Abstract
Depersonalization and derealization refer to an estranged state of mind that involves a profound feeling of detachment from one’s sense of self and the surrounding environment, respectively. The phenomena co-occur on a continuum of severity, ranging from a transient experience as a normal reaction to a traumatic event to a highly debilitating condition with persistent symptoms, formally described as depersonalization/derealization disorder (DPDR). Lack of awareness of DPDR is partly due to a limited neurobiological framework, and there remains a significant risk of misdiagnosis in clinical practice. Earlier literature has focused on several brain regions involved in the experience of depersonalization and derealization, including adaptive responses to stress via defense cascades comprising autonomic functioning, the hypothalamic-pituitary-adrenal (HPA) axis, and various other neurocircuits. Recent evidence has also demonstrated the role of more complex mechanisms that are bolstered by dissociative features, such as emotional dysregulation and disintegration of the body schema. This review intends to abridge the prevailing knowledge regarding structural and functional brain alterations associated with DPDR with that of its heterogenic manifestations. DPDR is not merely the disruption of various sensory integrations, but also of several large-scale brain networks. Although a comprehensive antidote is not available for DPDR, a holistic route to the neurobiological context in DPDR may improve general understanding of the disorder and help afflicted individuals re-establish their sense of personal identity. Such information may also be useful in the development of novel pharmacological agents and targeted psychological interventions.
Keywords: Depersonalization, derealization, dissociation, neurobiology, DPDR, trauma, frontal limbic inhibition, prefrontal cortex, insula, body schema, PNES, amygdala, default-mode network
First coined by Ludovic Dugas in 1898,1 the term depersonalization is defined as detachment from one’s sense of personal identity, wherein subjects experience themselves as outside observers of their own behaviors, emotions, and bodily sensations.2 Likewise, in derealization, one’s environment is perceived as “dreamlike, empty, lifeless, or visually distorted.”3 Because the two states typically co-occur and there is no demonstrable evidence to support that they exist independently of one another, a single identification, called depersonalization/derealization disorder (DPDR), has been formally acknowledged by the American Psychiatric Association (APA) and the International Classification of Diseases (ICD). Patients with DPDR often have great difficulty putting their experiences into words. There is often extremely diminished emotional responsivity, or deaffectualization.3,4 Important memories and personal values seem to no longer belong to oneself, which can provoke intrusive existential ruminations.5 Consider the account of one writer with DPDR: “…I viewed my actions, my internal and external lives, as if observing from the grave. I was visible but not present. And I could find no one, no other human who felt as I did.”6 Though patients with DPDR may have a drastic change in their subjective experience, this change is challenging to measure on psychiatric evaluation. These patients display intact reality testing, preserved emotional expression, and no evidence of psychosis. Since patients can interact and respond appropriately throughout the interview, appropriate assessment of the severity of the disorder is often missed by behavioral health providers.7 Nevertheless, patients are acutely aware of their problem. One stated, “I was not crazy because I knew that something was not right from the very moment that it became not right.”6 Interestingly, experimental psychiatrist Oscar Janiger described DPDR as the opposite of insanity: “It’s like being ’too’ sane, you become hypervigilant of your existence and the things around you.”6
The phenomenon of depersonalization/derealization has received some of the least attention in psychiatric research, despite there being a high rate of lifetime prevalence in the general population; epidemiological studies show between 26 and 74 percent of individuals have short-term symptoms.8 Brief and mild episodes of depersonalization/derealization, lasting from hours to days, are typically not abnormal and may ensue as a transient reaction toward excess fatigue and stress. Episodes may be apparent when there are circadian rhythm shifts, such as with jet lag, or during psychoactive drug use, including alcohol.9 Although these characteristics might be beneficial in endangering situations, as per evolutionary perspectives, they might be equally hindering in day-to-day life activities.3 Depersonalization/derealization can emerge as a secondary symptom of other psychiatric conditions, such as borderline personality disorder (BPD), obsessive compulsive disorder (OCD), major depressive disorder (MDD), or as a dissociative qualifier of posttraumatic stress disorder (PTSD+DS); however, it is only classified as DPDR when these symptoms are not better explained by another mental illness. The distinction of DPDR, as outlined by the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5), is assigned to individuals who “experience unreality or detachment” in respect to themselves (one’s thoughts, feelings, sensations, body, or action) and/or their surroundings, yet the criteria for the disorder does not have a specific duration of symptoms, only that episodes must be “persistent or recurrent.”10 Typically, there is a chronic, unremitting progression lasting months to years, and the disorder is associated with significant impairment in social and occupational functioning.
