Abstract
Experimental evidence supports the fact that changes in the bowel microflora due to environmental or dietary factors have been investigated as implicating factors in the etiopathogenesis of inflammatory bowel disease (IBD). The amassing knowledge that the inhabited microbiome regulates the gut physiology and immune functions in IBD, has led researchers to explore the effectiveness of prebiotics, probiotics, and synbiotics in treating IBD. This therapeutic approach focuses on restoring the dynamic balance between the microflora and host defense mechanisms in the intestinal mucosa to prevent the onset and persistence of intestinal inflammation. Numerous microbial strains and carbohydrate blends, along with their combinations have been examined in experimental colitis models and clinical trials, and the results indicated that it can be an attractive therapeutic strategy for the suppression of inflammation, remission induction, and relapse prevention in IBD with minimal side effects. Several mechanisms of action of probiotics (for e.g., Lactobacillus species, and Bifidobacterium species) have been reported such as suppression of pathogen growth by releasing certain antimicrobial mediators (lactic and hydrogen peroxide, acetic acid, and bacteriocins), immunomodulation and initiation of an immune response, enhancement of barrier activity, and suppression of human T-cell proliferation. Prebiotics such as lactulose, lactosucrose, oligofructose, and inulin have been found to induce the growth of certain types of host microflora, resulting in an enriched enteric function. These non-digestible food dietary components have been reported to exert anti-inflammatory effects by inhibiting the expression of tumor necrosis factor-α-related cytokines while augmenting interleukin-10 levels. Although pro-and prebiotics has established their efficacy in healthy subjects, a better understanding of the luminal ecosystem is required to determine which specific bacterial strain or combination of probiotics and prebiotics would prove to be the ideal treatment for IBD. Clinical trials, however, have given some conflicting results, requiring the necessity to cite the more profound clinical effect of these treatments on IBD remission and prevention. The purpose of this review article is to provide the most comprehensive and updated review on the utility of prebiotics, probiotics, and synbiotics in the management of active Crohn’s disease and ulcerative colitis/pouchitis.
Keywords: Ulcerative colitis, Crohn’s disease, Pouchitis, Dysbiosis, Microbiota, Inflammation
Core Tip: Current treatments for inflammatory bowel disease (IBD), such as corticosteroids and immunosuppressants, have potential adverse effects, and a significant proportion of patients dependent on these treatments are exposed to these associated long-term side effects. The discovery of novel and efficacious therapeutic strategies is a worldwide goal of IBD research, and probiotics, prebiotics, and synbiotics can offer viable solutions. These products offer a novel strategy to deliver beneficial components into the gut and emerge as promising new treatments for IBD, as intestinal dysbiosis has been reported as a major cause of his IBD. The review highlights the current state and action mechanism of these microbial therapies along with various studies that have reported their effectiveness in restoring balance in the gastrointestinal microbiota and thus eventually reducing intestinal inflammation.
INTRODUCTION
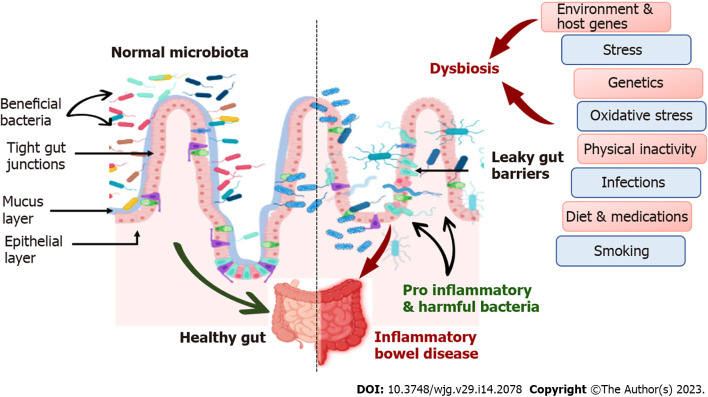
Inflammatory bowel disease (IBD) is an idiopathic disease resulting from a debilitating immunological response of the body to the host’s gastrointestinal (GI) microflora (mainly colon and duodenum). IBD classified as Crohn’s disease (CD) and ulcerative disease (UC), was previously anticipated to be triggered by adaptive immune responses, however, the recent research findings suggest the prominent role of the innate immune system in instigating an imbalance and disparity between the beneficial microbiome and commensal microflora harboring in the human gut. This imbalance, known as dysbiosis (Figure 1), leads to an aggravated inflammatory response, causing IBD (Figure 2). Alterations in the microbiome affect host homeostatic systems and interactions with luminal stimuli, which can ultimately lead to uncontrolled inflammation in the intestinal mucosa, leading to IBD (Figure 3). This suggests that the human gut microbiome is beginning to be recognized for its important role and potential therapeutic solution for IBD. A better comprehensive understanding of the synergy between host genetics, external environmental factors and gut microbiome has opened new paradigms for seeking alternative effective therapies[1].
Figure 1.
Comparison between normal microbiome and dysbiosis.
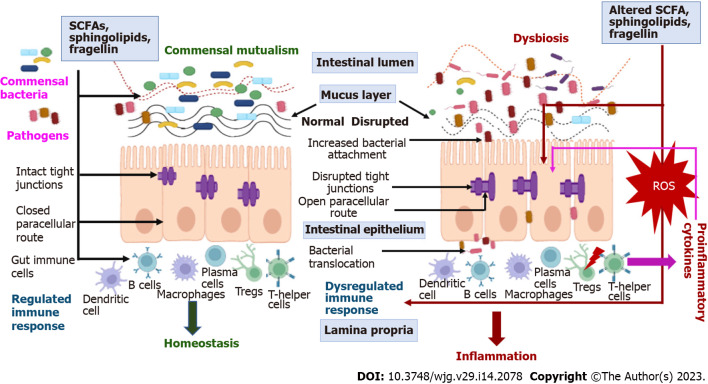
Figure 2.
Role of dysbiosis in inflammatory bowel disease pathophysiology. SCFA: Short chain fatty acids; Tregs: Regulatory T cells; ROS: Reactive oxygen species.
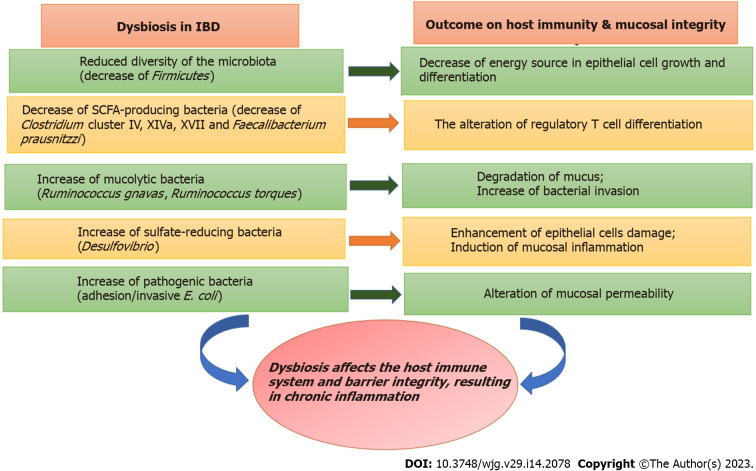
Figure 3.
Dysbiosis in inflammatory bowel disease and its pathological outcomes. IBD: Inflammatory bowel disease; SCFA: Short chain fatty acids.
PROBIOTICS, PREBIOTICS, AND SYNBIOTICS AS THERAPEUTIC STRATEGY
Standard clinical treatment of IBD consists of agents that modulate the inflammatory pattern of the GI tract (GIT), including mesalamine, azathioprine, anti-tumor necrosis factors (TNFs), and glucocorticoids. However, these drugs often appear to have serious side effects, and some patients require higher doses throughout the course of treatment. A significant proportion of patients with IBD either initially do not respond to treatment or lose response over time. Gut microbiota modulation has emerged as an attractive new therapeutic approach for IBD, and gut microbiota-targeted/based therapies have been intensively investigated with varying degrees of success. Although the exact etiology of IBD remains unknown, the critical role of the gut microbiota in the development and persistence of IBD highlights the importance of microbiota-host interactions in health and disease. Recent advances in assessing the therapeutic potential of microbiota in the treatment of IBD support the reconstitution of microbial resident populations by administration of appropriate microbes. The gut microbiota influences the host by modulating physiological, pathophysiological, and immunological processes. Experimental animal studies along with clinical data have confirmed the influence of the gut microbiome in ameliorating inflammation, highlighting its potential as a therapeutic strategy for treating inflammatory diseases. Numerous therapeutic strategies have been developed to modify and remodel the gut microbiome for the treatment of other GI diseases, including IBD. Prebiotics, probiotics, synbiotics, and fecal microbiota transplantation (FMT) are currently considered to be the most common treatments[2].
PROBIOTICS
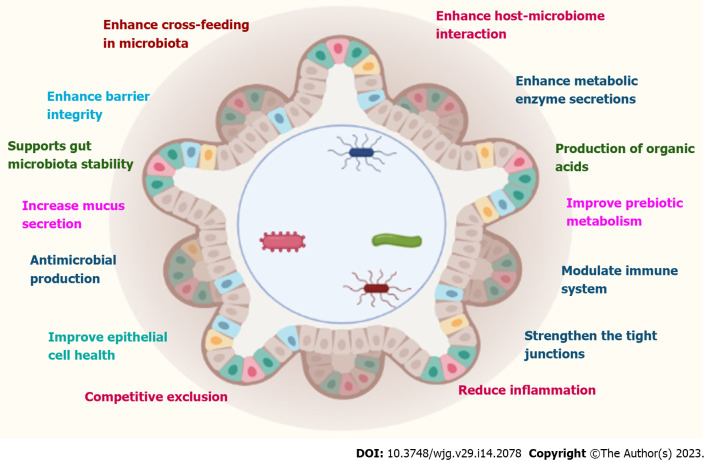
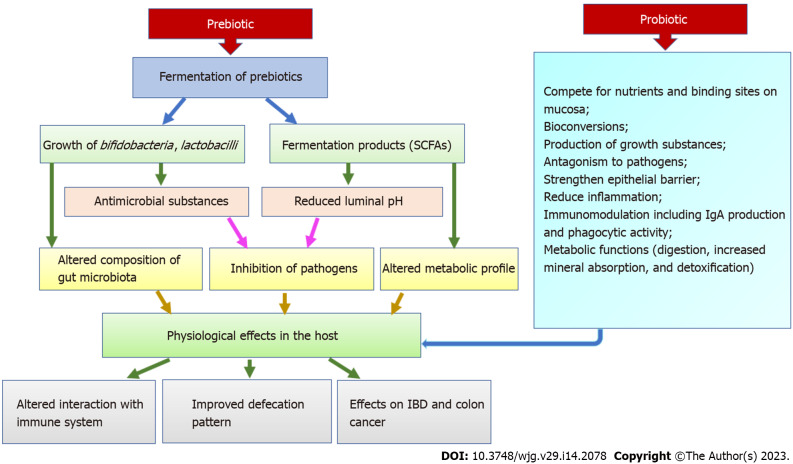
Probiotics are specific live microorganisms, when consumed in appropriate amount, are beneficial to the health of the host. Probiotic therapy involves the targeted introduction of beneficial microorganisms into the intestinal flora. This causes many beneficial bacteria to compete for nutrients and starve harmful bacteria. Probiotics participate in many positive health-promoting activities in human physiology, including the maintenance of a healthy gut. The most common strains currently available as probiotics and possessing beneficial health effects are Enterococcus faecium, Bifidobacterium, Bacillus, Saccharomyces boulardii (S. boulardii), Lactobacillus strains, and Pediococcus. Molecular mechanisms for the beneficial effects of these probiotics include (Figure 4): (1) Production of butyrate, immunoglobulin A (IgA), and short-chain fatty acid (SCFA) formation and stimulatory signaling proteins; (2) Reduced secretion of pro-inflammatory cytokines; (3) Increased mucin-2 expression; (4) Increased autophagy; and (5) Augmented upregulation of defensins. Although probiotics have shown promise both preclinically and clinically, the theoretical risks have been explained in several case reports, clinical trial results, and experimental models. These include systemic infections, adverse metabolic activity, overstimulation of the immune system in susceptible individuals, gene transfer, and GI side effects[3].
Figure 4.
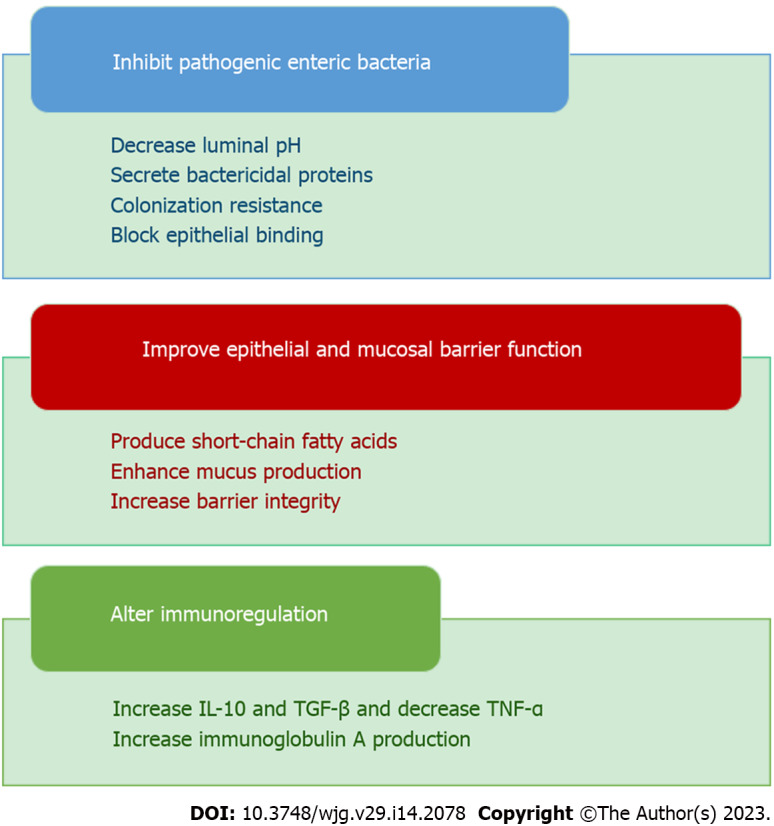
Various actions of probiotics. IL-10: Interleukin-10; TGF-β: Transforming growth factor-β; TNF-α: Tumor necrosis factor-alpha.
