Abstract
Gram-positive (G+) bacterial infection is a great burden to both healthcare and community medical resources. As a result of the increasing prevalence of multidrug-resistant G+ bacteria such as methicillin-resistant Staphylococcus aureus (MRSA), novel antimicrobial agents must urgently be developed for the treatment of infections caused by G+ bacteria. Endolysins are bacteriophage (phage)-encoded enzymes that can specifically hydrolyze the bacterial cell wall and quickly kill bacteria. Bacterial resistance to endolysins is low. Therefore, endolysins are considered promising alternatives for solving the mounting resistance problem. In this review, endolysins derived from phages targeting G+ bacteria were classified based on their structural characteristics. The active mechanisms, efficacy, and advantages of endolysins as antibacterial drug candidates were summarized. Moreover, the remarkable potential of phage endolysins in the treatment of G+ bacterial infections was described. In addition, the safety of endolysins, challenges, and possible solutions were addressed. Notwithstanding the limitations of endolysins, the trends in development indicate that endolysin-based drugs will be approved in the near future. Overall, this review presents crucial information of the current progress involving endolysins as potential therapeutic agents, and it provides a guideline for biomaterial researchers who are devoting themselves to fighting against bacterial infections.
Keywords: Bacteriophage, Endolysins, Gram-positive bacteria, Infectious diseases
Introduction
The extensive use of antibiotics promotes the crisis of antimicrobial resistance (AMR), which has made the clinical treatment of bacterial infections difficult and poses a challenge to global public health; the AMR problem requires immediate action, preferably one that is long term [1, 2]. Drug-resistant bacterial infections can result in at least 50,000 deaths every year in Europe and the United States and hundreds of thousands of victims in other regions of the world [3], leading to a loss of $3 trillion in gross domestic product annually [4]. In 2017, the World Health Organization published a list of global priority pathogens that require the exploration and development of novel antimicrobials [5]. Among these pathogens, Gram-positive (G+) bacteria occupy a large proportion in the clinical detection of drug-resistant bacteria, especially methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecium, and β-lactamase-resistant Streptococcus pneumoniae, which are major healthcare problems [5]. Therefore, novel antimicrobial agents must be urgently developed to combat the infections caused by drug-resistant G+ bacteria.
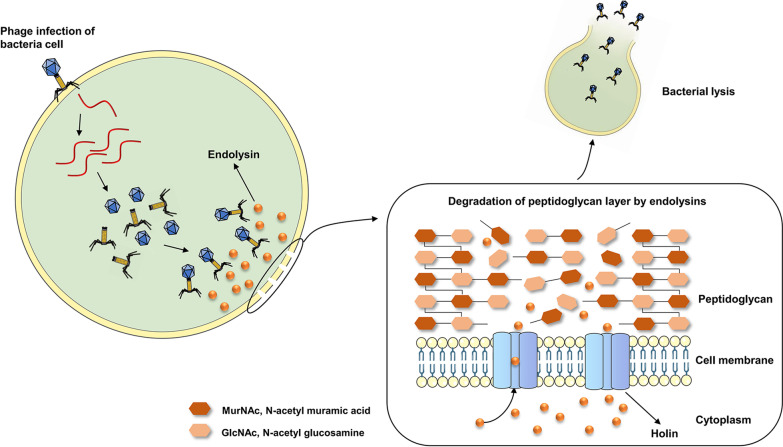
Bacteriophages (phages) are the most abundant biological entities on earth. They are widespread all over the biosphere from the soil to marine environments, the atmosphere, and the human body. Phages are viruses that can specifically infect and rapidly kill the bacterial hosts in their lytic life cycles [6, 7]. After replication inside the bacterial cells, phages need to exit from the bacterial hosts to release assembled progeny virions. The phages evolved a lytic system to digest the bacterial cell wall, thereby inducing bacterial lysis [8]. Phage endolysins are highly efficient molecules that have been used by phages for billions of years for this exact purpose. Endolysins can access the peptidoglycan through membrane lesions formed by the second phage-encoded proteins (holins); they degrade the integrity of the cell wall from the inside of the bacteria (Fig. 1) [9, 10]. About half of the bacteria on earth can be killed by their phages in 48 h, making endolysins the most effective and widespread bactericidal agents on the planet [7]. Although intact phages can also be an antibacterial option, endolysins have more advantages compared with phage particles, making them important candidates for use as alternatives to antibiotics [11, 12].
Fig. 1.
The role of endolysins and holins in the process of phage infection of a G+ bacterium. After replication inside the bacterial cell, progeny phages utilize a lytic system including endolysins and holins to destroy the integrity of the cell wall from the inside of the bacterium and release the assembled phage virions
The presence of the outer membrane of Gram-negative (G−) bacteria effectively presents a physical protective barrier against endolysins, which can directly target the bonds in the peptidoglycan and lyse the cell wall of G+ bacteria that do not have outer membranes [13–15]. This discovery prompted scientists to attempt to harness the bacteriolytic properties of endolysins to treat G+ bacterial infections. Many recombinant endolysins have already been expressed, identified, and purified; they sufficiently display potent bacteriolytic activity against G+ bacteria [15–17]. In addition, phage endolysins can eradicate staphylococcal and streptococcal biofilms in a short time [18–20]. For example, CF-301 removes all biofilms in catheters within 1 h [20], purified CHAPK completely eliminates the staphylococcal biofilms within 4 h [21], and ClyF decreases the 25.2–93.5% biofilm mass within 45 min [22]. Furthermore, multiple in vitro and in vivo experiments have demonstrated that endolysins, such as PlyC [23], PlyG [24], Cpl-1 [25], CHAPK [18], LysGH15 [26], and LysP108 [11], are effective against a variety of G+ bacterial infections. The endolysin-based candidate drugs such as P128 and N-Rephasin® SAL200 are being tested in phases II and IIa in the treatment of S. aureus bacteremia, respectively [27–29]. However, CF-301 failed in the phase III clinic trials. Here, we present an overview of the characteristics and antimicrobial potential of endolysins derived from phages and evaluate whether they can alternate or sensitize conventional antibiotics in the treatment of G+ bacterial infections.
Structure and classification of endolysins
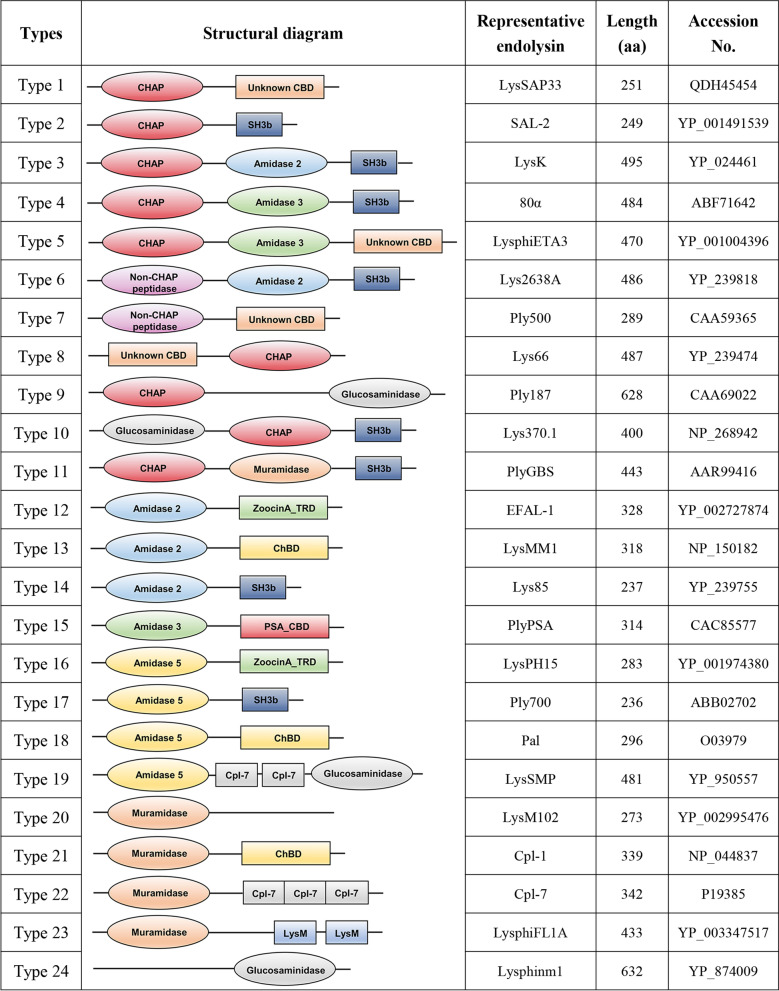
The structures of phage endolysins are determined by their origin. In General, endolysins produced by phages infecting G− bacteria (molecular weight, 15–20 kDa) have a simple globular configuration, whereas most of the endolysins derived from phages targeting G+ bacteria (molecular weight, 25–40 kDa) comprise two modular structures: an N-terminal catalytic domain (CD) joined by a flexible linker to a C-terminal cell wall-binding domain (CBD) [30–32]. Some of them feature a modular architecture comprising two different types of functional domains linked jointly to a single CBD, particularly staphylococcal endolysins [33, 34]. A central CBD can separate the two CDs, and this structure is presented in streptococcal endolysins λSA2 and PlySK1249 [35–37]. Specifically, a unique endolysin Ply187, from a S. aureus phage 187, has two CDs but lacks a CBD [38–40]. Therefore, the abundant modular structures of endolysins are diverse, and the function of different CDs and CBDs is distinct. To better understand these complex endolysins derived from phages targeting G+ bacteria (mainly including Staphylococcus, Streptococcus, Enterococcus, and Listeria), we propose a systematic classification of these endolysins based on their domain compositions (Fig. 2) and update other types of staphylococcal endolysins given that they were classified into six types [41, 42]. The information from the National Center for Biotechnology Information database on the representatives of different types of endolysins is shown in Fig. 2. In general, the N-terminal CD of endolysin is responsible for hydrolyzing various specific peptidoglycan bonds of G+ bacteria [14, 43, 44]. By contrast, the C-terminal CBD recognizes and non-covalently binds to different ligands (usually carbohydrate) in the cell wall for proper fixation of the CDs [13, 45, 46]. Although the C-terminal CBD is required to maintain the intact lytic activity of CDs [47, 48], truncation or deletion of the CBD can also result in equal or increased lytic activity of the mutants [43, 49, 50].
Fig. 2.
The typical modular structures of different types of endolysins derived from phages targeting G+ bacteria. 24 types of endolysins are proposed according to their molecule structures. CHAP cysteine- and histidine-dependent aminopeptidase/hydrolase, SH3 bacterial Src homology 3 domain, responsible for cell-wall peptidoglycan recognition and binding, ChBD choline-binding domain, PSA_CBD cell wall-binding domain, ZoocinA_TRD a target recognition domain, Cpl-7 Cpl-7-like cell wall-binding domain, LysM a small domain involved in binding peptidoglycan
Sequence comparison of endolysins of the same type shows high homology within the N-terminal enzymatically active domain and low similarity within the C-terminal cell binding region [14, 51]. The phages that infect G+ bacteria have naturally designed such distinct domain structures to better disseminate the progeny particles [8]. The similarity of the amino acid sequences of the endolysin CDs may be explained by the conserved peptidoglycan bonds of bacterial hosts, whereas most of the CBDs may have evolved to target unique components of the cell wall of the host bacteria at high affinity, thereby resulting in variability, high selectivity, and low propensity for developing resistance [15, 51, 52]. The modular structure of endolysins can be exploited for bioengineering, because different domains can be genetically swapped or shuffled among different endolysins, thereby generating novel fused enzymes with high specificity and catalytic activity [52–54]. For example, the recombinant chimeric endolysin PRF-119, which was designed with a CD, a cysteine- and histidine-dependent aminopeptidase/hydrolase (CHAP) domain from the endolysin of phage K, and a CBD from the lysostaphin, is highly active against S. aureus, including MRSA [55]. In addition, as a chimeric phage endolysin, Ply187AN-KSH3b exhibits strong antimicrobial activity against S. aureus, including disruption of biofilms and protection of mice from S. aureus endophthalmitis [56]. Therefore, the modular arrangement of endolysins has enormous potential in the creative design of important enzymes with specific functions or features.
