Abstract
Background
Refeeding syndrome (RFS) can occur in malnourished patients when normal, enteral, or parenteral feeding is resumed. The syndrome often goes unrecognized and may, in the most severe cases, result in death. The diagnosis of RFS can be crucially facilitated by the use of clinical decision support systems (CDSS).
Methods
The literature in PubMed was searched for current treatment recommendations, randomized intervention studies, and publications on RFS and CDSS. We also took account of insights gained from the development and implementation of our own CDSS for the diagnosis of RFS.
Results
The identification of high-risk patients and the recognition of manifest RFS is clinically challenging due to the syndrome’s unspecific symptoms and physicians’ lack of awareness of the risk of this condition. The literature shows that compared to patients without RFS, malnourished patients with RFS have significantly greater 6-month mortality (odds ratio 1.54, 95% confidence interval: [1.04; 2.28]) and an elevated risk of admission to intensive care (odds ratio 2.71 [1.01; 7.27]). In a prospective testing program, use of our own CDSS led to correct diagnosis in two thirds of cases.
Conclusion
RFS is difficult to detect and represents a high risk to the patients affected. Appropriate CDSS can identify such patients and ensure proper professional care.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. The questions on this article can be found at http://daebl.de/RY95. The submission deadline is 16 February 2024.
Participation is possible at cme.aerztebatt.de
Malnourished patients in whom feeding is resumed following significantly reduced or no caloric intake lasting at least 5 days are at risk for refeeding syndrome (RFS). Any form of feeding can cause RFS: oral, enteral, parenteral as well as simple normal meals. Enteral feeding poses the highest risk for RFS (1).
The cardinal symptom is considered to be hypophosphatemia with onset within 2–5 days of resumed feeding, accompanied by hypokalemia and/or hypomagnesemia (2). This can be associated with severe fluid shifts and cause central and peripheral edema. In addition, neuromuscular, cardiac, and central nervous complications such as tachycardia and impaired cognition may develop.
RFS often goes unrecognized due to the fact that its symptoms are nonspecific (3). By way of example, a recent survey of 281 physicians clinically active in Germany and based on sample case vignettes of RFS found that only 14 % of physicians and nutritionist teams correctly diagnosed RFS (4). Moreover, half of the respondents in audits (New Zealand and the United Kingdom) of prescribing practices in parenteral nutrition (PN) were unable to identify patients at risk or name risk factors for RFS despite correct determination of serum electrolytes (5, 6).
The clinical relevance of RFS is high: If unrecognized and untreated, fatal treatment outcomes are possible (7, 8). Treatment includes replacement therapy of the affected electrolytes and other vitamins (in particular, vitamin B1), as well as the reintroduction of feeding in a calorie-reduced manner. Against the background of the diagnostic challenges posed by RFS, a clinical decision support system (CDSS) for the automated diagnosis of the syndrome was newly developed and tested at the University of Leipzig Medical Center, Germany.
Methods
A selective literature search on RFS was carried out in the PubMed database on the basis of current treatment recommendations, meta-analyses, systematic review articles, randomized controlled studies, cohort studies, and observational studies. Publications in English and German from between 1990 and 2022 were taken into consideration. Particular focus was placed on publications relating to RFS in conjunction with CDSS. The following search terms were used: “refeeding syndrome” [AND] “diagnosis,” “recognition,” “incidence,” “management,” “prospective” “clinical decision support system,” and “CDSS.” Practical experience with the use of our own CDSS for RFS diagnosis in inpatient care was additionally taken into consideration.
Definition and epidemiology
There is no standardized definition of RFS still today. In April 2020, the American Society for Parenteral and Enteral Nutrition (ASPEN) published consensus recommendations on RFS, thereby offering for the first time standardized diagnostic criteria for future studies. RFS is defined as falls in serum levels of phosphate, potassium, and/or magnesium by 10–20% (mild), 20–30% (moderate), or > 30% (severe), with or without organ damage, within 5 days of resumption of previously strongly decreased caloric intake (2). A significantly decreased caloric intake is defined as a reduced food intake over at least 5 days, meeting less than 50% of the individual patient’s calorie requirements. For the identification of risk patients, application of the British National Institute for Health and Care Excellence (NICE) criteria (9) has become established in clinical practice (table 1).
Table 1. Criteria of the National Institute for Health Care and Excellence for the identification of patients at risk for the development of refeeding syndrome with main and secondary criteria (10)*.
| One of the following (main criteria) | Two of the following (secondary criteria) |
| BMI < 16 kg/m2 | BMI < 18.5 kg/m2 |
| Unintentional weight reduction of > 15% within 3–6 months | Unintentional weight reduction of > 10% within 3–6 months |
| Little or no food intake for > 10 days | Little or no food intake for > 5 days |
| Low to normal-low electrolyte levels for phosphate, potassium, and/or magnesium prior to refeeding | Positive history of alcohol abuse, treatment with diuretics, chemotherapeutic agents, or antacid agents |
*An increased risk for the development of refeeding syndrome is present if at least one main criterion or two secondary criteria are met.
BMI, body mass index
There are differing definitions of RFS in the literature, and reliable findings on the incidence of RFS are lacking. This is highlighted by the results of a recent meta-analysis of 35 observational studies (10). Malnourished individuals are at risk of developing RFS. According to the 2019 Nutrition Report of the German Nutrition Society (Deutsche Gesellschaft für Ernährung, DGE), 35% of patients in German hospitals are affected by malnutrition (15% to a moderate and 20% to a severe extent) (11). In the acute medical inpatient setting, multicenter randomized studies show an RFS incidence of 8–14.6% in the feeding of malnourished patients (12, 13).
Diseases associated with malnutrition increase the risk for RFS. These include consumptive diseases (for example, cancer, tuberculosis, HIV), malabsorption and malassimilation syndromes (chronic inflammatory bowel diseases, radiation enteritis, etc.), and psychiatric disorders (alcohol addiction, anorexia nervosa, etc.). Decreased food intake over several days (for example, due to multiple surgical procedures, loss of appetite) or chronic nutrient deficiency following bariatric surgery can also lead to RFS (2, 14, 15). The risk profile of oncology patients for RFS is characterized by weight loss, loss of appetite, persistent inflammation, and multiple medical interventions and is thus particularly unfavorable (16). The fact that chemotherapy is included in the NICE criteria takes this risk profile into account (table 1). According to current knowledge, the metabolic switch from catabolic to anabolic is relevant from a pathophysiological perspective (more on pathophysiology in Figure 1).
