Abstract
Infertility has been a major issue in our society for many years, and millions of couples all over the world are still experiencing it. There are several reasons for and causes of infertility in both men and women. Recent studies have shown that apoptosis, inflammation, and oxidative stress contribute immensely to infertility. The data regarding this report were obtained through a thorough review of scientific articles published in various databases, including Elsevier, Web of Science, PubMed, Scopus, and Google Scholar. Furthermore, PhD and MSc theses were also reviewed when compiling the data. Apoptosis, also known as "programmed cell death," is a natural and harmless process that occurs in human beings. Although it can become harmful if altered, Inflammation, on the other hand, is the body’s reaction to detrimental stimuli caused by toxic substances or compounds, while oxidative stress is a phenomenon that results in an imbalance between the generation and aggregation of reactive oxygen species (ROS) in the cells against antioxidants. These three factors interchangeably bring about several reproductive disorders in the body, resulting in infertility. This review aims at discussing how apoptosis, inflammation, and oxidative stress play a role in human infertility.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Keywords: Apoptosis, Infertility, Inflammation, Oxidative stress
Graphical Abstract
Highlights
-
•
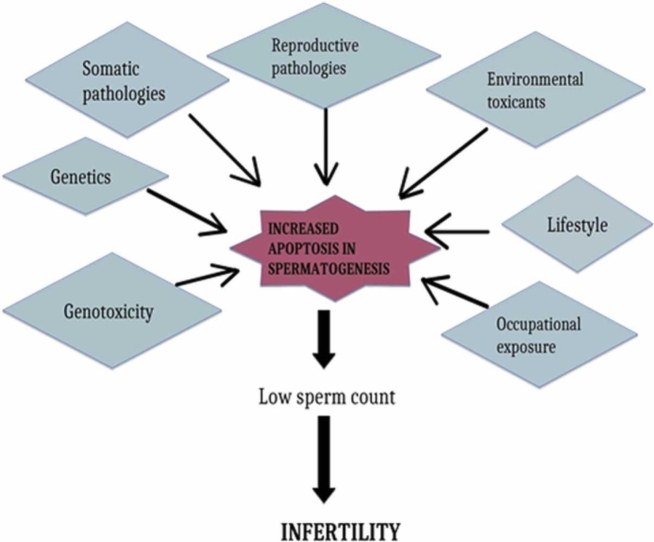
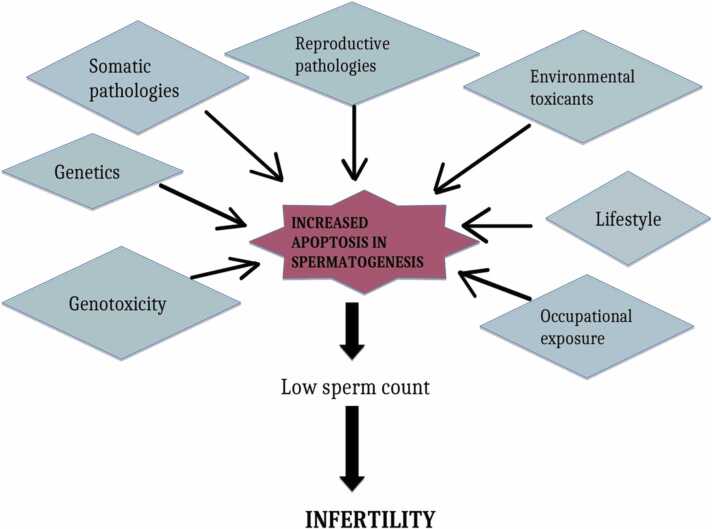
Increased apoptosis in both the sperm and ovaries gives off negative effects that contribute to the problem of infertility.
-
•
Inflammation performs physiological processes such as ovulation, as well as abnormal sperm production and function.
-
•
Overproduction of ROS results in an inflammatory injury in the reproductive tract.
1. Introduction
Infertility is a medical condition that means you cannot have a baby after 12 months of frequent, unprotected sexual activity [1]. This disorder affects both the male and female reproductive systems. It causes physical, mental, and emotional stress for both the individual and the couple [2], [3]. The rate of infertility globally suggests that about 48 million couples and 186 million people are affected by it [1]. Infertility can be categorized into primary and secondary infertility. The term "primary infertility" is used for a person who has never experienced pregnancy, while "secondary infertility" is used for someone who has previously experienced a successful pregnancy. In men, infertility is mostly the result of sperm ejaculation, oligozoospermia (low sperm count), weak sperm motility, and morphological differences in the sperm; in women, it could be the result of disorders or abnormalities in the ovaries, the uterus, and the fallopian tubes, hormonal imbalance, and endocrine factors [1]. Infertility has also been associated with factors such as medical, physical, emotional, and environmental factors [3].
Apoptosis, also known as "programmed cell death," is a process that takes place in living organisms as well as humans and involves events leading to morphological changes and the death of damaged cells [4]. Apoptosis can be activated by a minor injury to the cell by several factors that could be inside or outside the cell and that is disposed of in a controlled manner. The mechanism of apoptosis is usually characterized by cell shrinkage, nuclear DNA fragmentation, chromatin condensation, dynamic membrane blebbing, and loss of adhesion to extracellular matrices [5]. The effect on apoptosis in female infertility begins right from the ovaries, which in turn affects the female ovarian cycle [6]. In the ovary, early apoptosis begins in the nucleus of the granulosa cells, avoiding the mitochondria until the next stage of apoptosis. In pathological settings, uncontrolled and excessive apoptosis in the male germ cell can lead to alterations in sperm quality, which can lead to infertility. [7].
Inflammation is the body's response to harmful stimuli like toxic substances or compounds, pathogens, irritation, or cells that have been damaged [8]. Inflammation is a complicated defense mechanism that happens in response to harmful stimuli. It can cause abnormalities in the body's structure, which can lead to infertility and pelvic adhesions [9]. According to some research, inflammation is associated with reproductive tract disorders, which in turn affect fertility in both men and women. Oxidative stress is a condition that involves the imbalance between pro-oxidants such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) and antioxidants [10]. These pathological changes could lead to the development of the following: Parkinson’s disease, aging, cancer, heart failure, Alzheimer’s disease, depression, and atherosclerosis [11]; In men, this could lead to damage to spermatozoa, defects in the sperm, sperm DNA fragmentation, and possible cases of varicocele and testicular torsion, while in females, it could lead to polycystic ovary syndrome (PCOS), premature ovarian failure, and endometriosis [10], [11]. Apoptosis, inflammation, and oxidative stress are important factors in the pathophysiology of infertility in both males and females.
2. Methods
Using the keywords apoptosis, inflammation, oxidative stress, male infertility, female infertility, sperm, and ovary, a search was done in Google Scholar, Pubmed/Medline, and Scopus from the beginning of the databases to December 2022. The references of all papers were checked for cross-references that had not been found in the database search.
3. Infertility
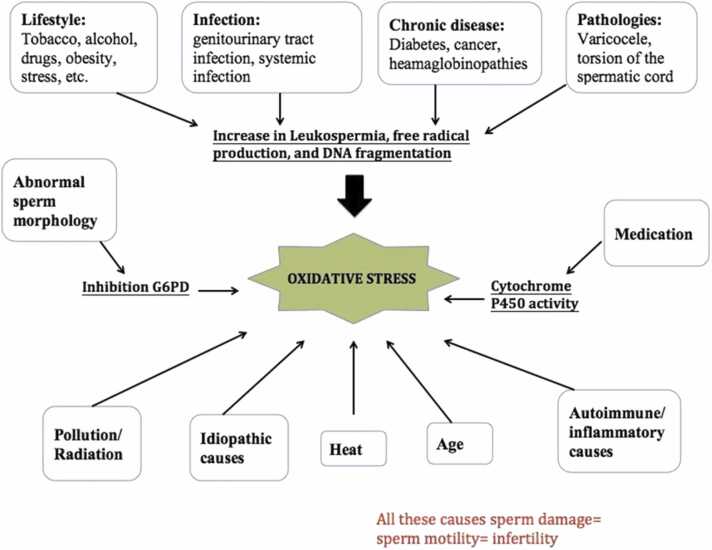
After a period of 12 months of unprotected sexual activity, infertility is defined as the inability to carry a pregnancy to term. It is estimated that around 7% of couples throughout the globe are unable to have a child, with men being responsible for 50% of these cases [12]. The etiology of male infertility is complicated and involves several variables, one of which is oxidative stress (OS). The presence of reactive oxygen species (ROS) has been shown to have a significant correlation with male infertility [13]. Free radicals such as superoxide anions (O2), hydrogen peroxide (H2O2), peroxyl (ROO), and hydroxyl (OH) are examples of extremely reactive free radicals that are included in ROS [14]. Capacitation of sperm, hyperactivation of sperm, acrosomal response, and fertilization of ovules by sperm all need low amounts of reactive oxygen species (ROS). However, OS is caused when there is an imbalance between the quantities of ROS and antioxidants in the seminal plasma. Damage to sperm caused by reactive oxygen species (ROS) is the primary reason for male infertility in 30–80% of cases, and these circumstances eventually induce DNA damage in the nucleus and mitochondria of cells [15]. Sperm cells are very vulnerable to the effects of oxidative damage. On the other hand, these cells contain cytoplasmic membranes that are high in unsaturated fatty acids; as a result, they are susceptible to lipid peroxidation when exposed to ROS because of the presence of these membranes [13]. In addition, since these cells suffer from a lack of critical cytoplasmic enzymes, they are unable to repair the damage that has been caused by oxidative stress. Moreover. Finally, sperm lose their ability to move and die as a result of the depletion of adenosine triphosphate (ATP), which is brought on by lipid peroxidation and is then followed by axonemal damage [16]. OS has an indirect effect on the axis of the hypothalamus and has the potential to impair the production of sex hormones. ROS lowers the levels of male sex hormones and restores their equilibrium, but it also has the potential to wreak havoc on the reproductive system [17].
Infertility in women can be caused by an increase in the level of ovarian steroidogenesis (OS) in the body, which can be caused by a wide variety of factors, including genetic, epigenetic, and lifestyle-related factors like excessive alcohol consumption, smoking, radiation, and obesity, as well as infections and varicoceles [18]. As a result, the function of the antioxidant system is very necessary to combat excessive ROS production. Plasma or natural sperm cells have a high concentration of antioxidant enzymes and compounds. Some examples of these are superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT) [19]. The genes NRF2, SOD, CAT, glutathione S-transferase (GST), and glutathione peroxidase (GPx) are responsible for encoding the majority of these enzymes. In males, infertility caused by OS is caused by genetic abnormalities in these genes, which, in turn, induce infertility [20]. As a consequence of these injuries, the rates of fertilization and implantation are decreased, fetal development is disrupted, and the probability of abortion is raised. Because redox homeostasis is maintained by a balance between the levels of antioxidants and ROS, the use of oral antioxidants in conjunction with herbal medicine may be beneficial in the treatment of infertility in males. These antioxidants get rid of any additional ROS and keep the redox equilibrium in check [21]. To overcome infertility, it is important to focus more attention on the processes that cause harm to sperm as well as the treatment approaches related to these mechanisms. Recent months have seen the publication of a few research reviews that describe the impact that OS has on the male reproductive system. For example, Bui et al. found that high levels of ROS were responsible for genomic and mitochondrial DNA damage, as well as a reduction in telomere length, Y chromosomal microdeletions, and epigenetic shifts [15]. Additionally, Agarwal et al. explored infection- and inflammation-induced OS as well as male infertility in their study [22]. The mechanisms of ROS-mediated alteration of male hormonal profiles were elucidated by Darbandi et al. in a different publication [17]. However, in this study, we used a more all-encompassing approach and explored the many effects of OS on male infertility, including its pathophysiology and genetic components, with a primary focus on the therapeutic use of antioxidants.
