Abstract
Objective
To assess whether an easy-to-use multifaceted intervention for children presenting to primary care with respiratory tract infections would reduce antibiotic dispensing, without increasing hospital admissions for respiratory tract infection.
Design
Two arm randomised controlled trial clustered by general practice, using routine outcome data, with qualitative and economic evaluations.
Setting
English primary care practices using the EMIS electronic medical record system.
Participants
Children aged 0-9 years presenting with respiratory tract infection at 294 general practices, before and during the covid-19 pandemic.
Intervention
Elicitation of parental concerns during consultation; a clinician focused prognostic algorithm to identify children at very low, normal, or elevated 30 day risk of hospital admission accompanied by antibiotic prescribing guidance; and a leaflet for carers including safety netting advice.
Main outcome measures
Rate of dispensed amoxicillin and macrolide antibiotics (superiority comparison) and hospital admissions for respiratory tract infection (non-inferiority comparison) for children aged 0-9 years over 12 months (same age practice list size as denominator).
Results
Of 310 practices needed, 294 (95%) were randomised (144 intervention and 150 controls) representing 5% of all registered 0-9 year olds in England. Of these, 12 (4%) subsequently withdrew (six owing to the pandemic). Median intervention use per practice was 70 (by a median of 9 clinicians). No evidence was found that antibiotic dispensing differed between intervention practices (155 (95% confidence interval 138 to 174) items/year/1000 children) and control practices (157 (140 to 176) items/year/1000 children) (rate ratio 1.011, 95% confidence interval 0.992 to 1.029; P=0.25). Pre-specified subgroup analyses suggested reduced dispensing in intervention practices with fewer prescribing nurses, in single site (compared with multisite) practices, and in practices located in areas of lower socioeconomic deprivation, which may warrant future investigation. Pre-specified sensitivity analysis suggested reduced dispensing among older children in the intervention arm (P=0.03). A post hoc sensitivity analysis suggested less dispensing in intervention practices before the pandemic (rate ratio 0.967, 0.946 to 0.989; P=0.003). The rate of hospital admission for respiratory tract infections in the intervention practices (13 (95% confidence interval 10 to 18) admissions/1000 children) was non-inferior compared with control practices (15 (12 to 20) admissions/1000 children) (rate ratio 0.952, 0.905 to 1.003).
Conclusions
This multifaceted antibiotic stewardship intervention for children with respiratory tract infections did not reduce overall antibiotic dispensing or increase respiratory tract infection related hospital admissions. Evidence suggested that in some subgroups and situations (for example, under non-pandemic conditions) the intervention slightly reduced prescribing rates but not in a clinically relevant way.
Trial registration
ISRCTN11405239ISRCTN registry ISRCTN11405239
Introduction
Unnecessary use of antibiotics is associated with the needless development and proliferation of antimicrobial resistance.1 2 Around 80% of antibiotics prescribed for human consumption are prescribed in primary care,3 and around 50% of antibiotic prescribing in this setting has been estimated to be unnecessary.4 Children with respiratory tract infections are the single most frequent patient group to make use of primary care and receive antibiotics,5 with clinical uncertainty about prognosis leading to defensive antibiotic use.6 Although prescribing for uncomplicated respiratory tract infection has declined in England over the past decade, more than a third of children were still given antibiotics for these infections.7
Previous qualitative research has also shown that clinicians prescribe antibiotics “just in case” to mitigate perceived risk of future hospital admission and complications,8 9 that parents want better information on the signs and symptoms of serious respiratory tract infections and when to consult,10 and that parents also want more useful advice on home management of symptoms.6 We hypothesised that improved identification of children at very low risk of future hospital admission might help to reduce clinical uncertainty and that, combined with improved parent communications, this might reduce antibiotic use.
In an attempt to reduce “just in case” prescribing, we previously developed and internally validated the STARWAVe prognostic algorithm, which uses seven clinical characteristics to stratify children’s risk of hospital admission in the following 30 days into “very low,” “normal,” and “high” risk (bootstrapped area under the receiver operating characteristic curve 0.81). Children in the very low risk stratum have a 0.3% (95% confidence interval 0.2% to 0.4%) post-test probability of needing hospital treatment in the following 30 days and thus are unlikely to need antibiotics.11 As no datasets known to contain STARWAVe variables were available for external validation, we considered that the next step was to investigate clinical effectiveness. The algorithm was combined with tools to improve patient-doctor communication and parent information,12 following the Medical Research Council’s framework guidelines for development of complex interventions.13 We then conducted an individual patient randomised feasibility trial of the resulting intervention in a similar practice population, concluding that a future trial should reduce the time needed in the consultation to recruit patients and differential recruitment of less unwell patients between study arms.14 The trial we now report overcomes these problems by randomising general practice clusters (thereby avoiding individual patient recruitment) and using routinely collected aggregate outcome data (thereby avoiding the need for parental consent, as well as being more resource efficient).15 The intervention was also embedded within the general practice electronic medical record system. The aim of this study was to assess whether a refined, multifaceted intervention could reduce antibiotic dispensing among children aged 0-9 years presenting with cough and respiratory tract infection, without increasing respiratory tract infection related hospital admissions.
Methods
Study design
The CHICO (CHIldren’s COugh) randomised controlled trial was an efficient, pragmatic, open label, two arm (intervention versus usual care) trial of children aged 0-9, clustered by primary care practices in England. The trial included an embedded qualitative study that will be reported in detail elsewhere and a health economic analysis. The CHICO trial randomised practices between October 2018 and October 2020, with a trial data collection period from November 2018 to September 2021, thus including the covid-19 pandemic that began in March 2020 and was still ongoing in September 2021. We recruited practices by using clinical commissioning groups (CCGs; renamed integrated care boards from 1 July 2022) and the National Institute for Health and Care Research’s Clinical Research Network. We did an internal pilot in three CCGs initially to identify any problems with the intervention. This lasted three months and included a further four CCGs to help to establish best practice for recruiting and communicating with practices before widening to the remaining CCGs. The trial protocol has been published.16
Recruitment
Recruitment comprised two elements. Firstly, as CCGs routinely collected the co-primary outcome of hospital admission for respiratory tract infections, we endeavoured to recruit CCGs to the study. We targeted CCG recruitment at those with 15 or more practices using the EMIS patient record system (used in 56% of English practices).17 Secondly, practices were recruited from within participating CCGs by enlisting the help of the 15 clinical research networks across England. We excluded practices if they were not using the EMIS system, were participating in any potentially confounding concurrent intervention studies (for example, antimicrobial stewardship), or were merging, or planning to merge, with another practice. Once a practice had agreed to take part in the trial and completed their baseline questionnaire, it was randomised. The trial manager used the Bristol Randomised Trials Collaboration clinical trials unit randomisation system to randomise practices, stratified by CCG, using a one to one allocation, to either the intervention or control (usual care) arm, and using minimisation to balance list size and past dispensing rate, in 0-9 year olds, in the 12 months before randomisation. Practices used the intervention over a 12 month period from randomisation. The clinicians from practices randomised to the control arm were asked to treat children presenting with cough or respiratory tract infection as they usually would. A practice champion was appointed at each intervention practice to distribute training materials within the practice, coordinate training of prescribing staff, encourage all clinicians to use the intervention appropriately, and report from the EMIS system how many times the intervention was used.
