Abstract
Aim of the study
In this study, we investigated the therapeutic potential of vitamin D (VITD) in OA Wistar rats induced by anterior cruciate ligament transection combined with medial meniscectomy (ACLT + MMx). In ACLT + MMx-induced OA rats, pain severity, cartilage destruction, inflammatory cytokines, and MMPs were all measured.
Materials and methods
ACLT + MMx methods were used to induce OA, and pain behavioral studies such as the weight bearing test and paw withdrawal test were performed while the knee width and body weights were also measured. Furthermore, Hematoxylin and Eosin (H&E) staining was used to determine knee histopathological studies, as well as OARSI scoring, cartilage thickness, cartilage width, and cartilage degradation scores. The enzyme-linked immunosorbent assay (ELISA) studies were used to check the serum levels of VITD, C-telopeptide of Type II collagen (CTX-II), and pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and anti-inflammatory cytokines interleukin-10 (IL-10), and MMPs (MMP-3, MMP-9, and MMP-13). Finally, the reverse transcription polymerase chain reaction (RT-PCR) test was used to determine the levels of MMPs, nuclear factor-kappa B (NF-κB), TNF-α, IL-6, and IL-10 in IL-1β stimulated chondrocytes.
Results
The oral VITD supplement significantly reduced OA pain, inflammation, cartilage destruction, and MMPs levels. Furthermore, serum VITD levels increased while CTX-II levels decreased, indicating that VITD reduced cartilage degradation effectively. Moreover, VITD supplementation reduced the expression of pro-inflammatory TNF-α, IL-1β, and IL-6 cytokines while increasing the expression of anti-inflammatory IL-10. The elevation of MMPs after ACLT + MMx surgery contributed to articular cartilage destruction, which was reduced by VITD supplementation. Finally, VITD supplementation significantly reduces serum levels of MMPs, IL-1β, TNF-α, and IL-6 while increasing IL-10 levels. Then, using the in-vitro cytotoxicity (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) MTT assay, examine the cytotoxicity profile of VITD in rat chondrocytes after stimulated with IL-1β, which shows no toxicity in the dose range of VITD 0–500 IU. Finally, RT-PCR studies in IL-1β stimulated rat chondrocytes revealed that VITD (50, 100, and 500 IU) significantly reduced the mRNA levels of MMPs, NF-κB, TNF-α, and IL-6, while increasing IL-10 levels, indicating that VITD reduced chondrocyte destruction and overcame harsh conditions in a dose-dependent manner.
Conclusion
Overall, the in vivo and in vitro findings show that VITD effectively reduces OA pain, inflammation, and chondrocyte destruction by lowering MMPs levels specifically.
Keywords: Knee osteoarthritis, Vitamin D, Knee pain, Chondrocytes, Inflammation, Matrix metalloproteases
Graphical abstract
1. Introduction
Knee osteoarthritis (OA) is a degenerative joint disorder that worsens over time. OA is described by articular cartilage deterioration, synovial membrane inflammation, subchondral bone defects, and joint space reduction, etc. The complexity of OA has hampered efforts to unravel its etiology, which remains a mystery [1]. Predominant OA is caused by a combination of risk factors, the most significant of which include increasing age and obesity. Treatment approaches are mostly concerned with symptom relief, and there are presently no disease-modifying OA therapeutics that can reduce symptoms while also slowing disease progression. The primary OA therapeutic agents focused on reducing inflammation and pain symptoms [2]. According to US nutritional research society, VITD, vitamin E, and vitamin C rich foods significantly reduced the advancement of OA disorder and its consequences [3,4]. VITD deficiency is still accompanied by OA disease advancement, chondrocytes, synoviocytes inflammation, and pain mechanisms. In some recent studies, VITD-supplemented patients had slower OA progression, less pain and stiffness than non-VITD-supplemented patients. There is no gold-stranded evidence of VITD's effect on chondrocyte protection and pain relief at the molecular level [[5], [6], [7]]. VITD has shown some adverse effects in clinical trial studies, and long-term administration reveals no meaningful benefit in terms of OA progression and pain relief [4,5,[8], [9], [10]].
According to recent studies, VITD deficiency is directly linked to oxidative stress damage and mitochondrial abnormalities, which worsens OA disease and increases pain [11]. In another study, VITD significantly increases muscle strength and function in the skeletal muscle, which has been extensively studied in the elderly. There is no evidence of pain relief or chondrocyte protection in their study [12,13]. The oxidative stress conditions and mitochondrial membrane potential changes have been influencing the VITD status and severity of OA, but this one has a direct impact on the VITD receptor functionality in the various organs. The VITD receptors play an important role in the regulation of VITD concentration in chondrocytes to keep the redox environment stable [14]. Another clinical trial study found that the less amount of VITD accompanied by chronic inflammation at joints and pain index varied by gender and age [15,16].