3.1. Toxicity effects of xenobiotics on infertility
Xenobiotics have the potential to build up in the body, which may have detrimental effects on a variety of organs and tissues [23]. Mammalian testicles are responsible for the production of spermatozoa as well as the discharge of testosterone into the bloodstream. Hematotesticular barriers are among the most densely packed in any of the mammalian species [24]. Several different heavy metals cause organ and system damage because of their acute and chronic toxicities. Intestinal and urinary tract dysfunction, neurological and endocrine system diseases [25], immune system dysfunction, birth deformities, and cancer are some of the health problems that can be caused by exposure to radiation. They are caused by the toxicity of heavy metals [26]. The cumulative effects of exposure to two or more metals may be harmful to the body [26]. Lead pollution is a consequence of industrialization. Children living in areas of Idaho that are close to lead plants have blood lead levels of 25 mg/dl and severe anemia. Therefore, lead poisoning over an extended period may interfere with reproduction [27].
According to the findings of Wang et al. [28], lead is responsible for the death of and toxicity to spermatogenic cells in the seminal tubules of the testicles. Both Mariola et al. [29] and Patrick [30] hold the view that an excessive amount of reactive oxygen species causes lead to poison the testicles. They came to this conclusion independently. During the process of testicular cell death, levels of reactive oxygen species and caspase-3 grow [31].
Products containing cadmium are quite common in industrialized countries, which makes the risks associated with them much greater. In high enough concentrations, cadmium may cause osteomalacia, hepatotoxicity, renal toxicity, neurotoxicity, and infertility. It can also cause cancer [32]. According to Zhu Q [33], when male animals, including humans, consume cadmium, it is claimed to cause damage to the testicles. This includes both humans and other male animals (2020). It causes significant structural damage to the seminal tubules of Sertoli cells as well as the hematotesticular barrier, which leads to the loss of sperm from the testicles as a final consequence. It prevents the production of Leydig cells, prevents these cells from performing their normal functions, and causes tumors to grow in these cells. In addition to this, it causes damage to the vascular system of the testicles.
Titanium dioxide nanoparticles have a detrimental effect on the morphological parameters of rat testicles, which affects important aspects of this structure, such as the proliferative activity of cells and their capacity to specialize into various parts of the male reproductive system. This study was conducted on rats [34].
According to a study that was carried out by Loebenstein et al. [35] exposure to xenobiotics is associated with abnormalities of the reproductive tract like cryptorchidism and hypospadias, as well as testicular cancer and hypo-fertility/infertility in men, which is known as testicular dysgenesis syndrome. Although its precise mechanism is not yet completely known, epigenetic regulation is assumed to be involved in testicular dysgenesis syndrome. This is even though there is no concrete evidence to support this hypothesis. This is related to the fact that male infertility in adulthood may be caused by toxicant exposure that occurred during the development of the fetus [36]. According to the findings of a study that was carried out by M.S. El-Neweshy [37], the manifestation of these changes was shown to be connected with a decline in testosterone levels. Heavy metal salts, as stated by Pandya C. et al. [38], have an effect on the spermatogenic and steroidogenic activities of the testicles, reduce male fertility and sperm quality, and promote degeneration of the testicles, which results in infertility. These negative effects of heavy metal salts on the testicles can be reversed by avoiding exposure to heavy metals. Intoxication with heavy metal salts is said to result in histological lesions of the testicles, interstitial edema, degeneration, and the development of intracellular giant cells, as stated by Manal M. Sayeda [39].
Compounds that are not naturally occurring may have harmful effects on the ovary, which can result in temporary or even permanent infertility. Because of the ovary's crucial function in the process of female reproduction, the presence of these substances is a cause for significant worry. The effect that temporary infertility has during a woman's prime reproductive years is a source of concern. It is estimated that 11% of women living in the United States have had problems with their fertility [40]. On the other hand, permanent infertility, often known as menopause in females, is a reason for concern because of the negative health implications that are known to be connected with ovarian failure [41], [42].
Oocytes, which are immature germ cells, are present in a limited number in the primordial follicles of female animals at the time of their birth. The primordial follicle consists of one oocyte that is encircled by a single layer of pregranulosa cells that are shaped like squamous cells. Because the oocyte is stopped in the prophase of the first meiotic division when germ cells are being formed in the fetal ovary, the amount of germ cells available throughout a person's lifespan is restricted. Primordial follicles transform primary follicles when they become activated, and then continue their development into secondary and antral follicles [42]; If an environmental factor damages all populations of follicles, this results in both disrupted cyclicity (temporary infertility) and early ovarian failure (permanent infertility). The antral preovulatory follicle is the most mature developmental stage. It can become ovulated and is the site of many environmental factors. The mechanisms of selective targeting may change based on the kind of follicle that is being targeted. Because of their potential to disrupt cell cycle control, cell signaling pathways, steroidogenesis, and genomic DNA, xenobiotics have the potential to cause harm to larger follicles. In addition, the destruction of primordial follicles might potentially come from a disturbance of the delicate balance that exists between the survival and activation of follicular cells [43].
3.2. Apoptosis
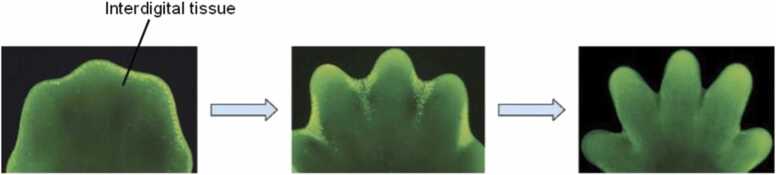
Apoptosis, also known as "programmed cell death," is a process that takes place in living organisms as well as humans and involves events leading to morphological changes and the death of damaged cells [4]. For example, apoptosis occurs in fetal development during the separation of the webbed fingers and toes of a fetus (Fig. 1). The cells between the fingers undergo apoptosis and disintegrate, causing the fingers and toes to properly separate. The mechanism of apoptosis is usually characterized by cell shrinkage, nuclear DNA fragmentation, chromatin condensation, dynamic membrane blebbing, and loss of adhesion to extracellular matrices [5]. Apoptosis activates cell death with high specificity and efficiency, resulting in the proper development and protection of organisms from developmental abnormalities and diseases [44]. However, excess apoptosis could lead to neurodegenerative diseases or cases of infertility, whereas insufficient apoptosis could cause uncontrolled proliferation of the cells, which could lead to cancer and autoimmune diseases. The biochemical alterations in apoptosis include the externalization of phosphatidylserine and the activation of cysteine aspartyl protease, also known as caspases, which lead to cell death [5]. The cascade of events that leads to apoptosis does not initiate inflammatory responses and is ignored by the tissues around it [44]. The process of apoptosis is regulated by several stimuli. Although not all cells die in response to the same stimuli [45], The stimuli could be drugs, toxins, stressors, heat, radiation, or reactive oxygen species (ROS). Two mechanisms lead to the initiation of apoptosis: the extrinsic pathway and the intrinsic pathway. These pathways are closely related, as some molecules from one pathway affect the other [46].
Fig. 1.
Showing the process of apoptosis in between the developing fingers in a foetus.
Modified from [47].
3.2.1. Apoptotic pathways
3.2.1.1. The extrinsic apoptotic pathway
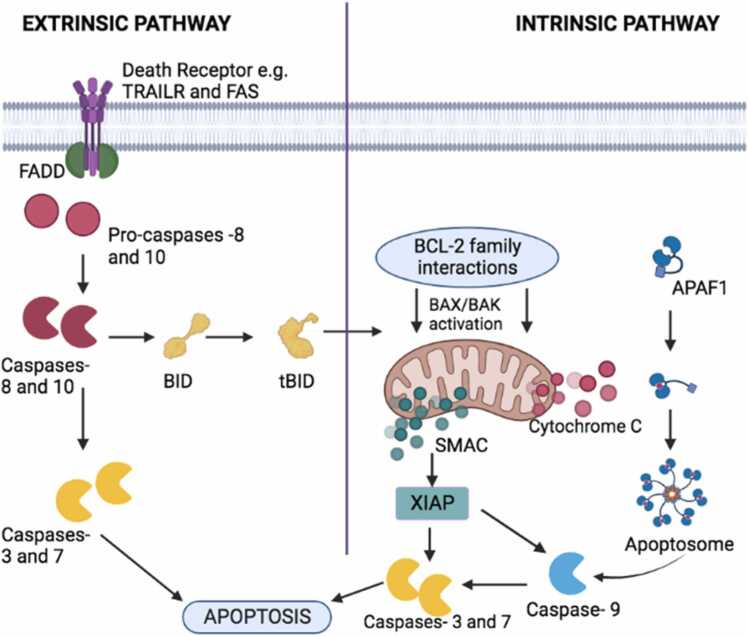
The death receptor pathways, which are part of the extrinsic apoptotic pathways (Fig. 2), are made up of transmembrane death receptors that depend on the interaction between the ligand and the receptor for cell death to happen [46]. Several receptors are part of the tumor necrosis factor (TNF) receptor genes involved in the extrinsic apoptotic pathways; however, FasL/Fas, TNF-α/TNF-1, and TRAIL receptors are the most studied [48]. When the ligands FasL and TRAIL bind to their respective receptors, it triggers their receptors to aggregate on the target cell, causing the recruitment of the adaptor proteins called Fas-associated death domain protein (FADD) on the cytoplasmic part of the receptors [48]. The FADD protein dimerizes with pro-caspase-8, leading to the formation of the Death-Inducing Signaling Complex (DISC), which activates pro-caspase-8, and this could either initiate apoptosis directly or activate the intrinsic apoptotic pathway to promote DNA fragmentation and cell death depending on the cell type [46].
Fig. 2.
Illustration of the extrinsic and intrinsic Pathways of apoptosis as modified from [49].
3.2.1.2. The intrinsic apoptotic pathway
The mitochondria control the activities inside the cell that are part of the intrinsic apoptosis pathway (Fig. 2). This pathway involves a cascade of events that initiates apoptosis. The proteins that are mainly involved in the control of the intrinsic pathway are the Bcl-2 family proteins, which have both pro-apoptotic and anti-apoptotic proteins [46]. The constituents of the pro-apoptotic Bcl-2 protein family are Bax, Bak, Bok, Bim, Bik, Bad, Noxa, and Puma, and the main anti-apoptotic members of the Bcl-2 protein family are Bcl-2 and Bcl-XL; others include A1, Bcl-w, and Mcl1 [48]. These anti-apoptotic members hinder apoptosis by stopping the release of cytochrome C from the mitochondria. When cytochrome is released from the mitochondria, caspase-9 is activated, and then it activates caspase-3 and caspase-7, which are responsible for the cell death process. An imbalance in the proportion of the pro-apoptotic and anti-apoptotic members can trigger different reactions, which could either lead to the inhibition of apoptosis or excessive apoptosis [48]. The pro-apoptotic members, Bax and Bak, are activated by members of their kind, such as Bid, which promotes the proteolytic activity that eventually leads to apoptosis, while other pro-apoptotic members such as Noxa and Puma, via the TP53 mediator, increase their response to chromosomal abnormalities. The absence of TP53 will not lead to apoptosis, which could cause several abnormalities in the male and female germ cells as well as some cancer diseases [49].
