Abstract
Background:
Each year, children die awaiting LT as the demand for grafts exceeds the available supply. Candidates with public health insurance are significantly less likely to undergo both deceased donor LT and D-LLD LT. ND-LLD is another option to gain access to a graft. The aim of this study was to evaluate if recipient insurance type is associated with likelihood of D-LLD versus ND-LLD LT.
Methods:
The SRTR/OPTN database was reviewed for pediatric LDLT performed between January 1, 2014 (Medicaid expansion era) and December 31, 2019 at centers that performed ≥1 ND-LLD LDLT during the study period. A multivariable logistic regression was performed to assess relationship between type of living donor (directed vs. non-directed) and recipient insurance.
Results:
Of 299 pediatric LDLT, 46 (15%) were from ND-LLD performed at 18 transplant centers. Fifty-nine percent of ND-LLD recipients had public insurance in comparison to 40% of D-LLD recipients (p = .02). Public insurance was associated with greater odds of ND-LLD in comparison to D-LLD upon multivariable logistic regression (OR 2.37, 95% CI 1.23–4.58, p = .01).
Conclusions:
ND-LLD allows additional children to receive LTs and may help address some of the socioeconomic disparity in pediatric LDLT, but currently account for only a minority of LDLT and are only performed at a few institutions. Initiatives to improve access to both D-LLD and ND-LLD transplants are needed.
Keywords: liver transplantation, living donor
INTRODUCTION
A significant gap exists between supply and demand of liver grafts for children awaiting LT. Each year, 4%–12% of children on the LT waiting list are removed because they die or become too sick for transplant.1,2 In addition, many children experience significant physical and cognitive morbidity while waiting for extended periods of time on the waiting list.3–5 LDLT helps bridge this gap, providing increased access to LT for children. In 2019, 79 pediatric LDLTs were performed in the USA, representing 14% of all pediatric LTs in the nation.6 Beyond expanding the donor pool, recent studies have demonstrated that LDLT achieves equal, if not superior, outcomes to DDLT in pediatric recipients.7–9 In addition, LDLT offers other advantages over DDLT, such as the ability to transplant recipients in better overall health, greater control over surgical timing for families, enhanced pre-operative donor imaging, and better graft to recipient size matching.
Socioeconomic disparities in pediatric LT are apparent and have most commonly been demonstrated with the use of type of health insurance (private vs. public) as proxy for socioeconomic status. Pediatric liver candidates without private insurance are less likely to receive exception requests for their MELD or PELD scores.10 Children with public insurance are also half as likely to undergo LDLT in comparison to dying on the list or DDLT.11 Reflecting this reduced access to both deceased and living donor transplant options, pediatric liver candidates with public insurance have significantly greater risk of mortality on the waiting list.11,12
Since 2000, pediatric LDLT from anonymous ND-LLD has been increasingly performed across North America.13 Although there is no formal policy regulating the allocation of ND-LLDs, the majority of programs performing LDLT from ND-LLD have reported allocating these unique donors based on blood type compatibility and medical urgency, with preference given to pediatric candidates.14–16 The impact of ND-LLDs on access to LDLT among pediatric candidates with public insurance has not been explored. The aim of this study was to compare types of living donors (ND-LLD vs. D-LLD), by recipient insurance type among pediatric LDLT recipients. We hypothesized that pediatric LT recipients with public insurance would be more likely to undergo ND-LLD than D-LLD LDLT in comparison to recipients with private insurance.
METHODS
The SRTR/OPTN database was reviewed for all pediatric (age <18 years) LDLT performed between January 1, 2014 (Medicaid expansion era) and December 31, 2019. Multi-organ transplants and re-transplants were excluded from the analysis. Recipients were categorized by type of insurance (public vs. private) and living donor (D-LLD vs. ND-LLD). Only transplants performed at centers that performed at least one ND-LLD in the study period were included to allow us to compare the likelihood of obtaining a directed versus non-directed living donor where ND-LLD was a possible alternative living donor option. Recipients with self-pay or foreign government as their primary payor were excluded from the analysis. This study was approved for institutional review board exemption.
