Summary
Dominance hierarchy is a fundamental social phenomenon in a wide range of mammalian species, critically affecting fitness and health. Here, we investigate the role of pheromone signals in the control of social hierarchies and individual personalities within groups of wild mice. For this purpose, we combine high-throughput behavioral phenotyping with computational tools in freely interacting groups of wild house mice, males and females, in an automated, semi-natural system. We show that wild mice form dominance hierarchies in both sexes but use sex-specific strategies, displaying distinct male-typical and female-typical behavioral personalities that were also associated with social ranking. Genetic disabling of VNO-mediated pheromone detection generated opposite behavioral effects within groups, enhancing social interactions in males and reducing them in females. Behavioral personalities in the mutated mice displayed mixtures of male-typical and female-typical behaviors, thus blurring sex differences. In addition, rank-associated personalities were abolished despite the fact that both sexes of mutant mice formed stable hierarchies. These findings suggest that group organization is governed by pheromone-mediated sex-specific neural circuits and pave the way to investigate the mechanisms underlying sexual dimorphism in dominance hierarchies under naturalistic settings.
Keywords: wild mice, personality, dominance hierarchy, group social behavior, pheromones, vomeronasal system, sexual dimorphism, Pareto task interface
Highlights
-
•
Male and female wild mice form dominance hierarchies under semi-natural settings
-
•
Males and females display sex-specific personalities associated with ranking
-
•
Dominance hierarchies are dependent on pheromone detection in a sex-specific manner
-
•
Genetic disabling of pheromone detection abolishes ranking-associated personalities
Zilkha et al. investigate dominance hierarchies and individual personalities in groups of wild mice. They show that males and females form hierarchies using different strategies and display sex-typical personalities. Disabling of VNO-mediated pheromone detection generated sexually dimorphic effects and abolished rank-associated personalities.
Introduction
Numerous species live in social groups, ranging from several individuals to even thousands.1,2,3 A basic feature of group dynamics is the formation of dominance hierarchies4,5 in which the ranking position within the hierarchical structure can greatly influence an individual’s physiology,6,7,8,9 disease susceptibility,8,9,10,11 reproductive success, and life span.9,10,12,13 In spite of the pivotal role of dominance hierarchies in group social organization, the mechanisms governing formation and dynamics of such hierarchies remain unclear.
Group dominance hierarchy, typically established by sequences of multiple competitive aggressive interactions, is naturally occurring in both sexes across species.2,6,14 Wild female house mice (Mus musculus) display robust conspecific aggressive behavior in various contexts at a level similar to males,15,16,17,18,19,20 unlike domesticated lab female mice of most strains.16,18,21 Concomitantly, wild male and female mice are also territorial and form structures of dominance hierarchy.20,22,23,24,25,26,27,28,29,30 Yet our knowledge of the mechanisms underlying dominance hierarchies is based predominantly on studies in lab males,31,32,33,34,35 often using standard lab settings restricted to dyadic interactions.33,36,37,38 Therefore, little is known regarding the manner by which dominance hierarchy is formed and maintained under naturalistic conditions in wild mammalian species39,40 and to what extent the governing mechanisms are sexually dimorphic.7,41,42,43
Accordingly, we hypothesize that, by studying groups of wild mice, we will gain new insights into the processes underlying dominance hierarchies and behavioral “personalities”44,45,46 unattainable in standard laboratory mouse strains and conditions.
In this study, we tracked same-sex groups of individually tagged wild house mice within large semi-natural enclosures, over 6 days,47 and quantified dozens of individual behaviors, pairwise interactions, and dynamic group organization. We performed a comprehensive phenotypic characterization and examined the potential sex differences and similarities in the dynamic construction of dominance hierarchies across same-sex groups. Moreover, we set out to determine the role of pheromone signals in dominance hierarchy and behavioral personalities. Signals from pheromones mediated through the vomeronasal organ (VNO) play an essential role in regulating various social and reproductive behaviors in mice. Importantly, in lab males, pheromone inputs were extensively shown to drive conspecific pairwise aggression (reviewed in Beny and Kimchi48). Similar findings were found in VNO-disabled wild mice (i.e., wild-backcrossed TrpC2 mutated mice) in females.16 However, the role of pheromonal inputs in the control of group organization and formation of dominance hierarchy, in either sex, is unknown.
We first show that domestication carries major effects on dominance hierarchies and behavioral personalities, in groups of both males and females. Wild mice were more anxious in approaching other individuals and were thus less engaged in social interaction, compared with lab mice. We also find that a stable dominance hierarchy is formed in lab males but not in lab females, whereas in wild mice, both sexes establish stable hierarchical structures. Next, using the Pareto task interface approach for analyzing high-dimensional datasets,49,50,51 we describe three sex-specific behavioral personalities (“archetypes”), as well as archetypes associated with individual social ranks, in groups of wild mice. Finally, we reveal that genetic disabling of VNO-mediated pheromone detection in wild mice did not prevent the formation of dominance hierarchies but produced opposite effects on the behavioral repertoires of males and females, both in terms of individual behaviors and dyadic social interactions within the group. Moreover, genetic disabling of VNO-mediated pheromone detection completely shuffled behavioral repertoires between sex-specific personalities and abolished any rank-associated archetype.
Results
Mice domestication hinders individual behaviors and dyadic social interactions within groups in both sexes
Domesticated lab mice were created through decades of artificial selection and inbreeding of their ancestral wild mice, a process which fundamentally altered numerous behavioral traits, including pairwise social interactions and specifically aggression.16,21 Here, we examined the effects of domestication on the behavioral repertoire of mice in the settings of a same-sex group.