3.3. Apoptosis in the ovaries and female infertility
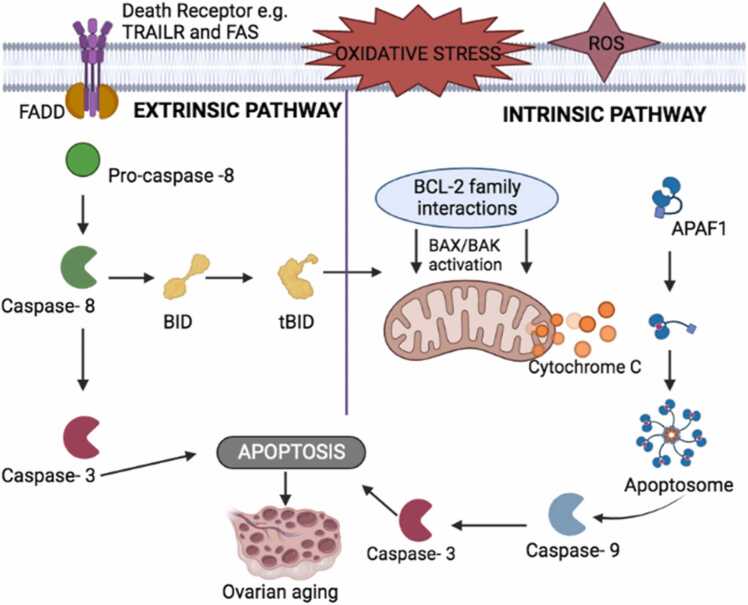
The effect of apoptosis in female infertility begins right in the ovaries, which in turn affects the female ovarian cycle. Early apoptosis in the ovary begins in the nucleus of the granulosa cells. Until the next phase of apoptosis [50], the mitochondria are not involved. Hormones like follicle-stimulating hormone (FSH), luteinizing hormone (LH), estrogen, progesterone, prolactin, laminin, leptin, glucocorticoids, and other growth factors like bone morphogenetic proteins (BMP), epidermal growth factors (EGF), and fibroblast growth factor (FGH) has anti-apoptotic factors that stop cells from dying at a certain time during folliculogenesis. Most of the time, apoptosis is stopped during the follicular phase [50] of the ovarian cycle. The follicular phase is the first phase in the ovarian cycle. It involves the growth of granulosa cells that surround the follicles that contain the developing ovum. The granulosa cells continuously proliferate, causing the size of the follicle to increase as a result of the continuous secretion of follicle-stimulating hormone (FSH). As this process goes on, a sheath known as the capillary follicle begins to form around the growing follicle. The theca follicle consists of two layers: the theca interna and theca externa. The theca interna is responsible for the secretion of estrogen. The theca externa consists of several vesicular follicles, one of which develops into a mature follicle known as the dominant follicle or the Graffian follicle, which later, in response to the secretion of the luteinizing hormone, ruptures and expels the ovum. The remaining vesicular follicles degenerate through the process of apoptosis [51]. The hormones produced by the theca and granulosa cells give off an anti-apoptotic effect at a particular point during the development of the follicles. Once the Graffian follicle has been selected, cholesterol gets converted to pregnenolone by cytochrome P450 in the mitochondria of the theca cells. The newly formed pregnenolone is released from the mitochondria and is converted to progesterone, which undergoes a series of reactions to form testosterone. The testosterone formed is deposited in the granulosa cells from the theca cells, where estrogen is produced. Estrogen prevents granulosa cell apoptosis by the actions of the anti-apoptotic signaling pathways [50]. If apoptosis does not occur or oxidative stress alters the apoptosis process (Fig. 3), there will be a continuous proliferation of the granulosa cells and vesicular follicles, leading to the development of several ovarian cystic disorders such as follicular cysts, corpus luteum cysts, polycystic syndrome, ovarian aging, or ovarian cancer, which are all associated with female infertility [52].
Fig. 3.
The altered apoptotic pathway in the female ovarian cycle as a result of oxidative stress.
Modified from [53].
3.4. Apoptosis in the sperm and male infertility
The process of apoptosis is important in the male germ cell because it keeps the ratio of germ cells to Sertoli cells stable and gets rid of damaged or abnormal germ cells as they develop [7]. Uncontrolled and too much apoptosis in the male germ cell could change the quality of the sperm, which could make it impossible to get pregnant (Fig. 4). Apoptosis usually occurs in the spermatocytes and not infrequently in the spermatogonia and spermatids. The research carried out by Martinic and colleagues shows that in infertile males, uncontrolled apoptosis occurs in the germ cells while the Sertoli cells do not go through apoptosis. Usually, Sertoli cells show FasL, which stimulates apoptosis in germ cells that are Fas-positive and exhibits paracrine interactivity between Sertoli cells and the male germ cell. Also, apoptosis increases as one gets older, leading to a reduction of the germ cells; it could also be associated with oxidative stress.
Fig. 4.
Factors that trigger an increase in male germ cell apoptosis.
Modified from [54].
4. Inflammation
Inflammation is the body's response to harmful stimuli like toxic substances or compounds, pathogens, irritation, or cells that have been damaged [8]. Inflammation is a complex defense mechanism that happens in response to harmful stimuli. It can cause abnormalities in the body's structure, which could be linked to infertility and pelvic adhesions [9]. Some research shows that inflammation is linked to problems with the reproductive tract, which in turn affect both men's and women's ability to have children. Some physiological processes, like ovulation, implantation, and pregnancy in women, and abnormal sperm production, function, and ejaculation [9], [55], are largely caused by inflammation. Inflammation mediators usually come from damaged tissue cells, the platelets, the leukocytes, the blood plasma, and the endothelial cells that line the blood vessels [56]. Infectious agent’s Infectious agents like bacteria, viruses, and protozoa can cause inflammatory effects on the reproductive tracts. Bacteria such as Chlamydia trachomatis, Neisseria gonorrhea, Traponema pallidum, Mycoplasma, and Ureaplasma play a role in inflammation. In comparison to less developed countries, Chlamydia trachomatis infection is the most common cause of infertility as a result of inflammation in developed countries [9].
4.1. Inflammation and male infertility
4.1.1. Disorders caused by inflammation to infertility
4.1.1.1. Varicocele
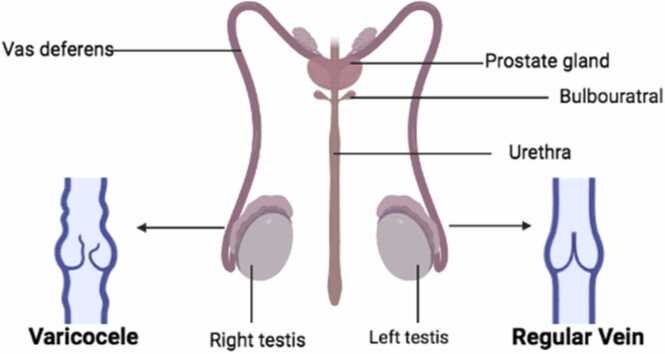
Varicocele is a condition that is characterized by an unusual enlargement of the vein in the scrotum that happens when the blood in the veins accumulates instead of flowing to all parts of the scrotum (Fig. 5) [57]. This condition is usually seen in cases of abnormally low sperm count and morphology as well as weak sperm motility. This is an endogenous cause of inflammation [58]. The restriction of blood flow in the testes causes an increase in the amounts of inflammatory cytokines and NO (nitric oxide) [58]. The functionality of the sperm cells in the testes is preserved by reasonable amounts of cytokines. Chemical mediators such as interleukin-1 (IL-1) also influence the function of the Leydig cells and Sertoli cells in the testes, while IL-37 and IL-18 are increased in the prostatic fluid of a patient diagnosed with varicocele, and this is also harmful to the function of the sperm cells in the testes. High amounts of endothelial NO synthase are used to improve the flow of blood into the tissues of the testicles. This makes up for the lack of oxygen in the accumulated blood pool in the veins, but if the required amount of NO is exceeded in this situation, it can become dangerous and react with the free radicals of superoxide, which could cause infertility [58].
Fig. 5.
Showing the difference between inflamed veins and normal veins in the testes as modified from [59].
4.1.1.2. Leukocytospermia
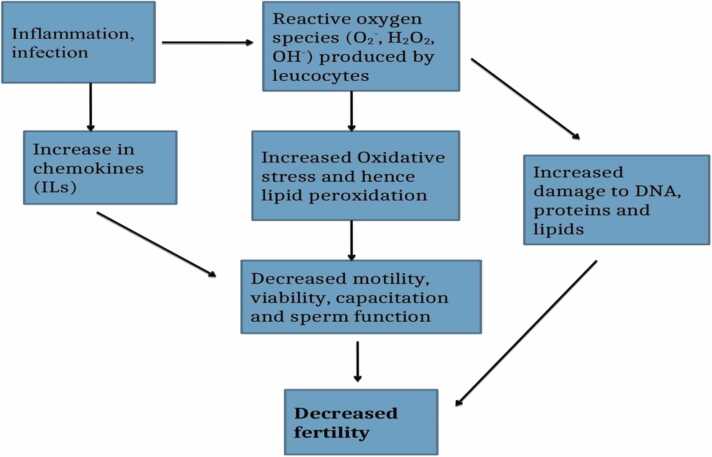
Leukocytospermia is also an endogenous cause of inflammation. Leukocytospermia, also known as pyospermia, can be defined as a condition in which there are over 1 million leukocytes found in 1 mL of sperm ejaculate and which occurs as a result of an infection in the urogenital system [60]. Leukocytospermia is a serious condition that can greatly affect the sperm's health and its genetic materials. In leukocytospermia, leukocytes generate high amounts of reactive oxygen species (ROS) that fight infections (Fig. 6). Its infectious agents increase the G6PDH process, which results in the production of reasonable amounts of NADPH [58]. NADPH oxidase from NADPH utilizes an electron to convert oxygen into toxic cells known as superoxide anion, which will give rise to a large amount of oxidative stress (OS) that will cause the chemotactic cytokines such as CXCL5, CXCL8, IL6, and IL8 to be activated. In pyospermia, the excess white blood cells begin to clump, resulting in a weakness in the motility of the sperm and making the process of impregnating a woman difficult and nearly impossible [60].
Fig. 6.
Illustrating the role of Leukocytospermia in male infertility as modified from [61].
4.1.1.3. Urethritis
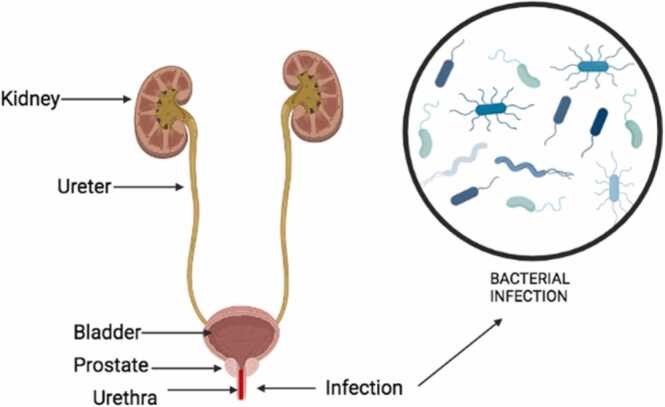
Urethritis is the inflammation of the urethra (Fig. 7). This inflammatory condition is usually linked with sexually transmitted infections, which are generally caused by infectious agents such as Chlamydia trachomatis, Neisseria gonorrhea, Mycoplasma genitalium, Trichomonas vaginalis, adenoviruses, and herpes simplex virus (62; 57). During the ejaculation of sperm cells across the urethra, the sperm cells usually get infected, and this infection may lead to infertility in men [57]. Also, the cicatrical tissue along the urethra limits the amount and quality of sperm cells to be deposited in the female reproductive parts.
Fig. 7.
Illustrating the infection and inflammation of the urethra as modified from [62].