Categorical variables are presented as quantity (percentage) and compared using the chi-squared test. Continuous variables are presented as mean (SD) and compared using two-sample Student’s t-test. Logistic regression was used to assess for an association between type of living donor and recipient insurance. Other recipient characteristics found to be significantly different between ND-LLD and D-LLD recipients on univariable comparisons were included in the multivariable logistic regression to determine adjusted ORs. Recipient and graft survival are demonstrated using Kaplan–Meier survival curves and compared with the log-rank test. Missing data were censored in pairwise fashion. A p-value of <.05 was set as the threshold of statistical significance for all tests of significance. All statistical analysis was performed using STATA® 16.0 (StataCorp). This study was exempt from review by the Colorado Institutional Review Board.
RESULTS
In total, 1232 pediatric LTs were performed that met inclusion criteria during the study period, including 299 pediatric LDLT. Forty-six (15%) of the LDLT were from ND-LLD performed at 18 centers. Of the 46 ND-LLD, 27 (59%) had public insurance in comparison to 102 (40%) of D-LLD recipients (p = .02; Table 1). A greater proportion of D-LLD recipients were status 1A or 1B, while a greater proportion of ND-LLD recipients had a MELD/PELD score of 20–30. ND-LLD and D-LLD recipients were similar in regard to age and weight at transplant, sex, race/ethnicity, ABO blood group, diagnosis, and hospitalization status.
TABLE 1.
Characteristics of pediatric LDLT recipients by living donor type
| N (%) | Directed living donor recipients (n = 253) | Non-directed living donor recipients (n = 46) | p-value |
|---|---|---|---|
| Public insurance | 102 (40%) | 27 (59%) | .02 |
| Age, years | 3.05 (4.81) | 3.26 (4.27) | .8 |
| Weight, kg | 16.16 (15.99) | 14.70 (8.74) | .5 |
| Female | 117 (46%) | 21 (46%) | .9 |
| Non-White race or Hispanic ethnicity | 97 (38%) | 21 (46%) | .4 |
| O blood group | 131 (52%) | 24 (52%) | 1.0 |
| Biliary atresia primary diagnosis | 136 (54%) | 24 (52%) | .8 |
| MELD/PELD with exception | .04 | ||
| <20 | 84 (33%) | 14 (30%) | |
| 20–30 | 30 (12%) | 12 (26%) | |
| >30 | 99 (39%) | 17 (37%) | |
| Status 1A or 1B | 40 (16%) | 3 (7%) | |
| Condition | .4 | ||
| Home | 166 (66%) | 35 (76%) | |
| Hospitalized, non-ICU | 62 (25%) | 8 (17%) | |
| Hospitalized, ICU | 25 (10%) | 3 (7%) |
Upon univariable logistic regression, public insurance was significantly associated with greater odds of undergoing a LDLT from an ND-LLD than a D-LLD (OR 2.10, 95% CI 1.11–3.98, p = .02). After adjusting for MELD/PELD score, public insurance remained significantly and independently associated with increased odds of ND-LLD LDLT (adjusted OR 2.37, 95% CI 1.23–4.58, p = .01; Table 2). Additionally, recipients with a MELD/PELD score of 20–30 also had significantly greater odds of receiving a LDLT from an ND-LLD than a D-LLD in comparison to recipients with a score <20 (adjusted OR 2.78, 95% CI 1.13–6.83, p = .03).
TABLE 2.
Multivariable logistic regression of living donor type
| Variable | Reference | Adjusted OR [95% CI] | p-value |
|---|---|---|---|
| Public insurance | Private insurance | 2.37 [1.23–4.58] | .01 |
| MELD/PELD with exception | |||
| 20–30 | <20 | 2.78 [1.13–6.83] | .03 |
| >30 | 1.14 [0.52–2.47] | .7 | |
| Status 1A or 1B | 0.44 [0.12–1.62] | .2 | |
There was no significant difference in recipient or graft survival by type of donor (p = .5 and .3, respectively; Figure 1). Similarly, no significant difference in recipient or graft survival was detected by type of insurance (p = .2 for both; Figure 2).
FIGURE 1.