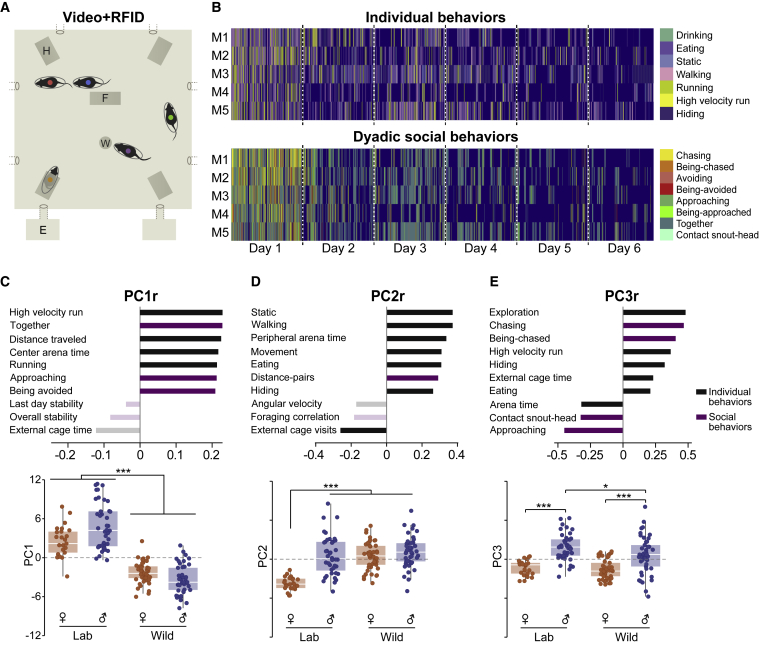
We analyzed a set of >40 parameters composed of individual, dyadic, and collective group behaviors using principal component analysis (PCA) in male and female lab and wild mice (Figure 1; Table S1; Videos S1 and S2). The PCA produced three components explaining 62% of the variance in the behavioral data. Principal component 1 (PC1, 35% of the variance) was mainly correlated with social behaviors, alongside movement activity (e.g., running, distance-traveled, and together), and showed significant effects of strain. That is, the levels of this set of movement and pro-social related behaviors were significantly lower in wild mice than in lab mice, in both sexes (p < 0.001; Figure 1C; Table S1A). Principal component 2 (PC2, 15% of the variance) was mainly correlated with individual behaviors (e.g., walking, being static inside the arena). ANOVA showed significant effects of mouse strain and sex in the scores of PC2, such that lab females were significantly lower than all the other groups (p < 0.001; Figure 1D; Table S1B). Namely, lab females spent significantly more time in the external cages and less time active in the arena compared with the other three groups. Principal component 3 (PC3, 12% of variance) was mainly correlated with increased aggressive chasing (Video S1), exploration, hiding, and eating, as well as reduced social (Video S2) and individual behaviors, and showed significant effects of strain and sex. Specifically, males engaged in significantly more agonistic and less pro-social behaviors compared with females, in both lab and wild mice (p < 0.001; Figure 1E; Table S1C). Also, lab males presented more of this behavioral set compared with wild males (p < 0.05).
Figure 1.
Sexually dimorphic effects of domestication on behavioral repertoires in groups of mice
(A) Schematic illustration of the semi-natural enclosures with 5 freely behaving mice, individually tagged and tracked automatically by fusion of video and RFID data.
(B) Representative ethograms of selected individual (up) and social (bottom) behaviors extracted automatically from each experiment. Blue background repesents all other behaviors.
(C–E) Principal component analysis (PCA) of >40 behavioral parameters explaining 62% of total variance in PC1 (C), PC2 (D), and PC3 (E) during the experimental period. For each PC, ten representative parameters are presented with their correlation to the respective PC (full array of behavioral parameters used with their respective correlations to each PC is presented in Table S1). Non-significant parameters are partly transparent. H, hiding box; F, food stand; W, water bottle; E, external cage. nLabMales = 40, nLabFemales = 25, nWildMales = 45, and nWildFemales = 45. Values are displayed as medians ±1.5 interquartile range. ∗p ≤ 0.05, ∗∗∗p ≤ 0.001.
Domestication induces robust impairments in the formation of dominance hierarchies within groups of female mice
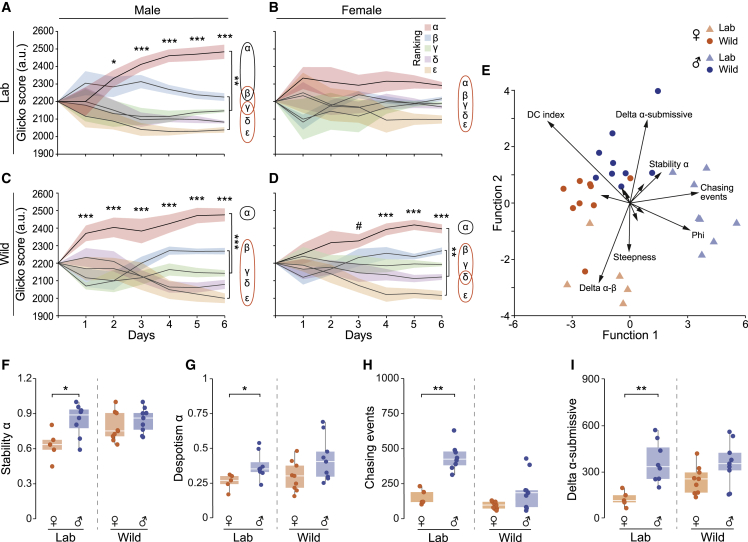
We next analyzed the social dynamics within groups of lab and wild mice of both sexes, using the Glicko rating algorithm.32,52,53 The algorithm extracts dynamic dominance rankings within the group based on the outcome of aggressive chasing interactions, with a higher Glicko score indicating a higher dominance rank (Figures 2A–2D). In lab male mice, after only 1 day, the dominant mouse, i.e., “alpha” was distinctive from the other mice, resulting in a clear dominance hierarchy that remained stable throughout the experiment (Figures 2A and S1A). Repeated-measures ANOVA of daily Glicko ratings between “alpha” and all other individuals (i.e., “submissives”) showed a significant interaction of rank × day × sex (p < 0.001). Post-hoc analysis revealed significant differences between dominant and submissive male mice, starting from day 2 up until the end of the experiment (Figure 2A).
Figure 2.
Sexually dimorphic effects of domestication on dominance hierarchies in groups of mice
(A–D) Mean daily Glicko scores of dominant (alpha) versus submissive (beta, gamma, delta, and epsilon) mice during 6 days of experiment in the semi-natural enclosures in groups of male (A and C) and female (B and D), lab (A and B) and wild (C and D) mice. Venn diagram to the right of each panel depicts statistical separation between all ranks.
(E) Flexible discriminant analysis (FDA) of various hierarchy parameters in lab and wild mice of both sexes. Each individual data point represents a single group of 5 mice (arena). For legibility of the entire plot, the arrows were plotted with 1.5 multiplication of the original (x, y) coordinates and only the relevant parameters are labeled. A full list of parameters with their loading coefficient for functions 1, 2, and 3 is presented in Figure S1G.