PREBIOTICS
Prebiotics are non-digestible food ingredients that selectively stimulate the growth of beneficial bacteria or promote the activity of a limited number of health-promoting bacteria. However, prebiotics can also help to improve the existence and effectiveness of ingested PRO bacteria. According to the most recent definition, “a prebiotic is a selectively fermented ingredient that allows specific changes, both in the composition and/or activity in the GI microbiota that confers benefits upon host well-being and health”. Now, the International Scientific Association of probiotics and Prebiotics has introduced a new definition of prebiotics as “a substrate which is selectively fermented by the gut microflora and bestows health benefits to the host”. This new definition states that non-carbohydrate constituents are also considered prebiotics and their applications are not constrained to the GIT only[4].
SYNBIOTICS
Combinations of probiotics and prebiotics are viewed as promising new approaches and provide an opportunity to explore their potential and efficacy in human IBD. When probiotics and prebiotics are combined in a product to achieve synergistic actions, they are commonly referred to as synbiotics. Many examples have demonstrated that prebiotics appears to be more efficacious when used along with a probiotic as a part of the synbiotic combination. The term synbiotic refers to synergism where the prebiotic component is selectively favoured by the live probiotic organism. The synbiotic combination is intended to enhance the in vivo survival and activity of proven probiotics to promote or enhance the beneficial properties of both products. However, recently the term ‘synbiotics’ has been re-defined as preparations favoring synergism, where the probiotics metabolize the complemented prebiotics to induce specific rebalancing of the dysbiotic gut and host health. Synergistic probiotics and prebiotics stimulate selective microbial growth or activate specific metabolism via gut flora. The presence of the readily fermentable substrate should enhance the survival of the probiotic. The prebiotic component should also protect the probiotic from gastric acidity and proteolysis, possibly through steric hindrance and coating of the probiotic. Therefore, it is important to select specific substrate and microbial combinations in synbiotic products that can enhance beneficial effects compared to products containing probiotics or prebiotics alone[5].
MECHANISM OF ACTION OF PROBIOTICS, PREBIOTICS, AND SYNBIOTICS
Probiotics
Probiotics, prebiotics, or synbiotics can achieve therapeutic effects in IBD through various mechanisms. The mechanisms of action of probiotics include competitive actions with commensal and pathogenic bacteria and effects on epithelial function and immune responses. By augmenting the production of SCFA, they can lower the pH of the intestinal environment, thereby inhibiting the growth of potentially pathogenic microorganisms. Some probiotics enhance the integrity of the mucosal barrier thereby normalizing intestinal permeability[6]. The effects of probiotics vary and depend on type and dose as well as on their interaction with the host in different ways. Some exhibit direct antibacterial action via the production of substances such as bacteriocins, hydroperoxides, lactic acid, and defensins. Others exhibit non-immunological action such as competing with pathogens for nutrients, increasing mucus production, changing intestinal pH, by promoting the formation of tight junctions (TJs), or enhancing tissue repair processes, thereby reducing intestinal mucosal permeability. Finally, probiotics can also modulate the immunological response (immunoglobulin production, pro-inflammatory cytokine production) by releasing cell wall fragments or DNA in the intestinal lumen (Figure 5). They also regulate the overactivation of the nuclear factor kappa light chain enhancer of activated B cells (NFκB) pathway, reduce the production and secretion of pro-inflammatory cytokines [such as interleukin (IL)-8, TNF-α, interferon gamma (IFN-γ)], and induce the production of anti-inflammatory cytokines such as IL-10 and transforming growth factor (TGF)-β[7].
Figure 5.
The mechanism of actions of probiotics in combating inflammatory bowel disease symptoms. IBD: Inflammatory bowel disease.
Prebiotics
Prebiotics exert beneficial effects on IBD through multiple mechanisms of action. Primarily they accelerate the selective proliferation of native bacteria of the gut microbiota. These ingredients provide a better breeding space for beneficial microorganisms due to their loose structure and large surface area and at the same time inhibit the growth of pathogens. Secondly, they increase the production of SCFAs such as acetate, butyrate, and propionate. These SCFAs are formed during the fermentation of prebiotics and play a very vital role in the proper functioning of the intestine. They accelerate the regeneration and healing process of the intestinal epithelial cell; augment mucus production; maintain the correct pH in the intestine. They also inhibit the attachment of pathogenic microbes to enterocytes. Acetate is commonly used as a cellular fuel for building muscle and colonic tissue. Butyrate exhibit various beneficial effects on the host, such as improving metabolism, modulating the host’s immune system, and promoting anti-inflammatory actions, therefore receives special attention[8].
Therefore, prebiotic consumption has been shown to boost host immune function, reduce infection rates, enhance colonic integrity, and downregulate allergic reactions. However, these effects are not directly imposed by ingesting prebiotics. It was suggested that the benefits of prebiotics are achieved indirectly. Prebiotics improves the mucosal barrier via encouraging the probiotics growth that can upregulate epithelial defense mechanisms[6].
Synbiotics
In vitro studies specify that synbiotics exert primarily anti-inflammatory actions along with some antiproliferative activities. The literature on synbiotics is difficult to interpret, as it is often impossible to distinguish whether the desired therapeutic benefits are attributable to prebiotics, probiotics, or synergistic interactions between them. Various studies have provided robust preliminary evidence that synbiotic administration to IBD patients results in beneficial therapeutic effects. In one study, prebiotic Synergy 1 in combination with Bifidobacterium longum improved sigmoidoscopy scores and reduced b-defensins, TNF-α, and IL-1α in biopsy samples from UC patients. In another study, patients who received Bifidobacterium longum and prebiotic Synergy 1 [with Fructooligosaccharides (FOS)/inulin blend] combination revealed a significant histological improvement in comparison to the placebo group. Synbiotics significantly reduced the TNF-α expression and thereby reported the potential beneficial effect of synbiotics in the management of IBD (Figure 6). Combinations of synbiotics may exert beneficial impacts on the intestinal mucosa. Therefore, evaluating the role of synbiotics as an alternative form of IBD treatment should be considered[9].
Figure 6.
Mechanisms of action of probiotics, prebiotics, and synbiotics. SCFA: Short chain fatty acids; IBD: Inflammatory bowel disease.
PROBIOTICS IN IBD
Research on the use of probiotics in the treatment of IBD has been conducted since 1997. A 50% increase in probiotic use has been reported in IBD patients. This is due to the belief that probiotics are safe and beneficial as adjunctive therapy for IBD patients in both exacerbation and remission. Despite the moderately huge data reports on the use of probiotics in IBD, the possibility to draw firm conclusions is significantly limited. This may be due to the small number of patients in the study groups, large differences in intervention types, or lack of standardization in study methods. There are also few published clinical studies on the effects of probiotics on inflammatory changes examined by GI endoscopy in IBD patients. However, the potential use of well-selected commensal microbial species with protective effects on the intestinal mucosa and modulation of immune responses offers hope for new treatment options for patients with IBD[9]. Various preclinical studies have been conducted to explore the efficiency of probiotics in the IBD are summarized in Table 1 and the clinical interventions are summarized in Table 2.
Table 1.
Summary of preclinical studies of probiotics
|
Ref.
|
Model
|
Treatment
|
Dose and duration
|
Parameters analyzed
|
Conclusion
|
| Yoo et al[26], 2022 | DSS-induced colitis in male C57BL/6 mice | Lactobacillus plantarum, Bifidobacterium longum, and Bifidobacterium bifidum | 1 × 109 CFU once daily for 5 d | Cytokines and corticosterone, colonic MPO activity, fecal LPS level | Suppressed colonic inflammation, and fatigue by the suppression of the IL-1β or IL-6 to IL-10 expression ratio and gut bacterial LPS production |
| Qin et al[27], 2022 | DSS-induced colitis in mice | Lactobacillus (Pediococcus pentosaceus, Lactobacillus plantarum, and Weissella cibaria) | - | DAI, colon length, pathological score, cytokine secretion | Showed the potential to treat IBD |
| Khan et al[28], 2022 | DSS-induced colitis in mice (C57BL/6) | Lactobacillus plantarum | - | Colitis indexes, IL-17A, IL-17F, IL-6, IL-22, and TNF-α and anti-inflammatory cytokines, i.e., TGF-β, IL-10 | Restored gut microbiota balance and modulated the resident gut microbiota and immune response |
| Fei et al[29], 2022 | DSS-induced colitis in mice (C57/BL6) | Ligilactobacillus salivarius Li01 and RSV | Li01 (109 CFU/d) and RSV (1.5 g/kg/d) | IL-1β and IL-6, TGF-β and IL-17A | An improved synergistic anti-inflammatory effect from the RSV and Li01 combination treatment |
| Liu et al[30], 2021 | DSS-induced colitis in mice (C57/BL6) | Goji juice fermented by Lactobacillus plantarum, Lactobacillus reuteri and Streptococcus thermophilus | 20 mL/kg/d for 30 d | Pro-inflammatory cytokines and total superoxide dismutase in serum and colon, MPO and glutathione peroxidase | Probiotics-fermentation enhanced the anti-ulcerative colitis function of goji berry juice and modulated gut microbiota |
| Kim et al[31], 2020 | DSS-induced colitis in male C57BL/6 mice | Lactobacillus plantarum CBT LP3 (KCTC 10782BP) | 1 × 108 bacteria in 0.1 mL PBS once daily for 16 d | DAI, analysis of macrophages and T cell subsets gene expression and cytokine profiles | Effective anti-inflammatory effects, with increased induction of Treg and restoration of goblet cells, suppression of proinflammatory cytokines |
| Chen et al[32], 2020 | DSS-induced colitis in C57BL/6J mice | Bifidobacterium breve, CCFM683 | 0.2 mL (1010 CFU/d CCFM683, once daily for 2 wk | Weight loss, stool consistency and fecal blood | Improved intestinal epithelial barriers, restored gut microbiota |
| Chen et al[33], 2020 | DSS-induced colitis in C57BL/6 mice | Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis with (quadruple probiotics, Pqua) or without (triple probiotics, P-tri) aerobic Bacillus cereus | B. infantis, L. acidophilus, and E. faecalis (1.5 × 109 CFU respectively) in 200 uL PBS and B. cereus (0.5 × 108 CFU) in 200 uL once daily for 45 d | Intestinal inflammation and functions of multiple barriers, including the mucus barrier, and epithelial barrier | Effective (Aerobe-contained Pqua was a powerful adjuvant therapy for chronic colitis, via restoring the intestinal microflora and recovering the multi-barriers in the inflamed gut) |
| Komaki et al[34], 2020 | Mice | Lactococcus lactis subsp. lactic JCM5805 | 1 mg, 5 mg, 10 mg, 15 mg, or 20 mg, once daily for 1 wk | The survival rate, length, histopathological parameters of the colon, and concentrations of inflammatory cytokines in serum | Effective (high-dose administration deteriorates intestinal inflammation) |
| Silveira et al[35], 2020 | C57BL/6 mice | Lactobacillus bulgaricus | 1 × 109 CFU was diluted in 200 mL of PBS, 3 times per week for 12 wk | Intestinal inflammation, cytokines levels were determined from colon and/or tumor | Regulates the inflammatory response and prevents Colitis-associated cancer |
DSS: Dextran sodium sulfate; CFU: Colony forming unit; LPS: Lipopolysaccharide; DAI: Disease activity Index; IBD: Inflammatory bowel disease; RSV: Resveratrol; MPO: Myeloperoxidase; IL: Interleukin; TNF-α: Tumor necrosis factor-α; TGF-β: Transforming growth factor-β; PBS: Phosphate buffer solution; Tregs: Regulatory T cells.
Table 2.
