Summary
Liver non-parenchymal cells (NPCs) play a critical role in the progression of non-alcoholic steatohepatitis (NASH). We aimed to explore the heterogeneity of NPCs and identify NASH-specific subpopulations contributing to NASH progression. Through single-cell RNA sequencing, we uncovered a proinflammatory subpopulation of Itgadhi/Fcrl5hi macrophages with potential function of modulating macrophage accumulation and promoting NASH development. We also identified subpopulations of Egr1hi and Ly6ahi liver sinusoidal endothelial cells (LSECs), which might participate in pathological angiogenesis and inflammation regulation. The Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs emerged in the early stage and expanded significantly along with pathological progression of liver injury during NASH. Cell-cell interactions between hepatic stellate cells (HSCs) and Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs or Ly6ahi LSECs were enhanced in NASH liver. Our results revealed that expansion of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs or Ly6ahi LSECs was strongly associated with NASH severity, suggesting these subpopulations might be involved in NASH progression.
Subject areas: Immunology, Cell biology, Transcriptomics
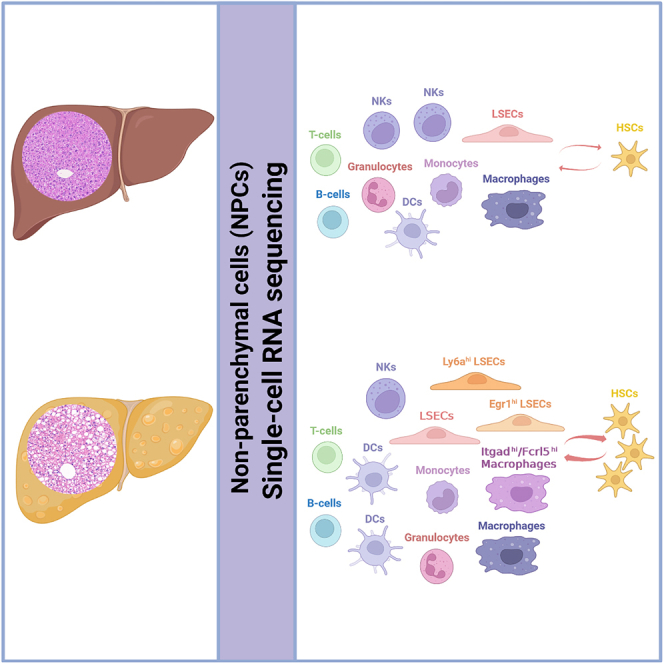
Graphical abstract
Highlights
-
•
Itgadhi/Fcrl5hi macrophages, Egr1hi and Ly6ahi LSECs emerged in the early stage of NASH
-
•
Itgadhi/Fcrl5hi macrophages, Egr1hi and Ly6ahi LSECs expanded during NASH progression
-
•
Cell-cell communications among non-parenchymal cells were enhanced in NASH liver
Immunology; Cell biology; Transcriptomics
Introduction
With a global increase in obesity, non-alcoholic fatty liver disease (NAFLD) is becoming a rapidly growing health problem, affecting about 25% of the adult population worldwide.1 It is estimated that 25–40% of individuals with NAFLD were in the stage of non-alcoholic steatohepatitis (NASH), which is featured by hepatic steatosis, hepatocellular ballooning, and lobular inflammation.2,3 Hepatic fibrosis could be absent or present in different stages of NASH progression, but eventually up to 25% of patients with NASH could progress to liver cirrhosis or even hepatocellular carcinoma.4 However, there is no approved drug for NASH treatment up to now.
Revealing the cellular and molecular mechanisms of NASH would be of great help in identifying therapeutic targets. Although experimental and clinical efforts have been made to elucidate the mechanisms, the NASH-specific elements remain unclear, and cell-cell interactions in NASH liver are needed to be further illustrated.5
Liver non-parenchymal cells (NPCs) are mainly composed of liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs), hepatic stellate cells (HSCs), lymphocytes, and other cells.6,7 It was suggested that cell-cell interactions between different NPCs played a critical role in the development and progression of NASH.8 LSECs are the gatekeeper of liver maintaining hepatic homeostasis. Under pathological conditions of NASH, LSECs would undergo capillarization and dysfunction that consequently activate KCs and HSCs.9,10,11 The activation of KCs could initiate inflammation responses and recruit blood-derived monocytes that would differentiate into proinflammatory macrophages and further aggravate NASH progression.12 Meanwhile, activation of HSCs leads to increased deposition of extracellular matrix (ECM) in the liver, which destroys the normal structure of liver and promotes the progression of NASH.13 In recent years, deeper understanding of cellular heterogeneity and cell-cell interactions under both homeostatic and pathogenic conditions has been achieved with the aid of single-cell RNA sequencing (scRNA-seq) technology.14,15 Through scRNA-seq, multiple studies have provided deep insights into heterogeneity of NPCs in NASH liver, although their functional roles remain unclear.16,17,18 For example, HSCs could be subdivided into four clusters in NASH mice, i.e., proliferative cluster, intermediate activation cluster, immune and inflammatory cluster, and the classical fibrogenic myofibroblast cluster, implying HSCs might play multifunctional roles during NASH.17 Analysis of intrahepatic ligand-receptor signaling network further revealed that HSCs primarily interact with endothelial cells (ECs) and immune cells in NASH.18 Distinct subpopulations of ECs have also been reported, and dysregulation of genes related to homeostasis, vascular development, lipid metabolism, and chemokine release was observed in ECs derived from NASH livers.18 Remarkably, an NASH-specific macrophage subpopulation, namely NASH-associated macrophages (NAMs), characterizing by high expression of triggering receptor expressed on myeloid cells 2 (Trem2) was identified to be associated with disease progression and pharmacological interventions in both NASH mice model and NASH patients.18 More investigations are needed to characterize NASH-specific cell subpopulations that would facilitate the identification of diagnostic and/or therapeutic targets for NASH.
In this study, we performed scRNA-seq analysis of liver NPCs derived from healthy and NASH mice, respectively. Besides Trem2hi NAMs reported in the previous study,18 we identified another NASH-specific macrophage subpopulation characterized by abundant expression of integrin, alpha D (Itgad), and Fc receptor-like 5 (Fcrl5), which was expanded during NASH progression. We also found two subpopulations of LSECs, characterized by highly expressed early growth response 1 (Egr1) and lymphocyte antigen 6 complex, locus A (Ly6a), respectively, emerged in the early stage of NASH and underwent dramatic expansion during pathological progression. In addition, enhanced cell-cell interactions among NPCs were observed in NASH liver, especially those between HSCs and ECs/macrophages. Our results indicated that Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs were strongly associated with NASH progression.
Results
RNA-seq and scRNA-seq analysis of liver NPCs from healthy and NASH mice
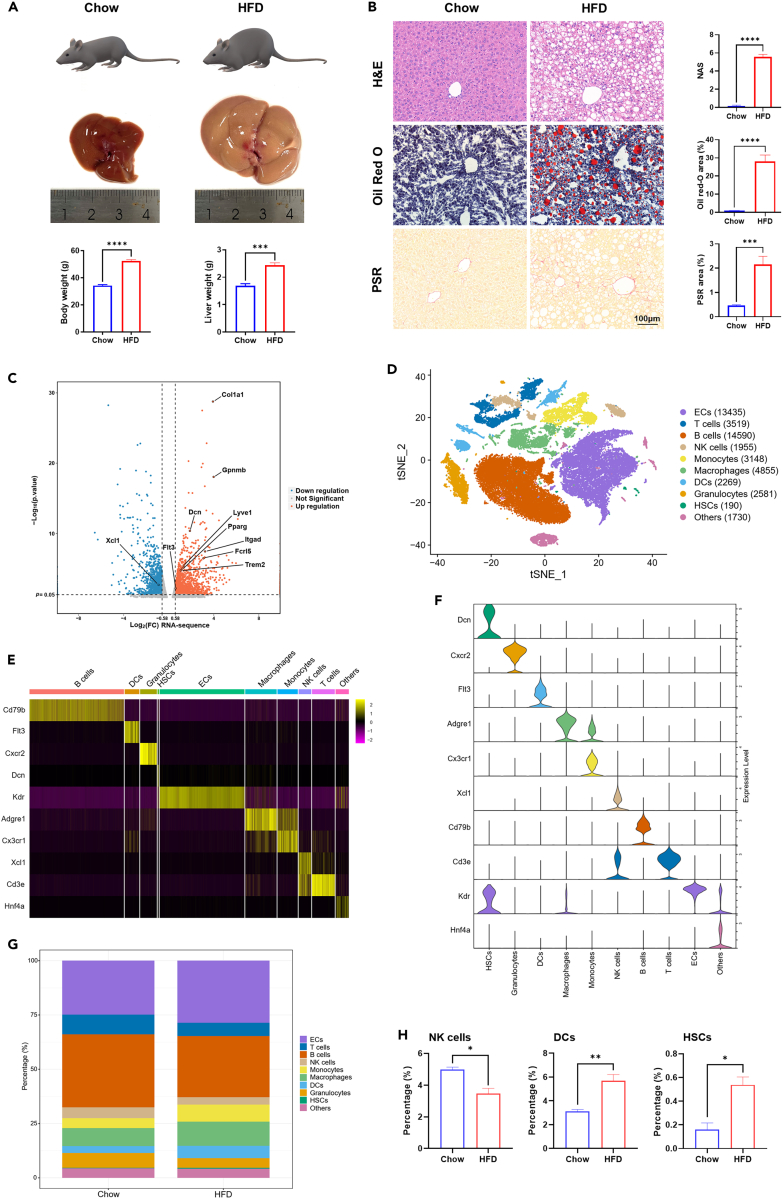
According to previous reports,19,20 we established mouse NASH model induced by high-fat and high-fructose diet (referred to as HFD group). After 20 weeks, the body weight, liver weight, and liver volume of HFD group were significantly increased compared to those fed chow diet (referred to as Chow group) (Figure 1A). HFD mice exhibited steatosis, hepatocellular ballooning, lobular inflammatory infiltration, and fibrosis in liver, which were consistent with the pathological manifestations of NASH (Figure 1B). Liver NPCs were isolated from both HFD (n = 3) and Chow (n = 3) mice and subjected to RNA-Seq. A total of 1,613 genes differentially expressed between HFD and chow were found, with 830 genes upregulated and 783 genes downregulated in HFD, including genes representative of NAMs (Trem2, Gpnmb), HSCs (Dcn, Col1α1), ECs (Lyve1), dendritic cells (DCs) (Flt3), and natural killer (NK) cells (Xcl1) (Figure 1C). These results implied that various types of NPCs might be affected during NASH progression.