MMPs are known to be essential in OA cartilage disruption, with chondrocytes expressing mRNA for a wide range of MMPs [[17], [18], [19]]. Cytokines have been linked to the complex regulation of MMPs generation by chondrocytes, and recent animal studies have shown that VITD plays a crucial role in cartilage metabolism [20]. VITD is known to play a pivotal role in calcium and phosphorous metabolism as well as a variety of bone and mineral disorders such as osteoporosis and rickets [21,22]. VITD also regulates cell proliferation, differentiation, and immune function through its binding to VITD receptors [[23], [24], [25]]. VITD is believed to modulate chondrocyte expression in different areas of the growth plate cartilage, with different effects on proteoglycan and collagen synthesis, plasminogen activator restrict the MMPs expression [[25], [26], [27]]. However, the majority of existing in vivo and in vitro studies are also using traditional therapeutic agents to investigate MMPs role in cartilage degradation, and inflammation. Moreover, there is no proper evidence of VITD impact on MMPs levels and how it contributes to reduced cartilage degradation, inflammation, and pain at the in vivo or in vitro levels studies [28].
The prospective relationship between VITD deficiency and OA progression, pain reduction, and chondrocyte protection remains unknown in vivo and in vitro studies. As shown in Fig. 1, we investigated the effects of VITD effectiveness in vivo and in vitro. Oral VITD supplementation reduced OA pain, cartilage destruction, joint inflammation, and MMPs levels, according to our findings.
Fig. 1.
The graphic representation of animal grouping, the oral administration of VITD, pain behavioral studies, the extraction of knee samples, and chondrocytes, and the testing of biological parameters are all part of the research.
2. Materials and methods
2.1. Animals
Male Wistar rats, weight 290–310 g (10 weeks old), were purchased from BioLASCO Taiwan Co., Ltd (Taipei, Taiwan). The rats were housed in 2 per cage at Catha Medical Research Institute (CMRI) with controlled temperature (22 ± 2 °C), humidity (55%), and light (12/12 h light/dark cycle), and had access to sterile food and water. All procedures were approved by Cathay Hospital Institutional Animal Care and Use Committee (IACUC) (IACUC mummer: CGH-IACUC-111-002).
2.2. OA induction and drug treatment
OA was induced in rats, as previously reported [[29], [30], [31]]. To induce OA in rats, the ACLT + MMx method was used on the right knee. Rats were anesthetized with isoflurane (5%, Panion, and BF, Biotech Inc., Taipei, Taiwan). In brief, the medial aspect of the joint capsule was incised, the ACLT was transected, and MMx was removed. To maintain hygienic conditions, cefazolin (100 mg/kg/day) (Cat: 097603.1566, Sigma-Aldrich, Neihu, Taipei, Taiwan) was administered intramuscularly (IM) for 4 days. Sham rats had a medical joint capsule incision made and closed but without the anterior cruciate ligament transection and MMx removal. One week after OA induction, VITD (Cat: 1131009, cholecalciferol, Sigma-Aldrich, NY, USA) were orally administered daily for up to 12 weeks (500 (VITD-H), 100 (VITD-M), and 50 (VITD-L) IU/kg/day). The sham and OA group animals were treated with an equivalent volume of saline containing 10% dimethyl sulfoxide (DMSO).
2.3. Assessment of pain behavior
As previous report [32], mechanical sensitivity was used to assess pain; a dynamic plantar Aesthesiometer (Ugo Basile, Gemonio, VA, Italy) was used to assess the pain response after OA induction and VITD supplementation. OA-treated rats were tested for hind paw response to masseter mechanical stimulation with rigid von Frey filaments and a force transducer (Electronic von Frey, Woodland Hills, CA, USA). The rat's paw withdrawal threshold was determined by gradually reducing the pressure from 1 to 50 g through a metal filament (0.5 mm) aimed at the plantar region, the average was set at 50 g as the cut-off threshold to prevent paw injury. Every 2 min, the paw reflexes were measured in triplicate, for analysis, the mean three values were used.
2.4. Weight-bearing test
An incapacitance tester (Linton instrument, Norfolk, UK) with a dual channel weight mean value was used to test weight bearing. The rats were carefully placed in a plastic chamber. The strength applied via a hind limb was averaged over a 3 s. The single data point was the average of three measurements. The weight distribution on the surgery hind limb (ipsilateral) was calculated using the weight difference between the non-surgical and surgical limb (△ Force, g) [33].
2.5. Histological studies
The effects of VITD supplement on cartilage degeneration in OA rats were determined using histological changes. After 12 weeks of study, rats were sacrificed and knee joints removed. The joints were resected and fixed for an additional 48 h at 4 °C, including the patella and joint capsule. The fixed joint specimens were decalcified for 12 days in a 5% collagenase solution at 40 °C, followed by 5 days in 2% formic acid. The samples were then embedded in paraffin and stained with Hematoxylin and Eosin (H&E). The Osteoarthritis Research Society International (OARSI) score, cartilage thickness, width, and degradation score were used to estimate OA histopathology [34].