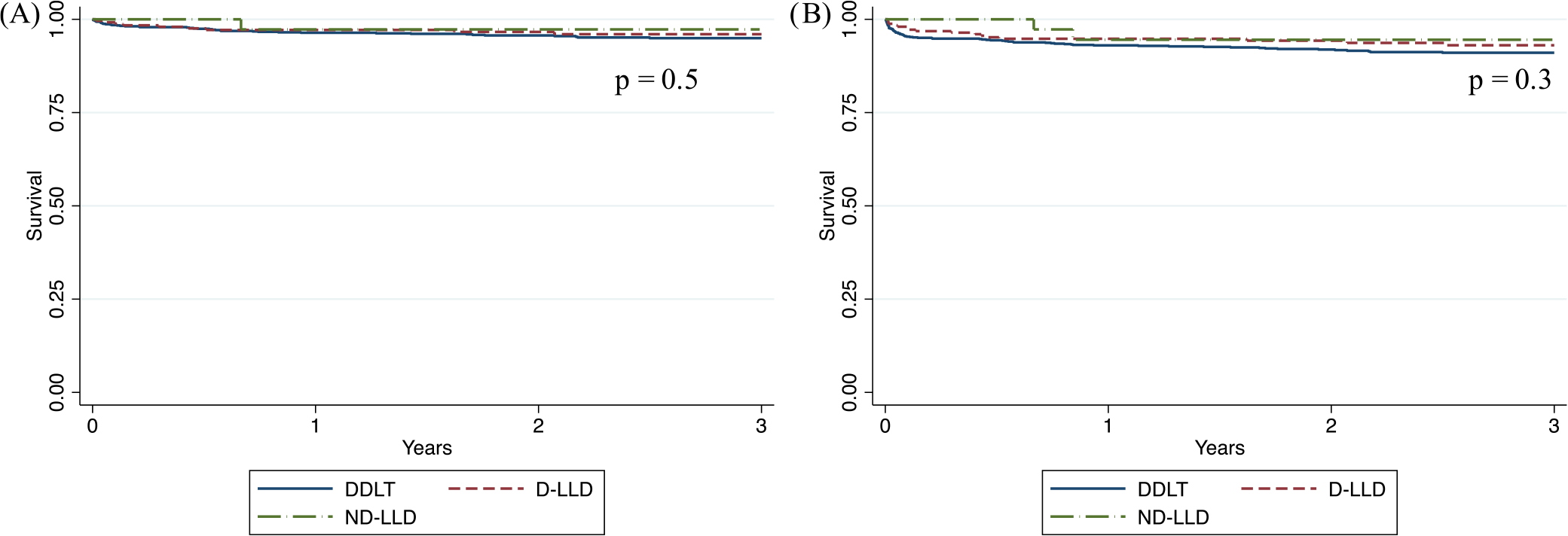
(A) Recipient and (B) graft survival by type of donor
FIGURE 2.
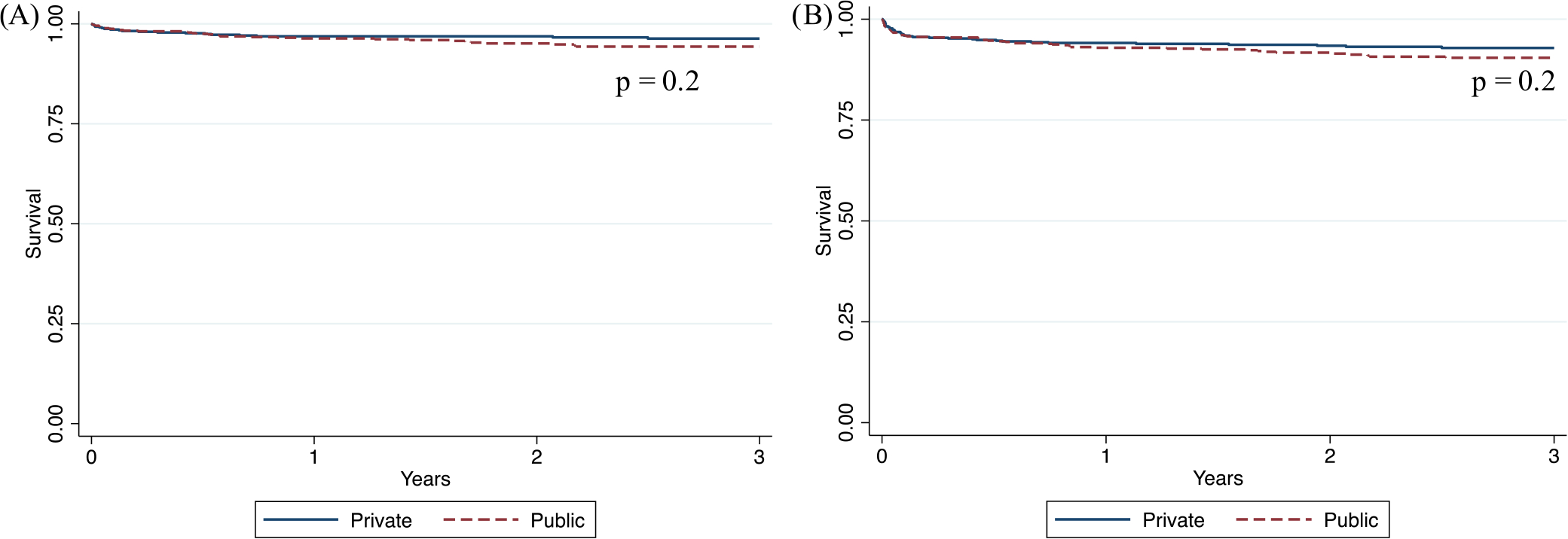
(A) Recipient and (B) graft Survival by Type of Insurance
DISCUSSION
In this retrospective analysis of pediatric LDLT performed in the USA since Medicaid expansion, we demonstrated that recipients with public insurance were twice as likely to receive a living donor graft from a non-directed donor rather than a directed donor. This finding is of dual importance, as it not only highlights the socioeconomic disparity in access to D-LLD LDLT but also illustrates the potential for ND-LLDs to help offset this disparity.
Mortality on the pediatric LT waiting list persists and is highest for children younger than 1 year of age.1,17 Split and living donor grafts expand the pool of size-matched organs for pediatric candidates but remain infrequently utilized in the USA despite achieving excellent outcomes.5,7–9,18 LDLT especially has been associated with superior survival outcomes in comparison to DDLT in recent years. Directed donation is the most commonly utilized living donation option. However, not every child has a directed donor. Potential living donors may not ultimately be able to donate due to ABO incompatibility or medical, anatomic, or psychological contraindications identified during the donor evaluation process.1,15 Additionally, potential donors may ultimately decide not to donate secondary to concerns about the financial implications about donation or lack of a sufficient support system to help them through the process. While the recipient’s insurance covers the living donor operation and hospitalization, additional