Abstract
In order to meet the growing demand for resources, there is a rising interest in macroalgae cultivation worldwide due to their potential as a source of food, fuel, and bio-products. However, large-scale and sustainable seaweed cultivation has been a persistent challenge. Specific fundamental issues need to be addressed to maximize the benefits of seaweed production. This article reviews a plan for transitioning to an environmentally sustainable aquaculture system incorporating non-toxic nanoparticles. It also provides an overview of genetic enhancement techniques for macroalgae species to realize their potential fully. Additionally, the article discusses the need for advanced tools and concepts to overcome the challenges in seaweed identification and cultivation and emphasizes the importance of a coordinated effort in fundamental and applied research using emerging technologies to ensure long-term practicality.
Keywords: Seaweed, Cultivation, Nanoparticles, Genetic engineering
1. Introduction
Seaweeds or macroalgae are multicellular photosynthetic organisms that can float freely or be attached to objects like rocks in the subtidal or intertidal part of the marine environment [1]. The production of seaweed has increased significantly over the past few decades. It is predicted that just 2% of the ocean's surface might be used to cultivate seaweed species, increasing current agricultural food production twofold [2]. Growing seaweed has received a lot of interest as a sustainable source of bioenergy feedstock as it is more productive (1600 g C m− 2 year− 1) than terrestrial plants (470 g C m− 2 year− 1) [3]. It now accounts for 51.3% (34.7 MMT, wet weight) of all marine aquaculture worldwide, with China and eight other Asian nations dominating output (99.6%) [4,5] (see Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6).
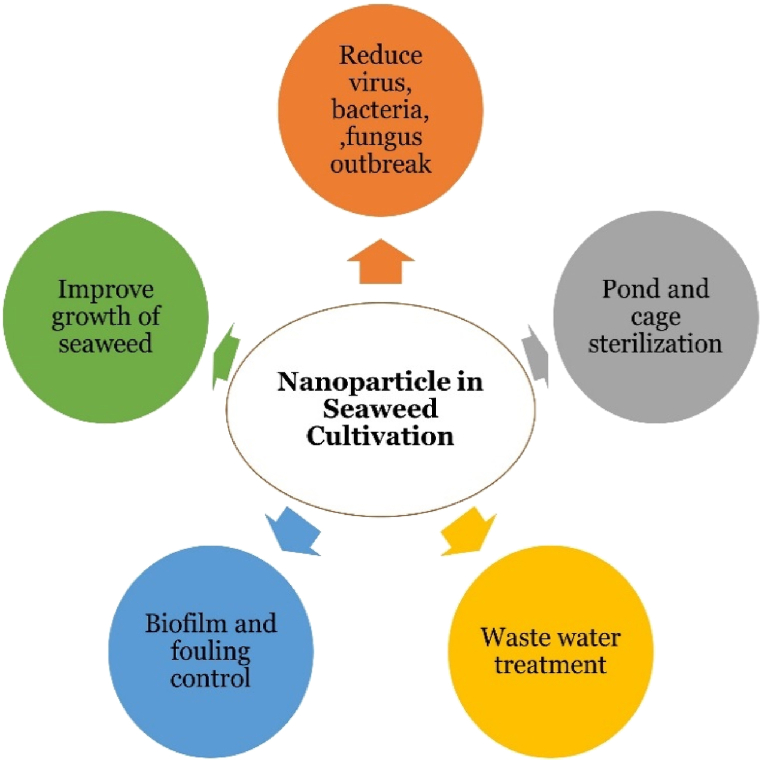
Fig. 1.
Application of nanoparticles.
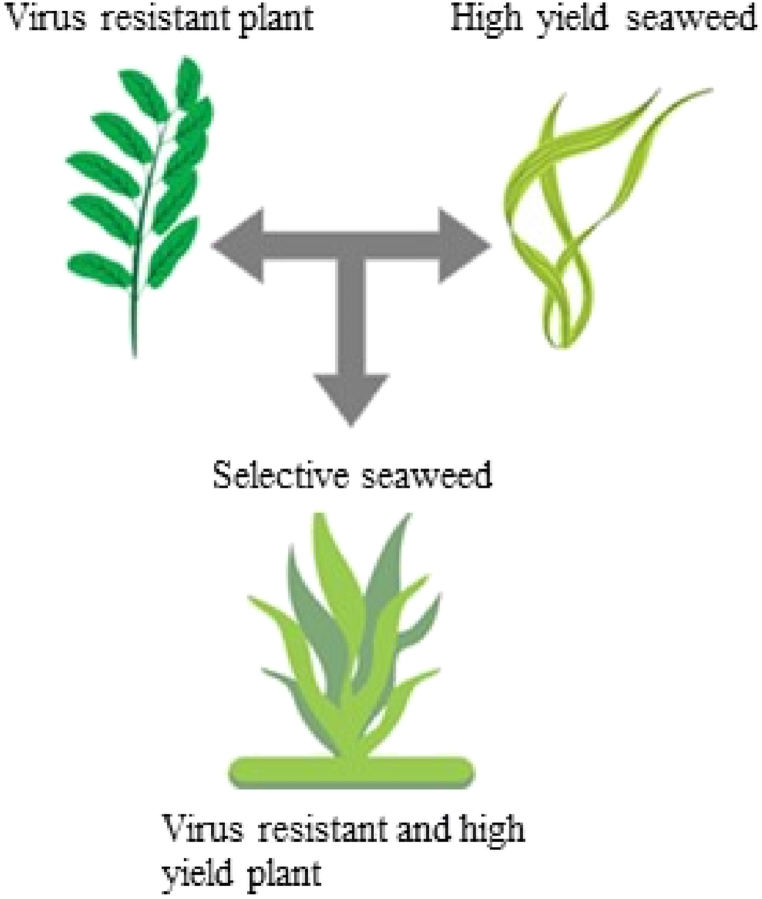
Fig. 2.
Diagram showing selective breeding.
Fig. 3.
DNA mutation by physical and chemical mutagen.
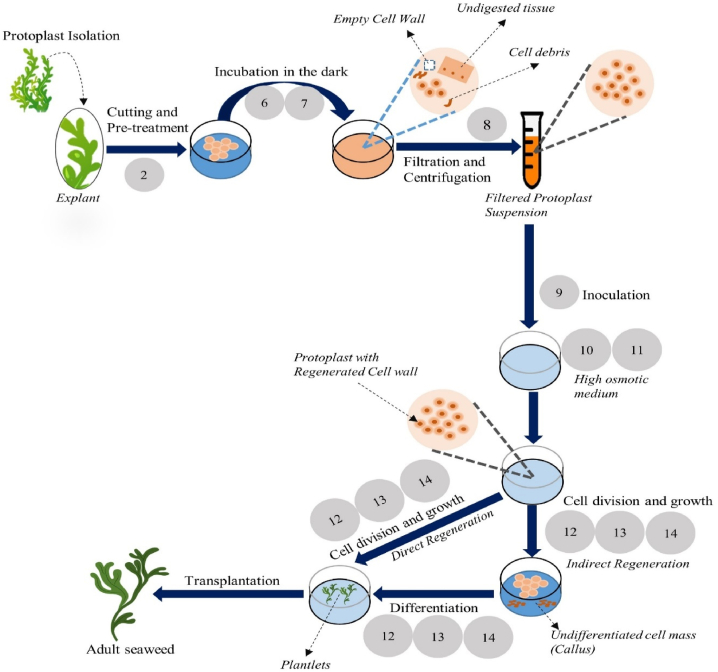
Fig. 4.
Macroalgae separation, cultivation, and regeneration [6].