Figure 1.
Transcriptional analysis of liver NPCs on the bulk and single-cell level in healthy and NASH mice
(A) Body weight and liver weight of chow and HFD mice. Mice were fed with chow diet (Chow) and HFD diet supplemented with fructose and sucrose in the drinking water (HFD), respectively for 20 weeks.
(B) Hepatic steatosis, lipid accumulation, inflammation, and fibrosis in each group were evaluated by hematoxylin-eosin (H&E), oil red O, picrosirius red (PSR), and NAFLD activity score (NAS). Scale bar, 100 μm. The statistics were shown on the right.
(C) Differentially expressed genes between chow and HFD group on the bulk level. Genes up- or downregulated by > 1.5-fold were shown in orange and blue, respectively, while those ≤ 1.5-fold were shown in gray.
(D) t-SNE visualization of scRNA-seq results. NPCs were classified into 10 distinct types. Cell counts for each cell type were shown in parentheses.
(E) Heatmap of cell type specific marker genes.
(F) Representative marker gene expression in each cell type.
(G) Percentage of each cell type in NPCs isolated from livers of chow and HFD mice.
(H) Differences in the percentage of hepatic NK cells, DCs, and HSCs between chow and HFD group. n = 3–5 per group. The data are expressed as mean ± SEM. Statistical significance is shown as ∗ for p < 0.05, ∗∗ for p < 0.01, ∗∗∗ for p < 0.001, and ∗∗∗∗ for p < 0.0001, as evaluated by Student’s t test.
To investigate the exact role of different types of NPCs and their subpopulations in the pathogenesis of NASH, the same batch of NPCs subjected to RNA-Seq were also put through scRNA-seq analysis. A total of 48,272 cells isolated from the liver of six mice were sequenced successfully, with 29,784 cells from HFD mice and 18,488 cells from chow mice. According to cell type specific marker genes, these cells could be classified into 10 distinct types including ECs, T cells, B cells, NK cells, monocytes, macrophages, DCs, granulocytes, HSCs, and other cells that were mainly composed of hepatocytes and red blood cells (Figures 1D–1F). Each cell type consisted of several clusters featured by their maker genes (Figure S1A and Table S1). B cells and ECs were the most abundant cell types in both HFD and chow mice, accounting for 28.0–33.6% and 25.3–29.5% of NPCs, respectively (Figures 1G and S1B). The average proportion of HSCs increased significantly from 0.2% in chow group to 0.5% in HFD group, while that of NK cells decreased from 5.0% to 3.5% (Figures 1H and S1B). We also found that the proportion of ECs and macrophages were higher in HFD mice than in chow mice, although the differences were not statistically significant (Figure S1B). In addition, DCs were significantly more abundant in HFD group than in chow group, which was further validated by flow cytometry analysis (Figures S1B and S1C). These findings further suggested that alterations in NPCs, especially HSCs, DCs, ECs, NK cells, and macrophages might be associated with NASH development.
Heterogeneity of hepatic macrophages and expansion of Itgadhi/Fcrl5hi macrophages in NASH liver
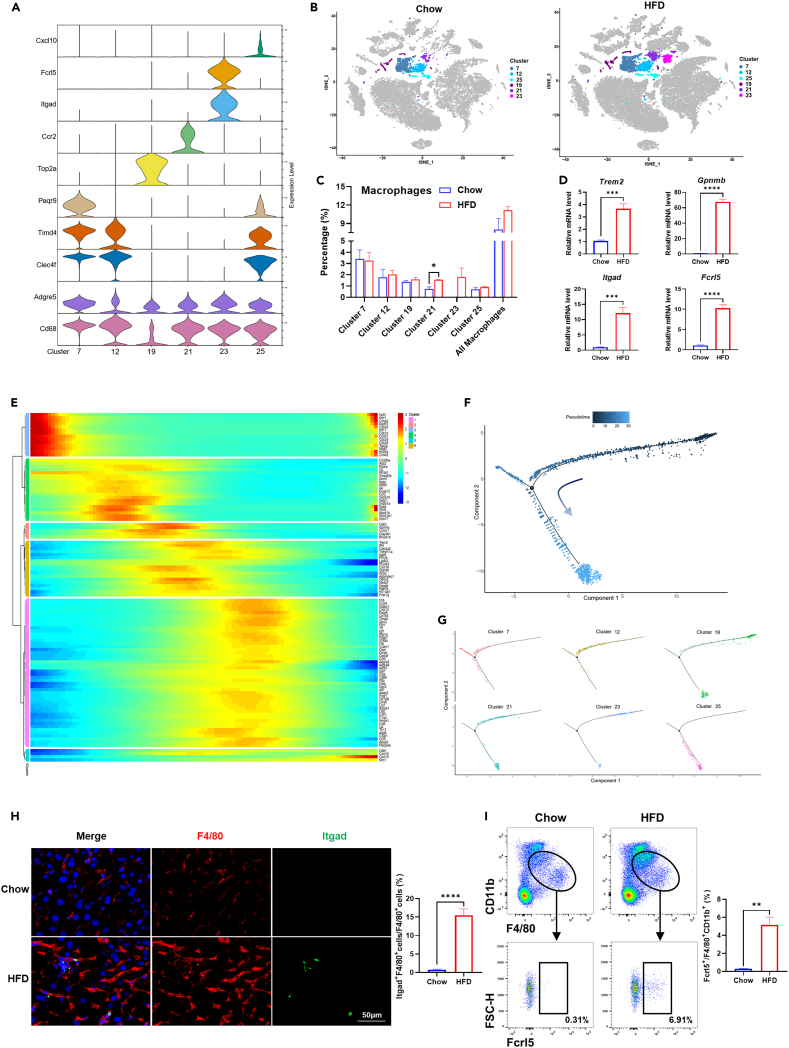
Macrophages in the liver can be divided into liver-resident KCs and monocyte-derived macrophages (MDMs), according to their different origin and gene expression profile.21 Our scRNA-Seq data revealed hepatic macrophages were composed of six clusters (cluster 7, 12, 19, 21, 23, and 25) (Figure S1A). Each cluster harbored its cluster-specific marker genes, as well as universal macrophage markers such as Adgre5 and Cd68 (Figure 2A and Table S1). Cluster 7, 12, and 25 were characterized by high expression of KC-specific marker genes such as Clec4f and Timd4,22 which were rarely expressed in cluster 19, 21, and 23. Thus, we classified the former clusters as KCs, and the latter clusters as MDMs (Figure S2A). Cluster 19 exhibited high expressions of Top2a and Mki67, indicating it might be a subpopulation that was undergoing division.23 In addition to Ccr2, cluster 21 was also featured by high expression of Trem2 (Table S1). Meanwhile, the majority of cells in cluster 21 were derived from HFD, and significant expansion of this subpopulation was observed in HFD mice compared to chow mice (Figures 2B and 2C and S2B), suggesting it is likely to be the subpopulation of NAMs as described previously.18 Cluster 23 was characterized by high expressions of Itgad and Fcrl5, while it did not harbor marker genes of NAMs such as Trem2 and Gpnmb (Figure 2A and Table S1). Notably, almost all of these Itgadhi/Fcrl5hi cells were derived from HFD mice, suggesting they might be a distinctive subpopulation of macrophage strongly associated with NASH progression (Figures 2B and 2C and S2B). Similar to cluster 21, expansion of cluster 23 was also observed in HFD group, although the difference was not significant when compared to chow group (Figures 2C and S2B). Upregulation of Trem2, Gpnmb, Itgad, and Fcrl5 in HFD mice was validated in liver NPCs by qPCR analysis, further indicating that subpopulations of cluster 21 and 23 expanded during NASH progression (Figure 2D). Pseudotime analysis showed that dividing cells featured by high expressions of Top2a and Mki67 were at the beginning of the trajectory path. Through an intermediate state characterized by Itgadhi/Fcrl5hi and Trem2hi/Gpnmbhi cells, macrophages reached the state of Timd4hi/Clec4fhi cells (Figures 2E–2G). Thus, we proposed that cluster 23 that was composed of Itgadhi/Fcrl5hi cells might emerge earlier than the Trem2hi/Gpnmbhi NAMs during NASH development.
Figure 2.
Identification of Itgadhi/Fcrl5hi macrophages in mice liver
(A) Violin plots showing marker gene expression for each cluster (cluster 7, 12, 19, 21, 23, and 25) of macrophages.
(B) t-SNE visualization of clusters of macrophages in chow and HFD group.
(C) The percentage of each cluster in liver NPCs in chow and HFD group.
(D) Expression of marker genes for cluster 21(Trem2, Gpnmb) and cluster 23 (Itgad, Fcrl5) in liver NPCs of chow and HFD group at mRNA level.
(E) Dynamic changes in gene expression along pseudotime in macrophages.
(F) Pseudotime trajectory of macrophages as a whole.
(G) Pseudotime trajectory of each cluster of macrophages.
(H) Detection of Itgad+ macrophages in chow and HFD mice liver by immunofluorescence staining. Scale bar, 50 μm.
