Abstract
Background
Dietary intake of pulses is associated with beneficial effects on body weight management and cardiometabolic health, but some of these effects are now known to depend on integrity of plant cells, which are usually disrupted by flour milling. Novel cellular flours preserve the intrinsic dietary fiber structure of whole pulses and provide a way to enrich preprocessed foods with encapsulated macronutrients.
Objectives
This study aimed to determine the effects of replacing wheat flour with cellular chickpea flour on postprandial gut hormones, glucose, insulin, and satiety responses to white bread.
Methods
We conducted a double-blind randomized crossover study in which postprandial blood samples and scores were collected from healthy human participants (n = 20) after they consumed bread enriched with 0%, 30%, or 60% (wt/wt) cellular chickpea powder (CCP, 50 g total starch per serving).
Results
Bread type significantly affected postprandial glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) responses (time × treatment, P = 0.001 for both). The 60% CCP breads elicited significantly elevated and sustained release of these anorexigenic hormones [between 0% and 60% CPP—GLP-1: mean difference incremental area under the curve (iAUC), 3101 pM/min; 95% CI: 1891, 4310; P-adjusted < 0.001; PYY: mean difference iAUC, 3576 pM/min; 95% CI: 1024, 6128; P-adjusted = 0.006] and tended to increase fullness (time × treatment, P = 0.053). Moreover, bread type significantly influenced glycemia and insulinemia (time × treatment, P < 0.001, P = 0.006, and P = 0.001 for glucose, insulin, and C-peptide, respectively), with 30% CCP breads eliciting a >40% lower glucose iAUC (P-adjusted < 0.001) than the 0% CCP bread. Our in vitro studies revealed slow digestion of intact chickpea cells and provide a mechanistic explanation for the physiologic effects.
Conclusions
The novel use of intact chickpea cells to replace refined flours in a white bread stimulates an anorexigenic gut hormone response and has potential to improve dietary strategies for prevention and treatment of cardiometabolic diseases.
This study was registered at clinicaltrials.gov as NCT03994276.
Keywords: dietary fiber, GLP-1, PYY, satiety, intact plant cells, randomized crossover, legumes, bioaccessibility
Introduction
Obesity and cardiometabolic disease are partially fueled by the increased intake of processed foods made from highly refined ingredients [1, 2]. Processing disrupts food microstructure and increases rates of carbohydrate (e.g., starch) digestion and absorption, resulting in high glycemic, less-satiating foods than minimally processed whole foods [3, 4]. In whole pulses, the dietary fiber structure, particularly the intact plant cell wall, helps to regulate macronutrient digestion [5, 6]. By designing new food ingredients that retain the cellular structure, it may be possible to slow macronutrient digestion for improved glucose homeostasis and satiety.
Glucagon-like peptide (GLP-1) and peptide YY (PYY) are anorexigenic gut hormones important in the regulation of glucose homeostasis and food intake [7] through the ileal brake [8]. These hormones are satiety-promoting pharmacologic targets, successfully used in obesity management [[9], [10], [11]]. GLP-1 and PYY are produced after meal ingestion when bioaccessible macronutrient digestion products bind nutrient-sensing receptors of specialized enteroendocrine cells [7]. The density of these cells increases distally in the intestine [12, 13]. Thus, rapidly digested foods have limited capacity to stimulate satiety through the distal gut and likely contribute to a positive energy balance leading to obesity [4, 14].
Food microstructure has a profound effect on nutrient absorption [15] and could offer new routes to optimize diet-mediated satiety. Cotyledon cells from legumes (including pulses such as chickpeas) are attracting interest for their natural bioencapsulation properties, providing a new route to deliver nutrients to the distal gut [5, 6, [16], [17], [18], [19]]. Unlike wheat and other cereals, owing to their primary plant cell wall (dietary fiber) structure and properties, cotyledon tissues of cooked pulses tend to separate into intact cells [20], such that macronutrients remain encapsulated by the cell walls [5, 20]. Laboratory [20, 21] and human intubation studies [22, 23] have shown that cotyledon cells from cooked pulses resist digestive conditions of the stomach and small intestine. The slow release of these encapsulated nutrients underpins the low glycemic properties of pulses [[24], [25], [26]] and contribute to their effects on subjective appetite [27]. Thus, although pulses are regarded as a source of dietary fiber and resistant starch (RS), the preservation of plant cell intactness may be critical to achieve beneficial effects on obesity and cardiometabolic disease risk [[28], [29], [30]]. By contrast, refined pulse ingredients such as conventionally milled pulse flours, which consist predominantly of ruptured plant cells, are highly digestible once hydrothermally processed [31] and may not be as satiating as whole pulses [27].
We have recently shown that pulses can be exploited to obtain a novel cellular ingredient with high levels of encapsulated starch (type 1 RS) [17, 26, 31, 32]. The ingredient retains starch resistance to amylolysis, even after baking, and lowers starch digestibility and glycemic potency of starch-rich foods [[26], [31]]. In addition, inclusion of intact chickpea cells (∼140 μm) within a white bread reduced postprandial interstitial fluid glucose concentrations while retaining acceptable product palatability [26]. Laboratory studies by our group [20, 31] and others [21, 33, 34] indicate that intact legume cell walls slow the release of encapsulated starch during simulated digestion. This is likely to influence the spatiotemporal concentration of digestion products in the intestinal lumen in vivo, affecting enteroendocrine nutrient-sensing and gut hormone secretion. Thus, plant cells with slow digestibility may have the potential to trigger postprandial gut hormone secretion. This study aimed to investigate the use of newly developed cellular flours to influence gut hormone secretion and satiety responses in healthy adults.
Materials and Methods
Bread products for in vitro and in vivo testing
Bread rolls were prepared in which 30% or 60% of the white wheat flour was replaced in the breadmaking recipe with a cellular chickpea powder (CCP, WO 2019/155190 A1, available commercially as PulseON®; PulseON Foods). Further details on the methods used to prepare the CCP and its technofunctional characteristics have been published previously [26, 31]. In addition, a control wheat bread (0% CCP) made with 100% white flour was prepared. The formulation and bread preparation method have been described in full previously [26]. In brief, the CCP bread rolls include on a %wet basis (ingredient mass/dough mass): 44.1%–50.3% water; 12.8%–33.4% white wheat bread flour (Taste the Difference Very Strong Canadian Bread Flour; Sainsbury’s); 1.0% sucrose (white caster sugar; Sainsbury’s); 0.8% NaCl (Saxa table salt; Premier Foods); 1.8% vegetable fat (Trex Vegetable Baking Fat; Princes Group); 2.4%–5.2% wheat gluten (Vital Wheat Gluten 75%–80% protein; Bob’s Red Mill); 0.1% ascorbic acid (Dove’s farm), purchased from Amazon; and 0.9% dry baker’s yeast (Ferminpan Red) provided by Lallemand. The entire dough was divided into matched bread rolls so that each bread roll would contain 50 g of potentially available starch per serving. Measured macronutrient composition of these breads is provided in Table 1, which includes a detailed breakdown of the carbohydrate fractions. All bread rolls contained a similar amount of wheat gluten per serving (11.2–13.5 g/serving), and the higher protein content of CCP bread rolls is owing to the cell-encapsulated protein within the CCP. Full details of the preparation and characteristics of these bread rolls has been reported previously [26]. In brief, the previous study showed no significant differences in the participant sensory scores of 0%, 30%, and 60% CCP bread rolls in taste or texture when these were consumed on separate occasions, indicating good palatability. At 60% CCP, the bread was noticeably less moist and more difficult to eat than the control. Although not detected by participants, the laboratory analysis did reveal harder texture and increased bulk density in CCP breads than those of the control [26]. For context, a photograph of bread rolls is shown alongside bread loaves in Supplemental Figure 1.
TABLE 1.
Nutrient composition of test meals (per served portion)
| 0% CCP | 30% CCP | 60% CCP | |
|---|---|---|---|
| Bread roll | |||
| Amount served (g)1 | 115.0 ± 2.7 | 150.0 ± 1.6 | 201.2 ± 1.3 |
| Moisture (g/serving)2 | 39.1 | 62.3 | 94.6 |
| Energy (kJ/serving)2 | 1301.8 | 1501.5 | 1823.1 |
| Protein (g/serving)2 | 12.88 | 17.7 | 27.16 |
| Fat (g/serving)2 | 3.33 | 5.55 | 8.84 |
| Starch (g/serving)3 | 45.3 | 44.9 | 42.5 |
| Digestible | 45.07 | 43.23 | 39.38 |
| Resistant | 0.25 | 1.63 | 3.13 |
| Total sugar (g/serving)3 | 2.76 | 3.45 | 5.67 |
| Potentially available carbohydrate (g/serving)4 | 48.1 | 48.3 | 48.2 |
| Dietary fiber (g/serving)2 | 2.64 | 6.15 | 10.65 |
| Sodium (g/serving)2 | 0.419 | 0.543 | 0.752 |
| Jam | |||
| Amount served (g) | 20 | 20 | 20 |
| Energy (kJ/serving)5 | 139 | 139 | 139 |
| Protein (g/serving)5 | 0.06 | 0.06 | 0.06 |
| Fat (g/serving)5 | 0.02 | 0.02 | 0.02 |
| Sugars (g/serving)5 | 0.4 | 0.4 | 0.4 |
| Polyols (g/serving)5 | 12.8 | 12.8 | 12.8 |
| Dietary fiber (g/serving)5 | 0.12 | 0.12 | 0.12 |
| Salt (g/serving)5 | 0.04 | 0.04 | 0.04 |
| Water | |||
| Amount served (g) | 381 | 358 | 325 |
| Total meal weight (g) | 516 | 528 | 546 |
Values are mean ± SD; 1 bread roll per serving; see further details in a recent publication [26].
