Abstract
Primary monophasic synovial sarcoma of the lung is an extremely rare malignant mesenchymal tumor that can develop at any anatomic site. Synovial sarcoma is considered a high grade tumor with a poor prognosis. Metastatic pulmonary sarcoma is much more common. Hence primary lesion elsewhere in the body needs exclusion. No clinical or radiological features are specific for pulmonary sarcoma, often it is confused with bronchogenic carcinoma. Therefore biopsy is needed to establish the diagnosis of this rare tumor. We hereby present two cases of histologically proven primary monophasic synovial sarcoma of lung. The imaging features of this rare disease is reviewed.
Keywords: Diagnostic Radiology, Pulmonary oncology, Monophasic synovial sarcoma, Chest imaging, Malignant mesenchymal tumor, Pulmoary synovial sarcoma
Introduction
Primary synovial sarcoma of lung is extremely rare tumor. Primary synovial sarcoma commonly originates from periarticular soft tissues, however origin of this tumor from variety of other locations are also mentioned [1]. In recent literature, lung and pleura has been reported as origin sites for synovial sarcoma [2,3]. It accounts for less than 0.5% of all lung tumors [4]. Metastases in lung from extra pulmonary synovial sarcoma is far more common than primary pulmonary synovial sarcoma. Our patients were thoroughly evaluated for any primary malignancy elsewhere in the body, however there were no such evidence. Very few case reports have been published in the literature describing the imaging features of this rare entity.
Case report
Case 1: A 27 years old young male presented to our institution for the evaluation of lung mass detected on chest radiography for assessment of difficulty in breathing and cough. The patient at first complained of dry cough with tightness of chest, for which chest radiograph was advised. Patient was treated symptomatically but the symptoms were gradually progressive. There was no associated fever or hemoptysis. Patient gave no history of smoking or radiation or chemical exposures. His vitals were stable, however patient appeared debilitated at the time of presentation at our institute. Sputum examination for tuberculosis was negative. As the clinical condition of the patient was not improving, patient was then further evaluated for the suspected lung mass. High resolution Computed tomography and CT guided biopsy was performed. Blood tumor markers were AFP- 7.6 IU/mL (reference-1.31-7.89 ng/mL), Beta HCG- 0.9 0 mIU/mL (reference- 0.1-5 mIU/mL), CA19.9-6.20 U/mL (reference-1.98-25.12 U/mL), and CEA- 0.32 ng/mL (reference-0.51-4.86 ng/mL).
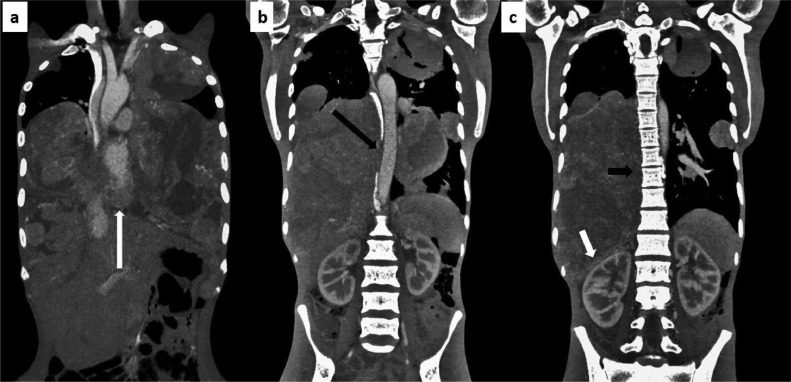
Computed tomography of thorax showed few well defined heterogeneously enhancing masses nearly occupying whole of bilateral hemithorax. The masses were showing heterogeneously enhancing soft tissue component with intermixed areas of necrosis. No evidence of calcification were seen within the masses. The masses were displacing the heart and great vessels anteriorly with stretching of left pulmonary vein. Right pulmonary vein was not visualized. Trachea was displaced towards right side with severe compression over esophagus. The largest mass on the right side was measuring ∼ 110 × 100 × 170 mm predominantly involving the right middle and lower lobes with partial collapse of right upper lobe. The mass was indenting on the right hemi diaphragm, displacing the liver inferiorly. A similar mass measuring ∼ 44 × 40 × 35 mm was seen in apical segment of right upper lobe. The largest mass on the left measures 110 × 90 × 140 mm and was predominantly involving anteromedial and lateral segment of left lower lobe. Two similar other masses were seen measuring ∼ 65 × 53 × 45 mm (in the left upper lobe) and 30 × 31 × 32 mm (in the lateral basal segment of left lower lobe). Pleural effusion or mediastinal lymphadenopathy were not evident. (Figs. 1, 2 and 3)
Fig. 1.
