Abstract
Background
Intimate partner violence (IPV) is common worldwide. However, the health effects of exposure to IPV during pregnancy are significantly more severe. We investigated the relationship between exposure to IPV during pregnancy and the risk of preterm and low birthweight births among women in Ghana's northern region.
Methods
We recruited 402 postnatal women aged 15–49 years from five selected public health facilities in the Tamale Metropolis of the northern region of Ghana. Using Kobo Collect, information on a wide range of factors, including exposure to IPV during the last pregnancy and pregnancy outcomes, was collected electronically. Multiple logistic regression analyses were conducted in Stata to determine the associations between prenatal exposure to IPV and binary measures of gestational age at birth and birthweight.
Results
Overall, 35.1% (95% CI: 30.5, 39.9) of the respondents experienced IPV during their recent pregnancy; 6.7% (95% CI: 4.6, 9.6) experienced physical IPV; and 34.8% (95% CI: 30.3, 39.6) experienced psychological IPV. The prevalence of preterm and low birthweight deliveries was 18.9% (95% CI: 15.4, 23.1) and 9.0% (95% CI: 6.5, 12.2), respectively. Prenatal exposure to IPV was linked to poor newborn outcomes by multivariable binary regression models. Women who suffered IPV during their last pregnancy were three times more likely to deliver low birthweight babies (AOR = 3.12: 95% CI: 1.42, 6.84). Exposed women were also about twice as likely to deliver prematurely, although this association was not statistically significant (AOR = 1.81; 95% CI: 0.97, 3.38).
Conclusion
Exposure to IPV during pregnancy increases a woman's risk of delivering prematurely and having a low birthweight baby. IPV screening should be a regular part of ANC, so that pregnant women who are experiencing IPV can be monitored and supported to avoid adverse outcomes for their babies.
Keywords: Intimate partner violence, Newborn outcomes, Pregnant women, Ghana
1. Introduction
Physical, emotional, and sexual violence against women is a major violation of human rights and a global public health issue of concern. Intimate Partner Violence (IPV) is defined as “behaviour within an intimate relationship that causes physical, sexual, or psychological harm, including acts of physical aggression, sexual coercion, psychological abuse, and controlling behaviours” [1]. While we recognise that women can also perpetrate violence against their partners and that IPV occurs in same-sex relationships, existing evidence indicates that men are more likely than women to perpetrate IPV [2]. It is estimated that 27% of ever-partnered women aged 15 or older have experienced IPV at some point in their lives [3]. There is also evidence of regional variations in the prevalence of IPV. A systematic review and meta-analysis of cross-sectional studies in sub-Saharan Africa (SSA) has reported that 44% of women in the region have experienced IPV at some time in their lives, with emotional violence being the highest (29.4%), followed by physical violence (25.9%), and the lowest being sexual violence (18.8%) [4]. Unfortunately, most people in the region, including some women, accept violence against women. A multi-country study reported that about 29% of women in SSA endorse the beating of women under certain circumstances, ranging from nearly 17% in Malawi to as high as 82.3% in Mali. The same study reported a rate of 30% for Ghana [5].
Although IPV can occur at any point in a woman's life, it is of particular concern during pregnancy due to the negative effects it can have on both the mother and her unborn child. The prevalence of IPV during pregnancy varies by country, but international comparisons have proven difficult due to varying study methodologies and definitions. Reviews, however, established that IPV during pregnancy ranged from 2 to 57% among African countries [6], 3–44% among Latin American and Caribbean countries [7], and 6–33% among Asian countries [8,9]. These statistics demonstrate that IPV during pregnancy is widespread. IPV during pregnancy is claimed to be influenced by numerous factors, including but not limited to unintended pregnancies, socioeconomic status, women's decision-making power, alcohol and drug use, pregnancy-related depression, the type of marriage, the partner's level of education, and acceptance of violence [[6], [7], [8],10].
Studies have demonstrated the multifaceted effects of violence on pregnant women. Women who experience IPV during pregnancy have more symptoms of depression and anxiety and overall poorer psychosocial health than those who do not [[11], [12], [13], [14], [15]]. In addition, pregnant women who are exposed have an increased risk of arterial hypertension, premature rupture of membranes, preeclampsia, intrauterine growth restriction (IUGR), placental abruption, risky sexual behaviours, and genitourinary tract infections [[16], [17], [18], [19]]. Women who experienced prenatal IPV were less likely to use antenatal care (ANC) services, or if they did, they made fewer contacts and were less likely to take iron and folic acid before delivery than if they did not experience IPV during pregnancy [[20], [21], [22], [23]].
Regarding IPV during pregnancy and the risk of adverse newborn outcomes, specifically preterm birth and having a low birthweight baby, the evidence is inconclusive. Several epidemiological studies have linked exposure to IPV during pregnancy to an increased risk of preterm birth and low birthweight [18,[24], [25], [26], [27], [28], [29]]. Other studies have found no significant association between maternal exposure to IPV during pregnancy and these outcomes [[30], [31], [32]]. Importantly, according to the literature, preterm delivery and low birthweight, are major causes of newborn morbidity and mortality in children under five years of age and contribute to various complications later in life [[33], [34], [35]].
Men's tolerance of IPV against women varies by region in Ghana. According to a recent study, men's justification for IPV in the form of wife-beating has decreased in the country from 32% in 2003 to 12.4% in 2014, with the northern region reporting the second highest rate of 30% after the upper east region in 2014 [36]. The study also found that northern men were significantly more likely than their southern counterparts to justify domestic violence. In addition to its tolerance for domestic abuse, the northern region is known for having a relatively high rate of poor neonatal outcomes. According to the literature, low birth weight, for example, is 30% in the northern region [37] compared to 14% in the upper east [38], 8.2% in the upper west [39] and 10–23% in the regions in the southern and coastal areas of the country [[40], [41], [42]]. However, it is not known if IPV during pregnancy is linked to adverse outcomes for babies in the region.
This study examined the association between prenatal exposure to IPV and the risk of adverse newborn outcomes, such as preterm birth and having a low birthweight baby, in the Tamale Metropolitan Area of northern Ghana.
2. Material and methods
2.1. Study setting and design
A health facility-based retrospective cross-sectional study was conducted in five selected public health facilities in the Tamale Metropolis in the northern region of Ghana. The Tamale Metropolis is one of the sixteen districts in the northern region, with Tamale as the capital. It shares boundaries with Savelugu Municipality to the North, East Gonja Municipal to the South, Central Gonja District to the South-West, Yendi Municipal Assembly to the East, and Tolon District to the West (Fig. 1) [43]. In terms of public health infrastructure, the Metropolis has three hospitals, including one teaching hospital, eight health centres, and eighteen Community-based Health Planning and Services (CHPS) serving a population of 374,744. More than 80% of the population in the Metropolis lives in urban areas.
Fig. 1.
Map of Tamale Metropolis. Source: Ghana statistical service, 2014.
In general, the northern region of Ghana, where the current study's setting is located, is one of the most disadvantaged regions in the country. According to national statistics [44], the northern region has the highest rate of illiteracy among both men and women, and more than half of the population in the region is in the lowest wealth quintile. It is also the region with the highest rate of polygynous unions in the country.