4.2. Inflammation and female infertility
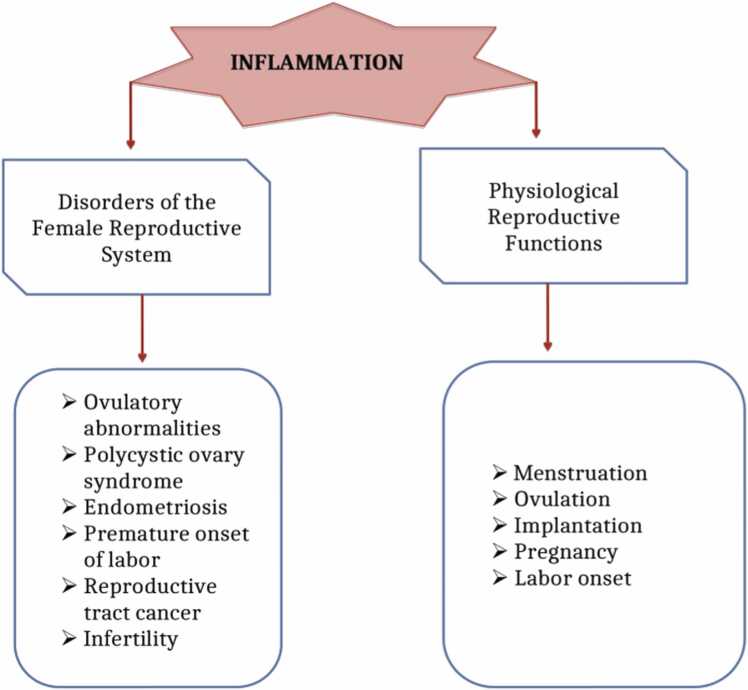
Inflammation in the female reproductive tract can lead to several reproductive disorders and physiological conditions that could all be associated with infertility (Fig. 8).
Fig. 8.
Showing the effects of inflammation in the female reproductive tract and physiology as modified from [9].
4.2.1. Disorders caused by inflammation in relation to female infertility
4.2.1.1. Ovulatory abnormalities
Premature ovarian failure and anovulation are a few of the ovulatory abnormalities that are related to female infertility because of their connection to inflammation [9]. Premature ovarian failure usually affects women who are 40 years of age and older. This causes a problem with the fertility rate of such women. Several other factors that are linked to premature ovarian failure are genetic abnormalities and chromosomal diseases, radiation, toxins, and ovarian surgeries. There are several factors responsible for the inflammation in the ovaries, and they include cytokines, leukotrienes, histamines, and prostaglandins. These inflammation mediators affect the function and quality of the ovaries [9].
4.2.1.2. Endometriosis
Endometriosis is a common condition that is associated with infertility and is strongly linked to inflammation [9], [63]. About 50% of infertile women suffer from endometriosis. It has been observed that the cytokines TNF-α, IL-1, IL-6, and IL-8 are all enhanced in women diagnosed with endometriosis. For instance, TNF-α takes part in the inflammatory process as its amount rises in the fluid in the peritoneal cavity. Also, pelvic inflammation occurs due to damage and adhesion as well as anatomical abnormalities in the female reproductive tract. Endometriosis creates an obstruction linked to endometriotic lesions that could lead to the inability of the embryo to be implanted and miscarriage.
4.2.1.3. Polycystic ovary syndrome (PCOS)
Polycystic ovary syndrome is a common endocrine disease that is linked to infertility as a result of anovulation [9]. During ovulation, deregulated inflammation could lead to PCOS. Some research has shown that the visceral adipose tissue in the uterine cavity contributes to inflammation in PCOS. Inflammation triggers the generation of larger amounts of androgen and hyperandrogenism, which can be linked to the low-chronic inflammation state. There are pro-inflammatory genotypes that encode cytokines such as IL-6 and TNF receptors that are also associated with PCOS and play a major role in inflammation.
5. Oxidative stress
Oxidative stress is the condition involving the imbalance between pro-oxidants such as reactive oxygen species (ROS) and antioxidants [10]. Oxidative stress occurs when a given cell, tissue, or fluid produces reactive oxygen species (ROS) that exceed the defensive capacity of the antioxidant system [11]. Usually, pro-oxidants and anti-oxidant mechanisms normally occur in a balanced state where biological oxidation reactions can cautiously take place, but if this balance is altered as a result of excess generation of ROS or a lack of antioxidant defense, it would lead to the oxidation of substrates in an uncontrolled manner and cause changes pathologically [11]; These pathological changes could lead to the development of the following: Parkinson’s disease, aging, cancer, heart failure, Alzheimer’s disease, depression, atherosclerosis, and vulnerability to both the male and female reproductive systems (Fig. 9). Cytotoxic substances and an exacerbated state of stress could be the causes of this type of oxidative vandalism. Oxidative stress as a result of the overproduction of ROS is a major cause of infertility in humans [10]. In men, this could lead to damage to spermatozoa, defects in the sperm, sperm DNA fragmentation, and possible cases of varicocele and testicular torsion, while in females, it could lead to polycystic ovary syndrome (PCOS), premature ovarian failure, and endometriosis [10], [11].
Fig. 9.
Showing the relationship between oxidative stress and infertility as modified from [10].
5.1. Oxidative stress in male
5.1.1. Precursor germ cells
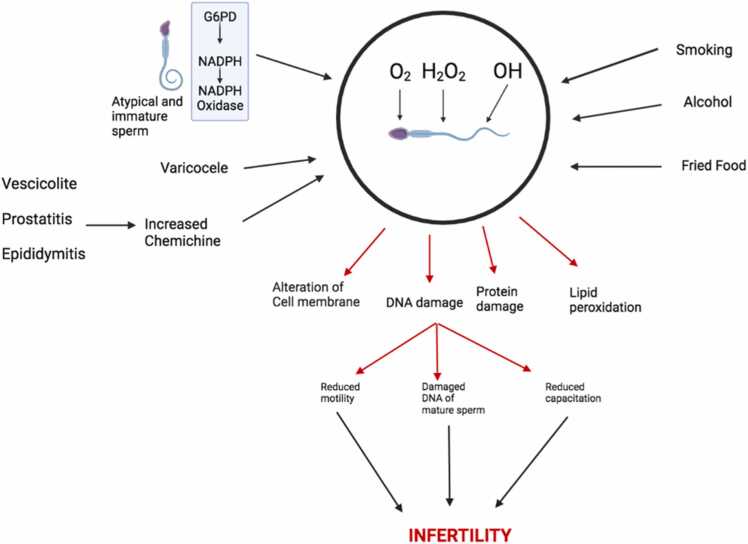
Because ionizing radiation can kill cells, male fertility was one of the first reproductive functions that were vulnerable to oxidative stress [11]. The effect of testicular heating on reproduction can also be seen when the precursor cells of germ cells are put under oxidative stress. The testes are located outside of the body because, for them to function, they need to be heated at a lower temperature than the normal body temperature. Heating the testes above their normal temperature could kill the male germ cells at the spermatocyte and spermatid stages of development. This would make men sterile. Also, the spermatids are specifically sensitive to lipid peroxidation and the cytotoxic effect of lipid aldehydes (Fig. 10).
Fig. 10.
The effects of oxidative stress on the sperm cell.
Modified from [64].
5.1.2. Spermatozoa
5.1.2.1. Damage or impairment of the spermatozoa
Damaged sperms are one of the major causes of infertility in men. The nucleohistone part of the sperm that consists of histone-bound DNA and the gene promoters for the development of the embryo is usually vulnerable to oxidative stress, which results in infertility [10] (Fig. 10). Also, the changes in the structure of the chromatin in the sperm as a result of mutations in the gene could also lead to infertility.
5.1.2.2. Fragmentation of sperm DNA
Some research shows that free radicals are what cause sperm DNA to break up [10]. The systems responsible for sperm DNA fragmentation include DNA crosslinking and fragmentation, as well as the initiation of pyrimidine, purine, and deoxyribose. Once DNA fragmentation occurs in the sperm, the sperm is no longer functional, which results in infertility.
5.1.2.3. Sperm damage or dysfunction
Sperm dysfunction could be the result of several reasons related to oxidative stress, such as damage to the integrity of the sperm DNA as a result of damage to the sperm cell membrane, leading to an adverse effect on the permeability and fluidity of the sperm cell, which results in infertility [10].
5.2. Oxidative stress in female
The female germ cells are more sensitive to oxidative stress compared to the male germ cells [11]. Oxidative stress is a major cause of Polycystic Ovary Syndrome (PCOS), endometriosis, and some other cases of infertility [10].
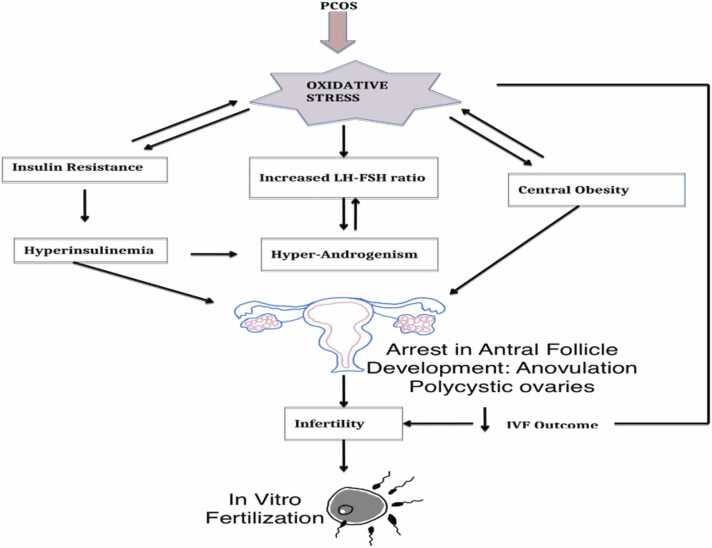
5.2.1. Oxidative stress and PCOS
PCOS refers to multiple cysts in the ovaries that occur as a result of oxidative stress [10]. Patients with PCOS undoubtedly have reactive oxygen species (ROS). Protein carbonyl (PC), which is a molecular marker of oxidative stress, and superoxide dismutase (SOD) are found to be more present in women with PCOS. The content of protein carbonyl in patients with PCOS possesses a positive correlation with fasting insulin and the connection between insulin resistance and protein oxidation. The higher levels of pro-inflammatory markers of oxidative stress, which are IL-6 and TNF-α are found in patients diagnosed with PCOS but are increased in PCOS-IR patients [10] (Fig. 11).
Fig. 11.
The relationship between oxidative stress and PCOS as modified from [65].
5.2.2. Endometriosis
Oxidative stress is thought to destroy the peritoneal cavity by making it easier for ectopic endometrium cells to stick together [66]. As we've already talked about, endometriosis is an inflammatory disease that causes endometrial tissue to grow on the outside of the uterus. No one knows for sure what causes endometriosis, but it is known that oxidative stress leads to general inflammation, which in turn leads to endometriosis. In women with endometriosis, the granulosa cells have oxidatively damaged DNA and display more occurrences of apoptotic bodies. The ovarian cortex is also affected by endometriosis, which is also a result of oxidative stress. The ovarian cortex in women diagnosed with endometriosis exhibits high amounts of 8-hydroxy-2-deoxyguanosine, which is a marker for calculating the result of oxidative stress on DNA fragmentation [67], [68].
5.3. The role of oxidative stress in apoptosis
Oxidative stress is the most common cause of infertility [7]. Oxidative stress takes place due to a disproportion in the generation of reactive oxygen species (ROS). When the mitochondrion of the sperm cell and ovaries is exposed to ROS, it triggers the apoptotic process, leading to disintegration. As one gets older, the production of ROS increases, and if the antioxidant capacity is low, it also triggers apoptosis and leads to oxidative damage. Research has highlighted the fact that infertile men have higher amounts of cytochrome C in the mitochondrion of their sperm cells.
