ABSTRACT
The rapid and accurate detection of viable probiotic cells in dairy products is important for assessing product quality in manufacturing. Flow cytometry is widely used for the rapid analysis of bacterial cells. However, further investigation is needed into the optimum property to use it for assessing cell viability. Here, we proposed using the efflux activity of a fluorescent dye, carboxyfluorescein (CF), as an indicator of cell viability. CF is generated from 5(6)-carboxyfluorescein diacetate as a result of cleavage by intracellular esterase. It generally accumulates in the cell, but certain bacterial species are known to extrude it. We found here that the probiotic strain Lacticaseibacillus paracasei strain Shirota (LcS) also extruded CF in the presence of energy sources, such as glucose. To investigate the mechanism of its CF-efflux activity, we screened CF-efflux-negative mutants from a random mutagenesis LcS library and examined the whole genome for genes responsible for CF efflux. We identified a base substitution in the pfkA gene in the glycolytic pathway, and we demonstrated that intact pfkA was essential for CF efflux, indicating that CF-efflux-positive cells must have uncompromised glycolytic activity. We also confirmed that there was a good correlation between the rate of CF-efflux-positive cells and that of colony-forming cells of LcS in a fermented milk product, whereas other properties, such as esterase activity and cell membrane integrity, lost their correlation with the colony-forming activity after long storage. We propose that CF-efflux activity could be an appropriate indicator of cell viability in certain probiotic strains.
IMPORTANCE To our knowledge, this is the first report to demonstrate that CF efflux requires uncompromised glycolytic activity in certain lactic acid bacteria. Compared with the cell properties currently widely used for cell viability assessment, such as intracellular esterase activity and membrane integrity, CF-efflux activity enables the accurate detection of culturable cells, especially in products stored for long periods at cold temperatures. These results indicate strongly that CF-efflux activity can be an adequate cell-viability indicator and that flow cytometric quantification could be an alternative to conventional CFU counting. Our findings should be especially informative for dairy/probiotic product manufacturing.
KEYWORDS: carboxyfluorescein, cell viability, efflux activity, flow cytometry, probiotics
INTRODUCTION
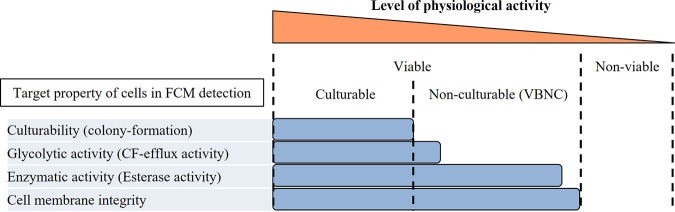
Lactic acid bacteria (LAB) are commonly used as probiotic strains in various fermented foods, such as dairy products. The rapid and accurate detection of viable LAB cells in fermented milk products is important for assessing the quality of both LAB cells and products during manufacturing and storage. Flow cytometry (FCM) enables the rapid detection of “active” bacterial cells by evaluating properties or activities, such as cell membrane integrity and esterase activity (1, 2). Traditionally, bacterial culturability based on conventional culture-dependent methods was regarded as an absolute criterion of cell viability. However, it possibly depends on the culture conditions whether cells in intermediate states, such as damaged cells, form colonies and are counted as viable. Moreover, the presence of cells that have biological activity but are nonculturable (viable but nonculturable [VBNC]) provoked wide discussion about extensions of the definition of viability. Davis et al. (3) proposed in their review article that viable microbes should be metabolically active and/or have intact membranes. Cell membrane integrity is one of the criteria most widely used for the selection of viable cells because its assessment procedure is simple and the results are easy to interpret. The international standard ISO 19344 adopts FCM for the detection of LAB in milk products, and it uses propidium iodide (PI) (an indicator of cell membrane integrity) in combination with carboxyfluorescein diacetate (cFDA) (an indicator of intracellular esterase activity) for the selective detection of active cells (4). This double staining, or similar combinations, is used to assess cells in growth culture (5) or cells that have been subjected to several kinds of stress (6–8). However, it is noteworthy that membrane integrity and esterase activity occasionally persist much longer than culturability. For example, Lahtinen et al. (9) reported that several strains of LAB in fermented milk stored at cold temperatures retained both cell membrane integrity and esterase activity, even after colony-forming activity had been impaired. The same research group also reported that PCR counts of probiotic cells with cell membrane integrity were dissociated from CFU counts in the course of cold storage (10). These studies suggested the presence of cells that had esterase activity and intact cell membranes but that had lost culturability, namely, they were VBNC cells. The reason why cell membrane integrity and esterase activity remained may be that mild environmental conditions, such as low temperatures, and the presence of milk proteins have a protective effect. We consider that membrane integrity and enzymatic activity are very fundamental properties required for cell vitality, although colony-forming activity (culturability) is a much more sophisticated property. Therefore, an appropriate indicator that can lessen the gap between those fundamental properties and culturability is required.
Previous reports have shown that certain species of LAB, such as Lactococcus lactis and Lacticaseibacillus rhamnosus, can extrude fluorescent dyes (11–13). cFDA is a reagent used in ISO 19344 (4) for detecting intracellular esterase activity by measuring the fluorescence of accumulated carboxyfluorescein (CF), which is generated by the cleavage of cFDA. However, CF is known to be extruded in the presence of some energy sources (12, 13). The fact that CF efflux is energy dependent implies that the efflux activity reflects an aspect of metabolism and could be used as an indicator of cell viability (14). However, the application of efflux activity to probiotic strains in milk products is still limited, and further characterization of efflux-based viability assessment is needed.
Here, we investigated the CF-efflux activity of Lacticaseibacillus paracasei strain Shirota (LcS) (formerly Lactobacillus casei strain Shirota). First, we collected basic data on CF efflux activity under various cell-treatment conditions, such as the presence or absence of sugars, changes in the concentration of the glucose, and exposure to stresses. Second, we demonstrated that the pfkA gene, which encodes ATP-dependent 6-phosphofructokinase (PFK), an enzyme involved in the third reaction of the glycolytic pathway, is essential for CF-efflux activity. Finally, we used a CF-efflux assay to detect the glycolytically active cells in fermented milk products during cold storage, and we compared the rates of these cells with the rates of membrane-integrity-positive cells, esterase-activity-positive cells, and colony-forming cells.
