Abstract
Background:
Hydroxychloroquine and ivermectin received widespread attention after initial studies suggested that they were effective against COVID-19. However, several of these studies were later discredited.
Objectives:
We explored the impact of scientific articles, public announcements, and social media posts on hydroxychloroquine and ivermectin purchases in the U.S. and Canada during the COVID-19 pandemic.
Methods:
We conducted a retrospective, population-based time series analysis of retail hydroxychloroquine and ivermectin purchases in the U.S. and Canada from February 2016 through December 2021, using IQVIA’s Multinational Integrated Data Analysis database. We fit the purchasing rates with interventional autoregressive integrated moving average models. We used Google Trends to identify the most influential interventions to include in the models.
Results:
There were significant pulse increases in hydroxychloroquine purchases in March 2020 in both the U.S. (p < 0.0001) and Canada (p < 0.0001). For ivermectin, there were no significant changes in April 2020 in either the U.S. (p = 0.41) or Canada (p = 0.16); however, significant pulse increases occurred from December 2020 to January 2021 in both the U.S. (p = 0.0006) and Canada (p < 0.0001), as well as significant ramp increases from April to August 2021 in both the U.S. (p < 0.0001) and Canada (p = 0.02). The increases in ivermectin purchases were larger in the U.S. than in Canada.
Conclusions:
Increases in hydroxychloroquine and ivermectin purchasing rates aligned with controversial scientific articles and social media posts. This highlights the importance of scientific integrity and disseminating accurate epidemiologic information during pandemics.
1. Introduction
Coronavirus disease 2019 (COVID-19) has been the subject of considerable scientific research and media attention. New agents for prevention or treatment take time to develop, and therefore much interest in the early parts of the pandemic was focused on the potential for drug repurposing for off-label use. In 2020 and 2021, speculation that hydroxychloroquine and ivermectin may prevent or treat COVID-19 led to their increasing popularity across North America despite a lack of rigorous data supporting their use.1–3 As additional evidence emerged that called into question the effectiveness of hydroxychloroquine and ivermectin, several of the initial studies were discredited.4,5
Hydroxychloroquine is an antimalarial and antirheumatic drug that showed early potential against COVID-19 by inhibiting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in vitro.6,7 In March 2020, a small, non-randomized study suggested that it reduced viral load in COVID-19 patients.8 Then-President of the United States (U.S.) Donald Trump endorsed hydroxychloroquine, referencing this study.9 The U.S. FDA subsequently issued an emergency use authorization for hydroxychloroquine in COVID-19.10 However, there were soon reports of serious arrhythmia from its use.11 When the interim results of several large randomized controlled trials showed no benefit in mortality in June 2020,12–14 the FDA revoked the emergency use authorization.10
By contrast, the timeline of events for ivermectin was relatively protracted. Ivermectin is an antiparasitic drug that also showed early potential by inhibiting SARS-CoV-2 in vitro.15 While some observational studies, randomized controlled trials, and meta-analyses reported its efficacy in COVID-19 patients throughout 2020 and 2021,16–25 concerns were raised in late 2021 about randomization failure and potential fraud among published randomized controlled trials.5 Several studies were subsequently retracted.17,20,25 One of these, a meta-analysis, was re-analyzed and found no benefit in survival if high-risk-of-bias studies were excluded.26
Neither the FDA nor Health Canada has approved hydroxychloroquine or ivermectin to prevent or treat COVID-19. In fact, both federal regulators have recommended against their use based on results from large randomized controlled trials.27–30 Nevertheless, over the course of the pandemic, these drugs received widespread attention. Controversial papers led to debate in the scientific community and, through the lay press and social media, influenced public opinion. Although some studies in the U.S. have described early trends in hydroxychloroquine and ivermectin prescribing,1–3,31 the evidence and public discourse has continued to evolve. There is a great deal of shared media across the U.S.-Canada border, but little is known about how purchasing patterns compare between the two countries. Understanding the impact of scientific and social discourse on drug purchasing is important for informing the conduct and dissemination of research. This is particularly relevant when considering the health risks of the widespread communication of studies with poor scientific rigour during a pandemic. Therefore, our objective was to evaluate the impact of influential scientific articles, public announcements, and social media posts on purchasing patterns of hydroxychloroquine and ivermectin in the U.S. and Canada.
2. Methods
2.1. Setting
We conducted a retrospective, population-based time series analysis of oral, solid units of hydroxychloroquine and ivermectin purchased in U.S. and Canadian outpatient settings between February 1, 2016 (earliest data available from IQVIA) and December 31, 2021. The start date for ivermectin in Canada was limited to November 2018 because it only entered the Canadian market then.32 Ethical approval was granted by the University of Pittsburgh Institutional Review Board (Ref. STUDY21060160).
2.2. Data sources
We obtained the monthly outpatient purchase quantities of hydroxychloroquine and ivermectin using IQVIA’s Multinational Integrated Data Analysis (MIDAS) database (Parsippany, NJ, USA). We restricted the data to oral, solid dosage forms, which were reported in units of single tablets for these two drugs. MIDAS includes all drug purchasing data for the U.S. and Canada, regardless of payer, by reporting annual transactions from pharmaceutical manufacturers to wholesaler distribution centers.33 IQVIA internally validates their data annually against alternate sources through a standardized quality assurance program.33 Data capture was 97% in the U.S. and 100% in Canada. We used monthly population estimates from the U.S. Census Bureau and Statistics Canada to population-adjust the drug purchasing data.34–36 Population data in Canada was only available quarterly, and therefore we used linear interpolation to obtain monthly estimates.
To capture social discourse around these medications, we extracted monthly national web search data from Google Trends for the terms “hydroxychloroquine” and “ivermectin” in all search categories, for the U.S. and Canada, from February 2016 to December 2021. Google Trends creates a score for each month on a scale from 0 to 100, with 100 representing the month of maximum search interest during the study period.37 This publicly available tool has been used to study social responses,9,38–40 as well as correlation with prescription patterns,41 during COVID-19. Finally, we obtained the number of monthly COVID-19 cases for the U.S. and Canada from the Johns Hopkins Coronavirus Resource Center.42
2.3. Outcomes
We measured the monthly rates of outpatient purchases of hydroxychloroquine and ivermectin in the U.S. and Canada, per 1000 population. These rates were overlaid against social discourse trends (using Google Trends) and COVID-19 case rates over the same period (see Table S1 for details on the dominant COVID-19 variants of concern during the study period).