The prevalence of DPDR in the general population is estimated to be as high as 1 to 2 percent worldwide.11 Studies of subjects with DPDR have revealed that the disorder has equal occurrence in both men and women, with a mean age of onset in adolescence; only five percent of cases start in persons above the age of 25 years, and occurrence beyond the age of 40 years is rare.12 However, due to a lack of recognition among medical and mental health professionals, DPDR can linger untreated for a long time; a correct diagnosis of DPDR takes an average of 7 to 12 years to obtain.8,9 Once diagnosed, DPDR typically has a poor response to pharmacotherapy.2,7
The current review will revisit the works of literature available on the neurobiology involved in DPDR. Discerning the diverse neural processes that underlie this prevalent, yet often-neglected, psychiatric disorder could aid in better diagnosis and treatment moving forward.
METHOD OF SEARCH
The methodology of this study is a scoping review. It differs from systematic reviews in both purpose and aim. This approach is useful when data on a topic of inquiry has yet to be comprehensively reviewed. The method is also appropriate when the subject of analysis is complex or heterogeneously diverse. The scoping review framework has several identifiable objectives, including 1) clarifying key concepts and definitions, 2) identifying and locating types of existing evidence, and 3) summarizing research results and identifying knowledge gaps, which was critical to the present study.13 This analysis was thus prepared based on existing or secondary data. Publications were chosen predominantly from 2014 to 2021, although some older articles were also included to establish the groundwork. PubMed, Google Scholar, Science Direct, and Web of Knowledge were used to search peer-reviewed articles to identify the neurobiological underpinnings of DPDR.
DEPERSONALIZATION/DEREALIZATION AS A RESPONSE TO TRAUMA
Dissociative symptoms, including depersonalization/derealization, are thought to be the result of a vestigial reaction to events perceived as life-threatening.1 It has been shown that there exists an especially strong relationship between early interpersonal trauma and dissociative disorders.14 In one study, 198 psychiatric patients, ranging from 11 to 19 years of age, were administered the Adolescent Dissociative Experience Scale to determine if the degree of dissociative experience correlated with childhood trauma, which was measured through a checklist of various kinds of traumatic events. The study revealed that children who had experienced a history of neglect, abuse (physical and sexual), and stressful life events had a much higher degree of dissociative experiences than those who did not. Emotional neglect was determined to be the most substantial pathogenic risk factor.15 It has been suggested that these highly stressful encounters fail to fit into the subject’s cognitive scheme regarding the self, others, and the world; therefore, they split off from consciousness. When seen in this way, dissociation is part of the brain’s effort to eliminate the salience of painful memories, with the unintended consequence of causing intrusive thoughts and emotional blunting.16
Recent research exploring disorders with a traumatogenic etiology, such as PTSD+DS, has shed light on the neural processes involved in DPDR.17 When traumatic memories surface, nondissociative patients with PTSD demonstrate activity associated with emotional hyperarousal, such as an accelerated heart rate, reduced activation of prefrontal regions, and increased activation of the amygdala.17,18 In contrast, the dissociative subtype of PTSD shows the opposite pattern, with slowed heart rate, increased prefrontal activity, and decreased activity of the amygdala. As a result, it is thought that dissociation might be used to regulate elevated arousal in PTSD through limbic hyperinhibition (Figure 1).18 This strategy represents a hard-wired survival response meant to reduce anxiety, while inducing a state of heightened attention through executive brain areas.19 However, when this response continues past the threatening situation, depersonalization/derealization may persist maladaptively, maintained by a negative feedback loop in response to the uncomfortable symptoms.20
FIGURE 1.
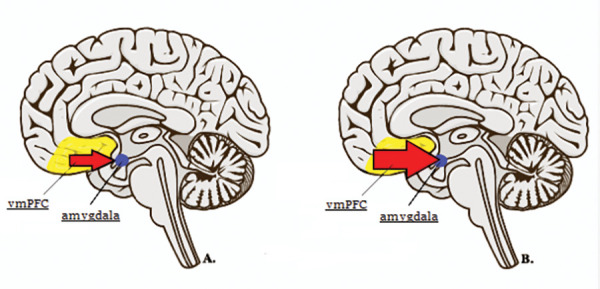
Recent studies on dissociation have investigated the neurophysiology of trauma responses. A. Classical or nondissociative patients with posttraumatic stress disorder (PTSD) demonstrate activity associated with hyperarousal; the corresponding brain activity is lessened ventromedial prefrontal cortex (vmPFC) inhibition of limbic activity. B. The dissociative subtype of posttraumatic stress disorder (PTSD+DS) demonstrates the opposite pattern of activity, involving overmodulation of limbic activity via vmPFC hyperactivation.