2.2. Sample
The study population was comprised of women who had delivered in the past 6–12 months prior to the study. This population was selected to minimise recall bias. In addition, according to Ghana's child welfare schedule, this is the time when mothers or caregivers actively bring their children to health facilities for growth monitoring and immunizations, which would allow the research team easy access to the participants. The sample size was determined using the formula:
| (1) |
where: ‘n’ is the sample size required for the study; ‘N’ is the estimated cumulative number of deliveries (4,292) for the facilities selected; and ‘e’ is the acceptable margin of error (5%). A total of 402 women were needed, which included a 10% non-response rate. The study included only partnered women who gave birth to single babies at the facility and had babies aged 6–12 months who were receiving postnatal or child welfare care services, had complete health records, and were willing to participate.
2.3. Selection of facilities and respondents
Multiple sampling methods were used to select the public health facilities and the respondents in this study. Two secondary public health facilities (i.e., Tamale Central and Tamale West hospitals) were purposefully selected because they provided ANC and delivery services to a significant proportion of reproductive women in the area. In addition to the two secondary-level facilities, three health centres (Datoyili Health Center, Bilpiela Health Center, and Vittin Reproductive and Child Health) were randomly selected from the eight in the Metropolis. The sample for the study was distributed to the facilities using the proportion-to-size technique.
Using a systematic random sampling technique, the respondents were then chosen. The postnatal register was used to compile a list of partnered women who had given birth in the 6–12 months prior to the survey at each of the chosen health facilities. Then a sampling interval was calculated using the sampling frame by dividing the total number of eligible women by the sample size required for the facility. As a starting point, a participant was chosen at random from the first ten names in the sampling frame. The sampling interval was then used to choose respondents until the desired sample size was reached.
2.4. Data collection and tool
The data were collected electronically using the Kobo Collect application installed on Android-powered mobile phones. The data collection tool was pretested among 40 women (10% of the total sample size) from a health facility outside the study setting. The pretesting ensured the validity of the questionnaire in gathering the required information. All ambiguous questions were revised. The questionnaire collected data on sociodemographic and economic characteristics, obstetric history, and other maternal health-related factors. Data on the woman's gestation at delivery, haemoglobin levels, and birthweight of the child at delivery were collected by reviewing the ANC record book. Trained research assistants with a degree in nursing collected the data under the supervision of the research team. The data collection took place in June 2022.
2.5. Measures
2.5.1. Assessing exposure to intimate partner violence during pregnancy (main exposure variable)
To measure exposure to violence, the respondents were asked a series of questions on violent acts that occurred during their recent pregnancies. The questions were adopted from the domestic violence module questionnaire used by the Demographic and Health Survey (DHS) program (https://dhsprogram.com/pubs/pdf/DHSQMP/domestic_violence_module.pdf.pdf). Information on experience of any physical violence during pregnancy was collected by asking women “whether her partner ever pushed, shook or had something thrown at her; slapped her; punched her with his fist or with something that could hurt her, kicked her; dragged her or beat her up; tried to choke her or burn her on purpose; threatened or attacked her with a knife, gun, or any other weapon; or twisted her arm or pulled her hair.” Information on psychological IPV was gathered from asking women “whether her partner ever humiliated, threatened with harm, or insulted or made to feel bad” during her most recent pregnancy. The response options were no and yes. We created dichotomous variables for the experience of physical and psychological IPV. The experience of any physical or psychological violence (coded as 1) was considered if a woman reported at least one act of violence from her partner during the index pregnancy. Women who reported never experiencing any of these incidents during pregnancy were not considered to have been exposed to physical or psychological violence (coded as 0).
2.6. Outcome variable
The dependent variables in the study were the gestation at birth and the birthweight of the baby. Specifically, the two adverse newborn outcomes examined were preterm birth and having a low birthweight baby. Information on these outcomes was retrieved from the ANC card of the mother. These variables were dichotomized to create preterm and low birthweight outcomes. Gestation at birth was coded as “1” (preterm) if the gestation at birth was below 37 weeks and coded as “0” if it was ≥37 weeks. Similarly, birth weight was coded as “1” if it was less than 2.5 kg (or 2500 g) and coded as “0” if it was ≥2.5 kg. The categorization of the variables was guided by the existing WHO literature [45].
2.7. Covariates
The analysis was adjusted for several potential confounding factors. These include women's sociodemographic and economic factors, obstetrical factors, and health-related behavioural factors. The sociodemographic and economic factors included women's current age (in years) and education. Obstetric factors included the number of pregnancies and parity. The gestation at ANC initiation, the number of ANC contacts before delivery, the experience of complications with the previous pregnancy (yes/no), the experience of any malaria episode during the previous pregnancy (yes/no), and anaemia status in the first and second trimesters (yes/no) were all health-related behavioural factors considered in the current study.
2.8. Data analysis
We first performed descriptive statistics on the background characteristics of the respondents and the IPV variables to examine the distribution of these variables. Then univariate analyses were performed using a Chi-square test or Fishers' test as appropriate to examine the association between the IPV variables and preterm birth and low birthweight. Lastly, we performed binary multiple logistic regression analyses to evaluate the association between women's experience of IPV during pregnancy and the risk of preterm and low birthweight births. We entered all the covariates into the multiple logistic regression models to adjust for potential confounding by these factors. The adjusted odds ratio (AOR) was reported with a 95% confidence interval (CI). Statistical significance was pegged at a probability value of less than 0.05. The reported P-values were two-tailed. All the statistical analyses were performed using Stata/IC 15.0 for Windows (StataCorp LLC, College Station, Texas, USA).
3. Results
3.1. Background characteristics of the respondents in the current study
The background characteristics of the respondents in the study, including sociodemographic and economic characteristics, as well as obstetric and other health-related characteristics, are presented in Table 1. The mean age was 31.3 (±5.1 years), ranging from 19 to 49 years. More than half (58.5%) of the respondents were in the age group of 25–34 years, 64.2% of them received no formal education, and 95.8% were affiliated with Islam. According to the results on obstetric and health-related characteristics, 17.2% had at least 5 pregnancies, and 21.6% had at least 4 living children. In addition, 11.9% of the respondents made their first ANC contact in the first trimester, and 37.6% made at least eight contacts before delivery. The results also showed that 20.4% and 32.1% of the women were anaemic in the first and second trimesters, respectively (Table 1).
Table 1.
