Abstract
The cutting-edge combination of aspirin (ASA) and sildenafil citrate (SIC) has been presented as a suggested dosage form for the treatment of thin endometrium and erectile dysfunction, particularly in patients with cardiovascular diseases. However, ASA is highly sensitive to degradation into its major deterioration product, known as salicylic acid (SA). Consequently, it is eminently essential to evolve approaches for the synchronous quantification of ASA and SIC in the presence of SA.
The main objective of this work is to develop three approaches for the synchronous quantification of ASA and SIC in the presence of SA in their commixtures and suggested formulations without any prior separation.
Three green UV-methods were employed for the synchronous quantification, namely: Dual Wavelength in Ratio Spectra (DW-RS), Advanced Amplitude Centering (AAC), and Double Divisor of Ratio Difference Derivative (DDRD-D1). In DW-RS and AAC two-wavelength manipulation was used for resolution, while in DDRD-D1 only an appropriate wavelength for the synchronous quantification of the triplex commixture was used. All approaches can be able to resolve the highly interfering spectrum of the three components presented in the triplex commixture.
Good linearity was inspected in the range of 20.0–100.0, 5.0–50.0, and 4.0–60.0 μg/mL for the ASA, SIC, and SA, respectively.
All developed approaches have been advocated in accordance with ICH guidelines.
All results from these approaches are presented and statistically reconciled with the proclaimed HPLC method, with no considerable differences.
Furthermore, the approaches' eco-friendliness was predestined by Analytical Greenness (AGREE), and the complex GAPI.
Moreover, the sustainability of the used solvent was evaluated by Green Solvents Selecting Tool (GSST); in addition, the greenness of the solvent was evaluated by Greenness Index tool with a spider diagram.
The suggested UV-methods may be employed for routine quality control studies of the suggested formulations ASA & SIC since they were considered sustainable, economical, and effective.
Keywords: Aspirin, Sildenafil citrate, Salicylic acid, UV-methods, AGREE, Complex GAPI, Green solvents selecting tool, Spider diagram
Graphical abstract
Highlights
-
•
The first UV approaches have been utilized for the synchronous quantification of aspirin and sildenafil citrate in the presence of salicylic acid.
-
•
Use of DDRD-D1 to increase the sensitivity of the approach.
-
•
GSST was used for the first time to evaluate the solvents sustainability in spectrophotometric analytical methods.
-
•
Green Engineering Design for evaluating Solvents via greenness index tool with spider diagram.
-
•
AGREE; and Complex GAPI to evaluate the greenness of the applied UV- method.
1. Introduction
The pharmaceutical formulation of ASA and SIC is utilized for the treatment of thin endometrium [1,2]. Moreover, ASA can be added to the therapy of erectile dysfunction, because of its synergistic action and defensive cardiac effect [3,4].
An important comparative study has shown that an improvement in the results and a curtailment of side effects can be achieved by the combination of aspirin and one of the phosphodiesterase-5 inhibitors in the treatment of vascular erectile dysfunction [5]. Further studies foreseen also includes an extensive study on a larger number of phosphodiesterase-5 inhibitors.
Acetylsalicylic acid (ASA) is 2-(Acetyloxy) benzoic acid, Fig. 1 A, which is an antiplatelet drug that lowers the risk of cardiovascular diseases mortality [[6], [7], [8]]. Some methods used for the quantification of ASA like HPLC [9], spectrophotometric [10], UHPLC [11], TLC [11], and UPLC [12]. These methods are shown in Table 1.
Fig. 1.
Structural formula of A- ASA, B– SIC and C- SA.
Table 1.
The previous methods of ASA and SIC quantification.
| Drug analyzed | Method studied | Publication year | Reference |
|---|---|---|---|
| Aspirin + Methocarbamol in the apparition of guaifenesin and salicylic acid. | HPLC-DAD method | 2019 | [9] |
| Aspirin + Omeprazole in the apparition of Salicylic Acid | Spectrophotometric methods | 2022 | [10] |
| Aspirin + Omeprazole in the presence of salicylic acid | UHPLC and TLC | 2021 | [11] |
| Aspirin + Salicylic acid + Salvianolic acid B | UPLC-MS/MS | 2021 | [12] |
| Sildenafil citrate + Dapoxetine + Vardenafil + Tadalafil | Spectrofluorimetric and Spectrophotometric methods | 2020 | [15] |
| Dapoxetine + Sildenafil | Spectrophotometric methods | 2022 | [16] |
| Sildenafil citrate | Infrared Spectroscopic method | 2021 | [17] |
| Sildenafil | HPLC | 2020 | [18] |
| Aspirin + Sildenafil citrate | Spectrophotometric method | 2015 | [19] |
| Aspirin + Sildenafil citrate | Spectrophotometric methods | 2022 | [20] |
| Aspirin + Sildenafil citrate + Salicylic acid | HPLC | 2014 | [4] |
Sildenafil citrate (SIC) is 5-[2-Ethoxy-5-[(4-methylpiperazin-1-yl) sulfonyl]phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo [4,3-d]pyrimidin-7-one dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate, Fig. 1 B, which is an effective therapy in the treatment of erectile dysfunction [6,13], and pulmonary arterial hypertension [14]. Some methods used for the quantification of SIC like spectrofluorimetric [15], spectrophotometric [15,16], IR spectroscopic [17], and HPLC [18]. These methods are shown in Table 1.
With respect to the quantification of ASA and SIC, a review of the literature found two spectrophotometric approaches for their synchronous quantification [19,20], as shown in Table 1. However, none of these preceding spectrophotometric studies analyzed these drugs in the presence of salicylic acid (SA) Fig. 1 C. SA is produced by the hydrolysis of ASA, which is considered the major metabolites of ASA [[21], [22], [23]].
Regarding the quantification of ASA, SIC and SA, only one chromatographic method has been proclaimed for their synchronous quantification [4], as shown in Table 1. The current trend in environmental greenness is minimizing or eliminating the usage and production of harmful substances during analysis through adapting an eco-friendly analytical technique [24]. Chromatographic analytical methods are widely utilized in pharmaceutical quality control due to their sensitivity and specificity. These techniques, which utilize a significant number of poisonous solvents, generate a lot of waste and take long time to analyze. As a result, this technique cannot be considered the best friend to the environment. In the light of green analytical chemistry (GAC) [25], UV-methods provide economic green analytical methods instead of chromatographic ones, because they do not use more solvents, do not generate a lot of waste, and do not need additional equipment. So they are considered as cost-saving methods [[26], [27], [28], [29], [30], [31]].
In this paper, ASA and SIC were quantified synchronously in the presence of SA by Dual Wavelength in Ratio Spectra (DW-RS) [32,33] which rely on choosing two wavelengths in which the intervention component has the same absorptivities. In addition, Advanced Amplitude Centering (AAC) [34,35] was employed for the synchronous quantification utilizing a single divisor; supplying a favorable way to educe the target component without any interference from the escorting components in their triplex commixture. Moreover, the Double Divisor of the Ratio Difference Derivative (DDRD-D1) [36,37] was assigned, supplying an exceptional way to elicit the signal of the target component without any interference from the escorting components in their triplex commixture. Unlike the preceding advanced approaches, this modern approach has the convenience of designating the best wavelength from anywhere in the spectrum for the synchronous quantification of the triplex combination.
We must point out that the key to achieving perfect greenness analytical methods is choosing the environmental solvent, so the greenness evaluation of the solvent includes safety, health, and environment (SHE) impact, all of which must be taken into account. This has been achieved through Greenness Index and spider diagram tools [[38], [39], [40]].
The methodology for assessing the greenness of solvent data is based on publicly-acknowledged principles, such as those included in the GAC 12 Principles and the Greenness Engineering 12 Principles [41].
The sustainability of the studied solvent was evaluated through Green Solvents Selecting Tool (GSST) for the first time in UV-methods [42].
To complete the greenness profiles of the suggested UV-methods, the Analytical Greenness (AGREE) tool was also used to give us an integrated idea about the greenness of the proposed spectral methods in this research [43].
