Abstract
Background:
Pancreatic adenocarcinoma (PDAC) remains a refractory disease, however, modern cytotoxic chemotherapeutics can induce tumor regression and extend life. We previously developed a blood-based, pharmacogenomic, ChemoSensitivity Assay using gene expression profiling of circulating tumor and invasive cells (CTICs) to predict treatment response.1 FOLFIRINOX and gemcitabine/nab-paclitaxel (G/nab-P) are established frontline approaches for treating advanced PDAC, however, there are no validated biomarkers for treatment selection. A similar unmet need exists for choosing second-line therapy.
Methods:
The ChemoSensitivity Assay was evaluated in metastatic PDAC patients presenting for frontline treatment. A prospective study enrolled patients (n=70) prior to receiving either FOLFIRINOX or G/nab-P at a 1:1 ratio. Six mL of peripheral blood was collected at baseline and at time of disease progression. CTICs were isolated, gene-expression profiling was performed and the Assay was used to predict effective and ineffective chemotherapeutic agents. Treating physicians were blinded to the Assay prediction results.
Results:
Patients receiving an effective regimen as predicted by the ChemoSensitivity Assay experienced significantly longer mPFS (7.8 mo v 4.2 mo, HR = 0.35, p = 0.0002) and mOS (21.0 mo v 9.7 mo, HR = 0.40, p = 0.005), compared with an ineffective regimen. Assay prediction for effective second-line therapy was explored. The entire study cohort experienced favorable outcomes compared with historical controls, 7.1 month median progression-free survival (mPFS) and 12.3 month median overall survival (mOS).
Conclusions:
ChemoSensitivity Assay profiling is a promising tool for guiding therapy in advanced PDAC. Further prospective validation is underway (ClinicalTrials.gov NCT03033927).
Keywords: Gene expression modeling, circulating tumor and invasive cells, pancreatic cancer, chemotherapy, personalized medicine
Precis:
We present results from a validation study of an innovative pharmacogenomic tool to predict effective chemotherapeutic drug therapy for patients with advanced pancreatic cancer based on profiling of circulating tumor and invasive cells isolated from peripheral blood. We show that a simple blood test can guide an individualized approach to treating pancreatic cancer, leading to improved survival across the two standard frontline chemotherapy regimens.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) currently represents the 2nd leading cause of cancer mortality in the U.S.1 Combination chemotherapy regimens such as 5-fluorouracil (5-FU), leucovorin (LV), irinotecan, oxaliplatin (FOLFIRINOX)2 and gemcitabine/nab-paclitaxel (G/nab-P)3 have become established and active frontline treatment options. Efforts to develop targeted and immune therapies have had limited impact, punctuated by a spate of negative randomized phase III trials.4–7 Although the PARP inhibitor olaparib benefits a small subset of patients harboring germline BRCA-pathway mutations,8 for the vast majority of patients, cytotoxic chemotherapy remains the mainstay of treatment. For individual patients, we are without tools to choose effective cytotoxic drugs and regimens. To address this significant unmet need, we have developed a blood-based assay using gene expression profiling of circulating tumor and invasive cells, termed ChemoSensitivity Assay. The Assay, as previously described,9 was developed based upon the connectivity mapping concept10 which hypothesizes that diverse biological systems can share key biological properties, in our case, drug response, which can be described by similar gene expression profiles. This concept has been validated for predicting drug response in other malignancies.11 Our Assay is based on the premise that gene expression pathways relevant to drug response are independent of, and more predictive than, tissue of origin. Our Assay was originally developed using the NCI-60 cell line panel,9 and our approach builds upon prior work using this cell line panel to model drug sensitivity based on common genes and pathways across tumor types. Scherf and colleagues demonstrated that profiling entirely different gene sets were required for and capable of segregating NCI-60 cell lines based upon drug response as opposed to tissue of origin.12 We applied our model in a recent study which provided proof of principle that the Assay is capable of predicting response in advanced PDAC patients receiving G/nab-P chemotherapy.9
In addition to applying an innovative gene expression algorithm, the ChemoSensitivity Assay leverages profiling of circulating tumor and invasive cells, which has distinct advantages over profiling tumor tissue. Tumor and related cells in circulation likely represent cancer cells in transit with high metastatic potential. Peripheral blood sampling can be performed serially during the course of treatment with greater ease than tumor sampling. Cells from peripheral blood are captured using a well-studied and reliable collagen matrix invasion platform. This approach has been shown to successfully isolate classical circulating tumor cells (CTCs) across a wide variety of malignancies, including breast13 and prostate14 cancers. Cells with tumorigenic properties (CD45−, EpCAM+ESA+cytokeratin+, and ability to degrade and ingest collagenous matrices) are captured,13–15 however, not all captured cells express these markers typical of classical tumor cells. Thus, we have coined this population of cells circulating tumor and invasive cells (CTICs).
In light of our prior work, we conducted a prospective trial to further evaluate the predictive value of the ChemoSensitivity Assay in patients presenting with advanced PDAC. The primary objective was to determine the median progression-free survival (mPFS) in patients receiving standard frontline chemotherapy predicted by the Assay to be effective versus ineffective. Other objectives included median overall survival (mOS) and Assay performance in the second-line.
MATERIALS AND METHODS
ChemoSensitivity Assay
The ChemoSensitivity Assay accurately measures the gene expression profile of 95 genes by quantitating mRNA levels for each gene in circulating tumor and invasive cells isolated from whole blood (6 mL). Seven chemotherapeutic agents chosen for modeling were gemcitabine, nab-paclitaxel, 5-FU, oxaliplatin, irinotecan, mitomycin C, and cisplatin. Gene expression templates were created for each of these chemotherapeutic agents using cell line expression patterns of NCI-60 cell lines, each line chosen based on sensitivity to one of these seven drugs. Gene expression data for the entire NCI-60 panel was generated using the Human Genome U133 Plus 2.0 Array (Affymetrix, Santa Clara, CA, USA) and are publicly available (NCBI Gene Expression Omnibus). From this set of over 40,000 transcripts, the current model uses 95 drug transport genes as classifiers in the algorithm (see Figure 1).
Figure 1.