Background characteristics of the respondents in the current study (N = 402).
| Variable | Frequency | Percentage |
|---|---|---|
| Age group (years) mean 31.3 (±5.1 years) | ||
| 15–24 | 47 | 11.7 |
| 25–34 | 235 | 58.5 |
| 35–49 | 120 | 29.9 |
| Education | ||
| No formal education | 258 | 64.2 |
| Some formal education | 144 | 35.8 |
| Religious affiliation | ||
| Christian | 17 | 4.2 |
| Islam | 385 | 95.8 |
| Ethnicity | ||
| Dagomba | 343 | 85.3 |
| Gonja | 34 | 8.5 |
| Mamprusi | 10 | 2.5 |
| Othersa | 15 | 3.7 |
| Employment | ||
| Unemployed | 96 | 23.9 |
| Employed | 306 | 76.1 |
| Number of pregnancies | ||
| 1 | 88 | 21.9 |
| 2 | 99 | 24.6 |
| 3 | 85 | 21.1 |
| 4 | 61 | 15.2 |
| ≥5 | 69 | 17.2 |
| Parity | ||
| 1 | 111 | 27.6 |
| 2 | 127 | 31.6 |
| 3 | 77 | 19.2 |
| ≥4 | 87 | 21.6 |
| Gestation at first ANC contact | ||
| 1st trimester | 48 | 11.9 |
| 2nd trimester | 339 | 84.3 |
| 3rd trimester | 15 | 3.7 |
| Number of ANC contacts before delivery | ||
| 1–3 | 20 | 5.0 |
| 4–7 | 231 | 57.5 |
| 8+ | 151 | 37.6 |
| Experienced complications with last pregnancy | ||
| No | 189 | 47.0 |
| Yes | 213 | 53.0 |
| Had malaria episode during recent pregnancy | ||
| No | 285 | 70.9 |
| Yes | 117 | 29.1 |
| Anaemia at 1st trimester | ||
| No | 320 | 79.6 |
| Yes | 82 | 20.4 |
| Anaemia at 2nd trimester | ||
| No | 273 | 67.9 |
| Yes | 129 | 32.1 |
Others: Frafra, Fulani, Akan, Wala, Hausa, and Dagaaba
3.2. Prevalence of physical and psychological intimate partner violence during pregnancy
Table 2 summarises the prevalence of each abuse variable. The results show that 6.7% (95% CI: 4.6, 9.6) of the women experienced acts of physical IPV and 34.5% (95% CI: 30.3, 39.6) experienced acts of psychological IPV during the last pregnancy. Overall, 35.1% (95% CI: 30.5, 39.9) of the sample experienced at least one form of IPV during pregnancy; 28.6% experienced only one form of IPV; and 6.5% experienced the two forms of IPV examined. The distribution of the responses used to construct these IPV variables is presented as supplementary material (see Supplemental Material Table 1).
Table 2.
Prevalence of physical and psychological intimate partner violence during pregnancy (N = 402).
| Scores for the number of acts of physical intimate partner violence during recent pregnancy | Frequency | Percentage |
|---|---|---|
| 0 | 375 | 93.3 |
| 1 | 20 | 5.0 |
| 2 | 5 | 1.2 |
| 3 | 2 | 0.5 |
| Exposure to any act of physical intimate partner violence | ||
| No | 375 | 93.3 |
| Yes | 27 | 6.7 |
| Scores for the number of acts of psychological intimate partner violence | ||
| 0 | 262 | 65.5 |
| 1 | 127 | 31.4 |
| 2 | 11 | 2.7 |
| 3 | 2 | 0.5 |
| Exposure to any act of psychological intimate partner violence | ||
| No | 262 | 65.5 |
| Yes | 140 | 34.5 |
| Number of forms of IPV experienced during the most recent pregnancy | ||
| 0 | 261 | 64.9 |
| 1 | 115 | 28.6 |
| 2 | 26 | 6.5 |
| Experience of any form of IPV during most recent pregnancy | ||
| No | 261 | 64.9 |
| Yes | 141 | 35.1 |
3.3. Prevalence of preterm delivery and low birthweight in the study
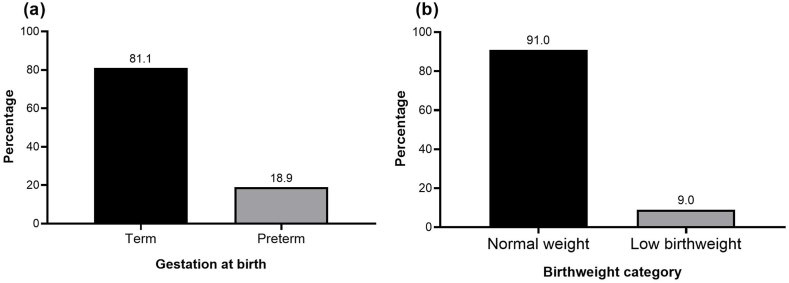
The findings revealed that of the 402 women studied, 76 had preterm births and 36 had low birthweight babies, giving a prevalence of 18.9% (95% CI: 15.4, 23.1) for preterm delivery (Fig. 2a) and 9.0% (95% CI: 6.5, 12.2) for low birthweight (Fig. 2b). Further analysis revealed that 17.9% (95% CI: 14.4, 22.0) of the women had only one adverse pregnancy outcome, while 5.0% (95% CI: 3.2, 7.6) had the two adverse neonatal outcomes.
Fig. 2.
Prevalence of preterm delivery and low birthweight in the current study (N = 402).
3.4. Distribution of preterm delivery by women's experience of intimate partner violence during their last pregnancy and other background characteristics
Women's exposure to IPV during pregnancy was associated with varying rates of preterm birth. A higher percentage of preterm births was recorded among women who suffered physical (59.3%), psychological (24.3%), and at least one form of IPV (24.8%) during their most recent pregnancy than those who did not (Table 3). Furthermore, the rate of preterm delivery also differed considerably by the women's age, number of pregnancies, parity, number of ANC contacts prior to delivery, a history of complications, and a malaria episode during the most recent pregnancy. Higher rates of preterm births were recorded among women in the age group of 15–24 (42.6%), women with one pregnancy (53.4%), and women who made 1–3 ANC visits during their most recent pregnancy (50.0%). In addition, preterm birth rates were higher among women who did not experience complications with their last pregnancy (31.2%) and those who did not experience any malaria episodes during their most recent pregnancy (12.0%).
Table 3.
Distribution of preterm delivery by women's experience of intimate partner violence during their most recent pregnancy and other background characteristics (N = 402).
| Variable | Preterm birth |
P-value | |
|---|---|---|---|
| No | Yes | ||
| Experienced physical IPV during pregnancy | <0.001 | ||
| No | 315 (84.0) | 60 (16.0) | |
| Yes | 11 (40.7) | 16 (59.3) | |
| Experienced psychological IPV during pregnancy | 0.044 | ||
| No | 220 (84.0) | 42 (16.0) | |
| Yes | 106 (75.7) | 34 (24.3) | |
| Exposure to any form of IPV during recent pregnancy | 0.026 | ||
| No | 220 (84.3) | 41 (15.7) | |
| Yes | 106 (75.2) | 35 (24.8) | |
| Age group | <0.001 | ||
| 15–24 | 27 (57.4) | 20 (42.6) | |
| 25–34 | 184 (78.3) | 51 (21.7) | |
| 35–49 | 115 (95.8) | 5 (4.2) | |
| Education | 0.461 | ||
| No formal education | 212 (82.2) | 46 (17.8) | |
| Some formal education | 114 (79.2) | 30 (20.8) | |
| Number of pregnancies | <0.001b | ||
| 1 | 41 (46.6) | 47 (53.4) | |
| 2 | 86 (86.9) | 13 (13.1) | |
| 3 | 82 (96.5) | 3 (3.5) | |
| 4 | 53 (86.9) | 8 (13.1) | |
| ≥5 | 64 (92.8) | 5 (7.2) | |
| Parity | <0.001b | ||
| 1 | 61 (55.0) | 50 (45.0) | |
| 2 | 112 (88.2) | 15 (11.8) | |
| 3 | 71 (92.2) | 6 (7.8) | |
| ≥4 | 82 (94.3) | 5 (5.7) | |
| Gestation at first ANC contact | 0.100 | ||
| 1st trimester | 34 (70.8) | 14 (29.2) | |
| 2nd trimester | 281 (82.9) | 58 (17.1) | |
| 3rd trimester | 11 (73.3) | 4 (26.7) | |
| Number of ANC contacts before delivery | <0.001 | ||
| 1–3 | 10 (50.0) | 10 (50.0) | |
| 4–7 | 178 (77.1) | 53 (22.9) | |
| 8+ | 138 (91.4) | 13 (8.6) | |
| Experienced complications with last pregnancy | <0.001 | ||
| No | 130 (68.8) | 59 (31.2) | |
| Yes | 196 (92.0) | 17 (8.0) | |
| Had malaria episode during recent pregnancy | 0.023 | ||
| No | 223 (78.2) | 62 (21.8) | |
| Yes | 103 (88.0) | 14 (12.0) | |
| Anaemia at 1st trimester | 0.155 | ||
| No | 264 (82.5) | 56 (17.5) | |
| Yes | 62 (75.6) | 20 (24.4) | |
| Anaemia at 2nd trimester | 0.660 | ||
| No | 223 (81.7) | 50 (18.3) | |
| Yes | 103 (79.8) | 26 (20.2) | |
bFisher's test.
