Abstract
Background and Objectives
To assess the clinical practice applicability of autoimmune encephalitis (AE) criteria (2016).
Methods
Medical records of 538 adults diagnosed with AE or related autoimmune encephalopathy at Mayo Clinic (not including pure movement disorders) were reviewed and AE guideline criteria applied.
Results
Of 538 patients, 288 were male (52%). The median symptom onset age was 55 years (range, 11–97 years; 16 had onset as children). All had other non-AE diagnoses reasonably excluded. Of 538 patients, 361 (67%) met at least possible criteria, having all 3 of subacute onset; memory deficits, altered mental status or psychiatric symptoms, and ≥1 supportive feature (new focal objective CNS finding, N = 285; new-onset seizures, N = 283; supportive MRI findings, N = 251; or CSF pleocytosis, N = 160). Of 361 patients, AE subgroups were as follows: definite AE (N = 221, 61%, [87% AE-specific IgG positive]), probable seronegative AE (N = 18, 5%), Hashimoto encephalopathy (N = 20, 6%), or possible AE not otherwise categorizable (N = 102, 28%). The 221 patients with definite AE had limbic encephalitis (N = 127, 57%), anti–NMDA-R encephalitis (N = 32, 15%), ADEM (N = 8, 4%), or other AE-specific IgG defined (N = 54, 24%). The 3 most common definite AE-IgGs detected were as follows: LGI1 (76, 34%), NMDA-R (32, 16%), and high-titer GAD65 (23, 12%). The remaining 177 patients (33%) not meeting possible AE criteria had the following: seizures only (65, 12% of all 538 patients), brainstem encephalitis without supratentorial findings (55, 10%; none had Bickerstaff encephalitis), or other (57, 11%). Those 57 “others” lacked sufficient supportive clinical, radiologic, or CSF findings (N = 26), had insidious or initially episodic onset of otherwise typical disorders (N = 21), or had atypical syndromes without clearcut memory deficits, altered mental status, or psychiatric symptoms (N = 10). Fifteen of 57 were AE-specific IgG positive (26%). Among the remaining 42, evidence of other organ-specific autoimmunity (mostly thyroid) was encountered in 31 (74%, ≥1 coexisting autoimmune disease [21, 50%] or ≥1 non–AE-specific antibodies detected [23, 53%]), and all but 1 had an objective immunotherapy response (97%).
Discussion
The 2016 AE guidelines permit autoimmune causation assessment in subacute encephalopathy and are highly specific. Inclusion could be improved by incorporating AE-IgG–positive patients with isolated seizures or brainstem disorders. Some patients with atypical presentations but with findings supportive of autoimmunity may be immune therapy responsive.
Certain neural autoantibodies have a very high specificity for autoimmune encephalitis (AE) and have led to an improved recognition of AE and related disorders in general.1 However, many patients are seronegative (possibly as high as 50%), lending further emphasis to the importance of accurate clinical history, examination, and supportive paraclinical diagnostic tests (such as MRI, inflammatory CSF parameters, and EEG).2,3 Guidelines to aid the prompt diagnosis of AE were published in 2016.4 These provide a framework for the assessment of patients presenting with subacute onset altered mental status and short-term memory loss principally using clinical assessment, MRI, and CSF white cell count.4 In that algorithmic assessment, to meet at least possible AE criteria, patients require subacute onset (rapid progression of less than 3 months) of working memory deficits (short-term memory loss), altered mental status (altered level of consciousness, lethargy, or personality change) or psychiatric symptoms with at least 1 of new focal CNS findings, new-onset seizures, CSF pleocytosis, and supportive MRI. These tests are complemented by AE-specific IgG antibody testing, CSF IgG index and oligoclonal bands, EEG, and brain biopsy to further refine diagnostic certainty. Specificity is assured by the requirement for reasonable exclusion of other diagnoses and differing levels of diagnostic certainty (possible, probable, and definite). Discerning the characteristics and relative frequencies in the clinical practice of different AE disorder subcategories (possible, probable, definite, and seropositive or seronegative) and related encephalopathies not meeting at least possible AE criteria (e.g., those with primarily seizure disorders without encephalopathy, brainstem disorders without altered awareness, and atypical presentations) would be informative for clinical treatment decisions, epidemiologic studies, and clinical trial design.
In this study, we applied the AE guidelines to 538 adult patients diagnosed with diverse autoimmune encephalopathies within our practice (Autoimmune Neurology Clinic, Mayo Clinic, Rochester, MN).4 The scope of this assessment did not include pure ataxias, stiff-person syndrome, or other pure movement disorders.5
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
This retrospective study was approved by the Mayo Clinic Institutional Review Board (IRB, 21-001297). Medical records of patients who consented to research review were included.
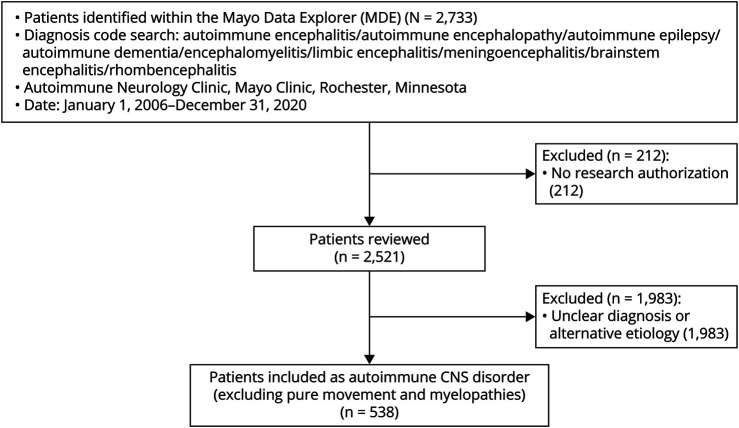
Initially surveyed medical records were from 2,733 adult patients evaluated clinically by one of the authors (January 1, 2006 until December 31, 2020) who had at least one of the following diagnoses recorded: encephalitis, encephalopathy, epilepsy, seizures, dementia, encephalomyelitis, limbic encephalitis, meningoencephalitis, brainstem encephalitis, rhombencephalitis, Bickerstaff encephalitis, Hashimoto encephalopathy, and its alternative moniker steroid-responsive encephalopathy associated with autoimmune thyroiditis (SREAT), Figure 1. Patients evaluated in the Autoimmune Neurology Clinic were either referred directly as outpatient or had been initially evaluated at the Mayo Clinic Hospital. Of those 2,733 patients, 212 lacking documented research authorization were excluded from further review. Of 2,521 charts reviewed, 1,983 patients were excluded from the study because they had one of the following: other nonautoimmune final diagnosis, uncertain diagnosis, isolated autoimmune movement disorder (myoclonic disorder, cerebellar ataxia, chorea, or stiff-person syndrome), or autoimmune myelopathy or autoimmune neuropathy occurring in isolation.