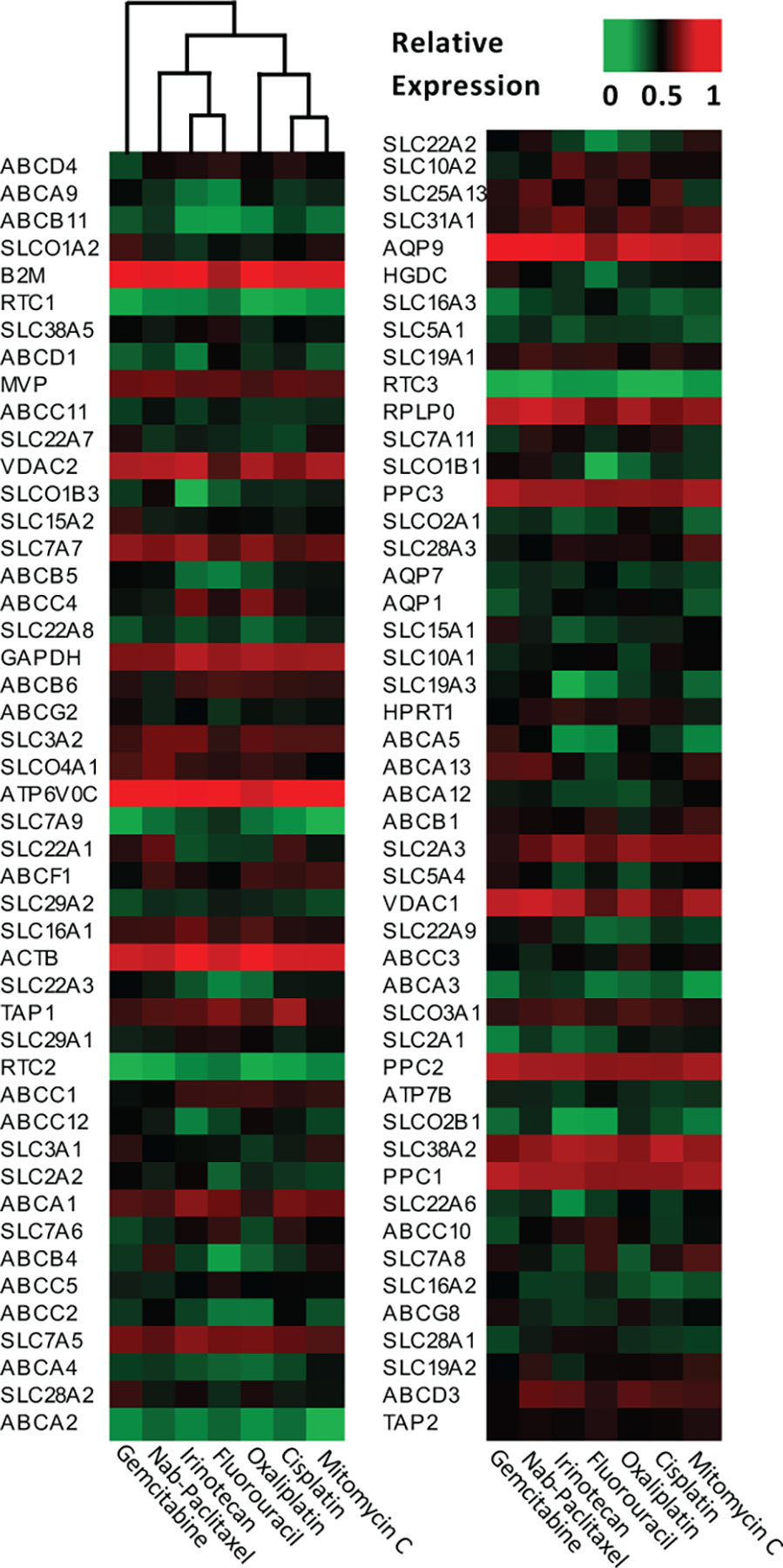
Gene expression templates for modeling of seven commonly used chemotherapy agents.
Nearest Template Prediction
To match patient blood samples to cell line derived drug sensitivity templates, Adera Biolabs utilized nearest template prediction analysis (previously described in References (33–35)) with its underlying premise that if two samples share similar expression profiles of key, relevant genes, they will have similar drug responses. A detailed description and earlier validation studies have been previously described.(9) Nearest template prediction methodology was used to match chemotherapeutic templates to the profile derived from individual patient blood samples and sort the templates in ranked-order to obtain sensitivity values for each of the 7 chemotherapeutic agents (Adera Biolabs, Germantown MD).
In the frontline setting, samples were classified as “sensitive” if patients were treated with the regimen, either FOLFIRINOX or G/nab-P, containing the single, highest scoring drug, otherwise samples were classified as “resistant”. In case of a tie, classification was “intermediate”. In the second-line setting, the same principle was used, with the patients receiving either 5-FU or gemcitabine based regimens, either as a single agent or as part of combination regimens.
Cell Enrichment
Coded and deidentified samples were shipped at 4 °C overnight to Adera Biolabs (Germantown, MD, USA) for CTIC isolation and enrichment. A collagen adhesion matrix (CAM) in a modified cell invasion assay was used to capture EPCAM+ invasive cells, a well-characterized approach for capturing CTCs.(13,14,21,36) 1.5 mL aliquots of whole blood were incubated with collagen-coated microcarriers (Pall Corporation, Port Washington, NY, USA) and cultured for 2 h in Dulbecco’s modified Eagle’s medium with F12 supplemented with 10% calf serum, 5% Nu-serum, 1 unit/mL penicillin, and 10 μg/mL streptomycin). Captured cells were then washed and lysed in situ.
qPCR and Expression Analysis
Lysed CTICs directly isolated from the invasion assay were subjected to qPCR analysis as previously reported.(9) Specifically, total RNA from lysed cells was purified by RNeasy Mini Kit (Qiagen, Valencia, CA, USA), cDNA was synthesized (Ovation Pico SL, Nugen Technologies, San Carlos, CA, USA) and then subjected to qPCR analysis. Gene expression of our 95 gene panel was measured by qPCR and processed at standard thermal cycling rates using standard SYBR Green and ROX Mastermix (Adera Biolabs, Germantown, MD, USA). Arrays with >5% error rates were discarded. Assay reliability is regulated and assessed in accordance with CLIA-certified mandates for molecular analysis controls and sample consistency.
Clinical Trial Design
A single-institution, prospective clinical trial was conducted (ClinicalTrials.gov Identifier NCT03033927). A total of 70 patients with metastatic, AJCC stage IV PDAC were enrolled. Patients planning on receiving frontline chemotherapy treatment with either FOLFIRINOX or G/nab-P were enrolled at a 1:1 FOLFIRINOX:G/nab-P ratio. All research was performed in accordance with MSKCC institutional guidelines/regulations, and informed consent was obtained from all participants and/or their legal guardians. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was fully reviewed and approved by the MSKCC Institutional Review Board (IRB) and its Ethics Committee. Patients were accrued between February 13th, 2017 and November 26th, 2019. Data cutoff for events was February 7th, 2021. The primary objective of the study was mPFS in patients with effective versus ineffective chemotherapy based on the ChemoSensitivity Assay. The secondary objective was mOS across these two groups. Exploratory objectives included second-line mPFS and mOS across these two groups. Key eligibility criteria included: histological or cytological confirmation of PDAC, radiographic confirmation of American Joint Committee on Cancer (AJCC) stage IV disease, planned treatment with FOLFIRINOX or G/nab-P and an Eastern Cooperative Oncology Group (ECOG) performance status of 0, 1 or 2.
Following written informed consent and prior to initiation of chemotherapy treatment, a 6 mL blood sample was obtained in a sodium-heparinized Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ) from each study participant using standard clinical procedures. Blood samples were collected at baseline and at the time of disease progression for ChemoSensitivity Assay analysis.
Statistical Analysis
The study was designed to accrue a total sample size of 80 patients requiring 69 events to determine a 3.5 difference in mPFS between effective vs ineffective group (7.5 months vs 4 months, HR=0.54) with 80% power and a one-sided type I error of 0.05. 10 patients were consented to the study but not included in the analysis due to: not receiving a standard regimen (2), no treatment and/or follow-up (4), non-PDAC malignancy (2) and no evidence of cancer at study enrollment (2). PFS and OS were calculated from date of frontline treatment until date of disease progression or death whichever occurred first (for PFS) or date of last follow (for OS) and estimated using the Kaplan-Meier method. To control for potential underlying differences due to the chemotherapy regimen received (FOLFIRINOX vs G/nab-P), a stratified Cox regression model was used to compare PFS of sensitive vs resistant patients, stratifying by regimen. Disease response was assessed by RECIST 1.1 criteria. Disease response defined as CR+PR was estimated using binomial proportions along with two-sided exact 95% confidence intervals (CI). Among the subset of patients treated with second-line therapy, OS and PFS were calculated from the date of second-line therapy and estimated using the Kaplan-Meier method. Patients who have not progressed, or were lost to follow-up were not captured. As a result, the included analysis was limited to patients receiving G/nab-P as the sample size for patients receiving FOLFIRINOX was limited. A linear regression model was created to study the relationship between change in predicted drug sensitivity versus time on frontline. For each patient, the time on frontline therapy (Δt) is plotted on the x-axis. For each drug profiled, the change in the ChemoSensitivity Assay result between baseline and at the time of progression (Δs) was plotted on the y-axis. A positive Δs predicts increased drug sensitivity; a negative Δs predicts decreased drug sensitivity. A linear regression model was created, Δs = a + βΔt, where Δs is the response variable and the Δt is the independent variable. Linear model fit is shown in the graphs as a solid line with y-axis = Δs and x-axis = Δt. The shaded area represents the 95% confidence interval of the regression estimate.