Proximate determinations by ALS Laboratories.
Direct measurements obtained using Megazyme total and resistant starch kits.
Potentially available carbohydrates is the sum of total starch and sugars.
Nutrition specification data from the manufacturer.
Acute postprandial study
Study participants
A human study (randomized double-blind crossover design) was conducted (Supplemental Figure 2), in which 20 fasted, healthy participants aged 18–45 y were recruited using advertisements around King’s College London, including circular e-mails and posters. Exclusion criteria included the following: BMI <18 or >35 kg/m2, blood pressure ≥160/100 mm Hg, fasted glucose >6.0 mmol/L, plasma cholesterol ≥7.8 mmol/L, plasma triacylglycerol ≥5.0 mmol/L, medications that may interfere with the study (e.g., antidiabetic or lipid-lowering drugs), allergy or sensitivity to wheat, alcohol intake >28 units/wk, and active or recent cessation of smoking (<6 months). Participants were healthy with no history of cardiovascular disease, diabetes, or gastrointestinal disorders, as confirmed by a full medical history (Table 2). BMI, blood pressure, liver function, blood cell count, fasting plasma glucose, and lipid concentrations were confirmed to be within limits during a screening clinical visit that took place before confirming enrolment. Individuals who met all inclusion and exclusion criteria were randomly allocated 1 of the 6 treatment orders (Table 2) using Sealed Envelope (www.sealedenvelope.com). The composition of treatment meals A, B, and C was blinded to the investigators, technicians performing analysis of blood samples, and participants. Investigators and participants remained blinded until the completion of the study and data analysis. The study was conducted in accordance with the Declaration of Helsinki and approved by the relevant research ethics committee (HR-18/19-8431, BDM Research Ethics Subcommittee at King’s College London) in the United Kingdom, and registered on clinicaltrials.gov (NCT03994276) as phase 2. The results of phase 1 have been previously reported [26]. All participants gave their written informed consent after being provided with oral and written information about the aims and protocol of the study. Data were stored in accordance with the General Data Protection Regulation 2018 as per the Data Protection Act [35], and biological samples were handled, stored, transported and disposed of in accordance with the Human Tissue Act (2004) [36]. Participants were reimbursed for their time and travel expenses on completion of the study.
TABLE 2.
Physical characteristics of enrolled study participants by the randomization sequence
| Treatment sequence |
||||||
|---|---|---|---|---|---|---|
| ABC | ACB | BAC | BCA | CAB | CBA | |
| Sex, n (%) | ||||||
| Male | 2 (66.6) | 1 (50) | 1 (33.3) | 2 (40) | 0 (0) | 4 (80) |
| Female | 1 (33.3) | 1 (50) | 2 (66.6) | 3 (60) | 2 (100) | 1 (20) |
| Age (y) | 26.33 ± 1.50 | 31.50 ± 10.61 | 26.00 ± 2.52 | 27.40 ± 3.44 | 25.00 ± 0.00 | 30.20 ± 5.98 |
| BMI (kg/m2) | 24.63 ± 3.62 | 22.65 ± 3.89 | 21.10 ± 0.50 | 24.20 ± 4.15 | 25.20 ± 0.99 | 23.52 ± 1.90 |
| Fasted plasma glucose (mM) | 5.00 ± 0.25 | 4.92 ± 0.40 | 4.53 ± 0.17 | 5.00 ± 0.48 | 4.65 ± 0.21 | 4.56 ± 0.16 |
| Fasted total cholesterol (mM) | 4.48 ± 0.65 | 4.91 ± 0.40 | 4.71 ± 0.43 | 4.76 ± 1.03 | 4.33 ± 1.68 | 4.50 ± 1.41 |
Values are given as mean ± SD unless otherwise specified.
Study design
A randomized, controlled, double-blind, crossover design study was undertaken at the Metabolic Research Unit (MRU), Department of Nutritional Sciences, King’s College London, between August 2019 and January 2020. The trial investigated the effects of 0%, 30% and 60% CCP breads on postprandial glucose, insulin, and gut hormone responses, using blood samples collected over a 240-min time course. Primary outcomes were glucose, insulin, and C-peptide levels, gut hormone responses [GLP-1, PYY, and glucose-dependent insulinotropic peptide (GIP)], and the postprandial incremental area under the curve (iAUC) between 0 and 60 min as the predefined primary outcome measure for each biomarker. Predefined secondary outcome measures investigated the iAUC in other time frames (0–120 min, 30–90 min, and 90–240 min) and peak concentrations (Cmax) and time to peak (Tmax). Additional exploratory outcomes included the first peak iAUC, subjective satiety responses, and amino acid responses over the 240-min period. On examination of the postprandial curves, it was apparent that the fixed periods chosen as the predefined primary and secondary outcome measures did not fully capture the postprandial responses for all individuals. Therefore, our analysis has focused more on the first peak area, which was a more appropriate descriptor of the postprandial response and, therefore, better suited to addressing the scientific aims of the study. Analyses relating to predefined primary and secondary outcome measures are included in the supplementary material (Supplemental Table 1).
Three bread rolls baked with different quantities of CCP (0%, 30%, or 60%, wt/wt, of refined wheat flour replaced with CCP) were consumed by participants in a random order, at 3 separate study visits and with at least 4 days washout between each visit. Replacement of wheat flour with CCP meant that ∼12 and 30 g of the total starch in the 0% bread roll was replaced by starch from CCP to make the 30% and 60% bread rolls, respectively. Each bread roll was served with 20 g of no-added sugar strawberry jam (energy-reduced strawberry jam with sweetener; Marillo Foods), providing <0.4 g sugars (mainly fructose) and 12.8 g polyols (mainly from sorbitol), to aid palatability. The total weight of drinking water served with each meal was adjusted to achieve a constant total meal weight of 420 g because the different type of breads had different weights, owing mostly to the differences in moisture content. Participants were instructed to consume the meal at their normal pace, which was standardized based on their first visit. Each participant received a different bread roll treatment on each of the 3 separate visits to the MRU.
On the morning of each intervention, the participants arrived at the MRU after a 12-h fast and having consumed a standard evening meal (350–450 kcal and <12 g fat per serving and < 3 g dietary fiber/100 g) the previous evening. A venous cannula was inserted in a vein in the antecubital fossa or a forearm vein by a trained phlebotomist. Baseline fasted blood samples (t = − 15 min) were collected before the allocated test meal was consumed at t = 0 min, and blood samples were subsequently taken at frequent intervals up to 4 h posttest meal, at t = 15, 30, 45, 60, 90, 120, 180, and 240 min after the first bite. Blood samples were collected into appropriate tubes [BD Vacutainer tubes: fluoride/oxalate tubes for glucose analysis; SST serum tubes for insulin and C-peptide analysis, and K2 EDTA tubes with DPP-IV (10 μL/mL blood; Merck Millipore) and aprotinin (500 KIU/mL blood; Nordic Pharma) for GIP, GLP-1, and PYY analysis]. All samples were centrifuged at 1300 × g, 4°C for 15 min, and plasma/serum aliquots were stored at −80°C until used for biochemical analyses, performed by a clinical pathology accredited biochemistry laboratory (Affinity Biomarkers Labs). Glucose was determined enzymatically on a Siemens Advia 1800 auto-analyzer and insulin and C-peptide by Siemens Centaur XP (Siemens Healthcare Diagnostics). Plasma gut hormone concentrations (GIP, GLP-1, and PYY) were determined by electrochemiluminescent multiplexed assay (Mesoscale Discovery). Amino acids were determined using UPLC-MS/MS method, described further. Of the 21 participants recruited, 20 completed the study (Supplemental Figure 2); however, one of the volunteers was unable to provide venous blood samples after consumption of one of the test meals (n = 20 for 0% CCP and 30% CCP; n = 19 for 60% CCP).
Visual analog scales (VAS) were completed at baseline (t = − 10 min), after the test meal (t = 10 min), and at t = 30, 60, 90, 120, 180, and 240 min. Participants marked responses to satiety questions (How hungry do you feel?, How full do you feel?, How strong is your desire to eat?) using 100-mm VAS, with questions that were anchored from “not at all” to “extremely.” In addition, participants were asked, “How would you rate your digestive comfort?” anchored from “very uncomfortable” to “very comfortable,” and “How much do you think you can eat?” anchored to “nothing at all” and “a lot.” The combined satiety score was calculated as follows: [(100 − Hunger) + (100 − Desire to eat) + (100 − Food Volume) + (1/Fullness)]/4 [37]. After the completion of the test period, at t = 255 min, participants were provided with an unlimited lunch, consisting of pasta, tomato-based sauce, and cheese. The unlimited energy intake was calculated from the total amount of food consumed (in grams) at the end of each test period.
