Abstract
Background
Beyond alcohol and coffee, the relationship between other dietary factors, including specific vegetables and fruits, and liver outcomes remains poorly understood.
Objective
To evaluate the associations between fruit and vegetable intake with the risk of liver cancer and chronic liver disease (CLD) mortality.
Methods
This study was based on the National Institutes of Health-American Association of Retired Persons Diet and Health Study, including 485,403 participants aged 50–71 y from 1995 to 1996. Fruit and vegetable intake was estimated using a validated food frequency questionnaire. Cox proportional hazards regression was used to estimate the multivariable hazard ratios (HR) and 95% confidence intervals (CI) for liver cancer incidence and CLD mortality.
Results
During a median follow-up of 15.5 y, 947 incident liver cancers and 986 CLD deaths (other than liver cancer) were confirmed. A higher intake of total vegetables was associated with a lower risk of liver cancer (HRQuintile 5 vs. Quintile 1 = 0.72, 95% CI: 0.59, 0.89; Ptrend < 0.001). When further subclassified into botanical groups, the observed inverse association was mainly driven by lettuce and the cruciferous family (broccoli, cauliflower, cabbage, etc.) (Ptrend < 0.005). Additionally, higher total vegetable intake was associated with a lower risk of CLD mortality (HRQuintile5 vs. Quintile1 = 0.61, 95% CI: 0.50, 0.76; Ptrend < 0.001). Inverse associations were observed for lettuce, sweet potatoes, cruciferous vegetables, legumes, and carrots with CLD mortality (all Ptrend < 0.005). In contrast, total fruit intake was not associated with liver cancer or CLD mortality.
Conclusions
Higher intakes of total vegetables, especially lettuce and cruciferous vegetables, were associated with lower liver cancer risk. Higher intakes of lettuce, sweet potatoes, cruciferous vegetables, legumes, and carrots were associated with a lower risk of CLD mortality.
Keywords: vegetables, lettuce, legumes, carrots, cruciferous vegetables, fruit, liver cancer, liver disease mortality, cohort study, epidemiological study
Introduction
Liver cancer was the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death globally in 2020 [1]. The incidence of liver cancer has tripled since the early 1980s, and 42,230 liver cancer cases were reported in 2021 in the United States [2, 3]. Current major known risk factors for liver cancer include chronic infections (hepatitis B virus [HBV], hepatitis C virus [HCV]), metabolic disorders (nonalcoholic fatty liver disease [NAFLD], type 2 diabetes [T2D], obesity), behavioral factors (alcohol consumption, tobacco), and aflatoxins [4]. The World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) reported that except for coffee and alcohol, the evidence concerning the relationship of dietary factors to liver cancer is still limited and calls for more research [5]. Fruits and vegetables, rich in vitamins and phytochemicals (e.g., carotenoids, flavonoids, polyphenols, anticarcinogenic compounds, and antioxidants), are hypothesized to protect against liver cancer. Certain vegetables, including cruciferous vegetables rich in glucosinolates, carrots rich in carotenoids, and legumes rich in phytoestrogens, have been inversely associated with risk of cancers, including lung and colorectal cancers [[6], [7], [8]]. A meta-analysis of 9 cohort studies recently showed that higher vegetable intake was associated with 39% lower liver cancer risk, whereas fruit intake was not associated with liver cancer risk [9]. However, only 2 out of 9 studies were conducted in Western populations where the etiology may differ from Asian countries because of the lower prevalence of HBV/HCV infection [9]. Specifically, the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort [10] and the NIH-AARP Diet and Health Study [11] reported an inverse association for total vegetables and a null association for fruits. However, what specific vegetables influence liver cancer risk remains unknown. Identification of specific vegetables may provide clues for experimental studies and inform precision nutrition suggestions for population intervention.
Chronic liver diseases (CLD), including cirrhosis, fibrosis, alcoholic liver disease, NAFLD, and chronic hepatitis, are the tenth leading cause of death worldwide, causing 2 million deaths each year [12]. The deaths from CLD steadily increased from 11.0 per 100,000 persons in 2017 to 13.8 per 100,000 persons in 2020 in the United States [13]. Most liver cancers also arise from CLD [14]. There is growing evidence that dietary factors can play an important role in the development of liver diseases due to the critical effect of diet on obesity, inflammation, and gut microbiota [15]. Evidence is accumulating on dietary factors and NAFLD [16], but the influence of diet on liver disease-related mortality remains poorly understood. Recently, one meta-analysis reported neither fruits nor vegetables were associated with NAFLD based on evidence from case-control or cross-sectional studies [17]. However, to date, no study has yet examined the associations between fruit and vegetable consumption and CLD mortality.
In this study, we evaluated the associations between the intake of specific fruits and vegetables and the risk of liver cancer and CLD mortality using the NIH-AARP Diet and Health Study.
Methods
Study population
The NIH-AARP Diet and Health Study is a large prospective cohort of US men and women conducted by the National Cancer Institute. Details about the cohort design were described previously [18]. Briefly, during the baseline survey from 1995 to 1996, a self-administered questionnaire on demographics, diet, and lifestyle was mailed to 3.5 million AARP members aged 50 to 71 y residing in 6 states (California, Florida, Louisiana, New Jersey, North Carolina, and Pennsylvania) and 2 metropolitan areas (Atlanta, Georgia, and Detroit, Michigan). Among 617,119 respondents, 566,398 participants (91.8%) satisfactorily completed the baseline questionnaire.
In this analysis, we excluded persons for the following reasons: [1] cancers except for nonmelanoma skin cancer diagnosis at or before baseline enrollment (n = 43,467); [2] zero person-years of follow-up (n = 9,859); [3] self-reported poor health status (n = 15,782); [5] unreliable energy intake (≥two interquartile ranges from the sex-specific median intake level for the cohort) (n = 11,887). After these exclusions, our current analyses included 485,403 persons (294,375 men and 191,028 women) (Supplemental Figure 1).
Cancer ascertainment
In the current study, we included 2 primary outcomes: incident liver cancer and CLD mortality. We further separated liver cancer into hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) based on the International Classification of Disease for Oncology (3rd edition) codes. Details about the definition of liver cancer and CLD mortality can be found in the Supplemental Methods.
Dietary assessment
Dietary information was collected using a self-administered semiquantitative food frequency questionnaire (FFQ) with 124 items by asking about the frequency of food intake over the 12 months before enrollment. [19] A validation study (n = 2053) showed that energy-adjusted correlation coefficients for total fruit and vegetables between FFQ and 2 nonconsecutive 24-hour dietary recalls were 0.72 and 0.61 in men and women, respectively [19].