Analyses were carried out using SAS Version 9.4 and R (4.0.4). One-sided confidence bounds were used to estimate mPFS according to ChemoSensivity status and a one-sided test was employed to compare PFS distributions between effective and ineffective regimens. Two-sided tests were used on all others secondary and exploratory objecives. P-values of 0.05 were indicative of statistical significance.
RESULTS
Prospective Clinical Trial
All key patient characteristics analyzed were well balanced across treatment cohorts (see Table 1). The median time of follow-up among surviving patients (n=16) at the time of data lock was 19.4 (range, 8.6 to 42.3) months. For the cohort as a whole, mPFS was 7.1 (95%CI, 5.5 to 7.7) months and mOS was 12.3 (95%CI, 9.7 to 19.8) months. The ChemoSensitivity Assay classified patients as follows: 34 (49%) sensitive, 12 (17%) intermediate and 24 (34%) as resistant. Groups classified by the Assay were well balanced with regards to regimen administered, key patient and disease characteristics (see Table 2). In 23/58 (39.7%) of patients classified as sensitive to one regimen, sensitivity to one or more drugs in the opposing regimen was predicted, although to a lesser extent.
Table 1.
Patient demographics.
| Characteristics | FOLFIRINOX | GA | Total |
|---|---|---|---|
|
| |||
| Total, N (%) | 34 (49) | 36 (51) | 70 (100) |
| Age, Y, median | 64 | 65 | 65 |
| Gender, N (%) | |||
| Male | 16 (47) | 21 (58) | 37 (53) |
| Female | 18 (53) | 15 (42) | 33 (47) |
| Race, N (%) | |||
| White | 30 (88) | 28 (78) | 58 (83) |
| Black | 2 (6) | 2 (6) | 4 (6) |
| Asian | 0 (0) | 5 (14) | 5 (7) |
| Other/unknown | 2 (6) | 1 (3) | 3 (4) |
| ECOG PS, N (%) | |||
| 0 | 12 (35) | 11 (31) | 23 (33) |
| 1 | 22 (65) | 24 (67) | 46 (66) |
| 2 | 0 (0) | 1 (3) | 1 (1) |
| Primary site | |||
| Head | 10 (29) | 12 (33) | 22 (31) |
| Body | 8 (24) | 14 (39) | 22 (31) |
| Tail | 16 (47) | 10 (28) | 26 (37) |
| Metastasis site | |||
| Liver | 27 (79) | 27 (75) | 54 (77) |
| Peritoneum | 13 (38) | 16 (44) | 29 (41) |
| Lung | 4 (12) | 7 (19) | 11 (16) |
| Other | 1 (3) | 3 (8) | 4 (6) |
| Number of metastatic sites | |||
| 1 | 24 (71) | 22 (61) | 46 (66) |
| 2 | 9 (26) | 11 (31) | 20 (29) |
| 3 | 1 (3) | 3 (8) | 4 (6) |
Table 2.
Key patient characteristics by ChemoSensitivity Assay result.
| ChemoSensitivity Assay Result | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Sensitive | Intermediate | Resistant | Total | ||||
|
| ||||||||
| Total, N (%) | 34 | (49) | 12 | (17) | 24 | (34) | 70 | (100) |
| Age, Y, median | 62 | 60 | 70 | 65 | ||||
| Gender, N (%) | ||||||||
| Male | 20 | (59) | 6 | (50) | 11 | (46) | 37 | (53) |
| Female | 14 | (41) | 6 | (50) | 13 | (54) | 33 | (47) |
| Race, N (%) | ||||||||
| White | 29 | (85) | 10 | (83) | 19 | (79) | 58 | (83) |
| Black | 1 | (3) | 0 | (0) | 3 | (13) | 4 | (6) |
| Asian | 2 | (6) | 1 | (8) | 2 | (8) | 5 | (7) |
| Other/unknown | 2 | (6) | 1 | (8) | 0 | (0) | 3 | (4) |
| ECOG PS, N (%) | ||||||||
| 0 | 15 | (44) | 4 | (33) | 4 | (17) | 23 | (33) |
| 1 | 19 | (56) | 7 | (58) | 20 | (83) | 46 | (66) |
| 2 | 0 | (0) | 1 | (8) | 0 | (0) | 1 | (1) |
| Primary site | ||||||||
| Head | 10 | (29) | 3 | (25) | 9 | (38) | 22 | (31) |
| Body | 11 | (32) | 3 | (25) | 8 | (33) | 22 | (31) |
| Tail | 13 | (38) | 6 | (50) | 7 | (29) | 26 | (37) |
| Metastasis site | ||||||||
| Liver | 24 | (71) | 10 | (83) | 20 | (83) | 54 | (77) |
| Peritoneum | 15 | (44) | 5 | (42) | 9 | (38) | 29 | (41) |
| Lung | 4 | (12) | 2 | (17) | 5 | (21) | 11 | (16) |
| Other | 0 | (0) | 1 | (8) | 3 | (13) | 4 | (6) |
| Number of metastatic sites | ||||||||
| 1 | 26 | (76) | 7 | (58) | 13 | (54) | 46 | (66) |
| 2 | 7 | (21) | 4 | (33) | 9 | (38) | 20 | (29) |
| 3 | 1 | (3) | 1 | (8) | 2 | (8) | 4 | (6) |
| Regimen | ||||||||
| FOLFIRINOX | 15 | (44) | 7 | (21) | 12 | (35) | 34 | (49) |
| G/nab-P | 19 | (53) | 5 | (14) | 12 | (33) | 36 | (51) |
ChemoSensitivity Assay Predicts Survival
Progression-free Survival (PFS)
Patients whose tumors were predicted to be sensitive to the chemotherapy regimen received experienced a mPFS of 7.8 months (one-sided 95% lower bound: 6.5 months) compared with only 4.2 months (one-sided 95% lower bound: 2.6 months) for the resistant cohort (unadjusted HR: 0.35, 95% one-sided upper bound: 0.58, p = 0.0002). This association remained significant after the analysis was stratified by the chemotherapy regimen administered (adjusted HR: 0.40, 95% one-sided upper bound: 0.75, p = 0.001, Figure 2A). Patients in the intermediate group experienced a median PFS in between, 7.1 months (one-sided 95% lower bound: 3.0 months). Comparison of mPFS for the three ChemoSensitivity groups is presented in Figure S1A.
Figure 2.