Summary of clinical studies of using probiotics in inflammatory bowel disease patients
|
Ref.
|
Treatment
|
Dose and duration
|
Parameters analyzed
|
Conclusion
|
| Agraib et al[43], 2022 | Lactobacillus and Bifidobacterium species | 3 × 1010 probiotic capsules | Partial mayo score, CRP, IgA, IL-10, hemoglobin, hematocrit, and RBC levels | Significantly induced remission in UC patients |
| Ojetti et al[44], 2022 | Limosilactobacillus reuteri ATCC PTA 4659 | Two times a day for 10 d | Inflammatory markers CRP and calprotectin | Significantly reduced both blood and fecal inflammatory marker |
| Wu et al[45], 2021 | Live bacillus licheniformis capsules and live combined Bifidobacterium, lactobacillus, and enterococcus capsules followed by Chamomile capsules | Bacillus licheniformis capsules two capsules, three times a day and live combined Bifidobacterium, lactobacillus, and enterococcus capsules two capsules twice a day. Probiotics consumed at a level of 107 CFU/mL | Leiden Index of Depression Sensitivity, Beck Depression Inventory questionnaires | Probiotics tend to improve cognitive reactivity to the sad mood in CD patients |
| Bamola et al[46], 2021 | Bacillus coagulans Unique IS-2 | 2 billion-CFU/capsule twice in day 4 wk | Presence of beneficial gut bacteria, serum cytokines, symptoms of the disease | Showed beneficial effect when administered along with standard medical treatment |
| Waal M van der et al[47], 2019 | Nine bacterial strains (Bifidobacterium bifidum W23; Bifidobacterium lactis W51; Bifidobacterium lactis W52; Lactobacillus acidophilus W22; Lactobacillus casei W56; Lactobacillus paracasei W20; Lactobacillus plantarum W62; Lactobacillus salivarius W24; Lactobacillus lactis W19) | 7.5 × 109 CFU per 3 g in powder form, once daily for 6 wk | Quality of life from a patient perspective (semi-structured interviews) | Effective |
| Kamarli Altun et al[48], 2022 | Enterococcus faecium, Lactobacillus Plantarum, Streptococcus thermophilus, Bifidobacterium lactis, Lactobacillus acidophilus, Bifidobacterium long, and FOS | Probiotic strains (3 × 109 CFU) and FOS (225 mg/ tablet), 1 tablet twice a day for 8 wk | Hemoglobin, leukocyte, neutrophil-to-lymphocyte ratio, sedimentation, and CRP, clinical and endoscopic activity indices | Effective |
| Sun et al[49], 2018 | Clostridium butyricum | 420 mg per capsule, 1.5 × 107 CFU/g, 3 capsules 3 times a day for 4 wk | Change from baseline in IBD symptoms, quality of life, stool consistency, and frequency | Effective |
| Matsuoka et al[50], 2018 | Bifidobacterium breve strain Yakult and Lactobacillus acidophilus | 10 billion bacteria of Bifidobacterium breve and 1 billion bacteria of Lactobacillus acidophilus, one pack per day for 48 wk | Clinical procedure (change in abdominal symptom score from baseline. Sutherland DAI subscore, change in abdominal symptom scores (passage of flatus and bloating), and intestinal microbiota | Less significant effect |
CRP: C-reactive protein; UC: Ulcerative colitis; CD: Crohn’s disease; CFU: Colony forming unit; FOS: Fructooligosaccharides; DAI: Disease activity index; IBD: Inflammatory bowel disease; IgA: Immunoglobulin A; RBC: Red blood cell; IL: Interleukin.
Effectiveness of probiotics in the animal models of colitis
Eradication of reactive oxygen species (ROS) by antioxidant enzymes such as catalase from the inflammatory site may efficiently restrain IBD pathogenesis. A genetically engineered probiotic E. coli Nissle 1917 (EcN), which acts by overexpressing the catalase and superoxide dismutase, was evaluated for the treatment of intestinal inflammation in a mouse IBD model induced by dextran sodium sulfate (DSS), 2,4,6-trinitrobenzene sulfonic acid (TNBS) and oxazolone. The probiotic bioavailability in the GIT was increased by the application of chitosan and sodium alginate effective biofilms. It effectively relieved inflammation and repaired epithelial barriers in the colon and restored the expression of TJ-associated proteins. It also regulated the gut microbial flora and augmented the abundance of vital microbes that helped in the maintenance of intestinal homeostasis such as Lachnospiraceae_NK4A136 and Odoribacter[10].
With remarkable advances in genetic engineering, scientists have recently developed bacterial/probiotic strains that are genetically engineered to function as ‘gut biosensors’ that can help to detect inflammatory markers or ‘resident cell factories’ of therapeutic molecules that will act as biotherapeutic drugs to improve the drug delivery at mucosal surfaces[10].
Wang et al[11] showed that an engineered EcN discharging the immunoregulatory protein Sj16 isolated from the helminth Schistosoma japonicum alleviated the DSS-induced colitis in mice by modifying the gut microbiota. The immunoregulatory protein exhibited a protective effect against colitis through its action on the peroxisome proliferator-activated receptor-alpha (PPAR-α) receptor, reestablishing populations of the Ruminococcaceae family, thereby augmenting intestinal butyrate levels.
Zhang et al[12] established a constitutively expressing IL-35 E. coli as a novel oral delivery system with immunosuppressive actions, facilitated by regulatory T cells and B cells. The IL-35-producing E. coli demonstrated a decrease in the inflammatory response in a mice model of colitis by downregulating Th17 cells.
Lactobacillus paracasei has been genetically engineered with the human N-acylphosphatidylethanolamine-specific phospholipase D gene and, when potentiated with an ultra-low exogenous dose of exogenous palmitate, it selectively induced palmitoylethanolamide in the GIT. PEA exerted potent anti-inflammatory effects, and it had showed improvement in inflammation in animal models of colitis[13].
Liu et al[14] designed an engineered Bifidobacterium longum R0175 (B. longum) that expresses the antioxidant enzyme manganese superoxide dismutase. The probiotic helped to improve colitis symptoms by attenuating the ROS-mediated oxidative stress and constraining endothelial cell activation. Following the treatment, attenuation in TNF-α, IL levels, as well as a complete improvement in the macroscopic and microscopic inflammatory markers was observed. Yet another study reported the beneficial effect of genetically modified B. longum that expresses αmelanocyte-stimulating hormone in a DSS model of colitis. The probiotic exhibited significant anti-inflammatory properties by suppressing the release of proinflammatory cytokines such as ILs, TNF-α, and NO, while increasing the anti-inflammatory cytokine (IL-10) release[15].
Feng et al[16] explored the ameliorating effects of pasteurized probiotic fermented milk on DSS-induced IBD in rats and found that intragastric gavage of milk prominently declined the disease activity index (DAI) scores and alleviated the colon tissue damage. The improvement was ascribed to the anti-inflammatory effect of the probiotic by decreasing TNF-α and IL-6 levels. The pasteurized probiotic fermented milk alleviated IBD by reducing the inflammatory response and restoring the gut microbiota.
Javed et al[17] showed that Bifidobacterium infantis had beneficial effects in alleviating TNBS-induced colitis. Supplementation with Bifidobacterium infantis demonstrated significantly less damage to mucosal cyto-structures and reduced the colitis symptoms. This demonstrates the importance of probiotics in protecting the goblet cells and epithelial cell layers[17]. Based on another research group, Bifidobacterium bifidum supplementation significantly increased IL-10 levels and decreased IL-1β levels in colonic sections, confirming anti-inflammatory effects. These results seem to support the regulatory properties of Bifidobacterium infantis and Bifidobacterium bifidum to reduce inflammation and clinical signs of colitis[18].
In IBD, a decrease in Firmicutes (F) abundance and an increase in the Bacteroidetes (B) bacteria are found to be associated with the disease progression. Early administration of L. reuteri DSM 17938 to C57BL/6J mice improved the abundance of F and diminished the abundance of B comparatively, thereby altering gut microbial homeostasis[19].
Live and dead L. plantarum AN1 administered to an IBD mouse model via drinking water exhibited intestinal regulatory and anti-inflammatory properties. A combination of two diverse probiotics (Bifidobacterium bifidum WBIN03 and L. plantarum ZDY2013) diminished the UC in mice by altering the microbiota and reducing oxidative stress as well as inflammation. The combination upregulated antioxidant factors and downregulated TNF-α in UC mice. A probiotic blend augmented the frequency of F and reduced the frequency of B[20].
The co-administration of the L. fermentum KBL374 and KBL375 amended gut dysbiosis and ameliorated colitis by decreasing the pro-inflammatory cytokine levels and augmented anti-inflammatory cytokines. These probiotics mechanism of action includes balancing the F/B ratio, epithelial cell barrier improvement and altering cytokine secretion[21].
Numerous data reports the utilization of multi-strain probiotic preparations. The VSL#3 is well known for its efficiency in IBD. VSL#3 is a commercial probiotic blend composed of 8 bacterial strains. Four lactobacillus strains (Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus delbrueckii subsp. bulgaricus), three Bifidobacterium strains (B. breve, Bifidobacterium longum, Bifidobacterium infantis), and a streptococcal strain (Streptococcus salivarius subsp. thermophilus). Several studies have demonstrated the effect of VSL#3 on DSS-induced colitis[22]. One study showed that VSL#3 (0.5 mL/d) reduced gut bacterial diversity associated with tissue injury. Conjugated linoleic acid was locally produced by VSL#3, which suppressed colitis by targeting myeloid cell PPARγ in the colon. Moreover, the anti-inflammatory effect remains the most important therapeutic mechanism of VSL#3 in DSS-induced rat colitis[23]. Both live and heat-killed VSL#3 decreased the expression of IL-23, IL-6, STAT3, and phosphorylated STAT3 (P-STAT3) in colonic tissue, thus reducing DSS-induced colitis in rats. The expression of inflammation-related mediators such as iNOS, NFκB was also inhibited by VSL#3[22].
Various experimental studies have concluded that VSL#3 normally acts on four components of the intestinal barrier: Biological, chemical, mechanical, and immune barriers. Regarding biological barriers, VSL#3 can increase the abundance of commensal gut bacteria and reduce the abundance of fungi. Regarding chemical barriers, VSL#3 can increase MUC2, MUC3, and MUC5AC gene expression and regulate mucus secretion. Regarding the mechanical barrier, VSL#3 can augment ZO-1 and occludin while attenuating claudin-2 to improve the function of TJs proteins. About the immune barrier, VSL#3 can inhibit the pro-inflammatory NFκB pathway while upregulating PPARα signalling[24].
A research group led by Hrdý et al[25] demonstrated that probiotic strains affect host cells in different ways. The mechanism of action of Bifidobacterium animalis species lactis B15764 and Lactobacillus reuteri Lr5454 were determined in mouse models of TNBS-induced colitis. Both strains exert beneficial effects on the host as expressed by body weight, gross indices of inflammation (Wallace score) and histopathological analysis (Ameho score), and lipocalin-2 levels in feces[25].
All of the above studies led to the inference that even a simplified preclinical model of colitis, which skips the genetic and external environmental influences, requires a broad and multidisciplinary approach.
Clinical study of probiotics among IBD patients
Several trials have reported the therapeutic action of the most common probiotic cocktail of proven efficacy, VSL#3 in adults with mild-to-moderate UC. In two clinical studies, VSL#3 was able to reduce DAI scores and significantly reduce clinical UC symptoms compared to the placebo. One study showed that 42.9% of patients treated with VSL#3 achieved remission in comparison to the placebo patients (15.7% remission only)[24]. Furthermore, VSL#3 and conventional drugs appear to have a synergistic effect. Although the mechanism is unknown, it is suggested that VSL#3 could enhance the anti-inflammatory effects of 5-aminosalicylic acid (5-ASA), inhibit free radical production, and suppress leukotriene and IL-1 production. A study also showed that combination therapy with VSL#3 and low-dose balsalazide was more efficacious than mesalazine or balsalazide alone in achieving remission of UC. Longer treatment with VSL#3 may result in greater improvement. Furthermore, in an open-label study, treatment with VSL#3 resulted in remission and improvement in 77% of patients with active mild-to-moderate disease UC. Two bacterial components B. infantis VSL#3 and S. salivarius subspecies thermophilus contributed a significant role in inducing remission by reaching the intestinal site of the disease. The effect of alkaline sphingomyelinase was also examined in 15 UC patients treated with VSL#3 for 5 wk and the outcome showed that VSL#3 upregulated mucosal alkaline sphingomyelinase activity and improved UC[25].
VSL#3 has also demonstrated valuable effects in children having IBD. A study showed that VSL#3 was operative in maintaining remission and reducing relapses in children with active UC. Apart from its role in maintaining remission, VSL#3 therapy also resulted in disease remission in children with mild to moderate acute UC as reported by another pilot study[36].
Jia et al[37] performed a meta-analysis of remission, relapse, and complication rates between EcN 1917 and mesalazine. The results demonstrated that there were no significant differences in the EcN1917 group or mesalazine-treated patients and were safe and well-tolerated. In summary, EcN 1917 has a comparable efficacy to mesalazine in terms of remission induction. This probiotic could be considered as an alternative for patients with IBD. Tamaki et al[38] reported that treatment with Bifidobacterium longum in patients with mild to moderate UC, prominently reduced DAI score and decreased rectal bleeding, as well as showed that they also achieved clinical remission. Treatment with probiotics and commonly used anti-inflammatory drugs together appears to be a more effective solution than treatment with probiotics alone. Palumbo et al[39] combined mesalazine with a probiotic mixture (Lactobacillus salivarius, Lactobacillus acidophilus, and Bifidobacterium bifidum strain). The combination showed beneficial effects among UC patients. The dual-treatment group demonstrated shorter recovery time, lower disease activity, and showed better endoscopic images. Another research group found that oral administration of Bifidobacterium infantis suppressed the CRP and TNF-α levels in both GI inflammatory diseases but did not specifically affect UC disease[40].