Psychological trauma has also been correlated with psychosomatic dissociative or somatoform symptoms (e.g., seizures). In epilepsy (ES), repetitive focal excitement, or kindling, irreversibly disrupts the balance of neural excitation and inhibition, which leads to a permanent state of excitability and spontaneous convulsions. Likewise, it is hypothesized that ongoing traumatic stress might play a role in kindling inhibitory systems implicated in sustained dissociative states, which might therefore bring about the development of seizure-like symptoms via a lowered excitatory threshold.16 Depersonalization/derealization may be a symptom evident in both psychogenic nonepileptic seizures (PNES) and ES. A mixed-methods, content analysis study differentiating PNES from ES showed that, compared to ES, PNES reported longer seizures, more anxiety symptoms, dissociative phenomena, and alexithymia, the reduced ability to recognize and express emotions.21 Estimates suggest about 75 percent of adults with PNES report prior physical, sexual, or emotional trauma, with those reporting prior sexual abuse having earlier event onset and more severe convulsions.21
NEUROBIOLOGY OF DPDR
Early explanations of depersonalization/derealization theorized that it was a vestigial brain response to life-threatening conditions. More recent discoveries suggest that it might be related to temporal lobe dysfunction. However, little is known about its biological underpinnings.1,7 Studies have opined that depersonalization and derealization might have distinct neuroanatomical correlates. For instance, one study investigated the relevance of the lesion technique in separating depersonalization and derealization. The study found that asomatognosia (the loss of conscious awareness of oneself and bodily processes) may serve as the biological model of depersonalization, as it can be attributed to lesions in several neurological conditions.22 Another study concluded that depersonalization is a distinct result of frontal lobe, as opposed to temporal lobe, misfiring, when comparing depersonalization and derealization postictal sequalae in ES.23 However, other studies have yielded outcomes that postulate left-hemispheric frontotemporal activation may be involved in both depersonalization and derealization.24 Although the features of depersonalization and derealization are phenomenologically distinct, little scholarly evidence demonstrates that depersonalization can manifest independently of derealization.25 As a result, this review examines depersonalization/derealization as an individual pathology.
FRONTOLIMBIC INHIBITION AND EMOTIONAL NUMBING
First posited by Geschwind,26 and later by Sierra and Berrios,1 the concept of corticolimbic disconnection is one model that explains the neurobiology of DPDR. This model presupposes that DPDR occurs because of excitatory amygdala circuits that modulate ascending arousal systems, leading to right prefrontal cortex (PFC) hyperactivation. A reciprocal inhibitory element mediated by PFC hyperactivation then impedes the limbic system, which is involved in experiencing emotions, and dampens sympathetic outputs.1,26 It is thought that activation of PFC functions inhibits the anterior cingulate cortex (ACC), an area that plays a key role in coordinating bottom-up and top-down processing within fear regulation circuitry. The ACC has extensive connections with cognitive, emotional, and motor processing areas.27 The sum of these activities is a hyperattentive state and a subsequent overmodulation of emotions—findings consistent with those in patients with PTSD+DS.17,18 Patients with PTSD+DS were also found to have enhanced top-down connectivity from the ventromedial PFC (vmPFC) to another key brain area, the periaqueductal gray (PAG). Consistent with other studies, patients with PTSD without predominant depersonalization and derealization showed the opposite pattern of connectivity.28 This dissonance between cortical and subcortical areas is corroborated by neuroimaging of patients with DPDR.
In a functional magnetic resonance imaging (fMRI) study, patients with DPDR who viewed images meant to elicit revulsion had less activation compared to controls in the occipitotemporal cortex, amygdala, and insula, all areas responsible for perceiving disgust. Instead, patients with DPDR showed activation of the right ventral PFC. The insula of patients with DPDR was shown to be active when viewing neutral images. This confirms that in depersonalization/derealization, circuitry involved in processing emotionally salient imagery may be downregulated by the PFC.29 These results were validated in a similar study that showed that patients with DPDR exhibited decreased activation of limbic areas, including the right amygdala and right hypothalamus, with comparatively increased activation in various prefrontal regions, compared to controls.30,31
What accounts for the feelings of unfamiliarity or alienation from one’s environment and sense of self in DPDR may also be explained by the frontolimbic inhibition model. The amygdala is not only involved in emotional responses to external stimuli, but also stores implicit procedural memories. A hypoactive limbic system would therefore indicate difficulty recalling firmly etched past experiences. Whereas the hippocampus primarily stores verbal, declarative memories, the amygdala stores their personal salience, or affective quality.4 When a memory reaches a traumatic threshold, the hippocampus cannot store an explicit memory trace, as in dissociative amnesia, because it cannot contextualize the threat.32,33 Furthermore, it is acknowledged in the literature that hippocampal volume shrinks in PTSD and chronic stress.34
MULTISENSORY INTEGRATION AND THE BODY SCHEMA