2.4. Statistical analysis
For our interrupted time series analyses, we fit the monthly population-adjusted prescription purchasing rates with interventional autoregressive integrated moving average (ARIMA) models. ARIMA models can evaluate the impact of population-level health interventions.43 We defined our interventions as major scientific articles, public announcements, or social media posts related to each drug. First, we compiled a list of influential articles, announcements, and posts about hydroxychloroquine or ivermectin from the literature and lay media; then, we plotted the Google Trends time series and used the peaks to identify the most influential interventions. All identified interventions are outlined in Tables 1 and 2. In cases where multiple interventions occurred in quick succession, we selected the date of the first intervention to fit in the models. We included pulse or ramp transfer functions to test for temporary or gradual changes, respectively, in purchasing rates after each intervention. A pulse function models a sudden change that returns to baseline afterwards. A ramp function models a gradual change that increases in magnitude linearly over time.
Table 1.
Major hydroxychloroquine events from the start of the COVID-19 pandemic to December 2021
| Intervention in model | Date in 2020 | Description | ||
|---|---|---|---|---|
| 1 | Pulsea | March | 11 | The World Health Organization (WHO) declared COVID-19 a pandemic.70 |
| 9, 18 | Yao et al. and Liu et al. suggested hydroxychloroquine inhibits SARS-CoV-2 in vitro.6,7 | |||
| 19 | Then-President Trump endorsed hydroxychloroquine in a White House press briefing.9 | |||
| 20 | Gautret et al. suggested hydroxychloroquine efficiently clears nasopharyngeal carriage of SARS-CoV-2 in a small, open-label, non-randomized clinical trial.8 | |||
| 21 | Then-President Trump cited Gautret et al. in a tweet supporting hydroxychloroquine use.9 | |||
| 28 | The FDA issued an emergency use authorization for hydroxychloroquine.10 | |||
| (i) | Not includeda | April | 24 | The FDA issued a drug safety communication for hydroxychloroquine (reports of serious arrhythmia).11 |
| (ii) | Not includeda | May | 18 | Then-President Trump said he was taking hydroxychloroquine prophylactically.9 |
| 22 | Mehra et al. suggested hydroxychloroquine increased in-hospital mortality in a large, multinational registry analysis (retracted on June 4).71 | |||
| (iii) | Not includeda | June | 4 | Mehra et al. retracted their paper because they were unable to independently verify their data set.72 |
| 5, 19, 19 | Three large randomized controlled trials (RECOVERY, Solidarity, and ORCHID) closed enrolment early because of no observed benefit in mortality.12–14 | |||
| 15 | The FDA revoked the emergency use authorization for hydroxychloroquine.10 | |||
| (iv) | Not includeda | July | 27 | Then-President Trump tweeted a viral video supporting hydroxychloroquine use that was later removed by major social media platforms.9 |
Major hydroxychloroquine events occurred every month from March to July 2020. It is difficult to model and interpret interventions when there are few or no time points separating them. Hence, we only included the first intervention (March 2020) in the models.
Table 2.
Major ivermectin events from the start of the COVID-19 pandemic to December 2021
| Intervention in model | Date in 2020 | Description | ||
| (v) | Not included | March | 11 | The World Health Organization (WHO) declared COVID-19 a pandemic.70 |
| 2 | Pulse | April | 3 | Caly et al. suggested ivermectin inhibits SARS-CoV-2 in vitro.15 |
| 6 | Patel et al. suggested ivermectin effectively reduces mortality in a multinational registry analysis (pre-print) (retracted for “further analysis” in May – exact date uncertain).16,56 | |||
| 3 | Pulse (December to January)a | November | 13 | Elgazzar et al. suggested ivermectin effectively reduces mortality in a large, randomized controlled trial (pre-print) (version 1 on November 13, version 2 on November 16, version 3 on December 28, 2020, and retracted on July 14, 2021).17 |
| Intervention in model | Date in 2021 | Description | ||
| 4 | Ramp (April to August)b | April | 22 | Kory et al.’s meta-analysis on ivermectin (which used pre-print data by Elgazzar et al. and Niaee et al.) suggested large reductions in mortality, time to clinical recovery, and time to viral clearance.23 |
| (vi) | May | 26 | Samaha et al. suggested ivermectin reduces symptoms, viral load, and hospital admissions in a randomized controlled trial (retracted on October 26).20 | |
| (vii) | June | 21 | Bryant et al.’s meta-analysis of ivermectin (which used pre-print data by Elgazzar et al. and Niaee et al., and published data by Samaha et al.) suggested large reductions in mortality.24 | |
| 25 | Niaee et al.’s article was published, which suggested ivermectin reduces mortality in a randomized controlled trial (there have been concerns of randomization failure, though).5,19 | |||
| (viii) | July | 6 | Hill et al.’s meta-analysis of ivermectin (which used data by Elgazzar et al., Samaha et al., and Niaee et al.) suggested large reductions in mortality (retracted in 2022; their re-analysis after excluding high risk of bias studies showed no benefit in survival).25,26 | |
| 14 | Elgazzar et al.’s pre-print version 3 was retracted by the pre-print platform after concerns of fraudulent data emerged.17 | |||
| 15 | The Guardian covered Lawrence et al.’s concerns about Elgazzar et al.’s study, such as duplicated patient records.73 | |||
| Intervention in model (continued) | Date in 2021 | Description | ||
| (viii) | Ramp (April to August)b | July | 28 | Popp et al.’s Cochrane systematic review did not support ivermectin use outside of randomized trials.74 |
| (ix) | Not includedc | September | 22 | Lawrence et al.’s letter to Nature Medicine’s editor about the Elgazzar et al. (potentially fraudulent data) and Niaee et al. (potential randomization failure) papers was published.5 |
| (x) | Not includedc | October | 26 | Samaha et al. retracted their paper after it was found that blocks of patient records were duplicated.75 |
Although their first version was posted in November 2020, based on the delayed Google Trends spike, we modeled this pulse from December 2020 (their third and last version was posted on December 28) to January 2021.