6. Antioxidants in the treatment of male infertility
The antioxidant system plays an important role in maintaining reproductive and other biological tissues below a crucial threshold of ROS, hence minimizing deleterious effects on reproduction [69]. This is accomplished by keeping ROS levels below the threshold at which ROS becomes harmful. Antioxidant-containing herbal remedies were able to lower ROS levels, shielding germ cells from the effects of OS-mediated apoptosis [70]. They have the potential to be used in complementary and alternative medicine fields to facilitate pregnancy [71]. It has been shown that many plant-derived pure molecules and herbal extracts provide some degree of protection against a variety of illnesses [72], including those that are associated with the reproductive system [73]. Recent research has revealed that giving male plant extracts improves sperm quality, as well as sperm parameters, androgen status, and fertility index (Table 1) [74], [75]. These findings demonstrate that plant extracts have a favorable impact on sperm quality. In female patients, herbal medication improves ovarian dysfunction and ovarian follicle [76] and increases endometrial receptivity [77], [78]. It also affects the underlying molecular process and inhibits estrogen-dependent endometrial hyperplasia. Additionally, an herbal treatment that has activities on the hypothalamic–pituitary–gonadal axis may alter reproductive physiology and alleviate certain types of infertility disorders [79]. The gonadotrophic-like effects of the extracts were characterized by the following biological parameters: an increase in the weight of the ovary and the uterus; the induction of ovulation; an increase in the levels of estradiol, progesterone, and protein; a decrease in the level of cholesterol; and so forth [80]. Many other groups of phytocompounds, most notably flavonoids, and phenolic compounds have been suggested to be responsible for the antimutagenic or protective actions [81]. Natural antioxidants that can scavenge free radicals have garnered a lot of interest as possible treatments for oxidative stress and aberrant hormone functioning [82]. Antioxidants can directly scavenge reactive oxygen species (ROS), therefore rendering them inactive and repairing the damage. In addition to this, they demonstrated a wide range of biological activities as a consequence of their capacity to imitate the effects of endogenous estrogen, block the actions of hormones, and modify the synthesis of hormones [82]. It has been shown time and time again, using a wide variety of in vitro and in vivo systems, that phenolic compounds, flavonoids, and foods rich in these compounds have powerful antioxidant activity. In the current research, our primary objective was to evaluate the impacts and underlying processes of several different plant extracts and natural products on the reproductive system. Around the globe, including in Korea, people have been using a broad variety of herbs for the treatment of infertility for thousands of years [83]. In addition, a wide variety of natural compounds, including extracts from plants, have been shown to have the capacity to operate as agents that may reverse the reproductive activity of both men and females (Table 2). The origin of natural goods may be traced back to plants, animals, or fungi, and their final forms can take the shape of compounds, extracts, or a variety of other recipes. The reproductive system has been shown to have undergone structural and functional changes, and studies have identified the particular processes responsible for the observed consequences.
Table 1.
Plant derived natural products and male infertility (in vitro studies).
| Classification | Compound/Extract | System | Source | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Plant | Date palm pollen extract |
In vitro | Phoenix dactylifera Linn. | Sertoli cells, spermatogonia stem cells from mice | 0.06, 0.25, 0.62 mg/mL; 14 days | Increase in proliferation of spermatogonia | [84] | |
| Plant | 5 H-purin-6-amine, Sedum sarmentosum extract |
In vitro | Sedum sarmentosum | Spermatogonial stem cells C57BL. 6-TG-EGFP |
0.01, 0.1, 1, 10 mg/mL; 1 week | Increase of self-renewal in SSCs |
↑ PLZF, GFRα1, VASA, Lhx1 ↓ Pgk2 |
[85] |
| Plant | Licorice extract | In vitro | Glycyrrhiza uralensis Fisch. | Testis tissue from C57BL/6 N mice | 0.2, 2, 20 µmol/L; 72 h | Increase in proliferation of spermatogonia | ↑ PCNA, SCP3, Spo11 | [86] |
| Plant | Lycium barbarum polysaccharide | In vitro | Lycium barbarum Linn. | Leydig MLTC-1 | 50 µg/mL; 48 h | Increase in cell viability |
↑ Testosterone, ↓ p-PERK/PERK, p-elF2α/elF2α, ATF4/β-actin, apoptosis rate, LC3II/I, Atg5/β-actin |
[87] |
| Plant | Morindae radix aqueous extract | In vitro | Morinda officinalis | TM3 cells, mouse Leydig cells | 10, 50, 100, 250 mg/mL; 24 h | Increase in testosterone production. Decrease of H2O2 induced cytotoxicity, and lipid peroxidation |
↑ SOD, CAT↓ MDA | [88] |
| Plant | Taraxacum officinale aqueous extract | In vitro | Taraxacum officinale | TM3, ATCCNoCRL | 1, 10, 25, 50 mg/mL; 12, 48 h | Increase in the levels of steroidogenic enzymes | ↑ STAR, CYP11A1, CYP17A1 | [89] |
| Plant | Typha capensis rhizome extract F1 fraction | In vitro | Typha capensis (Rohrb.) N.E.Br. | TM3-Leydig cells | 10, 100 µg/mL; 96 h | ↑ Testosterone | [90] |
PCNA, proliferating cell nuclear antigen; PERK, protein kinase-like endoplasmic reticulum kinase; p-PERK, phospho-PERK; elF2α, eukaryotic initiation factor 2; p-elF2α, phospho-elF2α; ATF4, activating transcription factor 4; SCP3, synaptonemal complex protein 3; SOD, superoxide dismutase; CAT, catalase; MDA, malondialdehyde; StAR, steroidogenic acute regulatory protein; CYP17A1, cytochrome P450 17A1; CYP11A1, cytochrome P450 11A1.
Table 2.
Plant derived natural products and male infertility (in vivo studies).
| Classification | Compound/Extract | System | Source | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Plant | Acacia hydaspica ethyl acetate extract | In vivo | Acacia hydaspica R. Parker | SD rats | 400 mg/kg; 21 days | Increase of seminiferous tubule diameter, area, epithelial height Decrease of width of tubular lumen, interstitial space, DNA damage |
↑ Testosterone, LH, FSH, SOD, POD, CAT, QR, GSH, GR, GST, GPx, γ-GT↓ H2O2, NO, MDA | [91] |
| Plant |
Achillea millefolium inflorescences ethanol extract |
In vivo | Achillea millefolium Linn. | Wistar mice | 120 mg/kg; 48 days | Increase of sperm motility, capsule thickness, epithelial thickness, tubule differentiation index |
↑ SOD, LH ↓ LDH, NO, MDA |
[92] |
| Plant | Aged garlic extract | In vivo |
Allium sativum for. pekinense MAKINO |
Wistar mice | 250 mg/kg; 14 days | Increase of testis weight, sperm count, motility, recovery of seminiferous tubules Decrease of the death of sperm, sperm abnormality |
↑ Testosterone, GSH, GSH-Px, CAT, SOD,↓ MDA | [93] |
| Plant | Angelica keiskei powder | In vivo | Angelica keiskei Koidz. | Self-breeding CD-1 mice | 57.5 mg/kg; 7 days | Increase of density of sperm, motility, motile sperm density, progressive sperm velocity, progressive sperm density Decrease of abnormal seminiferous tubules, DNA fragmentation |
↑ GSS, HO-1, Hspa11, Hspa2 Hsf1, Hsf2 | [94] |
| Plant | Banaba leaf and ginseng extract | In vivo | Lagerstroemia speciosa (L.) Pers. | Swiss mice | 150 mg/kg; 30 days | Increase of testis weight, epididymis weight, seminal vesicle weight, sperm density, sperm viability, progressive sperm motility Decrease of nonprogressive sperm motility, abnormal sperm morphology (head, tail, twisted body) |
↑ Testis glycogen, testis protein, testis fructose, seminal vesicle fructose, Epididymal fructose, testis protein, seminal vesicle protein, epididymal protein ↓ Testis cholesterol |
[82] |
| Plant | Bajijiasu | In vivo | Morinda officinalis F.C. | Normal Kunming mice kidney-yang-deficient Kunming mouse |
20, 40, 80,160,320 mg/kg; 30 days 80, 160, 320 mg/kg; 18 days |
Increase of the sexual behavior Decrease of DNA damage of sperm by H2O2 |
↑ Testosterone, ↓ Cortisol, SOD, GPx, CAT, MAD |
[95] |
| Plant | Balanites aegyptiaca sapogenin extract | In vivo | Balanites aegyptiaca | Albino rats | 25, 50, 100 mg/kg; 70 days | Increase of semen quality |
↑ LH, Estradiol, Testosterone, Glucose↓ FSH, Cholesterol, sAST, urea, creatinine |
[96] |
SOD, superoxide dismutase; GSH, glutathione; MDA, malondialdehyde; NO, nitric oxide, GSS, glutathione synthase, GST, glutathione-S-transferase; GPx, glutathione peroxidase; GSH-Px, glutathione peroxidase; Hsp70, heat shock protein 70, CAT, catalase; H2O2, hydrogen peroxide; POD, peroxidase; QR, quinone reductase; GR, glutathione reductase; γ-GT, γ-glutamyl transpeptidase; LH, luteinizing hormone; FSH, follicle-stimulating hormone; HO-1, heme oxygenase-1; LDH, lactate dehydrogenase
6.1. Antioxidants in female infertility management
Since the beginning of time, people have been using plants as a source of medicine to prevent, protect against, and treat a wide variety of health conditions. This practice dates back to ancient times. Herbal remedies and other natural items, including the many chemical derivatives of these natural products, account for nearly half of all the medicine that is being utilized around the globe right now [97]. In addition, traditional herbal treatments play a significant part in the healthcare systems of underdeveloped nations as well as wealthy ones [98], [99]. Medicinal herbs are used for the treatment of female infertility as an alternative therapy option to avoid the high costs and harmful health consequences connected with the many infertility treatment procedures that are accessible in the form of assisted reproductive technology (ART). Kaadaaga and his colleagues [100] have only recently investigated the prevalence of the use of herbal medicine by infertile women as well as the parameters linked with its use. Researchers have carried out numerous ethnopharmacological surveys in various regions of the world to discover the various medicinal plants that are used for the treatment of infertility or pathologies in females that cause infertility [101], [102]. Some of these medicinal plants are highlighted below (Table 3).
Table 3.