Also in one study, S. boulardii and VSL#3 in combination with conventional therapy in mild to moderate UC (involving 244 patients) showed no improvement in remission rates. However, only modest benefits were obtained in terms of reduction in disease activity[41]. EcN, S. boulardii, B. breve, and Bifidobacterium bifidum strains Yakult has demonstrated efficacy and safety similar to standard 5-ASA in maintaining remission in patients with mild to moderate UC based on histology, endoscopy, or quality of life. Presently, only four clinical trials have reported the use of S. boulardii as a probiotic therapy for IBD, with three reports of efficacy. Probiotic therapy using probiotic yeast such as S. boulardii could be a probable treatment for clinical trials, but the validation of reported efficacy in animal models requires multiple placebo trials[42].
An randomized controlled trial (RCT) evaluated the ability of VSL#3 to prevent the endoscopic recurrence of CD in humans after surgery. In this study, 10% of patients in the early VSL#3 group (VSL#3 administered throughout 365 d) had no severe lesions on day 90, but severe lesions developed on day 365 compared to the 26% of patients in the late VSL#3 group (administration of VSL#3 from day 90 to day 365). The finding suggested that VSL#3 exposure time was closely related to its therapeutic efficacy. However, DAI and IBD questionnaire (IBDQ) scores were similar in the two groups.
Few clinical studies have also shown that VSL#3 can prevent or maintain remission in chronic pouchitis. It was stated that after ileal pouch-anal anastomosis for UC, in the VSL#3 group 10% of patients had an onset of acute pouchitis when compared to the 40% of patients in placebo group. VSL#3-treated patients (17 patients, 85%) maintained antibiotic-induced pouchitis remission compared to placebo-treated patients (1 patient, 6%)[42].
PREBIOTICS IN IBD
Numerous studies have demonstrated the role of prebiotics on the intestinal flora and demonstrated that the use of prebiotics can enhance the metabolic function of the intestinal flora. Various studies reported that prebiotics diminishes the inflammatory cytokines, such as IL-1α, IL-1β, IL-6, IL-12, TNF-α, IFN-γ, and improve the natural intestinal barrier by increasing the mucinous layer and TJs between epithelial cells. Their ability to decrease pathological bacteria in the gut and provide commensal bacteria with substrates capable of being metabolized into substances that contribute to the production and secretion of anti-inflammatory cytokines make them interesting candidates for various researchers working in IBD management. Surprisingly, more research has been done preclinically and a low number of significant prebiotic-associated human clinical trials are reported. The restricted research studies number is a foremost drawback and not adequately sufficient to support the use of prebiotics to treat IBD. Most prebiotics used in animal studies were polysaccharides derived from grapes, mushrooms such as Ganoderma lucidum, and herbs. In contrast, most human clinical trials described the usage of FOS for prebiotic treatment of IBD[34].
Prebiotic effectiveness in animal model of colitis
The use of prebiotics has shown promise in the management of colitis and is also widely used in animal models (Table 3). Various prebiotic preparations have been tested in animal models of colitis. The prebiotic effects of inulin have been studied in DSS-induced distal colitis in a rat model histologically resembling human UC. Daily administration of inulin by the oral route increases the number of natural lactobacilli in the lumen of the cecum and also lowers colonic pH. In rats with DSS-induced colitis, mucosal inflammation and histological injury scores are reduced by oral administration of inulin. Furthermore, inulin-fed rats showed a lower degree of mucosal damage and less severe crypt damage compared to controls. Treatment with orally administered inulin showed similarly beneficial effects, regardless of whether treatment was provided before or during the DSS exposure[51].
Table 3.
Summary of preclinical studies of prebiotics
|
Ref.
|
Model
|
Type of treatment
|
Composition
|
Dose
|
Parameters analyzed
|
Conclusion
|
| Cui et al[57], 2021 | DSS-induced colitis in C57BL/6J mice | Polysaccharide from Scutellaria baicalensis Georgi | Mannose, ribose, rhamnose, glucuronic acid, glucose, xylose, arabinose, fucose | 50 and 200 mg/kg once daily for 10 d | Body weight, loose stools, morbidity, hematochezia, and the DAI | Effective (attenuated body weight loss, reduced DAI, ameliorated colonic pathological damage, and decreased MPO activity) |
| Tolonen et al[58], 2022 | DSS-induced colitis in male C57Bl/6 mice | Synthetic glycans | FOS, GOS, XOS, pullulan, and lactulose | 1% (v/v) glycans (days 7-14) | Weight loss, scores of diarrhea, endoscopy, and colonic histology | Synthetic glycans increase survival, reduce weight loss, and improve clinical scores in mouse models of colitis |
| Qian et al[59], 2022 | DSS-induced colitis in male C57Bl/6 mice | GOS, FOS along with FMT | FMT alone or combined with various ratios of GOS, and FOS | - | DAI scores, histology, protein or mRNA expression levels of FFAR3 and ZO-1, a tight junction protein | Treatment with FMT plus a prebiotic blend restores thestructure of the intestinal flora and increased the levels of acetic acid, butyric acid, FFAR3, and ZO-1 |
| Liu et al[60], 2016 | DSS-induced colitis in male C57BL/6 mice | Alpha D-glucan from marine fungus Phoma herbarum YS4108 | Glucopyranose | 40 mg/kg/d once daily for 1 wk | DAI scores, histology immunohistochemistry analysis, evaluation of SOD and MDA activities, and determinations of inflammatory cytokines | Effective (significantly increased butyrate, isovaleric acid levels, and prominent alterations on specific microbiota) |
| He et al[61], 2020 | DSS-induced colitis in male C57BL/6 mice | Stachyose | Stachyose | 1.5 g/kg/d for 28 d | Inflammatory cytokines including IL-6, IL-10, IL-17a, and TNF-α | Increased beneficial microbiota and bacterial diversity to alleviate acute colitis in mice |
| Kanwal et al[62], 2019 | Dictyophora indusiate polysaccharide | Glucose 59.84%, mannose 23.55%, and galactose 12.95% | Low dose 10 mg/kg and (high dose 33 mg/kg once daily for 2 wk | Assessment of DAI, histological, analysis of goblet cells and mucus layer thickness, cytokines | Effective | |
| Li et al[63], 2020 | Male C57BL/6 mice | FMG or dealcoholized muscadine wine | FMG: Fructose 34.7% glucose 31%, sucrose 9.9%. DMW: Fructose, sucrose, and glucose not detected | FMG (7%, w/w) or DMW (5.5%, v/ w) for 3 wk | Bodyweight, stool consistency and bleeding, DAI, short-chain fatty acids in feces, and Mucin 2 and IgA in feces | Effective (reduced dysbiosis in the colon) |
| K-da et al[64], 2020 | Male C57BL/6 mice | GFO | Monosaccharide composition in the GFO was D-galactose | 100, 500, or 1000 mg/kg once daily for 2 wk | GI transit time, ex vivo propulsive motility, in vitro colonic smooth muscle contractility, the composition of colonic microbiota, and production of SCFAs | Effective (prevented and attenuated colitis symptoms and GI dysmotility, reducing populations of harmful bacteria and increasing SCFAs) |
DSS: Dextran sodium sulfate; DAI: Disease activity Index; MPO: Myeloperoxidase; FOS: Fructooligosaccharides; GOS: Galactooligosaccharides; XOS: Xylooligosaccharides; FMT: Fecal microbiota transplantation; ZO-1: Zona occludens; SOD: Superoxide dismutase; MDA: Malondialdehyde; SCFAs: Short-chain fatty acids; GI: Gastrointestinal; IL: Interleukin; TNF: Tumor necrosis factor; FMG: Freeze-dried muscadine grapes; DMW: Dealcoholized muscadine wine; FFAR3: Free fatty acid receptor 3; GFO: Gracilaria fisheri oligosaccharides.
A nutritional combination of inulin and oligofructose at 5 g/kg body weight reduces intestinal inflammation in transgenic rats. An increase in Bifidobacteria and Lactobacilli was observed in the gut along with a decrease in pro-inflammatory cytokines and an increase in the growth factor-β that alters immune regulation. Taken together, these results suggest that combination therapy with different prebiotics may be more effective than monotherapy due to the fact that each drug has specific biological properties[52].
Pectic polysaccharides (PPS) are thought to be essential carbohydrates available to the gut microbiota, playing a dominant role in maintaining intestinal balance and are more potent than some conventional prebiotics. PPS can stimulate the growth of beneficial bacteria, such as Lactobacillus, Bacteroides, and Bifidobacterium, fully meeting the condition of a “stimulating probiotic”. Some aberrant PPS have shown specific immunological capabilities compared to the PPS on the market. For example, sweet cherry PPS significantly induces the expression of NO and some immune proteins such as IL-6 and IL-10. Moreover, PPS from silver linden flowers enhances immunity in mice by inducing ROS and NO and suppressing iNOS. One study shows that PPS from Gentiana crassicaulis can enhance host immunity in terms of immune complement fixation[53].
Lactulose is reported to reduce inflammation and instigate the growth of lactic acid bacteria in IL-10 knockout mice while administration of inulin and germinated barley foodstuff (GBF) reduced DSS-induced colitis in rats. It has been shown to increase the luminal concentration of SCFA, as well as increase the density of Lactobacillus and Bifidobacterium[54].
Mushrooms consist of diverse polysaccharides with prebiotic potential, including α-glucans, chitin, mannans, xylans, and galatians. Xie et al[55] stated that the prebiotics Ganoderma lucidum polysaccharide enhance SCFA-producing bacteria and augments SCFA production and suppresses DAI prominently. The study also described a decrease in infective microbiota such as Shigella and Escherichia in the rat model[55].
The effect of neoagarotetraose (NT), a hydrolytic product of agar by β-agarase, was evaluated in the DSS-induced murine model. The data show that NT intake improved intestinal integrity and inflammation scores. NT reversed the density of Proteobacteria from the DSS-induced increased levels[56].
Reduced clinical signs and increased MUC-3 expression were observed in rats that were nourished with goat’s milk oligosaccharides as compared to the DSS-induced colitis rats. In trinitrobenzene sulfonates that provoke colitis rats, the colonic inflammation and necrotic lesions are also reduced by goat’s milk oligosaccharides as compared with control rats[56].
Clinical study of prebiotic among IBD patients
Although there are few human studies using prebiotics, some new evidence suggests that the prebiotic treatment holds promise (Table 4). After colectomy for UC inulin showed a positive effect in the management of chronic pouchitis. A small, open-label study in 10 patients with active CD showed that the 21-d oral administration of 15 g of oligofructose and inulin significantly reduced the disease status. In UC patients, Plantago ovata (psyllium) outperformed placebo in reducing symptom severity and significantly increasing fecal concentrations of Bifidobacteria. Psyllium seeds formerly stimulated SCFA production, when tested as maintenance therapy for the UC patient in remission in an open randomized study. In this, patients received mesalamine (500 mg/d thrice daily for 1 year), psyllium seed alone (10 g twice daily), or a combination of both at the same doses. Remission were comparable in all groups, and a substantial augmentation in fecal butyrate concentration was detected after the administration of Plantago ovata seeds[65].
Table 4.
Summary of clinical studies of using prebiotics in inflammatory bowel disease patients
|
Ref.
|
Type of treatment
|
Dose
|
Parameters analyzed
|
Conclusion
|
| Valcheva et al[68], 2022 | β-fructans (oligofructose and inulin) | 15 g/d for 6 mo | Mayo score. FCP, along with stool metabolites | Did not prevent symptomatic relapses in UC patients but reduced the severity of biochemical relapse and increased anti-inflammatory metabolites |
| Pietrzak et al[69], 2022 | Sodium butyrate | 150 mg sodium butyrate twice a day for 12-wk | DAI, FCP | As adjunctive therapy, it did not show efficacy in newly diagnosed children and adolescents with IBD |
| Vernero et al[70], 2020 | Oral microencapsulated sodium butyrate (BLM) | Dose of two capsules/day for 12 mo (500 mg of BLM for each capsule) | DAI, FCP, CRP | BLM supplementation appears to be a valid add-on therapy to maintain remission in patients with UC |
| Valcheva et al[71], 2019 | Oligofructose-enriched inulin | 7.5 g (n = 12) or 15 g (n = 13) daily oral oligofructose-enriched inulin for 9 wk | Mayo score, endoscopic activity and FCP | 15 g/d dose inulin-type fructans produced functional but not compositional shifts of the gut microbiota. Controlled studies for the use of β-fructans as an adjunct therapy in patients with active UC are required |
| Azpiroz et al[72], 2017 | scFOS | 5 g per sachet, twice daily for 4 wk | Rectal sensitivity, anxiety/depression, quality of life scores, and composition of fecal microbiota | Less significant (scFOS on rectal sensitivity may require higher doses and may depend on the subgroup) |
FCP: Fecal calprotectin; DAI: Disease activity index; CRP: C-reactive protein; UC: Ulcerative colitis; scFOS: Short chain fructooligosaccharides.
GBF consists of an extract rich in glutamine and hemicellulose. In small pilot and placebo-controlled studies, its use was evaluated in UC patients having mild to moderate disease severity. GBF significantly increased fecal levels of Bifidobacteria and reduced clinical and endoscopic activities at a dose of 25-30 mg/d. Comparable outcomes were described by 24-wk open-label study. A combination of 15 g/d oligofructoses and inulin was investigated on ten patients with active CD in a small open-label study. A substantial decrease in DAI accompanied by a substantial increase in mucosal Bifidobacteria was observed. Interestingly, prebiotics amplified colonic dendritic cells expressing IL-10, toll-like receptors-2, and 4, signifying the mechanism of the prebiotics on the mucosal innate immune response.
