Abstract
South-East Asian countries report a high prevalence of extended-spectrum cephalosporin- (ESC-) and colistin-resistant Escherichia coli (Col-R-Ec). However, there are still few studies describing the molecular mechanisms and transmission dynamics of ESC-R-Ec and, especially, Col-R-Ec. This study aimed to evaluate the prevalence and transmission dynamics of Ec containing extended spectrum beta-lactamases (ESBL) and mobile colistin resistance (mcr) genes using a 'One Health' design in Thailand. The ESC-R-Ec and Col-R-Ec isolates of human stool samples (69 pig farmers, 155 chicken farmers, and 61 non-farmers), rectal swabs from animals (269 pigs and 318 chickens), and the intestinal contents of 196 rodents were investigated. Resistance mechanisms and transmission dynamics of Ec isolates (n=638) were studied using short and long read sequencing. We found higher rates of ESBL-Ec isolates among pig farmers (n=36; 52.2%) than among chicken farmers (n=58; 37.4 %; P<0.05) and the control group (n=61; 31.1 %; P<0.05). Ec with co-occurring ESBL and mcr genes were found in 17 (6.0 %), 50 (18.6 %) and 15 (4.7 %) samples from humans, pigs and chickens, respectively. We also identified 39 (13.7 %) human samples with non-identical Ec containing ESBL and mcr. We found higher rates of ESBL-Ec, in particular CTX-M-55, isolates among pig farmers than among non-pig farmers (P<0.01). 'Clonal' animal-human transmission of ESBL-Ec and Ec with mcr genes was identified but rare as we overall found a heterogenous population structure of Ec. The Col-R-Ec from human and animal samples often carried mcr-1.1 on conjugative IncX4 plasmids. The latter has been identified in Ec of many different clonal backgrounds.
Keywords: clonal transmission, Extended-spectrum beta-lactamase, mobile colistin resistance, occupational exposure, One Health, pig farm
Data Summary
The sequence reads were submitted to the European Nucleotide Archive (accession number: PRJNA707214). Individual accession numbers are listed in the Supplementary table 1.
Impact Statement.
Antimicrobial resistance (AMR) poses a great threat to human and animal health. It is therefore important that AMR is studied with a 'One Health' concept. Among the multidrug resistant bacteria, Escherichia coli (Ec) having ESBL and/or mcr genes is considered as a critical pathobiont.
Here, we investigated the prevalence of ESC-R-Ec and/or Col-R-Ec isolates from human (69 pig farmers, 155 chicken farmers and 61 non-farmers), farm animal (269 pigs and 318 chickens), and rodent samples (n=196) from Thailand. A total of 638 isolates were sequenced.
Overall, we revealed that (1) the prevalence of ESBL-producing-Ec and mcr-producing-Ec was very high (>30 %) in the pigs and humans. The latter is interesting considering that the first reports of Ec with mcr genes have only been published a few years ago. In addition, carriage of Ec with ESBL was found (2) to be associated with being a pig farmer. Therefore, programmes aiming at reducing antimicrobial usage should include the pig farms. However, mcr-producing-Ec was not primarily found in pig farmers anymore but all humans. Finally, there were (3) high proportions of co-occurrence of ESBL and mcr genes in Ec in our study. This indicates that above mentioned programmes should concurrently include several rather than single antibiotics in Thailand.
Introduction
The overuse and misuse of antimicrobial drugs in farm animals are suspected to contribute to the emergence of bacteria resistant to antibiotics [1]. Such bacteria may reside in food animals and can be transmitted to humans via direct or indirect contact. However, the transmission of bacteria with antimicrobial resistance (AMR) is difficult to demonstrate since its potential reservoirs and transmission routes are so diverse [2]. Fully elucidating the transmission dynamics of AMR between livestock and humans requires using a ‘One Health’ perspective involving looking at humans and animals simultaneously [3] and performing detailed genetic analyses of the resistant bacterial strains isolated from them [4]. South-East Asia maintains an important food animal production sector and is also currently considered a hot spot for AMR [5]. Among the biggest contemporary worldwide concerns involving AMR are the increase in extended-spectrum cephalosporin-resistance (ESC-R) [6, 7] and the emergence and rapid dissemination of plasmids carrying the mobile colistin resistance (mcr) gene in Enterobacterales [8, 9].
The prevalence of ESC-R Enterobacterales, most prominently Escherichia coli (Ec), is well studied in humans and animals. Risk factors surrounding the antimicrobial usage (AMU) for selecting intestinally carried extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales have been reported and analysed [10, 11]. During 2011–2014, the emergence of the first plasmid-mediated polymyxin resistance mechanism in Enterobacterales, mcr-1, was reported in China [12]. However, knowledge concerning the prevalence of Ec containing mcr genes is still largely incomplete due to a lack of recent studies, especially in South-East Asia. A high prevalence of mcr genes has been found in Laos [13], and the prevalence of mcr-1-positive E. coli (MCRPEC) has been reported to range from 3.7–32.7 % (average 15.0 %) in humans in China [14]. Thailand has been categorized as having a low rate of colistin-resistant E. coli (Col-R-Ec) [15], but more recent human and animal data are urgently needed.
Two important necessities for ‘One Health’ studies investigating the essential dynamics of the transmission of multidrug-resistant pathogens are a) the molecular characterization of AMR isolates and b) identifying the epidemiological links between humans, animals, and the environment. In this ‘One Health’ study, samples from animals, humans, and rodents living around those farms were collected in northern Thailand. Samples from rodents were considered as being representative for one aspect from the environment.
Our objective was to determine the prevalence of Ec with ESBL and mcr genes in pig and chicken farms. In addition, we aimed at scrutinizing the transmission dynamics of these isolates by whole genome sequencing (WGS) in very small- and small-sized farms, and the epidemiology of circulating plasmids containing mcr-1.
Methods
Study design and sample collection
The study’s overall design and samplingwere published previously [16]. Briefly, by using a cross-sectional design, human and animal samples were collected from very small- and small-sized pig and poultry farms (1–20 and 20–100 animals per farm, respectively) where animals lived in close contact with farmworkers. Up to four human faecal samples and up to 10 rectal swabs from animals were collected from each farm visited, and rodents were trapped nearby to collect their intestinal contents. Importantly, we also included samples from a human control population with no occupational contact with animals to compare the population structure of the E. coli isolates among different human groups. Questionnaires with metadata and AMU information in farms were administered to the participants. The study was undertaken during 2018 and 2019. Mahidol University’s Ethics Committee for the Faculty of Tropical Medicine approved the collection of biological samples and data from humans (Certification number: MUTM-2018-035-01). Kasetsart University’s Scientific Research Committee gave ethical approval for the animal section (ACKU 62-VTN-010) of this study.