Mode of endolysin action
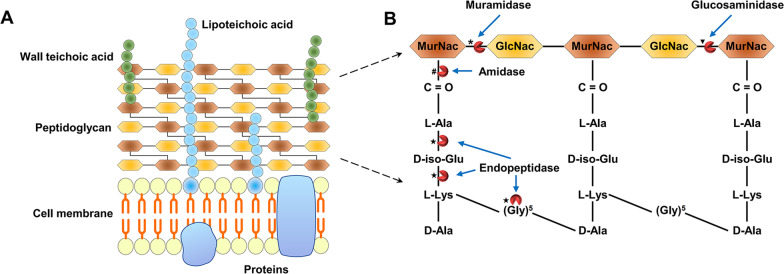
The modular structure of endolysins is closely related to their mode of action. With the individual binding specificity of CBD, endolysin CDs kill bacteria by enzymatically degrading the peptidoglycan of the bacterial cell wall, which protects the cell protoplast from mechanical damage and osmotic lysis and is essential to bacterial viability. Compared with G− bacteria, the cell walls of G+ bacteria are thicker (15–80 nm) and consist of tens of layers of peptidoglycan associated with teichoic acids (Fig. 3A) [51]. Apart from lytic transglycosylases (e.g., phage λ lysozyme), endolysins are peptidoglycan hydrolases that use a water molecule to catalyze the cleavage of different bonds (Fig. 3B), such as β-1,4 glycosidic bond, amide bond, and peptide bond [51]. Most staphylococcal phage endolysins have two catalytic domains: a CHAP domain with D-Ala-Gly activity and an amidase domain with MurNAc-L-Ala activity [57]. Electron microscopy revealed that endolysin-mediated peptidoglycan digestion leads to perforation in the cell wall, through which the high intracellular osmotic pressure squeezes the cytoplasmic membrane to cause hypotonic lysis of the bacteria within seconds. By contrast, antibiotics depend on the inhibition of a metabolic pathway and require more steps and time to arrest bacterial growth or kill bacterial cells [14, 16, 52, 58]. Moreover, endolysins can effectively eliminate staphylococcal biofilms and reduce bacterial persisters due to the active mode of action, resulting in the successful therapy of chronic infections after treatment failure by antibiotics [59, 60].
Fig. 3.
The function of endolysin catalytic domains encoded by phages infecting G+ bacteria. A Schematic representation of the G+ bacterial cell wall. B Diagram of the peptidoglycan bonds cleaved by different endolysins. MurNAc and GlcNAc are repeating units of the glycan strands that are linked to a stem peptide through an amide bond to the MurNAc. Stem peptides are then cross-linked through a pentaglycine (in the case of S. aureus) to adjacent stem peptides forming a tight stable net around the bacterium. Based on the cleaved chemical bonds within the peptidoglycan layer, endolysins have several enzyme activities, including muramidase (N-acetylmuramidase), glucosaminidase (N-acetyl-β-D-glucosaminidases), amidase (N-acetylmuramoyl-L-alanine amidase), and endopeptidase (L-alanoyl-D-glutamate endopeptidase or interpeptide bridge-specific endopeptidases). MurNAc N-acetyl muramic acid, GlcNAc N-acetyl glucosamine, L-Ala L-alanine, D-iso-Glu D-iso-glutamic acid, L-Lys L-lysine, D-Ala D-alanine. *β-1,4 glycosidic bond between MurNAc and GlcNAc. ▼β-1,4 glycosidic bond between GlcNAc and MurNAc. #amide bond between MurNAc and L-Ala. ★peptide bond between two amino acids
Efficacy of endolysins
Endolysins show bactericidal activity against certain bacterial species that are closely related to the bacterial hosts of the phages from which they were produced. Despite their strong specificity, the host range of endolysins can reach approximately two-thirds of the tested strains, and some even reach 100% (Table 1), which is significantly stronger than the host range of the phage itself [36]. For instance, a purified pneumococcal phage endolysin (Pal) can kill 15 common serotypes of pneumococci, including highly penicillin-resistant strains [16]. In many cases, endolysins might be identified with extended lytic activity (Table1). For instance, LysPBC2 was isolated from a Bacillus cereus phage and displayed very broad lytic activity against all Bacillus, Listeria, and Clostridium species tested [70]. An enterococcal phage endolysin PlyV12 reportedly kills not only enterococci but also several other G+ pathogens, such as streptococci and staphylococci [71]. Furthermore, endolysins have been successfully exploited to kill G+ pathogenic bacteria in a dose-dependent manner, including antibiotic-sensitive bacteria and antibiotic-resistant ones, such as B. anthracis and B. cereus [24], C. difficile [71], C. perfringens [72], E. faecalis and E. faecium [70], L. monocytogenes [58], S. aureus [73], S. agalactiae [74, 75], and S. pyogenes [76]. An earlier study found that 2 units (U) (2 μg) of recombinant phage endolysin PlyG can destroy 1.0 × 104 colony formation unit (CFU) of streptomycin-resistant B. cereus within 10 s [24]. In a separate kinetic assay, the addition of 2 U of PlyG to 1 mL of log-phase B. cereus cells resulted in a 17,000-fold decrease of bacterial numbers within 20 s and near sterilization at 2 min when compared with 50 mM Tris buffer treatment [24]. Therefore, endolysin has strong lytic efficacy against bacterial cells.
Tabel 1.
The host range of representative endolysins
| Endolysin and/or derivative | Origin | The number of tested strains | The number of lysed strains | Host range | References |
|---|---|---|---|---|---|
| SAL200 | Staphylococcal phage SAP-1 | 425 | 425 | 100% | [61] |
| Exebacase (CF-301 or PlySs2) | Prophage of Streptococcus suis | 477 | 365 | 77% | [62] |
| P128 | CHAP domain (TAME phage K) + SH3b (lysostaphin) | 62 | 62 | 100% | [63] |
| Staphefekt SA.100 | M23 endopeptidase (lysostaphin) + Amidase (Ply2638) + SH3b (Ply2638) | 11 | 10 | 91% | [64] |
| XZ.700 | Staphefekt SA.100 deleted 44 amino acids region | 120 | 107 | 89% | [64] |
| LysK | Staphylococcal phage K | 27 | 18 | 67% | [65] |
| CHAPK (truncated LysK) | Staphylococcal phage K | 31 | 28 | 90% | [66] |
| ClyF | CD domain (Ply187) + CBD domain (PlySs2) | 51 | 45 | 88% | [22] |
| LysGH15 | Staphylococcal phage GH15 | 57 | 52 | 91% | [67] |
| LysH5 | Staphylococcal phage PhiH5 | 90 | 77 | 86% | [68] |
| Pal | Streptococcal phage Dp-1 | 25 | 19 | 76% | [16] |
| Cpl-1 | Streptococcal phage Cp-1 | 27 | 22 | 81% | [69] |
| PlyG | B. anthracis γ-phage | 27 | 16 | 59% | [24] |
TAME tail-associated muralytic enzymes, CHAP cysteine- and histidine-dependent aminopeptidase/hydrolase, CD catalytic domain, CBD cell wall-binding domain
Unlike antibiotics, which are small molecules and generally non-immunogenic, one of the potential concerns with endolysin treatment is the adverse immune response induced by the generation of neutralizing antibodies that may reduce in vivo endolysin activity after systemic and mucosal application [8, 77]. Early studies have confirmed that although endolysins are immunogenic, antibodies against the corresponding endolysins specific for B. anthracis, S. aureus, S. pneumoniae, or S. pyogenes, obtained from rabbit hyperimmune serum do not remarkably diminish lytic activity in vitro [76, 78, 79]. For example, the bactericidal activity and binding capacity of staphylococcal-specific endolysin LysGH15 were not blocked even after incubation with anti-LysGH15-serum for 60 min [26]. Furthermore, experiments with pneumococcal-specific endolysin Cpl-1 in immunized rabbit serum (in vitro) and immunized mice (in vivo) did not affect its therapeutic efficacy [69]. These results were verified with endolysins MV-L and Pal [80, 81]. Collectively, endolysins are hardly affected by the immune response. Thus, they have almost no loss of efficacy or adverse effect when applied to treat bacterial infections, which may be partially explained by the strong binding affinity of an endolysin to its cell wall substrate and rapid bactericidal activity, which outcompetes the hosts’ immune response [8, 79].
Advantages of endolysins used as antimicrobial agents
Given the resistance crisis, phage therapy was proposed and served as a powerful regimen for clinical infections [36]. However, the narrow antimicrobial spectrum, complicated pre-clinical and clinical evaluation, and improper regulatory framework of phages hamper the wide application of phage therapy [27, 36]. Compared with active phages, endolysins develop considerably faster and have many advantages, such as non-proliferation, fast bactericidal activity, wide host spectrum, definite pharmacokinetics, and low possibility of resistance development (Table 2), making endolysins important candidates as the alternatives of antibiotics, especially for drug-resistant bacteria. Among these advantages, low possibility of resistance development is most prominent for endolysins to overwhelm phage therapy and antibiotics [78]. Phages have coevolved with bacteria for billions of years. To avoid being trapped in the host, the CBD of endolysins has evolved to target the highly conserved bonds within the peptidoglycan of the cell wall, which is necessary for bacterial viability; thus, resistance to these enzymes is a rare event [8, 52]. This speculation was confirmed by the evidence that the binding epitopes for endolysin CBD in the cell walls of pneumococci, Group A streptococci, and B. anthracis are choline [91], polyrhamnose [84], and neutral polysaccharide [24], respectively, which are important molecules for bacterial growth. To our knowledge, no case of resistance to endolysins has ever been reported; thus, corresponding mutants hardly survive. Even repeated exposure of staphylococci, pneumococci, and B. cereus to low concentrations of endolysins on agar plates or in broth culture does not identify spontaneously resistant mutants, whereas a concomitant 1,000-fold and 10,000-fold increase in novobiocin and streptomycin resistance could be observed [16, 24]. Endolysin ClyS displays a decreased potential for the development of resistance compared with mupirocin when MRSA or methicillin-sensitive S. aureus (MSSA) was exposed to increasing concentrations (1/32 × to 4 × minimal inhibitory concentration, MIC) of either agent for over 8 days in vitro [78]. LysGH15 also does not induce resistance in MRSA or MSSA strains after repeated treatment with sub-MIC [26]. The expression of thick polysaccharide capsules by streptococci or B. anthracis or the formation of dense biofilms by staphylococci or streptococci does not block endolysin lytic activity [74, 79, 92]. Therefore, the intrinsic endolysin resistance is seldom, which is a great advantage for the use of endolysin as a promising therapeutic agent.
Table 2.