RESULTS
CF-efflux-positive cells in fermented milk products.
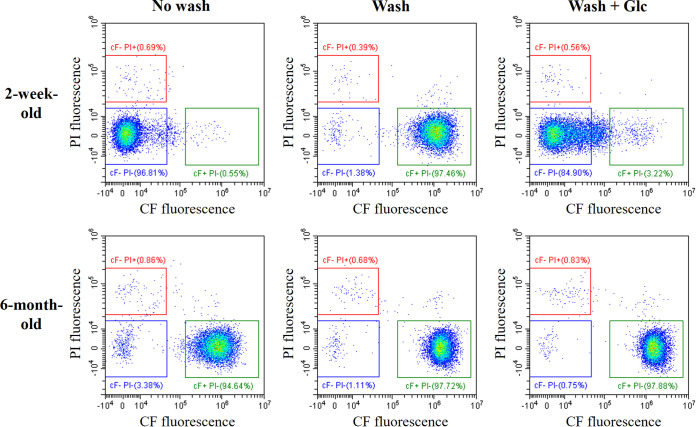
Fresh and old fermented milk products with LcS (LcS-milk) stored at 4°C were used for the detection of CF-efflux-positive LcS cells. The LcS-milk samples without washing, with washing (treatment with Dulbecco’s phosphate-buffered saline [DPBS] to eliminate saccharides from the LcS-milk sample), and with both washing and the addition of glucose were stained with cFDA and PI and then analyzed by FCM (Fig. 1). More than 90% of LcS cells in fresh (2 weeks after production) LcS-milk with the no-washing treatment were detected in the CF−PI− gate, while most LcS cells in the fresh LcS-milk sample with washing were detected in the CF+PI− gate. LcS cells with washing and the addition of glucose were detected in the CF−PI− gate. These results indicate that intracellular esterase activity was positive in the fresh LcS cells; CF accumulated in the cells without glucose but was extruded in the presence of energy sources, such as glucose. In other words, fresh LcS cells were esterase positive, membrane-integrity positive, and CF-efflux positive. In contrast, most LcS cells in the old (6 months after production) LcS-milk samples were consistently detected in the CF+PI− gate, indicating that the LcS in the old samples was esterase positive and membrane-integrity positive but CF-efflux negative. The LcS cells in the samples that were at least 6 months old were no longer culturable, namely, they were VBNC; percentages of culturable cells determined by a cell sorting analysis were less than the detection limit of 0.5% (data not shown). Therefore, CF-efflux activity is closer to culturability than membrane integrity and esterase activity and can be used as another new indicator of cell viability.
FIG 1.
CF efflux by LcS in a fermented milk product (LcS-milk). LcS cells in LcS-milk stored at 4°C for 2 weeks (within the due date of the product) or for 6 months were stained with cFDA and PI and then were analyzed by FCM. CF-efflux activity and cell membrane integrity were evaluated on a CF versus PI plot. In the presence of saccharide, viable LcS cells were identified in the CF−PI− gate, indicating efflux positivity and membrane-integrity positivity. “No wash” indicates no treatment of the LcS-milk before staining. “Wash” indicates wash treatment with DPBS to eliminate saccharides from the LcS-Milk sample. “Wash + Glc” indicates the addition of glucose after wash treatment with DPBS.
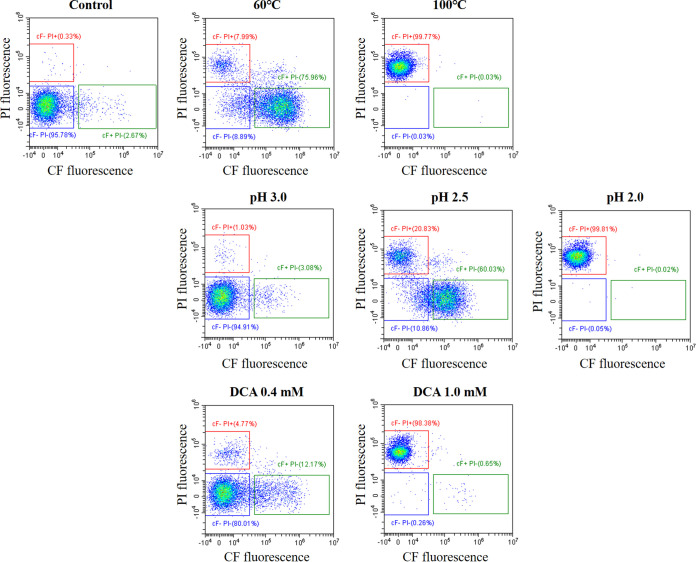
Next, LcS cells exposed to stressors, such as high temperature, low pH, or deoxycholic acid (DCA), were analyzed in the presence of an energy source (Fig. 2). Exposure to milder stressors, such as 60°C, pH 2.5, or 0.4 mM DCA, resulted in at least a partial shift from the CF−PI− gate to the CF+PI− gate, indicating the presence of damaged cells. More severe stressors, such as 100°C, pH 2.0, or 1.0 mM DCA, drove the LcS to the CF−PI+ gate, indicating the presence of membrane-compromised dead cells. We therefore observed that viable CF−PI− cells shifted to damaged CF+PI− cells, and finally to dead CF−PI+ cells. Our method of detecting CF-efflux-positive cells (i.e., CF−PI− cells) as viable cells is therefore applicable not only to the quality control of fermented milk products but also to the assessment of stress survival.
FIG 2.
CF-efflux by LcS exposed to various stressors. LcS cells in LcS-milk were subjected to stressors, namely, heat (60 or 100°C), low pH (3.0 to 2.0), or DCA (0.4 or 1.0 mM). The treated LcS cells were stained with cFDA and PI in the presence of saccharide and then analyzed by FCM.
Accumulation and efflux of CF.