2.6. ELISA studies
The total serum VITD levels were measured by the radioimmunoassay assay using a Cobas E411 analyzer (Cathay GeneralHospital, Taipei, Taiwan). To determine the concentrations of biological proteins in serum using commercially available ELISA kits. TNF-α, IL-6, and IL-10 (San Diego, CA, USA), MMP-3, MMP-9, IL-1β, and CTX-II (Cambridge, MA, USA) ELISA kits from Abcam Co., Ltd. All measurements were taken in triplicate as a directed manufacturer. For the detection of the concentrations of each independent study sample, a standard curve was calculated [30,35].
2.7. Extraction and culture of chondrocytes
Chondrocytes were isolated from the knee samples. The tissues were immersed in a sterilized phosphate buffer solution (pH7.4). The tissue fat and connective tissue were digested with 1% collagenase solution (Invitrogen, Thermo Fisher Scientific, Grand Isle, NY, USA), and kept at 37 °C for 50 min. The cells were isolated from tissue culture using 70 μM cell strainers. The cells were then cultured with Dulbecco Modified Eagle Medium (DMEM) (Gibco BRL, NY, USA) and antibiotics penicillin and streptomycin (100 ng/mL) (Sigma-Aldrich, Darmstadt, Germany), and incubated at 37 °C with 5% CO2. After reaching confluence, the cells were detached with 0.1% trypsin (Sigma-Aldrich, Darmstadt, Germany) and pretreated with (10 ng/mL) IL-1β before being treated with VITD for 24 h to simulate harsh conditions. MTT assay was used to determine the toxicity of VITD (Sigma-Aldrich, Darmstadt, Germany) [36,37].
2.8. RT-PCR test
Chondrocytes were cultured in 100 mm cell culture dishes (10 × 106 cells/dish) and used for RT-PCR experiments. Cells were washed twice with PBS solution (pH-7.4). After VITD treatment and total mRNA was isolated using TRIzol reagent. The total mRNA (1 g) was reverse-transcribed into cDNA using the prime-script RT reagent kit and the gDNA eraser (Takara Bio, Kusatsu, Shiga, Japan) as directed using manufacturer instructions. SYBER premix Ex TaqII was also used on an ABI prism 7500 fast RT-PCR system (Applied Biosystem, Wilmington, NC, USA). The expression levels were calculated using the −2△△CT method (Livak and Schmittgen) with actin serving as the reference gene [38]. The primer sequences were shown in Table 1.
Table 1.
Details of the RT-PCR primer sequences.
| Forward Primers (5’-3’) | Reverse Primers (3’-5’) | |
|---|---|---|
| MMP-3 | CATAATACACAGCTGACCTGTATAA | ATTTAAGAAATCATAGATAAACAGTTACTTA |
| MMP-9 | TAGTGAGAGACTCTACACAG | CCACTTCTTGTCAGTGTCGA |
| MMP-13 | TGATGATGAAACCTGGACAAGCA | GAACGTCATCTCTGGGAGCA |
| NF-κB | CATGCGTTTCCGTTACAAGTGCGA | TGGGTGCGTCTTAGTGGTATCTGT |
| TNF-α | AGCCCACGTCGTAGCAAACCACCAA | AACACCCATTCCCTTCACAGAGCAAT |
| IL-6 | GACAAAGCCAGAGTCCTTCAGA | AGGAGAGCAATTGGAAATTGGGG |
| IL-10 | TTGCCAAGCCTTGTCTGAGAT | TTCTCCCCCAGGGAGTTCAC |
| β-actin | GGAGATTACCTGCCCTGGCTCCTA | GACTCATCTACTCCTGCTTGCTG |
2.9. Statistical analysis
The data are presented as mean ± S.D., GraphPad Prism version 6 was used to create all of the figures. One-way ANOVA analysis was performed on all data sets, followed by a t-test for multiple comparisons among the groups. P-values less than 0.05 were considered significant. Significant differences between groups were found at *p < 0.05, **P < 0.01, and ***P < 0.001.
3. Results and discussions
OA is the most common type of knee arthritis that develops in middle age, and it is associated with many risk factors, including age, sex, genetics, and obesity. There is no cure for OA, but anti-inflammatory and analgesic drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids are frequently used. Although these therapeutic agents effectively relieve pain, they are not without risks. Steroid drugs can cause some side effects, making long-term use difficult, whereas NSAIDs can cause gastrointestinal issues as well as hepatotoxicity and renal toxicity. As a result, long-term medications without such complications are required. According to recent clinical reports, VITD is safe over time and may be useful in the treatment of OA patients. In this study, we investigated the effects of VITD in ACLT + MMx surgical method-induced OA rats by reducing inflammation and inhibiting MMPs to lessen articular cartilage damage.