(I) Percentage of Fcrl5+ cells in F4/80+CD11b+ double-positive macrophages isolated from chow and HFD mice liver. The statistics were shown on the right. n = 3–5 per group. The data are expressed as mean ± SEM. Statistical significance is shown as ∗ for p < 0.05, ∗∗ for p < 0.01, ∗∗∗ for p < 0.001, and ∗∗∗∗ for p < 0.0001, as evaluated by Student’s t test.
Next, we carried out immunofluorescence and flow cytometry analysis to confirm the existence of Itgadhi/Fcrl5hi macrophages in the liver. Consistent with the results of scRNA-seq, we found the subpopulation of Itgadhi/Fcrl5hi macrophage was expanded significantly in NASH livers. As shown in Figure 2H, Itgad+ F4/80+ double-positive cells were rarely detected in chow group, while they were increased significantly and accounted for 11.7–21.2% of F4/80+ cells in HFD mice. Moreover, among F4/80+CD11b+ double-positive macrophages, the proportion of Fcrl5+ cells were significantly higher in HFD group than in chow group (Figure 2I). Co-expression of Itgad and Fcrl5 was confirmed through double immunofluorescent staining (Figure S3). Our results demonstrated that besides NAMs, a subpopulation of Itgadhi/Fcrl5hi macrophages also emerged in mice liver, both of which expanded during NASH progression.
Heterogeneity of hepatic ECs and expansion of Egr1hi and Ly6ahi LSECs in NASH liver
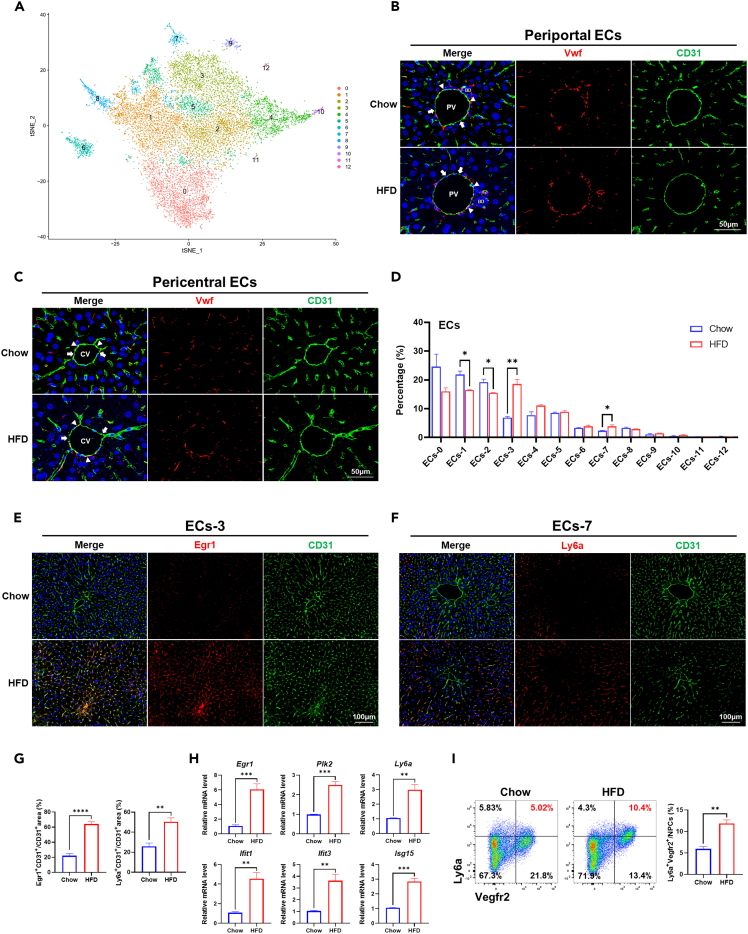
Previous studies demonstrated that liver ECs were also heterogeneous, and subpopulation-specific alterations of LSECs, such as expansion of scar-associated endothelial cells (SAEndo) characterized by CD34+PLVAP+ACKR1+, have been identified in cirrhotic livers.18,24,25,26 In this study, we found SAEndo also existed in the liver of NASH mice (cluster 24, Figure S1A and Table S1), suggesting SAEndo might emerge before the cirrhotic stage of liver disease. To further explore the heterogeneity of ECs in NASH mice, we re-clustered the ECs, i.e., cluster 1, 3, 4, 24, and 27 (Figure S1A) into 13 subpopulations (ECs-0 ∼ ECs-12) (Figure 3A). According to the specific transcriptomic signatures of different ECs subpopulations described previously,18,27,28 ECs-1 and ECs-8 exhibiting abundant expression of Dll4/Msr1 but low expression of Wnt2/Kit were considered to be periportal ECs, while ECs-0, ECs-2, ECs-4, and ECs-10 featured by moderate to high expression of Wnt2/Kit but extremely low expression of Dll4/Msr1 might belong to pericentral ECs. Moderate to high expression of Vwf were observed in ECs-4, 8, and 10, while it was very low in other ECs subpopulations (Figure S4). In line with this, we detected Vwfhi and Vwflow subpopulations in either periportal or pericentral ECs in livers of chow and HFD mice (Figures 3B and 3C). Comparing to periportal and pericentral ECs, ECs-3, ECs-5, and ECs-7 exhibited moderate expression of Dll4/Msr1 and Wnt2/Kit, but unique expression of Cxcl10 and were regarded as LSECs. ECs-6, ECs-9, ECs-11, and ECs-12 were likely to be doublets of ECs mixed with immune cells or red blood cells.
Figure 3.
Heterogeneity of ECs in mice liver
(A) t-SNE visualization of re-clustered hepatic ECs.
(B) Vwfhi and Vwflow ECs in the periportal area in chow and HFD mice liver. PV, portal vein. BD, bile duct. Arrowheads indicated Vwfhi ECs, and arrows indicated Vwflow ECs. Scale bar, 50 μm.
(C) Vwfhi and Vwflow ECs in the pericentral area in chow and HFD mice liver. CV: Central vein. Arrowheads indicate Vwfhi ECs, and arrows indicated Vwflow ECs. Scale bar, 50 μm.
(D) The percentage of each cluster in hepatic ECs in chow and HFD group.
(E) Detection of Egr1+ ECs (ECs-3) in chow and HFD mice liver by immunofluorescence staining. Scale bar, 100 μm.
(F) Detection of Ly6a+ ECs (ECs-7) in chow and HFD mice liver by immunofluorescence staining. Scale bar, 100 μm.
(G) The statistics of CD31+Egr1+ double-positive and CD31+Ly6a+ double-positive areas in chow and HFD mice liver.
(H) Expression of marker genes for ECs-3 (Egr1, Plk2) and ECs-7 (Ly6a, Ifit1, Ifit3, Isg15) in liver NPCs of chow and HFD group at mRNA level.
(I) Detection of Ly6ahi ECs (ECs-7) in chow and HFD mice liver by flow cytometry. Ly6ahi Vegfr2hi ECs were located on the top right panel of each plot, and their percentages in liver NPCs were shown in red. The statistics were shown on the right. n = 3–5 per group. The data are expressed as mean ± SEM. Statistical significance is shown as ∗ for p < 0.05, ∗∗ for p < 0.01, ∗∗∗ for p < 0.001, and ∗∗∗∗ for p < 0.0001, as evaluated by Student’s t test.
Another aspect of ECs heterogeneity was reflected by the fact that the proportion of different subpopulations could be either increasing or decreasing during NASH. As shown in Figure 3D, proportion of LSECs subpopulations ECs-3 and ECs-7 was increased, while that of periportal subpopulations ECs-1 and pericentral subpopulations ECs-2 was decreased significantly in HFD group. ECs-3 showed abundant expression of Egr1 that could promote inflammation in mice with cholestatic liver injury, as well as Plk2, which was strongly associated with cell proliferation29,30 (Table S2). ECs-7 was characterized by high expression of interferon-induced genes such as Isg15, Ifit1, Ifit2, Ifit3, Ifi44, Ifi47 et al. In addition, it also exhibited high expression of Ly6a. We confirmed the expansion of Egr1hi subpopulation and Ly6ahi subpopulation in livers of HFD mice by immunofluorescence (Figures 3E–3G). Besides, the mRNA expression level of Egr1, Plk2, Ly6a, Ifit1, Ifit3, and Isg15 in NPCs were also increased significantly in HFD mice (Figure 3H). Flow cytometry analysis showed that Ly6a+ ECs accounted for approximately 10% of NPCs in HFD mice, while it was only around 5% in chow mice (Figure 3I). These results demonstrated that Egr1hi and Ly6ahi LSECs subpopulations expanded during NASH progression.
