Abstract
Effective antiretroviral therapy (ART) reduces plasma HIV RNA viral load (VL) to undetectable levels and its effectiveness depends on consistent adherence. Consistent adherence and use of safe sex practices may substantially decrease the risk of HIV transmission. We sought to explore the potential association between self-reported nonadherence to ART and engaging in unsafe sexual practices capable of transmitting HIV. Using clinical and audio computer-assisted self-interview data from the prospective HIV Outpatient Study from 2007 to 2014, we assessed the frequency of self-reported ART nonadherence during the three days prior to the survey among HIV-infected persons in care and factors associated with self-reported ART nonadherence. Of 1729 patients included in this analysis (median age = 48 years, 74.3% men who have sex with men), 17% were nonadherent, 15% had a detectable VL, and 42% reported condomless anal or vaginal sex in the past six months. In multivariable analysis, self-reported nonadherence was independently associated with younger age (adjusted odds ratio [aOR] 0.8 per additional ten years, [95% CI] 0.7–1.0), non-Hispanic black race/ethnicity (aOR 1.9; 95% CI 1.4–2.6 versus white), public health insurance (aOR 1.6, 95% CI 1.2–2.3 compared with private), survey date in 2011–2014 versus 2007–2010 (aOR 0.7, 95% CI 0.5–0.9), CD4 cell count ≥ 500 versus < 200 cells/mm3 (aOR 0.3, 95% CI 0.2–0.5), greater number of ART regimen doses (aOR 1.6, 95% CI 1.3–2.2), and binge drinking (aOR 1.4, 95% CI, 1.1–1.9). In this analysis, self-reported nonadherence was not associated with engaging in condomless sex.
Keywords: HIV, risk behavior, condomless sex, adherence, antiretroviral therapy
Introduction
The medical management of HIV-infected persons requires consideration of psychosocial and behavioral risk factors, including alcohol and drug use, sexual risk behavior, and adherence to prescribed antiretroviral therapies.1 Effective antiretroviral therapy (ART) reduces plasma HIV RNA viral load (VL) to undetectable levels, decreasing the risk of HIV-associated morbidity and mortality as well as HIV transmission.2,3 However, effectiveness of ART depends on consistent adherence,4 which should be routinely assessed.5 In addition to adherence support to aid virologic suppression, patients in HIV care should be counseled to maintain consistent condom use if they are sexually active6,7 and be informed of other HIV prevention options, such as pre-exposure prophylaxis (PrEP) for their HIV-uninfected sexual partners to further reduce the risk of HIV transmission to the uninfected partner.8,9 Published reports exploring the association of sexual risk behavior and ART adherence vary widely in their findings. Although some studies have documented increased unsafe sexual behavior among persons on ART10,11 others have documented decreased sexual risk behaviors among persons receiving ART.12,13 A meta-analysis of the topic found that condomless sex was not associated with being on ART.14 Additional data suggest an association between ART nonadherence and condomless sex but vary on the strength of that association.1,15,16
The extent to which patients in routine HIV care who report being nonadherent versus adherent to ART engage in unsafe sexual practices capable of transmitting HIV has not been well described. Understanding the interplay between sexual risk behavior and ART adherence could provide insights to guide combination prevention strategies. We hypothesized that self-reported nonadherence may be associated with condomless sex, because some of the same underlying risk factors (e.g. drug and alcohol use, and nondisclosure of HIV serostatus to sexual partners) that may contribute to having sex without condoms15 may be associated with inconsistent use of ART.17 Such an association would point to the need for enhanced counseling of HIV-infected patients regarding the reduction of their sexual risk behaviors and improvements in ART adherence, both for their own health and for prevention of HIV transmission to sexual partners. Using data from the HIV Outpatient Study (HOPS), we sought to describe the frequency of self-reported antiretroviral nonadherence among HIV-infected persons in care and to identify factors associated with ART nonadherence.
Methods
The HOPS
The HOPS is an ongoing prospective observational cohort study of HIV-infected adults presently receiving care at nine participating HIV clinics (university based, public, and private) in six U.S. cities (Chicago, IL; Denver, CO; Stony Brook, NY; Philadelphia, PA; Tampa, FL; and Washington, DC) since 1993.3 The HOPS is an open cohort: patients may enter the study at any point after a diagnosis of HIV infection regardless of treatment history and may leave the study at any point for a variety of reasons such as patient request or loss to follow-up.18 Since its inception, the HOPS has been approved by institutional review boards at the Centers for Disease Control and Prevention (Atlanta, GA), Cerner Corporation (Kansas City, MO), and each of the local sites. Patient data, including sociodemographic characteristics, diagnoses, ART and other treatments, and laboratory values (including CD4+ cell count [CD4 cell count] and HIV viral load [HIV VL]) were abstracted from medical charts and entered into an electronic database by trained medical record abstractors with backgrounds in nursing or other healthcare-related fields.
