Abstract
Background:
There are currently no disease-targeted treatments for cognitive or behavioral symptoms in patients with behavioral variant frontotemporal dementia (bvFTD).
Objective:
To determine the effect of tolcapone, a specific inhibitor of Catechol-O-Methyltransferase (COMT), in patients with bvFTD.
Methods:
In this randomized, double-blind, placebo-controlled, cross-over study at two study sites, we examined the effect of tolcapone on 28 adult outpatients with bvFTD. The primary outcome was reaction time on the N-back cognitive test. As an imaging outcome, we examined differences in the resting blood oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) signal intensity between subjects on placebo versus tolcapone performing the N-back test. Secondary outcomes included measures of cognitive performance and behavioral disturbance using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Neuropsychiatric Inventory-Questionnaire (NPI-Q), and Clinical Global Impressions scale (CGI).
Results:
Tolcapone was well tolerated and no patients dropped out. The most frequent treatment-related adverse event during tolcapone treatment was elevated liver enzymes (21%). There were no significant differences between tolcapone treatment and placebo in the primary or imaging outcomes. However, there were significant differences between RBANS total scores (p < 0.01), NPI-Q total scores (p = 0.04), and CGI total scores (p = 0.035) between treatment conditions which were driven by differences between baseline and tolcapone conditions. Further, there was a trend toward significance between tolcapone and placebo on the CGI (p = 0.078).
Conclusions:
Further study of COMT inhibition and related approaches with longer duration of treatment and larger sample sizes in frontotemporal lobar degeneration-spectrum disorders may be warranted.
Keywords: COMT, dopamine, frontotemporal dementia, tolcapone, treatment
INTRODUCTION
Frontotemporal lobar degeneration (FTLD) is the third most common type of neurodegenerative dementia [1]. The most prevalent clinical syndrome associated with FTLD is behavioral-variant frontotemporal dementia (bvFTD), characterized by early changes in personality, emotion, and social cognition [2]. Many of the most problematic symptoms of bvFTD, including apathy and cognitive dysfunction, respond poorly to current medication treatment [3-5].
Tolcapone is a centrally acting, reversible inhibitor of Catechol-O-Methyltransferase (COMT) that has been shown to improve neurocognitive task performance [6]. Prior studies have demonstrated that in healthy subjects, after only 7 days of tolcapone treatment there are measurable differences in executive function and verbal episodic memory [7]. In particular, performance on the N-back, a test of working memory, was improved after 7 days of tolcapone treatment [7]. It is thought that tolcapone’s effects are due to augmented dopamine levels throughout the brain but perhaps especially in the prefrontal cortex where COMT plays a prominent role in dopamine regulation [8-10]. Previous studies suggest that FTLD patients have degeneration of the dopamine system [11-13]. However, no previous studies have examined whether altering dopamine tone with a COMT inhibitor would be beneficial in the treatment in bvFTD. Patients with bvFTD can have prominent impairment in working memory [2], and we hypothesized that 7 days of tolcapone treatment may be sufficient to improve working memory and other cognitive and behavioral symptoms in patients with bvFTD.
To determine whether tolcapone augmentation can improve cognitive and behavioral symptoms in bvFTD patients we performed a brief, proof of concept cross-over study. The study was designed to replicate a brief proof of concept study examining tolcapone in healthy control participants [7] with the goal of determining whether an expanded trial of tolcapone in bvFTD and related disorders is warranted. While certain COMT genotypes have been associated with greater degeneration of the dopamine system [12], this study on COMT inhibition was conceptualized as a symptomatic, rather than disease-modifying, trial for FTD, thus, the short duration of the current trial. The primary outcome of this study was performance on the N-back working memory task since prior reports have demonstrated that short term tolcapone treatment improves N-back performance in healthy controls [7]. To more generally assess differences in cognition during treatment phases, patients completed the Repeatable Battery Assessment of Neuropsychological Status (RBANS) [14]. Because behavioral symptoms directly impact quality of life and clinical outcomes for dementia patients and because current treatments are inadequate [15], we also assessed behavioral symptoms using the Neuropsychiatric Inventory-Questionnaire (NPI-Q). Finally, patients were evaluated on the Clinical Global Impressions scale (CGI).
MATERIALS AND METHODS
Study design
Twenty-eight participants with a diagnosis of bvFTD were enrolled in this phase II double-blind, placebo-controlled cross-over study in which all participants received both active treatment and placebo (Fig. 1). The study was registered at ClinicalTrials.gov (NCT00604591) before it was performed from January 30, 2008 to June 16, 2016. To obtain an adequate number of participants, patients were studied at two geographic locations, National Institute of Neurological Disorders and Stroke (NINDS) and Columbia University Medical Center (CUMC). We chose a cross-over design for several reasons. First, bvFTD is an uncommon illness and it is difficult to recruit the numbers of subjects required to sufficiently power a study in which subjects are randomized to only treatment or placebo. Also, the power of the within-subject analyses of a cross-over study is greater to detect small to medium effect sizes [16]. Using this design also allowed us to directly compare our results in bvFTD patients to a cross-over design study performed in healthy controls that had shown tolcapone improves cognition in association with functional magnetic resonance imaging (fMRI) changes [7].
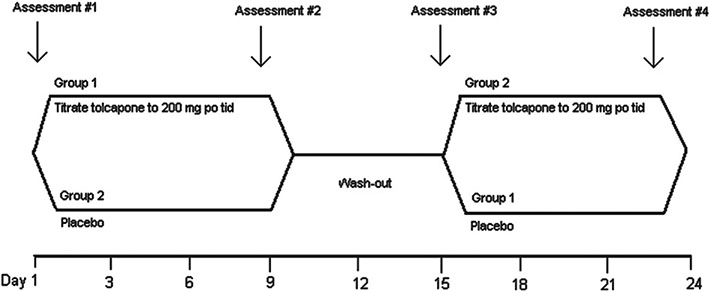
Fig. 1.
Study design. MRI scans performed at Assessments #2, 3, and 4. Clinical and neuropsychological study measures performed at screening and Assessments #2, 3, and 4. Side effect evaluation, vital signs, physical examination, Liver Function Tests, and AIMS performed at all assessment points. mg po tid, milligrams per oral three times a day.