In order to provide a complete idea related to the green chemistry evaluation of the developed UV-methods, the Complementary Green Analytical Procedure Index (Complex GAPI) is used to evaluate the greenness of the entire analytical procedure, starting with sample collection and ending with the final analysis [44].
2. Theoretical background
2.1. Dual Wavelength in Ratio Spectra (DW-RS)
This approach is adopted for the synchronous quantification of the triplex commixture (X, Y, and Z) by removing the interference of the two components in the triplex commixture for the selective determination of the first component. Firstly, it stands on Z spectrum as a divisor (Z′). Then, two wavelengths are chosen, where Y and Z have the same amplitudes. The difference of amplitudes (ΔP) at these wavelengths relates to X concentrations. Furthermore, additional two wavelengths are chosen, where X and Z have the same amplitudes. ΔP at these wavelengths relates to Y concentrations. Finally, Y spectrum is used as a second divisor (Y′) for the quantification of Z. Likewise, two wavelengths are chosen, where X and Y have the same amplitudes. ΔP at these wavelengths relates to Z concentrations.
2.2. Advanced Amplitude Centering (AAC)
This approach can be implemented for the synchronous quantification of the triplex commixture (X, Y, and Z) with severe overlap, utilizing Z as a single divisor (Z′).
This approach is implemented in three steps:
Step 1
amplitude difference was applied on the ratio spectra at λ1-λ2 and λ1- λ3, where Y and X emerge with the same amplitudes at these wavelengths, respectively.
Initially, the regression equalizations represent the interrelationship between ΔP of the ratio spectra at λ1-λ2 and λ1-λ3 of X and Y versus the amplitudes of the ratio spectra of X and Y with Z as a divisor at λ1.
| (1) |
| (2) |
where, ΔP (P1–P2) and ΔP (P1–P3) depict the amplitude differences λ1-λ2 and λ1-λ3, respectively.
(X/Z′)1 and (Y/Z′)1 depict the postulated amplitude accompanying X and Y at λ1, respectively.
Substitution in previously applied regression equalizations was applied, utilizing ΔP between λ1-λ2 in the matter of Eq (1) and λ1-λ3 in the matter of Eq (2) to get the postulated amplitude value accompanying X and Y, respectively, where the constant Z/Z′ show the same amplitudes at these chosen wavelengths.
Step 2
amplitude subtraction was applied, where the constant amplitude Z/Z′ could be calculated by subtracting the recorded amplitude of the commixture at λ1, from the previously calculated postulated amplitude accompanying X or Y at λ1.
Step 3
The concentrations of X, Y, and Z were calculated, utilizing the regression equalizations that depict the interrelationship between the amplitudes of the ratio spectra at the same wavelength λ1 and the accompanying concentration of X, Y, and Z.
2.3. Double Divisor of Ratio Difference Derivative (DDRD-D1)
Application of DDRD-D1 can be utilized for the synchronous quantification of the triplex commixture (X, Y, and Z). Firstly, it denotes the availability of the Y + Z spectrum as a double divisor, followed by D1 derivatization to get the final spectrum, which has no effect on Y and Z spectrum and only relates to X concentrations. Likewise, Y and Z concentrations can be determined.
3. Experimental
3.1. Apparatus and software
All UV analysis was carried out utilizing V-650 (JASCO, Japan), qualified with a pair of 1.0 cm quartz cells. Scans were executed at 200.0–400.0 nm.
3.2. Experimental
3.2.1. Pure samples
ASA and SA were gained from JQC (Huayin); China, SIC was gained from Xuhuang; China. ASA, SIC, and SA have a purity of 99.8% ± 0.74, 99.5% ± 0.72, and 100.0% ± 0.44, respectively, in agreement with BP official methods.
3.2.2. Pharmaceutical preparations
Since the co-formulated combination of ASA and SIC is not readily available, individual tablets containing ASA (150 mg) and SIC (50 mg) were prepared from tablets purchased from the pharmacy. These tablets are Aspirin® Bayer enteric-coated tablet labeled as consisting of 100 mg ASP, and Vega® film-coated tablet labeled as consisting of SIL equivalent to 50 mg base (Asia pharmaceutical industries, Syria).
3.2.3. Solvents
Absolute ethanol of analytical grade was purchased from Scharlab in Spain and used to make the standard and sample solutions.
3.2.4. Standard solutions
-
-
500.0 μg/mL Ethanolic stock standard solutions of ASA, SIC, and SA.
-
-
50.0 μg/mL Ethanolic working standard solutions of ASA, SIC, and SA
3.3. Procedures
3.3.1. Linearity and calibration curves
A series of six 5 mL volumetric flasks were created by relocating portions equivalent to 20.0–100.0 μg/mL of ASA, 5.0–50.0 μg/mL of SIC, and 4.0–60.0 μg/mL of SA separately from the working solution, and diluting with ethanol to the mark.
3.3.1.1. Dual Wavelength in Ratio Spectra (DW-RS)
ASA and SIC D0 spectra were subdivided by 50.0 μg/mL spectrum of SA, producing the ratio spectra ASA/SA and SIC/SA, respectively. ΔP were opted at 275.2–248.4 nm, and 253.7–275.2 nm for the quantification of ASA and SIC, respectively. Calibration curves were constructed utilizing the linear equalization between ΔP at 275.2–248.4 nm and 253.7–275.2 nm versus their accompanying concentrations of ASA and SIC, respectively.
Similarly, 40.0 μg/mL spectrum of SIC was used as a second divisor, in order to SA quantification. Calibration curves were constructed utilizing the linear equalization between ΔP at 308.0–325.0 nm versus their accompanying concentrations of SA.
3.3.1.2. Advanced Amplitude Centering (AAC)
As a single divisor, the D0 spectra of the triplex commixture were subdivided by the 50.0 μg/mL spectrum of SA.
ΔP of the ratio spectra was measured at 275.2–248.4 nm for ASA and 253.7 nm–275.2 nm for SIC. Then, the postulated value for each ASA and SIC was obtained separately by constructing the relationship between ΔP of ASA ratio spectra at 275.2–248.4 nm versus the accompanying ratio amplitude at 275.2 nm, and between ΔP of SIC ratio spectra at 253.7–275.2 nm versus the accompanying ratio amplitude at 275.2 nm.
Finally, the constant value SA/SA’ can be calculated by applying amplitude subtraction to the recorded amplitude of the mixture's ratio spectrum at 275.2 nm and the calculated postulated amplitude at 275.2 nm.
Calibration curves were constructed utilizing the linear equalization between the amplitude at 275.2 nm versus their accompanying concentrations of ASA, SIC, and SA, respectively.
3.3.1.3. Double Divisor of Ratio Difference Derivative (DDRD-D1)
ASA, SIC, and SA D0 spectra were subdivided by (40.0 μg/mL SIC+40.0 μg/mL SA), (40.0 μg/mL ASA+40.0 μg/mL SA), and (40.0 μg/mL ASA+40.0 μg/mL SIC) spectrum, respectively, producing ratio spectra. Then, D1 derivatization was applied (19 data points, scale factor 10). Calibration curves were constructed between signals at 262.0, 263.4, and 321.2 nm versus their accompanying concentrations of ASA, SIC, and SA, respectively.
3.3.2. Laboratory synthetic mixtures
Accurate ratios of ASA, SIC, and SA were mixed, and different dilutions were applied in the linearity range of each drug and transferred into a series of 5 mL volumetric flasks utilizing ethanol as a solvent.
3.3.3. Pharmaceutical formulations
Twenty tablets were accurately weighed. Tablet powder equivalent to 75.0 mg ASA and 25.0 mg SIC was weighed and transferred to 25 mL volumetric flask and dissolved in ethanol; the solution was then sonicated for 8 min, and the volume was filled up to the mark. Then it was filtered and 0.15 mL was transferred to 5.0 mL volumetric flask to get ultimate concentrations, which is claimed to consist of 90.0 μg/mL of ASA, and 30.0 μg/mL of SIC for the quantitative analysis of the drugs in their suggested formulation.