In 2007, a supplemental survey was introduced that collected information on patients’ sociodemographic characteristics and risk behaviors using a brief telephone audio computer-assisted self-interview (ACASI). In April 2014, a web version of the ACASI was implemented in addition to the telephone version. Patients’ responses were kept confidential and individual responses were not available to their HIV care providers. During an annual study visit, patients in HOPS are invited to participate in the ACASI interview. HOPS patients were asked to either complete the survey via phone by dialing a toll free number from a private location in the clinic or from home or by accessing a secure website via a web-enabled device such as a computer or tablet. Patient participation in the interview, as in the larger medical abstraction study, is completely voluntary and patients are not provided any incentives to complete the interview. Data included sociodemographic information; use of tobacco, alcohol, and ‘recreational’ drugs (per wording in the survey); adherence to ART medication regimen; types and frequency of sexual activity; condom use (past six months)19; and disclosure of HIV status to sexual partners. Examples of ACASI items included but were not limited to the following: ‘In the last six months have you used marijuana, also known as pot, hash, or cannabis?’; ‘In the last three days have you missed taking any of your HIV medicines?’; ‘Have you had sex in the past six months?’; and ‘In the last six months have you had another person’s penis in your anus without using a condom?’
Study population
Our analysis was limited to a cross-sectional convenience sample of HOPS patients who were surveyed via ACASI between March 2007 and December 2014 and who were either gay, bisexual, or other men who have sex with men (MSM) or heterosexual men and women (HET). Participants who declined to complete the entire survey or who did not answer the question regarding self-reported ART adherence were excluded, as were participants who were not prescribed an ART regimen at the time of the survey. An ACASI is offered to patients annually: if a participant completed multiple ACASIs, we analyzed the results of the most recent qualifying interview.
Variables
We analyzed behavioral and clinical factors associated with self-reported ART nonadherence. The participants were queried about their ART adherence during the three days preceding the ACASI. Nonadherence was defined as missing at least one HIV medication dose during the past three days. Additionally, we considered standard risk factor variables, all of which were ascertained by ACASI, including alcohol and drug use (the survey focused on illicit/nonprescribed drugs, by asking about ‘recreational drug use’), number of sexual partners, and HIV status disclosure. Condomless sex was defined as self-report of anal or vaginal sex without a condom during the six months prior to ACASI; among those who engaged in condomless sex, we also obtained information on possible discordance, i.e. whether any sexual partners were HIV-negative or had an unknown HIV status. Data on age, sex, race/ethnicity, insurance payer, current ART regimen information, CD4 cell count, and a detectable HIV VL, within six months prior or 30 days after ACASI survey date, were obtained through medical chart abstraction.
Statistical analyses
We used descriptive statistics to compare the characteristics of participants who reported being adherent versus non-adherent to ART (Table 1). We calculated p-values for categorical variables using the Chi square test, Fisher’s exact test, or Cochran–Armitage test for trend when appropriate; p-values for continuous variables were obtained from a two-sided Wilcoxon rank sum test. We used logistic regression to estimate the odds ratios (ORs) for the association of independent variables with ART nonadherence. Statistical associations with p < 0.05 were considered significant. Factors were selected for the multivariable logistic regression model via a forward selection process20 utilizing a boundary p-value of 0.05 for inclusion. For this process, all univariate models were fitted, and the variable with the lowest p-value < 0.05 (i.e. the most significant covariate) was selected for inclusion. Then all bivariate models were fitted including the first selected variable and one other variable; again, the variable with the lowest p-value < 0.05 was included. This process continued until there were no more variables to add with p-value < 0.05. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Table 1.
Characteristics of HIV-infected patients by self-reported ARTadherence, HIV Outpatient Study, USA 2007–2014, N = 1729.
| Characteristic | Adherent (n = 1444) | Nonadherenta (n = 285) | p-valueb | ||
|---|---|---|---|---|---|
| Age in years, median (IQR) | 48.7 (42.5, 55.0) | 46.0 (39.8, 52.1) | 0.001 | ||
| N (Column %) | Row % | N (Column %) | Row % | ||
| Gender, n (%) | <0.001 | ||||
| Female | 216 (15.0) | 74.0 | 76 (26.7) | 26.0 | |
| Male | 1228 (85.0) | 85.0 | 209 (73.3) | 14.5 | |
| Race/ethnicity, n (%) | <0.001 | ||||
| Non-Hispanic white | 904 (62.6) | 88.5 | 118 (41.4) | 11.5 | |
| Non-Hispanic black | 370 (25.6) | 74.3 | 128 (44.9) | 25.7 | |
| Hispanic | 133 (9.2) | 81.1 | 31 (10.9) | 18.9 | |
| Other/unknown | 37 (2.6) | 82.2 | 8 (2.8) | 17.8 | |
| HIV transmission risk group, n (%) | <0.001 | ||||
| MSM | 1108 (76.7) | 86.3 | 176 (61.8) | 13.7 | |