3.1. VITD alleviates pain in ACLT + MMx-induced OA rats
The most common symptoms of OA are pain and cartilage destruction. Thus, we tested tactile allodynia in ACLT + MMx induced OA rats to see if VITD can alleviate pain. The paw withdrawal thresholds (g) in orally treated VITD-H, VITD-M, and VITD-L group rats were significantly higher than in sham-group rats (Fig. 2A). When compared to sham-group rats, VITD-H, VITD-M, and VITD-L treated rats show a significant difference in weight-bearing (Force g) (Fig. 2B). In vivo studies revealed that VITD has significant anti-nociceptive activity in a dose-dependent manner.
Fig. 2.
The therapeutic effect of VITD in ACLT + MMx-induced OA rats. Pain behavior was analyzed by (A) paw withdrawal threshold method (g), and (B) weight bearing method, and were measured in sham-group (n = 5), OA-group (n = 5), VITD-H (500-IU/kg/day) (n = 5), VITD-M (100-IU/kg/day) (n = 5), and VITD-L (50-IU/kg/day) (n = 5) group animals. Data are presented as means ± S.D, *P < 0.05, **P < 0.01, and ***P < 0.001.
Throughout the experiment, consistently increased body weight (g) in all groups of rats (Fig. 3A). Supplement of VITD attributes no adverse or toxic effects. The knee width (nm) in VITD-treated rats shows better reduction compared to OA rats (Fig. 3B). This reveals that the knee inflammation was reduced, with knee width reduction, in a dose-dependent manner. Additionally, this data is supported by additional experimental findings, such as the serum pro-inflammatory and anti-inflammatory cytokines expression.
Fig. 3.
Therapeutic effect of VITD in ACLT + MMx-induced OA rats. Checking the (A) body weight changes (g), and (B) Knee with (nm) changes in sham- (n = 5), OA- (n = 5), VITD-H (500-IU/kg/day) (n = 5), VITD-M (100-IU/kg/day) (n = 5), and VITD-L (50-IU/kg/day) (n = 5) group animals. Data are presented as mean ± S.D.*P < 0.05, **P < 0.01, and ***P < 0.001.
3.2. VITD attenuates cartilage destruction in ACLT + MMx induced OA rats
In OA knee, articular cartilage destruction is caused by a combination of increased extracellular matrix degradation (ECM), decreased ECM production, and chondrocyte death. In addition, members of the MMPs and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) genes are throughout to be essential in cartilage degradation [39]. According to our previous, the ACLT + MMx-induced OA revealed that progressive reduced serum VITD and increased MMPs play a significant role in the progression of OA in a time-dependent manner [35].
For the chondroprotective property of VITD, H&E-stained revealed that VITD treatment (Fig. 4A c-e) reduced articular cartilage destruction compared to OA-group rats (Fig. 4A b). OARSI scores revealed that VITD-treated ACLT + MMX-induced rats (VITD-H, VITD-M, and VITD-L) had significantly less cartilage destruction than OA-group rats (Fig. 4A f). Furthermore, compared to OA-group rats, VITD-treated rats significantly increased cartilage thickness (μM) (Fig. 4B a), decreased cartilage width (μM) (Fig. 4B b), and decreased cartilage degradation score (Fig. 4B c). The overall results showed that VITD effectively reduced cartilage destruction in a dose-dependent manner.
Fig. 4.
The cartilage protective effect of VITD in ACLT + MMx-induced OA rats. (A) H&E staining studies of (a) sham-group (n = 5), (b) OA-group (n = 5), (c) VITD-L (n = 5), (d) VITD-M (n = 5), (e) VITD-H (n = 5) (scale bar 200 μM), and (f) OARSI score. (B) H&E studies estimated cartilage destruction parameters, (a) cartilage thickness (μM), (b) cartilage width (μM), and (c) cartilage degradation score. Data are presented as mens±S.D. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.3. The effect of VITD on serum levels of VITD and CTX-II in ACLT + MMx-induced OA rats
According to previous studies, lower serum VITD levels had been shown to hasten the progression of OA. The roles of VITD and CTX-II in cartilage degradation and OA progression are critical targets for detection and treatment. According to recent studies, higher serum levels of CTX-II enhanced the progression of OA [30,40]. In our study, serum VITD levels increased significantly in a dose-dependent manner when compared to OA-group animals (Fig. 5A). Furthermore, serum CTX-II, responsible for cartilage degradation, levels were significantly lower in VITD-treated groups compared with OA-group animals (Fig. 5B). These findings support the argument that VITD supplementation restores VITD levels while decreasing CTX-II levels, thereby reducing cartilage degradation.
Fig. 5.
VITD effect on the serum VITD and CTX-II levels. ELISA studies of (A) VITD concentration (ng/mL), and (B) CTX-II concentration (pg/mL) levels checked in the sham-group (n = 5), OA-group (n = 5), VITD-H (500-IU/kg/day) (n = 5), VITD-M (100-IU/kg/day) (n = 5), and VITD-L (50-IU/kg/day) (n = 5) group animals. Data are presented as means ± S.D. *P < 0.05. **P < 0.01, and ***P < 0.001.