In the current analyses, total vegetable intake included intake from 23 items (white potatoes were excluded from the current analyses). Total fruits included fruit juice (2 items) and whole fruit intake (12 items) [20]. We used the MyPyramid Food Guidance System to define equivalent cups. A 1-cup equivalent (8 oz, 225 g, or 237 mL) was defined as 1 cup raw or cooked vegetables or fruit, 1 cup vegetable or fruit juice, 0.5 cups dried fruit, or 2 cups leafy salad greens based on the MyPyramid Equivalents Database version 1.0 [21]. We further grouped fruit and vegetable into 13 selected botanical families based on botanical taxonomy to help identify specific phytochemical-rich food sources [22].
Covariates
We selected the following covariates a priori from the baseline questionnaire as potential confounders based on the literature: age at entry into cohort, sex, education level, race/ethnicity, alcohol intake, body mass index (BMI), smoking pack-years, total energy intake, moderate-intensity physical activity, self-reported diabetes, aspirin use, and coffee intake. The education level included “≤11 y,” “high school graduate,” “post-high school training,” “some college,” and “college or postgraduate.” Alcohol intake was estimated based on the consumption of beer, wine, wine coolers, and liquor or mixed drinks and converted to grams per day in analyses. Moderate-intensity physical activity was measured by asking about the frequency of physical activity at work or home in the past 12 months (including exercise, sports, and activities like carrying heavy loads) more than 20 minutes that caused increases in breathing or heart rate or sufficient to work up a sweat.
Statistical analysis
For liver cancer, follow-up time was calculated from the date of baseline enrollment to the date of diagnosis of liver cancer, date of death, or the end of the follow-up (31 December 2011), whichever came first. For CLD mortality, follow-up time was calculated from baseline to date of death or end of follow-up (December 31, 2011), whichever came first. Quintiles of intakes of fruit, vegetable, and specific botanical groups were computed based on the sex-specific distribution in the study population. Cox proportional hazards regression was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the associations of fruit and vegetable intake with the risk of liver cancer and CLD mortality. The proportional hazards assumption was tested by adding an interaction term between fruit or vegetable intake in continuous scale and follow-up time, with no evidence of violations observed. We provided both age adjusted and multivariable-adjusted HR and 95% CIs. We used the nutrient density model, in which daily intakes of fruits and vegetables were expressed as the number of pyramid cups per 1000 kcal of total energy. Multivariable models adjusted for aforementioned potential confounders and stratified by sex. Tests for trends across ordered categorical variables were calculated using the median value for each category. We also calculated the HR for one cup difference of fruit or vegetable intake per 1000 kcal of total energy and HR for one standard deviation (SD) difference of a specific botanical group of fruit or vegetable. We further examined the associations of fruit and vegetables with 2 common subtypes of liver cancer, HCC, and ICC. Several sensitivity analyses were performed to test the robustness of our main findings. Details of these analyses can be found in the Supplemental Methods. Due to the lack of HBV/HCV information in the NIH-AARP cohort, we further analyzed the associations between total fruit and vegetable intake and HBV surface antigen (HBsAg) and HCV using data from the National Health Nutrition and Examination Survey (NHANES) (Supplemental Methods).
All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, US). All tests were 2-sided, with P < 0.005 considered statistically significant, as suggested by Benjamin et al. [23], and 0.05< P ≤ 0.005 was considered suggestively significant.
Results
During 5,952,317 person-years of follow-up (median follow-up = 15.5 y), a total of 947 incident liver cancers (746 male and 201 female) and 986 CLD deaths (707 male and 279 female) were recorded among 485,403 participants. The median age at entry was 62.0 (interquartile range: 57.0, 66.0) y. The median age at liver cancer diagnosis was 71.7 y (interquartile range: 67.2, 75.7) and the median age at CLD death was 70.3 (interquartile range: 66.3, 74.6) y. Participants with higher fruit and vegetable intake were more likely to have attained a higher education level, exercised more, did not smoke, drank less alcohol, and had self-report diabetes (Table 1). These observations were similar when stratified by sex (Supplemental Table 1).
TABLE 1.
Characteristics of study participants by quintiles of total fruits and vegetables consumption in the NIH-AARP Diet and Health Study, United States, 1995-2011
| Characteristics | Total fruits consumption 1 |
Total vegetables consumption 1 |
||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| N | 97,080 | 97,081 | 97,080 | 97,080 | 97,081 | 97,080 |
| Age at entry, y | 60.5 (5.4) | 61.7 (5.3) | 62.0 (5.3) | 61.2 (5.4) | 61.5 (5.4) | 61.7 (5.3) |
| Female, % | 39.4 | 39.4 | 39.4 | 39.4 | 39.4 | 39.4 |
| White, % | 93.9 | 92.7 | 86.7 | 88.5 | 92.9 | 91.6 |
| College or above, % | 30.5 | 41.2 | 41.8 | 32.3 | 39.9 | 42.8 |
| BMI at baseline, kg/m2 | 27.2 (5.2) | 27.0 (4.8) | 26.6 (4.8) | 27 (5.1) | 27 (4.9) | 26.9 (5.0) |
| Physical activity≥5 times/wk, % | 13.7 | 19.0 | 25.9 | 15.8 | 18.7 | 24.9 |
| Never drinker, % | 5.3 | 5.5 | 13.0 | 7.4 | 5.5 | 10.8 |
| Alcohol intake, g/d | 22.2 (48.9) | 10.7 (22.6) | 5.8 (13.0) | 20.5 (49.9) | 10.7 (22.0) | 7.4 (14.6) |
| Never smoker, % | 28.7 | 39.7 | 46.3 | 35.8 | 39.3 | 40.1 |
| Smoking dose, pack-year | 1.9 (1.6) | 1.5 (1.6) | 1.3 (1.5) | 1.6 (1.6) | 1.5 (1.6) | 1.5 (1.6) |
| Self-reported history of diabetes, % | 7.7 | 9.0 | 8.8 | 7.7 | 8.1 | 10.4 |
| Aspirin use, % | 43.5 | 46.9 | 43.9 | 43.3 | 46.4 | 44.7 |
| Total coffee intake, g/d | 923 (705) | 771 (611) | 622 (579) | 808 (677) | 781 (622) | 720 (618) |
| Total fruit, cups/d | 0.6 (0.4) | 1.8 (0.7) | 4.1 (2.2) | 1.8 (1.8) | 2.0 (1.5) | 2.3 (1.7) |
| Total vegetables, cups/d | 2.0 (1.3) | 2.3 (1.3) | 2.3 (1.6) | 1.1 (0.5) | 2.1 (0.8) | 3.7 (1.8) |
Values are means (SD) for continuous variables; percentages for categorical variables and are standardized to the age distribution of the study population except for age at entry and sex.
