extraocular organelle (Fig. 3F), and the ‘hypo down’ genes were mostly enriched in the terms establishment of protein localization to ER and cytoplasmic translation (Fig. 3G).
The m6A-modified mRNA score is better than well-established signatures for predicting biochemical recurrence-free survival in PCa patients
The clinical value of m6A-modified mRNA in PCa is still unclear. In this study, we developed an m6A-modified mRNA-based model using the TCGAPRAD dataset, utilizing ridge regression and five-fold cross-validation. In the results mentioned above, a total of 264 differentially methylated genes were identified across the hyper-upregulated and hypo-downregulated groups. After the low abundance genes were filtered out (mean CPM >1), we obtained a 239-molecule signature and named it the m6A-modified mRNA score (the MMM score). We utilized the hazard ratio (HR) calculated by Kaplan–Meier (KM) analysis, the concordance index (C-index), and time-dependent receiver operating characteristics (ROC) curves to evaluate the performance of our signature. Furthermore, we introduced three other proven established signatures—the Klein signature, the Penney signature, and the Wu signature—for comparison. First, we generated a forest plot to demonstrate each signature's prognostic performance in 9 external datasets (Stockholm, DKFZ, Belfast, GSE54460, Cambridge, CPC-Gene, CancerMap, CIT, and Taylor) (Fig. 4A-D). The MMM score showed significantly higher HRs in more datasets (8 out of 9 datasets) than the other three signatures (Klein signature, 7 out of 9; Penney signature, 7 out of 9; Wu signature, 6 out of 9). Second, the MMM score outranked the Klein signature and Wu signature and was similar to the Penney signature in the average C-index (Penney signature, 0.705; MMM Score, 0.701; Klein signature, 0.670; Wu signature, 0.681; Fig. 4E). The detailed C-index profile of each signature in the aforementioned datasets, exhibited in the form of a heatmap, is shown in Fig. 4F. In the area under the ROC curves (AUC) analysis, the MMM score showed similar results to the Penney signature, and the Wu signature and Klein signature had slightly lower scores (Penney, 0.752; MMM score signature, 0.748; Klein signature, 0.710; Wu signature, 0.718; Fig. 4G). We generated a heatmap to demonstrate the AUCs of each signature in the 9 datasets (Fig. 4H). Overall, the MMM score showed outstanding prognostic value.
Fig. 4.
The MMM Score predicts biochemical recurrence-free survival in PCa patients.
(A) The MMM score's prognostic performance (hazard ratio, HR) in 9 external datasets (Stockholm, DKFZ, Belfast, GSE54460, Cambridge, CPC-Gene, CancerMap, CIT, and Taylor).
(B) The Klein Signature's prognostic performance (hazard ratio, HR) in 9 external datasets.
(C) The Penney Signature's prognostic performance (hazard ratio, HR) in 9 external datasets.
(D) The Wu Signature's prognostic performance (hazard ratio, HR) in 9 external datasets.
(E) The boxplot shows the pooled C-Indexes of the MMM Score, Klein Signature, Penney Signature, and Wu Signature.
(F) The heatmap demonstrates the independent C-Indexes of each signature in the 9 datasets.
(G) The boxplot shows the pooled AUCs of MMM Score, Klein Signature, Penney Signature, and Wu Signature.
(H) The heatmap demonstrates the independent C-Indexes of each signature in the 9 datasets.
Next, we inspected the 239 selected genes at the genomic level. Overall, 31.11% (154 out of 495) of the PCa patients had mutations of these genes, and we have presented the top 20 frequently mutated genes among the 239 differentially methylated genes, which include our signature, in Figure S6A. Each mutated gene corresponds to its own-colored bar on the right side of the diagram. The specific bar shows the proportion of each mutation type in a specific gene, and the most prominent type was missense mutation (the green parts in the bars). Figure S6B shows the top 30 genes by copy number variation (CNV) frequency. Each gene has a particular line with two colored endpoints; the red endpoint represents an increase in CNV, while the blue endpoint represents a decrease in CNV. We subsequently separated the TCGA-PRAD samples into two subgroups—the high MMM score group and the low MMM score group—based on the median MMM score. Then, we investigated the 239 selected genes in the two groups and displayed the top 30 mutated genes in Fig. S6C. A total of 74.35% of the samples in the high MMM score group exhibited mutations of the genes mentioned above, and the high MMM score group mutation rate was higher than that of the low MMM score group (56.71%). To explore the significance of the MMM score, we focused on the differentially expressed genes between the high MMM score group and the low MMM score group. We assessed the upregulated and downregulated differentially expressed genes with KEGG pathway and GO analysis, the top enriched terms for the two categories are shown in Figs. S6D-G (Table S14).