Analysis of amino acids in plasma samples
Plasma extraction for amino acids analysis was adopted from a previously published method [38] as described elsewhere [39]. In brief, isotope-labeled internal standards (canonical amino acid mix; Cambridge Isotope Laboratories) dissolved in 90 μL of 60% acetonitrile were added to 10 μL plasma sample/calibration standards, vortexed for 1 min, and kept at 4°C for 5 min. Then, samples were centrifuged at 13,000 × g (4°C) for 10 min. The supernatant was transferred into HPLC vials for LC-MS/MS analysis. The UPLC-MS/MS method was optimized for targeted amino acids analysis in the samples using a HILIC column in an Agilent 1290 LC system coupled to a 6490 mass spectrometer with a jet stream electrospray ionization source. Separation of amino acids was performed according to the chromatographic method described by Prinsen et al. [40]. Amino acids were detected in a 1-μL extracted plasma sample injection by multiple reaction monitoring mode using positive electrospray polarity. Quantification was performed using the concentration-to-peak area ratio (the integrated peak area of the analyte to that of the internal standard) calibration curve, and data were processed with MassHunter Workstation Quantitative Analysis software (version 10.0; Agilent Technologies). The total amount of free amino acids was calculated by adding all individual amino acids measured in each sample.
In vitro digestion of bread rolls
The digestibility of 0%, 30%, and 60% CCP breads was determined in vitro in accordance with the international consensus method published by Brodkorb et al [41], following the individual enzyme format. Digestions were performed in triplicate for the breads containing 30% and 60% of CCP and in duplicate for the 0% CCP bread for each time point at 37°C. In each digestion tube, 0.228 g of breadcrumbs (1–2 mm) and 0.572 mL water were mixed with 0.8 mL simulated oral (pH 7), 1.6 mL gastric (pH 3), and 3.2 mL intestinal (pH 7) fluids to mimic the conditions of electrolytes, pH, bile salts, and enzymes in the mouth, stomach, and small intestine. Individual enzymes for the oral phase (salivary α-amylase), gastric phase (pepsin), and intestinal phase (pancreatic α-amylase, trypsin, chymotrypsin) at the specified activities [based on enzyme activity assays as per [41] were added to each time point as per the standard protocol. Bile salts were added to the intestinal phase. A blank digestion containing all enzymes and fluids with water instead of bread was included for each time point in each bread to account for the background values of the fluids, enzymes, and bile salts used. The digestion was stopped (by addition of an equal volume of 0.3 M sodium carbonate solution and, for the intestinal samples only, 50 μL of pefabloc 0.1 M per mL of intestinal digesta) at the end of the oral phase, after 30 and 60 min of gastric digestion, and at 0, 5, 10, 20, 30, 60 and 120 min of small intestinal digestion, discarding 1 tube for each time point. Inactivated samples of digesta were frozen immediately and stored at −80°C until the subsequent analysis. All reagents were from Sigma-Aldrich.
Analysis of maltose and amino acids produced from in vitro digestion
Inactivated digesta from different time points were thawed and centrifuged at 3000 × g for 10 min at 4°C. The concentration of free amino acids and reducing sugars (mainly maltose) in the supernatant was measured in aliquots taken from the supernatant. Maltose concentrations were determined using the “pahbah” (p-hydroxybenzoic acid hydrazide) reagent method [42]. Free amino acids in the supernatant were measured by LC-MS/MS (Agilent 6490 mass spectrometry), following the same method as used for human plasma samples, described earlier. Concentrations present in the digesta from the blank runs were subtracted from concentrations present in the corresponding digesta from the bread runs. The resulting net concentration of maltose or free amino acids in the digesta was divided by the initial food sample (dry matter basis) and plotted over time to represent release of these starch and protein digestion products from each bread type.
Microscopy
Light micrographs were captured with an Olympus BX60 Microscope equipped with Jenoptik ProgRes camera and a ProgRes CapturePro software. Inactivated samples of in vitro digesta from the end of oral, gastric, and duodenal digestion were thawed and stained with Lugol iodine (I2/KI) and/or 1% toluidine blue solution (Sigma-Aldrich) before viewing.
Data and statistical analysis
Statistical analyses of in vitro digestion, VAS, and blood biochemistry data (including iAUC, Cmax, and Tmax) and graphical representations were performed using GraphPad Prism 9.3.1 software (GraphPad Software). Normality of raw data distributions were assessed using a Shapiro-Wilk the normality test. For a mixed-effects analysis, normality of the residuals was checked by visual examination of QQ plots. Equal variance in the differences between treatments was not assumed, and the Greenhouse–Geisser correction applied to correct for violations in sphericity. Unless otherwise specified, the iAUC values are the first peak areas (first peak iAUC), calculated using the trapezoid rule, in GraphPad Prism 9.3.1, for individualized data using the area below the first peak and above the fasted baseline. In vivo data analyzed by the mixed-effects model ANOVA, comparing all 3 treatment groups with time and treatment (e.g., bread roll type) as fixed effects and individual differences as random effects. It is noteworthy that the multiple primary and secondary outcomes could result in an inflated risk of type 1 statistical errors. The type 1 error rate for each outcome was controlled with the use of an omnibus test and post hoc testing performed with Tukey adjustment for multiple comparisons. Post hoc analyses were performed when significant treatment × time effects were detected and multiplicity adjusted P values reported (P-adjusted), with mean differences and 95% CIs. Statistically significant effects were accepted at the 95% confidence level. A priori power calculation was based on a previous study [43] where a 30% substitution of wheat flour with RS resulted in a 40% reduction in postprandial plasma glucose and insulin responses, respectively. Using mean and SD data from the previous study indicated that n = 20 had a 90% power to detect a 40% difference in postprandial responses (iAUC) between breads containing 0%, 30%, or 60% CCP at α = 0.05. All data are presented as means ± SEM with the exception of glucose, insulin, C-peptide, and gut hormone fasting, Cmax, and Tmax values, which are reported as geometric mean ± geometric mean SD factor, and the number of participants (n) whose data were included in each analysis is indicated in figure legends and throughout the text. A nonlinear regression analysis was performed on in vitro digestibility data by fitting to 1-phase and 2-phase association equations in GraphPad Prism 9.3.1 software (GraphPad Software). Outliers were not excluded.
Results
Intact plant cells lowered postprandial glycemia and insulinemia
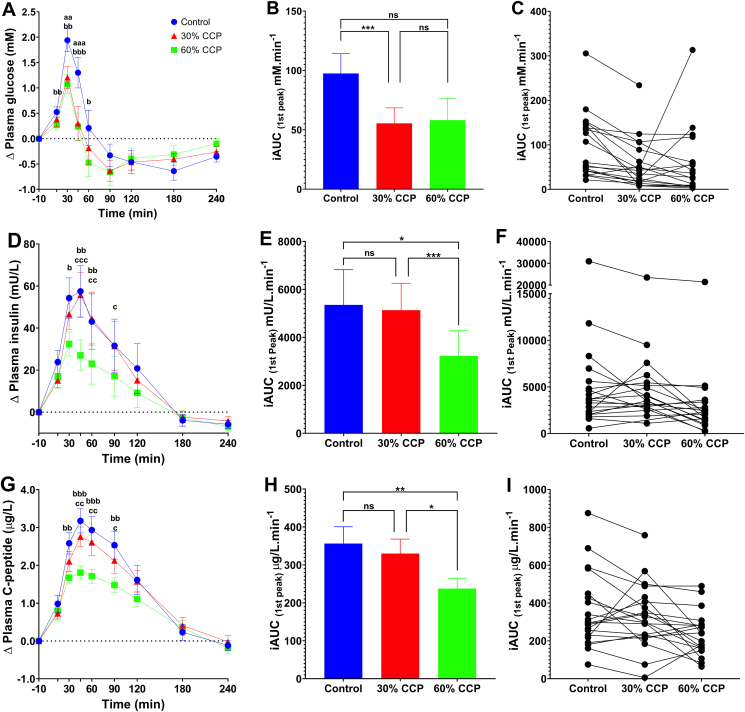
There was a significant main effect of bread type on postprandial plasma glucose responses (time × treatment, P < 0.001, ANOVA) (Figure 1A–C). Post hoc testing showed pairwise differences between the glucose responses to the 0% and those to 60% bread. The consumption of the 60% CCP bread elicited a significantly lower plasma glucose response than that of the 0% CCP bread, with a mean difference of 0.866 mmol/L (95% CI: 0.336, 1.397; P-adjusted = 0.002) at 30 min and 1.063 mmol/L (95% CI: 0.491, 1.640; P-adjusted < 0.001) at 45 min. No significant differences in the glycemic responses between 30% and 60% CCP breads were observed in the pairwise comparisons of the time-series data. Thus, analysis of time-series data (Figure 1A) revealed that effects of CCP enrichment are most pronounced during the early postprandial period (i.e., the first 60 min), with both CCP-enriched test breads eliciting a lower rise in blood glucose than the 0% CCP bread. Peak glucose concentrations (Cmax) were reached at ∼30 min and were significantly lower (P-adjusted = 0.002) for both CCP-enriched breads, relative to the 0% CCP control (Supplemental Table 2). Comparison of the mean differences in iAUC (first peak above the fasted baseline) for glucose (Figure 1B) demonstrated a significant reduction of more than 40% after consumption of 30% CCP that that of 0% CCP bread (P-adjusted < 0.001), with a mean difference of 41.97 mmol/L/min (95% CI: 18.34, 65.61). The difference in first peak iAUC for glucose between 0% and 60% CCP bread was not significant (P-adjusted = 0.115). From the individualized iAUC glucose data (Figure 1C), it is apparent that 3 participants showed a larger glucose response to the 60% CCP bread. In addition, significant differences between 0% CCP and both 30% and 60% CCP breads were observed in the iAUC over the first 60 min (P-adjusted < 0.001) (Supplemental Table 1). Thus, overall, our data showed that substitution of 30% wheat flour with CCP was sufficient to lower the glycemic response to bread, with no further attenuation at the higher level (60%) of CCP inclusion.
