Background:
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic is estimated to have claimed more than 6 million lives globally since it started in 2019. Germany was exposed to two waves of coronavirus disease 2019 in 2020, one starting in April and the other in October. To ensure sufficient capacity for coronavirus disease 2019 patients in intensive care units, elective medical procedures were postponed. The fraction of major abdominal cancer resections affected by these measures remains unknown, and the most affected patient cohort has yet to be identified.
Methods:
This is a register-based, retrospective, nationwide cohort study of anonymized ‘diagnosis-related group’ billing data provided by the Federal Statistical Office in Germany. Cases were identified using diagnostic and procedural codes for major cancer resections. Population-adjusted cancer resection rates as the primary endpoint were compared at baseline (2012–2019) to those in 2020.
Results:
A change in resection rates for all analyzed entities (esophageal, gastric, liver, pancreatic, colon, rectum, and lung cancer) was observed from baseline to 2020. Total monthly oncological resections dropped by 7.4% (8.7% normalized to the annual German population, P=0.011). Changes ranged from +3.7% for pancreatic resections (P=0.277) to −19.4% for rectal resections (P<0.001). Reductions were higher during lockdown periods. During the first lockdown period (April–June), the overall drop was 14.3% (8.58 per 100 000 vs. 7.35 per 100 000, P<0.001). There was no catch-up effect during the summer months except for pancreatic cancer resections. In the second lockdown period, there was an overall drop of 17.3%. In subgroup analyses, the elderly were most affected by the reduction in resection rates. There was a significant negative correlation between regional SARS-CoV-2 incidences and resections rates. This correlation was strongest for rectal cancer resections (Spearman’s r: −0.425, P<0.001).
Conclusions:
The pandemic lockdowns had a major impact on the oncological surgical caseload in Germany in 2020. The elderly were most affected by the reduction. There was a clear correlation between SARS-CoV-2 incidences regionally and the reduction of surgical resection rates. In future pandemic circumstances, oncological surgery has to be prioritized with an extra focus on the most vulnerable patients.
Keywords: abdominal cancer entities, oncological resection, pandemic, SARS-CoV-2
Introduction
Highlights
Monthly resections rate for malignancy dropped by 7.4% in Germany in 2020.
There was no catch-up effect during the summer of 2020 months.
Elderly patients were most affected by the reduction in resection rates due to coronavirus disease.
There was a correlation between regional coronavirus disease incidences and resection rates.
2020 saw the rapid spread of a new coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] around the world, causing coronavirus disease 2019 (COVID-19)1. The clinical picture ranged from asymptomatic cases to severe respiratory failure requiring invasive ventilation and extracorporeal membrane oxygenation, especially in the elderly population and patients with comorbidities2,3. Germany was exposed to two waves of COVID-19 in 2020, one in April/May and a second starting in October. The reaction to both waves was the implementation of national lockdowns from April to June and then from October to the following June. Elective medical procedures (including operations) were postponed to ensure sufficient treatment capacity for COVID-19 patients and to reduce the burden on intensive care units during the lockdown and high COVID-19 case periods4. Additionally, patients avoided medical consultations, and emergency departments saw a decrease in visits for various medical conditions5–7. There are several reports of a decrease in cancer diagnoses and therapies during the first COVID-19-related lockdowns in Germany as well as in other countries8–11. To this date, there is no objective analysis of the extent to which major oncological surgery was affected by pandemic circumstances in Germany.
The aim of this study was to investigate the impact of the COVID-19 pandemic on major abdominal and lung cancer resections in Germany by comparing population-adjusted resection rates during or in between lockdown periods to previous years.
Methods
This is a register-based, retrospective, nationwide cohort study of anonymized DRG (diagnosis-related group) billing data provided by the ‘Statistische Bundesamt’ (Federal Statistical Office in Germany). In accordance with data legislation in Germany with regard to secondary data analysis of anonymized data, no ethics approval was needed. Data were handled in accordance with the data safety protocols imposed by the Federal Statistical Office in Germany.
Identification of patients for this study was made using OPS (‘Operationen und Prozedurenschlüssel’; surgical and procedural coding system in Germany) codes and ICD (International Statistical Classification of Diseases and Related Health Problems) codes (Supplement Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). Due to the nature of the hospital billing system in Germany, all (private and public) hospital admissions meeting the above-named ICD and OPS codes criteria were included in this analysis. Application of these inclusion criteria identified a total of 745 971 patient records.
Each patient record contained data on age, gender, an anonymized institute identifier, procedural codes (OPS), main and secondary diagnoses (ICD-10-GM), length of stay and reason for admission and discharge. Duplicates were identified, and in cases of occurrence, one dataset was chosen randomly to minimize bias. We only analyzed complete data records. For clarity reasons, we did not analyze complication rates or failure to rescue. This, however, would be possible on the basis of the available data and has been performed elsewhere12,13.