Participants
Subjects were initially screened for suitability for the study. Inclusion criteria included diagnosis of bvFTD [2] between the ages of 40–85 year, assigned durable power of attorney, willingness of caregiver to accept responsibilities involved in the study, and Mattis Dementia Rating Scale-2 (MDRS2) rating score less than 136. Exclusion criteria included diagnosis of any type of dementia besides bvFTD including but not limited to Alzheimer’s disease, Lewy body dementia, vascular dementia, Parkinson’s disease, corticobasal syndrome, and supranuclear palsy. Other exclusion criteria included known allergy or serious adverse reaction to tolcapone, acute liver disease, any clinical or laboratory evidence of hepatic dysfunction, current alcohol abuse, active substance use, symptomatic cardiovascular disease (e.g., angina, transient ischemic attack, syncope), uncontrolled hypertension, uncontrolled hypotension, pregnant women, and patients with any known contraindication to tolcapone. Patient currently taking any medication that significantly affects the dopamine system including stimulants, antipsychotics, tolcapone or another COMT inhibitor, benserazide, alpha-methyldopa, dobutamine, apomorphine, iso-proterenol, a monoamine oxidase inhibitor (MAO-I), or clozapine were also excluded. The duration of the trial for all participants was 24 days since prior studies demonstrated effects of tolcapone on healthy controls over similar time periods [7, 17]. The CUMC subjects were recruited from The Lucy G. Moses Center for Memory and Behavioral Disorders in the Neurological Institute of Columbia University. The procedures and measures were identical between the two study sites.
Adverse events
Tolcapone has been approved by the FDA in the treatment of Parkinson’s disease as an adjunctive to levodopa/carbidopa. The most well-known and serious side effect related to tolcapone use is hepatotoxicity, including acute fulminant liver failure and increases in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [18]. Other side effects are dizziness, nausea, diarrhea, dyskinesia, dystonia, and sleep disturbance [19]. The risk of side effects was discussed with participants and caregivers before inclusion in the informed consent form. Trial continuation was to be stopped in individuals with a suspected serious adverse event of tolcapone including acute fulminant liver failure. There were no patients in which the trial was discontinued due to severe adverse side effects of tolcapone.
Procedures
Figure 1 illustrates the visit schedule. Briefly, participants were randomly assigned to start on 100 mg of either tolcapone (Tasmar) or placebo. After randomization, participants took 100 mg of the assigned treatment orally three times a day during the first day and then 200 mg orally for the next six days. After the dose was tapered on the seventh and eighth days, a wash-out period for one week followed. After the wash-out period, the drug schedule resumed, but with the alternative treatment (for example, if the participant had started with tolcapone, they were then given placebo). Notably, prior studies have shown that tolcapone treatment on the scale of 7–10 days can improve N-back performance in healthy control subjects [7, 17]. The washout period in the Apud et al. study was also 7 days [7]. Therefore, to be able to compare the current trial with prior trials of tolcapone, a similar drug administration and washout schedule were performed. Measures including N-Back, cognitive, and behavioral assessments were performed within 4 hours of tolcapone administration. The subjects were given 100 mg of Vitamin B2 with each dose of tolcapone or placebo to mask the urine discoloration sometimes observed with tolcapone treatment. Drug ingestion was monitored with pill counts which were performed at each assessment point.
Measures
We assessed the subjects at baseline and three time points thereafter during the trial (Fig. 1). We measured change in cognitive performance with the N-back cognitive test and RBANS [20]. Neuropsychiatric symptoms were assessed with the NPI-Q [21]. Finally, general clinical status was determined using the CGI scale.
N back cognitive test
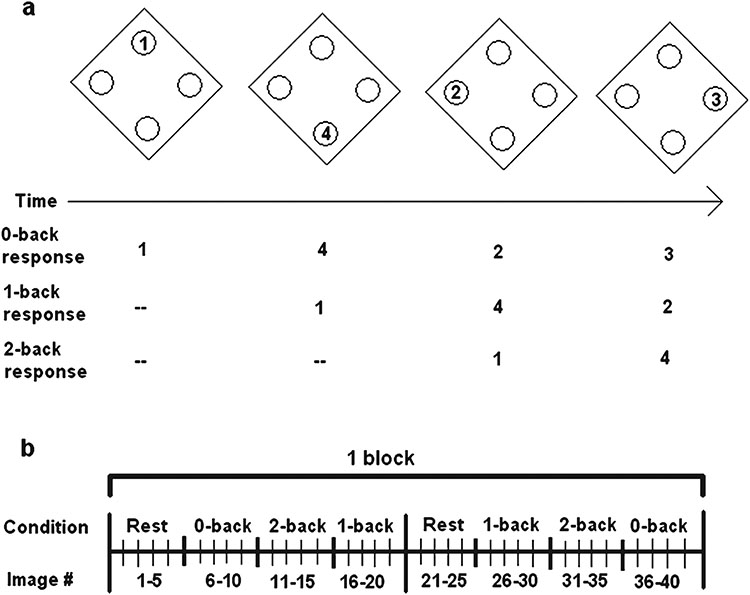
To assess executive function, and to facilitate the comparison of data obtained in this study to data collected in healthy controls [7], the subjects performed the N-back task in the MRI scanner at Assessment points #2, 3, and 4 (Fig. 1). In the N-back task, subjects are given a response pad with response buttons numbered 1, 2, 3, and 4 at the points of a diamond-shaped box and shown a random series of numbers from 1 to 4 appearing for 500 ms every 1.8 s at locations corresponding to the positions of the numbers on the response pad. Instructions presented on the screen above the diamond instruct patients to recall the stimulus seen “N” numbers previously. In the 0-back condition, the subjects are instructed to press the button with the number on the screen. In the 1-back condition, the subjects are instructed to report the number presented one number back from the number displayed on the screen. In the 2-back condition, they are to report the number 2 back from the one presented on the screen (Fig. 2). As “N” increases, so does the cognitive load and the degree of dorsal prefrontal cortex activation observed in healthy subjects [22]. Normal control subjects can perform the 2- and 3-back conditions with difficulty, but we found in pilot testing that the 2- and 3-back conditions were too difficult for most bvFTD patients to perform. Thus, we limited the conditions to the 0- and 1-back conditions. The order of conditions was counterbalanced to control for order effects. Three blocks of 40 images were administered during the N-back task for a total of 120 images [22, 23]. Response time and accuracy were measured following [7].
Fig. 2.
Structure of the N-back test. a) N-back test. b) One block of the N-back. Three counterbalanced blocks were administered. The rest condition had blank screens without a task.
Repeatable Battery for the Assessment of Neuropsychological Status [20]
The RBANS is a brief individually administered test that was initially developed as tool for diagnosing and tracking dementia. The RBANS captures multiple cognitive domains including attention, language, visuospatial/constructional abilities, immediate memory, and delayed memory. The RBANS has been extensively used in patients with dementias [20] and generates index scores for the 5 domains tested and a total index score. The RBANS has been used before as a cognitive outcome measure in bvFTD patients in prior studies [24].
