Abstract
Dysphagia has been classified as a “geriatric syndrome” and can lead to serious complications that result in a tremendous burden on population health and healthcare resources worldwide. A characteristic age-related change in swallowing is defined as “presbyphagia.” Medical imaging has shown some changes that seriously affect the safety and efficacy of swallowing. However, there is a general lack of awareness of the effects of aging on swallowing function and a belief that these changes are part of normal aging. Our review provides an overview of presbyphagia, which has been a neglected health problem for a long time. Attention and awareness of dysphagia in the elderly population should be strengthened, and targeted intervention measures should be actively implemented.
Keywords: Aging, Dysphagia, Presbyphagia, Geriatric syndromes, Swallowing
Core Tip: Dysphagia in older people is unfortunately considered a part of aging. Many factors contribute, such as decreased cognitive function, loss of teeth, reduced muscle strength, decreased taste and olfaction, altered salivary secretion, impaired cough and swallowing reflexes, altered hyoid bone and larynx position, reduced laryngeal adductor reflex, decreased tongue root retraction, incomplete esophageal sphincter opening, reduced pharyngeal constriction and sensation, reduced breathing and swallowing coordination, and decreased esophageal motility. Several may be amenable to therapeutic strategies, including rehabilitation, to improve, and even restore swallowing function at the anatomical and physiological levels. Improved screening, clinical assessment, and diagnostic procedures are needed.
INTRODUCTION
The effects of aging on swallowing are multifaceted, insidious, and frequently considered a natural part of aging. Older adults gradually develop characteristic changes in swallowing during aging; this phenomenon is called “presbyphagia”[1]. Dysphagia can affect the safety and effectiveness of swallowing, causing severe complications, and affect social interactions, quality of life, and mental health due to fear and anxiety about eating[2,3]. It also increases the financial burden on patients and the healthcare system. Bonilha et al[4] observed that yearly medical costs for patients with dysphagia were $4510 higher than those for patients without dysphagia.
However, the problem of presbyphagia continues to be overlooked by most patients, families, and medical practitioners due to the perception that these physiological changes constitute a part of aging. This review aims to raise awareness and understanding of presbyphagia as a critical health problem, improve the diagnostic criteria for presbyphagia, guide interventions, and enhance prevention and treatment performance.
EFFECTS OF AGING ON SWALLOWING FUNCTION
During aging, physiological functions continue to decline and gradually lose their integrity. This deterioration process is also a major contributor to the risk of dysphagia. Functional degradation is mediated by characteristic molecular and cellular phenomena associated with normal aging. These phenomena can be divided into three categories: Primary, antagonistic, and integrative hallmarks[5]. When environmental homeostasis mechanisms within the tissue cannot compensate for the cumulative damage caused by primary and antagonistic hallmarks, the integrative hallmarks arise and ultimately lead to age-related functional decline[5,6].
Nervous system changes
Changes in neural structure, brain chemistry, and related functions occur and accumulate with age, making aging a major risk factor for neurodegeneration. Malandraki et al[7] used functional magnetic resonance imaging to compare sites of central nervous activation during swallowing in healthy older adults with those in younger adults. They found that activation of the areas corresponding to sensory processing, sensorimotor integration, and motor coordination and control is diminished in healthy older adults compared to younger adults. The cerebral blood flow is reduced, the cortical sulcus is widened, and the ventricles are enlarged. Furthermore, the volume of some nuclei is reduced, and the ability to process neural information is diminished[8]. The functional and electrophysiological properties of the peripheral nervous system are affected by aging, manifesting as decreased nerve conduction velocity, autonomic responses, endoneurial blood flow, and sensory discrimination[9,10].
Skeletal changes
Cellular senescence increases with age, and senescent chondrocytes accumulate and produce a factor that inhibits cartilage regeneration[11-13]. Epiglottis and paired arytenoid cartilages play an important role in preventing aspiration. Aged cartilage changes shape and becomes less elastic, weakening the protective capacity of the airway. More than 75% of adults aged > 65 years suffer from degenerative changes in the cervical spine; cervical osteophytosis affects swallowing in older adults through various mechanisms, causing frequent coughing and choking[14,15].
Muscle changes
Research has shown that total muscle mass in older adults decreases by 0.5%–1.0% per year, with a cumulative decrease of 30%–50% by 80 years of age[16,17]. Forty muscles are involved in the complex physiological activity of swallowing[2,18,19]. The strength of muscle contraction decreases with age[20]. The tendon transmits the force of the muscle fiber contraction to the bone. During aging, the water and proteoglycan content of tendons decreases, while calcification and fat accumulation increases, leading to decreased tensile strength and stiffness in the tendons, impaired flexibility and coordination of physiological activities required for swallowing, and increased risk of serious complications in older adults[21-23].
Respiratory function
The number of lung epithelial cells gradually decreases with aging, the proportion of fibroblasts increases, and surfactant secretion is impaired, leading to a decline in lung volume and elasticity[24]. The change in thoracic shape serves to reduce chest wall compliance, as shown by altered spinal physiological curvature, increased sternal curvature, and thinning of chest wall muscles[25-27]. Significantly reduced pulmonary function and chest wall compliance decrease the ability of older adults to clear respiratory residue and increase the risk of food entering the respiratory tract.
Overall situation
Older adults are more susceptible to weight loss and malnutrition due to a diminished sense of smell and taste, which affects their appetite and dietary preferences[28,29]. Simultaneously, cortical decompensation reduces physiological sensitivity to osmotic pressure and volume stimuli in older adults, diminishes self-perception of thirst, causes defects in fluid regulation, and tends to result in dehydration[30]. Weakness and aging go hand in hand, manifesting as a weakening of the body's ability to resist and adapt[31]. Dehydration and malnutrition can worsen weakness and lead to muscle atrophy, decreased immunity, and reduced functional reserve. Weakness can, in turn, contribute to the deterioration of swallowing function, reducing the safety of swallowing and increasing the risk of aspiration.
STAGES OF PRESBYPHAGIA
Oral preparatory stage
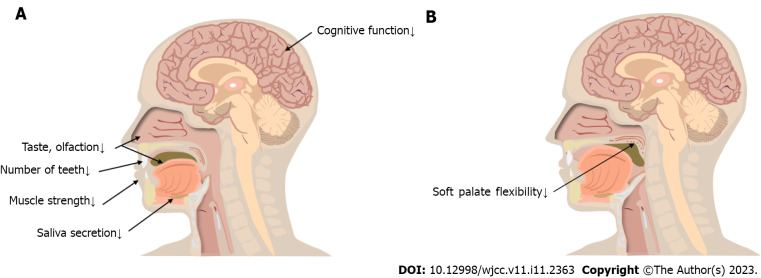
The oral preparatory stage is controlled by human will (i.e., food is placed in the mouth, stirred, chewed, and processed into a bolus) (Figure 1A). The main changes occurring during this stage are as follows.