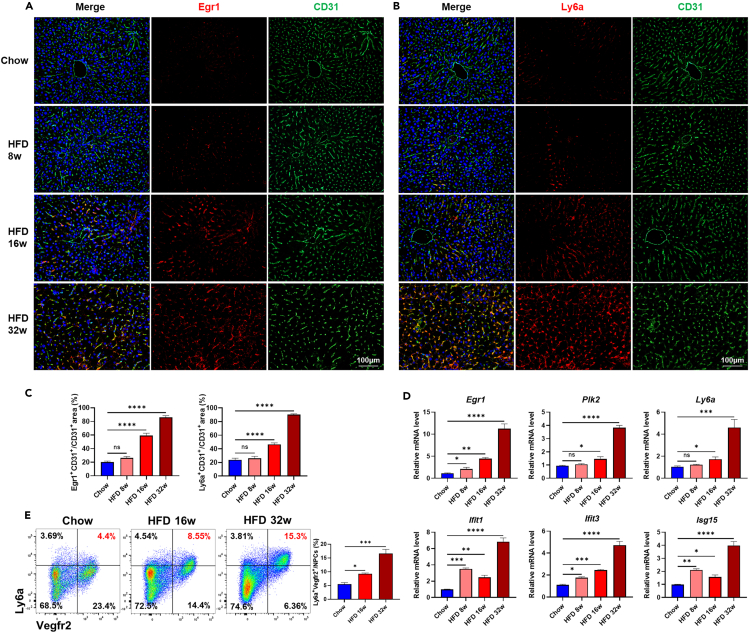
Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs emerged in the early stage of NASH progression
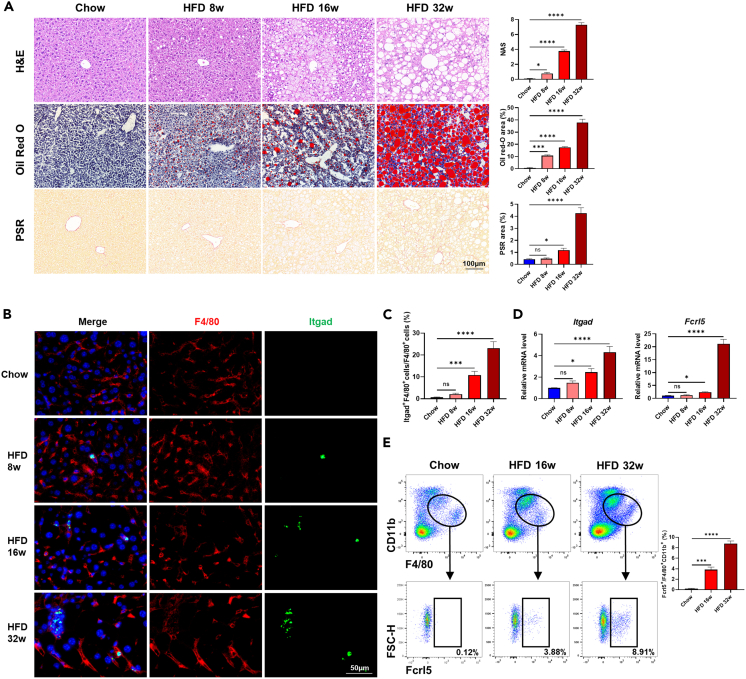
Based on our findings above, we next tried to figure out when did the subpopulations of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs emerge during NASH progression. As shown in Figure 4A, lipid accumulation, inflammatory infiltration, and fibrosis in the liver aggravated progressively along with prolonged high-fat and high-fructose diet for 8 weeks, 16 weeks, and 32 weeks. In fact, obvious lipid accumulation and inflammatory infiltration could be observed at 8 weeks, while significant alteration in fibrosis appeared at 16 weeks, both of which were dramatically enhanced at 32 weeks. In accordance with pathological progression of NASH, the amounts of Itgadhi/Fcrl5hi macrophages in mice liver increased continuously from week 8 to week 32 (Figures 4B and 4C). Significant and gradual upregulation of Itgad and Fcrl5 mRNA expression were observed in mice liver at 16 weeks and 32 weeks (Figure 4D). Expansion of Itgadhi/Fcrl5hi macrophages during NASH progression were further validated by flow cytometry analysis, with its proportion increasing from 3.88% at week 16 to 8.91% at week 32 (Figure 4E). Similarly, we also found a gradual increase in the amount of Egr1hi LSECs and Ly6ahi LSECs at week 8, week 16, and week 32 (Figures 5A–5C). The mRNA expression of Egr1, Ifit1, Ifit3, and Isg15 were significantly upregulated since week 8, while remarkable upregulation of Plk2 and Ly6a occurred at week 16, all of which reached their peak at week 32 (Figure 5D). Furthermore, flow cytometry analysis showed that the proportion of Ly6ahi LSECs increased from 8.55% at week 16 to 15.3% at week 32 (Figure 5E). These results indicated that subpopulations of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs emerged in the early stage of NASH and underwent dramatic expansion during pathological progression, implying they might be involved in the aggravation of NASH.
Figure 4.
Dynamic changes of Itgadhi/Fcrl5hi macrophages in NASH progression
(A) Representative images for H&E, oil red O and PSR stained liver tissues of chow and HFD (week 8, 16 and 32) mice. The statistics of NAS and the area of oil red O and PSR were shown on the right. Scale bar, 100 μm.
(B) Immunofluorescence staining of Itgad+ macrophages in liver tissues of chow and HFD (week 8, 16, and 32) mice. Scale bar, 50 μm.
(C) The statistics of Itgad+ macrophages in liver tissues of chow and HFD (week 8, 16, and 32) mice.
(D) Relative mRNA levels of Itgad and Fcrl5 in liver tissues of chow and HFD (week 8, 16, and 32) mice.
(E) Percentage of Fcrl5+ cells in F4/80+CD11b+ double-positive macrophages isolated from liver tissues of chow and HFD (week 16 and 32) mice. The statistics were shown on the right. n = 3–5 per group. The data are expressed as mean ± SEM. Statistical significance is shown as ∗ for p < 0.05, ∗∗∗ for p < 0.001, and ∗∗∗∗ for p < 0.0001, as evaluated by one-way ANOVA.
Figure 5.
Dynamic changes of Egr1hi LSECs and Ly6ahi LSECs in NASH progression
(A) Detection of Egr1hi ECs in chow and HFD (week 8, 16, and 32) mice liver by immunofluorescence staining. Scale bar, 100 μm.
(B) Detection of Ly6ahi ECs in chow and HFD (week 8, 16, and 32) mice liver by immunofluorescence staining. Scale bar, 100 μm.
(C) The statistics of CD31+Egr1+ double-positive and CD31+Ly6a+ double-positive areas in chow and HFD (week 8, 16, and 32) mice liver.
(D) Relative mRNA levels of Itgad, Fcrl5 and Ly6a, Ifit1, Ifit3, Isg15 in liver tissues of chow and HFD (week 8, 16, and 32) mice.
(E) Detection of Ly6ahi ECs in chow and HFD (week 16 and 32) mice liver by flow cytometry. Ly6ahi Vegfr2hi ECs were located on the top right panel of each plot, and their percentages in liver NPCs were shown in red. The statistics were shown on the right. n = 3–5 per group. The data are expressed as mean ± SEM. Statistical significance is shown as ∗ for p < 0.05, ∗∗ for p < 0.01, ∗∗∗ for p < 0.001, and ∗∗∗∗ for p < 0.0001, as evaluated by one-way ANOVA.
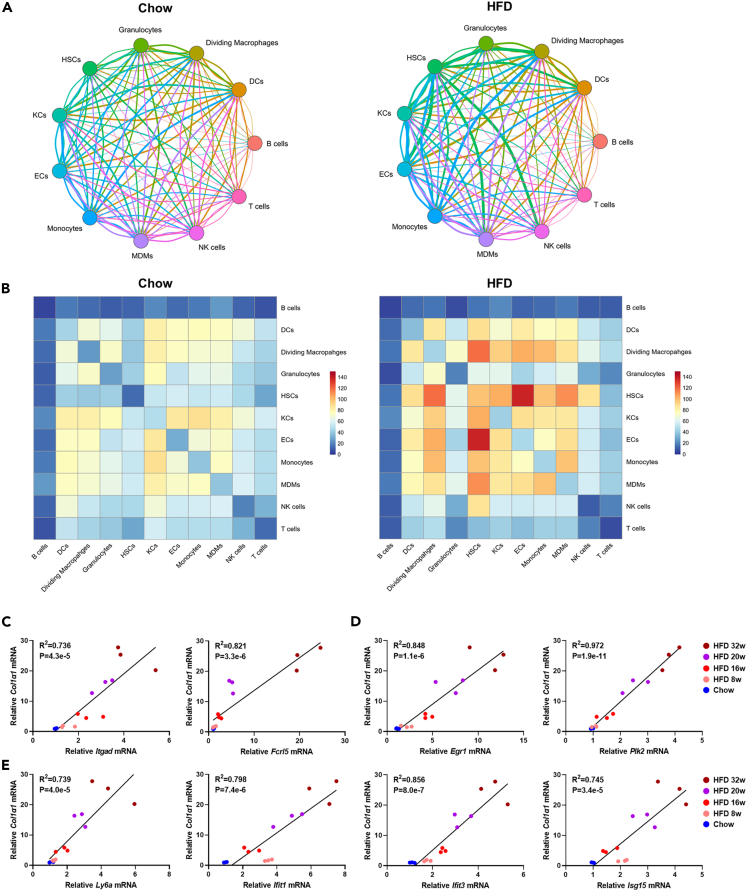
Enhanced cell-cell communication among NPCs in NASH liver
It was demonstrated that HSCs was a central hub of paracrine and autocrine signaling in liver NPCs, being capable of activating cytokine secretion by itself and modulating the function of LSECs and macrophages.18 We hypothesized the intercellular cross talk might be enhanced during NASH based on our findings that HSCs subpopulation was expanded significantly in HFD mice (Figures 1H and S1B). To test this hypothesis, we then performed analysis of cell-cell communication network based on ligand-receptor interactions. We found that compared to chow group, most of cell-cell communication were enhanced enormously in HFD group (Figures 6A and 6B). In HFD mice, HSCs communicated actively with multiple cell types, with the strongest interaction observed between HSCs and ECs. Moreover, stronger interactions were detected between HSCs and macrophages including KCs, MDMs, and dividing cells, while intercellular communication within HSCs themselves were also augmented remarkably in HFD. Notably, dynamic mRNA expression of Itgad, Fcrl5, Egr1, Plk2, Ly6a, Ifit1, Ifit3, and Isg15 were positively correlated with that of Col1α1 (Figures 6C–6E), suggesting intimate interplays may occur between HSC and subpopulations of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs during NASH progression. Together, our results indicated cell-cell interactions among NPCs, especially those between HSCs and ECs/macrophages were enhanced in NASH liver.
Figure 6.
Cell-cell communications among NPCs in mice liver
(A) Visualization of interacting pairs among different NPCs in chow and HFD mice.
(B) Visualization of the intensity of cell-cell communication among different NPCs in chow and HFD mice.