Neuropsychiatric Inventory-Questionnaire [21]
The NPI-Q is a retrospective caregiver/informant-based interview lasting around 20 min that assesses 12 neuropsychiatric symptom domains including delusions, hallucinations, agitation/aggression, depression, anxiety, euphoria/elation, apathy/indifference, disinhibition, irritability/lability, aberrant motor behaviors, night-time behavioral disturbances, and appetite/eating disturbances. Neuropsychiatric symptoms within a domain are rated by the caregiver in terms of severity (1 = mild; 2 = moderate; 3 = severe). Caregiver distress is also determined but was not used to calculate final scores in this study. Therefore, total scores ranged from 0 (no symptoms) to 36.
Clinical Global Impressions Scale [25]
The CGI is often used in treatment studies as a proxy for global functioning and is a subjective score assigned by the treating physician that incorporates elements of illness severity, patient distress, patient impairment, and functioning. It has been shown that the CGI correlates with well-known research drug efficacy scales widely used in psychiatry (where the CGI is most often implemented). The CGI is given a numerical ranking each visit after the first with 4 being no change, 3 mild improvement, 5 mild worsening, 2 major improvement, and 6 major worsening. Because the CGI reflects the severity of symptoms, lower is better (i.e., higher reflects an increase in symptoms).
Imaging
Echo-planar T2*-weighted images with blood-oxygenation-level-dependent (BOLD) contrast was acquired on a 3 Tesla General Electric (at NIH) and a Philips Achieva Quasar Dual Magnet (at CUMC) scanner equipped with a standard head coil, high order manual shimming to temporal and ventral frontal lobes, 3 mm slice thickness, 64 × 64 matrix, 37 slices, TR = 2.3 s, TE 20.5, FOV: 220 × 220, parallel to the anterior to posterior commissural line, whole brain coverage (not cerebellum). The first five volumes were discarded to allow for T1 equilibration effects. The combination of high-field MRI, thinner slices and high-order manual shimming were used to optimize the signal in anterior temporal and ventral frontal lobes. In addition, high resolution (1 mm3) T1-weighted 3D MP-RAGE (Magnetization-Prepared Rapid Acquisition Gradient Echo) structural images were collected (1mm slice thickness, 128 slices, matrix 224 × 224, TE 2.964; FOV: 220 X 222).
Resting BOLD time series were recorded for all patients at rest and during the N-back task. Data was collected for the on and off tolcapone conditions. For every time series, we temporally filtered both resting-BOLD time series and 6 motion parameters during the scan. The filter was a rectangular filter in the frequency range [0.009 Hz, 0.08 Hz] of order m = 20. As a set of nuisance voxels, we chose all voxels with gray-matter probability of p < 0.01 (= “nuisance” voxels) as judged by the probabilistic gray-matter mask supplied by SPM12; these voxels are most likely white-matter or CSF voxels. We performed Principal Component Analysis and took the time course of the first Principal Component as an additional motion regressor.
We then regressed the filtered signal at all voxel locations with gray-matter probability of p > 0.5 (= ‘signal” voxels) against the 7 motion regressors, i.e., the time series of the filtered white matter and CSF-signal plus the 6 filtered motion regressors, and formed the residual time series. The residual time series were used to compute 2 quantities: 1) the average amplitude of residual time series in the dorso-lateral prefrontal cortex, and 2) the functional connectome between all 34,716 possible pairs of 264 regions-of-interest, which were chosen according to the taxonomy put forth by Power et al. [26]. For (1), we averaged the standard deviation across time in the residual time series for all signal voxels in Brodmann areas 9 and 46, as indicated by the Anatomic Automated Labeling template [27]. To correct for arbitrary rescaling from scan to scan, we divided this mean amplitude by the mean amplitude of all signal voxels. We then checked whether this normalized amplitude correlated showed a significant difference as a function of medication status in a paired-sample T-test. For (2), we computed the all 34,716 pairwise temporal correlations between all signal voxels at the 264 locations provided by Power et al. [26]. No correlation for scaling was necessary, since correlation is already scale-invariant. We then performed 1,000 split-sample analysis for which the ON and OFF tolcapone data sets were randomly split in half. Principal Component Analysis was performed for data reduction to use up to 20 Principal Component scores, rather than the full 34,716 pairwise connectivity values. The set of Principal Components used was varied from 1, 1:2, 1:3, and so on, till 1:20, and a Support Vector Machine [28] was fitted in the training, with subsequent application and venturing of a prediction in of the 1/0 judgment about medication status in the test set. Predictive accuracy was recorded with specificity, sensitivity and the balanced accuracy rate, i.e., an average of sensitivity and specificity, with respect to the 1/0 judgment.
For each participant, the N-back task was preprocessed in statistical parametric mapping software (SPM12) with slice-timing, motion correction, registration to T1 structural, normalizing to standard space and smoothing with 8 mm full-width-at-half-maximum kernel. Then, the data were modeled with block design corresponding to each block of 0- or 1-back. Each visit was modeled separately and then contrasted at the group level with a covariate for the two scanners used for obtaining the MRI data. Of the 16 participants that performed the N-back task while undergoing fMRI, four participants were scanned at NINDS and twelve at Columbia. 0- and 1-back activations were analyzed in separate group analyses examining the within-subject effects of tolcapone versus placebo, with the covariate of site included in both analyses.
Statistical analyses for change in cognition and behavior
The means for the treatment, placebo, and washout conditions for the overall CGI were compared with a repeated measures analysis of variance (ANOVA) and post-hoc paired sample t-tests. Post-hoc sample t-tests were not corrected for multiple comparisons. For N-Back testing, despite the fact that no patients dropped out, some were unable to complete the N-Back even at the 0-back condition. Therefore, at each session patients were asked to perform as much as they were able and a mixed model analysis was performed to account for missing values.
Standard protocol approvals, registrations, and patient consents
All participants were required to assign a research durable power of attorney prior to admission, and the assigned individuals gave written informed consent for the study. The patients gave assent for the study. All aspects of the study and the consent procedure were approved by the NINDS and CUMC Institutional Review Boards. Clinicaltrials.gov identifier: NCT00604591.
Data availability statement
All data is available to qualified investigators upon request to the corresponding author.
RESULTS
Participant characteristics
Between January 30, 2008 and June 16, 2016, 28 patients (14 female, 14 male, ages 51–81) completed the study in 24 days. No patients dropped out before the completion of the study. All patients were able to participate in MRI scanning and behavioral measures. Patient demographics are described in Table 1.
Table 1.