We ended the ramp function before September 2021 because Lawrence et al.’s debunking article was published that month.
We did not model these events because there were not enough data points after them to know if their effects were sustained.
For each ARIMA model, we used differencing terms and the augmented Dickey-Fuller test to induce and confirm stationarity as well as seasonality. Seasonality refers to predictable fluctuations in rates that follow a repeated pattern, generally annually. Next, we selected model parameters using the autocorrelation function (ACF), partial autocorrelation function (PACF), and inverse autocorrelation function (IACF) plots. We chose the final model based on residual autocorrelation plots and the Ljung-Box chi-square test for white noise. Statistical analyses were conducted using SAS (Enterprise Guide 7.1, SAS Institute, Cary, NC) and a type 1 error rate of 0.05. To allow better visualization of interventions, we reported figures as of November 2018 (when ivermectin was first marketed in Canada). However, all ARIMA models were fit using the full data from February 2016 onwards.
3. Results
3.1. Hydroxychloroquine
During our study period from February 2016 to December 2021, U.S. outpatient pharmacies purchased 2,375,171,700 hydroxychloroquine tablets (average U.S. population 327,597,465; approximately 7 tablets per person over the entire study period), while Canadian outpatient pharmacies purchased 376,727,700 tablets (average Canadian population 37,255,419; approximately 10 tablets per person over the entire study period).
Prior to March 2020, the monthly rate of U.S. hydroxychloroquine purchases ranged from 72 to 114 tablets per 1000 population. In March 2020, hydroxychloroquine was shown to inhibit SARS-CoV-2 in vitro,6,7 appeared to reduce viral load in COVID-19 patients,8 and was endorsed by then-President Trump.9 That month, we observed a significant pulse change in the purchasing rate (increase of 117.50 tablets per 1000 population; 95% CI 102.03–132.97; p < 0.0001; Table 3), with the rate peaking at 231 tablets per 1000 population before returning to earlier trends in May 2020 (Figure 1A). The pattern was similar in Canada; prior to March 2020, the monthly rate of hydroxychloroquine purchases ranged from 119 to 151 tablets per 1000 population. In March 2020, there was also a significant pulse change in purchasing rate (increase of 111.55 tablets per 1000 population; 95% CI 95.60–127.50; p < 0.0001; Table 3), with the rate peaking at 246 tablets per 1000 population before returning to earlier trends in April 2020 (Figure 1B). Across both countries, there was a large spike in Google Trends in March and April 2020, and a smaller spike in July 2020. The purchasing spikes in March 2020 were aligned with the first COVID-19 case wave for both countries, but subsequent trends were not.
Table 3.
Results of the interventional ARIMA analyses
| Model 1: Hydroxychloroquine U.S. |
Model 2: Hydroxychloroquine Canada |
Model 3: Ivermectin U.S. |
Model 4: Ivermectin Canada |
|||||
|---|---|---|---|---|---|---|---|---|
| ARIMA model | (2,1,0) no intercept |
(4,1,1) no intercept |
(2,1,0) × (0,1,0)12 no intercept |
(2,1,0) no intercept |
||||
| Intervention: | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value | Estimate (95% CI) | p-value |
| 1 (pulse)a | 117.50 (102.03, 132.97) | <0.0001 | 111.55 (95.60, 127.50) | <0.0001 | – | – | – | – |
| 2 (pulse)a | – | – | – | – | 0.85 (−1.17, 2.87) | 0.41 | 0.07 (−0.03, 0.16) | 0.16 |
| 3 (pulse)a | – | – | – | – | 6.16 (2.66, 9.65) | 0.0006 | 0.44 (0.36, 0.51) | <0.0001 |
| 4 (ramp)a | – | – | – | – | 1.94 (1.11, 2.75) | <0.0001 | 0.02 (0.003, 0.04) | 0.02 |
