Abstract
Background
To investigate the sterilizing effect of antimicrobial photodynamic therapy (aPDT) based on PAD™ Plus on mixed biofilms of Candida albicans and Candida tropicalis.
Methods
A mature mixed biofilm model of C. albicans and C. tropicalis was constructed in vitro. FITC-concanavalin A staining was conducted to observe the formation of the extracellular matrix. MTT assay was performed to determine biofilm viability. The chromogenic medium was used to examine the Candida composition of the mixed biofilms. For aPDT treatment, based on PAD™ Plus, the biofilms were incubated with 1 mg/mL TBO for 1, 5, or 10 min, followed by 500 or 750 mW LED illumination for 1 or 2 min. The live/dead fungi were detected by SYTO9/propidium iodide staining. A multivariate factorial design was conducted to analyze the correlations of parameters with the inactivation effect of the mixed biofilms.
Results
Mature mixed biofilms formed at 24 h after seeding. Compared with untreated biofilms, following 1-min TBO incubation, 500 mW LED illumination for 1 min inactivated more than 90% of the fungi. Extending the incubation time did not significantly improve the inactivation effect. Application of 750 mW output power or 2 min LED illumination inactivated more than 99% of the fungi without increasing other parameters.
Conclusions
PAD™ Plus combined with 1 mg/mL TBO can rapidly inactivate the mature mixed biofilms of C. albicans and C. tropicalis, serving as a robust platform for the treatment of mixed infections of C. albicans and C. tropicalis.
Keywords: Candida albicans, Candida tropicalis, Biofilms, Antimicrobial photodynamic therapy, Toluidine blue O
1. Introduction
With the increasing number of patients with acquired immunodeficiency syndrome or malignant tumors and the wide application of antibiotics and immunosuppressants, the incidence of oral candidiasis caused by Candida albicans or non-albicans Candida has risen [1]. Mixed infection of C. albicans and C. tropicalis is common in patients with oral candidiasis [2,3]. Candida forms biofilm composed of yeast cells, hyphae or pseudohyphae, and extracellular matrix [4]. Compared with monospecies biofilms, mixed biofilms are more resistant to antifungal treatment [5]. Thus, it is of great importance to find an effective therapeutic strategy to treat mixed Candida infection.
Antimicrobial photodynamic therapy (aPDT) is a promising alternative to drug treatment in antimicrobial therapy against oral infection. In the presence of oxygen, photosensitizer is activated by the light with a specific wavelength to produce singlet oxygen and other reactive oxygen species. These cytotoxic products interact with the cell components (proteins, membrane lipids and nucleic acids, etc.) through different pathways, leading to irreversible damage and rapid necrosis of microorganisms [6]. Scholars have studied the effect of aPDT on the biofilm of Candida [7,8]. For example, Carmello et al. have found that incubation with 0.15 mg/mL photodiazine for 20 min followed by 9-min irradiation suppresses the growth of mixed biofilms of C. albicans and C. tropicalis [7]. Toluidine Blue O (TBO) is a phenothiazinium dye that has been widely studied for antimicrobial application owing to the advantages of low cost, low cytotoxicity to host cells, low excitation energy, singlet oxygen quantum yields, and high affinity to cellular components and membranes of target cells [9]. Incubation with 0.1 mg/mL TBO for 5 min or 2.5 mM TBO for 30 min inhibits the formation of C. albicans biofilm by at least 50% [[10], [11], [12]]. In addition, the relatively small concentrations of TBO require a long exposure time to the photosensitizer or the light source to achieve efficient disinfection, resulting in a long mouth opening time that may cause joint disorders or excess production of saliva to block the adherence of TBO to the biofilms. Thus, to treat oral candidiasis more efficiently, it is of great importance to reduce the time of oral manipulation.
PAD™ Plus is a microbe elimination system mainly used in disinfection of oral cavity, root canal, and periodontal pocket, supplied with a ready-to-use medical grade 1 mg/mL TBO solution and a light-emitting diode (LED) emitting 635 nm red light with an output power of 500 or 750 mW and the illumination time of 1 min or 2 min. TBO adheres to the microbes and reacts to the LED light, releasing singlet oxygen to kill the microbes. Some scholars have proposed that increasing the concentration of photosensitizer can enhance the inhibition effect on biofilm [13], and high-concentration toluidine blue-mediated aPDT can kill the lesion without long incubation [14]. PAD™ Plus guarantees fast and safe cleaning and disinfection owing to the high concentration of TBO and short TBO incubation time [15]. However, the effect of PAD™ Plus on the co-infection of C. albicans and C. tropicalis remains unknown. In this study, we, for the first time, tested the effect ofPAD™ Plus combined with 1 mg/mL TBO on mature mixed biofilms of C. albicans and C. tropicalis. Our results may provide a fast and highly efficient therapeutic approach to mixed C. albicans and C. tropicalis oral infection.
