Key Points
Question
Is engaging in moderate to vigorous physical activity (MVPA) associated with the risk of severe COVID-19–related outcomes?
Findings
In this case-control study of 6 396 500 patients in South Korea, MVPA showed an inverse association with SARS-CoV-2 infection but was mitigated in fully vaccinated patients. However, the association of MVPA with severe COVID-19 outcomes was limited.
Meaning
The study’s findings suggest that engagement in regular MVPA may lower the risk for severe COVID-19 outcomes but increase the risk for SARS-CoV-2 infection.
This case-control study investigates the association of changes in moderate to vigorous physical activity with risk of SARS-CoV-2 infection and severe COVID-19 outcomes in South Korea.
Abstract
Importance
The association of moderate to vigorous physical activity (MVPA) with COVID-19 outcomes is unclear and needs to be investigated.
Objective
To identify the association of longitudinal changes in MVPA with SARS-CoV-2 infection and severe COVID-19 outcomes.
Design, Setting, and Participants
This nested case-control study used data from 6 396 500 adult patients in South Korean who participated in National Health Insurance Service (NHIS) biennial health screenings from period 1 (2017-2018) to period 2 (2019-2020). Patients were followed from October 8, 2020, until the diagnosis of COVID-19 or December 31, 2021.
Exposure
Moderate to vigorous physical activity was measured by self-report on questionnaires during both NHIS health screenings and calculated by adding the frequency (times per week) of each moderate (≥30 minutes per day) and vigorous (≥20 minutes per day) physical activity.
Main Outcomes and Measures
The main outcomes were a positive diagnosis of SARS-CoV-2 infection and severe COVID-19 clinical events. Adjusted odds ratio (aORs) and 99% CIs were calculated using multivariable logistic regression analysis.
Results
A total of 183 350 patients with COVID-19 (mean [SD] age, 51.9 [13.8] years; female, 89 369 [48.7%]; male, 93 981 [51.3%]) among 2 110 268 participants were identified. For participants with vs without COVID-19, the proportion of MVPA frequency at period 2 was 35.8% vs 35.9% for physically inactive, 18.9% vs 18.9% for 1 to 2 times per week, 17.7% vs 17.7% for 3 to 4 times per week, and 27.5% vs 27.4% for 5 or more times per week. Among unvaccinated, physically inactive patients at period 1, the odds for infection increased when engaged in MVPA 1 to 2 times per week (aOR, 1.08; 99% CI, 1.01-1.15), 3 to 4 times per week (aOR, 1.09; 99% CI, 1.03-1.16), or 5 or more times per week (aOR, 1.10; 99% CI, 1.04-1.17) at period 2. Conversely, among unvaccinated patients with MVPA of 5 or more times per week at period 1, the odds for infection decreased when engaged 1 to 2 times per week (aOR, 0.90; 99% CI, 0.81-0.98) or physically inactive (aOR, 0.80; 99% CI, 0.73-0.87) at period 2. The trend of MVPA and incident infection was mitigated when participants were fully vaccinated. Furthermore, the odds for severe COVID-19 showed significant but limited associations with MVPA.
Conclusions and Relevance
The findings of this nested case-control study show a direct association of MVPA with risk of SARS-CoV-2 infection, which was mitigated after completion of the COVID-19 vaccination primary series. In addition, higher levels of MVPA were associated with a lower risk of severe COVID-19 outcomes to limited proportions.
Introduction
Since 2020, COVID-19 has spread worldwide, causing a global pandemic. To prioritize the high-risk patients, many researchers identified the risk factors for severe COVID-19, including age, sex, and physical inactivity,1,2 whereas other researchers investigated the association between physical activity (PA) and severe COVID-19 and reported the potential benefits of PA in reducing the risk of severe COVID-19.2,3,4,5,6 A recent study reported that moderate to vigorous PA (MVPA) was associated with a lower risk of severe COVID-19 than low PA, underscoring the protective effects of MVPA.7
However, the association between MVPA and SARS-CoV-2 infection remains unclear. Some researchers reported that MVPA reduced,3,5 increased,8 or was not associated with infection.9 In addition, evidence suggests that MVPA drives the transmission of COVID-19 through increased aerosol particle emission.10,11 Despite many health benefits, the association of MVPA with enhanced immunity but increased exposure risk is unclear and needs to be evaluated.12,13
In this study, we used cohort data from the Korean National Health Insurance Service (NHIS) and Korea Disease Control and Prevention Agency (KDCA) to investigate the change in MVPA between consecutive biennial health screenings with SARS-CoV-2 infection and severe COVID-19. We hypothesized that MVPA is associated with a lower risk of severe COVID-19 but a higher risk of SARS-CoV-2 infection.
Methods
Data Source and Study Population
Data for this nested case-control study are from the NHIS, a universal health management system that covers 97% of the Korean population.14 Following the outbreak of COVID-19, NHIS and KDCA generated a cohort of COVID-19–related registers merged with detailed medical information for scientific research. The characteristics of the data set are provided in the eMethods in Supplement 1.
The study included adults aged 19 years or older who participated in both biennial health screenings from period 1 (2017-2018) to period 2 (2019-2020). First, using the NHIS-KDCA 1:10 matched cohort, the data set consisted of patients diagnosed at least once with COVID-19 (n = 581 500), individuals without COVID-19 (n = 5 815 000), and individuals who participated in both health screenings (n = 2 128 608). Patients without COVID-19 who died before January 1, 2022 (n = 6234), had missing information on MVPA (n = 1998) or adjustment variables (n = 9847), or were diagnosed with SARS-CoV-2 infection before the second health screening (n = 261) were excluded from the study. The analytic cohort, therefore, comprised 2 110 268 patients. To investigate the risk of severe COVID-19 outcomes, those who died within 1 month before severe COVID-19 (n = 299) or who were diagnosed with COVID-19 within 1 month before the end of follow-up (n = 59 059) were excluded from the analysis (Figure 1). This study was approved by the institutional review board of CHA University Hospital (No. CHAMC 2022-05-052). The requirement for informed consent was waived because anonymized data were retrieved from the NHIS and KDCA databases. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Figure 1. Study Population Flow Diagram.
KDCA indicates Korea Disease Control and Prevention Agency; NHIS, National Health Insurance Service.