Comparison of the properties of antibiotic, phage, and endolysin as antibacterial therapeutic agents
| Properties | Antibiotic | Phage | Endolysin | References |
|---|---|---|---|---|
| Bacteriocidal specificity | Broad spectrum more common than narrow spectrum | Typically narrow, species or strain specificity | Relatively broad lytic activity | [82, 83] |
| Proliferation | Non-proliferation | Self-proliferation | Non-proliferation | [11, 84] |
| Mode of action | Applied from without, target specific sites, typically disrupts one bacterial process | Applied from without, disrupt many essential cellular processes | Applied from without, target bonds in the peptidoglycan | [83, 85] |
| Bacteriocidal speed | Short time between administration and eradication of bacteria | Long time between administration and eradication of bacteria | Rapid bacterial activity within seconds of contact | [14, 86] |
| Intracellular activity | Diffusion through membranes allows for treatment of intracellular bacteria | Unable to penetrate eukaryotic cells | Few or modified ones (e.g., CPP-fused endolysins) can enhance intracellular efficacy | [77, 86, 87] |
| Resistance development | Prone to develop resistance | Resistance occurs quite frequently | No resistance has ever been reported over number of treated generations | [88, 89] |
| Antibiofilm activity | Not very effective against biofilms | Effective antibiofilm agents with limited penetration | Relatively effective antibiofilm agents with higher destruction of biofilms | [13, 83] |
| Immune response | Generally non-immunogenic | Interaction with immune systems and susceptible to clearance by antibodies | Immunogenic, lower degree of antibody neutralization | [26, 51, 90] |
| Pharmacokinetics | Establish the relationship between concentration and the magnitude of killing activity | Little clinical evidence that defines optimal dosages and pharmacokinetic parameters of therapy | Defined concentration at site of infection and in blood circulation | [29, 88, 89] |
CPP cell-penetrating peptide
Endolysin therapy for G+ bacterial infections
Increasing interest in endolysins comes with emerging bacterial resistance and increasing need for novel antimicrobial agents. Given the natural structure of exposed bacterial cell wall without outer membrane barriers, endolysin therapy usually works best against infections caused by G+ bacteria [93]. Immediate lysis occurs without the need of holins or other partner enzymes when applied exogenously. Therefore, extensive experimental studies have focused on pathogenic G+ bacteria since the discovery of endolysins, especially after treatment failures increased considerably in S. aureus, Streptococcus sp., Enterococcus sp., and B. anthracis infections treated with antibiotic alone [17, 94, 95]. Most in vivo studies still concentrate on evaluating the efficacy of newly identified endolysins in the treatment of systemic infections, pneumonia, and nasal and skin infections caused by G+ bacteria; the majority of these studies involved mouse models and targeted MRSA and streptococcal species. Thus, this section mainly focused on the endolysins (including chimeolysins) in pre-clinical and clinical trial phases. Table 3 summarizes the different endolysins targeting G+ bacteria, and it will be discussed further.
Table 3.
Endolysins and related derivatives active against G+ bacteria
| Endolysin and/or derivative | Phage | Antimicrobial spectrum | Enzymatic activity | Type of infection treated in vivo | Clinical trial phase | Clinical trials identifier/accession No | References |
|---|---|---|---|---|---|---|---|
| SAL200 | SAP-1 | S. aureus | Amidase and endopeptidase | Bacteremia | IIa | NCT03089697 | [61] |
| Exebacase (CF-301 or PlySs2) | Prophage of S. suis | S. aureus, Streptococcus | Peptidase | Bacteremia | III | NCT04160468 | [28, 62] |
| P128 | CHAP domain (TAME phage K) + SH3b (lysostaphin) | S. aureus | CHAP | Bacteremia | II | NCT01746654 | [96–98] |
| Staphefekt SA.100 | M23 endopeptidase (lysostaphin) + Amidase (Ply2638) + SH3b (Ply2638) | S. aureus | Amidase and endopeptidase | Atopic dermatitis | I/II | NCT02840955 | [64, 99] |
| Medolysin® | – | S. aureus | – | Bacterial wound infections | – | – | [100] |
| XZ.700 | Staphefekt SA.100 deleted 44 amino acids region deleted | S. aureus | Amidase and endopeptidase | Skin infection | Pre-clinical | – | [64] |
| MV-L | MR11 | S. aureus, S. simulans | Amidase and endopeptidase | Nares infection, sepsis | Pre-clinical | BAF33253 | [81] |
| LysP108 | P108 | S. aureus | Amidase | Subcutaneous abscess | Pre-clinical | YP_009099525 | [17] |
| 80α | phi80α | S. aureus | Amidase and endopeptidase | Systemic infection | Pre-clinical | ABF71642 | [101] |
| phi11 | phi11 | S. aureus | Amidase and endopeptidase | Systemic infection | Pre-clinical | YP_500516 | [101] |
| LysK | K | S. aureus | Amidase and endopeptidase | Systemic infection | Pre-clinical | YP_024461 | [101] |
| Ply2638 | 2638A | S. aureus | Amidase and endopeptidase | Systemic infection | Pre-clinical | AAX90995 | [101] |
| Twort | Twort | S. aureus | Amidase and endopeptidase | Systemic infection | Pre-clinical | AAX92311 | [101] |
| phiSH2 | phiSH2 prophage | S. aureus | Amidase and endopeptidase | Systemic infection | Pre-clinical | BAE05642 | [101] |
| LysWMY | phiWMY | S. aureus | Amidase and endopeptidase | Systemic infection | Pre-clinical | BAD83402 | [101] |
| CHAPK | K | S. aureus | Endopeptidase | Nasal infection | Pre-clinical | 4CT3_D | [18, 102] |
| ClyF | CD domain (Ply187) + CBD domain (PlySs2) | S. aureus | CHAP | Bacteremia and burn wound infection | Pre-clinical | - | [22] |
| LysSS | SS3e | S. aureus, Salmonella, Escherichia coli | – | Systemic infection | Pre-clinical | AAW51228 | [103] |
| Ply6A3 | PD-6A3 | Acinetobacter baumannii, E. coli, S. aureus | – | Sepsis | Pre-clinical | ALM01856 | [104] |
| gp144 | ΦKZ | Pseudomonas aeruginosa, S. aureus, E. coli, B. cereus | Transglycosylase | – | – | AAL83045 | [105] |
| LysGH15 | GH15 | Staphylococcus | Amidase and CHAP | Bacteremia | Pre-clinical | ADG26756 | [26, 67, 106, 107] |
| PlyGRCS | GRCS | S. aureus, S. epidermidis | Endopeptidase | – | – | AHJ10590 | [19] |
| ClyH | CD domain (Ply187) + non-SH3b (phiNM3) | S. aureus | Amidase | Intraperitoneal infection | Pre-clinical | – | [108] |
| MR-10 | MR-10 | S. aureus | – | Subcutaneous | Pre-clinical | – | [109, 110] |
| LysH5 | PhiH5 | S. aureus, S. epidermidis | Amidase and endopeptidase | – | – | ACE77796 | [59, 68] |
| ClyS |
CD domain (Twort) + CBD domain (phiNM3) |
S. aureus | Endopeptidase | Intraperitoneal, nasal and skin infection | Pre-clinical | – | [73, 78] |
| ClyC | CD domain (Ply187) + CBD domain (LysSA97) | S. aureus | – | Bacteremia | Pre-clinical | – | [38] |
| Lys16 | P68 | S. aureus | CHAP | – | – | AAO83890 | [101, 111] |
| LysSAP33 | SAP33 | S. aureus | CHAP | – | – | QDH45454 | [42] |
| Pal | Dp-1 | S. pneumoniae | Amidase | Nasopharyngeal infection | Pre-clinical | O03979 | [16] |
| Cpl-1 | Cp1 | S. pneumoniae | Muramidase | Endocarditis, bacteremia, pneumonia, meningitis | Pre-clinical | NP_044837 | [69, 112–114] |
| Cpl-7 | CP-7 | S. pneumoniae, S. pyogenes, E. faecalis | Muramidase | Embryo infection | Pre-clinical | P19385 | [115, 116] |
| Cpl-711 | CD domain (Cpl-7) + CBD domain (Cpl-1) | S. pneumoniae | Muramidase | Bacteraemia | Pre-clinical | – | [117] |
| PL3 | CD domain (Cpl-7) + CBD domain (LytA) | S. pneumoniae | Amidase | Embryo infection | Pre-clinical | – | [118] |
| ClyJ |
CD domain (PlyC) + CBD domain (SPSL1) |
S. pneumoniae | CHAP | Bacteraemia | Pre-clinical | – | [119] |
| ClyJ-3 | ClyJ variant | S. pneumoniae | CHAP | Bacteraemia | Pre-clinical | – | [120] |
| ClyJ-3 m | ClyJ-3 variant | S. pneumoniae | CHAP | Bacteraemia | Pre-clinical | – | [121] |
| 23TH_48 | 23TH | S. pneumoniae | Amidase | – | – | QOI69927 | [122] |
| MSlys | MS1 | S. pneumoniae | Amidase | – | – | AQY55407 | [123] |
| PlyC | C1 | S. pyogenes | Amidase | Mucosal epithelium infection | Pre-clinical | AAP42310 | [76] |
| PlyPy | MGAS5005 prophage | S. pyogenes | Endopeptidase | Bacteremia | Pre-clinical | AAM79913 | [124] |
| PlyGBS | NCTC11261 | Group B streptococci, S. agalactiae | Endopeptidase and muramidase | Vaginal and oropharynx infection | Pre-clinical | AAR99416 | [74, 125] |
| PlySK1249 | SK1249 prophage | S. agalactiae, Streptococcus dysgalactiae | Amidase and endopeptidase | Bacteremia | Pre-clinical | EGL49245 | [126] |
| PlyG | γ-phage | B. anthracis, B. cereus | Amidase | Intraperitoneal infection | Pre-clinical | PFW40491 | [24] |
| PlyPH |
B. anthracis BA2805 genome |
B. anthracis, B. cereus | – | Intraperitoneal infection | Pre-clinical | WP_098639153 | [127] |
| LysPBC2 | PBC2 | B. cereus | Amidase | – | – | AKQ08512 | [128] |
| PlyV12 | Φ1 | E. faecalis, E. faecium | Amidase | – | – | YP_009814814 | [70] |
| LysEFm5 | IME-EFm5 | E. faecium | Amidase | – | – | YP_009200901 | [129] |
| LysIME-EF1 | IME-EF1 | E. faecalis | Endopeptidase | Intraperitoneal infection | Pre-clinical | YP_009042672 | [126] |
| LysEF-P10 | EF-P10 | E. faecalis | Endopeptidase | Balance of the gut microbiota | Pre-clinical | AQT27695 | [130] |
| Ply3626 | phi3626 | C. perfringens | Amidase | – | – | NP_612849 | [131] |
| Psa | St13 | C. perfringens | Amidase | – | – | WP_011010276 | [132] |
–, unknown or data not available
Endolysin therapy against staphylococcal infections
S. aureus is a representative of G+ bacteria that can cause skin and soft tissue infections, fetal pneumonia, pericarditis, brain abscess, bacteremia, and toxic shock syndrome [2, 133, 134]. Statistically, more than 10% of bloodstream S. aureus infections are caused by MRSA in 15 European countries, and the resistance rates are closer to 50% in some of these countries [3]. MRSA is a superbug that can cause various infections on the human body and is often acquired in the hospital. These nosocomial infections have been acquired by infected individuals, and they are often difficult to treat.
In the case of antibiotic treatment failure, endolysin is an effective option to control MRSA infections [93]. For example, the recombinant endolysin MV-L can rapidly and completely lyse MRSA within 15 min in vitro and efficiently protect mice from intranasal and intraperitoneal challenge with MRSA [81]. Similarly, a chimeric endolysin ClyS efficiently lysed MRSA, vancomycin-intermediate S. aureus, and MSSA strains by > 2-log10 in vitro and protected against death caused by MRSA in mouse nasal decolonization and bacteremia models [73]. Eight endolysins (80α, phi11, LysK, P68, 2638A, Twort, phiSH2, and LysWMY) display varied lytic activities against numerous staphylococcal strains in vitro, including cell surface mutants, drug-resistant strains, and their static biofilms. In a mouse model of systemic MRSA infection, these endolysins provide therapeutic potential and show no clinical symptoms at the end of treatment [61]. The above research results indicated that endolysin is highly effective in combating refractory infections caused by drug-resistant S. aureus.