(C) Correlation of mRNA expression level of Col1α1 and Itgad, Fcrl5 in chow and HFD (week 8, 16, and 32) mice liver.
(D) Correlation of mRNA expression level of Col1α1 and Egr1, Plk2 in chow and HFD (week 8, 16, and 32) mice liver.
(E) Correlation of mRNA expression level of Col1α1 and Ly6a, Ifit1, Ifit3, Isg15 in chow and HFD (week 8, 16, and 32) mice liver. n = 3 per group.
Discussion
Compared with tissue-wide genomics, single-cell genomics has obvious advantages in providing comprehensive information on cellular composition and interactions, as well as in revealing specific contributions of individual cell subpopulations. In this study, we applied scRNA-seq to explore the heterogeneity of liver NPCs isolated from diet-induced NASH mice, trying to figure out if there were specific subpopulations related to NASH progression. We identified subpopulations of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs in livers of NASH mice. More importantly, we found these subpopulations underwent dramatic expansion along with the increase in NASH severity, suggesting they were strongly associated with NASH progression.
Under homeostasis condition, KCs derived from fetal yolk sac were predominant in liver macrophage population, playing critical roles in sensing tissue damage and maintaining immunological tolerance.21 In responses to liver injury, MDMs were recruited from circulating monocytes to liver that could differentiate into KC-like cells when they entered into KCs niche.31,32 Expansion of MDMs in NASH liver has been reported by previous studies.25,31,33 Hepatic MDMs were found to enhance the severity of inflammation infiltration in obese mice liver, while the subpopulation of TREM2+CD9+ scar-associated macrophages originating from MDMs displayed a pro-fibrogenic phenotype, suggesting MDMs might participate in aggravation of liver injury.25,34 However, it was proposed that the subpopulation of NAMs, which were also characterized by high expression of Trem2 and Cd9, were likely to play a protective role in NASH progression.18 It remained uncertain whether NAMs originated from MDMs or from reprogrammed KCs.31 In this study, we also found the subpopulation of Trem2+ macrophages (cluster 21) were expanded significantly in NASH liver. As these macrophages exhibited high expression of Ccr2 but low expression of classical marker genes for KCs, such as Clec4f, Timd4, Vsig4, Hmox1, Cd5l, Folr2, and Cd163 (Table S1), we hypothesized they might come from MDMs. Furthermore, we identified another subpopulation featured by high expression of Itgad and Fcrl5 (cluster 23), which accumulated in liver gradually as liver injury aggravated. When mapping the transcriptional profile of macrophages along a pseudotemporal trajectory, we found these Itgadhi/Fcrl5hi cells (cluster 23) evolved from dividing cells (cluster 19), which were also lack of classical KC markers, and progressed to Trem2hi cells (cluster 21), suggesting both Itgadhi/Fcrl5hi cells and Trem2hi cells were MDM-derived. Of note, Itgadhi/Fcrl5hi cells emerged earlier than Trem2hi cells along the trajectory path, implying they might represent a subpopulation of macrophages in early response to liver injury induced by dietary intervention of high-fat and high-fructose. In fact, significant increase in proportion of Itgadhi/Fcrl5hi cells in hepatic macrophages could be observed at the early stage of NASH, which continued to increase as NASH progressed. Our results indicated that an MDM-derived subpopulation of Itgadhi/Fcrl5hi hepatic macrophages emerged early during NASH and expanded in parallel with the pathological progression of liver injury.
Itgad, also known as Cd11d, is an understudied member of the β2 integrin family mediating cell adhesion and inflammatory signaling.35,36,37 Significant upregulation of CD11d expression was detected in proinflammatory macrophages, which was supposed to be responsible for accumulation of macrophages at inflammatory sites and aggravation of chronic inflammation.35,38,39 Inflammatory macrophage infiltration was reduced in the aortas or adipose tissue of Cd11d−/− mice, which improved the outcomes of atherosclerosis or diabetes that were obtained compared to Cd11d+/+ mice, suggesting Cd11d may play an essential role in recruiting macrophages and promoting the progression of inflammatory diseases.39,40 Moreover, blockade of CD11d by anti-CD11d mAb could reduce the accumulation of macrophages in injured brain and lung.41,42 Although, Fcrl5 was preferentially expressed by B cells, myeloid-derived macrophages expressing Fcrl5 has been identified in splenocytes from mice with systemic lupus erythematosus, emerging before the development of pathologic autoimmunity.43,44 However, whether Fcrl5 is involved in the pathogenesis of NASH remains unclear. To our knowledge, either Cd11d- or Fcrl5-expressing hepatic macrophages have not been described previously in NASH mice. Our results showed a subpopulation of Itgadhi/Fcrl5hi macrophages, which also exhibited abundant expression of inflammation-related genes (such as Itgax, Lgals3, Fgr, Laptm5, Ccl3, Ccl4, Ctss, and Slc11a1), emerged in the early stage of NASH in mice. Besides, they were also characterized by high expression of Cd300e, an immune-activating receptor and Rgs1, a member of regulator of G-protein signaling family, which regulated macrophage accumulation.45,46 Taken together, we reasoned that these Itgadhi/Fcrl5hi cells might be a subpopulation of activated, proinflammatory macrophages with potential function of modulating macrophage accumulation and promoting the development of diet-induced NASH.
During liver injury, LSECs lost their protective role in maintaining macrophages and HSCs in a quiescent state and acquired proinflammatory function.9,10,47 It was demonstrated that alterations in LSECs occurred in the early stage of NAFLD even before appearance of extensive inflammation or activation of HSCs and contributed to the perpetuation of liver injury.47,48,49 Consistently, we found two subpopulations of LSECs, characterizing by highly expressed Egr1 and Ly6a, respectively, expanded in the early stage of NASH. More importantly, the expansion was enhanced and their interactions with HSCs were strengthened along with increased severity of NASH. Egr-1 is an immediate-early gene (IEG) that could sense extracellular stimuli and induce expression of growth factors and proinflammatory genes.50,51,52 It is also capable of promoting angiogenesis in case of wound healing, tumor and corneal neovascularization.53 The Egr1hi subpopulation of LSECs also expressed high level of other IEGs including Fos, Fosb, Ier2, Ier3, Ier5, Jun, Junb, Jund, Plk2, and Rhob et al. Fos, Fosb, Jun, Junb, and Jund belong to the activator protein-1 family that are critical in regulating cell proliferation and angiogenesis, while Plk2 and Rhob may potentiate angiogenesis under physiological or pathological conditions.54,55,56,57 Thus, we speculated that the Egr1hi subpopulation of LSECs might play a role in early response to the liver injury and contribute to pathological angiogenesis in NASH.
Ly6a, a specific marker for hematopoietic stem cells, is also expressed in LSECs promoting its proliferation and production of interleukin 6.58 We also found Ly6a was highly expressed in LSECs, while its expression was quite low in periportal and pericentral endothelial cells (Figures 5B and S5). However, Xiong et al. reported that the expression of Ly6a was low in LSECs and pericentral endothelial cells, but high in periportal endothelial cells.18 Such inconsistence might come from many experimental variables, which were not exactly the same in different labs. For example, amylin diet containing 40% fat (of which 18% was trans-fat), 22% fructose, and 2% cholesterol was applied to induce NASH mouse model in Xiong’s study, while mice were fed high-fat diet (60% fat) supplemented with 42 g/L of carbohydrates in the drinking water (55% fructose and 45% sucrose) in our study. Besides, the protocols for isolation of liver NPCs and the scRNA sequencing platform were also different between Xiong’s and our study. In this study, we identified a Ly6ahi subpopulation of LSECs in mice liver, which was characterized by abundant expression of interferon-induced genes (ISGs) such as Ifit1, Ifit2, Ifit3, Ifit3b, Ifi44, Ifi203, Ifi204, Ifi206, Ifi207, Ifi208, and Isg15, as well as genes regulating expression of ISGs such as Irf7, Stat1, and Stat2. Such expression pattern was in line with that of interferon-activated ECs that has been identified in healthy murine spleen, kidney, brain, muscle, and heart.24 Evidences from mice model showed that interferon signaling might be involved in pathogenesis of NAFLD, probably in a tissue-specific manner.59,60,61 However, the role of interferon-activated ECs in both healthy and diseased tissues remained unrevealed. Our results indicated interferon-activated Ly6ahi LSECs also existed in murine liver, expressing abundant interferon-inducible proinflammatory chemokines Cxcl9 and Cxcl10. Furthermore, these Ly6ahi LSECs underwent significant expansion in NASH liver, suggesting they might contribute to NASH progression by regulating hepatic inflammation via interferon pathway. Consistently, the expression of IFN-γ also increased in the liver tissues of HFD mice, which was positively correlated with the amount of Itgadhi macrophages (Figure S6).
In summary, our findings demonstrated that in liver NPCs, subpopulations of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs were accumulated in the early stage of NASH, and their expansion was strongly associated with disease severity, suggesting these subpopulations might be involved in NASH progression. Cell-cell interactions among NPCs were enhanced in NASH liver, with intimate interplays occurring between HSCs and Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs, implying these subpopulations may contribute to the activation of HSCs. Our study provided insights into the heterogeneity of NPCs in NASH progression. The function and significance of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs in healthy and diseased liver need to be investigated in follow-up studies.