Figure 1. Study Inclusion Algorithm.
The remaining 538 patients all had AE or related disorder diagnosis made by a Mayo Clinic neuroimmunologist (≥1 of the coauthors) and had a reasonable exclusion of other nonimmune-mediated diagnoses.4 The medical records for those 538 patients were further reviewed. Clinical and testing data were abstracted from the medical records and documented in a password-protected database. Data were evaluated for each patient to determine whether at least “possible AE” criteria were met (Table 1). For those meeting at least possible AE criteria, clinical, MRI, CSF, brain biopsy, and autoantibody data were reviewed to determine whether criteria were met for other AE subgroups (definite, seronegative but probable, Hashimoto encephalopathy/SREAT, acute disseminated encephalomyelitis [ADEM], and Bickerstaff brainstem encephalitis). The same data points were also recorded for the remaining patients not meeting possible criteria. In addition, for those cases, antibody status, coexisting autoimmune diseases, immune therapies used, and physician-observed responses were recorded.
Table 1.
Autoimmune Encephalitis Diagnostic Guidelines
All 538 patients had undergone evaluation in the Mayo Clinic Neuroimmunology Laboratory for neural antibodies pertinent to AE in both the serum and CSF (486), the CSF only (1), or the serum only (51). AE-specific neural IgG antibodies included in our analyses included those antibodies already described during publication of the 2016 AE Guidelines (e.g., n-methyl-d-aspartate [NMDA] receptor [R]-IgG [in the CSF]; high-titer [≥ 20 nmol/L, equivalent to >10,000 IU/mL] glutamic acid decarboxylase 65 kDa isoform [GAD65] antibody [in the serum or any titer detected in the CSF]; and leucine-rich glioma-inactivated [LGI11] antibody, contactin-associated protein 2 [CASPR2], or antineuronal nuclear antibody type 1 [ANNA-1, anti-Hu] detected in any specimen type).4 We excluded those antibodies with a limited specificity for AE (e.g., non-LGI-1/CASPR2 voltage-gated potassium channel antibodies, calcium channel antibodies, low-positive GAD65 antibody, and NMDA-R-IgG detected in the serum only).4,6-8 In addition, we included antibodies with high disease specificity reported since 2016 (glial fibrillary acidic protein [GFAP] and neuronal intermediate filament [NIF] IgGs detected in the CSF) and adenylate kinase 5 antibody.9-11 Because we included cases with brainstem encephalitis in our analyses, we also included ANNA-2 (anti-Ri), immune globulin–like family member 5 (IgLON5), and kelch-like protein 11 (KLHL11) IgGs as AE-specific biomarkers.12-14 Positivity in either the serum or CSF was acceptable except for NMDA-R, GFAP, and NIF IgGs, where CSF positivity was required. Paraneoplastic associations where pertinent were as previously reported (e.g., ANNA-1 and small cell carcinoma).15 Intergroup nonparametric categorical data were compared using the Fisher exact test (p < 0.05 was considered significant).
Data Availability
Anonymized data used for this study are available on request.
Results
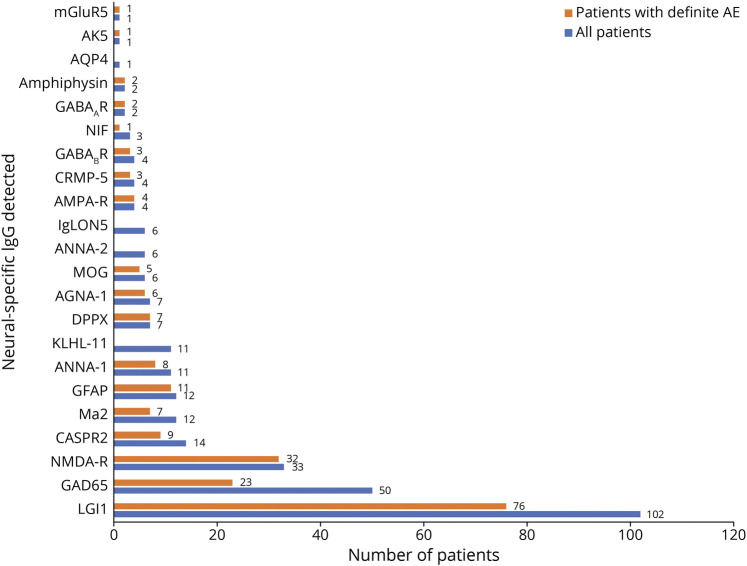
Of 538 included patients who were evaluated in our Autoimmune Neurology Clinic, 288 were male (52%). The median symptom onset age was 55 years (range, 11–97 years). Sixteen patients had symptom onset as children but were assessed in our clinic for AE diagnosis after turning 18 years. Two hundred ninety-one patients had ≥1 AE-specific IgG detected (54%), and 247 were seronegative (Figure 2). Of the 247 seronegative cases, 12 had an unclassified neural-restricted IgG antibody detected by tissue IFA (5%). These were detected in the CSF only where the serum showed negative results (9), in both the serum and CSF (2), and in the serum only where the CSF was not submitted for testing (1). The distribution of diagnoses of the 538 patients based on the 2016 AE criteria is summarized in Figure 3.
Figure 2. Autoantibody Findings Among All 538 Patients and 221 Cases With Definite AE.