Medicinal plant derived natural products and female infertility.
| Plant species | Part used | Study candidate/ animal | Treatment | Improved reproductive functions | References |
|---|---|---|---|---|---|
| Trigonella foenum-graecum | Seed | PCOS women | 2 capsules of 500 mg each/day for 90 days | A reduction in both the volume of the ovary and the number of ovarian cysts The restoration of normal menstrual periods Observable rises in both LH and FSH levels |
[103] |
| Healthy women | 600 mg/day seed extract for 2 menstrual cycles | Increases of both free testosterone and estradiol to a significant degree Enhancement of sexual desire as well as sexual arousal |
[104] | ||
| Justicia insularis | Leaf | Immature rat | 12.5, 50, and 100 mg/kg bw extract daily for 20 days | An earlier opening of the uterus. The quantity of hemorrhagic spots, the corpus luteum, the implantation sites, the weight of the ovary, and the uterine and ovarian proteins all increased (induces folliculogenesis) Decrease in the amount of cholesterol found in the ovary |
[105] |
| Acmella oleracea | Flowers | Rat | 88.91 mg/kg and 444.57 mg/kg daily for 21 days | Increased incidence of both the proestrous and the estrous phases Reduced incidence of the metaestrous and diestrus phases of the menstrual cycle |
[106] |
| Cimicifuga racemosa | Rhizome | Infertile women | 120 mg/day dry extract | increased levels of oestrogen and luteinizing hormone Increases in blood progesterone, endometrial thickness, and the percentage of clinical pregnancies |
[107], [108] |
| PCOS women | 20 mg dry extract daily for 10 days | A higher progesterone level, which indicates ovulation will be more successful Increased thickness of the endometrial layer Increased number of pregnancies |
[109] | ||
| Zingiber officinale | Rhizome | Rat | 100 mg ginger powder daily for 5 and 10 days | An increasing amount of antral follicles as well as ovarian stromal VEGF; an elevated number of endometrial VEGEF as well as ovarian stromal eNOS | [110] |
| 100 mg/kg/day of 6-gingerol-rich fraction for 35 days |
Guarded from an increase in OS that was caused by chlorpyrifos (H2O2 and NO) GSH levels in ovarian and uterine tissue were shown to have increased, along with the activity of the antioxidant enzymes CAT, SOD, GPx, and GST. |
[111] | |||
| Nigella sativa | Seed | Mice | 0.2, 0.5, and 1.0 mL/100 g N. sativa oil for 5 days | Cyclophosphamide treatment led to an elevation in the amount of normal, primary, and secondary follicles as well as the diameter of the ovary in rats. | [112] |
| Rat | 0.5 and 1.0 g/kg daily from day 3 to day 15 of lactation | Lactation results in enhanced milk yield as well as enhanced weight in the puppies. | [113] | ||
| PCOS women | 2 g/day powdered seed as capsules for 16 weeks |
A rise in both the average length of the menstrual cycle and the number of cycles that occur each month was observed. Reduced time between periods of menstruation |
[114] | ||
| Hypericum perforatum | Flowering tops | Women with premenstrual syndrome |
900 mg/day for 2 menstrual cycles |
a reduction in the severity of the premenstrual syndrome's behavior and physical characteristics | [115] |
| Crocus sativus | Stigma | Women with premenstrual syndrome |
30 mg/day for 2 menstrual cycles |
Efficient in the treatment of the discomforts associated with premenstrual syndrome | [116] |
| Cinnamomum zeylanicum | Bark | PCOS women | 3 cinnamon capsules (Each containing 500 mg cinnamon) for 8 weeks | an elevation in the capacity of total antioxidants (TAC) Reduced levels of MDA in the serum |
[117] |
| 1500 mg/day for 6 months | Improved menstrual cyclicity | [118] | |||
| Urtica dioica | Leaves and root | Women with hyperandrogenism |
300–600 mg dried extract daily for 4 months | Significant drop in complete testosterone level, free testosterone level, and DHEA level | [119] |
| Linum usitatissimum | Seed | PCOS women | 15 g flaxseed powder for 3 months | a decrease in ovarian volume as well as the number of follicles Enhancement of the menstrual cycle and the likelihood of pregnancy |
[120] |
7. Conclusion
Apoptosis, which is a process that takes place in living organisms as well as humans, involves events leading to morphological changes and the death of damaged cells. From the discussions in the previous sections, it has been shown that increased apoptosis in both the sperm and ovaries gives off negative effects that contribute to the problem of infertility in both men and women. To increase the rate of fertility, it is important to control the process of apoptosis. Furthermore, oxidative stress via ROS generation plays an important role in apoptosis by producing various reproductive abnormalities that lead to infertility. Inflammation, which plays a major role in some physiological processes such as ovulation, implantation, and pregnancy in women, as well as abnormal sperm production and function, and ejaculation of sperm, is the main contributor to inflammation. The mediators of inflammation, which are cytokines, chemokines, and growth factors, are the main contributors to inflammation. Reproductive tract diseases can be the result of excessive activation of inflammatory pathways. The overproduction of ROS is a result of an inflammatory injury in the reproductive tract. Infectious agents, which settle in the reproductive tract, also trigger the excessive generation of ROS and free radicals, which are associated with inflammation reactions. Xenobiotics are hazardous substances that people are exposed to in their environment and thus run the risk of developing infertility. They also contribute to the growing problem of infertility in both sexes. Additionally, antioxidants, when present in persons, they help to mop up the excess ROS generated by xenobiotic exposure. To maintain cell activity, antioxidants can regulate the excess generation of ROS.
Ethics approval and Consent to participate
Not applicable.
CRediT authorship contribution statement
All authors contributed to the study's conception and design. All authors read and approved the final manuscript.
Consent for publication
not applicable.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the Bowen University, Iwo for partly supporting this work.
Data availability
Data will be made available on request.
References
- 1.World Health Organization, 2020, September 14, Infertility. Www.who.int.〈https://www.who.int/news-room/fact-sheets/detail/infertility〉.
- 2.Walker, M.H., & Tobler, K.J., 2020, Female Infertility. PubMed; StatPearls Publishing. 〈https://www.ncbi.nlm.nih.gov/books/NBK556033/〉. [PubMed]
- 3.Alahmar A.T. Role of oxidative stress in male infertility: an updated review. J. Hum. Reprod. Sci. 2019;12(1):4–18. doi: 10.4103/jhrs.JHRS_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlesinger, P.H., 2022, September 29, apoptosis. Encyclopedia Britannica. 〈https://www.britannica.com/science/apoptosis〉.
- 5.Jan R., Chaudhry G.E. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019;9(2):205–218. doi: 10.15171/apb.2019.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regan S., Knight P.G., Yovich J.L., Leung Y., Arfuso F., Dharmarajan A. Granulosa cell apoptosis in the ovarian follicle-a changing view. Front. Endocrinol. 2018;9:61. doi: 10.3389/fendo.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asadi A., Ghahremani R., Abdolmaleki A., Rajaei F. Role of sperm apoptosis and oxidative stress in male infertility: A narrative review. Int. J. Reprod. Biomed. 2021;19(6):493–504. doi: 10.18502/ijrm.v19i6.9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204–7218. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaisi-Raygani A., Asgari R. Association of inflammation with female reproductive system disorders. Cent. Asian J. Med. Pharm. Sci. Innov. 2021;1(2):67–73. doi: 10.22034/CAJMPSI.2021.02.03. [DOI] [Google Scholar]
- 10.Banerjee P., Bhattacharya J. Impact of Oxidative stress on Infertility, with emphasis on infertility management strategies. Glob. J. Fertil. Res. 2019;4(1):010–018. doi: 10.17352/gjfr.000012. [DOI] [Google Scholar]
- 11.Aitken R.J. Impact of oxidative stress on male and female germ cells: implications for fertility. Reprod. (Camb., Engl. ) 2020;159(4):R189–R201. doi: 10.1530/REP-19-0452. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A., Mulgund A., Hamada A., Chyatte M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015;13(1):37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal A., Prabakaran S., Allamaneni S.S. Relationship between oxidative stress, varicocele and infertility: a metaanalysis. Reprod. Biomed. Online. 2006;12(5):630–633. doi: 10.1016/s1472-6483(10)61190-x. [DOI] [PubMed] [Google Scholar]
- 14.Wagner H., Cheng J.W., Ko E.Y. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J. Urol. 2018;16(1):35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bui A., Sharma R., Henkel R., Agarwal A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia. 2018;50(8) doi: 10.1111/and.13012. [DOI] [PubMed] [Google Scholar]
- 16.Gogol P., Szczęśniak-Fabiańczyk B., Wierzchoś-Hilczer A. The photon emission, ATP level and motility of boar spermatozoa during liquid storage. Reprod. Biol. 2009;9(1):39–49. doi: 10.1016/s1642-431x(12)60093-x. [DOI] [PubMed] [Google Scholar]
- 17.Darbandi M., Darbandi S., Agarwal A., Sengupta P., Durairajanayagam D., Henkel R., Sadeghi M.R. Reactive oxygen species and male reproductive hormones. Reprod. Biol. Endocrinol. 2018;16(1):87. doi: 10.1186/s12958-018-0406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum. Reprod. Update. 2008;14(3):243–258. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura B.N., Lawson G., Chan J.Y., Banuelos J., Cortés M.M., Hoang Y.D., Ortiz L., Rau B.A., Luderer U. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic. Biol. Med. 2010;49(9):1368–1379. doi: 10.1016/j.freeradbiomed.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meseguer M., Antonio Martinez-Conejero J., Muriel L., Pellicer A., Remohí J., Garrido N. The human sperm glutathione system: a key role in male fertility and successful cryopreservation. Drug Metab. Lett. 2007;1(2):121–126. doi: 10.2174/187231207780363633. [DOI] [PubMed] [Google Scholar]
- 21.Alahmar A.T. The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2018;45(2):57–66. doi: 10.5653/cerm.2018.45.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agarwal A., Rana M., Qiu E., AlBunni H., Bui A.D., Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018;50(11) doi: 10.1111/and.13126. [DOI] [PubMed] [Google Scholar]
- 23.Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M.R., Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021:227. doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng C.Y., Mruk D.D. The blood-testis barrier and its implications for male contraception. Pharmacol. Rev. 2012;64(1):16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes Azevedo B., Barros Furieri L., Peçanha F.M., Wiggers G.A., Frizera Vassallo P., Ronacher Simões M., Valentim Vassallo D. Toxic effects of mercury on the cardiovascular and central nervous systems. J. Biomed. Biotechnol. 2012;2012 doi: 10.1155/2012/949048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gazwi H.S., Yassien E.E., Hassan H.M. Mitigation of lead neurotoxicity by the ethanolic extract of Laurus leaf in rats. Ecotoxicol. Environ. Saf. 2020;192 doi: 10.1016/j.ecoenv.2020.110297. [DOI] [PubMed] [Google Scholar]
- 27.Giuliani R., Bettoni F., Leali D., Morandini F., Apostoli P., Grigolato P., Aleo M.F. Focal adhesion molecules as potential target of lead toxicity in NRK-52E cell line. FEBS Lett. 2005;579(27):6251–6258. doi: 10.1016/j.febslet.2005.09.097. [DOI] [PubMed] [Google Scholar]