The intervention
The intervention consisted of eliciting explicit concerns from carers during consultation, a clinician focused algorithm to predict risk (low, normal, or elevated) of hospital admission within 30 days for children consulting with a respiratory tract infection along with antibiotic prescribing guidance, and a carer focused personalised printout recording decisions made at the consultation, covering common concerns and providing safety netting information, which was based on a leaflet co-designed with parents.18 We sent intervention practices instructions including screen shots on how to install the intervention’s algorithm on the EMIS system. The algorithm included seven predictors, two of which (age of patient and history of asthma) were already available for automatic entry; the other five predictors (short illness duration, temperature, intercostal or subcostal recession on examination, wheeze, and moderate or severe vomiting) were entered during consultation. Table 1 provides the resulting comment associated with each score (each predictor scores one point). The training package for clinicians emphasised that the primary purpose of the algorithm was to identify the large proportion of children (69%) who have a very low risk of hospital admission and so could be safely managed at home. When a child in the eligible age range attended, the healthcare professional received a “soft” (that is, a reminder) screen alert (Quality and Outcomes Framework prompt style) asking if the child was presenting with a respiratory tract infection. The alert gave the option of opening the CHICO intervention template; alternatively, entry of specific respiratory tract infection codes triggered the launch of the template during the consultation. Each use of the template was automatically recorded using an EMIS code. Given the nature of the trial intervention, practices were not blinded to their allocation. The trial manager, administrative staff, and statistician were the only members of the trial management group who were unblinded.
Table 1.
Clinician advice associated with the algorithm output*
| CHICO result | Pop-up text |
|---|---|
| Low risk group | Very reassuring CHICO score: 0 or 1 CHICO predictors: >99.6% of children will recover from this illness with home care. Consider a no or delayed antibiotic prescribing strategy. CHICO leaflet and letter covers common concerns and safety netting advice |
| Average risk group | Reassuring CHICO score: 2 or 3 CHICO predictors: >98% of children will recover from this illness with home care. Consider no or delayed antibiotic prescribing strategy. CHICO leaflet and letter covers common concerns and safety netting advice |
| Elevated risk group | Safety netting needed: ≥4 CHICO predictors: This is more than average, but >87% of children will still recover from this illness with home care. Highlight safety netting advice in CHICO leaflet |
As presented in Seume et al, 2021.15
Outcomes
The co-primary outcomes for children aged 0-9 years over a 12 month period were the rate of dispensed amoxicillin and macrolide items prescribed, for all indications (superiority comparison) collected routinely by NHSBSA ePACT2 (Electronic Prescribing Analysis and Cost) system,19 and the rate of hospital admission for respiratory tract infection (non-inferiority comparison) routinely collected by CCGs. As no children were directly consented/recruited into the trial, we used proxy measures, for which the denominator was all 0-9 year old children registered at each practice. We collected baseline data on the characteristics of the practice and follow-up data after 12 months. Table 2 lists primary and secondary outcomes (including subgroup analyses).
Table 2.
Primary and secondary outcomes*
| Outcome No | Description |
|---|---|
| Primary outcomes | |
| P1 | Rate of amoxicillin and macrolide items dispensed, calculated using number of items dispensed to 0-9 year olds and number of children aged 0-9 registered at each practice over 12 month period (testing for superiority). Number of items dispensed, for all indications, was used as proxy measure owing to limitations of routine data |
| P2 | Rate of hospital admissions for RTI, calculated using number of admissions for RTI among children aged 0-9 years and number of children aged 0-9 registered at each practice over 12 month period (testing for non-inferiority) |
| Secondary outcomes | |
| S1 | Emergency department attendance rates for RTI, calculated using number of attendances for RTI among children aged 0-9 years and number of children aged 0-9 registered at each practice over 12 month period |
| S2 | Exploration into usage of intervention, in terms of both usage over 12 month period and seasonality, and effects it has on primary outcome P1 |
| S3 | Between arm comparison of mean NHS costs in cost-consequence analysis (health economics) |
| S4 | Acceptability of intervention and variation in use determined by qualitative interviews with clinicians |
| S5 | Comparison of primary outcome P1 stratified by categorisation of proportion of locums (median) used over 12 months practice is in study |
| S6 | Comparison of primary outcome P1 stratified by categorisation of proportion of nurses (median) used over 12 months practice is in study. This was originally planned as dichotomisation of practices into those with GP prescribers only and those with nurse or other prescribers as well. However, given that most practices had nurse prescribers, it seemed sensible to look at nurses as proportion of staff |
| S7 | Comparison of primary outcome P1 stratified by categorisation of practice dispensing rates taken from 12 months before data collection from each practice |
| S8 | Comparison of primary outcome P1 dichotomised by practices with one site only and those with multiple sites |
| S9 | Comparison of primary outcome P1 dichotomised by practices with follow-up periods before covid-19 pandemic and those with follow-up months on or after March 2020 |
| S10 | Comparison of primary outcome P1 dichotomised by practices with high level of deprivation versus those with low level of deprivation |
GP=general practitioner; RTI=respiratory tract infection.
As presented in Seume et al, 2021.15
Serious adverse events
The trial was considered low risk, so serious adverse events were reported only if fatal or serious and potentially related to trial participation. As one of the outcomes for the trial was hospital admission, we expected that some participants would be admitted to hospital (owing to a deterioration of their underlying illness, for example) so this was not subject to expedited reporting.
Sample size
The trial consisted of two co-primary outcomes, so we used a two sided α of 0.025 to take account of this. Both sample size calculations used a comparison of two proportions and assumed 90% power, an intra-cluster correlation coefficient of 0.03, a coefficient of variation of 0.65, and an estimated 750 children (registered 0-9 year olds) per practice. We estimated that 33 items would be dispensed and one hospital admission would occur per 100 children. To detect a 10% reduction, to 29 items, in dispensing data (superiority) and no more than an absolute 1% increase, to two, in hospital admissions (non-inferiority), we needed 155 practices per arm. We could conclude non-inferiority if the rate ratio, comparing the intervention arm with the control, had an upper confidence interval ≤1.01.