3.5. Distribution of low birthweight by women's experience of intimate partner violence during their most recent pregnancy and other background characteristics
There was a statistically significant association between women's exposure to IPV during pregnancy and the rate of low birthweight. The results showed that the rate of low birthweight was higher among women with a history of physical IPV (33.3%), psychological IPV (15.0%), or at least one form of IPV (14.9%). The rate of low birthweight delivery was higher among women in the age group of 15–24 (19.1%), women with one pregnancy (19.3%) and women of parity one (19.8%). Furthermore, a significant percentage of women who made 1–3 ANC contacts before delivery (20.0%) and women who were anaemic during the first trimester (15.9%) gave birth to low birthweight babies compared to their counterparts (Table 4).
Table 4.
Distribution of low birthweight by women's experience of intimate partner violence during their most recent pregnancy and other background characteristics (N = 402).
| Variable | Low birthweight |
P-value | |
|---|---|---|---|
| No | Yes | ||
| Experienced physical IPV during recent pregnancy | <0.001 | ||
| No | 348 (92.8) | 27 (7.2) | |
| Yes | 18 (66.7) | 9 (33.3) | |
| Experienced psychological IPV during recent pregnancy | 0.002 | ||
| No | 247 (94.3) | 15 (5.7) | |
| Yes | 119 (85.0) | 21 (15.0) | |
| Exposure to any form of IPV during recent pregnancy | 0.002 | ||
| No | 246 (94.3) | 15 (5.7) | |
| Yes | 120 (85.1) | 21 (14.9) | |
| Age group (years) | 0.034 | ||
| 15–24 | 38 (80.9) | 9 (19.1) | |
| 25–34 | 217 (92.3) | 18 (7.7) | |
| 35–49 | 111 (92.5) | 9 (7.5) | |
| Education | 0.744 | ||
| No formal education | 234 (90.7) | 24 (9.3) | |
| Some formal education | 132 (91.7) | 12 (8.3) | |
| Number of pregnancies | 0.006b | ||
| 1 | 71 (80.7) | 17 (19.3) | |
| 2 | 94 (94.9) | 5 (5.1) | |
| 3 | 81 (95.3) | 4 (4.7) | |
| 4 | 55 (90.2) | 6 (9.8) | |
| ≥5 | 65 (94.2) | 4 (5.8) | |
| Parity | <0.001b | ||
| 1 | 89 (80.2) | 22 (19.8) | |
| 2 | 122 (96.1) | 5 (3.9) | |
| 3 | 72 (93.5) | 5 (6.5) | |
| ≥4 | 83 (95.4) | 4 (4.6) | |
| Gestation at first ANC contact | 0.633 | ||
| 1st trimester | 42 (87.5) | 6 (12.5) | |
| 2nd trimester | 310 (91.4) | 29 (8.6) | |
| 3rd trimester | 14 (93.3) | 1 (6.7) | |
| Number of ANC contacts before delivery | 0.019b | ||
| 1–3 | 16 (80.0) | 4 (20.0) | |
| 4–7 | 206 (89.2) | 25 (10.8) | |
| 8+ | 144 (95.4) | 7 (4.6) | |
| Experienced complications with last pregnancy | 0.076 | ||
| No | 167 (88.4) | 22 (11.6) | |
| Yes | 199 (93.4) | 14 (6.6) | |
| Had malaria episode during recent pregnancy | 0.570 | ||
| No | 258 (90.5) | 27 (9.5) | |
| Yes | 108 (92.3) | 9 (7.7) | |
| Anaemia at 1st trimester | 0.014 | ||
| No | 297 (92.8) | 23 (7.2) | |
| Yes | 69 (84.1) | 13 (15.9) | |
| Anaemia at 2nd trimester | 0.836 | ||
| No | 248 (90.8) | 25 (9.2) | |
| Yes | 118 (91.5) | 11 (8.5) | |
bFisher's test.
3.6. Association between exposure to intimate partner violence during pregnancy and the risk of preterm birth and having a low birthweight baby
Table 5 shows the results of a binary logistic regression analysis examining the association between exposure to IPV during pregnancy and the risk of preterm and low birthweight births. After controlling for demographic, economic, and obstetric characteristics, the results indicated that women's exposure to IPV during pregnancy was related to the probability of delivering preterm and having a low birthweight baby. However, only the latter result was statistically significant at the alpha level of 5%. Women who experienced intimate partner violence during their most recent pregnancy had a higher chance of delivering preterm (AOR = 1.81; 95% CI: 0.97, 3.38) and low birthweight babies (AOR = 3.12: 95% CI: 1.42, 6.84). We found that, among the covariates, the number of ANC contacts prior to delivery was linked with the likelihood of both preterm and low birthweight births. The results demonstrated that the risk of preterm and low birthweight births decreases as the number of contacts increases. Mothers with at least eight contacts, for example, were 95% less likely to deliver preterm (AOR = 0.05; 95% CI: 0.01, 0.20) and 79% less likely to deliver a baby with a low birthweight (AOR = 0.21; 95% CI: 0.05, 0.96).
Table 5.