Demographics of the 28 patients included in the study
| Demographic | Tolcapone First (n = 14) |
Placebo First (n = 14) |
Total (n = 28) |
|---|---|---|---|
| Average Age (SD) | 62.7 (6.7) | 63.6 (9.1) | 63.1 (7.8) |
| Average Age of Onset (SD) | 54.2 (3.0) | 52.5 (9.0) | 53.3 (6.7) |
| Sex (M/F) | 6/8 | 8/6 | 14/14 |
| Race (white/other) | 13/1 (African American) | 12/2 (Asian-Indian) | 25/3 (one African American, two Asian-Indian |
| Average Education Years (SD) | 17.4 (1.6) | 16.8 (2.0) | 17.1 (1.8) |
| Average MDRS Baseline (SD) | 109.2 (23.3) | 105.0 (27.1) | 107.1 (24.8) |
| Site (NINDS/Columbia) | 4/10 | 5/9 | 9/19 |
SD, standard deviation; M, male; f, female; NINDS, National Institute of Neurological Disorders and Stroke.
Adherence to tolcapone
Overall, tolcapone was well-tolerated. Six subjects (21.4%) had at least one reading of a liver enzyme level above the upper end of normal range at some point in their participation. All of these returned back to the normal range by later measurements. No subjects had liver function tests greater than 100% of the upper normal limit during their participation in the study. No subjects had any clinical signs or symptoms of hepatic impairment at any point during the study. Three subjects (10.7%) reported involuntary movements at baseline, which either persisted or resolved during the study (none with reported worsening). The most common side effects reported were nausea (n = 4, 14.3%), diarrhea (n = 4, 14.3%), headache (n = 4, 14.3%), constipation (n = 3, 10.7%), upset stomach (n = 2, 7.1%), hyperactive bowel syndrome (n = 2, 7.1%), and falls (n = 2, 7.1%, moderate falls causing no injury, not requiring hospitalization). Nearly all reported side effects were mild. Two subjects (7.1%) entered the study with notable long-standing gastrointestinal problems. Two subjects (7.1%) entered the study with history of chronic coughing. Two subjects (7.1%) entered the study with chronic headaches. No subjects exhibited signs of liver toxicity, dyskinesia, or neuroleptic malignant syndrome while in the study. One adverse event occurred during the study. A single subject (3.5%) experienced a seizure while enrolled in the study. This occurred during the washout week of participation, thus resulting in the postponement of a study visit. The adverse event was not deemed to be the result of the study medication, and the patient completed the study protocol without further incident. None of the patients experienced serious adverse events that required them to be removed from the trial.
Differences in N-back test performance and imaging results
The primary outcome measure in this study was performance on the N-back test of working memory and updating. Reaction time for the 0- and 1- back conditions showed no change (Table 2). The mean reaction time for 0-back condition was 8322 ms (standard deviation, [SD] = 1940 ms) for tolcapone and 7935 ms (SD = 2563 ms) for placebo, while the mean reaction for 1-back condition was 8226 ms (SD = 2842 ms) for tolcapone and 7060 ms (SD = 2475 ms) for placebo. For both 0- and 1-back conditions, there was no significant effect of tolcapone treatment when compared to baseline or placebo. There were no significant differences in accuracy on the N-back test between the tolcapone or treatment conditions for 0-back (both 83% accurate) or the 1-back (37% accurate on placebo, 35% accurate on tolcapone). We did not find significant effects of tolcapone treatment on network connectivity or resting BOLD signal in any of the regions of interest examined (data not shown).
Table 2.
Characteristics of all 28 patients before and during treatment. Means and (SD) reported. For CGI, Wash-out rather than Baseline reported
| Outcomes | Baseline | Washout | Treatment (Tolcapone) |
Placebo |
p-value for difference between tolcapone and placebo |
|---|---|---|---|---|---|
| CGI | |||||
| CGI Total | — | 4.23 (0.59) | 3.75 (0.65) | 4.14 (0.76) | 0.078 |
| NPI-Q | |||||
| NPI-Q Total | 9.14 (4.76) | 9.46 (4.88) | 8.21 (4.50) | 8.68 (4.83) | 0.211 |
| Delusions | 0.11 (0.31) | 0.07 (0.26) | 0.11 (0.32) | 0.07 (0.26) | 0.326 |
| Hallucinations | 0.00 (0) | 0.00 (0) | 0.00 (0) | 0.00 (0) | >0.999 |
| Agitation | 0.71 (0.81) | 0.71 (0.90) | 0.61 (0.83) | 0.75 (0.89) | 0.443 |
| Depression | 0.75 (0.97) | 0.79 (0.92) | 0.46 (0.79) | 0.68 (1.09) | 0.110 |
| Anxiety | 0.96 (0.96) | 1.14 (1.04) | 0.79 (0.83) | 0.93 (0.98) | 0.355 |
| Euphoria | 0.18 (0.67) | 0.21 (0.57) | 0.14 (0.53) | 0.14 (0.53) | >0.999 |
| Apathy | 1.79 (0.99) | 1.78 (1.10) | 1.89 (0.99) | 1.61 (1.07) | 0.090 |
| Disinhibition | 1.00 (1.05) | 1.14 (1.01) | 0.86 (0.93) | 0.86 (0.97) | >0.999 |
| Irritability | 0.68 (0.77) | 0.68 (0.77) | 0.61 (0.74) | 0.79 (0.88) | 0.057 |
| Motor Behavior | 1.07 (1.15) | 1.14 (1.18) | 1.07 (0.98) | 1.07 (1.12) | >0.999 |
| Nighttime behaviors | 0.57 (0.92) | 0.54 (0.92) | 0.43 (0.84) | 0.57 (0.92) | 0.460 |
| Appetite and Eating | 1.32 (1.30) | 1.25 (1.27) | 1.29 (1.21) | 1.21 (1.29) | 0.537 |
| RBANS | |||||
| Total | 62.68 (14.96) | 67.57 (19.63) | 66.57 (18.32) | 67.89 (17.66) | 0.250 |
| Working memory | 61.75 (20.79) | 67.07 (25.25) | 67.11 (24.11) | 64.32 (22.55) | 0.164 |
| VS | 79.07 (18.10) | 84.00 (20.99) | 82.46 (23.55) | 82.82 (23.34) | 0.872 |
| Language | 67.25 (19.80) | 68.89 (19.95) | 74.18 (20.74) | 71.46 (18.89) | 0.084 |
| Attention | 70.82 (15.77) | 73.79 (18.05) | 75.61 (18.85) | 74.50 (19.31) | 0.487 |
| Delayed memory | 64.43 (22.10) | 69.93 (26.18) | 68.71 (24.29) | 67.75 (25.32) | 0.515 |
| NBACK | |||||
| 0-back accuracy (percentage correct) | 0.747 (0.280) | – | 0.831 (0.242) | 0.834 (0.196) | 0.948 |
| 1-back accuracy (percentage correct) | 0.351 (0.248) | – | 0.391 (0.303) | 0.380 (0.274) | 0.436 |
| 0-back reaction time (ms) | 9141 (2225) | 8322 (1940) | 7935 (2563) | 0.8624 | |
| 1-back reaction time (ms) | 7863 (2323) | 8226 (2842) | 7060 (2475) | 0.2011 |
CGI, Clinical Global Impressions scale; NPI-Q, Neuropsychiatric Inventory-Questionnaire; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; VS, visuospatial. Note: Total means a summary score for the particular scale. For RBANS, sub-scale scores were the totals of 2 items for working memory, 2 items for VS, 2 items for language, 2 for attention, and 4 for delayed memory. For NPI-Q, sub-scales scores were taken from a single item score for each domain. p-value was calculated using a t-test. There were no significant differences in any of the measures examined between baseline and washout.