Furthermore, several endolysins for the treatment of S. aureus infections are close to clinical application. P128 is an engineered endolysin (chimer) developed by the Indian company Gangagen, and it is currently in the clinical development stage. P128 is active against globally prevalent drug-resistant clinical S. aureus and S. epidermidis isolates. It exerts potent activity against sinus-derived S. aureus biofilms and is developed for clearing S. aureus nasal colonization and MRSA infection in mice and dogs [97, 98, 135, 136]. Staphefekt SA.100 and XDR.300 are commercially available recombinant endolysins that have been applied to patients with chronic skin infections caused by S. aureus [137]. Exebacase (also termed CF-301 or PlySs2) is considered an attractive agent that has rapid bacteriolytic activity, biofilm elimination capacity, and anti-staphylococcal potentials ranging from bacteremia to osteomyelitis when combined with other antibiotics. In addition to the above properties, exebacase has a minimal propensity for resistance development, no cross-resistance with antibiotics, and delayed post-antibiotic effect in vitro and in vivo [138]. Another endolysin, CHAPK, has the potential to reduce S. aureus colonization in the skin; thus, it may be used as a disinfecting agent in the healthcare environment [13]. Overall, endolysin treatment is a promising approach for staphylococcal infections.
Endolysin therapy against streptococcal infections
S. pneumoniae is another clinically important G+ bacterium that can cause different diseases ranging from a streptococcal pharyngitis to life-threatening pneumonia [77]. Endolysins have been successfully determined in a mouse model with streptococci. Cpl-1 and Pal alone or in combination have been used in the treatment of pneumococcal infections [80, 139]. A single dose of aerosolized Cpl-1 can rescue mice from fatal pneumococcal pneumonia [140]. Cpl-1 can reduce intranasal S. pneumoniae and ultimately prevent the development of acute otitis media following infection with influenza virus [141]. A single intracisternal injection of Cpl-1 (20 mg/kg) and intraperitoneal administration of Cpl-1 (200 mg/kg) decreased pneumococci in cerebrospinal fluid by 3-log10 and 2-log10, respectively, representing a promising alternative treatment option for pneumococcal meningitis [113]. Cpl-1 also shows therapeutic effects on S. pneumoniae rat endocarditis [112] and murine pneumococcal bacteremia [69]. In a mouse model of nasopharyngeal colonization, Pal was found to reduce S. pneumoniae to undetectable titers (log10 0 CFU/10 mL nasal wash) 5 h after a single dose treatment and did not induce Pal-resistant pneumococci after extensive exposure to the enzyme [16]. Therefore, Cpl-1 and Pal are potent antimicrobial agents for the prevention and treatment of mucosal and systemic pneumococcal infections. Moreover, PlySs2 derived from a S. suis phage has broad lytic activity against group A Streptococcus, group B Streptococcus, group G Streptococcus, group E Streptococcus, and S. pneumoniae. PlySs2 (128 μg/mL) led to a 3-log reduction in the growth of S. pyogenes within 1 h, and 2 mg of PlySs2 protected 92% (22/24) of mice from bacteremia caused by mixed MRSA and S. pyogenes infections [82]. Similar results were observed with endolysin PlyC and PlyGBS [23, 76, 125]. These promising results have led to great research interest in the treatment of streptococcal infections with endolysins.
Endolysin therapy for infections caused by other G+bacteria
Among G+ bacteria, Staphylococcus and Streptococcus are the most common in clinical settings. Therefore, many studies on endolysin-related treatment have been conducted, whereas research on endolysin therapy of other G+ bacteria is relatively lacking. However, an increasing number of studies on endolysin treatment of other G+ bacteria have been performed in recent years. For example, PlyV12 from an E. faecalis phage and LysEFm5 from an E. faecium phage have been described to be useful in the treatment of mucosal infections [70, 129]. B. cereus and B. anthracis are pathogenic bacilli that can cause serious harm to their hosts via food poisoning and anthrax toxicity, respectively [142]. Two recombinant endolysins PlyG and PlyPH are effective therapeutic agents for the control of B. cereus and B. anthracis both in vitro and in vivo [24, 127]. Moreover, endolysins have been recommended as impressive agents against drug-resistant pathogen C. perfringens, which can cause the infection of over 95% of chickens [13]. The endolysins Ply3626 and Psm are expected to be applied to poultry with broad lytic activity against C. perfringens [13, 131]. Interestingly, LysZ5 shows excellent activity against L. monocytogenes in soya milk and is greatly needed in food safety and food processing systems [13]. Therefore, these studies demonstrated that endolysins may be used to either eliminate or reduce G+ bacterial colonization from mucosal epithelium of either carriers or infected individuals and systemic infections, paving the way for endolysins to be applied as alternatives to the treatment of associated diseases in humans and animals.
Synergistic effects of endolysins to combat G+bacteria
In the treatment of G+ bacterial infections, the antimicrobial spectrum and antibacterial capacity of endolysins can be improved through the synergistic effect between the endolysins and antibiotics or other enzymes with diverse enzymatic specificities [17, 62, 143, 144]. Staphylococcal lysostaphin and LysK were found to exhibit strong synergistic activity on the MRSA strain USA300 and the mastitis-causing strain S. aureus 305 through checkerboard assay [145]. Two anti-pneumococcal endolysins Cpl-1 and Pal with muraminidase and amidase activities, respectively, were synergized in vitro and exhibited increased activity compared with either individual endolysin in a mouse pneumococcal infection model [80, 139, 146].
The synergistic effect between different endolysins or other cell wall hydrolases (i.e., lysostaphin) can be explained by the enhanced destructive effect generated when two different bonds were simultaneously cleaved within the 3D peptidoglycan meshwork. Alternatively, the cleavage of the first bond by one enzyme can result in better accessibility to the second target site by the other endolysin, causing a faster degradation of the substrate. A novel chimeric endolysin ClyS exhibited a typical pattern of synergistic action with both vancomycin and oxacillin in vitro. More importantly, ClyS and oxacillin at low doses that were no protective individually were found to present synergistic effects against MRSA septic death in a mouse model [73]. The combination of a staphylococcal endolysin Lys11 and an antimicrobial peptide R8K can enhance the bacteriolytic action against S. aureus, including MRSA clinical strains [147]. Animal experiments suggested that the synergistic antibacterial effects of LysP108 and vancomycin greatly reduce the area of subcutaneous abscess of mice infected with MRSA [11]. Concurrently, combinations of endolysins with antibiotics not only increase bactericidal efficacy but also resensitize drug-resistant bacteria, such as Cpl-1 and penicillin, MV-L and vancomycin, and SAL200 and standard-of-care antibiotics, such as nafcillin and vancomycin [81, 148, 149]. Therefore, the optimal combination of endolysin and other antimicrobial agents can help control the development of bacterial resistance and reduce the required antibiotic dosage [13, 150, 151].
Safety of endolysins
As for applications, endolysin safety is an inescapable issue. Numerous experimental studies have shown that endolysins are innocuous after both topical and systemic administration in mice [51, 69, 76]. Treatment with endolysin specific to Group A streptococci does not bring about any histopathologic abnormalities in the mucosa and skin tissues of mice when administered daily for 7 days [51]. SAL200 is an endolysin-based candidate against S. aureus; it shows no toxicity and adverse effects in mice, dogs, and monkeys under pre-clinical safety evaluation [29, 152, 153]. Furthermore, SAL200 was found to be well tolerated among healthy male volunteers in a human single dose-escalating (0.1–10 mg/kg) study. More than three participants had some adverse effects, such as fatigue, stiffness, headache, and myalgia; most adverse effects were transient, mild, and self-limiting [29]. A high-dose intravenous injection of LysGH15 (10 mg) did not induce remarkable side effects (after 10 days) or pathological changes in the tissues of mice infected with S. aureus [26]. No severe allergic reactions as adverse events were found in pre-clinical studies, such as SAL200 and CF-301 [77, 152, 154]. Eukaryotic cells did not have peptidoglycan; thus, endolysins are expected to be safe in humans [52]. However, the main concern with respect to the safety of endolysins is the release of pro-inflammatory factors and bacterial components (e.g., lipopolysaccharide) during bacteriolysis, which may be directly toxic for eukaryotic cells [155]. To date, the side effects of endolysins have not been reported, thereby strongly supporting the safety of endolysin-based drug treatments.
Future challenges and possible solutions
Given the global prevalence of multidrug-resistant bacterial infections, endolysins are considered as an attractive therapeutic option in clinical settings. Although endolysins show many advantages in the treatment of drug-resistant bacteria (Table 2), some challenges remain, and further research must be performed to consider their large-scale production, engineering, and drug delivery toward widespread utilization.
The large-scale production of phage endolysins has attracted great attention. However, the two main challenges that need to be solved are manufacturing cost and safety. Escherichia coli is the most common organism for the production of recombinant proteins, as this expression platform is well-established, and the cellular and molecular tools needed in the process of protein expression from gene cloning to protein purification are widely accessible [156, 157]. In general, S. aureus recombinant endolysins are expressed in E. coli [157]. However, some functional recombinant proteins are unavailable due to protein toxicity to the host or aggregation in inclusion bodies, even if numerous studies have attempted to optimize the E. coli expression system in the aspects of host engineering, expression vector design, and culture optimization [156]. Alternatively, the Pichia pastoris expression system has high recombinant protein yields; even some filamentous fungi or other systems can be considered for the production of recombinant endolysins [157, 158]. For example, the endolysins Cpl-1 and Pal, which are specific against S. pneumoniae, can be successfully expressed in chloroplasts of Chlamydomonas reinhardtii; this host has many advantages, such as the lack of endotoxins, no infectious potential, and low production costs [159]. Moreover, a platform for expressing an endolysin against Cutibacterium acnes in cyanobacteria can reduce production costs and avoid toxicity issues caused by toxic bacterial components such as endotoxin [157].
Furthermore, new strategies are needed to develop novel and suitable endolysins that possess enhanced bactericidal activity; expanded lytic spectrum; and increased solubility, stability, and circulating half-life. The modifications of native endolysins by molecular engineering and specific designing can completely create new enzymes with several improved features. Several approaches have been applied to modify endolysin enzymes, including domain deletion, addition, shuffling, and site-directed modifications. Virion-associated lysins (VALs) are another class of phage-encoded enzymes with antimicrobial activities that have been engineered to act on certain bacteria by fusing them into a chimeric lysin. EC300 and P128 are good examples of the VAL-derived chimeolysins that efficiently target E. faecalis and S. aureus, respectively [97, 160]. This breakthrough facilitates the generation of novel customized proteins that use not only VAL domains but also other agents, such as antimicrobial peptides, bacteriocins, and bacteriolysins [32]. A chimeric protein K-L, composed of the CHAP endopeptidase and amidase domains of LysK, as well as the glycyl-glycine endopeptidase domain of lysostaphin, displays increased stability in the presence of block copolymers of poly-L-glutamic acid and polyethylene glycol. The chimeric design of K-L reduces the immunogenicity of the enzyme [161]. A study demonstrated that the addition of cysteine to the C-terminal (CTC modification strategy) of antimicrobial peptide or lysin can increase the efficacy against both G+ and G− pathogens by at least twofold [161]. Perhaps the suitable formulation of therapeutic compounds containing various endolysins, such as phage cocktail therapy, can enhance the lytic activity against specific bacteria, extend lytic spectrum, and decrease the chance of bacterial resistance.