Association between exposure to IPV during pregnancy and the risk of preterm and low birthweight delivery among women (N = 402).
| Variable | Preterm delivery |
Low birthweight |
||
|---|---|---|---|---|
| AOR (95% CI) | P-value | AOR (95% CI) | P-value | |
| Exposure to any form of IPV | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.81 (0.97, 3.38) | 0.064 | 3.12 (1.42, 6.84) | 0.005 |
| Age group (years) | ||||
| 15–24 | 1.00 | 1.00 | ||
| 25–34 | 0.61 (0.27, 1.35) | 0.223 | 0.42 (0.15, 1.18) | 0.100 |
| 35–49 | 0.29 (0.07, 1.17) | 0.082 | 0.98 (0.22, 4.32) | 0.976 |
| Education | ||||
| No formal education | 1.00 | 1.00 | ||
| Some formal education | 0.84 (0.43, 1.64) | 0.610 | 0.75 (0.32, 1.76) | 0.506 |
| Number of pregnancies | 0.80 (0.45, 1.40) | 0.432 | 1.71 (0.93, 3.13) | 0.082 |
| Parity | 0.61 (0.30, 1.24) | 0.171 | 0.32 (0.15, 0.67) | 0.003 |
| Gestation at first ANC contact | ||||
| 1st trimester | 1.59 (0.32, 7.79) | 0.570 | 2.43 (0.23, 25.10) | 0.457 |
| 2nd trimester | 0.40 (0.09, 1.70) | 0.215 | 1.49 (0.17, 13.12) | 0.717 |
| 3rd trimester | 1.00 | 1.00 | ||
| Number of ANC contacts before delivery | ||||
| 1–3 | 1.00 | 1.00 | ||
| 4–7 | 0.21 (0.07, 0.70) | 0.010 | 0.54 (0.14, 2.15) | 0.383 |
| 8+ | 0.05 (0.01, 0.20) | <0.001 | 0.21 (0.05, 0.96) | 0.045 |
| Experienced complications with recent pregnancy | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.33 (0.15, 0.72) | 0.005 | 0.51 (0.19, 1.38) | 0.186 |
| Malaria during recent pregnancy | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.97 (0.42, 2.22) | 0.939 | 1.01 (0.38, 2.69) | 0.978 |
| Anaemia at 1st trimester | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.90 (0.91, 4.00) | 0.088 | 3.27 (1.39, 7.70) | 0.007 |
| Anaemia at 2nd trimester | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.01 (0.52, 1.96) | 0.981 | 1.26 (0.54, 2.98) | 0.594 |
4. Discussion
The purpose of the study was to examine the link between exposure to abuse by an intimate partner during pregnancy and the risk of adverse neonatal outcomes in a cross-section of reproductive women in Ghana's northern region. From our study, more than one in three women suffered psychological violence and about 7% suffered physical IPV during their last pregnancy. In total, more than one-third of the women suffered some form of IPV during their last pregnancy. The rate of IPV among pregnant women in this study is within the range of 2–57% among African countries [6], 3–44% in the Latin American and Caribbean region [7], and 6–33% among Asian countries [8,9]. The results of the current study confirm that IPV is a common occurrence during pregnancy, affecting many women. Also, from a review and meta-analysis study, more women in SSA suffer psychological abuse than any other form of abuse [4]. The current study found that psychological IPV is highly prevalent compared to physical IPV in the study setting. We acknowledge that comparing rates of IPV across countries has been challenging due, in part, to varying methodologies, violence measures, and context. A community-based study in Ethiopia, for instance, examined IPV during pregnancy based on exposure to physical, psychological, and sexual abuse and reported a rate of 44.5% [46]. Using the three forms of IPV, a facility-based study in Kenya revealed a rate of 37%, which is somewhat lower than the percentage observed in Ethiopia [10]. Despite the obvious limitations of comparison, violence against women by an intimate partner is a violation of human rights, and the health repercussions are more severe for pregnant women because of their vulnerability and the effects on both mother and unborn baby [7,16,17,47,48].
In this study, about 19% of babies were born prematurely, and 9% had low birthweight, which is comparable to what has been documented in other African and non-African countries as well as around the globe [[49], [50], [51]]. The key finding of our study is that exposure to physical and/or psychological abuse during pregnancy is associated with the risk of adverse newborn outcomes. We found that women who experienced prenatal IPV had a twofold increased risk of delivering prematurely and a threefold increased risk of delivering a baby with a low birthweight compared to those who did not experience any form of violence, although the evidence for the former was statistically insignificant. This finding on the association between exposure to IPV during pregnancy and the risk of adverse newborn outcomes is consistent with some studies [16,24,28,47,51], but not with others [[30], [31], [32]].
There are some viable scientific and psychosocial explanations for the link between prenatal exposure to IPV and foetal outcomes [52]. Physical or psychological IPV can influence neonatal outcomes through physiological reactions to violence-related stress by releasing prostaglandin, which can cause premature contractions and delivery, or vasoconstrictors or cortisol, which can result in IUGR [7,53,54]. Additionally, high levels of cortisol are necessary to trigger the physiological processes involved in parturition and prepare the uterus’ myometrium for the effects of oxytocin [55]. Stress, on the other hand, triggers the release of cortisol before term, which could result in preterm labour and delivery. Placental abruption and premature rupture of membranes are among some of the pregnancy complications caused by direct physical assault and are linked to poor newborn outcomes [19,48,56]. Violence-related stress during pregnancy raises the levels of norepinephrine and cytokines, which cause the uterine and placental blood vessels to narrow and stay that way. This long-term vasoconstriction makes it harder for oxygen and nutrients to get to the foetus, which slows foetal growth [54].
Other explanations for the association between prenatal IPV and premature birth and low birthweight are that abused women suffer from high stress and poor mental health, which can lead to poor nutrition patterns and their consequences, such as anaemia, underweight, and poor gestational weight gain, potentially resulting in IUGR and low birthweight [7,27,[57], [58], [59]]. Poor diet due to psychological stress can increase underlying illnesses like hypertension and gestational diabetes or lead to pregnancy disorders like preeclampsia and eclampsia, which are risk factors for premature labour and low birthweight [7,19,60]. Also, women who suffer IPV typically lack decision-making power over their own health and are more likely to not register, to commence ANC late, and to underutilize ANC services, decreasing their chances of obtaining health interventions that promote better maternal and newborn health prior to birth [21,49,51,61].
Our study also identified additional significant predictors of preterm and low birthweight births, including parity, number of ANC contacts, history of prenatal problems, and anaemia during the first trimester of pregnancy, which either support or refute other research. Higher parity has been linked to an increased risk of low birthweight in northern and southern Ghana [37,42]. The relationships between fewer ANC contacts, low haemoglobin levels, and an increased risk of bad newborn outcomes are also consistent with findings from other studies [42,51,59,62,63]. In contrast, the result that women who had problems during their previous pregnancy were less likely to deliver prematurely contrasts with what has been reported in Ethiopia, where women who had complications had an increased risk of premature birth [49].
4.1. Study limitations
Our study yields significant findings that can inform policy decisions. However, the results must be evaluated in light of a number of limitations. There are various types of IPV, including sexual violence and controlling behaviours. The current study, however, only looked at psychological and physical IPV. Furthermore, the current study did not take into account several adverse pregnancy outcomes, such as stillbirths, intrauterine foetal death, and antepartum haemorrhage (APH). These outcomes should be included in future research in the study setting. A cross-section of reproductive women who were in a union (married or cohabiting) at the time of the study were recruited from public health institutions in the northern area of Ghana for the current study. Consequently, our findings are restricted to this particular sample of women. In addition, the cross-sectional methodology restricts the conclusions to associations, not causal links. IPV is a sensitive subject in the majority of circumstances. We therefore believe that some women may have underreported their exposure. We also feel that the retrospective collection of this information was susceptible to recollection bias. Despite this, the short recollection interval may have reduced the magnitude of bias. Last but not least, there are numerous known factors that can potentially affect birth outcomes, such as heat and air pollution exposure, pregnancy interval, body mass index, human immunodeficiency virus infection, and history of abortion among others [[64], [65], [66], [67]]. However, in the present study, we were unable to take into account all of these variables.
