Abstract
The past 50 years have witnessed a massive expansion in the demand and application of pesticides. However, pesticides are difficult to be completely degraded without intervention hence the pesticide residue could pose a persistent threat to non-target organisms in many aspects. To aim at the problem of the abuse of pesticide products and excessive pesticide residues in the environment, chemical and biological degradation methods are widely developed but are scaled and insufficient to solve such a pollution. In recent years, bio-degradative tools instructed by synthetic biological principles have been further studied and have paved a way for pesticide degradation. Combining the customized design strategy and standardized assembly mode, the engineering bacteria for multi-dimensional degradation has become an effective tool for pesticide residue degradation. This review introduces the mechanisms and hazards of different pesticides, summarizes the methods applied in the degradation of pesticide residues, and discusses the advantages, applications, and prospects of synthetic biology in degrading pesticide residues.
Keywords: Synthetic biology, Degradation, Remediation, Biological engineering, Pesticide residue
1. Introduction
Since the middle of the 20th century, the global production in the traditional forms of agriculture has been inadequate to satisfy the escalation of the human population on the earth, which resulted in the issue that pesticides are widely used to achieve higher output and meet the growing food demand. According to the Food and Agriculture Organization of the United Nations (FAO), pesticide use increased by 36% from 2000 to 2019 [1]. However, in most situations, the main ingredients of pesticides are toxic to the fragile ecology [[2], [3], [4]], and the half-life of pesticide residue can be as long as a few years [5]. The pesticides enter into the ecosystem through water and air [[6], [7], [8], [9]] and concentrate along the food chain and eventually creates a potentially severe threat to biodiversity and human health [10]. Unfortunately, pesticides have become an indispensable part of modern agriculture, thus making the development of pesticide residue degradation technology necessary and emergent to provide a guarantee for both agricultural harvest and ecological civilization [11]. In this sense, comparing to restrict the pesticide utilization, the more sensible option is to develop robust degradation strategies of pesticide residue in a way that not only enhancing environmental restoration but also maintaining sustainable development of the survival environment [12,13].
According to the different targets, pesticides include, but are not limited to, insecticides, herbicides, fungicides, and nematicides [14]. They vary in target, efficiency, and chemical component, thus different degradation methods are required in different pesticide degradation situations. So, relevant research has been carried out to develop several methods for environmental remediation, including chemical degradation and biological degradation [15]. However, these methods still face dilemmas such as strict conditions, low degradation efficiency, and poor substrate sensitivity, which limit the degradation efficiency [16]. The rapid development of synthetic biology provides a powerful tool for pesticide degradation because of its advantage in diverse combination, precise control mode, and variable design strategy [17]. More and more scientists have devoted themselves to employing synthetic biology to degrade pesticide residues [[18], [19], [20], [21], [22]]. These works provide not only a variety of technical support for the degradation of pesticide residues beyond the limitations of original technology but also the insurance for environment and food safety.
This paper firstly introduces the properties, inhibition mechanisms, and environmental hazards of various pesticides, then summarizes available techniques for the degradation of pesticides, including chemical and biological routes; lastly, the design, application, and prospect of synthetic biology in pesticide residue degradation are detailed described, in hopes of offering some suggestions for researchers in a similar field.
2. Mechanisms and hazards of pesticides
2.1. Mechanisms of pesticides
Pesticides can be classified as insecticides, herbicides, fungicides, and nematicides according to the target and sharing diverse mechanisms (Fig. 1). As for the insecticidal mechanism, insecticides often intervene in the normal physiological activities of pests, resulting the death. A wide range of insecticides kills pests by inhibiting acetylcholinesterase and exciting the nervous system constantly, such as organophosphates and carbamates [23]. By contrast, neonicotinoids can combine with acetylcholine receptors as nicotinic acetylcholine receptor agonists [24]. Organochlorines and pyrethroids mainly prevent the closing process of the voltage-gated sodium channel (VGSC) in nerve cells [25]. Those pesticides targeting the nervous system often lead to muscle paralysis or rigidity and, then, a quick death. Without a specific target system, a series of insecticides focus on inhibiting electron transfer and/or oxidative phosphorylation to interfere with mitochondrial respiration, such as chlorfenapyr and tolfenpyrad [26]. Some insect growth regulators, like benzamide (a chitin synthesis inhibitor) and diacylhydrazine (a molt-accelerating compound) don't produce an instant effect [27].
Fig. 1.
Mechanisms of pesticides. (A) Ach: acetylcholine; AchR: acetylcholine receptors; VGSC: voltage-gated sodium channel. (D) AchE: acetylcholinesterase; CIC: chloride channels.
The mode of action of herbicide includes but is not limited to the following four different ways. (1) Disrupting photosynthesis, for example, phenylureas (including diuron and monouron) and S-triazines (including atrazine and simazine) [28]. (2) Affecting the ETC, for example, dinoseb and 2,6-dichlorobenzamide [29]. (3) Inhibiting critical biosynthesis such as cytochromes (e.g., pyrazlynate), amino acids (e.g., glyphosate and sulfonylureas), fatty acids (e.g., aryloxyphenoxypropionates) [30,31] (4) Preventing cell division (e.g., asulam). and growth (e.g., benzoylprop-ethyl) [32,33].
As respiratory inhibiting is a widely used mechanism among all kinds of pesticides, various fungicides possess the function of inhibiting respiratory function and disturbing ATP synthesis, such as strobilurins (an outer quinone inhibitor [34]), meptyldinocap (an uncoupler of oxidative phosphorylation) and fluopyram (a succinate dehydrogenase inhibitors [35]). Others are focusing on inhibiting biosynthesis. Dimethomorph can disrupt fungal cell wall formation. Amines (or morpholines) can inhibit sterol synthesis, while hydroxypyrimidines block inosine and adenosine nucleotide synthesis [36]. Methyl benzimidazole carbamates have a clear mode of action that is to combine with β-tubulin subunits and interfere with polymerization [35]. But there is a number of fungicides like aryl-phenyl ketones and cyflufenamids whose mechanisms remained unexplored.
Plant-parasitic nematodes cause significant damage to crops by direct or indirect damage to crops. Nematicides are pesticides that kill parasitic worms such as roundworms and threadworms and can be classified as fumigant and non-fumigant. Due to potential environmental hazards, fumigants are going out of focus, while biocontrol agents (e.g., abamectin) are gaining lots of attention [37]. Abamectin can release chloride ions into muscle cells, restrain muscle contraction, and paralyze and kill nematodes eventually. Some nematicides, such as oxamyl [38], fluopyram, and fluensulfone [39] can inhibit acetylcholinesterase, similar to some insecticides. Besides, aryl formamides can combine with enzymes or ETC to interfere with cell metabolism.
Pesticides mentioned above are all facing a similar dilemma of increasingly enhanced resistance owing to gene variation of the target protein. More resistance is coming with overdosed and broad-spectrum pesticide usage, leading to larger dosages of pesticides to realize the same effect compared with the past [40,41]. It is to say that the economic benefit brought by pesticides is decreasing while the environmental burden is increasing. Under this situation, degradation cannot be cast a veil, and synthetic biology methods are blooming to offer solutions. The application can be seen in biotic pesticides [42,43], and degradation in particular.
2.2. Hazards of pesticides
After spraying, numerous residual particles of pesticides are suspended in the environment through multiple approaches (Fig. 2). It can react with nitrogen oxides in the atmosphere to produce ozone and affect air quality [9]. Besides, a wide range of pesticides is detected in potable water, surface water, and groundwater [2,7]. Many water samples contain pesticides that far exceed the standard specified for drinking water [6]. Pesticide remaining in the soil is beyond the degradation ability of most microorganisms, resulting in their metabolic disturbance and stunted growth.
Fig. 2.
Harm of pesticide residue to ecology and environment.
Ordinarily, most trace pesticide residue shows no significant effects on non-target organisms due to their high selectivity. However, toxicological studies from vitro to vivo demonstrated that pesticides targeting different systems (such as nervous system, endocrine, reproduction, etc.) pose a persistent threat to non-target organisms in many aspects after long-term accumulation [44]. Results from epidemiology research, reappearance in model animals (e.g., zebrafish, rats, mice), and multi-omics data analysis demonstrated how pesticide residue affects each physiological system.
As to the nervous system, pesticides (e.g., glyphosate and chlorphoxim) interfere nerve cells via oxidative stress and mitochondrial damage, leading to DNA damage [45]. In the most serious situations, pesticides, especially organophosphorus pesticides, are related to Parkinson's disease and autism [46]. In particular, maneb, a widely used fungicide, can also cause Parkinson's disease by disturbing neurotransmitter biosynthesis and neurotransmitter signaling [47]. Most pesticides (e.g., pyrethroids) have low acute mammalian toxicity. However, long-term exposure can lead to nerve dysfunction, according to epidemiology research, especially for liposoluble pesticides, which can be accumulated in tissue with high-fat content [4].
As a large part of Endocrine Disruptor Chemicals (EDCs), pesticides can disturb endocrine homeostasis through various approaches. DDE (1,1-dichloro-2,2-bis(p-chlorophenyl) ethane) is an antagonist to the androgen receptor [48], and DDT (2Dichlorodiphenyltrichloroethane) can separate the thyrotropin receptor from lipid raft anchored in the cell membrane to inhibit downstream stimulation and internalization of thyroid hormone, causing thyroid dysfunction in rodents, birds, amphibians and fish [3]. Both can act as estrogen receptor agonists and promote estrogen receptor production. Besides, strong evidence proves that pyrethroid is also a thyroid hormone disruptor and has the hormone-like activity to interfere with reproduction [49]. Except for the endocrine way, pyrethroids can modify the expression of ovarian miRNAs to interfere with ovarian development and maturation more directly [50]. Moreover, the impact on reproduction can't be ignored in aquatic animals caused by water-soluble pesticides. Atrazine has a significant impact on aquatic animals and amphibians, which not only affects the reproductive capacity of frogs but also increases the risk of myocardial fibrosis in Xenopus laevis [51,52]. As a common EDC in water, triclosan fungicide can disrupt the intermediate steroidogenic cascade and induce antiandrogenic activity in fish [53].