Validating the role of the mmm score in stratifying patients based on PAM50 subtype and response to ARSI and chemotherapy
To better evaluate the MMM score's clinical value, we assessed the PAM50 subtype of the PCa samples. PAM50 is a molecular subtyping method, and we used this method because it has general utility in predicting prognosis and therapy response [16]. We retained three subtypes of PCa—luminal A, luminal B, and basal—for further analysis. The proportions of patients with each of the three PAM50 subtypes in each MMM score subgroup are shown in Fig. 5A. We also listed the sensitive treatments for each PAM50 subtype. Luminal A patients are sensitive to gemcitabine and androgen receptor signaling inhibitors (ARSIs), but luminal B patients are sensitive to androgen deprivation therapy (ADT) and ARSIs. Individuals of the basal subtype appear to be sensitive to docetaxel. We further detected a difference in the MMM score of the three subtypes (Fig. 5B). The luminal B patients had the highest score, while those in the luminal A subgroup scored the lowest. Then, we generated KM curves and ROC curves for the high and low MMM score subgroups for each of the three subtypes in the TCGA-PRAD cohort (Fig. 5C–F). The MMM score could accurately predict biochemical recurrence (BCR) in each molecular subtype. Regardless of the subtype, patients in the high MMM score group had a lower BCR-free rate than those in the low MMM score group. The AUCs of the MMM score in the three PAM50 subtypes exceeded 0.7 (basal: 0.74, luminal A: 0.81, luminal B: 0.82, luminal combined: 0.83), demonstrating that the MMM score performed well.
Fig. 5.
The MMM Score predicts the prognosis for PCa PAM50 subtypes and responses to ARSI and chemotherapy.
(A) The doughnut chart shows the proportions of patients with each of the three PAM50 subtypes in each MMM score subgroup. The outer doughnut with the red frame represents the high-MMM score group, and the inner one with the blue frame represents the low-MMM score group.
(B) The violin plot demonstrates a difference in the MMM scores of the three PAM50 subtypes.
(C-F) The KM plots and ROC curves illustrate the MMM score's performance in prognosis prediction in each PAM50 subtype.
(G) The KM plots curves inspect the MMM score's role in the response to ARSI therapy (overall survival, OS; progression-free survival, PFS).
(H) The diagram shows the correlation between the MMM score and the response to other drugs by using the Genomics of Drug Sensitivity in Cancer (GDSC) analysis. The horizontal axis represents the correlation coefficient, and the bar with gradient colors on the top left indicates the IC50 effect.
Furthermore, we employed an ARSI treatment cohort to observe the role of the MMM score in predicting the response to ARSI therapy (Fig. 5G). Consequently, patients in the low MMM score group presented a more favorable overall survival (OS) and progression-free survival (PFS) than those in the high MMM score group. Notably, the low MMM score group had a median OS time of 28 months, more than two times that of the high MMM score group (12 months). Likewise, the median PFS time of the high MMM score group was nearly one-third of that of the low MMM score group (4 months vs. 11 months). In addition to the ability of the score to predict ARSI response, we also investigated the correlation between the MMM score and the response to other drugs by using Genomics of Drug Sensitivity in Cancer (GDSC) analysis. The MMM score was positively correlated with resistance to ruxolitinib in six PCa cell lines (Fig. 5H). Overall, the MMM score is related to response to ARSIs and chemotherapy.