Patients were divided into several patient cohorts, defined by the time of the SARS-CoV-2-related lockdowns of public life and reduction of elective surgical procedures in Germany between mid-March (22nd of March was the official start of lockdown measures) and May (4th of May was the end of lockdown) 2020. We, therefore, defined a first observation period with a lag of 2 weeks from April 2020 to June 2020 and the same periods in 2012–2019 for reference, as well as a pre-lockdown period between January and March 2020 and an interim period from July to September 2020 with respective reference periods in 2012–2019. Furthermore, we defined the beginning of a ‘light lockdown’ from 2 November 2020 (with a previous announcement mid/end of October) as a second ‘lockdown’ period (October–December) with a respective reference period between 2012 and 2019 (https://www.bundesgesundheitsministerium.de/coronavirus/chronik-coronavirus.html).
Diagnoses were coded using the German modification of the 10th revision of the International Classification of Diseases (ICD-10-GM). All in-house patients between 2012 and 2020 with ICD codes and an associated procedure code for major abdominal cancer resection were included in the study (see Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). Procedures were considered hierarchically for each patient, and the most radical procedure was assigned as the defining procedure.
For each patient, a three-category frailty score based on the secondary diagnoses was computed, and on a cohort basis, a categorical rate (low, intermediate, and high risk) was determined14,15.
Incidences were calculated in rates per 100 000 people of the whole population (as officially stated by the Statistical Office Germany per year, https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Bevoelkerung/Bevoelkerungsvorausberechnung/_inhalt.html;jsessionid=5AFAD894070519218E9938F2ABA78B2E.live731#233982) per month to adjust for variation in the total number of insured persons. Mean values were reported with their standard deviation. Differences between reference and observation periods were compared using Student’s t test or ANOVA (analysis of variance). If the assumption of normality was wrong, the Wilcoxon rank-sum test was employed. Where appropriate, 95% CIs were computed.
The primary endpoint in this study was the incidence, that is the population-adjusted rates, of performed abdominal and lung resections for cancer. As secondary endpoints, we stratified the data by sex, age, and frailty.
Interrupted time series (ITS) studies were conducted using ordinary least-squares regression analysis as described elsewhere16. The segmentation was set between March/April 2020 and September/October 2020. Prior to regression analysis, we screened both visually and with a test as proposed by Cumby and Huizinga for autocorrelation17.
The work has been reported in line with the STROCSS (Strengthening the reporting of cohort, cross-sectional and case–control studies in surgery) criteria18, Supplemental Digital Content 1, http://links.lww.com/JS9/A131. It was registered retrospectively with a Research Registry UIN (researchregistry8458) (https://researchregistry.knack.com/research-registry#home/registrationdetails/6363b5f32c9a880022a70d5c/).
All calculations were performed with Microsoft Excel (version 2010) and Stata 16.1 (StataCorp LP, College Station, Texas, USA). A P value of 0.05 or less was considered significant.
Results
From 2012 until the end of 2020, a total of 745 971 cancer resections meeting the inclusion criteria (Supplementary Table 1, Supplemental Digital Content 2, http://links.lww.com/JS9/A138) were performed in Germany (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). Due to the fact that the analysis was performed on billing data, only complete patient records were available, and no exclusion was necessary.
The fraction of female patients ranged from between 20.5% for esophageal resections to 48.0% for pancreatic resections. The mean age ranged from between 64.8 years (esophageal resections) to 71.6 years (colon resections) (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). There was a significant trend toward lower in-house mortality and a significant trend toward the shorter length of stay in all entities over the study period (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). Also, the annual number of hospitals performing surgeries in the distinct entities decreased over time (Supplementary Table 2, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). Population-adjusted resection rates from 2012 until 2019 were stable in esophageal, gastric, colon, and liver cancer, while lung and pancreatic cancer resection rates increased and rectal cancer resections significantly decreased (nonparametric testing, cutoff P<0.05, P<0.001). 2020 marked a change of trend in all analyzed entities (Table 1). Compared to previous years, total monthly oncological resections were reduced by 7.4% (8.7% normalized to the annual German population, P=0.011). Changes ranged from +3.7% for pancreatic resections(P=0.277) to −19.4% for rectal resections (P<0.001) (Table 1).
Table 1.
Resections and rates with change: Periods of interest (lockdown).
| Previous years (01–12, 2012–2019) | The year 2020 (01–12, 2020) | Change (%) | P | |
|---|---|---|---|---|
| Average number of patients/month (±SD) | 6964.5 (699.1) | 6448.3 (1173.5) | −7.4 | |
| Number of patients/100 000 people | 8.49 (0.85) | 7.75 (1.41) | −8.7 | 0.011 |
| Mortality (mean number per 100 000 patients/month ±SD) | 0.44 (0.09) | 0.37 (0.12) | 0.024 | |
| Cancer location (mean no per 100 000 patients/month) | ||||
| Lung | 1.32 (0.15) | 1.29 (0.22) | −2.3 | 0.622 |
| Esophagus | 0.36 (0.05) | 0.34 (0.08) | −5.6 | 0.220 |
| Gastric | 0.88 (0.12) | 0.76 (0.16) | −13.6 | 0.003 |
| Pancreas | 0.54 (0.07) | 0.56 (0.13) | +3.7 | 0.277 |
| Liver | 0.92 (0.09) | 0.90 (0.14) | −2.2 | 0.429 |
| Colon | 3.24 (0.31) | 2.90 (0.55) | −10.5 | 0.002 |
| Rectum | 1.24 (0.14) | 1.00 (0.20) | −19.4 | <0.001 |
‘People’ represent total number of people in Germany in the respective year. P values stem from Student’s t test.