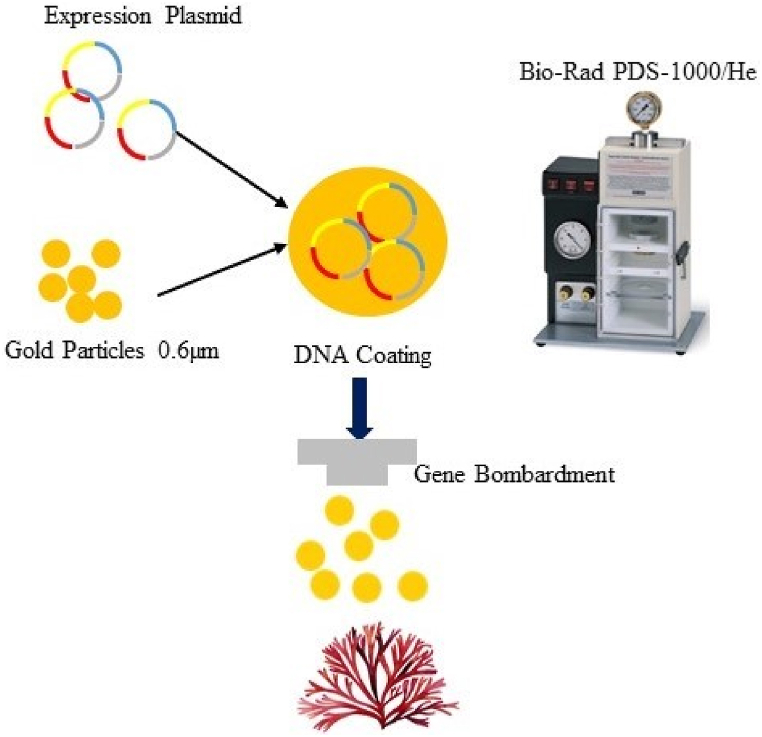
Fig. 5.
Gentic transformation technique in N. yezoensis (Rhodophyta) [7].
Fig. 6.
Application of Advanced tools in seaweed farming.
According to ([8], seaweed farming can accomplish various objectives while advancing several of the Sustainable Development Goals set forth by the United Nations (UN SDGs). These include eradicating hunger (SDG2), promoting health and well-being (SDG3), ensuring access to affordable and clean energy (SDG7), addressing climate change (SDG13), and preserving life in the oceans (SDG14), all of which have resounding advantages that support other SDGs [8]. In coastal countries, seaweed farming is a popular method for developing a sustainable blue economy [9]. Seaweed can also enhance the worldwide vegetable diet consumption and food supply chain.
Seaweed can be grown offshore, on land, and in integrated aquaculture systems. According to the species, location of the farm, and growing facilities, seaweed is chosen for cultivation. In Integrated multi-trophic aquaculture (IMTA), feed species are combined with extractive species, which thrive by consuming organic and inorganic waste from aquaculture. IMTA systems can be modified depending on the physical, chemical, environmental, biological, sociological, and economic factors unique to different world regions. IMTA can support sustainable aquaculture, which has advantages for the environment, the economy, and society. This can be accomplished through product variety and potential value payments. Higher financial benefits are brought on by increased production efficiency and nutrient recycling [10]; Osch et al., 2019).
Seaweed farming is based primarily on conventional methods, in which seaweeds are sensitive to diseases and environmental issues. Most seaweeds are grown from seedlings derived from cultivated germplasm via vegetative propagation to boost productivity. There is increasing attention among various stakeholders to engage in producing different macroalgae species broadly. Expansion of seaweed cultivation requires new associates and the enhancement of existing ones. A synergy between various growth parameters is necessary to ensure optimal seaweed yield from the cultivation systems.
Logistical issues for traditional seaweed growers include locating suitable agricultural sites, infection, and seed mortality brought on by extreme weather and polluted water environment. Increasing the efficiency of seaweed cultivation requires new technology and the enhancement of existing ones. Nanotechnology and Modern biotechnology can be regarded as one of the finest choices for increasing production by overcoming various issues.
The utilization of high-tech cultivation methods, such as smart engineering and genetic engineering techniques, offer promising opportunities for the commercial development of marine algae. By introducing modified morphological traits, enhanced characteristics, and improved growth rates, seaweed production capacity can be significantly expanded. For instance, researchers can use genome editing techniques to understand better the relationship between genotype and phenotype and gene transformation techniques and approaches to identify promoter sequences. In addition, synthetic biology tools, such as de novo genome design, can create new systems from individual modules [11].
This review considers various technical aspects of nanotechnology and genetic engineering strategies to enhance the macroalgae farming technique. Hence, the study focuses on the following objective.
-
•
To investigate the various innovative technologies in aquaculture, including nanotechnology and genetic engineering.
-
•
To discuss the prospects of remote sensing and advanced computational tools in seaweed farming.
-
•
To provide some recommendations and future directions for advanced seaweed farming techniques.
2. Application of nanotechnology
Nanotechnology is the study, advancement, synthesis, modification, and use of functional material devices and systems by controlling matter at the nanometre scale (1–100 nm, where 1 nm is equal to 10−9 of a meter), that is, at the atomic and molecular levels. Nanotechnology is quickly gaining traction as the scientific and technological foundation for the next generation of new advances. Many nanomaterials have been produced and applied to various research disciplines. Nanotechnology has also considerably boosted specialized activity in a variety of industrial applications. In aquaculture, there are several nanomaterials applications, including pond sanitation, water purification, control of marine pathogens, and effective delivery of nutrients and medications [12]; S [13]. Nanotechnology is considered a prolific road for addressing significant problems of seaweed farming, such as monitoring diseases and parasites multiplying, biofouling control, and the potential to improve aquaculture productivity. For instance, nanoparticles substantially impact the financial feasibility of microalgae-based products [[14], [15], [16]]; hence nanomaterial's ability promotes a new green movement with fewer threats for farmers also [17] reported that Ni–Cu/TiO2/Ti electrode is a promising candidate for a nanostructure sensor of glucose concentrations detected in polluted and unpolluted growth conditions of U. lactuca. Nanosensor devices can also detect low quantities of parasites, bacteria, and viruses in the water and polluting components. This is especially essential in commercial aquaculture outbreaks because it might take a long time for the etiological agent to produce an impact and be diagnosed, delaying pathogen control treatment and causing significant economic losses. In this context, nanotechnology can solve this problem in macroalgae farming by detecting and eradicating viruses early on.
An extensive literature frame provides a concise description of nanotechnology's applications in aquaculture; however, there is little evidence of the use of nanoparticles in seaweed production. This technology may provide innovative and economic development routes to improve seaweed cultivation in the long term. Without question, new and inventive approaches are critical to macroalgae cultivation's long-term success. Finally, we emphasized that the aquaculture industry's embrace of nanomaterials and nanotechnology will be slow. Many possibilities, however, point to a potential approach to tackling macroalgae cultivation's major issues shortly. Overall, incorporating nanotechnology into various aquaculture techniques appears to be unavoidable and will undoubtedly lead to further advancements in the future. Below are the different foreseeable roles of nanoparticles in terms of their applications in seaweed cultivation (Fig. 1).
2.1. Few works of nanotechnology on seaweed cultivation
With fewer farming risks, the potential of nanomaterials inspires a new green revolution. Finally, we stressed that adopting nanomaterials and nano-technology in aquaculture will be a gradual process. To address the increasing challenges of sustainable production and food security in the upcoming years, nano-technology will have several applications in seaweed growth. However, there is still a long way to go. Although it is difficult to predict how a nanodevice would function in seaweed, we must begin with actual field tests to address some of the issues raised. The use of nanomaterials in the growing and harvesting of seaweeds is becoming more and more critical, but the field is still young and needs space for both their practical application and the prevention of adverse side effects. Precautions must be taken due to the impact of various nanoparticles and how they interact with multiple seaweeds in changing environmental circumstances. According to Ref. [18] in terms of recycling and NM functionalization with biocompatible elements, magnetic nanoparticles may help lower the toxicity, This could aid in the cost-effective, large-scale production of seaweeds that is also environmentally beneficial. Magnetic nanoparticles increased dispersibility to reach significant harvesting capacity; yet, their oxides were difficult to recycle or reuse. Cubic spinel ferrites coupled Mn, Cu, and Zn divalent ions revealed strong chemical stability, excellent biocompatibility, and inexpensive production costs aside from magnetic nanoparticles [19,20]. Furthermore, the diversification of macroalgae farming could be aided by manufacturing a wide range of high-value-added goods. In addition, the process's techno-economic evaluation would help with operational improvement to achieve overall energy-efficient harvesting and production of seaweed.Table 1 provides the summary of selected works on Nano-technology use in Seaweed farming
Table 1.
nanoparticles application as emerging ‘smart’ technologies in aquaculture.
| Nanoparticles | Applications | Reference |
|---|---|---|
| Nanodevice | Nanonets can improve water quality attributes by raising the pH of the water. | [52] |
| nCuO, nZnO, nSi | Promising contenders with a high specific surface area ratio provide a more effective defence against fouling agents. | [53] |
| AgNPs | Reduce fungus infection in rainbow trout fertilized ovules. In the future, it might be utilized to treat fungal diseases in seaweed. | [54] |
| AgNPs | Experiments show that bacteria cannot develop resistance to AgNPs to antibiotics | [55,56]. |
| Fe2O3, zero-valent nano iron | Significantly increase in growth and biochemical composition with adequate dose of nano iron | [57]. |
| Ag, Au NPs | The antimicrobiofilm activity against common marine biofilm-forming bacteria such as A. hydrophila, Salmonella sp., and S. liquefaciens | [58] |
2.2. Adverse effect of nanoparticles
It has been revealed that components based on nanotechnology are destructive to the environment and human health. As a result, it's crucial to consider the damaging effects of nanomaterials on aquatic ecosystems and human health.