Figure 1.
Oral preparatory and transport stage changes. A: Oral preparatory stage changes; B: Oral transport stage changes.
Decreased cognitive function: Overall, the brain’s weight and cortical surface area decrease with age; a rigorous survey demonstrated that 40% of older adults show symptoms of cognitive impairment[32]. Compared with younger adults, hemispheric specialization is reduced or altered in older adults, necessitating the recruitment of a higher volume of brain areas to compensate for the increased task demands during swallowing[33,34]. The main manifestation of this stage is a cognitive bias regarding the texture, quantity, temperature, taste, and smell of food. Affected individuals may also show the following symptomology: Difficulty concentrating, lower responsivity, impaired casual eating movements, poor coordination, lack of flexibility in adjusting the speed of eating and food intake, and inability to judge in advance how the food will be handled in the mouth[35].
Decreased number of teeth: A controlled trial conducted by Yurkstas et al[36] found that individuals with a greater number of teeth (within an age-matched group of older individuals) chewed more efficiently than those with a smaller number of teeth. Missing teeth reduced masticatory efficiency (16%–50%), prolonged the residence time of food in the mouth, and affected older people’s choice of food. The prevalence of root caries in people aged > 60 years is twice as high as in young people, and 64% and 96% of people aged > 80 years have root and crown caries, respectively[37,38]. When the neurovascular structures in the dental pulp are involved, the teeth may become sensitive, painful, and even infected (i.e., bacteremia)[39]. This may lead to a reduced intake of vegetables, fruits, and nuts, thus affecting nutritional status. Hence, people without teeth are more likely to suffer from weight loss and malnutrition[37,40].
Reduced muscle strength: Reduced contraction of the orbicularis oris muscle frequently occurs during aging–approximately 20% of older adults are unable to keep their lips closed while swallowing, and food and liquids flow out of their mouths[41]. The cross-sectional area of the masticatory muscles (i.e., the masseterica, temporalis, pterygoideus medialis, pterygoideus lateralis, buccinator, zygomaticus, and mentalis) decreases during the aging process, resulting in a decrease in the force of muscle contraction. The tongue muscles show muscle atrophy and increased connective tissue, reduced muscle strength and range of motion, and decreased flexibility[1,42,43]. Moreover, older adults have a decreased swallowing pressure reserve and lower maximum tongue pressure compared to younger adults[44]. Hence, food spreads in the mouth and stays longer.
Decreased taste and olfaction: The number of nerve fibers and receptors on the olfactory bulb decreases significantly with age. Olfactory function deteriorates progressively with aging such that > 34.5% of older adults have olfactory impairment[45]. Older adults have thinner oral mucosa, weaker secretion, fewer chemoreceptors, and reduced taste perception[46]. During the oral preparation period, olfactory and gustatory information converges on specific brain neurons, activating the amygdala, insular cortex, and anterior cingulate cortex regions; reduced olfaction and gustation can lead to reduced appetite[47]. Compared to younger adults, older adults need two to three times the salt concentration to experience the same saltiness in their food. Older adults prefer sweet and salty foods due to a diminished sense of taste; this increases their risk of obesity, cardiovascular disease, and metabolic disorders[48,49]. Therefore, nutritional problems are important complications of olfactory and gustatory disorders. A 5-year follow-up study[50] showed that a reduced sense of taste and smell affects the quality of life of older persons and is detrimental to their mental health.
Altered salivary secretion: The prevalence of xerostomia increases with age, affecting approximately 30% of the population aged ³65 years[51,52]. Aging affects salivary gland secretion and decreases inorganic ion concentrations, thereby increasing the threshold for eliciting taste sensations. Further, the number of salivary secretory cells decreases with age, causing diminished sensitivity in taste receptor cells and affecting taste perception[53]. Reduced saliva production also affects the forward movement of food in the mouth, increases oropharyngeal residue, aggravates xerostomia, and increases the risk of poor oral hygiene in older adults.
Oral transport stage
The tongue pushes the bolus backward towards the pharynx in a process termed the oral transport stage. The soft palate plays an important role in this stage: It hangs between the oropharynx and nasopharynx, preventing food from falling into the pharynx prematurely and/or flowing backward into the nasopharynx during swallowing[47] (Figure 1B).
Diminished flexibility tension in the soft palate: The soft palate is composed of connective tissue and muscle. Muscle fiber number, length, and cross-sectional area all decrease due to aging, and increased connective tissue and lipid deposition in the soft palate reduce its flexibility and range of motion[54-56]. When the closure of the tongue and soft palate is inadequate, food can fall into the pharynx and the open expiratory pathways before the swallowing response is triggered, leading to pre-swallowing aspiration[57]. Aerodynamic studies have shown that older speakers exhibit a similar nasal airflow and air volume compared to younger speakers and that velopharyngeal function does not deteriorate with age[58].
Pharyngeal stage
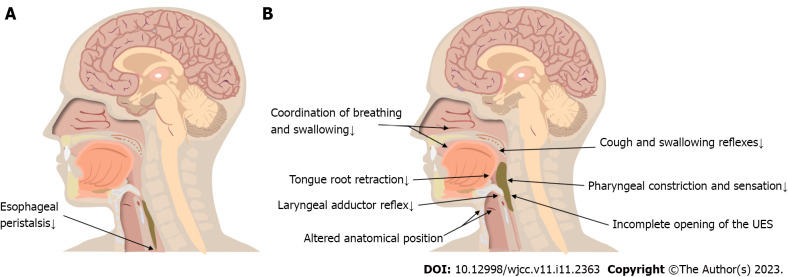
The pharyngeal stage represents the period when the bolus enters the pharynx and reaches the esophagus through the open upper esophageal sphincter (UES) (Figure 2A). Physiological activity during this period is rapid and complex. In a previous study, videofluoroscopic swallowing studies (VFSSs) were performed on 56 healthy older people, and only 16% of them had normal swallowing function[59]. Pharyngeal residue was the most frequent phenomenon during imaging, though it did not cause significant clinical symptoms in most cases[59-61]. Increased pharyngeal residue is usually associated with weak tongue extrusion and diminished pharyngeal clearance[62]. VFSSs revealed that frail older individuals had severely impaired swallowing and coughing reflexes. Up to 55% of older adults show fluid penetration into the laryngeal vestibule during swallowing; approximately 15% show fluid crossing the vocal cords to reach the trachea and bronchi, which eventually causes aspiration[41]. Studies[63-67] have also shown physiological changes in older adults, such as delayed swallowing response, altered distance and time with respect to hyoid and laryngeal elevation, delayed laryngeal closure, and incomplete opening of the UES. These age-related changes result in a higher incidence of permeation and aspiration, significantly reducing the safety of swallowing in older adults (Figure 2B).