Wilson et al[66] explored the effects of prebiotic Galactooligosaccharides (GOS) supplementation on colonic inflammation in 17 patients with active UC. Patients reported improved stool consistency, decreased incidence and severity of loose stools, and decreased urgency of defecation after administration of GOS at 2.8 g/d for 6 wk. The proportion of Bifidobacterium and Christensenelaceae increased only in patients with low disease activity, suggesting that prebiotic effects may depend on disease activity. A controlled study is required to validate these observations to essentially determine if the GOS prebiotic is a useful adjunct therapy in active UC[66].
The effect of enteral inulin on ileal pouch function was assessed by examining epithelial gene expression, cell turnover, and mucosal morphology. The authors found that enteric supplementation with 24 g/d inulin increased butyrate production, reduced inflammation-related factors, decreased secondary bile acids, and significantly reduced endoscopic and histological DAI scores[67].
SYNBIOTICS IN IBD
Synbiotics not only improve the survival of beneficial microorganisms added to food and feed but are also used to stimulate the growth of certain natural bacterial strains present in the GIT. Given a large number of possible combinations, the application of synbiotics to modulate the human gut microbiota appears promising.
Effectiveness of synbiotic in animal models of colitis
Recently, several preclinical studies have shown that the use of probiotics and prebiotics as a synbiotic combination alleviates intestinal inflammation more than either probiotics or prebiotics alone (Table 5). The effects of formulated prebiotic mixtures, probiotic mixtures, and synbiotics were investigated in the colitis model induced by DSS in mice. Results in Synbiotic-treated colitis mice showed the preservation of colonic histological architecture and mucin production, upregulation of occludin expression, and diminished cell infiltration. A significant decrease in plasma IL-6 levels was observed after treatment. Treatment also modified gut microbiome, improved colonic integrity, upregulated anti-inflammatory cytokines, and suppressed inflammation markers, possibly through inhibition of IL-6/STAT3 signaling. In addition, synbiotic-treated mice displayed the highest levels of anti-inflammatory mediator IL-10 among the treatment groups in colitis mice. Among the treatments, synbiotics showed the most pronounced effect, indicating the highest potential for prevention and treatment of IBD[73].
Table 5.
Summary of preclinical studies of synbiotics
|
Ref.
|
Model
|
Probiotics
|
Prebiotics
|
Dose
|
Parameters analyzed
|
Conclusion
|
| Xue et al[80], 2023 | DSS-induced colitis in mice | Lactobacillus plantarum LP90 | Soluble dietary fiber obtained from Lentinula edodes by products | 1 × 109 CFU/kg | DAI, histological studies, IL-10, IL-17, IgA levels, TNF-α | Alleviated colitis |
| Ivanovska et al[81], 2017 | DSS-induced colitis in C57BL/6J mice | Bifidobacterium infantis and Bifodobacterium longum | Equal parts FOS, GOS and XOS | 0.5 × 109 CFU Probiotics and 2.5 g of prebiotics for 1 mo | Gut microbiome, cecal and fecal SCFAs | Increased the diversity of the microbiome and be associated with more SCFAs, and less gut inflammation |
| Seong et al[82], 2020 | Chronic restraint stress in male Wistar rats | L. paracasei | Opuntia humifusa extract (mucilage + pectin) | L. paracasei (1 × 1010 CFU/g) & (10.0/30.0 mg%, w/w) of Opuntia humifusa extract once daily for 4 wk | Fecal microbial analysis, serum corticosterone levels, TNF-α levels in the colon tissue | Effective (greater abundance of L. paracasei in fecal microbial analysis, lower serum corticosterone levels, lower TNF-α levels in the colon tissue |
| dos Santos Cruz et al[83], 2020 | IL 10- knockout mice | VSL#3 | Yacon (6% FOS + inulin) | VSL#3 (109 CFU/d) + PBY (6% FOS and inulin) for 13 wk | Manifestations of colitis, colon histology, expression of antioxidant enzymes, production of organic acids, and intestinal microbiota | Preservation of intestinal architecture, improve intestinal integrity, increased expression of antioxidant enzymes and concentration of organic acids |
| Wang et al[84], 2019 | DSS-induced colitis in C57BL/6J mice | Lactobacillus acidophilus, L. Rhamnosus, and Bifidobacterium lactis | Inulin | Probiotics: 1.0 × 109 CFU per day per mice, and prebiotic 5 × 108 CFU/d | Pathologic scores, mucosal flora | Increased the proportion of helpful bacteria and regulated the balance of intestinal microbiota, reduced the degree of inflammation in acute colitis mice |
| Son et al[85], 2019 | DSS-induced colitis in female BALB/c mice | LGG | Tagatose | 109 CFU/mL of LGG and 25 mg of tagatose once in 2 d for 3 wk | Body weight, food intake, rectal bleeding, stool conditions, blood in stool, expression of proinflammatory cytokines | Effective (gut microbiota composition recovered from the dysbiosis caused by DSS treatment) |
| Ivanovska et al[81], 2017 | TNBS-induced colitis in Wistar rats | L. Casey 01 | Oligofructose-enriched inulin | 1 mL containing non-encapsulated probiotic/prebiotics once daily for 2 wk | Assessment of colonic damage, inflammation scoring, MPO and microbiological studies | Effective |
DAI: Disease activity index; IL: Interleukin; TNF: Tumor necrosis factor; FOS: Fructooligosaccharides; GOS: Galactooligosaccharides; XOS: Xylooligosaccharides; CFU: Colony forming unit; DSS: Dextran sodium sulfate; MPO: Myeloperoxidase; LGG: L. rhamnosus strain; SCFAs: Short-chain fatty acids.
Kangwan et al[74] demonstrated a protective effect of L. pentosus A14-6, CMY46 against DSS-induced intestinal inflammation. A14-6 and CMY46 are the novel strain of L. pentosus isolated from tea leaves (Miang) in Northern Thailand. The anti-inflammatory actions of L. pentosus CMY46 combined with GOS and L. pentosus A14-6 combined with XOS and were explored in C57BL/6 mice for 21 d. Synbiotics ameliorated DSS-induced colitis by preserving weight loss, reducing DAI, restoring colon length, and suppressing histopathological damage. Moreover, synbiotics enhanced intestinal barrier integrity and reduced colonic inflammation. Further, synbiotics possessed excellent anti-inflammatory and immunomodulatory activities, as evidenced by decreased inflammatory mediator expressions of TNF-α, IL-1β, IL-6, and cyclooxygenase-2 (COX-2) in the colon. Symbiotic CMY46 in combination with GOS markedly increased IL-10 expression. These results suggest that synbiotics isolated from Mian are more effective than sulfasalazine[74].
The efficacy of Bacillus amyloliquefaciens enriched camel milk (BEY) was evaluated in TNBS-induced colitis mice models. Results showed that BEY treatment attenuated the proinflammatory cytokines (IL-1β, IL-6, IL-8, and TNF-α) and myeloperoxidase levels. In addition, the protein markers such as phosphatase and tensin homolog, NFκB, COX-2, proliferation nuclear antigen, and occludin were substantially downregulated by BEY treatment. The BEY alleviated the colitis symptoms[75].
Bacillus coagulans FCYS01 spores in combination with chitooligosaccharides (COS) were evaluated for the possible ameliorating effects on DSS-induced colitis in mice. In comparison to the DSS group, the supplement significantly modulated the levels of CPR and cytokines IL-4, IL-6, IL-8, and IL-10. It significantly restored the TJ proteins and mucin protein expressions, thereby promoting the recovery of the intestinal barrier. Additionally, these dietary supplements improve SCFA production by modulating the composition of the gut microbiota and enhancing SCFA-producing bacteria. In conclusion, synbiotics mitigated the inflammatory status of the experimental UC model and showed better therapeutic efficacy than individual B. coagulans or COSs[76].
In another study, supplementation with synbiotics could substantially ameliorate the disease activity in DSS-induced acute colitis mice. The synbiotic significantly preserved the epithelial TJ proteins at colon, signifying the shielding of the intestinal barrier. The pro-inflammatory cytokines were reduced while augmentation of anti-inflammatory cytokines was mediated by the symbiotic treatment. The synbiotic used in the study was composed of 8 probiotic strains, including Bifidobacterium animal, Lactobacillus paracasei, Bifidobacterium lactis, Lactobacillus reuteri, Lactobacillus rhamnosus, B. breve, Lactobacillus fermentum, and Streptococcus thermophilus along with FOS[77].
In another study, a synbiotic consisting of Lactobacillus fermentum HFY06 and arabinoxylan showed that the synbiotic can prevent and treat DSS-induced colitis. The results exhibited their synergistic effect by inhibiting the activation of the NFκB signaling pathway, upregulating the mRNA expression of NFκB inhibitor-α, downregulating mRNA expressions of NFκB-p65, inhibiting the cytokines TNF-α, inducible NOS, and COX-2, and exerted anti- colitis effects[78].
Synbiotic supplement with probiotic Bacillus coagulans spores and prebiotic green banana resistant starch ameliorated intestinal inflammation in the murine IBD model induced by DSS. A considerable efficacy of synbiotic supplementation was highlighted as it reduced the colitic manifestations and its severity. A significant anti-inflammatory effect was produced by suppressing abnormal immunological responses and colonic damage induced by DSS. Synbiotic accounted for about 29% increase in IL-10 levels and about 37% suppression of CPR along with about 40% IL-1β suppression compared to that of the DSS-control. The combination also improved SCFA production. The synbiotic supplementation amended the complete inflammatory condition via synergistic actions[79].
Clinical study of synbiotic among IBD patients
The effects of daily supplementation of total gut repair (TGR) on microbial community composition and activity were investigated in the short-term-Quad-M-SHIME model inoculated with gut microbiota from two individual IBD donors. TGR comprises of probiotics, prebiotics along with combination of amino acids, immunoglobins and flavonoids. TGR supplementation increased SCFA production, increased beneficial bacterial density, decreased inflammation, and damage to the intestinal barrier from endotoxin exposure. Intestinal barrier function was improved compared to controls, and levels of the anti-inflammatory molecules IL-6 and IL-10 were elevated. TGR supplementation daily promoted changes in the gut microbiota of IBD patients.
The efficacy of synbiotic therapy has been evaluated in UC patients in a clinical study (Table 6). Patients received a synbiotic formulation consisting of prebiotic FOS along with 6 probiotic strains. The results showed a significant decrease in inflammation and improved disease status. The double-blind randomized, placebo clinical trial study by Liang et al[88] confirmed the efficacy of a synbiotic formulation in suppressing IBD symptoms. The synbiotic consisted of FOS and L. acidophilus, B. bifidum; B. longum; B. lactis; and L. rhamnosus. Rectal pain, bloating, incomplete bowel movements and diarrhea sensations were significantly improved in patients compared to placebo[88].
Table 6.
Summary of clinical studies of using synbiotics in inflammatory bowel disease patients
|
Ref.
|
Probiotics
|
Prebiotics
|
Dose
|
Parameters analyzed
|
Conclusion
|
| Amiriani et al[86], 2020 | Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, Streptococcus thermophiles | FOS | Lactocare capsule twice daily for 8 wk | DAI | Mitigated symptoms in patients with UC and suggested to use pre-probiotics in the standard treatment, particularly in those with more than five years of the disease |
| Kamarlı Altun et al[87], 2019 | Enterococcus faecium, Lactobacillus plantarum, Streptococcus thermophilus, Bifidobacterium lactis, Lactobacillus acidophilus, Bifidobacterium longum | FOS | 3 × 109 CFU probiotic and 225 mg/tablet prebiotic for 8 wk | Hemoglobin, leukocyte, neutrophil-to-lymphocyte ratio, sedimentation, and CRP and clinical and endoscopic activity indices | Improvement in clinical activity |
DAI: Disease activity index; FOS: Fructooligosaccharides; UC: Ulcerative colitis; CRP: C-reactive protein; CFU: Colony forming unit.
A recent randomized, double-blind, controlled trial observed synbiotic use in 18 subjects with functional UC. The treatment included the prebiotics inulin and oligofructose. Sigmoidoscopy inflammation scores were reduced in the synbiotic group compared to the placebo group. The TNF and IL-1α levels in the intestine were also reduced. Furthermore, rectal cultures showed greater epithelial regeneration and reduced inflammation in synbiotic-treated subjects. A tiny, open-labeled trial of 10 active CD subjects, 21 d of 15 g oligofructose and inulin oral administration also showed a substantial lowering of the disease symptoms[89].
Since it has been shown that obesity-induced gut microbiota aggravation can exacerbate IBD symptoms, BG from the Schizophyllum commune, a probiotic, and a synbiotics containing both BG and probiotic (SYN) may improve symptoms of obesity-related colitis. BG and probiotic protected intestinal TJs, but did not modulated the inflammatory markers (i.e., IL-6 and TNF-α) infiltration. In contrast, SYN displayed more prominent actions in attenuating colonic inflammation. SYN treatment group supported the growth of both indigenous and supplemented bacteria while maintaining bacterial diversity, thereby improving the obesity-associated colitis symptoms[90].