To sum up, most pesticides lack solid epidemiological evidence to support their impact on human health [45]. But the possibility cannot be ruled out. Through long-term accumulation, pesticides banned long ago (DDT), are still existed, harming the environment and organisms as persistent organic pollutants (POPs) [3]. Long-term application of pesticides also accumulates in crops, affecting imports, exports, and trade. Degradation methods are indispensable to being developed. Based on the principle and mode of pesticide degradation, the degradation methods can be simply classified as chemical degradation, biological degradation, and synthetic biological degradation methods, which will be detailed discussed in the following content in this paper.
3. Chemical degradation methods
Generally, pesticides are mostly organic molecules with specific functional groups, which can be degraded through a chemical route. Incineration is a simple and crude approach for disposing pesticide residue in pesticide wastewater. With a high temperature (about 1000 °C) and sufficient oxygen, non-volatile pesticides like methomyl can be degraded, whereas volatile pesticides incineration could emit organic compounds and carbon dioxide [54,55]. Thus, incineration is not a suitable method for pesticide degradation, which would lead to severe environmental containment. Nevertheless, it can still be a cost-effective way to treat wastewater from pesticide manufacturers instead of field or water.
Similar to incineration, hydrolysis is another traditional and easily-obtained chemical degradation method. Organophosphorus, organochlorine pesticides, and organonitrogen pesticides usually contain heteroatoms, which can be hydrolyzed in alkali conditions, in which metal compounds act as heterogeneous catalysts [56]. However, harmful compounds would be produced during the organophosphorus pesticides hydrolysis process [57]. Moreover, the alkaline condition and metal compounds were a requirement for pesticide residue degradation, which make it a limitation for the application in soil, food, and plant sample.
Diverse oxidants have been explored to degrade pesticide residues, such as δ-MnO2 [58], H2O2, and ozone. In the case of ozone, it is a widely available oxidant that can effectively oxidize pesticides by different technologies, such as enhanced ozonation processes (EOPs), advanced oxidation processes (AOPs), and the non-thermal plasma (NTP) technology. Hydroxyl radicals in AOPs can react with pesticides unselectively, while EOPs offer an advantage by producing highly oxidizing radicals to damage pesticides molecule [59,60]. The NTP technology can yield NTP by introducing an electric field to the gas, and then the field energy can drive the generated reactive species and free electrons to degrade pesticides [61]. Except for oxidation, ultrasonic treatment [62] and pH adjustment can degrade pesticides to a certain extent, but they also suffer from the disadvantage of limited efficiency and extent in degradation like oxidation.
In the last few years, photochemical degradation has become a new technology used for agricultural pollutants treatment. After being excited by ultraviolet light, pesticide molecules undergo the process of photoionization and homolytic fission, being converted into small, environmental-friendly molecules eventually [63]. Also, some photocatalysts, represented by TiO2, can generate positive holes after exposure to light, which have a high oxidation potential to oxidize pesticide pollutants, significantly accelerating the mineralization process of pesticide pollutants [64]. P–O and P–S bonds, the most common chemical bonds in pesticides, are easily oxidized by the semiconductor catalyst of TiO2. Taking pyrethroids as an example (Fig. 3), photochemical degradation usually contains a series of reactions, including isomerization, 3,3-dimethacrylate formation, ester bond cleavage (C–C, C–O bond cleavage), decarboxylation, and reductive dehalogenation [[65], [66], [67]]. Furthermore, beyond TiO2, many composite materials, such as Bi2MoO6, ZnIn2S4, CdS/BiOBr/Bi2O2CO3, and Fe–TiO2, also exhibit excellent performance in pesticides degradation (e.g., atrazine) [68].
Fig. 3.
Photochemical degradation pathways of pyrethroids [69].
Soil biology electrochemistry system (SBES) is a frontier concept in chemical biology, which was first proposed in 2015 [70]. In this system, cytochromes on the surface of microorganisms undergo extracellular electron transfer (EET), losing their electrons and converted to an oxidation state, through which the microorganism with high efficiency can oxidize pesticide pollutants. Electrodes are generally used as terminal electron acceptors for microorganisms, through which they can induce pollutants oxidation and generate electricity. However, this method is not suitable for compounds with a complete oxidation state. SBES was successful in the pentachlorophenol (PCP) oxidation, which owned a high reduction potential and could be easily oxidized. In 2020, Zhou's group developed a method using biochar (prepared by heating biomass) as an electrode, which not only increased the functional radius of the electrodes but also significantly sped up the degradation rate of PCP [71]. These successes were achieved on a laboratory scale, while it is still a long way to using this green technique to deal with pesticide residue degradation in a more effective and economical way.
These chemical degradation processes mentioned above all undergo violent chemical reactions, which not only exert a negative influence on the environment but also cause secondary pollution. Compared with chemical methods, biodegradation is considered to be a mild, cost-effective, and eco-friendly approach to degrading pesticide residue. Furthermore, pesticide residue can be degraded more thoroughly via microbial metabolism, which will be assimilation and become a part of the cell (Fig. 4).
Fig. 4.
Chemical degradation and biological degradation. Pesticides can be degraded into harmless molecules through multiple chemical degradation methods mentioned above while enzymes can degrade pesticides transported into the cell step by step. When pesticides can't be degraded completely by chemical methods, intermediates still can be transported into the cell and end up with harmless molecules.
4. Biological degradation methods
In contaminated soil, some microbes have evolved special pathways to assimilate several pesticides that share similar functional groups, which can be used as carbon, nitrogen, or phosphorus sources at last [72]. Thus, the critical enzyme related to pesticide degradation in these pathways was fundamental for biological degradation. This method generally aimed to degrade pesticides with reconstructed catalytic pathways in microorganisms. The basic flow of the methods contains the following processes: First, seek out the functional microbes through screening methods such as single carbon source selection [73]; second, identify the enzymes that catalyzed the distinctive steps in pesticide degradation; third, discover and screen out the genes that encode these enzymes; last, express heterologous enzyme and optimize expression systems in engineered bacteria for effective pesticide degradation [74,75].
Most organophosphorus pesticides in the soil can be completely mineralized into phosphorus and carbon sources for the growth of microorganisms [76]. Carbamate pesticide is a broad-spectrum benzimidazole fungicide with the properties of high solvency, strong toxicity, and thermal instability. It can be hydrolyzed, photolyzed, and oxidized easily to various decomposition products and derivatives [77]. Research showed that a variety of microorganisms could absorb and degrade carbamate for growth, such as Pseudomonas, Flavobacterium, non-pigmented bacteria, Neosphingomyces, Sphingomonas, nematodes, and Arthrobacter, etc. [78]. To a certain extent, chemical degradation methods can be integrated into biological, resulting in higher efficiency (Fig. 4). For instance, imidacloprid degradation efficiency reaches up to 94.6% by combining the TiO2 photocatalysis with the biodegradation method [68]. Sometimes, the degradation process can be achieved in a cell-free environment. As long as the pH and temperature are appropriate, biocatalysts like His6-OPH can be fixed to various carrier materials and degrade multiple water pollutants containing pesticides [79].
As for a mixture of multiple pesticides, constructing genetically engineered microorganisms was a preferred strategy. Diverse approaches can be employed to integrate multiple degradation abilities into one engineered bacterium, such as protoplast fusion, horizontal gene transfer, and homologous recombination. Some studies used the protoplast fusion technique for the biological degradation of various chlorophenols. Researchers employed a protoplast fusion technique to recombine the genome of Psathyrella candolleana and Pseudomonas putida, by which to construct a versatile degraded strain [80]. Eventually, it was tested and confirmed that the fusing strain has a strong degradation capability to the utmost 78.98% PCP degradation efficiency in polluted water [80]. At the same time, lots of efforts were made to turn research into practice. It is reported that the engineered bacteria were successfully assembled as a micro bioreactor and immobilized in the chamber as the stationary phase to degrade the pesticide residue in the contaminated water [81]. Meanwhile, microbial cell-immobilized biochar (MCB) is a cost-effective and environmentally friendly way to degrade pesticides in soil [82]. These methods can reduce the risk of engineered bacteria escaping and lay a fundamental for large-scale production in the industry.
Despite the genetic reconstruction in one strain, the co-culture of different degradative microbes with distinct functions also has been developed as a biological degradation strategy. For example, various microorganisms have been found to degrade imidacloprid, a representative one in organic nitrogen pesticides. As shown in Fig. 5 [83], most microorganisms can only perform one or two steps in the degrading process, leaving potentially toxic intermediate metabolites [84]. Although multi-bacterial cooperation is a possible option, a more systematic study was still needed to promote its application in imidacloprid degradation. The unstable cooperation relationship and inconsistent growth rates of different microorganisms are main limiting factors.
Fig. 5.
Various ways used by microorganisms to metabolize imidacloprid.
To sum up, biological degradation methods are limited by complex compounds and incomplete decomposition, as well as the incompatibility of various biological degradation modules. Notwithstanding, the previous research has paved the way for the development of synthetic biological methods, which can reduce the limitations of cultivating difficult, low abundance, and single function.
5. Synthetic biology in pesticide degradation
With the development of the toolbox of chemistry and biology, synthetic biology is not only the synonym of bioengineering but provides higher possibilities for further expansion of cell function. Compared to traditional methods, it significantly improves the application scope and applicability in the contaminated environment. Using synthetic biology methods, many shortcomings of biology and chemistry are overcome, which gives possibilities to realize the long-term and environmental adaptive bioremediation in the environment. The development of synthetic biology methods may lead it to a new level that can break the border of the species and even exceed the field of biology.