Figure 2.
Esophageal and pharyngeal stage changes. A: Esophageal stage changes; B: Pharyngeal stage changes. UES: Upper esophageal sphincter.
Impaired cough and swallowing reflexes: The airway protective reflexes include coughing and swallowing reflexes, which are important for preventing aspiration pneumonia[68]. Tracy et al[69] measured the initiation time of the swallowing reflex in different age groups; they found that the swallowing response initiation time was on average 0.4 s longer and the sensitivity of the swallowing reflex area was reduced in older adults. The decrease in the number of myelin sheaths with age leads to reduced sensory input, and older adults require a larger bolus volume to elicit a swallowing reflex[70-72]. Frequently, the bolus has fallen into the pharynx by the time older adults begin swallowing. Most older adults with impaired swallowing reflexes also have impaired cough reflexes. The cough reflex is significantly weakened and suppressed in older patients with aspiration pneumonia; this may be related to the disruption of the cortical facilitation pathway and the medullary reflex pathway of cough caused by aging[73,74].
Altered anatomical position of the hyoid bone and larynx: The anatomical positions of the hyoid bone and larynx are altered in older persons due to changes in spinal morphology and reduced muscle tone. Additionally, the larynx descends to a position close to C7 in older persons aged > 70 years. However, the distance traversed by the larynx during swallowing does not decrease[43]. Accordingly, older individuals need more time to complete the movement that mediates laryngeal elevation[75]. Functional reserve describes the difference between a maximal effort and a normal swallowing task[76]. Aging decreases the speed of hyoid bone and laryngeal elevation, reducing the functional reserve in older adults and increasing the risk of swallowing problems[66,75]. Changes in the anatomical positions weaken the protective mechanisms of the respiratory tract and affect the duration and degree of opening of the UES, hence preventing the smooth passage of the bolus from the pharynx into the esophagus[47,77,78]. The hyoid bone is connected to the larynx through the thyroid hyoid membrane; the movement of the hyoid bone generates a pulling force on the larynx, which is then transmitted to the UES by means of the cricoid cartilage. This is the initial force that causes the cricopharyngeal muscle to open by approximately 8 mm[75,79-81].
Reduced laryngeal adductor reflex: Superior laryngeal nerves play an important role in sensation and autonomic innervation. Mortelliti et al[82] used electron-microscopic morphometric techniques to observe the difference between the superior laryngeal nerves of older adults and younger people. They showed that the myelinated nerve fiber count in older people decreased by 31% compared to that in younger people (P = 0.032). The ammonia concentration required to trigger laryngeal closure is six times higher in older adults than in younger adults[83]. The occurrence of silent aspiration is not only related to motor abnormalities, but is also largely influenced by sensory abnormalities[84]. Randomized controlled trials have shown that patients with laryngopharyngeal sensory deficits have difficulty inducing the laryngeal adductor reflex (even under intense stimulation) and a significantly higher risk of penetration and aspiration than controls[85]. Patients lacking the laryngeal adductor reflex have a 6.8 times increased risk of pneumonia (odds ratio: 6.75; 95% confidence interval: 1.76–25.96)[86].
Decreased tongue root retraction: Retraction of the tongue root is one of the main driving forces on the bolus during the pharyngeal stage and is especially important for the smooth entry of the bolus into the pharynx. Cook et al[87] found that aging causes a decrease in tongue root retraction. It is speculated that this is a compensatory mechanism due to the increased threshold of the swallowing reflex in older persons; reductions in tongue root retraction movement can increase the accumulation of food in the oropharynx, making it easier to trigger pharyngeal-stage swallowing. On the other hand, the reduced retraction of the tongue root weakens its ability to remove food from the pharynx, resulting in increased pharyngeal residue[88,89].
Incomplete opening of the UES: The resting pressure of the UES in older persons is 43 ± 5 mmHg, which is significantly lower than that in younger persons (71 ± 8 mmHg; P < 0.05)[90]. With aging, the number of UES muscle fibers decreases, and the excitatory impulses that maintain cricopharyngeal tension also gradually reduce[91]. An inadequate UES pressure decrease during swallowing was observed in 15.4% of older adults aged 60–69 years and 30.4% of older adults aged 70–83 years, while 39% had inadequate UES opening[59,92]. The UES pressure decreased more slowly in older adults, and there was a delayed opening time and significantly fewer UES openings than in younger adults[92-94]. Aging increases muscle connective tissue, which results in a decrease in the elasticity and compliance of the UES[94]. The degree and duration of UES opening are abnormal, increasing the risk of pharyngeal residual and aspiration.
Reduced pharyngeal constriction and sensation: There is prolonged bolus passage through the pharynx when healthy older adults swallow their food[92]. The pharyngeal sensation is significantly reduced in older adults compared to younger adults. A disrupted pattern of cortical activation is observed in older adults, and their ability to find and remove residual food from the pharynx is reduced[90,95,96]. Increased pharyngeal residue is the most common imaging sign in older adults, and the amount of pharyngeal residue is positively correlated with age[87,89]. Reginelli et al[97] conducted a VFSS study on older adults and observed that they had a higher prevalence of post-swallowing aspiration than younger adults–this is usually caused by foods remaining in the pharynx.
Reduced coordination of breathing and swallowing: Swallowing and breathing share a common anatomy; hence, food and liquids must be secured as they enter the digestive tract by ensuring that the respiratory tract is closed. Shaker et al[98] reported reduced coordination between swallowing and breathing in older persons and found that aging leads to substantially longer pauses in breathing during swallowing. Older adults take longer to return to normal tidal breathing than young individuals[47]. Aging also reduces respiratory function and weakens airway clearance[24].
Esophageal stage
The esophageal stage begins at the tip of the esophagus. The bolus passes through the esophagus under peristaltic contraction and gravity, the lower esophageal sphincter opens, and the bolus enters the stomach (Figure 2A).
Decreased esophageal motility: Myenteric neurons in the esophagus steadily decline with age, causing dysmotility associated with denervation[99]. Secondary esophageal peristalsis removes refluxed contents from the esophagus and is one of the airway protective mechanisms[100]. Mei et al[101] found a reduced incidence of secondary esophageal peristalsis and a decreased esophageal response to low-volume ultra-slow reflux in older adults. In the absence of secondary peristalsis of the esophagus, reflux containing gastric acid will stay in the esophagus for a longer time and easily cause reflux esophagitis[102]. Biomechanical changes in the esophagus during aging were observed: The esophageal wall changed in stiffness, circumferentially and longitudinally, with age (P < 0.05 and P < 0.01, respectively)[103]. Although the percentage of muscle fiber types in the esophagus does not change meaningfully in older persons, a prior study demonstrated a statistically significant increase in muscle fiber diameter due to atrophy and compensatory hypertrophy[104].