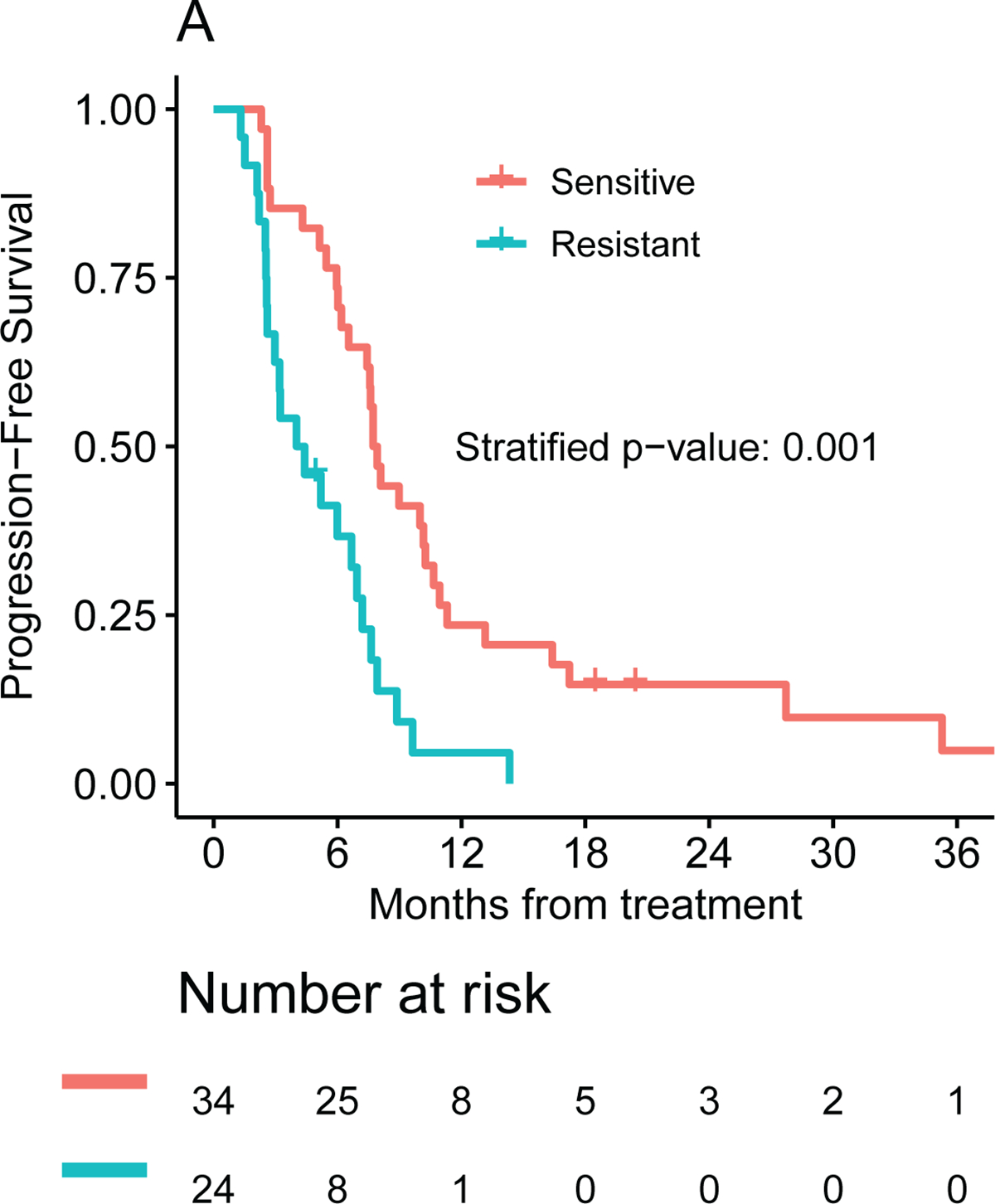
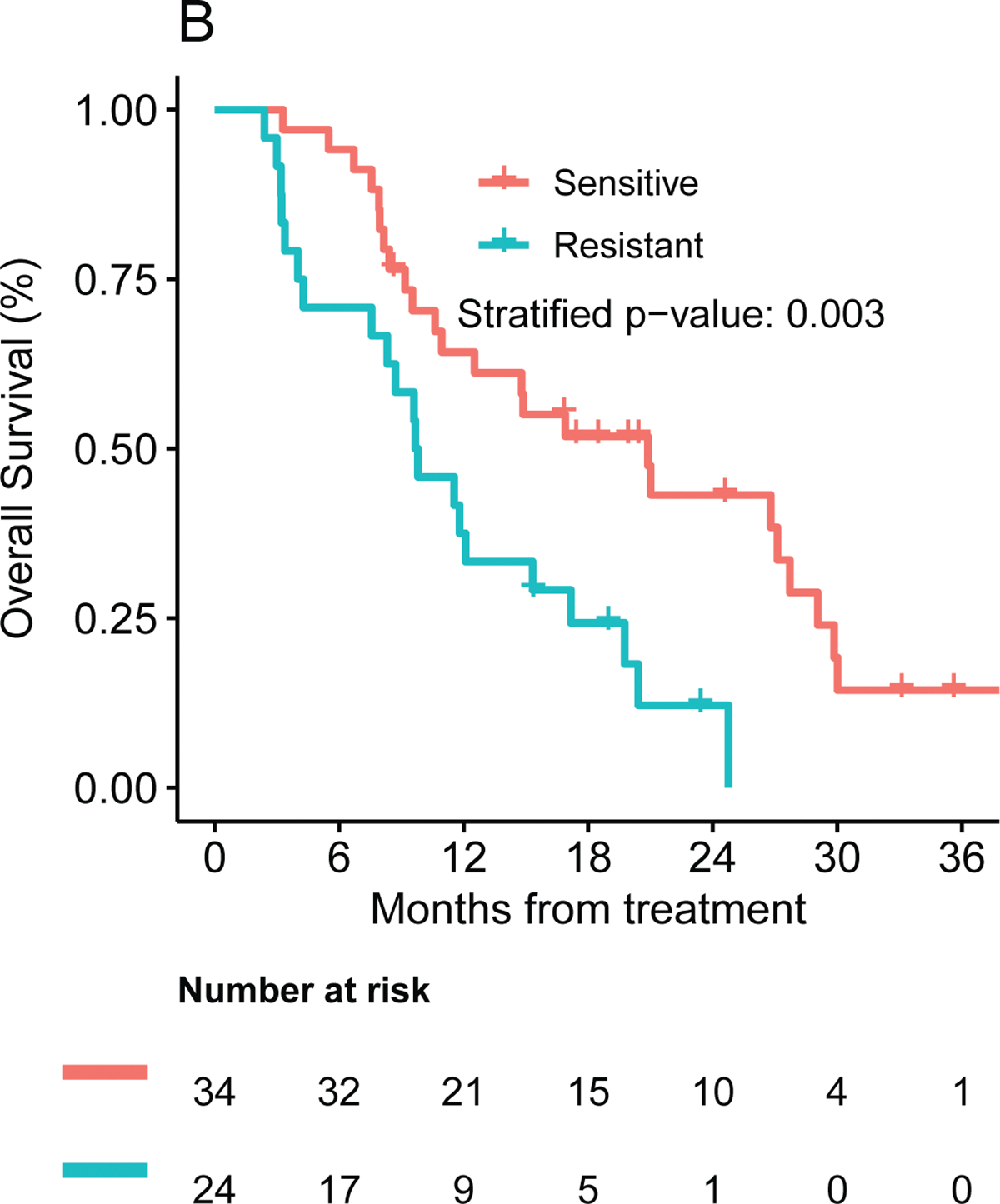
Frontline median (A) PFS and (B) OS by ChemoSensitivity Assay result.
No difference in median PFS was seen between patients treated with FOLFIRINOX versus G/nab-P, 7.3 (95% CI: 4.4 to 7.9) months and 6.7 (95% CI: 4.1 to 7.9) months. As an exploratory aim, performance of the ChemoSensitivity Assay was analyzed by regimen administered. Median PFS for the sensitive, intermediate and resistant cohorts was 7.9 (95% CI: 5.9 to 10.2), 4.1 (95% CI: 2.3 to not reached) and 3.6 months (95% CI: 1.5 to 7.6) months for patients receiving G/nab-P, and 7.7 (95% CI: 2.6 to 27.7), 7.8 (95% CI: 2.9 to not reached) and 4.8 (95% CI: 2.5 to 7.2) months for patients receiving FOLFIRINOX (Figure S2).