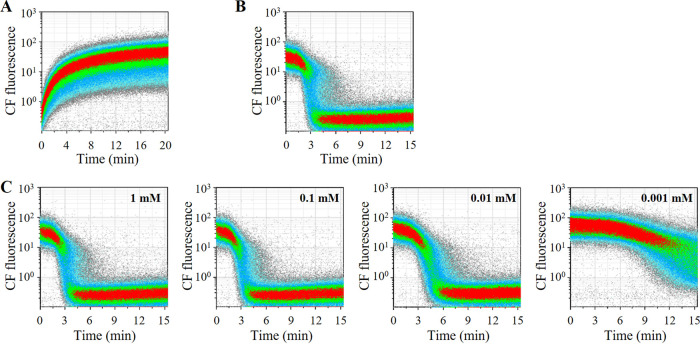
To study the kinetics of accumulation and efflux of CF, we subjected LcS pure culture in Man, Rogosa, and Sharpe (MRS) broth to a time-lapse analysis of CF fluorescence after the addition of cFDA without glucose and then after the addition of 10 mM glucose. CF began to accumulate immediately after the addition of cFDA and had fully accumulated in most LcS cells after 10 to 15 min (Fig. 3A). When glucose was added to the LcS with CF accumulation, LcS began to extrude CF in about 3 min, and extrusion was almost complete by 6 min (Fig. 3B). An experiment that varied the concentration of glucose added revealed that the efflux of CF was delayed considerably with glucose at 0.001 mM and was delayed slightly with glucose at 0.01 mM, indicating that 0.1 mM or more of glucose was needed for the fastest CF efflux (Fig. 3C).
FIG 3.
Accumulation and efflux of CF in LcS. Accumulation and efflux of CF were evaluated by time-lapse analysis of CF fluorescence by FCM. (A) LcS cells in MRS pure culture were washed with DPBS to remove saccharide, and then cFDA was added. The LcS cells were immediately analyzed by FCM, and the fluorescence of CF was monitored for 20 min. (B) LcS cells with CF accumulation were prepared by the same cFDA treatment for 15 min, and then 10 mM glucose was added. The fluorescence of CF was monitored by FCM for 15 min. (C) CF efflux was similarly evaluated with the addition of glucose at 0.001 mM to 1 mM.
Exploration of genes associated with CF efflux.
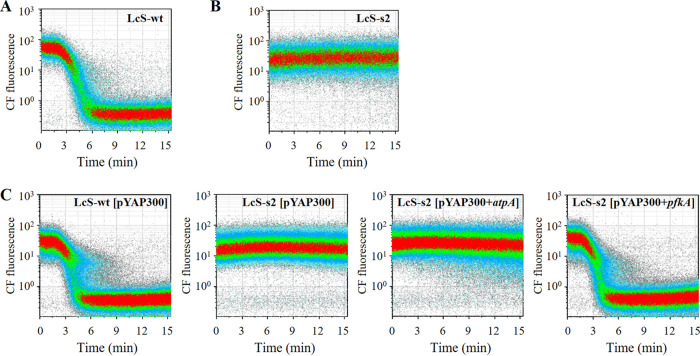
To identify the genes associated with CF-efflux activity, we obtained a CF-efflux-deficient LcS mutant and performed whole-genome sequencing for gene mutations. We first constructed a mutated LcS library by N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) treatment and used a cell sorter to collect, into an MRS broth plate, each single cell in the CF+PI− gate as a CF-efflux-deficient candidate. An analysis of the isolates revealed that the s2 strain lacked CF-efflux activity, unlike the wild type (LcS-wt) (Fig. 4A and B). Examination of the whole-genome sequence of the LcS-s2 strain revealed frameshift and missense mutations in 21 different genes (see Table S1 in the supplemental material). As the LcS-s2 strain had a lower growth rate than LcS-wt in MRS broth (data not shown), we speculated that the LcS-s2 strain had a malfunction of the genes involved in energy production. We then selected two genes, namely, ORF1247 (ATP synthase subunit alpha [atpA]) and ORF1442 (ATP-dependent 6-phosphofructokinase [pfkA]), as candidates responsible for CF-efflux deficiency, and we introduced the genes from the LcS-wt into the LcS-s2 strain. Complementation of the pfkA gene resulted in the recovery of CF-efflux activity to the same level as that in LcS-wt, whereas that of atpA made no difference (Fig. 4C). As PFK encoded by pfkA is a key enzyme in the glycolytic pathway, it is likely that a malfunction of glycolysis is attributable to the CF-efflux deficiency in the LcS-s2 strain. To examine whether PFK activity had actually deteriorated in the LcS-s2 strain, we compared the amounts of intracellular fructose-6-phosphate (F6P), a substrate of PFK, by quantifying F6P in the cell lysate of each LcS strain in MRS pure culture. A large amount of intracellular F6P remained in the LcS-s2 cells in MRS pure culture, whereas almost no F6P was detected in the LcS-wt cells and the LcS-s2+pfkA cells (Fig. 5), suggesting that the consumption of F6P by PFK did not proceed efficiently in the LcS-s2 strain. This result indicated that uncompromised PFK activity, which contributed to the prompt metabolism of glucose, was essential for the expression of CF-efflux activity.
FIG 4.
CF efflux activity in wild-type LcS, CF-efflux-deficient LcS mutant, and its recombinant mutant. The CF-efflux activity of LcS wild type (LcS-wt) (A) and CF-efflux-deficient LcS mutant (LcS-s2) (B) was evaluated by time-CF plot for 15 min after the addition of glucose. (C) The CF efflux activity of these recombinant LcS strains was evaluated. [pYAP300+atpA] and [pYAP300+pfkA] indicate complementation of atpA and pfkA genes, respectively, of the LcS wild type. [pYAP300] indicates the introduction of no-recombinant pYAP300 vector as a control.
FIG 5.
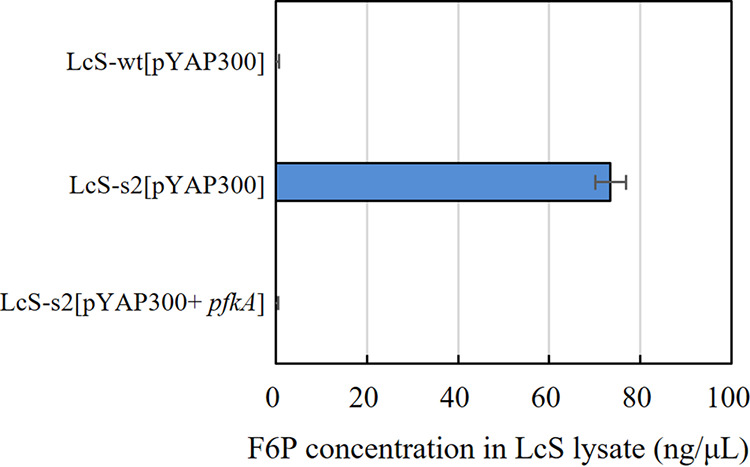
Amount of F6P in LcS cell lysates from MRS broth culture. The PFK activity of three LcS strains was evaluated by measuring the amount of F6P, a substrate of PFK, in the LcS lysate of a 1-day culture in MRS broth.