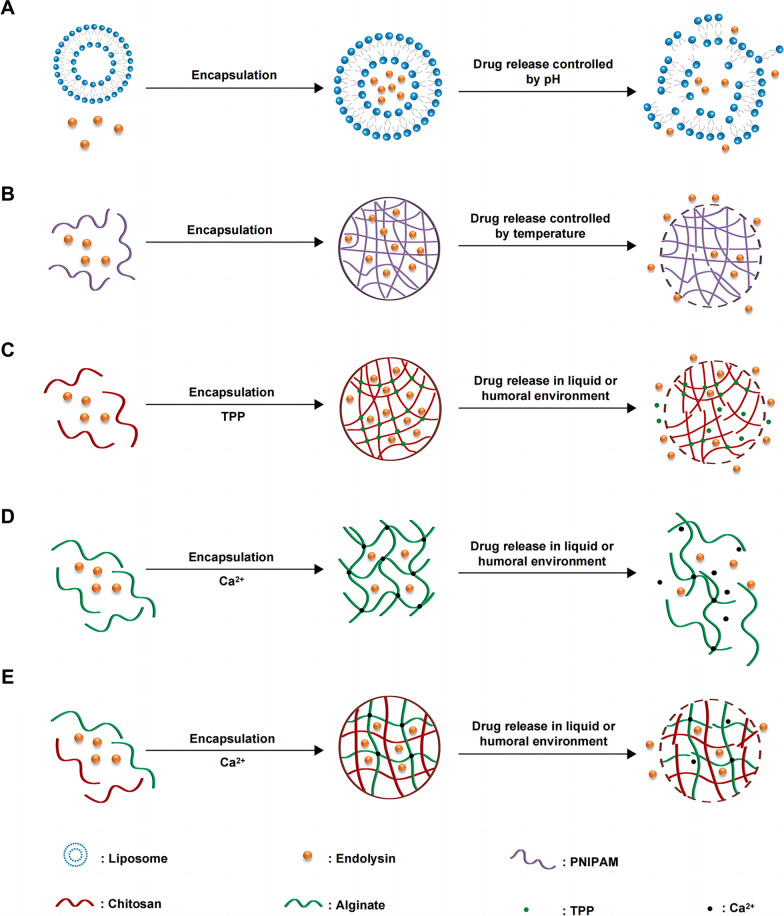
Finally, a considerable hurdle to the application of endolysin therapy is drug delivery. Many endolysins are used for topical treatment, such as skin bacterial infections, whereas the systemic application of endolysins remains challenging. In oral administration, endolysins can easily be degraded by stomach acids and proteases, which lead to poor bioavailability and irreversible damage to the integrity of the protein structure [93]. The encapsulation technique has offered a novel way of protecting endolysins until they reach their desired targets; this approach may enable unsuitable endolysins to become effective therapeutic agents. The release of the endolysin-encapsulated nanoparticles can be triggered by different environmental conditions (e.g., temperature or pH) or certain host- or pathogen-produced stimuli (e.g., cytokine, enzymes, secreted toxins, or signaling molecules) [137, 162]. Some successful results regarding the encapsulation of endolysins have been reported. The encapsulation of LysRODI endolysin in pH-sensitive liposomes can reduce planktonic S. aureus and its biofilm at pH 5 (Fig. 4A) [163]. The endolysin CHAPk and lysostaphin encapsulated in the thermally triggered poly(N-isopropylacrylamide) (PNIPAM) nanoparticles can be released in the S. aureus infection sites at 37 °C (Fig. 4B) [164]. Cpl-1-loaded chitosan nanoparticles are promising biocompatible candidates with increased bioavailability and in-vivo half-life for the treatment of S. pneumoniae infections (Fig. 4C) [25]. Moreover, chimeric ClyC-loaded alginate hydrogel (ClyC-AH) can retain the stability and activity of ClyC, decrease cytotoxicity, and reduce bacterial burden in a mouse S. aureus osteomyelitis model (Fig. 4D) [165]. In addition, the alginate-chitosan hydrogel delivery system can efficiently transfer the anti-staphylococcal endolysin LysMR-5 in vivo (Fig. 4E). Compared with the blank alginate-chitosan hydrogel, LysMR-5-loaded hydrogel shows enhanced bactericidal activity and good biocompatibility [166]. Apart from the examples described above, there are several studies about endolysin delivery, such as nanoparticles of chitosan derivatized with diethylaminoethyl (DEAE) groups encapsulating the Cpl-711 pneumococcal chimeric lysin [167], liposomes loaded with the endolysin MSlys [168], and pH-responsive nanoparticles of self-assembling peptide fusion with the endolysin P128 [169]. With further exploration of endolysins, these challenges can be overcome in the near future, facilitating the clinical application of phage endolysins.
Fig. 4.
The delivery strategies for endolysins. Endolysins can be encapsulated in pH-sensitive liposomes (A), thermally triggered PNIPAM nanoparticles (B), chitosan nanoparticles (C), alginate hydrogel (D), and alginate-chitosan hydrogel (E). These nanoparticles and hydrogels can release endolysins at infection sites triggered by different circumstances and displayed lytic activity both in vitro and in vivo. PNIPAM poly N-isopropylacrylamide, TPP sodium tripolyphosphate
Conclusion
The unique mode of action, the rapid killing activity against bacteria (including persisters), and the low probability of resistance development are appealing features of endolysins for their application as alternatives of antibacterial agents. Many studies have shown that endolysins are effective antimicrobial agents that have synergistic effects with diverse antibiotics and antimicrobial peptides. With the high priority for the development of novel agents against multidrug-resistant bacteria, phage endolysins are promising candidates that serve as therapeutic options for controlling G+ bacterial infections. In the face of challenges such as activity, stability, cost, and ready-to-use drug availability in endolysin therapy, engineering modification (e.g., chimeric endolysins), production process optimization, and drug delivery development can be used to enhance the potential of endolysins, making endolysins a clinically proven drug to combat the crisis of drug-resistant bacteria in the future.
Acknowledgements
Not applicable.
Author contributions
XR and Sl proposed the idea for the article. HL and ZH searched, analyzed, and summarized the literature. HL drafted this work and contributed to the original writing. ML and YY carried out the review and the proofreading of this article. XR and SL critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 31871251 to S.L.).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuguang Lu, Email: shulang88@126.com.
Xiancai Rao, Email: raoxiancai@126.com.
References
- 1.Nuti R, Goud NS, Saraswati AP, Alvala R, Alvala M. Antimicrobial peptides: a promising therapeutic strategy in tackling antimicrobial resistance. Curr Med Chem. 2017;24:4303–4314. doi: 10.2174/0929867324666170815102441. [DOI] [PubMed] [Google Scholar]
- 2.Venter H, Henningsen ML, Begg SL. Antimicrobial resistance in healthcare, agriculture and the environment: the biochemistry behind the headlines. Essays Biochem. 2017;61:1–10. doi: 10.1042/EBC20160053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutradhar I, Ching C, Desai D, Suprenant M, Briars E, Heins Z, et al. Computational model to quantify the growth of antibiotic-resistant bacteria in wastewater. mSystems. 2021;6:e0036021. doi: 10.1128/mSystems.00360-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins RR, Bonomo RA. Overview: the ongoing threat of antimicrobial resistance. Infect Dis Clin N Am. 2020;4:649–658. doi: 10.1016/j.idc.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Karaman R, Jubeh B, Breijyeh Z. Resistance of Gram-positive bacteria to current antibacterial agents and overcoming approaches. Molecules. 2020;25:2888. doi: 10.3390/molecules25122888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clokie MR, Millard AD, Letarov AV, Heaphy S. Phages in nature. Bacteriophage. 2011;1:31–45. doi: 10.4161/bact.1.1.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischetti VA. Development of phage lysins as novel therapeutics: a historical perspective. Viruses. 2018;10:310. doi: 10.3390/v10060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischetti VA. Bacteriophage endolysins: a novel anti-infective to control Gram-positive pathogens. Int J Med Microbiol. 2010;300:357–362. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vukov N, Moll I, Bläsi U, Scherer S, Loessner MJ. Functional regulation of the Listeria monocytogenes bacteriophage A118 holin by an intragenic inhibitor lacking the first transmembrane domain. Mol Microbiol. 2003;2003(48):173–186. doi: 10.1046/j.1365-2958.2003.03421.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang IN, Smith DL, Young R. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol. 2000;54:799–825. doi: 10.1146/annurev.micro.54.1.799. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y, Wang Y, Wang J, Zhao Y, Zhong Q, Li G, et al. Phage endolysin LysP108 showed promising antibacterial potential against methicillin-resistant Staphylococcus aureus. Front Cell Infect Microbiol. 2021;11:668430. doi: 10.3389/fcimb.2021.668430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson DC, Schmelcher M, Rodriguez-Rubio L, Klumpp J, Pritchard DG, Dong S, et al. Endolysins as antimicrobials. Adv Virus Res. 2012;83:299–365. doi: 10.1016/B978-0-12-394438-2.00007-4. [DOI] [PubMed] [Google Scholar]
- 13.Abdelrahman F, Easwaran M, Daramola OI, Ragab S, Lynch S, Oduselu TJ, et al. Phage-encoded endolysins. Antibiotics (Basel) 2021;10:124. doi: 10.3390/antibiotics10020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischetti VA. Bacteriophage lytic enzymes: novel anti-infectives. Trends Microbiol. 2005;13:491–496. doi: 10.1016/j.tim.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Loessner MJ. Bacteriophage endolysins–current state of research and applications. Curr Opin Microbiol. 2005;8:480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Loeffler JM, Nelson D, Fischetti VA. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 17.Love MJ, Bhandari D, Dobson RCJ, Billington C. Potential for bacteriophage endolysins to supplement or replace antibiotics in food production and clinical care. Antibiotics (Basel) 2018;7:17. doi: 10.3390/antibiotics7010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keary R, Sanz-Gaitero M, van Raaij MJ, O'Mahony J, Fenton M, McAuliffe O, et al. Characterization of a bacteriophage-derived murein peptidase for elimination of antibiotic-resistant Staphylococcus aureus. Curr Protein Pept Sci. 2016;17:183–190. doi: 10.2174/1389203716666151102105515. [DOI] [PubMed] [Google Scholar]
- 19.Linden SB, Zhang H, Heselpoth RD, Shen Y, Schmelcher M, Eichenseher F, et al. Biochemical and biophysical characterization of PlyGRCS, a bacteriophage endolysin active against methicillin-resistant Staphylococcus aureus. Appl Microbiol Biotechnol. 2015;99:741–752. doi: 10.1007/s00253-014-5930-1. [DOI] [PubMed] [Google Scholar]
- 20.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob Agents Chemother. 2017;61:e02666–e2716. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenton M, Keary R, McAuliffe O, Ross RP, O’Mahony J, Coffey A. Bacteriophage-derived peptidase CHAP(K) eliminates and prevents staphylococcal biofilms. Int J Microbiol. 2013;2013:625341. doi: 10.1155/2013/625341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang H, Zhang H, Wang J, Yu J, Wei H. A novel chimeric lysin with robust antibacterial activity against planktonic and biofilm methicillin-resistant Staphylococcus aureus. Sci Rep. 2017;7:40182. doi: 10.1038/srep40182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson D, Schuch R, Chahales P, Zhu S, Fischetti VA. PlyC: a multimeric bacteriophage lysin. Proc Natl Acad Sci U S A. 2006;103:10765–10770. doi: 10.1073/pnas.0604521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 25.Gondil VS, Dube T, Panda JJ, Yennamalli RM, Harjai K, Chhibber S. Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter S. pneumoniae infections. Int J Pharm. 2020;573:118850. doi: 10.1016/j.ijpharm.2019.118850. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Li D, Li X, Hu L, Cheng M, Xia F, et al. LysGH15 kills Staphylococcus aureus without being affected by the humoral immune response or inducing inflammation. Sci Rep. 2016;2016(6):29344. doi: 10.1038/srep29344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdelkader K, Gerstmans H, Saafan A, Dishisha T, Briers Y. The preclinical and clinical progress of bacteriophages and their lytic enzymes: the parts are easier than the whole. Viruses. 2019;11:96. doi: 10.3390/v11020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, et al. The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis. Antimicrob Agents Chemother. 2019;63:e02291–e2318. doi: 10.1128/AAC.02291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jun SY, Jang IJ, Yoon S, Jang K, Yu KS, Cho JY, et al. Pharmacokinetics and tolerance of the phage endolysin-based candidate drug SAL200 after a single intravenous administration among healthy volunteers. Antimicrob Agents Chemother. 2017;61:e02629–e2716. doi: 10.1128/AAC.02629-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Briers Y, Volckaert G, Cornelissen A, Lagaert S, Michiels CW, Hertveldt K, et al. Muralytic activity and modular structure of the endolysins of Pseudomonas aeruginosa bacteriophages phiKZ and EL. Mol Microbiol. 2007;65:1334–1344. doi: 10.1111/j.1365-2958.2007.05870.x. [DOI] [PubMed] [Google Scholar]