Validating the role of the MMM score in stratifying patients based on immune subtype and response to immunotherapies
In addition to our experiments based on PAM50 subtype, we also tested the utility of the MMM score in stratifying patients based on another molecular subtyping method, TME characteristics, and immune subtype. The TME can be classified into four subtypes—desert (D), fibrotic (F), immune-enriched and nonfibrotic (IE), and immune-enriched and fibrotic (IE/F)—and this classification to categorize PCa patients to predict which patients are likely to have a better prognosis [17]. The population structure in each MMM score subgroup is displayed in Fig. 6A. After that, the difference in the MMM score between the four TME subtypes is shown in Fig. 6B. Notably, the IE subtype had a significantly higher MMM score, while the difference in MMM score between the other three subgroups was not significant. Both KM curve and ROC curve analyses of each TME subtype showed that the MMM score was useful and accurate in predicting PCa patient BCR (Fig. 6C-F). In each immune subtype, the median BCR-free duration (months) in the low MMM score group was at least two times that in the high MMM score group. In line with this, the AUCs in all four groups were over 0.7 (D, 0.85; F, 0.82; IE, 0.73; IE/F, 0.84). We tested the MMM score in three other cohorts [[18], [19], [20]] (a clear cell renal cell carcinoma (ccRCC) cohort and two melanoma cohort) to further understand its role of in predicting immunotherapy response. An anti-PD-1 agent was administered to patients in the ccRCC cohort, and we divided patients into high and low MMM score groups to determine whether there was a difference in prognosis between the groups (Fig. 6G-H). The median OS time of the low MMM score group was almost twofold higher than that of the high MMM score group (36.20 months vs. 19.13 months, p = 0.008). The melanoma cohort consisted of two populations: one that had received CTLA-4 therapy and another that had received adoptive T-cell therapy (Fig. 6I–J). Although the OS difference between the two groups in the adoptive T-cell therapy population was statistically insignificant, there was a clinically significant difference in OS between the two groups in both subpopulations. In the CTLA4 population, the median OS time of the low MMM score group was six times that of the high MMM score group (32.84 months vs. 5.49 months, p < 0.01). On the other hand, in the adoptive T-cell therapy population, there was a 3-fold difference between the two MMM score groups (12.13 months in the high MMM score group vs. 35.13 months in the low MMM score group, p = 0.21). The MMM score demonstrated undeniable value in analyses of real clinical data from patients receiving immunotherapy.
Fig. 6.
The MMM score predicts the prognosis for PCa TME subtypes and responses to immunotherapies in several cohorts.
(A) The doughnut chart shows the proportions of patients with each of the three TME subtypes in each MMM score subgroup. The outer doughnut with the red frame represents the high-MMM score group, and the inner one with the blue frame represents the low-MMM score group.
(B) The violin plot displays a difference in the MMM scores of the four TME subtypes.
(C-F) The KM plots and ROC curves present the MMM score's survival prediction performance in each TME subtype.
(G-H) The KM plots investigate the MMM score's role in the response to anti-PD-1 immunotherapy (overall survival, OS; progression-free survival, PFS).
(I) The KM plots investigate the MMM score's role in the response to CTLA4 immunotherapy.
(J) The KM plots investigate the MMM Score's role in the response to adoptive T cell immunotherapy.
Experimental verification of the hub genes considered by the MMM score
To validate the hub genes considered in the MMM score, we ranked the 239 genes by the HR of the univariate Cox regression analysis of BCR. The top ten genes with HR>1 and limited relevant published studies in PubMed were selected (Table S15). These top ten genes were subjected to high-throughput screening transwell assays in which the genes were knocked down. As shown in the results, only those cells with downregulation of TLE1, PFKL, TTYH3 and AGRN had attenuated penetration compared to the respective control cells, which indicated that these four candidates might participate in the metastasis of PCa (Fig. 7A-B). To further verify our hypothesis, we first tested the transfection efficiency of the designed siRNAs targeting the indicated genes to select the most effective siRNA for further validation experiments (Fig. 7C). Based on our transwell results, decreased expression of TLE1 and PFKL significantly reduced cell invasion, while knockdown of TTYH3 and AGRN did not (Fig. 7D). Of note, TLE1 and PFKL expression were closely related to m6A modification in our MMM score analyses. Thus, we carried out a MeRIP assay, which showed that both TLE1 and PFKL mRNA could be significantly enriched by an anti-m6A antibody, demonstrating that TLE1 and PFKL were directly modified by m6A (Fig. 7E). Subsequently, we re-examined the MeRIP-seq data of our PCa samples. The m6A peaks of TLE1 and PFKL were visualized in UCSC Genome Browser (Fig. 7F). TLE1 and PFKL were both found to be highly expressed in plasmablasts in advanced PCa at the single-cell level (Fig. 7G).
Fig. 7.
The high content hub genes screening and verification in PCa.
(A) Transwell assay with Calcein AM cell staining was used to screen for high content metastasis-related genes among the top ten genes in MMR score.
(B) Analytical results of the high-content screening assay are displayed above. The blue bar represents the total cell number of every indicated well. The orange bar represents the difference in cell numbers between the target group and the control group.