Comparison of CF-efflux-positive cells, membrane-integrity-positive cells, esterase-activity-positive cells, and colony-forming cells.
We next treated LcS in LcS-milk samples stored at 4°C with four different staining conditions for the FCM-based analysis, namely, staining by cFDA in the presence of glucose for the detection of CF-efflux-positive cells, staining by PI for membrane-integrity-positive cells, staining by cFDA in the absence of glucose for esterase-activity-positive cells, and staining by an LcS-specific antibody for single-cell isolation using a cell sorter for the determination of colony-forming cells. The longitudinal changes of the rates of the four properties for 3 months of cold storage were compared (Fig. 6). Although the LcS-milk samples were frozen at various time points at −80°C and stored until use for the analysis, we had confirmed in advance that freezing and thawing up to two times made almost no difference to our study results (data not shown). The membrane-integrity-positive rate and esterase-activity-positive rate remained more than 90% throughout the storage from day −12 to day 75. On the other hand, the CF-efflux-positive rate and the colony-formation rate similarly decreased. Both of the rates gradually decreased until day 30 and then dropped sharply between day 40 and 60. The CF-efflux-positive rate was about 20% higher than the colony-formation rate from the beginning until just after day 30, suggesting the presence of LcS cells that showed CF-efflux activity but lacked colony-forming activity, namely, VBNC cells. We also compared the FCM counts of CF-efflux-positive cells or membrane-integrity-positive cells, as well as the CFU counts of LcS, from day −13 to day 107, and confirmed that the number of CF-efflux-positive cells correlated well with CFU (see Fig. S1 in the supplemental material).
FIG 6.
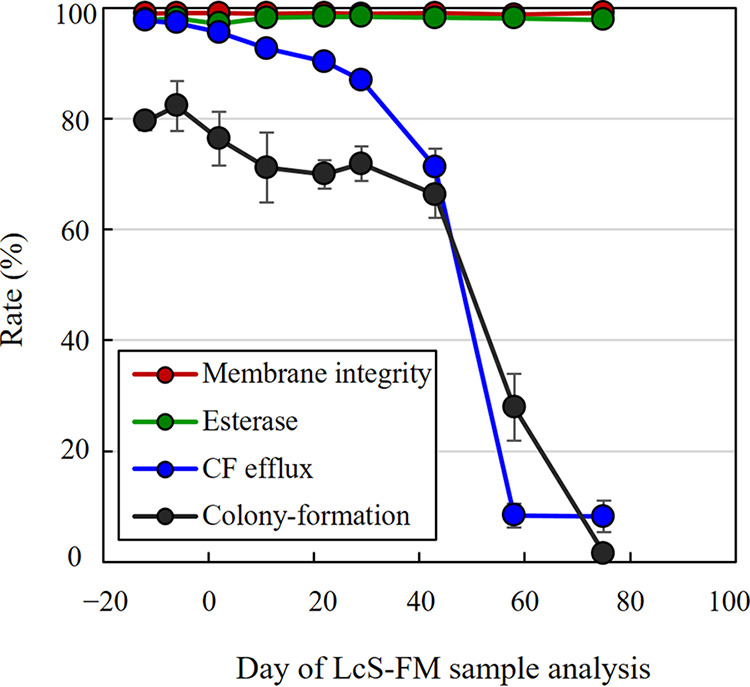
Comparison of CF-efflux activity, esterase activity, membrane integrity, and colony-forming activity. LcS-milk samples stored at 4°C for different periods were used for analysis. Day −14 means the day of production of LcS-milk, and day 0 means the best-before date of the product. The rates of CF-efflux positive (CF−PI− in the presence of glucose), membrane-integrity positive (PI−), esterase-activity positive (CF+ in the absence of glucose), and colony-formation were compared. The colony-formation rate was determined by single-cell sorting of L8+ cells into MRS agar by using a cell sorter and by calculating the percentage of colony-forming cells.
DISCUSSION
The efflux of fluorescent dyes by certain bacterial species is known to occur. Especially, the efflux activity of L. lactis was well studied in the 1990s (11, 12, 15, 16). As efflux happened in the presence of energy sources, such as glucose or lactose, it was considered to be probably related to energy production. Bunthof et al. (14) stated in their review article that CF efflux by L. lactis was probably ATP dependent and could be a good indicator of LAB viability. However, the detailed mechanism had remained unclear. Here, we demonstrated that PFK malfunction resulted in the loss of CF-efflux activity (Fig. 4 and 5). As PFK is one of the most important regulatory enzymes in glycolysis, its malfunction was suggested to delay subsequent energy production and eventually lead to the loss of CF efflux. We concluded that prompt metabolism of glucose in the glycolytic pathway is essential for CF efflux and that CF-efflux-positive cells can therefore be regarded as glycolysis-activity-positive cells. To our knowledge, this report is the first one to have established the association between glycolysis and CF-efflux activity. As glycolysis is one of the most fundamental and essential functions for bacterial growth, CF-efflux activity could be an accurate indicator of cell viability.
So far, no transporters responsible for CF efflux have been identified. A similar fluorescent dye, 2’,7’-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), is extruded by a heterodimeric ABC transporter, LmrCD, in L. lactis (17). We removed the homolog in LcS by gene deletion using a stepwise double-crossover method, as described previously (18), but it made no difference to the CF-efflux activity (data not shown). Efflux transporters can be classified into ATP-dependent and proton-motive force (PMF)-dependent types. CF-efflux was inhibited by uncoupling agents, nigericin, and carbonyl cyanide m-chlorophenylhydrazone (data not shown), suggesting that CF-efflux in LcS was associated with PMF-dependent transporters, unlike the case with L. lactis (14). Major facilitator superfamily (MFS) transporters are prevalent in Gram-positive bacteria (19) and can be the possible candidates. In this study, we also aimed to screen CF-efflux-deficient mutants that were ascribed to mutations in the transporters responsible for CF-efflux, but no such mutants were obtained. This result may be because two or more efflux transporters work complementarily, and a CF-efflux-negative phenotype was not constructed by this mutagenesis approach.