- 31.Fischetti VA. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol. 2008;11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira H, São-José C, Azeredo J. Phage-derived peptidoglycan degrading enzymes: challenges and future prospects for in vivo therapy. Viruses. 2018;10:292. doi: 10.3390/v10060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarre WW, Ton-That H, Faull KF, Schneewind O. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a D-alanyl-glycine endopeptidase activity. J Biol Chem. 1999;274:15847–15856. doi: 10.1074/jbc.274.22.15847. [DOI] [PubMed] [Google Scholar]
- 34.Becker SC, Foster-Frey J, Stodola AJ, Anacker D, Donovan DM. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene. 2009;443:32–41. doi: 10.1016/j.gene.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 35.Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl Environ Microbiol. 2007;73:7150–7154. doi: 10.1128/AEM.01783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rahman MU, Wang W, Sun Q, Shah JA, Li C, Sun Y, et al. Endolysin, a promising solution against antimicrobial resistance. Antibiotics (Basel) 2021;10:1277. doi: 10.3390/antibiotics10111277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oechslin F, Menzi C, Moreillon P, Resch G. The multidomain architecture of a bacteriophage endolysin enables intramolecular synergism and regulation of bacterial lysis. J Biol Chem. 2021;296:100639. doi: 10.1016/j.jbc.2021.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Wang S, Nyaruaba R, Liu H, Yang H, Wei H. A highly active chimeric lysin with a calcium-enhanced bactericidal activity against Staphylococcus aureus in vitro and in vivo. Antibiotics (Basel) 2021;10:461. doi: 10.3390/antibiotics10040461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong Q, Wang J, Yang H, Wei C, Yu J, Zhang Y, et al. Construction of a chimeric lysin Ply187N-V12C with extended lytic activity against staphylococci and streptococci. Microb Biotechnol. 2015;8:210–220. doi: 10.1111/1751-7915.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao J, Schmelcher M, Harty WJ, Foster-Frey J, Donovan DM. Chimeric Ply187 endolysin kills Staphylococcus aureus more effectively than the parental enzyme. FEMS Microbiol Lett. 2013;342:30–36. doi: 10.1111/1574-6968.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang Y, Ryu S. Characterization of a novel cell wall binding domain-containing Staphylococcus aureus endolysin LysSA97. Appl Microbiol Biotechnol. 2017;101:147–158. doi: 10.1007/s00253-016-7747-6. [DOI] [PubMed] [Google Scholar]
- 42.Yu JH, Park DW, Lim JA, Park JH. Characterization of staphylococcal endolysin LysSAP33 possessing untypical domain composition. J Microbiol. 2021;59:840–847. doi: 10.1007/s12275-021-1242-1. [DOI] [PubMed] [Google Scholar]
- 43.Low LY, Yang C, Perego M, Osterman A, Liddington RC. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J Biol Chem. 2005;280:35433–35439. doi: 10.1074/jbc.M502723200. [DOI] [PubMed] [Google Scholar]
- 44.Vollmer W, Bertsche U. Murein (peptidoglycan) structure, architecture and biosynthesis in Escherichia coli. Biochim Biophys Acta. 2008;1778:1714–1734. doi: 10.1016/j.bbamem.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Matamp N, Bhat SG. Phage endolysins as potential antimicrobials against multidrug resistant Vibrio alginolyticus and Vibrio parahaemolyticus: current status of research and challenges ahead. Microorganisms. 2019;7:84. doi: 10.3390/microorganisms7030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmelcher M, Donovan DM, Loessner MJ. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012;7:1147–1171. doi: 10.2217/fmb.12.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Donovan DM, Lardeo M, Foster-Frey J. Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett. 2006;265:133–139. doi: 10.1111/j.1574-6968.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 48.Sass P, Bierbaum G. Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol. 2007;2007(73):347–352. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaeng S, Scherer S, Neve H, Loessner MJ. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl Environ Microbiol. 2000;66:2951–2958. doi: 10.1128/AEM.66.7.2951-2958.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mayer MJ, Garefalaki V, Spoerl R, Narbad A, Meijers R. Structure-based modification of a Clostridium difficile-targeting endolysin affects activity and host range. J Bacteriol. 2011;193:5477–5486. doi: 10.1128/JB.00439-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borysowski J, Weber-Dabrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp Biol Med (Maywood) 2006;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- 52.Fischetti VA. Novel method to control pathogenic bacteria on human mucous membranes. Ann N Y Acad Sci. 2003;987:207–214. doi: 10.1111/j.1749-6632.2003.tb06050.x. [DOI] [PubMed] [Google Scholar]
- 53.Schmelcher M, Tchang VS, Loessner MJ. Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity. Microb Biotechnol. 2011;4:651–662. doi: 10.1111/j.1751-7915.2011.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Özal D, Arndt A, Thomé M. Bacteriophages and related endolysins for reduction of microorganisms in the human body—a systematic review. GMS Hyg Infect Control. 2022;17:Doc01. doi: 10.3205/dgkh000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Idelevich EA, von Eiff C, Friedrich AW, Iannelli D, Xia G, Peters G, et al. In vitro activity against Staphylococcus aureus of a novel antimicrobial agent, PRF-119, a recombinant chimeric bacteriophage endolysin. Antimicrob Agents Chemother. 2011;55:4416–4419. doi: 10.1128/AAC.00217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singh PK, Donovan DM, Kumar A. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob Agents Chemother. 2014;58:4621–4629. doi: 10.1128/AAC.00126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Becker SC, Dong S, Baker JR, Foster-Fre J, Pritchard DG, Donovan DM. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol Lett. 2009;294:52–60. doi: 10.1111/j.1574-6968.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- 58.Loessner MJ, Kramer K, Ebel F, Scherer S. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol. 2002;44:335–349. doi: 10.1046/j.1365-2958.2002.02889.x. [DOI] [PubMed] [Google Scholar]
- 59.Gutiérrez D, Ruas-Madiedo P, Martínez B, Rodríguez A, García P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE. 2014;9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meng X, Shi Y, Ji W, Meng X, Zhang J, Wang H, et al. Application of a bacteriophage lysin to disrupt biofilms formed by the animal pathogen Streptococcus suis. Appl Environ Microbiol. 2011;77:8272–8279. doi: 10.1128/AEM.05151-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jun SY, Jung GM, Yoon SJ, Oh MD, Choi YJ, Lee WJ, et al. Antibacterial properties of a pre-formulated recombinant phage endolysin, SAL-1. Int J Antimicrob Agents. 2013;41:156–161. doi: 10.1016/j.ijantimicag.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, et al. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis. 2014;209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poonacha N, Nair S, Desai S, Tuppad D, Hiremath D, Mohan T, et al. Efficient killing of planktonic and biofilm-embedded coagulase-negative staphylococci by bactericidal protein P128. Antimicrob Agents Chemother. 2017;61:e00457–e517. doi: 10.1128/AAC.00457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eichenseher F, Herpers BL, Badoux P, Leyva-Castillo JM, Geha RS, van der Zwart M, et al. Linker-improved chimeric endolysin selectively kills Staphylococcus aureus in vitro, on reconstituted human epidermis, and in a murine model of skin infection. Antimicrob Agents Chemother. 2022;66:e0227321. doi: 10.1128/aac.02273-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol. 2005;187:7161–7164. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fenton M, Ross RP, McAuliffe O, O'Mahony J, Coffey A. Characterization of the staphylococcal bacteriophage lysin CHAP(K) J Appl Microbiol. 2011;111:1025–1035. doi: 10.1111/j.1365-2672.2011.05119.x. [DOI] [PubMed] [Google Scholar]
- 67.Gu J, Xu W, Lei L, Huang J, Feng X, Sun C, et al. LysGH15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2011;49:111–117. doi: 10.1128/JCM.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Obeso JM, Martínez B, Rodríguez A, García P. Lytic activity of the recombinant staphylococcal bacteriophage PhiH5 endolysin active against Staphylococcus aureus in milk. Int J Food Microbiol. 2008;128:212–218. doi: 10.1016/j.ijfoodmicro.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 69.Loeffler JM, Djurkovic S, Fischetti VA. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun. 2003;71:6199–6204. doi: 10.1128/IAI.71.11.6199-6204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoong P, Schuch R, Nelson D, Fischetti VA. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J Bacteriol. 2004;186:4808–4812. doi: 10.1128/JB.186.14.4808-4812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayer MJ, Narbad A, Gasson MJ. Molecular characterization of a Clostridium difficile bacteriophage and its cloned biologically active endolysin. J Bacteriol. 2008;190:6734–6740. doi: 10.1128/JB.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmitz JE, Ossiprandi MC, Rumah KR, Fischetti VA. Lytic enzyme discovery through multigenomic sequence analysis in Clostridium perfringens. Appl Microbiol Biotechnol. 2011;89:1783–1795. doi: 10.1007/s00253-010-2982-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:1603–1612. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cheng Q, Nelson D, Zhu S, Fischetti VA. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother. 2005;49:111–117. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pritchard DG, Dong S, Baker JR, Engler JA. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology (Reading) 2004;150:2079–2087. doi: 10.1099/mic.0.27063-0. [DOI] [PubMed] [Google Scholar]
- 76.Nelson D, Loomis L, Fischetti VA. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci U S A. 2001;98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gerstmans H, Criel B, Briers Y. Synthetic biology of modular endolysins. Biotechnol Adv. 2018;6:624–640. doi: 10.1016/j.biotechadv.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Pastagia M, Euler C, Chahales P, Fuentes-Duculan J, Krueger JG, Fischetti VA. A novel chimeric lysin shows superiority to mupirocin for skin decolonization of methicillin-resistant and -sensitive Staphylococcus aureus strains. Antimicrob Agents Chemother. 2011;55:738–744. doi: 10.1128/AAC.00890-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pastagia M, Schuch R, Fischetti VA, Huang DB. Lysins: the arrival of pathogen-directed anti-infectives. J Med Microbiol. 2013;62:1506–1516. doi: 10.1099/jmm.0.061028-0. [DOI] [PubMed] [Google Scholar]
- 80.Jado I, López R, García E, Fenoll A, Casal J, García P. Phage lytic enzymes as therapy for antibiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother. 2003;52:967–973. doi: 10.1093/jac/dkg485. [DOI] [PubMed] [Google Scholar]