Differences in cognitive performance
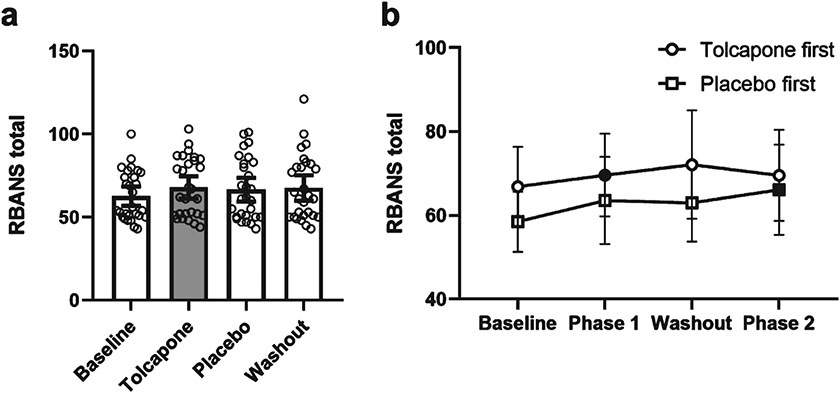
Overall, there was a significant difference in the RBANS total score between the treatment conditions [F (4,27) = 6.86, p < 0.001]. This estimated effect was driven by an improvement in the tolcapone treatment condition compared to baseline [t(27) = 3.86, p = 0.001, Effect Size, [ES] = 0.60, Fig. 3]. There were significant estimated sub-effects on working memory [t(27) = 2.55, p = 0.02], language [t(27) = 2.88, p = 0.01], and attention [t(27) = 2.45, p = 0.02], which all showed improvement on tolcapone, but not on visuospatial or delayed memory subtests (Supplementary Figure 1). There was not a significant difference on the total RBANS between tolcapone treatment and placebo conditions [tolcapone versus placebo: t(27) = 1.17, p = 0.25, ES = 0.22)]. There were no significant differences between tolcapone and placebo on RBANS subdomains (Table 2). There was a trend towards significance for language which was improved with tolcapone treatment compared to placebo [t(27) = 1.79, p = 0.084, ES = 0.14].
Fig. 3.
Effects of treatment phase on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). a) Mean score at Baseline, Treatment (Tolcapone, in gray), Placebo, and Washout phases. Open circles are individual data points. Error bars represent 95% confidence intervals. b) Mean score during each phase of the trial for Tolcapone first (circle) and Placebo first (square) conditions. Filled symbols indicate active (tolcapone) phase. Error bars represent 95% confidence intervals.
Differences in behavioral symptoms
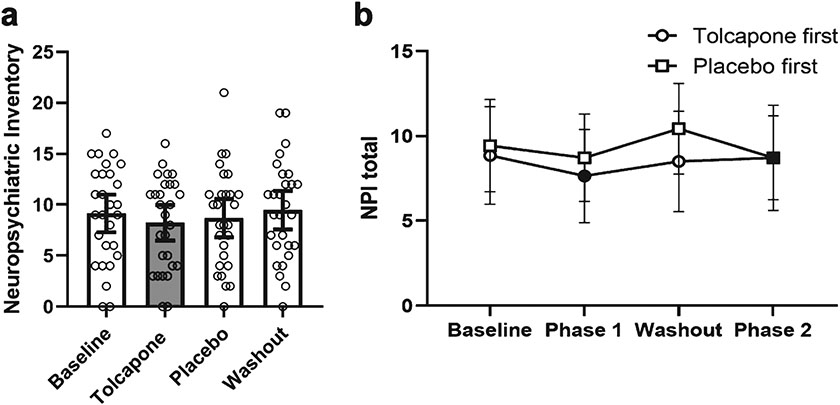
To explore whether tolcapone may improve behavioral disturbances in bvFTD patients, participants were assessed on the NPI-Q. There was a significant difference in total NPI-Q between the different treatment conditions [F (1,25) = 10.19, p = 0.004]. This was driven by a difference in NPI-Q scores between the tolcapone treatment and baseline conditions [t(25) = 3.19, p = 0.004, ES = 0.63; Table 2, Fig. 4]. In particular, there was a significant improvement in the depression subdomain when comparing tolcapone treatment to baseline [t(27) −2.51, p = 0.017, ES = 0.28]. There was no significant difference between tolcapone and placebo treatment in the NPI-Q [t(25) = 1.28, p = 0.21, ES = 0.25]. There was a trend toward improvement in the subdomain of depression [t(27) = 1.65, p = 0.11, ES = 0.27 tolcapone versus placebo] and a trend toward improvement in irritability [t(27) = 1.99, p = 0.057, ES = 0.22 tolcapone versus placebo] which were both decreased by treatment when compared to placebo (Table 2, Supplementary Figure 2). There was also a trend toward increased apathy with treatment [t(27) = 1.76, p = 0.090, ES = 0.24 tolcapone versus placebo] when compared to placebo.
Fig. 4.
Effects of treatment phase on the Neuropsychiatric Inventory-Questionnaire (NPI-Q). a) Mean score at Baseline, Treatment (Tolcapone, in gray), Placebo, and Washout phases. Open circles are individual data points. Error bars represent 95% confidence intervals. b) Mean score during each phase of the trial for Tolcapone first (circle) and Placebo first (square) conditions. Filled symbols indicate active (tolcapone) phase. Error bars represent 95% confidence intervals.