FIGURE 1.
Glycemic and insulinemic responses to control and CCP-enriched test bread. Postprandial responses are based on analysis of blood samples collected following consumption of white bread rolls containing 0% (control, n = 20), 30% (n = 20) or 60% (n = 19) cellular chickpea powder (CPP) and 50 g of available carbohydrate per serving. Time-course data show the change (relative to fasting concentrations) in postprandial plasma; (A) glucose (D) insulin , (G) C-peptide measured for 240 min. Bar charts show the integrated area under the curve (iAUC) calculated for the 1st peak (above baseline) of the time-course data for glucose (B), insulin (E) and C-peptide (H) responses. The scatter plots show the matched iAUC for individual participants for glucose (C), insulin (F) and C-peptide (I) following the consumption of each of the bread types; data points connected by a line were from the same individual. Significant time x treatment effects were detected for glucose (ABC, P < 0.001), insulin (DEF, P = 0.006), and C-peptide (GHI, P = 0.001). Data presented as means ± SEM, significance determined by mixed-effects ANOVA and Tukey’s post hoc analysis; Significant differences between 0% CCP and 30% CCP (aaP < 0.01 and aaaP < 0.001), significant differences between 0% CCP and 60% CCP (bP < 0.05, bbP < 0.01 and bbbP < 0.001) and significant differences between 30% CCP and 60% CCP (cP < 0.05, and ccP < 0.01). For iAUC values; ns P > 0.05, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Color legend is the same for all panels.
In addition, bread type exhibited significant main time × treatment effects on insulin (P = 0.006, ANOVA) and C-peptide (P = 0.001, ANOVA) responses. Post hoc analyses revealed that the main effects of CCP enrichment on postprandial insulinemia were due to the lower plasma insulin (Figure 1D–F) and C-peptide (Figure 1G–I) concentrations within the first 90 min after ingestion of 60% CCP bread than the 0% CCP bread, being reflected in the insulin first peak iAUC values (0% vs 60% CCP: iAUC mean difference, 2127 mU/min; 95% CI: 370, 3884; P-adjusted = 0.017) and C-peptide first peak iAUC values (0% vs 60% CCP: iAUC mean difference, 118.8 μg/L/min; 95% CI: 28.0, 209.5; P-adjusted = 0.010). Moreover, this was evident in the iAUC analysis over the initial postprandial period (Supplemental Table 1). Insulin and C-peptide responses to breads enriched with only 30% CCP were not significantly different from responses to the 0% CCP bread (0% vs 30% CCP bread: insulin iAUC mean difference, 220 mU/min; 95% CI: −1346, 1787; P-adjusted = 0.931; 0% vs 30% CCP bread: C-peptide iAUC mean difference, 26.3 μg/L/min; 95% CI: −52.3, 105.0; P-adjusted = 0.677) and remained significantly higher than that of 60% CCP bread (Figure 1E, H). Insulin iAUC values (Figure 1E) were 40% lower and peak concentrations (Cmax) (Supplemental Table 2) were 28% lower after the 60% CCP bread than those of the 0% CCP bread. Regardless of the type of bread consumed, the plasma insulin concentrations peaked between 30 and 40 min and returned to fasted levels within 180 min (Supplemental Table 2). Circulating levels of C-peptide (Figure 1G–I) reflected the observed differences in insulin and peak areas, and mean iAUC for C-peptide responses were 33% lower when participants consumed the 60% CCP bread than in those who consumed 0% CCP (P-adjusted = 0.009).
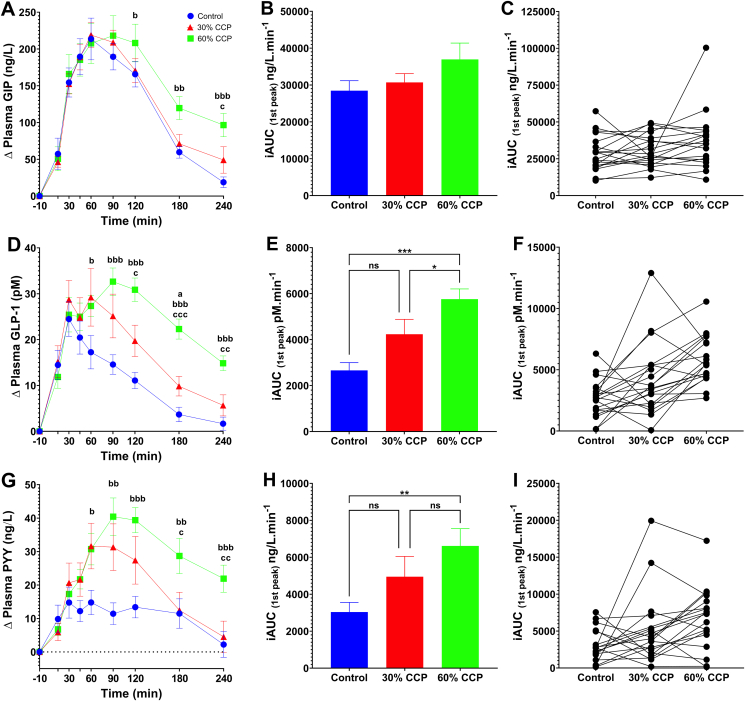
Intact plant cells produced a sustained release of glucose-dependent insulinotropic peptide, glucagon-like peptide-1, and peptide YY
The analysis of postprandial gut hormone concentrations in venous blood revealed that breads baked with intact plant cells gave rise to elevated and sustained release of satiety-promoting hormones (Figure 2). Plasma GIP responses (Figure 2A–C) showed a significant time × treatment effect (P = 0.007), and GIP concentrations tended to differ in the latter phase of the postprandial period (Figure 2A) following the different bread roll types; however, the iAUC were not significantly different between bread types (P > 0.05). The iAUC data and individualized data for GIP responses are shown in Figures 2B, C, respectively. Peak plasma GIP concentrations were similar and occurred after ∼70 min, regardless of meal type (Supplemental Table 2).
FIGURE 2.
Gut hormone responses to control and CCP-enriched test bread. Postprandial responses are based on analysis of blood samples collected following consumption of white bread rolls containing 0% (control, n = 20), 30% (n = 20) or 60% (n = 19) cellular chickpea powder (CPP) and 50 g of available carbohydrate per serving. Time-course data show the change (relative to fasting concentrations) to (A) GIP, (D) GLP-1 and (G) PYY, measured for 240 min. Bar charts show the integrated area under the curve (iAUC) calculated for the 1st peak (above baseline) of the time-course data for GIP (B), GLP-1 (E) and PYY (H) responses. The scatter plots show the matched iAUC for individual participants for GIP (C), GLP-1(F) and PYY (I) following the consumption of each of the bread types; data points connected by a line were from the same individual. Significant time x treatment effects were detected for GIP (ABC, P = 0.007), GLP-1 (DEF, P < 0.001), and PYY (GHI, P < 0.001). Data presented as means ± SEM, significance determined by mixed-effects ANOVA and Tukey’s post hoc analysis; Significant differences between 0% CCP and 30% CCP (aP< 0.05), significant differences between 0% CCP and 60% CCP (bP < 0.05, bbP < 0.01 and bbbP < 0.001) and significant differences between 30% CCP and 60% CCP (cP < 0.05, ccP < 0.01 and cccP < 0.001). For iAUC values; ns P > 0.05, ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Color legend is the same for all panels.
There was a significant main effect of bread type on plasma GLP-1 responses (treatment × time interaction, P = 0.001). GLP-1 concentrations were significantly higher after consumption of 30% and 60% CCP breads than those after the consumption of the 0% CCP bread (as evident from pairwise comparisons of time-series data) (Figure 2D), and differences were particularly pronounced during the late postprandial period (Supplemental Table 1). Although the peak plasma concentrations (Cmax) reached were similar for all bread types, the GLP-1 concentrations peaked >40 min later when participants consumed the 60% CCP bread than after the 0% CCP and 30% CCP breads (Supplemental Table 2). Overall, this prolonged plasma GLP-1 response to CCP-enriched bread is reflected in a doubling of the iAUC (Figure 2E) for the GLP-1 response after consumption of 60% CCP compared with that after the 0% CCP bread consumption, with a mean difference of 3101 pmol/L/min (95% CI: 1891, 4310; P-adjusted < 0.001), and 60% CCP compared with that after the 30% CCP, with a mean difference of 1528 pM/min (95% CI: 2.9, 3053.0; P-adjusted = 0.050). Although the overall mean iAUC for GLP-1 after 30% CCP was not different from the 0% CCP bread (mean difference, 1573 pmol/min; 95% CI: 240, 3386; P-adjusted = 0.096, Tukey post hoc), it is noteworthy that the GLP-1 response tended to increase in a dose-dependent manner for most individuals (Figure 2F). It may be that the application of iAUC to temporal data could mask important effects, as demonstrated by the inter-individual differences; notably, we identified nonresponders but did not exclude their data from the statistical analysis (see individualized data in Figure 2C, F, and I).