INTERVENTION MEASURES
Rehabilitation education
Rather than directly improving the physiological function of swallowing in older adults, rehabilitation education minimizes or eliminates the symptoms of swallowing dysfunction and reduces the occurrence of adverse events. These include postural adjustment, adjustment of eating strategies, use of assistive devices, and periodic oral care[105,106]. Swallowing forcefully in older adults can help to increase pharyngeal pressure, and the downward swallowing position of the lower jaw can change the biomechanical relationship during swallowing and reduce the occurrence of aspiration[106]. Adjustments to diet and eating strategies are the most common compensatory methods. For example, older people should eat slowly, not eat while watching TV, not talk while eating, avoid eating when tired or in a hurry, avoid taking too much in one bite, and avoid swallowing mixed foods and liquids. Single texture foods are easier to swallow. Foods that are relatively viscous and easy to form are also easier to swallow. Assistive devices, such as those used for placing, guiding and controlling food or fluids during swallowing, can increase or prolong eating independence. Paying attention to oral hygiene can also reduce the risk of developing aspiration pneumonia in older people with dysphagia.
Rehabilitation therapy
Rehabilitation training can improve the physiological function of swallowing and reduce the risk of complications[105], and ageing-induced muscle weakness can be improved by rehabilitation training. In a study involving older people, isometric resistance training of the buccal surface, lips, tongue and related oropharyngeal muscles for 8 wk significantly improved swallowing function[107]. In another study, ice stimulation improved sensitivity of the soft palate and pharynx, increased sensory input, excited neurons in the motor pathway and promoted axonal regeneration of neurons[108]. Additionally, respiratory training improved the coordination of swallowing and breathing and enhanced the ability of the respiratory tract to clear foreign bodies[109]. Finally, simple shaker training in older people improved cricopharyngeal opening, prolonged its opening time, and improved the strength of the supraglottis muscle group and the thyrohyoid muscle[105].
CONCLUSION
Many people, including healthcare practitioners, assume that some age-related changes in swallowing are part of natural aging. However, imaging signs show that some changes may affect the effectiveness and safety of swallowing and lead to severe complications[41,64,89]. Presbyphagia is a common and dangerous, but widely neglected health problem. First, improving the awareness of presbyphagia among medical staff is necessary. We need to understand the mechanisms through which aging affects swallowing function, as well as improve our ability to recognize presbyphagia and its manifestations. Second, screening and assessment of high-risk groups should be performed as early as possible. However, the current screening and clinical assessment process for dysphagia in older adults is not standardized. Further research is needed to improve and standardize screening tools, clinical assessments, and instrumental diagnostic procedures. Finally, it has been well documented that rehabilitation therapy positively affects the anatomical structures and sensory-cortical-motor circuits related to swallowing. Additionally, it can directly and effectively improve and restore swallowing function at the anatomical and physiological levels[105,110-112]. In recent years, non-invasive brain stimulation has been shown to induce plasticity changes in the swallowing motor cortex, resulting in increased cortical excitability and ultimately improving swallowing disorders in older adults[113,114]. Taste[115], olfaction[116], and vision[117] can also alter cortical excitability or activate the swallowing motor cortex. However, there is no standard intervention at present to manage dysphagia in older adults. Further clinical research is required to facilitate the development of clear guidelines to address this issue.
Footnotes
Conflict-of-interest statement: Wang Xiaowen has received research funding from China Disabled Persons' Federation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: January 17, 2023
First decision: January 31, 2023
Article in press: March 22, 2023
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Byeon H, South Korea; Huang Y, China; Liao Z, Singapore S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
Contributor Information
Hai-Yang Feng, School of Rehabilitation Medicine, Weifang Medical University, Weifang 261021, Shandong Province, China.
Ping-Ping Zhang, School of Rehabilitation Medicine, Weifang Medical University, Weifang 261021, Shandong Province, China.
Xiao-Wen Wang, School of Rehabilitation Medicine, Weifang Medical University, Weifang 261021, Shandong Province, China. 1832770656@qq.com.
References
- 1.Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- 2.Baijens LW, Clavé P, Cras P, Ekberg O, Forster A, Kolb GF, Leners JC, Masiero S, Mateos-Nozal J, Ortega O, Smithard DG, Speyer R, Walshe M. European Society for Swallowing Disorders - European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging. 2016;11:1403–1428. doi: 10.2147/CIA.S107750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gellrich D, Wechtenbruch J, Echternach M. [Swallowing disorders in the elderly] MMW Fortschr Med. 2019;161:45–48. doi: 10.1007/s15006-019-0961-2. [DOI] [PubMed] [Google Scholar]
- 4.Bonilha HS, Simpson AN, Ellis C, Mauldin P, Martin-Harris B, Simpson K. The one-year attributable cost of post-stroke dysphagia. Dysphagia. 2014;29:545–552. doi: 10.1007/s00455-014-9543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, Bohr VA. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 7.Malandraki GA, Perlman AL, Karampinos DC, Sutton BP. Reduced somatosensory activations in swallowing with age. Hum Brain Mapp. 2011;32:730–743. doi: 10.1002/hbm.21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertoni-Freddari C, Fattoretti P, Paoloni R, Caselli U, Galeazzi L, Meier-Ruge W. Synaptic structural dynamics and aging. Gerontology. 1996;42:170–180. doi: 10.1159/000213789. [DOI] [PubMed] [Google Scholar]