Evidence from randomized controlled studies
For over 50 years, case reports have been published describing the severe courses of RFS, such as cardiac arrhythmias, delirium, and death, in a number of different patient populations (17). A large proportion of scientific investigations into RFS are observational studies. Prospective randomized controlled studies on RFS and its treatment, especially in non-anorexic patients, are rare (14). In German-speaking countries, there is only one secondary analysis of a multicenter randomized controlled study investigating the effect on malnourished medical inpatients of individualized nutritional therapy compared to standard hospital food (18, 19). The secondary analysis of this study on RFS was a planned part of the study design, screened the largest sample to date for the occurrence of RFS (n = 967), and analyzed its clinical outcomes. The secondary analysis compares malnourished patients under individualized nutritional therapy that did not develop RFS (n = 826) with malnourished patients that did develop RFS (n = 141). Patients with RFS exhibited significantly higher 180-day mortality (42/141 [29.8%] versus 181/826 [21.9%], odds ratio [OR] 1.53; p < 0.05), an increased risk for admission to intensive care (6/141 [4.3%] versus 13/826 [1.6%], OR 2.71; p < 0.05), and longer hospital stay (10.5 ± 6.9 versus 9.0 ± 6.6 days; median additional days 1.57; p = 0.01) (12). In addition, a recent meta-analysis on mortality in RFS patients showed a significantly increased 6-month mortality rate (OR 1.54; 95% confidence interval: [1.04; 2.28]) (20).
The therapeutic effects of low-calorie to high-calorie nutritional protocols on RFS have been investigated in randomized controlled studies in intensive care patients, in the normal inpatient setting, in patients on geriatric units, as well as in patients receiving treatment for anorexia nervosa. These studies yielded partially conflicting results.
A multicenter randomized study conducted by Doig et al. investigated 60-day survival in intensive care patients that developed RFS 72 h following commencement of nutritional support and received either caloric restriction (n = 163) or standard nutritional support (n = 164). The group receiving caloric restriction had significantly better 60-day survival (standard nutritional support: 128/164 [78%] versus caloric restriction 149/163 [91%]; p = 0.002) (21). In 2021, Olsen et al. investigated the effect of an intensive nutritional protocol of 20 kcal/kg/day, whereby energy intake was increased to reach the targeted caloric intake within 3 days, compared to a reduced-calorie protocol of 10 kcal/kg/day, whereby energy intake was increased to reach targeted caloric intake within 7 days. The 85 geriatric patients were investigated for hand grip strength, 3-month mortality, and the development of RFS. All patients could be classified as high-risk patients for RFS according to NICE criteria. Nutrition was administered enterally via nasogastric tube feeding. Patients receiving intensive nutrition were more likely to develop RFS (group receiving 20 kcal/kg/day 17.1% versus group receiving 10 kcal/kg/day 9.3%; p = 0.29). The occurrence of RFS was not statistically significant, but patients were significantly more likely to experience respiratory distress (group receiving 20 kcal/kg/day 53.6% versus group receiving 10 kcal/kg/day 30.2%, p = 0.029). There were no differences in hand grip strength or mortality (22). Lower-calorie refeeding had a favorable effect on morbidity and mortality in the investigations carried out by Doig et al. and Olsen et al. (21, 23).
A UK interventional study conducted at two centers investigated the effect of different refeeding rates with a newly prescribed PN on the occurrence of electrolyte disturbances, ECG changes, infections, and hospital stay in patients at risk for RFS. Within the first 48 h, patients at risk for RFS received low-calorie (15 kcal/kg/day) or high-calorie (30 kcal/kg/day) PN, followed by a standard PN regimen of 30 kcal/kg/day in line with standard practice for the two centers. The different refeeding rates had no effect on the endpoints (24). However, the study protocol was significantly hampered by problems during the inclusion of patients. Instead of the planned 225 patients, ultimately only 48 were included in the final data analysis (24). This resulted in small samples in the study arms (for example, moderate RFS risk and high-calorie feeding with 30 kcal/kg/day = 10). It was unlikely that an effect would be demonstrated with this reduced sample size.
Investigations conducted in the US on patients with anorexia nervosa show contrasting results for caloric restriction as a therapeutic intervention in RFS. A multicenter study investigated the effect of oral refeeding on the medical stability of hospitalized patients receiving high-calorie (n = 60, starting at 2000 kcal/day, increasing daily by 200 kcal) compared to low-calorie refeeding (n = 51, starting at 1400 kcal/day, increasing daily by 200 kcal). High-calorie refeeding resulted in faster medical stability during hospitalization (hazard ratio 1.67 [1.10; 2.53]; p = 0.01) (25). Medical stability was determined by heart rate (HR) > 45/min, systolic RR > 90 mmHg, body temperature > 35.6° C, an intact orthostatic response (maximum increase in HR ≤ 35 and maximum decrease in systolic RR 20 mmHg), as well as reaching at least 75% of an age- and sex-adjusted median BMI. RFS did not develop more frequently in the high-calorie group than it did in the low-calorie group (four versus three individuals) (25). The stable long-term study effects that were published in 2022 confirm the robustness of these results (26).
Despite the conflicting results from interventional studies, caloric restriction is the therapeutic feeding intervention recommended in RFS. The reliability of the UK interventional study is reduced by its diminished sample size. It is unclear whether the results from anorexia nervosa patients can be extrapolated to other patients. The mean age of the 111 individuals included in the abovementioned study was 16.4 ± 2.5 years (mean ± standard deviation [SD]) (25). One can assume that the organism of a 16-year-old patient can better tolerate faster refeeding than can a multimorbid patient of more advanced age.