3.4. The effect of VITD on serum pro-inflammatory and inflammatory cytokines expression in ACLT + MMx-induced OA rats
The pro-inflammatory IL-1β, TNF-α, IL-6, and anti-inflammatory cytokine IL-10, in particular, regulate articular cartilage matrix degradation, making them prime targets for therapeutic strategies [41]. Recent animal and clinical studies support this treatment strategy of OA, via inhibiting the pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 (Macrophase type 1 (M1 type)), and enhancing the level of anti-inflammatory cytokines IL-10 level (Macrophase type 2 (M2 type)) [42]. According to recent studies, VITD is a powerful agonist of the transcription factor vitamin D receptor. The VITD has received widespread acclaim for its impact not only on mineral homeostasis, but also on metabolic diseases, such as immunological disease and cancer, among others [43,44]. However, VITD treatment modalities have demonstrated potent anti-inflammatory activity in both animal and cell culture models. In this study, VITD significantly reduced the serum pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 when compared to OA-group rats (Fig. 6A–C). Furthermore, VITD-treated rats exhibit a significant increase in serum anti-inflammatory cytokine (IL-10) concentrations in a dose-dependent manner when compared to OA-group rats (Fig. 6D). According to the present findings, the VITD supplement reduced the inflammation in OA; the width of the knee joints was reduced after treatment.
Fig. 6.
VITD effect on serum pro-inflammatory and anti-inflammatory cytokines levels. (A–C) ELISA studies of serum pro-inflammatory cytokines IL-1β (pg/mL), TNF-α (pg/mL), and IL-6 (pg/mL), and (D) anti-inflammatory cytokines IL-10 (pg/mL) concentration was determined in the sham-group (n = 5), OA-group (n = 5), VITD-H (500-IU/kg/day) (n = 5), VITD-M (100-IU/kg/day) (n = 5), and VITD-L (50-IU/kg/day) (n = 5) group animals. Data are presented as means ± S.D. *P < 0.05, **P < 0.01, and ***P < 0.001.
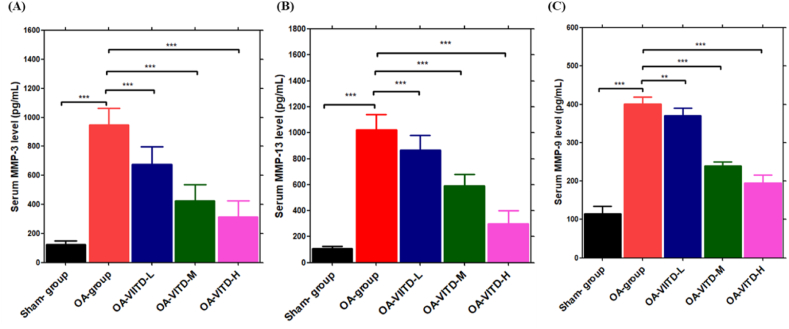
3.5. The effect of VITD on serum MMPs in ACLT + MMx-induced OA rats
In chronic OA, the pro-inflammatory cytokines stimulate the production of MMPs, which degrade all extracellular matrix components. MMP-3, MMP-9, and MMP-13 collagenases are rate-limiting in the collagen degradation process and thus play a significant role in OA progression. MMP-13, for example, is produced by chondrocytes in cartilage and degrades aggrecan, a proteoglycan molecule, as well as collagen, giving it a dual role in cellular matrix destruction. MMP-3 and MMP-9 are also elevated in OA, which degrades non-collagen matrix components of joints [[45], [46], [47], [48]]. When compared to the OA-group rats, the VITD supplement significantly lowered MMP-13 levels (Fig. 7B). Furthermore, MMP-3 and MMP-9 enzyme levels were significantly lower after VITD treatment compared to OA-group rats (Fig. 7A&C). Overall, the findings show that oral VITD supplement protects the cartilage against collagen degradation by lowering MMPs expression in joint chondrocytes.
Fig. 7.
VITD effect on MMPs enzymes levels: MMPs levels in the serum were measured using an ELISA test, (A) MMP-3, (B) MMP-13, and (C) MMP-9 enzyme levels in the sham-group (n = 5), OA-group (n = 5), VITD-H (500-IU/kg/day) (n = 5), VITD-M (100-IU/kg/day) (n = 5), and VITD-L (50-IU/kg/day) (n = 5) group animals. Data are presented as means ± S.D. *P < 0.05, **P < 0.01, and ***P < 0.001.
3.6. The effect of VITD on rat chondrocytes
Cartilage is a tissue made up of only one type of cell chondrocytes, which are wrapped in a collagen-rich extracellular matrix they produced. Chondrocytes can adopt different phenotypes in vivo and in vitro which are determined by the type of collagen they produced. When separated from their matrix, chondrocytes can differentiate and produce fibroblastic type I and III collagens. With the onset of OA and chondrocytes undergo several variations, including changes in proliferation, viability, and secretory profile [[49], [50], [51], [52]].