-
1.
Nanoparticles negatively affect DNA replication as a result of assimilation, which could result in genetic changes [21].
-
2
[22] demonstrated that nAl2O3 inhibited Dunaliella salina growth indicating a link between NP concentration. Furthermore, the rise in NP content was accompanied by a decrease in chlorophyll and carotenoid levels in microalgae.
-
3.
At low concentrations, ZnO nanoparticles are highly cytotoxic and have a high capacity for protein adsorption [23].
-
4.
Water-soluble stable NPs can accumulate in the aquatic systems, and the activity of marine organisms is impacted by their exposure to the aquatic food chain in a concentration-dependent approach [[24], [25], [26]].
3. Biological engineering
3.1. Breeding to improve seaweed quality
Collecting seaweed strains with favourable environmental characteristics of high development rates, carrageenan content, disease tolerance, and rapid development is the foundation of a successful seaweed breeding program. Seaweed breeding techniques adapt to keep the farming sector growing in an ever-changing environment. Breeding seeks to alter a population's genetic makeup to enhance its characteristics according to its intended use. The first step in developing breeding is deciding on the attribute(s) to improve, such as the composition, characteristics, developmental factors, and modes of action to biotic and abiotic, which influence yield and productivity.
3.2. Selective breeding
Over ten years of study, a method for artificially producing sporelings from filamentous sporophytes was broadly applied. In Japan, by the 1960s [27], selective breeding with spores isolated from blades with good development was made possible. Parents with a larger ratio of beneficial alleles and a lower proportion of less acceptable alleles are identified in selection-based breeding procedures. Although selective breeding is used to generate superior variations, mostly limited to macroalgae genera like Porphyra (Rhodophyta) and Laminaria (Phaeophyceae). Since 1949, when Porphyra's life cycle and reproduction control were discovered, only 30 cultivars have been created [28]. Several high-value seaweed genera, including Saccharina [29], Undaria (Phaeophyceae), and Porphyra (Rhodophyta) [30,31], successfully used selective breeding and genetic improvement of cultivars for improving output, flavor, and other factors. At the Hyogo Prefectural Fisherie, cultivar development via the selection of thalli generated from protoplasts of selected cultivars has undertaken to improve the effectiveness of selective breeding. This technology allows for the collection of a huge quantity of single cells at once, increasing the chances of detecting variations caused by mutations [32].
The 59 Neopyropia yezoensis (formerly Porphyra yezoensis) (Nori) (Rhodophyta) was studied for a selective breeding program of economically important nori crops using the ITS1 region. Selective breeding in Japan achieves most nori cultivar enhancement for aquaculture [33]. Eukaryo ABC (Algae Breeding Cultures) is a Norwegian organization that specializes in selective seaweed breeding to create cultivars or “varieties” with higher yields, better consistency, and stability than wild seaweed. In general, such breeding functions as an effective partnership with industry where progeny is produced for the sustainability of entire industries. A MARINER Category 5 project led by the Woods Hole Oceanographic Institution aims to create a selective breeding program for, Saccharina latissima (Phaeophyceae), one of the most economically significant kelp types. The project's objective is to boost seaweed farming's production and profitability. Selective breeding has not yet produced successful seaweeds from the agarophytes, carragenophytes, and green seaweeds groups. Selective breeding concept is presented in Fig. 2.
Recurrent self-hybridization and selection are used in linebreeding. Line-breeding is used by some plants and the few seaweed genetic enhancement projects that are currently underway. To establish lines and the superior crossbred or F1 individuals from those lines, trait selection is combined with inbreeding.
3.3. Gene modification through mutagenesis
Random mutagenesis generated by either a physical or a chemical mutagen is employed to cause genetic alterations in algae (Fig. 3).
Chemical mutagens are standard mutagenesis methods in a wide variety of organisms. It is the most common way to generate mutations in phenotypic traits. Mutagenic chemicals like N-methyl-N′-nitro-N-nitrosoguanidine and ethyl-methane sulfonate have been used in marine macroalgae. For example, EMS has been used to create mutants of the red seaweed Gracilaria tikvahiae [34], which exhibit varied increasing patterns and thallus thickness, probably due to variations in the size of the medullary and sub-cortical cells of thallus [35]. Still, there is a significant chance of contamination during sewage disposal, which is a huge downside.
Physical mutagens consist of several forms of radiation that fall into two groups. The first group includes X-rays and gamma rays, generally considered to travel in waves and are part of the electromagnetic spectrum. The second form of radiation is corpuscular radiation, which comprises moving particles such as protons, neutrons, and electrons [36]. Among those physical mutagens, gamma rays have been used intensively in mutation breeding research to improve crop species' agronomic characteristics and quality. Physical mutagens are more likely to produce chromosomal alterations and more prominent DNA. Irradiation used on the brown macroalgae Ectocarpus siliculosus (Phaeophyceae) and the green seaweed Ulva compressa (formerly U. mutabilis) (Chlorophyta) has proven to be safe and effective [37]. Despite advances in genomic studies in terrestrial plants, seaweeds have rarely been studied globally and are not included in approaches to unravel gene functions. As a result, long-term seaweed genetic improvement initiatives should be started and developed. These mutations were created at random and without being labeled, they can only be identified using forward genetic techniques and molecular instruments.
3.4. Protoplast fusion and somatic hybridization
An invitro genetic modification approach is more successful than traditional strain enhancement strategies like mutation and selection. Using protoplasts from multiple sources takes advantage of natural genetic diversity and creates new genetic pairings that boost various functional features. This method has been widely used in terrestrial plants, resulting in several agricultural varieties with increased productivity or tolerance to biotic/abiotic. Bicolor, red and green chimera seedlings result from the protoplast fusion between two Gracilaria species, namely G. tikvahiae (green pigmented) and G. chilensis (red pigmented) [38]. Researchers generated seaweed variations with superior agronomic qualities via protoplast fusion between Gayralia oxysperma (formerly Monostroma oxyspermum) and Ulva reticulata (Chlorophyta) [39]. Fig. 4 illustrates the gene modification approach through regeneration
Utilizing the inherent genetic variety of phylogenetically distant species, somatic hybridization by protoplast fusion is a practical method that promotes the recombination of economically significant features [40]. For the first time [41], demonstrated successful trans-divisional protoplast fusion and regeneration in Monostroma and Porphyra somatic hybrids [42,43] discovered protoplast-based methods for generating seedling stocks (micro thalli) that can survive in an incubator for several years while still having the ability to grow into Ulva and Monostroma leafy thalli. Although protoplast synthesis and regeneration are not financially viable, Porphyra, Laminaria, Undaria, and Gracilaria are a few species of seaweed that are ubiquitous adopting the procedure [44]. Despite significant protoplast separation and regeneration achievements in various seaweed species, seaweed research lags significantly behind terrestrial plants.
3.5. Genetic transformation
In contrast to agricultural plants, macroalgae genetic transformation processes are still in the early phases of development. Under the direction of the genes delivered into cells, transformations can be classified as genetic (stable) or temporary (transient). In contrast to transient transformation, where introduced foreign genes are typically lost quickly, genetic recombination preserves genes in the genome over generations of cells.