Total fruits and vegetables intake was adjusted for energy intake using density method and categorized into quintiles based on sex-specific distribution.
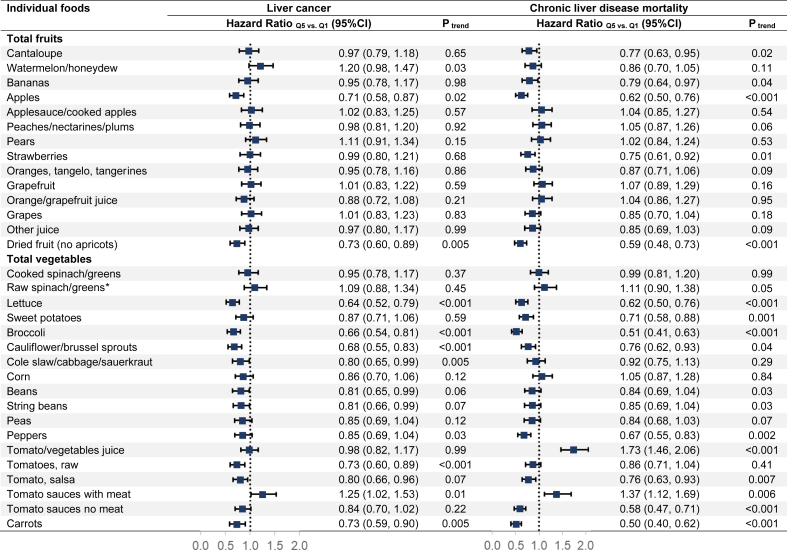
Total vegetable intake was significantly associated with a lower risk of liver cancer (HRQ5 vs. Q1 = 0.72, 95% CI: 0.59,0.89; Ptrend < 0.001) (Table 2). A one-cup difference per 1000 kcal daily in vegetable intake was associated with a 20% decreased risk of liver cancer (HRper 1 cup = 0.80, 95% CI: 0.71,0.89). Among the selected botanical groups of vegetables, significant associations with a lower risk of liver cancer for a higher intake of Compositae (lettuce) and Cruciferae (broccoli, cauliflower, brussels sprouts, turnip, cabbage, coleslaw, collard, mustard, and kale) (Ptrend < 0.005) and suggestive significant associations for Leguminosae (dried beans, string beans, and pea) and Umbelliferae (carrots) (Ptrend < 0.05) (Table 2). Mutual adjustment for the significant botanical groups showed similar results for lettuce and cruciferous vegetables (Supplemental Table 2). We did not find any significant associations between total and botanical groups of fruit intake and liver cancer. We further examined the associations between the intake of specific fruits or vegetables and liver cancer (Figure 1). We found lettuce, broccoli, cauliflower/brussels sprouts, cole slaw/cabbage/sauerkraut, raw tomatoes, and carrots were inversely associated with the risk of liver cancer (all Ptrend ≤ 0.005).
TABLE 2.
Hazard ratios of liver cancer according to quintile of fruits and vegetables consumption in NIH-AARP Diet and Health Study, 1995-2011 (n = 485,403)
| Fruits or vegetables | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | Per unit difference 2 |
|---|---|---|---|---|---|---|---|
| Total fruits1 | |||||||
| Cases | 207 | 211 | 173 | 169 | 187 | ||
| Age adjusted | 1 | 0.96 (0.79,1.16) | 0.76 (0.62,0.93) | 0.73 (0.59,0.89) | 0.80 (0.66,0.98) | 0.009 | 0.95 (0.88,1.03) |
| Multivariable adjusted | 1 | 1.03 (0.85,1.25) | 0.85 (0.69,1.05) | 0.83 (0.67,1.03) | 0.92 (0.74,1.13) | 0.21 | 1.00 (0.92,1.09) |
| Botanical groups | |||||||
| Cucurbitaceae | 1 | 1.05 (0.85,1.29) | 1.08 (0.88,1.32) | 1.03 (0.84,1.27) | 1.19 (0.97,1.46) | 0.10 | 1.03 (0.96,1.09) |
| Musaceae | 1 | 0.88 (0.72,1.07) | 0.83 (0.68,1.02) | 0.86 (0.70,1.06) | 0.93 (0.76,1.14) | 0.93 | 1.03 (0.97,1.10) |
| Rosaceae | 1 | 0.90 (0.74,1.10) | 0.90 (0.73,1.09) | 0.91 (0.75,1.12) | 0.83 (0.68,1.03) | 0.17 | 0.99 (0.92,1.06) |
| Rutaceae (citrus) | 1 | 0.94 (0.77,1.15) | 0.93 (0.76,1.14) | 0.95 (0.78,1.16) | 0.85 (0.69,1.04) | 0.15 | 0.99 (0.92,1.05) |
| Vitaceae | 1 | 1.05 (0.86,1.27) | 0.86 (0.70,1.06) | 0.91 (0.74,1.12) | 1.01 (0.82,1.23) | 0.80 | 1.00 (0.93,1.07) |
| Total vegetables1 | |||||||
| Cases | 235 | 216 | 171 | 161 | 164 | ||
| Age adjusted | 1 | 0.90 (0.75,1.08) | 0.70 (0.57,0.85) | 0.66 (0.54,0.80) | 0.66 (0.54,0.81) | <0.001 | 0.76 (0.67,0.85) |
| Multivariable adjusted | 1 | 0.98 (0.81,1.18) | 0.78 (0.64,0.95) | 0.73 (0.60,0.90) | 0.72 (0.59,0.89) | <0.001 | 0.80 (0.71,0.89) |
| Botanical groups | |||||||
| Chenopodiaceae | 1 | 0.99 (0.77,1.29) | 0.99 (0.82,1.19) | 1.09 (0.91,1.30) | 0.95 (0.78,1.15) | 0.54 | 1.00 (0.94,1.07) |
| Compositae | 1 | 0.81 (0.67,0.98) | 0.80 (0.66,0.96) | 0.63 (0.51,0.77) | 0.65 (0.53,0.79) | <0.001 | 0.86 (0.79,0.94) |
| Convolvulaceae | 1 | 1.02 (0.82,1.26) | 0.97 (0.80,1.18) | 0.94 (0.77,1.14) | 0.97 (0.80,1.18) | 0.67 | 0.97 (0.90,1.05) |
| Cruciferae | 1 | 1.02 (0.85,1.22) | 0.69 (0.57,0.85) | 0.77 (0.63,0.94) | 0.66 (0.53,0.81) | <0.001 | 0.83 (0.76,0.91) |
| Gramineae | 1 | 1.02 (0.84,1.24) | 0.99 (0.82,1.21) | 0.94 (0.77,1.15) | 0.87 (0.71,1.07) | 0.10 | 0.95 (0.88,1.02) |
| Leguminosae | 1 | 0.98 (0.81,1.18) | 0.81 (0.67,0.99) | 0.77 (0.63,0.95) | 0.82 (0.67,1.00) | 0.03 | 0.92 (0.85,0.99) |