| Heterosexual | 336 (23.3) | 75.5 | 109 (38.2) | 24.5 | |
| Insurance payer, n (%) | <0.001 | ||||
| Private | 820 (56.8) | 87.6 | 116 (40.7) | 12.4 | |
| Public | 412 (28.5) | 75.7 | 132 (46.3) | 24.3 | |
| Other or unknown | 212 (14.7) | 85.1 | 37 (13.0) | 14.9 | |
| Any condomless sex, n (%) | 0.96 | ||||
| Yes | 599 (41.5) | 83.3 | 120 (42.1) | 16.7 | |
| No | 615 (42.6) | 83.8 | 119 (41.8) | 16.2 | |
| No sexual partners | 210 (14.5) | 83.7 | 41 (14.4) | 16.3 | |
| Unknown | 20 (1.4) | 80.0 | 5 (1.8) | 20.0 | |
| Condomless sex with possible discordance,c n (%) | 0.98 | ||||
| At least one HIV− or unknown status partner | 429 (71.6) | 83.3 | 86 (71.7) | 16.7 | |
| All partners HIV+ | 168 (28.0) | 83.6 | 33 (27.5) | 16.4 | |
| No sexual partners | 210 (14.5) | 83.7 | 41 (14.4) | 16.3 | |
| Unknown | 2 (0.3) | 66.7 | 1 (0.8) | 33.3 | |
| Disclosed HIV status to…, n (%) | 0.059 | ||||
| None/some partners | 536 (43.4) | 81.5 | 122 (50.0) | 18.5 | |
| All partners | 698 (56.6) | 85.1 | 122 (50.0) | 14.9 | |
| Year of ACASI | 0.005 | ||||
| 2007–2010 | 493 (34.1) | 80.2 | 122 (42.8) | 19.8 | |
| 2011–2014 | 951 (65.9) | 85.4 | 163 (57.2) | 14.6 | |
| CD4 cell count (cells/mm3),d n (%) | <0.001 | ||||
| <200 | 55 (3.8) | 57.9 | 40 (14.0) | 42.1 | |
| 200–349 | 173 (12.0) | 82.0 | 38 (13.3) | 18.0 | |
| 350–499 | 224 (15.5) | 81.8 | 50 (17.5) | 18.2 | |
| ≥ 500 | 755 (52.3) | 87.3 | 110 (38.6) | 12.7 | |
| Unknown | 237 (16.4) | 83.5 | 47 (16.5) | 16.5 | |
| HIV RNA viral load,d n (%) | <0.001 | ||||
| Undetectablee | 1038 (71.9) | 86.4 | 163 (57.2) | 13.6 | |
| Detectable | 182 (12.6) | 68.4 | 84 (29.5) | 31.6 | |
| Unknown | 224 (15.5) | 85.5 | 38 (13.3) | 14.5 | |
| Regimen type, n (%) | 0.003 | ||||
| PI based | 435 (30.1) | 82.9 | 90 (31.6) | 17.1 | |
| NNRTI based | 524 (36.3) | 88.2 | 70 (24.6) | 11.8 | |
| Integrase/entry inhibitor based | 230 (15.9) | 81.0 | 54 (18.9) | 19.0 | |
| Based on two or more classesf | 203 (14.1) | 79.0 | 54 (18.9) | 21.0 | |
| Other antiretrovirals | 49 (3.4) | 75.4 | 16 (5.6) | 24.6 | |
| All other/unknown | 3 (0.2) | 75.0 | 1 (0.4) | 25.0 | |
| Hepatitis B coinfection, n (%) | 126 (8.7) | 84.0 | 24 (8.4) | 16.0 | 0.87 |
| Hepatitis C coinfection, n (%) | 144 (10.0) | 75.4 | 47 (16.5) | 24.6 | <0.001 |
| Years on ART, median (IQR) | 11.4 (5.6, 16.6) | 10.9 (6.5, 15.7) | 0.66 | ||
| Number of pills per day, median (IQR) | 3 (1, 4) | 3 (2, 5) | <0.001 | ||
| Number of daily doses in regimen, median (IQR) | 1 (1, 2) | 2 (1, 2) | <0.001 | ||
| Years since HIV diagnosis, median (IQR) | 13.7 (7.4, 19.5) | 13.4 (8.2, 18.1) | 0.68 | ||
ACASI: audio computer-assisted self-interview; ART: antiretroviral therapy; IQR: interquartile range; MSM: gay, bisexual, and other men who have sex with men; NNRTI: nonnucleoside reverse transcriptase inhibitor; PI: protease inhibitor.
Nonadherence: missed at least one HIV medication dose during the past three days.
P-values for comparison of categorical variables were obtained using a Chi square test, Fisher’s exact test, or Cochran–Armitage test of trend when appropriate; p-values for continuous variables were obtained from a two-sided Wilcoxon rank sum test.
Includes only patients who reported having sexual activity in the six months before interview.
Closest CD4 or viral load lab within six months prior and 30 days after date of interview
Defined as an undetectable viral load or < 50 copies/ml.
Regimen containing antiretrovirals in at least two of the following classes: protease inhibitor, nonnucleoside reverse transcriptase inhibitor, or integrase/entry inhibitors.
Results
Of 4625 patients actively receiving care in the HOPS during 2007–2014, 2047 completed at least one ACASI including questions regarding self-reported ART adherence and condomless vaginal or anal sex (44% survey acceptance and completion rate). When compared to those who were offered but declined to take the survey, those who were included in this study were similar with regard to age and gender, but were more likely to be of non-Hispanic white race/ethnicity. We restricted analysis to participants who were either MSM or HET (n = 1853); 124 participants were further excluded because they were not prescribed antiretrovirals at the time of the survey, leaving a total of 1729 qualifying participants. In this population (median age 48.3 years, median CD4 cell count 570 cells/mm3), 74.3% were MSM and 25.7% were HET. Participants who reported being adherent versus nonadherent differed in their characteristics by age, gender, race/ethnicity, HIV risk group, insurance payer, and other factors (Table 1). Of the 16.5% who were nonadherent, 29.5% had a detectable VL (≥ 50 copies/ml), 42.1% engaged in condomless anal or vaginal sex in the past six months, and 50.0% reported no or limited disclosure of their HIV status to sexual partners (Table 1). By comparison (p-values contrasting with a respective measure for nonadherent participants), of the 83.5% who were adherent, 12.6% (p < 0.001) had detectable VL, 41.5% (p = 0.96) reported condomless anal or vaginal sex, and 43.4% (p = 0.059) reported no or limited disclosure of their HIV status to sexual partners. MSM were more likely to report having had condomless anal or vaginal sex compared with HET participants (47.0% versus 25.8%); however, they also reported higher medication adherence (86.3% versus 75.5%) and were more likely to have an undetectable VL (71.6% versus 63.4%) (p < 0.001 for all, Figure 1).