SAFETY ASPECTS OF PRO, PREBIOTIC, AND SYNBIOTIC PRODUCTS
The Centers for Disease Control and Prevention advises that over-the-counter prebiotics and probiotics are generally safe for use by healthy people. The ignoring the importance of dose and strain specificity is a concern today. Probiotics manufactured as dietary supplements rather than pharmaceuticals are not subject to regulatory review because they are not required to support claims about the safety or efficacy of food or dietary supplements. This is one of the main reasons for the insufficient or nonexistent information on the efficacy and safety of most marketed products. Probiotic is characterized by a generally safe profile but should be used with caution in certain population groups such as pregnant women, neonates born prematurely, or with immune deficiency. A World Health Organization working group proposed several criteria that are to be considered in order to define strains with GRAS status: Resistance of probioticstrains to antibiotics; evaluation of metabolic properties (lactate production, bile deconjugation) monitoring of side effects in clinical trials, epidemiological studies on the occurrence of side effects after commercial approval, identification of all substances excreted by strains that are toxic to mammals, and determination of the hemolytic capacity of strains[91].
Risks and side effects
No serious side effects of probiotic interventions in IBD patients have been reported. Inadequate immunological stimulation, genes transfer, systemic infections, and fatal metabolic activities have been detected in certain individuals receiving probiotic supplementation. A mild dry cough has been reported in one UC patient with B. longum 536 supplementations. Septicemia, and certain cased of endocarditis, have been associated with certain probiotic strains, like L. acidophilus, L. rhamnosus, Bacillus subtilis, S. boulardii, L. Casey, and B. breve. Administration of Lactobacillus rhamnosus in 64-year-old UC patient who was treated with prednisone, caused bacteremia due to bacterial transfer from intestinal lumen to the blood. Probiotic interventions have been reported to induce an inflammatory response in the small intestinal region, leading to D-lactic acidosis. The complications in certain patient populations, especially those with compromised immune systems are accelerated by S. boulardii and Lactobacillus GG administration. Pregnant women, new-borns, and the elderly are at increased risk of potential probiotic infections due to their weakened immune systems[92].
STUDY LIMITATIONS
Significant heterogeneity between studies jeopardizes the interpretation of the current literature on probiotics and prebiotics in IBD. Choice of prebiotic or probiotic studied, the trial design, their doses, and the outcome were also reported to vary. Study populations varied, with some studies included active disease patients while others working on the remission maintenance persuaded by conventional therapy, antibiotics, or surgery. Most studies enrolled small numbers of patients, limiting their statistical power, which is especially important given the high placebo response rates observed in IBD clinical trials. Finally, no studies provided information on patient diet, which may have a significant impact on the effectiveness of microbial therapy. The exact mechanisms of action have not yet been elucidated. The understanding of the mechanisms responsible for the beneficial effects of probiotics, prebiotics, and synbiotics is rather superficial.
Inadequate evidence on probiotic dosages essential for specific clinical effects has amplified the necessity for molecular description of probiotics to establish health claims. Evidence for immunological mechanisms of probiotics is still limited. Evaluation of interactions between cocktail of probiotic strains in formulations such as #VSL-3 have not been considered and yet to be investigated. Clinical trials and validation studies planned with larger sample sizes require an understanding of the interactions between the microbiota, host, and prebiotic components. Due to the very limited published literature in the field of manufacturing processes and subsequent formulation, much needs to be done to improve strain viability during formulation and storage[93].
OBSTACLES, CHALLENGES, AND FUTURE PROSPECTS
The main barrier with the pre-, pro-and synbiotics is the “difficulty in demonstrating clinical efficacy”. The situation is complicated by the different levels of evidence essential to support health claims from country to country. The Food and Drug Administration now states that active ingredients, including probiotics, taken to cure, alleviate, treat, diagnose, or prevent disease must be classified as pharmaceuticals and go through the same approval process as new drugs. Eventually, high-quality human intervention studies are needed to substantiate health claims on products.
Probiotic supplements vary widely in composition, dosage, as well as in host interactions, and these should be specifically considered before recommending their use. Remarkably, several prebiotic and synbiotic products contain a slight amount of prebiotic ingredients (per serving), that may be too small to produce any health benefits. Lower doses are used in part to avoid unwanted GI discomfort, but possibly also for cost reasons. Developing clinically effective synbiotic combinations is a major challenge and must meet several requirements. It is generally expected that the minimum effective dose of each component must be determined.
Maintaining probiotic bacteria viability is a foremost marketing and technical challenge in probiotic applications. A basic prerequisite for probiotics is that the product comprises an adequate number of microorganisms by the expiration date. Therefore, probiotics should cover precise strains and maintain a specific number of viable cells to provide a health benefit to the host. Many viable cultures die during final product manufacturing, storage, transportation, and passage through the gut. As a result, the majority die before consumers can reap the health benefits. Market research has also shown that even before the expiry date, product show much lower count. Therefore, the shelf life of probiotics cannot be accurately predicted. As a result, the industry has to struggle alot to substantiate the label’s claims[94].
Also, for optimal effectiveness, probiotics must remain viable after contact with stomach acid, bile, and digestive enzymes to cross the upper GIT. This is a basic property that many products have not tested. Microorganisms may die while passing through the upper intestinal tract to the colon and thus may not be able to colonize the colon. Therefore, they must withstand the gastric acid and bile salts encountered during transit through the GIT.
Even when screening of synbiotic combinations was performed in vitro or in situ, the methods ignored the environmental factors that influence probiotic strains in vivo. Also, competition for the prebiotic substrate between the probiotic strain and members of the gut microbiota was not considered. Identifying prebiotics that specifically and selectively boost the probiotic strain of interest can be challenging.
Clinically effecacious synbiotic development remains a challenge and must meet numerous requirements. It is generally expected that the minimum effective dose of each component must be determined. Including adequate controls in synbiotic studies is particularly challenging. Prebiotic-only and probiotic-only controls must be included, in addition to standard control, for checking the synergistic or additive actions. Justification on how the probiotics and prebiotics were selected and combined should be included[95,96].
FMT IN IBD
For patients with metabolic syndromes linked with gut dysbiosis, FMT is an evolving microbial therapy. The technique involves the transfer of healthy fecal microbe population to patients with metabolic conditions. FMT’s technical approach involves oral capsules, nasogastric or nasojejunal tubes, and enemas that are utilized for restoring a healthy GI microbiome. FMT samples are carefully chosen from healthy donors who have undergone a standard screening procedure for avoiding the risk of transmission of unknown pathogens from donor to recipient. Donors are usually evaluated for their historical backgrounds such as health profiles, family history of autoimmune reactions, metabolic disorders, transfusion details, or any previous surgery. Other donor data include travel history, food intake, particularly alcohol and drugs, and sexual behavior. After selecting an appropriate donor, their stool and blood samples are tested for the presence of pathogens. Extensive support and education are provided to the patients undergoing FMT prior to the treatment. No fecal substances such be present in the colon. Patients may even consume loperamide prior to infusion to make sure that transplanted feces stay there for at least 4 h. The recipient is not allowed to consume antibiotics 48 h prior to infusion[97].
There are several methods of transferring fecal material to the recipients. Presently, fecal material is administered via the upper GIT or lower GIT route, or as oral capsules. For patients who are suffering from an inflamed colon, FMT is performed via nasogastric tube, esophagogastroduodenoscopy, nasojejunal tube, or upper GI route via nasoduodenal tube. Lower GI route FMT can be accomplished by retention enema or by colonoscopy. While colonoscopy aids in the successful recolonization of all the parts of the colon with favorable microflora, retention enemas are only restricted to the distal colon. Retention enemas are however much cheaper and less invasive than colonoscopy. For most reported treatments, FMT patients receive an average relative dose of 25 g via the upper GI route compared to an average of 90 g via colonoscopy. In an RCT, colonoscopy using a 152 g stool sample reported a 90% success rate in preventing recurrent infections. Another research group reported that consuming 17 g of frozen and thawed or fresh FMT showed 60% efficacy using the retention enema method. The fecal capsules can also aid in restoring ecosystem integrity and overcoming microbial loss in the GI environment[98].
Recently, a study reported that FMT in IBD patients showed a response rate of 53.8% and a complete response rate of 37%. Furthermore, it has been reported that FMT is a more practical treatment with safe and beneficial results for the treatment of active UC patients. Pooled results exhibited that FMT treatment might improve clinical and endoscopic rates of active UC. FMT also significantly alters the microbiota composition of UC patients as compared with control groups[98]. In UC patients, Tian et al[99] assessed the B proportion that exhibited a steady rising trend after FMT. Prevotella and Proteus were also prominently augmented as compared to healthy control. On other hand, it was found that the populations of Klebsiella and Streptococcus, which are pathogenic bacteria decreased significantly after FMT treatment. Reducing the abundance of Prevotella while increasing the proportion of Klebsiella and Streptococcus was a key factor in the development of UC. Unfortunately, several studies have stated conflicting results, with FMT therapy failing to ameliorate the disease severity and restore the gut microbiota.
In this regard, several studies have found that the efficacy of FMT for treating IBD is unpredictable. Therefore, it is still unclear whether FMT fits into the therapeutic paradigm. Despite reports of significant positive taxonomic changes in the GIT in patients diagnosed with FMT, observations remain conflicting and its functional and metabolic effects are not well documented. For UC, FMT may be a promising treatment, but for CD or pouchitis, very limited information have been available to draw good conclusions[100].
CONCLUSION
Although probiotics and prebiotics have been studied in many animal models and clinical trials of intestinal inflammation and offer health benefits, the individual efficacy of each probiotic strain and its administration remains uncertain. Large, rigorously designed, high-quality human studies need to be evaluated to examine dosage, duration of use, formulations containing one or more strains, and probiotics, prebiotics, antibiotics as well as simultaneous use of substances. For a thorough knowledge of the structure and function of the microbiome with regards to probiotics and prebiotics, modern approaches based on bioengineering, genetic engineering, system biology, molecular biology, multiomics, nanotechnology, and immunology must be employed. These investigations will aid in the comprehension of the relationship between human physiological processes and the microbiome. A potential area for future developments includes implementation of a personalized therapy for IBD, based on a detailed assessment of the gut microbiota and immune system profile in the individuals. Such personalized holistic therapy, that combines biotics with dietary and pharmaceutical therapy, would improve therapeutic efficacy while decreasing adverse effects. This approach will also enable a more thorough understanding of the pathophysiology of IBD and the adoption of targeted therapies for the preservation of the gut microbiome and rectification of bacterial metabolic activities, as well as the restoration of the regulatory immune system. This will enable the use of innovative treatment approaches to manage IBD patients in a safer and more effective manner.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: October 28, 2022
First decision: November 14, 2022
Article in press: March 21, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jovandaric MZ, Serbia; Maslennikov R, Russia; Lakatos PL, Canada S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
Contributor Information
Supriya Roy, Amity Institute of Pharmacy, Lucknow, Amity University Uttar Pradesh, Sector 125, Noida 201313, Uttar Pradesh, India.
Suneela Dhaneshwar, Amity Institute of Pharmacy, Amity University Maharashtra, Mumbai 410206, Maharashtra, India. suneeladhaneshwar@rediffmail.com.
