Abstract
Background
Although cases of respiratory bacterial infections associated with coronavirus disease 2019 (COVID-19) have often been reported, their impact on the clinical course remains unclear. Herein, we evaluated and analyzed the complication rates of bacterial infections, causative organisms, patient backgrounds, and clinical outcome in Japanese patients with COVID-19.
Methods
We performed a retrospective cohort study that included inpatients with COVID-19 from multiple centers participating in the Japan COVID-19 Taskforce (April 2020 to May 2021) and obtained demographic, epidemiological, and microbiological results and the clinical course and analyzed the cases of COVID-19 complicated by respiratory bacterial infections.
Results
Of the 1,863 patients with COVID-19 included in the analysis, 140 (7.5%) had respiratory bacterial infections. Community-acquired co-infection at COVID-19 diagnosis was uncommon (55/1,863, 3.0%) and was mainly caused by Staphylococcus aureus, Klebsiella pneumoniae and Streptococcus pneumoniae. Hospital-acquired bacterial secondary infections, mostly caused by Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia, were diagnosed in 86 patients (4.6%). Severity-associated comorbidities were frequently observed in hospital-acquired secondary infection cases, including hypertension, diabetes, and chronic kidney disease. The study results suggest that the neutrophil–lymphocyte ratio (> 5.28) may be useful in diagnosing complications of respiratory bacterial infections. COVID-19 patients with community-acquired or hospital-acquired secondary infections had significantly increased mortality.
Conclusions
Respiratory bacterial co-infections and secondary infections are uncommon in patients with COVID-19 but may worsen outcomes. Assessment of bacterial complications is important in hospitalized patients with COVID-19, and the study findings are meaningful for the appropriate use of antimicrobial agents and management strategies.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-023-02418-3.
Keywords: SARS-CoV-2 infection, Neutrophil–lymphocyte ratio, Mortality, Invasive mechanical ventilation, Intensive care unit
Background
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory coronavirus 2 (SARS-CoV-2) has been spreading worldwide. It has been reported that some patients developed rapid respiratory failure approximately one week post disease onset [1] or thrombotic complications later [2]. Bacterial co-infections or secondary infections in patients with COVID-19 are considered uncommon complications [3] compared to the co-infection rate in patients with severe influenza which is reportedly 20–30% [4] and associated with higher mortality [5].
In a retrospective study of 989 COVID-19 patients, co-infections and secondary infections were found in 3.1 and 4.7% of patients, respectively [6]. The incidence of hospital-acquired secondary infection varies among studies, ranging from 13 to 27% in adults with SARS-CoV-2 [7].
In one meta-analysis, the most common bacterial pathogens associated with influenza are Streptococcus pneumoniae and Staphylococcus aureus in 35 and 28% of cases, respectively [8]. The most frequently identified bacterial co-infections in patients with COVID-19 are caused by Staphylococcus aureus and Streptococcus pneumoniae [6], whereas Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus are frequently isolated microorganisms in secondary infections [6, 9].
The characteristics of patients with COVID-19, that are also affected by bacterial infections, as well as the predictive factors for bacterial co-infections remain unclear. Thus, a more detailed analysis of COVID-19 cases with bacterial infections will contribute to not only clarifying the unknown aspects of SARS-CoV-2 infection but also promoting the appropriate treatment with antimicrobial agents.
The Japan COVID-19 Task Force is the largest Japanese cohort with biospecimen resources [10], and the findings from this database are considered reliable. In the present study, we evaluated the patient background, frequency, causative organisms, and clinical outcomes affected by bacterial infections in Japanese patients hospitalized for COVID-19.
Methods
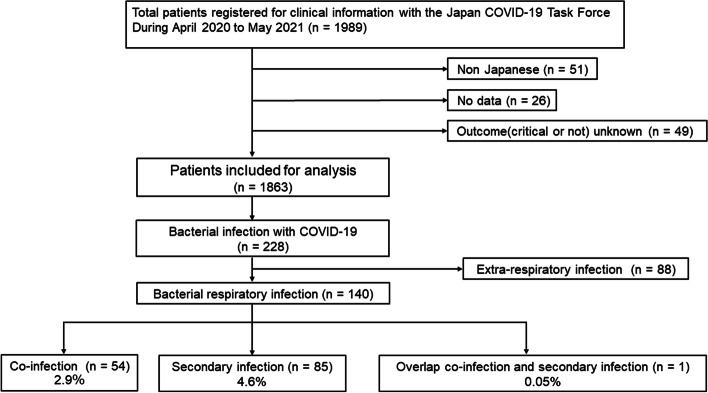
All patients were recruited through the Japan COVID-19 Task Force, and a retrospective cohort study was conducted [10, 11]. From April 2020 to May 2021, data from consenting consecutive inpatients aged 18 years or older who had been diagnosed with COVID-19 using SARS-CoV-2 polymerase chain reaction or antigen tests at one of the more than 100 affiliated hospitals were registered in an electronic case record form by the study subspecialist at the affiliated research institute. We excluded patients based on the following criteria: [i] non-Japanese patients and [ii] patients with incomplete medical records, such as inability to obtain critical outcome information (Fig. 1). We obtained written or oral informed consent from all enrolled patients, and the study was approved by the ethics committees of Keio University School of Medicine (20,200,061) and related research institutions.
Fig. 1.
Study flow chart of patient identification and selection. Study flow chart of patient identification and selection. A total of 125 records were excluded from the 1,989 cases registered in the coronavirus disease 2019 (COVID-19) taskforce database for the following reasons: 51 were not Japanese, 26 lacked essential clinical information, and 49 had an unknown outcome. Ultimately, 1,863 cases met the eligibility criteria, of which 228 had complications from bacterial infections. Of these, 140 were respiratory bacterial infections, comprising 54 co-infections, 85 secondary infections, and one case of multiple infections
Data collection
The following information was extracted from the electronic case record form: age, sex, body mass index (BMI), clinical signs and symptoms, laboratory findings on admission, comorbidities, and disease severity (ICU entry, use of IMV, and survival status). All laboratory tests were performed according to the clinical care needs of the patients. Signs and symptoms were checked on admission, whereas other parameters, such as clinical course and treatment, were collected during hospitalization. Laboratory and radiographic results were collected within 48 h of the initial visit or admission. Missing data in the patient background were noted as unknown.
Definitions
During hospitalization, standard microbiological tests were performed using sputum, urine, and blood samples. In patients receiving invasive mechanical ventilation, tracheal aspiration and bronchoalveolar lavage were performed as needed. The diagnosis of respiratory bacterial infection was determined by the attending clinician. Bacterial infections diagnosed at the time of admission due to COVID-19 or within 48 h of admission were defined as co-infections. Infections diagnosed after 48 h of COVID-19 admission were defined as secondary infections, as previously described [6].