2. Materials and methods
2.1. Candida strains and culture
C. albicans SC5314 (Taxonomy ID:237561) were obtained from Shijiazhuang Heya Biotechnology Co., LTD. (Hebei, China). C. tropicalis ATCC750 (Taxonomy ID: 5482) was purchased from Nanjing Yizhi Biotechnology Co., LTD. (Jiangsu, China). Both strains were stored at −80 °C until use. The strains were activated on Candida chromogenic medium (Comagal microbial technology Co., Shanghai, China). A single activated colony was picked, inoculated into a conical flask containing 20 mL yeast extract-peptone-dextrose medium, and incubated at 30 °C in a shaking water bath (150 r/min) for 24 h. Then, the fungal suspension was transferred to a 50 mL centrifuge tube and centrifuged at 3000 r/min for 15 min. The supernatant was discarded. The precipitate was resuspended in 20 mL phosphate-buffered saline (PBS) and centrifuged again. After repeating the centrifugation and PBS washing process 3 times, the supernatant was discarded, and the fungi were collected and could be retained for use for up to one week when stored at 4 °C.
2.2. FITC-concanavalin A (ConA) staining
Activated C. albicans and C. tropicalis were resuspended in RPMI 1640 medium at 1 × 106 yeast cells/mL, respectively, and then co-cultured in a 20 mm glass-bottom dish at a ratio of 1:1, 1 mL in total, at 37 °C as previously described [16]. FITC-ConA staining was performed at 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, 48 h, 72 h, and 96 h after culture to examine the formation of the extracellular polysaccharides. Briefly, after removing the medium and washing Candida with PBS, 100 μL fluorescent dye FITC-ConA (Kehua Jingwei Technology Co., Beijing, China) was added to the coculture and incubated in the dark for 20 min at room temperature. After removing the unbound dye by rinsing with PBS, the fluorescence was visualized, and images were acquired using a fluorescence microscope (Olympus, Japan).
2.3. MTT assay
Activated C. albicans and C. tropicalis were resuspended in RPMI 1640 medium at 1 × 106 yeast cells/mL and co-cultured in 96-well plates at a ratio of 1:1. The mixed biofilms viability was determined at 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, 48 h, 72 h, and 96 h after seeding using an MTT kit (Xavier Biotechnology Co., Wuhan, China). Briefly, 20 μL MTT solution was added to each well of the plate and incubated for 4 h at 37 °C, followed by incubation with 100 μL DMSO for additional 30 min. The optical density was measured at 490 nm (according to manufacturer's instructions) wavelength using a microplate reader (Thermoelectric, Japan). The growth curve of the biofilm was plotted.
2.4. Examination of the Candida composition of the mixed biofilms
The mixed biofilms were re-cultured to maturity in a 96-well plate. After removing the medium and washing Candidas with PBS, 100 μL PBS was added into the well, and the mixed biofilms at the bottom of the well were scraped off with a sterile toothpick, which could allowed the mixed biofilms to mix with PBS in the well. The suspension was transferred to a centrifuge tube, mixed evenly by swirls, and diluted by 10-fold. A small amount of suspension was inoculated into Candida chromogenic medium. It was placed in an incubator at 37 °C for 24 h to identify the composition of the strains in the mixed biofilms. The fungi were identified by the colors of the clones. Green clones were C. albicans, and blue clones were C. tropicalis.
2.5. aPDT treatment
aPDT treatment was conducted using a DX9001 PAD™ Plus system (Denfotex, UK). The mixed biofilms were seeded in glass-bottom cell culture dishes and cultured for 24 h until maturation. After removing the medium, the biofilms were incubated with 100 μL of 1 mg/mL TBO solution supplied with PAD™ Plus for 1, 5, or 10 min, followed by exposure to 635 nm LED red light with an output power of 500 mW or 750 mW at a distance of 1.5 cm for 1 min or 2 min. Untreated biofilms were used as a negative control. The live/dead fungi were detected by SYTO9 (green)/propidium iodide (PI; red) staining using a kit from Kehua Jingwei Technology (Beijing, China) following the manufacturer’s instruction. The fluorescence was observed using a confocal laser scanning microscope (Olympus, Japan) at the wavelength of 488/546 nm. The number of live/dead fungi was counted using Image J software. Inactivation effect of the biofilms was calculated as the number of dead fungi/(the number of dead fungi + the number of live fungi) × 100%.
2.6. Statistical analysis
All experiments were performed three times with three biological replicates. The inactivation effect of the biofilms was expressed as the mean ± standard deviation. A comparison between untreated and aPDT-treated biofilms was conducted using one-way ANOVA and Tamhane's T2 test. The correlation of aPDT parameters with the inactivation effect of the mixed biofilms was assessed using the 2 × 3 × 2 multivariate factorial design. Statistical analysis was conducted using SPSS 20.0 software (IBM, Armonk, NY, USA). A P value < 0.05 was considered statistically significant.