We confirmed the usefulness of CF-efflux activity for evaluating cell viability under stressors, such as heat, low pH, and DCA (Fig. 2), or long-term cold storage (Fig. 1 and 6). We summarized the states of cFDA- and PI-double-stained cells in the evaluation of the viability of bacteria, such as LcS, that have efflux ability (Fig. 7). The CF-efflux-positive cells in the CF−PI− gate can be regarded as glycolytic-activity-positive viable cells. Cells in milk products stored long term in cold storage remained physically intact and retained their functional enzyme activity but lost CF-efflux activity, resulting in VBNC cells in the CF+PI− gate. Several studies have also shown that probiotic strains in mild environments, such as milk products in cold storage, may become physically intact but nonculturable (9, 10). Damaged cells exposed to mild stressors (heat, low pH, or DCA) might be detectable in the same CF+PI− gate, suggesting impairment of CF-efflux activity. In our study, very few cells were detected in the CF+PI+ gate, although other studies using the cFDA and PI staining indicated that damaged cells were found in the CF+PI+ gate (5–7). This discrepancy might be due to the difference of the solvent-containing LcS cells. Since we used the LcS-milk sample for the exposure to the stressors (Fig. 2), milk protein may alleviate the damage to the cell membranes of LcS and lead a lower proportion of cells in the CF+PI+ gate. Cells exposed to the severe stress were physically damaged and lost their enzymatic activity, resulting in nonviable/dead cells in the CF−PI+ gate. The CF-efflux-based analysis enables the discrimination of those physiological states of LAB cells that have the CF-efflux ability. Moreover, we have confirmed the differences in longitudinal changes of the various activities of LcS in LcS-milk samples stored at cold temperature (Fig. 6). The CF-efflux-positive rates correlated with the colony-formation rates throughout the storage time but dissociated with the esterase-activity-positive rates and the membrane-integrity-positive rates after the long storage. Interestingly, the sustainability of the CF-efflux activity and esterase activity differed a lot, although both the glycolytic activity (CF efflux) and the enzymatic activity (esterase) are regarded to be in the same category of metabolic activity. Figure 8 describes a concept of the detectability of cells in different physiological states using flow cytometric analysis in this study. The detection of glycolytically active cells can provide additional profiles of bacterial properties for the evaluation of cell viability. Each FCM method can detect a different range of viable cells, and therefore, what method to use depends on what property of cells to detect for the evaluation of cell viability. We consider that the glycolytic activity may be closer to culturability and can be an informative indicator especially for analyzing probiotics in fermented milk products containing physically intact and enzymatically active but nonculturable cells. It should be noted that energy sources need to be supplied to cells for this analysis. The CF-efflux-based cell viability assessment has been applied before to L. lactis under various stressors (12), to L. rhamnosus under osmotic stress (13), and to Lacticaseibacillus casei and Bifidobacterium longum under salt stress (20). However, the application of CF efflux is not common. Our data support the importance of this method and its further application.
FIG 7.
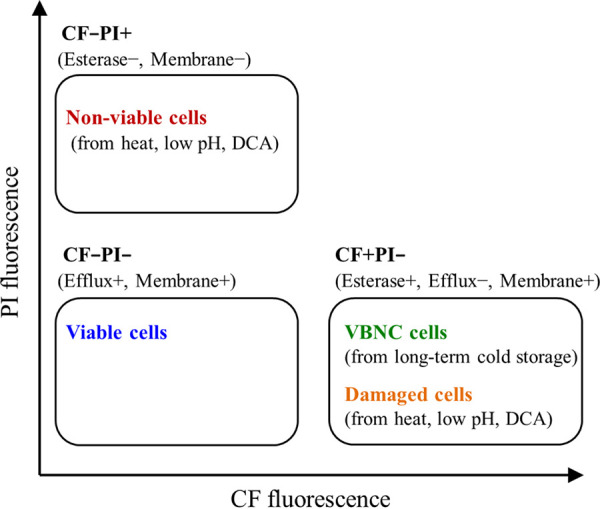
Summary of states of cFDA- and PI-double-stained cells in the presence of glucose in the evaluation of the viability of LcS.
FIG 8.
Summary of the detectability of cells in different physiological states using flow cytometry. Viable is defined as “metabolically active and/or having intact membranes” in accordance with the proposal by Davis et al. (3). Colony-formation rate was determined by single-cell isolation using a cell sorter and subsequent culture. CF, carboxyfluorescein.
A limitation of this method is that the bacterial strains to which it is applicable must have the ability to extrude CF. We investigated whether other LAB strains showed CF-efflux activity, and we found that some strains lacked this ability (see Fig. S2 in the supplemental material). The reason is unclear, but these strains may lack transporters or pumps to extrude CF. Since the responsible transporters have not been identified as mentioned above, we cannot predict from the genome information whether target bacterial strains have CF-efflux ability or not. The presence of CF-efflux ability first needs to be examined when new bacterial strains are employed for CF-efflux-based evaluation. Another difficulty of this method is that the target gate of active cells is double negative, namely, CF−PI−. This gate could include nonspecific particles, such as debris of milk products, or noise. Adjustment of the measurement conditions to remove those nonspecific particles is important, and additional specific staining of target bacteria, such as with the L8 antibody used here for LcS-specific staining, is effective if it can be done. It should be also noted that we had kept LcS-milk samples frozen until use for the FCM and culture-based analyses because it was necessary to compare multiple samples from different time points in the same experiment. However, in general, freezing samples prior to physiological analyses should be avoided. Even in this study, we performed sufficient preliminary experiments and confirmed that freezing the LcS-milk samples had almost no influence on the results.