The toxic and protective effects of VITD were tested in chondrocytes using the MTT assay. When chondrocytes were first treated with IL-1β, cell viability was drastically reduced due to reactive oxygen species (ROS) levels, as previously discovered. The VITD-treated chondrocytes show protection from harsh conditions at all drug concentrations after being stimulated with IL-1β. Furthermore, the VITD alone treatment shows constant cell viability for up to 24 h, indicating that the VITD supplement has no toxicity toward chondrocytes (Fig. 8B). Based on the findings from our study, we recommend VITD (Fig. 8A) as a good candidate for the protection of chondrocytes under the harsh conditions of OA.
Fig. 8.
Checking the VITD effect on chondrocytes. (A) Chemical structure of VITD, and (B) MTT assay studies on chondrocytes with VITD (500, 100, and 50 -IU) for 24 h of treatment before and after stimulation with IL-1β.
3.7. The effect of VITD on chondrocytes mRNA expression of MMPs, NF-kB, TNF-α, IL-6, and IL-10
VITD (dose-dependent) supplementation significantly reduced the mRNA expression of MMPs, TNF-α, NF-kB, and IL-6, and increased IL-10 mRNA expression in IL-1β stimulated rat chondrocytes (Fig. 9A–G), which indicates VITD successfully reduced the inflammation and protects from the harsh conditions.
Fig. 9.
The effect of VITD on chondrocyte mRNA levels was investigated using RT-PCR studies: (A) MMP-3, (B) MMP-9, (C) MMP-13, (D) NF-κB, (E) TNF-α, (F) IL-6, and (G) IL-10 levels with VITD (500, 100, and 50 -IU) for 24 h of treatment before and after stimulation with IL-1β. Data are presented as means ± S.D. *P < 0.05, **P < 0.01, and ***P < 0.001.
4. Conclusion
In this study, we discovered that VITD has a chondroprotective effect in ACLT + MMx-induced OA rats and IL-1β-stimulated rat chondrocytes. The pain behavioral studies show that VITD (dose-dependent) treatment significantly reduced pain severity, cartilage destruction, as well as inflammation. The ACLT + MMx-induced OA animals show low levels of VITD and increased levels of CTX-II levels; however, after VITD oral supplementation, the VITD and CTX-II levels are restored. According to the H&E studies, VITD treatment significantly reduced cartilage destruction. Furthermore, ELISA studies revealed that after VITD treatment, serum pro-inflammatory cytokines IL-1β, TNF-α, and IL-6 levels significantly reduced while anti-inflammatory cytokines IL-10 levels increased, indicating that VITD successfully reduced inflammation in OA rats. Furthermore, VITD also inhibited MMPs expression in chondrocytes (MMP-3, MMP-9, and MMP-13), which are required for cartilage protection against the harsh conditions of OA. Furthermore, the VITD toxicity study, using MTT assay, shows that VITD is not toxic before or after IL-1β stimulation. Finally, we further examined MMPs, IL-6, TNF-α, IL-10, and NF-κB mRNA expression in chondrocytes. Revealed a significant reduction in MMPs, IL-6, TNF-α, and NF-κB expression, and enhanced IL-10 mRNA expression after VITD treatment. This study found that VITD has anti-inflammatory, pain reduction, and protective effects on rat chondrocytes.
Author contribution statement
Prabhakar Busa: Conceived and desinged the experiemnts; Perfomed the experiments; Analyzed and interpreted the data; Wrote the paper. Niancih Huang, Yaswanth Kuthati: Contributed reagents, materials, analysis tools or data. Chih-Shung Wong: Conceived and desinged the experiemnts, Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Ministry of Science and Technology (Taiwan) [MOST 111-2314-B-281-010-MY2, and MOST 111-2314-B-281-007-MY2].
This work was supported by Cathay Medical Research Institute [CGH-MR-A11131].
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Contributor Information
Prabhakar Busa, Email: Prabhakar.busa01@gmail.com.
Niancih Huang, Email: niancih@hotmail.com.
Yaswanth Kuthati, Email: yaswanthk1992@gmail.com.
Chih-Shung Wong, Email: w82556@gmail.com.