Primary monophasic synovial sarcoma of lung (A,B) NCCT and (C)lung window axial images show soft tissue attenuation mass lesions in bilateral hemithorax, without any calcification or air bronchogram within the masses.
Fig. 2.
Primary monophasic synovial sarcoma of lung (A, B) axial and (C, D) sagittal contrast enhanced CT show few heterogeneously enhancing masses with areas of necrosis in bilateral hemithorax, with indistinct plane with heart (thin white arrow), abutting the main pulmonary artery (black long arrow) and displacing the abdominal aorta anteriorly (white long arrow), indenting the liver inferiorly (white short arrow).
Fig. 3.
Primary monophasic synovial sarcoma of lung, coronal: (A) heterogeneously enhancing masses with indistinct planes with the heart (long white arrow), (B) the mass is abutting the descending thoracic aorta (long black arrow), (C) it is inferiorly indenting the right kidney (short white arrow) and abutting the vertebral bodies with no obvious bony erosion seen (short black arrow).
Microscopically, tumor showed sheets and fascicles of fairly uniform spindle shaped cells. These cell had vesicular plump to ovoid nuclei with dispersed chromatin, inconspicuous nucleoli and moderate amount of pale cytoplasm set in delicate edematous stroma. Few mitoses were also evident. On Immunohistochemistry TLE-1, CD56, CD99 and Bcl2 were positive. Pan CK, NKX-2.2 and EMA were negative. Ki-67 proliferation index was 10 % (Fig. 4).
Fig. 5.
Primary monophasic synovial sarcoma of lung, lung window (coronal, sagittal and axial): an irregular soft tissue attenuation mass in right lung with surrounding ground glass opacity, no air bronchogram seen within the lesion.
Case 2: A 35-year old female, non-smoker presented with complaints of shortness of breath, right sided chest pain and cough with expectoration for 4 months. Chest pain was gradually progressing. She also gave a history of 1 episode of blood streaks in sputum. She had associated complaints of loss of appetite and weight loss. Patient gave no history of smoking or radiation or chemical exposures Sputum examination for tuberculosis was negative. Patient was advised high resolution computed tomography and CT guided biopsy for the suspected lung mass. Blood tumor markers were AFP- 5.9 IU/mL (reference-1.01-7.1 ng/mL), CA19.9-20.05 U/mL (reference-2.36-29.29 U/mL), and CEA- 0.52 ng/mL (reference-0.35-3.45 ng/mL).
Contrast enhanced computed tomography showed an enhancing mass measuring ∼51 × 52 × 25mm in middle lobe of right lung with cut off of bronchus and surrounding ground glass opacity. It was abutting adjacent costal pleura with no evidence of bony erosion. It was extending upto the right hilum. No evidence of any intratumoral calcification, lymphadenopathy or pleural effusion seen. (Fig. 6, Fig. 7 and 6)
Fig. 6.
Primary monophasic synovial sarcoma of lung, contrast enhanced CT: (A) coronal image showing an irregular heterogeneously enhancing mass in right lung, abutting right hilum and involving the adjacent costal pleura (white arrows) with focal eventration (yellow asterisk).
Fig. 7.
Primary monophasic synovial sarcoma of lung; (B, C) FDG PET scan 3D tracer uptake noted in right lung mass. (Thin long and thick short black arrows) and (A, D) fusion image showing uptake in right lung mass (thin long and thick short white arrows).