For further analysis, the year 2020 was divided into four equal periods according to pandemic development and COVID-19 incidence. During the ‘pre-lockdown period’ (January–March), the overall oncological resection rate was 8.82 (±0.81) per 100 000 people on average between 2012 and 2019 and 8.91 (±0.85) in the same period in 2020 (+1.0%, P=0.871). During the first lockdown period (April–June), there was an overall drop of 14.3% in the incidence rate of major oncological resections compared to the same months in previous years (8.58 per 100 000 vs. 7.35 per 100 000, P<0.001) (Table 2 and Fig. 1). In the interim period (July–September), there was an overall drop of 5.1% in the incidence rates in 2020 compared to the mean of the years 2012–2019 (P=0.202). Rectum and gastric resections remained significantly lower than in previous years (−20.3%, P<0.001 and −13.5%, P=0.012, respectively), while there were significantly more pancreatic resections performed in 2020 than in the same months between 2012 and 2019 (+14.5%, P=0.004) (Table 3 and Fig. 1). In the second lockdown period (October–December), there was an overall drop of 17.3% of major resections (P=0.065). Except for colon resections, the reduction of each entity was stronger than in the previous lockdown period, while the strongest drop was again in rectal cancer resections (−29.8%, P=0.003). (Table 1). Combining the two lockdowns and the interim period revealed an overall trend of lower rates of major abdominal cancer resections after the pandemic onset in 2020 in Germany (Supplementary Table 3, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). In an approximate calculation, the overall number of missed cases due to the pandemic reduction of resections was 7544 in Germany between April and December 2020 (Table 1, Supplementary Table 3, Supplemental Digital Content 2, http://links.lww.com/JS9/A138 and Supplement Table 4, Supplemental Digital Content 2, http://links.lww.com/JS9/A138).
Table 2.
Resections and rates with change: periods of interest (lockdown).
| Reference period 1 (04–06, 2012–2019) | Observation period 1 (04–06, 2020) | Change (%) | P | |
|---|---|---|---|---|
| Average number of patients/month (±SD) | 7034.5 (418.6) | 6110.3 (597.4) | −13.1 | |
| Number of patients/100 000 people | 8.58 (0.50) | 7.35 (0.72) | −14.3 | <0.001 |
| Mortality (mean no per 100 000 patients/month ±SD) | 0.43 (0.03) | 0.34 (0.02) | <0.001 | |
| Cancer location (mean no per 100 000 patients/month) | ||||
| Lung | 1.33 (0.10) | 1.25 (0.08) | −6.0 | 0.240 |
| Esophagus | 0.36 (0.03) | 0.36 (0.02) | −0.0 | 0.836 |
| Gastric | 0.89 (0.06) | 0.74 (0.12) | −16.9 | 0.002 |
| Pancreas | 0.55 (0.05) | 0.54 (0.07) | −1.8 | 0.905 |
| Liver | 0.93 (0.07) | 0.86 (0.10) | −7.5 | 0.132 |
| Colon | 3.29 (0.19) | 2.62 (0.35) | −20.4 | <0.001 |
| Rectum | 1.24 (0.11) | 0.98 (0.13) | −21.0 | <0.001 |
| Reference period 2 (10–12, 2012–2019) | Observation period 2 (10–12, 2020) | |||
| Average number of patients/month (±SD) | 6391.1 (834.7) | 5357.0 (1738.1) | −16.2 | |
| Number of patients/100 000 people | 7.79 (1.02) | 6.44 (2.09) | −17.3 | 0.065 |
| Mortality (mean no per 100 000 patients/month ±SD) | 0.41 (0.06) | 0.28 (0.12) | 0.0043 | |
| Cancer location (mean no per 100 000 patients/month) | ||||
| Lung | 1.20 (0.18) | 1.10 (0.34) | −8.3 | 0.425 |
| Esophagus | 0.33 (0.06) | 0.25 (0.11) | −24.2 | 0.033 |
| Gastric | 0.80 (0.13) | 0.65 (0.25) | −18.8 | 0.109 |
| Pancreas | 0.50 (0.08) | 0.43 (0.19) | −14.0 | 0.243 |
| Liver | 0.85 (0.11) | 0.77 (0.22) | −9.4 | 0.368 |
| Colon | 2.97 (0.33) | 2.44 (0.76) | −17.5 | 0.032 |
| Rectum | 1.14 (0.16) | 0.80 (0.23) | −29.8 | 0.003 |
‘People’ represent total number of people in Germany in the respective year. P values stem from Student’s t test. Visualization in Figure 1.