| Solanaceae | 1 | 0.79 (0.65,0.96) | 0.70 (0.57,0.86) | 0.82 (0.67,0.99) | 0.81 (0.66,0.98) | 0.20 | 0.99 (0.93,1.06) |
| Umbelliferae | 1 | 0.97 (0.80,1.17) | 0.82 (0.68,1.00) | 0.80 (0.65,0.98) | 0.74 (0.60,0.92) | 0.006 | 0.97 (0.90,1.05) |
Chenopodiaceae: raw spinach and cooked spinach; Compositae: lettuce; Convolvulaceae: sweet potatoes and yams; Cruciferae: broccoli, cauliflower, brussels sprouts, turnip, cabbage, coleslaw, collard, mustard and kale; Cucurbitaceae: cantaloupe, watermelon and honeydew melon; Gramineae: corn; Leguminosae: dried beans, string beans and peas; Musaceae: bananas; Rosaceae: apples, peach, nectarines, plums, pears and strawberries; Rutaceae (citrus): oranges, tangerines, tangelos and grapefruits; Solanaceae: tomatoes, peppers; Umbelliferae: carrots; Vitaceae: grapes.
Multivariable-adjusted model adjusted for age at entry into cohort, education level, race and ethnicity, alcohol intake, BMI, smoking, total energy intake, usual activity throughout the day, diabetes, aspirin use, coffee intake, and stratified by sex.
The median intake for fruits intake in each quintile is 0.30, 0.67, 1.01, 1.43, and 2.24 cups/1000 kcal per day; the median intake for vegetables intake in each quintile is 0.57, 0.87, 1.12, 1.43, and 2.06 cups/1000 kcal per day.
The unit is one cup per 1000 kcal per day for total fruit and vegetable and SD for botanical groups.
FIGURE 1.
Associations between individual fruits or vegetables and liver cancer and chronic liver disease mortality in the National Institutes of Health-American Association of Retired Persons Diet and Health Study (n = 485,403). Cox proportional hazard regression model adjusted for age, education, race and ethnicity, alcohol intake, body mass index, smoking, total energy intake, total physical activity, history of diabetes, aspirin uses, coffee intake and stratified by sex.∗
We examined these associations separately for 2 major liver cancer subtypes, HCC and ICC (Supplemental Tables 3 and 4). The results for HCC, but not ICC, were somewhat stronger than those for liver cancer overall although cases were limited. Intake of total vegetables was inversely associated with HCC risk (HRQ5 vs. Q1 = 0.64, 95% CI: 0.49,0.82; Ptrend < 0.001). Similar to the results for liver cancer overall, besides lettuce, cruciferous vegetables, and legumes, carrots intake was inversely associated with HCC risk. We did not observe strong significant associations for ICC risk.
Total vegetable intake (HRQ5 vs. Q1 = 0.61, 95% CI: 0.50,0.76; Ptrend < 0.001) was significantly associated with CLD mortality, but fruit intake was not (HRQ5 vs. Q1 = 0.89, 95% CI: 0.73,1.10; Ptrend= 0.15) (Table 3). Higher intake of lettuce, Convolvulaceae (sweet potatoes and yams), cruciferous vegetables, legumes, and carrots were associated with a lower risk of CLD mortality (all Ptrend<0.005). The strongest reduction of HR in the quintile analyses was for carrots (HRQ5 vs. Q1 = 0.49, 95% CI: 0.39,0.61). Additionally, we found Solanaceae (tomatoes and pepper) was suggestively positively associated with CLD mortality (HRQ5 vs. Q1=1.27, 95% CI: 1.04,1.54; Ptrend = 0.03). Mutual adjustment for these botanical groups did not materially change the results except for legumes (Supplemental Table 2). No overall association was found between total fruits and CLD mortality. Still, specific botanical groups, including Cucurbitaceae (cantaloupe, watermelon, and honeydew melon), Musaceae (bananas), Rosaceae (apples, peach, nectarines, plums, pears, and strawberries), were suggestively inversely associated with CLD mortality (Table 3). A significantly lower risk of CLD mortality was associated with a higher intake of apples, dried fruit, lettuce, sweet potatoes, broccoli, peppers, carrots, and a lower intake of tomato/vegetable juice and tomato sauces with meat (all Ptrend < 0.005) (Figure 1).
TABLE 3.