Selecting ESC-R-Ec and Col-R-Ec for molecular characterization of resistance genes
ESC-R and Col-R Enterobacterales were isolated from pig and chicken rectal swabs, small wild rodents’ intestinal contents, and human stool samples (farmers and non-farmers) and partially characterized as described [17]. Importantly, each sample was tested independently for both ESC-R and Col-R by inoculation onto a MacConkey agar plate supplemented with ceftriaxone (2 µg ml−1) and onto a MacConkey agar plate supplemented with colistin (1 µg ml−1) to detect antibiotic resistant Gram-negative bacteria [17]. All isolates were also tested for carbapenemase producers but they were non-existent. For this study, we only included ESC-Ec and Col-R-Ec which was the vast majority of received isolates and excluded K. pneumoniae, Enterobacter spp. and Citrobacter spp. According to CLSI guidelines, we considered an isolate as ESC-R-Ec or Col-R-Ec if MIC>=4 mg l−1 for ceftriaxone or MIC>=4 mg l−1 for colistin, respectively. In addition, we also found small numbers of Ec with >=2 mg l−1 but <4 mg l−1 for ceftriaxone (n=10; 2.5 %) and for colistin (n=9; 3.0 %). It was found, that, after genomic analyses (see below), all of these isolates also possessed ESBL and/or mcr genes and, therefore, these isolates were also included in the study. We used E. coli ATCC 25922 as a control strain in order to determine if the phenotyping test results of the tested isolates were valid.
Whole-genome sequencing (WGS) analyses and prevalence analyses of Ec with ESBL and/or mcr genes
Genomic DNA of E. coli isolates was extracted using QIAamp DNA Mini Kit with a QIAcube robot. The median quantity of DNA sent for sequencing was 5.5 µg and the ranges were 2–25 µg. This was initially quantified by Nanodrop but subsequently remeasured with Qubit. Additional purification after Qubit measurement was performed if deemed necessary. Isolates were sequenced with Illumina PE150 (paired-end) the specifications were Q30≥80 %. For the sequencing, a 350 bp insert DNA library was used and it was aimed at getting 100 X per sample. Quality control of the sequence data was performed with FastQC (Version 11.8). The summary of FastQC reports was performed with MultiQC (Version 1.6) [18]. The results of quality control were consistent with the reports of the sequencing facility.
Genomes were assembled (de novo assembly) using the default parameters of SPAdes genome assembler v3.14.0 [19]. Resistance genes were identified using ResFinder software v3.2 [20]. The output for ESBL and/or mcr genes was then 'manually' inspected and their presence was defined if there was >98 % sequence identity as recommended [21] and >95 % gene coverage. Multilocus sequence typing (MLST) characterizations for Ec were taken from the Centre for Genomic Epidemiology website (https://cge.food.dtu.dk/services/MLST/ accessed in October 2021).
For the calculation of prevalence of resistance genes, each ESBL and/or mcr-gene was only counted once per sample. Prevalence calculations were done for six different categories. This included a) samples with ESBL-Ec, b) samples with Ec with mcr genes, c) samples with ESBL-Ec only, d) samples with Ec with mcr only, e) samples with Ec with ESBL and mcr (different isolates) and f) samples with Ec with ESBL and mcr (same isolate).
The prevalence data from chicken and pig farms were compared using chi-square tests and a P-value less than 0.05 was considered as being statistically significant. Accession number for the sequencing reads is PRJNA707214.
Sequence analyses for investigating population heterogeneity
The subsequent analyses excluded all the isolates in which we found no ESBL or mcr genes. In addition, we also excluded non-Ec isolates and those that were received in duplicates (i.e. identical isolates). The assembled genomes were filtered using several parameters: number of contigs, genome coverage and contaminations. The number of contigs and genome coverage were assessed using the Quast tool, v5.0.2 [22]. For Quast quality control, a reference E. coli genome (accession number GCA_000005845.2) was used. Only samples with assemblies of fewer than 1200 contigs were used for core genome analysis. The fraction of coverage of the E. coli reference genome was set to 80 % to ensure that only Ec isolates were analysed (Fig. S1a, b, available in the online version of this article). Data was also inspected using the CheckM tool, v1.1.2 [23]. Based on using a set of marker genes of reference genomes, if the genome of our study was not assigned to the correct 'marker lineage' (i.e. Enterobacteriaceae), it was removed.
In total, 463 Ec isolates passed all the above-mentioned quality checks and were used for subsequent analyses. The concatenated alignment of the genes shared by ≥99 % of all the isolates (the core genome) was constructed using Roary, v3.11.0 [24]. The phylogenetic tree was produced using FastTree v2.1.10 [25]. Core genome single nucleotide variants (cgSNVs) were extracted from this concatenated alignment using SNP-sites software, v2.5.1 [26]. Sequence clusters (SCs) were identified using the FastBaps algorithm [27]. A core genome tree was created using the ggtree package, v2.4.2, in R software, v4.0.5.
Long-read sequencing, hybrid assembly of short and long reads and definition of clonal transmission
We also performed Nanopore sequencing to define the genetic backgrounds of ESBL and mcr genes for a subset of isolates. To investigate potential 'clonal' transmission events between animal to humans, we selected all the isolates with identical sequence types (STs) in humans and animals. We have previously described our long-read sequencing protocol [28]. In brief, isolates’ DNA were multiplexed, barcoded, and sequenced using a GridIon system. We then performed a hybrid assembly (of short- and long-read sequencing reads) using Unicycler v.0.4.8 [29]. The plasmids were received as fully closed contigs, annotated and compared using the blast Ring Image Generator [30].
A concatenated alignment of the genes shared by ≥99 % of all hybrid assemblies was constructed using Roary v3.11.0 and a phylogenetic tree was received with FastTree v2.1.10. Subsequently, cgSNVs were extracted using SNP-sites software, v2.5.1 and the differences i.e. ΔcgSNV between isolates were calculated from the alignment file created by Roary. Ultimately, clonal transmission was defined if pairwise cgSNVs differences were ≤99 as recently suggested [31] and identical plasmids containing identical resistance genes identified.
Analyses for investigating the position of mcr-1.1
By solely assembling short reads and subsequently investigating the resulting contigs, it is often difficult to define the correct genetic background of bla and/or mcr genes. Additional long read sequencing is recommended but quite costly to do for all isolates. We therefore followed a 'read mapping' approach which has been recently shown by Shen et al. [32] and focused on mcr-1.1. In more detail, the position of the mobilized colistin-resistance gene (mcr-1.1) was assessed in three different ways. First, we defined the contig on which the mcr-1.1 gene was located using the ResFinder database [20]. The contig containing the mcr-1.1 gene was selected using SAMtools utilities, v1.10 [33] and annotated using the blast. If the contig was longer than 10 kb and defined by blast as a ‘genome’, then that mcr-1.1 gene was defined as chromosomal for that sample. The second way involved annotating the contig with the mcr-1.1 gene using the PlasmidFinder, v2.1 [34], if it was longer than 10 kb. The third type of analysis was used for samples where the contig with the mcr-1.1 gene was shorter than 10 kb. For this, we first defined a set of reference plasmids from our data and retrieved some additional plasmids from a study by Shen et al. [32] (Table S2). All the samples’ reads were then mapped to these reference plasmids using SAMtools utilities, and the best mapped plasmid was selected [highest (maximum) coverage]. Those plasmids’ Inc-types were defined according to their sample if the coverage with the reference plasmid was greater than 60 %.