5.1. Surface display
The cell membrane separates the interior from the outside environment. It plays a more or less selective role for the substances while protecting the cell. Nevertheless, sometimes, the selectivity of the cell membrane of the engineered bacteria cannot support them to play their due role. Therefore, many surface display systems are designed for pesticide degradation [85]. Different anchoring motifs such as ice-nucleation protein (INP) [86], glycosylphosphatidylinositol (GPI) [87], and Lpp-OmpA [88] are used in these systems. One possible form is shown in Fig. 6. E. coli is usually used as engineering bacteria to construct a surface display system to display different functional enzymes so that various pesticides like carbaryl [89], λ-cyhalothrin [90], and paraoxon [91] can be degraded. By functionally displaying degradation enzymes on the surface of cells, the activity and stability will be promoted. Other than E. coli, pesticide degradation enzymes can also play their role in yeast surface display system with the glycosylphosphatidylinositol (GPI) and show a higher potential in pesticide degradation [87]. The whole cell activity of S. cerevisiae MT8-1 contains GPI-OPH system reaches 5-fold higher than E. coli XL1-Blue displaying OPH, and no obvious difference in growth can be observed between S. cerevisiae MT8-1 with and without GPI-OPH system [87].
Fig. 6.
Diagrammatic of a surface display system using INP [86].
The application of lab-born strains is facing challenges of the complexity of composition and the survival competition in the environment. As for the application in the environment, more modifications should be done to enhance the adaptability of engineered strains. Bacillus endospores can resist most physical assaults and survive in the soil environment for a long time. By displaying AtzA, an atrazine chlorohydrolase on the surface of B. thuringiensis spore with mCherry (an anchoring motif), the atrazine degradation rate in the soil is much higher than recombinant AtzA protein, and the AtzA-bearing spores show outstanding stability as the degradation rate decreased only 18% after 45 months [92]. Thus, the viability and stress resistance of the bacteria or fungi should be improved to survive and cope with the complicated environment before application.
As mentioned, there are many limitations in applying lab-born bacteria in a contaminated environment. Many pesticides are used in a mixture with other ones in the environment, which may result in potentiated toxicity. It not only threatens the viability of engineered bacteria but also imposes new requirements on their functions [93]. With the surface display systems, we can expand the function of some pesticide-degrading microorganisms to cope with multiple pesticide residues. As chlorpyrifos and other organophosphorus pesticides are usually more difficult to pass through the membrane, the common method is displaying an enzyme that can degrade organophosphorus on the surface of γ-HCH degrading strains like Sphingobium japonicum UT26 [[94], [95], [96]] while constructing a multi-function bacteria. With the application of fusion protein, engineered bacteria can expand other functions to improve their practicability. By combining OPH with EC20 to form a fusion protein that was displayed on the Sphingobium japonicum UT26, its degradation rate in the environment with cadmium recovers a lot though still lower than without cadmium [95]. OPH and green fluorescent protein (GFP) fusion provides a possibility that monitors the activity of engineered bacteria in the process of bioremediation at some specific expression level [18]. As a good tool, this combined structure can hlep with proving work in the study of the bioremediation process.
5.2. Degradation part development
Some pesticide-degrading enzymes, like OPH, are able to degrade a wide range of pesticides with the same characteristics, but their efficiency differs by the specific kind [97]. Many mutants with higher performance can be developed through DNA mutation and screening. Surface display systems can also assist the screening with the substrate transfer so that the authenticity won't be affected during the measurement of degradation activity. A mutant is screened out by directed evolution using the INPNC surface display system, whose activity is about 25-fold higher than that of the wild type in degrading parathion [97], and its activity in degrading paraoxon and coumaphos is also increased at different degrees [98].
Compared to the random mutagenesis strategy, the site-specific mutations possess a higher probability of success in getting positive ones. However, the selection of sites is also limited by the existing knowledge of the structure and catalytical mechanisms [98]. The analysis of the structure of mutants obtained by random mutagenesis may pay the way for successful site-specific mutations [98]. Applying saturation mutagenesis at position 283 of BphAEs, researchers obtained some effective dioxygenases which can metabolize a wide range of PCBs. Among them, Ser283Met mutation showed enhanced catalytic activity toward some biphenyl and highly chlorinated biphenyl [99]. As position 283 has been known as the entrance of the catalytic pocket, such studies often have a clearer purpose and estimate [99]. In addition to the expansion of degradation substrates, directed evolution is also employed to improve the catalytic activity in pesticide degradation [100], which will develop various degradation parts with stronger abilities for synthetic biology.
5.3. Multi-module integration strategy
Though some strains can degrade various pesticides, it is essential to make improvements for many strains to obtain excellent performance. Exogenous gene circuits provide a possibility to endow natural microorganisms with extrinsic degradation abilities. In some degradation pathways, pesticides would not be degraded into innoxious compounds but into intermediates that are even more harmful than the pesticide itself. Thus, it is possible to avoid producing these intermediates and further degrade them into innocuous compounds by multiple degradation modules integrated together. This process may need the participation of many enzymes and regulatory parts to realize the cascade reaction, which could be integrated into a complete system. At last, either by introducing this genetic circuit in the plasmid or assembling them into the chromosome, an engineered strain can be developed with stronger degradation ability and efficiency based on synthetic biology [19].
The MP and γ-HCH mineralization device can be integrated into the chromosome of the chassis (Pseudomonas putida KT2440) by scarless integration. Thus, an engineered strain has been developed with the potential for bioremediation (Fig. 7). This engineered strain exhibits excellent performance in hereditary stability and still retained the mineralization capability for MP and γ-HCH after 50 generations [101]. Moreover, this efficient construction strategy was also used to construct a γ-HCH and TCP (the main degradation products of herbicide triclopyr and insecticide chlorpyrifos) mineralization strain by Pseudomonas putidaKT2440 [102].
Fig. 7.
The engineered Pseudomonas putida KT2440 with MP and γ-HCH mineralization pathways.
In the past, many teams in a synthetic biology competition, international genetically engineered machine competition (iGEM) have made different attempts in the degradation of pesticides by multi-module integration strategy, and their works are listed in Table 1. Here, we would describe some representative work based on a multi-module integration strategy.
Table 1.
Summary of different methods of iGEM teams of pesticides degradation.
| Team (year) | Chassis | Gene (source) | Function |
|---|---|---|---|
| Team: METU_Turkey (2013) [103] | B. subtilis | CYG6G1 (Drosophila melanogaster) | degrade imidacloprid into 4, 5-hydroxyimidacloprid |
| sacB secretion label | secrete protein into bee intestines | ||
| Team: British_Columbia (2015) [104] | E.coli | CYG6G1, CYP2D6, CYP6CM1 (Drosophila melanogaster) | metabolize imidacloprid |
| Cch2 (Bradyrhizobiaceae SG-6C) | convert 6-CNA into harmless intermediate harmless intermediate | ||
| Team: Goettingen (2018) [105] | B. subtilis | GAT (Bacillus licheniformis) | glyphosateN-acetyltransferase, degrading glyphosate |
| aroA (Salmonella typhimurium) | ESPS enzyme, having tolerance to glyphosate | ||
| Team: UIUC_Illinois (2019) [106] | E.coli | glpA (Pseudomonas Pseudomallei) | phosphorylate glyphosate to reduce its toxicity to cells. |
| glpB (Pseudomonas Pseudomallei) | glyphosate oxidoreductase, decomposing glyphosate into AMPA by C–N bond cleavage | ||
| Team: CSL_Pittsburgh (2019) [107] | E.coli | CYP6AY1 (brown plant hopper) | metabolize imidacloprid |
| signal peptide pelB | target the protein to the periplasm of Escherichia coli | ||
| Team: Waterloo (2019) [108] | B.diazoefficiens | LibA (Variovorax sp. Strain SRS16) | converse linuron to 3,4-DCA |
| Nat1 (Mesorhizobium loti) | converse 3,4-DCA to N-acetyl-3,4-DCA | ||
| Team: XMU-China (2020) [109] | E.coli | phnE1, phnE2 (Sinorhizobium meliloti) | phosphonate acid transporter, transporting organophosphorus across the membrane |
| phnO (Salmonella typhimurium) | aminoalkylphosphonate N-acetyl-transferase, derivatizing AMPA into glyphosate analog | ||
| phnJ (Enterobacter cloacae) | C–P lyase, degrading organophosphorus to small molecule | ||
| Team: USP-Brazil (2021) [110] | micro-tomato | CYG6G1 (Drosophila melanogaster) | degrade imidacloprid into 4, 5-hydroxyimidacloprid |
| pLAT52 promoter | ensure CYG6G1 only can be expressed in pollen tissue | ||
| Team: Concordia-Montreal (2022) [111] | Cyanobacteria | oph (Pseudomonas putida strain HM01) | hydrolyzes organophosphorus compounds, phosphonic and phosphinic acids using divalent cations as cofactors |
| opdB (Burkholderia sp. FDS-1) | organophosphorus hydrolase which can degrade multiple pesticides, especially fenitrothion |
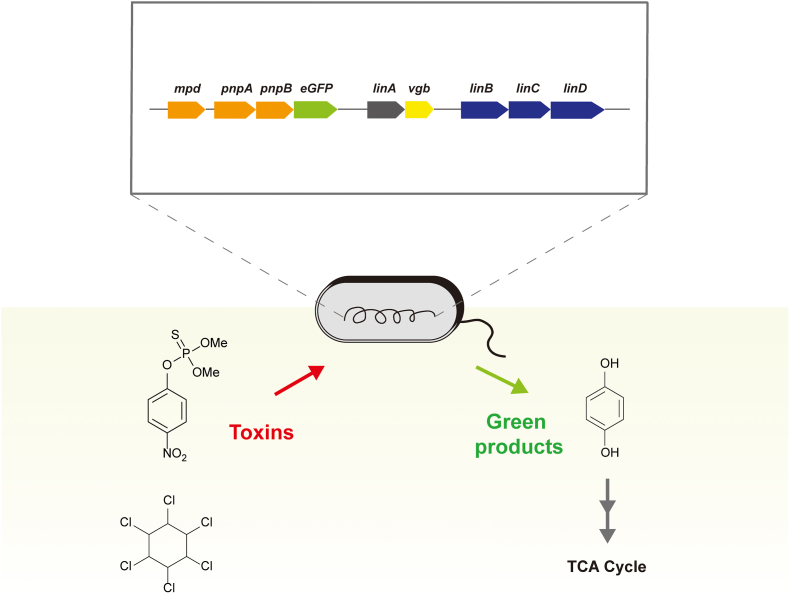
Team: British_Columbia also choose CYG6G1: They add two other genes from the same cytochrome P450 family, CYP2D6 and CYP6CM1. The signal peptide pelB can target the protein in the periplasm of E. coli. Besides, 6-chloronicotinic acid (6-CNA), one of the metabolites of imidacloprid is also toxic to bees. They further introduce Cch2, a chlorine hydrolase of BradyrhizobiaceaeSG-6C, to convert 6-CNA into a harmless intermediate 6-hydroxynicotinic acid (harmless intermediate). Thus, the bees with engineered bacteria can be resistant to both imidacloprid and 6-CNA at the same time [109].