Treatment and monitoring
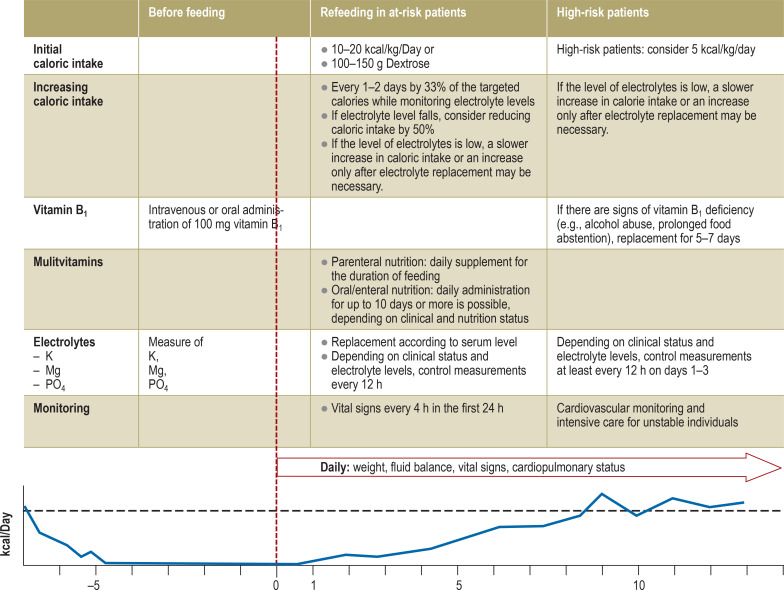
A number of different recommendations for the prevention and treatment of RFS have been published in Europe and the US (9, 27, 28). The most recent treatment recommendations on refeeding in patients at risk for RFS are the consensus recommendations of the American Society for Parenteral and Enteral Nutrition (ASPEN) published in 2020 (2). These recommend extensive replacement of the required electrolytes and vitamins (in particular vitamin B1) and a stepwise approach to refeeding with a reduced caloric intake (figure 2) (2, 9, 27– 29). The primary goal of a stepwise reintroduction of nutrition is to prevent RFS, much like prophylaxis. If manifest RFS develops, the further nutrition and replacement therapy follows the recommendations on the refeeding of high-risk patients (Figure2). Due to the above-mentioned conflicting results from interventional studies, the level of evidence for the recommendations on caloric restriction is based on expert consensus. The increase in caloric intake is determined by the risk for RFS, the patient’s clinical status during refeeding, and the intensity of occurring electrolyte disorders, in particular phosphate. The severity of electrolyte disturbances determines the frequency of electrolyte monitoring and simultaneously serves as a decision-making criterion for increases in caloric intake. In the intensive care setting, monitoring should be adapted to the individual needs of the patient. The initial caloric amount per kilogram body weight is 5–20 kcal/kg/day, depending on the specialist society, whereby the following applies: the higher the risk of RFS, the lower the initial caloric amount per kilogram bodyweight (2, 9, 27). The ASPEN recommends an initial caloric intake of 10–20 kcal/kg/day. There is no recommendation for a restriction of salt, fluid, or protein intake. Usually, the targeted caloric intake can be reached within 7 days.
Figure 2.
Recommendations on refeeding in patients at risk of refeeding syndrome and treatment recommendations in manifest refeeding syndrome according to the consensus recommendations (2) of the American Society for Parenteral and Enteral Nutrition
P, potassium; Mg, magnesium; PO4, phosphate
Diagnostic challenges and the potential offered by clinical decision support systems
As a result of the nonspecific symptoms of RFS, physicians often fail to recognize this clinical picture. If the risk for the development or manifestation of RFS goes unrecognized, it is not possible to initiate clinical steps. This results in vulnerable patients being put at risk (8, 30). Due to its characteristic electrolyte disturbances, RFS lends itself to the development of an automated diagnostic algorithm with a notification system. Automated diagnostic suggestions, complemented by treatment recommendations, can be represented in a CDSS. To date, CDSS are poorly established in clinical nutritional medicine. However, they have already been able to achieve improved compliance with protein and calorie targets (31) and improved glycemic control in clinical nutritional medicine (32– 34). As part of the AMPEL research project (35) (analysis and notification system to improve patient safety through real-time integration of laboratory findings, www.ampel.care), a CDSS of this kind has been newly developed for RFS at the University Medical Center Leipzig (UMCL). It was prospectively tested over a 6-month period (for inclusion and exclusion criteria as well as CDSS development, see the eMethods Section). The CDSS checks adult patients with hypophosphatemia (< 0.84 mmol/L) as to whether other states of electrolyte deficiency in relation to potassium and magnesium have been present in the preceding 5 days. In the case of electrolyte imbalances suspicious for RFS, the CDSS then checks exclusion criteria using laboratory values and coding data pointing to the presence of procedures and comorbidities (for example, terminal kidney failure with dialysis) that cause hypophosphatemia of other etiology (36– 38). In the 6-month exploratory prospective test phase, the CDSS was able to identify 21 individuals with suspected RFS (patient characteristics, Table 2). Their diagnoses were confirmed in subsequent bedside visits. RFS severity was determined on the basis of the ASPEN diagnostic criteria. The distribution of principal diagnoses shows that RFS patients encounter physicians in all specialties (table 2). According to a telephone survey of the treating physicians, 13 (62%) cases would not have been recognized without a CDSS notification (Table 2, patients above the cut-off line). Determination of the necessary serum electrolytes alone failed to establish the diagnosis in a relevant proportion of patients. Thus, an additional assessment by a CDSS can improve diagnosis, enabling affected individuals to receive nutritional medical support. Due to the low case numbers in the retrospective development phase (100 patients in 30 months, eFigure), neither randomization nor comparison to a control group could be performed in the prospective testing (6 months).
Table 2. Characteristics of patients with confirmed refeeding syndrome (RFS) in the test phase of a clinical decision support system (CDSS). All individuals in whom the CDSS resulted in a diagnosis are shown above the line. Those in whom RFS was already known are shown in the shaded portion.