It has been established that the incorporation of bodily sensations into conscious awareness is critical to the experience of emotional states.35 This finding shed light on the relationship of afferent somatosensory signals with the production of both subjective feelings and interoceptive awareness, or the perception of sensations from inside the body, related to internal organ functions (e.g., heartbeat, satiety, gastrointestinal motility, respiration, autonomic activity, etc.).36 Activation of the insula has been broadly implicated in a range of functional neuroimaging experiments involving emotional states and internal and external physical sensations. The insula is one of the most complex and least understood brain areas, mainly due to its location deep within the lateral sulcus, which makes it difficult to assess, as well as a low prevalence of lesion studies.37 The structure is known to be responsible for a wide array of functions, including pain processing, salience detection, sensorimotor functioning, and the interoceptive inputs implicated in generating the body schema.37,38 Reviews that have directly queried interoception have found anterior insula activity to be positively correlated with such awareness, as well as self-reported emotional experience.39 The posterior insula has been shown to be involved in the initial recognition of internal body changes via input from limbic, thalamic, and brainstem networks, while the midinsula interacts with both the anterior and posterior insula to aid in translating visceral sensory input into emotions. The core phenomenon of DPDR—the overmodulation of the subjective experience of emotion—remains evident in these studies. According to an earlier fMRI study in 2001 examining the brain activity of patients with DPDR while viewing aversive imagery, the anterior insula appears to be underactive during emotional stimulation, whereas prefrontal activity increases.29 Notably, the insula is of particular relevance in understanding the universal neurobiological correlates of psychopathology. A study examined over 15,000 MRI brain images with matched controls across six diagnostic groups (bipolar disorder, anxiety, depression, addiction, OCD, and schizophrenia) and found shared patterns of decreased gray matter in the anterior insula and dorsal anterior cingulate.40
Higher cortical areas involved in sensory and somatic integration are also likely to mediate depersonalization/derealization. Hypoemotionality may result from lesions in temporal-parietal regions.41 Using positron emission tomography (PET), one study compared patients with DPDR to healthy controls and found that activation in the temporal and parietal sensory association cortex differed significantly from that of the control group. These findings indicated abnormal communication between visual, auditory, and somatosensory cortices, as well as the inability to generate a fully integrated body schema.7
With the PTSD-dissociation relationship having been established, some studies sought to determine the degree to which patients with PTSD were vulnerable to altered perceptions of bodily ownership. For instance, one fMRI study focused on the link between PTSD and alterations in bodily self-consciousness using the Rubber Hand Illusion (RHI).42 RHI involves the manipulation of tactile, visual, and proprioceptive sensory inputs from a person’s hidden hand by brushing the hidden real hand and a believable, visible rubber hand simultaneously. The illusion is that the rubber hand seems to substitute for the hidden real hand. This experiment was administered to patients with PTSD, patients with PTSD+DS, and healthy controls. Patients with PTSD showed a lower illusion effect, depicting rigid body representation as a cognitive avoidance strategy that weakened the impact of body ownership manipulation, whereas the PTSD+DS group showed a state-dependent body representation, indicating higher susceptibility to manipulation of embodiment.42
DEPERSONALIZATION/DEREALIZATION AND SEIZURES
The link between depersonalization/derealization and seizures is well-established in the clinical literature.24 There are reports of depersonalization/derealization across other neurological conditions as well, such as postencephalitic stage, migraine, cerebrovascular disease, and head trauma. However, the correlations to neural mechanisms remain limited.1 A review of studies examining the co-occurrence of neuropsychiatric disorders with organic disorders found dissociative symptoms, such as fugue and trance-like states, delusions of possession, delusions of self-misidentification, and out-of-body experiences, were dominant in migraine and ES, especially of the temporal lobe.43 An examination of aura in the pathology of temporal lobe ES found derealization, dreamy states, altered time, and dysmnesic symptoms (déjà vu and jamais vu).1,44
The Dissociative Experiences Scale (DES-II) has been a popular tool in uncovering the link between depersonalization/derealization, ES, and other types of seizures. In patients with ES, dissociative symptoms show a positive correlation with shorter duration and higher frequency of seizures.45 As aforementioned, patients with PNES have a longer duration of seizures than those with ES; these patients are more likely to report a history of interpersonal trauma, with earlier life abuse correlated with the severity of convulsions.21 In one study, the DES-II was administered to patients with concurrent ES and PNES. The results showed significantly higher DES-II scores in patients with both ES and PNES than the other two groups (epilepsy-only and healthy controls).46
Factors of the DES-II used in these studies comparing ES and PNES include depersonalization-derealization (e.g., other people and objects do not seem real, feeling as though one’s body is not one’s own, not recognizing oneself in the mirror, being in a familiar place but feeling it is strange and unfamiliar), inattentiveness, absorption-imaginative factors, amnesia, and reality distortion.47 In some survey studies, the amnesia factor showed evidence of memory loss being attributable to solely neurological or medical causes, as the ES group had higher scores in this domain. An important stipulation to point out is that this result is noted to have several flaws.48 Two of the seven amnesia elements (“approached by people who one doesn’t know or who call one by a different name” and “feeling of watching oneself as if looking at another person”) might not solely constitute a lapse of memory, but could instead be a profound disturbance of self-identity. Furthermore, as mentioned in the previous section on frontolimbic inhibition in DPDR, the item, “told of one not recognizing friends and family people,” might also be related to amygdaloid disconnections of the dissociative process. Notably, if the survey results looking at the DES-II amnesia factor indicated memory loss related to ES alone, the studies would have revealed a significant difference between the PNES and ES groups independent of child abuse histories, which they did not.