Since the early 1990s, researchers have worked hard to find approaches to genetic transformation in seaweeds to create new strains with beneficial traits for business and medicine. But because it's a laborious process, most early studies were disclosed in conference abstracts rather than full publications. This condition has impeded scientists' ability to learn about gene activities in many physiological processes and the use of seaweeds in biotechnological applications. Creating an effective transformation process and selecting appropriate reporter genes and promoters are the foundations for building a seaweed transformation system [45]. To use these regulatory domains as prospective algal promoters for efficient and steady expression of foreign genes, considerable work was done to understand the gene regulatory domains in the algal system. Therefore, choosing influential endogenous promoters is essential for limiting the high expression of alien genes as promoter determines how well foreign genes are expressed in transformants, which is critical for genetic engineering. The NOS (nopaline synthase) promoter from Agrobacterium tumefaciens T-DNA and the 35S cauliflower mosaic virus (CaMV) are the two most common used promoters for constitutive expression [46]. used a fragment of 18SrDNA in a vector pQD-GUS for the first time to use homologous promoters for foreign gene expression in Porphyra protoplasts, comparing GUS protein expression to that of the original pBS-GUS vector. Another study using the SV40 promoter (from the simian virus) found that the lacZ reporter gene was expressed uniformly in regenerated Laminaria sporophytes, demonstrating a high transcription recognition efficiency regardless of tissue specificity [47]. Functionality of the Cauliflower Mosaic Virus 35S promoter in Chondrus which has also worked well in, Gracilaria changii [48], Kappaphycus alvarezii [49] and Wildemania miniata (formerly Porphyra miniata) (Rhodophyta) [50] (
Additionally, the selection of beneficial transformants can be sped up by using the right reporter genes. Macroalgae have previously been utilized as reporters for the bacterial -galactosidase lacZ and -glucuronidase (GUS) genes. According to Ref. [51], fluorescent proteins can be expressed briefly in Neopyropia yezoensis (Rhodophyta) gametophytic cells. This raises the possibility of using fluorescent proteins as reporter genes in upcoming genetic transformation studies. Particle bombardment, particle guns, or biolistics is one of the most common methods for achieving nuclear and organelle transformation in organisms for algae andplants, fungi, insects, animals, and even bacteria. Below are different methods applied to seaweed for genetic transformation, as shown in Table 2.
Table 2.
Seaweeds that were genetically transformed.
| Method | Technique | Seaweed | Promotors | Marker | Reference |
|---|---|---|---|---|---|
| Electroporation | Electrodes create voltage imbalance across the cell membrane, enabling substances to travel through the phospholipid bilayer into the cell. |
Neopyropia yezoensis (formerly Porphyra yezoensis), (Rhodophyta) |
CaMV 35S | GUS | [46]. |
| Microparticle Bombardment | Target cells collide with accelerated DNA-coated non-reactive metal (gold or tungsten) micro projectiles | Gracilaria changii (Rhodophyta) | SV40 | lacZ | [59] |
| Glass bead agitation | Algal cells are activated by glass beads when DNA and polyethylene glycol are together. | NeoPorphyra haitanensis (formerly Pyropia haitanensis) (Rhodophyta) | SV40 | lacZ; EGFP | [60] |
| Electroporation | – | Ulva lactuca (Chlorophyta) | CaMV 35S | GUS | (X [61]. |
| Particle bombardment | – | Undaria pinnatifida (Phaeophyceae) | CaMV 35S | GUS | [45] |
| Particle bombardment | – | Ulva australis (formerly Ulva pertusa) (Chlorophyta) | UprbcS | EGFP | [62] |
Although genetic transformation techniques are positively practical to several microalgae, very little research on seaweed has been published because microalgae genomic information is abundant and comprehensive, whereas seaweed genomic information is limited. Seaweed molecular biological studies are now proceeding more slowly than land green plant molecular biological studies due to challenges with efficient genetic transformation methods. A genetic transformation system's development would improve genetic engineering and our biological knowledge (Fig. 5).
3.6. Less acceptability of genetically modified algae
Genetic engineering has the potential to address several of these tech issues and environmental concerns, but still, there is less acceptability of genetically modified algae. GE algal research is only permitted in accredited labs and pilot plants in the EU. Safety procedures must be taken to prevent any hazards that may ultimately arise from the uncontrolled release of these GE algae into the environment. The legal status of GE algae (and genetically engineered organisms in general) in the United States was reviewed in 2014. The authors concentrate on the difficulties in controlling modified microorganisms, one of which is the research and development phase, where it's possible that the risks of application won't be understood until the research is over [63]. Recent instances of approved outdoor GE algae growing at facilities run by Sapphire Energy in California, United States, and StelaGenomics México in Guanjuato, Mexico. Maintaining secondary containment, netting, and checking bucket traps outside the main growing pond for the development of the GE algae were among the TERA's requirements for Sapphire Energy [64]under the Biosafety Law of GMOs, Mexico made similar demands [65]. According to Ref. [66], there is no proof of doubts or even resistance to the GE algae. This can be due to the lack of coverage in the media and the limited public awareness of GE algae.
A coordinated effort in fundamental research is urgently needed to assess genetic resources and develop adequate genetic conservation policies, which are, to date, almost totally absent, to ensure the sustainability of GE algal cultivation and its public acceptance as an environmentally sustainable activity. The federal government of a country must be notified and, in some situations, approved using genetically modified algae in contained or uncontained applications. Most nations have specific legislation for actions involving altered microorganisms that apply to algae. According to a US Environmental Protection Agency (EPA) judgment (TSCA), any altered algal biomass is governed by the Toxic Substances Control Act. Before using the modified algae for commercial purposes, importing it, or conducting outside research and development (R&D), the authorities must be notified. The final biosafety evaluation of a genetically modified seaweed requires interdisciplinary research on algal genetics, physiology, reproductive biology, and ecology from the molecular to at least the local aquatic ecosystem level through mathematics, biology, chemistry, physics, and even sociology when taking into account environmental issues. These developments will open up countless opportunities for seaweed genetic engineering, which is still in its early stages.
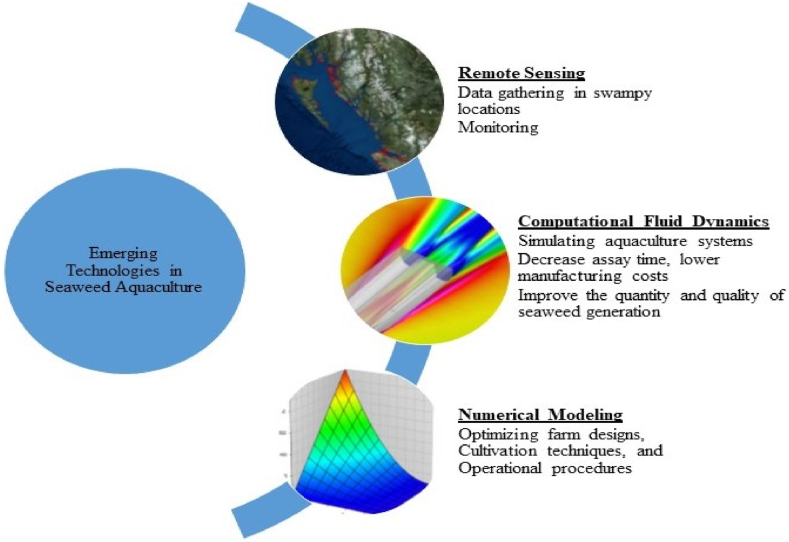
4. Emerging methods facilitating seaweed aquaculture
4.1. Remote sensing
Data gathering in swampy locations, particularly macroalgal bloom mapping, can be complex since it typically requires special vehicles such as hovercrafts that are highly labour-intensive. Hence, new scientific and technological paths have surfaced to address these aquaculture challenges. One of the most efficient tools for monitoring is remote sensing, a supplement to field-based environmental monitoring. Long-term monitoring of the floating surface canopy of giant kelp was possible because of multispectral Landsat data [67]. Many kelp forest habitats, which provide critical services to both people and the environment, have experienced historical and continuous reductions, prompting the creation of this web tool. Using three Landsat sensors [68],created an enormous kelp canopy spatiotemporal dataset to investigate the impact of diverse period and site scales on long-term biomass changes.