Outcome and statistics
The primary outcome was death. Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as numbers (percentages). Data were compared among three groups defined by cases with co-infections, those with secondary infections, and cases without bacterial infections. ANOVA and Dunnet’s tests were used as indicated. To assess the impact of bacterial infection on COVID-19 severity, univariate and multivariate logistic regression analyses adjusted for patient characteristics, including BMI group, age, sex, chronic obstructive pulmonary disease (COPD), and presence of comorbidities associated with the severity of COVID-19 [12–18], were performed for ICU treatment, IMV use, and death. In intensive care, shock, IMV use, myocardial injury, acute kidney injury (AKI), and acute respiratory distress syndrome (ARDS) are also factors associated with death in COVID-19 [19–21]. In the multivariate analysis with death as the outcome, these variables were also added in a separate model. In the present analysis, shock was defined as cases in which patients were treated with vasopressors; for ARDS, these were substituted with cases with bilateral infiltrative shadows on chest radiographs. Odds ratio (OR) and adjusted OR are presented along with 95% confidence intervals. In all outcome analyses, the group without bacterial infection was defined as the control group. Moreover, univariate analysis was also performed for the frequency of thrombosis and myocardial injury during treatment, comparing the groups with and without bacterial infection as complications.
All P-values were two-sided, and statistical significance was set at P < 0.05. Data were analyzed using the JMP 16 program (SAS Institute Japan Ltd., Tokyo, Japan).
Results
We assessed 1,989 consecutive adult patients with COVID-19 who had either been discharged or died during the study period at one of the hospitals that participated in the Japan COVID-19 Task Force. Of these, 1,863 patients with COVID-19 met the inclusion criteria, and 228 (12.2%) had bacterial infections concomitant with COVID-19. Respiratory bacterial infections with COVID-19 were diagnosed in 140 patients (7.5%), of which 54 (2.9%) were co-infections, and 85 (4.6%) were secondary infections. Only one case had an overlap of co-infection and secondary infection (Fig. 1).
Patient characteristics at the time of admission
The main patient characteristics of each group are shown in Tables 1, 2, 3, and 4. Comparing the clinical backgrounds among the three groups comprising bacterial co-infections, secondary bacterial infections, and no bacterial infections, the parameters, such as age, COPD and percentage of steroid users were significantly higher in patients with bacterial infections than in those without (P < 0.05). The proportions of patients with male sex, hypertension, diabetes mellitus, chronic kidney disease, hyperuricemia, and cardiovascular disease, which are factors associated with the severity of COVID-19 [12–15, 18, 22], were exclusively increased in the group with secondary infections, as compared to that without bacterial infections (Table 1). Patients with bacterial co-infections showed significantly increased body temperatures at the time of admission (P < 0.05). In addition, patients with co-infections and secondary infections had higher prevalence of sputum (P = 0.005), disturbance of consciousness (P < 0.0001), and dyspnea (P < 0.0001). By contrast, the rates of dysgeusia and dysosmia were decreased in secondary infected patients, compared to the group without bacterial infections (P < 0.05) (Table 2).
Table 1.
Patient characteristics at the time of admission
| No Bacterial Infection (n = 1635) | Respiratory Co-infection (n = 54) | Respiratory Secondary Infection (n = 85) | P value | |
|---|---|---|---|---|
| Age | 58.0 ± 18.0 | 65.9 ± 15.5 | 70.0 ± 13.9 | < 0.0001a=**/b=** |
| Sex (male) | 1131 (64.9%) | 41 (75.9%) | 71 (83.5%) | 0.0006 |
| BMI | 24.5 ± 4.68 | 23.3 ± 4.57 | 24.6 ± 6.97 | 0.19 |
| Smoker | 260 (16.0%) | 6 (13.6%) | 5 (7.04%) | 0.0001 |
| Hypertension | 607 (35.4%) | 18 (34. 0%) | 43 (51.2%) | 0.02 |
| Cardiovascular disease | 167 (9.67%) | 5 (9.26%) | 23 (27.1%) | < 0.0001 |
| Cancer | 114 (6.65%) | 5 (9.43%) | 7 (8.43%) | 0.61 |
| Diabetes | 381 (22.1%) | 11 (20.4%) | 32 (37.7%) | 0.004 |
| Asthma | 112 (6.58%) | 3 (5.88%) | 5 (6.02%) | 0.96 |
| Hyperuricemia | 178 (10.4%) | 6 (11.1%) | 20 (23.8%) | 0.0001 |
| COPD | 74 (4.31%) | 6 (11.3%) | 8 (9.52%) | 0.01 |
| Chronic liver disease | 53 (3.19%) | 1 (1.89%) | 4 (4.71%) | 0.63 |
| Chronic kidney disease | 117 (7.09%) | 6 (11.1%) | 19 (22.6%) | < 0.0001 |
| Steroid users | 62 (3.60%) | 7 (13.4%) | 9 (10.6%) | < 0.0001 |
| Immunosuppressive drug users | 34 (1.98%) | 0 (0%) | 3 (3.53%) | 0.35 |
Data are expressed as number (percentage) or mean ± standard deviation (SD)
**p < 0.01
aComparison of patients without infection versus patients with community-acquired co-infection
bComparison of patients without infection versus patients with hospital-acquired secondary infection
Table 2.
Symptoms and vital signs at the time of admission
| No Bacterial Infection (n = 1635) | Respiratory Co-infection (n = 54) | Respiratory Secondary Infection (n = 85) | P value | |
|---|---|---|---|---|
| Fever | 37.2 ± 0.92 | 37.6 ± 1.03 | 37.4 ± 1.15 | 0.004 a=** |
| Cough | 1009 (58.8%) | 27 (50.0%) | 44 (58.7%) | 0.43 |
| Sputum | 402 (23.5%) | 21 (38.9%) | 25 (33.8%) | 0.005 |
| Sore throat | 409 (24.0%) | 9 (17.0%) | 9 (12.00%) | 0.03 |
| Nasal discharge | 260 (15.2%) | 4 (7.69%) | 5 (6.67%) | 0.04 |
| Dysgeusia | 318 (18.6%) | 4 (7.84%) | 6 (8.11%) | 0.01 |
| Dysosmia | 283 (16.6%) | 5 (9.80%) | 4 (5.41%) | 0.02 |
| Dyspnea | 513 (30.4%) | 28 (51.9%) | 35 (48.0%) | < 0.0001 |
| Disturbance of consciousness | 50 (2.91%) | 8 (13.0%) | 9 (12.2%) | < 0.0001 |
| Malaise | 835 (48.7%) | 31 (57.4%) | 42 (56.8%) | 0.19 |
| Systolic blood pressure | 129.2 ± 19.6 | 129.8 ± 21.9 | 130.6 ± 27.4 | 0.83 |
| Diastolic blood pressure | 80.6 ± 13.5 | 79.6 ± 13.7 | 77.1 ± 13.9 | 0.09 |
| Heart rate | 87.2 ± 16.6 | 93.7 ± 19.6 | 86.7 ± 18.8 | 0.02a=* |
| Respiratory Rate | 19.2 ± 4.58 | 20.7 ± 5.47 | 21.8 ± 6.47 | < 0.0001b=** |
| SpO2 | 96.0 ± 3.07 | 93.7 ± 4.95 | 95.3 ± 3.04 | < 0.0001a=** |
Data are expressed as number (percentage) or mean ± SD
*p < 0.05
**p < 0.01
aComparison of patients without infection versus patients with community-acquired co-infection
bComparison of patients without infection versus patients with hospital-acquired secondary infection
Table 3.