Analytical methods
A full CHICO statistical, health economic, and qualitative analysis plan was developed and agreed by an independent Trial Steering Committee and Data Monitoring Committee.20 All primary and secondary analyses were conducted on an intention-to-treat basis. General practices were the unit of randomisation/analysis in this cluster randomised trial, with the randomisation stratified by CCGs. Any variation between CCGs was accommodated by using CCG level random effects—that is, a two level model. We used mixed effects Poisson regression models to derive the rate ratio for both co-primary outcomes, including the practice list size (of 0-9 year old children) as the exposure/time variable, the baseline rate as a covariate, and random effects at the CCG level. We used the dispensing record of the practices in the 12 months before randomisation as a minimisation variable, thus balancing the dispensing records at baseline. We adjusted for this, in the primary analysis of dispensing rates, to resolve any residual difference. Although rates are described throughout, these are a calculation of dispensed items divided by list size of 0-9 year old children. Therefore, to aid interpretation in the text, rates have been converted to number per year per 1000 children. We analysed the hospital admission outcome in a similar way to the dispensing outcome but tested for non-inferiority, whereby a difference of no more than 1% higher in the intervention arm indicated non-inferiority, with emphasis placed on the confidence interval. We adjusted for baseline hospital admission rates in this analysis. We compared baseline characteristics descriptively, between the arms, and planned an adjusted sensitivity analysis if these differed by more than 10% (categorical variables), half a standard deviation, or half an interquartile range (continuous variables). We calculated the index of multiple deprivation by using the postcode of the practice in which the practice champion was based, using online routine data to group the practices into tenths of deprivation; data were available from 2019.21 Other sensitivity analyses have been described fully in the supplementary material. Subgroup analyses were pre-specified and investigated the treatment effect within subgroups of practices (dichotomised). To determine whether the treatment effect differed across the levels of the subgroup (for example, proportion of locums), we used likelihood ratio tests to compare models with and without the interaction term included, treating the subgroup of interest as a continuous variable wherever possible. We note that these subgroup analyses do not have sufficient power and should be used as guides for future research rather than confirmatory evidence. We used Stata 17.0 for all analyses and described the results in terms of strength of evidence rather than significance. We compared mean differences in NHS costs between arms by using two way mixed effect linear regression.
Patient and public involvement
The CHICO intervention was developed in collaboration with both a Parent Advisory Group and a Clinician and Practitioner Advisory Group, which we met several times during the final year of the CHICO feasibility trial. The Parent Advisory Group thought that conducting a national study and using a whole practice intervention were important and that using a prediction tool on the computer during consultation would provide reassurance. They strongly endorsed and encouraged us to use the parent leaflet. Patient and public involvement was maintained throughout the main trial through a group of three parents, two of whom (in rotation) attended and contributed to all the Trial Steering Committee meetings, including the final results reveal meeting. As the intervention focus was on general practices and not on recruiting individual patients, we made extensive contact with the Clinician and Practitioner Advisory Group during the trial period. We sought advice on the use of alert style and trigger codes in a consultation, the format of the template, the personalised letter, and questionnaires used to collect the data. We also ran some “think aloud” sessions with several general practitioners to see the intervention in action in EMIS and gather their thoughts about any problems or changes needed. The findings were disseminated to the patient and public involvement group and Trial Steering Committee members for comment and presented at primary care conferences, and a summary will be fed back to practices as the data are published.
Results
Recruitment and ascertainment
Of approximately 200 CCGs in England in October 2018, 110 were assessed as being eligible (≥15 practices using EMIS); 52 consented to take part, and 47 provided at least one practice (fig 1). Recruitment took 24 rather than 12 months, continuing to October 2020 (owing to slow response of some CCGs and effects of the covid-19 pandemic). Of the 310 practices needed, 294 (95%) were recruited (144 intervention and 150 controls), representing 336 496 registered 0-9 year-olds (5% of all 0-9 year old children in England). Of the 294 practices, 12 (4%) subsequently withdrew (six owing to the pandemic). Four serious adverse events (three intervention; one control) were reported, none of them related to the intervention.
Fig 1.
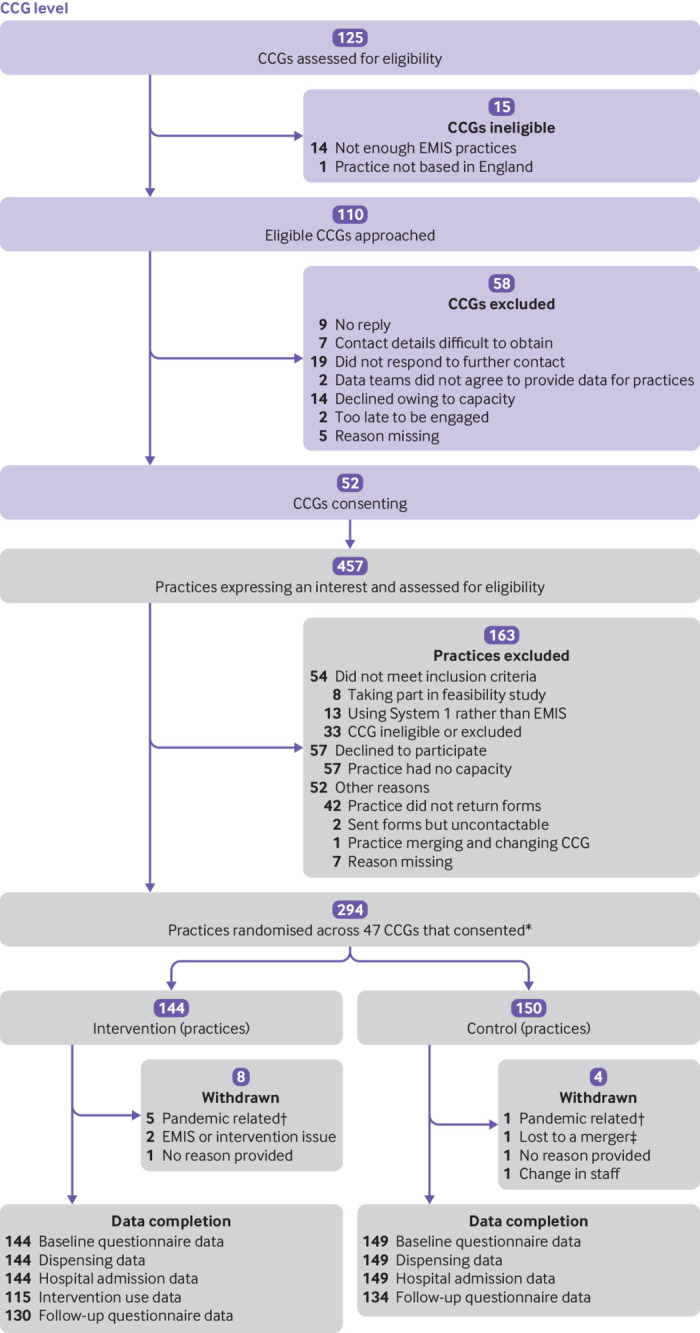
CHICO trial Consolidated Standards of Reporting Trials flowchart. CCG=clinical commissioning group; EMIS=electronic health record system. *One practice randomised was made up of two practices that work closely and were due to merge. †Reasons include lack of staff, lack of resources, and lack of consultations for children with cough. ‡Two practices in control arm merged, so outcome data were compiled into one practice
Baseline characteristics
The two randomised arms were well balanced with respect to baseline characteristics (table 3) and did not meet pre-specified criteria for sensitivity analysis. Of the practices randomised, 62% had a high list size (of 0-9 year old children) relative to other practices in their CCG, suggesting that smaller practices were less likely to be recruited. Similarly, randomised practices had lower baseline dispensing rates and were located in areas of higher socioeconomic deprivation.