Figure 1.
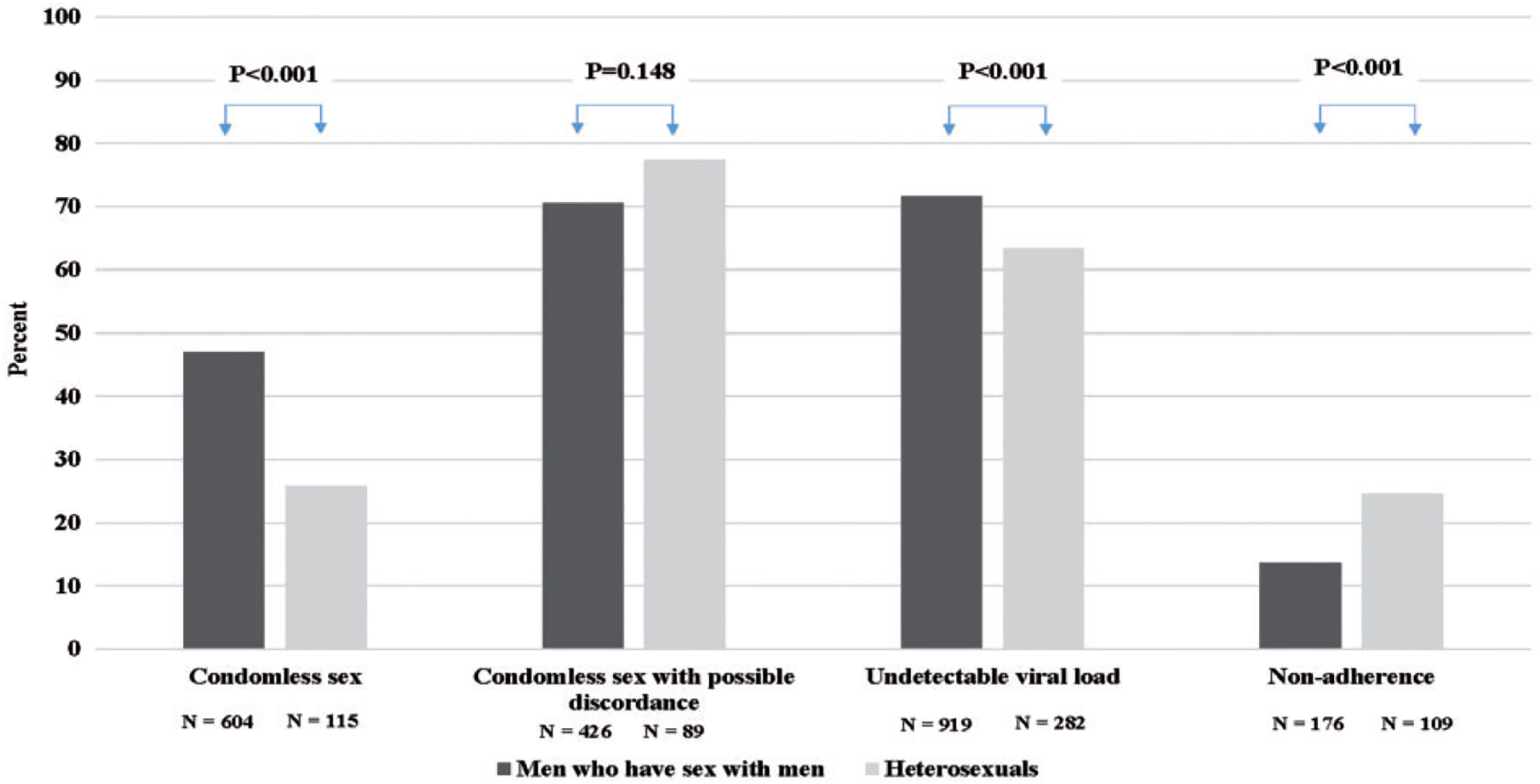
Characteristics of MSM and heterosexual patients completing an ACASI, HIV Outpatient Study, USA, 2007–2014 (N = 1729).
ACASI: audio computer-assisted self-interview; MSM: gay, bisexual and other men who have sex with men.
Condomless sex was defined as self-report of anal or vaginal sex without a condom in the six months before interview.
Discordance was defined as having condomless sex with partners of unknown HIV status or who were known to be HIV-negative and was only defined for those who have reported having condomless sex.
Viral load was the closest viral load to the date of ACASI within six months prior and one month after the survey.
Nonadherence was defined as missing at least one HIV medication dose during the three days prior to ACASI.
P-values for differences in categories were obtained using a Chi square test; N shows number with the characteristic (numerator).
Participants who reported nonadherence to their ART were more likely to report binge drinking (≥5 drinks on at least one occasion in the last 30 days) than participants who were adherent (37.2% versus 30.1%, p = 0.045) (Table 2). Similarly, participants who reported nonadherence were more likely to be current tobacco smokers (39.6% versus 23.5%, p < 0.001). In univariate logistic regression analysis (Table 3), the following factors were associated with nonadherence (p < 0.05 for all): younger age, female gender, non-Hispanic black and Hispanic versus non-Hispanic white race/ethnicity, HET versus MSM transmission risk group, earlier survey date, lower CD4 cell counts, a greater number of doses in the ART regimen, a greater number of pills per day in the ART regimen, binge drinking, and drug use.