Exposures
Leisure time PA was measured by self-report questionnaires during biennial health screenings. Based on the responses, the intensity of PA was categorized into 2 levels: moderate PA defined as exercise that causes mild shortness of breath (eg, fast cycling, doubles tennis) and vigorous PA defined as exercise that causes severe shortness of breath (eg, running, hiking). If patients engaged in any moderate PA for at least 30 minutes per day or any vigorous PA for at least 20 minutes per day, they answered each question with an average frequency of days per week. We then calculated the frequency of MVPA by adding these values. For the analyses, we categorized MVPA as follows: (1) physically inactive, (2) 1 to 2 times per week, (3) 3 to 4 times per week, and (4) 5 or more times per week. Reliability and validity of the questionnaire are described elsewhere.15 The method of calculating metabolic equivalent task (MET) score from MVPA is presented in the eMethods in Supplement 1.
Outcomes
We investigated the association of change in MVPA with COVID-19 outcomes. The primary outcome was a first positive diagnosis of SARS-CoV-2 infection based on reverse transcriptase–polymerase chain reaction assays performed on either nasopharyngeal or oropharyngeal swab. The secondary outcomes were defined as acute clinical events of COVID-19 after hospitalization, including requirement of oxygen supply with conventional oxygen therapy (COT), high-flow nasal cannula (HFNC), continuous positive airway pressure, admission to the intensive care unit (ICU), requirement of mechanical ventilation, and extracorporeal membrane oxygenation. Each outcome was assessed until the following month from the diagnosis month. The definitions of reinfection and breakthrough infection are listed in the eMethods in Supplement 1.
Key Variables
Key variables were identified before the start of follow-up, including age, sex, household income, body mass index, fasting serum glucose, smoking, alcohol consumption, medical condition (history of hypertension, diabetes, dyslipidemia), medication history (antihypertensive, antidiabetic, lipid lowering), comorbidities, and COVID-19 vaccination status. Information of key variables is provided in the eMethods in Supplement 1.
Statistical Analysis
We analyzed the change in MVPA between periods 1 and 2 with the odds for SARS-CoV-2 infection and severe COVID-19. In addition, the association of MVPA at period 2 with COVID-19–related outcomes was analyzed to account for current MVPA. The index date for the outcome was set at October 9, 2020, and patients were followed until the diagnosis of COVID-19 or the study end date of December 31, 2021. Distributions of continuous variables were presented as mean (SD) and continuous variables as No. (%). Baseline characteristics were calculated using the χ2 test for categorical variables, nonparametric test for continuous variables, and effect sizes for each variable (eMethods in Supplement 1). Total and event numbers were presented for each change in MVPA. Adjusted odds ratios (aORs) and 99% CIs were calculated using age and multivariable-adjusted logistic regression analysis. For the multivariable regression, odds were calculated after adjusting for key variables, including age, sex, household income, fasting serum glucose, smoking, alcohol consumption, medical condition (history of hypertension, diabetes, dyslipidemia), medication history (antihypertensive, antidiabetic, lipid lowering), and Charlson Comorbidity Index (CCI). COVID-19 vaccination status was used to stratify the analytic cohort by unvaccinated or completion of the primary series, since it is the most powerful determinant for COVID-19–related outcomes. To take advantage of the longitudinal data set, we additionally analyzed the risk of COVID-19–related outcomes by Cox proportional hazard regression models in the same manner. Adjusted hazard ratios and 99% CIs were calculated by the same adjustment. For the sensitivity analyses, MET scores were used as an exposure variable for the multivariable logistic regression model. To minimize bias by small sample and rare event rates on severe COVID-19, reinfection, and breakthrough infection, we used penalized likelihood logistic regression based on the Firth method, which is effective for reducing the bias from maximum likelihood estimation.16 For the subgroup analyses, we stratified the patients by age (<65 years, ≥65 years), sex (female, male), and CCI (0, 1, ≥2) and examined the change in MVPA with SARS-CoV-2 infection. The P values for interaction were calculated using the specified categorical variables. In addition, tests for trend were performed for all subgroups. To account for multiple comparisons, we set a 2-sided P < .01 as statistically significant and calculated 99% CIs. All statistical analyses were performed using SAS, version 9.4 software (SAS Institute Inc).
Results
Baseline Characteristics
We identified 183 350 patients with positive results for SARS-CoV-2 infection among 2 110 268 participants (Table 1). Of the patients with COVID-19, the mean (SD) age was 51.9 (13.8) years, 93 981 were men (51.3%), and 89 369 were women (48.7%). The proportion of MVPA frequency at period 2 for COVID-19 vs no COVID-19 was 35.8% vs 35.9% for physically inactive, 18.9% vs 18.9% for 1 to 2 times per week, 17.7% vs 17.7% for 3 to 4 times per week, and 27.5% vs 27.4% for 5 or more times per week, respectively. The effect size of MVPA levels did not differ between patients with and without COVID-19 between periods 1 and 2. However, COVID-19 vaccination status significantly differed between the patients with and without COVID-19 (no vaccination, 40.1% vs 3.6%; completion of the primary series, 18.1% vs 95.1%; P < .001; effect size = 0.69). Other baseline characteristics are described in Table 1.
Table 1. Participant Characteristicsa.