Figure 1.
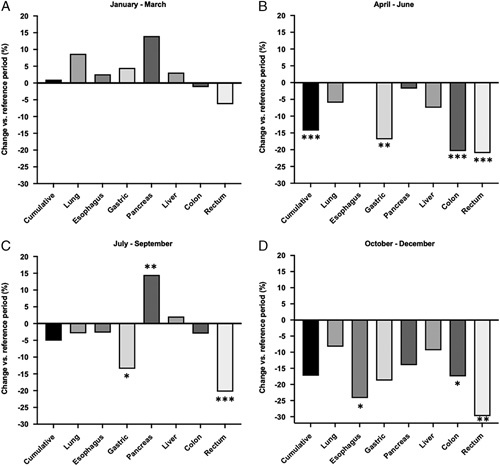
Change of standardized resections rates: pre-lockdown (A), first lockdown (B), interim period (C), and beginning of second (D) lockdown. Numbers of *designate significance (*P<0.05, **P<0.01, ***P<0.001; nonsignificance is not stated).
Table 3.
Resections and rates with change: pre-lockdown and interim period.
| Pre-lockdown (reference) (01–03, 2012–2019) | Pre-lockdown period (observation) (01–03, 2020) | Change (%) | P | |
|---|---|---|---|---|
| Average number of patients/month (±SD) | 7237.1 (662.4) | 7405.7 (705.5) | +2.3 | |
| Number of patients/100 000 people | 8.82 (0.81) | 8.91 (0.85) | +1.0 | 0.871 |
| Cancer location (mean no per 100 000 patients/month) | ||||
| Lung | 1.38 (0.13) | 1.50 (0.14) | +8.7 | 0.154 |
| Esophagus | 0.39 (0.06) | 0.40 (0.04) | +2.6 | 0.673 |
| Gastric | 0.89 (0.06) | 0.93 (0.03) | +4.5 | 0.667 |
| Pancreas | 0.57 (0.08) | 0.65 (0.06) | +14.0 | 0.150 |
| Liver | 0.96 (0.02) | 0.99 (0.09) | +3.1 | 0.561 |
| Colon | 3.32 (0.06) | 3.28 (0.32) | −1.2 | 0.798 |
| Rectum | 1.28 (0.13) | 1.20 (0.14) | −6.3 | 0.341 |
| Interim period (reference) (07–09, 2012–2019) | Interim period (observation) (07–09, 2020) | |||
| Average number of patients/month (±SD) | 7195.2 (476.3) | 6920 (178.0) | −3.8 | |
| Number of patients/100 000 people | 8.77 (0.58) | 8.32 (0.21) | −5.1 | 0.202 |
| Cancer location (mean number per 100 000 patients/month) | ||||
| Lung | 1.36 (0.12) | 1.32 (0.04) | −2.9 | 0.512 |
| Esophagus | 0.37 (0.03) | 0.36 (0.05) | −2.7 | 0.482 |
| Gastric | 0.89 (0.07) | 0.77 (0.05) | −13.5 | 0.012 |
| Pancreas | 0.55 (0.04) | 0.63 (0.04) | +14.5 | 0.004 |
| Liver | 0.95 (0.06) | 0.97 (0.06) | +2.1 | 0.653 |
| Colon | 3.36 (0.27) | 3.26 (0.13) | −3.0 | 0.532 |
| Rectum | 1.28 (0.12) | 1.02 (0.03) | −20.3 | <0.001 |
‘People’ represent total number of people in Germany in the respective year. P values stem from Student’s t test. Visualization in Figure 1.
In the descriptive analysis, there was a clear seasonality of resections in all entities for each year (Fig. 2). There was a two-peaked trend of higher resections rates at the beginning of each year and in each summer, while resection rates were lower during winter months (Fig. 2 and Supplementary Table 4, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). In the same descriptive analysis, there was a clear surge in resection rates at the beginning of 2020 (April 2020, the beginning of the first lockdown). However, seasonality was also observed in 2020 with similar peaks (Fig. 2). For a quantitative analysis of seasonal trends, an analysis19 was conducted (Fig. 3 and Supplementary Table 5, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). Choosing both the beginning of the first and the second lockdown periods as the point of intervention (i.e. lockdown), level changes were significantly reduced for most entities (Supplementary Table 5, Supplemental Digital Content 2, http://links.lww.com/JS9/A138).
Figure 2.
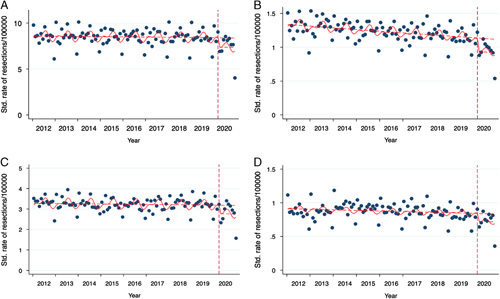
Seasonality and intercept as of 04/2020: expected rates vs. real rates before and after the first lockdown. Dots represent individual months. (A) Cumulative cancer resections, (B) rectum cancer resections, (C) colon cancer resections, and (D) gastric cancer resections. See Supplementary Figure 1 for the remaining entities.