- 9.Adinolfi AM, Yamuy J, Morales FR, Chase MH. Segmental demyelination in peripheral nerves of old cats. Neurobiol Aging. 1991;12:175–179. doi: 10.1016/0197-4580(91)90058-r. [DOI] [PubMed] [Google Scholar]
- 10.Verdú E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- 11.Williams PD, Day T. Antagonistic pleiotropy, mortality source interactions, and the evolutionary theory of senescence. Evolution. 2003;57:1478–1488. doi: 10.1111/j.0014-3820.2003.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 12.Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T. DNA damage response and cellular senescence in tissues of aging mice. Aging Cell. 2009;8:311–323. doi: 10.1111/j.1474-9726.2009.00481.x. [DOI] [PubMed] [Google Scholar]
- 13.Carlo MD Jr, Loeser RF. Increased oxidative stress with aging reduces chondrocyte survival: correlation with intracellular glutathione levels. Arthritis Rheum. 2003;48:3419–3430. doi: 10.1002/art.11338. [DOI] [PubMed] [Google Scholar]
- 14.Chen YR, Sung K, Tharin S. Symptomatic Anterior Cervical Osteophyte Causing Dysphagia: Case Report, Imaging, and Review of the Literature. Cureus. 2016;8:e473. doi: 10.7759/cureus.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel-Aziz M, Azab N, El-Badrawy A. Cervical osteophytosis and spine posture: contribution to swallow disorders and symptoms. Curr Opin Otolaryngol Head Neck Surg. 2018;26:375–381. doi: 10.1097/MOO.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 16.Siparsky PN, Kirkendall DT, Garrett WE Jr. Muscle changes in aging: understanding sarcopenia. Sports Health. 2014;6:36–40. doi: 10.1177/1941738113502296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, Saltness RA, Jeganathan KB, Verzosa GC, Pezeshki A, Khazaie K, Miller JD, van Deursen JM. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahrilas PJ, Lin S, Chen J, Logemann JA. Oropharyngeal accommodation to swallow volume. Gastroenterology. 1996;111:297–306. doi: 10.1053/gast.1996.v111.pm8690194. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki M, Asada Y, Ito J, Hayashi K, Inoue H, Kitano H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. 2003;18:71–77. doi: 10.1007/s00455-002-0088-x. [DOI] [PubMed] [Google Scholar]
- 20.Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M. Autophagy impairment in muscle induces neuromuscular junction degeneration and precocious aging. Cell Rep. 2014;8:1509–1521. doi: 10.1016/j.celrep.2014.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vafek EC, Plate JF, Friedman E, Mannava S, Scott AT, Danelson KA. The effect of strain and age on the mechanical properties of rat Achilles tendons. Muscles Ligaments Tendons J. 2017;7:548–553. doi: 10.11138/mltj/2017.7.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornton GM, Lemmex DB, Ono Y, Beach CJ, Reno CR, Hart DA, Lo IK. Aging affects mechanical properties and lubricin/PRG4 gene expression in normal ligaments. J Biomech. 2015;48:3306–3311. doi: 10.1016/j.jbiomech.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Peffers MJ, Thorpe CT, Collins JA, Eong R, Wei TK, Screen HR, Clegg PD. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J Biol Chem. 2014;289:25867–25878. doi: 10.1074/jbc.M114.566554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Islam MN, Boostanpour K, Aran D, Jin G, Christenson S, Matthay MA, Eckalbar WL, DePianto DJ, Arron JR, Magee L, Bhattacharya S, Matsumoto R, Kubota M, Farber DL, Bhattacharya J, Wolters PJ, Bhattacharya M. Molecular programs of fibrotic change in aging human lung. Nat Commun. 2021;12:6309. doi: 10.1038/s41467-021-26603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MR. Structural and physiological age-associated changes in aging lungs. Semin Respir Crit Care Med. 2010;31:521–527. doi: 10.1055/s-0030-1265893. [DOI] [PubMed] [Google Scholar]
- 26.Kado DM, Prenovost K, Crandall C. Narrative review: hyperkyphosis in older persons. Ann Intern Med. 2007;147:330–338. doi: 10.7326/0003-4819-147-5-200709040-00008. [DOI] [PubMed] [Google Scholar]
- 27.Hochhegger B, Meirelles GS, Irion K, Zanetti G, Garcia E, Moreira J, Marchiori E. The chest and aging: radiological findings. J Bras Pneumol. 2012;38:656–665. doi: 10.1590/s1806-37132012000500016. [DOI] [PubMed] [Google Scholar]
- 28.Wysocki CJ, Pelchat ML. The effects of aging on the human sense of smell and its relationship to food choice. Crit Rev Food Sci Nutr. 1993;33:63–82. doi: 10.1080/10408399309527613. [DOI] [PubMed] [Google Scholar]
- 29.Ahmed T, Haboubi N. Assessment and management of nutrition in older people and its importance to health. Clin Interv Aging. 2010;5:207–216. doi: 10.2147/cia.s9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips PA, Rolls BJ, Ledingham JG, Forsling ML, Morton JJ, Crowe MJ, Wollner L. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311:753–759. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 31.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou X, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24:1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanguilder HD, Freeman WM. The hippocampal neuroproteome with aging and cognitive decline: past progress and future directions. Front Aging Neurosci. 2011;3:8. doi: 10.3389/fnagi.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dziewas R, Sörös P, Ishii R, Chau W, Henningsen H, Ringelstein EB, Knecht S, Pantev C. Neuroimaging evidence for cortical involvement in the preparation and in the act of swallowing. Neuroimage. 2003;20:135–144. doi: 10.1016/s1053-8119(03)00285-4. [DOI] [PubMed] [Google Scholar]
- 34.Teismann IK, Steinstraeter O, Schwindt W, Ringelstein EB, Pantev C, Dziewas R. Age-related changes in cortical swallowing processing. Neurobiol Aging. 2010;31:1044–1050. doi: 10.1016/j.neurobiolaging.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Michel A, Verin E, Hansen K, Chassagne P, Roca F. Buccofacial Apraxia, Oropharyngeal Dysphagia, and Dementia Severity in Community-Dwelling Elderly Patients. J Geriatr Psychiatry Neurol. 2021;34:150–155. doi: 10.1177/0891988720915519. [DOI] [PubMed] [Google Scholar]