Ab = antibody; AGNA = antiglial/neuronal nuclear antibody; AK5 = adenylate kinase 5; AMPA = α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; Amphi = amphiphysin; ANNA = antineuronal nuclear antibody; AQP4 = aquaporin-4; CASPR = contactin-associated protein; CRMP = collapsin-response mediator protein; DPPX = dipeptidyl peptidase; GABA = gamma amino butyric acid; GAD65 = glutamic acid decarboxylase 65 kDa isoform; GFAP = glial fibrillary acidic protein; IgLON = immunoglobulin-like cell adhesion molecule; KLHL = kelch-like protein; LGI1 = leucine-rich glioma-inactivated 1; mGluR5 = metabotropic glutamate receptor-5; MOG = myelin oligodendrocyte glycoprotein; NIF = neuronal intermediate filaments; NMDA = n-methyl-d-aspartate; R = receptor.
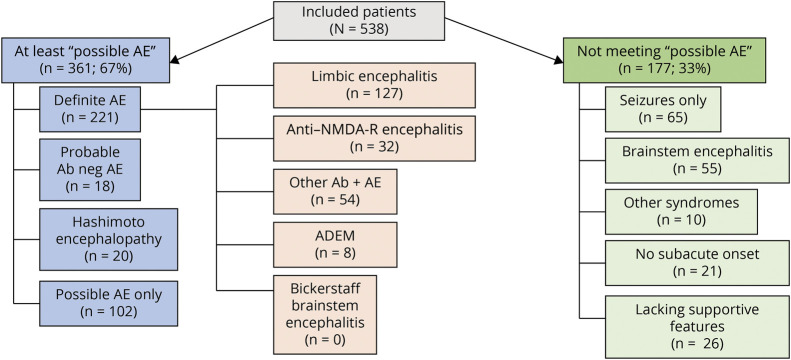
Figure 3. Diagnostic Categories for 538 Patients After Application of 2016 Autoimmune Encephalitis Criteria.
Ab = antibody; ADEM = acute disseminated encephalomyelitis; AE = autoimmune encephalitis; NMDA-R = n-methyl-d-aspartate receptor.
Two-Thirds of Patients Fulfilled at Least Possible AE Criteria, Most of Whom Had Definite AE
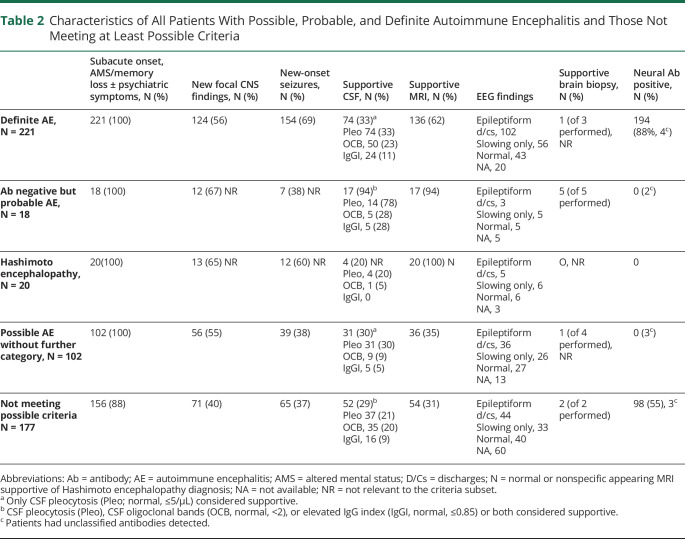
Of 538 patients, 361 (67%) met at least possible criteria, having all 3 of subacute onset, memory deficits, altered mental status or psychiatric symptoms, and had at least 1 supportive feature (new focal objective CNS finding, 285 [79%]; new-onset seizures, 283 [78%]; supportive MRI findings, 251 [70%], or CSF pleocytosis, 160 [44%]). Of the 361 patients, 221 (61%) had definite AE, 18 (5%) had probable AE, and 102 (28%) had possible AE not otherwise categorized. Twenty patients (6%) met Hashimoto encephalopathy criteria, Table 2.
Table 2.
Characteristics of All Patients With Possible, Probable, and Definite Autoimmune Encephalitis and Those Not Meeting at Least Possible Criteria
Patients With Definite AE Were Usually AE-Specific IgG Positive (87%)
The 221 patients with definite AE had limbic encephalitis (N = 127, 57%), had anti–NMDA-R encephalitis (N = 32, 15%), had ADEM (N = 8, 4%), or were classified as “other” (N = 54, 24%), Figure 3. Of 221 cases with definite AE, 193 were neural antibody positive (87%). Among seropositive patients, 18 different neural antibodies were detected in the serum, the CSF, or both, the 3 most common being leucine-rich glioma-inactivated protein 1 (LGI1; 76, 34%), NMDA-R (32, 16%), and high-titer GAD65 (23, 12%), Figure 2, eTable 1, links.lww.com/CPJ/A412. Coexisting antibodies in 8 patients were in 3 with anti-NMDA-R encephalitis with either GFAP-IgG (2 patients) or myelin oligodendrocyte glycoprotein (MOG)–IgG (1 patient) coexisting, all with typical anti–NMDA-R encephalitis; 4 with limbic encephalitis (with 1 each of gamma amino butyric acid [GABA]AR and GAD65; LGI1 and contactin-associated protein-2 [CASPR2]; ANNA-1, amphiphysin and GABABR, or antiglial/neuronal nuclear antibody type 1 [AGNA-1, also known as SOX-1]; and GABABR); and 1 with typical GABAAR encephalitis and coexisting GAD65 antibody.