Serological data at the time of admission
| No Bacterial Infection (n = 1635) | Respiratory Co-infection (n = 54) | Respiratory Secondary Infection (n = 85) | P value | |
|---|---|---|---|---|
| WBC (/μL) | 5627.5 ± 2693.5 | 7579.8 ± 3868.5 | 7927.3 ± 4971.6 | < 0.0001a=**/b=** |
| Neutrophil (/ μL) | 3633.8 ± 2440.9 | 6109.9 ± 4275.7 | 6689.5 ± 4737.9 | < 0.0001a=**/b=** |
| Lymphocyte(/μL) | 1059.8 ± 616.3 | 705.2 ± 438.1 | 742.0 ± 462.9 | < 0.0001a=**/b=** |
| Hb (g/dL) | 14.1 ± 1.88 | 13.5 ± 1.98 | 13.1 ± 2.15 | < 0.0001a=*/b=** |
| PLT (× 103 /μL) | 19.9 ± 7.65 | 20.4 ± 7.50 | 19.5 ± 8.95 | 0.80 |
| Alb (g/dL) | 3.78 ± 0.61 | 3.29 ± 0.92 | 3.12 ± 0.62 | < 0.0001a=**/b=** |
| TB (mg/dL) | 0.66 ± 0.36 | 0.78 ± 0.65 | 0.69 ± 0.43 | 0.08 |
| ALP (IU/L) | 175.5 ± 124.0 | 220.3 ± 142.3 | 197.3 ± 100.6 | 0.02a=* |
| AST (IU/L) | 40.9 ± 69.1 | 54.7 ± 53.1 | 53.0 ± 91.3 | 0.13 |
| ALT (IU/L) | 38.6 ± 91.2 | 50.7 ± 63.5 | 43.8 ± 54.4 | 0.56 |
| BUN (mg/dL) | 16.5 ± 10.6 | 27.6 ± 25.5 | 31.4 ± 41.2 | < 0.0001a=**/b=** |
| Cr (mg/dL) | 1.06 ± 1.43 | 1.68 ± 2.34 | 1.38 ± 1.47 | 0.0018 a=** |
| LDH (IU/L) | 268.7 ± 136.6 | 337.1 ± 150.4 | 368.2 ± 184.1 | < 0.0001a=**/b=** |
| UA (mg/dL) | 4.88 ± 1.81 | 5.55 ± 2.34 | 5.11 ± 2.21 | 0.04a=* |
| CK (IU/L) | 149.7 ± 456.3 | 248.7 ± 347.8 | 218.9 ± 387.1 | 0.14 |
| Na (mEq/L) | 138.3 ± 3.62 | 137.7 ± 5.53 | 137.4 ± 3.91 | 0.08 |
| K (mEq/L) | 3.98 ± 0.46 | 4.03 ± 0.54 | 4.20 ± 0.55 | 0.0002b=** |
| Cl (mEq/L) | 101.8 ± 3.97 | 100.7 ± 5.56 | 102.1 ± 3.80 | 0.11 |
| BNP (pg/mL) | 69.4 ± 401.9 | 157.1 ± 403.0 | 104.2 ± 169.6 | 0.51 |
| IgG(mg/dL) | 1151.2 ± 359.4 | 1128.4 ± 293.3 | 1197.8 ± 293.6 | 0.27 |
| Ferritin (ng/mL) | 531.1 ± 583.0 | 814.4 ± 712.5 | 876.7 ± 982.0 | < 0.0001a=*/b=** |
| KL-6 (IU/L) | 315.9 ± 302.4 | 422.0 ± 308.9 | 488.2 ± 439.1 | < 0.0001 b=** |
| HbA1c (%) | 6.37 ± 1.31 | 6.76 ± 1.60 | 6.88 ± 1.39 | 0.002 b=** |
| CRP (mg/dL) | 4.48 ± 5.35 | 10.8 ± 9.61 | 9.93 ± 10.1 | < 0.0001a=**/b=** |
| Procalcitonin (ng/mL) | 0.16 ± 0.62 | 2.81 ± 9.57 | 1.05 ± 2.83 | < 0.0001a=**/b=* |
Data are shown as mean ± SD
WBC white blood cell, Hb hemoglobin, PLT platelet, Alb albumin, TB total bilirubin, ALP alkaline phosphatase, AST aspartate aminotransferase, ALT alanine aminotransferase, BUN blood urea nitrogen, Cr creatinine, LDH lactate dehydrogenase, UA uric acid, CK creatinine kinase, Na sodium, K potassium, Cl chlorine, BNP brain natriuretic peptide, IgG Immunoglobulin G, KL-6 Krebs von den Lungen-6, CRP C-reactive protein
*p < 0.05
**p < 0.01
aComparison of patients without infection versus patients with community-acquired co-infection
bComparison of patients without infection versus patients with hospital-acquired secondary infection
Table 4.