(F–I) Comparison of specific hierarchy parameters between males and females in lab and wild mice. (F and G) Stability (F) and despotism (G) of the dominant alpha mouse.
(H) Number of aggressive chasing events per arena of 5 mice.
(I) Delta between the Glicko scores of the dominant alpha mouse and its respective submissive mice, per arena of 5 mice. nLabMales = 40, nLabFemales = 25, nWild Males = 45, nWildFemales = 45.
Values are displayed as mean ± SEM (A–D) or as medians ±1.5 interquartile range (F–I).
(A–D) Parameters were calculated per mouse.
(E–I) Parameters were calculated per group of mice (arena). #p = 0.08, ∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001.
See also Figure S1.
Groups of lab females, in contrast to males, showed no difference between the alpha and submissives, nor were there any differences between the ranks on any specific experimental day (Figures 2B and S1B). A similar effect was observed by calculating the David scores as an additional measure for social dominance in which scores significantly differed between mice of different ranks, but only for males (p < 0.001; Figure S1E, left), and not for females (p = 0.17; Figure S1E, right).
On the other hand, in groups of wild mice, both males and females formed clear dominance hierarchies (rank × day, p < 0.01; Figures 2C, 2D, S1C, and S1D). However, the Glicko scores of dominant wild males were significantly distinctive from the submissive group as early as the 1st day of the experiment, whereas dominant females displayed a clear separation starting on day 4 (Figures 2C and 2D). Analysis of David scores showed significant differences between the ranks in both males (p < 0.001; Figure S1F, left) and females (p < 0.001; Figure S1F, right). Detailed comparisons between all ranks in each sex of wild mice showed main effects of rank for both males (p < 0.001; Figure 2C, right) and females (p < 0.001; Figure 2D, right), with the alpha mice displaying significant differences in their Glicko score from all other individual ranks in both sexes. The submissive mice were not significantly different from their following ranking mice, suggesting that a despotic form of hierarchy is established. Further analyses per day showed that in wild males, the alpha individuals were significantly separated from beta mice on days 5–6 (p = 0.028), from delta mice on days 3–6 (p < 0.001), and from gamma and epsilon mice on days 1–6 (p = 0.033). In the wild females, the alpha individuals were significantly separated from gamma and delta mice on days 4–6 (p = 0.036), and from epsilon mice on days 3–6 (p = 0.001). These findings indicate that wild males and females both form dominance hierarchies, yet it is sexually dimorphic in the temporal dynamics and strength.
We further analyzed group dynamics using parameters developed for characterizing dominance hierarchies of whole groups32 (STAR Methods), such that each cohort of mice “arena” constitutes an individual subject. We first applied flexible discriminant analysis (FDA)54 on the four types of groups (males-females, wild-lab) using hierarchy-related parameters (STAR Methods; Figures 2E and S1G). The analysis revealed a marked and clear separation in the distribution of parameters between the lab groups and the wild groups. Furthermore, it showed separation in the distribution of parameters between males and females, in both strains of mice. Further analysis of the specific hierarchy parameters between males and females revealed significant differences only in lab mice, whereas wild males and females showed similar levels in each hierarchy parameter. Specifically, lab male mice showed higher levels of stability of the alpha mouse (p < 0.05; Figure 2F), despotism (i.e., the proportion of wins made by the alpha mouse, p < 0.05; Figure 2G), number of chasing events (p < 0.01; Figure 2H), and the calculated delta of Glicko scores between alpha and the submissive mice (p < 0.01; Figure 2I) compared with lab females. Other hierarchical parameters, including steepness of hierarchy, Phi, DC index, Landau’s h, triangle transitivity, and stability of the epsilon mouse (STAR Methods), did not show any significant difference between males and females in either strain (Figures S1H–S1N).
Because lab males establish a clear dominance hierarchy, and lab females fail to do so, whereas in wild mice both sexes establish hierarchies, we further focused our experiments on wild mice.
Wild mice in groups show distinct behavioral personalities associated with sex and social ranking
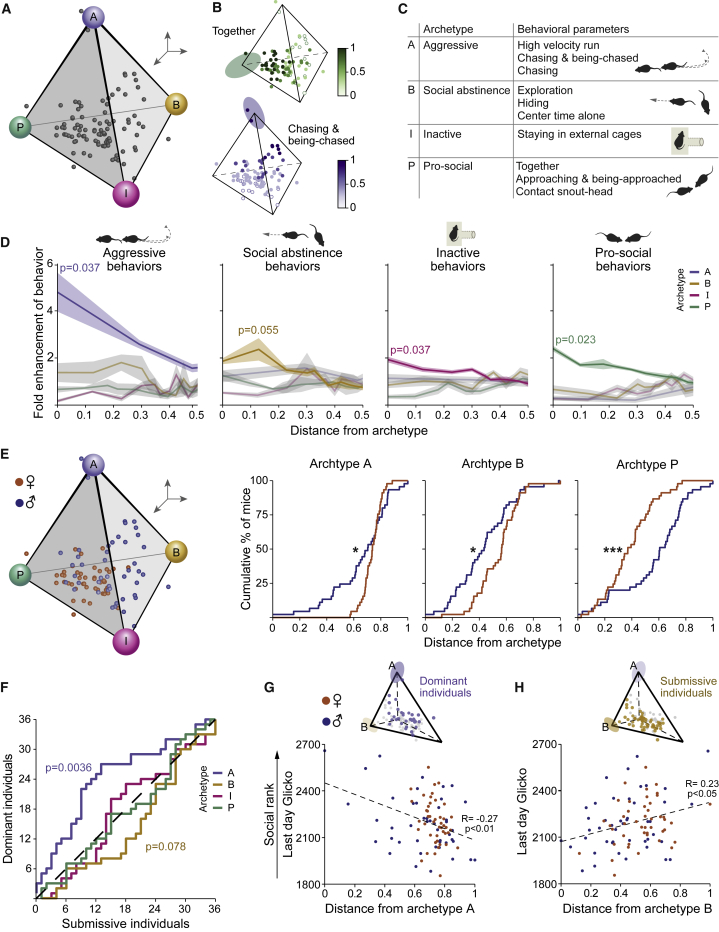
In order to assess and define unique behavioral individualities within groups of males and females, we performed Pareto task interface analysis.49,50,55,56,57 Unlike standard clustering methods that split data points into groups, the Pareto approach predicts phenotypes, which combine the behavioral parameters into “archetypes” (i.e., behavioral “personalities”) located at the vertices of low dimension polytopes, which confine the data.31,51,55,58,59