Of 221 definite cases, 141 (60%) required antibody positivity to fulfill definite criteria (Figure 3, eTable 1, links.lww.com/CPJ/A412). Subgroups of patients requiring antibody positivity to meet the definite criteria were as follows: limbic encephalitis, 64/127 (50%, including 56 of 76 cases with LGI1, 74%); anti–NMDA-R encephalitis, 23/32 (72%), ADEM, 1/8 (13%, MOG-IgG positive), and all 54 “other” cases fulfilling criteria based on meeting possible criteria and being AE-specific antibody positive, eTable 1, links.lww.com/CPJ/A412. Among 127 cases that met definite limbic encephalitis criteria, 11 (9%) did not meet criteria in the absence of antibody positivity because T2 MRI changes were unilateral only (LGI1, 5; GAD65, 4; ANNA-1, and Ma2, 1 each), Figure 4. The 54 “other” patients not meeting definite limbic encephalitic criteria but still had antibody positivity to meet definite AE criteria had either: nonlimbic syndromes (16 patients [30%], with autoimmune GFAP astrocytopathy [9, with radiologic features of meningoencephalitis], dipeptidyl peptidase-6 [DPPX] autoimmunity [6, with multifocal encephalomyelitis, with gastrointestinal symptoms and weight loss], and GABAAR encephalitis [1, encephalopathy with extratemporal seizures]) or lack of sufficient supportive findings despite having a typical limbic syndrome (38, 70%). While those 38 patients without sufficient supportive findings had temporal lobe EEG changes in most cases (33, 89%), they all lacked supportive CSF and MRI findings (pleocytosis and bilateral limbic encephalitic changes). Of note, 6 of those patients did have unilateral T2 changes (5 cases with LGI1 and a single case with metabotropic glutamate receptor-5 [mGluR5]).
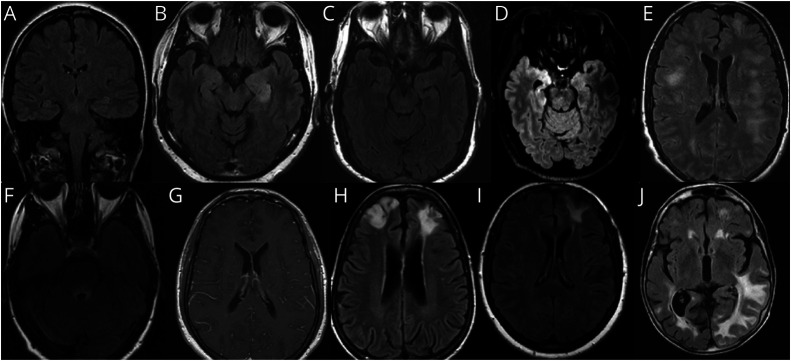
Figure 4. Illustrative MRI Brain Findings.
Images are T2 axial FLAIR, except A (T2 coronal FLAIR) and G (T1 axial postgadolinium). (A) Normal appearing hippocampal formations in a patient with anti–NMDA-receptor encephalitis. (B) Bilateral hyperintensity of hippocampal formations in a patient with LGI1 encephalitis. (C) Subtle left hippocampal hyperintensity in a patient with LGI1 encephalitis. (D) Prominent right amygdalar hyperintensity in a patient with anti-Ma2 encephalitis. (E) Multifocal white matter lesions with ill-defined borders in a patient with MOG antibody–associated disease and an ADEM attack. (F) Peri-IVth ventricular hyperintensity in a patient with aquaporin-4-IgG–positive neuromyelitis optica spectrum disorder with brainstem encephalitis; (G) Periventricular leptomeningeal enhancement in a patient with autoimmune GFAP astrocytopathy. (H) Bifrontal lobar hyperintensities in a patient with GABAA receptor encephalitis. (I) Left frontal hyperintensity in a patient with possible autoimmune encephalitis. (J) Diffuse T2 signal abnormalities in a patient with probable autoimmune encephalitis. ADEM = acute disseminated encephalomyelitis; FLAIR = fluid-attenuated inversion recovery; GABA = gamma amino butyric acid; GFAP = glial fibrillary acidic protein; LGI1 = leucine-rich glioma-inactivated 1; MOG = myelin oligodendrocyte glycoprotein; NMDA-R = n-methyl-d-aspartate receptor.
No cases of Bickerstaff brainstem encephalitis were encountered. A wider search of the Mayo medical records beyond the scope of our Autoimmune Neurology group (2006–2020) yielded 1 case with Bickerstaff encephalitis case evaluated in our department 2 years after hospitalization elsewhere (ganglioside GQ1B antibody positive). Twenty patients met 2016 criteria for Hashimoto encephalopathy (Table 2).
Patients Meeting Probable Seronegative or Possible AE Criteria Had Diverse Findings
All 18 patients with probable but seronegative AE had both MRI and CSF findings supportive of the diagnosis, 5 of whom also underwent brain biopsy (all 5 had supportive histologic evidence of inflammatory infiltrates, Table 2). The other 102 patients met possible criteria only (Table 2), one of whom did not meet definite limbic encephalitis criteria owing to unilateral rather than bilateral hippocampal T2 hyperintensity. Inflammatory-appearing MRI findings were diverse among the probable and possible group patients (examples shown in Figure 4).
Two-Thirds of Patients Not Meeting at Least Possible AE Criteria Had Isolated Seizures or Brainstem Encephalitis
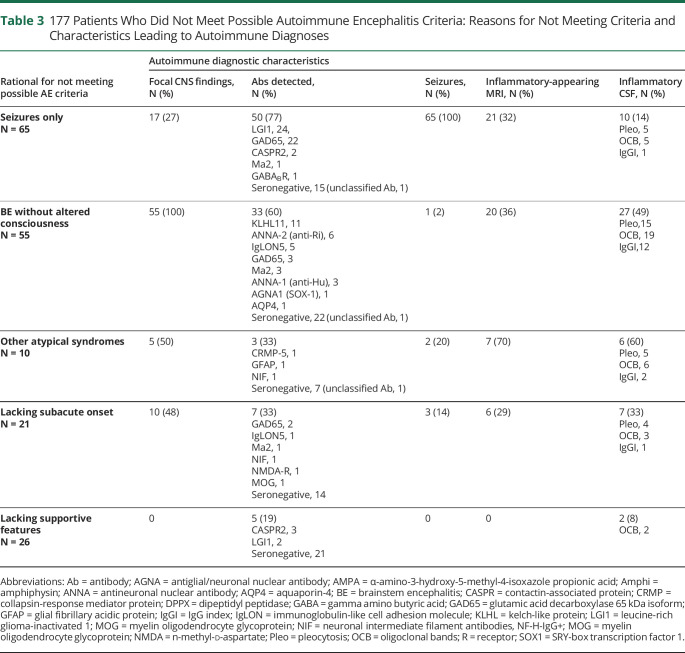
Of the 177 patients (33% of 538) evaluated and diagnosed with autoimmune encephalopathy who did not meet at least 2016 possible AE criteria (Tables 2 and 3, Figure 3), 120 (68%) had either seizures only (65, 12% of all patients) or brainstem encephalitis without supratentorial findings (55, 10% of all patients), and 83 cases were neural antibody positive (69%, Table 3). Patients with brainstem encephalitis had diverse combinations of eye movement disorders (35), ataxic (28) or postural (32) difficulties, vestibulocochlear symptoms (18, including deafness in 5), upper motor neuron signs (13), dysarthria (13), dysphagia (9), other cranial neuropathies (7), and tremulousness (5). Seven of 11 patients with KLHL-11-IgG positivity had vestibulocochlear symptoms. One patient had AQP4-IgG positivity and isolated (radiologically inflammatory appearing) brainstem encephalitis, thus meeting NMO diagnostic criteria.16 Twenty-one patients without pleocytosis in the CSF had oligoclonal bands (20) or an elevated IgG index (8) detected, 4 of whom did not meet possible criteria for lack of supportive findings. Epileptiform EEG abnormalities in the “seizure-only” patients (captured in 36 of 65, 55%) were temporal (28), extratemporal (4), or both (4).