In summary, the DES-II has separate domains that relate distinctively to depersonalization/derealization, neurological impairment, and childhood and/or interpersonal trauma.49 The heterogeneous item content of the DES-II is a potential confound that should be appreciated when studying dissociation within neuropsychiatric populations. Though dissociative symptoms have related processes, depersonalization/derealization should still be viewed as distinct when the scale is used. Overall, there are positive correlations between DPDR and neurological impairment, which are likely mediated by trauma.48
STRUCTURAL CHANGES AND LARGE-SCALE ATTENTIONAL NETWORKS
Neuroimaging studies on DPDR have also revealed variations in cortical thickness. Symptom severity was positively correlated with gray matter structural alterations.46 Certain structural MRI studies showed decreased cortical thickening in the right middle temporal region and increased size of the gyrus rectus, left dorsomedial PFC, and right somatosensory region in these patients.46 Further supporting evidence showed that severe depersonalization was especially correlated with greater precuneus volume.50,51 The precuneus is a higher level cortical area dedicated to a wide variety of integrative complex tasks. It is considered a hub for multimodal sensory processing, playing a major role in generating visuospatial imagery and coordinating goal-directed motor movement.52 The structure is thus known to be highly implicated in self-referential processing operations, such as generating body image representation.52,25 In corroboration, another study also analyzing cortical/subcortical thickness and volume within dissociative and functional neurologic disorders showed a positive correlation between DPDR severity and cortical thickness in visual association areas.53
Several studies have demonstrated the role of specific DPDR-associated alterations in several brain structures and their functions.1,23,28,33,54 The first known study on aberrations of structural white matter connections in patients with DPDR showed that, compared to controls, patients with DPDR had disrupted connections between the left superior temporal gyrus and left temporal poles, as well as between the right middle temporal gyrus and right supramarginal gyrus.55 The degree of disconnection correlated with dissociative symptom severity in the DPDR group. This research strongly points to the role of local neuronal changes that may be arbitrated by altered interregional connections of white matter. These irregularities of communication between fiber tracts may not solely be a consequence of volume loss concerning the gray matter, but rather primary pathophysiology. Therefore, the heterogenic symptomology of DPDR may be linked to network-level dysfunctions representing abnormal connectivity of the fiber tracts, thereby informing a transdiagnostic perspective.55
Studies on the role of the orbitofrontal cortex (OFC) provide further insight into the absence of self-concept in DPDR. This region has nerve fibers descending to the amygdala and is generally implicated in social and emotional reasoning.56,57 In an analysis examining functional connectivity of the precuneus in 282 patients with MDD and 254 controls, 125 patients receiving medication for depression demonstrated lessening activity between the lateral OFC and precuneus to levels similar to those of controls.56 Other studies showed evidence that connections between the OFC and precuneus provide emotional information involved in recalling autobiographical memory.52,58 In healthy subjects, the OFC responds to many punishing stimuli that are typically meant to elicit visceral responses of disgust or revulsion via the anterior insula. It is important to note that, regarding the connections involved in autonomic responses, the anterior insula is overactive in depression and underactive in DPDR. These findings corroborate the notion that the lateral OFC’s nonreward or penal system has enhanced effects on self-representational regions, including the precuneus, which might help explain poor self-esteem in patients with depression.57 Interestingly, these regions have been shown to be underactive in DPDR, which might represent an overmodulation effect to these same self-representational areas.29
Distorted self-image has been investigated within many different regions, including the right frontal, orbitofrontal, and medial-frontal regions, along with the limbic system, corpus callosum, and subcortical-cortical midline structures, including hypothalamus/hypophysis, temporoparietal junction, bilateral temporal poles, insular cortex, frontal region, ventro- and dorsolateral and medial PFC, parietal cortex, and frontolimbic networks. Other regions evident in self-image include the brain stem; PAG; colliculi; sub-, pre-, and supragenual anterior cingulate cortex; retrosplenial cortex; and posterior cingulate cortex.54 This provides evidence for the role of large-scale brain networks, most notably the default mode network (DMN), for the mental expression of one’s sense of self, given the multiple processes involved.
One study compared brain activity during externalized cognition involving visuospatial planning (attention to the environment) to brain activity during internalized cognition involving autobiographical planning (attention to personal information).59 The study showed that autobiographical planning activated the DMN, whereas visuospatial planning engaged another large-scale circuit, the dorsal attentional network (DAN). The critical finding, however, was that a third network, the frontoparietal network (FPN), was highly active during both forms of planning. It was previously thought that the activity of the DMN and DAN had an intrinsically competitive relationship. However, the FPN is now believed to be involved in the mediation of planning across different attentional domains.60
Largely found in the posterior parietal cortex and dorsolateral PFC, the FPN serves as a cortical mediator, guiding goal-directed cognition across major neurocircuits.59 Because it interacts with both the DAN and DMN, it is responsible for the active manipulation and maintenance of working memory, utilizing both environmental and autobiographical information.60 The DMN is located throughout the medial posterior cingulate cortex, angular gyrus, medial temporal lobe, and vmPFC. It is most active during internal mental-state tasks, such as interoception and episodic memory retrieval, and is suppressed during focused attention to external stimuli. Notably, the precuneus is a functional core of the DMN.61,62 Though the interaction of the DMN and FPN is necessary to regulate goal-directed behavior, the literature now demonstrates that hyperconnectivity between these networks is correlated with high degrees of dissociation.59,63–65 This represents the maladaptive integration of internal mental processes involved in DPDR within higher level neurocircuitry.