Radiographic findings at the time of admission
| No Bacterial Infection (n = 1635) | Respiratory Co-infection (n = 54) | Respiratory Secondary Infection (n = 85) | P value | |
|---|---|---|---|---|
| GGO on chest X-ray images | 1176 (65.8%) | 44 (83.0%) | 70 (84.3%) | < 0.0001 |
| Infiltrative shadow on chest X-ray images | 423 (26.2%) | 32 (64.0%) | 45 (54.2%) | < 0.0001 |
| GGO on chest CT images | 1261 (81.2%) | 47 (92.2%) | 76 (97.4%) | 0.0002 |
| Infiltrative shadow on chest CT images | 586 (38.4%) | 31 (67.4%) | 47 (61.8%) | < 0.0001 |
GGO ground glass opacity, CT Computed Tomography
Patient laboratory and radiographic results at the time of admission
The clinical laboratory values of the enrolled patients are presented in Table 3. Patients with co-infections or secondary infections had higher levels of white blood cells, neutrophils, blood urea nitrogen, lactate dehydrogenase, serum ferritin, Krebs-von-den-Lungen-6, C-reactive protein, and procalcitonin (all P < 0.0001) than COVID-19 patients without bacterial infections. Conversely, lymphocytes, hemoglobin, and albumin values (all P < 0.0001) were significantly lower in patients with bacterial infections than in those without (Table 3). Regarding imaging findings on chest X-ray and chest CT scans, ground-glass opacities and infiltrated shadows were significantly more frequent in the groups with bacterial co-infections and secondary infections than in those without bacterial infections (all P < 0.0001) (Table 4).
Causative pathogens of co-infections and secondary infections
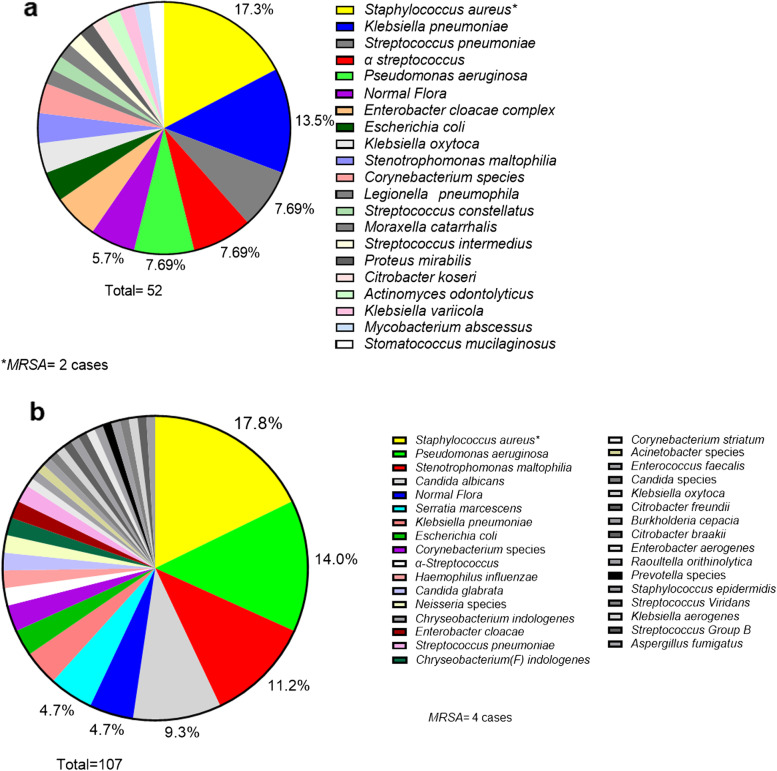
Of the 55 co-infected patients with COVID-19, bacteria were detected in the sputum culture of 31 patients; bacterial species could not be isolated in the sputum culture of 24 patients, and for these patients, the diagnosis was based on clinical judgment.
The most frequently isolated microorganisms in respiratory bacterial co-infections were Staphylococcus aureus (n = 9) and Klebsiella pneumoniae (n = 7) (Fig. 2a). Methicillin-resistant Staphylococcus aureus (MRSA) was isolated from two patients. One or more bacterial species were identified in the sputum culture of 82 of the 86 patients with secondary infection. No bacterial species were identified in the sputum culture of four patients. The most frequently isolated microorganisms were Staphylococcus aureus (n = 19), Pseudomonas aeruginosa (n = 15), and Stenotrophomonas maltophilia (n = 12). MRSA was detected in four cases. Figure 2b details the pathogens associated with respiratory bacterial secondary infections. Tracheal aspirates were obtained in most cases of ventilator-associated pneumonia (VAP); moreover, bronchoalveolar lavage was also performed to identify the causative organisms in one case. Pseudomonas aeruginosa, Staphylococcus aureus, and Stenotrophomonas maltophilia were frequently detected in these patients (Additional file 1).
Fig. 2.
Bacterial pathogens identified in respiratory bacterial co-infection and secondary infection cases with coronavirus disease 2019. a All identified organisms as a proportion of total number of organisms per pathogen in sputum culture and urinary antigen of co-infection pneumonia with coronavirus disease 2019 (COVID-19). Bacterial pathogens detected in COVID-19 patients with respiratory bacterial co-infections, as a proportion (%) of the total number of isolates (n = 47). Some patients had multiple bacterial infection. In 24 cases, no causative organism was detected in sputum cultures. MRSA, Methicillin-Resistant Staphylococcus aureus. b All identified organisms as a proportion of total number of organisms per pathogen in sputum, tracheal aspirate, or bronchoalveolar lavage culture of secondary infection pneumonia with COVID-19. Bacterial pathogens detected in COVID-19 patients with respiratory bacterial secondary infection, as a proportion (%) of the total number of isolates (n = 107). Some patients had more than one bacterial infection. MRSA, Methicillin-Resistant Staphylococcus aureus
Predictive factors of respiratory co-infections
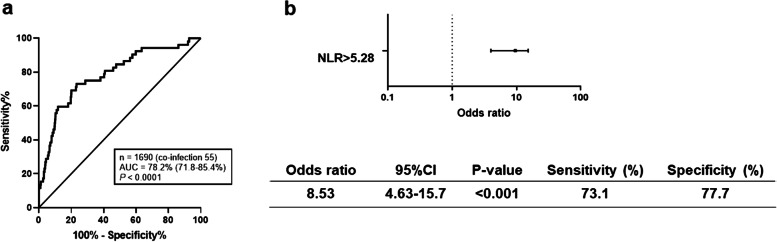
In laboratory parameters of co-infected patients, white blood cells, neutrophil count, lymphocyte count, neutrophil–lymphocyte ratio (NLR), C-reactive protein, and procalcitonin showed significant associations. The receiver operating characteristic curve was determined for each item, and the area under the curve (AUC) was calculated. The parameter NLR (AUC = 0.78) showed the highest value. The OR of NLR in patients with co-infections was 8.53 (4.63–15.7), with a sensitivity of 73.1% and a specificity of 77.7% at the cutoff value of 5.28 (Fig. 3, Additional file 2). Furthermore, we plotted ROC curves to determine predictors of co-infections using the NLR values in steroid users and non-users separately (Additional file 3). The results suggested NLR as a stronger predictive factor of co-infection complications in non-steroid users than in steroid users.
Fig. 3.