Figure 3.
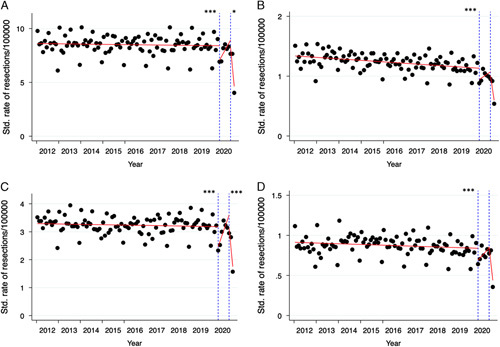
Interrupted time series analysis: the impact of the first vs. the first and second lockdown. (A) Cumulative cancer resections, (B) rectum cancer resections, (C) colon cancer resections, and (D) gastric cancer resections. See Supplementary Figure 2 for the remaining entities. The stated number of *designate P as in Supplementary Table 5 for level change (left-hand *for 04/2020, right-hand for 10/2020; no designation for nonsignificance).
When analyzing specific patient cohorts, no differences were observed between males and females except for lung cancer resections, while grouping patients according to their age revealed significant differences. In fact, it was the elderly whose resection rates were most influenced in both lockdowns: the most pronounced drop in an age-adjusted resection rate analysis of both lockdowns showed that those who were most affected was the older than 75 years age group (Table 4).
Table 4.
Stratification by age, frailty, and sex.
| Reference period 1 (04–06, 2012–2019) | Observation period 1 (04–06, 2020) | Change (%) | P | Reference period 2 (10–12, 2012–2019) | Observation period 2 (10–12, 2020) | Change (%) | P | |
|---|---|---|---|---|---|---|---|---|
| Gastric cancer | ||||||||
| Age | ||||||||
| ≤59 | 0.27 | 0.24 | −11.1 | 0.173 | 0.25 | 0.21 | −16.0 | 0.157 |
| 60–74 | 2.08 | 1.70 | −18.3 | 0.008 | 1.87 | 1.47 | −21.4 | 0.057 |
| ≥75 | 3.20 | 2.37 | −25.9 | <0.001 | 2.83 | 2.13 | −24.7 | 0.077 |
| Females | 0.69 | 0.58 | −15.9 | 0.007 | 0.63 | 0.49 | −22.2 | 0.071 |
| Males | 1.09 | 0.90 | −17.4 | 0.004 | 0.97 | 0.81 | −17.5 | 0.161 |
| Colon cancer | ||||||||
| Age | ||||||||
| ≤59 | 0.72 | 0.65 | −9.7 | 0.042 | 0.68 | 0.64 | −5.9 | 0.326 |
| 60–74 | 7.23 | 5.22 | −27.9 | <0.001 | 6.54 | 4.80 | −26.8 | <0.001 |
| ≥75 | 14.34 | 10.95 | −23.6 | <0.001 | 12.75 | 10.08 | −20.9 | 0.047 |
| Females | 3.14 | 2.38 | −24.2 | <0.001 | 2.78 | 2.29 | −17.6 | 0.038 |
| Males | 3.44 | 2.87 | −16.9 | <0.001 | 3.18 | 2.60 | −18.2 | 0.030 |
| Rectal cancer | ||||||||
| Age | ||||||||
| ≤59 | 0.40 | 0.36 | −10.0 | 0.140 | 0.38 | 0.29 | −23.7 | 0.006 |
| 60–74 | 3.13 | 2.38 | −24.0 | 0.001 | 2.88 | 1.90 | −34.0 | 0.002 |
| ≥75 | 3.96 | 2.69 | −32.1 | <0.001 | 3.59 | 2.34 | −34.8 | 0.004 |
| Females | 0.93 | 0.73 | −22.6 | 0.006 | 0.86 | 0.65 | −25.6 | 0.015 |
| Males | 1.56 | 1.23 | −21.2 | <0.001 | 1.44 | 0.96 | −32.6 | 0.001 |
| Esophageal cancer | ||||||||
| Age | ||||||||
| ≤59 | 0.16 | 0.15 | −6.3 | 0.372 | 0.14 | 0.10 | −28.6 | 0.028 |
| 60–74 | 1.06 | 1.06 | 0 | 0.966 | 0.97 | 0.72 | −25.8 | 0.057 |
| ≥75 | 0.63 | 0.63 | 0 | 0.964 | 0.62 | 0.42 | −33.3 | 0.043 |
| Females | 0.14 | 0.15 | +7.1 | 0.507 | 0.14 | 0.09 | −35.7 | 0.040 |
| Males | 0.58 | 0.58 | 0 | 0.898 | 0.53 | 0.40 | −24.5 | 0.042 |
| Pancreatic cancer | ||||||||
| Age | ||||||||
| ≤59 | 0.17 | 0.16 | −5.9 | 0.389 | 0.15 | 0.13 | −13.3 | 0.400 |
| 60–74 | 1.54 | 1.37 | −11.0 | 0.026 | 1.40 | 1.03 | −25.7 | 0.039 |
| ≥75 | 1.53 | 1.68 | −9.8 | 0.306 | 1.44 | 1.34 | −6.9 | 0.625 |
| Females | 0.52 | 0.53 | +1.9% | 0.815 | 0.47 | 0.39 | −17.0 | 0.183 |
| Males | 0.57 | 0.56 | −3.5 | 0.589 | 0.53 | 0.47 | −11.3 | 0.347 |
| Lung cancer | ||||||||
| Age | ||||||||
| ≤59 | 0.47 | 0.42 | −10.6 | 0.087 | 0.43 | 0.34 | −18.6 | 0.077 |
| 60–74 | 4.27 | 3.90 | −8.9 | 0.068 | 3.81 | 3.46 | −9.4 | 0.353 |