The integration of optical and acoustic sensors in new autonomous vehicle technologies can provide quick, repeatable facilities across the surface of the water [69,70]. MODIS (Moderate Resolution Imaging Spectroradiometer) pictures were also an excellent source for mapping floating elements in different aquatic systems (C [[71], [72], [73]].
To determine the optimal location for seaweed cultivation systems to prevent harmful algal blooms, using multi-sensor remote sensing data from satellite systems and in-situ observation, multi-temporal and multi-spatial evolution of N. yezoensis cultivation in the southern Yellow Sea [74].
The traditional remote sensing data processing technique has been transformed by the Google Earth Engine (GEE), a virtual solution for extensive geospatial information study. This platform gives customers access to advanced satellite image data (Landsat and Sentinel series satellites) and does image pre-processing on the cloud platform. One can track the spread of algae over time by incorporating the algae extraction algorithm. From 2007 to 2015 [75] used the mean distribution of Ulva prolifera in the South Yellow Sea using the FAI algorithm on MODIS imagery and statistical approaches according to the findings, U. prolifera had the most widespread coverage in 2015.
4.2. Computational fluid dynamics (CFD) analysis
CFD technique is an essential tool for simulating aquaculture systems by constructing aquaculture model systems. It can decrease assay time, lower manufacturing costs, and increase the quantity and quality of seaweed generation. Furthermore, the CFD model can account for biotic and abiotic elements influencing the seaweed habitat. More reliable data and evidence can be used to build a better solution for the proposed seaweed system. Hence it is an effective instrument for conducting parametric analyses to improve aquaculture systems.
[76] constructed a CFD model that enabled them to predict the velocity profile related to the flow of nutrients from monoline to tube net, demonstrating good nutrient availability in monoline mode, which led to better production. According to (Haro et al., 2020), CFD Simulation helps to speed up the process of a seaweed harvesting vessel by upgrading the conventional designs. Significant improvements in the shape of seaweed harvesting vessels can help to reduce lateral displacement, improve worker safety, and test novel strategies. CFD simulations and model creation for the most significant cultivation parameters must be confirmed through laboratory/field experiments; the numerical results can aid in developing a better experimental design. These innovations will bring unlimited possibilities in seaweed genetic engineering, which is still in its infancy. Combining these innovations with the rapidly developing fields of systems biology and metabolic engineering will satisfy the demands related to energy, the environment, and human health.
4.3. Numerical modeling
Numerical modeling can help to maximize production by optimizing farm designs, cultivation techniques, and operational procedures and anticipating impacts on coastal ecosystems. Fig. 6 illustrates the application of advanced tools in seaweed farming.
For Gelidium corneum (formerly Gelidium sesquipedale) (Rhodophyta [77] developed a dynamic model that forecasted NPP. Depth, production in relation to heat, respiration rates, brightness, output exudation, frond breakage, fatality, and a carbon-to-dry-weight conversion factor are considered in the model. The metabolism and growth rate of Ulva sp. were investigated using a mathematical model [78] in which one output file was generated for each month of the year while the model was running on a global 1° grid. Light intensity (I), temperature (T), salinity (S), dissolved nutrients (nitrate and phosphate), and respiration rate were used to compute the algal growth rate. The model presumption was that the rate of biomass increase was influenced differently by each environmental component [79]. developed a multi-scale model with variables such as climate, sunlight, and essential nutrients on an intense commercial farm for Ulva sp. (Chlorophyta) seaweed production and nitrogen absorption. It provides a critical alternative for environmental agencies and seaweed enthusiasts looking to extend their phytoremediation and seaweed production farms, enhancing ocean sustainability and the macroalgae-based economy [80]. developed the Macroalgal Cultivation Modeling System MACMODS, a macroalgae-specific computational modeling framework for evaluating within-farm fluctuations in sunlight, salinity flow, and nutrition fields on spatial and temporal scales. Models can help site selection for aquaculture activities, increasing blue growth.
5. Outlook and future perspectives
-
•
Activities like urbanization, rapid industrialization, pollution of water bodies, and tourism in coastal areas affect seaweed growth. Even though seaweed production is economically viable, the blue economy concept is still a convincing argument for growing macroalgae.
-
•
The intersection of biotech and aquaculture is positioned to cultivate macroalgae in a strategic convergence to keep up with the rising demand for food, fuel, and bio-product needs.
-
•
The advances in macroalgae biology and structural composition, microbiome sequencing, and computational approaches have substantially improved our understanding of microbiome diversity and the genes involved.
-
•
Genome-based techniques are becoming increasingly prominent for examining and identifying novel gene functions in bacterial communities.
-
•
Gene mutations are feasible by recognizing the genetic variants in producing metabolites and compounds. This must be done and applied with caution.
6. Conclusion
Seaweed can be grown in various ways; however, fundamental problems must be addressed for consistent yields to realize the prospective benefits of macroalgae farming. The following are the key outcomes from the review study:
-
•
It is still challenging to adopt economically feasible and sustainable methods of seaweed cultivation in several regions of the globe while maintaining productivity. The macroalgae aquaculture industry has extensive challenges in terms of innovation.
-
•
Nano-technology undoubtedly plays an essential role in aquaculture's growth and the longstanding feasibility of underutilizing seaweed cultivation. Using nanoparticles in macroalgae agriculture is a viable technique to achieve desired biomass output and high-value goods. We believe that nano-technology will alter aquaculture by focusing research and development toward achieving sustainable macroalgae farming with targeted efforts from governments and academics. However, its use will be recognized with further study and continued use.
-
•
New possibilities for incorporating nano-technologies in the seaweed cultivation method should be investigated while considering potential environmental or human health risks. However, further investigation is strongly suggested to increase seaweed growth and the potential risk of using nanoparticles in macroalgae farming.
-
•
To address biological challenges in seaweed cultivation, it is crucial to establish long-term genetic improvement strategies. Genetic engineering holds promise in bridging the gap between fundamental and applied seaweed science. By leveraging these advancements, new avenues can be explored in seaweed cultivation.
-
•
Competency in seaweed biology, genetics, and metabolomics is required to optimize seaweed composition to reach large-scale production demands. In addition, it provides informed decisions regarding species selection, propagation, disease prevention, and sustainability.
-
•
A new multidisciplinary approach must be evolved, combining existing seaweed scientific knowledge with computational tools and electronic sensors to efficiently control the complex biological process. It can reduce overall costs and enhance aquaculture productivity and efficiency. Numerous obstacles still exist to overcome in research and acceptance among the scientific community.However, continous innovations in Seaweed farming are needed to cope with the changing world due to global climate change
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
No data was used for the research described in the article.
Declaration of interest’s statement
The authors declare no competing interests.
Acknowledgements
This research is funded by the Universiti Malaysia Pahang (UMP)www.ump.edu.my) Postgraduate Research Grant Scheme (PGRS) grant number: PGRS210348.