| Variable | No. (%) | P valueb | Cohen d or Cramér Vc | |
|---|---|---|---|---|
| COVID-19 (n = 183 350) | No COVID-19 (n = 1 926 918) | |||
| MVPA at health screening period 1 (2017-2018), times/wk | ||||
| Physically inactive | 65 700 (35.8) | 691 694 (35.9) | .61 | <.001 |
| 1-2 | 34 667 (18.9) | 363 901 (18.9) | ||
| 3-4 | 32 486 (17.7) | 343 007 (17.8) | ||
| ≥5 | 50 497 (27.5) | 528 316 (27.4) | ||
| MVPA at health screening period 2 (2019-2020), times/wk | ||||
| Physically inactive | 49 770 (26.6) | 510 189 (26.5) | <.001 | 0.01 |
| 1-2 | 31 242 (17.0) | 321 080 (16.7) | ||
| 3-4 | 35 845 (19.6) | 379 601 (19.7) | ||
| ≥5 | 67 493 (36.8) | 716 048 (37.2) | ||
| Age, mean (SD), y | 51.9 (13.8) | 51.6 (14.1) | <.001 | 0.02 |
| Sex | ||||
| Female | 89 369 (48.7) | 924 384 (48.0) | <.001 | 0.01 |
| Male | 93 981 (51.3) | 1 002 534 (52.0) | ||
| Household income, quartile | ||||
| First (lowest) | 39 181 (21.4) | 398 448 (20.7) | <.001 | 0.01 |
| Second | 38 119 (20.8) | 386 119 (20.0) | ||
| Third | 47 466 (25.9) | 510 762 (26.5) | ||
| Fourth (highest) | 58 584 (32.0) | 631 589 (32.8) | ||
| Body mass indexd | ||||
| <18.5 | 3798 (2.1) | 47 137 (2.5) | <.001 | 0.02 |
| 18.5-25 | 100 118 (54.6) | 1 092 357 (56.7) | ||
| 25-30 | 66 216 (36.1) | 660 913 (34.3) | ||
| ≥30 | 13 224 (7.2) | 126 511 (6.6) | ||
| Waist circumference, mean (SD), cm | 83.0 (10.3) | 82.5 (10.5) | <.001 | 0.05 |
| Systolic blood pressure, mean (SD), mm Hg | 124.8 (14.7) | 124.8 (14.6) | .65 | 0.0 |
| Diastolic blood pressure, mean (SD), mm Hg | 77.1 (10.2) | 77.1 (10.0) | .23 | 0.0 |
| Triglycerides, mean (SD), mg/dL | 132.7 (99.6) | 134.3 (99.9) | .33 | 0.02 |
| Fasting blood glucose, mean (SD), mg/dL | 103.8 (25.1) | 103.5 (24.5) | <.001 | 0.01 |
| Cigarette smoking | ||||
| Ever smoker | 120 001 (65.6) | 1 208 914 (62.7) | <.001 | 0.02 |
| Never smoker | 63 349 (34.6) | 718 004 (37.3) | ||
| Alcohol consumption | ||||
| Yes | 113 268 (61.8) | 1 117 257 (61.1) | <.001 | 0.01 |
| No | 70 082 (38.2) | 749 661 (38.9) | ||
| Hypertension | 44 056 (24.2) | 450 457 (23.4) | <.001 | <.01 |
| Diabetes | 18 945 (10.3) | 185 152 (9.6) | <.001 | 0.01 |
| Dyslipidemia | 21 139 (11.5) | 218 764 (11.4) | .02 | <.01 |
| Charlson Comorbidity Index | ||||
| 0 | 66 209 (36.1) | 776 338 (40.3) | <.001 | 0.02 |
| 1 | 59 554 (32.5) | 598 665 (31.1) | ||
| ≥2 | 57 587 (31.4) | 551 915 (28.6) | ||
| COVID-19 vaccination | ||||
| No | 73 483 (40.1) | 67 159 (3.5) | <.001 | 0.69 |
| Completion of primary series | 33 186 (18.1) | 1 832 142 (95.1) | ||
Abbreviation: MVPA, moderate to vigorous physical activity.
Baseline was defined as the National Health Insurance Service health screening period 2 (2019-2020). Each time of MVPA was defined as more than 20 to 30 minutes based on self-report from health screening records.
Calculated using the χ2 test for categorical and nonparametric test for continuous variables.
The effect size was calculated using Cramér V (small, >0.1; medium, >0.3; large, >0.5) for categorical variables and Cohen d (small, >0.2; medium, >0.5; large, >0.8) for continuous variables.
As measured by weight in kilograms divided by height in meters squared.
Association of Increase in MVPA With SARS-CoV-2 Infection
For MVPA at period 2, the odds for infection among unvaccinated patients increased as the frequency increased compared with physically inactive patients (eTable 1 in Supplement 1). In addition, the increase in MVPA showed a nonlinear association with SARS-CoV-2 infection. From the unvaccinated patients who were physically inactive at period 1, the odds for infection increased when engaged in MVPA 1 to 2 times per week (aOR, 1.08; 99% CI, 1.01-1.15), 3 to 4 times per week (aOR, 1.09; 99% CI, 1.03-1.16), or 5 or more times per week (aOR, 1.10; 99% CI, 1.04-1.17) at period 2, with a dose-response trend (P < .001 for trend) compared with persistently physically inactive patients (Table 2). However, no association was found between increased MVPA and patients who completed the primary series or were reinfected with SARS-CoV-2, whereas decreased ORs were found at period 2 as MVPA frequency increased (eTables 1 and 2 in Supplement 1). The association was similar with Cox regression models (eTables 3 and 4 in Supplement 1) and with MET score (eTable 5 in Supplement 1) as an explanatory variable.
Table 2. Association of Changes in Moderate to Vigorous Physical Activity (MVPA) Between Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection by Vaccination Statusa.