| Sex, age | Main diagnosis | BMI [kg/m2] | Nutrition | Electrolytes [mmol/L] | NRS | NICEcriteria | RFS severity* | ||
| PO4 | P | Mg | |||||||
| F, 84 | Cardiogenic shock in AV block III | 23.5 | ON | 0.41 | 3.21 | 0.52 | 2 | Positive | Severe |
| M, 78 | NSCLC, pneumonia | 20.3 | PN | 0.59 | 4.18 | 0.79 | 5 | Positive | Mild |
| F, 65 | Hypopharyngeal cancer | 11.9 | PN | 0.3 | 2.7 | 0.63 | 5 | Positive | Severe |
| M, 74 | COVID-19 | 16.4 | ON+LD | 0.5 | 2.98 | 0.76 | 6 | Positive | Moderate |
| F, 79 | Exsiccosis, flu | 30.8 | ON | 0.58 | 3.1 | n.a. | 3 | Negative | Moderate |
| F, 56 | Breast cancer | 27.1 | ON | 0.55 | 2.93 | 0.76 | 3 | Positive | Moderate |
| F, 84 | Staphylococcus aureus sepsis | 20.5 | ON+LD | 0.53 | 3.06 | 0.74 | n.a. | Positive | Moderate |
| M, 44 | Good syndrome | 17.4 | PN | 0.44 | 2.21 | 0.86 | 3 | Positive | Severe |
| F, 54 | Anastomosis after Roux-en-Y bypass | 24.7 | PN | 0.56 | 3.12 | 0.7 | 3 | Positive | Moderate |
| M, 86 | Sigmoid diverticulitis | 21.6 | PN | 0.31 | 5.13 | 0.55 | 4 | Positive | Moderate |
| M, 61 | DLBCL, diarrhea | 23.5 | ON+LD | 0.48 | 2.16 | 0.71 | 4 | Negative | Severe |
| M, 64 | Liver cirrhosis, malnutrition | 23.9 | ON | 0.15 | 2.7 | 0.7 | 2 | Negative | Moderate |
| M, 55 | Depression | 18.6 | ON+LD | 0.25 | 2.94 | 0.66 | 4 | Positive | Moderate |
| F, 68 | Depression | 19.5 | ON+LD | 0.5 | 2.96 | 0.85 | 5 | Positive | Moderate |
| M, 57 | Poisoning (alcohol) | 20.7 | ON | 0.43 | 2.33 | n.a. | 4 | Positive | Severe |
| F, 59 | Clostridium difficile enteritis | 22.6 | PN | 0.34 | 3.1 | 0.45 | 2 | Negative | Severe |
| F, 69 | COVID-19, DLBCL | 27.5 | ON | 0.5 | 3.08 | 0.77 | 5 | Positive | Moderate |
| M, 66 | COVID-19 | 21.6 | ON | 0.53 | 3.09 | n.a. | 6 | Negative | Moderate |
| M, 47 | Poisoning (alcohol) | 24.7 | ON | 0.2 | 3.4 | 0.56 | 3 | Positive | Moderate |
| F, 72 | Urinary tract infection | 19.2 | ON | 0.59 | 3.56 | n.a. | 4 | Negative | Mild |
| F, 46 | Hypokalemia, alcohol abuse | 19.5 | ON | 0.36 | 3.08 | 0.48 | 3 | Positive | Severe |
* Classification into severity levels is in accordance with the RFS diagnostic criteria of the American Society for Parenteral and Enteral Nutrition (ASPEN).
AV, atrioventricular; BMI, body mass index; DLBCL, diffuse large B cell lymphoma; P, potassium; M, male; Mg, magnesium; NICE, National Institute for Health and Care Excellence; NRS, Nutritional Risk Score 2002; NSCLC, non-small-cell lung cancer; n.a., not available; ON, oral nutrition; PN; parenteral nutrition; PO 4, phosphate; LD, liquid diet; F, female
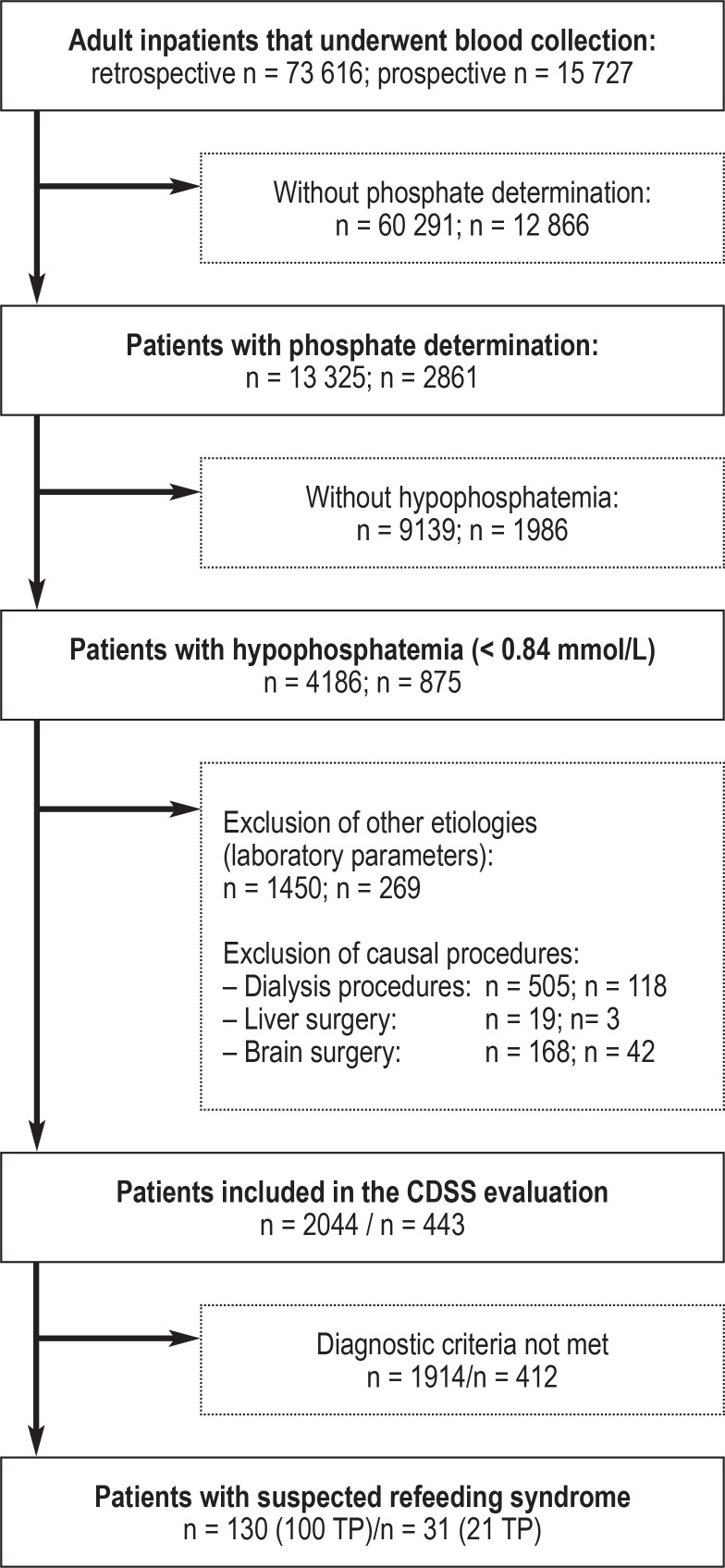
The greatest limitation of the CDSS is the detection of hypophosphatemia as the initiator of the subsequent automated electrolyte analysis. Phosphate determinations are not routine in clinical practice: Only 18% of investigated individuals (n = 13,325, eFigure) had this checked in the retrospective CDSS development phase. Hypophosphatemia was found in approximately a third of these (n = 4186, eFigure). The CDSS is unable to identify patients with RFS without phosphate determination. Thus, it is not possible to determine the proportion of patients recognized as false-negative by the CDSS. The electrolyte disturbances in RFS show a frequency peak 72 h after nutrition therapy (12, 39). The use of the RFS-CDSS in combination with a standardized determination of serum phosphate 72 h following the initiation of feeding, at least in malnourished patients, would be a cost-effective approach to screening (laboratory costs according to the German Uniform Evaluation Standard [einheitlicher Bewertungsmaßstab, EBM] 32 086: 0.40 Euros) to improve the effectiveness of the CDSS. It is also necessary that physicians caring for people at increased risk for the development of RFS educate themselves about this clinical picture. This would enable at-risk patients to be promptly identified and correspondingly treated prior to the initiation of refeeding. Only then is it possible to prevent a hazardous drop in serum electrolyte levels and the development of RFS.