Table 2.
Drug, alcohol, and tobacco use among patients adherent and nonadherent to ART, HIV Outpatient Study, USA, 2007–2014, N = 1729.
| Characteristic | Adherent | Nonadherenta | P-valueb | ||
|---|---|---|---|---|---|
| N (Column %) | Row % | N (Column %) | Row % | ||
| Total | 1444 | 83.5 | 285 | 16.5 | |
| Alcohol use, n (%) | 0.228 | ||||
| Yes | 1035 (71.7) | 84.3 | 193 (67.7) | 15.7 | |
| No | 398 (27.6) | 81.9 | 88 (30.9) | 18.1 | |
| Unknown | 11 (0.8) | 73.3 | 4 (1.4) | 26.7 | |
| Days per week drinking, median (IQR) | 1 (0, 3) | 1 (0, 3) | 0.79 | ||
| Number of drinks per day, median (IQR) | 2 (1, 3) | 2 (1, 3) | 0.007 | ||
| Binge drinking,c n (%) | 0.045 | ||||
| Yes | 435 (30.1) | 80.4 | 106 (37.2) | 19.6 | |
| No | 948 (65.7) | 84.3 | 176 (61.8) | 15.7 | |
| Unknown | 61 (4.2) | 95.3 | 3 (1.1) | 4.7 | |
| Number of days of binge drinking in past 30 days, n (%) | 0.18 | ||||
| 0 days | 948 (65.7) | 84.3 | 176 (61.8) | 15.7 | |
| 1–3 days | 319 (22.1) | 79.6 | 82 (28.8) | 20.4 | |
| 4–7 days | 78 (5.4) | 82.1 | 17 (6.0) | 17.9 | |
| 8 or more days | 38 (2.6) | 84.4 | 7 (2.5) | 15.6 | |
| Unknown | 61 (4.2) | 95.3 | 3 (1.1) | 4.7 | |
| Current tobacco smoker, n (%) | <0.001 | ||||
| Yes | 339 (23.5) | 75.0 | 113 (39.6) | 25.0 | |
| No | 1101 (76.2) | 86.5 | 172 (60.4) | 13.5 | |
| Unknown | 4 (0.3) | 100.0 | 0 (0.0) | 0.0 | |
| Number of cigarettes per day (median),d n (%) | 10 (4, 15) | 8 (4, 15) | 0.49 | ||
| Drug use in past six months, n (%) | 0.019 | ||||
| Yes | 784 (54.3) | 83.1 | 160 (56.1) | 16.9 | |
| No | 492 (34.1) | 87.5 | 70 (24.6) | 12.5 | |
| Unknown | 168 (11.6) | 75.3 | 55 (19.3) | 24.7 | |
| Drug used during past six months, n (%) | |||||
| Marijuana | 421 (29.2) | 82.1 | 92 (32.3) | 17.9 | 0.28 |
| Cocaine | 79 (5.5) | 68.1 | 37 (13.0) | 31.9 | <0.001 |
| Poppers | 317 (22.0) | 85.0 | 56 (19.6) | 15.0 | 0.43 |
| Heroin | 8 (0.6) | 50.0 | 8 (2.8) | 50.0 | 0.002 |
| Methamphetamines | 83 (5.7) | 83.0 | 17 (6.0) | 17.0 | 0.89 |
| Club/party drugs | 41 (2.8) | 78.8 | 11 (3.9) | 21.2 | 0.35 |
| Viagra, Cialis, etc.e | 344 (28.0) | 88.4 | 45 (21.5) | 11.6 | 0.092 |
| Injected drugs not prescribed by a doctor | 35 (2.4) | 71.4 | 14 (4.9) | 28.6 | 0.021 |
| Most common dual combinations of drug use in past six months (not mutually exclusive), n (%) | |||||
| Poppers/Viagra, Cialis, or Levitra | 137 (9.5) | 84.6 | 25 (8.8) | 15.4 | 0.70 |
| Marijuana/poppers | 133 (9.2) | 86.9 | 20 (7.0) | 13.1 | 0.23 |
| Marijuana/Viagra, Cialis, or Levitra | 124 (8.6) | 90.5 | 13 (4.6) | 9.5 | 0.022 |
| Poppers/methamphetamines | 58 (4.0) | 85.3 | 10 (3.5) | 14.7 | 0.69 |
| Methamphetamines/Viagra, Cialis, or Levitra | 47 (3.3) | 81.0 | 11 (3.9) | 19.0 | 0.60 |
| Marijuana/cocaine | 37 (2.6) | 64.9 | 20 (7.0) | 35.1 | <0.001 |
| Cocaine/poppers | 33 (2.3) | 84.6 | 6 (2.1) | 15.4 | 0.29 |
| Marijuana/methamphetamines | 35 (2.4) | 77.8 | 10 (3.5) | 22.2 | 0.85 |
ART: antiretroviral therapy; IQR: interquartile range.
Nonadherence: missed at least one HIV medication dose during the past three days.
P-values for comparison of categorical variables were obtained using a Chi square test, Fisher’s exact test, or Cochran–Armitage test of trend when appropriate; p-values for continuous variables were obtained from a two-sided Wilcoxon Rank Sum Test.
Binge drinking was defined as self-report of having ≥ 5 drinks on at least one occasion in the last 30 days.
Defined only for current smokers.
Defined only for men.
Table 3.