Predictive factor of respiratory co-infection. a Receiver operating characteristic curve for NLRs calculated from blood tests within 48 h of admission. b Sensitivity, specificity, and odds ratio for the NLR cutoff of 5.28 for complications of respiratory bacterial co-infections. CI, confidence interval; AUC, area under the curve
Details of the respiratory secondary bacterial infections
Of the 86 secondary infection cases, 54 required the use of IMV, 41 of which developed VAP (Additional file 4a). Fourteen of the 41 VAP cases were repeated cases and had significantly higher mortality rates than those of non-repeated VAP and other secondary infection cases (Additional file 4b, c).
Impact of bacterial infections on clinical prognosis
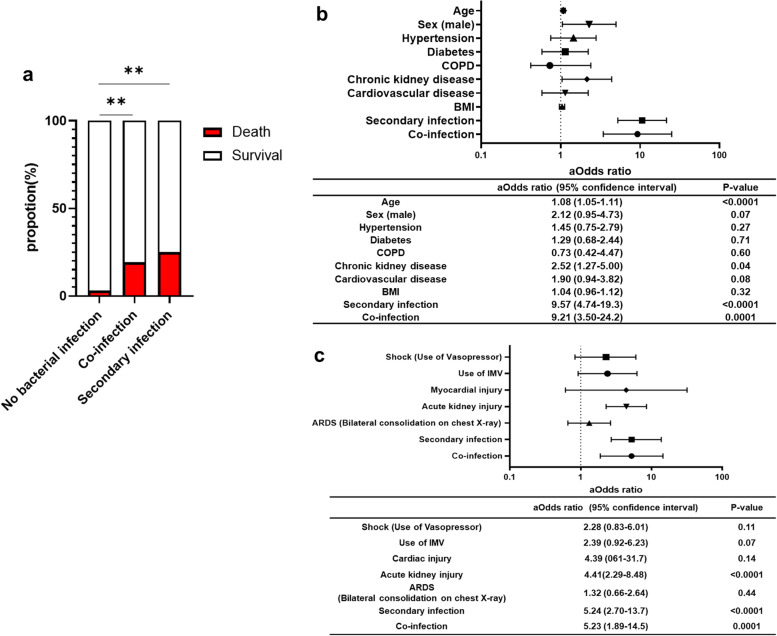
Of the 55 co-infected patients, 18 (37.2%) were admitted to the ICU, 14 (25.5%) were intubated, and 10 (18.1%) died. Furthermore, among the 86 patients with secondary infections, 58 (67.4%) were admitted to the ICU, 54 (62.8%) were intubated, and 63 (75.0%) died. In the univariate analysis, patients with co-infections or secondary infections had higher mortality rates than those without bacterial infections (Fig. 4a). Multivariate logistic regression analysis showed that compared to patients without bacterial infections, those who had co-infections (OR = 9.21 (3.50–24.2)) or secondary infections (OR = 9.57 (4.74–19.3)) were at higher risk of death (Fig. 4b). Moreover, both co-infections (OR = 5.23 (1.89–14.5)) and secondary infections (OR = 5.24 (2.70–13.7)) were risk factors associated with death in the multivariate analysis of model 2, which included IMV use and the complications of AKI, myocardial injury, shock, and ARDS during hospitalization as variables (Fig. 4c). In this study, 141 patients were treated with anti-IL6 receptor antibodies as immunosuppressive therapy. The incidence of secondary infections was significantly increased in the group treated with anti-IL6 receptor antibodies (Additional file 5a). However, in the multivariate analysis, the use of anti-IL6 receptor antibodies was not associated with death (Additional file 5b).
Fig. 4.
Clinical outcome of bacterial respiratory infection with COVID-19. a Proportions of deaths in bacterial co-infection, secondary infection, and non-bacterial infection cases with coronavirus disease 2019 (COVID-19) patients. b Forest plot of adjusted odds (aOdds) ratio via multivariate logistic regression analysis of risk factors of comorbidities associated with death in coronavirus disease 2019 (COVID-19) patients with bacterial infections. BMI, body mass index. c Forest plot of aOdds ratios via multivariate logistic regression analysis of risk factors of critical care category associated with death in coronavirus disease 2019 (COVID-19) patients with bacterial infections. IMV, invasive mechanical ventilation; ARDS, acute respiratory distress syndrome
The univariate analysis also showed that patients with co-infections or secondary infections excluding VAP were more frequently admitted to the ICU and underwent IMV more frequently compared to those without bacterial infections. Both univariate analyses of ICU admission and IMV use were performed in cases of secondary infection occurring prior to these events (Additional file 6a, b). In a multivariate analysis that evaluated risk factors for ICU admission and IMV use, secondary infection excluding VAP and co-infection were independent risk factors for these severe conditions (Additional file 6c). Furthermore, the multivariate analysis of risk factors for death with nosocomial pneumonia and VAP separately revealed that both were independent risk factors (Additional file 7).
The incidence of thrombosis and myocardial injury significantly increased in both the co-infection and secondary infection groups compared to those in the non-bacterial infection group (Additional file 8).
Discussion
In this study, we present a large series of Japanese patients with COVID-19 focusing on the description of respiratory bacterial community-acquired co-infections and hospital-acquired secondary infections in these patients. The incidence of complications from bacterial pneumonia in patients hospitalized due to COVID-19 was less than that from influenza [23]. The frequency of SARS-CoV-2 co-infections with other respiratory pathogens has been reported to be generally low [1, 23]; in the present study of Japanese patients, the rate of respiratory bacterial co-infections was 3.0%. The incidence of bacterial infections secondary to COVID-19 varies in previous studies [6, 23, 24]. In this study, the frequency of secondary infection was 4.6%. The frequency of both co-infections and secondary infections in a previous Japanese study is comparable to that in this study (2.86 and 5.59% vs. 3.0 and 4.6%, respectively) [24].
Interestingly, severity-associated comorbidities such as hypertension, diabetes, and chronic kidney disease were frequently observed in secondary infection cases. For patients with these predisposing factors, it is important to consider the possibility complications associated with secondary bacterial infections. We have shown, using multivariate analysis, that both co-infections and secondary infections were associated with death at a high odds ratio independent of other severity factors. These facts indicate that it is crucial for clinicians to properly assess bacterial complications and initiate appropriate antibiotic therapy.
Among cases with respiratory bacterial co-infections in this study, Staphylococcus aureus, Klebsiella pneumoniae, and other causative organisms like those previously reported were detected in sputum cultures [6, 25]. Staphylococcus aureus is a frequent concomitant in patients with influenza infection [8]. Previous studies have hypothesized that excessive use of broad-spectrum antibiotics, especially for early bacterial co-infections, can induce changes in the microbiota, leading to VAP in the late stages of hospitalization with poor prognosis [9]. Therefore, the choice of antibiotics in the early stage of the treatment is important. If bacterial co-infections are suspected, the use of antibiotics with coverage of Staphylococcus aureus is recommended. There is an urgent need to develop prospective evidence to support the development of antimicrobial strategies and appropriate stewardship interventions specific to COVID-19 treatment, as previously suggested [26].