CONCLUSION
DPDR is thought to be a complex behavioral response to psychological trauma. Though the underlying mechanisms are not entirely understood, several theories using existing models of neural circuitry have identified mechanisms that are thought to contribute to the symptomatic profile observed in those who experience chronic and persistent dissociation. It is unlikely that any one of these mechanisms alone can fully explain DPDR; instead, it is likely that many of these neural pathways together contribute to its pathogenesis. Currently, no definitive treatment exists to alleviate symptoms of dissociation. Pharmacologic interventions should be part of a comprehensive formulation of a treatment plan addressing the biopsychosocial elements at play. Different psychotherapies may endeavor to strengthen the relationship between the cerebral cortex and subcortical regions by integrating the various aspects of the self, bodily, emotionally, and representationally. Further research is needed on the relevant neural networks and neurotransmitters to better understand the mechanism behind depersonalization/derealization.
REFERENCES
- Sierra M, Berrios GE. Depersonalization: neurobiological perspectives. Biol Psychiatry. 1998;44(9):898–908. doi: 10.1016/s0006-3223(98)00015-8. [DOI] [PubMed] [Google Scholar]
- Simeon D, Guralnik O, Schmeidler J et al. Fluoxetine therapy in depersonalisation disorder: randomised controlled trial. Br J Psychiatry. 2004;185:31–36. doi: 10.1192/bjp.185.1.31. [DOI] [PubMed] [Google Scholar]
- Adler J, Beutel ME, Knebel A et al. Altered orientation of spatial attention in depersonalization disorder. Psychiatry Res. 2014;216(2):230–235. doi: 10.1016/j.psychres.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Medford N. Emotion and the unreal self: depersonalization disorder and de-affectualization. Emot Rev. 2012;4(2):139–144. [Google Scholar]
- Medford N, Sierra M, Baker D et al. Understanding and treating depersonalisation disorder. Adv Psychiatr Treat. 2005;11(9):92–100. [Google Scholar]
- Abugel J. Stranger to Myself: Inside Depersonalization: The Hidden Epidemic. 2011. Johns Road Publishing.
- Simeon D, Guralnik O, Hazlett EA et al. Feeling unreal: a PET study of depersonalization disorder. Am J Psychiatry. 2000;157(11):1782–1788. doi: 10.1176/appi.ajp.157.11.1782. [DOI] [PubMed] [Google Scholar]
- Hunter ECM, Sierra M, David AS. The epidemiology of depersonalisation and derealisation. A systematic review. Soc Psychiatry Psychiatr Epidemiol. 2004;39(1):9–18. doi: 10.1007/s00127-004-0701-4. [DOI] [PubMed] [Google Scholar]
- Medford N, Baker D, Hunter E et al. Chronic depersonalization following illicit drug use: a controlled analysis of 40 cases. Addiction. 2003;98(12):1731–1736. doi: 10.1111/j.1360-0443.2003.00548.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition. American Psychiatric Association; 2013.
- Jinyan Y, Millman LS, David AS, Hunter E. The prevalence of depersonalization-derealization disorder: a systematic review. J Trauma Dissociation. 2023;24(1):8–41. doi: 10.1080/15299732.2022.2079796. [DOI] [PubMed] [Google Scholar]
- Spiegel D. Depersonalization/derealization disorder. Merck Manuals. https://www.merckmanuals.com/professional/psychiatric-disorders/dissociative-disorders/depersonalization-derealization-disorder Modified Sep 2022. Accessed 20 Jan 2023.