PET-CT showed heterogeneously increased FDG uptake [SUV max 8.09] in the mass measuring ∼ 39 × 36 × 20 mm. Distant metastases or any primary lesion elsewhere in the body were absent (Fig. 7).
Fig. 4.
Primary monophasic synovial sarcoma of lung:(A- 20x, B-40x) Hematoxylin & Eosin stain shows fairly uniform spindle shaped cells with dispersed chromatin, inconspicuous nucleoli and moderate amount of pale cytoplasm. Few mitoses are also evident, (C) Pan cytokeratin negativity (D) TLE-1 positivity (E) Bcl2 positivity (F) vimentin positivity [20x].
Microscopically, tumor showed sheets and fascicles of fairly uniform spindle shaped cells with dispersed chromatin, inconspicuous nucleoli and moderate amount of pale cytoplasm. No necrosis were seen. On Immunohistochemistry TLE-1, and Bcl2 were positive. EMA was negative. Ki-67 proliferation index was 12 % (Fig. 8).
Fig. 8.
Primary monophasic synovial sarcoma of lung:(A-20x, B-40x) Hematoxylin & Eosin stain shows spindle shaped cells with inconspicuous nucleoli and moderate amount of pale cytoplasm, (C) TLE-1 positivity (D) Bcl2 positivity (E) EMA negativity [20x].
Discussion
Synovial sarcoma is a malignant tumor which accounts nearly 10% of all primary soft tissue tumors. Periarticular soft tissues of the extremities are mostly affected [5]. It originates from pluripotent mesenchymal cells, and is potential of aberrant epithelial differentiation, [2,6,7]. This theory has been supported by various studies suggesting other locations of origin including esophagus, head and neck, mediastinum and heart [8]. Few cases of primary pleura and pulmonary synovial sarcoma have been reported in newer literatures [2,3]. In recent times the cases are on rise due to increasing ability of diagnosing the tumor by histochemical and cytogenetics techniques [9]. Leiomyosarcoma, malignant fibrous histiocytoma, fibrosarcoma are the most common histologic types described and now synovial sarcoma [10]. It was first reported by Zeren et al [2] in 1995. Few cases were published in the literature, however not many have described the radiologic imaging features of the tumor [10], [11], [12], [13], [14]. Frazier et al has detailed the imaging features of primary synovial sarcoma in recent study. He reported as radio-opacity of the affected hemi thorax with ipsilateral pleural effusion on chest radiographs. At CT, it appeared as a well-defined, heterogeneously enhancing mass with areas of necrosis associated with pleural effusion. It was difficult to determine the site of origin between lung and pleura on CT, especially with large lesion [3,11,[15], [16], [17]]. Lymphadenopathy has been rarely reported in primary synovial sarcoma of the lung [11,18]. One case reported peripheral ground glass opacity around the mass, which was also seen in our case [19]. Lung cancer usually present with lymphadenopathy which is less likely seen in primary pulmonary synovial sarcoma. As in our cases, lymphadenopathy was not evident. Synovial sarcoma metastasize to the lung, which is far more common than primary pulmonary synovial sarcoma. Our patient had no primary malignancy elsewhere in the body. The other primary and metastatic lung neoplasms, pleuro-pulmonary blastoma and localized fibrous tumors of lung and pleura are considered as radiological differential diagnosis of primary pulmonary sarcoma [10,11]. Metastatic disease usually does not exhibit as a large solitary pulmonary lesion.
Histologically, Primary pulmonary synovial sarcomas are of 4 subtypes- monophasic epithelial, monophasic fibrous (spindle), biphasic and poorly differentiated. Monophasic is the most common subtype. Spindle cells variant of monophasic subtype is frequently seen in the lung. The biphasic subtype comprises of combination of epithelial and spindle cells [20,21]. Both the cases in our report showed monophasic subtype (spindle cells variant) with TLE-1, Bcl2 and Vimentin positivity. Hemangiopericytoma, leiomyosarcoma, fibrosarcoma, and spindle cell variant of SCC are considered as differential diagnosis of monophasic subtypes. Hence to differentiate it from others immunohistochemistry is needed. On immunohistochemistry, synovial sarcomas are positive for cytokeratin, BCL2, EMA and Vimentin and negative for S100, desmin, and vascular tumor marker [22].