Hazard ratios of chronic liver disease mortality according to quintile of fruits and vegetables consumption in NIH-AARP Diet and Health Study, 1995-2011 (n = 485,403)
| Fruits or vegetables | Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P for trend | Per unit difference 2 |
|---|---|---|---|---|---|---|---|
| Total fruits1 | |||||||
| Cases | 267 | 210 | 180 | 156 | 173 | ||
| Age adjusted | 1 | 0.74 (0.62,0.89) | 0.62 (0.51,0.75) | 0.53 (0.43,0.65) | 0.59 (0.48,0.71) | <0.001 | 0.83 (0.76,0.90) |
| Multivariable adjusted | 1 | 0.95 (0.79,1.15) | 0.86 (0.71,1.05) | 0.78 (0.63,0.96) | 0.89 (0.73,1.10) | 0.15 | 0.99 (0.91,1.08) |
| Botanical groups | |||||||
| Cucurbitaceae | 1 | 0.87 (0.72,1.05) | 0.90 (0.74,1.09) | 0.85 (0.70,1.03) | 0.76 (0.62,0.93) | 0.02 | 0.99 (0.92,1.06) |
| Musaceae | 1 | 0.87 (0.72,1.05) | 0.96 (0.80,1.17) | 0.89 (0.73,1.09) | 0.77 (0.62,0.95) | 0.03 | 0.95 (0.88,1.01) |
| Rosaceae | 1 | 0.79 (0.66,0.95) | 0.74 (0.61,0.90) | 0.82 (0.67,0.99) | 0.71 (0.58,0.87) | 0.02 | 0.92 (0.85,0.99) |
| Rutaceae (citrus) | 1 | 0.71 (0.58,0.86) | 0.87 (0.72,1.05) | 0.79 (0.65,0.96) | 0.93 (0.77,1.13) | 0.78 | 1.08 (1.02,1.15) |
| Vitaceae | 1 | 0.92 (0.76,1.11) | 0.98 (0.81,1.19) | 0.91 (0.75,1.11) | 0.86 (0.70,1.06) | 0.19 | 1.00 (0.94,1.07) |
| Total vegetables1 | |||||||
| Cases | 299 | 195 | 171 | 178 | 143 | ||
| Age adjusted | 1 | 0.64 (0.54,0.77) | 0.55 (0.46,0.67) | 0.57 (0.48,0.69) | 0.46 (0.38,0.56) | <0.001 | 0.67 (0.60,0.76) |
| Multivariable adjusted | 1 | 0.80 (0.66,0.96) | 0.73 (0.60,0.88) | 0.77 (0.63,0.93) | 0.61 (0.50,0.76) | <0.001 | 0.82 (0.73,0.91) |
| Botanical groups | |||||||
| Chenopodiaceae | 1 | 0.96 (0.76,1.22) | 0.95 (0.79,1.13) | 0.95 (0.80,1.14) | 0.92 (0.76,1.11) | 0.47 | 0.98 (0.91,1.06) |
| Compositae | 1 | 0.89 (0.74,1.06) | 0.89 (0.74,1.07) | 0.62 (0.50,0.76) | 0.63 (0.51,0.78) | <0.001 | 0.84 (0.77,0.91) |
| Convolvulaceae | 1 | 0.93 (0.77,1.13) | 0.79 (0.66,0.95) | 0.78 (0.65,0.94) | 0.71 (0.58,0.86) | <0.001 | 0.86 (0.78,0.95) |
| Cruciferae | 1 | 0.93 (0.77,1.11) | 0.78 (0.64,0.94) | 0.70 (0.57,0.86) | 0.66 (0.53,0.81) | <0.001 | 0.87 (0.80,0.95) |
| Gramineae | 1 | 1.08 (0.90,1.30) | 0.95 (0.78,1.15) | 0.90 (0.73,1.10) | 1.07 (0.88,1.30) | 0.87 | 1.04 (0.98,1.10) |
| Leguminosae | 1 | 1.18 (0.99,1.42) | 0.93 (0.76,1.13) | 0.82 (0.67,1.00) | 0.83 (0.67,1.02) | 0.003 | 0.89 (0.82,0.96) |
| Solanaceae | 1 | 1.14 (0.94,1.39) | 1.05 (0.86,1.29) | 1.04 (0.85,1.28) | 1.27 (1.04,1.54) | 0.03 | 1.09 (1.05,1.13) |
| Umbelliferae | 1 | 0.89 (0.75,1.06) | 0.71 (0.59,0.85) | 0.68 (0.55,0.82) | 0.49 (0.39,0.61) | < 0.001 | 0.72 (0.64,0.82) |
Chenopodiaceae: raw spinach and cooked spinach; Compositae: lettuce; Convolvulaceae: sweet potatoes and yams; Cruciferae: broccoli, cauliflower, brussels sprouts, turnip, cabbage, coleslaw, collard, mustard and kale; Cucurbitaceae: cantaloupe, watermelon and honeydew melon; Gramineae: corn; Leguminosae: dried beans, string beans and peas; Musaceae: bananas; Rosaceae: apples, peach, nectarines, plums, pears and strawberries; Rutaceae (citrus): oranges, tangerines, tangelos and grapefruits; Solanaceae: tomatoes, peppers; Umbelliferae: carrots; Vitaceae: grapes.
Multivariable-adjusted model adjusted for age at entry into cohort, education level, race and ethnicity, alcohol intake, BMI, smoking, total energy intake, usual activity throughout the day, diabetes, aspirin use, coffee intake, and stratified by sex.
The median intake for fruits intake in each quintile is 0.30, 0.67, 1.01, 1.43, and 2.24 cups/1000 kcal per day; the median intake for vegetables intake in each quintile is 0.57, 0.87, 1.12, 1.43, and 2.06 cups/1000 kcal per day.
The unit is one cup per 1000 kcal per day for total fruit and vegetable and SD for botanical groups.
In general, we observed similar results for fruit and vegetable intakes in relation to liver cancer, HCC, and CLD mortality by strata of selected baseline factors, including sex, age, race, education, BMI, smoking status, drinking status, physical activity, self-reported diabetes, and aspirin use (Supplemental Figure 2–4). When we conducted sensitivity analyses by excluding liver cancer or CLD death cases that occurred in the first 2 or 5 y, the association of vegetable intake and liver cancer and CLD mortality remained (Supplemental Tables 5 and 6). We observed similar results when we further excluded participants with missing values in covariates (Supplemental Table 7). Additional adjustments for dietary quality (modified HEI-2015) did not materially change our main findings (Supplemental Table 8). Using residual methods to control for total energy intake provided similar results (Supplemental Table 9).
The correlations between HBsAg positivity and fruit and vegetable intake were 0.009 and 0.001, respectively (P values are 0.14 and 0.80 for fruits and vegetables, respectively) in the NHANES. We also evaluated the correlation between HCV antibody positivity and the consumption of fruits and vegetables, and little correlation was observed (Spearman coefficient of -0.029 for fruit and -0.012 for vegetables with P values of <0.001 and 0.14, respectively).
Discussion
Principal findings
In the current cohort study with 485,403 US adults, we found higher intake of total vegetables, as well as the subtypes of lettuces and cruciferous vegetables, were significantly associated with a lower risk of liver cancer. In contrast, fruits were not associated with liver cancer risk in general. Our results also indicated that lettuces, sweet potatoes, cruciferous vegetables, legumes, and carrots were inversely associated with CLD mortality.