| MVPA change from period 1 to 2 | Unvaccinated | Completion of the primary series | ||||
|---|---|---|---|---|---|---|
| No. of events/total | OR (99% CI)b | No. of events/total | OR (99% CI)b | |||
| Age adjusted | Multivariable adjustedc | Age adjusted | Multivariable adjustedc | |||
| Physically inactive at period 1 (2017-2018) | ||||||
| Persistently physically inactive | 13 137/25 828 | 1.00 [Reference] | 1 [Reference] | 13 669/308 348 | 1 [Reference] | 1[Reference] |
| Physically inactive to MVPA 1-2 times/wk | 4977/9508 | 1.11 (1.04-1.18)d | 1.08 (1.01-1.15)e | 4530/108 729 | 1.03 (0.99-1.07) | 1.04 (0.98-1.09) |
| Physically inactive to MVPA 3-4 times/wk | 4119/7751 | 1.12 (1.04-1.20)d | 1.09 (1.03-1.16)e | 4347/100 558 | 1.00 (0.97-1.04) | 1.00 (0.97-1.04) |
| Physically inactive to MVPA ≥5 times/wk | 6341/11 852 | 1.11 (1.04-1.18)d | 1.10 (1.04-1.17)d | 7741/167 890 | 1.03 (1.00-1.06) | 1.02 (1.00-1.05) |
| P value for trend | NA | <.001 | <.001 | NA | .20 | .13 |
| MVPA 1-2 times/wk at period 1 (2017-2018) | ||||||
| Decrease of MVPA from 1-2 times/wk to physically inactive | 3328/6392 | 0.86 (0.78-0.93)d | 0.90 (0.82-0.98)e | 3009/74 092 | 0.93 (0.85-0.99)e | 0.91 (0.85-0.98)d |
| Persistently 1-2 times/wk | 4867/8945 | 1.00 [Reference] | 1 [Reference] | 4123/103 056 | 1 [Reference] | 1 [Reference] |
| Increase from 1-2 to 3-4 times/wk | 3826/6958 | 1.01 (0.95-1.07) | 1.02 (0.96-1.09) | 3471/86 028 | 0.99 (0.94-1.03) | 0.98 (0.93-1.03) |
| Increase from 1-2 to ≥5 times/wk | 4126/7394 | 1.03 (0.96-1.09) | 1.05 (0.99-1.12) | 4019/95 883 | 1.00 (0.95-1.04) | 0.99 (0.94-1.03) |
| P value for trend | NA | <.001 | <.001 | NA | .01 | .001 |
| MVPA 3-4 times/wk at period 1 (2017-2018) | ||||||
| Decrease of from 3-4 times/wk to physically inactive | 2109/4054 | 0.80 (0.64-1.00) | 0.83 (0.75-0.92)d | 2301/50 991 | 0.99 (0.94-1.05) | 0.98 (0.93-1.03) |
| Decrease of from 3-4 to 1-2 times/wk | 2688/4911 | 0.98 (0.91-1.05) | 0.97 (0.90-1.05) | 2419/57 904 | 1.04 (0.99-1.09) | 1.04 (0.99-1.10) |
| Persistently 3-4 times/wk | 4057/7255 | 1.00 [Reference] | 1 [Reference] | 4065/97 555 | 1 [Reference] | 1 [Reference] |
| Increase from 3-4 to ≥5 times/wk | 5445/9495 | 1.04 (0.98-1.11) | 1.04 (0.98-1.11) | 5850/134 927 | 1.03 (0.99-1.07) | 1.03 (0.98-1.07) |
| P value for trend | NA | <.001 | <.001 | NA | .27 | .14 |
| MVPA ≥5 times/wk at period 1 (2017-2018) | ||||||
| Decrease from ≥5 times/wk to physically inactive | 2746/5193 | 0.77 (0.72-0.83)d | 0.80 (0.73-0.87)d | 3369/71 155 | 0.94 (0.88-0.99)e | 0.93 (0.88-0.98)d |
| Decrease from ≥5 to 1-2 times/wk | 2048/3739 | 0.91 (0.84-0.98)e | 0.90 (0.81-0.98)e | 2045/46 709 | 0.99 (0.95-1.04) | 0.99 (0.94-1.03) |
| Decrease from ≥5 to 3-4 times/wk | 3859/6728 | 0.98 (0.93-1.04) | 0.96 (0.91-1.02) | 4230/93 378 | 0.99 (0.96-1.03) | 0.99 (0.95-1.02) |
| Persistently ≥5 times/wk | 12 172/21 001 | 1 [Reference] | 1 [Reference] | 14 728/318 855 | 1 [Reference] | 1 [Reference] |
| P value for trend | NA | <.001 | <.001 | NA | .01 | .003 |
Abbreviations: NA, not applicable; OR, odds ratio.
Each time of MVPA was defined as more than 20 to 30 minutes based on the self-report from National Health Insurance Service health screening records. COVID-19 vaccination status was defined as the number of COVID-19 inoculations that were available in South Korea, including BNT162b2 (Pfizer-BioNTech), mRNA-1273 (Moderna), ChAdOx1 nCoV-19 (AstraZeneca), NVX-CoV2373 (Novavax), and Ad26.COV2.S (Janssen Pharmaceuticals/Johnson & Johnson). The vaccination status included homologous and heterologous primary schedules for the completion of the primary series except for Ad26.COV2.S, which was a single dose approved for the completion of the primary series.
Calculated using logistic regression.
Adjusted for age, sex, household income, body mass index, fasting blood glucose, hypertension, diabetes, dyslipidemia, Charlson comorbidity index, smoking, alcohol consumption, and dose of COVID-19 vaccination.
P < .001.
P < .01.
Association of Decrease in MVPA With SARS-CoV-2 Infection
A decrease in MVPA also showed a nonlinear association with the incidence of SARS-CoV-2 infection. For unvaccinated patients who engaged in MVPA 5 or more times per week at period 1, the odds for infection decreased when they engaged in MVPA 1 to 2 times per week (aOR, 0.90; 99% CI, 0.81-0.98) or were physically inactive (aOR, 0.80; 99% CI, 0.73-0.87) at period 2, compared with those who persistently engaged in 5 or more times per week across both periods. Similarly, for the patients who completed the primary vaccination series, the odds for infection decreased when MVPA radically decreased to physically inactive (aOR, 0.93; 99% CI, 0.88-0.98) (Table 2). However, there was no significant association for any specific vaccine type or with breakthrough infection after receiving booster doses (eTables 6 and 7 in Supplement 1). The decrease in MVPA with SARS-CoV-2 infection showed a dose-response association among unvaccinated patients (P < .001 for trend), but this was attenuated for patients who completed the primary series (P = .003 for trend) (Table 2).