Results
Prevalence of Ec with ESBL and/or mcr genes in samples from pig and chicken farms
Doing short read sequencing, we analysed the molecular mechanisms of ESC-R-Ec and/or Col-R-Ec isolates (n=638) from human (69 pig farmers, 155 chicken farmers and 61 non-farmers), farm animal (269 pigs and 318 chickens), and rodent samples (n=196) from a northern province of Thailand. In total, 122 and 280 ESC-Ec were sequenced from 285 human and 783 animal samples, respectively. We found an ESBL gene in 114 (93.4 %) human and 267 (88.8 %) animal ESC-R-Ec. We also sequenced 298 Col-R-Ec from the total of 1068 samples (Table S3).
The overall prevalence of samples having Ec with ESBL and/or mcr genes among human participants was generally >30 % (Table 1). We identified higher rates of ESBL-Ec isolates among pig farmers (n=36; 52.2%) than among chicken farmers (n=58; 37.4 %; Χ2=4.3; P<0.05) and the control group (n=61; 31.1 %; Χ2=5.8; P<0.05) (Table 1). In contrast, we found no significant association (difference between pig farmers, chicken farmers and non-pig farmers) for Ec with mcr. Furthermore, we found higher ESBL-Ec and mcr-Ec rates among pigs than among chickens (n=164; 61.0 % versus n=35; 11.0 % for ESBL-Ec; P<0.001 and n=112; 41.6 % versus n=27; 8.5 % for mcr-Ec; P<0.001) (Table 1). Based on information related to AMU in farms, penicillin and streptomycin and amoxicillin are often given to sows after the farrowing which may select for a high prevalence of ESBL-Ec in these pig farms (Table S4).
Table 1.
Molecular characterization of samples with ESC-R-Ec and Col-R-Ec isolates with ESBL and/or mcr genes
|
Sample origin |
Farm size |
N |
Samples with ESBL-Ec |
Samples with Ec with mcr genes |
Samples with ESBL-Ec only |
Samples with Ec with mcr only |
Samples with Ec with ESBL and mcr (different isolates)* |
Samples with Ec with ESBL and mcr (identical isolate)† |
Total‡ |
|---|---|---|---|---|---|---|---|---|---|
|
Human Farmers |
|||||||||
|
Pig farmers |
69 |
36 (52.2 %) |
22 (31.9 %) |
21 (30.4 %) |
7 (10.1 %) |
8 (11.6 %) |
7 (10.1 %) |
43 (62.3 %) |
|
|
Chicken farmers |
155 |
58 (37.4 %) |
48 (31.0 %) |
26 (16.8 %) |
16 (10.3 %) |
24 (15.5 %) |
8 (5.1 %) |
74 (47.7 %) |
|
|
Control group |
61 |
19 (31.1 %) |
15 (24.6 %) |
10 (16.4 %) |
6 (9.8 %) |
7 (11.5 %) |
2 (3.3 %) |
25 (41.0 %) |
|
|
Total |
285 |
113 (42.0 %) |
75 (26.3 %) |
57 (20.0 %) |
29 (10.25) |
39 (13.7 %) |
17 (6.0 %) |
142 (49.8 %) |
|
|
Animals |
|||||||||
|
Pigs |
Very small |
169 |
99 (58.6 %) |
71 (42.0 %) |
46 (27.2 %) |
18 (10.7 %) |
18 (10.7 %) |
35 (20.7 %) |
117 (69.2 %) |
|
Pigs |
Small |
100 |
65 (65 %) |
41 (41 %) |
35 (35 %) |
11 (11 %) |
15 (15 %) |
15 (15 %) |
76 (76 %) |
|
Total |
269 |
164 (61.0 % |
112 (41.6 %) |
81 (30.1 %) |
29 (10.8 %) |
33 (12.3 %) |
50 (18.6 %) |
193 (71.7 %) |
|
|
Chickens |
Very small |
218 |
17 (7.8 %) |
23 (10.6 %) |
2 (0.9 %) |
8 (3.7 %) |
1 (0.5 %) |
14 (6.4 %) |
25 (11.4 %) |
|
Chickens |
Small |
100 |
18 (18 %) |
4 (4 %) |
17 (17 %) |
3 (3 %) |
0 |
1 (1 %) |
21 (21 %) |
|
Total |
318 |
35 (11.0 %) |
27 (8.5 %) |
19 (6.0 %) |
11 (3.5 %) |
1 (0.3 %) |
15 (4.7 %) |
46 (14.4 %) |
|
|
Rodents |
None |
196 |
4 (2.0 %) |
4 (2.0 %) |
3 (1.5 %) |
3 (1.5 %) |
0 |
1 (0.5 %) |
7 (3.6 %) |
*Each sample was screened independently for Col-R-Ec and ESC-R-Ec. Therefore, the detection of multiple isolates in samples was feasible. Each isolate has been sequenced.
†This could be as the genes are a) on the same plasmid, b) one on the chromosome and one on a plasmid or c) on two different plasmids.
‡'Total' includes all samples with Ec with mcr and/or ESBL genes.
We found no significant associations between rates of Ec with ESBL or mcr genes and different sizes of pig farms. In contrast, significant differences in prevalences of ESBL-Ec were found between small-sized and very small-sized chicken farms (18 % versus 7.8 % Χ2=5.6; P<0.05) (Table 1). The prevalence of Ec with ESBL or mcr genes in rodents were low (around 2 %; Table 1).
Detection of strain heterogeneity in samples
We next investigated the absence or presence of strain heterogeneity in human and animal samples. For doing this, we defined four additional categories in Table 1 (i.e. samples with Ec with ESBL and mcr found in different or same isolates). As for non-heterogenic samples we found significantly more samples with Ec with ESBL but no mcr genes in pig farmers (31.9 %) as compared to chicken farmers (14.2 %) and the control group (16.4 %) (P<0.05; P<0.05). There were also significantly more samples with isolates having co-occurring ESBL and mcr genes in pigs (n=50; 18.6 %) as compared to humans (n=17; 6.0 %) (P<0.05). As for heterogenic samples, we identified 38 (13.3 %) human and 33 (12.3 %) pig samples with non-identical Ec containing ESBL and mcr. This indicates a considerable Ec strain heterogeneity in human and pig samples of Thailand. However, of note, we did not additionally conduct non-selective growth on MacConkey to gauge a systematic understanding of the full diversity of Ec-types within the animal and/or human populations.
Characterization of ESBL and mcr genes in samples from pig and chicken farms
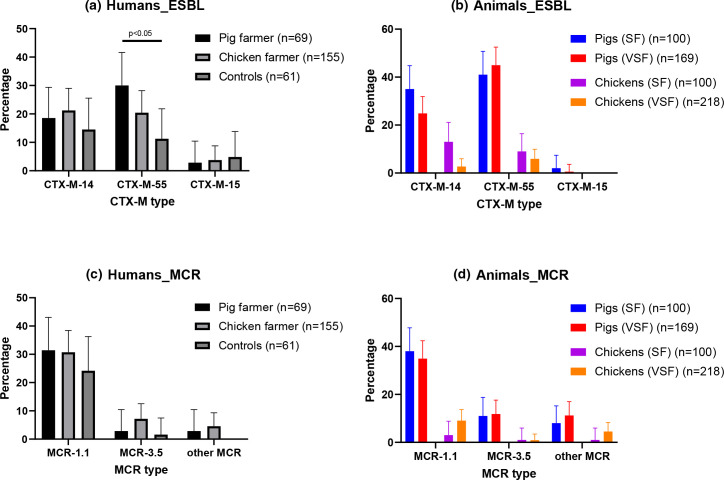
As for the in-depth characterization of ESBL genes, we exclusively found bla CTX-M but no bla TEM and/or bla SHV. Overall, we identified a significantly higher rate of bla CTX-M-55 among pig farmer (n=21; 30.4%) than among non-pig farmer samples (n=34; 15.7 %, Χ2=6.3, P=0.01; Fig. 1a). In addition, bla CTX-M-55 was the most frequent ESBL gene in pigs’ rectal swabs (Fig. 1b). As for the mcr-genes, the mcr-1.1 gene was the most frequent mcr gene in all three human groups with no significant differences among the groups (Fig. 1c). As expected, mcr-1.1 was also highly prevalent in pig but not chicken rectal swabs, but other mcr genes were also detected (Fig. 1d).