Conclusion
RFS receives insufficient clinical attention. It can occur in malnourished individuals across the entire spectrum of medicine and is characterized by nonspecific symptoms that hamper diagnosis. In severe cases, RFS can cause death. The ability to recognize this clinical picture and identify at-risk patients is relevant to all those treating malnourished patients. The use of CDSS can assist clinically active physicians in establishing its diagnosis. Evidence on the prevention and treatment of RFS is sparse. Current treatment recommendations based on expert consensus include replacement therapy of the necessary electrolytes and other vitamins (in particular, vitamin B1), as well as the reintroduction of feeding in an initially calorie-reduced form. Prospective randomized analyses are needed in the future in order to systematically improve RFS treatment guidelines while taking into account appropriate CDSS.
Supplementary Material
eMethods
Development of the clinical decision support system
A diagnostic algorithm was drawn up on the basis of the diagnostic criteria published in 2020 by the American Society for Parenteral and Enteral Nutrition (ASPEN). The description of the development of the algorithm, the final algorithm, the design of the clinical decision support system (CDSS), and its implementation in clinical workflow are currently in the process of being published, meaning that the algorithm used is currently not publicly available. A description of the content of the diagnostic steps is given in the section “Process of automated patient evaluation.” The algorithm was retrospectively tested using a dataset from laboratory data from January 2019 to June 2021 and further developed for adults. The dataset contained all serum levels and blood gas analyses as well as point-of-care testing of whole blood concentrations of the electrolytes phosphate, magnesium, and potassium at the University Medical Center Leipzig (UMCL), Germany. The algorithm thus developed was then incorporated in a CDSS together with action and treatment recommendations according to the severity of refeeding syndrome (RFS). The recommendations were displayed in the electronic medical records of identified patients via warning icons. Physicians were able to view the treatment recommendations by clicking on the warning icon in a pop-up window. The CDSS was prospectively tested over a 6-month test phase, and the suspected diagnosis expressed in an automated manner could be confirmed by a nutrition medicine specialist or nutrition therapist in a bedside visit of individuals detected by the system. The data collection periods were determined by the availability of data; electrolyte data were retrospectively available for the period from January 2019 to June 2021. Prospective testing was scientifically supervised for 6 months and terminated when the project period came to an end. The exploratory study was approved by the ethics committee of the University of Leipzig Medical Center (No. 214/18-ek).
Process of automated patient evaluation
All adult patients that had undergone blood collection and phosphate determination were included in the patient evaluation. The CDSS is initiated by a phosphate determination below the lower reference range (0.84 mmol/L) and performs, in a first step, an automated verification of exclusion criteria (eFigure). Exclusion criteria are verified by the presence of laboratory values and coding data in the electronic medical record suggestive of hypophosphatemia of other etiology and are designed to prevent incorrect notifications. The detection of procedure codes for acute dialysis treatments and surgical procedures on the liver and brain results in exclusion, as does the detection of certain laboratory parameters such as ketone bodies in urine (suggestive of decompensated diabetes mellitus), paracetamol in serum (paracetamol poisoning), hyperphosphatemia and hypermagnesemia (suggestive of severe acute or chronic renal dysfunction), an elevated PTH level (hyperparathyroidism), and secretion of PTH-related peptides (lung and neuroendocrine carcinomas). Electrolyte imbalances triggered by these diseases and interventions and which are unrelated to nutrition but resemble RFS have been described in the literature (35– 38). For included patients, further electrolyte assessments of magnesium and potassium are performed for up to a total of 5 days in the past. Depending on the percentage decrease in concentration, an alarm is triggered for mild, moderate, or severe RFS. Individuals identified by the CDSS are flagged up with a warning icon in the digital inpatient unit overview. In moderate RFS, an electrolyte imbalance alone results in a patient being flagged up, while in mild RFS, decreased energy intake additionally needs to have been recorded in the coding data at the time of admission to the unit. By clicking on the warning notification, a pop-up window opens showing critical laboratory results together with an interpretation text module for clinical use and a link to in-house treatment guidelines. In total, 100 cases were retrospectively confirmed as RFS in 130 notifiable patients based on a review of electronic medical records by two independent physicians. In the prospective test phase, 21 of 31 individuals for whom notifications were triggered were confirmed as correct RFS cases. Incorrect false-positive notifications were due to liver dysfunction (n = 3), brain trauma (n = 2), dialysis with coding pending (n = 3), or multiorgan failure (n = 2). These confounders in electrolyte interpretation in the case of suspected RFS are already known from the literature (36– 38).