Table 3.
Practice level baseline characteristics, by arm. Values are numbers (percentages) unless stated otherwise
| Characteristic | Intervention | Control | |||
|---|---|---|---|---|---|
| No* | Value | No* | Value | ||
| Randomisation variables † | |||||
| High list size (0-9 year olds), relative to others in their CCG | 144 | 92 (64) | 150 | 91 (61) | |
| High dispensing rate, relative to others in their CCG | 144 | 60 (42) | 150 | 66 (44) | |
| Baseline practice level ‡ | |||||
| Median (IQR) list size§: all ages 0-9 | 144 | 975 (701-1422) | 150 | 997 (645-1375) | |
| Median (IQR) list size§: age 0-4 epoch | 144 | 480 (339-682) | 150 | 474 (308-62) | |
| Median (IQR) list size§: age 5-9 epoch | 144 | 485 (349-780) | 150 | 515 (334-723) | |
| Median (IQR) dispensing rate, per 100 list size (0-9 year olds) | 144 | 19.0 (14.3-24.9) | 150 | 17.7 (14.3-23.9) | |
| CHICO leaflet in use at practice | 142 | 12 (8) | 142 | 10 (7) | |
| Median (IQR) distance to nearest children’s emergency department, miles | 142 | 4.7 (2.3-9.0) | 145 | 4.0 (2.2-11.5) | |
| IMD fifth based on practice postcode: | |||||
| 1 (most deprived) | 144 | 37 (26) | 150 | 40 (27) | |
| 2 | 29 (20) | 31 (21) | |||
| 3 | 31 (22) | 29 (19) | |||
| 4 | 28 (19) | 26 (17) | |||
| 5 (least deprived) | 19 (13) | 24 (16) | |||
| Median (IQR) income deprivation affecting children index score | 144 | 0.15 (0.08-0.25) | 150 | 0.15 (0.08-0.26) | |
| Practice staff | |||||
| Median (IQR) No of general practitioners | 143 | 6.0 (4.0-10.0) | 147 | 6.0 (4.0-9.0) | |
| Median (IQR) No of salaried nurses | 114 | 2.0 (1.0-4.0) | 124 | 2.0 (1.0-4.0) | |
| Median (IQR) No of sessional nurses | 68 | 0.0 (0.0-1.0) | 65 | 0.0 (0.0-0.0) | |
| Median (IQR) No of pharmacist prescribers | 89 | 1.0 (0.0-1.0) | 101 | 1.0 (0.0-1.0) | |
| Median (IQR) No of locums | 102 | 4.0 (1.0-6.0) | 101 | 3.0 (2.0-6.0) | |
| Patient management | |||||
| Patient management (not mutually exclusive): | |||||
| No triage | 141 | 35 (25) | 145 | 46 (32) | |
| Nurse face to face | 141 | 17 (12) | 145 | 20 (14) | |
| GP face to face | 141 | 47 (33) | 145 | 47 (32) | |
| Receptionist telephone triage | 141 | 44 (31) | 145 | 48 (33) | |
| Nurse telephone triage | 141 | 17 (12) | 145 | 26 (18) | |
| GP telephone triage | 141 | 74 (52) | 145 | 81 (56) | |
| Other¶ | 141 | 16 (11) | 145 | 20 (14) | |
| Proportion of RTIs consulted over phone: | |||||
| None | 142 | 22 (15) | 146 | 26 (18) | |
| Very few | 35 (25) | 34 (23) | |||
| Some cases | 39 (27) | 40 (27) | |||
| Most cases | 32 (23) | 29 (20) | |||
| Always | 14 (10) | 17 (12) | |||
| Of these, how many are completely dealt with on phone? | |||||
| None | 138 | 25 (18) | 140 | 33 (24) | |
| Very few | 55 (40) | 46 (33) | |||
| Some cases | 44 (32) | 55 (39) | |||
| Most cases | 13 (9) | 5 (4) | |||
| Always | 1 (1) | 1 (1) | |||
CCG=clinical commissioning group; GP=general practitioner; IMD= index of multiple deprivation; IQR=interquartile range; RTI=respiratory tract infection.
Number of practices with data (denominator).
When CCGs accepted participation in trial, previous 12 months of list sizes and dispensing figures (in 0-9 year olds) was used to split practices into high and low categories, relative to other practices within their CCG. This information was used in randomisation minimisation process.
Collected from baseline practice questionnaires unless stated otherwise.
Collected from NHS digital data.
Includes online (askmyGP), emergency care practitioner, and pharmacist.
Intervention usage
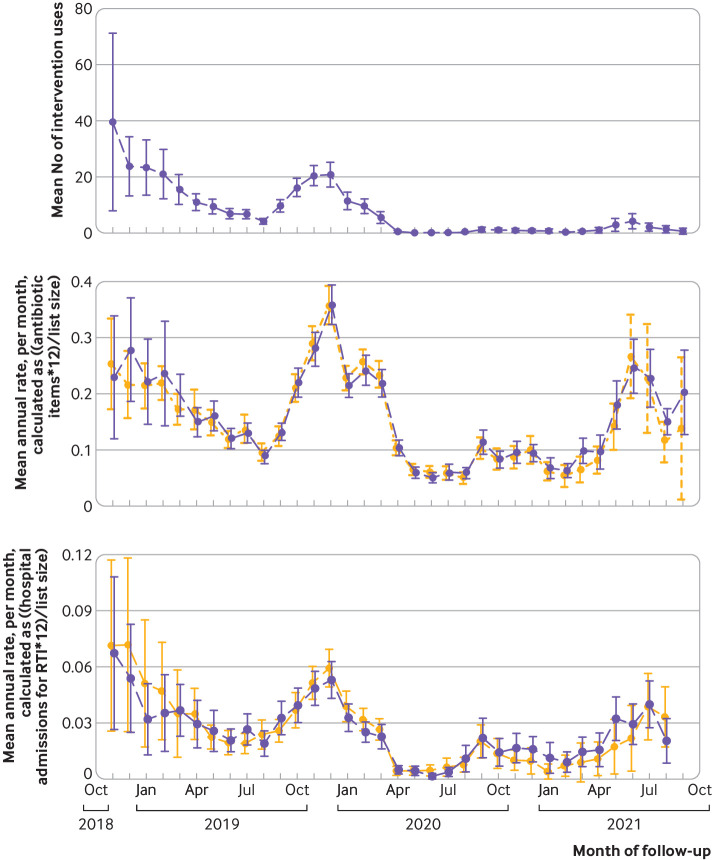
Across the 121 (84%) intervention practices that provided at least one month of intervention usage data, a total of 11 944 intervention uses were started and recorded (practice median 70 (interquartile range 9-142)). Twenty (17%) practices recorded zero usage over the 12 month period, of which 13 provided data wholly within the covid-19 pandemic period. The median number of clinical staff at intervention practices was 13, and the median number of intervention users per practice was 9 (3-16). Of staff whose job title was captured (n=1339), 74% (n=994) were general practitioners, 14% (187) were nurses, 6% (77) were office staff, 3% (40) were clinicians, 3% (34) were locum general practitioners, and 1% (7) were pharmacists. The baseline and follow-up data collection periods spanned October 2017 to September 2021 and thus included the covid-19 pandemic that began in the spring of 2020. Both use of the intervention and antibiotic dispensing data followed the expected seasonal winter peak until the pandemic, during which the intervention usage dramatically fell (table 4) and seasonal patterns disappeared.