A meta-analysis of 9 cohorts reported that total vegetable intake was inversely associated with liver cancer risk but with moderate heterogeneity (relative risk highest vs. lowest = 0.61, 95% CI: 0.50,0.75; I2 = 47.1%) [9]. Evidence on specific types of vegetables is limited. In the Shanghai Women’s and Men’s Health Studies, the inverse association with liver cancer was mainly driven by legumes and legume products, celery, allium vegetables, mushrooms, and composite vegetables (including asparagus, lettuce, and garland chrysanthemum) [24]. A Japanese cohort found green-yellow vegetables and green-leafy vegetables were inversely associated with the risk of HCC (Ptrend = 0.06 and 0.04, respectively) [25]. Results from these abovementioned studies are consistent with our findings. As mentioned earlier, the most common green-leafy or green-yellow vegetables in the studies were lettuces, cruciferous vegetables, legumes, and carrots [24, 25].
Evidence on the association between vegetables and CLD is limited to one prospective study. The study reported inverse associations of both fruit and vegetable intake and NAFLD in a dose-response manner among Korean women but not among men [26]. This study also showed inverse associations between spinach intake and NAFLD risk. No study has yet examined the associations between fruit and vegetables and CLD mortality.
Several potential mechanisms have been proposed to support the inverse associations between specific vegetables and liver disease outcomes [6, 8, 27]. First, vegetables are nutrient-rich foods with lower energy density, which have been hypothesized to help prevent obesity and T2D, 2 major risk factors of liver cancer and liver disease, although our results were independent of BMI and diabetes. Second, some bioactive compounds in vegetables have been found to reduce body inflammation and oxidation and thus might inhibit cancer progression and promote liver health [28]. Importantly, vegetables are a rich source of phytochemicals, many of which have shown preventive and/or therapeutic activities against liver cancers or diseases [29]. This may at least partially explain the observed inverse associations between vegetables and liver outcomes that are mainly driven by lettuce and cruciferous vegetables (broccoli, cabbage, etc.). In animal models, lettuce may increase the total cholesterol end-product excretion and improve antioxidant status [30]. Isothiocyanates are hydrolyzed products of glucosinolates, the primary phytochemicals in cruciferous vegetables. Isothiocyanates modulate carcinogen metabolism and inhibit carcinogenesis and tumor growth in liver cancer models [31]. Carrots are a good source of β-carotene, showing possible protective effects against hepatic steatosis, fibrosis, and inflammation [27, 32]. In addition, vegetables are sources of nutrients such as vitamin C, carotenoids, potassium, and dietary fiber, all of which have shown inverse associations with the risk of liver cancer and liver diseases [33, 34]. In a recently reported analysis in the NIH-AARP cohort, our group also found that fiber from vegetables was inversely associated with liver cancer and CLD mortality [35]. Our study also found raw tomato intake was inversely associated with a lower risk of liver cancer, which was consistent with previous studies either in animal models [36] or cohort studies [37], indicating the anticancer effects of lycopene in liver carcinogenesis. Future experimental studies focused on these bioactive compounds are needed to clarify the clinical importance of these foods for the prevention of liver cancer.
Four studies investigated the association between fruit intake and liver cancer incidence or mortality, none of which found significant associations [10, 11, 25, 38]. The null association between total fruit intake and liver cancer may be explained by the potential adverse effects of chemicals in fruits like fructose, which may counterbalance the effect of protective nutrients like polyphenols, vitamins, and fibers [39, 40]. Further studies are needed to explain the null associations between fruit intake and liver cancer risk. However, we found higher intakes of apples or dried fruits were associated with a lower risk of liver cancer. Our findings were in line with previous meta-analyses, which found inverse associations of apples with cancers of the lung, colorectum, breast, and overall digestive tract [41].
In our study, even though there was no association between total fruit intake and CLD mortality, we found specific fruits were inversely associated with CLD mortality, including cantaloupes, bananas, apples, strawberries, and dried fruits. In animal studies, melons and bananas showed hepatoprotective effects by significantly decreasing aspartate transaminase, alanine transferase levels in blood, or hepatic superoxide dismutase levels [42]. In addition, ellagic acid, a polyphenol found in strawberries, has been reported to mitigate oxidative stress, inflammatory response, steatosis, and gut microbiota dysbiosis in mice with alcoholic liver disease [43].
Strengths and limitations
Our study has several strengths. First, the study had a large sample size with 947 liver cancer cases and 986 CLD deaths among 485,403 US adults. Second, our study had a relatively long follow-up time of 15.5 y, allowing for sufficient case events and ensuring that observed associations were unlikely to be based on reverse causation. Third, we have controlled for many potential confounders.
Several limitations of our study should also be noted. Measurement errors in dietary assessment based on FFQ cannot be avoided even though there was an adequate correlation of over 0.5 for most of the 29 food groups between the FFQ and the 24-hour recalls [44]. Moreover, our dietary data were collected at the baseline and did not consider the long-term changes during follow-up. In addition, we did not have information on the HBV and HCV status of the participants. However, using data from the NHANES, we found that neither HBV nor HCV status was associated with fruit and vegetable intake in the US population. Over 90% of the study population were of European ancestry; thus, our results may not be generalizable to other racial/ethnic groups. Finally, we cannot rule out the residual confounding of other dietary factors, such as beverage intake or animal foods intake. However, we adjusted coffee intake and dietary quality in our multivariable-adjusted models, and similar results were observed.
Conclusions
Examining a large cohort study in the US, we found higher intake of specific types of vegetables, such as lettuces and cruciferous vegetables, was associated with a lower risk of liver cancer and CLD mortality. However, additional research in diverse racial/ethnic populations with objective measurements, such as certain biomarkers of fruit and vegetable intake, is needed to gain further insight into the associations between specific fruit and vegetable and liver cancer risk and CLD mortality.
Funding
Dr. Zhang is supported by the NIH/NCI MERIT Award (R37 CA262299).
Author Contributions
The authors’ responsibilities were as follows: Study concept and design: LZ, XZ. Development and implementation of literature search: LZ. Data analyses: LZ. Interpretation of results: All authors. Drafting of the manuscript: LZ. Critical revision of the manuscript for important intellectual content: All authors. Study supervision: XZ.
Data Availability
The full dataset and statistical code are available from the corresponding author.