(C) Verification of the transfection efficiency of siRNAs targeting TLE1, PFKL, TTYH3 and AGRN.
(D) Transwell experiments with 0.1 percent crystal violet were used to test the metastatic efficacy of siRNAs transfected into PCa cells. Each experiment was repeated three times in a row.
(E) TLE1 and PFKL enrichment in the MeRIP assay was confirmed using qPCR and agarose gel electrophoresis.
(F) The genome browser exhibits TLE1 and PFKL's MeRIP-Seq peaks in our PCa samples.
(G) TLE1 and PFKL expression levels were found in single-cell transcriptomes from 14 advanced prostate cancer patients.
Discussion
It is becoming increasingly evident that nonmutational epigenetic reprogramming regulates cancer vulnerabilities [21]. As the most abundant chemical modification in eukaryotic mRNA, the role of m6A-modified mRNAs in PCa is largely unclear. This study is the first to determine the m6A transcriptome-wide profile in BPH, PCa primary tumor and PCa metastatic samples.
We found that significant differences in m6A modification status among the benign, primary, and metastasis groups. The primary group exhibited fewer m6A modification peaks compared to the benign group, suggesting that most genes were hypomethylated in the primary group. This finding indicates that m6A hypomethylation might contribute to PCa initiation. Conversely, the metastasis group displayed more m6A modification peaks than the primary group, implying that a majority of genes were hypermethylated in the metastasis group. This observation reveals that hypermethylation might influence PCa progression and metastasis. In summary, genes were predominantly hypermethylated in the benign and metastasis groups, whereas hypomethylation was more common in the primary group. By considering our MeRIP-seq and mRNA-seq data together, we found a positive association between the benign group and the primary group and the primary group and the metastasis group. Moreover, some cancer-related pathways were found to be enriched in the functional analyses. These results indicate that m6A-modified mRNAs might play a critical role in PCa metastasis.
To determine the clinical value of these specific m6A-modified mRNAs, we used them to build a scoring system via Cox-Ridge regression to predict BCR in PCa patients. According to our previous study, Cox-Ridge regression commonly shows better performance than other algorithms in signature construction [9]. As such, the TCGA-PRAD dataset was used as a training set to develop the MMM scoring system. Experimentally generated prognostic signatures are not clinically useful without numerous external validations. Therefore, we tested the scoring system in nine external multicenter cohorts, and a high MMM score indicated faster biochemical recurrence. More importantly, the predictive ability of the MMM score was better than that of three other established signatures, which were tested in our earlier report [9]. These results indicate the powerful prognosis-predicting ability of the MMM score.
In addition to radical prostatectomy (RP), ADT, ARSIs, chemotherapy and immunotherapy are vital treatment options for PCa. Moreover, the MMM score can predict the clinical response to these therapies. The PAM50 classification scheme in PCa has been proven to be related to sensitivity to ADT, gemcitabine, docetaxel and ARSIs [16]. In the PAM50 subgroup analysis, the higher MMM score group always had the worst clinical outcome among the three subtypes. This finding suggests that we should perform more active intervention in patients with higher MMM scores regardless of PAM50 subtype with therapies to which they are more sensitive. Furthermore, we verified the utility of the MMM score in an ARSI cohort. The results revealed that the high MMM score group may not be sensitive to ARSIs given the OS and PFS results.
As noted in some previous articles, PCa is a “cold tumor” [17]. It is not surprising that approximately 40% of patients have desert subtype disease according to the TME classification proposed for the TCGA-PRAD cohort. Interestingly, the ability of the MMM score to predict prognosis remained in the D, IE, F and IE/F immune subgroups. However, there are no public datasets with follow-up information for PCa immunotherapy. In addition, we verified the MMM score in some immunotherapy ccRCC and melanoma cohorts. In the anti-PD-1, CTLA-4 and adoptive T-cell therapy cohorts, the lower MMM score group may have a better response in terms of OS and/or PFS. In general, the above analysis results may promote the clinical application of the MMM score in PCa according to its ability to predict the prognosis of distinct molecular subtypes and guide precision therapies.