Table 3.
177 Patients Who Did Not Meet Possible Autoimmune Encephalitis Criteria: Reasons for Not Meeting Criteria and Characteristics Leading to Autoimmune Diagnoses
The Remaining 57 Patients Did Not Meet at Least Possible AE Criteria for Diverse Reasons
The remaining 57 of 177 patients (32%) lacked supportive clinical, radiologic, or CSF findings (26), had insidious or initially episodic onset of otherwise typical disorders (21), or atypical multifocal syndromes with cognitive symptoms in the absence of memory deficits or altered mental status (10), Table 3. Characteristic features supporting an autoimmune diagnosis in the 57 patients were diverse. These were typical neurocognitive syndrome by AE criteria (47, 82%), subacute onset (36, 63%), new focal CNS findings (15, 26%), inflammatory CSF abnormalities (15, 26%; 1 or more of CSF-exclusive oligoclonal bands [11], pleocytosis [9], or an elevated IgG index [3]), AE-specific antibodies (15, 26%: CASPR2, 3; GAD65, 2; LGI1, 2; NIF, 2; GFAP, NMDA-R, collapsin-response mediator protein-5, Ma2, MOG, and IgLON5, 1 each), inflammatory MRI findings (13), seizures (5), and supportive brain biopsy findings (2). In addition, physician-reported immune therapy responses were recorded in 52 of 57 patients (91%). One of the 57 patients had 6 of these 8 abovementioned characteristics (2%), 6 had 5 (11%), 14 had 4 (26%), 32 had 3 (56%), and 3 had 2 (5%). When compared with the 102 patients meeting 2016 possible AE criteria only, the 57 patients were as likely to have coexisting seizures (39/102 v 14/57, p = 0.083), supportive MRI findings (36/102 v 13/57, p = 0.150), or CSF pleocytosis (31/102 v 9/57, p = 0.057) but were less likely to have new focal CNS findings (56/102 v 14/57, p = 0.004).
Coexisting Autoimmunity and Treatment Responses Were Diagnostically Useful in 8% of the Cohort
Among 42 (of the 57) patients who were neural IgG negative, other evidence of diverse organ-specific autoimmunity was encountered in 31 (74%), eTable 2, links.lww.com/CPJ/A412. Twenty-one had ≥1 coexisting autoimmune disease (50%, autoimmune thyroid disease, 14; B12 deficiency, 4; type 1 diabetes, 2; celiac disease, 2; and 1 each of autoimmune hemolytic anemia, immune thrombocytopenic purpura, sclerosing cholangitis, ulcerative colitis, systemic lupus erythematous, rheumatoid arthritis, leukocytoclastic vasculitis, and preexisting Lambert-Eaton syndrome) and 23 had ≥1 non-neurological disease–specific antibodies detected (55%, thyroid, 17 [thyroid peroxidase, 16; thyroglobulin, 1]; low-positive [≤ 20 nmol/L] GAD65, 5; p-ANCA, 1; phospholipid antibody, 1), and 1 had an unclassified neural antibody detected in the CSF. Twenty-five of 42 patients had clinical or serologic evidence of thyroid autoimmunity (60%). All but 1 of 42 patients had objective improvements in neurologic examination reported by the treating clinician (97%), supported by improvements on formal bedside cognitive or neuropsychometric testing (14), EEG (7), MRI (4), or PET-CT (1).
Among the 3 most common seropositive subgroups in the entire cohort of 538 patients (LGI1, GAD65, and NMDA-R), 54 of 160 patients did not meet at least possible 2016 criteria (34%): LGI1 26/76 patients (34%, seizures only, 24; and lack of supportive features, 2); GAD65 27/51 patients (53%, seizures only 22, brainstem encephalitis, 3, lack of subacute onset, 2); NMDA-R 1/33 patients (3%, lack of supportive features), Table 3.
Psychiatric Symptom–Predominant Phenotypes Were Uncommon and Not Typical of Primary Psychiatric Disorders
Thirteen of 538 patients presented with predominant or exclusive psychiatric symptoms (2.5%). They had subacute symptom onset of multimodal neuropsychiatric presentations consisting of altered mental status (all 13), behavior or personality change (10), paranoid delusions (5), auditory and/or visual hallucinations (4), agitation (3), catatonia (3), and suicidal ideation (2). Nine of 13 (69%) had focal objective CNS findings (abnormal cognitive examination [8], aphasia [1], hypomimia [1], and apraxia with hyperreflexia [1]), 4 had supportive MRI findings, and 3 had supportive CSF pleocytosis (an additional 2 had CSF-exclusive oligoclonal bands). All but 1 of 13 met at least possible AE criteria. Diagnoses were anti–NMDA-R encephalitis, 6 (all were NMDA-R antibody positive in the CSF); anti-DPPX encephalitis, 1, and Hashimoto encephalopathy/SREAT, 1. Of the 5 remaining patients (seronegative), 4 met only possible criteria, and 1 met definite limbic encephalitis criteria. Immune therapy responses were evident in all 13.