| ≥75 | 2.46 | 2.32 | −5.7 | 0.484 | 2.31 | 2.16 | −6.5 | 0.628 |
| Females | 1.00 | 1.05 | +5 | 0.513 | 0.92 | 0.93 | +1.1 | 0.920 |
| Males | 1.66 | 1.46 | −12.0 | 0.019 | 1.48 | 1.27 | −14.2 | 0.188 |
| Liver cancer | ||||||||
| Age | ||||||||
| ≤59 | 0.36 | 0.34 | −5.6 | 0.364 | 0.33 | 0.30 | −9.1 | 0.318 |
| 60–74 | 2.67 | 2.33 | −12.4 | 0.018 | 2.39 | 2.10 | −12.6 | 0.183 |
| ≥75 | 2.04 | 1.78 | −12.3 | 0.109 | 1.86 | 1.66 | −10.8 | 0.382 |
| Females | 0.70 | 0.65 | −7.1 | 0.211 | 0.65 | 0.58 | −9.2 | 0.345 |
| Males | 1.17 | 1.07 | −8.5 | 0.130 | 1.05 | 0.97 | −7.6 | 0.407 |
Rates are calculated based on population data of that age group (i.e. per 100 000 people of that age group, https://service.destatis.de/bevoelkerungspyramide/#!y).
When analyzing the distribution of patients between low, medium, and high-volume centers, a stronger reduction of cancer resections in low-volume centers was observed (Supplementary Table 6, Supplemental Digital Content 2, http://links.lww.com/JS9/A138).
The incidence of COVID-19 infections demonstrated strong regional differences in Germany in the first and second waves. To analyze the association between the actual regional SARS-CoV-2 incidence and the reduction of surgical volume in the same region, SARS-CoV-2 incidences (Supplementary Table 7, Supplemental Digital Content 2, http://links.lww.com/JS9/A138) were correlated with the reduction of surgical resection rates (Fig. 4). In high incidence states, the overall reduction of resection rates was significantly higher than in medium and low incidence states (data not shown). There was a significant negative correlation between monthly incidence rates and resections rates in all entities except lung cancer resections (Fig. 4 and Supplementary Figure 3, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). This correlation was strongest for rectal cancer resections (Spearman’s r: −0.425; −0.275 to −0.556, P<0.001).
Figure 4.
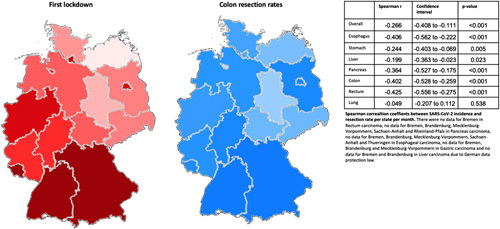
The impact of the first lockdown per state within Germany. Higher incidences are depicted in darker red. Coronavirus disease incidences per month and state in Supplementary Table 6. High resection rates in brighter blue and darker blue designate lower (‘higher reduction’) resection rates. Resection rates are depicted for colon resections. Correlation factors with 95% CI are in the accompanying table. The second lockdown is in Supplementary Figure 3. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Discussion
In this retrospective population-based cohort study and time series analysis, we demonstrated that the overall rate of major cancer resections significantly dropped during the two COVID-19-related lockdown periods in Germany in 2020. Interestingly, there were profound differences between cancer entities. Those normally diagnosed due to severe symptoms, such as icterus for pancreatic cancer or dysphagia for esophageal cancer, showed no overall changes in 2020. In contrast, cancer entities which often present with more unspecific symptoms or are diagnosed during screening programs, such as colorectal carcinomas, were markedly reduced. This may be due to postponement or cancellation of screening procedures. Also, the rate of lung cancer resection did not change. This could partly be attributed to the fact that more chest CT scans were performed due to COVID-19, revealing lung cancer.