Tumor size and patient age are dominant criteria for prognosis. Other factors includes male gender, tumor necrosis, high grade, increase number of mitotic figures and SSX translocation [23]. The present treatment includes surgical resection followed by adjunctive chemotherapy and radiotherapy however no definite treatment protocol is available due to its rarity [24]. Primary pulmonary synovial sarcoma is a very aggressive tumor indicating poor prognosis with a 5 year survival rate of 50% [25].
Conclusion
Primary pulmonary synovial sarcoma is an extremely rare tumor with poor prognosis. It may mimic imaging features of typical lung malignancy. The diagnosis of primary pulmonary synovial sarcoma requires pathological and immunohistochemically investigations to exclude alternative primary tumors of lung and metastasis.
Patient consent
Written informed consent for the publication of this case report was obtained from the father of the patient in case 1 and from the patient in case 2.
Footnotes
Competing Interests: There is no interest to declare.
References
- 1.Raney RB. Synovial sarcoma in young people: background, prognostic factors, and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207–211. doi: 10.1097/01.mph.0000161764.60798.60. [DOI] [PubMed] [Google Scholar]
- 2.Zeren H, Moran CA, Suster S, Fishback NF, Koss MN. Primary pulmonary sarcomas with features of monophasic synovial sarcoma: a clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum Pathol. 1995;26(5):474–480. doi: 10.1016/0046-8177(95)90242-2. [DOI] [PubMed] [Google Scholar]
- 3.Gaertner E, Zeren EH, Fleming MV, Colby TV, Travis WD. Biphasic synovial sarcomas arising in the pleural cavity. A clinicopathologic study of five cases. Am J Surg Pathol. 1996;20(1):36–45. doi: 10.1097/00000478-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Keel SB, Bacha E, Mark EJ, Nielsen GP, Rosenberg AE. Primary pulmonary sarcoma: A clinicopathologic study of 26 cases. Mod Pathol. 1999;12(12):1124–1131. [PubMed] [Google Scholar]
- 5.Cadman N, Soule E, Kelly PJ. Synovial sarcoma: an analysis of 34 tumors. Cancer. 1965;18:613–627. doi: 10.1002/1097-0142(196505)18:5<613::aid-cncr2820180510>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Dickersin GR. Synovial sarcoma: a review and update, with emphasis on the ultrastructural characterization of the nonglandular component. Ultrastruct Pathol. 1991;15(4-5):379–402. doi: 10.3109/01913129109016247. [DOI] [PubMed] [Google Scholar]
- 7.Fisher C. Synovial sarcoma: ultrastructural and immunohistochemical features of epithelial differentiation in monophasic and biphasic tumors. Hum Pathol. 1986;17(10):996–1008. doi: 10.1016/s0046-8177(86)80083-1. [DOI] [PubMed] [Google Scholar]
- 8.Essary LR, Vargas SO, Fletcher CD. Primary pleuropulmonary synovial sarcoma: reappraisal of a recently described anatomic subset. Cancer. 2002;94(2):459–469. doi: 10.1002/cncr.10188. [DOI] [PubMed] [Google Scholar]
- 9.Bégueret H, Galateau-Salle F, Guillou L, Chetaille B, Brambilla E, Vignaud JM, et al. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol. 2005;29(3):339–346. doi: 10.1097/01.pas.0000147401.95391.9a. [DOI] [PubMed] [Google Scholar]
- 10.Frazier AA, Franks TJ, Pugatch RD, Galvin JR. From the archives of the AFIP: pleuropulmonary synovial sarcoma. Radiographics. 2006;26(3):923–940. doi: 10.1148/rg.263055211. [DOI] [PubMed] [Google Scholar]