4. Results and discussion
4.1. Spectral characteristics
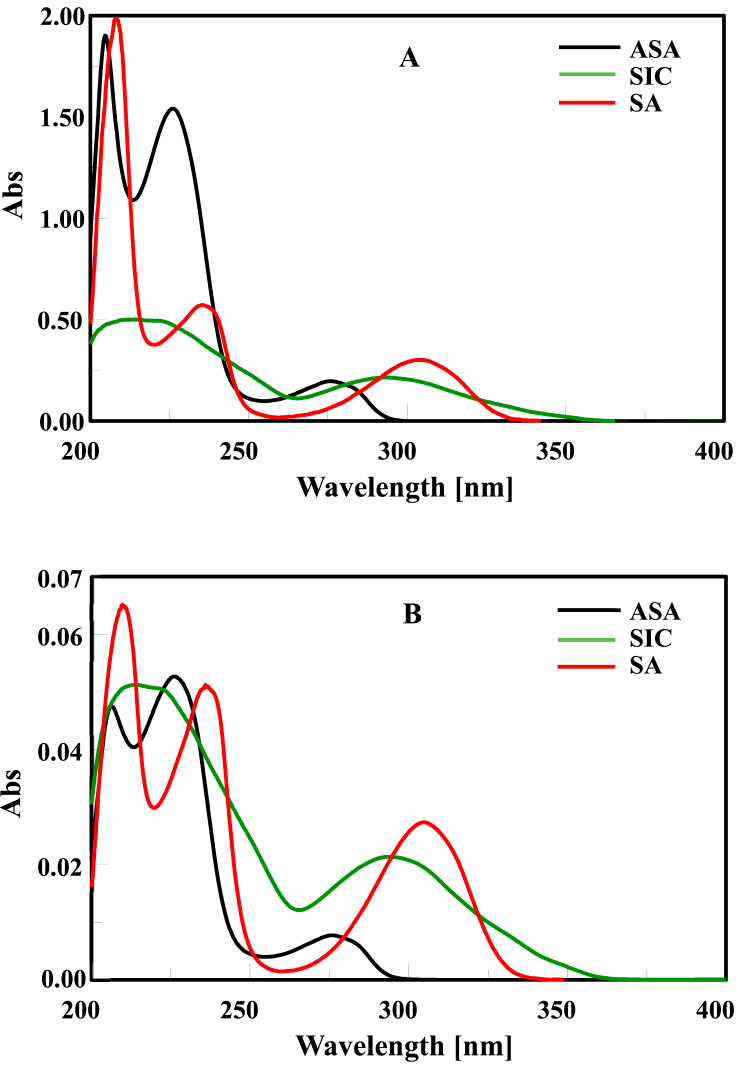
The D0 spectra of ASA, SIC, and SA show severe overlap with no spectral features for quantifiable analysis. The spectrum of ASA is strongly overlapped with that of SIC and SA, while in the region of 300–400 nm we find only the spectrum of SIC and SA without any interference from ASA.
The strong overlap of D0 spectra of the triplex components of ASA, SIC, and SA in the ratio of (3:1:1), as laid out in Fig. 2 A, hinders the direct quantification of multi-component formulations, making the process more difficult .
Fig. 2.
A- D0 spectra of ASA (30 μg/mL), SIC (10 μg/mL) and SA (10 μg/mL) parading severe overlap.
B- Normalized spectra of ASA (1 μg/mL), SIC (1 μg/mL) and SA (1 μg/mL) parading the absorptivity value of each ingredient in commixture.
This interference can be tackled by applying mathematical procedures based on UV- mathematical steps depending on ratio spectrum and derivation, which allow the quantification of ASA and SIC in the presence of SA.
The absorptivity value of each ingredient was expounded via the normal spectra, as laid out in Fig. 2 B.
4.2. Dual Wavelength in Ratio Spectra (DW-RS)
-
•
A number of SA concentrations were examined to elect the best of them as a divisor. The spectrum of 50.0 μg/mL of SA was adopted so that it gives the best results for ASA and SIC quantification in the triplex commixture of ASA, SIC, and SA. The resulting ratio spectra were ASA + SIC + SA/SA. Likewise, 40 μg/mL spectrum of SIC was utilized as a second divisor, in order to achieve SA quantification as shown in Table 2.
-
•
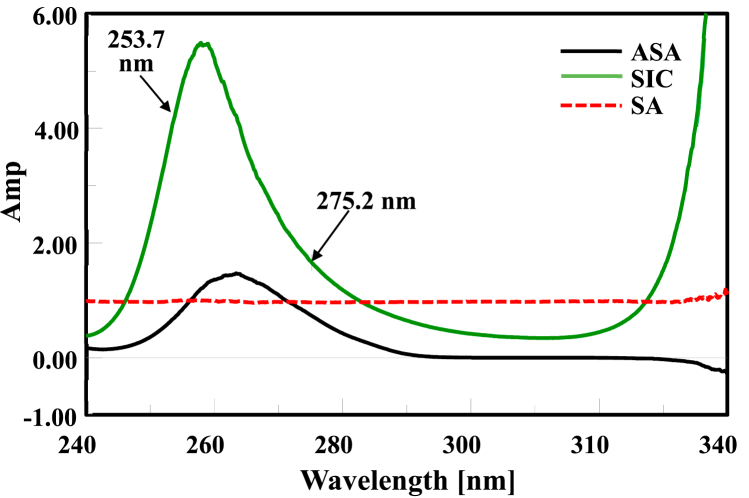
A number of wavelengths were examined to elect the best of them. ΔP was opted at 275.2–248.4 nm, where SA exhibits a straight line and SIC gives the selfsame amplitudes. Thus, ΔP at 275.2–248.4 nm will disclose the ASA concentrations as laid out in Fig. 3.
Table 2.
The elected divisors of the approaches.
| ASA | SIC | SA | ||
|---|---|---|---|---|
| DW-RS | Two divisors | 50.0 μg/mL SA | 50.0 μg/mL SA | 40.0 μg/mL SIC |
| AAC | Single divisor | 50.0 μg/mL SA | 50.0 μg/mL SA | 50.0 μg/mL SA |
| DDRD-D1 | Double divisor | 40.0 μg/mL SIC+ 40.0 μg/mL SA |
40.0 μg/mL ASA+ 40.0 μg/mL SA |
40.0 μg/mL ASA+ 40.0 μg/mL SIC |
Fig. 3.
Ratio spectra of ASA (30 μg/mL) utilizing SA (50 μg/mL) as a divisor, showing the same amplitudes of SIC (30 μg/mL) at 275.2–248.4 nm.
In addition, ΔP was opted at 253.7–275.2 nm, where SA exhibits a straight line and ASA gives the selfsame amplitudes. Thus, P at 253.7–275.2 nm will disclose the SIC concentrations as laid out in Fig. 4.
Fig. 4.
Ratio spectra of SIC (30 μg/mL) utilizing SA (50 μg/mL) as a divisor, showing the same amplitudes of ASA (30 μg/mL) at 275.2–253.7 nm.
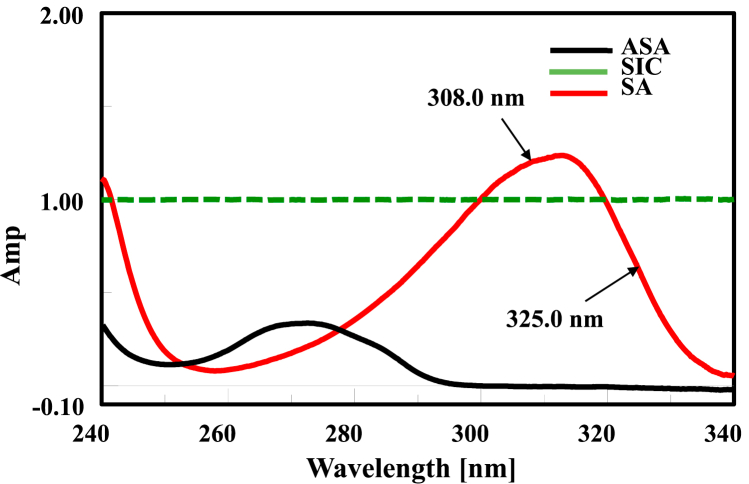
Correspondingly, two wavelengths were opted at 308.0–325.0 nm, where ASA has no absorption after 300.0 nm, and SIC has a straight line as laid out in Fig. 5. Calibration curves were constructed as shown in Table 3.