- Sucharew H, Macaluso M. Progress notes: methods for research evidence synthesis: the scoping review approach. J Hosp Med. 2019;14(7):416–418. doi: 10.12788/jhm.3248. [DOI] [PubMed] [Google Scholar]
- Simeon D, Guralnik O, Knutelska M et al. Hypothalamic-pituitary-adrenal axis dysregulation in depersonalization disorder. Neuropsychopharmacology. 2001;25(5):793–795. doi: 10.1016/S0893-133X(01)00288-3. [DOI] [PubMed] [Google Scholar]
- Brunner R, Parzer P, Schuld V et al. Dissociative symptomatology and traumatogenic factors in adolescent psychiatric patients. J Nerv Ment Dis. 2000;188(2):71–77. doi: 10.1097/00005053-200002000-00002. [DOI] [PubMed] [Google Scholar]
- Bob P: Pain, dissociation and subliminal self-representations. Conscious Cogn. 2008;17(1):355–369. doi: 10.1016/j.concog.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Vermetten E, Loewenstein RJ et al. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry. 2010;167(6):640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Huijstee J, Vermetten E. The dissociative subtype of post-traumatic stress disorder: research update on clinical and neurobiological features. Curr Top Behav Neurosci. 2018;38:229–248. doi: 10.1007/7854_2017_33. [DOI] [PubMed] [Google Scholar]
- Gentile JP, Snyder M, Marie Gillig P. Stress and trauma: psychotherapy and pharmacotherapy for depersonalization/derealization disorder. Innov Clin Neurosci. 2014;11(7–8):37–41. [PMC free article] [PubMed] [Google Scholar]
- Hunter ECM, Phillips ML, Chalder T et al. Depersonalisation disorder: a cognitive-behavioural conceptualisation. Behav Res Ther. 2003;41(12):1451–1467. doi: 10.1016/s0005-7967(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Perez DL, LaFrance WC., Jr Nonepileptic seizures: an updated review. CNS Spectr. 2016;21(3):239–246. doi: 10.1017/S109285291600002X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M, Senior C, Dalton J et al. Autonomic response in depersonalization disorder. Arch Gen Psychiatry. 2002;59(9):833–838. doi: 10.1001/archpsyc.59.9.833. [DOI] [PubMed] [Google Scholar]
- Hollander E, Carrasco JL, Mullen LS et al. Left hemispheric activation in depersonalization disorder: a case report. Biol Psychiatry. 1992;31(11):1157–1162. doi: 10.1016/0006-3223(92)90161-r. [DOI] [PubMed] [Google Scholar]
- Heydrich L, Marillier G, Evans N et al. Depersonalization- and derealization-like phenomena of epileptic origin. Ann Clin Transl Neurol. 2019;6(9):1739–1747. doi: 10.1002/acn3.50870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra M, Berrios GE. The phenomenological stability of depersonalization: comparing the old with the new. J Nerv Ment Dis. 2001;189(9):629–636. doi: 10.1097/00005053-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I Brain. 1965;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Stevens FL. The anterior cingulate cortex in psychopathology and psychotherapy: effects on awareness and repression of affect. Neuropsychoanalysis. 2016;18(1):53–68. [Google Scholar]
- Harricharan S, Rabellino D, Frewen PA. et al. fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype. Brain Behav. 2016. 6 12e00579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Medford N, Senior C et al. Depersonalization disorder: thinking without feeling. Psychiatry Res. 2001;108(3):145–160. doi: 10.1016/s0925-4927(01)00119-6. [DOI] [PubMed] [Google Scholar]
- Medford N, Sierra M, Stringaris A et al. emotional experience and awareness of self: functional MRI studies of depersonalization disorder. Front Psychol. 2016;7:432. doi: 10.3389/fpsyg.2016.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemche E, Sierra-Siegert M, David AS et al. Cognitive load and autonomic response patterns under negative priming demand in depersonalization-derealization disorder. Eur J Neurosci. 2016;43(7):971–978. doi: 10.1111/ejn.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Brierley B, Brammer M et al. Emotional memory in depersonalization disorder: a functional MRI study. Psychiatry Res. 2006;148(2–3):93–102. doi: 10.1016/j.pscychresns.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Boyd JE, McKinnon MC et al. A review of the neurobiological basis of trauma-related dissociation and its relation to cannabinoid- and opioid-mediated stress response: a transdiagnostic, translational approach. Curr Psychiatry Rep. 2018;20(12):118. doi: 10.1007/s11920-018-0983-y. [DOI] [PubMed] [Google Scholar]
- Logue MW, van Rooij SJH, Dennis EL et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83(3):244–253. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio A. Feelings of emotion and the self. Ann N Y Acad Sci. 2003;1001:253–261. doi: 10.1196/annals.1279.014. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Nomi JS, Hébert-Seropian B et al. Structure and function of the human insula. J Clin Neurophysiol. 2017;34(4):300–306. doi: 10.1097/WNP.0000000000000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harricharan S, Nicholson AA, Thome J et al. PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology. 2020;57(1):e13472. doi: 10.1111/psyp.13472. [DOI] [PubMed] [Google Scholar]