- 28.Wang C., Zhang Y., Liang J., Shan G., Wang Y., Shi Q. Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin. Chim. Acta. 2006;370(1–2):82–88. doi: 10.1016/j.cca.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Marchlewicz M., Michalska T., Wiszniewska B. Detection of lead-induced oxidative stress in the rat epididymis by chemiluminescence. Chemosphere. 2004;57(10):1553–1562. doi: 10.1016/j.chemosphere.2004.08.102. [DOI] [PubMed] [Google Scholar]
- 30.Patrick L. Lead toxicity part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Altern. Med. Rev. 2006;11(2) [PubMed] [Google Scholar]
- 31.Wang X., Sharma R.K., Sikka S.C., Thomas A.J., Jr, Falcone T., Agarwal A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil. Steril. 2003;80(3):531–535. doi: 10.1016/s0015-0282(03)00756-8. [DOI] [PubMed] [Google Scholar]
- 32.Benoff S., Jacob A., Hurley I.R. Male infertility and environmental exposure to lead and cadmium. Hum. Reprod. Update. 2000;6(2):107–121. doi: 10.1093/humupd/6.2.107. [DOI] [PubMed] [Google Scholar]
- 33.Zhu Q., Li X., Ge R.S. Toxicological effects of cadmium on mammalian testis. Front. Genet. 2020;11:527. doi: 10.3389/fgene.2020.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan A.M., Ibrahim M.A., Noshy P.A. Reproductive toxicity provoked by titanium dioxide nanoparticles and the ameliorative role of Tiron in adult male rats. Biochem. Biophys. Res. Commun. 2017;486(2):595–600. doi: 10.1016/j.bbrc.2017.03.098. [DOI] [PubMed] [Google Scholar]
- 35.Loebenstein M., Thorup J., Cortes D., Clasen-Linde E., Hutson J.M., Li R. Cryptorchidism, gonocyte development, and the risks of germ cell malignancy and infertility: a systematic review. J. Pediatr. Surg. 2020;55(7):1201–1210. doi: 10.1016/j.jpedsurg.2019.06.023. [DOI] [PubMed] [Google Scholar]
- 36.Skinner A., Khan S.J., Smith-Bolton R.K. Trithorax regulates systemic signaling during Drosophila imaginal disc regeneration. Development. 2015;142(20):3500–3511. doi: 10.1242/dev.122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Neweshy M.S., El-Shemey T.M., Youssef S.A. Pathologic and immunohistochemical findings of natural lumpy skin disease in Egyptian cattle. Pak. Vet. J. 2013;33(1):60–64. [Google Scholar]
- 38.Pandya C., Pillai P., Nampoothiri L.P., Bhatt N., Gupta S., Gupta S. Effect of lead and cadmium co‐exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia. 2012;44:813–822. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 39.El-Sadaawy M.M., El-Said G.F. Assessment of fluoride in three selected polluted environments along the Egyptian Mediterranean Sea: Effects on local populations. Hum. Ecol. Risk Assess.: Int. J. 2014;20(6):1643–1658. [Google Scholar]
- 40.Cedars M.I., Taymans S.E., DePaolo L.V., Warner L., Moss S.B., Eisenberg M.L. The sixth vital sign: what reproduction tells us about overall health. Proceedings from a NICHD/CDC workshop. Hum. Reprod. Open. 2017;2017(2) doi: 10.1093/hropen/hox008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Devine P.J., Hoyer P.B. Endocrine Disruptors. CRC Press,; 2004. Ovotoxic environmental chemicals: indirect endocrine disruptors; pp. 67–100. [Google Scholar]
- 42.Mark-Kappeler C.J., Hoyer P.B., Devine P.J. Xenobiotic effects on ovarian preantral follicles. Biol. Reprod. 2011;85(5):871–883. doi: 10.1095/biolreprod.111.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoyer P.B., Keating A.F. Xenobiotic effects in the ovary: temporary versus permanent infertility. Expert Opin. Drug Metab. Toxicol. 2014;10(4):511–523. doi: 10.1517/17425255.2014.880690. [DOI] [PubMed] [Google Scholar]
- 44.Bejarano I., Rodríguez A.B., Pariente J.A. Apoptosis Is a Demanding Selective Tool During the Development of Fetal Male Germ Cells. Front. Cell Dev. Biol. 2018;6:65. doi: 10.3389/fcell.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019;20(3):175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belkacemi L. Exploiting the extrinsic and the intrinsic apoptotic pathways for cancer therapeutics. J. Cancer Cure. 2018;1(1):1004. [Google Scholar]
- 47.Pérez-Garijo A., Steller H. Spreading the word: Non-autonomous effects of apoptosis during development, regeneration and disease. Development. 2015;142(19):3253–3262. doi: 10.1242/dev.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Alem L.F., Baker A.T., Pandya U.M., Eisenhauer E.L., Rueda B.R. Understanding and Targeting Apoptotic Pathways in Ovarian Cancer. Cancers. 2019;11(11):1631. doi: 10.3390/cancers11111631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalkavan H., Green D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2018;25(1):46–55. doi: 10.1038/cdd.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regan S., Knight P.G., Yovich J.L., Leung Y., Arfuso F., Dharmarajan A. Granulosa Cell Apoptosis in the Ovarian Follicle-A Changing View. Front. Endocrinol. 2018;9:61. doi: 10.3389/fendo.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sembulingam K. ESSENTIALS Med. Physiol.: Free Rev. Med. Physiol. 2019:516–517. 8th ed. [Google Scholar]
- 52.Cole L.A., Kramer P.R. Academic Press,; 2015. Human Physiology, Biochemistry and Basic Medicine; pp. 219–221. [Google Scholar]
- 53.Yang L., Chen Y., Liu Y., Xing Y., Miao C., Zhao Y., Chang X., Zhang Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021:11. doi: 10.3389/fphar.2020.617843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moreno R.D., Urriola-Muñoz P., Lagos-Cabré R. The emerging role of matrix metalloproteases of the ADAM family in male germ cell apoptosis. Spermatogenesis. 2011;1(3):195–208. doi: 10.4161/spmg.1.3.17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hasan H., Bhushan S., Fijak M., Meinhardt A. Mechanism of Inflammatory Associated Impairment of Sperm Function, Spermatogenesis and Steroidogenesis. Front. Endocrinol. 2022;13 doi: 10.3389/fendo.2022.897029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pant, P., & Chitme, H.R., 2020b, Inflammation and Ovulation. In www.intechopen.com. IntechOpen. 〈https://www.intechopen.com/chapters/73458〉.
- 57.Leslie, S.W., Sajjad, H., & Siref, L.E., 2020, Varicocele. PubMed; StatPearls Publishing. 〈https://www.ncbi.nlm.nih.gov/books/NBK448113/〉. [PubMed]
- 58.Dutta S., Sengupta P., Slama P., Roychoudhury S. Oxidative stress, testicular inflammatory pathways, and male reproduction. Int. J. Mol. Sci. 2021;22(18):10043. doi: 10.3390/ijms221810043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giragani, D.S., 2020, How to treat varicoceles without surgery? Yashoda Hospitals. 〈https://www.yashodahospitals.com/blog/how-to-treat-varicoceles-without-surgery/〉.
- 60.Gestor, F.C., 2021, Everything you need to know about leukocytospermia. IVIRMA Innovation. 〈https://www.ivi-rmainnovation.com/everything-you-need-to-know-about-leukocytospermia/〉.
- 61.Puri V., Gaur K., Hooda S., Shukla S., Sharma S. Papanicolaou-Stained Cytosmear Preparations in the Evaluation of Leucocytospermia: A Tertiary Centre Experience and Assessment of Utility. J. Lab. Physicians. 2021;13(3):208–213. doi: 10.1055/s-0041-1730846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young, A., Toncar, A., & Wray, A.A. (2020). Urethritis. PubMed; StatPearls Publishing. 〈https://www.ncbi.nlm.nih.gov/books/NBK537282〉. [PubMed]
- 63.Chauhan, D.M., 2019, June 6, Herbal Remedies for Urethritis, Ayurvedic Treatment - Causes & Symptoms. Planet Ayurveda. 〈https://www.planetayurveda.com/library/urethritis/〉.
- 64.Mohammed Rasheed H.A., Hamid P. Inflammation to infertility: panoramic view on endometriosis. Cureus. 2020;12(11) doi: 10.7759/cureus.11516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raimondo S., Gentile T., Cuomo F., De Filippo S., Aprea G.E., Guida J. Quantitative evaluation of p53 as a new indicator of DNA damage in human spermatozoa. J. Hum. Reprod. Sci. 2014;7(3):212–217. doi: 10.4103/0974-1208.142490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gharaei R., Mahdavinezhad F., Samadian E., Asadi J., Ashrafnezhad Z., Kashani L., Amidi F. Antioxidant supplementations ameliorate PCOS complications: a review of RCTs and insights into the underlying mechanisms. J. Assist. Reprod. Genet. 2021;38(11):2817–2831. doi: 10.1007/s10815-021-02342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scutiero G., Iannone P., Bernardi G., Bonaccorsi G., Spadaro S., Volta C.A., Greco P., Nappi L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017;2017 doi: 10.1155/2017/7265238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moga M.A., Bălan A., Dimienescu O.G., Burtea V., Dragomir R.M., Anastasiu C.V. Circulating miRNAs as Biomarkers for Endometriosis and Endometriosis-Related Ovarian Cancer—An Overview. J. Clin. Med. 2019;8(5):735. doi: 10.3390/jcm8050735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arafa N.M. Efficacy of Echinacea on the action of cyproterone acetate in male rats. Pak. J. Biol. Sci. 2010;13(20):966. doi: 10.3923/pjbs.2010.966.976. [DOI] [PubMed] [Google Scholar]
- 70.Yari A., Sarveazad A., Asadi E., Raouf Sarshoori J., Babahajian A., Amini N., Shams A. Efficacy of Crocus sativus L. on reduction of cadmium‐induced toxicity on spermatogenesis in adult rats. Andrologia. 2016;48(10):1244–1252. doi: 10.1111/and.12568. [DOI] [PubMed] [Google Scholar]
- 71.Nam E.Y., Kim S.A., Kim H., Kim S.H., Han J.H., Lee J.H., Kim D.I. Akt activation by Evodiae Fructus extract protects ovary against 4-vinylcyclohexene diepoxide-induced ovotoxicity. J. Ethnopharmacol. 2016;194:733–739. doi: 10.1016/j.jep.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 72.Rahmouni F., Daoud S., Rebai T. Teucrium polium attenuates carbon tetrachloride‐induced toxicity in the male reproductive system of rats. Andrologia. 2019;51(2) doi: 10.1111/and.13182. [DOI] [PubMed] [Google Scholar]
- 73.Askaripour M., Hasanpour A., Hosseini F., Moshrefi M., Moshtaghi G., Hasannejad M., Nematollahi-Mahani S.N. The effect of aqueous extract of Rosa damascena on formaldehyde-induced toxicity in mice testes. Pharm. Biol. 2018;56(1):12–17. doi: 10.1080/13880209.2017.1413663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park H.J., Koo Y.K., Park M.J., Hwang Y.K., Hwang S.Y., Park N.C. Restoration of spermatogenesis using a new combined herbal formula of epimedium koreanum nakai and angelica gigas nakai in an luteinizing hormone-releasing hormone agonist-induced rat model of male infertility. World J. Men. 'S. Health. 2017;35(3):170–177. doi: 10.5534/wjmh.17031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fakher S., Seghatoleslam A., Noorafshan A., Karbalay-Doust S., Rahmanifard M., Rashidi M. The impact of echium amoenum distillate on naturally boosting fertility: Potential ameliorative role in male mice reproductive parameters. Iran. J. Med. Sci. 2019;44(3):227–235. [PMC free article] [PubMed] [Google Scholar]
- 76.Orkhon B., Kobayashi K., Javzan B., Sasaki K. Astragalus root induces ovarian β oxidation and suppresses estrogen dependent uterine proliferation. Mol. Med. Rep. 2018;18(6):5198–5206. doi: 10.3892/mmr.2018.9493. [DOI] [PubMed] [Google Scholar]
- 77.Choi H.J., Chung T.W., Park M.J., Jung Y.S., Lee S.O., Kim K.J., Ha K.T. Water-extracted tubers of Cyperus rotundus L. enhance endometrial receptivity through leukemia inhibitory factor-mediated expression of integrin αVβ3 and αVβ5. J. Ethnopharmacol. 2017;208:16–23. doi: 10.1016/j.jep.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 78.Kim E.Y., Choi H.J., Chung T.W., Choi J.Y., Kim H.S., Jung Y.S., Ha K.T. Water-extracted Perilla frutescens increases endometrial receptivity though leukemia inhibitory factor-dependent expression of integrins. J. Pharmacol. Sci. 2016;131(4):259–266. doi: 10.1016/j.jphs.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Ajuogu P.K., Mgbere O.O., Bila D.S., McFarlane J.R. Hormonal changes, semen quality and variance in reproductive activity outcomes of post pubertal rabbits fed Moringa oleifera Lam. leaf powder. J. Ethnopharmacol. 2019;233:80–86. doi: 10.1016/j.jep.2018.12.036. [DOI] [PubMed] [Google Scholar]
