Abstract
This study uses data from an all-payer database of prescriptions dispensed in US retail pharmacies to assess trends in buprenorphine initiation and retention during 2016-2022.
Increasing initiation of therapy with buprenorphine, a highly effective medication for opioid use disorder, is important in light of record US opioid-related mortality.1,2 Ensuring retention in therapy after buprenorphine initiation is also important because retention is strongly associated with decreased opioid-related mortality.3
Prior studies have examined buprenorphine initiation and retention rates through the end of 2020.4,5 However, buprenorphine access may have since improved owing to the implementation of policies to increase use, such as the elimination of training requirements for buprenorphine waivers in April 2021. Additionally, the relaxation of social distancing measures may have alleviated barriers to health care visits, thus increasing opportunities to diagnose and initiate treatment for opioid use disorder. This study assessed trends in buprenorphine initiation and retention during 2016-2022.
Methods
We analyzed the IQVIA Longitudinal Prescription Database, an all-payer database that reports 92% of prescriptions dispensed in US retail pharmacies. Because data were deidentified, the institutional review board of the University of Michigan exempted analyses from review.
We initially included buprenorphine prescriptions dispensed from January 2016 through October 2022 to patients residing in 1 of the 50 US states or the District of Columbia. Buprenorphine products included immediate-release and extended-release formulations approved for opioid use disorder but not formulations primarily used to treat pain. We excluded prescriptions for patients who had any buprenorphine prescription with missing or potentially invalid data on days supplied (≤0 days or >90 days).
We calculated the monthly buprenorphine initiation rate, defined as the number of US patients initiating buprenorphine therapy per 100 000 individuals. We identified these patients based on the occurrence of an “initial” buprenorphine prescription, defined as a prescription for a patient without buprenorphine dispensing in the prior 180 days. Population denominators came from the US Census Bureau (eMethods in Supplement 1).
Among patients initiating buprenorphine therapy, we calculated the monthly proportion retained in therapy. Following a National Quality Forum–endorsed quality measure,6 we defined retention as 180 days or more of continuous buprenorphine treatment following the dispensing date of the initial buprenorphine prescription, without any gaps exceeding 7 days (eMethods in Supplement 1). Retention analyses stopped in April 2022 owing to the need for a 180-day look-forward period.
Using Joinpoint version 4.9.0.0 (National Cancer Institute), we assessed for slope changes in buprenorphine initiation and retention rates. Analyses used 2-sided hypothesis tests, with α = .05.
Results
Among 94 106 548 buprenorphine prescriptions initially included, 393 385 (0.4%) were excluded, leaving 93 713 163 prescriptions. During the study period, 3 006 629 patients initiated buprenorphine therapy; 1 289 039 (42.9%) were female.
During January 2016 through September 2018, the monthly buprenorphine initiation rate increased from 12.5 to 15.9 per 100 000 (monthly percentage change, 0.62% [95% CI, 0.40% to 0.84%]; P < .001). During October 2018 through October 2022, the slope was flat (monthly percentage change, −0.03% [95% CI, −0.16% to 0.09%]; P = .62). The median monthly buprenorphine initiation rate during March 2020 through December 2020 (14.4 per 100 000) was slightly lower than during January 2019 through February 2020 (15.5 per 100 000) and January 2021 through October 2022 (15.0 per 100 000), but no joinpoint in 2020 was detected (Figure, A).
Figure. Monthly Trends in Buprenorphine Initiation and Retention in the US, January 2016 Through October 2022.