References
- 1.Pati M.P., Sharma S. Das, Nayak L., Panda C.R. Uses of seaweed and its application to human welfare: a review. Int. J. Pharm. Pharmaceut. Sci. 2016;8(10):12–20. doi: 10.22159/IJPPS.2016V8I10.12740. [DOI] [Google Scholar]
- 2.Forster J., Radulovich R. Seaweed and food security. Seaweed Sustainability. Food Non-Food App. 2015:289–313. doi: 10.1016/B978-0-12-418697-2.00011-8. [DOI] [Google Scholar]
- 3.Balina K., Romagnoli F., Pastare L., Blumberga D. Use of macroalgae for bioenergy production in Latvia: review on potential availability of marine coastline species. Energy Proc. 2017;113:403–410. doi: 10.1016/j.egypro.2017.04.022. [DOI] [Google Scholar]
- 4.Cai J., Lovatelli A., Aguilar-Manjarrez, Cornish L., Dabbadie L. 2021. Seaweeds And Microalgae: An Overview For Unlocking Their Potential In Global Aquaculture Development. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development. [DOI] [Google Scholar]
- 5.Chopin T., Tacon A.G.J. 2020. Importance of Seaweeds and Extractive Species in Global Aquaculture Production; pp. 139–148. 10.1080/23308249.2020.1810626, 29(2) [DOI] [Google Scholar]
- 6.Avila-Peltroche J. International Society for Applied Phycology Newsletter. 2021. Protoplasts: a promising biotechnological tool for seaweed research.https://www.appliedphycologysoc.org/resources/Documents/ISAP newsletter 1-2021_July2021.pdf. [Google Scholar]
- 7.Mikami K. 2013. Current Advances in Seaweed Transformation. An Integrated View Of the Molecular Recognition And Toxinology - from Analytical Procedures To Biomedical Applications. [DOI] [Google Scholar]
- 8.Duarte C.M., Bruhn A., Krause-Jensen D. A seaweed aquaculture imperative to meet global sustainability targets. Nat. Sustain. 2022;5(3):185–193. doi: 10.1038/S41893-021-00773-9. [DOI] [Google Scholar]
- 9.Sarker S., Mahmudul Islam M. 2021. pp. 1–15. (Marine Protected Area and Biodiversity Conservation). [DOI] [Google Scholar]
- 10.Chopin T., Cooper J.A., Reid G., Cross S., Moore C. Open-water integrated multi-trophic aquaculture: environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev. Aquacult. 2012;4(4):209–220. doi: 10.1111/J.1753-5131.2012.01074.X. [DOI] [Google Scholar]
- 11.Purnick P.E.M., Weiss R. The second wave of synthetic biology: from modules to systems. Nat. Rev. Mol. Cell Biol. 2009;(6):410–422. doi: 10.1038/nrm2698. 2009 10:6, 10. [DOI] [PubMed] [Google Scholar]
- 12.Atanu B., Janardana R.S., Md Mahbub H., Adeyemi M.M., Regina M.R., Raja N.H. Nano-technology - a unique future technology in aquaculture for the food security. Biol. Eng. Int. J. Bioass. 2015 https://www.semanticscholar.org/paper/Nanotechnology-a-unique-future-technology-in-for-Bhattacharyya-Reddy/6d10f86a818b8848467a825ad6cd356631f444ec [Google Scholar]
- 13.Huang S., Wang L., Liu L., Hou Y., Li L. Nano-technology in agriculture, livestock, and aquaculture in China. A review. Agron. Sustain. Dev. 2015;35(2):369–400. doi: 10.1007/S13593-014-0274-X/TABLES/6. [DOI] [Google Scholar]
- 14.Duman F., Sahin U., Atabani A.E. Harvesting of blooming microalgae using green synthetized magnetic maghemite (γ-Fe2O3) nanoparticles for biofuel production. Fuel. 2019;256 doi: 10.1016/J.FUEL.2019.115935. [DOI] [Google Scholar]
- 15.Javed F., Aslam M., Rashid N., Shamair Z., Khan A.L., Yasin M., Fazal T., Hafeez A., Rehman F., Rehman M.S.U., Khan Z., Iqbal J., Bazmi A.A. Microalgae-based biofuels, resource recovery and wastewater treatment: a pathway towards sustainable biorefinery. Fuel. 2019;255 doi: 10.1016/J.FUEL.2019.115826. [DOI] [Google Scholar]
- 16.Lau A.K.S., Bilad M.R., Nordin N.A.H.M., Faungnawakij K., Narkkun T., Wang D.K., Mahlia T.M.I., Jaafar J. Effect of membrane properties on tilted panel performance of microalgae biomass filtration for biofuel feedstock. Renew. Sustain. Energy Rev. 2020;120 doi: 10.1016/J.RSER.2019.109666. [DOI] [Google Scholar]
- 17.Alsufyani T., Fadlallah S.A. Detection of glucose in the growth media of Ulva lactuca using a Ni-Cu/TiO2/Ti self-assembly nanostructure sensor under the influence of crude oil. Sens. Bio-Sens. Res. 2017;14:7–16. doi: 10.1016/J.SBSR.2017.03.005. [DOI] [Google Scholar]
- 18.Jain U., Chauhan N. Nano-technology for enhancing sea food production and its application in coastal agriculture. Nanosci. Sustain. Agri. 2019:639–657. doi: 10.1007/978-3-319-97852-9_25/COVER. [DOI] [Google Scholar]
- 19.Lee Y.C., Lee K., Oh Y.K. Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: a review. Bioresour. Technol. 2015;184:63–72. doi: 10.1016/J.BIORTECH.2014.10.145. [DOI] [PubMed] [Google Scholar]
- 20.Solano E., Perez-Mirabet L., Martinez-Julian F., Guzmán R., Arbiol J., Puig T., Obradors X., Yañez R., Pomar A., Ricart S., Ros J. Facile and efficient one-pot solvothermal and microwave-assisted synthesis of stable colloidal solutions of MFe2O4 spinel magnetic nanoparticles. J. Nanoparticle Res. 2012;14(8):1–15. doi: 10.1007/S11051-012-1034-Y. 2012 14:8. [DOI] [Google Scholar]
- 21.Suppan S. Institute for Agriculture and Trade Policy; 2011. Racing Ahead: U.S. Agri-Nanotechnology in the Absence of Regulation.https://www.iatp.org/documents/racing-ahead-us-agri-nanotechnology-absence-regulation [Google Scholar]
- 22.Shirazi M., Shariati F., Keshavarz A.K., Ramezanpour Z. Toxic effect of aluminum oxide nanoparticles on green micro-algae Dunaliella salina. Int. J. Environ. Res. 2015 https://www.cabdirect.org/cabdirect/abstract/20153234153 [Google Scholar]
- 23.Suman T.Y., Radhika Rajasree S.R., Kirubagaran R. Evaluation of zinc oxide nanoparticles toxicity on marine algae chlorella vulgaris through flow cytometric, cytotoxicity and oxidative stress analysis. Ecotoxicol. Environ. Saf. 2015;113:23–30. doi: 10.1016/J.ECOENV.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Gilroy K.D., Neretina S., Sanders R.W. Behavior of gold nanoparticles in an experimental algal–zooplankton food chain. J. Nanoparticle Res. 2014;16(5):1–8. doi: 10.1007/S11051-014-2414-2. 2014 16:5. [DOI] [Google Scholar]
- 25.Rinke K.H., Simon M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids (8 pp) Environ. Sci. Pollut. Control Ser. 2006;13(4):225–232. doi: 10.1065/ESPR2006.06.311. 2006 13:4. [DOI] [PubMed] [Google Scholar]
- 26.Safari J., Zarnegar Z. Advanced drug delivery systems: nano-technology of health design A review. J. Saudi Chem. Soc. 2014;18(2):85–99. doi: 10.1016/J.JSCS.2012.12.009. [DOI] [Google Scholar]
- 27.Miura A. Undefined; 1984. A New Variety and a New Form of Porphyra (Bangiales,Rhodophyta) from Japan:Porphyra Tenera Kjellman var.Tamatsuensis Miura,var.nov.and P.Yezoensis Ueda form.Narawaensis Miura,form.Nov. [Google Scholar]
- 28.Robinson N., Winberg P., Kirkendale L. Genetic improvement of macroalgae: status to date and needs for the future. J. Appl. Phycol. 2012;25(3):703–716. doi: 10.1007/S10811-012-9950-X. 2012 25:3. [DOI] [Google Scholar]