Fig. 5.
Ratio spectra of SA (30 μg/mL) utilizing SIC (40 μg/mL) as a divisor, showing the same amplitude of ASA (30 μg/mL) at 308.0–325.0 nm.
Table 3.
The calibration curves of the approaches.
| ASA | SIC | SA | |
|---|---|---|---|
| DW-RS | The difference of amplitudes at 275.2–248.4 nm will relate to ASA concentrations. | The difference of amplitudes at 253.7–275.2 nm will relate to SIC concentrations. | The difference of amplitudes at 308.0–325.0 nm will relate to SA concentrations. |
| AAC | The amplitude at 275.2 nm will relate to ASA concentrations. | The amplitude at 275.2 nm will relate to SIC concentrations. | The amplitude at 275.2 nm will relate to SA concentrations. |
| DDRD- D1 | D1 of ratio spectra at 262.0 nm will relate to ASA concentrations. | D1 of ratio spectra at 263.4 nm will relate to SIC concentrations. | D1 of ratio spectra at 321.2 nm will relate to SA concentrations. |
4.3. Advanced Amplitude Centering (AAC)
-
•
A number of SA concentrations were examined to elect the best of them as a single divisor. As shown in Table 2, the spectrum of 50.0 μg/mL SA yielded the best results for ASA, SIC, and SA quantification in their commixtures.
-
•
A number of wavelengths were examined to elect the best of them. ΔP was opted at 275.2–248.4 nm for ASA, 253.7 nm–275.2 nm for SIC as laid out in Fig. 3, Fig. 4, respectively.
-
•
The postulated value for each ASA and SIC was obtained from the ratio amplitude at 275.2 nm, where SIC and ASA have no amplitude at this wavelength.
-
•
Amplitude subtraction was applied on the recorded amplitude of the mixture's ratio spectrum at 275.2 nm, and the calculated postulated amplitude at 275.2 nm. Thus, the constant value SA/SA’ can be calculated as shown in Table 3.
4.4. Double Divisor of Ratio Difference Derivative (DDRD-D1)
This approach is intended for the application of a double divisor, which is the sum of the D0 spectra of the interference components in the triplex commixture and which was used to get the ratio spectra, followed by D1 derivatization (19 data points, scale factor 10) to get the final spectra. The elected double divisors are betokened in Table 2. The wavelengths were chosen to have a high selectivity and sensitivity for the quantification. Measurements were done at 262.0, 263.4, 321.2 nm for the quantification of ASA, SIC and SA, respectively, as shown in Table 2. The D1 ratio spectra of all components in their commixture are depicted in Fig. 6 A, B, and C. Calibration curves were constructed as shown in Table 3.
Fig. 6.
A- D1 ratio spectra of ASA using (SIC + SA) as a double divisor.
B- D1 ratio spectra of SIC using (ASA + SA) as a double divisor.
C- D1 ratio spectra of SA using (ASA + SIC) as a double divisor.
5. Validation and analysis of statistics
Validation of the preceding approaches was evaluated in accordance to ICH guidelines [45] for linearity, accuracy, precision, and specificity. Linearity was affirmed for the preceding UV-spectroscopy approaches by analyzing six diverse concentrations of ASA, SIC, and SA under the experimental circumstances reached and approved. The linearity ranges and regression equalization parameters are shown in Table 4. Accuracy was practiced on pure materials by analyzing three different concentrations at 30.0, 50.0, and 70.0 μg/mL for ASA, 15.0, 25.0, 35.0 μg/mL for SIC, and 15.0, 25.0, 35.0 μg/mL for SA, in triplicates, and on pharmaceutical formulations by applying the method of standard addition, which depends on adding known amounts of standard solution of ASA and SIC at three different concentrations (80.0, 100.0, and 120.0%) as shown in Table 5. Precision was checked by analyzing three different concentrations of each component in triplicates within the same day and on three successive days to assess the repeatability and intermediate precision of the preceding UV-spectroscopy approaches. The used concentrations were 40.0, 60.0, and 80.0 μg/mL for ASA, 20.0, 30.0, and 40.0 μg/mL for SIC, and 20.0, 30.0, and 40.0 μg/mL for SA. Repeatability and intermediate precision showed good results with RSD%<2. These results are shown in Table 4.
Table 4.
Validation sheet for ASA, SIC and SA quantification.
| Parameters | ASA |
SIC |
SA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| DW-RS | AAC | DDRD-D1 | DW-RS | AAC | DDRD-D1 | DW-RS | AAC | DDRD-D1 | |
| Intercept | −0.019 | −0.0451 | 0.0045 | −0.061 | −0.0314 | −0.0271 | 0.0218 | 0.036 | 0.0125 |
| Slope | 0.016 | 0.0259 | −0.0054 | 0.0876 | 0.0578 | 0.036 | 0.0192 | 0.0184 | 0.0227 |
| Linearity (μg/mL) | 20.0–100.0 | 20.0–100.0 | 20.0–100.0 | 5.0–50.0 | 5.0–50.0 | 5.0–50.0 | 4.0–60.0 | 4.0–60.0 | 4.0–60.0 |
| Accuracy (mean%±SD) | 100.27 ± 0.92 | 100.38 ± 0.92 | 101.07 ± 1.72 | 100.33 ± 0.93 | 100.41 ± 0.94 | 100.97 ± 1.39 | 101.63 ± 1.71 | 100.56 ± 1.52 | 98.21 ± 1.59 |
| Correlation coefficient | 0.9999 | 0.9999 | 0.9999 | 0.9997 | 0.9992 | 0.9997 | 0.9995 | 0.9996 | 0.999 |
| Repeatability ± RSD% | 0.82 | 0.82 | 1.18 | 1.24 | 1.24 | 1.96 | 1.69 | 1.34 | 1.49 |
| Intermediate precision ± RSD% | 1.63 | 1.63 | 1.93 | 1.17 | 1.17 | 1.75 | 1.57 | 1.66 | 1.96 |
Table 5.
Application of standard addition technique.
| Raw material added (μg/mL) | Drug sample (μg/mL) | Drug recovery%±SD |
||
|---|---|---|---|---|
| DW-RS | AAC | DDRD-D1 | ||
| ASA | ||||
| 36 | 45 | 103.19 ± 2.13 | 103.30 ± 2.13 | 98.35 ± 1.55 |
| 45 | 45 | 103.33 ± 0.75 | 103.44 ± 0.75 | 101.03 ± 1.55 |
| 54 | 45 | 101.88 ± 1.46 | 101.98 ± 1.46 | 97.94 ± 1.28 |
| SIC | ||||
| 12 | 15 | 97.92 ± 0.88 | 98.09 ± 0.88 | 99.88 ± 1.30 |
| 15 | 15 | 99.85 ± 2.31 | 100.02 ± 2.31 | 100.74 ± 1.79 |
| 18 | 15 | 97.62 ± 2.61 | 97.79 ± 2.62 | 98.40 ± 1.67 |
Method specificity was assessed by applying the preceding UV-spectroscopy approaches on drug commixtures with different mixing proportion within the linearity range of the components in triplicates.
Table 6 indicates the capability to quantify the multi-component drugs with diverse ratios within the linearity range for each component. Gratifying results were achieved, affirming the vacancy of intervention from the other escorting components in their triplex commixture.
Table 6.