5. Conclusions
According to our study, more than one-third of pregnant women in the study setting suffered physical and/or psychological abuse from their intimate partner during their most recent pregnancy. In addition, we found that women who suffered IPV during their most recent pregnancy had a higher chance of having a preterm or low birthweight baby compared to those who did not experience any form of violence. IPV screening should be a regular part of ANC, so that pregnant women who are experiencing IPV can be monitored and supported to avoid adverse outcomes for their babies.
Ethics and consent
Ethical clearance was obtained from the Ethics Review Committee of the Ghana Health Service, Accra, Ghana. Official permission was obtained from the Tamale Metropolitan Health Directorate. In addition, the study was conducted while taking into consideration the principles and ethics of health research. Participation was strictly voluntary, and written informed consent was obtained from respondents prior to administering the questionnaires. The nature of the study, including the objectives, risks, and potential benefits, was clearly explained to the respondents. Respondents were also informed about their right to withdraw at any time during the study. Confidentiality and anonymity were ensured throughout the execution of the study by excluding any information that could be linked to the respondents.
Author contribution statement
Michael Boah; Abdul-Nasir Issah; Daudi Yeboah; Mary Rachael Kpordoxah; Jevaise Aballo: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Nashiru Abdulai; Martin Nyaaba Adokiya: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding
The study received no funding from any organization.
Data availability statement
The data underlining the conclusions drawn in this study are contained within the manuscript. The dataset, however, can be made available on reasonable request from the corresponding author.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e15391.
Contributor Information
Michael Boah, Email: mboah@uds.edu.gh, boahmichael@gmail.com.
Abdul-Nasir Issah, Email: anissah@uds.edu.gh.
Jevaise Aballo, Email: jaballo@unicef.org.
Abbreviations
- ANC
Antenatal care
- AOR
Adjusted odds ratio
- APH
Antepartum Haemorrhage
- CHPS
Community-based Health Planning and Services
- CI
Confidence interval
- DHS
Demographic and Health Survey
- IPV
Intimate Partner Violence
- IRB
Institutional Review Board
- IUGR
Intrauterine Growth Restriction
- LMICs
Low and Middle-Income Countries
- SSA
sub-Saharan Africa
- WHO
World Health Organization
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Orgainization/London School of Hygiene and Tropical Medicine Preventing intimate partner and sexual violence against women: taking action and generating evidence, Geneva. 2010. https://apps.who.int/iris/bitstream/handle/10665/44350/9789241?sequence=1 (accessed December 5, 2022)
- 2.World Health Organization . 2005. WHO Multi-Country Study on Women's Health and Domestic Violence against Women : Summary Report of Initial Results on Prevalence, Health Outcomes and Women's Responses, Geneva. [Google Scholar]
- 3.World Health Organization on behalf of the United Nations Inter-Agency Working Group on Violence Against Women Estimation and Data (UNICEF UNFPA UNODC UNWomen) Violence against women prevalence estimates, 2018. Global, regional and national prevalence estimates for intimate partner violence against women and global and regional prevalence estimates for non-partner sexual violence against women, Geneva, Switzerland. 2021. https://www.who.int/publications/i/item/9789240022256 (accessed November 4, 2021)
- 4.Muluneh M.D., Stulz V., Francis L., Agho K. Gender based violence against women in sub-saharan Africa: a systematic review and meta-analysis of cross-sectional studies. Int. J. Environ. Res. Publ. Health. 2020;17:903. doi: 10.3390/ijerph17030903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zegeye B., Olorunsaiye C.Z., Ahinkorah B.O., Ameyaw E.K., Budu E., Seidu A.-A., Yaya S. Understanding the factors associated with married women's attitudes towards wife-beating in sub-Saharan Africa. BMC Wom. Health. 2022;22:242. doi: 10.1186/s12905-022-01809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shamu S., Abrahams N., Temmerman M., Musekiwa A., Zarowsky C. A systematic review of african studies on intimate partner violence against pregnant women: prevalence and risk factors. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han A., Stewart D.E. Maternal and fetal outcomes of intimate partner violence associated with pregnancy in the Latin American and Caribbean region. Int. J. Gynecol. Obstet. 2014;124:6–11. doi: 10.1016/j.ijgo.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 8.Wang T., Liu Y., Li Z., Liu K., Xu Y., Shi W., Chen L. Prevalence of intimate partner violence (IPV) during pregnancy in China: a systematic review and meta-analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0175108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do H.P., Tran B.X., Nguyen C.T., Van Vo T., Baker P.R.A., Dunne M.P. Inter-partner violence during pregnancy, maternal mental health and birth outcomes in Vietnam: a systematic review. Child. Youth Serv. Rev. 2019;96:255–265. doi: 10.1016/j.childyouth.2018.11.039. [DOI] [Google Scholar]
- 10.Makayoto L.A., Omolo J., Kamweya A.M., Harder V.S., Mutai J. Prevalence and associated factors of intimate partner violence among pregnant women attending Kisumu district hospital, Kenya, matern. Child Health J. 2013;17:441–447. doi: 10.1007/s10995-012-1015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnbogadóttir H., Baird K., Thies-Lagergren L. Birth outcomes in a Swedish population of women reporting a history of violence including domestic violence during pregnancy: a longitudinal cohort study. BMC Pregnancy Childbirth. 2020;20:183. doi: 10.1186/s12884-020-02864-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahenge B., Likindikoki S., Stöckl H., Mbwambo J. Intimate partner violence during pregnancy and associated mental health symptoms among pregnant women in Tanzania: a cross-sectional study. BJOG Int. J. Obstet. Gynaecol. 2013;120:940–947. doi: 10.1111/1471-0528.12185. [DOI] [PubMed] [Google Scholar]
- 13.Van Parys A.-S., Deschepper E., Michielsen K., Galle A., Roelens K., Temmerman M., Verstraelen H. Intimate partner violence and psychosocial health, a cross-sectional study in a pregnant population. BMC Pregnancy Childbirth. 2015;15:278. doi: 10.1186/s12884-015-0710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari A., Chan K., Fong D., Leung W., Brownridge D., Lam H., Wong B., Lam C., Chau F., Chan A., Cheung K., Ho P. The impact of psychological abuse by an intimate partner on the mental health of pregnant women. BJOG Int. J. Obstet. Gynaecol. 2008;115:377–384. doi: 10.1111/j.1471-0528.2007.01593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferraro A.A., Rohde L.A., Polanczyk G.V., Argeu A., Miguel E.C., Grisi S.J.F.E., Fleitlich-Bilyk B. The specific and combined role of domestic violence and mental health disorders during pregnancy on new-born health. BMC Pregnancy Childbirth. 2017;17:257. doi: 10.1186/s12884-017-1438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Afkhamzadeh A., Rahmani K., Yaghubi R., Ghadrdan M., Faraji O. Adverse perinatal outcomes of intimate partner violence during pregnancy. Int. J. Hum. Rights Healthcare. 2021;14:465–476. doi: 10.1108/IJHRH-08-2019-0067. [DOI] [Google Scholar]