We (Team: XMU-China, 2020) also constructed an engineered bacteria to degrade glyphosate effectively. The gene circuits combined with different gene modules can realize cooperative control of the glyphosate degradation system [109]. At first, we found that E. coli has an endogenous glyphosate degradation system, but the frame-shift mutation in the phnE gene restricted glyphosate transportation efficiency. In addition, phnF inhibits the expression of the C–P lyase gene phnGHIKJ through promoter regulation. Therefore, glyphosate degradation ability in E. coli is relatively weak. Thus, we introduce a series of exogenous C–P lyases, namely phnJ, phnO, phnE1, and phnE2, and also design an RNAi system targeted to endogenous phnF and phnJ. The engineered bacteria show a certain ability of glyphosate degradation [109].
Some novel ideas about imidacloprid degradation were proposed by Team: USP-Brazil in 2021 iGEM. They constructed a transgenic plant to protect bees from pesticides. Gene CYG6G1, an imidacloprid degradation enzyme, was introduced into micro-tomato but the pLAT52 promoter which controlled its expression only opened in pollen. Thus, a win-win situation is realized as the bees can collect imidacloprid-free pollen from fields which are applied imidacloprid to avoid pests [110].
5.4. Develop collaboration in microorganisms
For some recalcitrant organic chemicals, multiple steps were essential for complete degradation to avoid producing some toxic intermediates. However, an enormous genetic circuit will be a burden for a single microorganism to degrade these pesticides completely. By contrast, it is a better strategy to use a microbial consortium, in which each microorganism can take responsibility for degradation in corresponding steps [112]. Different kinds of microorganisms can play a complementary or synergistic role in the constructed microbial consortium, boosting the degradation process.
The constructed microbial consortium plays its role as a system, and every coorperation process should be taken into consideration apart from the individual function of each microorganism when constructing a microbial consortium. The transport efficiency of different microorganisms for different intermediates is such a crucial factor because improper microbial collocation may lead to the accumulation of intermediate products and cause a reduction of efficiency in the whole consortium [113]. In addition, differing from each kind of microorganism, the influence of environmental factors for the whole consortium will be different from that for a single kind of microorganism. So the preferred conditions of constructed microbial consortium and the influence of the original community in the environment also need to be considered in the design [20]. A successful microbiome design should balance a blend of top-down and bottom-up strategies, which means individual function should fit with the overall effect [114,115].
As an example, p-nitrophenol, a toxic intermediate produced in the degradation process of parathion, can be successfully degraded by a co-culture system. The system consists of engineered Escherichia coli SD2 and Pseudomonas putida KT2440. The former contains plasmids encoding genes of parathion hydrolase enzyme and green fluorescent protein, while the latter contains plasmid encoding genes for p-nitrophenol mineralization. P-nitrophenol can inhibit the growth of microorganisms, however, Pseudomonas putida KT2440 could reduce the accumulation of p-nitrophenol to an extremely low level [112]. The constructed degradation system can also be seen in the co-culture of E. coli DH5α and Sphingobium chlorophenolicum ATCC 39723 and exhibits excellent performance for hexachlorobenzene degradation [113].
Building a mutually-beneficial symbiosis among microorganisms can support the degradation of pesticides in different environments. For example, the microalgal can be used as an oxygen provider for bacterial consortium when working in polluted water to degrade glyphosate, and the carbon dioxide produced by bacterial consortium can be used by microalgal as well. These synergies resulted in a relatively stable state of mutually-beneficial symbiosis and an effective glyphosate degradation rate [116]. Beyond that, the application of materials shows the potential for improving the stability and effect of the consortium, which can protect the consortium against enduring adverse external environments [117].
6. Conclusions and prospects
The degradation of pesticide residue has been brought to insights for a long time. Techniques and methods in this field emerge one after another. From chemical degradation and biodegradation to synthetic biology degradation, to be more multi-dimensional and harmless degradation has become the general trend of pesticide residue research. As a powerful tool, synthetic biology shows integrated characteristics of multiple disciplines to construct diverse solutions towards pesticides residue degradation. An increasing number of microorganisms have been discovered with the ability to degrade pesticide residue, which broad our vision on more natural parts with regulatory and catalytic functions and chassis cells for synthetic biology. On these bases, synthetic biologists can build the modules, design a multi-module integration genetic circuit that merges the degradation ability of different kinds of pesticides and create multiple degradation systems, to provide effective tools and techniques for pesticide treatment. In addition to a higher degradation efficiency, synthetic biology shows strong customizability for specific problems. For different application scenarios, researchers can also choose different tools to construct various engineered bacteria to achieve the goal of degradation of pesticides with the highest efficiency in the safest range.
Many synthetic biology degradation methods have achieved success in the laboratory stage, while barriers still exist in application processing. On the one hand, biosafety is a limitation for practical applications, in which drug resistance genes in engineered bacteria may escape to the natural environment with the transfer of plasmids, leading to the generation of super-weed and super-bacteria. On the other hand, insufficient attention is paid to the problems of pesticide residue in vast farmland. The broad field and microbial communities may not be suitable for the reproduction and growth of engineering bacteria, which could not reach a working density within a short time. Nevertheless, biobricks that are essential for constructing gene circuits in engineering bacteria to deal with new and complex pesticide residues are always inefficient and incomplete. Moreover, the size of the plasmid used for genetic transformation is limited, which would restrict the number of parts and make the degradation inadequate. All these questions have challenged the application of synthetic biology in pesticide residue degradation.
Nevertheless, various strategies can also to developed to overcome these problems. For biosafety, many iGEM teams have provided different Kill Switches to limit the escaping probability of engineered bacteria and prevent the horizontal gene transfer. To enhance public awareness of pesticide residue, the government is supposed to disseminate the hazard of pesticide residue to the environment, human health, and sustainable development. Researcher teams also should boost the synthetic biology strategies in an actual environment, for example, soil. In addition, the process from the laboratory to the market also depends on the continuous improvement of supporting policies for the degradation of pesticide residue in the future. What's more, in order to co-degrade varieties of pesticide residue, we can design more than one type of engineered bacteria in a cooperation way, which needs to consider the survival competition among different strains. If the number of genes required for complete degradation is too large to be put into a single kind of engineered bacteria, synthetic biological microbial consortium is a wise choice.
For versatile function and stability, the consortium may show better potential than a single kind of microorganism, and there are many synthetic biology tools to engineer microbial communities. With these tools, the complex network can be constructed on the basis of intercellular signaling, syntrophic interactions, and exogenous molecules [22]. The existing applications include the expansion of orthogonality of quorum sensing systems [118], construction of co-auxotrophies community [119], and enhanced bioproduction [120]. Different tools and processes should be designed for the purpose of the consortium [22,114]. In addition, the appropriate method design needs to be based on sufficient data support; the simulation and analysis of environmental conditions will provide a basis for the expansion of synthetic biology application in pesticide degradation [121,122]. All in all, synthetic biological methods will head in a more multi-dimensional direction, combining chemical and biological degradation methods, breaking technical barriers and species restrictions to achieve a more integrated system. We believe synthetic biology methods will become the main force of pesticide bioremediation in the future.
CRediT authorship contribution statement
Bi Ruomeng: Writing – original draft, preparation. Ou Meihao: Writing – original draft, preparation. Zhou Siru: Writing – original draft, preparation. Geng Shichen: Writing – original draft, preparation. Zheng Yixian: Investigation, Validation, and, Visualization. Chen Junhong: Investigation, Validation, and, Visualization. Mo Ruijie: Investigation, Validation, and, Visualization. Li Yuan: Investigation, Validation, and, Visualization. Xiao Gezhi: Investigation, Validation, and, Visualization. Chen Xingyu: Investigation, Validation, and, Visualization. Zhai Shiyi: Investigation, Validation, and, Visualization. Zhang Aihui: Project administration, Writing – review & editing. Baishan Fang: Conceptualization, Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We also thanked very much the other members of the iGEM team XMU-China 2020 (https://2020.igem.org/Team:XMU-China/Team). This work was financially supported by the National Natural Science Foundation of China (No. 22278343) and Shenzhen Basic Research Project (JCYJ20190809160209449).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Zhang Aihui, Email: zhangaihui@xmu.edu.cn.
Fang Baishan, Email: fbs@xmu.edu.cn.