- 11.Duran-Mendicuti A, Costello P, Vargas SO. Primary synovial sarcoma of the chest: radiographic and clinicopathologic correlation. J Thorac Imaging. 2003;18(2):87–93. doi: 10.1097/00005382-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Gladish GW, Sabloff BM, Munden RF, Truong MT, Erasmus JJ, Chasen MH. Primary thoracic sarcomas. Radiographics. 2002;22(3):621–637. doi: 10.1148/radiographics.22.3.g02ma17621. [DOI] [PubMed] [Google Scholar]
- 13.Nakajo M, Ohkubo K, Nandate T, Shirahama H, Yanagi M, Anraku M, et al. Primary synovial sarcoma of the sternum: computed tomography and magnetic resonance imaging findings. Radiat Med. 2005;23(3):208–212. [PubMed] [Google Scholar]
- 14.Zamarrón C, Abdulkader I, Alvarez UC, Barón FJ, Prim JM, Ledo RL, et al. Primary synovial sarcoma of the lung. Intern Med. 2006;45(10):679–683. doi: 10.2169/internalmedicine.45.1508. [DOI] [PubMed] [Google Scholar]
- 15.Aubry MC, Bridge JA, Wickert R, Tazelaar HD. Primary monophasic synovial sarcoma of the pleura: five cases confirmed by the presence of SYT-SSX fusion transcript. Am J Surg Pathol. 2001;25(6):776–781. doi: 10.1097/00000478-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Zaring RA, Roepke JE. Pathologic quiz case. Pulmonary mass in a patient presenting with a hemothorax. Diagnosis: primary pulmonary biphasic synovial sarcoma. Arch Pathol Lab Med. 1999;123(12):1287–1289. doi: 10.5858/1999-123-977b-MBPACA. [DOI] [PubMed] [Google Scholar]
- 17.Eichelberger L, Jones TD, Vance G, Saxena R. A 37-year-old man with a pleural mass. Arch Pathol Lab Med. 2005;129(8):1063–1064. doi: 10.5858/2005-129-1063-AYMWAP. [DOI] [PubMed] [Google Scholar]
- 18.Roberts CA, Seemayer TA, Neff JR, Alonso A, Nelson M, Bridge JA. Translocation (X;18) in primary synovial sarcoma of the lung. Cancer Genet Cytogenet. 1996;88(1):49–52. doi: 10.1016/0165-4608(95)00298-7. [DOI] [PubMed] [Google Scholar]
- 19.Mikami Y, Nakajima M, Hashimoto H, Kuwabara K, Sasao Y, Manabe T. Primary poorly differentiated mono phasic synovial sarcoma of the lung. A case report with immunohistochemical and genetic studies. Pathol Res Pract. 2003;199(12):827–833. doi: 10.1078/0344-0338-00502. [DOI] [PubMed] [Google Scholar]
- 20.Weiss S, Goldblum J. 4th ed. Mosby; St. Louis, MO: 2001. Enzinger and Weiss's soft tissue tumors; pp. 1502–1504. [Google Scholar]
- 21.Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M. SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med. 1998;338:153–160. doi: 10.1056/NEJM199801153380303. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson G, Skytting B, Xie Y, Brodin B, Perfekt R, Mandahl N, et al. The SYT-SSX1 variant of synovial sarcoma is associated with a high rate of tumor cell proliferation and poor clinical outcome. Cancer Res. 1999;59:3180–3184. [PubMed] [Google Scholar]
- 23.Trassard M, Le Doussal V, Hacène K, Terrier P, Ranchère D, Guillou L, et al. Prognostic factors in localized primary synovial sarcoma: A multicenter study of 128 adult patients. J Clin Oncol. 2001;19:525–534. doi: 10.1200/JCO.2001.19.2.525. [DOI] [PubMed] [Google Scholar]
- 24.Treglia G, Caldarella C, Taralli S. A rare case of primary pulmonary synovial sarcoma in a pediatric patient evaluated by (18) F-FDG PET/CT. Clin Nucl Med. 2014;39:e166–e168. doi: 10.1097/RLU.0b013e318286bade. [DOI] [PubMed] [Google Scholar]
- 25.Bunch K, Deering SH. Primary pulmonary synovial sarcoma in pregnancy. Case Rep Obstet Gynecol. 2012;2012 doi: 10.1155/2012/326031. [DOI] [PMC free article] [PubMed] [Google Scholar]