Figure 1. Population-adjusted hydroxychloroquine purchasing rates, Google Trends, and COVID-19 case rates in (A) the United States and (B) Canada.
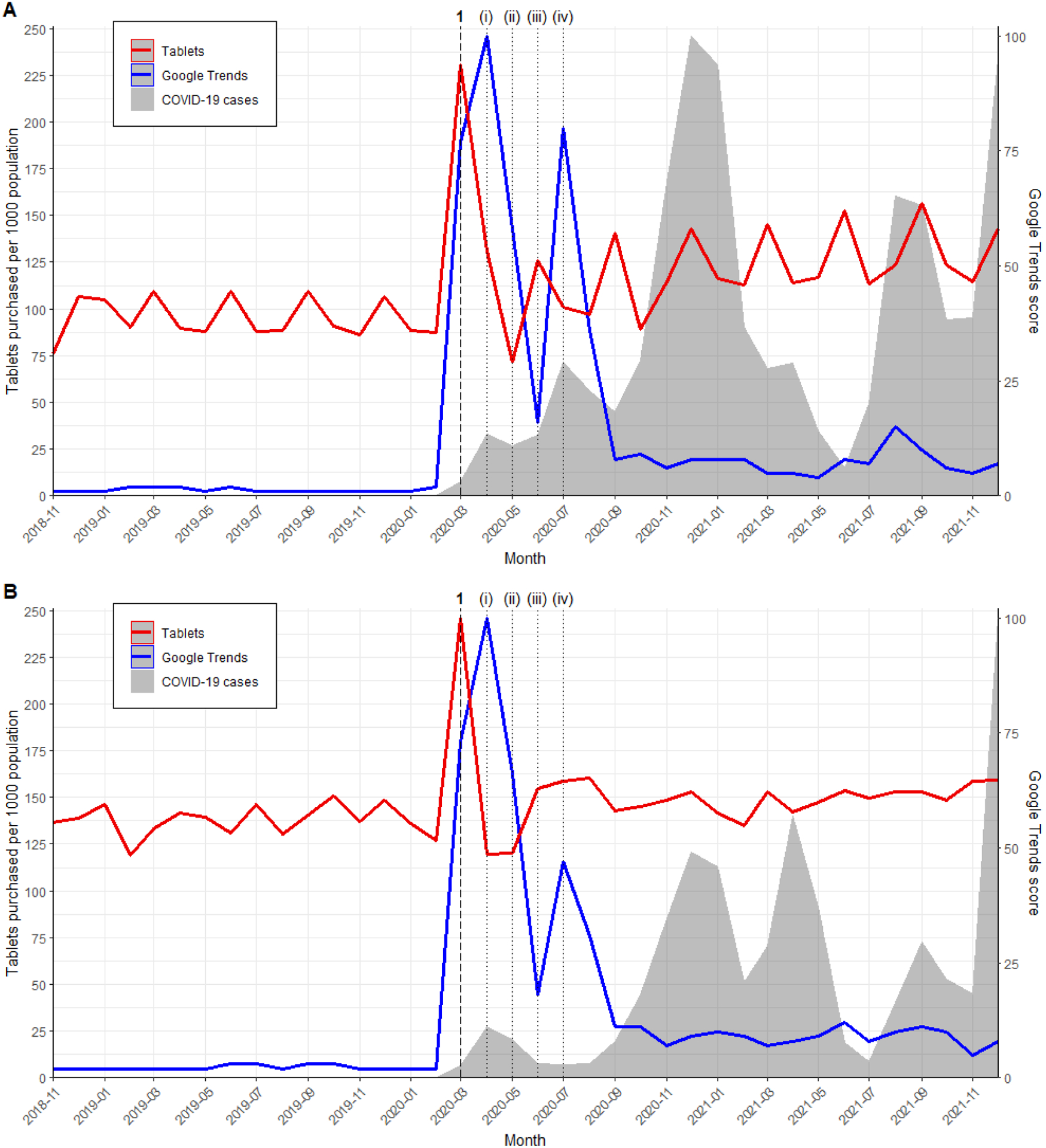
The Google Trends score ranges from 0 to 100. The y-axes for COVID-19 case rates are not shown, but cases in the U.S. began in January 2020 and peaked in December 2020 (19.8 per 1000 population). COVID-19 cases in Canada began in January 2020 and peaked in December 2021 (11.1 per 1000 population). Vertical lines represent major events such as in vitro studies, clinical studies, public announcements, and social media posts (details in Table 1).
3.2. Ivermectin
During our study period, U.S. outpatient pharmacies purchased 54,239,000 ivermectin tablets (approximately 0.2 tablets per person over the entire study period), while Canadian outpatient pharmacies purchased 354,164 tablets (approximately 0.01 tablets per person over the entire study period).
The results of the interrupted time series analysis were consistent across countries. In April 2020, after Caly et al.’s in vitro study and Patel et al.’s multinational registry analysis, there was no significant pulse change in monthly purchasing rate in both the U.S. (increase of 0.85 tablets per 1000 population; 95% CI −1.17–2.87; p = 0.41) and Canada (increase of 0.07 tablets per 1000 population; 95% CI −0.03–0.16; p = 0.16). In December 2020 and January 2021, after Elgazzar et al.’s pre-print, we observed a significant pulse change in rate of ivermectin purchasing in the U.S. (increase of 6.16 tablets per 1000 population; 95% CI 2.66–9.65; p = 0.0006) and Canada (increase of 0.44 tablets per 1000 population; 95% CI 0.36–0.51; p < 0.0001). This was followed by a significant ramp change between April and August 2021, when a series of meta-analysis supported ivermectin use for COVID-19, in both the U.S. (increase of 1.94 tablets per 1000 population; 95% CI 1.11–2.75; p < 0.0001) and Canada (increase of 0.02 tablets per 1000 population; 95% CI 0.003–0.04; p = 0.02) (Table 3). Purchasing rate trends mirrored the Google Trends and COVID-19 case rate trends across both countries. Despite similarities in responses to interventions across the U.S. and Canada, the population-adjusted rates of ivermectin purchasing differed considerably between the countries. Specifically, the monthly rate of ivermectin purchases reached a high of 30 tablets per 1000 population in the U.S. (August 2021), compared to a high of 0.8 tablets per 1000 population in Canada (January 2021; Figure 2; Figure S1).
Figure 2. Population-adjusted ivermectin purchasing rates, Google Trends, and COVID-19 case rates in (A) the United States and (B) Canada.
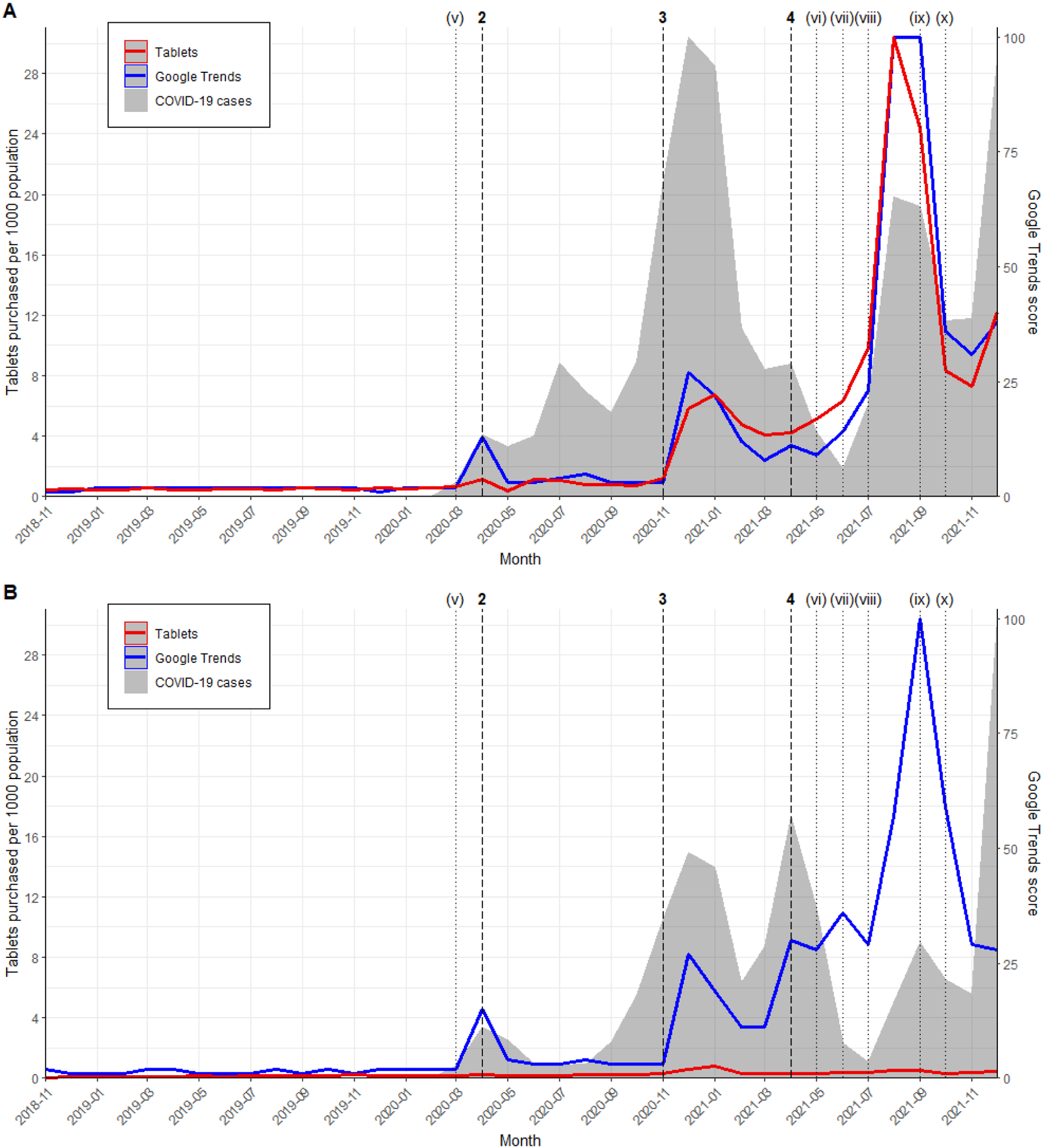
The Google Trends score ranges from 0 to 100. The y-axes for COVID-19 case rates are not shown, but cases in the U.S. began in January 2020 and peaked in December 2020 (19.8 per 1000 population). COVID-19 cases in Canada began in January 2020 and peaked in December 2021 (11.1 per 1000 population). Vertical lines represent major events such as in vitro studies, clinical studies, review articles, and public announcements (details in Table 2). Figure S1 in the supplement is an expanded view of Figure 2B.
4. Discussion
In this large study comparing the U.S. and Canada, we observed significantly increased outpatient purchasing rates of hydroxychloroquine and ivermectin during the COVID-19 pandemic. For hydroxychloroquine, despite initially increased sales associated with early announcements and publications, later reports of serious arrhythmia and negative interim results from several large randomized controlled trials appeared to swiftly return purchasing rates to baseline levels. By contrast, ivermectin had protracted increases in sales tied to COVID-19 case counts and publications supporting ivermectin’s efficacy, despite the later retraction of several of these articles due to concerns of fraudulent data.