Overall Survival (OS)
Patients whose tumors were predicted to be sensitive to the treatment received experienced a mOS of 21.0 (95% CI: 10.6 to 27.2) months compared with only 9.7 (95% CI: 4.3 to 15.3) months for the resistant cohort (p = 0.002, HR = 0.42, 95% CI: 0.22 to 0.81). The benefit in OS for the sensitive compared to the resistant group remained statistically significant after stratifying by the chemotherapy regimen administered (adjusted HR: 0.38 (95% CI: 0.20–0.72), p=0.003, Figure 2B). The intermediate group experiencing an intermediate mOS of 11.3 (95% CI: 3.88 to not reached) months (see Figure S1B). Median OS for the FOLFIRINOX and G/nab-P cohorts were 16.9 (95% CI: 9.8 to 27.7) and 10.6 (95% CI: 8.7 to 17.2) months, respectively; this difference does not meet statistical significance. The median OS for the sensitive, intermediate and resistant cohorts was 14.8 (95% CI: 9.2 to 27.1), 9.6 (95% CI: 7.13 to not reached), and 9.2 (95%CI: 3.2 to 17.2) months for patients receiving G/nab-P, and 26.8 (95% CI: 7.6 to not reached), 12.3 (95%CI: 3.71 to not reached), and 11.7 (95%CI: 3.0 to not reached) months for patients receiving FOLFIRINOX (Figure S2). Beyond survival endpoints, treatment response was analyzed; there appeared to be an improvement in disease response (defined as CR+PR) comparing patients in the sensitive vs resistant cohorts, 53% (95% CI: 35%-70%) vs 30% (13%-51%, p=0.072).
Longitudinal Testing Predictive for Second-line Therapy
ChemoSensitivity Assay profiles performed at baseline and at the time of disease progression were compared to characterize longitudinal changes for individual patients. For individual drugs, the change in predicted sensitivity was correlated with time on therapy. For patients treated with G/nab-P, linear regression analysis found a negative correlation between time on therapy and change in drug sensitivity for both gemcitabine (Figure S3, panel A) and nab-paclitaxel (panel B). By contrast, there was a positive correlation for drugs not used as part of frontline therapy, such as 5-FU (panel C) and irinotecan (panel D). Though exploratory, these results suggest that predicted drug sensitivity changes from baseline to time of disease progression based on both time on frontline therapy and whether the profiled drug was used as part of the frontline therapy.
The ChemoSensitivity Assay was also characterized for predicting treatment response in the second line. Thirty-four of the 70 study participants were profiled at the time of disease progression and went on to receive second-line therapy at the time of the analysis. Nineteen of the 34 (56%) patients were predicted to be sensitive to the chemotherapy regimen received, 15 (44%) patients were predicted to be resistant. Second-line therapies administered were FOLFIRI or 5-FU/LV/nanoliposomal irinotecan (12, 35%), FOLFIRINOX (10, 29%), G/nab-P (10, 29%) and 5-FU alone (2, 6%). The study was not powered to evaluate differences in outcomes based on the Assay for second-line therapy. The median PFS for the sensitive cohort from the beginning of second line therapy was 5.1 (95% CI: 2.3–11.2) months versus 2.7 (95% CI: 1.2 to 6.3) months in the resistant cohort (p = 0.14, see Figure 3A). Overall survival for the sensitive and resistant cohorts was 16.2 (95% CI: 5.1 to 22.0) months and 7.3 (95% CI: 3.2 to 10.8) months, respectively (see Figure 3B). As more study participants move from first-line to second-line therapy, we may be able to more definitively address the utility of the ChemoSensitivity Assay for predicting effective second-line therapy.
Figure 3.
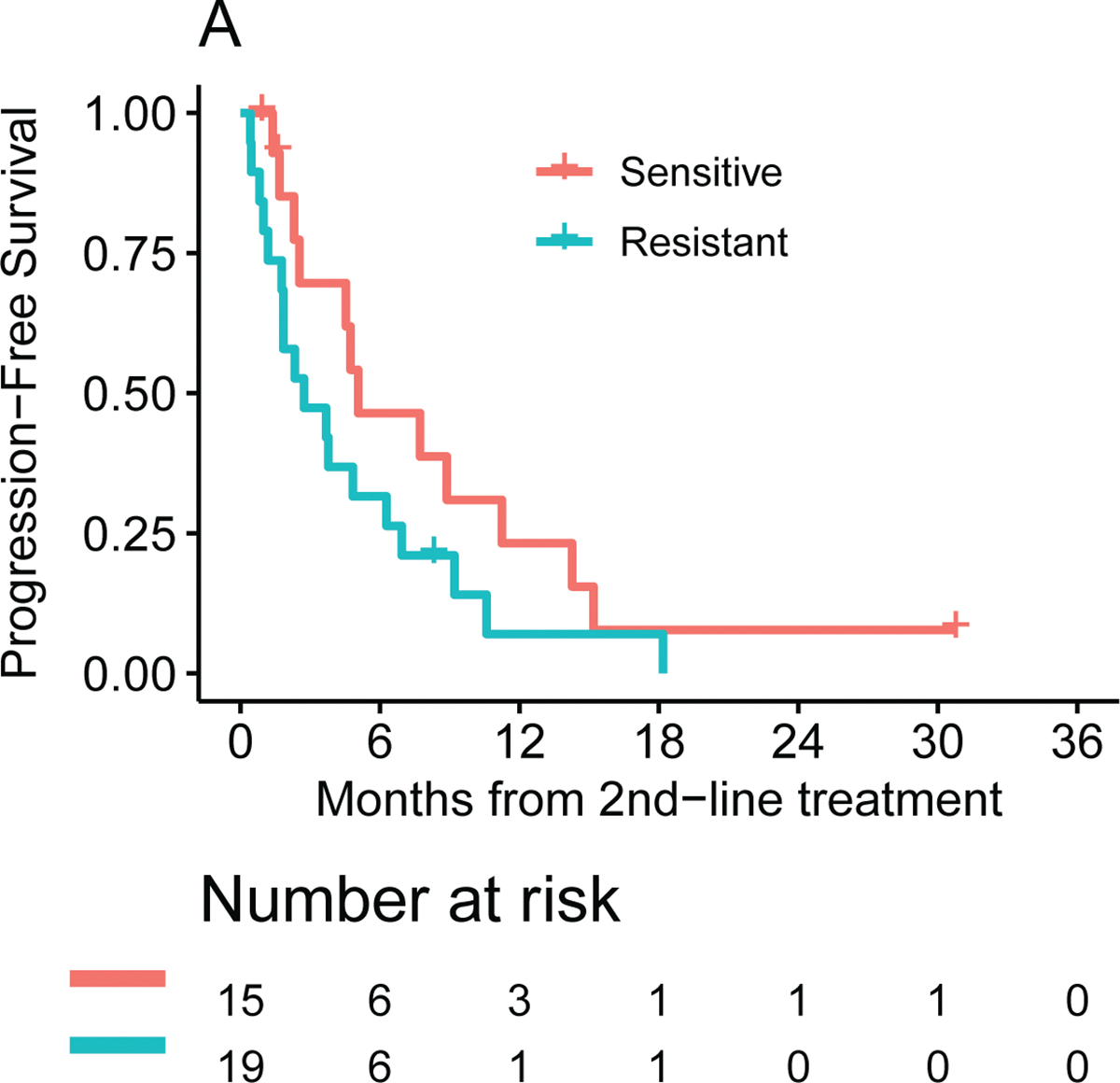
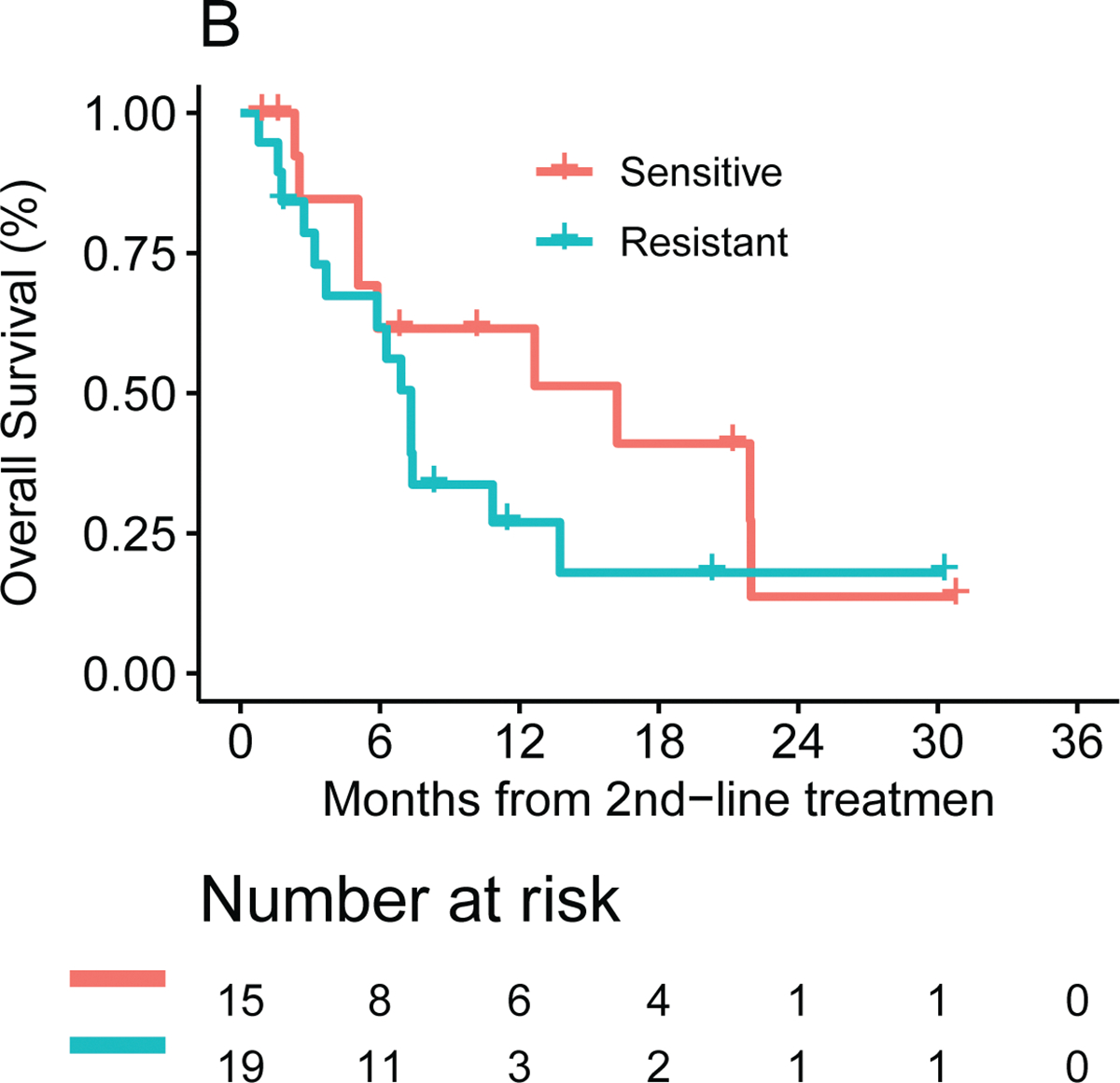
Second line median (A) PFS and (B) OS by ChemoSensitivity Assay result.
Tumor Genomics do not Predict Chemotherapeutic Drug Response
All 70 patients underwent genomic sequencing on both tumor tissue and germline DNA using our internal MSK-IMPACT™ platform (ClinicalTrials.gov Identifier NCT01775072);16 sequencing was successful in 57 (81%) patients. Gene mutations and frequency were consistent with what is known and has been reported in the literature (Table S1).
The six most commonly mutated somatic genes were similarly distributed across the three ChemoSensitivity cohorts. Mutations in these genes were not associated with median PFS. Mutations in TP53 and CDKN2Ap16 appeared to have worse prognosis with diminished median OS across the entire cohort (Figure S4). No relationship was found between somatic gene mutations and drug response or ChemoSensitivity profile (data not shown).