- 81.Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis. 2007;196:1237–1247. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 82.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57:2743–2750. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy. Bacteriophage. 2011;1:111–114. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fischetti VA, Nelson D, Schuch R. Reinventing phage therapy: are the parts greater than the sum? Nat Biotechnol. 2006;24:1508–1511. doi: 10.1038/nbt1206-1508. [DOI] [PubMed] [Google Scholar]
- 85.Zhu Y, Hao W, Wang X, Ouyang J, Deng X, Yu H, et al. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med Res Rev. 2022;42:1377–1422. doi: 10.1002/med.21879. [DOI] [PubMed] [Google Scholar]
- 86.Kortright KE, Chan BK, Koff JL, Turner PE. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe. 2019;25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 87.Röhrig C, Huemer M, Lorgé D, Luterbacher S, Phothaworn P, Schefer C, et al. Targeting hidden pathogens: cell-penetrating enzybiotics eradicate intracellular drug-resistant Staphylococcus aureus. MBio. 2020;11:e00209-20. doi: 10.1128/mBio.00209-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yılmaz Ç, Özcengiz G. Antibiotics: pharmacokinetics, toxicity, resistance and multidrug efflux pumps. Biochem Pharmacol. 2017;133:43–62. doi: 10.1016/j.bcp.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 89.Hatfull GF, Dedrick RM, Schooley RT. Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med. 2022;73:197–211. doi: 10.1146/annurev-med-080219-122208. [DOI] [PubMed] [Google Scholar]
- 90.Górski A, Dąbrowska K, Międzybrodzki R, Weber-Dąbrowska B, Łusiak-Szelachowska M, Jończyk-Matysiak E, et al. Phages and immunomodulation. Future Microbiol. 2017;12:905–914. doi: 10.2217/fmb-2017-0049. [DOI] [PubMed] [Google Scholar]
- 91.Yother J, Leopold K, White J, Fischer W. Generation and properties of a Streptococcus pneumoniae mutant which does not require choline or analogs for growth. J Bacteriol. 1998;180:2093–2101. doi: 10.1128/JB.180.8.2093-2101.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kokai-Kun JF, Chanturiya T, Mond JJ. Lysostaphin eradicates established Staphylococcus aureus biofilms in jugular vein catheterized mice. J Antimicrob Chemother. 2009;64:94–100. doi: 10.1093/jac/dkp145. [DOI] [PubMed] [Google Scholar]
- 93.Murray E, Draper LA, Ross RP, Hill C. The advantages and challenges of using endolysins in a clinical setting. Viruses. 2021;13:680. doi: 10.3390/v13040680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fowler VG, Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 95.Gerstmans H, Rodríguez-Rubio L, Lavigne R, Briers Y. From endolysins to Artilysin®s: novel enzyme-based approaches to kill drug-resistant bacteria. Biochem Soc Trans. 2016;44:123–128. doi: 10.1042/BST20150192. [DOI] [PubMed] [Google Scholar]
- 96.Channabasappa S, Durgaiah M, Chikkamadaiah R, Kumar S, Joshi A, Sriram B. Efficacy of novel antistaphylococcal ectolysin P128 in a rat model of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2018;62:e01358–e1417. doi: 10.1128/AAC.01358-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vipra AA, Desai SN, Roy P, Patil R, Raj JM, Narasimhaswamy N, et al. Antistaphylococcal activity of bacteriophage derived chimeric protein P128. BMC Microbiol. 2012;12:41. doi: 10.1186/1471-2180-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Junjappa RP, Desai SN, Roy P, Narasimhaswamy N, Raj JR, Durgaiah M, et al. Efficacy of anti-staphylococcal protein P128 for the treatment of canine pyoderma: potential applications. Vet Res Commun. 2013;37:217–228. doi: 10.1007/s11259-013-9565-y. [DOI] [PubMed] [Google Scholar]
- 99.de Wit J, Totté JEE, van Mierlo MMF, van Veldhuizen J, van Doorn MBA, Schuren FHJ, et al. Endolysin treatment against Staphylococcus aureus in adults with atopic dermatitis: a randomized controlled trial. J Allergy Clin Immunol. 2019;144:860–863. doi: 10.1016/j.jaci.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 100.Ho MKY, Zhang P, Chen X, Xia J, Leung SSY. Bacteriophage endolysins against gram-positive bacteria, an overview on the clinical development and recent advances on the delivery and formulation strategies. Crit Rev Microbiol. 2022;48:303–326. doi: 10.1080/1040841X.2021.1962803. [DOI] [PubMed] [Google Scholar]
- 101.Schmelcher M, Shen Y, Nelson DC, Eugster MR, Eichenseher F, Hanke DC, et al. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J Antimicrob Chemother. 2015;70:1453–1465. doi: 10.1093/jac/dku552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fenton M, Casey PG, Hill C, Gahan CG, Ross RP, McAuliffe O, et al. The truncated phage lysin CHAP(k) eliminates Staphylococcus aureus in the nares of mice. Bioeng Bugs. 2010;1:404–407. doi: 10.4161/bbug.1.6.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim S, Lee DW, Jin JS, Kim J. Antimicrobial activity of LysSS, a novel phage endolysin, against Acinetobacter baumannii and Pseudomonas aeruginosa. J Glob Antimicrob Resist. 2020;22:32–39. doi: 10.1016/j.jgar.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Wu M, Hu K, Xie Y, Liu Y, Mu D, Guo H, et al. A novel phage PD-6A3, and its endolysin Ply6A3, with extended lytic activity against Acinetobacter baumannii. Front Microbiol. 2019;9:3302. doi: 10.3389/fmicb.2018.03302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Paradis-Bleau C, Cloutier I, Lemieux L, Sanschagrin F, Laroche J, Auger M, et al. Peptidoglycan lytic activity of the Pseudomonas aeruginosa phage phiKZ gp144 lytic transglycosylase. FEMS Microbiol Lett. 2007;266:201–209. doi: 10.1111/j.1574-6968.2006.00523.x. [DOI] [PubMed] [Google Scholar]
- 106.Yan J, Yang R, Yu S, Zhao W. The application of the lytic domain of endolysin from Staphylococcus aureus bacteriophage in milk. J Dairy Sci. 2021;104:2641–2653. doi: 10.3168/jds.2020-19456. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Cheng M, Zhang H, Dai J, Guo Z, Li X, et al. Antibacterial effects of phage lysin LysGH15 on planktonic cells and biofilms of diverse staphylococci. Appl Environ Microbiol. 2018;84:e00886–e918. doi: 10.1128/AEM.00886-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang H, Zhang Y, Yu J, Huang Y, Zhang XE, Wei H. Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother. 2014;58:536–542. doi: 10.1128/AAC.01793-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chopra S, Harjai K, Chhibber S. Potential of sequential treatment with minocycline and S. aureus specific phage lysin in eradication of MRSA biofilms: an in vitro study. Appl Microbiol Biotechnol. 2015;99:3201–3210. doi: 10.1007/s00253-015-6460-1. [DOI] [PubMed] [Google Scholar]
- 110.Chopra S, Harjai K, Chhibber S. Potential of combination therapy of endolysin MR-10 and minocycline in treating MRSA induced systemic and localized burn wound infections in mice. Int J Med Microbiol. 2016;306:707–716. doi: 10.1016/j.ijmm.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Manoharadas S, Witte A, Bläsi U. Antimicrobial activity of a chimeric enzybiotic towards Staphylococcus aureus. J Biotechnol. 2009;139:118–123. doi: 10.1016/j.jbiotec.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob Agents Chemother. 2005;49:4789–4792. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. Phage lytic enzyme Cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis. 2008;197:1519–1522. doi: 10.1086/587942. [DOI] [PubMed] [Google Scholar]
- 114.Witzenrath M, Schmeck B, Doehn JM, Tschernig T, Zahlten J, Loeffler JM, et al. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia. Crit Care Med. 2009;37:642–649. doi: 10.1097/CCM.0b013e31819586a6. [DOI] [PubMed] [Google Scholar]
- 115.Bustamante N, Campillo NE, García E, Gallego C, Pera B, Diakun GP, et al. Cpl-7, a lysozyme encoded by a pneumococcal bacteriophage with a novel cell wall-binding motif. J Biol Chem. 2010;285:33184–33196. doi: 10.1074/jbc.M110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Díez-Martínez R, de Paz HD, Bustamante N, García E, Menéndez M, García P. Improving the lethal effect of cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob Agents Chemother. 2013;57:5355–5365. doi: 10.1128/AAC.01372-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Díez-Martínez R, De Paz HD, García-Fernández E, Bustamante N, Euler CW, Fischetti VA, et al. A novel chimeric phage lysin with high in vitro and in vivo bactericidal activity against Streptococcus pneumoniae. J Antimicrob Chemother. 2015;70:1763–1773. doi: 10.1093/jac/dkv038. [DOI] [PubMed] [Google Scholar]
- 118.Blázquez B, Fresco-Taboada A, Iglesias-Bexiga M, Menéndez M, García P. PL3 amidase, a tailor-made lysin constructed by domain shuffling with potent killing activity against pneumococci and related species. Front Microbiol. 2016;7:1156. doi: 10.3389/fmicb.2016.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang H, Gong Y, Zhang H, Etobayeva I, Miernikiewicz P, Luo D, et al. ClyJ is a novel pneumococcal chimeric lysin with a cysteine- and histidine-dependent amidohydrolase/peptidase catalytic domain. Antimicrob Agents Chemother. 2019;63:e02043–e2118. doi: 10.1128/AAC.02043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang H, Luo D, Etobayeva I, Li X, Gong Y, Wang S, et al. Linker editing of pneumococcal lysin ClyJ conveys improved bactericidal activity. Antimicrob Agents Chemother. 2020;64:e01610–e1619. doi: 10.1128/AAC.01610-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luo D, Huang L, Gondil VS, Zhou W, Yang W, Jia M, et al. A choline-recognizing monomeric Lysin, ClyJ-3m, shows elevated activity against Streptococcus pneumoniae. Antimicrob Agents Chemother. 2020;64:e00311–e320. doi: 10.1128/AAC.00311-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van der Kamp I, Draper LA, Smith MK, Buttimer C, Ross RP, Hill C. A new phage lysin isolated from the oral microbiome targeting Streptococcus pneumoniae. Pharmaceuticals (Basel) 2020;13:478. doi: 10.3390/ph13120478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Silva MD, Oliveira H, Faustino A, Sillankorva S. Characterization of MSlys, the endolysin of Streptococcus pneumoniae phage MS1. Biotechnol Rep (Amst) 2020;28:e00547. doi: 10.1016/j.btre.2020.e00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lood R, Raz A, Molina H, Euler CW, Fischetti VA. A highly active and negatively charged Streptococcus pyogenes lysin with a rare D-alanyl-L-alanine endopeptidase activity protects mice against streptococcal bacteremia. Antimicrob Agents Chemother. 2014;58:3073–3084. doi: 10.1128/AAC.00115-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng Q, Fischetti VA. Mutagenesis of a bacteriophage lytic enzyme PlyGBS significantly increases its antibacterial activity against group B streptococci. Appl Microbiol Biotechnol. 2007;74:1284–1291. doi: 10.1007/s00253-006-0771-1. [DOI] [PubMed] [Google Scholar]