Table 4.
Median number of times intervention was used, per month, over 12 months of follow-up
| Practice | Month of follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| All intervention practices (n=115) | 4 | 6 | 4 | 5 | 5 | 2 | 2 | 2 | 0 | 1 | 1 | 0 |
| Pre-covid: completed before March 2020 (n=29) | 22 | 18 | 11 | 7 | 8 | 4 | 6 | 6 | 7 | 11 | 10 | 7 |
| Partial covid: ≥1 month from March 2020 (n=57) | 4 | 9 | 7 | 9 | 10 | 6 | 5 | 2 | 0 | 0 | 0 | 0 |
| All covid: randomised on/after March 2020 (n=29) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Comparison of dispensing rates (co-primary outcome)
The main intention-to-treat analysis (table 5) showed no evidence that the antibiotic dispensing rate in the intervention practices (0.155, 95% confidence interval 0.138 to 0.174) differed from that of the controls (0.157, 0.140 to 0.176), with a rate ratio of 1.011 (95% confidence interval 0.992 to 1.029; P=0.25). On average, this translates into 155 versus 157 items dispensed a year per 1000 children. A rate ratio above 1 signifies that the rate in the intervention practices was higher than in the controls, which may seem to contradict the rates quoted above. Adjustment for baseline dispensing rates caused this change and was influential throughout all analyses.
Table 5.
Primary and sensitivity analyses for CHICO trial dispensing outcome
| Analysis | No (I:C) | Rate* (95% CI) | Adjusted rate ratio (95% CI)† | P value† | |
|---|---|---|---|---|---|
| Intervention (I) | Control (C) | ||||
| Primary analysis | 144:149 | 0.155 (0.138 to 0.174) | 0.157 (0.140 to 0.176) | 1.011 (0.992 to 1.029) | 0.25 |
| Pre-specified sensitivity analyses: | |||||
| Per protocol: excluding non-compliers in intervention arm‡ | 78:149 | 0.162 (0.143 to 0.184) | 0.161 (0.142 to 0.183) | 1.052 (1.029 to 1.076) | <0.001 |
| 0-4 year olds only | 144:149 | 0.225 (0.200 to 0.253) | 0.225 (0.200 to 0.253) | 1.037 (1.014 to 1.060) | 0.001 |
| 5-9 year olds only | 144:149 | 0.093 (0.083 to 0.103) | 0.096 (0.087 to 0.107) | 0.965 (0.935 to 0.997) | 0.03 |
| Worst case scenario: including “age unknown” as aged 0-9 | 144:149 | 0.183 (0.162 to 0.206) | 0.184 (0.163 to 0.208) | 1.002 (0.986 to 1.018) | 0.84 |
| Excluding internal pilot practices (n=48) | 117:128 | 0.150 (0.133 to 0.169) | 0.153 (0.136 to 0.183) | 1.026 (1.006 to 1.048) | 0.012 |
| Adjusting for No of months affected by covid-19 (0-12)§ | 144:149 | 0.155 (0.138 to 0.174) | 0.157 (0.140 to 0.176) | 1.002 (0.984 to 1.021) | 0.79 |
| Post hoc sensitivity analyses: | |||||
| Complier average causal effect analysis¶ | 144:145 | 1.023 (0.897 to 1.166) | 0.74 | ||
| Excluding individual practice months of data from March 2020** | 105:121 | 0.231 (0.208 to 0.257) | 0.249 (0.224 to 0.277) | 0.967 (0.946 to 0.989) | 0.003 |
| Amoxicillin items only (macrolides excluded) | 144:149 | 0.120 (0.107 to 0.136) | 0.124 (0.110 to 0.139) | 0.994 (0.974 to 1.014) | 0.56 |
| Adjusting for random effects at PCN level | 144:149 | 0.150 (0.141 to 0.161) | 0.151 (0.141 to 0.162) | 0.942 (0.916 to 0.969) | <0.001 |
| Adjusting for delayed start (number of months)†† | 144:149 | 0.155 (0.138 to 0.174) | 0.157 (0.140 to 0.176) | 1.043 (1.023 to 1.063) | <0.001 |
CCG=clinical commissioning group; CI=confidence interval; PCN=primary care network.
Rates taken from random effects Poisson regression, incorporating CCG as random effect.
Random effects Poisson regression, adjusting for baseline dispensing rate and incorporating CCG as random effect.
Excluding practices in intervention arm that did not comply with intervention ([number of uses/list size of children]<0.05) or for which compliance was unknown; excludes practice that merged with another practice that had taken part in control arm.
Including numerical variable (0-12) to indicate how many months were affected by covid-19.
Adjusting for baseline rate and number of months affected by covid-19.
Excluding follow-up months affected by covid-19.
Some practices asked to delay their start date and covariate was included to indicate number of months they delayed.
Comparison of dispensing rates in pre-specified sensitivity analyses
We did five pre-specified sensitivity analyses (table 5) for the dispensing rate primary outcome. The per protocol analysis produced strong evidence of increased dispensing in the intervention arm (162 v 161 items a year per 1000 children), although many non-compliant practices joined in the second half of the study, when covid-19 had lowered all dispensing rates leading to a surplus of “low dispensing” practices in the control arm. When we analysed 5-9 year olds only, the dispensing rate was lower in the intervention arm. Conversely, in 0-4 year olds, the dispensing rate was higher in the intervention arm. Other pre-specified sensitivity analyses did not materially change the treatment effect for dispensing rates, including adjusting for the number of months affected by covid-19, assuming “age unknown” was children aged 0-9 years, and excluding the internal pilot practices (n=48).
Comparison of dispensing rates in post hoc sensitivity analyses
We did five post hoc sensitivity analyses (table 5) that were added, largely because of the pandemic. Across all practices the pre-pandemic dispensing rate in the 12 month period before 1 March 2020 was 205 (196 to 215) items per 1000 children, with the usual seasonal patterns (fig 2). In the subsequent 12 months, the dispensing rate halved to 98 (92 to 105) items per 1000 children. We did a complier average causal effect analysis in an attempt to overcome the bias in the per protocol analysis; this produced similar results to the primary outcome. A post hoc sensitivity analysis that excluded any months after March 2020 resulted in a reduced dispensing rate in the intervention arm (table 5). Including the primary care network as the random effects variable, rather than CCG, also led to results that were in favour of the intervention. A delayed start occurred for four practices in the control arm and 36 practices in the intervention arm. Incorporating the number of months delayed as a covariate led to a treatment effect in favour of the control arm. A focus on amoxicillin items only (excluding macrolides) did not materially change dispensing rates.