expenses from missed work, travel and lodging, and child care arrangements are frequently out of pocket.19–27 Finally, the families of certain pediatric candidates may have difficulty or hesitancy communicating their child’s need for a living donor through social media and other outlets.28,29 These barriers may be more significant for socioeconomically disadvantaged and racial and ethnic minorities, as evident by significantly lower rates of LDLT among these groups.11
ND-LLD is uniquely situated to address disparities in LDLT as their evaluation and donation process is independent of the recipient, as is their financial situation and social support system. ND-LLDs are most often allocated to candidates with the highest medical urgency that are without an eligible directed living donor.16 Therefore, while ND-LLDs are not actively allocated to adjust for disparities in LDLT, our analysis demonstrates they “passively” do so because disadvantaged groups, such as those with public insurance, are less likely to have a directed living donor and therefore have a higher likelihood of receiving an ND-LLD.11 While recipients with public insurance were more likely to receive an ND-LLD than a D-LLD, the same pattern was not seen with racial and ethnic minorities, who have also been shown to have lower rates of directed LDLT.11 This may be due to the “sickest first” MELD/PELD allocation of ND-LLDs in which minorities are known to be disadvantaged due to lower rates of exception point appeals.10 It is critical to develop a standardized method for allocating ND-LLD grafts and be transparent with all families about how non-directed grafts will be allocated.