The frequency of co-infections and secondary infections associated with COVID-19, the identification of their causative organisms, and their association with clinical outcomes [7, 27]. However, in addition to these things, we consider this study valuable as we simultaneously revealed the clinical characteristics of co-infection and secondary infection cases and identified the utility of NLR as a diagnostic parameter for co-infection.
At the time of diagnosis, it is often difficult to discriminate between SARS-CoV-2 infection alone and SARS-CoV-2 infection with concomitant bacterial pneumonia. It remains controversial whether antimicrobial agents should be routinely used. In our study, the parameter NLR was found to be the best indicator of complications from bacterial co-infection with COVID-19 (cutoff, 5.28; AUC, 0.78; sensitivity, 73.1%; specificity, 77.7%). An increase in NLR can be caused by a systemic inflammatory response that disrupts the immunological functions of the body, resulting in a simultaneous increase in neutrophil count and decrease in lymphocyte count. NLR is an indicator of systemic inflammation and is widely used in many diseases, including the prediction of mortality in septic patients [28]. A meta-analysis of multiple observational studies showed that a high NLR is associated with severity and high mortality in COVID-19 [29], possibly attributed to mechanisms by which inflammatory factors, such as interleukin-6, interleukin-8, and granulocyte colony-stimulating factor, that are elicited in SARS-CoV-2 infections may stimulate the production of neutrophils [30].
The increased NLR in COVID-19 patients with bacterial infections might also be the result of a more aggressive neutrophil migration induced by immune responses to bacteria. Various previous studies have suggested optimal cutoff values for the NLR. Four studies aiming at the prediction of illness severity determined differing values ranging from 3.3 to 5.9 [30–32]. Two additional studies that sought to predict mortality established NLR cutoff values of 7.9 and 11.8 [33, 34]. In the present study, the best cutoff value of NLR for predicting complications of respiratory bacterial co-infections was 5.28, which is slightly higher than the value for predicting COVID-19 severity and lower than the value for death in patients with COVID-19. We found that respiratory bacterial co-infections in patients with COVID-19 were associated with ICU admission, IMV use, and high mortality. Thus, the cutoff value of 5.28 for NLR in this study is reasonable. Compared to other prognostic parameters for COVID-19, such as interleukin-6, D-dimers, C-reactive protein, and erythrocyte sedimentation rate [35, 36], NLR is more practical for clinical application since it can easily be obtained via routine blood examinations.
Our study is the first to suggest that NLR may help diagnose cases of bacterial co-infection in patients with COVID-19, and our calculated NLR cutoff value will be useful in making decisions regarding the concomitant use of antibiotics. Importantly, since steroids increase blood neutrophil counts and decrease lymphocyte counts, an elevated NLR can be confused with an infectious complication. In this study, the predictive accuracy of co-infection by NLR in the non-steroid users was superior to that in steroid users, suggesting that clinicians need to be cautious in this regard.
Regarding the organisms isolated in cases with secondary infections, the same previously reported species, such as Staphylococcus aureus, Pseudomonas aeruginosa, and Stenotrophomonas maltophilia, were detected [6]. Infections with Staphylococcus aureus secondary to influenza infections are common, which may be due to excessive mucus secretion, decreased clearance of mucosal cilia, and destruction of epithelial cells [8]. The mechanisms involved in secondary Staphylococcus aureus infections following SARS-CoV-2 infection remain poorly understood and require further research.
In a study analyzing the association between upper respiratory tract flora and clinical prognosis in patients with COVID-19, streptococcus-dominated microbiota was associated with lower severity of illness and lower mortality [37]. However, how the microbiota of the respiratory tract is altered by COVID-19 remains largely unknown [38]. Many of the organisms isolated from the sputum of secondary infection cases in our study are known to cause hospital-acquired pneumonia. Since secondary infections are independently associated with poor outcomes such as high mortality, further research, especially on how bacterial infections occur during hospitalization, is essential to reduce the incidence of secondary infections and provide appropriate therapeutic management.
Our study has several limitations. First, this study included only hospitalized patients with COVID-19, which might have resulted in a biased sample due to the high severity of the disease. Second, the diagnosis of bacterial complications was entirely dependent on the clinical judgment of the physician, and microbiological tests were not necessarily required to prove the causative pathogens. Two studies that strictly analyzed the frequency of VAP and causative organisms in patients who required IMV reported much higher rates of bacterial co-infections compared to those reported by studies that employed only the usual methods of care to identify bacterial pathogens [39]. This is a major limitation of our study. Regrettably, we were unable to prospectively assess all cases for the rigorous approach to detect bacterial pathogens in this multicenter study. Moreover, bacteriological evaluation, including evaluation of urinary antigens and endotracheal aspiration, as well as molecular biological evaluation, such as comprehensive viral and bacterial PCR tests, were not performed; thus, the identification of the causative organisms might have been underestimated. Third, there might not have been standardized management practices among different facilities during the study period. In particular, antibiotic administration and treatment methods have not been standardized, which may have affected the clinical outcomes. Fourth, this study included only Japanese patients; therefore, it may be inappropriate to generalize or extrapolate the present results to populations of patients with COVID-19 in other countries. Fifth, the sample size of this study was determined incidentally and was not designed to be statistically sufficient to detect the effects of complications from bacterial infections on severe illness and death. Further studies are needed to address these limitations and develop optimal treatment strategies.
Conclusions
In this study, we determined the frequency of bacterial co-infections and secondary infections in Japanese patients with COVID-19 and the causative organisms, as well as their associations with clinical outcomes. We also revealed the characteristics of patients with bacterial co-infections and secondary infections. Although co-infections and secondary infections in COVID-19 are infrequent, they are significantly associated with disease severity and high mortality, suggesting that the evaluation of bacterial infections and appropriate antimicrobial therapies are crucial in the treatment of COVID-19. Notably, our results suggest that NLR may be useful in the diagnosis of bacterial co-infections with COVID-19.
Supplementary Information
Additional file 1. Identification of organisms in ventilator-associated pneumoniacases
Additional file 2. Evaluation of white blood cells, neutrophils, lymphocytes, neutrophil-lymphocyte ratio, C-reactive protein, and procalcitonin on admission as predictors of respiratory bacterial co-infection based on the area under the curve
Additional file 3. Neutrophil-lymphocyte ratioas a predictor of co-infection in steroid and non-steroid users
Additional file 4. Details of respiratory secondary infection.