Fig. 1.
Prevalence of Ec with ESBL and mcr genes in humans and animals. The prevalence of samples with Ec with the three most frequent bla ESBL genes (CTX-M-14, CTX-M-55, and CTX-M-15) are shown in pig farmers, chicken farmers, and non-farmers (control) (Fig. 1a). The numbers for animal samples are illustrated for small- (SF) and very small-sized farms (VSF) (Fig. 1b). The prevalence of samples with Ec with mcr genes (MCR-1.1, MCR-3.5, and other mcr) are also shown for humans (Fig. 1c) and animals (Fig. 1d). The 95 % confidence intervals are indicated. Statistical significances were determined using a chi-square test.
Population structure of Ec with ESBL and/or mcr genes using whole-genome sequencing
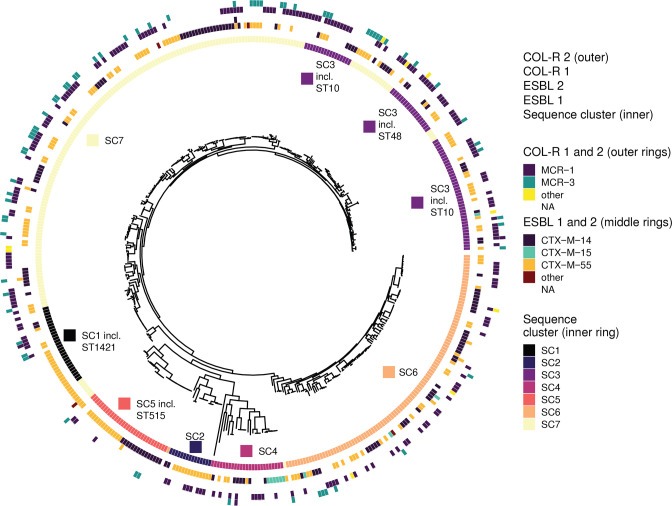
We then discarded Ec which had no ESBL or mcr genes and isolates with low quality sequencing data for population structure analyses. In total, we used 463 (72.6 %) assembled genomes of sufficiently high quality to perform core genome phylogenetic analyses. We identified 146 distinct STs, with ST10 (n=37), ST515 (n=21), and ST48 (n=18) being the most prevalent, detected in both humans and animals. We then investigated the core genome population structure across all the isolates. We obtained 2359 core genes for >99 % of the isolates. We also obtained 256 713 cgSNVs, which we clustered into eight major sequence clusters (Fig. 2). We found that the majority of isolates only possessed one resistance gene (ESBL or mcr), but there were also isolates having two or more of the resistance genes coding for the identical resistance phenotypes. To illustrate the latter, we found that mcr-3-variant genes were not found as a single resistance gene but rather jointly with a mcr or bla gene in their isolates and that this is independent of the STs (Fig. 2). Short read sequencing data is not able to resolve if the genes are a) on the same plasmid, b) one on the chromosome and one on a plasmid or c) on two different plasmids. However, for at least one isolate, co-occurrence of mcr 3.5 and CTX-M-55 have been found on an IncP1 plasmid as data from short (Illumina) and long (Nanopore) sequencing technologies were available (see results below). Sequence clusters were neither host-specific nor correlated with farm size (very small versus small-sized farms) (Fig. S2).
Fig. 2.
Population structure of Ec with ESBL and mcr genes from Thailand. Phylogeny of Ec isolates was obtained from an alignment of 2359 core genes. We defined eight major sequence clusters (inner ring) and identified 146 distinct sequence types (ST). The most frequently detected STs were ST10 (n=37), ST515 (n=21) and ST48 (n=18) and their SC affiliations are shown. The different ESBL (middle rings) and mcr genes (outer rings) are also indicated.
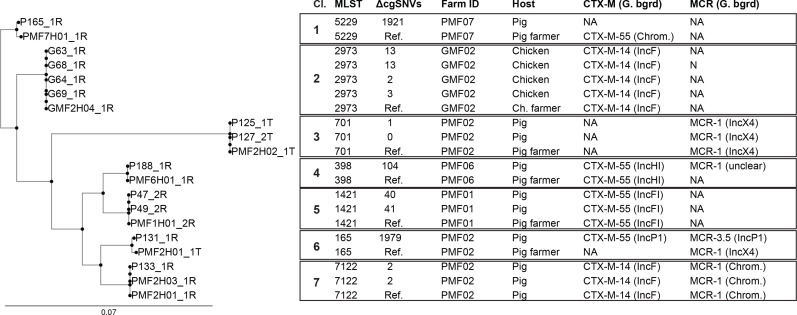
‘Clonal’ transmission of Ec isolates with ESBL and/or mcr genes
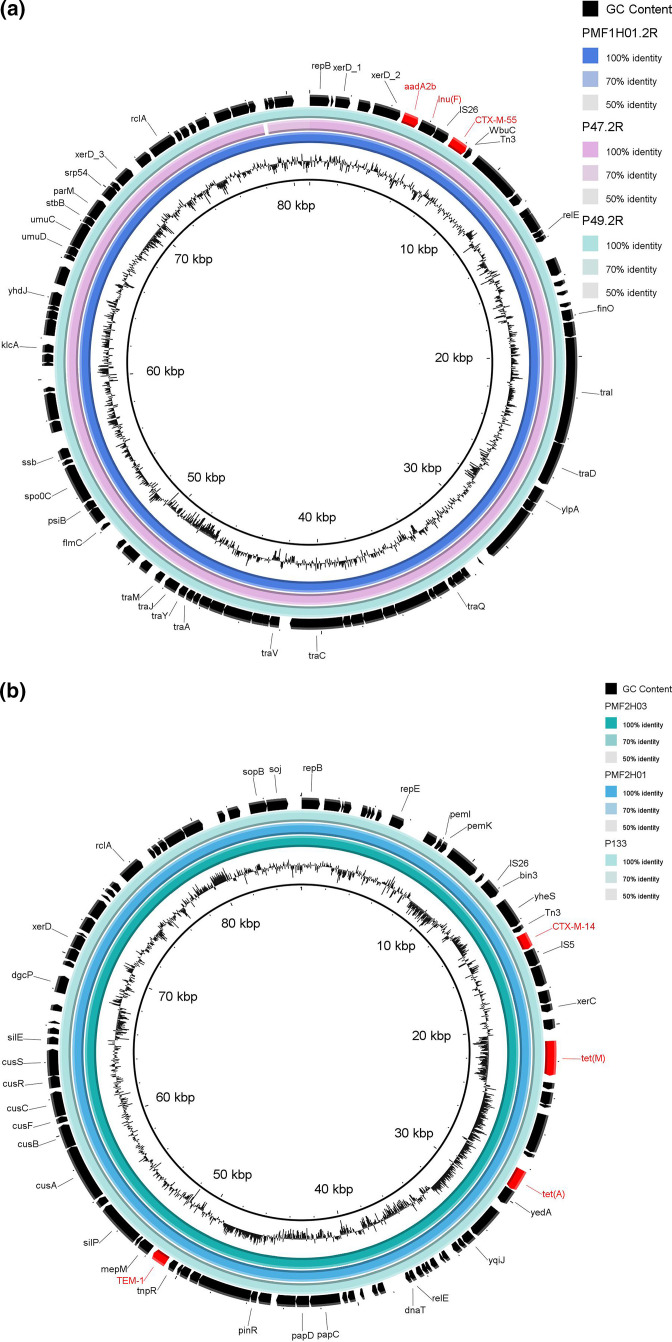