References
- 1.Lucas López R, Grande Burgos MJ, Gálvez A, Pérez Pulido R. The human gastrointestinal tract and oral microbiota in inflammatory bowel disease: a state of the science review. APMIS. 2017;125:3–10. doi: 10.1111/apm.12609. [DOI] [PubMed] [Google Scholar]
- 2.Britton RA, Hoffmann DE, Khoruts A. Probiotics and the Microbiome-How Can We Help Patients Make Sense of Probiotics? Gastroenterology. 2021;160:614–623. doi: 10.1053/j.gastro.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 3.Mishra J, Stubbs M, Kuang L, Vara N, Kumar P, Kumar N. Inflammatory Bowel Disease Therapeutics: A Focus on Probiotic Engineering. Mediators Inflamm. 2022;2022:9621668. doi: 10.1155/2022/9621668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naseer M, Poola S, Uraz S, Tahan V. Therapeutic Effects of Prebiotics on Constipation: A Schematic Review. Curr Clin Pharmacol. 2020;15:207–215. doi: 10.2174/1574884715666200212125035. [DOI] [PubMed] [Google Scholar]
- 5.Li HY, Zhou DD, Gan RY, Huang SY, Zhao CN, Shang A, Xu XY, Li HB. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients. 2021;13 doi: 10.3390/nu13093211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guandalini S, Sansotta N. Probiotics in the Treatment of Inflammatory Bowel Disease. Adv Exp Med Biol. 2019;1125:101–107. doi: 10.1007/5584_2018_319. [DOI] [PubMed] [Google Scholar]
- 7.Celiberto LS, Bedani R, Rossi EA, Cavallini DC. Probiotics: The scientific evidence in the context of inflammatory bowel disease. Crit Rev Food Sci Nutr. 2017;57:1759–1768. doi: 10.1080/10408398.2014.941457. [DOI] [PubMed] [Google Scholar]
- 8.Guarino MPL, Altomare A, Emerenziani S, Di Rosa C, Ribolsi M, Balestrieri P, Iovino P, Rocchi G, Cicala M. Mechanisms of Action of Prebiotics and Their Effects on Gastro-Intestinal Disorders in Adults. Nutrients. 2020;12 doi: 10.3390/nu12041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora 'on the scope'. Br J Clin Pharmacol. 2008;65:453–467. doi: 10.1111/j.1365-2125.2008.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Li M, Chen Q, Li X, Chen L, Dong Z, Zhu W, Yang Y, Liu Z. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. 2022;13:3432. doi: 10.1038/s41467-022-31171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Liao Y, Yang R, Zhu Z, Zhang L, Wu Z, Sun X. An engineered probiotic secreting Sj16 ameliorates colitis via Ruminococcaceae/butyrate/retinoic acid axis. Bioeng Transl Med. 2021;6:e10219. doi: 10.1002/btm2.10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, Liu Y, Lan X, Xu X, Zhang X, Li X, Zhao Y, Li G, Du C, Lu S, Wang H. Oral Escherichia coli expressing IL-35 meliorates experimental colitis in mice. J Transl Med. 2018;16:71. doi: 10.1186/s12967-018-1441-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couch DG, Cook H, Ortori C, Barrett D, Lund JN, O'Sullivan SE. Palmitoylethanolamide and Cannabidiol Prevent Inflammation-induced Hyperpermeability of the Human Gut In Vitro and In Vivo-A Randomized, Placebo-controlled, Double-blind Controlled Trial. Inflamm Bowel Dis. 2019;25:1006–1018. doi: 10.1093/ibd/izz017. [DOI] [PubMed] [Google Scholar]
- 14.Liu M, Li S, Zhang Q, Xu Z, Wang J, Sun H. Oral engineered Bifidobacterium longum expressing rhMnSOD to suppress experimental colitis. Int Immunopharmacol. 2018;57:25–32. doi: 10.1016/j.intimp.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Wei P, Yang Y, Ding Q, Li X, Sun H, Liu Z, Huang J, Gong Y. Oral delivery of Bifidobacterium longum expressing α-melanocyte-stimulating hormone to combat ulcerative colitis. J Med Microbiol. 2016;65:160–168. doi: 10.1099/jmm.0.000197. [DOI] [PubMed] [Google Scholar]
- 16.Feng C, Zhang W, Zhang T, Li B, He Q, Kwok LY, Zhang H. Oral administration of pasteurized probiotic fermented milk alleviates dextran sulfate sodium-induced inflammatory bowel disease in rats. J Funct Foods. 2022;94:105140. [Google Scholar]
- 17.Javed NH, Alsahly MB, Khubchandani J. Oral Feeding of Probiotic Bifidobacterium infantis: Colonic Morphological Changes in Rat Model of TNBS-Induced Colitis. Scientifica (Cairo) 2016;2016:9572596. doi: 10.1155/2016/9572596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakubczyk D, Leszczyńska K, Górska S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)-A Critical Review. Nutrients. 2020;12 doi: 10.3390/nu12071973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stojanov S, Berlec A, Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020;8 doi: 10.3390/microorganisms8111715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokota Y, Shikano A, Kuda T, Takei M, Takahashi H, Kimura B. Lactobacillus plantarum AN1 cells increase caecal L. reuteri in an ICR mouse model of dextran sodium sulphate-induced inflammatory bowel disease. Int Immunopharmacol. 2018;56:119–127. doi: 10.1016/j.intimp.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Jang YJ, Kim WK, Han DH, Lee K, Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10:696–711. doi: 10.1080/19490976.2019.1589281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theriot C, Thanissery R, O’Flaherty S, Barrangou R. Probiotic colonization dynamics after oral consumption of VSL#3® by antibiotic-treated mice. Microbiome Res Rep. 2022;1:21. doi: 10.20517/mrr.2022.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Carbo A, Shaykhutdinov R, Jobin C, Arthur JC, Corl BA, Vogel H, Storr M, Hontecillas R. Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR γ to suppress colitis. PLoS One. 2012;7:e31238. doi: 10.1371/journal.pone.0031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng FS, Pan D, Chang B, Jiang M, Sang LX. Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases. World J Clin Cases. 2020;8:1361–1384. doi: 10.12998/wjcc.v8.i8.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hrdý J, Alard J, Couturier-Maillard A, Boulard O, Boutillier D, Delacre M, Lapadatescu C, Cesaro A, Blanc P, Pot B, Ryffel B, Chamaillard M, Grangette C. Lactobacillus reuteri 5454 and Bifidobacterium animalis ssp. lactis 5764 improve colitis while differentially impacting dendritic cells maturation and antimicrobial responses. Sci Rep. 2020;10:5345. doi: 10.1038/s41598-020-62161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo JW, Shin YJ, Ma X, Son YH, Jang HM, Lee CK, Kim DH. The Alleviation of Gut Microbiota-Induced Depression and Colitis in Mice by Anti-Inflammatory Probiotics NK151, NK173, and NK175. Nutrients. 2022;14 doi: 10.3390/nu14102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin S, Huang Z, Wang Y, Pei L, Shen Y. Probiotic potential of Lactobacillus isolated from horses and its therapeutic effect on DSS-induced colitis in mice. Microb Pathog. 2022;165:105216. doi: 10.1016/j.micpath.2021.105216. [DOI] [PubMed] [Google Scholar]
- 28.Khan I, Wei J, Li A, Liu Z, Yang P, Jing Y, Chen X, Zhao T, Bai Y, Zha L, Li C, Ullah N, Che T, Zhang C. Lactobacillus plantarum strains attenuated DSS-induced colitis in mice by modulating the gut microbiota and immune response. Int Microbiol. 2022;25:587–603. doi: 10.1007/s10123-022-00243-y. [DOI] [PubMed] [Google Scholar]
- 29.Fei Y, Zhang S, Han S, Qiu B, Lu Y, Huang W, Li F, Chen D, Berglund B, Xiao H, Li L, Yao M. The Role of Dihydroresveratrol in Enhancing the Synergistic Effect of Ligilactobacillus salivarius Li01 and Resveratrol in Ameliorating Colitis in Mice. Research (Wash D C) 2022;2022:9863845. doi: 10.34133/2022/9863845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Fang H, Liu H, Cheng H, Pan L, Hu M, Li X. Goji berry juice fermented by probiotics attenuates dextran sodium sulfate-induced ulcerative colitis in mice. J Funct Foods. 2021;83:104491. [Google Scholar]
- 31.Kim DH, Kim S, Ahn JB, Kim JH, Ma HW, Seo DH, Che X, Park KC, Jeon JY, Kim SY, Lee HC, Lee JY, Kim TI, Kim WH, Kim SW, Cheon JH. Lactobacillus plantarum CBT LP3 ameliorates colitis via modulating T cells in mice. Int J Med Microbiol. 2020;310:151391. doi: 10.1016/j.ijmm.2020.151391. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Jin Y, Stanton C, Ross RP, Wang Z, Zhao J, Zhang H, Yang B, Chen W. Dose-response efficacy and mechanisms of orally administered CLA-producing Bifidobacterium breve CCFM683 on DSS-induced colitis in mice. J Funct Foods. 2020;75:104245. [Google Scholar]
- 33.Chen Y, Zhang L, Hong G, Huang C, Qian W, Bai T, Song J, Song Y, Hou X. Probiotic mixtures with aerobic constituent promoted the recovery of multi-barriers in DSS-induced chronic colitis. Life Sci. 2020;240:117089. doi: 10.1016/j.lfs.2019.117089. [DOI] [PubMed] [Google Scholar]
- 34.Komaki S, Haque A, Miyazaki H, Matsumoto T, Nakamura S. Unexpected effect of probiotics by Lactococcus lactis subsp. lactis against colitis induced by dextran sulfate sodium in mice. J Infect Chemother. 2020;26:549–553. doi: 10.1016/j.jiac.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Silveira DSC, Veronez LC, Lopes-Júnior LC, Anatriello E, Brunaldi MO, Pereira-da-Silva G. Lactobacillus bulgaricus inhibits colitis-associated cancer via a negative regulation of intestinal inflammation in azoxymethane/dextran sodium sulfate model. World J Gastroenterol. 2020;26:6782–6794. doi: 10.3748/wjg.v26.i43.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- 37.Jia K, Tong X, Wang R, Song X. The clinical effects of probiotics for inflammatory bowel disease: A meta-analysis. Medicine (Baltimore) 2018;97:e13792. doi: 10.1097/MD.0000000000013792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, Kusaka T, Uose S, Hisatsune H, Tojo M, Noda T, Arasawa S, Izuta M, Kubo A, Ogawa C, Matsunaka T, Shibatouge M. Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: A randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc. 2016;28:67–74. doi: 10.1111/den.12553. [DOI] [PubMed] [Google Scholar]
- 39.Palumbo VD, Romeo M, Marino Gammazza A, Carini F, Damiani P, Damiano G, Buscemi S, Lo Monte AI, Gerges-Geagea A, Jurjus A, Tomasello G. The long-term effects of probiotics in the therapy of ulcerative colitis: A clinical study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160:372–377. doi: 10.5507/bp.2016.044. [DOI] [PubMed] [Google Scholar]
- 40.Basson AR, Lam M, Cominelli F. Complementary and Alternative Medicine Strategies for Therapeutic Gut Microbiota Modulation in Inflammatory Bowel Disease and their Next-Generation Approaches. Gastroenterol Clin North Am. 2017;46:689–729. doi: 10.1016/j.gtc.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abraham B, Quigley EMM. Antibiotics and probiotics in inflammatory bowel disease: when to use them? Frontline Gastroenterol. 2020;11:62–69. doi: 10.1136/flgastro-2018-101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iheozor-Ejiofor Z, Kaur L, Gordon M, Baines PA, Sinopoulou V, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2020;3:CD007443. doi: 10.1002/14651858.CD007443.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agraib LM, Yamani MI, Tayyem R, Abu-Sneineh AT, Rayyan YM. Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: A pilot, randomized, double-blind, placebo-controlled study. Clin Nutr ESPEN. 2022;51:83–91. doi: 10.1016/j.clnesp.2022.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Ojetti V, Saviano A, Brigida M, Petruzziello C, Caronna M, Gayani G, Franceschi F. Randomized control trial on the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in reducing inflammatory markers in acute uncomplicated diverticulitis. Eur J Gastroenterol Hepatol. 2022;34:496–502. doi: 10.1097/MEG.0000000000002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu L, Shen F, Wang W, Qi C, Wang C, Shang A, Xuan S. The effect of multispecies probiotics on cognitive reactivity to sad mood in patients with Crohn’s disease. J Funct Foods. 2021;82:104431. [Google Scholar]
- 46.Bamola VD, Dubey D, Samanta P, Kedia S, Madempudi RS, Neelamraju J, Ahuja V, Chaudhry R. Effect of Bacillus coagulans Unique IS-2 in Inflammatory Bowel Disease (IBD): A Randomized Controlled Trial. 2021 Preprint. Available from: medRxiv:2021.07.18.21260556.