Univariate and multivariable analysis of factors associated with self-reported nonadherence to ART in the past three days, HIV Outpatient Study, USA 2007–2014, N = 1729.
| Characteristic | Univariate analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age (per 10 years) | 0.8 (0.7, 0.9) | < 0.001 | 0.8 (0.7, 1.0) | 0.018 |
| Gender | ||||
| Female | 2.1 (1.5, 2.8) | < 0.001 | ||
| Male | Referent | |||
| Race/ethnicity | ||||
| Non-Hispanic white | Referent | Referent | ||
| Non-Hispanic black | 2.7 (2.0, 3.5) | < 0.001 | 1.9 (1.4, 2.6) | <0.001 |
| Hispanic | 1.8 (1.2, 2.8) | 0.009 | 1.4 (0.9, 2.2) | 0.17 |
| Other/unknown | 1.7 (0.8, 3.6) | 0.209 | 1.3 (0.5, 3.3) | 0.53 |
| HIV risk | ||||
| MSM | Referent | |||
| Heterosexual | 2.0 (1.6, 2.7) | < 0.001 | ||
| Insurance payer | ||||
| Private | Referent | Referent | ||
| Public | 2.3 (1.7, 2.0) | < 0.001 | 1.6 (1.2, 2.3) | 0.002 |
| Other or unknown | 1.2 (0.8, 1.8) | 0.30 | 1.1 (0.7, 1.8) | 0.58 |
| Any condomless sex | ||||
| Yes | 1.0 (0.8, 1.4) | 0.81 | ||
| No | Referent | |||
| No sexual partners | 1.0 (0.7, 1.5) | 0.96 | ||
| Unknown | 1.3 (0.5, 3.5) | 0.62 | ||
| Condomless sex with possible discordance | ||||
| At least one HIV or unknown status partner | Referent | |||
| All partners HIV+ | 1.0 (0.6, 1.5) | 0.93 | ||
| No sexual partners | 1.0 (0.7, 1.3) | 0.86 | ||
| Unknown | 2.5 (0.2, 27.8) | 0.46 | ||
| Disclosed HIV status to… | ||||
| None/some sexual partners | 1.3 (1.0, 1.7) | 0.060 | ||
| All sexual partners | Referent | |||
| Had no sexual partners | 1.1 (0.8, 1.6) | 0.57 | ||
| Year of ACASI | ||||
| 2007–2010 | Referent | Referent | ||
| 2011–2014 | 0.7 (0.5, 0.9) | 0.005 | 0.7 (0.5, 0.9) | 0.010 |
| CD4+ cell count (cells/mm3) | ||||
| <200 | Referent | Referent | ||
| 200–349 | 0.3 (0.2, 0.5) | < 0.001 | 0.4 (0.2, 0.7) | 0.001 |
| 350–499 | 0.3 (0.2, 0.5) | < 0.001 | 0.4 (0.3, 0.8) | 0.004 |
| 500+ | 0.2 (0.1, 0.3) | < 0.001 | 0.3 (0.2, 0.5) | <0.001 |
| Unknown | 0.2 (0.2, 0.5) | < 0.001 | 0.4 (0.2, 0.8) | 0.004 |
| Years on ART (per five years)a | 1.0 (0.9, 1.1) | 0.70 | ||
| Years from HIV diagnosis (per five years) | 1.0 (0.9, 1.1) | 0.58 | ||
| Regimen type | ||||
| PI based | Referent | |||
| NNRTI based | 0.6 (0.5,0.9) | 0.011 | ||
| Integrase/entry inhibitor based | 1.1 (0.8, 1.6) | 0.51 | ||
| Based on two or more classesa | 1.3 (0.9, 1.9) | 0.19 | ||
| Other antiretrovirals | 1.6 (0.9, 2.9) | 0.14 | ||
| # Doses in regimenb | 1.6 (1.3, 2.1) | <0.001 | 1.6 (1.3, 2.2) | < 0.001 |
| # Pills per dayb | 1.1 (1.0, 1.1) | 0.012 | ||
| Binge drinkingc | ||||
| Yes | 1.3 (1.0, 1.7) | 0.045 | 1.4 (1.1, 1.9) | 0.021 |
| No | Referent | Referent | ||
| Unknown | 0.3 (0.1, 0.9) | 0.026 | 0.5 (0.1, 1.6) | 0.22 |
| Drug used | ||||
| Yes | Referent | |||
| No | 0.7 (0.5, 0.9) | 0.020 | ||
| Unknown | 1.6 (1.1, 2.3) | 0.008 | ||
ART: antiretroviral therapy; CI: confidence interval; dx: diagnosis; MSM: males who have sex with males; NNRTI: nonnucleoside reverse transcriptase inhibitor; OR: odds ratio; PI: protease inhibitor.
Regimen containing antiretrovirals in at least two of the following classes: protease inhibitor, nonnucleoside reverse transcriptase inhibitor, or integrase/entry inhibitors.
Reduced sample size due to missing/unknown information.
Binge drinking is defined as self-report of having ≥ 5 drinks on at least one occasion in the last 30 days.
Drug use during the past six months.