Moreover, there was a significant main effect of bread type on PYY responses (time × treatment, P < 0.001, ANOVA). Similar to the GLP-1 responses, the post hoc analysis revealed that postprandial plasma PYY responses increased significantly after consumption of the 30% and 60% CCP breads relative to 0% CCP (Figure 2G–I), with PYY concentrations reaching higher levels and remaining elevated for longer after the consumption of CCP-enriched breads, with a mean difference in iAUC between 0% and 60% CPP of 3576 pmol/min (95% CI: 1024, 6128; P-adjusted = 0.006). For the 0% CCP bread, PYY concentrations peaked within the first 24 min of meal consumption, but when participants consumed CCP-enriched breads, the peak PYY concentrations reached ∼40 to 76 min later (at ∼60–120 min after meal consumption) and the magnitude of the peak PYY response (change from fasted levels) was up to ∼170% higher than that elicited by the 0% CCP bread (Supplemental Table 2 and Figure 2G). Furthermore, after consuming the 60% CCP bread, PYY concentrations at 240 min (the last time point sampled) after the meal were considerably higher than the maximum PYY response to the 0% CCP bread. Overall, our analyses of postprandial blood samples demonstrated that the 30% CCP bread elicited significantly lower glycemic responses, and the 60% CCP bread elicited significantly lower insulinemic responses, in addition to significantly increasing and prolonging the postprandial release of anorexigenic gut hormones, compared with the control 0% CCP bread, for at least 4 h after meal consumption.
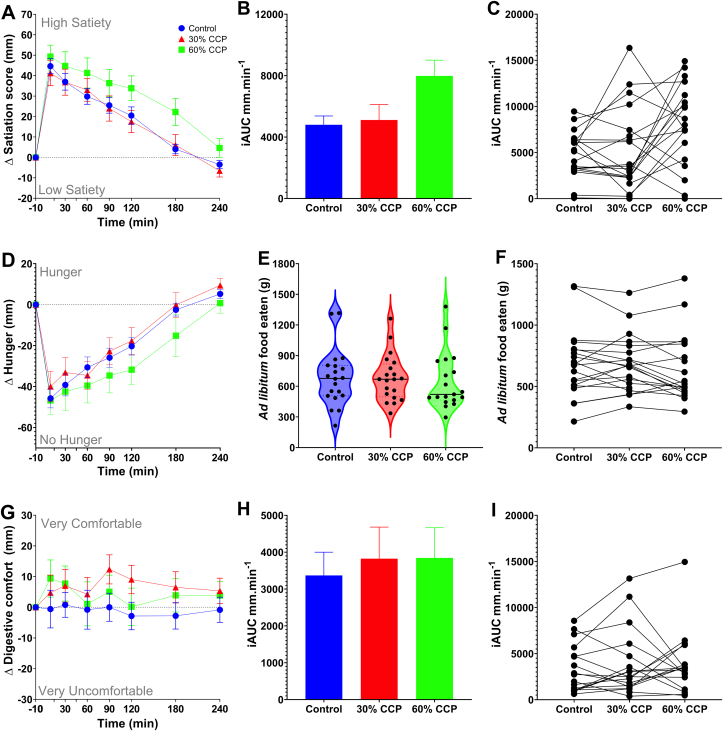
High levels of intact cells are needed to influence subjective satiety
There was a borderline significant time × treatment effect (P = 0.053) on the combined satiety score, with a tendency for the combined satiety score iAUC to be higher after the 60% CCP than after the 0% CCP consumption (iAUC, combined satiety score 0% vs 60% CCP: mean difference, 3184 mm/min, 95% CI: 491, 5878) (Figure 3B, C). The different components used for assessing appetite and satiety in this study (i.e., hunger, desire to eat and food volume) suggested a tendency for increased satiety after consumption of the bread meal containing 60% CCP, as shown by the hunger score (Figure 3D). The unlimited meal provided to participants after the experimental period did not demonstrate a significant reduction in food intake at the subsequent meal (no significant treatment effect, P = 0.176) (Figure 3E, F). Although the total meal weight (bread roll and drink) was matched for all bread types, the VAS responses to the question on meal volume shows that the participants did notice the larger bread roll size at the 60% dose, but this had no adverse effect on digestive comfort (Figure 3G–I). Overall, the replacement of 60% wheat flour with CCP resulted in a bread that elicited higher and sustained circulating levels of anorexigenic gut hormones (Figure 2), with a tendency (time × treatment, P = 0.053) to increase participant reported sensations of satiety (Figure 3).
FIGURE 3.
Effects of CCP-enrichment on participant satiety and food intake. The change in postprandial (A) combined satiety score, (D) hunger and (G) digestive comfort measured for 240 min following consumption of bread rolls, containing 0% (control), 30% or 60% chickpea powder (CPP) as measured by anchored visual analog scales. Barcharts show the integrated area under the curve (iAUC), calculated from the time-course data, for the negative peak of the combined satiety score (B), and total for digestive comfort (H). The matched iAUC for individual participants for combined satiety score (C), and digestive comfort (I) following the consumption of the control and CCP test meals. Time x treatment effects were not statistically significant for the combined satiety score (ABC, P = 0.053), hunger (D, P = 0.330) and digestive comfort (GH, P = 0.559). Changes in the amount of food eaten during a second, ad libitum meal provided to participants after 240 min (E) and comparison within individuals (F) was recorded for control and CCP test meals and meal effects on food intake were not statistically significant (main treatment effect, P = 0.180). Data presented as means ± SEM.
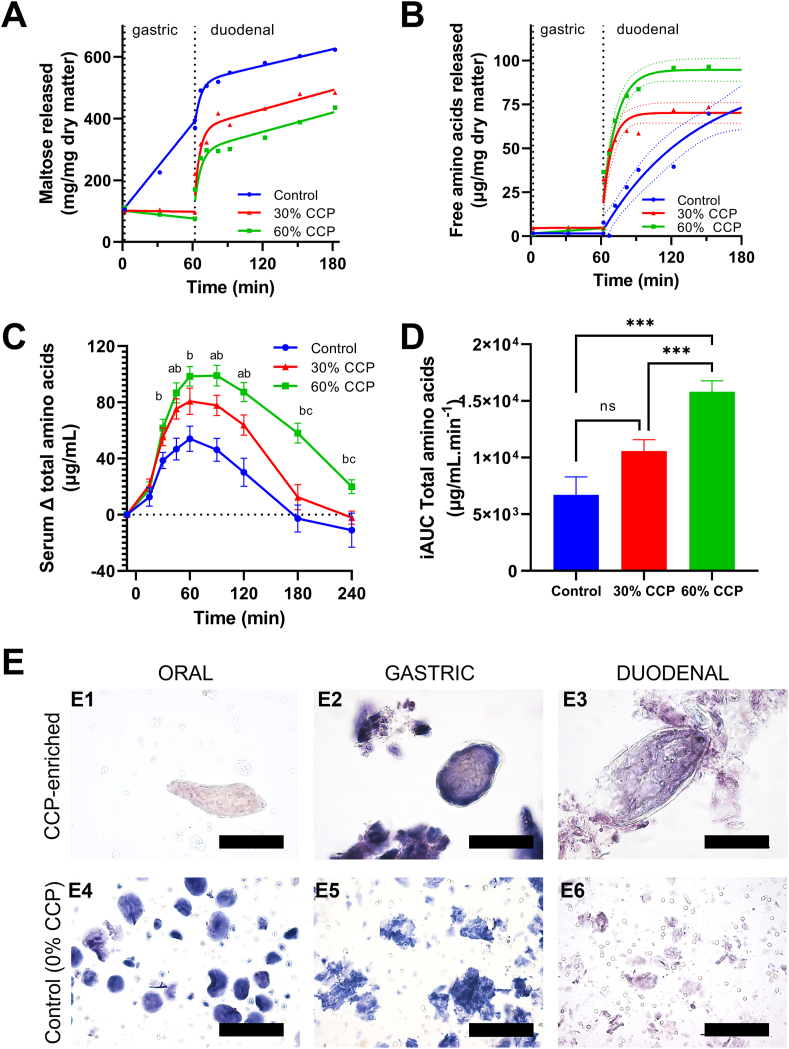
Differences in nutrient release during digestion underpin effects on postprandial responses
Because gut hormone production can be stimulated by nutrient concentrations in the intestinal lumen, we explored and compared the luminal release (bioaccessibility) of starch and protein digestive products from breads under simulated oral, gastric, and small intestinal digestive conditions (INFOGEST 2.0 protocol). We found that the main release of amylolytic products of starch digestion (expressed as maltose equivalents) occurred rapidly during the early small intestinal phase and tended to be higher in the 0% CCP bread than in the 30 and 60% CCP breads (Figure 4A)—this is in good agreement with higher plasma glucose concentration observed for the 0% CCP bread (Figure 1A) and consistent with our previous observations of slow rates of starch release from intact plant cells [26]. However, higher concentrations of maltose were observed in the gastric phase of the 0% CCP samples than those for both 30% and 60% CCP samples, being reflected in the Lugol iodine–stained starch observed in the corresponding samples (Figure 4E2, E5). This indicates residual salivary amylase activity in the gastric phase for the 0% CCP breads only [44].
FIGURE 4.