3. Results
3.1. C. albicans and C. tropicalis form mature mixed biofilms in vitro
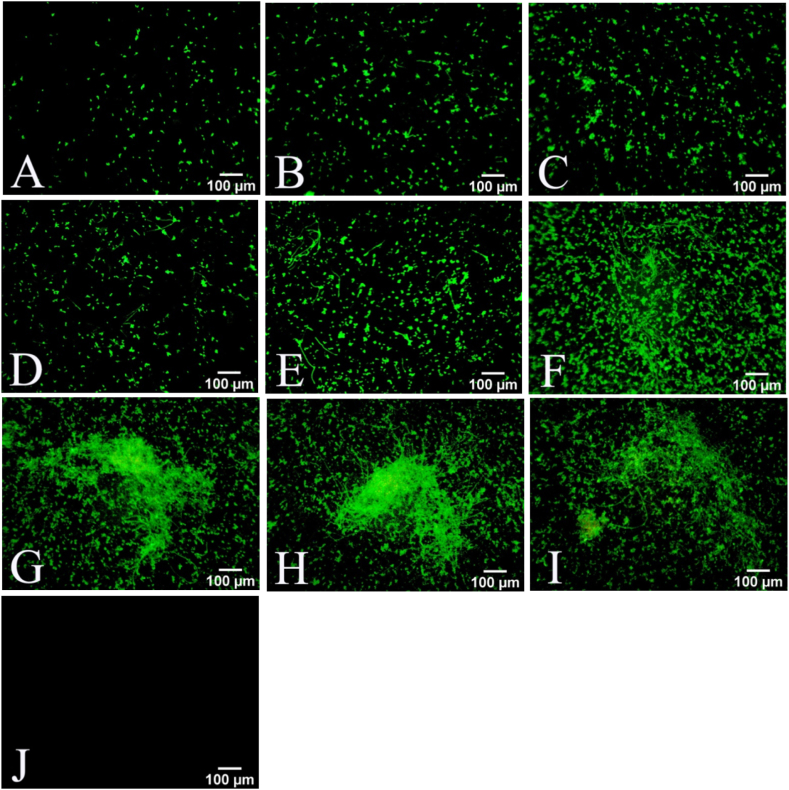
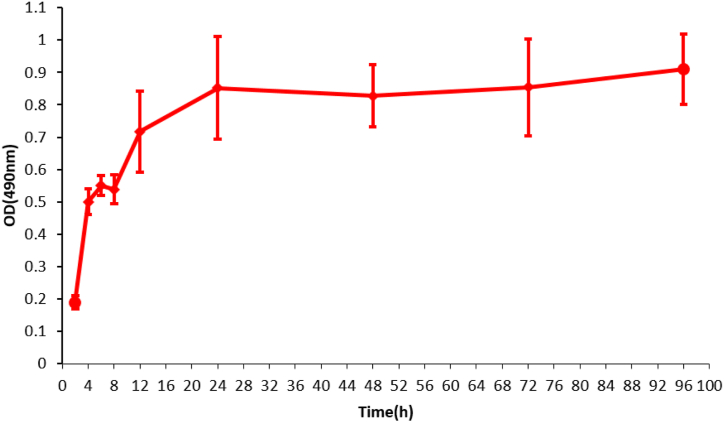
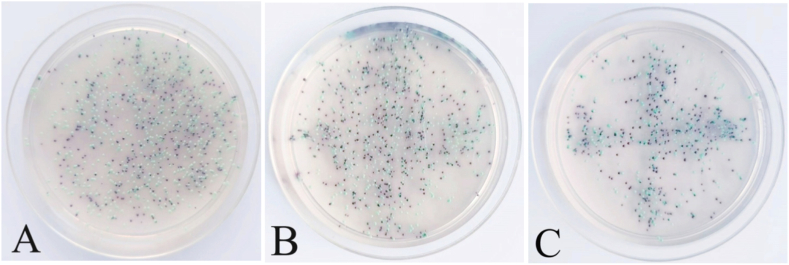
Polysaccharides are an important part of the extracellular matrix of Candida biofilm and also an important factor affecting the permeability of antifungal drugs. FITC-ConA fluorescein can bind with extracellular polysaccharides of biofilm and emit green light at specific wavelength. Therefore, we used FITC-ConA staining to indirectly observe the formation process of extracellular matrix of the mixed biofilms [17,18]. As shown in Fig. 1A–I, the fluorescent intensity in a fungus-containing well was significantly increased in a time-dependent manner compared with that in the negative control well (Fig. 1J). It appears that the extracellular matrix began to form at 2 h after seeding, gradually densified at 24 h, and dispersed at 96 h. MTT assay showed that the viability of C. albicans and C. tropicalis coculture was rapidly and time-dependently increased within 24 h after seeding, reaching a plateau thereafter for at least additional 72 h (Fig. 2). Mature biofilm is characterized by a structured mixture of cells and hyphae wrapped by dense extracellular matrix [19]. These findings suggest that the mature mixed biofilms basically form at about 24 h after seeding, and the metabolic activity of cells in the biofilms is maintained at a relatively stable level after 24 h.To examine the composition of the mixed biofilms, we collected the mature biofilm in Candida chromogenic medium to visualize the colonies. As shown in Fig. 3, there were approximately the same number of green (C. albicans) and blue (C. tropicalis) colonies in the medium at 24 h after seeding, suggesting that the mature biofilm was composed of equal amount of C. albicans and C. tropicalis (Fig. 3).