The way in which scientific evidence emerged may explain the differences between hydroxychloroquine (abrupt, short-term increase) and ivermectin (gradual, more sustained increase) purchasing trends. As suggested by Englund et al.,9 interest in hydroxychloroquine was likely driven by then-President Trump’s endorsement. His tweet on March 21, 20209 cited a non-randomized study by Gautret et al. which has been criticized for having numerous issues such as potential confounding, small sample size, and overinterpretation of results.4,44 This weak evidence base may explain why, upon reports of serious arrhythmia11 and results of several large randomized controlled trials that showed no benefit in mortality,12–14 the hydroxychloroquine pulse quickly dissipated. Unlike hydroxychloroquine, ivermectin was initially supported by randomized controlled trials17–22 and meta-analyses.23–25 However, randomized controlled trials by Elgazzar et al.17 and Samaha et al.20 were retracted after concerns of fraudulent data, and there have been concerns of randomization failure in the trial by Niaee et al.5 While the article by Elgazzar et al.17 was associated with higher ivermectin purchases, the highest peak in the U.S. was reached following the series of meta-analyses that incorporated the flawed data.23–25 Ivermectin purchases declined after Lawrence et al.5 published concerns about the quality of ivermectin trials in September 2021.
Despite the shared media across the U.S.-Canada border, there were differences between the two countries’ trends in hydroxychloroquine and ivermectin purchasing. Between-country differences were more apparent for ivermectin than hydroxychloroquine. For ivermectin, after Elgazzar et al. posted their now-retracted pre-print in November 2020, we observed a 5-fold increase in purchases in the U.S. compared to a 2-fold increase in purchases in Canada. The U.S. had an even greater increase in purchases after the publication of several meta-analyses supporting ivermectin efficacy starting in April 2021. By August 2021, the U.S. reached 30 tablets sold per 1000 population, versus just 0.5 in Canada. The U.S.’s acceleration to much higher purchasing rates may be tied to the contrasting stances between healthcare bodies and the government regarding inappropriate prescribing. U.S. national healthcare bodies and state medical boards have discouraged ivermectin use for COVID-19.28,45–48 However, some state legislatures have prohibited disciplinary action against physicians based on COVID-19 prescribing practices, such as in Tennessee and North Dakota.49 Meanwhile, Canada’s message has been more specific and actionable. Canadian national healthcare bodies and regulators have advised Canadians to not use ivermectin for COVID-19, and Health Canada has taken action against ivermectin advertisers.30,50 Canadian medical, pharmacy, and nursing licensing bodies have also taken strong stances against ivermectin use, and have restricted or suspended licenses due to inappropriate ivermectin prescribing for COVID-19.51,52 By contrast, between-country differences for hydroxychloroquine were relatively small (a slightly larger increase in March 2020 in the U.S. than in Canada), perhaps because interest only surged for one month, at the start of the pandemic when governments and health agencies were unprepared.