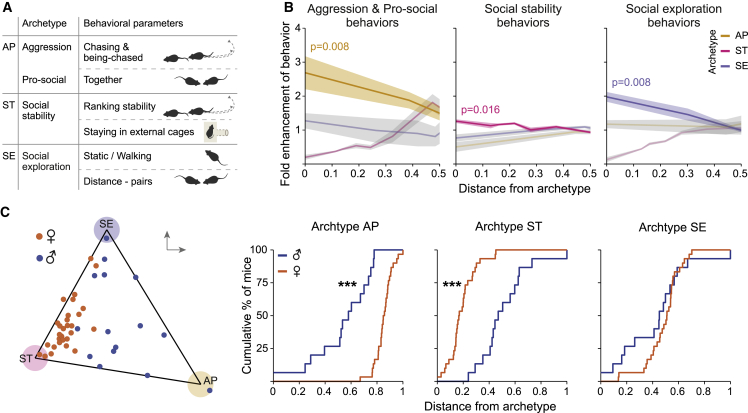
In groups of male and female wild mice, the model recommended by the algorithm, which significantly enclosed the dataset, was a tetrahedron with 4 distinguished vertices (i.e., archetypes) (p = 0.01; Figures 3A–3C and S2A; Table S2A), which can be viewed using 3 principal components. Alternative models with 3 or 2 vertices produced non-significant results (p = 0.11 and p = 0.63, respectively), i.e., the data could not be explained well with only 2 or 3 vertices. For each of the 4 archetypes reflecting a distinguished “behavioral personality,” we analyzed the set of behavioral parameters, which are enhanced near the specific archetype and depleted near the other archetypes (i.e., fold enhancement; STAR Methods). Behavioral enrichments for each archetype were plotted as a function of normalized distance from the archetype51,56 (Figures 3C and 3D). Archetype A (i.e., aggressive) was enriched by parameters associated with agonistic behavior, including “high velocity run,” “chasing and being-chased,” and “chasing” (p = 0.037). Archetype B (i.e., social abstinence) was marginally enriched by parameters associated with evasion of social interactions, including “hiding,” and “center time alone” (p = 0.055). Archetype I (i.e., inactive archetype) was enriched by parameters associated with passive behaviors such as spending time in the external cage (p = 0.037). Archetype P (i.e., pro-social) was enriched by parameters associated with non-aggressive social interactions, including “together,” “approaching and being-approached,” and “contact snout-head” (p = 0.023).
Figure 3.
Personality space analysis reveals sex and rank-typical archetypes, describing distinct behavioral individualities within a group
Pareto optimality analysis of individual male and female wild mice.
(A) A 3D representation of personality space, forming a tetrahedron of four distinguished behavioral archetypes (termed A, B, I, and P).
(B) Representative heatmaps for together (top) and chasing and being-chased (bottom) behaviors, depicting the level of behavior for each mouse. Behaviors were chosen based on the correlation with proximity to archetypes P and A, respectively. Ellipsoids represent the error of each archetype (STAR Methods).
(C) Characterization of each archetype according to its enriched behavioral parameters (i.e., behaviors most correlated).
(D) For each archetype-typical set of behaviors described in (C), the fold enhancement (STAR Methods) for each mouse is plotted against its distance from the different archetypes. High fold-enhancement near an archetype means that the archetype is enriched with this set of behaviors, whereas the other archetypes are depleted.
(E) A 3D representation of the personality space, displaying male (blue) and female (orange) mice, with accumulation curves as a function of distance from archetype, for the three archetypes with significant differences between the sexes. Males are significantly closer to archetypes A and B, whereas females are closer to archetype P.
(F) A classifier ROC curve describing the accumulation of most dominant (alpha and beta) versus most submissive (delta and epsilon) mice, for each archetype.
(G and H) Correlation of the last day Glicko score and the distance from archetypes A (G) and B (H), presented for of all the mice. Upper insets highlight dominant (alpha and beta) mice, which are closer to archetype A (G), and submissive (gamma, delta, and epsilon) mice, which are closer to archetype B (H). For optimal demonstration, the 3D tetrahedrons enclosing the data points were rotated in space to demonstrate the relevant archetypes in relation to the data. ∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001.
Comparing males to females as a function of their distance from each archetype, we found that males are significantly closer to archetype A and to archetype B, whereas females are closer to archetype P (Figures 3E and S2B). Archetype I did not show any sex bias (Figures S2B and S2C). We also examined whether social ranking is related to any specific archetype. First, we divided the mice into 2 equal-sized groups of the highest and lowest ranks and compared their accumulation relative to each archetype using a receiver operating characteristic (ROC) classifier curve (Figures 3F and S2D–S2G). The more dominant individuals were significantly closer to archetype A, whereas submissive individuals were marginally closer to archetype B. Accordingly, the Glicko score of each mouse (i.e., all ranks) at the last day of the experiment was negatively correlated with the distance from archetype A (p = 0.0096; Figure 3G) and positively correlated with the distance from archetype B (p = 0.031; Figure 3H). In contrast, archetypes I and P were not significantly close to either rank and their distances did not show any correlation to the Glicko score of the mice (Figure S2H).