Discussion
We applied the 2016 AE guidelines to our patients diagnosed with diverse autoimmune encephalopathies encountered in our clinical practice to understand their utility in assisting diagnostic decision-making.4 We found two-thirds of our patients diagnosed with an autoimmune brain disease (excluding ataxias and other movement disorders) met at least possible criteria. The guidelines permit structured assessment of patients with subacute-onset brain disorders using clinical and paraclinical data points to determine differing levels of diagnostic certainty (probable, definite, or possible, not otherwise specified). These are helpful in delineating the key diagnostic features for clinical practice and have also been used in the design of clinical trials and other research studies.3,17 Although our study was not designed to address the specificity of criteria among a control cohort, in our clinical experience, the definite AE criteria are highly specific, which is assured by the “reasonable exclusion of other diagnoses” requirement. In addition, we did not find alternative diagnoses among the cases where there was less certainty (possible, probable, or not meeting 2016 criteria). A longitudinal data collection to assess for any changes in diagnoses over time will be conducted in the future.
These 2016 AE criteria focused on being as specific as possible and were moderately sensitive in our patients. We respect the work conducted by others to date and acknowledge the impossibility of capturing all patients with disparate autoimmune encephalopathies using a single set of criteria without losing specificity. Some modification of the criteria could improve inclusion of AE-specific IgG-positive patients with seizure-only presentations, brainstem encephalitides, and perhaps some atypical presentations. This would ensure broader inclusion of affected patients in relation to epidemiologic studies, clinical trials, treatment guidelines, healthcare policies, and insurance rules developed in the future.
From our experience, the most common presentation beyond those specified in the AE guidelines was isolated seizures (12%), similar to the frequency previously reported for isolated seizures in general for LGI1 encephalitis (13%) and anti–NMDA-receptor encephalitis (12%).18,19 In our experience, LGI1 encephalitis presents with seizures in the absence of the full clinical picture of limbic encephalitis in one-third of cases (and thus would not be included in AE criteria). By contrast, the AE guidelines were sufficiently sensitive to detect anti–NMDA-R encephalitis except in 1 patient, who lacked subacute onset. The next most common category of patients not meeting criteria were those with brainstem encephalitis (10%), with neither altered awareness nor bilateral external ophthalmoplegia typical of Bickerstaff encephalitis, which was included in the 2016 guidelines.4,20 Despite being included in the diagnostic criteria, we never encountered a patient with Bickerstaff encephalitis in our practice, and only encountered 1 within our institution who met AE diagnostic guidelines during the study epoch. In our experience, brainstem encephalitis most commonly presents with subacute onset of cerebellar ataxic signs, postural instability, and symptomatic eye movement abnormalities. One or more of deafness, vertigo, speech and swallow difficulties, jaw dystonia, upper motor neuron signs, spasms, bowel or bladder dysfunction, myoclonus, or tremor were other accompaniments. Vestibulocochlear symptoms are frequent in KLHL11 autoimmunity.21
Though highly specific, the 2016 guidelines were not designed to capture the heterogenous group of patients (10% of our entire cohort) who did not meet possible AE criteria yet had a constellation of features suspicious for an autoimmune cause and who responded to immune therapy in almost all cases. One of the defining features of the 2016 AE criteria (subacute onset, typical neurocognitive syndrome, and sufficient supportive features) was absent in those 57 patients, yet suspicion for an autoimmune cause persisted. For 15 (25%) of those patients, autoimmune diagnoses were straightforward to confirm because AE-specific IgG testing in the serum or CSF was positive. However, for the remaining 42 seronegative patients, the diagnoses were less certain and illustrative of the importance of continued biomarker discovery for seronegative autoimmune neurologic disorders. Additional features we have found helpful over the years include a preexisting personal history of autoimmune disease (most commonly thyroid disease), CSF markers beyond pleocytosis (elevated IgG index, IgG synthesis rate, and oligoclonal bands), research-based testing for novel unclassified neural IgGs, and occasionally brain biopsy.22 Furthermore, all but 1 of those 42 patients had a physician-observed response to immune therapy. Although ideal to ascertain an autoimmune diagnosis before treatment, our experience and these data portend a lack of feasibility in some cases.23 Where there is uncertainty, before an immune therapy trial, we emphasize the importance of reasonable exclusion of other reversible disorders, such as neoplastic, infectious, toxic, and metabolic disorders, so as to leave 2 main possibilities remaining: an immune-mediated disorder or a neurodegenerative diagnosis with limited treatment options. Patients should be followed up expeditiously to determine whether there is an objective response to immune therapy and treatment promptly tapered and discontinued where there is none.
Some of our patients met criteria for Hashimoto encephalopathy (2016) while others (among our 42 seronegative cases not meeting possible criteria) met the less stringent SREAT criteria.24 Either way, the significance of thyroid autoantibodies (and other non-neural IgGs such as low-positive GAD65 antibody) should be interpreted with caution because they can be encountered in healthy people also. Three-quarters of patients referred to our practice for Hashimoto encephalopathy/SREAT received a final nonautoimmune diagnosis.25 In general, in this study, we diagnosed autoimmune encephalopathy in 1 in 5 of all patients assessed.
From a practical perspective, it would be difficult to capture all diverse patients from our practice we have described in this study in diagnostic criteria without losing specificity. Nonetheless, the sensitivity for possible AE could be improved by including some well-recognized seropositive “seizure-only” presentations, as previously described.26 A recent review of conceptual definitions suggested the terms “acute symptomatic seizures secondary to AE” to refer to seizures occurring in the setting of the active phase of immune-mediated encephalitis and provided a detailed summary of typical features of new-onset seizures in that context.27 The 2016 AE guidelines could be modified to include isolated new-onset, frequent, focal seizures among the clinical presentations, while retaining the other supportive criteria and requirement to exclude competing diagnoses.27 Similarly, a category could be added to current AE guidelines to include brainstem encephalitides, especially if seropositive for relevant neural autoantibodies, though the presentations are sufficiently diverse, distinct, and nascent in their recognition, that development of a separate brainstem-specific set of criteria could also be considered.28,29
Similar to our previous hospital-based experience, and the experience of others, psychiatric symptom–predominant autoimmune encephalopathies were distinct in presentation from primary psychiatric disorders. Our patients had multimodal psychiatric presentations, usually admixed with neurocognitive signs.30,31 In addition, those patients almost always had supportive findings and met AE diagnostic criteria. Therefore, a separate category of “autoimmune psychiatric” diagnoses seems unnecessary.32 Similarly, autoimmune dementia, rather than being a separate diagnostic category, serves as “aide memoire” to cases of AE where rapidly progressive cognitive decline, without delirium, might mimic CJD or rapidly progressive Alzheimer disease or diffuse Lewy body disease.23 The most challenging clinical presentations in our practice from a diagnostic standpoint include the 10% of patients with 1 of 3 central features atypical: nonsubacute onset (typically insidious with fluctuations), those with atypical symptomatology (such as admixed vague cognitive symptoms, language difficulties, and motor symptoms, where altered mental status and memory loss are not apparent), and those with a dearth of supportive clinical and paraclinical test findings. Nonetheless, those patients met 2 of the 3 central features, and if were AE-specific Ab negative, three-quarters had other clues supporting autoimmunity. Though not readily addressable in diagnostic criteria, in general within our practice, those patients would be diagnosed with possible autoimmune encephalopathy initially, and a likely diagnosis subsequently, if an objective immune therapy response occurred. We have found 1 or more of “before and after” MRI, EEG, and neuropsychometric testing to be of assistance in documenting treatment responses.