Fig. 1.
Formation of the mature mixed biofilms of Candida albicans and Candida tropicalis. C. albicans and C. tropicalis were cocultured at a ratio of 1:1. FITC- concanavalin A staining was performed at 2 h (A), 4 h (B), 6 h (C), 8 h (D), 12 h (E), 24 h (F), 48 h (G), 72 h (H), and 96 (I) after cultivation to detect the extracellular matrix of the biofilms, dishes stained without co-culture of Candida albicans and Candida tropicalis were used as a negative control (J). Scale bar = 100 μm. The experiment was performed three times with three biological replicates.
Fig. 2.
The growth curve of the mature mixed biofilm. C. albicans and C. tropicalis were cocultured at a ratio of 1:1. MTT assay was conducted to determine the biofilm viability of the cocultures at different time points after cultivation. Data are expressed as the mean ± standard deviation. The experiment was performed three times with three biological replicates.
Fig. 3.
Identification of the Candida composition of the mixed biofilms. The green (C. albicans) and blue (C. tropicalis) colonies were observed after the suspension of the mixed biofilms was cultured in chromogenic medium for 24 h (A–C). The experiment was performed three times with three biological replicates. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.2. PAD™ Plus showed significant inactivation effect on fungi in the mixed biofilms
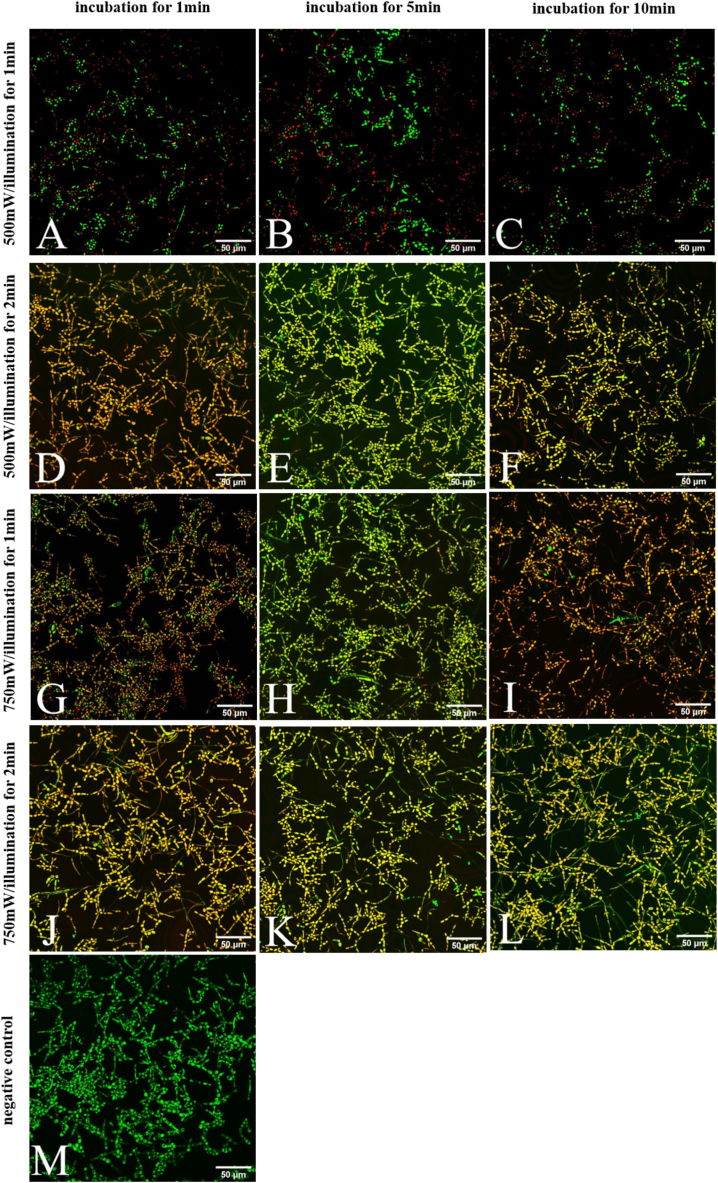
To evaluate the effect of PAD™ Plus combined with 1 mg/mL TBO on the mature mixed biofilms, we treated the biofilms with 1 mg/mL TBO for 1, 5, or 10 min, followed by 1 or 2-min LED illumination with a 500 or 750-mW output power (Table 1). SYTO9/PI staining showed that compared with untreated biofilms, PAD™ Plus with different combinations of parameters inactivated more than 90% of the fungi in the biofilms (Fig. 4, Table 2). Extending TBO incubation time from 1 min to 5 or 10 min did not significantly change the inactivation effect, suggesting that a 1-min TBO incubation is sufficient to inactivate more than 90% of the fungi in the mature mixed biofilms using PAD™ Plus. We further found that increasing the output power to 750 mW or illumination time to 2 min enhanced the inactivation effect to nearly 100% with TBO incubation time remaining the same. Thus, after incubating with 1 mg/mL TBO for 1 min, 750-mW LED illumination for 1 min or 500-mW LED illumination for 2 min may achieve the maximum inactivation effect in the mature mixed biofilms of C. albicans and C. tropicalis.