Pathogenic or likely pathogenic germline mutations were seen in 10 of 58 (17%) patients. Germline mutations in DNA damage repair genes such as BRCA1, BRCA2 and ATM are known predictors for increased sensitivity to platinum containing regimens such as FOLFIRINOX, although the response is not uniform.17–19 All 4 patients harboring BRCA1 and BRCA2 mutations received FOLFIRINOX chemotherapy. Two profiled as sensitive, with one each profiling as intermediate and resistant. The patient harboring an ATM mutation received G/nab-P chemotherapy and profiled as intermediate. Median PFS was 7.1 months (sensitive, intermediate and resistant groups with median PFS of 16.4 mo, 8.0 mo and 2.5 mo, respectively), and median OS has not been reached (sensitive, intermediate and resistant groups with median OS of not yet reached, 8 mo and 3 mo, respectively). Overall, genomic analysis is limited by the small size of this study, and larger data sets are required.
DISCUSSION
In the current study, we show for the first time that in a balanced cohort of patients with advanced PDAC, the ChemoSensitivity Assay can predict effective frontline therapy resulting in clinically meaningful improvements in progression free and overall survival. Beyond predicting effective frontline therapy, the assay has promising capabilities for longitudinal monitoring of emerging drug resistance and for predicting effective therapy in the second-line setting. The benefit of the ChemoSensitivity Assay was maintained even after stratifying for the regimen administered. Unlike the ChemoSensitivity Assay, genomic testing did not predict effective systemic chemotherapy.
The ChemoSensitivity Assay has numerous notable features, including the ability to generate profiles from a small volume of peripheral blood and provide actionable results within days delivering a clinically useful tool to determine an optimal treatment regimen within a clinically meaningful timeframe and longitudinally with sequential profiling. CTICs captured and profiled using our platform represent a heterogeneous population of cells, with a small proportion of cells representing classical EPCAM(+) CTCs, together with EPCAM(−) mesenchymal cells and invasive immune cells. Prior studies have characterized distinct subpopulations of CTCs isolated using this platform with features of epithelial lineage and others with stem or progenitor cells,13 with only a small number of cells isolated, between 0.03 to 0.07%, representing classic EPCAM(+) CTCs.20 We have previously shown a remarkable correlation between tumor and CTICs gene expression profiles,21 and our current study builds upon a growing body of literature supporting the utility of a mixed, CTIC population, particularly for predicting drug therapy in patients.22
The 95 genes profiled as part of the ChemoSensitivity Assay have been described previously,9 playing important roles in drug transport. ABC superfamily and solute carrier (SLC) genes are well-described active drug transporters, expression of which reduce accumulation of chemotherapy drugs within resistant cancer cells.23–28 Orthogonal platforms to classify PDAC have been studied, notably “classical” and “basal-like” gene expression sub-typing,29 however, there is limited data supporting an association with drug response. Tumor expression of GATA6 has been shown to discriminate classical and basal-like PDAC subtypes and may predict response to FOLFIRINOX.30 Patient-derived organoids are also being studied for predicting drug response.31 Tumor mutational profiling does not provide effective guidance for therapy in the vast majority of patients with advanced PDAC. Even in the subset of patient with germline mutations in DNA damage repair genes, the ChemoSensitivity Assay may be able to identify those patients more likely to benefit from platinum-based regimens, although more studies enriched for these patients are warranted.
The emergence of drug resistance is inevitable in advanced PDAC. The first evidence that CTICs profiles change in response to therapy was seen in earlier studies.21 Longitudinal profiling in the current trial showed not only the development of resistance to drugs used in the frontline regimen, but also increased sensitivity to drugs not yet utilized. Treatment with these drugs in the second-line was associated with a trend to improved survival, however, larger studies are required and are planned to confirm these results. The ability to use this Assay to guide therapy in multiple lines is of increasing importance as active frontline regimens allow more patients to receive subsequent lines of therapy, and with the approval of regimens in this setting.32
Although the current study design provides strong evidence that the Assay can be predictive, and is not simply prognostic, a prospective, guided trial will be necessary for confirmation, and is planned. The ChemoSensitivity Assay profiles sensitivity to individual drugs rather than combination regimens, and work to tailor individualized chemotherapy regimens for patients based on Assay results is being studied (ClinicalTrials.gov Identifier: NCT02555735). The Assay is one of the key biomarkers being tested in the PASS-01 trial, an international study randomizing frontline advanced PDAC patients to either FOLFIRINOX or G/nab-P chemotherapy (ClinicalTrials.gov Identifier: NCT04469556).