Association of Changes in MVPA With Severe Clinical COVID-19 Outcomes
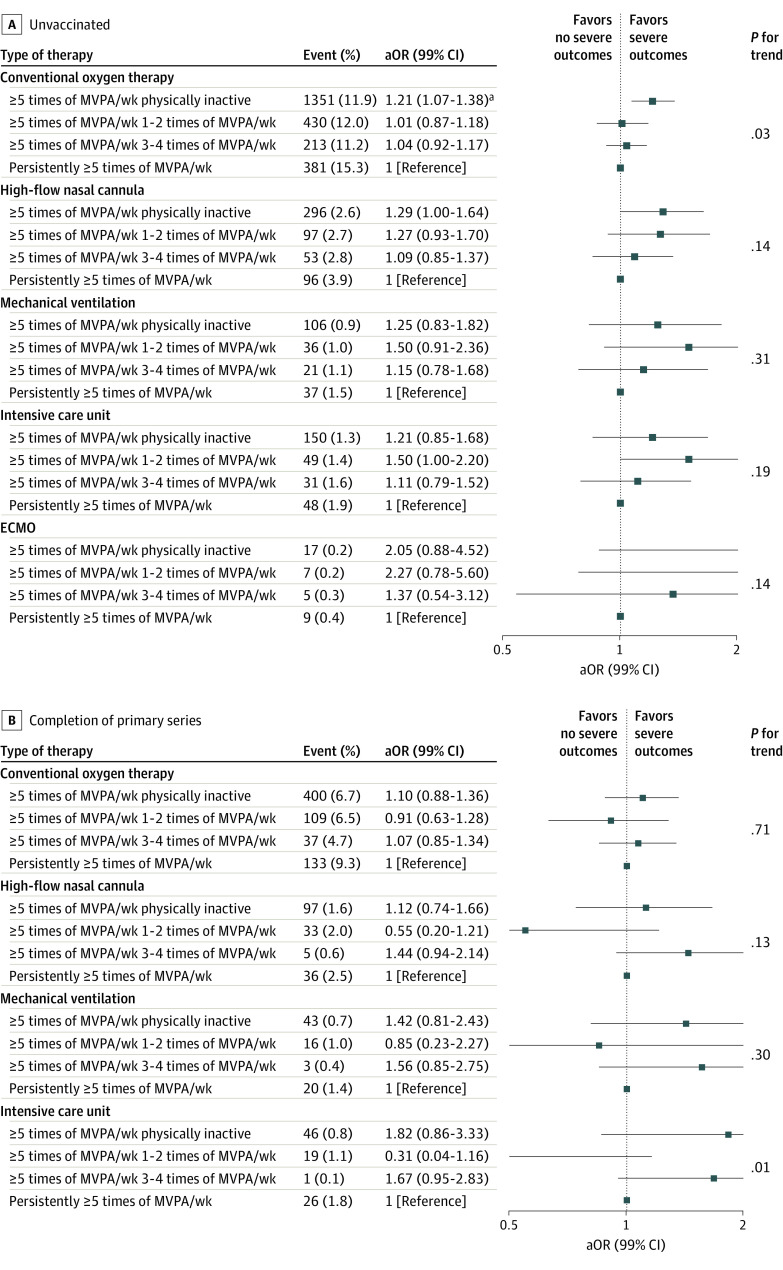
For MVPA at period 2, the odds for COT and HFNC among unvaccinated patients decreased as the frequency increased, whereas only the odds for COT decreased among patients with completion of the primary series (eTables 8 and 9 in Supplement 1). Compared with the unvaccinated patients who were persistently physically inactive, the odds for severe COVID-19 clinical outcomes decreased when MVPA increased from physically inactive to MVPA 3 to 4 times per week for HFNC (aOR, 0.68; 99% CI, 0.53-0.86) and mechanical ventilation (aOR, 0.64; 99% CI, 0.42-0.96) (Figure 2A), whereas the patients with completion of the primary series only showed a decreased odds for COT (aOR, 0.81; 99% CI, 0.65-1.00) (Figure 2B). Conversely, compared with unvaccinated patients who engaged persistently in MVPA 5 or more times per week, the odds for COT increased when MVPA decreased from 5 or more times per week to physically inactive (aOR, 1.21; 99% CI, 1.07-1.38) (Figure 3A). No association with decreased MVPA was found among patients who completed the primary series (Figure 3B). Clinical events experienced by fewer than 5 patients are listed separately in eTables 10 to 13 in Supplement 1. A similar association was found with MET score as an explanatory variable (eTables 14-17 in Supplement 1).
Figure 2. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MVPA From Physically Inactive at NHIS Health Screening Period 1 (2017-2018) by Vaccination Status.
Adjusted odds ratios (aORs) and 99% CIs were calculated using logistic regression after adjustments for age, sex, household income, body mass index, fasting blood glucose, hypertension, diabetes, dyslipidemia, Charlson comorbidity index, smoking, and alcohol consumption. Each MVPA session was defined as more than 20 to 30 minutes based on self-report from the NHIS health screening records. ECMO indicates extracorporeal membrane oxygenation; NHIS, National Health Insurance Service.
aP < .001.
bP < .01.
Figure 3. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MVPA from at Least 5 Times per Week at NHIS Health Screening Period 1 (2017-2018) by Vaccination Status.
Adjusted odds ratios (aORs) and 99% CIs were calculated using logistic regression after adjustments for age, sex, household income, body mass index, fasting blood glucose, hypertension, diabetes, dyslipidemia, Charlson comorbidity index, smoking, and alcohol consumption. Each MVPA session was defined as more than 20 to 30 minutes based on self-report from the NHIS health screening records. ECMO indicates extracorporeal membrane oxygenation; NHIS, National Health Insurance Service.
aP < .001.
Subgroup Analyses
In the subgroup analyses, the odds significantly differed with age between unvaccinated participants and those who completed the primary vaccine series when MVPA increased. Patients 65 years or older showed a higher odds for infection when unvaccinated, but the trend was mitigated and showed a lower odds with completion of the primary vaccine series (eTables 18 and 19 in Supplement 1). In contrast, a decrease in MVPA with SARS-CoV-2 infection showed consistent results when stratified by age, sex, and CCI (eTables 20 and 21 in Supplement 1).
Discussion
Main Findings
In this large, nationwide, population-based, nested case-control study using the linked NHIS-KDCA cohort of 6.3 million patients, increase in MVPA was positively associated with SARS-CoV-2 infection, but negatively associated with severe COVID-19 outcomes. To our knowledge, this study is the first to examine the longitudinal changes in MVPA with COVID-19–related outcomes stratified by COVID-19 vaccination status. This evidence of an association between MVPA and an increased risk of SARS-CoV-2 infection but a lower the risk of severe COVID-19 outcomes is striking and can be controversial compared with previous studies.