References
- 1.FAO World food and agriculture – statistical yearbook 2021. Rome, Italy; 2021. 2021;p:1–10. [Google Scholar]
- 2.Yu J., Xia Y., Tian X., Zhang R., Bian Z. Atrazine and its metabolites (ATZs) in source water, finished water, and tap water from drinking water treatment plants and its human risk assessment in Zhoukou City, China. Hum Ecol Risk Assess. 2021;27:1926–1938. https://doi.org/10.1080/10807039.2021.19338966 [Google Scholar]
- 3.Rossi M., Taddei A.R., Fasciani I., Maggio R., Giorgi F. The cell biology of the thyroid-disrupting mechanism of dichlorodiphenyltrichloroethane (DDT) J Endocrinol Invest. 2018;41:67–73. doi: 10.1007/s40618-017-0716-9. https://doi.org/10.1007/s40618-017-0716-9 [DOI] [PubMed] [Google Scholar]
- 4.Pitzer E.M., Williams M.T., Vorhees C.V. Effects of pyrethroids on brain development and behavior: deltamethrin. Neurotoxicol Teratol. 2021;87:106983. doi: 10.1016/j.ntt.2021.106983. https://doi.org/10.1016/j.ntt.2021.106983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Concha C, Manzano CA. Priority pesticides in Chile: Predicting their environmental distribution, bioaccumulation, and transport potential. Integrated Environ Assess Manag. 2022;00:1–8. doi: 10.1002/ieam.4680. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharyya S., Poi R., Mandal S., Baskey M., Hazra D.K., Saha S., et al. Method development, validation, monitoring, seasonal effect and risk assessment of multiclass multi pesticide residues in surface and ground water of new alluvial zone in eastern India. Environ Sci Pollut Res. 2022;29:17174–17187. doi: 10.1007/s11356-021-16959-9. https://doi.org/10.1007/s11356-021-16959-9 [DOI] [PubMed] [Google Scholar]
- 7.Thompson D.A., Hruby C.E., Vargo J.D., Field R.W. Occurrence of neonicotinoids and sulfoxaflor in major aquifer groups in Iowa. Chemosphere. 2021;281:130856 doi: 10.1016/j.chemosphere.2021.130856. https://doi.org/10.1016/j.chemosphere.2021.130856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mali H., Shah C.D., Raghunandan B.H., Prajapati A.S., Patel D.H., Trivedi U., et al. Organophosphate pesticides an emerging environmental contaminant: pollution, toxicity, bioremediation progress, and remaining challenges. J Environ Sci. 2023;127:234–250. doi: 10.1016/j.jes.2022.04.023. https://doi.org/10.1016/j.jes.2022.04.023 [DOI] [PubMed] [Google Scholar]
- 9.Gunier R.B., Raanan R., Castorina R., Holland N.T., Harley K.G., Balmes J.R., et al. Residential proximity to agricultural fumigant use and respiratory health in 7-year old children. Environ Res. 2018;164:93–99. doi: 10.1016/j.envres.2018.02.022. https://doi.org/10.1016/j.envres.2018.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sulaiman N.S., Rovina K., Joseph V.M. Classification, extraction and current analytical approaches for detection of pesticides in various food products. J Consum Prot Food Saf. 2019;14:209–221. https://doi.org/10.1007/s00003-019-01242-4 [Google Scholar]
- 11.Tarfeen N., Ul Nisa K., Hamid B., Bashir Z., Yatoo A.M., Dar M.A., et al. Microbial remediation: a promising tool for reclamation of contaminated sites with special emphasis on heavy metal and pesticide pollution: a review. Processes. 2022;10:1358. doi: 10.3390/pr10071358. [DOI] [Google Scholar]
- 12.Bolan S., Padhye L.P., Mulligan C.N., Alonso E.R., Saint -Fort R., Jasemizad T., et al. Surfactant-enhanced mobilization of persistent organic pollutants: potential for soil and sediment remediation and unintended consequences. J Hazard Mater. 2023;443:130189. doi: 10.1016/j.jhazmat.2022.130189. https://doi.org/10.1016/j.jhazmat.2022.130189 [DOI] [PubMed] [Google Scholar]
- 13.Bokade P., Gaur V.K., Tripathi V., Bobate S., Manickam N., Bajaj A. Bacterial remediation of pesticide polluted soils: exploring the feasibility of site restoration. J Hazard Mater. 2023;441:129906. doi: 10.1016/j.jhazmat.2022.129906. https://doi.org/10.1016/j.jhazmat.2022.129906 [DOI] [PubMed] [Google Scholar]
- 14.Wahab S., Muzammil K., Nasir N., Khan M.S., Ahmad M.F., Khalid M., et al. Advancement and new trends in analysis of pesticide residues in food: a comprehensive review. Plants-Basel. 2022;11:1106. doi: 10.3390/plants11091106. https://doi.org/10.3390/plants11091106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marican A., Durán-Lara E.F. A review on pesticide removal through different processes. Environ Sci Pollut Res. 2018;25:2051–2064. doi: 10.1007/s11356-017-0796-2. https://doi.org/10.1007/s11356-017-0796-2 [DOI] [PubMed] [Google Scholar]
- 16.Singh Y., Saxena M.K. Insights into the recent advances in nano-bioremediation of pesticides from the contaminated soil. Front Microbiol. 2022;13:982611. doi: 10.3389/fmicb.2022.982611. https://doi.org/10.3389/fmicb.2022.982611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain M.H., Mohsin M.Z., Zaman W.Q., Yu J., Zhao X., Wei Y., et al. Multiscale engineering of microbial cell factories: a step forward towards sustainable natural products industry. Synth Syst Biotechnol. 2022;7:586–601. doi: 10.1016/j.synbio.2021.12.012. https://doi.org/10.1016/j.synbio.2021.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C., Liu R.H., Yuan Y.L., Liu J.L., Cao X.Y., Qiao C.L., et al. Construction of a green fluorescent protein (GFP)-Marked multifunctional pesticide-degrading bacterium for simultaneous degradation of organophosphates and gamma-hexachlorocyclohexane. J Agric Food Chem. 2013;61:1328–1334. doi: 10.1021/jf304976h. https://doi.org/10.1021/jf304976h [DOI] [PubMed] [Google Scholar]
- 19.Liang P.X., Zhang Y.T., Xu B., Zhao Y.X., Liu X.S., Gao W.X., et al. Deletion of genomic islands in the Pseudomonas putida KT2440 genome can create an optimal chassis for synthetic biology applications. Microb Cell Factories. 2020;19:70 doi: 10.1186/s12934-020-01329-w. https://doi.org/10.1186/s12934-020-01329-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C.Y., Lv T.Y., Liu W.J., Zang H.L., Cheng Y., Li D.P. Efficient degradation of chlorimuron-ethyl by a bacterial consortium and shifts in the aboriginal microorganism community during the bioremediation of contaminated. Ecotoxicol Environ Saf. 2017;139:423–430. doi: 10.1016/j.ecoenv.2017.02.005. https://doi.org/10.1016/j.ecoenv.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 21.Li X., Wen C., Liu C., Lu S.Y., Xu Z.B., Yang Q.E., et al. Herbicide promotes the conjugative transfer of multi-resistance genes by facilitating cellular contact and plasmid transfer. J Environ Sci (China) 2022;115:363–373. doi: 10.1016/j.jes.2021.08.006. https://doi.org/10.1016/j.jes.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 22.McCarty N.S., Ledesma-Amaro R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019;37:181–197. doi: 10.1016/j.tibtech.2018.11.002. https://doi.org/10.1016/j.tibtech.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thapa S., Lv M., Xu H. Acetylcholinesterase: a primary target for drugs and insecticides. Mini Rev Med Chem. 2017;17:1665–1676. doi: 10.2174/1389557517666170120153930. https://doi.org/10.2174/1389557517666170120153930 [DOI] [PubMed] [Google Scholar]
- 24.Ihara M. Ligand-gated ion channels as targets of neuroactive insecticides. Biosci Biotechnol Biochem. 2022;86:157–164. doi: 10.1093/bbb/zbab202. [DOI] [PubMed] [Google Scholar]
- 25.Benavent-Albarracín L., Alonso M., Catalán J., Urbaneja A., Davies T.G.E., Williamson M.S., et al. Mutations in the voltage-gated sodium channel gene associated with deltamethrin resistance in commercially sourced Phytoseiulus persimilis. Insect Mol Biol. 2020;29:373–380. doi: 10.1111/imb.12642. https://doi.org/10.1111/imb.12642 [DOI] [PubMed] [Google Scholar]
- 26.Syromyatnikov M., Nesterova E., Smirnova T., Popov V. Methylene blue can act as an antidote to pesticide poisoning of bumble bee mitochondria. Sci Rep. 2021;11:14710 doi: 10.1038/s41598-021-94231-3. https://doi.org/10.1038/s41598-021-94231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva J.E., Silva W.M., Silva T.B.M., Campos M.R., Esteves A.B., de Siqueira H.A.A. High resistance to insect growth disruptors and control failure likelihood in Brazilian populations of the tomato pinworm Tuta absoluta. Phytoparasitica. 2021;49:689–701. https://doi.org/10.1007/s12600-021-00895-y [Google Scholar]
- 28.de Albuquerque F.P., de Oliveira J.L., Moschini-Carlos V., Fraceto L.F. An overview of the potential impacts of atrazine in aquatic environments: perspectives for tailored solutions based on nanotechnology. Sci Total Environ. 2020;700:134868 doi: 10.1016/j.scitotenv.2019.134868. https://doi.org/10.1016/j.scitotenv.2019.134868 [DOI] [PubMed] [Google Scholar]
- 29.Zhang X., Ivantsova E., Perez-Rodriguez V., Cao F., Souders C.L., Martyniuk C.J. Investigating mitochondria-immune responses in zebrafish, Danio rerio (Hamilton, 1822): a case study with the herbicide dinoseb. Comp Biochem Physiol C Toxicol Pharmacol. 2022;257 doi: 10.1016/j.cbpc.2022.109357. https://doi.org/10.1016/j.cbpc.2022.109357 [DOI] [PubMed] [Google Scholar]