Differences in clinical improvement between tolcapone and placebo
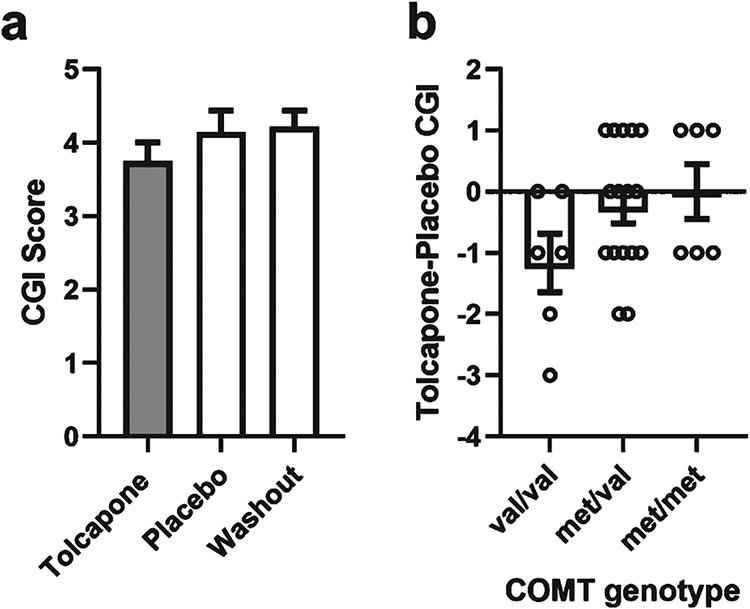
To evaluate for clinical improvement in bvFTD patients treated with tolcapone, we administered the CGI to all the subjects at each assessment point. There was a significant effect of treatment on the overall CGI [F(2,27) = 3.59, p = 0.035; Fig. 5]. Post-hoc t-tests showed a significant difference on the CGI between tolcapone and baseline [t(27) = 2.90, p = 0.008, ES = 0.57] and a trend toward improvement on tolcapone when compared to placebo [t(27) = 1.83, p = 0.078, ES = 0.35].
Fig. 5.
Effects of treatment phase on the Clinical Global Impressions Scale. a) Mean score at Treatment (Tolcapone, Gray), Placebo, and Washout phases. CGI of 4 is no change, scores lower than 4 represent improvement, greater than 4 represent worsening. Error bars represent 95% confidence intervals. b) Mean within-subject CGI difference of Treatment - Placebo CGI by genotype. Negative Treatment-Placebo CGI is an improvement. Open circles represent individual data points. Error bars represent standard error of the mean.
Effect of COMT genotype on association between tolcapone treatment and cognitive, behavioral, and clinical improvement
COMT inactivates released dopamine through enzymatic conversion to 3-methoxytyramine and is a key enzyme regulating dopamine in the prefrontal cortex [29-31]. The COMT gene has a common functional methionine (met) for valine (val) substitution at codon 158, referred to as the val158met polymorphism. In general populations, the met and val allele frequencies are approximately equal [32, 33]. The enzyme in individuals with the met/met genotype is less active than in individuals with the val/val genotype [34]. Thus, healthy individuals with the met allele have greater amounts of available dopamine and functionally demonstrate better performance on tests of frontal cognitive function in an allele dose-dependent fashion [22, 35-40]. Prior studies have suggested that a patient’s COMT genotype may be relevant to the effectiveness of tolcapone treatment [7] (although see [41] as a counterexample). We found that there was no significant effect of COMT genotype on the effects of tolcapone treatment on the RBANS or NPI-Q. There was a trend in CGI data whereby COMT val dosage was associated with improved CGI with tolcapone treatment (r = −0.343, p = 0.074, Fig. 5). This trend was not seen for other measures (data not shown).
DISCUSSION
This is the first double-blinded, placebo-controlled proof of concept trial of tolcapone in bvFTD. Tolcapone was well-tolerated in all participants, without serious adverse effects. The results of this study were negative for the efficacy of short term tolcapone treatment in improving N-Back performance (primary outcome). There were no significant differences in total RBANS, NPI-Q, or CGI scores between tolcapone treatment and placebo conditions. However, compared to baseline, tolcapone treatment was associated with a small to medium effect size short-term improvement in clinical status and a small effect size improvement in neuropsychiatric symptoms when compared to baseline (Table 3). There was no significant effect of COMT genotype on the effects of tolcapone on cognitive or behavioral symptoms, which is consistent with some prior studies [42]. However, there was a trend for improvement of clinical functioning in tolcapone treated patients that appeared to be related to COMT genotype. This is consistent with prior data demonstrating that healthy patients with the val/val COMT genotype showed greater improvement in cognitive performance with tolcapone treatment [43].
Table 3.
Summary of results
| Domain | Measure | Tolcapone effect size versus placebo |
Predicted COMT genotype effect |
|---|---|---|---|
| Clinical | CGI | 0.35 (small to medium sized) | Yes |
| Cognitive out of scanner | RBANS | 0.22 (small) | No |
| Behavioral | NPI | 0.25 (small) | Yes |
| Cognitive (in scanner) | N-back | – | – |
CGI, Clinical Global Impressions scale; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; NPI, Neuropsychiatric Inventory-Questionnaire; COMT, catechol-o-methyltransferase.
When NPI scores were broken down by subdomain, there were trends toward differences between tolcapone and placebo conditions. Interestingly, depression and irritability were decreased with tolcapone treatment but apathy was increased. The fact that there were bidirectional changes in the NPI sub-scales may explain, at least in part, why there were not significant differences in the NPI overall when treatment was compared to placebo. These findings may also speak to the potential use of tolcapone in treatment of specific psychiatric symptoms in FTLD including irritability and depression. Indeed, one animal study has suggested that tolcapone administration may prevent the development of depression-like symptoms in chronically-stressed rats [44] and one small open label study showed patients with major depressive disorder improved on tolcapone [45]. Although there have not been any studies that specifically report on the effect of tolcapone on irritability prior to our trial, one report demonstrated that addition of tolcapone could significantly improve non-motor symptoms in Parkinson’s disease with improved quality of life and reduced caregiver burden [46]. This may speak to the ability of tolcapone to improve multiple, but perhaps restricted, cognitive and behavioral domains that ultimately result in improved function across neurological disorders; as was seen in [46] and suggested in our study. Further work should be conducted to replicate these findings and to elucidate the relationship between tolcapone and behavioral measures.
There are multiple possibilities for why there were significant differences between tolcapone treatment and baseline but not between tolcapone and placebo. First, in the group treated with tolcapone first, there were improvements in clinical and behavioral symptoms that persisted through the washout and placebo period which may have confounded comparisons (Figs. 3 and 4). While the half-life of tolcapone is thought to be short [47], some studies suggest that tolcapone may have long-term effects [48, 49]. Therefore, further studies exploring long-term effects of tolcapone treatment may be warranted. That being said, the improvement observed with tolcapone treatment compared to baseline is approximately the same as that for cholinesterase-inhibitor treatment for Alzheimer’s disease [50]. Therefore, this study may be interpreted as providing some evidence that tolcapone could be effective at improving symptoms in bvFTD despite the fact that there was no significant difference between tolcapone treatment and placebo.