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute. Cancer incidence data from the Atlanta metropolitan area were collected by the Georgia Center for Cancer Statistics, Department of Epidemiology, Rollins School of Public Health, Emory University. Cancer incidence data from California were compiled by the California Department of Health Services, Cancer Surveillance Section. Cancer incidence data from the Detroit metropolitan area were collected by the Michigan Cancer Surveillance Program, Community Health Administration, State of Michigan. The Florida cancer incidence data used in this report were collected by the Florida Cancer Data System (FCDS) under contract with the Florida Department of Health (FDOH). Cancer incidence data from Louisiana were collected by the Louisiana Tumor Registry, Louisiana State University Medical Center in New Orleans. Cancer incidence data from New Jersey were collected by the New Jersey State Cancer Registry, Cancer Epidemiology Services, and the New Jersey State Department of Health and Senior Services. Cancer incidence data from North Carolina were collected by the North Carolina Central Cancer Registry. Cancer incidence data from Pennsylvania were supplied by the Division of Health Statistics and Research, Pennsylvania Department of Health, Harrisburg, Pennsylvania. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions. Cancer incidence data from Arizona were collected by the Arizona Cancer Registry, Division of Public Health Services, and Arizona Department of Health Services. Cancer incidence data from Texas were collected by the Texas Cancer Registry, Cancer Epidemiology and Surveillance Branch, Texas Department of State Health Services. Cancer incidence data from Nevada were collected by the Nevada Central Cancer Registry, Center for Health Data and Research, Bureau of Health Planning and Statistics, State Health Division, and State of Nevada Department of Health and Human Services. (The above information can also be found at: https://dietandhealth.cancer.gov/acknowledgement.html).
Footnotes
The first 2 quintiles for raw spinach and greens were collapsed into one category as the comparison group (nonconsumers of raw spinach and greens)
This work was presented as a poster at the American Association for Cancer Research Annual Meeting 2022 (Apr 8, 2022-Apr 13, 2022) New Orleans, Louisiana, USA.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2022.12.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Ozakyol A. Global epidemiology of hepatocellular carcinoma (HCC epidemiology) J Gastrointest Cancer. 2017;48(3):238–240. doi: 10.1007/s12029-017-9959-0. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 4.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinton S.K., Giovannucci E.L., Hursting S.D. The World Cancer Research Fund/American Institute for Cancer Research third expert report on diet, nutrition, physical activity, and cancer: impact and future directions. J Nutr. 2020;150(4):663–671. doi: 10.1093/jn/nxz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X., Huang H., Childs H., Wu Y., Yu L., Pehrsson P.R. Glucosinolates in brassica vegetables: characterization and factors that influence distribution, content, and intake. Annu Rev Food Sci Technol. 2021;12:485–511. doi: 10.1146/annurev-food-070620-025744. [DOI] [PubMed] [Google Scholar]
- 7.Goh Y.X., Jalil J., Lam K.W., Husain K., Premakumar C.M. Genistein: a review on its anti-inflammatory properties. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.820969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanini S., Marzotto M., Giovinazzo F., Bassi C., Bellavite P. Effects of dietary components on cancer of the digestive system. Crit Rev Food Sci Nutr. 2015;55(13):1870–1885. doi: 10.1080/10408398.2012.732126. [DOI] [PubMed] [Google Scholar]
- 9.Guo X.F., Shao X.F., Li J.M., Li S., Li K.L., Li D. Fruit and vegetable intake and liver cancer risk: a meta-analysis of prospective cohort studies. Food Funct. 2019;10(8):4478–4485. doi: 10.1039/c9fo00804g. [DOI] [PubMed] [Google Scholar]
- 10.Bamia C., Lagiou P., Jenab M., Aleksandrova K., Fedirko V., Trichopoulos D., et al. Fruit and vegetable consumption in relation to hepatocellular carcinoma in a multi-centre, European cohort study. Br J Cancer. 2015;112(7):1273–1282. doi: 10.1038/bjc.2014.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George S.M., Park Y., Leitzmann M.F., Freedman N.D., Dowling E.C., Reedy J., et al. Fruit and vegetable intake and risk of cancer: a prospective cohort study. Am J Clin Nutr. 2009;89(1):347–353. doi: 10.3945/ajcn.2008.26722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171. doi: 10.1016/j.jhep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Kim D., Bonham C.A., Konyn P., Cholankeril G., Ahmed A. Mortality trends in chronic liver disease and cirrhosis in the united states, before and during COVID-19 pandemic. Clin Gastroenterol Hepatol. 2021;19(12):2664–6 e2. doi: 10.1016/j.cgh.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West J., Card T.R., Aithal G.P., Fleming K.M. Risk of hepatocellular carcinoma among individuals with different aetiologies of cirrhosis: a population-based cohort study. Aliment Pharmacol Ther. 2017;45(7):983–990. doi: 10.1111/apt.13961. [DOI] [PubMed] [Google Scholar]
- 15.Simpson S.J., Raubenheimer D., Cogger V.C., Macia L., Solon-Biet S.M., Le Couteur D.G., et al. The nutritional geometry of liver disease including non-alcoholic fatty liver disease. J Hepatol. 2018;68(2):316–325. doi: 10.1016/j.jhep.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Tsompanaki E., Thanapirom K., Papatheodoridi M., Parikh P., Chotai de Lima Y., Tsochatzis E.A. Systematic review and meta-analysis: The role of diet in the development of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.11.026. S1542–3565(21):01264–7. [DOI] [PubMed] [Google Scholar]
- 17.He K., Li Y., Guo X., Zhong L., Tang S. Food groups and the likelihood of non-alcoholic fatty liver disease: a systematic review and meta–analysis. Br J Nutr. 2020;124(1):1–13. doi: 10.1017/S0007114520000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schatzkin A., Subar A.F., Thompson F.E., Harlan L.C., Tangrea J., Hollenbeck A.R., et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119–1125. doi: 10.1093/aje/154.12.1119. [DOI] [PubMed] [Google Scholar]
- 19.Thompson F.E., Kipnis V., Midthune D., Freedman L.S., Carroll R.J., Subar A.F., et al. Performance of a food-frequency questionnaire in the US NIH-AARP (National Institutes of Health-American Association of Retired Persons) Diet and Health Study. Public Health Nutr. 2008;11(2):183–195. doi: 10.1017/S1368980007000419. [DOI] [PubMed] [Google Scholar]