Limitations of the study
While the present study reveals expansion of Itgadhi/Fcrl5hi macrophages, Egr1hi LSECs, and Ly6ahi LSECs in the progression of NASH, there are several limitations that should be considered. First, whether the expansion of these distinctive subpopulations is a cause or consequence of NASH needs to be addressed. Second, the functions of these subpopulations remain to be undetermined. Third, the interaction among these subpopulations is to be clarified. Further studies are needed to gain a comprehensive understanding of these subpopulations.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD45 | Biolegend | Cat# 103105; RRID: AB_312970 |
| F4/80 | Biolegend | Cat# 123116; RRID: AB_893481 |
| CD11b | Biolegend | Cat# 101227; RRID: AB_893233 |
| Fcrl5 | R&D | Cat# FAB6757G; RRID: AB_2927459 |
| CD11c | Biolegend | Cat# 117318; RRID: AB_493568 |
| MHCII | Biolegend | Cat# 107606; RRID: AB_313321 |
| VEGFR2 | Biolegend | Cat# 136413; RRID: AB_2561606 |
| Ly-6a | Santa Cruz | Cat# sc-52601; RRID: AB_630228 |
| F4/80 | Santa Cruz | Cat# sc-377009; RRID:AB_2927461 |
| Itgad/Cd11d | Abbexa | Cat# abx101639; RRID: AB_2927460 |
| CD31 | Abcam | Cat# ab222783; RRID: AB_2905525 |
| vWF | Santa Cruz | Cat# sc-365712; RRID: AB_10842026 |
| Egr1 | Santa Cruz | Cat# sc-101033; RRID: AB_1122474 |
| Chemicals, peptides, and recombinant proteins | ||
| High-fat diet | Research Diets | Cat# D12492 |
| Fructose | Sigma-Aldrich | Cat# F0127 |
| Sucrose | Sigma-Aldrich | Cat# V900116 |
| Haematoxylin and Eosin dye | Servicebio | Cat# G1005 |
| Oil Red O dye | Sigma-Aldrich | Cat# 1320-06-5 |
| Picrosirius red dye | Servicebio | Cat# GC307014 |
| TRIzol | Takara | Cat# 9108 |
| SYBR Premix Ex Taq II | Takara | Cat# RR820A |
| Red blood cell lysate buffer | Miltenyi Biotec | Cat# 130-094-183 |
| DAPI | Yeason | Cat# 40728ES03 |
| Critical commercial assays | ||
| Liver Dissociation Kit | Miltenyi Biotec | Cat# 130-105-807 |
| Fixation/Permeabilization Kit | BD Biosciences | Cat# 554722 |
| PrimeScript RT Master Mix Kit | Takara | Cat# RR036A |
| BD Rhapsody cDNA Kit | BD Biosciences | Cat# 633773 |
| BD Rhapsody Targeted mRNA&AbSeq Amplification Kit | BD Biosciences | Cat# 633801 |
| Deposited data | ||
| Raw sequencing data | National Omics Data Encyclopedia | OEP003311 |
| Oligonucleotides | ||
| qPCR primers, see Table S1 | This paper | N/A |
| Software and algorithms | ||
| FlowJo 10 | Treestar | https://www.flowjo.com/solutions/flowjo |
| Leica software package | Leica | N/A |
| ImageJ software | NIH | https://imagej.nih.gov/ij/ |
| GraphPad Prism 8 | GraphPad Software | https://www.graphpad.com/ |
| R package | R CRAN | http://www.R-project.org/ |
| Seurat | Butler et al., 201862; Stuart et al., 201963 | https://satijalab.org/seurat/ |
| SingleR (v1.4.1) | Aran et al., 201964 | https://github.com/dviraran/SingleR |
| Monocle2 | Qiu et al., 201765 | https://github.com/cole-trapnell-lab/monocle-release |
| CellphoneDB | Efremova et al., 202066 | https://www.cellphonedb.org/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Lungen Lu (lungen.lu@shgh.cn).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Experimental animals
Healthy 6-week-old male C57BL/6J mice were purchased from Vital River Laboratory (Beijing, China). Mice were maintained under specific pathogen free facilities and under 12h light-dark cycles with free access to chow diet and water (referred to as Chow group), or high-fat diet (HFD) (60% kcal% fat, D12492, Research Diets) supplemented with 42 g/L of carbohydrates in the drinking water (55% fructose (F0127), 45% sucrose (V900116); Sigma-Aldrich) (referred to as HFD group) for 8, 16, 20 and 32 weeks as described in the previous study.19,20 Animal experiments in the present study were performed following the Guide for the Care and Use of Laboratory Animals and were approved by the Committee on the Ethics of animal experiments of Shanghai General Hospital.
Method details
Isolation of liver NPCs
Liver tissues were collected from mice after they were euthanized. Liver dissociation was performed according to the instructions on Liver Dissociation Kit provided by the manufacturer (Miltenyi Biotec, Cat No. 130-105-807). After dissociation, the liver cell suspension was centrifuged at 50 × g for 2 min to remove hepatocytes. The supernatant containing NPCs was then passed through a 70 μm nylon cell strainer followed by treatment with red blood cell lysate buffer (Miltenyi Biotec, Cat No.130-094-183) to lyse red blood cells. The NPCs suspension was kept on ice for follow-up experiments.
RNA-seq analysis of liver NPCs
Total RNA was extracted from liver NPCs isolated above using TRIzol Reagent (ThermoFisher Scientific), and mRNA was isolated using Dynabeads mRNA DIRECT Micro Purification kit (ThermoFisher Scientific). RNA-Seq libraries were constructed using KAPA Stranded RNA-Seq Library Preparation Kit (KAPA Biosystems) and sequenced in a PE150 mode (Pair-End for 150bp read) on NovaSeq platform (Illumina). Differential gene expression analysis was performed using DESeq2 in the R package (http://www.R-project.org/). A differentially expressed gene should meet the following criteria: average expression abundance of the gene at least in one group is more than one Read Per Kilobase per Million mapped reads (RPKM); false discovery rate (fdr, an adjusted p value after multiple testing of Benjamini-Hochberg (BH) approach) < 0.1 and fold change (FC) > 1.5 (up-regulated) or < -1.5 (down-regulated).
Single cell RNA sequencing of liver NPCs
Cells were stained with Calcein AM and Draq7, and examined by BD Rhapsody Scanner (BD Biosciences) to determine cell concentration and viability. Qualified cell suspension was loaded into BD Rhapsody micro-well cartridge as described previously.67 Cell capture beads were then loaded excessively to ensure that nearly every micro-well contains one bead, and the excess beads were washed away from the cartridge. After lysing cells with lysis buffer, cell capture beads were retrieved and applied to generating cDNA library which contained both cell labels and unique molecular identifiers (UMI). All procedures were performed following the manufacturers’ instructions on BD Rhapsody cDNA Kit (BD Biosciences, Cat No. 633773) and BD Rhapsody Targeted mRNA&AbSeq Amplification Kit (BD Biosciences, Cat No. 633801). All the libraries were sequenced in a PE150 mode (Pair-End for 150bp read) on NovaSeq platform (Illumina).
ScRNA-seq data processing
Raw sequencing data were processed by BD Rhapsody Whole Transcriptome Assay Analysis Pipeline (v1.8), which included read quality filtering, read annotation, molecule annotation, putative cell determination and single-cell expression matrix generation. Matrix of UMI counts for each gene per cell was used for following analysis. Genome Reference Consortium Mouse Build 38 (GRCm38) was used as reference for BD pipeline.
Seurat was utilized for dimensionality reduction, clustering and visualization.62,63 Cells with more than 25% mitochondrial UMI, or less than 500 UMI or 200 genes were removed. PCA was performed based on top 2000 highly variable features after scaling the data with respect to UMI counts. Clustering was performed at resolution 0.6 and data was visualized using t-Distributed Stochastic Neighbor Embedding (t-SNE).
Cell type annotation
SingleR (v1.4.1) was applied in unbiased annotation of cell types for each cluster in the combined dataset.64 FindMarkers from Seurat package was also used to identify specific markers for each cluster and annotate cell types by searching PanglaoDB (https://panglaodb.se/). Final adjustment for cell type annotation was done based on biological knowledge. Featureplot, Violinplot and Heatmap were used to visualize the expression of marker genes for each cluster.
Pseudotime trajectory analysis
Single cell pseudotime analysis was performed using Monocle2 with DDR-Tree reduction method.65 Briefly, a Monocle object was created according to the expression matrix and metadata information stored in the Seurat object. Top marker genes of Seurat clusters were set as the ordering genes for feature selection. Batch effect were eliminated during dimensionality reduction. Trajectory plots and Heatmap were utilized to visualize the results.
Cell-cell communication analysis
CellphoneDB was used to analyze cell-cell communication with default parameters.66 The cutoff for expression proportion of any ligand or receptor in a given cell type was set to 10%, and the times of permutations was set to 1000. Heatmap was used to visualize the intensity of cell-cell communication, and Dotplot was used to visualize the interacting pairs among different cell types.
Histological analysis
Liver tissues were fixed with 4% neutral buffered formaldehyde solution, embedded in paraffin and cut into 5 μm thickness. After deparaffinization and rehydration, paraffin sections were stained with hematoxylin and eosin (H&E) and Picrosirius red (PSR). Frozen sections were prepared using the Tissue-Tek OCT compound (Sakura) and cut into 8 μm thickness for Oil Red O staining (Sigma-Aldrich). The NAFLD activity score (NAS) was applied to evaluate the severity of histological alterations including steatosis (0–3), lobular inflammation (0–3) and hepatocellular ballooning (0–2).68 Images were captured using a Leica light microscope and quantified using Image J.
Flow cytometry
The NPCs suspensions were centrifuged at 300 × g for 5 min. The pelleted cells were washed and re-suspended in cold PBS containing 1% BSA. For cell-surface staining, 1–5 x 106 cells were stained with 100 μl of indicated antibodies diluted at optimal concentrations for 30 min at 4°C. For intracellular staining, the cells were fixed and permeabilized using Fixation/Permeabilization Kit according to manufacturer’s protocol (BD Biosciences, Cat No.554722). The cells were assayed with the BD LSRFortessa flow cytometer, and the data were analyzed using FlowJo software. The fluorochrome-conjugated antibodies against CD45 (Biolegend), F4/80 (Biolegend), CD11b (Biolegend), Fcrl5 (R&D), CD11c (Biolegend), MHCII (Biolegend), Vegfr2 (Biolegend) and Ly6a (Santa Cruz) were used to examine macrophages, DCs and ECs respectively.