Genetic disabling of the VNO produces a sexually dimorphic effect on the formation of dominance hierarchies in wild mice
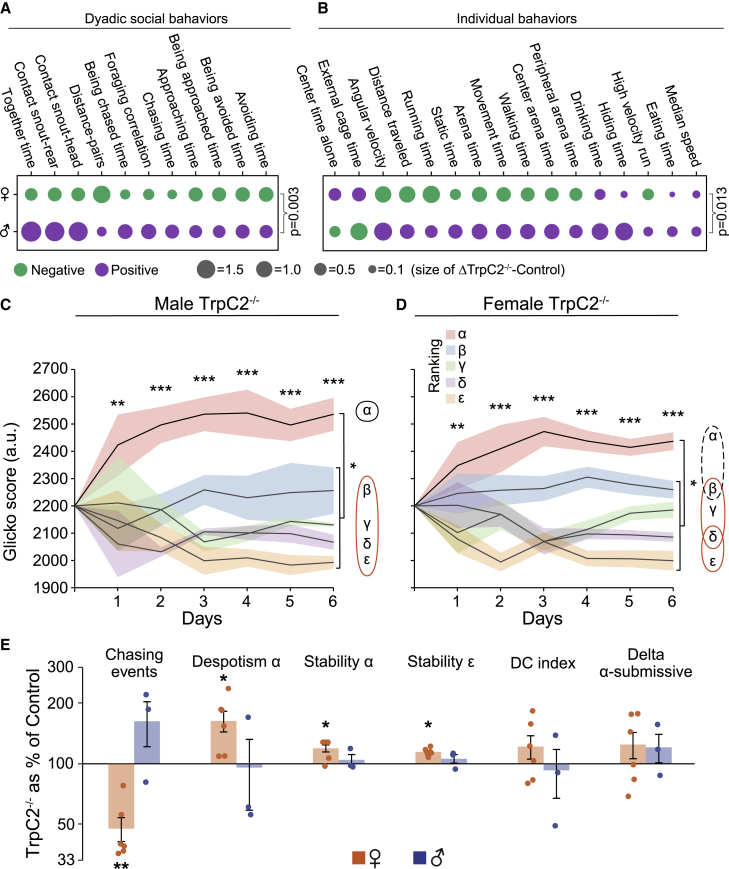
We next examined the role of VNO-mediated pheromones in social interactions within groups of males and females and in the formation of dominance hierarchies (Figures 4, S3, and S4) using groups of wild-backcrossed TrpC2−/− mutant mice (STAR Methods).16 These mice harbor a genetic mutation, which disables VNO-mediated pheromone detection.60,61,62,63,64 First, we analyzed specific behavioral parameters of male and female TrpC2−/− mice relative to their respective control groups. Amazingly, the effects of the mutation were robustly sexually dimorphic, displaying opposite patterns between males and females in almost all of the parameters measured, and specifically enhancing social interactions in males, whereas reducing them in females (Figures 4A and 4B). Analysis of group organization showed that, unexpectedly, the wild-backcrossed TrpC2−/− mutant groups formed clear dominance hierarchies within the 1st day, resembling those presented by the groups of wild-backcrossed (hereafter “control”) mice, in both males and females (rank main effect, p < 0.001; Figures 4C and 4D). Further analysis showed that in the male groups, VNO deficiency did not affect the formation of a stable social hierarchy compared with the control groups (Figure 4C). However, in the females, VNO deficiency promoted the formation of a stable social hierarchy. The wild-backcrossed TrpC2−/− dominant females showed significant separation from their submissive counterparts as early as the 1st day (Figure 4D), compared with the 4th day in the wild control mice (Figure 2D).
Figure 4.
TrpC2−/− wild mice establish dominance hierarchy, with a sexually dimorphic effect of gene disabling
(A and B) Behavioral repertoires of TrpC2−/− wild-backcrossed male and female mice, relative to their respective control groups. For each social (A) and individual (B) normalized behavioral parameter, the color of the circle represents the direction (green—reduced levels compared with controls, purple—increased levels compared with controls), and the size represents the magnitude of change (i.e., Δ) from female or male controls.
(C and D) Mean daily Glicko scores of dominant (alpha) versus submissives (beta, gamma, delta, and epsilon) during 6 days in the semi-natural enclosures, in groups of male (C) and female (D) TrpC2−/− wild mice. Venn diagram to the right of each panel depicts statistical separation between all ranks.
(E) Specific hierarchy parameters in groups of TrpC2−/− female/male wild mice, presented as a percentage of their respective wild control group. nWild TrpC2−/−Males = 15, nWild TrpC2−/−Females = 30. Results are displayed as mean ± SEM.
(A–D) Parameters were calculated per mouse.
(E) Parameters were calculated per group of mice (arena). ∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001.
See also Figures S3 and S4.
Using FDA, we analyzed the set of hierarchy-related parameters in the wild-backcrossed groups (TrpC2−/− mutants and controls, males and females; Figure S3B). The FDA classified the four experimental groups correctly with 3 discriminant functions. The first two functions explained 64% and 92% of the classification accuracy, cumulatively. In males, hierarchy of the Trpc2−/− groups was mostly driven by the “phi” parameter, which indicates the exclusiveness of aggression by the dominant individuals, and by the number of chasing events, compared with the control males which were mainly driven by the “steepness” of hierarchy, and the difference (i.e., “delta”) between alpha and beta mice. In contrast, the hierarchy of mutant female groups was mostly driven by the delta between the alpha and the epsilon mouse, whereas the control females were mainly driven by the DC index, which represents the directionality of agonistic interactions.
Further detailed analysis revealed that wild-backcrossed TrpC2−/− mutant females engaged in significantly fewer chasing interactions (p < 0.01; Figure 4E) than the control females. In addition, despotism of the dominant mouse was higher in the mutants compared with the control wild female groups (p < 0.05; Figure 4E), similarly there was greater stability of both alpha (p < 0.05; Figure 4E) and epsilon (p < 0.05; Figure 4E) in the mutant female mice than the controls. In wild-backcrossed males, no differences were found between TrpC2−/− and control groups (Figures 4E and S3C). In lab mice, TrpC2 mutation delayed hierarchy formation in males by 1 day and had no significant effect on the dynamics of hierarchy formation in females (Figure S3D).
To examine the behavioral mechanisms underlying differences in hierarchy formation, we analyzed patterns of social interactions, including chasing and being chased, and approaching and being approached, throughout the experimental period (Figures S3E–S3H). We found high differences in the levels and dynamics of all tested interactions, with significant interactions of genotype × day with sex (chasing/being chased: p < 0.001; Figures S3E and S3F; approaching/being approached: p < 0.001; Figures S3G and S3H), and with type (i.e., lab versus wild, chasing/being chased, p < 0.001; approaching/being approached, p < 0.05).
To elucidate the nature of chasing behavior among TrpC2 mutants in the semi-natural arena, we manually analyzed chasing events in a separate cohort of lab males during the 1st day of the experiment. We monitored the behavior to determine whether chasing events resulted in an aggressive attack or in pro-social behavior (e.g., social investigation or sexual pursuit). Interestingly, although the vast majority of chasing events in control mice led to aggressive attacks (75.6%), most chases in TrpC2−/− mice were followed by playful/sexual mounts (59.2%, U = 0, p = 0.012). In addition, we used the open field assay on a separate cohort of mice to measure social-independent exploration features in our mice groups (Figure S4A). We found that wild mice show reduced locomotion, visit the center of the open field less frequently, and spend more time rearing compared with lab mice. TrpC2−/− females showed similar exploration behaviors compared with controls in lab and wild mice (Figure S4A). Finally, we quantified initial olfactory investigation within the semi-natural environment between all experimental groups during the first day of the experiment. Our results show that lab mice display significantly more olfactory investigations compared with wild mice, in both sexes; however, the effect is more robust in males (Figure S4B).