- 36.Yurkstas A, Emerson WH. Dietary selections of persons with natural and artificial teeth. J Prosthet Dent. 1964;14:695–697. [Google Scholar]
- 37.Heath MR. The effect of maximum biting force and bone loss upon masticatory function and dietary selection of the elderly. Int Dent J. 1982;32:345–356. [PubMed] [Google Scholar]
- 38.MacDonald DE. Principles of geriatric dentistry and their application to the older adult with a physical disability. Clin Geriatr Med. 2006;22:413–34; x. doi: 10.1016/j.cger.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Hahn CL, Liewehr FR. Innate immune responses of the dental pulp to caries. J Endod. 2007;33:643–651. doi: 10.1016/j.joen.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Amella EJ. Feeding and hydration issues for older adults with dementia. Nurs Clin North Am. 2004;39:607–623. doi: 10.1016/j.cnur.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Rofes L, Arreola V, Romea M, Palomera E, Almirall J, Cabré M, Serra-Prat M, Clavé P. Pathophysiology of oropharyngeal dysphagia in the frail elderly. Neurogastroenterol Motil. 2010;22:851–858, e230. doi: 10.1111/j.1365-2982.2010.01521.x. [DOI] [PubMed] [Google Scholar]
- 42.Robbins J, Humpal NS, Banaszynski K, Hind J, Rogus-Pulia N. Age-Related Differences in Pressures Generated During Isometric Presses and Swallows by Healthy Adults. Dysphagia. 2016;31:90–96. doi: 10.1007/s00455-015-9662-x. [DOI] [PubMed] [Google Scholar]
- 43.Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- 44.Pitts LL, Stierwalt JAG, Hageman CF, LaPointe LL. The Influence of Oropalatal Dimensions on the Measurement of Tongue Strength. Dysphagia. 2017;32:759–766. doi: 10.1007/s00455-017-9820-4. [DOI] [PubMed] [Google Scholar]
- 45.Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The Prevalence of Olfactory Dysfunction in the General Population: A Systematic Review and Meta-analysis. Am J Rhinol Allergy. 2021;35:195–205. doi: 10.1177/1945892420946254. [DOI] [PubMed] [Google Scholar]
- 46.Krull IS, Mazzeo JR. Capillary electrophoresis: the promise and the practice. Nature. 1992;357:92–94. doi: 10.1038/357092a0. [DOI] [PubMed] [Google Scholar]
- 47.Ekberg O, Editor . Dysphagia Diagnosis and Treatment. 2nd ed. Berlin: Springer International Publishing, 2019: 247-267. [Google Scholar]
- 48.O'Keeffe M, Kelly M, O'Herlihy E, O'Toole PW, Kearney PM, Timmons S, O'Shea E, Stanton C, Hickson M, Rolland Y, Sulmont Rossé C, Issanchou S, Maitre I, Stelmach-Mardas M, Nagel G, Flechtner-Mors M, Wolters M, Hebestreit A, De Groot LCPGM, van de Rest O, Teh R, Peyron MA, Dardevet D, Papet I, Schindler K, Streicher M, Torbahn G, Kiesswetter E, Visser M, Volkert D, O'Connor EM MaNuEL consortium. Potentially modifiable determinants of malnutrition in older adults: A systematic review. Clin Nutr. 2019;38:2477–2498. doi: 10.1016/j.clnu.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Imoscopi A, Inelmen EM, Sergi G, Miotto F, Manzato E. Taste loss in the elderly: epidemiology, causes and consequences. Aging Clin Exp Res. 2012;24:570–579. doi: 10.3275/8520. [DOI] [PubMed] [Google Scholar]
- 50.Gopinath B, Russell J, Flood VM, Burlutsky G, Mitchell P. Adherence to dietary guidelines positively affects quality of life and functional status of older adults. J Acad Nutr Diet. 2014;114:220–229. doi: 10.1016/j.jand.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Rhodus NL, Moller K, Colby S, Bereuter J. Dysphagia in patients with three different etiologies of salivary gland dysfunction. Ear Nose Throat J. 1995;74:39–42, 45. [PubMed] [Google Scholar]
- 52.Ship JA, Pillemer SR, Baum BJ. Xerostomia and the geriatric patient. J Am Geriatr Soc. 2002;50:535–543. doi: 10.1046/j.1532-5415.2002.50123.x. [DOI] [PubMed] [Google Scholar]
- 53.Xu F, Laguna L, Sarkar A. Aging-related changes in quantity and quality of saliva: Where do we stand in our understanding? J Texture Stud. 2019;50:27–35. doi: 10.1111/jtxs.12356. [DOI] [PubMed] [Google Scholar]
- 54.Veldi M, Vasar V, Hion T, Kull M, Vain A. Ageing, soft-palate tone and sleep-related breathing disorders. Clin Physiol. 2001;21:358–364. doi: 10.1046/j.1365-2281.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 55.Michalowski R, Chibowska M. Lipid deposition in soft palate of man and its relation to aging. Pol Med Sci Hist Bull. 1965;8:141–144. [PubMed] [Google Scholar]
- 56.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 57.Ortega O, Martín A, Clavé P. Diagnosis and Management of Oropharyngeal Dysphagia Among Older Persons, State of the Art. J Am Med Dir Assoc. 2017;18:576–582. doi: 10.1016/j.jamda.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 58.Zajac DJ. Velopharyngeal function in young and older adult speakers: evidence from aerodynamic studies. J Acoust Soc Am. 1997;102:1846–1852. doi: 10.1121/1.420091. [DOI] [PubMed] [Google Scholar]
- 59.Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR Am J Roentgenol. 1991;156:1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- 60.Kendall KA, Leonard RJ, McKenzie S. Common medical conditions in the elderly: impact on pharyngeal bolus transit. Dysphagia. 2004;19:71–77. doi: 10.1007/s00455-003-0502-z. [DOI] [PubMed] [Google Scholar]
- 61.Clavé P, Rofes L, Carrión S, Ortega O, Cabré M, Serra-Prat M, Arreola V. Pathophysiology, relevance and natural history of oropharyngeal dysphagia among older people. Nestle Nutr Inst Workshop Ser. 2012;72:57–66. doi: 10.1159/000339986. [DOI] [PubMed] [Google Scholar]
- 62.Cook IJ. Oropharyngeal dysphagia. Gastroenterol Clin North Am. 2009;38:411–431. doi: 10.1016/j.gtc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 63.Nawaz S, Tulunay-Ugur OE. Dysphagia in the Older Patient. Otolaryngol Clin North Am. 2018;51:769–777. doi: 10.1016/j.otc.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 64.Robbins J. Normal swallowing and aging. Semin Neurol. 1996;16:309–317. doi: 10.1055/s-2008-1040989. [DOI] [PubMed] [Google Scholar]