Determination of ASA, SIC, and SA in laboratory synthetic commixtures.
| ASA:SIC:SA (μg/mL) | ASA |
SIC |
SA |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Recovery%±SD | |||||||||
| DW-RS | AAC | DDRD-D1 | DW-RS | AAC | DDRD-D1 | DW-RS | AAC | DDRD-D1 | |
| 100 + 25+25 | 97.52 ± 0.19 | 97.63 ± 0.19 | 98.55 ± 1.89 | 97.00 ± 1.59 | 97.09 ± 1.59 | 103.86 ± 2.77 | 100.25 ± 1.10 | 100.16 ± 1.36 | 104.05 ± 0.35 |
| 40 + 40+40 | 98.44 ± 1.59 | 98.55 ± 1.59 | 99.61 ± 3.47 | 99.86 ± 1.08 | 99.98 ± 1.09 | 101.72 ± 1.15 | 99.51 ± 0.79 | 97.83 ± 1.25 | 102.48 ± 0.77 |
| 70 + 50+30 | 101.22 ± 1.72 | 101.33 ± 1.72 | 101.54 ± 1.59 | 98.38 ± 1.66 | 98.51 ± 1.66 | 99.84 ± 1.54 | 99.75 ± 2.14 | 101.05 ± 1.19 | 101.15 ± 2.14 |
| 30 + 30+40 | 95.90 ± 0.87 | 96.01 ± 0.87 | 100.51 ± 1.55 | 99.29 ± 1.45 | 99.39 ± 1.45 | 102.02 ± 2.37 | 97.90 ± 1.24 | 99.87 ± 1.97 | 98.99 ± 0.65 |
| 60 + 30+30 | 99.38 ± 1.56 | 99.48 ± 1.56 | 99.54 ± 0.93 | 97.40 ± 0.56 | 97.50 ± 0.57 | 101.83 ± 1.11 | 102.75 ± 0.36 | 101.85 ± 1.48 | 102.33 ± 0.89 |
All advanced approaches were able to quantify ASA and SIC in their suggested formulation, as shown in Table 6. These results were statistically compared with the proclaimed HPLC method [4] by calculating f and t tests and applying one-way ANOVA. The results indicate that no significant variation is found between the advanced approaches and the proclaimed one, as shown in Table 7, Table 8. The advantages & outcomes of the methods were compared in Table 9.
Table 7.
Statistical analysis of the advanced approaches and the proclaimed HPLC method for the quantification of ASA and SIC in suggested formulation.
| Parameters | ASA |
SIC |
||||||
|---|---|---|---|---|---|---|---|---|
| DW-RS | AAC | DDRD-D1 | HPLC | DW-RS | AAC | DDRD-D1 | HPLC | |
| Mean | 101.53 | 101.64 | 100.10 | 101.02 | 102.71 | 102.82 | 101.70 | 101.74 |
| SD | 0.80 | 0.80 | 1.36 | 1.19 | 1.41 | 1.41 | 1.53 | 1.86 |
| Variance | 0.64 | 0.64 | 1.86 | 1.41 | 1.99 | 2.00 | 2.35 | 3.47 |
| N | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| t-testa (3.182) | 0.79 | 0.97 | 1.13 | – | 0.93 | 1.03 | 0.04 | – |
| F-testa (9.280) | 2.20 | 2.20 | 1.32 | – | 1.74 | 1.73 | 1.47 | – |
The tabulated values of t and F test at p = 0.05.
Table 8.
Results of one-way ANOVA.
| Source | Degree of freedom | Sum of squares | Mean Square |
P valuea | F valuea | F criticala | |
|---|---|---|---|---|---|---|---|
| ASA | Between groups | 3 | 5.8990 | 1.9663 | 0.2149 | 1.7251 | 3.49 |
| Within groups | 12 | 13.6782 | 1.1398 | ||||
| Total | 15 | 13.5772 | – | ||||
| SIC | Between groups | 3 | 4.4064 | 1.4688 | 0.6287 | 0.5975 | 3.49 |
| Within groups | 12 | 29.4982 | 2.4582 | ||||
| Total | 15 | 33.9046 | – |
There was no significance variance among all approaches using one-way ANOVA at p < 0.05.
Table 9.
Advantages & outcomes of the methods.
| Methods | Advantages & outcomes |
|---|---|
| DW-RS |
|
| |
| |
| |
| |
| AAC |
|
| |
| |
| |
| |
| DDRD-D1 |
|
| |
| |
| |
|
6. Greenness protocols
6.1. Green Solvents Selecting Tool (GSST)
Define green solvent, what exactly is a “green solvent”? It is difficult to provide a short answer to this question, but qualified companies may probably agree that a certified green solvent should have a ratio of low health risk, high safety, and little environmental effect over its whole life cycle.
In order to achieve sustainability in the pharmaceutical industry, many pharmaceutical companies -including Pfizer, Sanofi, and GlaxoSmithKline-have criteria to choose appropriate green sustainable solvents. To decide which solvents are recommended, GSK Solvent Sustainability Guidelines has available solvent sustainability guides. By comparing the benefits and drawbacks of various solvents from their information published in solvent Safety Data Sheets (SDSs).
By evaluating the greenness score provided by the GSK Solvent Sustainability Guidelines, which offers a quantitative measurement of solvents based on a wide range of different criteria in the form of a Composite Score, Christian Larsen research created a new chemometric tool to choose the green and sustainable solvent (G) [42].
According to the equation: , (G) reflects the numerical value of the fourth root generated by multiplying four primary sustainable factors: Health (H), Safety (S), Environment (E), and Waste Disposal (W). More information can be found in this manuscript [42], and a free calculator for many solvents is available at this link: http://green-solvent-tool.herokuapp.com/
A lower value of G indicates that the used solvent's features are not sustainable, and a higher score indicates a solvent's desirable sustainability and greenness. All values range from 1 to 10.
By comparing the obtained G values that were calculated online directly by the GSST tool, the developed UV-methods used Ethanol solvent, while in reported HPLC the acetonitrile solvent was used.
The developed UV-methods score is (GEthanol = 6.6 with category scores: W = 4.2, H = 8.9, E = 6.7, S = 7), while the score for the reported HPLC is (GAcetonitrile = 5.8 with category scores: W = 2.8, H = 5.9, E = 8.9, S = 7), as laid out in Fig. 7. This score is reflected an effort from the analyzer to achiev more environmentally-friendly alternative solvents with (high G value) which can be used in the analytical method.
Fig. 7.
(G) scores by GSST for ethanol and acetonitrile attained by online tool.
The GSST tool offers a simple, fast and free tool for evaluating solvents in accordance with the GSK Solvent Sustainability Guidelines, and this tool was used for the first time in studying the sustainability of the used solvents in analytical procedures.
The Green Solvent Selection tool is simple to use and quick to get results online when compared different solvents but we must emphasize that not all chemical solvents are available in this tool because it is restricted to a small number of commonly used solvents and depends on evaluating sustainability according to a particular mathematical equation.
6.2. Spider diagram for assessment of the greenness index
The reagents utilized in the proposed UV methods and the reported HPLC method were thoroughly reviewed using an evaluation tool called the Greenness Index with spider diagram. This tool depends on data from (SDSs), which describes a reagent's numerous properties and how they influence the SHE throughout the entire process.
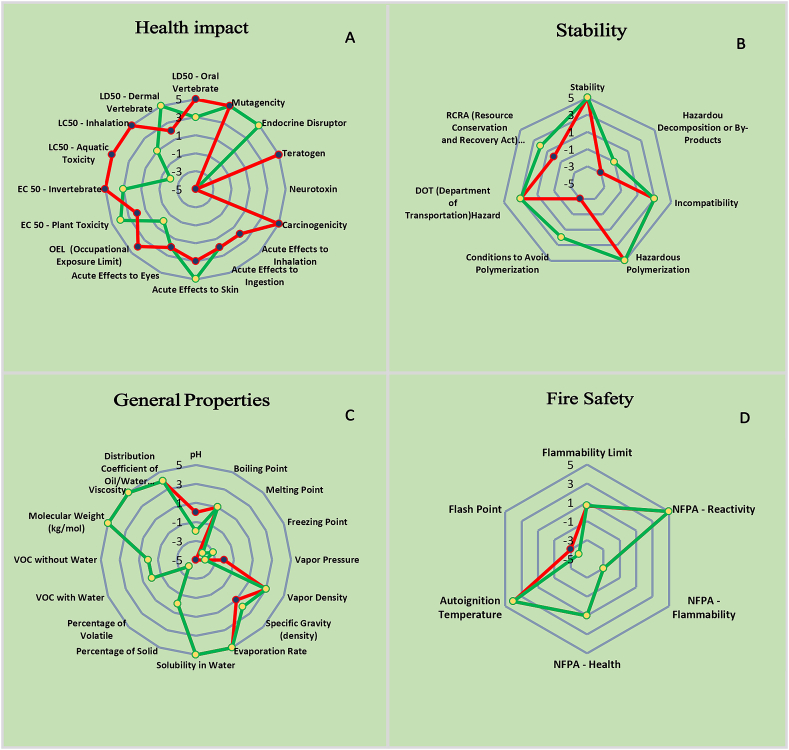
Five subcategories of evaluation criteria (Health Impact, Overall Characteristics, Odor, Fire Prevention, and Stability) were combined in a hierarchical spider diagram to provide a visual depiction of the overall sustainability level for the used chemicals. The results for these criteria ranged from −5 to +5. There are further spider charts that provide more information on each of the five subcategories described above.