Other studies have already shown a decrease in the incidence of cancer diagnoses and cancer resections during the 2020 COVID-19 lockdown in Germany9,10. For instance, a comprehensive prospective cohort study of 20 006 adult patients in 61 countries identified the fragility of worldwide cancer surgery during the pandemic. The COVIDSurg Collaborative demonstrated that full lockdowns were a major risk factor for surgical postponement11. Despite the retrospective nature of this analysis, to our knowledge, this is the first time that data on the whole German population of 2020 as the first pandemic year was compared to previous years.
The decrease in major resections was linked to patient age and hospital volume. Elderly patients, as well as lower volume hospitals, experienced a greater reduction in major abdominal and thoracic resections. This may partly be linked, however, since for some entities, patients of the geriatric population are more likely to be operated on in low-volume hospitals20. It may also be speculated that, out of fear of contracting the virus, the geriatric population actively avoided healthcare facilities more than other age groups during the COVID-19 waves.
Interestingly, the mortality of patients undergoing major cancer resections during the COVID-19 pandemic in 2020 was lower compared to previous years. There are several possible explanations for this finding. We observed a disproportionately greater decrease in operations performed on elderly patients, who in general have higher overall in-house mortality rates20–23. We also observed that patients were more likely to be operated on in high-volume centers during the pandemic than before. For several cancer entities such as colon, rectum, gastric and lung cancer, a strong association between decreased mortality and treatment in high-volume centers has been demonstrated in the German population21,22,24,25.
During the whole year 2020, there was an increase in pancreatic cancer resections (Table 1). This has to be interpreted in light of an overall increase in cases over the study period (Supplementary Fig. 1D, Supplemental Digital Content 2, http://links.lww.com/JS9/A138) in this entity. While there was a decrease of 1.8% and 14.0% in the two lockdowns, respectively, there was an increase of 14.0% in the pre-lockdown period and a catch-up effect of 14.5% in the interim period, which was the only detected catch-up effect among all entities. This catch-up effect might be due to the morbidity burden of pancreatic cancer patients. In addition, January was the month with the overall highest procedural rates for pancreatic resections during the entire time frame of the study, and there was another increase in resections in January 2020 (592 vs. 553.5 between 2012 and 2019, Supplementary Table 4, Supplemental Digital Content 2, http://links.lww.com/JS9/A138). These aspects explain the overall increase of pancreatic resections in 2020 despite the reduction during lockdowns.
The major strength of the study is the use of a complete population dataset reflecting the entire German population independent of insurance status. In addition, data validity was very high as hospital billing data is subject to intense external auditing by the Medical Service of the Health Insurance companies.
A further strength of this study is the use of an ITS design leading to a quasi-experimental situation. This type of study design is generally less susceptible to biases typically found in observational and retrospective designs, as potential confounding is limited to simultaneously occurring events. We further adjusted for seasonal trends in the data, as well as for general trends over time, such as a general decrease in the use of certain surgical interventions or disease entities.
While the results of the ITS analysis alone support a causal relation between COVID-19 waves and a decrease in major surgical procedures, this is further corroborated by the strong geographical association between COVID-19 incidence and the decrease in surgical volume.
Study limitations include that data was not specifically collected for scientific purposes but for hospital reimbursement. Due to its nature, no definitive causal inference could be made, even though this issue was partly addressed by ITS analysis. In addition, information on the tumor stage was missing. One could speculate that patients undergoing major resection for cancer present later than normal, with worse disease outcomes. Data on long-term survival or patient readmission, however, is lacking due to its nature. In addition, we only investigated the incidence of surgical resection. Other treatments of cancer were also affected by the COVID-19-related lockdown and delayed patient presentation, and may have impacted patient outcomes10. Finally, the database lacks information on socioeconomic status, which influences health-seeking behavior26. Moreover, the German context may not be generalizable to other geographical and political settings.
It will be interesting to see whether evidence can demonstrate an increase in advanced tumor stages in midterm analyses after pandemic lockdown periods.
In conclusion, we were able to demonstrate that operative procedures for several tumor entities dropped significantly during the first 9 months of the COVID-19 pandemic in Germany. The reduced rates in colorectal cancer resections, in particular, argue for a more intense screening approach to compensate for patients who are currently undiagnosed, and who are still in a potentially curable tumor stage. In future pandemic circumstances, oncological surgery will have to be prioritized with an extra focus on the most vulnerable patients.
Ethical approval
Diagnosis-related group (DRG) data were analyzed through the Federal Statistical Office in accordance with the ethical and legal data protection regulations of Germany. Because of complete anonymity, no approval from the Federal or University’s ethics committee is required (# n.a.).
Sources of funding
This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
Author contribution
K.L.U., J.D., C.-T.G., and A.W.: study design; K.L.U., J.D., P.B., M.H., and A.W.: data collection; L.K., J.D., P.B., and A.W.: data analysis; L.K., M.H., C.-T.G., and A.W.: writing.
Conflicts of interest disclosure
The authors declare no conflicts of interest.
Research registration unique identifying number (UIN)
Name of the registry: German clinical trial registry (DRKS).
Unique identifying number or registration ID: DRKS00029661.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.drks.de/drks_web/
Guarantor
Armin Wiegering.
Data availability statement
All data are provided on request from the authors.