- 80.Lienou L.L., Telefo B.P., Bale B., Yemele D., Tagne R.S., Goka S.C., Moundipa P.F. Effect of the aqueous extract of Senecio biafrae (Oliv. & Hiern) J. Moore on sexual maturation of immature female rat. BMC Complement. Altern. Med. 2012;12(1):1–9. doi: 10.1186/1472-6882-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saleem M.A., Al-Attar M.S. Protective effects of Mentha spicata aqueous extract against ifosfamide induced chromosomal aberrations and sperm abnormalities in male albino mice. Trends Biotechnol. Res. 2013;2(1) [Google Scholar]
- 82.Sm S., Mahaboob Basha P. Fluoride exposure aggravates the testicular damage and sperm quality in diabetic mice: protective role of ginseng and banaba. Biol. Trace Elem. Res. 2017;177:331–344. doi: 10.1007/s12011-016-0893-y. [DOI] [PubMed] [Google Scholar]
- 83.Noh S., Go A., Kim D.B., Park M., Jeon H.W., Kim B. Role of antioxidant natural products in management of infertility: a review of their medicinal potential. Antioxidants. 2020;9(10):957. doi: 10.3390/antiox9100957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mahaldashtian M., Naghdi M., Ghorbanian M.T., Makoolati Z., Movahedin M., Mohamadi S.M. In vitro effects of date palm (Phoenix dactylifera L.) pollen on colonization of neonate mouse spermatogonial stem cells. J. Ethnopharmacol. 2016;186:362–368. doi: 10.1016/j.jep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 85.Jung S.E., Kim Y.H., Cho S., Kim B.J., Lee H.S., Hwang S., Ryu B.Y. A phytochemical approach to promotion of self-renewal in murine spermatogonial stem cell by using Sedum sarmentosum extract. Sci. Rep. 2017;7(1):11441. doi: 10.1038/s41598-017-11790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang C., Jin Y., Jin Y. Promoting effect of licorice extract on spermatogonial proliferation and spermatocytes differentiation of neonatal mice in vitro. Vitr. Cell. Dev. Biol. -Anim. 2016;52:149–155. doi: 10.1007/s11626-015-9966-z. [DOI] [PubMed] [Google Scholar]
- 87.Yang F., Wei Y., Liao B. Lycium barbarum Polysaccharide Prevents Cisplatin Induced MLTC-1 Cell Apoptosis and Autophagy via Regulating Endoplasmic Reticulum Stress Pathway [Retraction] Drug Des., Dev. Ther. 2022;16(799):799. doi: 10.2147/DDDT.S367469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang M.S., Kim W.N., Yang W.M., Kim H.Y., Oh J.H., Park S.K. Cytoprotective effects of Morinda officinalis against hydrogen peroxide‐induced oxidative stress in Leydig TM3 cells. Asian J. Androl. 2008;10(4):667–674. doi: 10.1111/j.1745-7262.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 89.Chung H.J., Noh Y., Kim M.S., Jang A., Lee C.E., Myung S.C. Steroidogenic effects of Taraxacum officinale extract on the levels of steroidogenic enzymes in mouse Leydig cells. Anim. Cells Syst. 2018;22(6):407–414. doi: 10.1080/19768354.2018.1494628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ilfergane A., Henkel R.R. Effect of Typha capensis (Rohrb.) NE Br. rhizome extract F1 fraction on cell viability, apoptosis induction and testosterone production in TM 3–Leydig cells. Andrologia. 2018;50(2) doi: 10.1111/and.12854. [DOI] [PubMed] [Google Scholar]
- 91.Afsar T., Razak S., Almajwal A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer. 2017;17(1):1–14. doi: 10.1186/s12885-017-3898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Salahipour M.H., Hasanzadeh S., Malekinejad H. Ameliorative effects of Achillea millefolium inflorescences alcoholic extract against nicotine-induced reproductive failure in rat. Exp. Toxicol. Pathol. 2017;69(7):504–516. doi: 10.1016/j.etp.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 93.Nasr A.Y. The impact of aged garlic extract on adriamycin-induced testicular changes in adult male Wistar rats. Acta Histochem. 2017;119(6):648–662. doi: 10.1016/j.acthis.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Kokubu D., Ooba R., Abe Y., Ishizaki H., Yoshida S., Asano A., Miyazaki H. Angelica keiskei (Ashitaba) powder and its functional compound xanthoangelol prevent heat stress-induced impairment in sperm density and quality in mouse testes. J. Reprod. Dev. 2019;65(2):139–146. doi: 10.1262/jrd.2018-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wu Z.Q., Chen D.L., Lin F.H., Lin L., Shuai O., Wang J.Y., Zhang P. Effect of bajijiasu isolated from Morinda officinalis FC how on sexual function in male mice and its antioxidant protection of human sperm. J. Ethnopharmacol. 2015;164:283–292. doi: 10.1016/j.jep.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 96.NS A.E.R., Al-Ahmary S.H. Effect of sapogenin extract on anti-infertility induced by aluminium chloride in male rats. Adv. Med. Plant Res. 2013;1(4):77–85. [Google Scholar]
- 97.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33(8):1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Towns A.M., Mengue Eyi S., van Andel T. Traditional medicine and childcare in Western Africa: Mothers’ knowledge, folk illnesses, and patterns of healthcare-seeking behavior. PloS One. 2014;9(8) doi: 10.1371/journal.pone.0105972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Licata M., Tuttolomondo T., Leto C., Virga G., Bonsangue G., Cammalleri I., La Bella S. A survey of wild plant species for food use in Sicily (Italy)–results of a 3-year study in four regional parks. J. Ethnobiol. Ethnomed. 2016;12:1–24. doi: 10.1186/s13002-015-0074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kaadaaga H.F., Ajeani J., Ononge S., Alele P.E., Nakasujja N., Manabe Y.C., Kakaire O. Prevalence and factors associated with use of herbal medicine among women attending an infertility clinic in Uganda. BMC Complement. Altern. Med. 2014;14:1–6. doi: 10.1186/1472-6882-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Telefo P.B., Lienou L.L., Yemele M.D., Lemfack M.C., Mouokeu C., Goka C.S., Moundipa F.P. Ethnopharmacological survey of plants used for the treatment of female infertility in Baham, Cameroon. J. Ethnopharmacol. 2011;136(1):178–187. doi: 10.1016/j.jep.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 102.Akour A., Kasabri V., Afifi F.U., Bulatova N. The use of medicinal herbs in gynecological and pregnancy-related disorders by Jordanian women: a review of folkloric practice vs. evidence-based pharmacology. Pharm. Biol. 2016;54(9):1901–1918. doi: 10.3109/13880209.2015.1113994. [DOI] [PubMed] [Google Scholar]
- 103.Swaroop A., Jaipuriar A.S., Gupta S.K., Bagchi M., Kumar P., Preuss H.G., et al. Efficacy of a novel fenugreek seed extract (Trigonella foenum-graecum, Furocyst™) in polycystic ovary syndrome (PCOS) Int J. Med Sci. 2015;12:825–831. doi: 10.7150/ijms.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rao A., Steels E., Beccaria G., Inder W.J., Vitetta L. Influence of a specialized Trigonella foenum-graecum seed extract (Libifem), on testosterone, estradiol and sexual function in healthy menstruating women, a randomised placebo-controlled study. Phytother. Res. 2015;29(8):1123–1130. doi: 10.1002/ptr.5355. [DOI] [PubMed] [Google Scholar]
- 105.Telefo P.B., Tagne S.R., Koona O.E.S., Yemele D.M., Tchouanguep F.M. Effect of the aqueous extract of Justicia insularis T. Anders (Acanthaceae) on ovarian folliculogenesis and fertility of female rats. Afr. J. Tradit. Complement Alter. Med. 2012;9:197–203. doi: 10.4314/ajtcam.v9i2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.da Rocha C.F., de Medeiros Souza Lima Y., Carvalho H.O., Pinto R.C., Ferreira I.M., Castro A.N., et al. Action of the hydroethanolic extract of the flowers of Acmella oleracea (L.) R.K. Jansen on the reproductive performance of Wistar female rats: a popular female aphrodisiac from the Amazon. J. Ethnopharmacol. 2018;214:301–308. doi: 10.1016/j.jep.2017.12.024. [DOI] [PubMed] [Google Scholar]
- 107.Shahin A.Y., Ismail A.M., Zahran K.M., Makhlouf A. Adding phytoestrogens to clomiphene induction in unexplained infertility– a randomized trial. Reprod. BioMed. Online. 2008;16:580–588. doi: 10.1016/s1472-6483(10)60465-8. [DOI] [PubMed] [Google Scholar]
- 108.Shahin A.Y., Mohammed S.A. Adding the phytoestrogen Cimicifugae racemosae to clomiphene induction cycles with timed intercourse in polycystic ovary syndrome improves cycle outcomes and pregnancy rates–a randomized trial. Gynecol. Endocrinol. 2014;30(7):505–510. doi: 10.3109/09513590.2014.895983. [DOI] [PubMed] [Google Scholar]
- 109.Kamel H.H. Role of phyto-oestrogens in ovulation induction in women with polycystic ovarian syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;168(1):60–63. doi: 10.1016/j.ejogrb.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 110.Yılmaz N., Seven B., Timur H., Yorganci A., Inal H.A., Kalem M.N., et al. Ginger (Zingiber officinale) might improve female fertility: a rat model. J. Chin. Med Assoc. 2018;81(905):11. doi: 10.1016/j.jcma.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 111.Abolaji A.O., Ojo M., Afolabi T.T., Arowoogun M.D., Nwawolor D., Farombi E.O. Protective properties of 6-gingerol-rich fraction from Zingiber officinale (Ginger) on chlorpyrifos-induced oxidative damage and inflammation in the brain, ovary and uterus of rats. Chem. -Biol. Inter. 2017;270:15–23. doi: 10.1016/j.cbi.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 112.Kamarzaman S., Shaban M., AbdulRahman S. The prophylactic effect of Nigella sativa against cyclophosphamide in the ovarian follicles of matured adult mice: a preliminary study. J. Anim. Plant Sci. 2014;24:81–88. [Google Scholar]
- 113.Hosseinzadeh H., Tafaghodi M., Mosavi M.J., Taghiabadi E. Effect of aqueous and ethanolic extracts of Nigella sativa seeds on milk production in rats. J. Acupunct. Meridian Stud. 2013;6:18–23. doi: 10.1016/j.jams.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 114.Naeimi S.A., Tansaz M., Sohrabvand F., Hajimehdipoor H., Nabimeybodi R., Saber S., et al. Assessing the effect of processed Nigella sativa on oligomenorrhea and amenorrhea in patients with polycystic ovarian syndrome: a pilot study. Int J. Pharm. Sci. Res. 2018;9(11):4716–4722. [Google Scholar]
- 115.Canning S., Waterman M., Orsi N., Ayres J., Simpson N., Dye L. The efficacy of Hypericum perforatum (St John’s wort) for the treatment of premenstrual syndrome: a randomized, double-blind, placebo-controlled trial. CNS Drugs. 2010;24(3):207–225. doi: 10.2165/11530120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 116.Agha-Hosseini M., Kashani L., Aleyaseen A., Ghoreishi A., Rahmanpour H., Zarrinara A.R., et al. Crocus sativus L. (saffron) in the treatment of premenstrual syndrome: a double-blind, randomized and placebo-controlled trial. BJOG. 2008;115(4):515–519. doi: 10.1111/j.1471-0528.2007.01652.x. [DOI] [PubMed] [Google Scholar]
- 117.Bhardwaj J.K., Panchal H., Saraf P. Ameliorating effects of natural antioxidant compounds on female infertility: a review. Reprod. Sci. 2021;28:1227–1256. doi: 10.1007/s43032-020-00312-5. [DOI] [PubMed] [Google Scholar]
- 118.Kort D.H., Lobo R.A. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: a randomized controlled trial. Am. J. Obstet. Gynecol. 2014;211(487):e1–e6. doi: 10.1016/j.ajog.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 119.Najafipour F., Rahimi A.O., Mobaseri M., Agamohamadzadeh N., Nikoo A., Aliasgharzadeh A. Therapeutic effects of stinging nettle (Urtica dioica) in women with hyperandrogenism. Int J. Curr. Res Aca Rev. 2014;2(7):153–160. [Google Scholar]
- 120.Farzana F., Sulaiman A., Ruckmani A., Vijayalakshmi K., Karunya Lakshmi G., Shri R.S. Effects of flax seeds supplementation in polycystic ovarian syndrome. J. Res Med Sci. 2015;31(1):113–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.