References
- 1.Mora J.C., Przkora R., Cruz-Almeida Y. Knee osteoarthritis: pathophysiology and current treatment modalities. J. Pain Res. 2018;11:2189–2196. doi: 10.2147/JPR.S154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jang S., Lee K., Ju J.H. Recent updates of diagnosis, pathophysiology, and treatment on osteoarthritis of the knee. Int. J. Mol. Sci. 2021;22(5) doi: 10.3390/ijms22052619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidari B., Babaei M. Therapeutic and preventive potential of vitamin D supplementation in knee osteoarthritis. ACR Open Rheumatol. 2019;1(5):318–326. doi: 10.1002/acr2.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park C.Y. Vitamin D in the prevention and treatment of osteoarthritis: from clinical interventions to cellular evidence. Nutrients. 2019;11(2) doi: 10.3390/nu11020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanghi D., et al. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clin. Orthop. Relat. Res. 2013;471(11):3556–3562. doi: 10.1007/s11999-013-3201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alabajos-Cea A., et al. The role of vitamin D in early knee osteoarthritis and its relationship with their physical and psychological status. Nutrients. 2021;13(11) doi: 10.3390/nu13114035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin X., et al. Effectiveness of vitamin D supplementation on knee osteoarthritis - a target trial emulation study using data from the Osteoarthritis Initiative cohort. Osteoarthritis Cartilage. 2022;30(11):1495–1505. doi: 10.1016/j.joca.2022.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Diao N., Yang B., Yu F. Effect of vitamin D supplementation on knee osteoarthritis: a systematic review and meta-analysis of randomized clinical trials. Clin. Biochem. 2017;50(18):1312–1316. doi: 10.1016/j.clinbiochem.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Hussain S., et al. Vitamin D supplementation for the management of knee osteoarthritis: a systematic review of randomized controlled trials. Rheumatol. Int. 2017;37(9):1489–1498. doi: 10.1007/s00296-017-3719-0. [DOI] [PubMed] [Google Scholar]
- 10.Alkan G., Akgol G. Do vitamin D levels affect the clinical prognoses of patients with knee osteoarthritis? J. Back Musculoskelet. Rehabil. 2017;30:897–901. doi: 10.3233/BMR-160589. [DOI] [PubMed] [Google Scholar]
- 11.Uchitomi R., Oyabu M., Kamei Y. Vitamin D and sarcopenia: potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. 2020;12(10) doi: 10.3390/nu12103189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girgis C.M. Vitamin D and muscle function in the elderly: the elixir of youth? Curr. Opin. Clin. Nutr. Metab. Care. 2014;17(6):546–550. doi: 10.1097/MCO.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 13.Dawson-Hughes B. Vitamin D and muscle function. J. Steroid Biochem. Mol. Biol. 2017;173:313–316. doi: 10.1016/j.jsbmb.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Halfon M., Phan O., Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/953241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeck A.D., Pall M.L. Will vitamin D supplementation ameliorate diseases characterized by chronic inflammation and fatigue? Med. Hypotheses. 2011;76(2):208–213. doi: 10.1016/j.mehy.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Wöbke T.K., Sorg B.L., Steinhilber D. Vitamin D in inflammatory diseases. Front. Physiol. 2014;5:244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehana E.E., Khafaga A.F., El-Blehi S.S. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234 doi: 10.1016/j.lfs.2019.116786. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y.S., et al. The matrix metalloproteinases as pharmacological target in osteoarthritis: statins may be of therapeutic benefit. Med. Hypotheses. 2007;69(3):557–559. doi: 10.1016/j.mehy.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 19.Dhar S., Ray B.K., Ray A. In: Proteases in Health and Disease. Chakraborti S., Dhalla N.S., editors. Springer New York; New York, NY: 2013. Escalated expression of matrix metalloproteinases in osteoarthritis; pp. 313–325. [Google Scholar]
- 20.Li S., et al. Vitamin D inhibits activities of metalloproteinase-9/-13 in articular cartilage in vivo and in vitro. J. Nutr. Sci. Vitaminol. 2019;65(2):107–112. doi: 10.3177/jnsv.65.107. [DOI] [PubMed] [Google Scholar]
- 21.Norman A.W. Vitamin D metabolism and calcium absorption. Am. J. Med. 1979;67(6):989–998. doi: 10.1016/0002-9343(79)90640-5. [DOI] [PubMed] [Google Scholar]
- 22.Fukumoto S. Phosphate metabolism and vitamin D. BoneKEy Rep. 2014;3:497. doi: 10.1038/bonekey.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samuel S., Sitrin M.D. Vitamin D's role in cell proliferation and differentiation. Nutr. Rev. 2008;66(10 Suppl 2):S116–S124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 24.Aranow C. Vitamin D and the immune system. J. Invest. Med. 2011;59(6):881–886. doi: 10.231/JIM.0b013e31821b8755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umar M., Sastry K.S., Chouchane A.I. Role of vitamin D beyond the skeletal function: a review of the molecular and clinical studies. Int. J. Mol. Sci. 2018;19(6) doi: 10.3390/ijms19061618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallett S.A., Ono W., Ono N. Growth Plate chondrocytes: skeletal development, growth and beyond. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akkiraju H., Nohe A. Role of chondrocytes in cartilage formation, progression of osteoarthritis and cartilage regeneration. J. Dev. Biol. 2015;3(4):177–192. doi: 10.3390/jdb3040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kamisan N., et al. Chondrocyte density, proteoglycan content and gene expressions from native cartilage are species specific and not dependent on cartilage thickness: a comparative analysis between rat, rabbit and goat. BMC Vet. Res. 2013;9:62. doi: 10.1186/1746-6148-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busa P., et al. Carnosine alleviates knee osteoarthritis and promotes synoviocyte protection via activating the Nrf2/HO-1 signaling pathway: an in-vivo and in-vitro study. Antioxidants. 2022;11(6) doi: 10.3390/antiox11061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang N.-C., et al. Detection and evaluation of serological biomarkers to predict osteoarthritis in anterior cruciate ligament transection combined medial meniscectomy rat model. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen I.-J., Wong C.-S. Shea nut oil extracts enhance the intra-articular sodium hyaluronate effectiveness on surgically induced OA progression in rats. Nutrients. 2020;12 doi: 10.3390/nu12040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen I.-J., Lin S.-H., Wong C.-S. Oral shea nut oil triterpene concentrate supplement ameliorates pain and histological assessment of articular cartilage deterioration in an ACLT injured rat knee osteoarthritis model. PLoS One. 2019;14(4):e0215812. doi: 10.1371/journal.pone.0215812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen I.-J., et al. The circadian hormone melatonin inhibits morphine-induced tolerance and inflammation via the activation of antioxidative enzymes. Antioxidants. 2020;9 doi: 10.3390/antiox9090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuthati Y., et al. Mesoporous polydopamine nanoparticles attenuate morphine tolerance in neuropathic pain rats by inhibition of oxidative stress and restoration of the endogenous antioxidant system. Antioxidants. 2021;10(2) doi: 10.3390/antiox10020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busa P., et al. Carnosine alleviates knee osteoarthritis and promotes synoviocyte protection via activating the Nrf2/HO-1 signaling pathway: an in-vivo and in-vitro study. Antioxidants. 2022;11 doi: 10.3390/antiox11061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tetlow L.C., Woolley D.E. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartilage. 2001;9(5):423–431. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- 37.Tetlow L.C., Woolley D.E. Expression of vitamin D receptors and matrix metalloproteinases in osteoarthritic cartilage and human articular chondrocytes in vitro. Osteoarthritis Cartilage. 2001;9(5):423–431. doi: 10.1053/joca.2000.0408. [DOI] [PubMed] [Google Scholar]
- 38.Cataldo D.D., et al. Matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases mRNA transcripts in the bronchial secretions of asthmatics. Lab. Invest. 2004;84(4):418–424. doi: 10.1038/labinvest.3700063. [DOI] [PubMed] [Google Scholar]
- 39.Okubo M., Okada Y. [Destruction of the articular cartilage in osteoarthritis] Clin. Calcium. 2013;23(12):1705–1713. [PubMed] [Google Scholar]
- 40.Duclos M.E., et al. Significance of the serum CTX-II level in an osteoarthritis animal model: a 5-month longitudinal study. Osteoarthritis Cartilage. 2010;18(11):1467–1476. doi: 10.1016/j.joca.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Vojinovic J. Vitamin D receptor agonists' anti-inflammatory properties. Ann. N. Y. Acad. Sci. 2014;1317:47–56. doi: 10.1111/nyas.12429. [DOI] [PubMed] [Google Scholar]
- 42.E L.B., et al. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus. 2021;5(1) doi: 10.1002/jbm4.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mousa A., et al. Effect of vitamin D supplementation on inflammation: protocol for a systematic review. BMJ Open. 2016;6(4):e010804. doi: 10.1136/bmjopen-2015-010804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashemi R., et al. Anti-inflammatory effects of dietary vitamin D3 in patients with multiple sclerosis. Neurology Genetics. 2018;4(6):e278. doi: 10.1212/NXG.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dean D.D., et al. Vitamin D regulation of metalloproteinase activity in matrix vesicles. Connect. Tissue Res. 1996;35(1–4):331–336. doi: 10.3109/03008209609029208. [DOI] [PubMed] [Google Scholar]
- 46.Dean D.D., et al. Vitamin D metabolites regulate matrix vesicle metalloproteinase content in a cell maturation-dependent manner. Calcif. Tissue Int. 1996;59(2):109–116. doi: 10.1007/s002239900096. [DOI] [PubMed] [Google Scholar]
- 47.Kim S.H., et al. Vitamin D inhibits expression and activity of matrix metalloproteinase in human lung fibroblasts (HFL-1) cells. Tuberc. Respir. Dis. 2014;77(2):73–80. doi: 10.4046/trd.2014.77.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zawilla N.H., et al. Matrix metalloproteinase-3, vitamin D receptor gene polymorphisms, and occupational risk factors in lumbar disc degeneration. J. Occup. Rehabil. 2014;24(2):370–381. doi: 10.1007/s10926-013-9472-7. [DOI] [PubMed] [Google Scholar]
- 49.Moqbel S.A.A., et al. Rat chondrocyte inflammation and osteoarthritis are ameliorated by madecassoside. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/7540197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filip A., et al. A simple two dimensional culture method to study the hypertrophic differentiation of rat articular chondrocytes. Bio Med. Mater. Eng. 2015;25(1):87–102. doi: 10.3233/BME-141252. [DOI] [PubMed] [Google Scholar]
- 51.Sun F.F., et al. Tricetin protects rat chondrocytes against IL-1β-induced inflammation and apoptosis. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/4695381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fei J., et al. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019;109:1586–1592. doi: 10.1016/j.biopha.2018.09.161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.