Table 1.
The parameters of aPDT treatment groups.
| aPDT group | TBO incubation time (min) | Output power |
LED illumination time (min) |
|---|---|---|---|
| (mW) | |||
| A | 1 | 500 | 1 |
| B | 5 | 500 | 1 |
| C | 10 | 500 | 1 |
| D | 1 | 500 | 2 |
| E | 5 | 500 | 2 |
| F | 10 | 500 | 2 |
| G | 1 | 750 | 1 |
| H | 5 | 750 | 1 |
| I | 10 | 750 | 1 |
| J | 1 | 750 | 2 |
| K | 5 | 750 | 2 |
| L | 10 | 750 | 2 |
TBO, toluidine blue O; aPDT, antimicrobial photodynamic therapy; LED, light-emitting diode.
Fig. 4.
Photoactivated disinfection treatment. The mixed biofilms cultured for 24 h were incubated with 1 mg/mL toluidine blue O (TBO) for 1 min (A,D,G,J), 5 min (B,E,H,K), or 10 min (C,F,I,L), followed by exposure to LED for 1 min (A,B,C,G,H,I) or 2 min (D,E,F,J,K,L) with output power of 500 mW (A–F) or 750 mW (G–L). Output power/illumination time was arranged in each row, and incubation time was arranged in each column.Untreated biofilms were used as a negative control (M). SYTO9/propidium iodide staining was performed to identify live/dead fungi. Fungi were observed using a confocal laser scanning microscope. Green (A–M) fluorescence indicated live fungi and red (A,B,C,M) fluorescence indicated dead fungi. Orange (D,F,G,I,J) or Yellow (E,H,K,L) fluorescence is an overlay of green and red, indicating dead fungi. When Image J software was used to count the live/dead fungi on the image, the green channel and the red channel were counted respectively. Scale bar = 50 μm. The experiment was performed three times with three biological replicates. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Comparison of the inactivation effect between untreated and aPDT-treated biofilms.
| Group | Output power |
LED illumination time (min) | TBO incubation time (min) | inactivation |
|---|---|---|---|---|
| (mW) | effect (%) | |||
| Negative control | 0 | 0 | 0 | 1.43 ± 0.67 |
| aPDT | 500 | 1 | 1 | 92.41 ± 3.84a |
| 5 | 92.93 ± 3.61a | |||
| 10 | 92.97 ± 3.87a | |||
| 2 | 1 | 99.82 ± 0.29a | ||
| 5 | 99.95 ± 0.07a | |||
| 10 | 99.54 ± 0.64a | |||
| 750 | 1 | 1 | 99.75 ± 0.20a | |
| 5 | 99.92 ± 0.09a | |||
| 10 | 99.83 ± 0.25a | |||
| 2 | 1 | 99.74 ± 0.23a | ||
| 5 | 99.63 ± 0.30a | |||
| 10 | 99.86 ± 0.14a | |||
| P value | <0.01 | |||
TBO, toluidine blue O; aPDT, antimicrobial photodynamic therapy; LED, light-emitting diode; a, P < 0.05 vs. negative control.
3.3. Output power and LED illumination time significantly correlated with the inactivation effect of the mixed biofilms
To further evaluate the correlation of TBO incubation time, output power, and LED illumination time with the antifungal effect of PAD™ Plus, we performed a multivariate factorial design. The results showed that the output power and illumination time, individually or in combination, significantly correlated with the inactivation effect of the biofilms (Ps < 0.01; Table 3). No significant correlation was observed between the inactivation effect of the biofilms and the incubation time, individually or in combination with other parameters.
Table 3.
Multivariate factorial design.
| df | Mean Square | F value | P value | |
|---|---|---|---|---|
| Power | 1 | 334.54 | 92.21 | <0.01 |
| Incubation time | 2 | 0.30 | 0.08 | 0.92 |
| Illumination time | 1 | 322.13 | 88.79 | <0.01 |
| Power × incubation time | 2 | 0.23 | 0.06 | 0.94 |
| Power × illumination time | 1 | 339.35 | 93.54 | <0.01 |
| incubation time × illumination time | 2 | 0.42 | 0.12 | 0.89 |
| Power × incubation time × illumination time | 2 | 0.52 | 0.14 | 0.87 |
df, degrees of freedom.