We demonstrated here that glycolytically active bacterial cells were detected by targeting CF-efflux activity by FCM analysis in a probiotic strain, LcS. We also showed that glycolytic activity was a superior indicator of cell viability, especially in the analysis of bacteria in milk products, and it could be an alternative to colony-forming activity, which is the absolute indicator of live cells. This FCM method would be a powerful tool, not only for the quality assessment of probiotic cells in milk products but also for the further investigation of cell viability in bacterial research.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
Lacticaseibacillus paracasei YIT 9029 (strain Shirota [LcS]) was used in this study. LcS was cultured in MRS broth aerobically at 37°C for 16 to 24 h.
Fermented milk product.
LcS-milk containing at least 3.1 × 108 cells of LcS/mL was used. The products were purchased and stored at 4°C until use.
Elimination of saccharides from LcS samples.
Saccharides contained in the MRS broth culture and LcS-milk were eliminated differently before staining for FCM analysis, when necessary. MRS broth culture was washed with DPBS as follows. One hundred microliters of MRS broth culture was centrifuged at 15,000 × g for 3 min, and the bacterial pellet was suspended in 1 mL of DPBS. After another centrifugation of 15,000 × g for 3 min, the pellet was suspended again in 1 mL of DPBS to make an LcS suspension of approximately 108 cells/mL.
The LcS-milk sample was washed in combination with the degradation of milk proteins by a protease, as follows. One hundred microliters of LcS-milk was diluted with 400 μL of DPBS. Next, 150 μL of 1 M Tris-HCl (pH 8.0) containing 38.3 mg/mL of Protease P Amano 3DS (Amano Enzyme Inc., Nagoya, Japan) was added, and the mixture was incubated at 45°C for 2 min. The treated LcS suspension was then centrifuged at 15,000 × g for 3 min, and the bacterial pellet was suspended in 1 mL of DPBS. After another centrifugation of 15,000 × g for 3 min, the pellet was finally suspended in 100 μL of DPBS to make an LcS suspension of approximately 108 cells/mL.
Staining with cFDA, PI, and SYTOX orange.
The MRS broth culture of LcS, LcS-milk, or their LcS suspension was diluted with DPBS to make an approximately 100-fold dilution containing approximately 106 cells/mL of LcS. LcS cells were first treated with 50 μM cFDA for 15 min at 30°C and then with 30 μM PI for 15 min at 30°C. When necessary, LcS cells were treated with L8 antibody (a mouse IgM antibody for LcS-specific polysaccharide [21]), and then with goat anti-mouse IgM secondary antibody (DyLight 650; ThermoFisher Scientific, Waltham, MA), before being stained with cFDA and PI. A staining step with SYTOX orange (ThermoFisher Scientific), a reagent widely used for membrane integrity assay, was similarly performed by treating the LcS suspension with 0.1 μM SYTOX orange for 5 min instead of staining with cFDA and PI.
FCM analysis.
Stained samples were analyzed by using a cell analyzer, namely, a Gallios flow cytometer (Beckman Coulter, Brea, CA) or CytoFLEX S cytometer (Beckman Coulter), for CF-efflux-positive LcS cells. LcS cells were separated from noise or debris on the forward scatter versus side scatter plot and then plotted on the green fluorescence (for CF) versus red fluorescence (for PI) plot. Cells in the CF−PI− gate (CF-efflux positive and membrane-integrity positive) were defined as viable, and those in the CF−PI+ gate (esterase negative and membrane-integrity negative) were defined as dead. Cells in the CF+PI− gate (esterase positive, CF-efflux negative, and membrane-integrity positive) included viable or damaged cells. If LcS cells were stained with the L8 antibody, the L8-positive cells were first selected and then plotted on a CF versus PI plot to select L8+CF−PI− as CF-efflux-positive cells. LcS cells stained with SYTOX orange were similarly analyzed, and L8+Sytox− cells were selected as membrane-integrity-positive cells.
Exposure to stressors.
LcS cells in LcS-milk were exposed to stressors, such as high temperature, low pH, and DCA, as follows. LcS-milk was incubated at 60°C or 100°C for 10 min. LcS-milk was treated with artificial gastric juice (pH 2.0, 2.5, and 3.0) containing commercially available peptone (0.5% [wt/wt]), mucin (0.15% [wt/wt]), NaCl (0.5% [wt/wt]), NaHCO3 (0.3% [wt/wt]), and KH2PO4 (0.1% [wt/wt]) or with DCA solution (0.4 mM or 1.0 mM) for 60 min at 37°C.
Time-lapse analysis of the accumulation and efflux of CF.
A washed LcS cells suspension containing no saccharides and prepared from MRS broth culture was diluted with DPBS to make a 1,000-fold dilution (containing approximately 106 cells/mL). To analyze the accumulation of CF, cFDA was added to the LcS suspension at a concentration of 50 μM (time [T] = 0), and CF fluorescence was measured by FCM for 20 min (T = 0 to 20). To analyze CF efflux, the same 1,000-fold-diluted LcS cell suspension was first treated with 50 μM cFDA for 15 min to complete the accumulation of CF in the LcS cells. Afterward, glucose was added at a concentration of 10 mM (T = 0) and CF fluorescence was measured by FCM for 15 min (T = 0 to 15). The rate of accumulation and the efflux of CF were evaluated on a time versus CF plot.
Screening for CF-efflux-deficient cells in a mutated LcS library.
A fresh MRS culture of LcS was treated with 500 μg/mL MNNG for 1 h at room temperature, and a random mutagenesis LcS library was constructed. The LcS library was then subjected to sequential 15-min treatments with 50 μM cFDA at 30°C, with 30 μM PI at 30°C, and with 10 mM glucose at 30°C. The stained and energized LcS library was analyzed by a cell sorter (FACSAria Fusion; BD Biosciences, San Jose, CA), and each single cell in the CF+ PI− gate, which was expected to be an CF-efflux-compromised cell, was collected in MRS broth. The actual CF-efflux phenotype of pure cultures of these isolates was confirmed by FCM with cFDA.
Whole-genome sequencing and mutation analysis.