- 47.Waal M van der, Flach J, Browne PD, Vaart IB, Claassen E, Burwal LHM. Probiotics for improving quality of life in ulcerative colitis: Exploring the patient perspective. Pharmanutrition. 2019;7:100139. [Google Scholar]
- 48.Kamarli Altun H, Akal Yildiz E, Akin M. Impact of Synbiotic Therapy on the Quality of Life in Patients with Mild-to-Moderately Active Ulcerative Colitis. J Gastrointestin Liver Dis. 2022;31:417–423. doi: 10.15403/jgld-4345. [DOI] [PubMed] [Google Scholar]
- 49.Sun YY, Li M, Li YY, Li LX, Zhai WZ, Wang P, Yang XX, Gu X, Song LJ, Li Z, Zuo XL, Li YQ. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep. 2018;8:2964. doi: 10.1038/s41598-018-21241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuoka K, Uemura Y, Kanai T, Kunisaki R, Suzuki Y, Yokoyama K, Yoshimura N, Hibi T. Efficacy of Bifidobacterium breve Fermented Milk in Maintaining Remission of Ulcerative Colitis. Dig Dis Sci. 2018;63:1910–1919. doi: 10.1007/s10620-018-4946-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Hintze KJ, Ward RE. Effect of supplemental prebiotics, probiotics and bioactive proteins on the microbiome composition and fecal calprotectin in C57BL6/j mice. Biochimie. 2021;185:43–52. doi: 10.1016/j.biochi.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Floch MH. The Role of Prebiotics and Probiotics in Gastrointestinal Disease. Gastroenterol Clin North Am. 2018;47:179–191. doi: 10.1016/j.gtc.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 53.Chengxiao Y, Dongmei W, Kai Z, Hou L, Xiao H, Ding T, Liu D, Ye X, Linhardt RJ, Chen S. Challenges of pectic polysaccharides as a prebiotic from the perspective of fermentation characteristics and anti-colitis activity. Carbohydr Polym. 2021;270:118377. doi: 10.1016/j.carbpol.2021.118377. [DOI] [PubMed] [Google Scholar]
- 54.Laurell A, Sjöberg K. Prebiotics and synbiotics in ulcerative colitis. Scand J Gastroenterol. 2017;52:477–485. doi: 10.1080/00365521.2016.1263680. [DOI] [PubMed] [Google Scholar]
- 55.Xie J, Liu Y, Chen B, Zhang G, Ou S, Luo J, Peng X. Ganoderma lucidum polysaccharide improves rat DSS-induced colitis by altering cecal microbiota and gene expression of colonic epithelial cells. Food Nutr Res. 2019;63 doi: 10.29219/fnr.v63.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu F, Liu J, Wang TTY, Liu Z, Xue C, Mao X, Tang Q, Li RW. Molecular and Microbial Signatures Predictive of Prebiotic Action of Neoagarotetraose in a Dextran Sulfate Sodium-Induced Murine Colitis Model. Microorganisms. 2020;8:995. doi: 10.3390/microorganisms8070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui L, Guan X, Ding W, Luo Y, Wang W, Bu W, Song J, Tan X, Sun E, Ning Q, Liu G, Jia X, Feng L. Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int J Biol Macromol. 2021;166:1035–1045. doi: 10.1016/j.ijbiomac.2020.10.259. [DOI] [PubMed] [Google Scholar]
- 58.Tolonen AC, Beauchemin N, Bayne C, Li L, Tan J, Lee J, Meehan BM, Meisner J, Millet Y, LeBlanc G, Kottler R, Rapp E, Murphy C, Turnbaugh PJ, von Maltzahn G, Liu CM, van Hylckama Vlieg JET. Synthetic glycans control gut microbiome structure and mitigate colitis in mice. Nat Commun. 2022;13:1244. doi: 10.1038/s41467-022-28856-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian X, Jiang H, Wu Y, Shao MH, He JW, Bao X, He JL, Jia LY, Xu YZ. Fecal microbiota transplantation combined with prebiotics ameliorates ulcerative colitis in mice. 2022 Preprint. Available from: PPR: PPR518073. [DOI] [PubMed]
- 60.Liu R, Li Y, Zhang B. The effects of konjac oligosaccharide on TNBS-induced colitis in rats. Int Immunopharmacol. 2016;40:385–391. doi: 10.1016/j.intimp.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 61.He L, Zhang F, Jian Z, Sun J, Chen J, Liapao V, He Q. Stachyose modulates gut microbiota and alleviates dextran sulfate sodium-induced acute colitis in mice. Saudi J Gastroenterol. 2020;26:153–159. doi: 10.4103/sjg.SJG_580_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanwal S, Joseph TP, Aliya S, Song S, Saleem MZ, Nisar MA, Wang Y, Meyiah A, Ma Y, Xin Y. Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways. J Funct Foods. 2019;64:103641. [Google Scholar]
- 63.Li P, Xiao N, Zeng L, Xiao J, Huang J, Xu Y, Chen Y, Ren Y, Du B. Structural characteristics of a mannoglucan isolated from Chinese yam and its treatment effects against gut microbiota dysbiosis and DSS-induced colitis in mice. Carbohydr Polym. 2020;250:116958. doi: 10.1016/j.carbpol.2020.116958. [DOI] [PubMed] [Google Scholar]
- 64.K-da S, Peerakietkhajorn S, Siringoringo B, Muangnil P, Wichienchot S, Khuituan P. Oligosaccharides from Gracilaria fisheri ameliorate gastrointestinal dysmotility and gut dysbiosis in colitis mice. J Funct Foods. 2020;71:104021. [Google Scholar]
- 65.Wong C, Harris PJ, Ferguson LR. Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson B, Eyice Ö, Koumoutsos I, Lomer MC, Irving PM, Lindsay JO, Whelan K. Prebiotic Galactooligosaccharide Supplementation in Adults with Ulcerative Colitis: Exploring the Impact on Peripheral Blood Gene Expression, Gut Microbiota, and Clinical Symptoms. Nutrients. 2021;13 doi: 10.3390/nu13103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LeBlanc JF, Segal JP, de Campos Braz LM, Hart AL. The Microbiome as a Therapy in Pouchitis and Ulcerative Colitis. Nutrients. 2021;13 doi: 10.3390/nu13061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valcheva R, Armstrong H, Kovic O, Bording-Jorgensen M, Veniamin S, Elisa Pérez-Muñoz M, Haskey N, Silva M, Peerani F, Wong K, Kao DH, Veldhuyzen S, Zanten V, Kroeker KI, Gibson DL, Wine E, Gänzle M, Walter J, Dieleman LA. Double blind placebo-controlled trial for the prevention of ulcerative colitis relapses by β-fructan prebiotics: efficacy and metabolomic analysis. 2022 Preprint. Available from: medRxiv: 2022.01.16.22269376.
- 69.Pietrzak A, Banasiuk M, Szczepanik M, Borys-Iwanicka A, Pytrus T, Walkowiak J, Banaszkiewicz A. Sodium Butyrate Effectiveness in Children and Adolescents with Newly Diagnosed Inflammatory Bowel Diseases-Randomized Placebo-Controlled Multicenter Trial. Nutrients. 2022;14 doi: 10.3390/nu14163283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vernero M, De Blasio F, Ribaldone DG, Bugianesi E, Pellicano R, Saracco GM, Astegiano M, Caviglia GP. The Usefulness of Microencapsulated Sodium Butyrate Add-On Therapy in Maintaining Remission in Patients with Ulcerative Colitis: A Prospective Observational Study. J Clin Med. 2020;9 doi: 10.3390/jcm9123941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valcheva R, Koleva P, Martínez I, Walter J, Gänzle MG, Dieleman LA. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes. 2019;10:334–357. doi: 10.1080/19490976.2018.1526583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azpiroz F, Dubray C, Bernalier-Donadille A, Cardot JM, Accarino A, Serra J, Wagner A, Respondek F, Dapoigny M. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.12911. [DOI] [PubMed] [Google Scholar]
- 73.Wong WY, Chan BD, Leung TW, Chen MX, Tai WCS. Beneficial and anti-inflammatory effects of formulated prebiotics, probiotics, and synbiotics in normal and acute colitis mice. J Funct Foods. 2022;88:104871. [Google Scholar]
- 74.Kangwan N, Kongkarnka S, Boonkerd N, Unban K, Shetty K, Khanongnuch C. Protective Effect of Probiotics Isolated from Traditional Fermented Tea Leaves (Miang) from Northern Thailand and Role of Synbiotics in Ameliorating Experimental Ulcerative Colitis in Mice. Nutrients. 2022;14 doi: 10.3390/nu14010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khalifa A, Sheikh A, Ibrahim HIM. Bacillus amyloliquefaciens Enriched Camel Milk Attenuated Colitis Symptoms in Mice Model. Nutrients. 2022;14 doi: 10.3390/nu14091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu Z, Jiang Z, Zhang Z, Liu T, Fan Y, Peng N. Bacillus coagulans in Combination with Chitooligosaccharides Regulates Gut Microbiota and Ameliorates the DSS-Induced Colitis in Mice. Microbiol Spectr. 2022;10:e0064122. doi: 10.1128/spectrum.00641-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao M, Zhang Y, Qiu Y, Wu Z, Zhong Z, Zeng X, Zeng Y, Xiong L, Wen Y, Liu R. Fructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in DSS-induced acute colitis mice. Food Funct. 2021;12:9844–9854. doi: 10.1039/d1fo01147b. [DOI] [PubMed] [Google Scholar]
- 78.Li F, Huang H, Zhu F, Zhou X, Yang Z, Zhao X. A Mixture of Lactobacillus fermentum HFY06 and Arabinoxylan Ameliorates Dextran Sulfate Sodium-Induced Acute Ulcerative Colitis in Mice. J Inflamm Res. 2021;14:6575–6585. doi: 10.2147/JIR.S344695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shinde T, Perera AP, Vemuri R, Gondalia SV, Beale DJ, Karpe AV, Shastri S, Basheer W, Southam B, Eri R, Stanley R. Synbiotic supplementation with prebiotic green banana resistant starch and probiotic Bacillus coagulans spores ameliorates gut inflammation in mouse model of inflammatory bowel diseases. Eur J Nutr. 2020;59:3669–3689. doi: 10.1007/s00394-020-02200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xue Z, Li R, Liu J, Zhou J, Zhang X, Zhang T, Zhang M, Yang Y, Chen H. Preventive and synbiotic effects of the soluble dietary fiber obtained from Lentinula edodes byproducts and Lactobacillus plantarum LP90 against dextran sulfate sodium-induced colitis in mice. J Sci Food Agric. 2023;103:616–626. doi: 10.1002/jsfa.12173. [DOI] [PubMed] [Google Scholar]
- 81.Ivanovska TP, Mladenovska K, Zhivikj Z, Pavlova MJ, Gjurovski I, Ristoski T, Petrushevska-Tozi L. Synbiotic loaded chitosan-Ca-alginate microparticles reduces inflammation in the TNBS model of rat colitis. Int J Pharm. 2017;527:126–134. doi: 10.1016/j.ijpharm.2017.05.049. [DOI] [PubMed] [Google Scholar]
- 82.Seong G, Lee S, Min YW, Jang YS, Park SY, Kim CH, Lee C, Hong SN, Chang DK. Effect of a Synbiotic Containing Lactobacillus paracasei and Opuntia humifusa on a Murine Model of Irritable Bowel Syndrome. Nutrients. 2020;12 doi: 10.3390/nu12103205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dos Santos Cruz BC, da Silva Duarte V, Giacomini A, Corich V, de Paula SO, da Silva Fialho L, Guimarães VM, de Luces Fortes Ferreira CL, Gouveia Peluzio MDC. Synbiotic VSL#3 and yacon-based product modulate the intestinal microbiota and prevent the development of pre-neoplastic lesions in a colorectal carcinogenesis model. Appl Microbiol Biotechnol. 2020;104:8837–8857. doi: 10.1007/s00253-020-10863-x. [DOI] [PubMed] [Google Scholar]
- 84.Wang YN, Meng XC, Dong YF, Zhao XH, Qian JM, Wang HY, Li JN. Effects of probiotics and prebiotics on intestinal microbiota in mice with acute colitis based on 16S rRNA gene sequencing. Chin Med J (Engl) 2019;132:1833–1842. doi: 10.1097/CM9.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Son SJ, Koh JH, Park MR, Ryu S, Lee WJ, Yun B, Lee JH, Oh S, Kim Y. Effect of the Lactobacillus rhamnosus strain GG and tagatose as a synbiotic combination in a dextran sulfate sodium-induced colitis murine model. J Dairy Sci. 2019;102:2844–2853. doi: 10.3168/jds.2018-15013. [DOI] [PubMed] [Google Scholar]
- 86.Amiriani T, Rajabli N, Faghani M, Besharat S, Roshandel G, Akhavan Tabib A, Joshaghani H. Effect of Lactocare® Synbiotic on Disease Severity in Ulcerative Colitis: A Randomized Placebo-Controlled Double-Blind Clinical Trial. Middle East J Dig Dis. 2020;12:27–33. doi: 10.15171/mejdd.2020.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kamarlı Altun H, Akal Yıldız E, Akın M. Effects of synbiotic therapy in mild-to-moderately active ulcerative colitis: A randomized placebo-controlled study. Turk J Gastroenterol. 2019;30:313–320. doi: 10.5152/tjg.2019.18356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang Y, Liu M, Pu J, Zhu Z, Gao Z, Zhou Q, Gu Q, Li P, Van Der Veen S. Probiotics and Their Metabolites Ameliorate Inflammatory Bowel Disease: A Critical Review. Infect Microbes Dis. 2021;3:4–13. [Google Scholar]
- 89.Akram W, Garud N, Joshi R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov Ther. 2019;13:1–8. doi: 10.5582/ddt.2019.01000. [DOI] [PubMed] [Google Scholar]
- 90.Vu V, Muthuramalingam K, Singh V, Hyun C, Kim YM, Unno T, Cho M. Effects of β-glucan, probiotics, and synbiotics on obesity-associated colitis and hepatic manifestations in C57BL/6J mice. Eur J Nutr. 2022;61:793–807. doi: 10.1007/s00394-021-02668-z. [DOI] [PubMed] [Google Scholar]
- 91.Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. doi: 10.1038/s41575-020-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dore MP, Bibbò S, Fresi G, Bassotti G, Pes GM. Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients. 2019;11 doi: 10.3390/nu11122913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Astó E, Méndez I, Audivert S, Farran-Codina A, Espadaler J. The Efficacy of Probiotics, Prebiotic Inulin-Type Fructans, and Synbiotics in Human Ulcerative Colitis: A Systematic Review and Meta-Analysis. Nutrients. 2019;11 doi: 10.3390/nu11020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar D, Lal MK, Dutt S, Raigond P, Changan SS, Tiwari RK, Chourasia KN, Mangal V, Singh B. Functional Fermented Probiotics, Prebiotics, and Synbiotics from Non-Dairy Products: A Perspective from Nutraceutical. Mol Nutr Food Res. 2022;66:e2101059. doi: 10.1002/mnfr.202101059. [DOI] [PubMed] [Google Scholar]
- 95.Yadav MK, Kumari I, Singh B, Sharma KK, Tiwari SK. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl Microbiol Biotechnol. 2022;106:505–521. doi: 10.1007/s00253-021-11646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, Tan B, Wang XY. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24:5–14. doi: 10.3748/wjg.v24.i1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reinisch W. Fecal Microbiota Transplantation in Inflammatory Bowel Disease. Dig Dis. 2017;35:123–126. doi: 10.1159/000449092. [DOI] [PubMed] [Google Scholar]
- 98.Lopez J, Grinspan A. Fecal Microbiota Transplantation for Inflammatory Bowel Disease. Gastroenterol Hepatol (N Y) 2016;12:374–379. [PMC free article] [PubMed] [Google Scholar]
- 99.Tian Y, Zhou Y, Huang S, Li J, Zhao K, Li X, Wen X, Li XA. Fecal microbiota transplantation for ulcerative colitis: a prospective clinical study. BMC Gastroenterol. 2019;19:116. doi: 10.1186/s12876-019-1010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pigneur B, Sokol H. Fecal microbiota transplantation in inflammatory bowel disease: the quest for the holy grail. Mucosal Immunol. 2016;9:1360–1365. doi: 10.1038/mi.2016.67. [DOI] [PubMed] [Google Scholar]