Fig 2.
Mean and 95% confidence interval of intervention usage (top), dispensing rates (middle), and hospital admission rates (bottom), over course of CHICO trial follow-up period for intervention and control arms. RTI=respiratory tract infection
Comparison of dispensing rates in subgroup analyses
Some pre-defined subgroup analyses did interact with the treatment effect, with evidence of increased dispensing rates in the intervention arm among practices located in areas with a higher level of deprivation (P=0.004), practices with more than one site (P<0.001), and practices with a higher proportion of prescribing nursing staff (P<0.001) (table 6; supplementary figure A). We found some evidence to suggest that practices with more locums had a treatment effect in favour of the intervention (P=0.005), but, as with many of the other subgroups, the magnitude of effect is difficult to measure in analyses that are underpowered. We found no evidence of an interaction when looking at previous dispensing habits or separating practices into those affected or not affected by covid-19. However, given that some practices may have been affected for one month of follow-up and some for 12 months, this did not successfully capture the effect; thus, we included more post hoc sensitivity analyses, as described in the supplementary material.
Table 6.
Subgroup analyses for dispensing outcome
| Variable* | No (I:C) | Rate (95% CI) | Subgroup specific adjusted rate ratio (95% CI)‡ |
P value§ | |
|---|---|---|---|---|---|
| Intervention (I)† | Control (C)† | ||||
| S5. Proportion of locums: | |||||
| <25% | 54:41 | 0.147 (0.129 to 0.168) | 0.157 (0.137 to 0.179) | 0.937 (0.904 to 0.971) | 0.005 |
| ≥25% | 48:58 | 0.156 (0.135 to 0.180) | 0.174 (0.151 to 0.200) | 0.958 (0.925 to 0.993) | |
| S6. Proportion of nurses: | |||||
| <17% | 81:72 | 0.154 (0.137 to 0.173) | 0.165 (0.147 to 0.186) | 0.921 (0.896 to 0.947) | <0.001 |
| ≥17% | 62:74 | 0.149 (0.128 to 0.172) | 0.149 (0.129 to 0.173) | 1.051 (1.021 to 1.081) | |
| S7. Past dispensing rate: | |||||
| <18% | 68:80 | 0.112 (0.098 to 0.129) | 0.110 (0.096 to 0.126) | 1.031 (1.001 to 1.063) | 0.56 |
| ≥18% | 76:69 | 0.182 (0.165 to 0.199) | 0.194 (0.177 to 0.213) | 0.992 (0.967 to 1.018) | |
| S8. Practice site¶: | |||||
| 1 site | 95:90 | 0.149 (0.129 to 0.172) | 0.160 (0.139 to 0.185) | 0.928 (0.903 to 0.954) | <0.001 |
| ≥2 sites | 34:37 | 0.163 (0.141 to 0.188) | 0.158 (0.137 to 0.182) | 1.028 (0.990 to 1.067) | |
| S9. Follow-up completed before covid-19 pandemic: | |||||
| Yes | 31:26 | 0.171 (0.138 to 0.212) | 0.163 (0.131 to 0.202) | 0.994 (0.955 to 1.036) | 0.39 |
| No | 113:123 | 0.148 (0.130 to 0.167) | 0.152 (0.134 to 0.172) | 1.012 (0.991 to 1.035) | |
| S10. Level of deprivation: | |||||
| Low (rank** ≥14 387) | 72:74 | 0.150 (0.134 to 0.167) | 0.155 (0.139 to 0.173) | 0.955 (0.929 to 0.981) | 0.004 |
| High (rank** <14 387) | 72:75 | 0.154 (0.132 to 0.178) | 0.150 (0.129 to 0.174) | 1.061 (1.032 to 1.090) | |
CCG=clinical commissioning group; CI=confidence interval.
All subgroup analyses were pre-specified in statistical analysis plan,20 although outcomes S8-S10 were introduced after trial started.
Dichotomised on basis of median for continuous subgroups.
Rates taken from random effects Poisson regression, incorporating CCG as random effect.
Random effects Poisson regression, adjusting for baseline dispensing rate and incorporating CCG as random effect.
Taken from likelihood ratio test comparing random effects Poisson models with/without interaction term included, treating subgroup of interest as continuous variable where possible.
Taken from practice follow-up questionnaire.
Index of deprivation ranks every neighbourhood in England from 1 (most deprived area) to 32 844 (least deprived area).
Comparison of hospital admission rates (co-primary outcome)
We found no difference in the rate of hospital admissions at 0.013 (0.010 to 0.018) and 0.015 (0.012 to 0.020) for the intervention and control arms, respectively. This translates into 13 or 15 admissions a year per 1000 children, and the rate ratio was 0.952 (0.905 to 1.003). As 1.003 lies below the 1.01 non-inferiority margin we set, the intervention was considered non-inferior. Pre-specified sensitivity analyses that incorporated hospital admissions with “missing diagnosis” did not change these results (supplementary table A). The seasonal winter peak of hospital admissions was absent during the pandemic (fig 2). The secondary outcome of emergency department attendance rates were 0.045 (0.038 to 0.054) and 0.044 (0.037 to 0.052) for the intervention and control arms, respectively. This translates into approximately 49 and 45 attendances a year per 1000 children; the rate ratio was 1.013 (0.980 to 1.047; P=0.44). Pre-specified sensitivity analyses that incorporated “missing diagnosis” admissions and emergency department attendances are shown in the supplementary material.
Economic evaluation
We found no evidence of a difference in mean NHS costs (-£1999, –£6627 to £2630) in practices randomised to use the intervention compared with those that did not. This overall conclusion held under various sensitivity analyses, including a per protocol analysis.
Discussion
This complex intervention, designed for use in pre-pandemic conditions, did not reduce overall dispensing of amoxicillin and macrolide antibiotics for children with respiratory tract infections presenting before and during the covid-19 pandemic. Neither did it increase the rate of hospital admissions for respiratory tract infection. Sensitivity and subgroup analyses showed decreased dispensing rates in the intervention arm for older children, practices restricted to one site, and practices with proportionally fewer nurse practitioners and in less deprived areas, although, given the number of sensitivity analyses carried out, these may have been chance findings. After the introduction of covid-19 restrictions in March 2020, average antibiotic dispensing halved in both study arms until the end of the trial. A post hoc sensitivity analysis suggested possible effectiveness of the intervention before the pandemic, although the magnitude of any effect was difficult to measure.