The CF-efflux-negative mutant (named LcS-s2) was subjected to whole-genome sequencing. DNA was extracted with a Quick-DNA fungal/bacterial kit (Zymo Research, Irvine, CA) and then fragmented to approximately 800 bp by using an M220 DNA shearing system (Covaris, Brighton, UK). The DNA fragments were ligated with adaptors by using a TruSeq DNA PCR-free sample prep low-throughput (LT) kit (Illumina, San Diego, CA), and the DNA library was used for 250-bp paired-end sequencing by MiSeq (Illumina). The sequence reads of LcS-s2 were mapped to the complete whole-genome sequence of LcS-wt to identify mutations, and genes with amino acid change were selected. Gene products were estimated by BLAST homology search with parameters as follows: E value, <0.00001; identity, ≥60%; and query coverage, ≥80%. We used the DFAST default reference database v1.1.6 provided by the DDBJ Fast Annotation and Submission Tool (22, 23).
Complementation of pfkA and atpA into CF-efflux-deficient LcS mutant.
The plasmid pYAP300, which is an Escherichia coli cloning vector carrying the p15A ori region, the bacteriophage phiFSW integrase gene (int) and its attachment site (attP), a multicloning site, and a synthetic promoter sequence active in lactobacilli (24), was used for gene complementation analysis. Two candidate LcS-wt genes, namely, pfkA and atpA, which we had identified as being possibly involved in CF efflux, were introduced separately into the LcS-s2 strain. The amplicon of the target genes of LcS-wt was digested by XmaI and XbaI and linked to the multicloning site of pYAP300. The recombinant plasmid, namely, pYAP300+pfkA or pYAP300+atpA, was introduced into the LcS-s2 strain by electroporation and then integrated into the attB site in the chromosome in the LcS-s2 strain to give the LcS-s2[pYAP300+pfkA] strain and the LcS-s2[pYAP300+atpA] strain. To create controls, pYAP300 without the target genes was introduced into the LcS-wt and LcS-s2 strains to give LcS-wt[pYAP300] and LcS-s2[pYAP300].
Evaluation of PFK activity.
To evaluate the PFK enzymatic activity of the three LcS strains, namely, LcS-wt[pYAP300], LcS-s2[pYAP300], and LcS-s2[pYAP300+pfkA], the amount of F6P, a substrate of PFK, accumulated in LcS cells was measured. LcS bacterial pellets from 1 day of culture in MRS broth were washed with 20 mM HEPES buffer. The cell suspension was vigorously shaken with 0.1-mm glass beads (BioSpec Products, Bartlesville, OK) by using a FastPrep-24 homogenizer (MP Biomedicals, Santa Ana, CA), and the supernatant after centrifugation at 15,000 × g for 3 min was collected as a crude extract. The crude extract was employed in a bicinchoninic acid (BCA) protein assay kit (TaKaRa Bio Inc., Shiga, Japan) to determine the protein concentration and then diluted with 20 mM HEPES buffer to make a 0.5-mg protein/mL extracted solution. The concentration of F6P in the extract was determined by using a fructose-6-phosphate assay kit (Merck, Darmstadt, Germany) in accordance with the manufacturer’s instructions.
Comparison of CF-efflux-positive cell count and CFU count.
LcS-milk kept at 4°C was sampled on various days and stored at −80°C until use. Sampling days are indicated by defining the best-before date of the product as day 0. The thawed samples were treated with the set of L8 antibody, cFDA, and PI. The samples were analyzed by FCM for the rates of membrane-integrity-positive cells (PI−), esterase-activity-positive cells (CF+ in the absence of glucose), and CF-efflux-positive cells (CF−PI− in the presence of glucose). The rate of colony-forming positivity was also determined by using a combination of single-cell sorting and the count of colonies obtained. An LcS suspension of the LcS-milk sample treated with L8 antibody, cFDA, and PI was analyzed with a FACSAria fusion flow cytometer (BD Biosciences). A total of 192 single cells in the L8+ gate were sorted on an MRS agar plate by using single-cell sorting mode; they were then cultured anaerobically at 37°C for 3 days. The percentage of colony-forming cells (number of CFU/total number of cells sorted × 100%) was calculated.
For the quantitative analysis, the thawed samples were treated with the set of L8 antibody, cFDA, and PI or with the set of L8 antibody and SYTOX orange, and then MP-count beads (Biocytex, Marseille, France) were added to quantify the target cells by FCM. The same samples were spread onto MRS agar for CFU counts. The FCM count of CF-efflux-positive cells (L8+CF−PI−), that of membrane-integrity-positive cells (L8+Sytox−), and the CFU count were compared. It had been confirmed in advance that the initial treatment of LcS-milk with a set of freezing at −80°C and thawing made almost no difference to the FCM and culture results (data not shown).
Data availability.
Nucleotide sequences of the pfkA and atpA genes of the LcS-wt and LcS s2 strains have been deposited in the DDBJ/EMBL-Bank/GenBank database under accession numbers LC718890 to LC718893.
ACKNOWLEDGMENTS
We acknowledge Fumiyasu Ishikawa, Masanobu Nanno, and Masato Nagaoka of the Yakult Central Institute (YCI) for their helpful advice. We also acknowledge Akira Kushiro (former general manager of the Microbiological Research Department of YCI) for meaningful discussions and Eiji Ishikawa of YCI for instructions and technical advice on the construction of the random mutagenesis LcS library.
Footnotes
Supplemental material is available online only.
Contributor Information
Hiroyuki Kubota, Email: hiroyuki-kubota@yakult.co.jp.
Johanna Björkroth, University of Helsinki.
REFERENCES
- 1.Bunthof CJ, Bloemen K, Breeuwer P, Rombouts FM, Abee T. 2001. Flow cytometric assessment of viability of lactic acid bacteria. Appl Environ Microbiol 67:2326–2335. doi: 10.1128/AEM.67.5.2326-2335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunthof CJ, Abee T. 2002. Development of a flow cytometric method to analyze subpopulations of bacteria in probiotic products and dairy starters. Appl Environ Microbiol 68:2934–2942. doi: 10.1128/AEM.68.6.2934-2942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis C. 2014. Enumeration of probiotic strains: review of culture-dependent and alternative techniques to quantify viable bacteria. J Microbiol Methods 103:9–17. doi: 10.1016/j.mimet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 4.ISO. 2015. Milk and milk products—starter cultures, probiotics and fermented products—quantification of lactic acid bacteria by flow cytometry. International Standard ISO19344 [IDF232]. https://www.iso.org/standard/64658.html.