Quantitative real-time PCR
Total RNA was extracted from NPCs or whole liver using Trizol (Takara) and reverse transcription was performed using the PrimeScript RT Master Mix Kit (Takara). Real-time PCR was performed with SYBR Premix Ex Taq II (Takara). The mRNAs expression was quantified using the 2-ΔΔCt method. The primer sequences used for Quantitative real-time PCR (qPCR) were listed in Table S3.
Immunofluorescence staining
The paraffin sections were boiled in EDTA antigen retrieval solution for 10 min. After washing with PBS, sections were blocked with PBS containing 5% BSA for 1h at room temperature, and incubated with the primary antibodies against F4/80 (Santa Cruz), Itgad/Cd11d (R&D), CD31 (Abcam), vWF (Santa Cruz), Egr1 (Santa Cruz) or Ly6a (Santa Cruz) overnight at 4°C. Thereafter, the sections were rinsed 3 times for 5 min in PBS and stained with fluorochrome-conjugated secondary antibodies (1:500, CST) for 1 h at room temperature, and counterstained with 6-diamidino-2-phenylindole (DAPI, Yeason). Images were captured using a Leica fluorescence microscope and quantified using Image J.
Quantification and statistical analysis
Statistical analysis and visualization were performed using GraphPad Prism 8 software. All data were presented as means ± SEM. Differences between two groups were analyzed using Student’s t-test. Differences among multiple groups were analyzed using one-way ANOVA. Pearson’s correlation analysis was employed to evaluate the correlations of expression level of two genes. Statistical significance was set at 0.05 and denoted by ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 and ∗∗∗∗p < 0.0001.
Acknowledgments
Single cell RNA sequencing was performed by Sinotech Genomics Co., Ltd. Shanghai, China. The authors thank Dr. Quanli Zou and Guangni Li from Sinotech Genomics Co., Ltd for valuable suggestions and technical supports on data analysis and visualization. We also acknowledge BioRender.com for usage in the design of the graphical abstract. Dr. Hui Dong was funded by Shanghai Municipal Science and Technology Commission Research Project (22ZR1449700) and Shanghai General Hospital Start-up Fund (02.06.01.20.01). Dr. Lungen Lu was funded by National Natural Science Foundation of China (82170620).
Author contributions
L.L., H.D., and X.C. conceived and supervised the project. Z.S, H.D., B.S., and W.D. performed the experiments and analyzed the data; C.Z., X.L., Y.G., J.W., X.X., Z.S. assisted in animal experiments. Z.S., H.D., and L.L. wrote the manuscript with comments from all authors. All authors reviewed results and revised the manuscript.
Declaration of interests
The authors declared no potential conflicts of interests.
Published: April 5, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.106572.
Contributor Information
Xiaobo Cai, Email: xiaobo.cai@shgh.cn.
Hui Dong, Email: kerwinc2002@hotmail.com.
Lungen Lu, Email: lungen.lu@shgh.cn.
Supplemental information
Data and code availability
-
•
Raw sequencing data has been deposited at the National Omics Data Encyclopedia (https://www.biosino.org/node). Accession number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Brunt E.M., Wong V.W.S., Nobili V., Day C.P., Sookoian S., Maher J.J., Bugianesi E., Sirlin C.B., Neuschwander-Tetri B.A., Rinella M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers. 2015;1 doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 4.Angulo P., Machado M.V., Diehl A.M. Fibrosis in nonalcoholic Fatty liver disease: mechanisms and clinical implications. Semin. Liver Dis. 2015;35:132–145. doi: 10.1055/s-0035-1550065. [DOI] [PubMed] [Google Scholar]
- 5.Friedman S.L., Pinzani M. Hepatic fibrosis 2022: unmet needs and a blueprint for the future. Hepatology. 2022;75:473–488. doi: 10.1002/hep.32285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Si-Tayeb K., Lemaigre F.P., Duncan S.A. Organogenesis and development of the liver. Dev. Cell. 2010;18:175–189. doi: 10.1016/j.devcel.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Malik R., Selden C., Hodgson H. The role of non-parenchymal cells in liver growth. Semin. Cell Dev. Biol. 2002;13:425–431. doi: 10.1016/s1084952102001301. [DOI] [PubMed] [Google Scholar]
- 8.Kumar S., Duan Q., Wu R., Harris E.N., Su Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammoutene A., Rautou P.E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019;70:1278–1291. doi: 10.1016/j.jhep.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Furuta K., Guo Q., Hirsova P., Ibrahim S.H. Emerging roles of liver sinusoidal endothelial cells in nonalcoholic steatohepatitis. Biology. 2020;9 doi: 10.3390/biology9110395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim S.H. Sinusoidal endotheliopathy in nonalcoholic steatohepatitis: therapeutic implications. Am. J. Physiol. Gastrointest. Liver Physiol. 2021;321 doi: 10.1152/ajpgi.00009.2021. G67–g74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazankov K., Jørgensen S.M.D., Thomsen K.L., Møller H.J., Vilstrup H., George J., Schuppan D., Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 13.Schwabe R.F., Tabas I., Pajvani U.B. Mechanisms of fibrosis development in nonalcoholic steatohepatitis. Gastroenterology. 2020;158:1913–1928. doi: 10.1053/j.gastro.2019.11.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramachandran P., Matchett K.P., Dobie R., Wilson-Kanamori J.R., Henderson N.C. Single-cell technologies in hepatology: new insights into liver biology and disease pathogenesis. Nat. Rev. Gastroenterol. Hepatol. 2020;17:457–472. doi: 10.1038/s41575-020-0304-x. [DOI] [PubMed] [Google Scholar]
- 15.Saviano A., Henderson N.C., Baumert T.F. Single-cell genomics and spatial transcriptomics: discovery of novel cell states and cellular interactions in liver physiology and disease biology. J. Hepatol. 2020;73:1219–1230. doi: 10.1016/j.jhep.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu A.L., Schilling J.D., King K.R., Feldstein A.E. The power of single-cell analysis for the study of liver pathobiology. Hepatology. 2021;73:437–448. doi: 10.1002/hep.31485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthal S.B., Liu X., Ganguly S., Dhar D., Pasillas M.P., Ricciardelli E., Li R.Z., Troutman T.D., Kisseleva T., Glass C.K., Brenner D.A. Heterogeneity of HSCs in a mouse model of NASH. Hepatology. 2021;74:667–685. doi: 10.1002/hep.31743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong X., Kuang H., Ansari S., Liu T., Gong J., Wang S., Zhao X.Y., Ji Y., Li C., Guo L., et al. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Mol. Cell. 2019;75:644–660.e5. doi: 10.1016/j.molcel.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohli R., Kirby M., Xanthakos S.A., Softic S., Feldstein A.E., Saxena V., Tang P.H., Miles L., Miles M.V., Balistreri W.F., et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52:934–944. doi: 10.1002/hep.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen B., Wang J., Guo Y., Gu T., Shen Z., Zhou C., Li B., Xu X., Li F., Zhang Q., et al. Dextran sulfate sodium salt-induced colitis aggravates gut microbiota dysbiosis and liver injury in mice with non-alcoholic steatohepatitis. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.756299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krenkel O., Tacke F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017;17:306–321. doi: 10.1038/nri.2017.11. [DOI] [PubMed] [Google Scholar]
- 22.Guillot A., Tacke F. Liver macrophages: old dogmas and new insights. Hepatol. Commun. 2019;3:730–743. doi: 10.1002/hep4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadler K.C., Krahn K.N., Gaur N.A., Ukomadu C. Liver growth in the embryo and during liver regeneration in zebrafish requires the cell cycle regulator, uhrf1. Proc. Natl. Acad. Sci. USA. 2007;104:1570–1575. doi: 10.1073/pnas.0610774104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalucka J., de Rooij L., Goveia J., Rohlenova K., Dumas S.J., Meta E., Conchinha N.V., Taverna F., Teuwen L.A., Veys K., et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–779.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 25.Ramachandran P., Dobie R., Wilson-Kanamori J.R., Dora E.F., Henderson B.E.P., Luu N.T., Portman J.R., Matchett K.P., Brice M., Marwick J.A., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su T., Yang Y., Lai S., Jeong J., Jung Y., McConnell M., Utsumi T., Iwakiri Y. Single-cell transcriptomics reveals zone-specific alterations of liver sinusoidal endothelial cells in cirrhosis. Cell. Mol. Gastroenterol. Hepatol. 2021;11:1139–1161. doi: 10.1016/j.jcmgh.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpern K.B., Shenhav R., Massalha H., Toth B., Egozi A., Massasa E.E., Medgalia C., David E., Giladi A., Moor A.E., et al. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat. Biotechnol. 2018;36:962–970. doi: 10.1038/nbt.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poisson J., Lemoinne S., Boulanger C., Durand F., Moreau R., Valla D., Rautou P.E. Liver sinusoidal endothelial cells: physiology and role in liver diseases. J. Hepatol. 2017;66:212–227. doi: 10.1016/j.jhep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Allen K., Jaeschke H., Copple B.L. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma S., Charron J., Erikson R.L. Role of Plk2 (Snk) in mouse development and cell proliferation. Mol. Cell Biol. 2003;23:6936–6943. doi: 10.1128/mcb.23.19.6936-6943.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daemen S., Gainullina A., Kalugotla G., He L., Chan M.M., Beals J.W., Liss K.H., Klein S., Feldstein A.E., Finck B.N., et al. Dynamic shifts in the composition of resident and recruited macrophages influence tissue remodeling in NASH. Cell Rep. 2021;34 doi: 10.1016/j.celrep.2020.108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott C.L., Zheng F., De Baetselier P., Martens L., Saeys Y., De Prijck S., Lippens S., Abels C., Schoonooghe S., Raes G., et al. Bone marrow-derived monocytes give rise to self-renewing and fully differentiated Kupffer cells. Nat. Commun. 2016;7 doi: 10.1038/ncomms10321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krenkel O., Hundertmark J., Abdallah A.T., Kohlhepp M., Puengel T., Roth T., Branco D.P.P., Mossanen J.C., Luedde T., Trautwein C., et al. Myeloid cells in liver and bone marrow acquire a functionally distinct inflammatory phenotype during obesity-related steatohepatitis. Gut. 2020;69:551–563. doi: 10.1136/gutjnl-2019-318382. [DOI] [PubMed] [Google Scholar]