Many chemical reagents do not provide all of the necessary information regarding the five aforementioned subgroups on an SDS, so this missed data had a zero score in calculation. The portion of accessible data required to generate the Greenness Index values, along with all the missing data, are released in a data table referred to as the “Greenness Index Table.” The degree of confidence in the greenness judgment is indicated by these references [[38], [39], [40]].
The Greenness Index with spider diagram was employed in the analytical UV-method as well as in the proclaimed HPLC method in terms of providing a full review for the used solvents.
The primary spider chart, Fig. 8, shows that e the overall greenness index of ethanol is on the safe side, and that acetonitrile and potassium phosphate monobasic (KH2PO4) were elevated lower point totals, which reflected a decrease in the safety area. The secondary spider charts in Fig. 9 A-D display the supporting information for the other scores. Table 10 Greenness Index shows the average scores and the percentage of valuable data that are currently available for the used solvents. This spider approach provides visual evaluating reagents, whose comparison between them become easier . In UV-methods, just ethanol was utilized, but the proclaimed HPLC method used two reagents: KH2PO4 and acetonitrile; however, KH2PO4 is considered as safe, not dangerous, solvent . So the acetonitrile is responsible for the danger score. Therefore, KH2PO4 can be considered 24 not affect the evaluation of the greenness; however, the acetonitrile one plays the main role in determining the greenness solvent scores in the chromatographic method.
Fig. 8.
Primary spider diagram for ethanol and acetonitrile.
Fig. 9.
Secondary spider diagram for: A- Health impact, B- Stability, C- General properties, and D- Fire Safety.
Table 10.
Greenness Index Table for ethanol and acetonitrile.
| Evaluation parameters | Health Effect | Characteristics in general | Fire Safety | Odor | Stability | Score average |
|---|---|---|---|---|---|---|
| Acetonitrile score | 1.88 | 0.49 | 0.77 | 0.00 | 1.43 | 0.91 |
| Available information% | 100.00 | 87.50 | 100.00 | 100.00 | 100.00 | 97.50 |
| Ethanol score | 2.63 | 0.69 | 0.61 | 0.00 | 2.71 | 1.33 |
| Available information% | 100.00 | 87.50 | 100.00 | 100.00 | 85.71 | 97.50 |
By comparing the ethanol solvent with the acetonitrile ones, we can see from the previous spider diagram results and the Greenness Index table that the ethanol solvent with an average score 1.33 is safer for the environment and human health, and it is preferable to use UV-methods in the light of green chemistry.
It is clear that applying the Greenness Index method is not as easy as the GSST method; the implementation of the Spider Greenness Index technique is more challenging since it necessitates individual effort and involves viewing several SDSs in order to get the most beneficial information. Nevertheless, this approach offers a visual representation of the solvent greenness comparison because it explains the linked sub-points with each criterion of greenness through secondary spider charts. The study provides the greenness points for each solvent employed, and the greenness table index provides a broad overview. We must emphasize that any utilized solvent may be used with this technique.
6.3. (AGREE) Analytical Greenness diagram
The free, open-source AGREE software offers a circle pictogram in the shape of a clock with 12 digits, that each represents one of the 12 GAC principles on a scale from 0 to 1. The color range for this scale runs from dark green to dark red. The ultimate average calculated value, which was obtained from the 12 data, was represented by the middle value in the AGREE pictogram, that is colored correspondingly. These manuscripts [[46], [47], [48], [49], [50], [51]] have further information about this green metric instrument.
The AGREE program offers transparent data that meets all 12 GAC standards and gives the analyst the freedom to decide how the data should be weighed.
The UV-methods AGREE scoring is (0.84) in Fig. 10 A compared to the proclaimed HPLC method AGREE scoring of (0.71) as laid out in Fig. 10 B, confirming the viability of the suggested UV-method.
Fig. 10.
A- Results of AGREE scheme for the UV-methods.
B- Results of AGREE scheme for the HPLC proclaimed method.
Graphs show the full scores for the UV-methods and the proclaimed HPLC method, showing whether they were attained and whether they were also related to the greenness evaluation. These scores are used to represent all of the twelve GAC principles.
6.4. (ComplexGAPI) Complimentary green analytical procedure index
With this approach, each stage of the analytical process is evaluated using criteria from four broad categories and a total of fifteen factors.
Along with the method's qualitative or quantitative goals, factors to consider include transport, preservation, handling, chemical products and compounds utilized, and instruments. A color scheme pictogram of GAPI has been used, with each stage having two or three degrees of evolution.
The term “Complex GAPI” refers to a developed or extended GAPI matrix with an extra visualization tool at the bottom of the GAPI pictogram, showing the summary of GAPI evolution outcomes as well as the environmental impact of the pre-analysis procedure. The new tool is displayed as a hexagonal zone, containing subdivisions for selectivity and experimental settings, cost, the effects of chemicals and solvents, instrument setup, and purification. It also handles the waste produced when the e-factor is calculated; the higher the factor is (near to 1), the larger the trash is produced, and the more severe the environmental impact is.
Only three factors in the GAPI pictogram produced for the suggested UV-methods. Fig. 11 A displayed yellow color. Due to the method's use of solvent, waste has been produced, and no waste processing technology has been created. The waste produced was somewhat below 10 mL and biodegradable; nevertheless, a tiny quantity of ethanol, a green solvent, was employed as the solvent. Furthermore, the e-factor is lower than 0.1 due to the fact that there is no prior preparation for the analysis, which confirms the total environmental friendliness of the suggested UV-methods.
Fig. 11.
A- Complex GAPI pictogram for greenness assessment of the UV-methods.
B- Complex GAPI pictogram for greenness assessment of the proclaimed HPLC method.
However, the proclaimed HPLC method is not environmental-friendly according to produced results by complex GAPI in several ways, Fig. 11 B, including reagent usage, waste production, and conditions employed during analytical procedures. The analysis entails a number of steps, the application of a high temperature about 40 °C, and a high volume of toxic chemicals were used. The process has a higher E-factor and does not assist the green economy. Because of this, the final procedure receives the lowest score when compared to UV- methods in terms of the green character according to GAPI complex tool, and further changes are advised [44,52].
7. Conclusion
To the highest of our findings, no preceding UV spectroscopy studies have been recorded in a review of the literature for the synchronous quantification of this triplex commixture. Therefore, the major abridgment of this work is to develop three accurate, sensitive, and eco-friendly approaches for the synchronous quantification of ASA and SIC in the presence of SA.
Firstly, DW-RS was applied as a simple and accurate approach. Then, AAC was established as an alternative approach, utilizing a single divisor and a single wavelength for synchronous quantification. Finally, DDRS-D1 was created as a remarkable approach for electing the best wavelength throughout the spectrum for the synchronous quantification of the triplex commixture. Furthermore, four innovative greenness methods have been assessed utilizing GSST, spider diagram, AGREE, and GAPI complex metrics.
GSST has been developed for the first time to measure the influence of the utilized reagents in the UV-methods in the context of green and sustainable chemistry because reagents are crucial to the majority of analytical processes. The comparison of the two green metrics demonstrates that the AGREE and GAPI complex metrics are simple to use when analyzing information on how the methods reviewed affect the environment because both metrics are easy to use, and both of them are provided with an open program that can be downloaded for free and is easy to use.
The results from the AGREE metrics are obvious, and its free software version provides a digitally colored pictogram, which provides quick visual impression.