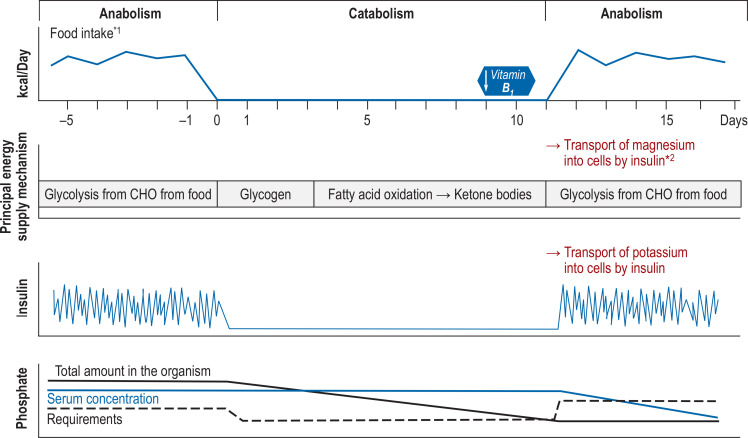
Figure 1.
Pathophysiology of refeeding syndroms (RFS), modified from Nguyen et al (40). In catabolism, energy production takes place via glycogenolysis and gluconeogenesis. Liver glycogen is generally used up after 12–24 h. Thereafter, primarily ketones from fatty acid oxidation serve as substrates of gluconeogenesis. Intracellular reserves of phosphate, potassium, and magnesium are used to maintain electrolyte homeostasis and become depleted in the absence of food intake. Serum electrolyte concentrations within the reference range mask a growing intracellular deficit. Upon reintroduction of feeding, insulin is secreted, thereby stimulating sodium–potassium ATPase and enabling the intracellular influx of glucose and phosphate. Thus, carbohydrates are once again available as substrates for glycolysis. Carbohydrate metabolism requires magnesium and thiamine (vitamin B1) as essential cofactors. The half-life of physiological vitamin B1 storage is 7–10 days and is quickly exhausted in the case of low food intake. The onset of glycolysis triggers a rapidly increasing requirement for vitamin B1 and electrolytes. Acute deficiency causes RFS complications.
*1 Balanced diet, e.g., 50% calories from carbohydrates, 30% fats, 20% protein; *2 intracellularly used for pyruvate entry into the cycle
kcal: kilocalories; CHO, carbohydrates.
eFigure 1.
Flow diagram showing the data analysis of retrospective data from January 2019 to June 2021 (left) and the prospective test phase (right) of the clinical decision support system (CDSS) using inclusion and exclusion criteria and the subsequent automated evaluation of decreases in the level of electrolytes (phosphate, potassium, magnesium) in refeeding syndrome. TP, true positive
Questions on the article in issue 7/2023:
Refeeding Syndrome—Diagnostic Challenges and the Potential of Clinical Decision Support Systems
The submission deadline is 16 February 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
Which of the following criteria is one of the main criteria for the identification of at-risk patients according to the National Institute for Health and Care Excellence?
Unintentional weight loss of > 5% within 3–6 months
Little or no food intake for > 10 days
Little or no food intake for > 3 days
Unintentional weight loss of > 10% within 3–6 months
BMI < 19 kg/m2
Question 2
Which electrolyte shift is associated with refeeding syndrome?
Hypokalemia and hypophosphatemia
Hyperkalemia and hypophosphatemia
Hypernatremia and hyperphosphatemia
Hyponatremia and hyperphosphatemia
Hyperkalemia and hyperphosphatemia
Question 3
Which vitamin is an essential component of replacement therapy in refeeding syndrome?
Vitamin D3
Vitamin A
Vitamin E
Vitamin B12
Vitamin B1
Question 4
According to the 2019 Nutrition Report of the German Nutrition Society, what is the overall percentage of patients in German hospitals affected by malnutrition?
5%
15%
25%
35%
45%
Question 5
Which one of the following elements is transported into cells at an increased rate when adequate nutrition is reintroduced after a phase of catabolic energy production lasting several days?
Calcium
Sodium
Magnesium
Selenium
Copper
Question 6
In patients at risk for the development of refeeding syndrome, the reintroduction of nutrition following a catabolic phase should be started cautiously. According to the American Society for Parenteral and Enteral Nutrition, which initial caloric intake should be used?
10–20 kcal/kg/Day
70–150 kcal/kg/Day
200–280 kcal/kg/Day
300–500 kcal/kg/Day
700–850 kcal/kg/Day
Question 7
What is the approximate time frame within which refeeding can be expected to reach the targeted caloric intake?
2 Days
1 Week
2 Weeks
1 Month
2 Months
Question 8
What does the abbreviation CDSS stand for in the text?
Clinical decompensation support system
Critical decompensation support system
Clinical delirium support system
Clinical decision support system
Clinical diagnostic support system
Question 9
Which laboratory value is named in the text as a limiting step in the CDSS for the recognition of refeeding syndrome and could be used for screening purposes in at-risk patients?
Lactate
Serum albumin
Creatinine
TSH value
Serum phosphate
Question 10
Which time point is particularly suited to screening for refeeding syndrome following the reintroduction of nutrition?
12 h
24 h
36 h
48 h
72 h
Acknowledgments
Translated from the original German by Christine Rye.
Acknowledgments
We would like to thank Kristin Gutsmuths, Carsten Güttich, and Anna-Sophie Junge for carrying out the study visits.
Footnotes
Conflict of interest statement
Dr. Heuft, PD Dr. Kaiser, and Dr. Voigt were involved in the development of the CDSS, the clinical implementation of which is reported in this article.
Other research partners in this project publicly funded under the eHealthSax guideline No.: 100331796 include the Muldental Clinics in Grimma and Wurzen as well as Xantas AG. Upon completion of the project, the option exists to transform the CDSS into a product. All research partners have independent and autonomous access to the knowledge gained.
The remaining authors declare that no conflict of interest exists.
Funding
This initiative (eHealthSax guideline No.: 100331796) is co-financed by public funds based on the budget passed by members of the Saxon State Parliament.