Until more deceased grafts are split, living donation will be the only way to increase the graft pool available for pediatric candidates. Currently, only about 14% of pediatric LTs come from living donors and fewer than 20 centers have performed an LDLT from an ND-LLD. Initiatives to support utilization of living donation are needed.28 First, educational opportunities such as training seminars, coursework, cadaver models, and/or simulation experiences should be designed to help more surgeons gain experience and comfort with living donation. Similar educational tools have been utilized in kidney transplant to increase expertise with living donation.30,31 Second, societal efforts to limit the financial burden of living donation (such as paid time off from work) are imperative and could increase both D-LLD and ND-LLD.24 Third, research utilizing stakeholder engagement is desperately needed to help understand the best processes for educating all families about the opportunity for living donation for their child.28 Each family with a pediatric LT candidate in the USA should be made aware of the possibility of both directed and non-directed living liver donation and centers that offer it. Additional research on the impact of social determinants of health on living donation rates and transplant outcomes is required to identify further initiatives and interventions to address persistent disparities.32–37
This study is limited by its retrospective nature and the relatively small number of centers across the USA that perform both D-LLD and ND-LLD. Furthermore, type of healthcare insurance was assumed to be an indicator of socioeconomic status, which is an imperfect, but commonly used, surrogate and is a limitation of the variables collected in the SRTR/OPTN database.10–12,38–40 Future studies assessing more granular data on social determinants of health are required to further describe disparities in LDLT and the role of ND-LLD in mitigating these disparities.
Socioeconomic disparities in pediatric LDLT from directed living donors persist. The novel phenomenon of non-directed living liver donation may help provide grafts to those candidates from lower socioeconomic status who do not have access to a directed living donor but currently only account for a minority of pediatric LDLTs performed in the USA. Initiatives to improve access to both D-LLD and ND-LLD transplants are needed to increase overall graft supply for pediatric candidates and to address disparities in pediatric LT.
ACKNOWLEDGMENTS
This work was supported in part by Health Resources and Services Administration contract HHSH250-2019-00001C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
FUNDING INFORMATION
DY is supported by NIH/NCATS Colorado CTSA Grant Number TL1 TR002533. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Abbreviations:
- CI
confidence interval
- DDLT
deceased donor liver transplant
- D-LLD
directed living liver donor
- ICU
intensive care unit
- LDLT
living donor liver transplantation
- LT
liver transplant
- MELD
model for end-stage liver disease
- ND-LLD
non-directed living liver donor
- OPTN
Organ Procurement and Transplantation Network
- OR
odds ratio
- PELD
pediatric end-stage liver disease
- SD
standard deviation
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
CONFLICT OF INTEREST
The authors of this manuscript have no conflicts of interest to disclose as described by Pediatric Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from SRTR/OPTN. Restrictions apply to the availability of these data. Data are available from the authors with the permission of SRTR/OPTN.
REFERENCES
- 1.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 annual data report: liver. Am J Transplant. 2021;21(Suppl 2):208–315. doi: 10.1111/ajt.16494 [DOI] [PubMed] [Google Scholar]
- 2.Hsu EK, Mazariegos GV. Global lessons in graft type and pediatric liver allocation: a path toward improving outcomes and eliminating wait-list mortality. Liver Transpl. 2017;23(1):86–95. doi: 10.1002/lt.24646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohammad S, Alonso EM. Approach to optimizing growth, rehabilitation, and neurodevelopmental outcomes in children after solid-organ transplantation. Pediatr Clin North Am. 2010;57(2):539–557. doi: 10.1016/j.pcl.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 4.Ng VL, Mazariegos GV, Kelly B, et al. Barriers to ideal outcomes after pediatric liver transplantation. Pediatr Transplant. 2019;23(6):e13537. doi: 10.1111/petr.13537 [DOI] [PubMed] [Google Scholar]