4. Discussion
In this study, we demonstrated that after a 1-min incubation with 1 mg/mL TBO, more than 99% of the fungi in the biofilms were inactivated when exposed to 750 mW LED for 1 min or 500 mW LED for 2 min using PAD™ Plus. Our results suggest that PAD™ Plus combined with 1 mg/mL TBO can rapidly and high-efficiently inactivate the fungi in the mature mixed biofilms of C. albicans and C. tropicalis.
Biofilm formation is well accepted as a major virulence feature implicated in the pathogenesis of candidiasis. Biofilms are 1000-fold more resistant to conventional antifungal drugs than planktonic cells due to the denser structure resulting from more biomass in the membrane and extracellular matrix [13,20,21]. In monospecies biofilm studies, a mature C. albicans biofilm forms at 24 h after seeding the fungi on a solid surface [22], whereas a mature biofilm of C. tropicalis forms at 48 h [23]. In this study, we monitored the development of the mature mixed biofilms of C. albicans and C. tropicalis for 96 h. We found that the extracellular matrix began to form at 2 h after seeding, gradually densified at 24 h, and dispersed at 96 h. These data suggest that the mature mixed biofilms basically form at around 24 h after seeding. MTT assay showed that the viability of the coculture peaked at 24 h post seeding. Thus, we further investigated the effect of PAD™ Plus on the mature mixed biofilms at 24 h after cultivation. Plate counting is the most basic method to determine the number of living cells in biofilm. This method however, requires scraping off the biofilm with a sterile toothpick or other auxiliary means, which can lead to non-uniform collection of the biofilm. In addition, fungal clumps can result in error-prone enumeration of colony counts. The SYTO9/PI-ClSM technique is a modern technique that overcomes the pitfalls associated with colony counting of biofilms. It does not require collection and re-suspension of biofilm, and can distinguish between live and dead fungi. Image J software is used to count the collected images and calculate the proportion of inactivated fungi [[24], [25], [26]]. Therefore, SYTO9/PI combined with CLSM technology was selected for quantitative analysis of the inactivation effect of biofilm.
Antibacterial photodynamic therapy based on PAD™ Plus was used in this study. The antimicrobial efficacy of aPDT results from the interaction of the photosensitizer, the light source, and oxygen. In this study, we used a photosensitizer of 1 mg/mL TBO, which is a ready-to-use medical grade solution supplied with the PAD™ Plus device. After being excited by 635 nm LED red light, TBO gets converted into a photoexcited form, producing singlet oxygen that interacts with a large number of molecules in the cells to generate oxidized products [27,28], Owing to the small molecule, positive charges, good water solubility, and strong hydrophilicity, TBO has a high affinity to the microbial cell membrane [11]. In addition, TBO exhibits significantly different affinities between Candida and host cells, showing a high selectivity for killing microorganisms [11]. Studies have shown that TBO-mediated aPDT suppresses biofilm growth by inactivating the yeast cells, reducing the number of hyphae, and inhibiting Candida adhesion [10,13]. The concentration of TBO affects the maximum absorption and emission wavelength, and thus plays a critical role in the inactivation effect. The relatively high concentration of photosensitizer contributes to strong antimicrobial activity. However, a high photosensitizer concentration may cause intermolecular aggregation of photosensitizer due to electrostatic and hydrophobic effects and inhibit the propagation of light [29], thereby reducing the antimicrobial efficacy.
The antimicrobial efficacy of aPDT is associated with multiple factors, including photosensitizer incubation time, illumination time, and output power [13]. The photosensitizer incubation time affects its penetration depth into the biofilm. Gu et al. have reported that aPDT with 1-min incubation with 1 mg/mL TBO and 1-min exposure to 750 mW LED red light has a good inactivation effect on C. albicans on the dorsal tongue of mice [15]. In our preliminary study, we did not observe a significant difference in the inactivation effects between 1 and 2-min TBO incubations. Therefore, we assessed the inactivation effects of 1, 5, and 10-min TBO incubations. Fumes et al. have suggested that a short pre-irradiation time promotes the photosensitive reaction of photosensitizer [30], and the output power affects the excitation state of photosensitizers [31]. Therefore, we examined the inactivation effects of 1 or 2-min illumination as well as 500 or 750-mW output power according to the radiation duration mode of our device. We found that with the minimum illumination time and output power, 1-min TBO incubation inactivated more than 90% of the fungi in the biofilms. Extending the incubation time did not significantly change the inactivation effect regardless of the illumination time or output power. Furthermore, when the output power reached 750 mW or illumination time reached 2 min, aPDT treatment inactivated nearly 100% of the fungi in the biofilms with the other two variables set at the minimum levels. The multivariate factorial analysis further showed that the output power and illumination time, individually or in combination, significantly correlated with the inactivation effect. This finding may provide important guidance for clinical practices in the treatment of mixed oral biofilms.