References
- 1.Zeki S, Culkin A, Gabe SM, Nightingale JM. Refeeding hypophosphataemia is more common in enteral than parenteral feeding in adult in patients. Clin Nutr. 2011;30:365–368. doi: 10.1016/j.clnu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 2.da Silva JSV, Seres DS, Sabino K, et al. ASPEN Consensus recommendations for refeeding syndrome. Nutr Clin Pract. 2020;35:178–195. doi: 10.1002/ncp.10474. [DOI] [PubMed] [Google Scholar]
- 3.Ponzo V, Pellegrini M, Cioffi I, Scaglione L, Bo S. The refeeding syndrome: a neglected but potentially serious condition for inpatients. A narrative review. Intern Emerg Med. 2021;16:49–60. doi: 10.1007/s11739-020-02525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen G, Pourhassan M, Lenzen-Großimlinghaus R, et al. The refeeding syndrome revisited: you can only diagnose what you know. Eur J Clin Nutr. 2019;73:1458–1463. doi: 10.1038/s41430-019-0441-x. [DOI] [PubMed] [Google Scholar]
- 5.Larsen S. The investigation of parenteral nutrition - Aotearoa (IPNA) setting up the 1st phase of a clinical audit of the delivery of parenteral nutrition (PN) in New Zealand (NZ) Masterarbeit. Albany, New Zealand. 2012 [Google Scholar]
- 6.Dyson JK, Thompson N. Adult parenteral nutrition in the North of England: a region-wide audit. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-012663. e012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vignaud M, Constantin JM, Ruivard M, et al. Refeeding syndrome influences outcome of anorexia nervosa patients in intensive care unit: an observational study. Crit Care. 2010;14 doi: 10.1186/cc9274. R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koekkoek WAC, van Zanten ARH. Is refeeding syndrome relevant for critically ill patients? Curr Opin Clin Nutr Metab Care. 2018;21:130–137. doi: 10.1097/MCO.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence (NICE) (ed.) Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition. 2006 [PubMed] [Google Scholar]
- 10.Cioffi I, Ponzo V, Pellegrini M, et al. The incidence of the refeeding syndrome A systematic review and meta-analyses of literature. Clin Nutr. 2021;40:3688–3701. doi: 10.1016/j.clnu.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 11.Volkert D, Weber J, Kiesswetter E, Sulz I, Hiesmayr M. Ernährungssituation in Krankenhäusern und Pflegeheimen - Auswertung der nutritionDay-Daten für Deutschland. 14 DGE Ernährungsbericht. 2019 V2-V669. [Google Scholar]
- 12.Friedli N, Baumann J, Hummel R, et al. Refeeding syndrome is associated with increased mortality in malnourished medical inpatients: secondary analysis of a randomized trial. Medicine (Baltimore) 2020;99 doi: 10.1097/MD.0000000000018506. e18506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraaijenbrink B, Lambers WM, Mathus-Vliegen E, Siegert C. Incidence of refeeding syndrome in internal medicine patients. Neth J Med. 2016;74:116–121. [PubMed] [Google Scholar]
- 14.Friedli N, Stanga Z, Sobotka L, et al. Revisiting the refeeding Syndrome: Results of a systematic review. Nutrition. 2017;35:151–160. doi: 10.1016/j.nut.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Wirth R, Diekmann R, Janssen G, et al. Refeeding-Syndrom Pathophysiologie, Risikofaktoren, Prophylaxe und Therapie. Internist. 2018;59:326–333. doi: 10.1007/s00108-018-0399-0. [DOI] [PubMed] [Google Scholar]
- 16.Marinella MA. Refeeding syndrome in cancer patients. Int J Clin Pract. 2008;62:460–465. doi: 10.1111/j.1742-1241.2007.01674.x. [DOI] [PubMed] [Google Scholar]
- 17.Boateng AA, Sriram K, Meguid MM, Crook M. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition. 2010;26:156–167. doi: 10.1016/j.nut.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Schuetz P, Fehr R, Baechli V, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393:2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 19.Schuetz P, Fehr R, Baechli V, et al. Design and rationale of the effect of early nutritional therapy on frailty, functional outcomes and recovery of malnourished medical inpatients trial (EFFORT): a pragmatic, multicenter, randomized-controlled trial. Int J Clin Trials. 2018;5 [Google Scholar]
- 20.Bioletto F, Pellegrini M, Ponzo V, et al. Impact of refeeding syndrome on short- and medium-term all-cause mortality: a systematic review and meta-analysis. Am J Med. 2021;134:1009–1018e1. doi: 10.1016/j.amjmed.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Doig GS, Simpson F, Heighes PT, et al. Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med. 2015;3:943–952. doi: 10.1016/S2213-2600(15)00418-X. [DOI] [PubMed] [Google Scholar]
- 22.Olsen SU, Hesseberg K, Aas A-M, Pripp AH, Ranhoff AH, Bye A. A comparison of two different refeeding protocols and its effect on hand grip strength and refeeding syndrome: a randomized controlled clinical trial. Eur Geriatr Med. 2021;12:1201–1212. doi: 10.1007/s41999-021-00520-5. [DOI] [PubMed] [Google Scholar]
- 23.Olthof LE, Koekkoek WACK, van Setten C, Kars JCN, van Blokland D, van Zanten ARH. Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: a retrospective study. Clin Nutr. 2018;37:1609–1617. doi: 10.1016/j.clnu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Ambrose T, de Silva A, Naghibi M, et al. Refeeding risks in patients requiring intravenous nutrition support: results of a two-centre, prospective, double-blind, randomised controlled trial. Clin Nutr ESPEN. 2021;41:143–152. doi: 10.1016/j.clnesp.2020.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Garber AK, Cheng J, Accurso EC, et al. Short-term outcomes of the study of refeeding to optimize inpatient gains for patients with anorexia nervosa: a multicenter randomized clinical trial. JAMA Pediatr. 2021;175:19–27. doi: 10.1001/jamapediatrics.2020.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden NH, Cheng J, Kapphahn CJ, et al. Higher-calorie refeeding in anorexia nervosa: 1-year outcomes from a randomized controlled trial. Pediatrics. 2021;147 doi: 10.1542/peds.2020-037135. e2020037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boland K, Solanki D, O’Hanlon C (ed.) Prevention and treatment of refeeding syndrome in the acute care setting: Irish Society for Clinical Nutrition & Metabolism 2013. IrSPEN Guideline Document No. 1 [Google Scholar]
- 28.Stanga Z, Brunner A, Leuenberger M, et al. Nutrition in clinical practice—the refeeding syndrome: illustrative cases and guidelines for prevention and treatment. Eur J Clin Nutr. 2008;62:687–694. doi: 10.1038/sj.ejcn.1602854. [DOI] [PubMed] [Google Scholar]
- 29.Aubry E, Aeberhard C, Leuenberger M, et al. Refeeding-Syndrom: Ein konsensusbasierter Algorithmus für stationäre Patienten. Aktuel Ernahrungsmed. 2019;44:33–42. [Google Scholar]