- 5.Perito ER, Roll G, Dodge JL, Rhee S, Roberts JP. Split liver transplantation and pediatric waitlist mortality in the United States: potential for improvement. Transplantation. 2019;103(3):552–557. doi: 10.1097/TP.0000000000002249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organ Procurement and Transplantation Network. Accessed August 28, 2020. https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/
- 7.Mogul DB, Luo X, Bowring MG, et al. Fifteen-year trends in pediatric liver transplants: split, whole deceased, and living donor grafts. J Pediatr. 2018;196:148–153 e2. doi: 10.1016/j.jpeds.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montenovo MI, Bambha K, Reyes J, Dick A, Perkins J, Healey P. Living liver donation improves patient and graft survival in the pediatric population. Pediatr Transplant. 2019;23(1):e13318. doi: 10.1111/petr.13318 [DOI] [PubMed] [Google Scholar]
- 9.Kehar M, Parekh RS, Stunguris J, et al. Superior outcomes and reduced wait times in pediatric recipients of living donor liver transplantation. Transplant Direct. 2019;5(3):e430. doi: 10.1097/TXD.0000000000000865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu EK, Shaffer M, Bradford M, Mayer-Hamblett N, Horslen S. Heterogeneity and disparities in the use of exception scores in pediatric liver allocation. Am J Transplant. 2015;15(2):436–444. doi: 10.1111/ajt.13089 [DOI] [PubMed] [Google Scholar]
- 11.Mogul DB, Luo X, Chow EK, et al. Impact of race and ethnicity on outcomes for children waitlisted for pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 2018;66(3):436–441. doi: 10.1097/MPG.0000000000001793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnon R, Annunziato RA, Willis A, et al. Liver transplantation for children with biliary atresia in the pediatric end-stage liver disease era: the role of insurance status. Liver Transpl. 2013;19(5):543–550. doi: 10.1002/lt.23607 [DOI] [PubMed] [Google Scholar]
- 13.Raza MH, Aziz H, Kaur N, et al. Global experience and perspective on anonymous nondirected live donation in living donor liver transplantation. Clin Transplant. 2020;34(4):e13836. doi: 10.1111/ctr.13836 [DOI] [PubMed] [Google Scholar]
- 14.Reichman TW, Fox A, Adcock L, et al. Anonymous living liver donation: donor profiles and outcomes. Am J Transplant. 2010;10(9):2099–2104. doi: 10.1111/j.1600-6143.2010.03244.x [DOI] [PubMed] [Google Scholar]
- 15.Yoeli D, Jackson WE, Adams MA, et al. Challenging the traditional paradigm of supply and demand in pediatric liver transplantation through nondirected living donation: a case series. Liver Transpl. 2021;27(10):1392–1400. doi: 10.1002/lt.26108 [DOI] [PubMed] [Google Scholar]
- 16.Fox AN, Liapakis A, Batra R, et al. The use of nondirected donor organs in living donor liver transplantation: perspectives and guidance. Hepatology. 2021;75:1579–1589. doi: 10.1002/hep.32260 [DOI] [PubMed] [Google Scholar]
- 17.Hsu EK, Shaffer ML, Gao L, et al. Analysis of liver offers to pediatric candidates on the transplant wait list. Gastroenterology. 2017;153(4):988–995. doi: 10.1053/j.gastro.2017.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbetta A, Butler C, Barhouma S, et al. Living donor versus deceased donor pediatric liver transplantation: a systematic review and meta-analysis. Transplant Direct. 2021;7(10):e767. doi: 10.1097/TXD.0000000000001219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colaneri J, Billman R, Branch J, Derkowski D, Frey G, Woodard A. Dissolving disincentives to living kidney donation. Nephrol Nurs J. 2021;48(5):481–488. [PubMed] [Google Scholar]
- 20.Gill J, Dong J, Gill J. Population income and longitudinal trends in living kidney donation in the United States. J Am Soc Nephrol. 2015;26(1):201–207. doi: 10.1681/ASN.2014010113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill J, Joffres Y, Rose C, et al. The change in living kidney donation in women and men in the United States (2005–2015): a population-based analysis. J Am Soc Nephrol. 2018;29(4):1301–1308. doi: 10.1681/ASN.2017111160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigue JR, Schold JD, Mandelbrot DA, Taber DJ, Phan V, Baliga PK. Concern for lost income following donation deters some patients from talking to potential living donors. Prog Transplant. 2016;26(4):292–298. doi: 10.1177/1526924816661332 [DOI] [PubMed] [Google Scholar]