In vitro digestibility, microstructural changes, and in vivo serum amino acid responses after ingestion of control and CCP-enriched breads. Digestibility curves show maltose (A) and free amino acid (B) release during in vitro digestion of control (0% CCP) and 30% and 60% CCP-enriched breads. Each of the data points in (A) and (B) are the mean of at least duplicate digestions, with curves fitted by nonlinear regression (solid line) with 95% confidence bands (dashed lines) shown for the latter. In vivo human study data (C) show postprandial increases in total serum amino acid concentrations after consumption of 0% (control, n = 20), 30% (n = 20), and 60% (n = 19) CCP breads, plotted over time as means ± SEM. Bar charts show incremental area under the curve (iAUC, mean ± SEM) for serum amino acid responses (D). Time × treatment effects for amino acid data (C, D) were statistically significant, P < 0.001, as determined by the mixed-effects ANOVA with Tukey post hoc analysis. Significant differences were noted between 0% CCP and 30% CCP (aP < 0.05), between 0% CCP and 60% CCP (bP < 0.05), and between 30% CCP and 60% CCP (cP < 0.05). ns, P > 0.05; ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001. Light micrographs (E), stained with Lugol iodine (E1–E6) and toluidine blue (E3, E6) show presence of intact plant cells with encapsulated starch from CCP-enriched breads during oral (E1) and gastric (E2) digestion, with some cell rupture evident at the end of the duodenal phase (E3). No intact cells were present in control bread, and extensive wheat starch granule digestion is evident (E4–E6). Scale bars = 100 μm.
Data from in vitro digestion of bread indicate that the production of free amino acids from protein hydrolysis also occurred mainly in the early intestinal phase and tended to increase with higher doses of CCP inclusion (Figure 4B). This is likely because of the higher protein content of 25.5 mg protein/100 mg dry matter for the 60% CCP bread than that of 17 mg protein/100 mg dry matter for the 0% CCP bread, rather than the former’s intrinsic protein digestibility. When this is considered, the free amino acids measured in simulated luminal fluid at the end of digestion accounted for ∼40% of the initial total protein mass, regardless of bread type and could indicate that although breads were digested at different rates, a similar proportion of protein would eventually be available for absorption (Figure 4B). The amount of maltose released from starch at the end of the small intestinal phase accounted for approximately 82% of the initial mass of starch within each bread type. Thus, it seems that a similar proportion of total starch would eventually be digested regardless of bread type.
The higher amount of total free amino acids released from the CCP breads during in vitro digestion (Figure 4B) is consistent with our analysis of postprandial serum amino acid responses in humans reported in Figure 4C and included as an exploratory outcome. Bread type affected the overall total serum amino acid concentrations (significant overall time × treatment effect, P < 0.001). Amino acid concentrations were found to increase in a dose-dependent manner and remained elevated for longer after the 30% and 60% CCP bread than after the 0% CCP bread consumption. The total serum free amino acid concentrations peaked at ∼60 min, and the overall amino acid iAUC (Figure 4D) was significantly higher when participants consumed the 60% CCP bread than that after the 0% CCP bread consumption (0% vs 60% CCP: 9094 μg/mL/min; 95% CI: 13,851, 4338; P-adjusted < 0.001). The higher mean amino acid iAUC after the 30% CCP bread consumption was not significantly different from that after the 0% CCP bread consumption (P-adjusted = 0.100). The ingested 0%, 30%, and 60% CCP breads contained 12.9, 17.7, and 27.2 g total protein/serving, respectively, of which ∼12 g was wheat-derived protein, whereas the chickpea protein contributed 0, 5, and 12 g, respectively. It is therefore likely that the higher serum amino acid response to the 60% CCP bread reflects the higher amount of total protein ingested with this meal.
A microscopic analysis of in vitro digesta (Figure 4E) confirmed the presence of intact plant cells with encapsulated starch and protein within the CCP breads and showed that free wheat starch granules (present in all breads) were digested earlier than the encapsulated starch in CCP. At the end of duodenal digestion, all wheat starch granules had been digested, and some cells from the CCP were showing damage and release of encapsulated nutrients, whereas other cells appeared intact with macronutrients still enclosed. This is consistent with our interpretation of the in vitro digestibility curves. Thus, our in vitro digestion experiments revealed how incorporation of CCP into bread flour lowers the rate of release of malto-oligosaccharides from cell-encapsulated starch, in addition to providing a higher amount of bioavailable total free amino acids (derived from protein digestion) when compared with that of the 0% CCP bread.
Discussion
The increased prevalence of obesity worldwide is partly because of the accessibility of processed high glycemic foods with only transient hunger suppression. A transformative dietary shift is needed, but it is challenging for people to change their diet when it is interlinked with cultural and societal behaviors. Improving the metabolic effect of staple foods such as white bread “by stealth” through microstructural modulation is an appealing diet-based strategy for improving population health. This study uses a novel cellular legume flour (CCP) to demonstrate for the first time, to our knowledge, that replacement of significant amounts of wheat flour by CCP in bread increases circulating levels of satiety-promoting hormones (GLP-1 and PYY) and fullness sensation, in addition to lowering blood glucose and insulin responses. These beneficial effects were clearly attributed to the slow digestion behavior of the intact cells, which are not present in conventionally milled white or whole meal cereal or legume flours.
Our studies provide evidence for a plausible mechanism by which intact legume plant cells influence appetite regulation [27] in the early postprandial state through the anorexigenic gut hormones and does not seem to be dependent on the delivery of RS to the distal gut (i.e., microbial fermentation). The observed stimulatory effects of CCP enrichment on circulating anorexigenic gut hormones is likely explained by the differential spatiotemporal release of cell-encapsulated nutrients from these meals during digestive transit. Indeed, given the increased tendency for prolonged satiety after consumption of CCP-enriched breads, the elevated circulating anorexigenic gut hormones may suppress feelings of hunger after dips in plasma glucose levels reported previously [45].
The slower release of starch from chickpea cells (as seen in this in vitro study) would result in higher luminal concentrations of monosaccharides and disaccharides toward the distal gut and stimulate local enteroendocrine cells with GLP-1– and PYY-producing capacity [4, 11, 13]. The highest density of these enteroendocrine cells is in the colon, and others have suggested that the colonic microbial fermentation of RS into short-chain fatty acids within this region may stimulate gut hormone production [46, 47], although evidence for this mechanism in humans remains equivocal [[48], [49], [50], [51]]. However, considering that the mean transit time to the ileocecal junction in humans is 3–6 h [8], we believe the acute differences in gut hormone responses observed within the present 4-h postprandial study are driven mainly by non–microbiome-mediated digestion in the small intestine. Thus, our study points to a different mechanism to previous studies in which fermentation of RS into short-chain fatty acids has been suggested to stimulate satiety. Our observations are better described by the ileal brake mechanism—a combination of processes mediated by GLP-1 and PYY in which macronutrients downregulate digestion and suppress food intake [8].
Although encapsulated starch has been the main focus of our previous studies, the novel chickpea cell flours studied in this study also contain ∼ 20% protein, and the digestion behavior of protein within the chickpea cells is less well understood. Recent evidence suggests that the cell wall barrier mechanism applies to protein as well [52]; however, our analyses suggests that some of the encapsulated chickpea protein within these breads was in fact bioaccessible and bioavailable [39]. The postprandial satiety-promoting effects observed in response to intact plant cells (type 1 RS) may not only be due to the delayed starch digestion alone but also be attributed to the release and digestion of peptides from co-located encapsulated protein. Thus, although evidence for RS effects on acute satiety in humans is limited and mechanisms unconfirmed [49], there is strong evidence for acute effects of protein in satiety regulation [53].
The observed effects on postprandial glucose, insulin, and GIP responses are explained by the early luminal appearance of macronutrient digestion products within the upper small intestine. Blood glucose responses are strongly influenced by availability of luminal glucose from starch digestion [42, 54], so the limited bioaccessibility and digestion (orogastric and duodenal) of intracellular starch provides an explanation for the attenuated postprandial glycemic responses to the CCP breads [44]. Insulin (and C-peptide) responses tend to reflect the changes to plasma glucose levels and, thus, were reduced or unchanged in response to intact plant cell intake. Similar mechanisms are likely to underpin the observed GIP responses; increasing luminal glucose concentrations in the duodenum are known to be detected by K-cells with GIP production capacity. Glucose derived from the rapidly digested wheat starch (present in all meals) is likely to have stimulated the initial GIP response, but the prolonged GIP response seen from 90 min onward after intact plant cell consumption from CCP breads could reflect continued stimulation due to slower availability of hydrolyzed products of starch and/or proteolytic products from intact plant cells [53].
A limitation of our study is that, owing to the complex nature of RS1, the meals inevitably varied not only in encapsulated starch but also in encapsulated protein content. Our original hypothesis focused on the effect of the cell wall barrier mechanism on starch digestion, but our results provide evidence of slow release and digestion of both starch and protein digestion products. Nevertheless, our observations of gut hormone response to plant cells in bread complement the findings of a recent study where nutrient-matched chickpea purees differing in plant cell intactness elicited different effects on subjective appetite [27]. In addition, the postprandial glucose and insulin responses measured in venous blood were less pronounced than differences in capillary and interstitial glucose responses to these breads, as reported previously [26], which is consistent with participants being healthy and with a good glycemic control. A greater sample size would have more power to detect small differences in venous glucose and insulin responses, particularly between 30% and 60% CCP breads. Regarding the statistical analyses, we acknowledge the inflated risk of type 1 statistical errors due to having multiple primary and secondary outcomes. The prespecified analysis plan included several related outcomes measures (iAUCs over different time segments) calculated from each postprandial curve. However, we made a data-driven decision to deviate from this analysis plan by focusing on the first peak iAUC and its parameters. Postprandial responses to a control meal are known to vary in duration and magnitude between individuals, so assessing the response based on the fixed, arbitrary period could have been misleading [55]. However, the observed postprandial responses did not deviate so strongly from the time course on which the original outcome measures were based, so the prespecified analyses (presented in Supplementary Table 1) would have led us to the same main conclusions. Nevertheless, the first peak iAUC and its parameters are preferrable and recommended as an outcome measure for future studies because they capture more completely the postprandial response.