This study has several limitations. With regard to the N-back task specifically, we were forced to use a modified and simplified version of the task because patients with bvFTD were largely incapable of performing the original version. However, in the original study that demonstrated a difference in N-back performance with tolcapone treatment in healthy controls, this difference was only noted on the 3- and 4-back conditions, possibly related to higher cognitive demand [7]. Therefore, the modified N-back used in this study may not have been cognitively demanding enough for a difference due to tolcapone treatment to have been detected. Also, one of our measures was N-back reaction time, and since reaction time can be affected by motor effects which we did not evaluate, this is another limitation of the study. More generally, it is notable that in our sample patients had symptoms for a number of years prior to enrolling in the trial (see Table 1). While there is no clear consensus regarding when symptomatic treatment should be started in FTD [4], it is possible that tolcapone may have more of an effect on cognitive and psychiatric symptoms in patients who are in an earlier stage of the disorder. Another limitation of this study was the relatively brief duration of treatment conditions and washout. While these durations were chosen strategically to mimic the conditions of the Apud et al. study, any subsequent studies may want longer treatment durations and washout as there is some evidence that tolcapone may have longer-term effects on the brain that remain poorly understood. Also, it is worth noting that some of the NPI-Q and RBANS subdomains were close to significance and future studies with higher sample sizes may be able to determine whether the trends noted in this study are reproducible and clinically meaningful as the trend toward improved CGI might suggest. One other limitation of this study is that some of the measures used involved neuropsychiatric testing which, in short intervals, can lead to significant practice effects. However, since we used the RBANS, we used different forms of the test for each point of evaluation in the trial which should minimize this concern.
In the current trial, we assessed the short-term effects of COMT inhibition on the symptoms of bvFTD. The effects of long-term COMT inhibition are unknown, although previous studies have shown that high COMT activity is associated with greater degeneration of dopaminergic brain structures in bvFTD [12]. Tolcapone has disadvantages for use, including the potential for hepatotoxicity. However, in this trial, tolcapone was well-tolerated. The other available COMT inhibitors developed for use in Parkinson’s disease have a better adverse effect profile than tolcapone, but do not have as good central nervous system (CNS) penetration. COMT inhibitors with good CNS penetration that are not associated with hepatotoxicity are currently under development. In this study, there was no significant effect of tolcapone treatment when compared to placebo, but there were differences between tolcapone and placebo treatment in certain sub-domains of the NPI. Also, there were significant differences between tolcapone treatment and baseline levels of cognitive, behavioral, and clinical measures. Trials involving a longer exposure to tolcapone, or using newer COMT inhibitors, may be warranted in FTLD-spectrum disorders. The results of this study support further investigation into the effect of tolcapone on cognitive, neuropsychiatric, and clinical features of FTLD-spectrum disorders. An important aspect for future studies will be determining an appropriate and meaningful primary outcome measure which will both capture the complexity of symptoms in these patients and be sensitive to changes in those symptoms.
Supplementary Material
ACKNOWLEDGMENTS
We thank Daniel Weinberger for scientific input.
This work was funded by NIH/NINDS R00NS 060766 and the Intramural program of NIH/NINDS.
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/19-1265r1).
Footnotes
Clinicaltrials.gov identifier: NCT00604591
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JAD-191265.
REFERENCES
- [1].Onyike CU, Diehl-Schmid J (2013) The epidemiology of frontotemporal dementia. Int Rev Psychiatry 25, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL (2011) Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tsai RM, Boxer AL (2014) Treatment of frontotemporal dementia. Curr Treat Options Neurol 16, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huey ED, Putnam KT, Grafman J (2006) A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology 66, 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Boxer AL, Lipton AM, Womack K, Merrilees J, Neuhaus J, Pavlic D, Gandhi A, Red D, Martin-Cook K, Svetlik D, Miller BL (2009) An open-label study of memantine treatment in 3 subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc Disord 23, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Leegwater-Kim J, Waters C (2006) Tolcapone in the management of Parkinson’s disease. Expert Opin Pharmacother 7, 2263–2270. [DOI] [PubMed] [Google Scholar]
- [7].Apud JA, Mattay V, Chen J, Kolachana BS, Callicott JH, Rasetti R, Alce G, Iudicello JE, Akbar N, Egan MF, Goldberg TE, Weinberger DR (2007) Tolcapone improves cognition and cortical information processing in normal human subjects. Neuropsychopharmacology 32, 1011–1020. [DOI] [PubMed] [Google Scholar]
- [8].Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M (1998) Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A 95, 9991–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Huotari M, Gogos JA, Karayiorgou M, Koponen O, Forsberg M, Raasmaja A, Hyttinen J, Mannisto PT (2002) Brain catecholamine metabolism in catechol-O-methyltransferase (COMT)-deficient mice. Eur J Neurosci 15, 246–256. [DOI] [PubMed] [Google Scholar]
- [10].Huotari M, Santha M, Lucas LR, Karayiorgou M, Gogos JA, Mannisto PT (2002) Effect of dopamine uptake inhibition on brain catecholamine levels and locomotion in catechol-O-methyltransferase-disrupted mice. J Pharmacol Exp Ther 303, 1309–1316. [DOI] [PubMed] [Google Scholar]
- [11].Engelborghs S, Vloeberghs E, Le Bastard N, Van Buggenhout M, Marien P, Somers N, Nagels G, Pickut BA, De Deyn PP (2008) The dopaminergic neurotransmitter system is associated with aggression and agitation in frontotemporal dementia. Neurochem Int 52, 1052–1060. [DOI] [PubMed] [Google Scholar]
- [12].Gennatas ED, Cholfin JA, Zhou J, Crawford RK, Sasaki DA, Karydas A, Boxer AL, Bonasera SJ, Rankin KP, Gorno-Tempini ML, Rosen HJ, Kramer JH, Weiner M, Miller BL, Seeley WW (2012) COMT Val158Met genotype influences neurodegeneration within dopamine-innervated brain structures. Neurology 78, 1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Butler PM, Chiong W, Perry DC, Miller ZA, Gennatas ED, Brown JA, Pasquini L, Karydas A, Dokuru D, Coppola G, Sturm VE, Boxer AL, Gorno-Tempini ML, Rosen HJ, Kramer JH, Miller BL, Seeley WW (2019) Dopamine receptor D4 (DRD4) polymorphisms with reduced functional potency intensify atrophy in syndrome-specific sites of frontotemporal dementia. Neuroimage Clin 23, 101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].O’Donnell BF, Drachman DA, Barnes HJ, Peterson KE, Swearer JM, Lew RA (1992) Incontinence and troublesome behaviors predict institutionalization in dementia. J Geriatr Psychiatry Neurol 5, 45–52. [DOI] [PubMed] [Google Scholar]