In multivariable analysis (Table 3), factors independently associated with self-reported nonadherence included younger age (adjusted odds ratio [aOR] 0.8 per additional ten years, 95% confidence interval [95% CI] 0.7–1.0), non-Hispanic black versus non-Hispanic white race/ethnicity (aOR 1.9; 95% CI 1.4–2.6), public versus private insurance (aOR 1.7, 95% CI 1.2–2.3), survey date in 2011–2014 versus 2007–2010 (aOR 0.7, 95% CI 0.5–0.9), CD4 cell count ≥ 500 versus < 200 cells/mm3 (aOR 0.3, 95% CI 0.2–0.5), a greater number of doses per day in the ART regimen (aOR 1.7, 95% CI 1.3–2.2), and binge drinking (aOR 1.4, 95% CI, 1.1–1.9). All p-values were < 0.05. Gender of participant, HIV transmission risk, condomless sex, condomless sex with possible discordance, HIV status disclosure, years of ART use, years HIV diagnosed, ART regimen class, number of pills per day in the ART regimen, and drug use were not associated with self-reported nonadherence.
Among the 285 participants who self-reported being nonadherent to ART, 84 (29%) had a detectable VL near the time of the survey. Of the 84 participants who were both nonadherent to ART and had a detectable VL, 32 (38%) engaged in condomless anal or vaginal sex, 40 (48%) only had sex with condoms, and 12 (14%) did not engage in sexual activity in the six months prior to the survey.
Discussion
In this analysis of HIV-infected adults in care, nonadherence to ART was relatively uncommon, with 16.5% of participants reporting missing at least one dose of their HIV medication within the three days prior to completing the ACASI. Among participants reporting nonadherence, about 42% reported having had anal or vaginal condomless sex in the previous six months. Although self-reported nonadherence was significantly associated with having detectable VL in our population, there was no association between nonadherence and the likelihood of engaging in anal or vaginal condomless sex or in condomless sex with HIV-negative or unknown status partners.
We identified several factors associated with self-reported HIV medication nonadherence. Younger HIV-infected participants were more likely to report nonadherence than older HIV-infected participants, as has been found in prior studies.21–23 This difference may be due to older participants surviving the early stages of the HIV epidemic in the US, and therefore being more attentive to and more experienced in regularly taking medications than their younger counterparts. Persons reporting binge drinking were also more likely to report being nonadherent than those reporting no binge drinking; this may be because binge drinking may lead to episodes of impairment that interfere with consistent HIV medication adherence, as documented previously.24–26 Additionally, those patients with a greater number of doses in their ART regimen, as well as those with a public insurance provider, were more likely to report missing at least one dose of ART during the past three days. Once-daily ART regimens may significantly reduce risk of nonadherence.27
Our results are subject to some limitations. We analyzed self-reported behaviors, including self-reported adherence, and did not measure adherence by pill count or other methods. However, ACASI surveys facilitate honest responses and others have found that reported sexual behaviors did not differ significantly by length of the behavior recall window,28–31 and VL findings corroborated nonadherence in our study population. The ACASI survey was an optional component of HOPS study participation; since 2009 we have systematically tracked ACASI offer rates, and in 2014 added a web version to provide different options for patients to participate, to facilitate survey participation and disclosure. Data for this analysis were largely from the era before PrEP use, and we recognize that behaviors related to sexual activity and adherence may change over time with more widespread use of PrEP.
Our findings highlight the potential for transmission posed by participants who report being nonadherent, of whom close to one-third had detectable HIV VL at a proximal measurement. In this analysis, although viremic participants were more likely to report being nonadherent, nonadherence was not associated with reporting condomless vaginal or anal sex, contrary to our original hypothesis. Given that condomless sex was prevalent among patients engaged in HIV care, our findings remain relevant and can inform provider and patient conversations on the importance of HIV medication adherence and safer sexual practices and the design of comprehensive HIV prevention interventions.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Centers for Disease Control and Prevention (contract nos. 200-2001-00133, 200-2006-18797 and 200-2011-41872).
Appendix 1. HOPS Investigators
The HOPS Investigators include the following persons and sites: Kate Buchacz, Marcus D. Durham, Division of HIV/AIDS Prevention, National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, GA; Stacey Purinton, Kate Shelton, Nabil Rayeed, Carl Armon, Thilakavathy Subramanian, Cheryl Akridge, Linda Battalora Cerner Corporation, Kansas City, MO; Frank J. Palella, Saira Jahangir, Conor Daniel Flaherty, Patricia Bustamante, Feinberg School of Medicine, Northwestern University, Chicago, IL; John Hammer, Kenneth S. Greenberg, Barbara Widick, Rosa Franklin, Rose Medical Center, Denver, CO; Bienvenido G. Yangco, Kalliope Chagaris, Infectious Disease Research Institute, Tampa, FL; Douglas J. Ward, Troy Thomas, Cheryl Stewart, Dupont Circle Physicians Group, Washington, DC; Jack Fuhrer, Linda Ording-Bauer, Rita Kelly, Jane Esteves, State University of New York (SUNY), Stony Brook, NY; Ellen M. Tedaldi, Ramona A. Christian, Faye Ruley, Dania Beadle, Princess Davenport, Lewis Katz School of Medicine at Temple University, Philadelphia, PA; Richard M. Novak, Andrea Wendrow, University of Illinois at Chicago, Chicago, IL; Benjamin Young, Mia Scott, Barbara Widick, Billie Thomas, APEX Family Medicine, Denver, CO.
Footnotes
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Remien RH, Dolezal C, Wagner GJ, et al. The association between poor antiretroviral adherence and unsafe sex: differences by gender and sexual orientation and implications for scale-up of treatment as prevention. AIDS Behav 2014; 18: 1541–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8: e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338: 853–860. [DOI] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Department of Health and Human Services. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, https://aidsinfo.nih.gov/guidelines (2016, accessed 12 July 2016).