In this study, we established a mixed biofilm system grown in a static environmental condition in vitro. There may be competition between the two fungi [32,33], which may be the reason why CLSM was unable to obtain biofilm thickness. A thicker, continuous flow biofilm model is required to solve this problem and verify our findings [34], which we will examine in future studies. In addition, more complex biofilms may be generated by different fungi and bacteria in clinical practice, and the effect of PAD™ Plus combined with 1 mg/mL TBO on the mixed complex biofilms needs to be further studied. Referring to Pinto, Shi et al. [13,35], the standard strains of Candida albicans Candida tropicalis were used in this study. Previous studies have demonstrated that PAD™ Plus-based aPDT is effective on a single Candida biofilm [15], so clinical strains and a single biofilm were not evaluated in this study. Additional studies on clinical strains and single biofilms are needed to improve the validity of the conclusions, and further studies will be carried out in the follow-up work.
In summary, we established a mature mixed biofilm model of C. albicans and C. tropicalis in vitro. We found that a 1-min incubation with 1 mg/mL TBO followed by 1-min 750 mW LED illumination or 2-min 500 mW LED illumination inactivated more than 99% of the fungi in the mature mixed biofilms based on PAD™ Plus. This study provides a promising therapeutic strategy for rapidly and efficiently combating the mixed infections of C. albicans and C. tropicalis.
Author contribution statement
Lifang Zhang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Na Liu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Qing Liu: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Qiaoyu Hu: Performed the experiments; Analyzed and interpreted the data.
Ying Zhang: Performed the experiments; Analyzed and interpreted the data.
Yanan Wang: Performed the experiments; Analyzed and interpreted the data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
Acknowledgements
This work was supported by the S&T Program of Hebei [grant number 20377799D]; the academic leader training program of Hebei Provincial government [grant number 2018133206-2]; the Medical Science Research subject of Health Commission of Hebei Province [grant number 20191079]; the Innovative experiment project for College Students of Hebei Medical University [grant number USIP 2021115].
Contributor Information
Na Liu, Email: liuna@hebmu.edu.cn.
Qing Liu, Email: liuqing@hebmu.edu.cn.
References
- 1.Muadcheingka T., Tantivitayakul P. Distribution of Candida albicans and non-albicans Candida species in oral candidiasis patients: correlation between cell surface hydrophobicity and biofilm forming activities. Arch. Oral Biol. 2015;60(6):894–901. doi: 10.1016/j.archoralbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Menezes Rde P., et al. Related factors for colonization by Candida species in the oral cavity of HIV-infected individuals. Rev. Inst. Med. Trop. Sao Paulo. 2015;57(5):413–419. doi: 10.1590/S0036-46652015000500008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu L., et al. Characterization of oral candidiasis and the Candida species profile in patients with oral mucosal diseases. Microb. Pathog. 2019;134 doi: 10.1016/j.micpath.2019.103575. [DOI] [PubMed] [Google Scholar]
- 4.Taff H.T., et al. Mechanisms of Candida biofilm drug resistance. Future Microbiol. 2013;8(10):1325–1337. doi: 10.2217/fmb.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vipulanandan G., et al. Dynamics of mixed- Candida species biofilms in response to antifungals. J. Dent. Res. 2018;97(1):91–98. doi: 10.1177/0022034517729351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmello J.C., et al. Antimicrobial photodynamic therapy reduces adhesion capacity and biofilm formation of Candida albicans from induced oral candidiasis in mice. Photodiagnosis Photodyn. Ther. 2019;27:402–407. doi: 10.1016/j.pdpdt.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Carmello J.C., et al. Photoinactivation of single and mixed biofilms of Candida albicans and non-albicans Candida species using Photodythazine((R)) [corrected] Photodiagnosis Photodyn. Ther. 2017;17:194–199. doi: 10.1016/j.pdpdt.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Davies A., et al. Cationic porphyrin-mediated photodynamic inactivation of Candida biofilms and the effect of miconazole. J. Physiol. Pharmacol. 2016;67(5):777–783. [PubMed] [Google Scholar]
- 9.NoryMariño-Ocamp J.S.R., Germán Günther, Belinda Heyne, Denis Fuentealba. Thiol-reacting toluidine blue derivatives: synthesis, photophysical properties and covalent conjugation with human serum albumin. Dyes Pigments. 2022;201 [Google Scholar]