- 65.Namasivayam-MacDonald AM, Barbon CEA, Steele CM. A review of swallow timing in the elderly. Physiol Behav. 2018;184:12–26. doi: 10.1016/j.physbeh.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kendall KA, Leonard RJ. Pharyngeal constriction in elderly dysphagic patients compared with young and elderly nondysphagic controls. Dysphagia. 2001;16:272–278. doi: 10.1007/s00455-001-0086-4. [DOI] [PubMed] [Google Scholar]
- 67.Aviv JE. Effects of aging on sensitivity of the pharyngeal and supraglottic areas. Am J Med. 1997;103:74S–76S. doi: 10.1016/s0002-9343(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 68.Pitts T. Airway protective mechanisms. Lung. 2014;192:27–31. doi: 10.1007/s00408-013-9540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tracy JF, Logemann JA, Kahrilas PJ, Jacob P, Kobara M, Krugler C. Preliminary observations on the effects of age on oropharyngeal deglutition. Dysphagia. 1989;4:90–94. doi: 10.1007/BF02407151. [DOI] [PubMed] [Google Scholar]
- 70.Ebihara S, Ebihara T, Kohzuki M. Effect of aging on cough and swallowing reflexes: implications for preventing aspiration pneumonia. Lung. 2012;190:29–33. doi: 10.1007/s00408-011-9334-z. [DOI] [PubMed] [Google Scholar]
- 71.Nakajima J, Karaho T, Kawahara K, Hayashi Y, Nakamura M, Matsuura N, Kohno N. Latent changes in the pharyngeal stage of swallowing in non-aspirating older adults. Eur Geriatr Med. 2022;13:655–661. doi: 10.1007/s41999-021-00604-2. [DOI] [PubMed] [Google Scholar]
- 72.Ebihara S, Ebihara T. Cough in the elderly: a novel strategy for preventing aspiration pneumonia. Pulm Pharmacol Ther. 2011;24:318–323. doi: 10.1016/j.pupt.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Yamanda S, Ebihara S, Ebihara T, Yamasaki M, Asamura T, Asada M, Une K, Arai H. Impaired urge-to-cough in elderly patients with aspiration pneumonia. Cough. 2008;4:11. doi: 10.1186/1745-9974-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sekizawa K, Ujiie Y, Itabashi S, Sasaki H, Takishima T. Lack of cough reflex in aspiration pneumonia. Lancet. 1990;335:1228–1229. doi: 10.1016/0140-6736(90)92758-a. [DOI] [PubMed] [Google Scholar]
- 75.Yokoyama M, Mitomi N, Tetsuka K, Tayama N, Niimi S. Role of laryngeal movement and effect of aging on swallowing pressure in the pharynx and upper esophageal sphincter. Laryngoscope. 2000;110:434–439. doi: 10.1097/00005537-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 76.Jones CA, Colletti CM. Age-Related Functional Reserve Decline Is Not Seen in Pharyngeal Swallowing Pressures. J Speech Lang Hear Res. 2021;64:3734–3741. doi: 10.1044/2021_JSLHR-21-00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kletzien H, Cullins MJ, Connor NP. Age-related alterations in swallowing biomechanics. Exp Gerontol. 2019;118:45–50. doi: 10.1016/j.exger.2019.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grote C, Reinhardt D, Zhang M, Wang J. Regulatory mechanisms and clinical manifestations of musculoskeletal aging. J Orthop Res. 2019;37:1475–1488. doi: 10.1002/jor.24292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leonard R, Kendall K, McKenzie S. UES opening and cricopharyngeal bar in nondysphagic elderly and nonelderly adults. Dysphagia. 2004;19:182–191. doi: 10.1007/s00455-004-0005-6. [DOI] [PubMed] [Google Scholar]
- 80.Im I, Kim Y, Oommen E, Kim H, Ko MH. The Effects of Bolus Consistency in Pharyngeal Transit Duration during Normal Swallowing. Ann Rehabil Med. 2012;36:220–225. doi: 10.5535/arm.2012.36.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mendell DA, Logemann JA. Temporal sequence of swallow events during the oropharyngeal swallow. J Speech Lang Hear Res. 2007;50:1256–1271. doi: 10.1044/1092-4388(2007/088). [DOI] [PubMed] [Google Scholar]
- 82.Mortelliti AJ, Malmgren LT, Gacek RR. Ultrastructural changes with age in the human superior laryngeal nerve. Arch Otolaryngol Head Neck Surg. 1990;116:1062–1069. doi: 10.1001/archotol.1990.01870090078013. [DOI] [PubMed] [Google Scholar]
- 83.Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68:560–566. doi: 10.1378/chest.68.4.560. [DOI] [PubMed] [Google Scholar]
- 84.Kikuchi R, Watabe N, Konno T, Mishina N, Sekizawa K, Sasaki H. High incidence of silent aspiration in elderly patients with community-acquired pneumonia. Am J Respir Crit Care Med. 1994;150:251–253. doi: 10.1164/ajrccm.150.1.8025758. [DOI] [PubMed] [Google Scholar]
- 85.Aviv JE, Spitzer J, Cohen M, Ma G, Belafsky P, Close LG. Laryngeal adductor reflex and pharyngeal squeeze as predictors of laryngeal penetration and aspiration. Laryngoscope. 2002;112:338–341. doi: 10.1097/00005537-200202000-00025. [DOI] [PubMed] [Google Scholar]
- 86.Kaneoka A, Pisegna JM, Inokuchi H, Ueha R, Goto T, Nito T, Stepp CE, LaValley MP, Haga N, Langmore SE. Relationship Between Laryngeal Sensory Deficits, Aspiration, and Pneumonia in Patients with Dysphagia. Dysphagia. 2018;33:192–199. doi: 10.1007/s00455-017-9845-8. [DOI] [PubMed] [Google Scholar]
- 87.Cook IJ, Weltman MD, Wallace K, Shaw DW, McKay E, Smart RC, Butler SP. Influence of aging on oral-pharyngeal bolus transit and clearance during swallowing: scintigraphic study. Am J Physiol. 1994;266:G972–G977. doi: 10.1152/ajpgi.1994.266.6.G972. [DOI] [PubMed] [Google Scholar]
- 88.Hosseini P, Tadavarthi Y, Martin-Harris B, Pearson WG Jr. Functional Modules of Pharyngeal Swallowing Mechanics. Laryngoscope Investig Otolaryngol. 2019;4:341–346. doi: 10.1002/lio2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehraban-Far S, Alrassi J, Patel R, Ahmad V, Browne N, Lam W, Jiang Y, Barber N, Mortensen M. Dysphagia in the elderly population: A Videofluoroscopic study. Am J Otolaryngol. 2021;42:102854. doi: 10.1016/j.amjoto.2020.102854. [DOI] [PubMed] [Google Scholar]
- 90.Shaker R, Ren J, Podvrsan B, Dodds WJ, Hogan WJ, Kern M, Hoffmann R, Hintz J. Effect of aging and bolus variables on pharyngeal and upper esophageal sphincter motor function. Am J Physiol. 1993;264:G427–G432. doi: 10.1152/ajpgi.1993.264.3.G427. [DOI] [PubMed] [Google Scholar]
- 91.Shaker R, Lang IM. Effect of aging on the deglutitive oral, pharyngeal, and esophageal motor function. Dysphagia. 1994;9:221–228. doi: 10.1007/BF00301914. [DOI] [PubMed] [Google Scholar]