To explore the biological relevance of the MMM score, we assessed the hub genes in this signature with laboratory experiments. Our results showed that two of the hub genes, TLE1 and PFKL, not only had in silico data supporting their oncogene status but also promoted cell invasion, and their m6A sites was assessed in vitro. TLE1 is a member of the TLE family that acts as a transcriptional corepressor. Diseases associated with TLE1 include hematological malignancies, glomus tumor, breast cancer, synovial sarcoma and gastric carcinoma [22]. TLE1 modulation in cancers is linked to the NF-kB and Wnt/beta-catenin signaling pathways [23]. However, the mechanism of TLE1 is unknown due to the limited number of studies. TLE-1 may also have potential roles as a counterregulator of inflammation in various inflammatory diseases [24]. On the other hand, PFKL is a phosphohexokinase that acts on a key step in glycolysis. Its tumor-promoting effect via metabolic reprogramming in breast cancer, neuroblastoma and glioma has been identified [25]. What's more, PFKL has potential clinical implications as a therapeutic target for diseases characterized by excessive NETosis and inflammation, and small-molecule compounds that activate PFKL and inhibit NOX2-dependent oxidative burst, such as LDC7559 and NA-11, have shown promise as potential treatments for such diseases [26,27]. Coincidentally, this study reported the m6A characteristics of TLE1 and PFKL in PCa for the first time.
There were several shortcomings in our study. First, we did not verify the signature in our follow-up data because our samples are still incomplete. Second, we could not test all 239 genes due to limited time and research funding.
Conclusions
The MMM score, a novel m6A-modified mRNA-based scoring system, has potential clinical value in many aspects and provides new insights into precision medicine in PCa.
CRediT authorship contribution statement
Jianming Lu: Conceptualization, Visualization, Resources, Formal analysis. Jiahong Chen: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. Zhuoyuan Lin: Project administration, Software, Writing – original draft, Writing – review & editing. Qinwei Liu: Formal analysis, Writing – original draft, Writing – review & editing. Chuanfan Zhong: Project administration, Software, Writing – original draft, Writing – review & editing. Zhouda Cai: Resources, Writing – original draft, Writing – review & editing. Zhenyu Jia: Project administration, Software, Writing – original draft, Writing – review & editing. Weide Zhong: Project administration, Software, Writing – original draft, Writing – review & editing. Yingke Liang: Formal analysis, Writing – original draft, Writing – review & editing. Chao Cai: Conceptualization, Visualization, Resources, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Ethics approval and consent to participate
The Ethics Committee of Guangzhou Medical University's First Affiliated Hospital authorized the current study's experimental procedures. Each participant signed an informed consent form for the sample collection and application.
Consent for publication
Not applicable.
Availability of data and material
Data are available on reasonable request.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No.82073294,82003271,82103358,82072813,81802555). The Science and Technology Development Fund (FDCT) of Macau SAR (0031/2021/A). Education Department of Guangdong Province (2020KTSCX102). Natural Science Foundation of Guangdong Province (2022A1515010344). The Guangzhou Planned Project of Science and Technology (202102010021, 202102010152).
Acknowledgments
Not applicable.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101670.
Appendix. Supplementary materials
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J. Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. 2021. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Research Program NCI. SEER*explorer: an interactive website for SEER cancer statistics [Internet]. 2021 [Available from: https://seer.cancer.gov/explorer/.
- 3.National Cancer Institute D, surveillance research program, surveillance systems branch. Surveillance, epidemiology, and end results (SEER) program SEER*stat database: incidence – SEER 18 regs research data 2020 [updated April 2021. Available from: https://seer.cancer.gov/canques/survival.html.