- 5.El Arbi A, Ghorbal S, Delacroix-Buchet A, Bouix M. 2011. Assessment of the dynamics of the physiological states of Lactococcus lactis ssp. cremoris SK11 during growth by flow cytometry. J Appl Microbiol 111:1205–1211. doi: 10.1111/j.1365-2672.2011.05114.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Cao Y, Ferguson LR, Shu Q, Garg S. 2012. Flow cytometric assessment of the protectants for enhanced in vitro survival of probiotic lactic acid bacteria through simulated human gastro-intestinal stresses. Appl Microbiol Biotechnol 95:345–356. doi: 10.1007/s00253-012-4030-3. [DOI] [PubMed] [Google Scholar]
- 7.Papadimitriou K, Pratsinis H, Nebe-von-Caron G, Kletsas D, Tsakalidou E. 2006. Rapid assessment of the physiological status of Streptococcus macedonicus by flow cytometry and fluorescence probes. Int J Food Microbiol 111:197–205. doi: 10.1016/j.ijfoodmicro.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 8.Amor KB, Breeuwer P, Verbaarschot P, Rombouts FM, Akkermans AD, De Vos WM, Abee T. 2002. Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead bifidobacterium cells during bile salt stress. Appl Environ Microbiol 68:5209–5216. doi: 10.1128/AEM.68.11.5209-5216.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lahtinen SJ, Ouwehand AC, Reinikainen JP, Korpela JM, Sandholm J, Salminen SJ. 2006. Intrinsic properties of so-called dormant probiotic bacteria, determined by flow cytometric viability assays. Appl Environ Microbiol 72:5132–5134. doi: 10.1128/AEM.02897-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahtinen SJ, Gueimonde M, Ouwehand AC, Reinikainen JP, Salminen SJ. 2005. Probiotic bacteria may become dormant during storage. Appl Environ Microbiol 71:1662–1663. doi: 10.1128/AEM.71.3.1662-1663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molenaar D, Bolhuis H, Abee T, Poolman B, Konings WN. 1992. The efflux of a fluorescent probe is catalyzed by an ATP-driven extrusion system in Lactococcus lactis. J Bacteriol 174:3118–3124. doi: 10.1128/jb.174.10.3118-3124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunthof CJ, van den Braak S, Breeuwer P, Rombouts FM, Abee T. 1999. Rapid fluorescence assessment of the viability of stressed Lactococcus lactis. Appl Environ Microbiol 65:3681–3689. doi: 10.1128/AEM.65.8.3681-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunny-Roberts EO, Knorr D. 2008. Evaluation of the response of Lactobacillus rhamnosus VTT E-97800 to sucrose-induced osmotic stress. Food Microbiol 25:183–189. doi: 10.1016/j.fm.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Bunthof CJ, van den Braak S, Breeuwer P, Rombouts FM, Abee T. 2000. Fluorescence assessment of Lactococcus lactis viability. Int J Food Microbiol 55:291–294. doi: 10.1016/s0168-1605(00)00170-7. [DOI] [PubMed] [Google Scholar]
- 15.Breeuwer P, Drocourt J, Rombouts FM, Abee T. 1996. A novel method for continuous determination of the intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol 62:178–183. doi: 10.1128/aem.62.1.178-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molenaar D, Abee T, Konings WN. 1991. Continuous measurement of the cytoplasmic pH in Lactococcus lactis with a fluorescent pH indicator. Biochim Biophys Acta 1115:75–83. doi: 10.1016/0304-4165(91)90014-8. [DOI] [PubMed] [Google Scholar]
- 17.Lubelski J, Mazurkiewicz P, van Merkerk R, Konings WN, Driessen AJ. 2004. ydaG and ydbA of Lactococcus lactis encode a heterodimeric ATP-binding cassette-type multidrug transporter. J Biol Chem 279:34449–34455. doi: 10.1074/jbc.M404072200. [DOI] [PubMed] [Google Scholar]
- 18.Serata M, Iino T, Yasuda E, Sako T. 2012. Roles of thioredoxin and thioredoxin reductase in the resistance to oxidative stress in Lactobacillus casei. Microbiology (Reading) 158:953–962. doi: 10.1099/mic.0.053942-0. [DOI] [PubMed] [Google Scholar]
- 19.Lorca GL, Barabote RD, Zlotopolski V, Tran C, Winnen B, Hvorup RN, Stonestrom AJ, Nguyen E, Huang LW, Kim DS, Saier MH, Jr. 2007. Transport capabilities of eleven gram-positive bacteria: comparative genomic analyses. Biochim Biophys Acta 1768:1342–1366. doi: 10.1016/j.bbamem.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi A, Shah NP. 2015. Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microbiol 49:197–202. doi: 10.1016/j.fm.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Yuki N, Watanabe K, Mike A, Tagami Y, Tanaka R, Ohwaki M, Morotomi M. 1999. Survival of a probiotic, Lactobacillus casei strain Shirota, in the gastrointestinal tract: selective isolation from faeces and identification using monoclonal antibodies. Int J Food Microbiol 48:51–57. doi: 10.1016/s0168-1605(99)00029-x. [DOI] [PubMed] [Google Scholar]
- 22.Tanizawa Y, Fujisawa T, Kaminuma E, Nakamura Y, Arita M. 2016. DFAST and DAGA: web-based integrated genome annotation tools and resources. Biosci Microbiota Food Health 35:173–184. doi: 10.12938/bmfh.16-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanizawa Y, Fujisawa T, Nakamura Y. 2018. DFAST: a flexible prokaryotic genome annotation pipeline for faster genome publication. Bioinformatics 34:1037–1039. doi: 10.1093/bioinformatics/btx713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasuda E, Serata M, Sako T. 2008. Suppressive effect on activation of macrophages by Lactobacillus casei strain Shirota genes determining the synthesis of cell wall-associated polysaccharides. Appl Environ Microbiol 74:4746–4755. doi: 10.1128/AEM.00412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.02156-22-s0001.pdf, PDF file, 0.3 MB (331.9KB, pdf)
Data Availability Statement
Nucleotide sequences of the pfkA and atpA genes of the LcS-wt and LcS s2 strains have been deposited in the DDBJ/EMBL-Bank/GenBank database under accession numbers LC718890 to LC718893.