Overall, this study adds to a growing body of evidence [[5], [55], [56], [57], [58]] that dietary fiber structure in the form of intact plant cell walls plays a critically important role in altering macronutrient bioavailability and postprandial metabolism. Current findings that plant cell structure influences the anorexigenic response to food is highly relevant to understanding the relationship between dietary fiber intake, obesity, and cardiometabolic risk reported in epidemiological studies [28]. An important implication of our study is that dietary fiber supplementation with disrupted cells may not be as effective in supporting cardiometabolic health as the consumption of whole foods, where the plant cell structures are intact. This is an important consideration in designing effective strategies for dietary fiber supplementation.
In conclusion, this study demonstrates how incorporation of a novel cellular powder into a staple food (bread) has beneficial effects on glycemia, insulinemia, and release of satiety-promoting gut hormones, particularly at a 60% substitution of white flour. Overall, the intact legume cell powders reveal a slow digestion behavior, and their incorporation into bread provides a new dietary strategy of stimulating release of satiety-promoting gut hormones. Because the mechanisms are dependent on the slow-release properties of intact plant cells, conventionally milled flours such as pulse flours or whole meal flours are unlikely to exert similar effects. Considering the magnitude of the observed effects seen acutely in healthy individuals and our recent encouraging findings regarding sensory characteristics [26], we believe further studies are required to: 1) investigate consumer acceptance and further optimize the bread formulation and 2) assess the therapeutic potential of legume cell powders in body weight and diabetes management in chronic intervention studies.
Author contribution
The authors’ responsibilities were as follows—BHB, SEB, PRE, CHE: conceptualized the research; BHB, AMP, SS, CHE: performed the formal analysis; BHB, AMP, NP-M, SS, PR, JA-J, AvdS, CB, CHE: data curation; BHB, CHE: visualized the study; BHB, AMP, NP-M, PR, AvdS, CB: performed the investigations; BHB, AMP, NP-M, CHE: wrote the original draft; BHB, SEB, PRE, CHE: reviewed and edited the manuscript; BHB, SEB, PRE, CHE: supervised and administrated the project; PR, JA-J: acquired the resources; SEB, PRE, CHE: were responsible for funding acquisition; and all authors: read and approved the final manuscript.
CE and PE are named inventors on the patent application WO 2019/155190 A1. SB receives consultancy and shares from ZOE Global Ltd. The other authors have no conflicts of interest to declare.
Funding
This research was funded by the Biotechnology and Biological Sciences Research Council, UK (BBSRC) Super Follow-On Fund grant, BB/PO23770/1; the BBSRC Institute Strategic Programme BB/R012512/1 and its constituent project BBS/E/F/000PR10343; and BBS/E/F/00044427.
Data Availability
The data described in the manuscript, code book, and analytic code will be made available on request pending approval by the corresponding authors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2022.12.008.
Contributor Information
Balazs H. Bajka, Email: Balazs.Bajka@kcl.ac.uk.
Cathrina H. Edwards, Email: Cathrina.edwards@quadram.ac.uk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Swinburn B.A., Sacks G., Hall K.D., McPherson K., Finegood D.T., Moodie M.L., et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds A., Mann J., Cummings J., Winter N., Mete E., Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–445. doi: 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 3.Fardet A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: a preliminary study with 98 ready-to-eat foods. Food Funct. 2016;7(5):2338–2346. doi: 10.1039/c6fo00107f. [DOI] [PubMed] [Google Scholar]
- 4.Dagbasi A., Lett A.M., Murphy K., Frost G. Understanding the interplay between food structure, intestinal bacterial fermentation and appetite control. Proc Nutr Soc. 2020:1–17. doi: 10.1017/S0029665120006941. [DOI] [PubMed] [Google Scholar]
- 5.Grundy M.M.-L., Edwards C.H., Mackie A.R., Gidley M.J., Butterworth P.J., Ellis P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br J Nutr. 2016;116(5):816–833. doi: 10.1017/S0007114516002610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiong W., Devkota L., Zhang B., Muir J., Dhital S. Intact cells: “nutritional capsules” in plant foods. Compr Rev Food Sci Food Saf. 2022;21(2):1198–1217. doi: 10.1111/1541-4337.12904. [DOI] [PubMed] [Google Scholar]
- 7.Pais R., Gribble F.M., Reimann F. Stimulation of incretin secreting cells. Ther Adv Endocrinol Metab. 2016;7(1):24–42. doi: 10.1177/2042018815618177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maljaars P.W.J., Peters H.P.F., Mela D.J., Masclee A.A.M. Ileal brake: a sensible food target for appetite control. A review. Physiol Behav. 2008;95(3):271–281. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Knudsen L.B., Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne) 2019;10:155. doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinert R.E., Feinle-Bisset C., Asarian L., Horowitz M., Beglinger C., Geary N. Ghrelin, CCK, GLP-1, and PYY(3-36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev. 2017;97(1):411–463. doi: 10.1152/physrev.00031.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eissele R., Göke R., Willemer S., Harthus H.-P., Vermeer H., Arnold R., Göke B. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur J Clin Invest. 1992;22(4):283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 13.Beumer J., Puschhof J., Bauzá-Martinez J., Martínez-Silgado A., Elmentaite R., James K.R., et al. High-resolution mRNA and secretome atlas of human enteroendocrine cells. Cell. 2020;181(6):1291–1306. doi: 10.1016/j.cell.2020.04.036. e19. [DOI] [PubMed] [Google Scholar]
- 14.Qin W., Ying W., Hamaker B., Zhang G. Slow digestion-oriented dietary strategy to sustain the secretion of GLP-1 for improved glucose homeostasis. Compr Rev Food Sci Food Saf. 2021;20(5):5173–5196. doi: 10.1111/1541-4337.12808. [DOI] [PubMed] [Google Scholar]
- 15.Novotny J.A., Gebauer S.K., Baer D.J. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am J Clin Nutr. 2012;96(2):296–301. doi: 10.3945/ajcn.112.035782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards C.H. Diabetes and Nutritional Sciences; King’s College London: 2014. The role of plant cell walls in influencing starch bioaccessibility. [Google Scholar]
- 17.Butterworth P.J., Edwards C.H., Ellis P.R., Hill S., Marson A., Obuchowicz J. WIPO; 2019. Medium/low glycaemic index products and methods. [Google Scholar]
- 18.Verkempinck S., Pallares A.P., Hendrickx M., Grauwet T. Processing as a tool to manage digestive barriers in plant-based foods: recent advances. Curr Opin Food Sci. 2020;35:1–9. [Google Scholar]
- 19.Pallares Pallares A., Gwala S., Pälchen K., Duijsens D., Hendrickx M., Grauwet T. Pulse seeds as promising and sustainable source of ingredients with naturally bioencapsulated nutrients: literature review and outlook. Compr Rev Food Sci Food Saf. 2021;20(2):1524–1553. doi: 10.1111/1541-4337.12692. [DOI] [PubMed] [Google Scholar]
- 20.Edwards C.H., Ryden P., Mandalari G., Butterworth P.J., Ellis P.R. Structure–function studies of chickpea and durum wheat uncover mechanisms by which cell wall properties influence starch bioaccessibility. Nat Food. 2021;2(2):118–126. doi: 10.1038/s43016-021-00230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pallares Pallares A., Alvarez Miranda B., Truong N.Q.A., Kyomugasho C., Chigwedere C.M., Hendrickx M., et al. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food Funct. 2018;9(12):6544. doi: 10.1039/c8fo01619d. 6454. [DOI] [PubMed] [Google Scholar]
- 22.Petropoulou K., Salt L.J., Edwards C.H., Warren F.J., Garcia-Perez I., Chambers E.S., et al. A natural mutation in Pisum sativum L. (pea) alters starch assembly and improves glucose homeostasis in humans. Nat Food. 2020;1:693–704. doi: 10.1038/s43016-020-00159-8. [DOI] [PubMed] [Google Scholar]
- 23.Noah L., Guillon F., Bouchet B., Buleon A., Molis C., Gratas M., et al. Digestion of carbohydrate from white beans (Phaseolus vulgaris L.) in healthy humans. J Nutr. 1998;128(6):977–985. doi: 10.1093/jn/128.6.977. [DOI] [PubMed] [Google Scholar]
- 24.Golay A., Coulston A.M., Hollenbeck C.B., Kaiser L.L., Wursch P., Reaven G.M. Comparison of metabolic effects of white beans processed into 2 different physical forms. Diabetes Care. 1986;9(3):260–266. doi: 10.2337/diacare.9.3.260. [DOI] [PubMed] [Google Scholar]
- 25.Tovar J., Granfeldt Y., Björck I.M. Effect of processing on blood glucose and insulin responses to starch in legumes. J Agric Food Chem. 1992;40(10):1846–1851. [Google Scholar]
- 26.Bajka B.H., Pinto A.M., Ahn-Jarvis J., Ryden P., Perez-Moral N., van der Schoot A., et al. The impact of replacing wheat flour with cellular legume powder on starch bioaccessibility, glycaemic response and bread roll quality: a double-blind randomised controlled trial in healthy participants. Food Hydrocoll. 2021;114 doi: 10.1016/j.foodhyd.2020.106565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pälchen K., Bredie W.L.P., Duijsens D., Isaac Alfie Castillo A., Hendrickx M., Van Loey A., et al. Effect of processing and microstructural properties of chickpea-flours on in vitro digestion and appetite sensations. Food Res Int. 2022;157 doi: 10.1016/j.foodres.2022.111245. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.J., de Souza R.J., Choo V.L., Ha V., Cozma A.I., Chiavaroli L., et al. Effects of dietary pulse consumption on body weight: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2016;103(5):1213–1223. doi: 10.3945/ajcn.115.124677. [DOI] [PubMed] [Google Scholar]
- 29.Viguiliouk E., Glenn A.J., Nishi S.K., Chiavaroli L., Seider M., Khan T., et al. Associations between dietary pulses alone or with other legumes and cardiometabolic disease outcomes: an umbrella review and updated systematic review and meta-analysis of prospective cohort studies. Adv Nutr. 2019;10(Suppl 4):S308–S319. doi: 10.1093/advances/nmz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sievenpiper J.L., Kendall C.W.C., Esfahani A., Wong J.M.W., Carleton A.J., Jiang H.Y., et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52(8):1479. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- 31.Edwards C.H., Ryden P., Pinto A.M., van der Schoot A., Stocchi C., Perez-Moral N., et al. Chemical, physical and glycaemic characterisation of PulseON®: A novel legume cell-powder ingredient for use in the design of functional foods. J Funct Foods. 2020;68 [Google Scholar]
- 32.Delamare G.Y.F., Butterworth P.J., Ellis P.R., Hill S., Warren F.J., Edwards C.H. Incorporation of a novel leguminous ingredient into savoury biscuits reduces their starch digestibility: implications for lowering the glycaemic index of cereal products. Food Chem X. 2020;5 doi: 10.1016/j.fochx.2020.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rovalino-Córdova A.M., Fogliano V., Capuano E. A closer look to cell structural barriers affecting starch digestibility in beans. Carbohydr Polym. 2018;181:994–1002. doi: 10.1016/j.carbpol.2017.11.050. [DOI] [PubMed] [Google Scholar]
- 34.Melito C., Tovar J. Cell walls limit in vitro protein digestibility in processed legume seeds. Food Chem. 1995;53(3):305–307. [Google Scholar]
- 35.Data Protection Act 2018, c12. (UK Parliament) [Internet]. Available from: https://www.legislation.gov.uk/ukpga/2018/12/contents. (Accessed 14 October 2022).