- 30.Bai S., Yin M., Lyu Q., Jiang B., Li L. Cytochrome P450 BsCYP99A44 and BsCYP704A177 confer metabolic resistance to ALS herbicides in Beckmannia syzigachne. Int J Mol Sci. 2022;23(20):12175. doi: 10.3390/ijms232012175. https://doi.org/10.3390/ijms232012175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X.Y., Wang J.H., Yu T., Nian H.H., Zhang H., Wang G.L., et al. Characterization of a novel aryloxyphenoxypropionate herbicide-hydrolyzing carboxylesterase with R-enantiomer preference from Brevundimonas sp. QPT-2. Process Biochem. 2019;82:102–109. https://doi.org/10.1016/j.procbio.2019.03.013 [Google Scholar]
- 32.Alizade S., Keshtkar E., Mokhtasi-Bidgoli A., Sasanfar H., Streibig J.C. Effect of water deficit stress on benzoylprop-ethyl performance and physiological traits of winter wild oat (Avena sterilis subsp. ludoviciana) Crop Protect. 2020;137:105292 https://doi.org/10.1016/j.cropro.2020.105292 [Google Scholar]
- 33.Vadlamani G., Sukhoverkov K.V., Haywood J., Breese K.J., Fisher M.F., Stubbs K.A., et al. Crystal structure of Arabidopsis thaliana HPPK/DHPS, a bifunctional enzyme and target of the herbicide asulam. Plant Commun. 2022;3:100322 doi: 10.1016/j.xplc.2022.100322. https://doi.org/10.1016/j.xplc.2022.100322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C., Zhou T., Xu Y., Du Z., Li B., Wang J., et al. Ecotoxicology of strobilurin fungicides. Sci Total Environ. 2020;742:140611. doi: 10.1016/j.scitotenv.2020.140611. https://doi.org/10.1016/j.scitotenv.2020.140611 [DOI] [PubMed] [Google Scholar]
- 35.Kunova A., Pizzatti C., Saracchi M., Pasquali M., Cortesi P. Grapevine powdery mildew: fungicides for its management and advances in molecular detection of markers associated with resistance. Microorganisms. 2021;9(7):1541. doi: 10.3390/microorganisms9071541. https://doi.org/10.3390/microorganisms9071541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J.-Y., Li J.-X., Ning J., Huo X.-K., Yu Z.-L., Tian Y., et al. Human cytochrome P450 3A-mediated two-step oxidation metabolism of dimethomorph: implications in the mechanism-based enzyme inactivation. Sci Total Environ. 2022;822 doi: 10.1016/j.scitotenv.2022.153585. https://doi.org/10.1016/j.scitotenv.2022.153585 [DOI] [PubMed] [Google Scholar]
- 37.Nguyen V., Park A.R., Duraisamy K., Vo D.D., Kim J.C. Elucidation of the nematicidal mode of action of grammicin on Caenorhabditis elegans. Pestic Biochem Physiol. 2022;188:105244 doi: 10.1016/j.pestbp.2022.105244. https://doi.org/10.1016/j.pestbp.2022.105244 [DOI] [PubMed] [Google Scholar]
- 38.Biancardi A., Aimo C., Piazza P., Lo Chiano F., Rubini S., Baldini E., et al. Acetylcholinesterase (AChE) reversible inhibitors: the role of +Oxamyl in the production of poisoned baits. Toxics. 2022;10(8):432. doi: 10.3390/toxics10080432. https://doi.org/10.3390/toxics10080432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oka Y. Effects of fluensulfone, fluopyram and their combination on Meloidogyne incognita and M. javanica hatching and infectivity. Eur J Plant Pathol. 2022;164:269–281. doi: 10.1007/s10658-022-02557-z. [DOI] [Google Scholar]
- 40.Cruse C., Moural T.W., Zhu F. Dynamic roles of insect carboxyl/cholinesterases in chemical adaptation. Insects. 2023;14:194. doi: 10.3390/insects14020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montull J.M., Torra J. Herbicide resistance is increasing in Spain: concomitant management and prevention. Plants. 2023;12:469. doi: 10.3390/plants12030469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrone P.G. Status of the biopesticide market and prospects for new bioherbicides. Pest Manag Sci. 2023 doi: 10.1002/ps.7403. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y.-Q., Sun P., Chen Y., Liu H.-X., Hao G.-F., Song B.-A. Bioinformatics toolbox for exploring target mutation-induced drug resistance. Briefings Bioinf. 2023;24(2):bbad033. doi: 10.1093/bib/bbad033. [DOI] [PubMed] [Google Scholar]
- 44.Tan H.D., Wu Q.M., Hao R., Wang C.M., Zhai J.L., Li Q.F., et al. Occurrence, distribution, and driving factors of current-use pesticides in commonly cultivated crops and their potential risks to non-target organisms: a case study in Hainan, China. Sci Total Environ. 2023:854. doi: 10.1016/j.scitotenv.2022.158640. https://doi.org/10.1016/j.scitotenv.2022.158640 [DOI] [PubMed] [Google Scholar]
- 45.Xiong Y., Wang C., Dong M., Li M., Hu C., Xu X. Chlorphoxim induces neurotoxicity in zebrafish embryo through activation of oxidative stress. Environ Toxicol. 2022;38:566–578. doi: 10.1002/tox.23702. [DOI] [PubMed] [Google Scholar]
- 46.Madani N.A., Carpenter D.O. Effects of glyphosate and glyphosate-based herbicides like RoundupTM on the mammalian nervous system: a review. Environ Res. 2022;214 doi: 10.1016/j.envres.2022.113933. https://doi.org/10.1016/j.envres.2022.113933 [DOI] [PubMed] [Google Scholar]
- 47.Liu C., Liu Z., Fang Y., Du Z., Yan Z., Yuan X., et al. Exposure to the environmentally toxic pesticide maneb induces Parkinson’s disease-like neurotoxicity in mice: a combined proteomic and metabolomic analysis. Chemosphere. 2022;308 doi: 10.1016/j.chemosphere.2022.136344. https://doi.org/10.1016/j.chemosphere.2022.136344 [DOI] [PubMed] [Google Scholar]
- 48.Molina E.M., Kavazis A.N., Mendonça M.T., Akingbemi B.T. Effects of different DDE exposure paradigms on testicular steroid hormone secretion and hepatic oxidative stress in male Long-Evans rats. Gen Comp Endocrinol. 2022;317 doi: 10.1016/j.ygcen.2021.113963. https://doi.org/10.1016/j.ygcen.2021.113963 [DOI] [PubMed] [Google Scholar]
- 49.Andersen H.R., David A., Freire C., Fernández M.F., D’Cruz S.C., Reina-Pérez I., et al. Pyrethroids and developmental neurotoxicity - a critical review of epidemiological studies and supporting mechanistic evidence. Environ Res. 2022;214 doi: 10.1016/j.envres.2022.113935. https://doi.org/10.1016/j.envres.2022.113935 [DOI] [PubMed] [Google Scholar]
- 50.Song J., Ma X., Li F., Liu J. Exposure to multiple pyrethroid insecticides affects ovarian follicular development via modifying microRNA expression. Sci Total Environ. 2022;828 doi: 10.1016/j.scitotenv.2022.154384. https://doi.org/10.1016/j.scitotenv.2022.154384 [DOI] [PubMed] [Google Scholar]
- 51.Brunetti I.A., Pereira P.C., Oliveira D.S., Silva V.A.O., Reis R.M., Americo-Pinheiro J.H.P., et al. Histological biomarkers and protein expression in Hyphessobrycon eques fish exposed to atrazine. Water Air Soil Pollut. 2022;233:97 https://doi.org/10.1007/s11270-022-05569-x [Google Scholar]
- 52.Johnson J.A., Ihunwo A., Chimuka L., Mbajiorgu E.F. Cardiotoxicity in African clawed frog (Xenopus laevis) sub-chronically exposed to environmentally relevant atrazine concentrations: implications for species survival. Aquat Toxicol. 2019;213:105218 doi: 10.1016/j.aquatox.2019.06.001. https://doi.org/10.1016/j.aquatox.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 53.Liu Y.-H., Yao L., Huang Z., Zhang Y.-Y., Chen C.-E., Zhao J.-L., et al. Enhanced prediction of internal concentrations of phenolic endocrine disrupting chemicals and their metabolites in fish by a physiologically based toxicokinetic incorporating metabolism (PBTK-MT) model. Environ Pollut. 2022;314 doi: 10.1016/j.envpol.2022.120290. https://doi.org/10.1016/j.envpol.2022.120290 [DOI] [PubMed] [Google Scholar]
- 54.Lin Z.Q., Zhang W.P., Pang S.M., Huang Y.H., Mishra S., Bhatt P., et al. Current approaches to and future perspectives on methomyl degradation in contaminated soil/water environments. Molecules. 2020;25(3):738 doi: 10.3390/molecules25030738. https://doi.org/10.3390/molecules25030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang N., Shi M., Wu S., Guo X., Zhang X., Ni N., et al. Study on volatile organic compound (VOC) emission control and reduction potential in the pesticide industry in China. Atmosphere. 2022;13(8):1241 https://doi.org/10.3390/atmos13081241 [Google Scholar]
- 56.Dannenberg A., Pehkonen S.O. Investigation of the heterogeneously catalyzed hydrolysis of organophosphorus pesticides. J Agric Food Chem. 1998;46:325–334. doi: 10.1021/jf970368o. https://doi.org/10.1021/jf970368o [DOI] [PubMed] [Google Scholar]
- 57.Hong F., Pehkonen S.O., Brooks E. Pathways for the hydrolysis of phorate: product studies by P-31 NMR and GC-MS. J Agric Food Chem. 2000;48:3013–3017. doi: 10.1021/jf990558u. https://doi.org/10.1021/jf990558u [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Yue W., Teng Y., Zhai Y., Zhu H. Degradation kinetics and transformation pathway of methyl parathion by δ-MnO2/oxalic acid reaction system. Chemosphere. 2023;320 doi: 10.1016/j.chemosphere.2023.138054. https://doi.org/10.1016/j.chemosphere.2023.138054 [DOI] [PubMed] [Google Scholar]