- 17.Ferdos J., Rahman M.M., Jesmin S.S., Rahman M.A., Sasagawa T. Association between intimate partner violence during pregnancy and maternal pregnancy complications among recently delivered women in Bangladesh. Aggress. Behav. 2018;44:294–305. doi: 10.1002/ab.21752. [DOI] [PubMed] [Google Scholar]
- 18.Martin-de-las-Heras S., Velasco C., Luna-del-Castillo J. de D., Khan K.S. Maternal outcomes associated to psychological and physical intimate partner violence during pregnancy: a cohort study and multivariate analysis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Audi C.A.F., Segall-Corrêa A.M., Santiago S.M., Pérez-Escamilla R. Adverse health events associated with domestic violence during pregnancy among Brazilian women. Midwifery. 2012;28:416–421. doi: 10.1016/j.midw.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Gashaw B.T., Magnus J.H., Schei B. Intimate partner violence and late entry into antenatal care in Ethiopia. Women Birth. 2019;32 doi: 10.1016/j.wombi.2018.12.008. e530–e537. [DOI] [PubMed] [Google Scholar]
- 21.Singh J.K., Evans-Lacko S., Acharya D., Kadel R., Gautam S. Intimate partner violence during pregnancy and use of antenatal care among rural women in southern Terai of Nepal. Women Birth. 2018;31:96–102. doi: 10.1016/j.wombi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Bahati C., Izabayo J., Niyonsenga J., Sezibera V., Mutesa L. Intimate partner violence as a predictor of antenatal care services utilization in Rwanda. BMC Pregnancy Childbirth. 2021;21:754. doi: 10.1186/s12884-021-04230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamieson B. Exposure to interpersonal violence during pregnancy and its association with women's prenatal care utilization: a meta-analytic review. Trauma Violence Abuse. 2020;21:904–921. doi: 10.1177/1524838018806511. [DOI] [PubMed] [Google Scholar]
- 24.Berhanie E., Gebregziabher D., Berihu H., Gerezgiher A., Kidane G. Intimate partner violence during pregnancy and adverse birth outcomes: a case-control study. Reprod. Health. 2019;16:22. doi: 10.1186/s12978-019-0670-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demelash H., Nigatu D., Gashaw K. A case-control study on intimate partner violence during pregnancy and low birth weight, southeast Ethiopia. Obstet. Gynecol. Int. 2015;2015:1–6. doi: 10.1155/2015/394875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaye D.K., Mirembe F.M., Bantebya G., Johansson A., Ekstrom A.M. Domestic violence during pregnancy and risk of low birthweight and maternal complications: a prospective cohort study at Mulago Hospital, Uganda. Trop. Med. Int. Health. 2006;11:1576–1584. doi: 10.1111/j.1365-3156.2006.01711.x. [DOI] [PubMed] [Google Scholar]
- 27.Nunes M.A.A., Camey S., Ferri C.P., Manzolli P., Manenti C.N., Schmidt M.I. Violence during pregnancy and newborn outcomes: a cohort study in a disadvantaged population in Brazil. Eur. J. Publ. Health. 2011;21:92–97. doi: 10.1093/eurpub/ckp241. [DOI] [PubMed] [Google Scholar]
- 28.Rahman M., Uddin H., Lata L.N., Uddin J. Associations of forms of intimate partner violence with low birth weight in India: findings from a population-based Survey. J. Matern. Neonatal Med. 2021;0:1–8. doi: 10.1080/14767058.2021.1940129. [DOI] [PubMed] [Google Scholar]
- 29.Sigalla G.N., Mushi D., Meyrowitsch D.W., Manongi R., Rogathi J.J., Gammeltoft T., Rasch V. Intimate partner violence during pregnancy and its association with preterm birth and low birth weight in Tanzania: a prospective cohort study. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urquia M.L., O'Campo P.J., Heaman M.I., Janssen P.A., Thiessen K.R. Experiences of violence before and during pregnancy and adverse pregnancy outcomes: an analysis of the Canadian Maternity Experiences Survey. BMC Pregnancy Childbirth. 2011;11:42. doi: 10.1186/1471-2393-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero-Gutiérrez G., Cruz-Arvizu V.H., Regalado-Cedillo C.A., Ponce-Ponce de León A.L. Prevalence of violence against pregnant women and associated maternal and neonatal complications in Leon, Mexico. Midwifery. 2011;27:750–753. doi: 10.1016/j.midw.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Audi C.A.F., Corrêa A.M.S., do Latorre M.R.D.O., Santiago S.M. The association between domestic violence during pregnancy and low birth weight or prematurity. J. Pediatr. 2008;84:60–67. doi: 10.2223/JPED.1744. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Oza S., Hogan D., Chu Y., Perin J., Zhu J., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz J., Lee A.C.C., Kozuki N., Lawn J.E., Cousens S., Blencowe H., Ezzati M., Bhutta Z.A., Marchant T., Willey B.A., Adair L., Barros F., Baqui A.H., Christian P., Fawzi W., Gonzalez R., Humphrey J., Huybregts L., Kolsteren P., Mongkolchati A., Mullany L.C., Ndyomugyenyi R., Nien J.K., Osrin D., Roberfroid D., Sania A., Schmiegelow C., Silveira M.F., Tielsch J., Vaidya A., Velaphi S.C., Victora C.G., Watson-Jones D., Black R.E. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382:417–425. doi: 10.1016/S0140-6736(13)60993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.United Nations Inter-agency Group for Child Mortality Estimation (UN IGME) Levels & trends in child mortality: report 2020, estimates developed by the united nations inter-agency group for child mortality estimation, New York. 2020. https://cdn.who.int/media/docs/default-source/mca-documents/child/levels-and-trends-in-child-mortality-igme-english_2020_.pdf (accessed September 13, 2021) [DOI] [PMC free article] [PubMed]
- 36.Ola B.E. What factors are associated with recent changes in men's attitudes towards intimate partner violence across regional, rural, and urban spaces of Ghana? Findings from three waves of Ghana national surveys from 2003 to 2014. J. Interpers Violence. 2022;37 doi: 10.1177/0886260520974070. NP8190–NP8225. [DOI] [PubMed] [Google Scholar]
- 37.Abubakari A., Kynast-Wolf G., Jahn A. Prevalence of abnormal birth weight and related factors in Northern region, Ghana. BMC Pregnancy Childbirth. 2015;15:335. doi: 10.1186/s12884-015-0790-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agorinya I.A., Kanmiki E.W., Nonterah E.A., Tediosi F., Akazili J., Welaga P., Azongo D., Oduro A.R. Socio-demographic determinants of low birth weight: evidence from the Kassena-nankana districts of the upper east region of Ghana. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abubakari A., Taabia F.Z., Ali Z. Maternal determinants of low birth weight and neonatal asphyxia in the Upper West region of Ghana. Midwifery. 2019;73:1–7. doi: 10.1016/j.midw.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Afaya A., Afaya R.A., Azongo T.B., Yakong V.N., Konlan K.D., Agbinku E., Agyabeng-Fandoh E., Akokre R., Karim J.F., Salia S.M., Kaba R.A., Ayanore M.A. Maternal risk factors and neonatal outcomes associated with low birth weight in a secondary referral hospital in Ghana. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Axame W.K., Binka F.N., Kweku M. Prevalence and factors associated with low birth weight and preterm delivery in the Ho municipality of Ghana. Adv. Public Heal. 2022;2022:1–11. doi: 10.1155/2022/3955869. [DOI] [Google Scholar]