Limitations of our study include the potential for referral bias, favoring patients with unusual presentations not meeting AE criteria, given our tertiary outpatient subspecialty practice. In addition, our institution's emphasis on US national ambulatory practice likely influenced LGI1 encephalitis being more common than anti–NMDA-R encephalitis. While we did encounter occasional patients without subacute symptom onset, it is also possible that some of those may have been artifacts of patient and family recall or documentation. In addition, antibody diagnostic evaluations were incremental in their sensitivity over time as new AE-specific IgGs were characterized. The largest AE subgroup was limbic encephalitis, 50% of whom required AE-specific IgG positivity to fulfill definite criteria, emphasizing the importance of antibody biomarkers in clinical practice.1 Although patients with occasional limbic encephalitis in our series had unilateral limbic MRI changes, only 1 seronegative patient with possible AE did not fulfill limbic encephalitis criteria based on not having bilateral findings, and glioma should also be considered in these cases.33
In summary, for patients with a subacute onset cerebral syndrome, key details from the clinical history and examination, CSF, MRI findings and EEG, and utilization of the 2016 AE guidelines criteria assist in assessing the likelihood of an autoimmune cause. Antibody testing assists in confirming the diagnosis and may provide guidance for cancer evaluation in those with identification of a high-risk paraneoplastic antibody. In our view, it is also important to have a low threshold for EEG, MRI, CSF, and serum/CSF antibody testing in a patient with an “outlier” clinical presentation in whom an alternative diagnosis is not immediately apparent and to consider an immune therapy trial. Future iterations of AE guidelines could capture more patients, particularly seropositive patients with isolated seizures and brainstem encephalitides. This would ensure optimized estimation of the epidemiologic burden of autoimmune encephalopathies and inform clinical trial design.
Appendix. Authors
Footnotes
Editorial, page e200155
Study Funding
National Institute of Neurologic Disorders and Stroke RO1NS126227 U01NS120901.
Disclosure
E. Orozco and C. Valencia-Sanchez report no disclosures relevant to the manuscript; J.W. Britton has consulted for UCB pharmaceuticals; D. Dubey has research support from the Department of Defence (CA210208), Centers of Multiple Sclerosis and Autoimmune Neurology and Clinical and Translational Science, Mayo Clinic, and Grifols pharmaceuticals, has consulted for UCB, Immunovant, Argenx, and Astellas pharmaceuticals (compensation for consulting activities paid directly to Mayo Clinic), and has patents pending for KLHL11-IgG, LUZP4-IgG, and cavin-4-IgG as markers of neurologic autoimmunity; E.P. Flanagan has funding from NIH (R01NS113828), has served on advisory boards for Alexion, Genentech, Horizon Therapeutics, and UCB, has received honoraria from Pharmacy Times and UpToDate, and has a patent pending for DACH1-IgG as a biomarker of paraneoplastic autoimmunity; A.S. Lopez-Chiriboga has consulted for Horizon Therapeutics and Genentech; N. Zalewski reports no disclosures relevant to the manuscript. A. Zekeridou has patent applications pending on PDE10A-IgG and DACH1-IgG as biomarkers of paraneoplastic neurologic autoimmunity and has received research funding from Genentech; S.J. Pittock is a named inventor on filed patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker, has patents pending for KLHL11-IgG and Septin-5-IgG and issued for MAP1B-IgG as markers of neurologic autoimmunity and paraneoplastic disorders, has consulted for Alexion and Medimmune, and has received research support from Genentech, Grifols, Medimmune, and Alexion; A. McKeon reports research funding from the NIH (NIH: RO1NS126227, U01NS120901), patents issued for GFAP and MAP1B-IgGs and patents pending for PDE10A, Septins-5 and Septins-7, and KLCHL11-IgGs, and has consulted for Janssen and Roche pharmaceuticals, without personal compensation. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Budhram A, Dubey D, Sechi E, et al. . Neural antibody testing in patients with suspected autoimmune encephalitis. Clin Chem. 2020;66(12):1496-1509. doi. 10.1093/clinchem/hvaa254. [DOI] [PubMed] [Google Scholar]
- 2.Hacohen Y, Wright S, Waters P, et al. . Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry. 2013;84(7):748-755. doi. 10.1136/jnnp-2012-303807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey D, Pittock SJ, Kelly CR, et al. . Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166-177. doi. 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graus F, Titulaer MJ, Balu R, et al. . A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391-404. doi. 10.1016/s1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honorat JA, McKeon A. Autoimmune movement disorders: a clinical and laboratory approach. Curr Neurol Neurosci Rep. 2017;17(1):4. doi. 10.1007/s11910-017-0709-2. [DOI] [PubMed] [Google Scholar]
- 6.Pittock SJ, Yoshikawa H, Ahlskog JE, et al. . Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc. 2006;81(9):1207-1214. doi. 10.4065/81.9.1207. [DOI] [PubMed] [Google Scholar]
- 7.Zalewski NL, Lennon VA, Lachance DH, Klein CJ, Pittock SJ, McKeon A. P/Q- and N-type calcium-channel antibodies: oncological, neurological, and serological accompaniments. Muscle Nerve. 2016;54(2):220-227. doi. 10.1002/mus.25027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budhram A, Freeman E, Bhayana V, Yang L. Positive predictive value of anti-GAD65 ELISA cut-offs for neurological autoimmunity. Can J Neurol Sci. 2022:1-3. doi. 10.1017/cjn.2022.276. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan EP, Hinson SR, Lennon VA, et al. . Glial fibrillary acidic protein immunoglobulin G as biomarker of autoimmune astrocytopathy: Analysis of 102 patients. Ann Neurol. 2017;81(2):298-309. doi. 10.1002/ana.24881. [DOI] [PubMed] [Google Scholar]