It is well known that MVPA is beneficial in reducing the severity of COVID-19, including a reduced risk of hospitalization,17,18 requirement for COT,3,19,20 and admission to the ICU.2,3,7 However, the association of MVPA with SARS-CoV-2 infection was inconsistent with previous studies. Lee et al3 reported that aerobic and muscle-strengthening activities were associated with a lower risk of SARS-CoV-2 infection and severe COVID-19 outcomes. However, they reported no association between MVPA and COVID-19–related outcomes, which is inconsistent with our study. The authors also did not include data on COVID-19 vaccination, which has the strongest effect on COVID-19 outcomes and, therefore, could have misled the association. In addition, their participants who engaged in aerobic and muscle-strengthening activities tended to be younger, which could subject the results to healthy volunteer bias. Latorre-Román et al21 investigated PA patterns before severe COVID-19 outcomes and reported that vigorous PA lowered the risk of respiratory distress (relative risk, 0.45; 95% CI, 0.22-0.90), which is similar to our association of MVPA (period 2) with COT provided as an initial treatment for respiratory distress. However, in our study, risk of ICU admission was not significant in highly active participants, which is inconsistent with the findings of Sallis et al.2 They identified patients with COVID-19 from January 1 through October 21, 2020; therefore, it is likely that most of those infections were based on original virus, whereas our data included newer variants that have different characteristics and prognoses.
COVID-19 Vaccination and Severe COVID-19
In our analyses, the longitudinal risk of SARS-CoV-2 infection was mitigated and reversed at a single point of MVPA on completion of the primary series of COVID-19 vaccination. For severe COVID-19, the completion of the primary series mitigated or reversed the risk of COT, HFNV, and mechanical ventilation but not ICU admission and extracorporeal membrane oxygenation. The findings of a reduced risk for COVID-19–-related outcomes when vaccinated are noteworthy. Being vaccinated not only protects from the disease but also encourages regular MVPA by the sense of security against COVID-19, which has numerous health benefits. Therefore, to mitigate the exposure risk caused by MVPA, being fully vaccinated could be important for preventing SARS-CoV-2 infection. However, the limited association with severe COVID-19 could be attributable, in part, to differences in pathogenicity and severity of variants. The severity of the Omicron (B.1.1.529) variant is known to be lower than for the Delta (B.1.617.2) variant, which was the dominant variant in 2021 before Omicron was detected in South Korea.20,22,23 Therefore, we postulate that there may have been no additional risk-lowering associations with vaccination because the variant already had lower severity. Additional studies regarding the variants are warranted to support our hypothesis.
Possible Mechanisms for MVPA
Because the transmission of SARS-CoV-2 occurs via respiratory droplets and proximity is the key determinant of transmission, engagement in MVPA may increase vulnerability for exposure to the pathogen.24 A retrospective study reported that total PA showed higher odds for nonsevere SARS-CoV-2 infection (aOR, 1.10; 95% CI, 1.04-1.16), suggesting a high correlation of PA with exposure risk.8 Moderate to vigorous PA may reflect the exposure risk because we calculated it based on frequencies (days per week) and used the latest health screening, which can be interpreted as current behavior, based on studies reporting SARS-CoV-2 transmission dynamics through PA.11,13,25 On the other hand, MVPA may be beneficial in reducing sarcopenia,26 boosting the immune system,27 and enhancing cardiopulmonary function28 and, thus, reducing the severity of COVID-19 outcomes. In addition to host immunocompetence, MVPA inhibits the angiotensin-converting enzyme (ACE)/angiotensin (Ang) II/Ang II type 1 receptor pathway and stimulates the ACE2/Ang-(1-7)/Mas receptor axis, which is known for its association with severe COVID-19.29,30 Therefore, MVPA may act as a protective factor for severe COVID-19.
Strengths and Limitations
A key strength of our study is the large size of the analytic cohort, which numbered approximately 6.3 million patients with COVID-19–related records. The large sample size allowed us to examine the association of MVPA with sufficient statistical power. Another key strength is the longitudinal design to fully examine MVPA with COVID-19–related outcomes.
This study also has several limitations. First, MVPA was measured based on self-report questionnaires, which can cause information bias or misclassification. From the responses, however, we were able to classify MVPA intensity and frequency, and by adding duration, we could calculate the MET score to add to the robustness of our findings.15 Second, despite being statistically significant, the effect size of the association was small in magnitude. However, engaging in regular MVPA has many other health benefits, including improvements in mental health and chronic comorbidities in addition to an immunosurveillance effect, which are crucial in pandemic situations.28,31 Third, MVPA at period 2 (2019-2020) was assessed before and during implementation of social distancing in South Korea, which may have confounded the results. In South Korea, social distancing was first implemented on March 22, 2020, and continued with a stepwise strategy to control the spread of the virus. Depending on the social distancing stage, the policy on group activity rapidly changed, and therefore, MVPA measurement may be prone to recall bias. However, the effect size of MVPA levels did not differ between participants with and without COVID-19 infection in both periods. Fourth, only leisure time PA, not occupational PA, was considered. Fifth, our data did not provide information on the spike gene mutation to stratify the infection with variants. Sixth, the proportion of reinfection (<0.1%) was very low compared with other studies. Since a recent study reported that most cases of reinfection occurred during the Omicron period, the very low proportion could be accounted for by the follow-up period ending on December 31, 2021, which was the beginning of the Omicron period.32 Seventh, due to the limitations of an observational study, reverse causality could not be fully solved with MVPA and COVID-19 outcomes.
Conclusion
In this case-control study, we found evidence that MVPA was directly associated with SARS-CoV-2 infection and inversely associated with severe clinical outcomes of COVID-19. The findings contrast with the current thought that MVPA would lower the risk of SARS-CoV-2 infection. However, the association of MVPA with infection was mitigated when patients were fully vaccinated, further encouraging MVPA for vaccinated patients. Our results showed a limited association with risk of severe COVID-19 outcomes and, therefore, need further investigation regarding the variants. The findings also suggest further consideration of adjusting public health interventions regarding COVID-19, including exercise and vaccination.