- 126.Oechslin F, Daraspe J, Giddey M, Moreillon P, Resch G. In vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia. Antimicrob Agents Chemother. 2013;57:6276–6283. doi: 10.1128/AAC.01701-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoong P, Schuch R, Nelson D, Fischetti VA. PlyPH, a bacteriolytic enzyme with a broad pH range of activity and lytic action against Bacillus anthracis. J Bacteriol. 2006;188:2711–2714. doi: 10.1128/JB.188.7.2711-2714.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kong M, Na H, Ha NC, Ryu S. LysPBC2, a novel endolysin harboring a Bacillus cereus spore binding domain. Appl Environ Microbiol. 2019;85:e02462–e2518. doi: 10.1128/AEM.02462-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gong P, Cheng M, Li X, Jiang H, Yu C, Kahaer N, et al. Characterization of Enterococcus faecium bacteriophage IME-EFm5 and its endolysin LysEFm5. Virology. 2016;492:11–20. doi: 10.1016/j.virol.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 130.Cheng M, Zhang Y, Li X, Liang J, Hu L, Gong P, et al. Endolysin LysEF-P10 shows potential as an alternative treatment strategy for multidrug-resistant Enterococcus faecalis infections. Sci Rep. 2017;7:10164. doi: 10.1038/s41598-017-10755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zimmer M, Vukov N, Scherer S, Loessner MJ. The murein hydrolase of the bacteriophage phi3626 dual lysis system is active against all tested Clostridium perfringens strains. Appl Environ Microbiol. 2002;68:5311–5317. doi: 10.1128/AEM.68.11.5311-5317.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sekiya H, Okada M, Tamai E, Shimamoto T, Shimamoto T, Nariya H. A putative amidase endolysin encoded by Clostridium perfringens St13 exhibits specific lytic activity and synergizes with the muramidase endolysin Psm. Antibiotics (Basel) 2021;10:245. doi: 10.3390/antibiotics10030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gould FK, Brindle R, Chadwick PR, Fraise AP, Hill S, Nathwani D, et al. Guidelines (2008) for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the United Kingdom. J Antimicrob Chemother. 2009;63:849–861. doi: 10.1093/jac/dkp065. [DOI] [PubMed] [Google Scholar]
- 134.Chen J, Zhou H, Huang J, Zhang R, Rao X. Virulence alterations in Staphylococcus aureus upon treatment with the sub-inhibitory concentrations of antibiotics. J Adv Res. 2021;31:165–175. doi: 10.1016/j.jare.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Drilling AJ, Cooksley C, Chan C, Wormald PJ, Vreugde S. Fighting sinus-derived Staphylococcus aureus biofilms in vitro with a bacteriophage-derived muralytic enzyme. Int Forum Allergy Rhinol. 2016;6:349–355. doi: 10.1002/alr.21680. [DOI] [PubMed] [Google Scholar]
- 136.Paul VD, Rajagopalan SS, Sundarrajan S, George SE, Asrani JY, Pillai R, et al. A novel bacteriophage tail-associated muralytic enzyme (TAME) from phage K and its development into a potent antistaphylococcal protein. BMC Microbiol. 2011;11:226. doi: 10.1186/1471-2180-11-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Haddad Kashani H, Schmelcher M, Sabzalipoor H, Seyed Hosseini E, Moniri R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: current status of research and novel delivery strategies. Clin Microbiol Rev. 2018;31:e00071–e117. doi: 10.1128/CMR.00071-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McCarthy MW. Exebacase: a novel approach to the treatment of staphylococcal infections. Drugs R D. 2022;22:113–117. doi: 10.1007/s40268-022-00383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Loeffler JM, Fischetti VA. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother. 2003;47:375–377. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Doehn JM, Fischer K, Reppe K, Gutbier B, Tschernig T, Hocke AC, et al. Delivery of the endolysin Cpl-1 by inhalation rescues mice with fatal pneumococcal pneumonia. J Antimicrob Chemother. 2013;68:2111–2117. doi: 10.1093/jac/dkt131. [DOI] [PubMed] [Google Scholar]
- 141.McCullers JA, Karlström A, Iverson AR, Loeffler JM, Fischetti VA. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3:e28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nakonieczna A, Cooper CJ, Gryko R. Bacteriophages and bacteriophage-derived endolysins as potential therapeutics to combat Gram-positive spore forming bacteria. J Appl Microbiol. 2015;119:620–631. doi: 10.1111/jam.12881. [DOI] [PubMed] [Google Scholar]
- 143.Wittekind M, Schuch R. Cell wall hydrolases and antibiotics: exploiting synergy to create efficacious new antimicrobial treatments. Curr Opin Microbiol. 2016;33:18–24. doi: 10.1016/j.mib.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 144.Schmelcher M, Powell AM, Camp MJ, Pohl CS, Donovan DM. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Appl Microbiol Biotechnol. 2015;99:8475–8486. doi: 10.1007/s00253-015-6579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Becker SC, Foster-Frey J, Donovan DM. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol Lett. 2008;287:185–191. doi: 10.1111/j.1574-6968.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- 146.Rodríguez-Cerrato V, García P, Del Prado G, García E, Gracia M, Huelves L, et al. In vitro interactions of LytA, the major pneumococcal autolysin, with two bacteriophage lytic enzymes (Cpl-1 and Pal), cefotaxime and moxifloxacin against antibiotic-susceptible and -resistant Streptococcus pneumoniae strains. J Antimicrob Chemother. 2007;60:1159–1162. doi: 10.1093/jac/dkm342. [DOI] [PubMed] [Google Scholar]
- 147.Gouveia A, Pinto D, Veiga H, Antunes W, Pinho MG, São-José C. Synthetic antimicrobial peptides as enhancers of the bacteriolytic action of staphylococcal phage endolysins. Sci Rep. 2022;12:1245. doi: 10.1038/s41598-022-05361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Djurkovic S, Loeffler JM, Fischetti VA. Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme Cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. Antimicrob Agents Chemother. 2005;49:1225–1228. doi: 10.1128/AAC.49.3.1225-1228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim NH, Park WB, Cho JE, Choi YJ, Choi SJ, Jun SY, et al. Effects of phage endolysin SAL200 combined with antibiotics on Staphylococcus aureus infection. Antimicrob Agents Chemother. 2018;62:e00731–e818. doi: 10.1128/AAC.00731-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Haddad Kashani H, Fahimi H, Dasteh Goli Y, Moniri R. A novel chimeric endolysin with antibacterial activity against methicillin-resistant Staphylococcus aureus. Front Cell Infect Microbiol. 2017;7:290. doi: 10.3389/fcimb.2017.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nair S, Desai S, Poonacha N, Vipra A, Sharma U. Antibiofilm activity and synergistic inhibition of Staphylococcus aureus biofilms by bactericidal protein P128 in combination with antibiotics. Antimicrob Agents Chemother. 2016;60:7280–7289. doi: 10.1128/AAC.01118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jun SY, Jung GM, Yoon SJ, Youm SY, Han HY, Lee JH, et al. Pharmacokinetics of the phage endolysin-based candidate drug SAL200 in monkeys and its appropriate intravenous dosing period. Clin Exp Pharmacol Physiol. 2016;43:1013–1016. doi: 10.1111/1440-1681.12613. [DOI] [PubMed] [Google Scholar]
- 153.Jun SY, Jung GM, Yoon SJ, Choi YJ, Koh WS, Moon KS, et al. Preclinical safety evaluation of intravenously administered SAL200 containing the recombinant phage endolysin SAL-1 as a pharmaceutical ingredient. Antimicrob Agents Chemother. 2014;58:2084–2088. doi: 10.1128/AAC.02232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Burgin DJ, Liu R, Hsieh C, Heinzinger L, Otto, M. Investigational agents for the treatment of methicillin-resistant Staphylococcusaureus (MRSA) bacteremia: progress in clinical trials. Expert Opin Investig Drugs. 2022;31263–79. [DOI] [PMC free article] [PubMed]
- 155.Nau R, Eiffert H. Modulation of release of proinflammatory bacterial compounds by antibacterials: potential impact on course of inflammation and outcome in sepsis and meningitis. Clin Microbiol Rev. 2002;15:95–110. doi: 10.1128/CMR.15.1.95-110.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rosano GL, Morales ES, Ceccarelli EA. New tools for recombinant protein production in Escherichia coli: a 5-year update. Protein Sci. 2019;28:1412–1422. doi: 10.1002/pro.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gutiérrez D, Fernández L, Rodríguez A, García P. Are phage lytic proteins the secret weapon to kill Staphylococcus aureus? MBio. 2018;9:e01923–e2017. doi: 10.1128/mBio.01923-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adrio JL, Demain AL. Recombinant organisms for production of industrial products. Bioeng Bugs. 2010;1:116–131. doi: 10.4161/bbug.1.2.10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stoffels L, Taunt HN, Charalambous B, Purton S. Synthesis of bacteriophage lytic proteins against Streptococcus pneumoniae in the chloroplast of Chlamydomonas reinhardtii. Plant Biotechnol J. 2017;15:1130–1140. doi: 10.1111/pbi.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Proença D, Leandro C, Garcia M, Pimentel M, São-José C. EC300: a phage-based, bacteriolysin-like protein with enhanced antibacterial activity against Enterococcus faecalis. Appl Microbiol Biotechnol. 2015;99:5137–5149. doi: 10.1007/s00253-015-6483-7. [DOI] [PubMed] [Google Scholar]
- 161.Filatova LY, Donovan DM, Ishnazarova NT, Foster-Frey JA, Becker SC, Pugachev VG, et al. A chimeric LysK-lysostaphin fusion enzyme lysing Staphylococcus aureus cells: a study of both kinetics of inactivation and specifics of interaction with anionic polymers. Appl Biochem Biotechnol. 2016;180:544–557. doi: 10.1007/s12010-016-2115-7. [DOI] [PubMed] [Google Scholar]
- 162.Gondil VS, Chhibber S. Bacteriophage and endolysin encapsulation systems: a promising strategy to improve therapeutic outcomes. Front Pharmacol. 2021;12:675440. doi: 10.3389/fphar.2021.675440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Portilla S, Fernández L, Gutiérrez D, Rodríguez A, García P. Encapsulation of the antistaphylococcal endolysin LysRODI in pH-sensitive liposomes. Antibiotics (Basel) 2020;9:242. doi: 10.3390/antibiotics9050242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hathaway H, Ajuebor J, Stephens L, Coffey A, Potter U, Sutton JM, et al. Thermally triggered release of the bacteriophage endolysin CHAPK and the bacteriocin lysostaphin for the control of methicillin resistant Staphylococcus aureus (MRSA) J Control Release. 2017;245:108–115. doi: 10.1016/j.jconrel.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yao F, Wu X, Liao Y, Yan Q, Li Y. Smart chimeric lysin ClyC loaded alginate hydrogel reduces Staphylococcusaureus induced bone infection. Front Mater. 2021;8.
- 166.Kaur J, Kour A, Panda JJ, Harjai K, Chhibber S. Exploring endolysin-loaded alginate-chitosan nanoparticles as future remedy for staphylococcal infections. AAPS PharmSciTech. 2020;21:233. doi: 10.1208/s12249-020-01763-4. [DOI] [PubMed] [Google Scholar]
- 167.Vázquez R, Caro-León FJ, Nakal A, Ruiz S, Doñoro C, García-Fernández L, et al. DEAE-chitosan nanoparticles as a pneumococcus-biomimetic material for the development of antipneumococcal therapeutics. Carbohydr Polym. 2021;273:118605. doi: 10.1016/j.carbpol.2021.118605. [DOI] [PubMed] [Google Scholar]
- 168.Silva MD, Paris JL, Gama FM, Silva BFB, Sillankorva S. Sustained release of a Streptococcus pneumoniae endolysin from liposomes for potential otitis media treatment. ACS Infec Dis. 2021;7:2127–2137. doi: 10.1021/acsinfecdis.1c00108. [DOI] [PubMed] [Google Scholar]
- 169.Dzuvor CKO, Shanbhag BK, Younas T, Shen HH, Haritos VS, He L. Engineering self-assembled endolysin nanoparticles against antibiotic-resistant bacteria. ACS Appl Bio Mater. 2022. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.