Most previous studies of hydroxychloroquine and ivermectin trends during the COVID-19 pandemic have focused on the first year of the pandemic and used descriptive analytic approaches. Nevertheless, our results are broadly consistent with purchasing and dispensing trends previously reported in the U.S.1–3,31 as well as India,53 Germany,54 and Australia.55 Unlike the U.S. and Canada, Latin America experienced a large ivermectin increase at the start of the pandemic, which appeared to be linked to Patel et al.’s now-retracted multinational registry analysis in April 2020.56 This article was cited by researchers, clinicians, and governments in Latin America.56 It may have influenced the Peruvian and Bolivian governments’ decisions to recommend using ivermectin for COVID-19 in May 2020.57 This, combined with ivermectin not requiring a prescription58 and increased production,57 may explain why ivermectin use increased in Latin America.
There are many lessons to be learned from the increased use of hydroxychloroquine and ivermectin during the COVID-19 pandemic. High demand for these drugs have caused shortages in the U.S. and Canada.59–61 Shortages place patients who need these drugs for on-label use at risk of uncontrolled disease. This has especially affected patients with lupus and arthritis, who chronically require hydroxychloroquine.59 There have also been safety concerns. The FDA has noted reports of serious arrhythmia in patients with COVID-19 treated with hydroxychloroquine11 and there have been large increases in the number of ivermectin poisonings.46,62 Lastly, people may have been taking these drugs as a substitute for standard care, for example not being vaccinated or not seeking care after contracting COVID-19. This highlights the importance of consistent policies and evidence-based medicine during urgent, widespread public health crises, such as this pandemic. Furthermore, there are opportunities to improve the reporting of emerging research. For example, enhancing the ability for journals to request full disclosure of data for external review and accelerating the peer-review process may help facilitate high-quality, timely publications during crises. Media outlets can play an important role by being prudent with their coverage of pre-prints and emphasizing the uncertainty around findings that have not been peer-reviewed.63
The main strength of this study was its use of a large database that comprehensively captured hydroxychloroquine and ivermectin purchases in outpatient settings across the U.S. and Canada. Unlike many previous studies which were descriptive, we used ARIMA modeling to analyze the impact of interventions. These interventions were chosen based on peaks in Google Trends, which reflected surges in social interest of these two drugs. We also had a long follow-up period, until the end of 2021, allowing us to study multiple interventions. However, there were some limitations that require discussion. First, we did not have demographic, social, and clinical data on medication recipients, and thus could not explore variability in trends across ages, gender, vaccination status, or state/province. We also could not distinguish between off-label use of medications for COVID-19 versus on-label use, although the large increases in purchases compared to baseline in the absence of malarial or parasitic outbreaks supports the hypothesis that these were driven by off-label, COVID-19-related use. Second, we did not have data on veterinary ivermectin usage. The FDA received multiple reports of patients who required medical attention after taking veterinary ivermectin45 meaning that we have likely underestimated the increase in ivermectin usage. Third, there were many competing events that occurred throughout the study period making it difficult to disentangle their individual impacts. For example, a scientific article may have been referenced at various stages of development, including as a pre-print, e-publication, full publication, and as part of a systematic review. Another example was the impact of COVID-19 waves on ivermectin use. Although scientific articles were tied to ivermectin purchasing and discourse trends, so were COVID-19 case rates. This ambiguity made it difficult to conclusively link specific interventions with ivermectin purchasing rates. For hydroxychloroquine, the March-2020 pulse may not only be explained by then-President Trump’s endorsement, but also by the stockpiling behaviour seen for other chronic medications.54,55,64 However, the rapid increase in purchasing observed was more dramatic than stockpiling patterns seen with other medications,65 suggesting additional factors were at play. Finally, we only had purchasing data until December 2021 meaning that we were unable to explore the impacts of more recent interventions, such as the approval of nirmatrelvir/ritonavir (Paxlovid),66,67 or the publication of the I-TECH and TOGETHER trials which did not support ivermectin use.68,69 Future work can build on the findings of this study by exploring more recent trends and studying important patient subgroups (e.g. age, vaccination status).
Conclusion
We observed increased outpatient purchases in two purported treatments in the U.S. and Canada during the COVID-19 pandemic. The short-term increase in hydroxychloroquine purchases was associated with then-President Trump’s endorsement, while the protracted increase in ivermectin purchases was aligned with the release of flawed scientific articles. Although the trends were similar across both countries, important differences were also present, with the U.S. exhibiting much higher increases in ivermectin purchasing rates compared to Canada. COVID-19 has not just been a pandemic, but also an infodemic that has ensnared primary research, meta-analyses, clinical practice, and health policy. It has reinforced the importance of evidence-based decision making and disseminating accurate information to the public in the face of urgent public health crises.
Supplementary Material
Acknowledgements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The opinions expressed are those of the authors and do not represent those of the U.S. Department of Veterans Affairs, the U.S. government, or IQVIA or any of its affiliated entities. The statements, findings, conclusions, views, and opinions contained and expressed in this study are based in part on data obtained under license from IQVIA: MIDAS February 2016 to December 2021, IQVIA, Inc. The study sponsor had no role in the design or conduct of the study; data collection, management, analysis, or interpretation of the data; preparation, review, or appraisal of the manuscript; or decision to submit the manuscript for publication. The authors would like to acknowledge Daniel McCormack who supported some elements of the data analysis.
Funding
Research reported was support by the Agency for Healthcare Research and Quality under Grant R01HS027985 (Principal Investigator: KJS).
Footnotes
Transparency declarations
None to declare.