eTable 1. Association of MVPA at Period 2 (2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Were Unvaccinated or Completed the Primary Series
eTable 2. Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Reinfection Among Participants Who Were Unvaccinated
eTable 3. Cox Regression Model of the Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Were Unvaccinated
eTable 4. Cox Regression Model of the Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Completed the Primary Series
eTable 5. Association of Changes in MET Score Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Were Unvaccinated or Completed the Primary Series
eTable 6. Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants With Completion of Homologous and Heterologous Primary Vaccine Schedules
eTable 7. Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Breakthrough Infection Among Participants Who Completed the Primary Series
eTable 8. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to MVPA at Health Screening Period 2 (2019-2020) Among Unvaccinated Patients With COVID-19
eTable 9. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to MVPA at Health Screening Period 2 (2019-2020) Among Patients With COVID-19 Who Completed the Primary Series
eTable 10. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MVPA From Physically Inactive Among Unvaccinated Patients With COVID-19
eTable 11. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MVPA From Physically Inactive Among Patients With COVID-19 Who Completed the Primary Series
eTable 12. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MVPA From 5 or More Times of MVPA Per Week Among Unvaccinated Patients With COVID-19
eTable 13. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MVPA From 5 or More Times of MVPA Per Week Among Patients With COVID-19 Who Completed the Primary Series
eTable 14. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MET Score From Physically Inactive Among Unvaccinated Patients With COVID-19
eTable 15. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MET Score From Physically Inactive Among Patients With COVID-19 Who Completed the Primary Series
eTable 16. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MET Score From Physically Inactive Among Unvaccinated Patients With COVID-19
eTable 17. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MET Score From Physically Inactive Among Patients With COVID-19 Who Completed the Primary Series
eTable 18. Stratified Analyses on the Association of Increase in MVPA With Risk of COVID-19 Infection Among Unvaccinated Patients
eTable 19. Stratified Analyses on the Association of Increase in MVPA With Risk of COVID-19 Infection Among Those Who Completed the Primary Series
eTable 20. Stratified Analyses on the Association of Decrease in MVPA With Risk of COVID-19 Infection Among Unvaccinated Patients
eTable 21. Stratified Analyses on the Association of Decrease in MVPA With Risk of COVID-19 Infection Among Those Who Completed the Primary Series
eMethods.
eReferences.
Data Sharing Statement
References
- 1.Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428-455. doi: 10.1111/all.14657 [DOI] [PubMed] [Google Scholar]
- 2.Sallis R, Young DR, Tartof SY, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. 2021;55(19):1099-1105. doi: 10.1136/bjsports-2021-104080 [DOI] [PubMed] [Google Scholar]
- 3.Lee SW, Lee J, Moon SY, et al. Physical activity and the risk of SARS-CoV-2 infection, severe COVID-19 illness and COVID-19 related mortality in South Korea: a nationwide cohort study. Br J Sports Med. 2022;56(16):901-912. doi: 10.1136/bjsports-2021-104203 [DOI] [PubMed] [Google Scholar]
- 4.Ezzatvar Y, Ramírez-Vélez R, Izquierdo M, Garcia-Hermoso A. Physical activity and risk of infection, severity and mortality of COVID-19: a systematic review and non-linear dose-response meta-analysis of data from 1 853 610 adults. Br J Sports Med. 2022;56(20):1188-1193. doi: 10.1136/bjsports-2022-105733 [DOI] [PubMed] [Google Scholar]
- 5.Cho DH, Lee SJ, Jae SY, et al. Physical activity and the risk of COVID-19 infection and mortality: a nationwide population-based case-control study. J Clin Med. 2021;10(7):1539. doi: 10.3390/jcm10071539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieman DC. Exercise is medicine for immune function: implication for COVID-19. Curr Sports Med Rep. 2021;20(8):395-401. doi: 10.1249/JSR.0000000000000867 [DOI] [PubMed] [Google Scholar]
- 7.Steenkamp L, Saggers RT, Bandini R, et al. Small steps, strong shield: directly measured, moderate physical activity in 65 361 adults is associated with significant protective effects from severe COVID-19 outcomes. Br J Sports Med. 2022;56(10):568-576. doi: 10.1136/bjsports-2021-105159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rowlands AV, Dempsey PC, Gillies C, et al. Association between accelerometer-assessed physical activity and severity of COVID-19 in UK Biobank. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):997-1007. doi: 10.1016/j.mayocpiqo.2021.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowlands AV, Kloecker DE, Chudasama Y, et al. Association of timing and balance of physical activity and rest/sleep with risk of COVID-19: a UK Biobank study. Mayo Clin Proc. 2021;96(1):156-164. doi: 10.1016/j.mayocp.2020.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutsch B, Heiber M, Grätz F, et al. Aerosol particle emission increases exponentially above moderate exercise intensity resulting in superemission during maximal exercise. Proc Natl Acad Sci U S A. 2022;119(22):e2202521119. doi: 10.1073/pnas.2202521119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bak A, Mugglestone MA, Ratnaraja NV, et al. SARS-CoV-2 routes of transmission and recommendations for preventing acquisition: joint British Infection Association (BIA), Healthcare Infection Society (HIS), Infection Prevention Society (IPS) and Royal College of Pathologists (RCPath) guidance. J Hosp Infect. 2021;114:79-103. doi: 10.1016/j.jhin.2021.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chastin SFM, Abaraogu U, Bourgois JG, et al. Effects of regular physical activity on the immune system, vaccination and risk of community-acquired infectious disease in the general population: systematic review and meta-analysis. Sports Med. 2021;51(8):1673-1686. doi: 10.1007/s40279-021-01466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider IE, Lindsey G, Petesch M, et al. An integrated approach to monitoring and estimating COVID-19 risk exposure among leisure-time physical activity participants. J Transp Health. 2021;22:101088. doi: 10.1016/j.jth.2021.101088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the Korean National Health Insurance and Health Insurance Review & Assessment Database. Diabetes Metab J. 2020;44(5):671-678. doi: 10.4093/dmj.2020.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chun MY. Validity and reliability of Korean version of international physical activity questionnaire short form in the elderly. Korean J Fam Med. 2012;33(3):144-151. doi: 10.4082/kjfm.2012.33.3.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doerken S, Avalos M, Lagarde E, Schumacher M. Penalized logistic regression with low prevalence exposures beyond high dimensional settings. PLoS One. 2019;14(5):e0217057. doi: 10.1371/journal.pone.0217057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brawner CA, Ehrman JK, Bole S, et al. Inverse relationship of maximal exercise capacity to hospitalization secondary to coronavirus disease 2019. Mayo Clin Proc. 2021;96(1):32-39. doi: 10.1016/j.mayocp.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamer M, Kivimäki M, Gale CR, Batty GD. Lifestyle risk factors, inflammatory mechanisms, and COVID-19 hospitalization: a community-based cohort study of 387,109 adults in UK. Brain Behav Immun. 2020;87:184-187. doi: 10.1016/j.bbi.2020.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uz C, Umay E, Gundogdu I, et al. Predisease physical activity level and current functional capacity in patients with COVID-19: relationship with pneumonia and oxygen requirement. J Phys Act Health. 2021;18(11):1358-1363. doi: 10.1123/jpah.2021-0008 [DOI] [PubMed] [Google Scholar]