Supplementary Material
Financial support:
Kenneth H. Yu (NIH R01 CA202762), Cancer Center grant (NIH P30 CA008748)
Footnotes
Conflict of Interest:
The following authors report no conflicts of interest: JFLC, MC, JL and AV. KHY: Research Funding to MSK (Ipsen, Halozyme, BMS); KHY serves as an advisor to Adera Biolabs, has no financial interest in and has not received compensation. BC, AB and BM are employees of Adera Biolabs. EOR and GA: Research Funding to MSK (Arcus, Astra Zeneca, BioNtech, BMS, Celgene, Flatiron, Genentech/Roche, Genoscience, Incyte, Polaris, Puma, QED, Silenseed and Yiviva); Consulting Role (Adicet, Alnylam, Astra Zeneca, Autem, Beigene, Berry Genomics, Boehringer Ingelheim, Celgene, Cend, CytomX, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Genoscience, Helio, Helsinn, Incyte, Ipsen, Merck, Nerviano, Newbridge, Novartis, QED, Redhill, Rafael, Servier, Silenseed, Sobi, Vector and Yiviva). AE: Consulting (UpToDate). DI: Research Funding to MSK (Astellas, Eli Lilly, Pieris, and Taiho); Consulting Role (AstraZeneca, Amgen, Bayer, Bristol-Myers Squibb, and Roche). DK: Research Funding to MSK (Thompson Family Foundation; Applebaum Foundation; STARR Grant); Consulting Role (TR Therapeutics; Merck; Bristol Myers Squibb; Lilly). GYK Research Funding to MSK (AstraZeneca, Zymeworks, and Daiichi Sankyo); Consulting Role (Merck, Bristol-Myers Squibb, and Pieris). WP: Research Funding to MSK (Astellas, Gossamer Bio, and Merck); Consulting Role (Onconics, Aegle, Cerner Enviza).
Data Availability Statement Format Guidelines
The data that support the findings of this study are available from Adera Biolabs, Germantown MD, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Adera Biolabs, Germantown MD.
REFERENCES
- 1.Matrisian LM; Aizenberg R; Rosenzweig A The Alarming Rise of Pancreatic Cancer Deaths in the United States: Why We Need to Stem the Tide Today; Pancreatic Cancer Action Network: http://www.pancan.org/section_research/reports/pdf/incidence_report_2012.pdf, 2012.
- 2.Conroy T; Desseigne F; Ychou M; Bouche O; Guimbaud R; Becouarn Y; Adenis A; Raoul JL; Gourgou-Bourgade S; de la Fouchardiere C; Bennouna J; Bachet JB; Khemissa-Akouz F; Pere-Verge D; Delbaldo C; Assenat E; Chauffert B; Michel P; Montoto-Grillot C; Ducreux M, FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011, 364 (19), 1817–25. [DOI] [PubMed] [Google Scholar]
- 3.Von Hoff DD; Ervin T; Arena FP; Chiorean EG; Infante J; Moore M; Seay T; Tjulandin SA; Ma WW; Saleh MN; Harris M; Reni M; Dowden S; Laheru D; Bahary N; Ramanathan RK; Tabernero J; Hidalgo M; Goldstein D; Van Cutsem E; Wei X; Iglesias J; Renschler MF, Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013, 369 (18), 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tempero M; Van Cutsem E; Sigal D; D.-Y. O; Fazio N; Macarulla T; Hitre E; Hammel P; Hendifar A; Bates SE; C.-P. Li; de la Fouchardiere C; Heinemann V; Maraveyas A; Bahary N; Layos L; Sahai V; Zheng L; Lacy J; Bullock AJ, HALO 109–301: A randomized, double-blind, placebo-controlled, phase 3 study of pegvorhyaluronidase alfa (PEGPH20) + nab-paclitaxel/gemcitabine (AG) in patients (pts) with previously untreated hyaluronan (HA)-high metastatic pancreatic ductal adenocarcinoma (mPDA). J Clin Oncol 2020, 38 (suppl 4; abstr 638). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boston Biomedical, Inc. Announces Update on Phase 3 CanStem111P Study of Napabucasin in Patients with Metastatic Pancreatic Cancer Following Interim Analysis. www.prnewswire.com 2019.
- 6.Hecht JR; Lonardi S; Bendell JC; Sim H-W; Macarulla T; Lopez CD; Van Cutsem E; Munoz Martin AJ; Park JO; Greil R; Lin Y; Rao S; Ryoo B-Y In Randomized Phase III Study of FOLFOX Alone and with Pegilodecakin as Second-line Therapy in Patients with Metastatic Pancreatic Cancer (SEQUOIA). G.I. Symposium, J Clin Oncol: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tempero M; Oh D; Macarulla T; Reni M; Van Cutsem E; Hendifar A; Waldschmidt D; Starling N; Bachet J; Chang H; Maurel J; Lonardi S; Coussens L; Fong L; Tsao L; Cole G Jr.; James D; Tabernero J, Ibrutinib in combination with nab-paclitaxel and gemcitabine as first-line treatment for patients with metastatic pancreatic adenocarcinoma: results from the phase 3 RESOLVE study. Ann Oncol 2019, 30 Suppl 4, iv126. [DOI] [PubMed] [Google Scholar]
- 8.Golan T; Hammel P; Reni M; Van Cutsem E; Macarulla T; Hall MJ; Park JO; Hochhauser D; Arnold D; Oh DY; Reinacher-Schick A; Tortora G; Algul H; O’Reilly EM; McGuinness D; Cui KY; Schlienger K; Locker GY; Kindler HL, Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med 2019, 381 (4), 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu KH; Ricigliano M; McCarthy B; Chou JF; Capanu M; Cooper B; Bartlett A; Covington C; Lowery MA; O’Reilly EM, Circulating Tumor and Invasive Cell Gene Expression Profile Predicts Treatment Response and Survival in Pancreatic Adenocarcinoma. Cancers (Basel) 2018, 10 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb J; Crawford ED; Peck D; Modell JW; Blat IC; Wrobel MJ; Lerner J; Brunet JP; Subramanian A; Ross KN; Reich M; Hieronymus H; Wei G; Armstrong SA; Haggarty SJ; Clemons PA; Wei R; Carr SA; Lander ES; Golub TR, The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313 (5795), 1929–35. [DOI] [PubMed] [Google Scholar]
- 11.Wei G; Twomey D; Lamb J; Schlis K; Agarwal J; Stam RW; Opferman JT; Sallan SE; den Boer ML; Pieters R; Golub TR; Armstrong SA, Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 2006, 10 (4), 331–42. [DOI] [PubMed] [Google Scholar]
- 12.Scherf U; Ross DT; Waltham M; Smith LH; Lee JK; Tanabe L; Kohn KW; Reinhold WC; Myers TG; Andrews DT; Scudiero DA; Eisen MB; Sausville EA; Pommier Y; Botstein D; Brown PO; Weinstein JN, A gene expression database for the molecular pharmacology of cancer. Nature genetics 2000, 24 (3), 236–44. [DOI] [PubMed] [Google Scholar]
- 13.Lu J; Fan T; Zhao Q; Zeng W; Zaslavsky E; Chen JJ; Frohman MA; Golightly MG; Madajewicz S; Chen WT, Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int J Cancer 2010, 126 (3), 669–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paris PL; Kobayashi Y; Zhao Q; Zeng W; Sridharan S; Fan T; Adler HL; Yera ER; Zarrabi MH; Zucker S; Simko J; Chen WT; Rosenberg J, Functional phenotyping and genotyping of circulating tumor cells from patients with castration resistant prostate cancer. Cancer Lett 2009, 277 (2), 164–73. [DOI] [PubMed] [Google Scholar]