- 92.Nishikubo K, Mise K, Ameya M, Hirose K, Kobayashi T, Hyodo M. Quantitative evaluation of age-related alteration of swallowing function: Videofluoroscopic and manometric studies. Auris Nasus Larynx. 2015;42:134–138. doi: 10.1016/j.anl.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 93.Fulp SR, Dalton CB, Castell JA, Castell DO. Aging-related alterations in human upper esophageal sphincter function. Am J Gastroenterol. 1990;85:1569–1572. [PubMed] [Google Scholar]
- 94.Shaw DW, Cook IJ, Gabb M, Holloway RH, Simula ME, Panagopoulos V, Dent J. Influence of normal aging on oral-pharyngeal and upper esophageal sphincter function during swallowing. Am J Physiol. 1995;268:G389–G396. doi: 10.1152/ajpgi.1995.268.3.G389. [DOI] [PubMed] [Google Scholar]
- 95.Aviv JE, Martin JH, Sacco RL, Zagar D, Diamond B, Keen MS, Blitzer A. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann Otol Rhinol Laryngol. 1996;105:92–97. doi: 10.1177/000348949610500202. [DOI] [PubMed] [Google Scholar]
- 96.Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. J Auton Nerv Syst. 1984;10:225–233. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- 97.Reginelli A, D'Amora M, Del Vecchio L, Monaco L, Barillari MR, Di Martino N, Barillari U, Motta G, Cappabianca S, Grassi R. Videofluoroscopy and oropharyngeal manometry for evaluation of swallowing in elderly patients. Int J Surg. 2016;33 Suppl 1:S154–S158. doi: 10.1016/j.ijsu.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 98.Shaker R, Li Q, Ren J, Townsend WF, Dodds WJ, Martin BJ, Kern MK, Rynders A. Coordination of deglutition and phases of respiration: effect of aging, tachypnea, bolus volume, and chronic obstructive pulmonary disease. Am J Physiol. 1992;263:G750–G755. doi: 10.1152/ajpgi.1992.263.5.G750. [DOI] [PubMed] [Google Scholar]
- 99.Eckardt VF, LeCompte PM. Esophageal ganglia and smooth muscle in the elderly. Am J Dig Dis. 1978;23:443–448. doi: 10.1007/BF01072928. [DOI] [PubMed] [Google Scholar]
- 100.Shaker R. Airway protective mechanisms: current concepts. Dysphagia. 1995;10:216–227. doi: 10.1007/BF00431413. [DOI] [PubMed] [Google Scholar]
- 101.Mei L, Dua A, Kern M, Gao S, Edeani F, Dua K, Wilson A, Lynch S, Sanvanson P, Shaker R. Older Age Reduces Upper Esophageal Sphincter and Esophageal Body Responses to Simulated Slow and Ultraslow Reflux Events and Post-Reflux Residue. Gastroenterology. 2018;155:760–770.e1. doi: 10.1053/j.gastro.2018.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kawami N, Iwakiri K, Sano H, Tanaka Y, Sakamoto C. Effects of aging and acid reflux on esophageal motility. Digestion. 2015;91:181–186. doi: 10.1159/000367650. [DOI] [PubMed] [Google Scholar]
- 103.Zhao J, Gregersen H. Esophageal morphometric and biomechanical changes during aging in rats. Neurogastroenterol Motil. 2015;27:1638–1647. doi: 10.1111/nmo.12661. [DOI] [PubMed] [Google Scholar]
- 104.Leese G, Hopwood D. Muscle fibre typing in the human pharyngeal constrictors and oesophagus: the effect of ageing. Acta Anat (Basel) 1986;127:77–80. doi: 10.1159/000146241. [DOI] [PubMed] [Google Scholar]
- 105.Ney DM, Weiss JM, Kind AJ, Robbins J. Senescent swallowing: impact, strategies, and interventions. Nutr Clin Pract. 2009;24:395–413. doi: 10.1177/0884533609332005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christmas C, Rogus-Pulia N. Swallowing Disorders in the Older Population. J Am Geriatr Soc. 2019;67:2643–2649. doi: 10.1111/jgs.16137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanders DS, Carter MJ, D'Silva J, James G, Bolton RP, Willemse PJ, Bardhan KD. Percutaneous endoscopic gastrostomy: a prospective audit of the impact of guidelines in two district general hospitals in the United Kingdom. Am J Gastroenterol. 2002;97:2239–2245. doi: 10.1111/j.1572-0241.2002.05778.x. [DOI] [PubMed] [Google Scholar]
- 108.Adeyemo BO, Simis M, Macea DD, Fregni F. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Front Psychiatry. 2012;3:88. doi: 10.3389/fpsyt.2012.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ghannouchi I, Speyer R, Doma K, Cordier R, Verin E. Swallowing function and chronic respiratory diseases: Systematic review. Respir Med. 2016;117:54–64. doi: 10.1016/j.rmed.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 110.Marik PE, Kaplan D. Aspiration pneumonia and dysphagia in the elderly. Chest. 2003;124:328–336. doi: 10.1378/chest.124.1.328. [DOI] [PubMed] [Google Scholar]
- 111.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78-84 and 23-32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 112.Schulte JN, Yarasheski KE. Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int J Sport Nutr Exerc Metab. 2001;11 Suppl:S111–S118. doi: 10.1123/ijsnem.11.s1.s111. [DOI] [PubMed] [Google Scholar]
- 113.Pisegna JM, Kaneoka A, Pearson WG Jr, Kumar S, Langmore SE. Effects of non-invasive brain stimulation on post-stroke dysphagia: A systematic review and meta-analysis of randomized controlled trials. Clin Neurophysiol. 2016;127:956–968. doi: 10.1016/j.clinph.2015.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiang CF, Lin MT, Hsiao MY, Yeh YC, Liang YC, Wang TG. Comparative Efficacy of Noninvasive Neurostimulation Therapies for Acute and Subacute Poststroke Dysphagia: A Systematic Review and Network Meta-analysis. Arch Phys Med Rehabil. 2019;100:739–750.e4. doi: 10.1016/j.apmr.2018.09.117. [DOI] [PubMed] [Google Scholar]
- 115.Mistry S, Rothwell JC, Thompson DG, Hamdy S. Modulation of human cortical swallowing motor pathways after pleasant and aversive taste stimuli. Am J Physiol Gastrointest Liver Physiol. 2006;291:G666–G671. doi: 10.1152/ajpgi.00573.2005. [DOI] [PubMed] [Google Scholar]
- 116.Ebihara T, Ebihara S, Maruyama M, Kobayashi M, Itou A, Arai H, Sasaki H. A randomized trial of olfactory stimulation using black pepper oil in older people with swallowing dysfunction. J Am Geriatr Soc. 2006;54:1401–1406. doi: 10.1111/j.1532-5415.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 117.Vicario CM, Rafal RD, Borgomaneri S, Paracampo R, Kritikos A, Avenanti A. Pictures of disgusting foods and disgusted facial expressions suppress the tongue motor cortex. Soc Cogn Affect Neurosci. 2017;12:352–362. doi: 10.1093/scan/nsw129. [DOI] [PMC free article] [PubMed] [Google Scholar]