Additional file 5. Association of anti-IL-6 receptor antibody use with incidence of secondary infection and death.
Additional file 6. Admission to intensive care unitand use of invasive mechanical ventilationof bacterial respiratory infection with coronavirus disease 2019.
Additional file 7. Forest plot of adjusted oddsratios by multivariate logistic regression analysis of risk factors of death in patients of coronavirus disease 2019with secondary infection except ventilator-associated pneumoniaand VAP.
Additional file 8. Proportion of thrombosis and myocardial injury in bacterial respiratory co-infection and secondary infection with coronavirus disease 2019.
Acknowledgements
We would like to thank all the participants involved in this study, and all members of the Japan COVID-19 Task Force engaged in clinical and research work on COVID-19 every day. All members contributed cases to this study.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory coronavirus 2
- ICU
Intensive care unit
- IMV
Invasive mechanical ventilation
- ARDS
Acute respiratory distress syndrome
- BMI
Body mass index
- OR
Odds ratio
- COPD
Chronic obstructive pulmonary disease
- MRSA
Methicillin-resistant Staphylococcus aureus
- VAP
Ventilator-associated pneumonia
- NLR
Neutrophil-lymphocyte ratio
- AUC
Area under the curve
Authors’ contributions
Conceptualization: K.N., H.K., S.C., H.N., K.M., H.K., M.I., N.H., K.F. Data curation and interpretation: K.N., H.K., S.C., H.N., H.T., H.L., S.O., T.F., T.K., A.M., S.A., M.W., K.M., T.A, M.I., A.E., R.K., H.I., T.T., Y.M., N.H., H.K., T.Y., K.K., Y.M., M.M., S.U., H.O., T.O., T.S., H.M., K.K., N.M., T.M., M.F., Y.C., Y.N., M.O., M.A., T.O., M.T., K.N., Y.S., R.E., A.A, N. H., S.O., Y.K., T.K., T.K., Y.K., N.M., T.T., S.F., K.M., H.S., Y.S., Y.T., R.O., S.M., M.K., Y.H., Y.H., T.U., Y.T., T.I., A.F., N.K., H.K., E.H., Y.N., F.S., Y.K., S.A., T.I., T.O., Y.K., H.W., M.M., H.W., Y.K., A.K., Y.K., S.H., Y.O., T.T., K.K., M.A., A.K., T.S., K.T., S.I., Y.K., A.K., S.M., N.H., S.O., T,K., K.F. Formal analysis: K.N., H.K., S.C., H.N. Methodology: K.N., H.K., S.C., H.N. Supervision: K.N., H.K., S.C., H.N. K.M., M.I., N.H., N.H., T.U., S.U., T.I., K.A., F.S., T.Y., Y.N., Y.M., Y.S., K.M., Y.O., R.K., Y.K., A.K., S.I., S.M., S.O., T.K., K.F. Visualization: H.K., S.C., H.N. Writing—original draft: K.N., H.K. Writing—review and editing: K.N., H.K., S.O., H.T., H.L., A.M., T.F., M.W., T.K., R.K., T.T., N.H., T.Y.,Y.M., S.U., H.O., T.S., K.K., T.M., Y.O., T.T., M.O., T.O., K.N., R.E., N.H., Y.K., T.K., N.M., T.Y., K.M., M.Y., Y.S., Y.S., S.M., S.H., Y.H., T.U., T.I., N.K., T.K.,T.O., F.S., Y.K., S.A., T.I., Y.K., H.W., H.W., M.N., Y.K., S.H., Y.O., T.T., K.K., M.A., A.K., T.S., K.T., S.I., Y.K., A.K., S.M., N.H., S.O., T.K., K.F. The author(s) read and approved the final manuscript.
Funding
This work was supported by Japan Agency for Medical Research and Development (AMED), Ministry of Health, Labour and Welfare (MHLW), Japan Science and Technology Agency (JST), Takeda Science Foundation, Mitsubishi Foundation and Bioinformatics Initiative of Osaka University Graduate School of Medicine, Osaka University. K.F. received fundings from AMED (JP20fk0108415) and MHLW (20CA2054). T.K. received funding from AMED (JP20nk0101612). S.I. received funding from JST CREST (JPMJCR20H2). H.N. received funding from JST PREST (JPMJPR21R7). Y.O. received fundings from AMED (JP21jk0210034, JP21km0405211 and JP21km0405217), Takeda Science Foundation, Mitsubishi Foundation and Bioinformatics Initiative of Osaka University Graduate School of Medicine, Osaka University.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Keio University School of Medicine [20200061] and affiliated institutes. Written or oral informed consent was obtained from all patients. All aspects of the study conformed to the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–7. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah NS, Greenberg JA, McNulty MC, Gregg KS, Riddell J, Mangino JE, et al. Bacterial and viral co-infections complicating severe influenza: incidence and impact among 507 U.S. patients, 2013–14. J Clin Virol. 2016;80:12–9. doi: 10.1016/j.jcv.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martín-Loeches I, Sanchez-Corral A, Diaz E, Granada RM, Zaragoza R, Villavicencio C, et al. Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A[H1N1] virus. Chest. 2011;139:555–562. doi: 10.1378/chest.10-1396. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS One. 2021;16:e0251170. [DOI] [PMC free article] [PubMed]
- 8.Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh Y-H, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10:394–403. doi: 10.1111/irv.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Scaravilli V, Mangioni D, Scudeller L, Alagna L, Bartoletti M, et al. Hospital-acquired infections in critically ill patients with COVID-19. Chest. 2021;160:454–465. doi: 10.1016/j.chest.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka H, Lee H, Morita A, Namkoong H, Chubachi S, Kabata H, et al. Clinical characteristics of patients with Coronavirus Disease [COVID-19]: preliminary baseline report of Japan COVID-19 task force, a nationwide consortium to investigate host genetics of COVID-19. Int J Infect Dis. 2021;113:74–81. doi: 10.1016/j.ijid.2021.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Namkoong H, Edahiro R, Fukunaga K, Shirai Y, Sonehara K, Tanaka H, et al. A nation-wide consortium to elucidate Host Genetics of COVID-19 Pandemic in Japan. medRxiv. 2021;2021.