Strengths and limitations of study
Strengths of the study include widespread geographical recruitment of general practices, rigorous study design and conduct, and near 100% completeness for the primary outcome. Using different recruitment strategies,22 and existing networks such as the clinical research networks, helped us to recruit some research naive practices and gave us practices located in more socioeconomically deprived areas, which mirrors the distribution of practices at the national level. Conducting the trial at the practice level removed the need for recruitment of patients and potential for differential recruitment between arms while focusing the clinician’s time on using the intervention, reflecting real life practice. Dispensed antibiotics is a good measure of antibiotic use in that it reflects parental as well as clinicians’ perception of the need for an antibiotic: “delayed” and “immediate” prescriptions will not be collected by unconvinced parents.
Limitations of the study include that the March 2020 and subsequent covid-19 lockdowns meant a wholesale change from face-to-face to remote consultations in primary care, as well as greatly reduced capacity to support non-covid research. The CHICO intervention was designed for use in face-to-face consultations, and clinicians may have been reluctant to use it remotely. Secondly, recorded intervention use was low in relation to the likely number of 0-9 year old children in whom it could have been used; interviews with clinicians suggested that they often used the tool for “borderline” cases, and usage also dramatically fell after March 2020. We estimated use assuming that the dispensed items were all for respiratory tract infection consultations and that 50% of the children consulting were given these. The dispensing rate in the intervention arm was 15.5 items per 100 children; given that the median number of 0-9 year olds was 975 children in 144 intervention practices, this yields 21 762 dispensed items and 43 524 consultations for respiratory tract infection. This is speculative but would suggest that the 11 944 uses of the intervention was around one in every four consultations. This could have been higher if clinicians were able to determine prognostic risk group by memory. Thirdly, less than half of the eligible CCGs approached took part in the study; the difficulty in contacting some CCGs or lack of response makes assessing what bias this may have introduced difficult, although we did have at least one CCG in each of the 15 clinical research network areas in England. Fourthly, just over a quarter of the practices that expressed an interest and met the eligibility criteria subsequently declined, citing lack of capacity or failing to return forms. The impact of the pandemic may have played a part, but this questions the generalisability of the findings. Fifthly, around 2.5% of practices close or merge each year in England, and the number of multiple site practices was larger than anticipated (28%), substantially increasing the number of registered patients. which has implications for cluster trials and sample size calculations. The contrast in antibiotic dispensing rates between arms in multiple site practices compared with single site practices was notable and questions whether a single practice champion for multiple sites was able to promote intervention use effectively. Sixthly, a further limitation was use of the number of children aged 0-9 registered at the practice as the denominator rather than the number of children consulting for respiratory tract infection. A detailed record of how many children consulted for respiratory tract infection and whether the intervention was used would have been difficult to obtain, but its absence limits our ability to comment on whether the intervention was used as planned or whether a lack of use affected our findings. Finally, general advice is that algorithms should be externally validated before an evaluation of clinical effectiveness. However, this was not possible given the absence of a suitable dataset.
Comparison with other studies
A similar trial randomising general practices to receive a decision support tool embedded in electronic medical records with the aim of reducing antibiotic prescribing for respiratory infections in a wider age range found evidence that the intervention was effective in adults (15-84 years).23 However, as with our study, it did not find evidence of reduced prescribing in children (0-14 years), nor in adults aged >84 years. We speculate that this could be because clinicians and/or parents are more risk averse and unwilling to withhold antibiotic treatment when managing children rather than adults. This study also monitored safety by reporting hospital admissions for serious bacterial infections, finding no evidence that these were increased in the intervention group. The reduction in rates of consultation and antibiotic dispensing that we observed between pre-pandemic and covid-19 pandemic periods is consistent with that reported in several other studies, both in the UK and globally.24 25 26
Implications for clinical practice and future research
We did not find evidence to support the widespread use of the intervention, but our subgroup and sensitivity analyses suggest that the intervention may hold promise in some clinical groups. Our post hoc analyses were underpowered but provided a signal that the intervention held some promise under non-pandemic conditions. Future research should seek to confirm these signals and develop interventions tailored to the clinical needs of nurses, larger sites, younger children, and socioeconomically deprived communities, in post-pandemic conditions.
Conclusions
Embedding a multifaceted intervention into general practice for children presenting with acute cough and respiratory tract infection did not reduce antibiotic dispensing or affect hospital attendance for respiratory tract infections. Remote consulting during the pandemic may have affected the effectiveness of the intervention. More research is needed to confirm potential effects seen in practices with fewer prescribing nurses, restricted to a single site, or located in areas of lower deprivation and in younger children and post-pandemic conditions.
What is already known on this topic
Inappropriate use of antibiotics needlessly contributes to antimicrobial resistance
Children with acute cough and respiratory tract infection, usually with viral aetiology, are the most frequent patient group seen in primary care, with up to half treated with antibiotics
Uncertainty about poor prognosis is an important driver of indiscriminate antimicrobial use in children
What this study adds
The multifaceted intervention investigated included eliciting parental concerns, an algorithm to identify children at very low risk of hospital admission, and safety netting advice for carers
The intervention, designed for pre-pandemic conditions, did not reduce overall antibiotic dispensing or increase respiratory tract infection related hospital admissions
Subgroup and sensitivity analyses suggested that the intervention may be effective for older children and practices with fewer prescribing nurses, on a single site, or located in less deprived areas
Acknowledgments
This study was designed and delivered in collaboration with the Bristol Randomised Trials Collaboration, part of the Bristol Trials Centre, which receives National Institute for Health and Care Research (NIHR) clinical trials unit support funding. The University of Bristol is acting as the sponsor for this trial, and the trial is hosted by the NHS Bristol, North Somerset, and South Gloucestershire Clinical Commissioning Group (CCG). We thank all general practices, clinical commissioning groups, and clinical research networks for their involvement in CHICO. We also to thank the members of the Trial Steering Committee and Data Monitoring Committee.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary materials
Contributors: AH, PSB, PJL, NAF, and JI were responsible for developing the research questions. PSB, AH, JI, PJL, CCabral, CClement, EB, MG, JH, SC, JAL, and NAF were responsible for the study design and collection of data. PS, SB, and JT were responsible for study management and coordination. GY, PD, and CClement were responsible for the analysis of the data. PB drafted the paper and is the guarantor. All authors read, commented on, and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This research is funded by the NIHR Health Technology Assessment programme (funder ref: 16/31/98, ISRCTN11405239). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The funders had no role in considering the study design or in the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the NIHR Health Technology Assessment programme; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; AH is a member of the NIHR Efficacy and Mechanism Evaluation Committee; JAL is a member of a clinical trials unit in receipt of NIHR support funding; no other relationships or activities that could appear to have influenced the submitted work.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Patients and clinicians were involved in the design, conduct, reporting, and dissemination plans of this research. Refer to the Methods section for further details. Participants will be contacted with a summary of the findings when the embargo for the published main findings is lifted.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study received approval by the North of Scotland Research Ethics Committee on 29 October 2018 and by the Health Research Authority at London-Camden and Kings Cross Research Ethics Committee (ref: 18/LO/0345) on 14 November 2018.