- Lemche E, Brammer MJ, David AS et al. Interoceptive-reflective regions differentiate alexithymia traits in depersonalization disorder. Psychiatry Res. 2013;214(1):66–72. doi: 10.1016/j.pscychresns.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkind M, Eickhoff SB, Oathes DJ et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305–315. doi: 10.1001/jamapsychiatry.2014.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V, Andermann F, Rasmussen T et al. Parietal lobe epilepsy. Clinical manifestations and outcome in 82 patients treated surgically between 1929 and 1988. Brain. 1995;118(Pt 3):607–627. doi: 10.1093/brain/118.3.607. [DOI] [PubMed] [Google Scholar]
- Rabellino D, Densmore M, Harricharan S et al. Resting-state functional connectivity of the bed nucleus of the stria terminalis in post-traumatic stress disorder and its dissociative subtype. Hum Brain Mapp. 2018;39(3):1367–1379. doi: 10.1002/hbm.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MV, Sierra M, Phillips ML et al. The spectrum of organic depersonalization: a review plus four new cases. J Neuropsychiatry Clin Neurosci. 2002;14(2):141–154. doi: 10.1176/jnp.14.2.141. [DOI] [PubMed] [Google Scholar]
- Mula M, Pini S, Cassano GB. The neurobiology and clinical significance of depersonalization in mood and anxiety disorders: a critical reappraisal. J Affect Disord. 2007;99(1–3):91–99. doi: 10.1016/j.jad.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Hara K, Adachi N, Akanuma N et al. Dissociative experiences in epilepsy: effects of epilepsy-related factors on pathological dissociation. Epilepsy Behav. 2015;44:185–191. doi: 10.1016/j.yebeh.2014.12.018. [DOI] [PubMed] [Google Scholar]
- Özdemir O, Cilingir V, Özdemir PG et al. Dissociative experiences in patients with epilepsy. Arq Neuro-Psiquiatr. 2016;74(3):189–194. doi: 10.1590/0004-282X20160045. [DOI] [PubMed] [Google Scholar]
- Yokokawa K, Ito T, Takahata K et al. Neuromolecular basis of faded perception associated with unreality experience. Sci Rep. 2018;8(1):1–9. doi: 10.1038/s41598-018-26382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bob P. Dissociation and neuroscience: history and new perspectives. Int J Neurosci. 2003;113(7):903–914. doi: 10.1080/00207450390220376. [DOI] [PubMed] [Google Scholar]
- Alper K, Devinsky O, Perrine K et al. Dissociation in epilepsy and conversion nonepileptic seizures. Epilepsia. 1997;38(9):991–997. doi: 10.1111/j.1528-1157.1997.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Daniels JK, Gaebler M, Lamke J-P et al. Grey matter alterations in patients with depersonalization disorder: a voxel-based morphometry study. J Psychiatry Neurosci. 2015;40(1):19–27. doi: 10.1503/jpn.130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irle E, Lange C, Weniger G et al. Size abnormalities of the superior parietal cortices are related to dissociation in borderline personality disorder. Psychiatry Res. 2007;156(2):139–149. doi: 10.1016/j.pscychresns.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Krause-Utz A, Frost R, Winter D et al. Dissociation and alterations in brain function and structure: implications for borderline personality disorder. Curr Psychiatry Rep. 2017;19(1):6. doi: 10.1007/s11920-017-0757-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Sierra M, Nestler S, Jay E-L et al. A structural MRI study of cortical thickness in depersonalisation disorder. Psychiatry Res. 2014;224(1):1–7. doi: 10.1016/j.pscychresns.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Coyle JT, Rowland LP. Neurobiology of Brain Disorders: Biological Basis of Neurological and Psychiatric Disorders. 2015. Academic Press.
- Perez DL, Matin N, Williams B et al. Cortical thickness alterations linked to somatoform and psychological dissociation in functional neurological disorders. Hum Brain Mapp. 2018;39(1):428–439. doi: 10.1002/hbm.23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B, Cohen D. Neuroscience, Selflessness, and Spiritual Experience: Explaining the Science of Transcendence. 2019. Academic Press.
- Sierk A, Daniels JK, Manthey A et al. White matter network alterations in patients with depersonalization/derealization disorder. J Psychiatry Neurosci. 2018;43(4):170110. doi: 10.1503/jpn.170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W, Rolls ET, Qiu J et al. Increased functional connectivity of the posterior cingulate cortex with the lateral orbitofrontal cortex in depression. Transl Psychiatry. 2018;8(1):90. doi: 10.1038/s41398-018-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Dissociation by network integration. Am J Psychiatry. 2021;178(2):110–112. doi: 10.1176/appi.ajp.2020.20121728. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Cheng W, Du J et al. Functional connectivity of the right inferior frontal gyrus and orbitofrontal cortex in depression. Soc Cogn Affect Neurosci. 2020;15(1):75–86. doi: 10.1093/scan/nsaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34(3):932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather Hsu C-C, Rolls ET, Huang C-C et al. Connections of the human orbitofrontal cortex and inferior frontal gyrus. Cereb Cortex. 2020;30(11):5830–5843. doi: 10.1093/cercor/bhaa160. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP et al. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebois LAM, Li M, Baker JT et al. Large-scale functional brain network architecture changes associated with trauma-related dissociation. Am J Psychiatry. 2021;178(2):165–173. doi: 10.1176/appi.ajp.2020.19060647. [DOI] [PMC free article] [PubMed] [Google Scholar]