- 10.McKeon A, Shelly S, Zivelonghi C, et al. . Neuronal intermediate filament IgGs in CSF: autoimmune axonopathy biomarkers. Ann Clin Transl Neurol. 2021;8(2):425-439. doi. 10.1002/acn3.51284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muniz-Castrillo S, Hedou JJ, Ambati A, et al. . Distinctive clinical presentation and pathogenic specificities of anti-AK5 encephalitis. Brain. 2021;144(9):2709-2721. doi. 10.1093/brain/awab153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabater L, Gaig C, Gelpi E, et al. . A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: a case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014;13(6):575-586. doi. 10.1016/s1474-4422(14)70051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luque FA, Furneaux HM, Ferziger R, et al. . Anti-Ri: an antibody associated with paraneoplastic opsoclonus and breast cancer. Ann Neurol. 1991;29(3):241-251. doi. 10.1002/ana.410290303. [DOI] [PubMed] [Google Scholar]
- 14.Mandel-Brehm C, Dubey D, Kryzer TJ, et al. . Kelch-like protein 11 antibodies in seminoma-associated paraneoplastic encephalitis. N Engl J Med. 2019;381(1):47-54. doi. 10.1056/nejmoa1816721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology. 2011;76(12):1108-1110. doi. 10.1212/wnl.0b013e318211c379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. doi. 10.1212/wnl.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blackburn KM, Denney DA, Hopkins SC, Vernino SA. Low recruitment in a double-blind, placebo-controlled trial of ocrelizumab for autoimmune encephalitis: a case series and review of lessons learned. Neurol Ther. 2022;11(2):893-903. doi. 10.1007/s40120-022-00327-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez A, Klein CJ, Sechi E, et al. . LGI1 antibody encephalitis: acute treatment comparisons and outcome. J Neurol Neurosurg Psychiatry. 2022;93(3):309-315. doi. 10.1136/jnnp-2021-327302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabilondo I, Saiz A, Galan L, et al. . Analysis of relapses in anti-NMDAR encephalitis. Neurology. 2011;77(10):996-999. doi. 10.1212/wnl.0b013e31822cfc6b. [DOI] [PubMed] [Google Scholar]
- 20.Merwick A, Dalmau J, Delanty N. Bickerstaff encephalitis and atypical features–bickerstaff's papers revisited. J Neurol Sci. 2014;341(1-2):173. doi. 10.1016/j.jns.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Dubey D, Wilson MR, Clarkson B, et al. . Expanded clinical phenotype, oncological associations, and immunopathologic insights of paraneoplastic kelch-like protein-11 encephalitis. JAMA Neurol. 2020;77(11):1420. doi. 10.1001/jamaneurol.2020.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKeon A. Autoimmune encephalopathies and dementias. Continuum. 2016;22(2, Dementia):538-558. doi. 10.1212/con.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 23.Flanagan EP, McKeon A, Lennon VA, et al. . Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc. 2010;85(10):881-897. doi. 10.4065/mcp.2010.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castillo P, Woodruff B, Caselli R, et al. . Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol. 2006;63(2):197-202. doi. 10.1001/archneur.63.2.197. [DOI] [PubMed] [Google Scholar]
- 25.Valencia-Sanchez C, Pittock SJ, Mead-Harvey C, et al. . Brain dysfunction and thyroid antibodies: autoimmune diagnosis and misdiagnosis. Brain Commun. 2021;3(2):fcaa233. doi. 10.1093/braincomms/fcaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubey D, Pittock SJ, McKeon A. Antibody prevalence in epilepsy and encephalopathy score: increased specificity and applicability. Epilepsia. 2019;60(2):367-369. doi. 10.1111/epi.14649. [DOI] [PubMed] [Google Scholar]
- 27.Steriade C, Britton J, Dale RC, et al. . Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia. 2020;61(7):1341-1351. doi. 10.1111/epi.16571. [DOI] [PubMed] [Google Scholar]
- 28.Graus F. Towards a better recognition of paraneoplastic brainstem encephalitis. J Neurol Neurosurg Psychiatry. 2021;92(11):1141. doi. 10.1136/jnnp-2021-327386. [DOI] [PubMed] [Google Scholar]
- 29.Orozco E, Guo Y, Chen JJ, et al. . Clinical reasoning: a 43-year-old man with subacute onset of vision disturbances, jaw spasms, and balance and sleep difficulties. Neurology. 2022;99(9):387-392. doi. 10.1212/wnl.0000000000200950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruse JL, Lapid MI, Lennon VA, et al. . Psychiatric autoimmunity: N-Methyl-D-Aspartate receptor IgG and beyond. Psychosomatics. 2015;56(3):227-241. doi. 10.1016/j.psym.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti-N-methyl-d-aspartate receptor encephalitis. JAMA Neurology. 2013;70(9):1133-1139. doi. 10.1001/jamaneurol.2013.3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollak TA, Lennox BR, Muller S, et al. . Autoimmune psychosis: an international consensus on an approach to the diagnosis and management of psychosis of suspected autoimmune origin. Lancet Psychiatry. 2020;7(1):93-108. doi. 10.1016/S2215-0366(19)30290-1. [DOI] [PubMed] [Google Scholar]
- 33.Zoccarato M, Valeggia S, Zuliani L, et al. . Conventional brain MRI features distinguishing limbic encephalitis from mesial temporal glioma. Neuroradiology. 2019;61(8):853-860. doi. 10.1007/s00234-019-02212-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data used for this study are available on request.