The analyzer in GAPI complex selects parameters from drop-down boxes that correspond to the pre-analysis operations, the sample preparation, and the analysis stages, which resulted in generating Complex GAPI pictogram. This criterion is not present in the AGREE tool.
There are many instrumental techniques that can be used for the analysis of pharmaceutical drugs, including UV-spectrophotometry methods, fluorimetry, IR, and chromatography. The UV-methods are typically simpler and safer, and we can also say that the UV-methods succeeded in getting more green points because they do not use more energy and solvent like the proclaimed HPLC method.
The developed UV- methods can be beheld as a vigorous alternative to chemometric and HPLC approaches in quality control laboratories because of their rapid quantification without the need for complicated apparatuses.
Author contribution statement
Zahraa Kayali: performed the experiments; contributed reagents, materials, analysis tools or data; wrote the paper. Reem Obaydo: analyzed and interpreted the data; contributed reagents, materials, analysis tools or data; wrote the paper. Amir Alhaj Sakur: conceived and designed the experiments; analyzed and interpreted the data; contributed reagents, materials, analysis tools or data.
Funding statement
Not applicable.
Data availability statement
Data will be made available on request.
Additional information
Not available.
Conflicts of interest
The authors declare that they have no conflicts of interests.
Acknowledgement
Not applicable.
References
- 1.Eftekhar M., Tabibnejad N., Tabatabaie A.A. The thin endometrium in assisted reproductive technology: an ongoing challenge. Middle East Fertil. Soc. J. 2018;23:1–7. [Google Scholar]
- 2.Kunicki M., Łukaszuk K., Woclawek-Potocka I., Liss J., Kulwikowska P., Szczyptańska J. Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. BioMed Res. Int. 2014;2014 doi: 10.1155/2014/913235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irfan M., Ismail S.B., Noor N.M., Hussain N.H.N. Efficacy of aspirin for vasculogenic erectile dysfunction in men: a meta-analysis of randomized control trials. Am. J. Men's Health. 2020;14 doi: 10.1177/1557988320969082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Žegarac M., Lekšić E., Šket P., Plavec J., Bogdanović M.D., Bučar D., Dumić M., Meštrović E. A sildenafil cocrystal based on acetylsalicylic acid exhibits an enhanced intrinsic dissolution rate. CrystEngComm. 2014;16:32–35. doi: 10.1039/c3ce42013b. [DOI] [Google Scholar]
- 5.Bayraktar Z., Albayrak S. Efficacy and safety of combination of tadalafil and aspirin versus tadalafil or aspirin alone in patients with vascular erectile dysfunction: a comparative randomized prospective study. Int. Urol. Nephrol. 2019;51:1491–1499. doi: 10.1007/s11255-019-02211-4. [DOI] [PubMed] [Google Scholar]
- 6.British Pharmacopoeia . - References - Scientific Research Publishing; 2016. Stationery Office on Behalf of the Medicines and Healthcare Products Regulatory Agency (MHRA)- Crown Copyright. [Google Scholar]
- 7.Patrono C. Aspirin as an antiplatelet drug. N. Engl. J. Med. 1994;330:1287–1294. doi: 10.1056/NEJM199405053301808. [DOI] [PubMed] [Google Scholar]
- 8.Catella-Lawson F., Reilly M.P., Kapoor S.C., Cucchiara A.J., DeMarco S., Tournier B., Vyas S.N., FitzGerald G.A. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N. Engl. J. Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 9.El-Yazbi F.A., Amin O.A., El-Kimary E.I., Khamis E.F., Younis S.E. Simultaneous determination of methocarbamol and aspirin in presence of their pharmacopeial-related substances in combined tablets using novel HPLC-DAD method. Drug Dev. Ind. Pharm. 2019;45:265–272. doi: 10.1080/03639045.2018.1535603. [DOI] [PubMed] [Google Scholar]
- 10.Elmasry M.S., Serag A., Hassan W.S., El-Mammli M.Y., Badrawy M. Spectrophotometric determination of aspirin and omeprazole in the presence of salicylic acid as a degradation product: a comparative evaluation of different univariate/multivariate post processing algorithms. J. AOAC Int. 2021;105:309–316. doi: 10.1093/jaoacint/qsab105. [DOI] [PubMed] [Google Scholar]
- 11.Abdelaleem E.A., Abou El Ella D.A., Mahmoud A.M., Abdelhamid N.S. Green analysis of newly approved binary omeprazole/aspirin mixture in presence of aspirin impurity using ultra‐high‐performance liquid chromatography and thin‐layer chromatography methods. Biomed. Chromatogr. 2021;35 doi: 10.1002/bmc.4986. [DOI] [PubMed] [Google Scholar]
- 12.Cao W., Yao H., Qian Z., Wu Y., Wang S., Li R., Rui Gao R. Simultaneous quantification of aspirin, its metabolite salicylic acid, and salvianolic acid B in human plasma using UPLC-MS/MS. Int. J. Anal. Chem. 2021:2021. [Google Scholar]
- 13.Salonia A., Rigatti P., Montorsi F. Sildenafil in erectile dysfunction: a critical review. Curr. Med. Res. Opin. 2023;19:241–262. doi: 10.1185/030079903125001839. [DOI] [PubMed] [Google Scholar]
- 14.Bhogal S., Khraisha O., Al Madani M., Treece J., Baumrucker S.J., Paul T.K. Sildenafil for pulmonary arterial hypertension. Am. J. Therapeut. 2019;26 doi: 10.1097/MJT.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 15.Salem H., Abdel Aziz B.E. Utility of gold nanoparticles for spectrofluorimetric and spectrophotometric determination of sildenafil citrate, dapoxetine, vardenafil and tadalafil in their dosage forms and biological fluids. Anal. Chem. Lett. 2020;10:321–335. [Google Scholar]
- 16.Ramin M., Sohrabi M.R., Motiee F. Smart spectrophotometric methods for enhancement spectral resolution and rapid simultaneous determination of dapoxetine and sildenafil in commercial tablets using fuzzy inference system and continuous wavelet transform. Chemometr. Intell. Lab. Syst. 2022;230 [Google Scholar]
- 17.Bui V.T., Doan C.S., Tong T.T.V., Le D.C. Development and validation of a simple, green infrared spectroscopic method for quantitation of sildenafil citrate in siloflam tablets of unknown manufacturing formula. J. Anal. Methods Chem. 2021:2021. doi: 10.1155/2021/6616728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dural E. Investigation of the presence of sildenafil in herbal dietary supplements by validated HPLC method. Turkish J. Pharm. Sci. 2020;17:56. doi: 10.4274/tjps.galenos.2018.91249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khristi A., Mardia R., Suhagia B. Uv spectrophotometric method development and validation of first derivative method for simultaneous estimation of sildenafil citrate (sil) and aspirin (asp) in bulk and tablet dosage form. Indo Am. J. Pharm. Res. 2015;2015:5. [Google Scholar]
- 20.Sakur A.A., Kayali Z. Development of four UV-spectrometric techniques for concurrent estimation of aspirin and sildenafil citrate in their binary mixture and pharmaceutical formulations. Bull. Pharm. Sci. Assiut. 2022;45:761–773. [Google Scholar]
- 21.Bae S.K., Seo K.A., Jung E.J., Kim H., Yeo C., Shon J., Park K., Liu K., Shin J. Determination of acetylsalicylic acid and its major metabolite, salicylic acid, in human plasma using liquid chromatography–tandem mass spectrometry: application to pharmacokinetic study of Astrix® in Korean healthy volunteers. Biomed. Chromatogr. 2008;22:590–595. doi: 10.1002/bmc.973. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y., Xu P.P., Li X.X., Nie K., Tuo M.F., Kong B., Chen J. Monitoring the hydrolyzation of aspirin during the dissolution testing for aspirin delayed-release tablets with a fiber-optic dissolution system. J. Pharm. Anal. 2012;2:386–389. doi: 10.1016/j.jpha.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolores M., Morales-Hipólito E.A., Garduño-Rosas J.A., Villaseñor A., López-Arellano R. Development and validation of an alternate stability-indicating UV spectrophotometric analytical method for aspirin in tablets. Indian J. Pharmaceut. Sci. 2017;78:810–817. [Google Scholar]