- 59.Joshi R., Ratpukdi T., Knutson K., Bhatnagar A., Khan E. Bromate formation control by enhanced ozonation: a critical review. Crit Rev Environ Sci Technol. 2022;52:1154–1198. https://doi.org/10.1080/10643389.2020.1850169 [Google Scholar]
- 60.Liu Z., Hosseinzadeh S., Wardenier N., Verheust Y., Chys M., Van Hulle S. Combining ozone with UV and H2O2 for the degradation of micropollutants from different origins: lab-scale analysis and optimization. Environ Technol. 2019;40:3773–3782. doi: 10.1080/09593330.2018.1491630. https://doi.org/10.1080/09593330.2018.1491630 [DOI] [PubMed] [Google Scholar]
- 61.Kyere-Yeboah K., Bique I.K., Qiao X-c. Advances of non-thermal plasma discharge technology in degrading recalcitrant wastewater pollutants. A comprehensive review. Chemosphere. 2023;320 doi: 10.1016/j.chemosphere.2023.138061. https://doi.org/10.1016/j.chemosphere.2023.138061 [DOI] [PubMed] [Google Scholar]
- 62.Li X.H., Xu H., Liu F.M., Peng Q.R., Chen F., Guo Y.Y. Utilizing Plackett-Burman design and response surface analysis to optimize ultrasonic cleaning of pesticide residues from rape. J Sci Food Agric. 2022;102:2061–2069. doi: 10.1002/jsfa.11545. https://doi.org/10.1002/jsfa.11545 [DOI] [PubMed] [Google Scholar]
- 63.Burrows H.D., Canle M., Santaballa J.A., Steenken S. Reaction pathways and mechanisms of photodegradation of pesticides. J Photochem Photobiol B Biol. 2002;67:71–108. doi: 10.1016/s1011-1344(02)00277-4. https://doi.org/10.1016/S1011-1344(02)00277-4 [DOI] [PubMed] [Google Scholar]
- 64.Fouad K., Bassyouni M., Alalm M.G., Saleh M.Y. Recent developments in recalcitrant organic pollutants degradation using immobilized photocatalysts. Appl Phys A: Mater Sci Process. 2021;127:612 https://doi.org/10.1007/s00339-021-04724-1 [Google Scholar]
- 65.Fernandez-Alvarez M., Sanchez-Prado L., Lores M., Llompart M., Garcia-Jares C., Cela R. Alternative sample preparation method for photochemical studies based on solid phase microextraction: synthetic pyrethroid photochemistry. J Chromatogr A. 2007;1152:156–167. doi: 10.1016/j.chroma.2006.12.095. https://doi.org/10.1016/j.chroma.2006.12.095 [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Alvarez M., Lores M., Llompart M., Garcia-Jares C., Cela R. The photochemical behaviour of five household pyrethroid insecticides and a synergist as studied by photo-solid-phase microextraction. Anal Bioanal Chem. 2007;388:1235–1247. doi: 10.1007/s00216-007-1292-1. https://doi.org/10.1007/s00216-007-1292-1 [DOI] [PubMed] [Google Scholar]
- 67.Segal-Rosenheimer M., Dubowski Y. Photolysis of methyl-parathion thin films: products, kinetics and quantum yields under different atmospheric conditions. J Photochem Photobiol A-Chem. 2010;209:193–202. https://doi.org/10.1016/j.jphotochem.2009.11.014 [Google Scholar]
- 68.Sharma T., Kaur M., Sobti A., Rajor A., Toor A.P. Sequential microbial-photocatalytic degradation of imidacloprid. Environ Eng Res. 2020;25:597–604. https://doi.org/10.4491/eer.2019.150 [Google Scholar]
- 69.Zhu Q., Yang Y., Zhong Y., Lao Z., O’Neill P., Hong D., et al. Synthesis, insecticidal activity, resistance, photodegradation and toxicity of pyrethroids (A review) Chemosphere. 2020;254 doi: 10.1016/j.chemosphere.2020.126779. https://doi.org/10.1016/j.chemosphere.2020.126779 [DOI] [PubMed] [Google Scholar]
- 70.Yu B., Tian J., Feng L. Remediation of PAH polluted soils using a soil microbial fuel cell: influence of electrode interval and role of microbial community. J Hazard Mater. 2017;336:110–118. doi: 10.1016/j.jhazmat.2017.04.066. https://doi.org/10.1016/j.jhazmat.2017.04.066 [DOI] [PubMed] [Google Scholar]
- 71.Cai X., Yuan Y., Yu L., Zhang B., Li J., Liu T., et al. Biochar enhances bioelectrochemical remediation of pentachlorophenol-contaminated soils via long-distance electron transfer. J Hazard Mater. 2020;391 doi: 10.1016/j.jhazmat.2020.122213. https://doi.org/10.1016/j.jhazmat.2020.122213 [DOI] [PubMed] [Google Scholar]
- 72.Li Y., Yu X.T., Zheng J.R., Gong Z.L., Xu W.L. Diversity and phosphate solubilizing characteristics of cultivable organophosphorus-mineralizing bacteria in the sediments of sancha lake. Int J Environ Res Publ Health. 2022;19(4): 2320 doi: 10.3390/ijerph19042320. https://doi.org/10.3390/ijerph19042320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su X.J., Liu G.P., Zhang L., Zhou X.Y., Qiao W.J., Jiang J.D. Rhizobium flavescens sp. nov., isolated from a chlorothalonil-contaminated soil. Curr Microbiol. 2021;78:2165–2172. doi: 10.1007/s00284-021-02462-4. https://doi.org/10.1007/s00284-021-02462-4 [DOI] [PubMed] [Google Scholar]
- 74.Zhang Y.X., Xu Z.X., Chen Z.J., Wang G.J. Simultaneous degradation of triazophos, methamidophos and carbofuran pesticides in wastewater using an Enterobacter bacterial bioreactor and analysis of toxicity and biosafety. Chemosphere. 2020;261:128054 doi: 10.1016/j.chemosphere.2020.128054. https://doi.org/10.1016/j.chemosphere.2020.128054 [DOI] [PubMed] [Google Scholar]
- 75.Sajaphan K., Shapir N., Wackett L.P., Palmer M., Blackmon B., Tomkins J., et al. Arthrobacter aurescens TC1 atrazine catabolism genes trzN, atzB, and atzC are linked on a 160-kilobase region and are functional in Eschetichia coli. Appl Environ Microbiol. 2004;70:4402–4407. doi: 10.1128/AEM.70.7.4402-4407.2004. Go to ISI>://BIOSIS:PREV200400379231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Y., Yu X., Zheng J., Gong Z., Xu W. Diversity and phosphate solubilizing characteristics of cultivable organophosphorus-mineralizing bacteria in the sediments of sancha lake. Int J Environ Res Publ Health. 2022;19(4):2320 doi: 10.3390/ijerph19042320. https://doi.org/10.3390/ijerph19042320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Porto A., Melgar G., Kasemodel M., Nitschke M. Biodegradation of pesticides. 1th ed. Universidade de São Paulo, Instituto de Química de São Carlos, Brazil; Brazil: 2011. [Google Scholar]
- 78.Yan Q.X., Hong Q., Han P., Dong X.J., Shen Y.J., Li S.P. Isolation and characterization of a carbofuran-degrading strain Novosphingobium sp FND-3. FEMS Microbiol Lett. 2007;271:207–213. doi: 10.1111/j.1574-6968.2007.00718.x. https://doi.org/10.1111/j.1574-6968.2007.00718.x [DOI] [PubMed] [Google Scholar]
- 79.Efremenko E., Lyagin I., Aslanli A., Stepanov N., Maslova O., Senko O. Carrier variety used in immobilization of His6-OPH extends its application areas. Polymers. 2023;15:591. doi: 10.3390/polym15030591. https://doi.org/10.3390/polym15030591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen H.L., Zhan H.Y., Chen Y.C., Fu S.Y. Construction of engineering microorganism degrading chlorophenol efficiently by protoplast fusion technique. Environ Prog Sustain Energy. 2013;32:443–448. https://doi.org/10.1002/ep.11626 [Google Scholar]
- 81.Angelucci D.M., Piscitelli D., Tomei M.C. Pentachlorophenol biodegradation in two-phase bioreactors operated with absorptive polymers: Box-Behnken experimental design and optimization by response surface methodology. Process Saf Environ Protect. 2019;131:105–115. https://doi.org/10.1016/j.psep.2019.09.005 [Google Scholar]
- 82.Manikandan S.K., Pallavi P., Shetty K., Bhattacharjee D., Giannakoudakis D.A., Katsoyiannis I.A., et al. Effective usage of biochar and microorganisms for the removal of heavy metal ions and pesticides. Molecules. 2023;28:719. doi: 10.3390/molecules28020719. https://doi.org/10.3390/molecules28020719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lu T.Q., Mao S.Y., Sun S.L., Yang W.L., Ge F., Dai Y.J. Regulation of hydroxylation and nitroreduction pathways during metabolism of the neonicotinoid insecticide imidacloprid by Pseudomonas putida. J Agric Food Chem. 2016;64:4866–4875. doi: 10.1021/acs.jafc.6b01376. https://doi.org/10.1021/acs.jafc.6b01376 [DOI] [PubMed] [Google Scholar]
- 84.Ding F., Peng W. Biological assessment of neonicotinoids imidacloprid and its major metabolites for potentially human health using globular proteins as a model. J Photochem Photobiol B Biol. 2015;147:24–36. doi: 10.1016/j.jphotobiol.2015.03.010. https://doi.org/10.1016/j.jphotobiol.2015.03.010 [DOI] [PubMed] [Google Scholar]
- 85.Tang X., Liang B., Yi T., Manco G., Palchetti I., Liu A. Cell surface display of organophosphorus hydrolase for sensitive spectrophotometric detection of p-nitrophenol substituted organophosphates. Enzym Microb Technol. 2014;55:107–112. doi: 10.1016/j.enzmictec.2013.10.006. https://doi.org/10.1016/10.1016/j.enzmictec.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 86.Shimazu M., Mulchandani A., Chen W. Cell surface display of organophosphorus hydrolase using ice nucleation protein. Biotechnol Prog. 2001;17:76–80. doi: 10.1021/bp0001563. https://doi.org/10.1021/bp0001563 [DOI] [PubMed] [Google Scholar]
- 87.Takayama K., Suye S., Kuroda K., Ueda M., Kitaguchi T., Tsuchiyama K., et al. Surface display of organophosphorus hydrolase on Saccharomyces cerevisiae. Biotechnol Prog. 2006;22:939–943. doi: 10.1021/bp060107b. https://doi.org/10.1021/bp060107b [DOI] [PubMed] [Google Scholar]