- 29.Yan L., Jianzhou C., Xueyan S., Tao L., Qingli G. The application of dna molecular marker technique to heritable breeding of laminaria. Trans. Oceanol. Limnol. 2006 http://en.cnki.com.cn/Article_en/CJFDTotal-HYFB200601010.htm [Google Scholar]
- 30.Chaoyuan W., Guangheng L. Progress in the genetics and breeding of economic seaweeds in China. Hydrobiologia. 1987;151(1):57–61. doi: 10.1007/BF00046105. 1987 151:1. [DOI] [Google Scholar]
- 31.Dai J., Zhang Q., Bao Z. Genetic breeding and seedling raising experiments with Porphyra protoplasts. Aquaculture. 1993;111(1–4):139–145. doi: 10.1016/0044-8486(93)90032-T. [DOI] [Google Scholar]
- 32.Kyoung Hwang E., Yotsukura N., Jun Pang S., Su L., Feng Shan T. Seaweed breeding programs and progress in eastern Asian countries. Phycologia. 2019;58(5):484–495. doi: 10.1080/00318884.2019.1639436. [DOI] [Google Scholar]
- 33.Hu Z.M., Liu F.L., Shao Z.R., Yao J.T., Duan D.L. NrDNA internal transcribed spacer revealed molecular diversity in strains of red seaweed Porphyra yezoensis and genetic insights for commercial breeding. Genet. Resour. Crop Evol. 2010;57(5):791–799. doi: 10.1007/S10722-009-9519-Y. 2010 57:5. [DOI] [Google Scholar]
- 34.Meer J.P., van der. 2019. Genetics of Gracilaria sp. (rhodophyceae, gigartinales). V. Isolation and Characterization of Mutant Strains; pp. 47–54. 10.2216/I0031-8884-18-1-47.1, 18(1) [DOI] [Google Scholar]
- 35.Patwary M.U., Meer van der J.P. 2011. Genetics of Gracilaria tikvahiae (rhodophyceae). VIII. Phenotypic and genetic characterization of some selected morphological mutants; pp. 2556–2564. 10.1139/B82-310, 60(12) [DOI] [Google Scholar]
- 36.Worland A.J. Mutation breeding: theory and practical applications. J. Agric. Sci. 1999;133(4):459–462. doi: 10.1017/S0021859699257073. by A. M. Van Harten. xiv+353 pp. Cambridge: Cambridge University Press (1998). £75.00 or $120.00 (hardback). ISBN 0 521 47074 9. [DOI] [Google Scholar]
- 37.Bail A. Le, Charrier B. Culture methods and mutant generation in the filamentous brown algae Ectocarpus siliculosus. Methods Mol. Biol. 2013;959:323–332. doi: 10.1007/978-1-62703-221-6_22. [DOI] [PubMed] [Google Scholar]
- 38.Donald P C. In: Economically Important Marine Plants of the Atlantic: Their Biology and Cultivation. Yarish Charles, Pennimen Clayton A., Van Patten Peg., editors. 1990. Genetic improvement of seaweed through protoplast fusion; pp. 27–33.https://repository.library.noaa.gov/view/noaa/40726 Connecticut Sea Grant College Program. [Google Scholar]
- 39.Gupta V., Kumari P., Reddy C.R.K. Development and characterization of somatic hybrids of Ulva reticulata forsskål (×) Monostroma oxyspermum (Kutz.)Doty. Front. Plant Sci. 2015;6:1–15. doi: 10.3389/FPLS.2015.00003/ABSTRACT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodge F.J., Buchanan J., Zuccarello G.C. Hybridization between the endemic brown algae Carpophyllum maschalocarpum and Carpophyllum angustifolium (Fucales): genetic and morphological evidence. Phycol. Res. 2010;58(4):239–247. doi: 10.1111/J.1440-1835.2010.00583.X. [DOI] [Google Scholar]
- 41.Kito H., Kunimoto M., Kamanishi Y., Mizukami Y. Protoplast fusion between Monostroma nitidum and Porphyra yezoensis and subsequent growth of hybrid plants. J. Appl. Phycol. 1998;10(1):15–21. doi: 10.1023/A:1008063415548. 1998 10:1. [DOI] [Google Scholar]
- 42.Chen Y.C. Development of protoplasts from holdfasts and vegetative thalli of Monostroma latissimum (Chlorophyta, Monostromatacae) for algal seed stock. J. Phycol. 1998;34(6):1075–1081. doi: 10.1046/J.1529-8817.1998.341075.X. [DOI] [Google Scholar]
- 43.Chen Y.C., Shih H.C. Development of protoplasts of Ulva fasciata (chlorophyta) for algal seed stock. J. Phycol. 2000;36(3):608–615. doi: 10.1046/J.1529-8817.2000.99128.X. [DOI] [PubMed] [Google Scholar]
- 44.Reddy C.R.K., Gupta M.K., Mantri V.A., Jha B. Seaweed protoplasts: status, biotechnological perspectives and needs. J. Appl. Phycol. 2007;(5):619–632. doi: 10.1007/S10811-007-9237-9. 2007 20:5, 20. [DOI] [Google Scholar]
- 45.Song Q., Peng J., Xin-ping L., Xi-hua W., Cheng-kui Z. A transformation model for Laminaria japonica (Phaeophyta, Laminariales) Chin. J. Oceanol. Limnol. 1998;16(1):50–55. doi: 10.1007/BF02849080. 1998 16:1. [DOI] [Google Scholar]
- 46.Liu H., Yu W., Dai J., Gong Q., Yang K., Zhang Y. Increasing the transient expression of GUS gene in Porphyra yezoensis by 18S rDNA targeted homologous recombination. J. Appl. Phycol. 2003;00:1–7. http://www.paper.edu.cn [Google Scholar]
- 47.Jiang P., Qin S., Tseng C.K. Expression of the lacZ reporter gene in sporophytes of the seaweed Laminaria japonica (Phaeophyceae) by gametophyte-targeted transformation. Plant Cell Rep. 2003;21(12):1211–1216. doi: 10.1007/S00299-003-0645-2. [DOI] [PubMed] [Google Scholar]
- 48.Mizuta H., Yasui H., Saga N. Transient expression of lacZ in particle bombarded Gracilaria changii (Gracilariales, Rhodophyta) J. Appl. Phycol. 2003;15(4):345–349. doi: 10.1023/A:1025141913156. 2003 15:4. [DOI] [Google Scholar]
- 49.Kurtzman A.M., Cheney D.P. Direct gene transfer and transient gene expression in a marine red alga using the biolistic method | CiNii Research. J. Phycol. 1991:27–42. https://cir.nii.ac.jp/crid/1571698599608946432 [Google Scholar]
- 50.Kübler J.E., Minocha S.C., Mathieson A.C. Transient expression of the GUS reporter gene in proto-plasts of Porphyra miniata (Rhodophyta) | CiNii Research. J. Mar. Biotechnol. 1994:165–169. https://cir.nii.ac.jp/crid/1572824499675081984 [Google Scholar]
- 51.Uji T., Takahashi M., Saga N., Mikami K. Visualization of nuclear localization of transcription factors with cyan and green fluorescent proteins in the red alga Porphyra yezoensis. Mar. Biotechnol. 2010;12(2):150–159. doi: 10.1007/S10126-009-9210-5. [DOI] [PubMed] [Google Scholar]
- 52.Junqiang W., Dewen C., Yongliang D., Lushan Y., Junwen H. Summary report on experiment of strong nanometer 863 biological assistant growth unit in sea shrimp farming. Mod. Fish. Inf. 2003;18(10):12–15. http://open.oriprobe.com/articles/6657794/Summary_Report_on_Experiment_of_Strong_Nanometer_863_Biological_Assist.htm [Google Scholar]
- 53.Rather M.A., Sharma R., Aklakur M., Ahmad S., Kumar N., Khan M., Ramya V.L. Fisheries and Aquaculture Journal. 2011. Nano-technology: a novel tool for aquaculture and fisheries development. A prospective mini-review.http://astonjournals.com/faj 2011. [Google Scholar]
- 54.Johari S.A., Kalbassi M.R., Soltani M., Yu I.J. Application of nanosilver-coated zeolite as water filter media for fungal disinfection of rainbow trout (Oncorhynchus mykiss) eggs. Aquacult. Int. 2016;24(1):23–38. doi: 10.1007/s10499-015-9906-7. [DOI] [Google Scholar]
- 55.Rai M.K., Deshmukh S.D., Ingle A.P., Gade A.K. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012;112(5):841–852. doi: 10.1111/j.1365-2672.2012.05253.x. [DOI] [PubMed] [Google Scholar]