- 4.Cornford P., van den Bergh R.C.N., Briers E., Van den Broeck T., Cumberbatch M.G., De Santis M., et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate Cancer. Eur. Urol. 2021;79(2):263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 5.Fu Y., Dominissini D., Rechavi G., He C. Gene expression regulation mediated through reversible m⁶A RNA methylation. Nat. Rev. Genet. 2014;15(5):293–306. doi: 10.1038/nrg3724. [DOI] [PubMed] [Google Scholar]
- 6.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169(7):1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boccaletto P., Machnicka M.A., Purta E., Piatkowski P., Baginski B., Wirecki T.K., et al. MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018;46(D1):D303–D307. doi: 10.1093/nar/gkx1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang H., Weng H., Chen J. mA modification in coding and non-coding RNAs: roles and therapeutic implications in Cancer. Cancer Cell. 2020;37(3):270–288. doi: 10.1016/j.ccell.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R., Zhu J., Zhong W.D., Jia Z. Comprehensive evaluation of machine learning models and gene expression signatures for prostate cancer prognosis using large population cohorts. Cancer Res. 2022;82(9):1832–1843. doi: 10.1158/0008-5472.CAN-21-3074. [DOI] [PubMed] [Google Scholar]
- 10.Klein E.A., Cooperberg M.R., Magi-Galluzzi C., Simko J.P., Falzarano S.M., Maddala T., et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur. Urol. 2014;66(3):550–560. doi: 10.1016/j.eururo.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Penney K.L., Sinnott J.A., Fall K., Pawitan Y., Hoshida Y., Kraft P., et al. mRNA expression signature of Gleason grade predicts lethal prostate cancer. J. Clin. Oncol. 2011;29(17):2391–2396. doi: 10.1200/JCO.2010.32.6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C.L., Schroeder B.E., Ma X.J., Cutie C.J., Wu S., Salunga R., et al. Development and validation of a 32-gene prognostic index for prostate cancer progression. Proc. Natl. Acad. Sci. U. S. A. 2013;110(15):6121–6126. doi: 10.1073/pnas.1215870110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellón E.A., Indo S., Contreras H.R. Cancer stemness/epithelial-mesenchymal transition axis influences metastasis and castration resistance in prostate Cancer: potential therapeutic target. Int. J. Mol. Sci. 2022;23(23) doi: 10.3390/ijms232314917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawler J. Counter regulation of tumor angiogenesis by vascular endothelial growth factor and thrombospondin-1. Semin. Cancer Biol. 2022;86(Pt 2):126–135. doi: 10.1016/j.semcancer.2022.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Mirzaei S., Saghari S., Bassiri F., Raesi R., Zarrabi A., Hushmandi K., et al. NF-κB as a regulator of cancer metastasis and therapy response: a focus on epithelial-mesenchymal transition. J. Cell. Physiol. 2022;237(7):2770–2795. doi: 10.1002/jcp.30759. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S.G., Chang S.L., Erho N., Yu M., Lehrer J., Alshalalfa M., et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017;3(12):1663–1672. doi: 10.1001/jamaoncol.2017.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagaev A., Kotlov N., Nomie K., Svekolkin V., Gafurov A., Isaeva O., et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39(6):845–865. doi: 10.1016/j.ccell.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Braun D.A., Hou Y., Bakouny Z., Ficial M., Sant' Angelo M., Forman J., et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat. Med. 2020;26(6):909–918. doi: 10.1038/s41591-020-0839-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder A., Makarov V., Merghoub T., Yuan J., Zaretsky J.M., Desrichard A., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lauss M., Donia M., Harbst K., Andersen R., Mitra S., Rosengren F., et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat. Commun. 2017;8(1):1738. doi: 10.1038/s41467-017-01460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D. Hallmarks of Cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 22.Aref S., El-Ghonemy M.S., Atia D.M., Elbaiomy M.M., Abdelsalam S.A., Tawfik A., et al. Prognostic Value of TLE1 Gene Expression in Patients with T-cell Acute Lymphoblastic Leukemia. Asian Pac. J. Cancer Prev. 2021;22(5):1653–1658. doi: 10.31557/APJCP.2021.22.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madakshira M.G., Radotra B.D., Kaman L., Saikia U.N. Expression of TLE1, INI1, β-catenin, Claudin1, CK7, CK19, SS18 and calponin in synovial sarcoma. Indian J. Pathol. Microbiol. 2021;64(4):707–716. doi: 10.4103/IJPM.IJPM_1137_20. [DOI] [PubMed] [Google Scholar]
- 24.Ramasamy S., Saez B., Mukhopadhyay S., Ding D., Ahmed A.M., Chen X., et al. Tle1 tumor suppressor negatively regulates inflammation in vivo and modulates NF-κB inflammatory pathway. Proc. Natl. Acad. Sci. U. S. A. 2016;113(7):1871–1876. doi: 10.1073/pnas.1511380113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li K., Zhu X., Yuan C. Inhibition of miR-185-3p confers erlotinib resistance through upregulation of PFKL/MET in Lung Cancers. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.677860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amara N., Cooper M.P., Voronkova M.A., Webb B.A., Lynch E.M., Kollman J.M., et al. Selective activation of PFKL suppresses the phagocytic oxidative burst. Cell. 2021;184(17):4480–4494. doi: 10.1016/j.cell.2021.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Z., Huang D., Tang C., Zeng M., Hu X. PFKL, a novel regulatory node for NOX2-dependent oxidative burst and NETosis. J. Zhejiang Univ. Sci. B. 2022;23(7):607–612. doi: 10.1631/jzus.B2101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request.