- 20.Kabat G.C., Park Y., Hollenbeck A.R., Schatzkin A., Rohan T.E. Intake of fruits and vegetables, and risk of endometrial cancer in the NIH-AARP Diet and Health Study. Cancer Epidemiol. 2010;34(5):568–573. doi: 10.1016/j.canep.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subar A.F., Crafts J., Zimmerman T.P., Wilson M., Mittl B., Islam N.G., et al. Assessment of the accuracy of portion size reports using computer-based food photographs aids in the development of an automated self-administered 24-hour recall. J Am Diet Assoc. 2010;110(1):55–64. doi: 10.1016/j.jada.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith S.A., Campbell D.R., Elmer P.J., Martini M.C., Slavin J.L., Potter J.D. The University of Minnesota Cancer Prevention Research Unit vegetable and fruit classification scheme (United States) Cancer Causes Control. 1995;6(4):292–302. doi: 10.1007/BF00051404. [DOI] [PubMed] [Google Scholar]
- 23.Benjamin D.J., Berger J.O., Johannesson M., Nosek B.A., Wagenmakers E.J., Berk R., et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6–10. doi: 10.1038/s41562-017-0189-z. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W., Xiang Y.B., Li H.L., Yang G., Cai H., Ji B.T., et al. Vegetable-based dietary pattern and liver cancer risk: results from the Shanghai women's and men's health studies. Cancer Sci. 2013;104(10):1353–1361. doi: 10.1111/cas.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurahashi N., Inoue M., Iwasaki M., Tanaka Y., Mizokami M., Tsugane S., et al. Vegetable, fruit and antioxidant nutrient consumption and subsequent risk of hepatocellular carcinoma: a prospective cohort study in Japan. Br J Cancer. 2009;100(1):181–184. doi: 10.1038/sj.bjc.6604843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S.A., Shin S. Fruit and vegetable consumption and non-alcoholic fatty liver disease among Korean adults: a prospective cohort study. J Epidemiol Community Health. 2020;74(12):1035–1042. doi: 10.1136/jech-2020-214568. [DOI] [PubMed] [Google Scholar]
- 27.Elvira-Torales L.I., Garcia-Alonso J., Periago-Caston M.J. Nutritional importance of carotenoids and their effect on liver health: a review. Antioxidants (Basel) 2019;8(7):229. doi: 10.3390/antiox8070229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan R., Van Le Q., Yang H., Zhang D., Gu H., Yang Y., et al. A review of dietary phytochemicals and their relation to oxidative stress and human diseases. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2020.129499. [DOI] [PubMed] [Google Scholar]
- 29.Nishino H. Phytochemicals in hepatocellular cancer prevention. Nutr Cancer. 2009;61(6):789–791. doi: 10.1080/01635580903285031. [DOI] [PubMed] [Google Scholar]
- 30.Nicolle C., Cardinault N., Gueux E., Jaffrelo L., Rock E., Mazur A., et al. Health effect of vegetable-based diet: lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin Nutr. 2004;23(4):605–614. doi: 10.1016/j.clnu.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Crowley E., Rowan N.J., Faller D., Friel A.M. Natural and synthetic isothiocyanates possess anticancer potential against liver and prostate cancer in vitro. Anticancer Res. 2019;39(7):3469–3485. doi: 10.21873/anticanres.13493. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz B., Sahin K., Bilen H., Bahcecioglu I.H., Bilir B., Ashraf S., et al. Carotenoids and non-alcoholic fatty liver disease. Hepatobiliary Surg Nutr. 2015;4(3):161–171. doi: 10.3978/j.issn.2304-3881.2015.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang W.S., Zeng X.F., Liu Z.N., Zhao Q.H., Tan Y.T., Gao J., et al. Diet and liver cancer risk: a narrative review of epidemiological evidence. Br J Nutr. 2020;124(3):330–340. doi: 10.1017/S0007114520001208. [DOI] [PubMed] [Google Scholar]
- 34.George E.S., Sood S., Broughton A., Cogan G., Hickey M., Chan W.S., et al. The association between diet and hepatocellular carcinoma: a systematic review. Nutrients. 2021;13(1):172. doi: 10.3390/nu13010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X., Yang W., Petrick J.L., Liao L.M., Wang W., He N., et al. Higher intake of whole grains and dietary fiber are associated with lower risk of liver cancer and chronic liver disease mortality. Nat Commun. 2021;12(1):6388. doi: 10.1038/s41467-021-26448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mekuria A.N., Tura A.K., Hagos B., Sisay M., Abdela J., Mishore K.M., et al. Anti-cancer effects of lycopene in animal models of hepatocellular carcinoma: a systematic review and meta-analysis. Front Pharmacol. 2020;11:1306. doi: 10.3389/fphar.2020.01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas C.E., Luu H.N., Wang R., Adams-Haduch J., Jin A., Koh W.P., et al. Association between dietary tomato intake and the risk of hepatocellular carcinoma: The Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2020;29(7):1430–1435. doi: 10.1158/1055-9965.EPI-20-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauvaget C., Nagano J., Hayashi M., Spencer E., Shimizu Y., Allen N. Vegetables and fruit intake and cancer mortality in the Hiroshima/Nagasaki Life Span Study. Br J Cancer. 2003;88(5):689–694. doi: 10.1038/sj.bjc.6600775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao S., Jang C., Liu J., Uehara K., Gilbert M., Izzo L., et al. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579(7800):586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim J.S., Mietus-Snyder M., Valente A., Schwarz J.M., Lustig R.H. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol. 2010;7(5):251–264. doi: 10.1038/nrgastro.2010.41. [DOI] [PubMed] [Google Scholar]
- 41.Fabiani R., Minelli L., Rosignoli P. Apple intake and cancer risk: a systematic review and meta-analysis of observational studies. Public Health Nutr. 2016;19(14):2603–2617. doi: 10.1017/S136898001600032X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y.J., Wang F., Zhou Y., Li Y., Zhou T., Zheng J., et al. Effects of 20 selected fruits on ethanol metabolism: p32eotential health benefits and harmful impacts. Int J Environ Res Public Health. 2016;13(4):399. doi: 10.3390/ijerph13040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao L., Mehmood A., Soliman M.M., Iftikhar A., Iftikhar M., Aboelenin S.M., et al. Protective effects of ellagic acid against alcoholic liver disease in mice. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.744520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Midthune D., Schatzkin A., Subar A.F., Thompson F.E., Freedman L.S., Carroll R.J., et al. Validating an FFQ for intake of episodically consumed foods: application to the National Institutes of Health-AARP Diet and Health Study. Public Health Nutr. 2011;14(7):1212–1221. doi: 10.1017/S1368980011000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset and statistical code are available from the corresponding author.