- 42.Mohammed S., Bonsing I., Yakubu I., Wondong W.P. Maternal obstetric and socio-demographic determinants of low birth weight: a retrospective cross-sectional study in Ghana. Reprod. Health. 2019;16:70. doi: 10.1186/s12978-019-0742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghana Statistical Service, 2010 Population and Housing Census: District Analytical Report. Tamale Metropolis; Accra: 2014. https://www2.statsghana.gov.gh (accessed July 24, 2022) [Google Scholar]
- 44.Ghana Statistical Service Ghana health service, ICF international, Ghana demographic and health survey, 2014, rockville, Maryland, USA. 2015. https://dhsprogram.com/pubs/pdf/FR307/FR307.pdf (accessed July 10, 2022)
- 45.United Nations Children’s Fund, World Health Organization UNICEF-WHO Low birthweight estimates: levels and trends 2000-2015, Geneva. 2019. https://www.unicef.org/media/96976/file/UNICEF-WHO-Low-Birthweight-estimates-2000-2015.pdf (accessed June 13, 2022)
- 46.Abate B.A., Admassu Wossen B., Tilahun Degfie T. Determinants of intimate partner violence during pregnancy among married women in Abay Chomen district, Western Ethiopia: a community based cross sectional study. BMC Wom. Health. 2016;16:16. doi: 10.1186/s12905-016-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill A., Pallitto C., McCleary-Sills J., Garcia-Moreno C. A systematic review and meta-analysis of intimate partner violence during pregnancy and selected birth outcomes. Int. J. Gynecol. Obstet. 2016;133:269–276. doi: 10.1016/j.ijgo.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 48.Pastor-Moreno G., Ruiz-Pérez I., Henares-Montiel J., Escribà-Agüir V., Higueras-Callejón C., Ricci-Cabello I. Intimate partner violence and perinatal health: a systematic review. BJOG Int. J. Obstet. Gynaecol. 2020;127:537–547. doi: 10.1111/1471-0528.16084. [DOI] [PubMed] [Google Scholar]
- 49.Kassahun E.A., Mitku H.D., Getu M.A. Adverse birth outcomes and its associated factors among women who delivered in North Wollo zone, northeast Ethiopia: a facility based cross-sectional study. BMC Res. Notes. 2019;12:357. doi: 10.1186/s13104-019-4387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chawanpaiboon S., Vogel J.P., Moller A.-B., Lumbiganon P., Petzold M., Hogan D., Landoulsi S., Jampathong N., Kongwattanakul K., Laopaiboon M., Lewis C., Rattanakanokchai S., Teng D.N., Thinkhamrop J., Watananirun K., Zhang J., Zhou W., Gülmezoglu A.M. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Global Health. 2019;7 doi: 10.1016/S2214-109X(18)30451-0. e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan M.M.A., Mustagir M.G., Islam M.R., Kaikobad M.S., Khan H.T. Exploring the association between adverse maternal circumstances and low birth weight in neonates: a nationwide population-based study in Bangladesh. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-036162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray A.L., Kaiser D., Valdebenito S., Hughes C., Baban A., Fernando A.D., Madrid B., Ward C.L., Osafo J., Dunne M., Sikander S., Walker S., Van Thang V., Tomlinson M., Eisner M. The intergenerational effects of intimate partner violence in pregnancy: mediating pathways and implications for prevention. Trauma Violence Abuse. 2020;21:964–976. doi: 10.1177/1524838018813563. [DOI] [PubMed] [Google Scholar]
- 53.Wadhwa P.D., Entringer S., Buss C., Lu M.C. The contribution of maternal stress to preterm birth: issues and considerations. Clin. Perinatol. 2011;38:351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cardwell M.S. Stress: pregnancy considerations. Obstet. Gynecol. Surv. 2013;68:119–129. doi: 10.1097/OGX.0b013e31827f2481. [DOI] [PubMed] [Google Scholar]
- 55.Léonhardt M., Matthews S.G., Meaney M.J., Walker C.-D. Psychological stressors as a model of maternal adversity: Diurnal modulation of corticosterone responses and changes in maternal behavior. Horm. Behav. 2007;51:77–88. doi: 10.1016/j.yhbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Abdollahi F., Abhari F.R., Delavar M.A., Charati J.Y. Physical violence against pregnant women by an intimate partner, and adverse pregnancy outcomes in Mazandaran Province, Iran. J. Fam. Community Med. 2015;22:13. doi: 10.4103/2230-8229.149577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chia A.-R., Chen L.-W., Lai J.S., Wong C.H., Neelakantan N., van Dam R.M., Chong M.F.-F. Maternal dietary patterns and birth outcomes: a systematic review and meta-analysis. Adv. Nutr. 2019;10:685–695. doi: 10.1093/advances/nmy123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kibret K.T., Chojenta C., Gresham E., Tegegne T.K., Loxton D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: a systematic review and meta-analysis. Publ. Health Nutr. 2019;22:506–520. doi: 10.1017/S1368980018002616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kargbo D.K., Nyarko K., Sackey S., Addo-Lartey A., Kenu E., Anto F. Determinants of low birth weight deliveries at five referral hospitals in Western Area Urban district, Sierra Leone. Ital. J. Pediatr. 2021;47:212. doi: 10.1186/s13052-021-01160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dassah E.T., Kusi-Mensah E., Morhe E.S.K., Odoi A.T. Maternal and perinatal outcomes among women with hypertensive disorders in pregnancy in Kumasi, Ghana. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alwan N.A., Roderick P.J., Macklon N.S. Is timing of the first antenatal visit associated with adverse birth outcomes? Analysis from a population-based birth cohort. Lancet. 2016;388:S18. doi: 10.1016/S0140-6736(16)32254-1. [DOI] [Google Scholar]
- 62.Jung J., Rahman M.M., Rahman M.S., Swe K.T., Islam M.R., Rahman M.O., Akter S. Effects of hemoglobin levels during pregnancy on adverse maternal and infant outcomes: a systematic review and meta‐analysis. Ann. N. Y. Acad. Sci. 2019;1450 doi: 10.1111/nyas.14112. nyas. [DOI] [PubMed] [Google Scholar]
- 63.Mahmood T., Rehman A.U., Tserenpil G., Siddiqui F., Ahmed M., Siraj F., Kumar B. The association between iron-deficiency anemia and adverse pregnancy outcomes: a retrospective report from Pakistan. Cureus. 2019;11 doi: 10.7759/cureus.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laelago T., Yohannes T., Tsige G. Determinants of preterm birth among mothers who gave birth in East Africa: systematic review and meta-analysis. Ital. J. Pediatr. 2020;46:1–14. doi: 10.1186/s13052-020-0772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Endalamaw A., Engeda E.H., Ekubagewargies D.T., Belay G.M., Tefera M.A. Low birth weight and its associated factors in Ethiopia: a systematic review and meta-analysis. Ital. J. Pediatr. 2018;44:141. doi: 10.1186/s13052-018-0586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bekkar B., Pacheco S., Basu R., DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chersich M.F., Pham M.D., Areal A., Haghighi M.M., Manyuchi A., Swift C.P., Wernecke B., Robinson M., Hetem R., Boeckmann M., Hajat S. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: systematic review and meta-analysis. BMJ. 2020;371:m3811. doi: 10.1136/bmj.m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlining the conclusions drawn in this study are contained within the manuscript. The dataset, however, can be made available on reasonable request from the corresponding author.