- 6.Durham MD, Buchacz K, Richardson J, et al. Sexual risk behavior and viremia among men who have sex with men in the HIV Outpatient Study, United States, 2007–2010. J Acquir Immune Defic Syndr 2013; 63: 372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention, Health Resources and Services Administration, National Institutes of Health, American Academy of HIV Medicine, Association of Nurses in AIDS Care, International Association of Providers of AIDS Care, the National Minority AIDS Council, and Urban Coalition for HIV/AIDS Prevention Services. Recommendations for HIV Prevention with Adults and Adolescents with HIV in the United States, 2014, http://stacks.cdc.gov/view/cdc/26062 (accessed 15 June 2016).
- 8.Grant RM. Antiretroviral agents used by HIV-uninfected persons for prevention: pre- and postexposure prophylaxis. Clin Infect Dis 2010; 50: S96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC). Pre-exposure prophylaxis for the prevention of HIV infection in the United States – 2014 clinical practice guideline, http://www.cdc.gov/hiv/pdf/PrEPguidelines2014.pdf (accessed 12 April 2017).
- 10.McGowan JP, Shah SS, Ganea CE, et al. Risk behavior for transmission of human immunodeficiency virus (HIV) among HIV-seropositive individuals in an urban setting. Clin Infect Dis 2004; 38: 122–127. [DOI] [PubMed] [Google Scholar]
- 11.Osmond DH, Pollack LM, Paul JP, et al. Changes in prevalence of HIV infection and sexual risk behavior in men who have sex with men in San Francisco: 1997–2002. Am J Public Health 2007; 97: 1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond C, Richardson JL, Milam J, et al. Use of and adherence to antiretroviral therapy is associated with decreased sexual risk behavior in HIV clinic patients. J Acquir Immune Defic Syndr 2005; 39: 211–218. [PubMed] [Google Scholar]
- 13.Paz-Bailey G, Mendoza MC, Finlayson T, et al. Trends in condom use among MSM in the United States: the role of antiretroviral therapy and seroadaptive strategies. AIDS 2016; 30: 1985–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crepaz N, Hart TA and Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA 2004; 292: 224–236. [DOI] [PubMed] [Google Scholar]
- 15.Thanawuth N and Rojpibulstit M. Sexual risk behaviors among HIV-patients receiving antiretroviral therapy in Southern Thailand: roles of antiretroviral adherence and serostatus disclosure. AIDS Care 2016; 28: 612–619. [DOI] [PubMed] [Google Scholar]
- 16.Remien RH, Exner TM, Morin SF, et al. Medication adherence and sexual risk behavior among HIV-infected adults: implications for transmission of resistant virus. AIDS Behav 2007; 11: 663–675. [DOI] [PubMed] [Google Scholar]
- 17.Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC Med 2014; 12: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moorman AC, Holmberg SD, Marlowe SI, et al. Changing conditions and treatments in a dynamic cohort of ambulatory HIV patients: the HIV outpatient study (HOPS). Ann Epidemiol 1999; 9: 349–357. [DOI] [PubMed] [Google Scholar]
- 19.Crepaz N, Marks G, Liau A, et al. Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: a meta-analysis. AIDS 2009; 23: 1617–1629. [DOI] [PubMed] [Google Scholar]
- 20.Kleinbaum DG, Klein M and Pryor ER. Logistic regression: a self-learning text. 3rd ed. Berlin: Springer, 2010. [Google Scholar]
- 21.Cox LE. Predictors of medication adherence in an AIDS clinical trial: patient and clinician perceptions. Health Soc Work 2009; 34: 257–264. [DOI] [PubMed] [Google Scholar]
- 22.Halkitis. Analysis of HIV medication adherence in relation to person and treatment characteristics using hierarchical linear modeling. AIDS Patient Care STDs 2008; 22: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverberg MJ, Leyden W, Horberg MA, et al. Older age and the response to and tolerability of antiretroviral therapy. Arch Intern Med 2007; 167: 684–691. [DOI] [PubMed] [Google Scholar]
- 24.Beer L, Heffelfinger J, Frazier E, et al. Use of and adherence to antiretroviral therapy in a large U.S. sample of HIV-infected adults in care, 2007–2008. Open AIDS J 2012; 6: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chander G, Lau B and Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr 2006; 43: 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazo M. Patterns and predictors of changes in adherence to highly active antiretroviral therapy: longitudinal study of men and women. Clin Infect Dis 2007; 45: 1377. [DOI] [PubMed] [Google Scholar]
- 27.Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58: 1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schackman BR, Dastur Z, Rubin DS, et al. Feasibility of using audio computer-assisted self-interview (ACASI) screening in routine HIV care. AIDS Care 2009; 21: 992–999. [DOI] [PubMed] [Google Scholar]
- 29.Turner CF, Al-Tayyib A, Rogers SM, et al. Improving epidemiological surveys of sexual behaviour conducted by telephone. Int J Epidemiol 2009; 38: 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villarroel MA, Turner CF, Rogers SM, et al. T-ACASI reduces bias in STD measurements: the National STD and Behavior Measurement Experiment. Sex Transm Dis 2008; 35: 499–506. [DOI] [PubMed] [Google Scholar]
- 31.Crepaz N, Hart TA and Marks G. Highly active antiretroviral therapy and sexual risk behavior – a meta-analytic review. JAMA 2004; 292: 224–236. [DOI] [PubMed] [Google Scholar]