We then more thoroughly investigated the isolates with identical STs originating from humans and animals from the same farm to investigate transmission. The core genome analysis of these isolates is illustrated as a phylogenetic tree in Fig. 3. We identified seven potential animal-to-human (or vice versa) transmission clusters, with three of them occurring in the same farm (PMF2). However, we additionally performed long-read sequencing with subsequent hybrid assembly for all these isolates in order to study in-depth the 'clonal' transmission and the genetic background of the ESBL and mcr genes. We found a single plasmid containing bla CTX-M-14 among all the isolates within cluster two. The resistance gene in cluster five, bla CTX-M-55, was found on the identical IncFI plasmid in two pigs and one pig farm worker (Fig. 4a). bla CTX-M-55 was also identified in the large (228 400 bp) IncHI1 plasmid in one pig and one pig farm worker from cluster four (Fig. S3A). However, despite using long-read sequencing, we were unable to locate the mcr-1 gene in pig P188_1R. Finally, clusters 3, 6, and 7 were all found in the small-sized pig farm two (PMF02; Fig. 3). We revealed that the mcr-1 gene for cluster seven’s isolates was located on the chromosome (Fig. S3B), whereas bla CTX-M-14 was found on the IncF plasmid (Fig. 4b). Considering ΔcgSNVs of ≤99 to define clonal transmission [31], this has been recovered in clusters 2, 3, 5 and 7. We did not find transmission in cluster six but identified one isolate with mcr 3.5 and CTX-M-55 being located on a single IncP1 plasmid.
Fig. 3.
Analyses of Col-R-Ec and ESBL-Ec transmission events in Thailand. All isolates from small chicken (GMF) and pig (PMF) farms for which identical STs were found in both humans and animals were selected. Seven clusters (Cl.) of isolates with identical STs were found. The core genome phylogeny of the isolates’ fully closed genomes is plotted (left side) as are the bla ESBL and mcr genes. Pair wise core genome Single Nucleotide Variant (cgSNV) differences are shown as compared to the 'reference' (Ref.) strain in each cluster. The genomic backgrounds (G. bgrd) of the bla ESBL and mcr genes were identified thanks to additional long-read sequencing and subsequent hybrid assembly. Hybrid assembly analyses combined with epidemiological linkage enabled the definition of transmission from humans to animals (or vice versa).
Fig. 4.
Characterization of plasmids of Ec isolates using long- and short-read sequencing (hybrid assembly). The resistance gene (bla CTX-M-55) of cluster five from Fig. 3 was found on the identical IncFI plasmid for the three isolates P49_2R, PMF1H01_2R and P47_2R (PMF01) (Fig. 4a). The bla CTX-M-14’s IncF plasmid were identified in two pig farmers and one pig from farm ID PMF02 (Fig. 4b). The plasmids’ hybrid sequences are visualized using the blast Ring Image Generator. AMR genes are indicated in red.
Defining the genetic background of mcr-1.1
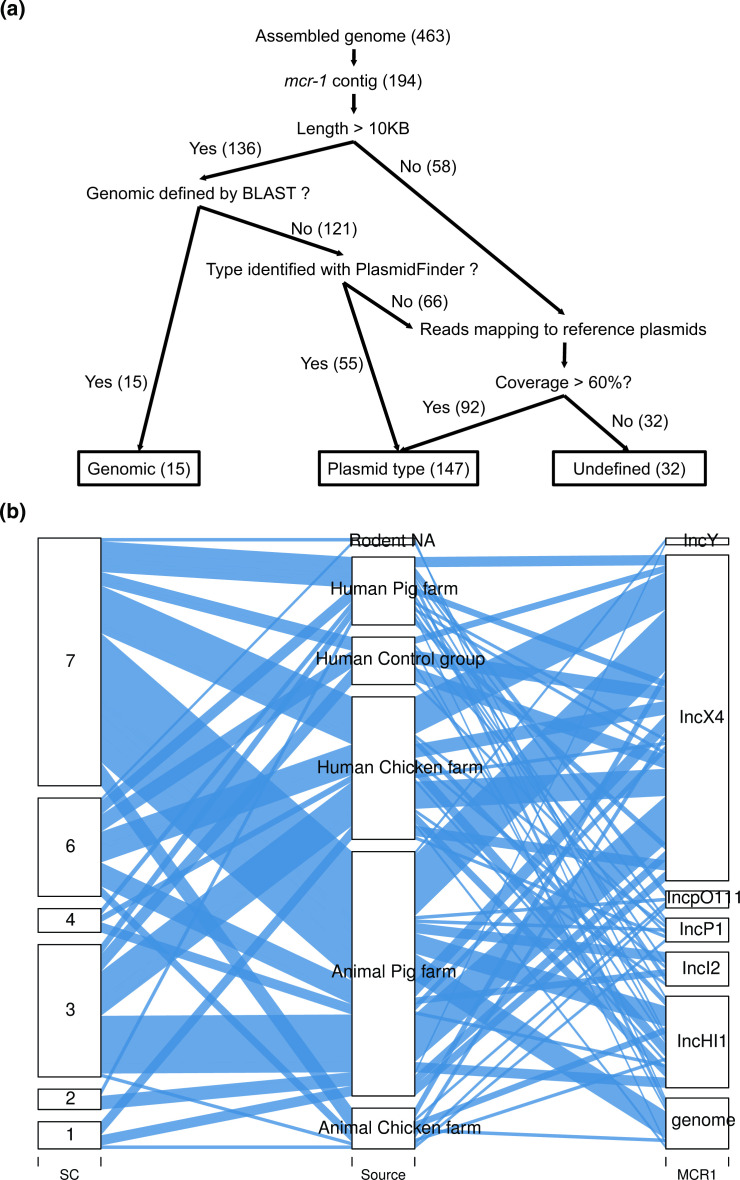
As mcr 1.1 was the most frequent mcr-gene and in order to investigate if this gene is primarily located on well-known conjugative plasmids, we subsequently inspected the genetic background of 194 Ec isolates with mcr-1.1 by a mapping approach. The plasmid or chromosomal location of mcr-1.1 was identified in 162 Ec isolates (83.5 %) (Fig. 5a and Table S2). A chromosomal location was revealed in 15 (7.7 %) of 194 isolates containing mcr-1.1. The proportions of Ec with mcr-1.1 isolates located in the IncX4 (46; 59.9 %), IncHI1 (13; 17.9 %), and IncI2 (5; 6.4 %) plasmids in human samples were comparable to those in animal samples, with IncX4 (50; 59.5 %), IncHI1 (13; 15.5 %), and IncI2 (5; 6.0 %) (Fig. 5b). The sequence clusters were not associated to specific plasmids containing the mcr-1.1 gene. Taken together, neither sequence clusters nor plasmids containing mcr-1.1 were associated to a distinct host or source in this study.