- 24.Mohamed H.M. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. TrAC Trends Anal. Chem. 2015;66:176–192. [Google Scholar]
- 25.Armenta S., Garrigues S., De la Guardia M., Esteve-Turrillas F.A. 3rd ed. 2019. Green Analytical Chemistry; pp. 497–511. [DOI] [Google Scholar]
- 26.Obaydo R.H., Sakur A.A. A green analytical method using algorithm (PCCA) for extracting components' contribution from severely overlapped spectral signals in pharmaceutical mixtures. Res. J. Pharm. Technol. 2019;12:4332–4338. [Google Scholar]
- 27.El-Hanboushy S., Marzouk H.M., Fayez Y.M., Abdelkawy M., Lotfy H.M. Eco-friendly spectrophotometric evaluation of triple-combination therapies in the treatment strategy of patients suffering from hypertension during coronavirus pandemic–Spectralprint recognition study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022:280. doi: 10.1016/j.saa.2022.121523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Hanboushy S., Marzouk H.M., Fayez Y.M., Abdelkawy M., Lotfy H.M. Sustainable spectrophotometric determination of antihypertensive medicines reducing COVID-19 risk via paired wavelength data processing technique-Assessment of purity, greenness and whiteness. Sustain. Chem. Pharm. 2022;29 doi: 10.1016/j.scp.2022.100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed D.A., Lotfy H.M. Sticking-pulling strategy for assessment of combined medicine for management of tough symptoms in COVID-19 Pandemic using different windows of spectrophotometric Platform-Counterfeit products' detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022:277. doi: 10.1016/j.saa.2022.121256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotfy H.M., Ahmad D.A., Rahman M.K.A., Weshahy S.A.F. Study of efficiency and spectral resolution for mathematical filtration technique using novel unlimited derivative ratio and classical univariate spectrophotometric methods for the multicomponent determination-stability analysis. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lotfy H.M., El-Hanboushy S., Fayez Y.M., Abdelkawy M. Smart spectrophotometric methods for concurrent determination of furosemide and spironolactone mixture in their pharmaceutical dosage forms. Braz. J. Pharm. Sci. 2022;58 [Google Scholar]
- 32.Marzouk H.M., Ibrahim E.A., Hegazy M.A., Saad S.S. Eco-friendly resolution of spectrally overlapping signals of a combined triple-action over-the-counter pharmaceutical formulation for symptomatic management of COVID-19 pandemic: application to content uniformity testing. BMC Chem. 2022;16:73. doi: 10.1186/s13065-022-00868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saad A.S. Novel spectrophotometric method for selective determination of compounds in ternary mixtures (dual wavelength in ratio spectra) Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;147:257–261. doi: 10.1016/j.saa.2015.03.095. [DOI] [PubMed] [Google Scholar]
- 34.Lotfy H.M., Saleh S.S. Advanced amplitude centering as an invigorating manipulation for unified wavelength spectral resolution of ternary mixtures. Int. J. Pharm. Pharmaceut. Sci. 2017;9 doi: 10.22159/ijpps.2017v9i5.16960. [DOI] [Google Scholar]
- 35.Fayed A.S., Youssif R.M., Salama N.N., Hendawy H.A., Elzanfaly E.S. Two-wavelength manipulation stability-indicating spectrophotometric methods for determination of meropenem and ertapenem: greenness consolidation and pharmaceutical product application. Chem. Pap. 2019;73:2723–2736. doi: 10.1007/s11696-019-00824-8. [DOI] [Google Scholar]
- 36.Pandey S., Karmakar N.K., Pandey R.K., Shukla S.S. A double divisor ratio-spectra derivative method for the ternary mixture of aspirin, clopidogrel bisulphate and rosuvastatin calcium. Anal. Chem. Lett. 2021;11:696–707. doi: 10.1080/22297928.2021.1929470. [DOI] [Google Scholar]
- 37.Sakur A.A., Zakri D.A. A new selective colorimetric method coupled with a high-resolution UV method for the consecutive quantification of three drugs in semi-solid preparations. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Y., Lo C., Nagaraj D.R., Farinato R., Essenfeld A., Somasundaran P. Development of greenness index as an evaluation tool to assess reagents: evaluation based on SDS (safety data sheet) information. Miner. Eng. 2016;94:1–9. [Google Scholar]
- 39.Abou-Taleb N.H., El-Enany N.M., El-Sherbiny D.T., El-Subbagh H.I. Spider diagram and Analytical GREEnness metric approach for assessing the greenness of quantitative 1H-NMR determination of lamotrigine: taguchi method based optimization. Chemometr. Intell. Lab. Syst. 2021;209 doi: 10.1016/j.chemolab.2020.104198. [DOI] [Google Scholar]
- 40.Lotfy H.M., Obaydo R.H., Nessim C.K. Spider chart and whiteness assessment of synergistic spectrophotometric strategy for quantification of triple combination recommended in seasonal influenza–Detection of spurious drug. Sustain. Chem. Pharm. 2023:32. [Google Scholar]
- 41.Anastas P.T., Zimmerman J.B. ACS Publications; 2003. Peer Reviewed: Design through the 12 Principles of Green Engineering. [DOI] [PubMed] [Google Scholar]
- 42.Larsen C., Lundberg P., Tang S., Ràfols-Ribé J., Sandström A., Mattias Lindh E., Wang J., Edman L. A tool for identifying green solvents for printed electronics. Nat. Commun. 2021;12 doi: 10.1038/s41467-021-24761-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pena-Pereira F., Wojnowski W., Tobiszewski M. AGREE—analytical GREEnness metric approach and software. Anal. Chem. 2020;92:10076–10082. doi: 10.1021/acs.analchem.0c01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Płotka-Wasylka J., Wojnowski W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021;23:8657–8665. [Google Scholar]
- 45.International Conference on Harmonization. Rockville U.S.A. 1996. Guidance for Industry Q2B Validation of Analytical Procedures: Methodology. [Google Scholar]
- 46.El-Yazbi A.F., Aboukhalil F.M., Khamis E.F., Youssef R.M., El-Sayed M.A. In situ green analytical methods for the rapid and sensitive determination of a newly launched orphan anticancer drug; Tigecycline in infusion bags: comparative study. Beni-Suef Univ. J. Basic Appl. Sci. 2022;11:1–15. [Google Scholar]
- 47.El-Yazbi A.F., Aboukhalil F.M., Khamis E.F., Youssef R.M., El-Sayed M.A. Simultaneous determination of Mometasone Furoate and salicylic acid in complex matrix using green analytical method. Microchem. J. 2021;163 [Google Scholar]
- 48.Lotfy H.M., Obaydo R.H., Sakur A.A. Evaluation of assay and in-vitro dissolution profile of certain fixed-dose combination using green analytical method. Ann. Pharm. Fr. 2021;79:3–15. doi: 10.1016/j.pharma.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 49.El-Yazbi A.F., Aboukhalil F.M., Khamis E.F., Youssef R.M., El-Sayed M.A. Greenness assessment of a stability indicating simple inexpensive high-performance thin-layer chromatography–dual wavelength method for simultaneous determination of mometasone furoate and salicylic acid in complex matrix using analytical eco-scale. JPC–Journal Planar Chromatogr. -Mod. TLC. 2021;34:455–466. [Google Scholar]
- 50.El-Yazbi A.F., Khamis E.F., Youssef R.M., El-Sayed M.A., Aboukhalil F.M. Green analytical methods for simultaneous determination of compounds having relatively disparate absorbance; application to antibiotic formulation of azithromycin and levofloxacin. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kannaiah K.P., Sugumaran A., Chanduluru H.K., Rathinam S. Environmental impact of greenness assessment tools in liquid chromatography–A review. Microchem. J. 2021;170 [Google Scholar]
- 52.Attimarad M., Chohan M.S., Narayanaswamy V.K., Nair A.B., Sreeharsha N., Shafi S., David M., Balgoname A.A., Altaysan A.I., Molina E.P., et al. Mathematically processed UV spectroscopic method for quantification of chlorthalidone and azelnidipine in bulk and formulation: evaluation of greenness and whiteness. J. Spectrosc. 2022:2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.