Genetic disabling of the VNO blurs sex differences and abolishes rank-related personalities in groups of males and females
We used the Pareto task interface analysis to examine the role of VNO-mediated pheromones in shaping behavioral personalities of wild mice within a group.
Unlike the control groups, the best model enclosing the dataset of the wild-backcrossed TrpC2−/− mutant mice was a triangle with 3 distinguished archetypes (p < 0.001), which can be viewed using 2 principal components (Figures 5A, 5B, S5A, and S5B; Table S2B). Next, we analyzed the enriched behaviors for each of the three archetypes. Archetype AP was enriched by parameters associated with aggression alongside pro-sociality, including “chasing and being-chased,” “high velocity run,” and “together” (p = 0.0079); archetype ST was enriched by parameters associated with social ranking stability, including “last day stability,” “overall stability,” and “stay at external cage” (p = 0.0159); and archetype SE was enriched by parameters associated with sociality and exploration, including “static,” “walking,” “arena time,” and “distance pairs” (p = 0.0079).
Figure 5.
Personality space analysis of TrpC2−/− wild mice shows blurring of sex differences in behaviors within a group
Pareto optimality analysis of individual male and female wild TrpC2−/− mice, distinguishing three separate archetypes.
(A) Characterization of each archetype according to its enriched behavioral parameters (i.e., behaviors most correlated). Behaviors are shuffled within the archetypes, containing mixtures of male-typical and female-typical behaviors.
(B) For each archetype-typical cluster of behaviors described in (A), the fold enhancement (STAR Methods) of each mouse is plotted against its distance from the different archetypes. High fold-enhancement near an archetype means that the archetype is enriched with this set of behaviors, whereas the other archetypes are depleted.
(C) A 2D representation of the personality space, displaying male (blue) and female (orange) mice, with accumulation curves as a function of distance from archetype, for each of the three archetypes. Males are significantly closer to archetype AP, whereas females are closer to ST.
∗p ≤ 0.05, ∗∗p ≤ 0.01, and ∗∗∗p ≤ 0.001.
Upon characterizing the mice closest to each archetype, we found that the mutant males were significantly closer to archetype AP, whereas the mutant females were closer to archetype ST (Figure 5C). Unlike in the control wild mice, we did not find any rank-related archetype in the TrpC2−/− groups (Figures S5C–S5F).
Notably, archetype ST, which was closest to females, manifested a behavioral repertoire that was opposite to the one presented by the control females (i.e., fewer social interactions, increased ranking stability). On the other hand, archetype AP, which was closest to the mutant males, was correlated with agonistic chasing behaviors (similar to male controls) and with pro-social behaviors (i.e. together). Thus, although the archetypes of TrpC2−/− mice showed clear geometric differentiation between males and females, the archetypes themselves contained a mixture of female-typical and male-typical behaviors (as defined in the control mice).
Discussion
House mice reside in various types of habitats, ranging from huge feral islands to high-density commensal populations.65,66 In nature, both male and female mice are driven to seek social contact, and in commensal populations, these various social interactions often result in stable hierarchical groups.2,67 Yet the majority of studies on social hierarchy have focused on lab males,32,33,54,68,69,70 and very little is known regarding hierarchy formation in females,7,41,71 or in wild mice in general. In addition, several studies examined the mechanisms underlying social dominance using pairwise encounters in the classic “tube test,”33,38,72,73,74 or in similar pairwise short assays.75,76 Such assays have produced valuable insights into the underlying neural mechanisms of dyadic social relationships (i.e. “winner”/”loser”).70,75,77,78,79 However, only a sparse number of studies attempted to employ ethologically relevant approaches in which the behavior of several mice in a group is analyzed over extended periods of time in a complex environment (reviewed in Milewski et al., Zilkha et al., and Peters et al.6,21,80).
We found that domestication has produced a sexually dimorphic effect on construction of dominance hierarchy, specifically impairing hierarchy formation in freely behaving groups of females. Although both wild and lab males establish clear hierarchies, only wild females, but not lab females, establish a stable hierarchical structure in which an alpha individual is clearly dominating the other four mice in the group. Yet even in wild mice, we identify a quantitative sexual dimorphism in which female hierarchies take more time to stabilize. These results are consistent with previous studies, which showed robust naturalistic aggressive behavior in wild female mice, but at a lower level compared with wild males.16,18,22,81,82,83,84,85,86 Our results are also in line with previous studies, which introduced outbred male and female CD1 mice into semi-natural enclosures. These studies have shown that outbred lab females establish dominance hierarchies in groups of same-sex mice, similar to males; however, their hierarchies were substantially weaker than those of the male groups.7,41
Beyond the effects of mouse domestication, our results indicate robust sexual dimorphism in patterns of social interactions and social organization within groups. Notably, we show for the first time that in a group setting, despite the fact that wild males and females both formed clear dominance hierarchies, there are compelling sex differences in the dynamics and strength of the hierarchy. Formation of social hierarchy in the male groups occurred faster and was better organized than in the female groups, as often seen in nature.3,87
Interestingly, males and females harness sex-specific behavioral strategies to establish a stable hierarchy within groups. Wild females are characterized by more “pro-social” behavioral traits, whereas wild males seem to be characterized by agonistic behaviors, social abstinence, and hiding. Notably, these sex differences are found even though both sexes establish clear dominance hierarchies, mainly through agonistic chasing/being-chased interactions. These findings are well in line with previous knowledge from pairwise behavioral assays in which females engage in more social interactions and find them more rewarding.88,89,90,91 Our current results extend these past findings into the context of a group and show that sex differences in social motivation, including greater sociability in females, are maintained in complex social structures under naturalistic settings. Similarly, extensive sex differences in the dynamics and behavioral strategies undertaken to form hierarchies were also found in other mammalian species, including hyenas,87,92 foxes,93 rhesus monkeys,94 baboons,95,96 and even humans.97
Despite the extensive sex differences between behavioral personalities of males and females in groups, there were sex similarities in the traits characterizing the social rank of individuals. In both males and females, the higher-ranked (dominant) mice were clustered closer to archetype A, and the dominance score was correlated with the proximity to the archetype. This archetype includes enhancement of agonistic chasing behaviors, as well as defensive being-chased behaviors and locomotive running, alongside depletion of pro-social together interactions and of being-approached. In contrast, the lower-ranked (subordinate) mice were characterized by traits of social abstinence, i.e., hiding and time alone, alongside depletion of approaching behaviors. Indeed, previous studies in various mammalian species have shown that dominant individuals show increased aggression,2,98,99,100,101,102,103,104,105 greater exploratory behavior,41,106,107 and lower anxiety,108,109 whereas subordinates tend to avoid social interactions,79,98,104,107,110 show greater fear responses,99 and have increased susceptibility to stress factors.3,111,112