- 36.https://www.legislation.gov.uk/ukpga/2004/30/contents Human Tissue Act 2004, c. 30. (UK Parliament) [Internet]. Available from:
- 37.Gibbons C., Hopkins M., Beaulieu K., Oustric P., Blundell J.E. Issues in measuring and interpreting human appetite (satiety/satiation) and its contribution to obesity. Curr Obes Rep. 2019;8(2):77–87. doi: 10.1007/s13679-019-00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kok M.G.M., Nix C., Nys G., Fillet M. Targeted metabolomics of whole blood using volumetric absorptive microsampling. Talanta. 2019;197:49–58. doi: 10.1016/j.talanta.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Perez-Moral N., Saha S., Pinto A.M., Bajka B.H., Edwards C.H. In vitro protein bioaccessibility and human serum amino acid responses to white bread enriched with intact plant cells. Food Chem. 2023;404:134538. doi: 10.1016/j.foodchem.2022.134538. [DOI] [PubMed] [Google Scholar]
- 40.Prinsen H., Schiebergen-Bronkhorst B.G.M., Roeleveld M.W., Jans J.J.M., de Sain-van der Velden M.G.M., Visser G., et al. Rapid quantification of underivatized amino acids in plasma by hydrophilic interaction liquid chromatography (HILIC) coupled with tandem mass-spectrometry. J Inherit Metab Dis. 2016;39(5):651–660. doi: 10.1007/s10545-016-9935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brodkorb A., Egger L., Alminger M., Alvito P., Assunção R., Ballance S., et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat Protoc. 2019;14(4):991–1014. doi: 10.1038/s41596-018-0119-1. [DOI] [PubMed] [Google Scholar]
- 42.Edwards C.H., Cochetel N., Setterfield L., Perez-Moral N., Warren F.J. A single-enzyme system for starch digestibility screening and its relevance to understanding and predicting the glycaemic index of food products. Food Funct. 2019;10(8):4751–4760. doi: 10.1039/c9fo00603f. [DOI] [PubMed] [Google Scholar]
- 43.Stewart M.L., Wilcox M.L., Bell M., Buggia M.A., Maki K.C. Type-4 resistant starch in substitution for available carbohydrate reduces postprandial glycemic response and hunger in acute, randomized, double-blind, controlled study. Nutrients. 2018;10(2):129. doi: 10.3390/nu10020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Freitas D., Le Feunteun S., Panouillé M., Souchon I. The important role of salivary α-amylase in the gastric digestion of wheat bread starch. Food Funct. 2018;9(1):200–208. doi: 10.1039/c7fo01484h. [DOI] [PubMed] [Google Scholar]
- 45.Wyatt P., Berry S.E., Finlayson G., O’Driscoll R., Hadjigeorgiou G., Drew D.A., et al. Postprandial glycaemic dips predict appetite and energy intake in healthy individuals. Nat Metab. 2021;3(4):523–529. doi: 10.1038/s42255-021-00383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chambers E.S., Morrison D.J., Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74(3):328–336. doi: 10.1017/S0029665114001657. [DOI] [PubMed] [Google Scholar]
- 47.Canfora E.E., van der Beek C.M., Jocken J.W.E., Goossens G.H., Holst J.J., Olde Damink S.W.M., et al. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep. 2017;7(1):2360. doi: 10.1038/s41598-017-02546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Mana N.M., Robertson M.D. Acute effect of resistant starch on food intake, appetite and satiety in overweight/obese males. Nutrients. 2018;10(12):1993. doi: 10.3390/nu10121993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai M., Dou B., Pugh J.E., Lett A.M., Frost G.S. The impact of starchy food structure on postprandial glycemic response and appetite: a systematic review with meta-analysis of randomized crossover trials. Am J Clin Nutr. 2021;114(2):472–487. doi: 10.1093/ajcn/nqab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christiansen C.B., Gabe M.B.N., Svendsen B., Dragsted L.O., Rosenkilde M.M., Holst J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol. 2018;315(1):G53–G65. doi: 10.1152/ajpgi.00346.2017. [DOI] [PubMed] [Google Scholar]
- 51.Christiansen C.B., Veedfald S., Hartmann B., Gauguin A.M., Møller S., Moritz T., et al. Colonic lactulose fermentation has no impact on glucagon-like peptide-1 and peptide-YY secretion in healthy young men. J Clin Endocrinol Metab. 2021;107(1):77–87. doi: 10.1210/clinem/dgab666. [DOI] [PubMed] [Google Scholar]
- 52.Pälchen K., Michels D., Duijsens D., Gwala S., Pallares Pallares A., Hendrickx M., et al. In vitro protein and starch digestion kinetics of individual chickpea cells: from static to more complex in vitro digestion approaches. Food Funct. 2021;12(17):7787–7804. doi: 10.1039/d1fo01123e. [DOI] [PubMed] [Google Scholar]
- 53.Santos-Hernández M., Miralles B., Amigo L., Recio I. Intestinal signaling of proteins and digestion-derived products relevant to satiety. J Agric Food Chem. 2018;66(39):10123–10131. doi: 10.1021/acs.jafc.8b02355. [DOI] [PubMed] [Google Scholar]
- 54.Ellis P.R., Roberts F.G., Low A.G., Morgan L.M. The effect of high-molecular-weight guar gum on net apparent glucose absorption and net apparent insulin and gastric inhibitory polypeptide production in the growing pig: relationship to rheological changes in jejunal digesta. Br J Nutr. 1995;74(4):539–556. doi: 10.1079/bjn19950157. [DOI] [PubMed] [Google Scholar]
- 55.Matthan N.R., Ausman L.M., Meng H., Tighiouart H., Lichtenstein A.H. Estimating the reliability of glycemic index values and potential sources of methodological and biological variability. Am J Clin Nutr. 2016;104(4):1004–1013. doi: 10.3945/ajcn.116.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ellis P.R., Kendall C.W., Ren Y., Parker C., Pacy J.F., Waldron K.W., et al. Role of cell walls in the bioaccessibility of lipids in almond seeds. Am J Clin Nutr. 2004;80(3):604–613. doi: 10.1093/ajcn/80.3.604. [DOI] [PubMed] [Google Scholar]
- 57.Capuano E., Pellegrini N. An integrated look at the effect of structure on nutrient bioavailability in plant foods. J Sci Food Agric. 2019;99(2):493–498. doi: 10.1002/jsfa.9298. [DOI] [PubMed] [Google Scholar]
- 58.Parada J., Aguilera J.M. Review: Starch matrices and the glycemic response. Food Sci Technol Int. 2011;17(3):187–204. doi: 10.1177/1082013210387712. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript, code book, and analytic code will be made available on request pending approval by the corresponding authors.