- 23.Tushla L, Rudow DL, Milton J, et al. Living-donor kidney transplantation: reducing financial barriers to live kidney donation-recommendations from a consensus conference. Clin J Am Soc Nephrol. 2015;10(9):1696–1702. doi: 10.2215/CJN.01000115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hays R, Rodrigue JR, Cohen D, et al. Financial neutrality for living organ donors: reasoning, rationale, definitions, and implementation strategies. Am J Transplant. 2016;16(7):1973–1981. doi: 10.1111/ajt.13813 [DOI] [PubMed] [Google Scholar]
- 25.Killian AC, Shelton B, MacLennan P, et al. Evaluation of community-level vulnerability and racial disparities in living donor kidney transplant. JAMA Surg. 2021;156(12):1120–1129. doi: 10.1001/jamasurg.2021.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hays RE, Thomas AE, Mathias E, Mezrich J, Mandelbrot DA. Barriers to the use of a federal travel grant by living kidney donors. Clin Transplant. 2017;31(2). doi: 10.1111/ctr.12876 [DOI] [PubMed] [Google Scholar]
- 27.Siegel JT, O’Brien EK, Alvaro EM, Poulsen JA. Barriers to living donation among low-resource Hispanics. Qual Health Res. 2014;24(10):1360–1367. doi: 10.1177/1049732314546869 [DOI] [PubMed] [Google Scholar]
- 28.Mogul DB, Lee J, Purnell TS, et al. Barriers to access in pediatric living-donor liver transplantation. Pediatr Transplant. 2019;23(6):e13513. doi: 10.1111/petr.13513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kranenburg LW, Zuidema WC, Weimar W, et al. Psychological barriers for living kidney donation: how to inform the potential donors? Transplantation. 2007;84(8):965–971. doi: 10.1097/01.tp.0000284981.83557.dc [DOI] [PubMed] [Google Scholar]
- 30.Raque J, Billeter AT, Lucich E, Marvin MM, Sutton E. Training techniques in laparoscopic donor nephrectomy: a systematic review. Clin Transplant. 2015;29(10):893–903. doi: 10.1111/ctr.12592 [DOI] [PubMed] [Google Scholar]
- 31.Sutton ERH, Billeter A, Druen D, Roberts H, Rice J. Development of a human cadaver model for training in laparoscopic donor nephrectomy. Clin Transplant. 2017;31(6). doi: 10.1111/ctr.12979 [DOI] [PubMed] [Google Scholar]
- 32.Wadhwani SI, Ge J, Gottlieb L, et al. Racial/ethnic disparities in wait-list outcomes are only partly explained by socioeconomic deprivation among children awaiting liver transplantation. Hepatology. 2022;75(1):115–124. doi: 10.1002/hep.32106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wadhwani SI, Gottlieb L, Bucuvalas JC, Lyles C, Lai JC. Addressing social adversity to improve outcomes for children after liver transplant. Hepatology. 2021;74(5):2824–2830. doi: 10.1002/hep.32073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wadhwani SI, Huang CY, Gottlieb L, et al. Center variation in long-term outcomes for socioeconomically deprived children. Am J Transplant. 2021;21(9):3123–3132. doi: 10.1111/ajt.16529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadhwani SI, Brokamp C, Rasnick E, Bucuvalas JC, Lai JC, Beck AF. Neighborhood socioeconomic deprivation, racial segregation, and organ donation across 5 states. Am J Transplant. 2021;21(3):1206–1214. doi: 10.1111/ajt.16186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadhwani SI, Bucuvalas JC, Brokamp C, et al. Association between neighborhood-level socioeconomic deprivation and the medication level variability index for children following liver transplantation. Transplantation. 2020;104(11):2346–2353. doi: 10.1097/TP.0000000000003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadhwani SI, Beck AF, Bucuvalas J, Gottlieb L, Kotagal U, Lai JC. Neighborhood socioeconomic deprivation is associated with worse patient and graft survival following pediatric liver transplantation. Am J Transplant. 2020;20(6):1597–1605. doi: 10.1111/ajt.15786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nobel YR, Forde KA, Wood L, et al. Racial and ethnic disparities in access to and utilization of living donor liver transplants. Liver Transpl. 2015;21(7):904–913. doi: 10.1002/lt.24147 [DOI] [PubMed] [Google Scholar]
- 39.Vagefi PA, Ascher NL, Freise CE, Dodge JL, Roberts JP. Use of living donor liver transplantation varies with the availability of deceased donor liver transplantation. Liver Transpl. 2012;18(2):160–165. doi: 10.1002/lt.22455 [DOI] [PubMed] [Google Scholar]
- 40.Emamaullee JA, Aljehani M, Hogen RVT, et al. Potential association between public medical insurance, waitlist mortality, and utilization of living donor liver transplantation: an analysis of the scientific registry of transplant recipients. Clin Transplant. 2021;35(10):e14418. doi: 10.1111/ctr.14418 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from SRTR/OPTN. Restrictions apply to the availability of these data. Data are available from the authors with the permission of SRTR/OPTN.