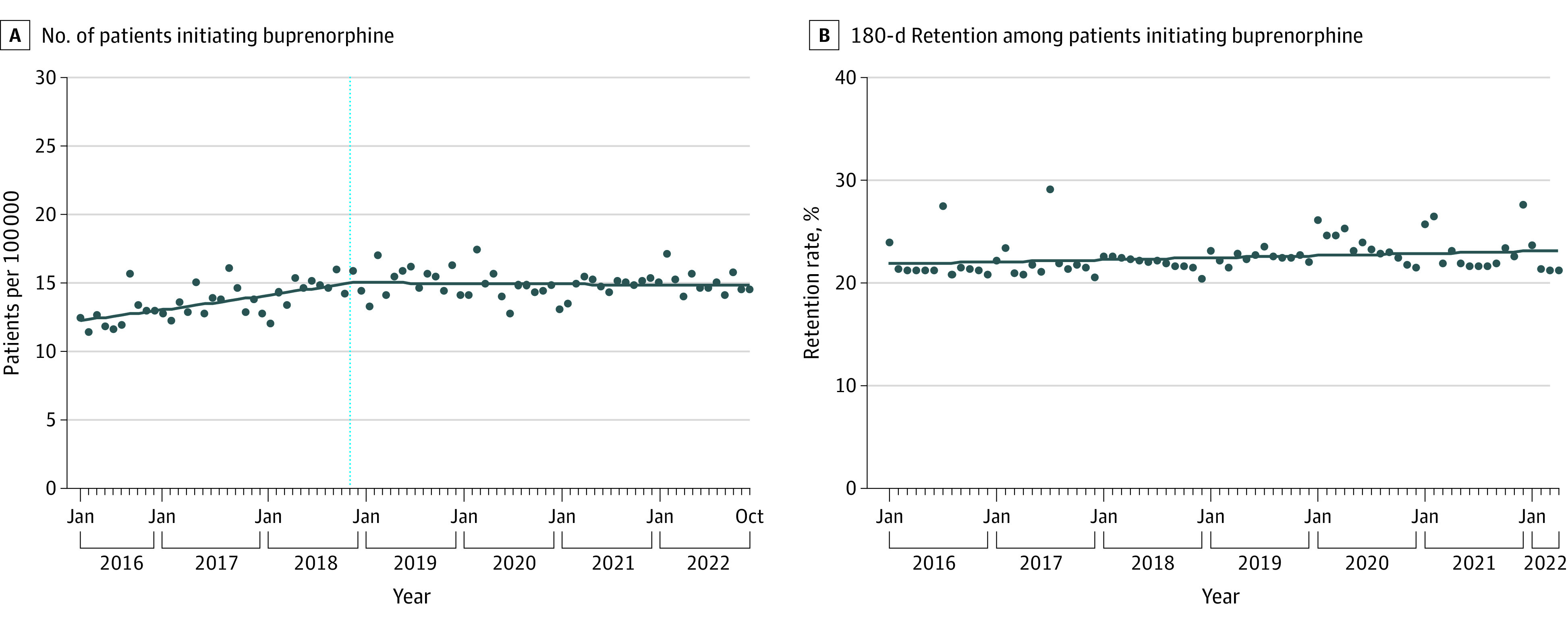
A, Monthly number of patients initiating buprenorphine therapy per 100 000 US individuals. Vertical blue dotted line denotes a slope change detected by joinpoint regression. B, Monthly 180-day rate of retention among patients initiating buprenorphine therapy. Analyses of retention stopped in April 2022 owing to the need for a 180-day look-forward period to assess retention. No slope change in the rate of retention was detected by joinpoint regression.
During January 2016 through April 2022, the median monthly retention rate was 22.2% (IQR, 21.5%-23.0%). The retention rate increased minimally without any slope changes (monthly percentage change, 0.08% [95% CI, 0.005% to 0.15%]; P = .04) (Figure, B).
Discussion
During January 2016 through October 2022, the monthly buprenorphine initiation rate increased, then flattened. This flattening occurred prior to the COVID-19 pandemic, suggesting that factors other than the pandemic were involved. Throughout the study period, including in 2021-2022, only 1 in 5 patients who initiated buprenorphine were retained in therapy for at least 180 days, a rate similar to that found in a prior study examining data through the end of 2020.4,5
Limitations include lack of data on race and ethnicity, in-clinic buprenorphine administration, and buprenorphine dispensing in methadone outpatient treatment programs. Additionally, some patients may have initiated buprenorphine to treat pain.
These findings suggest that recent clinical and policy efforts to increase buprenorphine use have been insufficient to meet the need for this medication. A comprehensive approach is needed to eliminate barriers to buprenorphine initiation and retention, such as stigma and uneven access to prescribers.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
eMethods
Data Sharing Statement
References
- 1.Centers for Disease Control and Prevention . Provisional drug overdose death counts. Published 2021. Accessed March 1, 2022. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 2.National Academies of Sciences, Engineering, and Medicine . Medications for Opioid Use Disorder Save Lives. National Academies Press; 2019. [PubMed] [Google Scholar]
- 3.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein BD, Landis RK, Sheng F, et al. Buprenorphine treatment episodes during the first year of COVID: a retrospective examination of treatment initiation and retention. J Gen Intern Med. 2023;38(3):733-737. doi: 10.1007/s11606-022-07891-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Stringfellow EJ, Russell WA, Jalali MS. Racial and ethnic disparities in buprenorphine treatment duration in the US. JAMA Psychiatry. 2023;80(1):93-95. doi: 10.1001/jamapsychiatry.2022.3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Quality Forum . Continuity of Pharmacotherapy for Opioid Use Disorder (OUD)—National Quality Strategy Domain: Effective Clinical Care; Meaningful Measure Area: Prevention and Treatment of Opioid and Substance Use Disorders. Published 2019. Accessed October 31, 2022. https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-Measures/2019_Measure_468_MIPSCQM.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Data Sharing Statement