Data availability statement
Data may be obtained from a third party (clinical commissioning groups and Public Health England) and are not publicly available.
References
- 1. Priest P, Yudkin P, McNulty C, Mant D. Antibacterial prescribing and antibacterial resistance in English general practice: cross sectional study. BMJ 2001;323:1037-41. 10.1136/bmj.323.7320.1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010;340:c2096. 10.1136/bmj.c2096 [DOI] [PubMed] [Google Scholar]
- 3. Public Health England . English Surveillance programme for antimicrobial utilisation and resistance (ESPAUR). PHE, 2016. [Google Scholar]
- 4. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of Inappropriate Antibiotic Prescriptions Among US Ambulatory Care Visits, 2010-2011. JAMA 2016;315:1864-73. 10.1001/jama.2016.4151 [DOI] [PubMed] [Google Scholar]
- 5. Hay AD, Heron J, Ness A, ALSPAC study team . The prevalence of symptoms and consultations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): a prospective cohort study. Fam Pract 2005;22:367-74. 10.1093/fampra/cmi035 [DOI] [PubMed] [Google Scholar]
- 6. Horwood J, Cabral C, Hay AD, Ingram J. Primary care clinician antibiotic prescribing decisions in consultations for children with RTIs: a qualitative interview study. Br J Gen Pract 2016;66:e207-13. 10.3399/bjgp16X683821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bou-Antoun S, Costelloe C, Honeyford K, et al. Age-related decline in antibiotic prescribing for uncomplicated respiratory tract infections in primary care in England following the introduction of a national financial incentive (the Quality Premium) for health commissioners to reduce use of antibiotics in the community: an interrupted time series analysis. J Antimicrob Chemother 2018;73:2883-92. 10.1093/jac/dky237 [DOI] [PubMed] [Google Scholar]
- 8. Lucas PJ, Cabral C, Hay AD, Horwood J. A systematic review of parent and clinician views and perceptions that influence prescribing decisions in relation to acute childhood infections in primary care. Scand J Prim Health Care 2015;33:11-20. 10.3109/02813432.2015.1001942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cabral C, Lucas PJ, Ingram J, Hay AD, Horwood J. “It’s safer to …” parent consulting and clinician antibiotic prescribing decisions for children with respiratory tract infections: An analysis across four qualitative studies. Soc Sci Med 2015;136-137:156-64. 10.1016/j.socscimed.2015.05.027 [DOI] [PubMed] [Google Scholar]
- 10. Ingram J, Cabral C, Hay AD, Lucas PJ, Horwood J, TARGET team . Parents’ information needs, self-efficacy and influences on consulting for childhood respiratory tract infections: a qualitative study. BMC Fam Pract 2013;14:106. 10.1186/1471-2296-14-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hay AD, Redmond NM, Turnbull S, et al. Development and internal validation of a clinical rule to improve antibiotic use in children presenting to primary care with acute respiratory tract infection and cough: a prognostic cohort study. Lancet Respir Med 2016;4:902-10. 10.1016/S2213-2600(16)30223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lucas PJ, Ingram J, Redmond NM, Cabral C, Turnbull SL, Hay AD. Development of an intervention to reduce antibiotic use for childhood coughs in UK primary care using critical synthesis of multi-method research. BMC Med Res Methodol 2017;17:175. 10.1186/s12874-017-0455-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skivington K, Matthews L, Simpson SA, et al. A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance. BMJ 2021;374:n2061. 10.1136/bmj.n2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blair PS, Turnbull S, Ingram J, et al. Feasibility cluster randomised controlled trial of a within-consultation intervention to reduce antibiotic prescribing for children presenting to primary care with acute respiratory tract infection and cough. BMJ Open 2017;7:e014506. 10.1136/bmjopen-2016-014506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blair PS, Ingram J, Clement C, et al. Can primary care research be conducted more efficiently using routinely reported practice-level data: a cluster randomised controlled trial conducted in England? BMJ Open 2022;12:e061574. 10.1136/bmjopen-2022-061574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Seume P, Bevan S, Young G, et al. Protocol for an ‘efficient design’ cluster randomised controlled trial to evaluate a complex intervention to improve antibiotic prescribing for CHIldren presenting to primary care with acute COugh and respiratory tract infection: the CHICO study. BMJ Open 2021;11:e041769. 10.1136/bmjopen-2020-041769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kontopantelis E, Stevens RJ, Helms PJ, Edwards D, Doran T, Ashcroft DM. Spatial distribution of clinical computer systems in primary care in England in 2016 and implications for primary care electronic medical record databases: a cross-sectional population study. BMJ Open 2018;8:e020738. 10.1136/bmjopen-2017-020738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabral C, Ingram J, Redmond N, et al. Caring for children with coughs: Information for parents. 2016. http://child-cough.bristol.ac.uk.
- 19. NHS Business Services Authority . ePACT”. https://www.nhsbsa.nhs.uk/epact2.
- 20.Young G, Dixon PC, Clement C, Seume P, Blair PS. CHICO The CHIldren with COugh study Statistical, Health Economic and Qualitative Analysis Plan. 2021. https://research-information.bris.ac.uk/ws/portalfiles/portal/278667871/CHICO_SAP_draft_v1.0_26.05.21_SIGNED.pdf.pdf.
- 21.Ministry of Housing. Communities & Local Government. 2019. English indices of deprivation 2019. https://imd-by-postcode.opendatacommunities.org/imd/2019.
- 22. Ellis SD, Bertoni AG, Bonds DE, et al. Value of recruitment strategies used in a primary care practice-based trial. Contemp Clin Trials 2007;28:258-67. 10.1016/j.cct.2006.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gulliford MC, Prevost AT, Charlton J, et al. Effectiveness and safety of electronically delivered prescribing feedback and decision support on antibiotic use for respiratory illness in primary care: REDUCE cluster randomised trial. BMJ 2019;364:l236. 10.1136/bmj.l236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrews A, Bou-Antoun S, Guy R, Brown CS, Hopkins S, Gerver S. Respiratory antibacterial prescribing in primary care and the COVID-19 pandemic in England, winter season 2020-21. J Antimicrob Chemother 2022;77:799-802. 10.1093/jac/dkab443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khouja T, Mitsantisuk K, Tadrous M, Suda KJ. Global consumption of antimicrobials: impact of the WHO Global Action Plan on Antimicrobial Resistance and 2019 coronavirus pandemic (COVID-19). J Antimicrob Chemother 2022;77:1491-9. 10.1093/jac/dkac028 [DOI] [PubMed] [Google Scholar]
- 26. Rezel-Potts E, L’Esperance V, Gulliford MC. Antimicrobial stewardship in the UK during the COVID-19 pandemic: a population-based cohort study and interrupted time-series analysis. Br J Gen Pract 2021;71:e331-8. 10.3399/BJGP.2020.1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary materials
Data Availability Statement
Data may be obtained from a third party (clinical commissioning groups and Public Health England) and are not publicly available.