Complex social organization requires social communication and the ability to identify specific social traits in other individuals.54 In most rodents, social information is primarily conveyed by chemosensory stimuli detected by the vomeronasal system,113,114,115 whereas non-social olfactory stimuli are primarily detected through the main olfactory system.116,117 We have previously shown that genetic disabling of VNO-mediated pheromone detection in wild-backcrossed TrpC2−/− mice substantially impaired dyadic social interactions, specifically aggression, in both males and females.16 Thus, it was reasonable to assume that obstruction of such a critical pathway for social communication60,118,119,120 might severely impair the formation of dominance relationships. To our surprise, wild-backcrossed TrpC2−/− mutant mice of both sexes were able to establish a solid dominance hierarchy, resembling the control wild groups. These findings suggest that social dominance and ranking information might also be conveyed in a TrpC2 independent manner,121 likely via main olfactory signals.48,113,115,121,122,123,124 The hypothesis that social discrimination is not entirely dependent on VNO-mediated signals is supported by previous findings, which showed that TrpC2−/− lab males are capable of distinguishing males from females despite presenting no sex preference.64,123
However, we observed a sexually dimorphic effect for the genetic disabling of the VNO, which produced opposite behavioral effects within groups, in both social and individual behaviors. Specifically, mutating the TrpC2 gene enhanced social interactions and general locomotion in males and reduced them in females. Moreover, male groups showed no effect on hierarchy construction in TrpC2−/− mice, whereas females displayed enhancement of hierarchy formation in the TrpC2−/− groups. This effect might imply sex differences in the role of VNO-mediated pheromones in conspecific social communication. Indeed, the social organization formed in male mice is mostly dependent on their degree of aggression.125,126,127,128 In contrast, social organization formed in female mice seems to depend more on social factors such as familiarity22,81,84 and physiological factors.125,129 Because detection of TrpC2-mediated pheromones is also crucial for social recognition and familiarity,130,131 their absence in TrpC2−/− females might increase dominance formation.
Behavioral personality analysis in groups of wild-backcrossed TrpC2−/− males and females revealed major sex differences and a clear geometric differentiation between archetypes of males and females. However, the archetypes themselves contained a mixture of female-typical and male-typical behaviors (as defined in the control mice). Specifically, TrpC2−/− males became more pro-social (alongside agonistic behavior), thus presenting a female-typical shift compared with the control males. Likewise, TrpC2−/− females became more stable in their hierarchies and less social, thus presenting a male-typical shift compared with the control females. This conclusion is also supported by previous findings in lab61,64,123,132 and wild16 TrpC2−/− mice, indicating that VNO-mediated signals repress the neural circuits governing social behavior typical of the opposite sex (i.e., male-typical behaviors in females and female-typical behaviors in males). Overall, these findings indicate that VNO-mediated pheromones are a crucial factor in determining social status and in shaping individualities within the group, in both sexes. In addition, our results in males might imply that the increase in chasing interactions seen in our TrpC2 mutants stems from null events, which are not necessarily aggressive but rather more related to social investigation/sexual behavior. This notion is supported by the fact that there is no difference in chasing interactions between TrpC2−/− and WT males after the 1st day and by the fact that dominant TrpC2−/− males did not differ from their submissive counterparts in the full personality analysis. Our results are summarized by a suggested model, showing that even within the complex settings of group organization, sex-specific neural circuits drive behavioral repertoires typical to males and females. Yet both sexes retain the neural circuits driving behaviors typical of the opposite sex, which are dormant. In the absence of VNO-mediated pheromones, the repression of neural circuits governing behaviors of the opposite-sex is released, thus attenuating overall sex differences. That is, males present pro-social behaviors alongside aggressive chasing, whereas females display reduced sociality and enhanced hierarchical stability (Figures S5G and S5H).
In summary, we were able to show for the first time that in wild mice both sexes are capable of establishing clear and stable dominance hierarchies, emphasizing the crucial role of this behavioral trait to both females and males in nature. Yet profound sex differences remain between complex social structures of males and females and between individual personalities of males and females within a group. This indicates that the neuronal circuits governing group organization might be sex specific and might serve different ecologically relevant functions.
We place our findings as a critical reference point for future studies on the neural mechanisms underlying ethologically relevant repertoires of social behaviors. This should lead to a better understanding of neural mechanisms underlying complex social behaviors and formation of social groups in nature.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Video-RFID tracking data | This paper | https://doi.org/10.5281/zenodo.7601926 |
| Wild-derived mice | Kimchi lab | Chalfin et al.16 |
| Software and algorithms | ||
| Tracking algorithm | This paper | https://github.com/ArenaZilkha2023/Zilkha_2023_TrackingSoftware.git |
| Glicko rating and behavioral parameters algorithm | This paper | https://github.com/ArenaZilkha2023/Arena_parameters |
| 'Compete' R package | So et al.,53 Williams et al.32 | https://github.com/jalapic/compete |
| Pareto Task interface | Hart et al.,49 Shoval et al.50 | https://github.com/vitkl/ParetoTI; RRID: SCR_022991 |
| MATLAB R2020b | MathWorks | https://www.mathworks.com/products/matlab.html; RRID: SCR_001622 |
| Python | Python Software Foundation | https://www.python.org/; RRID: SCR_008394 |
| R 4.2 | R Studio | https://www.r-project.org/; RRID: SCR_001905 |
| STATISTICA 14 | Tibco | https://www.tibco.com/products/data-science; RRID: SCR_014213 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Tali Kimchi (tali.kimchi@weizmann.ac.il).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Animals
Sexually naive adult mice of both sexes were used in all experiments, according to the following details: (1) C57BL/6Jx129sv inbred lab mice ("lab mice"), (2) wild mice, derived from wild house mice trapped in the fields near livestock barns (Idaho, USA), and served as an outbred stock of pathogen-free wild mice ("wild-derived mice").133 In both lab and wild mice, we tested either backcrossed TrpC2-/- mutant mice or TrpC2+/+ control mice.16 Wild-backcrossed TrpC2-/- is a mouse line that we have previously generated by repeated breeding of the lab TrpC2 knockout mouse strain to wild mice. This breeding process yields a knockout mouse line carrying a wild genetic background and presenting a wild behavioral and physiological phenotype. To maintain high genetic and phenotypic diversity in all of our wild-derived groups, we avoided inbreeding by preventing crosses between siblings or between parents and offspring. Mice were housed in groups of 4-5 in standard mouse cages under SPF conditions, with food and water ad libitum. Mice were maintained on a reversed 12/12 hours light/dark cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Weizmann Institute of Science.