Fig. 5.
Analyses of Genetic Background of mcr-1.1. The position of the mobilized colistin-resistance gene (mcr-1.1) was assessed in three different ways (Fig. 5a). First, we defined the contig on which the mcr-1.1 gene was located. Second, we annotated the contig with the mcr-1.1 gene using the PlasmidFinder, if it was >10 kb. Third, we mapped the samples’ reads to the reference plasmids (see main text for details). The alluvial plot is illustrating plasmids containing the mcr-1.1 gene (right column) and the isolates’ origins (middle column) (Fig. 5b). The sequence clusters (SC) are illustrated in the left column.
Discussion
The present study investigated the prevalence of Ec with ESBL and/or mcr genes in pigs, chickens, rodents, pig farmers, chicken farmers, and non-farmers in Thailand. In addition, we reported on clonal transmission events between animals and humans by co-analysing epidemiological links and WGS data. However, we speculate that horizontal transmission is more frequent as indicated by the found heterogenous population structure of Ec in our study.
Similarly to our study which found that ESBL-Ec carriage was associated with being a pig farmer, human ESBL carriage was found to be associated with average number of hours working on the farm per week and presence of ESBLs in pigs in the Netherlands [35]. The increase of the latter was due to the enhanced prevalence of bla CTX-M-55, which was also the most frequent ESBL gene in pigs and pig farmers in our study. In contrast, an analysis of Ec in Vietnam showed different bla CTX-M genes in humans and pigs and, therefore, the authors concluded that ESBL-Ec from pigs or their ESBL/AmpC encoding plasmids were not commonly spread to workers in close contact with the animals [36]. In 2013, it was hypothesized that the same STs of ESC-R-Ec were being transmitted between humans and food animals and that there was also parallel, independent evolution in the two host populations of E. coli strains [1]. Considering the high heterogeneity of the STs in the E. coli strains observed in our study and that certain STs, like ST10, were being found in both humans and animals, the above hypothesis may also be plausible for our setting.
During our visits to the farms, we collected information on AMU in animals but it was difficult to obtain accurate and detailed data as farmers usually don't want to disclose that information. However, the use of a drug containing both penicillin and streptomycin and/or a drug containing amoxicillin has been reported in almost all very-small pig farms. These drugs are used to prevent postpartum infections in sows and may act as selective pressure for the emergence of Ec with ESBL genes in pig farms. In contrast, the use of penicillin and streptomycin were reported in only one chicken farm while the use of erythromycin seemed to be very frequent in very small chicken farms but certainly without acting on the selection of Ec with ESBL genes.
As for colistin, Thailand approved a ban on using this antibiotic medication as a feed additive and for the prevention and control of infection in food animals in 2017 [37]. However, there are strong suggestions that farmers do not comply with these regulations, as a recent study revealed that 94 % of 51 small-sized pig farms in Thailand still used antibiotics like colistin [38]. Interestingly, we found that the prevalence of Ec with mcr genes was comparable in pig farmers, chicken farmers, and non-farmers. This was remarkable considering that the prevalence of Ec with mcr genes was significantly higher in pigs than in chickens. The high carriage of Ec with mcr genes in our control group (24%) indicated that sources other than pig farming could be relevant for acquiring Ec with mcr genes. Alternatively, humans may acquire such AMR bacteria by consuming contaminated meat or food animal products or from direct contact with live animals, although contamination rates from fresh food with Col-R bacteria in Thailand have been found to be low [39].
The predominant STs containing mcr-1 belonged to clonal complex 10 (i.e. ST10, ST48, and ST7122). Generally, the observed population structure is comparable to that seen in China [32]. As for the plasmids, we frequently identified IncX4, but we also found IncI2, IncP1, and IncH1 plasmids containing mcr-1 which have been found to be conjugative [40]. It was recently hypothesized that the efficient transfer of the IncX4 plasmid at different temperatures might indicate its ability to be an important vehicle for disseminating AMR bacteria [41] and that IncX4 is relevant for the dissemination of the mcr-1 gene along the food chain and in humans [42, 43]. Unfortunately, due to the low genetic heterogeneity of circulating IncX4 [13], it is difficult to undoubtedly prove horizontal transmission of IncX4 plasmids from animals to humans even if WGS and epidemiological linkage data of isolates are available like in our study.
Data from China have shown that the withdrawal of colistin reduced the incidence of Col-R-Ec, more specifically, the IncX4-type plasmids harbouring mcr-1 [32] which have been reported to have little genetic variability [43]. Considering the striking similarities between Col-R-Ec epidemiology in Thailand and that reported in China before the ban on colistin use in animal feed, we speculate that full compliance with regulations on the veterinary use of colistin in Thailand would also lead to a rapid, ecosystem-wide decline in Col-R-Ec, especially Ec with mcr genes. However, we also observed a high proportion of co-occurrence of ESBL and mcr genes in Ec in our study, and, therefore, it could be possible that a ban of colistin alone may not be as effective as in China. Co-occurrence has also been reported in pig samples from Egypt [44]. Interestingly, studies reporting co-occurrence mainly mention ESBL and mcr-1 [40, 45, 46] while we detected a particular high co-occurrence of ESBL with mcr-3.5 genes.
One strength of our study is the reporting of detailed molecular mechanisms and epidemiological links of ESC-R-Ec and/or Col-R-Ec.s. Importantly, the samples were collected from animals from different sizes of farms, farmers, non-farmers, and rodents in the same northern province in Thailand. One limitation of our study was that we did not fully explore all the possibilities of 'clonal' animal to human transmission of strains. Indeed, with few exceptions, we selected only one or two colonies per plate for ESC-R-Ec and/or Col-R-Ec. Due to the high workload involved, we also decided to focus on the epidemiologically important Ec with ESBL and/or mcr genes and, therefore, we did not investigate other resistance mechanisms. In addition, our study design has not been longitudinal. It is known that carriage duration of e.g. ESC-R-Ec is limited within both people and pigs [28, 47]. Therefore, detection of clonal transmission might be missed if the transmitted Ec isolates have been lost at the time of the visit or the relevant plasmid has been transferred to another Ec isolate in the human gut.
In conclusion, we found that the prevalences of ESBL-producing-Ec and mcr-producing-Ec were very high in the animals and humans and that ESBL-Ec carriage was associated with the occupation of pig farmer in northern Thailand. We also detected clonal transmission from animals to humans but this has probably happened infrequently as indicated by the heterogenous population structure of Ec isolates. Therefore, 'horizontal' transmission of highly prevalent plasmids like IncX4 is more likely. We identified compelling similarities in the epidemiology and prevalence of the circulating plasmids containing mcr-1 in Ec isolated from animals and humans in Thailand and China, especially when compared to the situation before colistin’s withdrawal in China and the latter should probably also be more encouraged in Thailand. However, the high proportions of co-occurrence of ESBL and mcr genes, especially mcr-3.5, in human and animal samples are of great concern and may more and more jeopardize the success of AMU reduction programmes, especially if exclusively focused on colistin.