- 10.Soares B.M., et al. In vitro photodynamic inactivation of Candida spp. growth and adhesion to buccal epithelial cells. J. Photochem. Photobiol., B. 2009;94(1):65–70. doi: 10.1016/j.jphotobiol.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Wiench R., et al. Efficacy of toluidine blue-mediated antimicrobial photodynamic therapy on Candida spp. A systematic review. Antibiotics (Basel) 2021;10(4) doi: 10.3390/antibiotics10040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang M.C., et al. Photodynamic inactivation potentiates the susceptibility of antifungal agents against the planktonic and biofilm cells of Candida albicans. Int. J. Mol. Sci. 2018;19(2) doi: 10.3390/ijms19020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.AP P., et al. Photodynamic Antimicrobial Chemotherapy (PACT), using Toluidine blue O inhibits the viability of biofilm produced by Candida albicans at different stages of development. Photodiagnosis Photodyn. Ther. 2018;21:182–189. doi: 10.1016/j.pdpdt.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 14.J L., et al. Toluidine blue mediated photodynamic therapy of oral wound infections in rats. Laser Med. Sci. 2010;25(2):233–238. doi: 10.1007/s10103-009-0700-5. [DOI] [PubMed] [Google Scholar]
- 15.Gu Y., et al. An experimental study of photoactivated disinfection in the treatment of acute pseudomembranous stomatitis. Photochem. Photobiol. 2022;98(6):1418–1425. doi: 10.1111/php.13637. [DOI] [PubMed] [Google Scholar]
- 16.Pierce C.G., et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008;3(9):1494–1500. doi: 10.1038/nport.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Fattani M.A., Douglas L.J. Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J. Med. Microbiol. 2006;55(Pt 8):999–1008. doi: 10.1099/jmm.0.46569-0. [DOI] [PubMed] [Google Scholar]
- 18.Strathmann M., Wingender J., Flemming H.C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods. 2002;50(3):237–248. doi: 10.1016/s0167-7012(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 19.Lohse M.B., et al. Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 2018;16(1):19–31. doi: 10.1038/nrmicro.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra J., Mukherjee P.K. Candida biofilms: development, architecture, and resistance. Microbiol. Spectr. 2015;3(4) doi: 10.1128/microbiolspec.MB-0020-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai J.V., Mitchell A.P. Candida albicans biofilm development and its genetic control. Microbiol. Spectr. 2015;3(3) doi: 10.1128/microbiolspec.MB-0005-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gulati M., Nobile C.J. Candida albicans biofilms: development, regulation, and molecular mechanisms. Microb. Infect. 2016;18(5):310–321. doi: 10.1016/j.micinf.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bizerra F.C., et al. Characteristics of biofilm formation by Candida tropicalis and antifungal resistance. FEMS Yeast Res. 2008;8(3):442–450. doi: 10.1111/j.1567-1364.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilson C., et al. Quantitative and qualitative assessment methods for biofilm growth: a mini-review. Res. Rev. J. Eng. Technol. 2017;6(4) [PMC free article] [PubMed] [Google Scholar]
- 25.Honraet K., Goetghebeur E., Nelis H.J. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J. Microbiol. Methods. 2005;63(3):287–295. doi: 10.1016/j.mimet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Peeters E., Nelis H.J., Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods. 2008;72(2):157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 27.J G., et al. Photosensitizers in antibacterial photodynamic therapy: an overview. Laser Ther. 2018;27:293–302. doi: 10.5978/islsm.27_18-RA-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.S B., et al. Production of reactive oxygen species from photosensitizers activated with visible light sources available in dental offices. Photomed. Laser Surg. 2010;28:519–525. doi: 10.1089/pho.2009.2505. [DOI] [PubMed] [Google Scholar]
- 29.da Collina G.A., et al. Controlling methylene blue aggregation: a more efficient alternative to treat Candida albicans infections using photodynamic therapy. Photochem. Photobiol. Sci. 2018;17(10):1355–1364. doi: 10.1039/c8pp00238j. [DOI] [PubMed] [Google Scholar]
- 30.Fumes A.C., et al. Influence of pre-irradiation time employed in antimicrobial photodynamic therapy with diode laser. Laser Med. Sci. 2018;33(1):67–73. doi: 10.1007/s10103-017-2336-1. [DOI] [PubMed] [Google Scholar]
- 31.Abrahamse H., Hamblin M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016;473(4):347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathirana R.U., et al. Filamentous non-albicans Candida species adhere to Candida albicans and benefit from dual biofilm growth. Front. Microbiol. 2019;10:1188. doi: 10.3389/fmicb.2019.01188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Barros P.P., et al. Candida tropicalis affects the virulence profile of Candida albicans: an in vitro and in vivo study. Pathog. Dis. 2018;76(2) doi: 10.1093/femspd/fty014. [DOI] [PubMed] [Google Scholar]
- 34.Uppuluri P., Chaturvedi A.K., Lopez-Ribot J.L. Design of a simple model of Candida albicans biofilms formed under conditions of flow: development, architecture, and drug resistance. Mycopathologia. 2009;168(3):101–109. doi: 10.1007/s11046-009-9205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi H., et al. The inhibitory activity of 5-aminolevulinic acid photodynamic therapy (ALA-PDT) on Candida albicans biofilms. Photodiagnosis Photodyn. Ther. 2021;34 doi: 10.1016/j.pdpdt.2021.102271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.