- 34.Morinaga H., Mayoral R., Heinrichsdorff J., Osborn O., Franck N., Hah N., Walenta E., Bandyopadhyay G., Pessentheiner A.R., Chi T.J., et al. Characterization of distinct subpopulations of hepatic macrophages in HFD/obese mice. Diabetes. 2015;64:1120–1130. doi: 10.2337/db14-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Vieren M., Le Trong H., Wood C.L., Moore P.F., St John T., Staunton D.E., Gallatin W.M. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity. 1995;3:683–690. doi: 10.1016/1074-7613(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 36.Miyazaki Y., Vieira-de-Abreu A., Harris E.S., Shah A.M., Weyrich A.S., Castro-Faria-Neto H.C., Zimmerman G.A. Integrin αDβ2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakubenko V.P., Belevych N., Mishchuk D., Schurin A., Lam S.C.T., Ugarova T.P. The role of integrin alpha D beta2 (CD11d/CD18) in monocyte/macrophage migration. Exp. Cell Res. 2008;314:2569–2578. doi: 10.1016/j.yexcr.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas A.P., Dunn T.N., Oort P.J., Grino M., Adams S.H. Inflammatory phenotyping identifies CD11d as a gene markedly induced in white adipose tissue in obese rodents and women. J. Nutr. 2011;141:1172–1180. doi: 10.3945/jn.110.127068. [DOI] [PubMed] [Google Scholar]
- 39.Aziz M.H., Cui K., Das M., Brown K.E., Ardell C.L., Febbraio M., Pluskota E., Han J., Wu H., Ballantyne C.M., et al. The upregulation of integrin α(D)β(2) (CD11d/CD18) on inflammatory macrophages promotes macrophage retention in vascular lesions and development of atherosclerosis. J. Immunol. 2017;198:4855–4867. doi: 10.4049/jimmunol.1602175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui K., Ardell C.L., Podolnikova N.P., Yakubenko V.P. Distinct migratory properties of M1, M2, and resident macrophages are regulated by α(D)β(2) and α(M)β(2) integrin-mediated adhesion. Front. Immunol. 2018;9:2650. doi: 10.3389/fimmu.2018.02650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weaver L.C., Bao F., Dekaban G.A., Hryciw T., Shultz S.R., Cain D.P., Brown A. CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats. Exp. Neurol. 2015;271:409–422. doi: 10.1016/j.expneurol.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utagawa A., Bramlett H.M., Daniels L., Lotocki G., Dekaban G.A., Weaver L.C., Dietrich W.D. Transient blockage of the CD11d/CD18 integrin reduces contusion volume and macrophage infiltration after traumatic brain injury in rats. Brain Res. 2008;1207:155–163. doi: 10.1016/j.brainres.2008.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis R.S. Fc receptor-like molecules. Annu. Rev. Immunol. 2007;25:525–560. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 44.Akama-Garren E.H., Carroll M.C. Lupus susceptibility loci predispose mice to clonal lymphocytic responses and myeloid expansion. J. Immunol. 2022;208:2403–2424. doi: 10.4049/jimmunol.2200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isobe M., Izawa K., Sugiuchi M., Sakanishi T., Kaitani A., Takamori A., Maehara A., Matsukawa T., Takahashi M., Yamanishi Y., et al. The CD300e molecule in mice is an immune-activating receptor. J. Biol. Chem. 2018;293:3793–3805. doi: 10.1074/jbc.RA117.000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel J., McNeill E., Douglas G., Hale A.B., de Bono J., Lee R., Iqbal A.J., Regan-Komito D., Stylianou E., Greaves D.R., Channon K.M. RGS1 regulates myeloid cell accumulation in atherosclerosis and aortic aneurysm rupture through altered chemokine signalling. Nat. Commun. 2015;6:6614. doi: 10.1038/ncomms7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyao M., Kotani H., Ishida T., Kawai C., Manabe S., Abiru H., Tamaki K. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab. Invest. 2015;95:1130–1144. doi: 10.1038/labinvest.2015.95. [DOI] [PubMed] [Google Scholar]
- 48.Pasarín M., La Mura V., Gracia-Sancho J., García-Calderó H., Rodríguez-Vilarrupla A., García-Pagán J.C., Bosch J., Abraldes J.G. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francque S., Laleman W., Verbeke L., Van Steenkiste C., Casteleyn C., Kwanten W., Van Dyck C., D'Hondt M., Ramon A., Vermeulen W., et al. Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab. Invest. 2012;92:1428–1439. doi: 10.1038/labinvest.2012.103. [DOI] [PubMed] [Google Scholar]
- 50.Arner E., Daub C.O., Vitting-Seerup K., Andersson R., Lilje B., Drabløs F., Lennartsson A., Rönnerblad M., Hrydziuszko O., Vitezic M., et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015;347:1010–1014. doi: 10.1126/science.1259418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khachigian L.M. Early growth response-1, an integrative sensor in cardiovascular and inflammatory disease. J. Am. Heart Assoc. 2021;10 doi: 10.1161/jaha.121.023539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harja E., Bucciarelli L.G., Lu Y., Stern D.M., Zou Y.S., Schmidt A.M., Yan S.F. Early growth response-1 promotes atherogenesis: mice deficient in early growth response-1 and apolipoprotein E display decreased atherosclerosis and vascular inflammation. Circ. Res. 2004;94:333–339. doi: 10.1161/01.Res.0000112405.61577.95. [DOI] [PubMed] [Google Scholar]
- 53.Fahmy R.G., Dass C.R., Sun L.Q., Chesterman C.N., Khachigian L.M. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat. Med. 2003;9:1026–1032. doi: 10.1038/nm905. [DOI] [PubMed] [Google Scholar]
- 54.Shaulian E., Karin M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 55.Yoshitomi Y., Ikeda T., Saito-Takatsuji H., Yonekura H. Emerging role of AP-1 transcription factor JunB in angiogenesis and vascular development. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22062804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerald D., Adini I., Shechter S., Perruzzi C., Varnau J., Hopkins B., Kazerounian S., Kurschat P., Blachon S., Khedkar S., et al. RhoB controls coordination of adult angiogenesis and lymphangiogenesis following injury by regulating VEZF1-mediated transcription. Nat. Commun. 2013;4:2824. doi: 10.1038/ncomms3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang H., Fang L., Zhan R., Hegarty J.M., Ren J., Hsiai T.K., Gleeson J.G., Miller Y.I., Trejo J., Chi N.C. Polo-like kinase 2 regulates angiogenic sprouting and blood vessel development. Dev. Biol. 2015;404:49–60. doi: 10.1016/j.ydbio.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luna G., Paez J., Cardier J.E. Expression of the hematopoietic stem cell antigen Sca-1 (LY-6A/E) in liver sinusoidal endothelial cells: possible function of Sca-1 in endothelial cells. Stem Cells Dev. 2004;13:528–535. doi: 10.1089/scd.2004.13.528. [DOI] [PubMed] [Google Scholar]
- 59.Ghazarian M., Revelo X.S., Nøhr M.K., Luck H., Zeng K., Lei H., Tsai S., Schroer S.A., Park Y.J., Chng M.H.Y., et al. Type I interferon responses drive intrahepatic T cells to promote metabolic syndrome. Sci. Immunol. 2017;2 doi: 10.1126/sciimmunol.aai7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitsumoto K., Watanabe R., Nakao K., Yonenaka H., Hashimoto T., Kato N., Kumrungsee T., Yanaka N. Time-course microarrays reveal early activation of the immune transcriptome in a choline-deficient mouse model of liver injury. Life Sci. 2017;184:103–111. doi: 10.1016/j.lfs.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 61.Wieser V., Adolph T.E., Grander C., Grabherr F., Enrich B., Moser P., Moschen A.R., Kaser S., Tilg H. Adipose type I interferon signalling protects against metabolic dysfunction. Gut. 2018;67:157–165. doi: 10.1136/gutjnl-2016-313155. [DOI] [PubMed] [Google Scholar]
- 62.Butler A., Hoffman P., Smibert P., Papalexi E., Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stuart T., Butler A., Hoffman P., Hafemeister C., Papalexi E., Mauck W.M., 3rd, Hao Y., Stoeckius M., Smibert P., Satija R. Comprehensive integration of single-cell data. Cell. 2019;177:1888–1902.e21. doi: 10.1016/j.cell.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aran D., Looney A.P., Liu L., Wu E., Fong V., Hsu A., Chak S., Naikawadi R.P., Wolters P.J., Abate A.R., et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 2019;20:163–172. doi: 10.1038/s41590-018-0276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qiu X., Mao Q., Tang Y., Wang L., Chawla R., Pliner H.A., Trapnell C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods. 2017;14:979–982. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Efremova M., Vento-Tormo M., Teichmann S.A., Vento-Tormo R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 2020;15:1484–1506. doi: 10.1038/s41596-020-0292-x. [DOI] [PubMed] [Google Scholar]
- 67.Fan H.C., Fu G.K., Fodor S.P.A. Expression profiling. Combinatorial labeling of single cells for gene expression cytometry. Science. 2015;347 doi: 10.1126/science.1258367. [DOI] [PubMed] [Google Scholar]
- 68.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Raw sequencing data has been deposited at the National Omics Data Encyclopedia (https://www.biosino.org/node). Accession number is listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.