Declarations
There was no funding for this study.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Supplementary Material
Footnotes
K.L.U. and J.D. contributed equally to this article.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.journal-surgery.net.
Published online 14 March 2023
Contributor Information
Konstantin L. Uttinger, Email: konstantin@uttinger.com.
Johannes Diers, Email: johannes.diers@gmx.de.
Philip Baum, Email: Philip.Baum@med.uni-heidelberg.de.
Mohammed Hankir, Email: Hankir_M@ukw.de.
Christoph-Thomas Germer, Email: germer_c@ukw.de.
Armin Wiegering, Email: Wiegering_A@ukw.de.
References
- 1. Robert Koch Institut. SARS-CoV-2-Inzidenz und Todesfälle; 2022. https://wwwrkide/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlenhtml
- 2. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie J, Tong Z, Guan X, et al. Clinical characteristics of patients who died of coronavirus disease 2019 in China. JAMA Netw Open 2020;3:e205619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flemming S, Hankir M, Ernestus RI, et al. Surgery in times of COVID-19-recommendations for hospital and patient management. Langenbecks Arch Surg 2020;405:359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kohler F, Acar L, van den Berg A, et al. Impact of the COVID-19 pandemic on appendicitis treatment in Germany – a population-based analysis. Langenbecks Arch Surg 2021;406:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slagman A, Behringer W, Greiner F, et al. Medical emergencies during the COVID-19 pandemic. Dtsch Arztebl Int 2020;117:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kohler F, Muller S, Hendricks A, et al. Changes in appendicitis treatment during the COVID-19 pandemic – a systematic review and meta-analysis. Int J Surg 2021;95:106148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform 2020;4:1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diers J, Acar L, Baum P, et al. Fewer operations for cancer in Germany during the first wave of COVID-19 in 2020. Dtsch Arztebl Int 2021;118:481–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Diers J, Acar L, Wagner JC, et al. Cancer diagnosis is one quarter lower than the expected cancer incidence in the first year of COVID-19 pandemic in Germany: a retrospective register-based cohort study. Cancer Commun (Lond) 2022;42:673–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glasbey J, Ademuyiwa A, Adisa A, et al. COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol 2021;22:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diers J, Baum P, Matthes H, et al. Mortality and complication management after surgery for colorectal cancer depending on the DKG minimum amounts for hospital volume. Eur J Surg Oncol 2021;47:850–857. [DOI] [PubMed] [Google Scholar]
- 13. Uttinger KL, Diers J, Baum P, et al. Mortality, complications and failure to rescue after surgery for esophageal, gastric, pancreatic and liver cancer patients based on minimum caseloads set by the German Cancer Society. Eur J Surg Oncol 2022;48:924–932. [DOI] [PubMed] [Google Scholar]
- 14. Eckart A, Hauser SI, Haubitz S, et al. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: results of a prospective, observational study. BMJ Open 2019;9:e026923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet 2018;391:1775–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner AK, Soumerai SB, Zhang F, et al. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. [DOI] [PubMed] [Google Scholar]
- 17. Cumby RE, Huizinga J. Testing the autocorrelation structure of disturbances in ordinary least squares and instrumental variables regressions. Econometrica 1992;60:185–195. [Google Scholar]
- 18. Mathew G, Agha R, Albrecht J, et al. STROCSS Group. STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case–control studies in Surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 19. Campbell DT, Stanley JC. Experimental and Quasi-Experimental Designs for Research. Rand McNally; 1966. [Google Scholar]
- 20. Diers J, Baum P, Lehmann K, et al. Disproportionately high failure to rescue rates after resection for colorectal cancer in the geriatric patient population – a nationwide study. Cancer Med 2022;11:4256–4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baum P, Diers J, Haag J, et al. Nationwide effect of high procedure volume in lung cancer surgery on in-house mortality in Germany. Lung Cancer 2020;149:78–83. [DOI] [PubMed] [Google Scholar]
- 22. Diers J, Baum P, Wagner JC, et al. Hospital volume following major surgery for gastric cancer determines in-hospital mortality rate and failure to rescue: a nation-wide study based on German billing data (2009–2017). Gastric Cancer 2021;24:959–969. [DOI] [PubMed] [Google Scholar]
- 23. Lehmann KS, Klinger C, Diers J, et al. Safety of anastomoses in colorectal cancer surgery in octogenarians: a prospective cohort study with propensity score matching. BJS Open 2021;5:zrab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diers J, Wagner J, Baum P, et al. Nationwide in-hospital mortality following colonic cancer resection according to hospital volume in Germany. BJS Open 2019;3:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diers J, Wagner J, Baum P, et al. Nationwide in-hospital mortality rate following rectal resection for rectal cancer according to annual hospital volume in Germany. BJS Open 2020;4:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan CQH, Lee KH, Low LL. A systematic review of health status, health seeking behaviour and healthcare utilisation of low socioeconomic status populations in urban Singapore. Int J Equity Health 2018;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided on request from the authors.