- 15.Fan T; Zhao Q; Chen JJ; Chen WT; Pearl ML, Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecologic oncology 2009, 112 (1), 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DT; Mitchell TN; Zehir A; Shah RH; Benayed R; Syed A; Chandramohan R; Liu ZY; Won HH; Scott SN; Brannon AR; O’Reilly C; Sadowska J; Casanova J; Yannes A; Hechtman JF; Yao J; Song W; Ross DS; Oultache A; Dogan S; Borsu L; Hameed M; Nafa K; Arcila ME; Ladanyi M; Berger MF, Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. The Journal of molecular diagnostics : JMD 2015, 17 (3), 251–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowery MA; Wong W; Jordan EJ; Lee JW; Kemel Y; Vijai J; Mandelker D; Zehir A; Capanu M; Salo-Mullen E; Arnold AG; Yu KH; Varghese AM; Kelsen DP; Brenner R; Kaufmann E; Ravichandran V; Mukherjee S; Berger MF; Hyman DM; Klimstra DS; Abou-Alfa GK; Tjan C; Covington C; Maynard H; Allen PJ; Askan G; Leach SD; Iacobuzio-Donahue CA; Robson ME; Offit K; Stadler ZK; O’Reilly EM, Prospective Evaluation of Germline Alterations in Patients With Exocrine Pancreatic Neoplasms. J Natl Cancer Inst 2018, 110 (10), 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wattenberg MM; Asch D; Yu S; O’Dwyer PJ; Domchek SM; Nathanson KL; Rosen MA; Beatty GL; Siegelman ES; Reiss KA, Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. British journal of cancer 2020, 122 (3), 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguirre AJ; Nowak JA; Camarda ND; Moffitt RA; Ghazani AA; Hazar-Rethinam M; Raghavan S; Kim J; Brais LK; Ragon D; Welch MW; Reilly E; McCabe D; Marini L; Anderka K; Helvie K; Oliver N; Babic A; Da Silva A; Nadres B; Van Seventer EE; Shahzade HA; St Pierre JP; Burke KP; Clancy T; Cleary JM; Doyle LA; Jajoo K; McCleary NJ; Meyerhardt JA; Murphy JE; Ng K; Patel AK; Perez K; Rosenthal MH; Rubinson DA; Ryou M; Shapiro GI; Sicinska E; Silverman SG; Nagy RJ; Lanman RB; Knoerzer D; Welsch DJ; Yurgelun MB; Fuchs CS; Garraway LA; Getz G; Hornick JL; Johnson BE; Kulke MH; Mayer RJ; Miller JW; Shyn PB; Tuveson DA; Wagle N; Yeh JJ; Hahn WC; Corcoran RB; Carter SL; Wolpin BM, Real-time Genomic Characterization of Advanced Pancreatic Cancer to Enable Precision Medicine. Cancer Discov 2018, 8 (9), 1096–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Premasekharan G; Gilbert E; Okimoto RA; Hamirani A; Lindquist KJ; Ngo VT; Roy R; Hough J; Edwards M; Paz R; Foye A; Sood R; Copren KA; Gubens M; Small EJ; Bivona TG; Collisson EA; Friedlander TW; Paris PL, An improved CTC isolation scheme for pairing with downstream genomics: Demonstrating clinical utility in metastatic prostate, lung and pancreatic cancer. Cancer Lett 2016, 380 (1), 144–52. [DOI] [PubMed] [Google Scholar]
- 21.Yu KH; Ricigliano M; Hidalgo M; Abou-Alfa GK; Lowery MA; Saltz LB; Crotty JF; Gary K; Cooper B; Lapidus R; Sadowska M; O’Reilly EM, Pharmacogenomic modeling of circulating tumor and invasive cells for prediction of chemotherapy response and resistance in pancreatic cancer. Clin Cancer Res 2014, 20 (20), 5281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearl ML; Dong H; Zhao Q; Tulley S; Dombroff MK; Chen WT, iCTC drug resistance (CDR) Testing ex vivo for evaluation of available therapies to treat patients with epithelial ovarian cancer. Gynecol Oncol 2017, 147 (2), 426–432. [DOI] [PubMed] [Google Scholar]
- 23.Deeley RG; Westlake C; Cole SP, Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 2006, 86 (3), 849–99. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman MM; Ling V, The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS letters 2006, 580 (4), 998–1009. [DOI] [PubMed] [Google Scholar]
- 25.Ross DD; Nakanishi T, Impact of breast cancer resistance protein on cancer treatment outcomes. Methods in molecular biology 2010, 596, 251–90. [DOI] [PubMed] [Google Scholar]
- 26.Li Q; Shu Y, Role of solute carriers in response to anticancer drugs. Mol Cell Ther 2014, 2, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L; Vasiliou K; Nebert DW, Analysis and update of the human solute carrier (SLC) gene superfamily. Hum Genomics 2009, 3 (2), 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemstrova R; Soucek P; Melichar B; Mohelnikova-Duchonova B, Role of solute carrier transporters in pancreatic cancer: a review. Pharmacogenomics 2014, 15 (8), 1133–45. [DOI] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research Network. Electronic address, a. a. d. h. e.; Cancer Genome Atlas Research, N., Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32 (2), 185–203 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aung KL; Fischer SE; Denroche RE; Jang GH; Dodd A; Creighton S; Southwood B; Liang SB; Chadwick D; Zhang A; O’Kane GM; Albaba H; Moura S; Grant RC; Miller JK; Mbabaali F; Pasternack D; Lungu IM; Bartlett JMS; Ghai S; Lemire M; Holter S; Connor AA; Moffitt RA; Yeh JJ; Timms L; Krzyzanowski PM; Dhani N; Hedley D; Notta F; Wilson JM; Moore MJ; Gallinger S; Knox JJ, Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res 2018, 24 (6), 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiriac H; Belleau P; Engle DD; Plenker D; Deschenes A; Somerville TDD; Froeling FEM; Burkhart RA; Denroche RE; Jang GH; Miyabayashi K; Young CM; Patel H; Ma M; LaComb JF; Palmaira RLD; Javed AA; Huynh JC; Johnson M; Arora K; Robine N; Shah M; Sanghvi R; Goetz AB; Lowder CY; Martello L; Driehuis E; LeComte N; Askan G; Iacobuzio-Donahue CA; Clevers H; Wood LD; Hruban RH; Thompson E; Aguirre AJ; Wolpin BM; Sasson A; Kim J; Wu M; Bucobo JC; Allen P; Sejpal DV; Nealon W; Sullivan JD; Winter JM; Gimotty PA; Grem JL; DiMaio DJ; Buscaglia JM; Grandgenett PM; Brody JR; Hollingsworth MA; O’Kane GM; Notta F; Kim E; Crawford JM; Devoe C; Ocean A; Wolfgang CL; Yu KH; Li E; Vakoc CR; Hubert B; Fischer SE; Wilson JM; Moffitt R; Knox J; Krasnitz A; Gallinger S; Tuveson DA, Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov 2018, 8 (9), 1112–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang-Gillam A; Li CP; Bodoky G; Dean A; Shan YS; Jameson G; Macarulla T; Lee KH; Cunningham D; Blanc JF; Hubner RA; Chiu CF; Schwartsmann G; Siveke JT; Braiteh F; Moyo V; Belanger B; Dhindsa N; Bayever E; Von Hoff DD; Chen LT; Group N-S, Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016, 387 (10018), 545–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Adera Biolabs, Germantown MD, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Adera Biolabs, Germantown MD.