- 30.Kagansky N, Levy S, Koren-Morag N, Berger D, Knobler H. Hypophosphataemia in old patients is associated with the refeeding syndrome and reduced survival. J Intern Med. 2005;257:461–468. doi: 10.1111/j.1365-2796.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 31.Ettori F, Henin A, Zemmour C, et al. Impact of a computer-assisted decision support system (CDSS) on nutrition management in critically ill hematology patients: the NUTCHOCO study (nutritional care in hematology oncologic patients and critical outcome) Ann Intensive Care. 2019;9 doi: 10.1186/s13613-019-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benyo B, Illyés A, Némedi NS, et al. Pilot study of the SPRINT glycemic control protocol in a Hungarian medical intensive care unit. J Diabetes Sci Technol. 2012;6:1464–1477. doi: 10.1177/193229681200600628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pielmeier U, Rousing ML, Andreassen S, Nielsen BS, Haure P. Decision support for optimized blood glucose control and nutrition in a neurotrauma intensive care unit: preliminary results of clinical advice and prediction accuracy of the Glucosafe system. J Clin Monit Comput. 2012;26:319–328. doi: 10.1007/s10877-012-9364-y. [DOI] [PubMed] [Google Scholar]
- 34.Kwan JL, Lo L, Ferguson J, et al. Computerised clinical decision support systems and absolute improvements in care: meta-analysis of controlled clinical trials. BMJ. 2020;370 doi: 10.1136/bmj.m3216. m3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter Costa MB, Wernsdorfer M, Kehrer A, et al. The clinical decision support system AMPEL for laboratory diagnostics: implementation and technical evaluation. JMIR Med Inform. 2021;9 doi: 10.2196/20407. e20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta HK, Malik M, Neely RDG. Hepatic surgery-related hypophosphatemia. Clinica Chimica Acta. 2007;380:13–23. doi: 10.1016/j.cca.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 37.Leung J, Crook M. Disorders of phosphate metabolism. J Clin Pathol. 2019;72:741–747. doi: 10.1136/jclinpath-2018-205130. [DOI] [PubMed] [Google Scholar]
- 38.Varudo R, Mota AM, Pereira E, Dias C. Impact of phosphatemia variability in neurological outcomes in patients with spontaneous subarachnoid hemorrhage. Cureus. 2021;13 doi: 10.7759/cureus.18257. e18257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedli N, Stanga Z, Culkin A, et al. Management and prevention of refeeding syndrome in medical inpatients: an evidence-based and consensus-supported algorithm. Nutrition. 2018;47:13–20. doi: 10.1016/j.nut.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen P, Schlögl H, Selig L, Baerwald C. Refeeding-Syndrom Pathophysiologie, Therapie und welche rheumatologischen Patienten besonders gefährdet sind. Z Rheumatol. 2021;80:263–269. doi: 10.1007/s00393-020-00952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Development of the clinical decision support system
A diagnostic algorithm was drawn up on the basis of the diagnostic criteria published in 2020 by the American Society for Parenteral and Enteral Nutrition (ASPEN). The description of the development of the algorithm, the final algorithm, the design of the clinical decision support system (CDSS), and its implementation in clinical workflow are currently in the process of being published, meaning that the algorithm used is currently not publicly available. A description of the content of the diagnostic steps is given in the section “Process of automated patient evaluation.” The algorithm was retrospectively tested using a dataset from laboratory data from January 2019 to June 2021 and further developed for adults. The dataset contained all serum levels and blood gas analyses as well as point-of-care testing of whole blood concentrations of the electrolytes phosphate, magnesium, and potassium at the University Medical Center Leipzig (UMCL), Germany. The algorithm thus developed was then incorporated in a CDSS together with action and treatment recommendations according to the severity of refeeding syndrome (RFS). The recommendations were displayed in the electronic medical records of identified patients via warning icons. Physicians were able to view the treatment recommendations by clicking on the warning icon in a pop-up window. The CDSS was prospectively tested over a 6-month test phase, and the suspected diagnosis expressed in an automated manner could be confirmed by a nutrition medicine specialist or nutrition therapist in a bedside visit of individuals detected by the system. The data collection periods were determined by the availability of data; electrolyte data were retrospectively available for the period from January 2019 to June 2021. Prospective testing was scientifically supervised for 6 months and terminated when the project period came to an end. The exploratory study was approved by the ethics committee of the University of Leipzig Medical Center (No. 214/18-ek).
Process of automated patient evaluation
All adult patients that had undergone blood collection and phosphate determination were included in the patient evaluation. The CDSS is initiated by a phosphate determination below the lower reference range (0.84 mmol/L) and performs, in a first step, an automated verification of exclusion criteria (eFigure). Exclusion criteria are verified by the presence of laboratory values and coding data in the electronic medical record suggestive of hypophosphatemia of other etiology and are designed to prevent incorrect notifications. The detection of procedure codes for acute dialysis treatments and surgical procedures on the liver and brain results in exclusion, as does the detection of certain laboratory parameters such as ketone bodies in urine (suggestive of decompensated diabetes mellitus), paracetamol in serum (paracetamol poisoning), hyperphosphatemia and hypermagnesemia (suggestive of severe acute or chronic renal dysfunction), an elevated PTH level (hyperparathyroidism), and secretion of PTH-related peptides (lung and neuroendocrine carcinomas). Electrolyte imbalances triggered by these diseases and interventions and which are unrelated to nutrition but resemble RFS have been described in the literature (35– 38). For included patients, further electrolyte assessments of magnesium and potassium are performed for up to a total of 5 days in the past. Depending on the percentage decrease in concentration, an alarm is triggered for mild, moderate, or severe RFS. Individuals identified by the CDSS are flagged up with a warning icon in the digital inpatient unit overview. In moderate RFS, an electrolyte imbalance alone results in a patient being flagged up, while in mild RFS, decreased energy intake additionally needs to have been recorded in the coding data at the time of admission to the unit. By clicking on the warning notification, a pop-up window opens showing critical laboratory results together with an interpretation text module for clinical use and a link to in-house treatment guidelines. In total, 100 cases were retrospectively confirmed as RFS in 130 notifiable patients based on a review of electronic medical records by two independent physicians. In the prospective test phase, 21 of 31 individuals for whom notifications were triggered were confirmed as correct RFS cases. Incorrect false-positive notifications were due to liver dysfunction (n = 3), brain trauma (n = 2), dialysis with coding pending (n = 3), or multiorgan failure (n = 2). These confounders in electrolyte interpretation in the case of suspected RFS are already known from the literature (36– 38).