- 88.Richins R.D., Kaneva I., Mulchandani A., Chen W. Biodegradation of organophosphorus pesticides by surface-expressed organophosphorus hydrolase. Nat Biotechnol. 1997;15:984–987. doi: 10.1038/nbt1097-984. https://doi.org/10.1038/nbt1097-984 [DOI] [PubMed] [Google Scholar]
- 89.Liu Y., Wang X., Nong S., Bai Z., Han N., Wu Q., et al. Display of a novel carboxylesterase CarCby on Escherichia coli cell surface for carbaryl pesticide bioremediation. Microb Cell Factories. 2022;21:97. doi: 10.1186/s12934-022-01821-5. https://doi.org/10.1186/s12934-022-01821-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ding J., Liu Y., Gao Y., Zhang C., Wang Y., Xu B., et al. Biodegradation of lambda-cyhalothrin through cell surface display of bacterial carboxylesterase. Chemosphere. 2022;289 doi: 10.1016/j.chemosphere.2021.133130. https://doi.org/10.1016/j.chemosphere.2021.133130 [DOI] [PubMed] [Google Scholar]
- 91.Latifi A.M., Karami A., Khodi S. Efficient surface display of diisopropylfluorophosphatase (DFPase) in E. coli for biodegradation of toxic organophosphorus compounds (DFP and cp) Appl Biochem Biotechnol. 2015;177:624–636. doi: 10.1007/s12010-015-1766-0. https://doi.org/10.1007/s12010-015-1766-0 [DOI] [PubMed] [Google Scholar]
- 92.Hsieh H.Y., Lin C.H., Hsu S.Y., Stewart G.C. A Bacillus spore-based display system for bioremediation of atrazine. Appl Environ Microbiol. 2020;86:18 doi: 10.1128/AEM.01230-20. https://doi.org/10.1128/AEM.01230-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hernandez A.F., Parron T., Tsatsakis A.M., Requena M., Alarcon R., Lopez-Guarnido O. Toxic effects of pesticide mixtures at a molecular level: their relevance to human health. Toxicology. 2013;307:136–145. doi: 10.1016/j.tox.2012.06.009. https://doi.org/10.1016/j.tox.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 94.Cao X., Yang C., Liu R., Li Q., Zhang W., Liu J., et al. Simultaneous degradation of organophosphate and organochlorine pesticides by Sphingobium japonicum UT26 with surface-displayed organophosphorus hydrolase. Biodegradation. 2013;24:295–303. doi: 10.1007/s10532-012-9587-0. https://doi.org/10.1007/s10532-012-9587-0 [DOI] [PubMed] [Google Scholar]
- 95.Yang C., Yu H., Jiang H., Qiao C., Liu R. An engineered microorganism can simultaneously detoxify cadmium, chlorpyrifos, and gamma-hexachlorocyclohexane. J Basic Microbiol. 2016;56:820–826. doi: 10.1002/jobm.201500559. https://doi.org/10.1002/jobm.201500559 [DOI] [PubMed] [Google Scholar]
- 96.Yang C., Liu R., Yuan Y., Liu J., Cao X., Qiao C., et al. Construction of a green fluorescent protein (GFP)-marked multifunctional pesticide-degrading bacterium for simultaneous degradation of organophosphates and gamma-hexachlorocyclohexane. J Agric Food Chem. 2013;61:1328–1334. doi: 10.1021/jf304976h. https://doi.org/10.1021/jf304976h [DOI] [PubMed] [Google Scholar]
- 97.Cho C.M., Mulchandani A., Chen W. Bacterial cell surface display of organophosphorus hydrolase for selective screening of improved hydrolysis of organophosphate nerve agents. Appl Environ Microbiol. 2002;68:2026–2030. doi: 10.1128/AEM.68.4.2026-2030.2002. https://doi.org/10.1128/AEM.68.4.2026-2030.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mee-Hie Cho C., Mulchandani A., Chen W. Functional analysis of organophosphorus hydrolase variants with high degradation activity towards organophosphate pesticides. Protein Eng Des Sel. 2006;19:99–105. doi: 10.1093/protein/gzj007. https://doi.org/10.1093/protein/gzj007 [DOI] [PubMed] [Google Scholar]
- 99.Li J.D., Min J., Wang Y., Chen W.W., Kong Y.C., Guo T.Y., et al. Engineering Burkholderia xenovorans LB400 BphA through site-directed mutagenesis at position 283. Appl Environ Microbiol. 2020;86:19 doi: 10.1128/AEM.01040-20. https://doi.org/10.1128/AEM.01040-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X., Liang M., Liu Y., Fan X. Directed evolution and secretory expression of a pyrethroid-hydrolyzing esterase with enhanced catalytic activity and thermostability. Microb Cell Factories. 2017;16:81. doi: 10.1186/s12934-017-0698-5. https://doi.org/10.1186/s12934-017-0698-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gong T., Liu R., Zuo Z., Che Y., Yu H., Song C., et al. Metabolic engineering of Pseudomonas putida KT2440 for complete mineralization of methyl parathion and gamma-hexachlorocyclohexane. ACS Synth Biol. 2016;5:434–442. doi: 10.1021/acssynbio.6b00025. https://doi.org/10.1021/acssynbio.6b00025 [DOI] [PubMed] [Google Scholar]
- 102.Zhao Y., Che Y., Zhang F., Wang J., Gao W., Zhang T., et al. Development of an efficient pathway construction strategy for rapid evolution of the biodegradation capacity of Pseudomonas putida KT2440 and its application in bioremediation. Sci Total Environ. 2021;761 doi: 10.1016/j.scitotenv.2020.143239. https://doi.org/10.1016/j.scitotenv.2020.143239 [DOI] [PubMed] [Google Scholar]
- 103.Team: METU_TURKEY 2013. https://2013.igem.org/Team:METU_Turkey
- 104.Team: British_COLUMBIA 2015. https://2015.igem.org/Team:British_Columbia
- 105.Team: Goettingen 2018. http://2018.igem.org/Team:Goettingen
- 106.Team: UIUC_Illinois 2019. https://2019.igem.org/Team:UIUC_Illinois
- 107.Team: CSL_PITTSBURGH 2019. https://2019.igem.org/Team:CSL_Pittsburgh
- 108.Team: Waterloo 2019. https://2019.igem.org/Team:Waterloo
- 109.Team: XMU-China 2020. https://2020.igem.org/Team:XMU-China
- 110.Team: USP-Brazil 2021. https://2021.igem.org/Team:USP-Brazil
- 111.Team: Concordia-Montreal 2022. https://2022.igem.wiki/concordia-montreal;
- 112.Gilbert E.S., Walker A.W., Keasling J.D. A constructed microbial consortium for biodegradation of the organophosphorus insecticide parathion. Appl Microbiol Biotechnol. 2003;61:77–81. doi: 10.1007/s00253-002-1203-5. https://doi.org/10.1007/s00253-002-1203-5 [DOI] [PubMed] [Google Scholar]
- 113.Yan D.Z., Mao L.Q., Li C.Z., Liu J. Biodegradation of hexachlorobenzene by a constructed microbial consortium. World J Microbiol Biotechnol. 2015;31:371–377. doi: 10.1007/s11274-014-1789-7. [DOI] [PubMed] [Google Scholar]
- 114.Lawson C.E., Harcombe W.R., Hatzenpichler R., Lindemann S.R., Loffler F.E., O’Malley M.A., et al. Common principles and best practices for engineering microbiomes. Nat Rev Microbiol. 2019;17:725–741. doi: 10.1038/s41579-019-0255-9. https://doi.org/10.1038/s41579-019-0255-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rocca J.D., Muscarella M.E., Peralta A.L., Izabel-Shen D., Simonin M. Guided by microbes: applying community coalescence principles for predictive microbiome engineering. mSystems. 2021;6 doi: 10.1128/mSystems.00538-21. https://doi.org/10.1128/mSystems.00538-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Borella L., Novello G., Gasparotto M., Renella G., Roverso M., Bogialli S., et al. Design and experimental validation of an optimized microalgae-bacteria consortium for the bioremediation of glyphosate in continuous photobioreactors. J Hazard Mater. 2023;441 doi: 10.1016/j.jhazmat.2022.129921. https://doi.org/10.1016/j.jhazmat.2022.129921 [DOI] [PubMed] [Google Scholar]
- 117.Liu J., Zhou X., Wang T., Fan L., Liu S., Wu N., et al. Construction and comparison of synthetic microbial consortium system (SMCs) by non-living or living materials immobilization and application in acetochlor degradation. J Hazard Mater. 2022;438 doi: 10.1016/j.jhazmat.2022.129460. https://doi.org/10.1016/j.jhazmat.2022.129460 [DOI] [PubMed] [Google Scholar]
- 118.Stephens K., Bentley W.E. Synthetic biology for manipulating quorum sensing in microbial consortia. Trends Microbiol. 2020;28:633–643. doi: 10.1016/j.tim.2020.03.009. https://doi.org/10.1016/j.tim.2020.03.009 [DOI] [PubMed] [Google Scholar]
- 119.Mee M.T., Collins J.J., Church G.M., Wang H.H. Syntrophic exchange in synthetic microbial communities. Proc Natl Acad Sci U S A. 2014;111(20): E2149-2156 doi: 10.1073/pnas.1405641111. https://doi.org/10.1073/pnas.1405641111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou K., Qiao K., Edgar S., Stephanopoulos G. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol. 2015;33:377–383. doi: 10.1038/nbt.3095. https://doi.org/10.1038/nbt.3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sjostrand J., Tofigh A., Daubin V., Arvestad L., Sennblad B., Lagergren J. A Bayesian method for analyzing lateral gene transfer. Syst Biol. 2014;63:409–420. doi: 10.1093/sysbio/syu007. https://doi.org/10.1093/sysbio/syu007 [DOI] [PubMed] [Google Scholar]
- 122.Schwarz E., Khurana S., Chakrawal A., Chavez Rodriguez L., Wirsching J., Streck T., et al. Spatial control of microbial pesticide degradation in soil: a model-based scenario analysis. Environ Sci Technol. 2022;56:14427–14438. doi: 10.1021/acs.est.2c03397. https://doi.org/10.1021/acs.est.2c03397 [DOI] [PMC free article] [PubMed] [Google Scholar]