- 56.Shrestha A., Zhilong S., Gee N.K., Kishen A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J. Endod. 2010;36(6):1030–1035. doi: 10.1016/j.joen.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Kadar E., Rooks P., Lakey C., White D.A. The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Sci. Total Environ. 2012;439:8–17. doi: 10.1016/j.scitotenv.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Vijayan S.R., Santhiyagu P., Singamuthu M., Kumari Ahila N., Jayaraman R., Ethiraj K. Synthesis and characterization of silver and gold nanoparticles using aqueous extract of seaweed, turbinaria conoides, and their antimicrofouling activity. Sci. World J. 2014 doi: 10.1155/2014/938272. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gan S.-Y., Qin S., Othman R.Y., Phang S.-M. The 4th Annual Seminar of National Science Fellowship; 2004. [BIO08] Development of a Transformation System for Gracilaria Changii (Gracilariales, Rhodophyta), a Malaysian Red Alga via Microparticle Bombardment. 2004. [Google Scholar]
- 60.Wang J., Jiang P., Cui Y., Guan X., Qin S. 2019. Gene Transfer into Conchospores of Porphyra haitanensis (Bangiales, Rhodophyta) by Glass Bead Agitation; pp. 355–360. 10.2216/09-91.1, 49(4) [DOI] [Google Scholar]
- 61.Huang X., Weber J.C., Hinson T.K., Mathieson A.C., Minocha S.C. Transient expression of the GUS reporter gene in the protoplasts and partially digested cells of Ulva lactuca L. (Chlorophyta) Bot. Mar. 1996;39(5):467–474. doi: 10.1515/BOTM.1996.39.1-6.467/MACHINEREADABLECITATION/RIS. [DOI] [Google Scholar]
- 62.Kimura M., Tominaga H., Coury D., Ikeda M. 2009. Isolation and Characterization of the rbcS Genes from a Sterile Mutant of Ulva Pertusa (Ulvales, Chlorophyta) and Transient Gene Expression Using the rbcS Gene Promoter.https://agris.fao.org/agris-search/search.do?recordID=JP2009004506 [Google Scholar]
- 63.Gregory N.M., Gary E.M. 2014. The living Regulatory Challenges Of Synthetic Biology - Iowa Law Review - The University Of Iowa College Of Law.https://ilr.law.uiowa.edu/print/volume-100-issue-1/the-living-regulatory-challenges-of-synthetic-biology/ Iowa Law Review. [Google Scholar]
- 64.Szyjka S.J., Mandal S., Schoepp N.G., Tyler B.M., Yohn C.B., Poon Y.S., Villareal S., Burkart M.D., Shurin J.B., Mayfield S.P. Evaluation of phenotype stability and ecological risk of a genetically engineered alga in open pond production. Algal Res. 2017;24:378–386. doi: 10.1016/J.ALGAL.2017.04.006. [DOI] [Google Scholar]
- 65.Morales S.I.G., Gutiérrez N.B.P., Rodríguez C.A.R., Bello A.A.B., Hernández P.E., Estrella L.H., Arredondo D.L.L. Metabolic engineering of phosphite metabolism in Synechococcus elongatus PCC 7942 as an effective measure to control biological contaminants in outdoor raceway ponds. Biotechnol. Biofuels. 2020;13(1):1–19. doi: 10.1186/S13068-020-01759-Z/FIGURES/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varela J.V., Burgués C., Rösch C. Acceptability of genetically engineered algae biofuels in Europe: opinions of experts and stakeholders. Biotechnol. Biofuels. 2020;13(1):1–21. doi: 10.1186/S13068-020-01730-Y/TABLES/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woodcock C.E., Allen R., Anderson M., Belward A., Bindschadler R., Cohen W., Gao F., Goward S.N., Helder D., Helmer E., Nemani R., Oreopoulos L., Schott J., Thenkabail P.S., Vermote E.F., Vogelmann J., Wulder M.A., Wynne R. Free access to Landsat imagery. Science (New York, N.Y.) 2008;320(5879):1011. doi: 10.1126/SCIENCE.320.5879.1011A. [DOI] [PubMed] [Google Scholar]
- 68.Bell T.W., Allen J.G., Cavanaugh K.C., Siegel D.A. Three decades of variability in California's giant kelp forests from the Landsat satellites. Rem. Sens. Environ. 2020;238 doi: 10.1016/J.RSE.2018.06.039. [DOI] [Google Scholar]
- 69.Ackleson S.G., Smith J.P., Rodriguez L.M., Moses W.J., Russell B.J. Autonomous coral reef survey in support of remote sensing. Front. Mar. Sci. 2017;4(OCT):325. doi: 10.3389/FMARS.2017.00325/BIBTEX. [DOI] [Google Scholar]
- 70.Hardin P.J., Lulla V., Jensen R.R., Jensen J.R. Small Unmanned Aerial Systems (sUAS) for environmental remote sensing. Challeng. Opport. Rev. 2018;56(2):309–322. doi: 10.1080/15481603.2018.1510088. 10.1080/15481603.2018.1510088. [DOI] [Google Scholar]
- 71.Huang C., Li Y., Yang H., Sun D., Yu Z., Zhang Z., Chen X., Xu L. Detection of algal bloom and factors influencing its formation in Taihu Lake from 2000 to 2011 by MODIS. Environ. Earth Sci. 2014;71(8):3705–3714. doi: 10.1007/S12665-013-2764-6. [DOI] [Google Scholar]
- 72.Kahru M., Mitchell B.G., Diaz A., Miura M. MODIS detects a devastating algal bloom in Paracas Bay, Peru. Eos. Transactions American Geophysical Union. 2004;85(45):465–472. doi: 10.1029/2004EO450002. [DOI] [Google Scholar]
- 73.Liang Q., Zhang Y., Ma R., Loiselle S., Li J., Hu M., Zhang Y., Giardino C., Li L., Mishra D.R., Gloaguen R., Thenkabail P.S. A MODIS-based novel method to distinguish surface cyanobacterial scums and aquatic macrophytes in lake taihu. Rem. Sens. 2017;9(2):133. doi: 10.3390/RS9020133. 2017. Page 133, 9. [DOI] [Google Scholar]
- 74.Xing Q., An D., Zheng X., Wei Z., Wang X., Li L., Tian L., Chen J. Monitoring seaweed aquaculture in the Yellow Sea with multiple sensors for managing the disaster of macroalgal blooms. Rem. Sens. Environ. 2019;231 doi: 10.1016/J.RSE.2019.111279. [DOI] [Google Scholar]
- 75.Qi L., Hu C., Xing Q., Shang S. Long-term trend of Ulva prolifera blooms in the western Yellow Sea. Harmful Algae. 2016;58:35–44. doi: 10.1016/J.HAL.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Kavale M.G., Yadav A., Mantri V.A. Initial comparison between monoline and tube net method of farming in red agarophyte Gracilaria dura: evidence from hydrodynamic CFD simulations. Aquaculture. 2021;536 doi: 10.1016/J.AQUACULTURE.2021.736485. [DOI] [Google Scholar]
- 77.Duarte P.M., Ferreira J.G. A model for the simulation of macroalgal population dynamics and productivity. Ecol. Model. 1997;98(2–3):199–214. doi: 10.1016/S0304-3800(96)01915-1. [DOI] [Google Scholar]
- 78.Lehahn Y., Ingle K.N., Golberg A. Global potential of offshore and shallow waters macroalgal biorefineries to provide for food, chemicals and energy: feasibility and sustainability. Algal Res. 2016;17:150–160. doi: 10.1016/J.ALGAL.2016.03.031. [DOI] [Google Scholar]
- 79.Zollmann M., Rubinsky B., Liberzon A., Golberg A. Multi-scale modeling of intensive macroalgae cultivation and marine nitrogen sequestration. Commun. Biol. 2021;4(1):1–11. doi: 10.1038/s42003-021-02371-z. 2021 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frieder C.A., Yan C., Chamecki M., Dauhajre D., McWilliams J.C., Infante J., McPherson M.L., Kudela R.M., Kessouri F., Sutula M., Arzeno-Soltero I.B., Davis K.A. A macroalgal cultivation modeling system (MACMODS): evaluating the role of physical-biological coupling on nutrients and farm yield. Front. Mar. Sci. 2022;9:214. doi: 10.3389/FMARS.2022.752951/BIBTEX. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.