References
- 1.Shehab N, Lovegrove M, Budnitz DS. US Hydroxychloroquine, Chloroquine, and Azithromycin Outpatient Prescription Trends, October 2019 Through March 2020. JAMA Intern Med 2020; 180: 1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bull-Otterson L, Gray EB, Budnitz DS et al. Hydroxychloroquine and Chloroquine Prescribing Patterns by Provider Specialty Following Initial Reports of Potential Benefit for COVID-19 Treatment — United States, January–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lind JN, Lovegrove MC, Geller AI et al. Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis. J Gen Intern Med 2021; 36: 2909–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim AHJ, Sparks JA, Liew JW et al. A Rush to Judgment? Rapid Reporting and Dissemination of Results and Its Consequences Regarding the Use of Hydroxychloroquine for COVID-19. Ann Intern Med 2020; 172: 819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence JM, Meyerowitz-Katz G, Heathers JAJ et al. The lesson of ivermectin: meta-analyses based on summary data alone are inherently unreliable. Nat Med 2021; 27: 1853–1854. [DOI] [PubMed] [Google Scholar]
- 6.Yao X, Ye F, Zhang M et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71: 732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Cao R, Xu M et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 2020; 6: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautret P, Lagier J-C, Parola P et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agent 2020; 56: 105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Englund TR, Kinlaw AC, Sheikh SZ. Rise and Fall: Hydroxychloroquine and COVID‐19 Global Trends: Interest, Political Influence, and Potential Implications. ACR Open Rheumatol 2020; 2: 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food & Drug Administration. Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
- 11.U.S. Food & Drug Administration. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or
- 12.The RECOVERY Collaborative Group, Horby P, Mafham M et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med 2020; 383: 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO Solidarity Trial Consortium. Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results. N Engl J Med 2021; 384: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Self WH, Semler MW, Leither LM et al. Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19. JAMA 2020; 324: 2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caly L, Druce JD, Catton MG et al. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res 2020; 178: 104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AN, Desai SS, Grainger DW et al. Ivermectin in COVID-19 Related Critical Illness. SSRN 2020. [Google Scholar]
- 17.Elgazzar A, Eltaweel A, Youssef SA et al. Efficacy and Safety of Ivermectin for Treatment and prophylaxis of COVID-19 Pandemic. Research Square Platform LLC 2020. [Google Scholar]
- 18.Mahmud R, Rahman MM, Alam I et al. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res 2021; 49: 3000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niaee MS, Namdar P, Allami A et al. Ivermectin as an adjunct treatment for hospitalized adult COVID-19 patients: A randomized multi-center clinical trial. Asian Pac J Trop Med 2021; 14: 266. [Google Scholar]
- 20.Samaha AA, Mouawia H, Fawaz M et al. Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon. Viruses 2021; 13: 989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahbaznejad L, Davoudi A, Eslami G et al. Effects of Ivermectin in Patients With COVID-19: A Multicenter, Double-blind, Randomized, Controlled Clinical Trial. Clin Ther 2021; 43: 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoumann WM, Hegazy AA, Nafae RM et al. Use of Ivermectin as a Potential Chemoprophylaxis for COVID-19 in Egypt: A Randomised Clinical Trial. J Clin Diagn Res 2021; 15: OC27–OC32. [Google Scholar]
- 23.Kory P, Meduri GU, Varon J et al. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. Am J Ther 2021; 28: e299–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryant A, Lawrie TA, Dowswell T et al. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am J Ther 2021; 28: e434–e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hill A, Garratt A, Levi J et al. Retracted: Meta-analysis of Randomized Trials of Ivermectin to Treat SARS-CoV-2 Infection. Open Forum Infect Dis 2021; 8: ofab358. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Hill A, Mirchandani M, Ellis L et al. Ivermectin for the prevention of COVID-19: addressing potential bias and medical fraud. J Antimicrob Chemother 2022; 77: 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Institutes of Health. Chloroquine or Hydroxychloroquine and/or Azithromycin. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/chloroquine-or-hydroxychloroquine-and-or-azithromycin/
- 28.National Institutes of Health. Ivermectin. https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ivermectin/
- 29.Government of Canada. Chloroquine and hydroxychloroquine can have serious side effects. These drugs should be used only under the supervision of a physician. https://recalls-rappels.canada.ca/en/alert-recall/chloroquine-and-hydroxychloroquine-can-have-serious-side-effects-these-drugs-should-be
- 30.Government of Canada. Ivermectin not authorized to prevent or treat COVID-19; may cause serious health problems. https://recalls-rappels.canada.ca/en/alert-recall/ivermectin-not-authorized-prevent-or-treat-covid-19-may-cause-serious-health-problems
- 31.Vaduganathan M, Van Meijgaard J, Mehra MR et al. Prescription Fill Patterns for Commonly Used Drugs During the COVID-19 Pandemic in the United States. JAMA 2020; 323: 2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Government of Canada. Product Information - Stromectol. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=97110
- 33.IQVIA. 2021 ACTS Annual Report: Statistical Quality Assurance applied to IQVIA’s Information Offerings. https://www.iqvia.com/-/media/iqvia/pdfs/library/publications/2021-acts-annual-report.pdf?_=1664680348796
- 34.United States Census Bureau. National Population Totals and Components of Change: 2010–2019 – Monthly Population Estimates for the United States: April 1, 2010 to December 1, 2020 (NA-EST2019–01). https://www.census.gov/data/datasets/time-series/demo/popest/2010s-national-total.html
- 35.United States Census Bureau. National Population Totals and Components of Change: 2020–2021 – Monthly Population Estimates for the United States: April 1, 2020 to December 1, 2022 (NA-EST2021-POP). https://www.census.gov/data/datasets/time-series/demo/popest/2020s-national-total.html
- 36.Statistics Canada. Table: 17-10-0009-01 Population estimates, quarterly. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000901
- 37.Google. FAQ about Google Trends data. https://support.google.com/trends/answer/4365533?hl=en-GB%26ref_topic=6248052
- 38.Beytía P, Cruz Infante C. Digital Pathways, Pandemic Trajectories. Using Google Trends to Track Social Responses to COVID-19. SSRN 2020. [Google Scholar]