- 20.Hyams C, Challen R, Marlow R, et al. ; AvonCAP Research Group . Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: A prospective cohort study in Bristol, United Kingdom. Lancet Reg Health Eur. 2023;25:100556. doi: 10.1016/j.lanepe.2022.100556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Latorre-Román PÁ, Guzmán-Guzmán IP, Delgado-Floody P, et al. Protective role of physical activity patterns prior to COVID-19 confinement with the severity/duration of respiratory pathologies consistent with COVID-19 symptoms in Spanish populations. Res Sports Med. 2023;31(1):74-85. doi: 10.1080/15438627.2021.1937166 [DOI] [PubMed] [Google Scholar]
- 22.Park AK, Kim IH, Man Kim H, et al. SARS-CoV-2 B.1.619 and B.1.620 lineages, South Korea, 2021. Emerg Infect Dis. 2022;28(2):415-419. doi: 10.3201/eid2802.211653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee D-W, Kim J-M, Park AK, et al. Genomic epidemiology of SARS- CoV-2 Omicron variants in the Republic of Korea. Sci Rep. 2022;12(1):22414. doi: 10.1038/s41598-022-26803-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyerowitz EA, Richterman A, Gandhi RT, Sax PE. Transmission of SARS-CoV-2: a review of viral, host, and environmental factors. Ann Intern Med. 2021;174(1):69-79. doi: 10.7326/M20-5008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae S, Kim H, Jung T-Y, et al. Epidemiological characteristics of COVID-19 outbreak at fitness centers in Cheonan, Korea. J Korean Med Sci. 2020;35(31):e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier NF, Lee DC. Physical activity and sarcopenia in older adults. Aging Clin Exp Res. 2020;32(9):1675-1687. doi: 10.1007/s40520-019-01371-8 [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Liu S, Li G, Xiao J. Exercise regulates the immune system. Adv Exp Med Biol. 2020;1228:395-408. doi: 10.1007/978-981-15-1792-1_27 [DOI] [PubMed] [Google Scholar]
- 28.Pinckard K, Baskin KK, Stanford KI. Effects of exercise to improve cardiovascular health. Front Cardiovasc Med. 2019;6:69. doi: 10.3389/fcvm.2019.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arazi H, Falahati A, Suzuki K. Moderate intensity aerobic exercise potential favorable effect against COVID-19: the role of renin-angiotensin system and immunomodulatory effects. Front Physiol. 2021;12:747200. doi: 10.3389/fphys.2021.747200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fagyas M, Fejes Z, Sütő R, et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int J Infect Dis. 2022;115:8-16. doi: 10.1016/j.ijid.2021.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ai X, Yang J, Lin Z, Wan X. Mental health and the role of physical activity during the COVID-19 pandemic. Front Psychol. 2021;12:759987. doi: 10.3389/fpsyg.2021.759987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guedes AR, Oliveira MS, Tavares BM, et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci Rep. 2023;13(1):712. doi: 10.1038/s41598-022-25908-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association of MVPA at Period 2 (2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Were Unvaccinated or Completed the Primary Series
eTable 2. Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Reinfection Among Participants Who Were Unvaccinated
eTable 3. Cox Regression Model of the Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Were Unvaccinated
eTable 4. Cox Regression Model of the Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Completed the Primary Series
eTable 5. Association of Changes in MET Score Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants Who Were Unvaccinated or Completed the Primary Series
eTable 6. Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Infection Among Participants With Completion of Homologous and Heterologous Primary Vaccine Schedules
eTable 7. Association of Changes in MVPA Between 2 Biennial Health Screening Periods (2017-2018 and 2019-2020) With Risk of SARS-CoV-2 Breakthrough Infection Among Participants Who Completed the Primary Series
eTable 8. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to MVPA at Health Screening Period 2 (2019-2020) Among Unvaccinated Patients With COVID-19
eTable 9. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to MVPA at Health Screening Period 2 (2019-2020) Among Patients With COVID-19 Who Completed the Primary Series
eTable 10. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MVPA From Physically Inactive Among Unvaccinated Patients With COVID-19
eTable 11. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MVPA From Physically Inactive Among Patients With COVID-19 Who Completed the Primary Series
eTable 12. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MVPA From 5 or More Times of MVPA Per Week Among Unvaccinated Patients With COVID-19
eTable 13. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MVPA From 5 or More Times of MVPA Per Week Among Patients With COVID-19 Who Completed the Primary Series
eTable 14. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MET Score From Physically Inactive Among Unvaccinated Patients With COVID-19
eTable 15. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to an Increase in MET Score From Physically Inactive Among Patients With COVID-19 Who Completed the Primary Series
eTable 16. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MET Score From Physically Inactive Among Unvaccinated Patients With COVID-19
eTable 17. Adjusted Odds Ratios for Severe Clinical Outcomes of COVID-19 Within 1 Month According to a Decrease in MET Score From Physically Inactive Among Patients With COVID-19 Who Completed the Primary Series
eTable 18. Stratified Analyses on the Association of Increase in MVPA With Risk of COVID-19 Infection Among Unvaccinated Patients
eTable 19. Stratified Analyses on the Association of Increase in MVPA With Risk of COVID-19 Infection Among Those Who Completed the Primary Series
eTable 20. Stratified Analyses on the Association of Decrease in MVPA With Risk of COVID-19 Infection Among Unvaccinated Patients
eTable 21. Stratified Analyses on the Association of Decrease in MVPA With Risk of COVID-19 Infection Among Those Who Completed the Primary Series
eMethods.
eReferences.
Data Sharing Statement