Supplementary Data
Funding information
The research was funded by the Swiss National Science Foundation (Southeast Asia – Europe Joint Funding Scheme for Research and Innovation, grant IZJFZ3-177614 to AO, MH, VT, JMR and SM); Thailand Centre of Excellence for Life Sciences (TCELS) and National Research Council of Thailand (NRCT) to VT and DS; AK, KC and SM report funds from the French ANR FutureHealthSEA (ANR-17-CE35-0003-02); CT and SM receive support from the Thailand International Cooperation Agency (TICA) project ‘Innovative Animal Health’.
Acknowledgements
We thank the help of the Livestock Department and the Department of Health, province of Nan (Thailand).
Author contribution
D.S., N.M., V.T., J.M. R., S.M., A.O. and M.H. were investigators in this study and contributed to the study design. D.S., S.T., R.A. and S.A.B. performed laboratory work and data curation. D.S., K.C., A.K., C.T., V.T. and S.M. were involved in sample acquisition and project administration resources. D.S., N.M. and M.H. performed (sequencing) data analyses and visualization. V.T., J. M. R., S.M., A.O. and M.H. were responsible for funding acquisition and supervision. D.S., N.M., A.O. and M.H. were writing the original draft. All authors contributed to the writing, review and editing of the manuscript.
Conflicts of interest
All authors declared no conflict of interests.
Ethical statement
Mahidol University’s Ethics Committee for the Faculty of Tropical Medicine approved the collection of biological samples and data from humans (Certification number: MUTM-2018-035-01). Kasetsart University‘s Scientific Research Committee gave ethical approval for the animal section (ACKU 62-VTN-010) of this study. All individuals have given oral informed consent to participate in the study.
Consent to publish
Not applicable
Footnotes
Abbreviations: AMR, antimicrobial resistiance; AMU, antimicrobial usage; cgSNV, core genome single nucleotide variant; Col-R, colistin-resistance; Ec, Escherichia coli; ESBL, extended-spectrum beta-lactamase; ESC-R, extended-spectrum cephalosporin-resistance; mcr, mobile colistin resistance.
All supporting data, code and protocols have been provided within the article or through supplementary data files.
Three supplementary figures and four supplementary tables are available with the online version of this article.
References
- 1.Seiffert SN, Hilty M, Perreten V, Endimiani A. Extended-spectrum cephalosporin-resistant Gram-negative organisms in livestock: an emerging problem for human health? Drug Resist Updat. 2013;16:22–45. doi: 10.1016/j.drup.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Smyth C, Leigh RJ, Delaney S, Murphy RA, Walsh F. Shooting hoops: globetrotting plasmids spreading more than just antimicrobial resistance genes across One Health. Microb Genom. 2022;8:mgen000858. doi: 10.1099/mgen.0.000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amuasi JH, Lucas T, Horton R, Winkler AS. Reconnecting for our future: the lancet one health commission. Lancet. 2020;395:1469–1471. doi: 10.1016/S0140-6736(20)31027-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludden C, Raven KE, Jamrozy D, Gouliouris T, Blane B, et al. One health genomic surveillance of Escherichia coli demonstrates distinct lineages and mobile genetic elements in isolates from humans versus livestock. mBio. 2019;10:e02693-18. doi: 10.1128/mBio.02693-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuya-Kanamori L, Stone J, Yakob L, Kirk M, Collignon P, et al. Risk factors for acquisition of multidrug-resistant Enterobacterales among international travellers: a synthesis of cumulative evidence. J Travel Med. 2020;27:taz083. doi: 10.1093/jtm/taz083. [DOI] [PubMed] [Google Scholar]
- 6.Pitout JDD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8:159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 7.Kiratisin P, Chattammanat S, Sa-Nguansai S, Dansubutra B, Nangpatharapornthawee P, et al. A 2-year trend of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in Thailand: an alert for infection control. Trans R Soc Trop Med Hyg. 2008;102:460–464. doi: 10.1016/j.trstmh.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Olaitan AO, Diene SM, Kempf M, Berrazeg M, Bakour S, et al. Worldwide emergence of colistin resistance in Klebsiella pneumoniae from healthy humans and patients in Lao PDR, Thailand, Israel, Nigeria and France owing to inactivation of the PhoP/PhoQ regulator mgrB: an epidemiological and molecular study. Int J Antimicrob Agents. 2014;44:500–507. doi: 10.1016/j.ijantimicag.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Liu Z, Yao Z, Fan Y, Ye X, et al. The prevalence and influencing factors of methicillin-resistant Staphylococcus aureus carriage in people in contact with livestock: a systematic review. Am J Infect Control. 2015;43:469–475. doi: 10.1016/j.ajic.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Luvsansharav U-O, Hirai I, Nakata A, Imura K, Yamauchi K, et al. Prevalence of and risk factors associated with faecal carriage of CTX-M β-lactamase-producing Enterobacteriaceae in rural Thai communities. J Antimicrob Chemother. 2012;67:1769–1774. doi: 10.1093/jac/dks118. [DOI] [PubMed] [Google Scholar]
- 11.Niumsup PR, Tansawai U, Na-Udom A, Jantapalaboon D, Assawatheptawee K, et al. Prevalence and risk factors for intestinal carriage of CTX-M-type ESBLs in Enterobacteriaceae from a Thai community. Eur J Clin Microbiol Infect Dis. 2018;37:69–75. doi: 10.1007/s10096-017-3102-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y-Y, Wang Y, Walsh TR, Yi L-X, Zhang R, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. 2016;16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 13.Moser AI, Kuenzli E, Campos-Madueno EI, Büdel T, Rattanavong S, et al. Antimicrobial-resistant Escherichia coli strains and their plasmids in people, poultry, and chicken meat in laos. Front Microbiol. 2021;12:708182. doi: 10.3389/fmicb.2021.708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Y, Zhou H, Xu J, Wang Y, Zhang Q, et al. Anthropogenic and environmental factors associated with high incidence of mcr-1 carriage in humans across China. Nat Microbiol. 2018;3:1054–1062. doi: 10.1038/s41564-018-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malchione MD, Torres LM, Hartley DM, Koch M, Goodman JL. Carbapenem and colistin resistance in Enterobacteriaceae in Southeast Asia: review and mapping of emerging and overlapping challenges. Int J Antimicrob Agents. 2019;54:381–399. doi: 10.1016/j.ijantimicag.2019.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Sudatip D, Chasiri K, Kritiyakan A, Phanprasit W, Thinphovong C, et al. A One Health approach to assessing occupational exposure to antimicrobial resistance in Thailand: the farmresist project. PLoS One. 2021;16:e0245250. doi: 10.1371/journal.pone.0245250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudatip D, Tiengrim S, Chasiri K, Kritiyakan A, Phanprasit W, et al. One health surveillance of antimicrobial resistance phenotypes in selected communities in Thailand. Antibiotics. 2022;11:556. doi: 10.3390/antibiotics11050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewels P, Magnusson M, Lundin S, Käller M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 2016;32:3047–3048. doi: 10.1093/bioinformatics/btw354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prjibelski A, Antipov D, Meleshko D, Lapidus A, Korobeynikov A. Using SPAdes de novo assembler. Curr Protoc Bioinformatics. 2020;70:e102. doi: 10.1002/cpbi.102. [DOI] [PubMed] [Google Scholar]