- 39.Higgins TS, Wu AW, Sharma D et al. Correlations of Online Search Engine Trends With Coronavirus Disease (COVID-19) Incidence: Infodemiology Study. JMIR Public Health Surveill 2020; 6: e19702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niburski K, Niburski O. Impact of Trump’s Promotion of Unproven COVID-19 Treatments on Social Media and Subsequent Internet Trends: Observational Study. J Med Internet Res 2020; 22: e20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuentes-Gonzalez MF, Ordinola Navarro A, Carmona-Aguilera Z et al. Outpatient prescription patterns of COVID-19 drugs in the metropolitan area of Mexico City. Fam Pract 2022; 39: 515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Center for Systems Science and Engineering. CSSE COVID-19 Time Series. https://github.com/CSSEGISandData/COVID-19/tree/master/csse_covid_19_data/csse_covid_19_time_series
- 43.Schaffer AL, Dobbins TA, Pearson S-A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: a guide for evaluating large-scale health interventions. BMC Med Res Methodol 2021; 21: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machiels JD, Bleeker-Rovers CP, Ter Heine R et al. Reply to Gautret et al: hydroxychloroquine sulfate and azithromycin for COVID-19: what is the evidence and what are the risks? Int J Antimicrob Agents 2020; 56: 106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Food & Drug Administration. Why You Should Not Use Ivermectin to Treat or Prevent COVID-19. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19
- 46.Centers for Disease Control and Prevention. Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID-19. https://emergency.cdc.gov/han/2021/han00449.asp
- 47.American Medical Association. AMA, APhA, ASHP statement on ending use of ivermectin to treat COVID-19. https://www.ama-assn.org/press-center/press-releases/ama-apha-ashp-statement-ending-use-ivermectin-treat-covid-19
- 48.Federation of State Medical Boards. FSMB: Spreading COVID-19 vaccine misinformation may put medical license at risk. https://www.fsmb.org/advocacy/news-releases/fsmb-spreading-covid-19-vaccine-misinformation-may-put-medical-license-at-risk/
- 49.Baron RJ, Emanuel EJ. Politicians should not be deciding what constitutes good medicine. Stat News. https://www.statnews.com/2022/03/07/politicians-should-not-be-deciding-what-constitutes-good-medicine/
- 50.Canadian Pharmacists Association. CPhA statement on ivermectin. https://www.pharmacists.ca/news-events/news/cpha-statement-on-ivermectin/
- 51.Loriggio P Restrictions imposed on doctor accused of spreading COVID misinformation. CP24 News. https://www.cp24.com/news/restrictions-imposed-on-doctor-accused-of-spreading-covid-misinformation-1.5603511
- 52.Stewart A Action launched against 3 more Toronto doctors for COVID-19 misconduct. Global News. https://globalnews.ca/news/8578677/action-launched-toronto-doctors-covid-misconduct/
- 53.Sulis G, Batomen B, Kotwani A et al. Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: An interrupted time series analysis. PLoS Med 2021; 18: e1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Enners S, Gradl G, Kieble M et al. Utilization of drugs with reports on potential efficacy or harm on COVID‐19 before, during, and after the first pandemic wave. Pharmacoepidemiol Drug Saf 2021; 30: 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaffer AL, Henry D, Zoega H et al. Changes in dispensing of medicines proposed for re-purposing in the first year of the COVID-19 pandemic in Australia. PLoS One 2022; 17: e0269482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Offord C Surgisphere Sows Confusion About Another Unproven COVID-19 Drug. The Scientist. https://www.the-scientist.com/news-opinion/surgisphere-sows-confusion-about-another-unproven-covid19-drug-67635
- 57.Mega ER. Latin America’s embrace of an unproven COVID treatment is hindering drug trials. Nature 2020; 586: 481–482. [DOI] [PubMed] [Google Scholar]
- 58.Schwalb A, Armyra E, Méndez‐Aranda M et al. COVID‐19 in Latin America and the Caribbean: Two years of the pandemic. J Intern Med 2022; 292: 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lupus Foundation of America. Joint Statement Urging White House Coronavirus Task Force, the leadership of the U.S. Congress and Nation’s Governors to Ensure Hydroxychloroquine Access During COVID-19 Crisis. https://www.lupus.org/news/joint-statement-urging-white-house-coronavirus-task-force-and-nation-s-governors-to-ensure
- 60.Drug Shortages Canada. Drug Shortage Report for APO-HYDROXYQUINE. https://www.drugshortagescanada.ca/shortage/109087
- 61.Drug Shortages Canada. Drug Shortage Report for STROMECTOL. https://www.drugshortagescanada.ca/shortage/131914
- 62.CBC News. Calls spike to Alberta poison hotline about ivermectin after anti-parasitic drug touted as COVID-19 treatment. https://www.cbc.ca/news/canada/calgary/ivermectin-poison-hotline-covid-drug-alberta-1.6203269
- 63.Fleerackers A, Riedlinger M, Moorhead L et al. Communicating Scientific Uncertainty in an Age of COVID-19: An Investigation into the Use of Preprints by Digital Media Outlets. Health Commun 2022; 37: 726–738. [DOI] [PubMed] [Google Scholar]
- 64.Karlsson P, Nakitanda AO, Löfling L et al. Patterns of prescription dispensation and over-the-counter medication sales in Sweden during the COVID-19 pandemic. PLoS One 2021; 16: e0253944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ODPRN. COVID-19 Ontario Prescription Drug Utilization Tool. https://odprn.ca/covid19-ontario-prescription-drug-utilization-tool/
- 66.U.S. Food & Drug Administration. FDA Updates on Paxlovid for Health Care Providers. https://www.fda.gov/drugs/news-events-human-drugs/fda-updates-paxlovid-health-care-providers
- 67.Government of Canada. Paxlovid. https://covid-vaccine.canada.ca/paxlovid/product-details
- 68.Lim SCL, Hor CP, Tay KH et al. Efficacy of Ivermectin Treatment on Disease Progression Among Adults With Mild to Moderate COVID-19 and Comorbidities. JAMA Intern Med 2022; 182: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reis G, Silva EASM, Silva DCM et al. Effect of Early Treatment with Ivermectin among Patients with Covid-19. N Engl J Med 2022; 386: 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19 – 11 March 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- 71.Mehra MR, Desai SS, Ruschitzka F et al. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet 2020; S0140–6736: 31180–6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Ledford H, Van Noorden R. High-profile coronavirus retractions raise concerns about data oversight. Nature. 2020; 582: 160. [DOI] [PubMed] [Google Scholar]
- 73.Davey M Huge study supporting ivermectin as Covid treatment withdrawn over ethical concerns. The Guardian. https://www.theguardian.com/science/2021/jul/16/huge-study-supporting-ivermectin-as-covid-treatment-withdrawn-over-ethical-concerns
- 74.Popp M, Stegemann M, Metzendorf M-I et al. Ivermectin for preventing and treating COVID‐19. Cochrane Database Syst Rev 2021; 7: CD015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schraer R, Goodman J. Ivermectin: How false science created a Covid ‘miracle’ drug. BBC News. https://www.bbc.com/news/health-58170809
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