- 12.Geng L, He C, Kan H, Zhang K, Mao A, Zhang C, et al. The association between blood pressure levels and mortality in critically ill patients with COVID-19 in Wuhan, China: a case-series report. Hypertens Res. 2021;44:368–370. doi: 10.1038/s41440-020-00594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santoso A, Pranata R, Wibowo A, Jibril Al-Farabi M, Huang I, Budhi Antariksa B, et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2021;44:352–357. doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh J, Malik P, Patel N, Pothuru S, Israni A, Chakinala RC, et al. Kidney disease and COVID-19 disease severity-systematic review and meta-analysis. Clin Exp Med. 2022;22:125–35. [DOI] [PMC free article] [PubMed]
- 16.Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 cardiovascular disease registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 17.O'Driscoll M,Ribeiro Dos Santos G, Wang L, Azman AS, Paireau J, Fontanet A,, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–5. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 18.Jin JM, Bai P, He W, Wu F, Liu X-F, Han D-M, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang D, Lian X, Song F, Ma H, Lian Z, Liang Y, et al. Clinical features of severe patients infected with 2019 novel coronavirus: a systematic review and meta-analysis. Ann Transl Med. 2020;8:576. doi: 10.21037/atm-20-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu Y, Sun J, Dai Z, Deng H, Li X, Huang Q, et al. Prevalence and severity of corona virus disease 2019 [COVID-19]: a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang R, Elhusseiny KM, Yeh YC, Sun WZ. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes-A systematic review and metaanalysis. PLoS One. 2021;16:e0246318. [DOI] [PMC free article] [PubMed]
- 22.Ishii M, Terai H, Kabata H, Masaki K, Chubachi S, Tateno H, et al. Clinical characteristics of 345 patients with coronavirus disease 2019 in Japan: a multicenter retrospective study. J Infect. 2020;81:e3–e5. doi: 10.1016/j.jinf.2020.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langford BJ, So M, Raybardhan S, Leung V, Westwood D, MacFadden DR, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takazono T, Mukae H, Izumikawa K, Kakeya H, Ishida T, Hasegawa N, et al. Empirical antibiotic usage and bacterial superinfections in patients with COVID-19 in Japan: a nationwide survey by the Japanese respiratory society. Respir Investig. 2021. [DOI] [PMC free article] [PubMed]
- 25.Lai CC, Wang CY, Hsueh PR. Co-infections among patients with COVID-19: the need for combination therapy with non-anti-SARS-CoV-2 agents? J Microbiol Immunol Infect. 2020;53:505–512. doi: 10.1016/j.jmii.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawson TM, Moore LSP, Zhu N, Ranganathan N, Skolimowska K, Gilchrist M, et al. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Wang J, Yang Y, Cai P, Cai P, Cao J, Cai X, et al. Etiology and antimicrobial resistance of secondary bacterial infections in patients hospitalized with COVID-19 in Wuhan, China: a retrospective analysis. Antimicrob Resist Infect Control. 2020;9:153. doi: 10.1186/s13756-020-00819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarı R, Karakurt Z, Ay M, Celik ME, Tekan UY, Ciyiltepe F, et al. Neutrophil to lymphocyte ratio as a predictor of treatment response and mortality in septic shock patients in the intensive care unit. Turk J Med Sci. 2019;49:1336–1349. doi: 10.3906/sag-1901-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simadibrata DM, Calvin J, Wijaya AD, Ibrahim NAA. Neutrophil-to-lymphocyte ratio on admission to predict the severity and mortality of COVID-19 patients: a meta-analysis. Am J Emerg Med. 2021;42:60–69. doi: 10.1016/j.ajem.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun S, Cai X, Wang H, Hed G, Line Y, Lue B, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou. China Clin Chim Acta. 2020;507:174–180. doi: 10.1016/j.cca.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng ZY, Feng SD, Chen GP, Wu JN. Predictive value of the neutrophil to lymphocyte ratio for disease deterioration and serious adverse outcomes in patients with COVID-19: a prospective cohort study. BMC Infect Dis. 2021;21:80. doi: 10.1186/s12879-021-05796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou J, Huang L, Chen J, Yuan X, Shen Q, Dong S, et al. Clinical features predicting mortality risk in older patients with COVID-19. Curr Med Res Opin. 2020;36:1753–1759. doi: 10.1080/03007995.2020.1825365. [DOI] [PubMed] [Google Scholar]
- 34.Yan X, Li F, Wang X, Yan J, Zhu F, Tang S, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study. J Med Virol. 2020;92:2573–2581. doi: 10.1002/jmv.26061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simadibrata DM, Lubis AM. D-dimer levels on admission and all-cause mortality risk in COVID-19 patients: a meta-analysis. Epidemiol Infect. 2020;148:e202. doi: 10.1017/S0950268820002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tjendra Y, Al Mana AF, Espejo AP, Akgun Y, Millan NC, Gomez-Fernandez C, et al. Predicting disease severity and outcome in COVID-19 patients: a review of multiple biomarkers. Arch Pathol Lab Med. 2020;144:1465–1474. doi: 10.5858/arpa.2020-0471-SA. [DOI] [PubMed] [Google Scholar]
- 37.Ren L, Wang Y, Zhong J, Li X, Xiao Y, Li J, et al. Dynamics of the upper respiratory tract microbiota and its association with mortality in COVID-19. Am J Respir Crit Care Med. 2021;204(12):1379–1390. doi: 10.1164/rccm.202103-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu R, Lu R, Zhang T, Wu Q, Cai W, Han X, et al. Temporal association between human upper respiratory and gut bacterial microbiomes during the course of COVID-19 in adults. Commun Biol. 2021;4:240. doi: 10.1038/s42003-021-01796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouzé A, Martin-Loeches I, Povoa P, Makris D, Artigas A, Bouchereau M, et al. Relationship between SARS-CoV-2 infection and the incidence of ventilator-associated lower respiratory tract infections: a European multicenter cohort study. Intensive Care Med. 2021;47:188–198. doi: 10.1007/s00134-020-06323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Identification of organisms in ventilator-associated pneumoniacases
Additional file 2. Evaluation of white blood cells, neutrophils, lymphocytes, neutrophil-lymphocyte ratio, C-reactive protein, and procalcitonin on admission as predictors of respiratory bacterial co-infection based on the area under the curve
Additional file 3. Neutrophil-lymphocyte ratioas a predictor of co-infection in steroid and non-steroid users
Additional file 4. Details of respiratory secondary infection.
Additional file 5. Association of anti-IL-6 receptor antibody use with incidence of secondary infection and death.
Additional file 6. Admission to intensive care unitand use of invasive mechanical ventilationof bacterial respiratory infection with coronavirus disease 2019.
Additional file 7. Forest plot of adjusted oddsratios by multivariate logistic regression analysis of risk factors of death in patients of coronavirus disease 2019with secondary infection except ventilator-associated pneumoniaand VAP.
Additional file 8. Proportion of thrombosis and myocardial injury in bacterial respiratory co-infection and secondary infection with coronavirus disease 2019.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.