- 20.Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75:3491–3500. doi: 10.1093/jac/dkaa345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, et al. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother. 2017;72:2764–2768. doi: 10.1093/jac/dkx217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikheenko A, Prjibelski A, Saveliev V, Antipov D, Gurevich A. Versatile genome assembly evaluation with QUAST-LG. Bioinformatics. 2018;34:i142–i150. doi: 10.1093/bioinformatics/bty266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page AJ, Cummins CA, Hunt M, Wong VK, Reuter S, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31:3691–3693. doi: 10.1093/bioinformatics/btv421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price MN, Dehal PS, Arkin AP. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5:e9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, et al. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom. 2016;2:e000056. doi: 10.1099/mgen.0.000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonkin-Hill G, Lees JA, Bentley SD, Frost SDW, Corander J. Fast hierarchical Bayesian analysis of population structure. Nucleic Acids Res. 2019;47:5539–5549. doi: 10.1093/nar/gkz361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moor J, Aebi S, Rickli S, Mostacci N, Overesch G, et al. Dynamics of extended-spectrum cephalosporin-resistant Escherichia coli in pig farms: a longitudinal study. Int J Antimicrob Agents. 2021;58:106382. doi: 10.1016/j.ijantimicag.2021.106382. [DOI] [PubMed] [Google Scholar]
- 29.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. doi: 10.1186/1471-2164-12-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falgenhauer L, Imirzalioglu C, Oppong K, Akenten CW, Hogan B, et al. Detection and characterization of ESBL-producing Escherichia coli from humans and poultry in Ghana. Front Microbiol. 2018;9:3358. doi: 10.3389/fmicb.2018.03358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen C, Zhong L-L, Yang Y, Doi Y, Paterson DL, et al. Dynamics of mcr-1 prevalence and mcr-1-positive Escherichia coli after the cessation of colistin use as a feed additive for animals in China: a prospective cross-sectional and whole genome sequencing-based molecular epidemiological study. Lancet Microbe. 2020;1:e34–e43. doi: 10.1016/S2666-5247(20)30005-7. [DOI] [PubMed] [Google Scholar]
- 33.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carattoli A, Hasman H. Plasmidfinder and in silico pMLST: identification and typing of plasmid replicons in Whole-Genome Sequencing (WGS) Methods Mol Biol. 2020;2075:285–294. doi: 10.1007/978-1-4939-9877-7_20. [DOI] [PubMed] [Google Scholar]
- 35.Dohmen W, Bonten MJM, Bos MEH, van Marm S, Scharringa J, et al. Carriage of extended-spectrum β-lactamases in pig farmers is associated with occurrence in pigs. Clin Microbiol Infect. 2015;21:917–923. doi: 10.1016/j.cmi.2015.05.032. [DOI] [PubMed] [Google Scholar]
- 36.Hounmanou YMG, Bortolaia V, Dang STT, Truong D, Olsen JE, et al. ESBL and AmpC β-Lactamase encoding genes in E. coli from Pig and Pig farm workers in Vietnam and their association with mobile genetic elements. Front Microbiol. 2021;12:629139. doi: 10.3389/fmicb.2021.629139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olaitan AO, Dandachi I, Baron SA, Daoud Z, Morand S, et al. Banning colistin in feed additives: a small step in the right direction. Lancet Infect Dis. 2021;21:29–30. doi: 10.1016/S1473-3099(20)30915-4. [DOI] [PubMed] [Google Scholar]
- 38.Hallenberg GS, Jiwakanon J, Angkititrakul S, Kang-Air S, Osbjer K, et al. Antibiotic use in pig farms at different levels of intensification-Farmers’ practices in northeastern Thailand. PLoS One. 2020;15:e0243099. doi: 10.1371/journal.pone.0243099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tangkoskul T, Thamthaweechok N, Seenama C, Thamlikitkul V. Antibiotic-resistant bacteria and antibiotic residue contamination in fresh raw foods sold at wholesale markets in Thailand. J Med Assoc Thai. 2021;104:654–662. doi: 10.35755/jmedassocthai.2021.04.12327. [DOI] [Google Scholar]
- 40.Sadek M, Ortiz de la Rosa JM, Abdelfattah Maky M, Korashe Dandrawy M, Nordmann P, et al. Genomic features of MCR-1 and extended-spectrum β-Lactamase-producing Enterobacterales from retail raw chicken in Egypt. Microorganisms. 2021;9:195. doi: 10.3390/microorganisms9010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo W-U, Chow K-H, Law PY, Ng K-Y, Cheung Y-Y, et al. Highly conjugative IncX4 plasmids carrying blaCTX-M in Escherichia coli from humans and food animals. J Med Microbiol. 2014;63:835–840. doi: 10.1099/jmm.0.074021-0. [DOI] [PubMed] [Google Scholar]
- 42.Irrgang A, Roschanski N, Tenhagen BA, Grobbel M, Skladnikiewicz-Ziemer T, et al. Prevalence of mcr-1 in E. coli from livestock and food in Germany, 2010-2015. PLoS One. 2016;11:e0159863. doi: 10.1371/journal.pone.0159863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zurfluh K, Nüesch-Inderbinen M, Klumpp J, Poirel L, Nordmann P, et al. Key features of mcr-1-bearing plasmids from Escherichia coli isolated from humans and food. Antimicrob Resist Infect Control. 2017;6:91. doi: 10.1186/s13756-017-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadek M, Ortiz de la Rosa JM, Ramadan M, Nordmann P, Poirel L. Molecular characterization of extended-spectrum ß-lactamase producers, carbapenemase producers, polymyxin-resistant, and fosfomycin-resistant Enterobacterales among pigs from Egypt. J Glob Antimicrob Resist. 2022;30:81–87. doi: 10.1016/j.jgar.2022.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Dhaouadi S, Soufi L, Hamza A, Fedida D, Zied C, et al. Co-occurrence of mcr-1 mediated colistin resistance and β-lactamase-encoding genes in multidrug-resistant Escherichia coli from broiler chickens with colibacillosis in Tunisia. J Glob Antimicrob Resist. 2020;22:538–545. doi: 10.1016/j.jgar.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 46.Haenni M, Poirel L, Kieffer N, Châtre P, Saras E, et al. Co-occurrence of extended spectrum β lactamase and MCR-1 encoding genes on plasmids. Lancet Infect Dis. 2016;16:281–282. doi: 10.1016/S1473-3099(16)00007-4. [DOI] [PubMed] [Google Scholar]
- 47.Pires J, Kuenzli E, Kasraian S, Tinguely R, Furrer H, et al. Polyclonal intestinal colonization with extended-spectrum cephalosporin-resistant Enterobacteriaceae upon traveling to India. Front Microbiol. 2016;7:1069. doi: 10.3389/fmicb.2016.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.