- [15].Manoochehri M, Huey ED (2012) Diagnosis and management of behavioral issues in frontotemporal dementia. Curr Neurol Neurosci Rep 12, 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cleophas TJ, Zwinderman AH (2002) Crossover studies with continuous variables: Power analysis. Am J Ther 9, 69–73. [DOI] [PubMed] [Google Scholar]
- [17].Ashare RL, Valdez JN, Ruparel K, Albelda B, Hopson RD, Keefe JR, Loughead J, Lerman C (2013) Association of abstinence-induced alterations in working memory function and COMT genotype in smokers. Psychopharmacology (Berl) 230, 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Borges N (2005) Tolcapone in Parkinson’s disease: Liver toxicity and clinical efficacy. Expert Opin Drug Saf 4, 69–73. [DOI] [PubMed] [Google Scholar]
- [19].Bonifati V, Meco G (1999) New, selective catechol-O-methyltransferase inhibitors as therapeutic agents in Parkinson’s disease. Pharmacol Ther 81, 1–36. [DOI] [PubMed] [Google Scholar]
- [20].Randolph C, Tierney MC, Mohr E, Chase TN (1998) The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol 20, 310–319. [DOI] [PubMed] [Google Scholar]
- [21].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J (1994) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- [22].Mattay VS, Goldberg TE, Fera F, Hariri AR, Tessitore A, Egan MF, Kolachana B, Callicott JH, Weinberger DR (2003) Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci U S A 100, 6186–6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mattay VS, Callicott JH, Bertolino A, Heaton I, Frank JA, Coppola R, Berman KF, Goldberg TE, Weinberger DR (2000) Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 12, 268–275. [DOI] [PubMed] [Google Scholar]
- [24].Huey ED, Garcia C, Wassermann EM, Tierney MAM, Grafman J (2008) Stimulant treatment of frontotemporal dementia in 8 patients. J Clin Psychiatry 69, 1981–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Guy W (1976) Clinicians’ Global Impression of Change (CGIC), National Institute of Mental Health, Rockville, MD. [Google Scholar]
- [26].Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011) Functional network organization of the human brain. Neuron 72, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- [28].Hastie T, Tibshirani R, Friedman JH (2009) The elements of statistical learning: Data mining, inference, and prediction, Springer, New York. [Google Scholar]
- [29].Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A (2001) Dopamine transporter immunoreactivity in monkey cerebral cortex: Regional, laminar, and ultrastructural localization. J Comp Neurol 432, 119–136. [DOI] [PubMed] [Google Scholar]
- [30].Mazei MS, Pluto CP, Kirkbride B, Pehek EA (2002) Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res 936, 58–67. [DOI] [PubMed] [Google Scholar]
- [31].Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J Neurosci 22, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Munafo MR, Bowes L, Clark TG, Flint J (2005) Lack of association of the COMT (Val(158/108) Met) gene and schizophrenia: A meta-analysis of case-control studies. Mol Psychiatry 10, 765–770. [DOI] [PubMed] [Google Scholar]
- [33].Rujescu D, Giegling I, Gietl A, Hartmann AM, Moller HJ (2003) A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biol Psychiatry 54, 34–39. [DOI] [PubMed] [Google Scholar]
- [34].Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J (1995) Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34, 4202–4210. [DOI] [PubMed] [Google Scholar]
- [35].Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A 98, 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR (2003) Executive subprocesses in working memory: Relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch Gen Psychiatry 60, 889–896. [DOI] [PubMed] [Google Scholar]
- [37].Diamond A, Briand L, Fossella J, Gehlbach L (2004) Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry 161, 125–132. [DOI] [PubMed] [Google Scholar]
- [38].Nolan KA, Bilder RM, Lachman HM, Volavka J (2004) Catechol O-methyltransferase Val158Met polymorphismin schizophrenia: Differential effects of Val and Met alleles on cognitive stability and flexibility. Am J Psychiatry 161, 359–361. [DOI] [PubMed] [Google Scholar]
- [39].Rosa A, Peralta V, Cuesta MJ, Zarzuela A, Serrano F, Martinez-Larrea A, Fananas L (2004) New evidence of association between COMT gene and prefrontal neurocognitive function in healthy individuals from sibling pairs discordant for psychosis. Am J Psychiatry 161, 1110–1112. [DOI] [PubMed] [Google Scholar]
- [40].Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR (2005) Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci 25, 5038–5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chong DJ, Suchowersky O, Szumlanski C, Weinshilboum RM, Brant R, Campbell NR (2000) The relationship between COMT genotype and the clinical effectiveness of tolcapone, a COMT inhibitor, in patients with Parkinson’s disease. Clin Neuropharmacol 23, 143–148. [DOI] [PubMed] [Google Scholar]
- [42].Bhakta SG, Light GA, Talledo JA, Balvaneda B, Hughes E, Alvarez A, Rana BK, Young JW, Swerdlow NR (2017) Tolcapone-enhanced neurocognition in healthy adults: Neural basis and predictors. Int J Neuropsychopharmacol 20, 979–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Apud JA, Weinberger DR (2007) Treatment of cognitive deficits associated with schizophrenia: Potential role of catechol-O-methyltransferase inhibitors. CNS Drugs 21, 535–557. [DOI] [PubMed] [Google Scholar]
- [44].Moreau JL, Borgulya J, Jenck F, Martin JR (1994) Tolcapone: A potential new antidepressant detected in a novel animal model of depression. Behav Pharmacol 5, 344–350. [PubMed] [Google Scholar]
- [45].Fava M, Rosenbaum JF, Kolsky AR, Alpert JE, Nierenberg AA, Spillmann M, Moore C, Renshaw P, Bottiglieri T, Moroz G, Magni G (1999) Open study of the catechol-O-methyltransferase inhibitor tolcapone in major depressive disorder. J Clin Psychopharmacol 19, 329–335. [DOI] [PubMed] [Google Scholar]
- [46].Muller T, Investigators TS (2014) Tolcapone addition improves Parkinson’s disease associated nonmotor symptoms. Ther Adv Neurol Disord 7, 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Huotari M, Gainetdinov R, Mannisto PT (1999) Microdialysis studies on the action of tolcapone on pharmacologically-elevated extracellular dopamine levels in conscious rats. Pharmacol Toxicol 85, 233–238. [DOI] [PubMed] [Google Scholar]
- [48].Maser T, Rich M, Hayes D, Zhao P, Nagulapally AB, Bond J, Saulnier Sholler G (2017) Tolcapone induces oxidative stress leading to apoptosis and inhibition of tumor growth in Neuroblastoma. Cancer Med 6, 1341–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Di Giovanni S, Eleuteri S, Paleologou KE, Yin G, Zweckstetter M, Carrupt PA, Lashuel HA (2010) Entacapone and tolcapone, two catechol O-methyltransferase inhibitors, block fibril formation of alpha-synuclein and beta-amyloid and protect against amyloid-induced toxicity. J Biol Chem 285, 14941–14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rockwood K (2004) Size of the treatment effect on cognition of cholinesterase inhibition in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 75, 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available to qualified investigators upon request to the corresponding author.