Method details
Animal tagging
Three weeks prior to their introduction into the semi-natural enclosures, mice were anesthetized with a 1:1 solution of ketamine (100mg/kg) and xylazine (23mg/kg). After validation of complete anesthesia, they were subcutaneously implanted with two radio-frequency identification (RFID) microchips (ID-100, operating frequency 125–135kHz; Trovan), as previously detailed.47 Following chip implantation, mice were allowed to recover in single cages until the beginning of the experiment. Implantation of microchips did not interfere with the health or typical behavior of the mice.47
Experimental procedure and the semi-natural set-up
The mice were allowed 4-5 hours habituation to the experimental room prior to initiation of the experiment. Each arena was occupied by 5 unfamiliar adult mice of the same type (lab or wild-derived), sex, and genotype (TrpC2-/- or TrpC2+/+) for 6 days. Total recording time for each experimental day consisted of 4 hours in accumulation out of the 12 hours comprising the dark period of the day. As previously described,47 the semi-natural enclosure contained a large central exploratory arena (LxWxH, 119.2x119.2x80 cm) surrounded by 8 standard mouse cages that served as external chambers that were connected with short Perspex tubes to the central arena. The central arena was videotaped from the ceiling by infrared-sensitive cameras, and the floor contained RFID antennas connected to a central computer. The mice were allowed to freely roam the arena and the external cages throughout the experiment. The arena floor was covered with bedding and equipped with transparent hiding shelter boxes, bridges, a free-access feeder (containing standard chow) and a water container (Videos S1 and S2). In the experiments of female groups (wild and lab), two males enclosed in small, perforated cages were placed in opposite sides of the arena in order to increase territoriality among the mice.
Automatic mice tracking
The tracking system consisted of time-synchronized video and RFID data sets, as previously described.47 Mouse trajectories, as well as behavioral parameters described below, were detected automatically offline based on our previously published algorithm,47 which was upgraded and improved using a custom-designed MATLAB algorithm (R2020b, MathWorks, Natick, MA, USA). Briefly, the detection algorithm integrated data of video segments with the 10-digit number of the detected RFID microchips to produce the full trajectory of each mouse. First, for each video frame, a video-based mouse segmentation was extracted. Then the extracted positions were assigned to mice identities by matching their position with the closest detecting RFID antenna. If the RFID detection fails, the positions/orientations are predicted from the adjacent frames and matched with the positions found by the segmentation. In order to optimize the position assignment of each mouse, we also applied the Munkres–Hungarian assignment algorithm.134
Classification of behaviors
A basic set of individual and social pairwise behaviors was extracted from the tracked trajectory as previously described.47 These, as well as additional behaviors, were characterized as follows:
Individual behaviors
Running: when mouse velocity is between 40-100cm/sec. High velocity run: when mouse velocity is higher than 100cm/sec. Walking: when mouse velocity is between 0.5-40cm/sec. Static: when mouse velocity is lower than 0.5cm/sec. Movement: when mouse velocity is between 0.5-100cm/sec. External cage: when the mouse is in one of the external chambers outside the central arena. Eating: when the mouse is positioned within the area of the food stand, with velocity <5cm/sec. Drinking: when the mouse is in a 4cm radius around the drinking bottle tip, with velocity <5cm/sec. Hiding: when the mouse stays in a shelter box inside the central arena. Arena time: when the mouse is not in one of the external cages or hiding shelters. Alone: when the mouse is not in one of the external cages or hiding shelters (i.e. 'arena time'), and there are no other mice inside the arena. Peripheral / center arena: when the mouse is outside / inside the central part of the arena (as defined by the four hiding boxes, see Videos S1 and S2), respectively. Speed: median speed (cm/sec) during time spent in the central part of the arena. Acceleration: median acceleration (cm/secˆ2) during time spent in the central part of the arena. Distance-traveled: total distance (m) during time spent in the central part of the arena. Angular velocity: The rate of change in the mouse's direction (radians/sec). ROI exploration (bits/hour): entropy in selecting regions of interest inside the arena (i.e. shelter boxes, bridges, feeder, and water container), as previously described.135
Social pairwise behaviors
Foraging correlation: correlation of time spent inside the central arena with the other mice. Together: when a specific mouse is less than 10cm apart from another mouse, while both are not in either the external cages or shelter boxes. Chasing / being chased: Mouse A, running after Mouse B, was defined as the ‘chasing’ mouse, while Mouse B was defined as the ‘being chased’ mouse. Behavior was classified as chasing when two mice were moving forward in the same direction and time, the two interacting mice traveled at least 60cm, the distance between the two mice was shorter than 30cm, and the trajectory correlation between the interacting mice was higher than 0.7. Distance pairs (cm): The mean distance between a specific mouse and each of the other mice. Approaching / being approached: when a mouse moves towards another mouse that is in the central arena and their final distance is <5cm while the velocity of the approaching mouse is >10cm/sec and the velocity of the approached mouse is <1cm/sec. Avoiding / being avoided: when Mouse A approaches Mouse B, and Mouse B changes velocity and direction. Mouse A is defined as the ‘being avoided’, while Mouse B is defined as the ‘avoiding’ mouse. Behavior was classified as avoidance if the velocity of the avoiding mouse increases to >10 cm/sec and its angular direction changes by >50 degrees. Contact snout-head: when two mice are less than 2cm apart inside the central arena, facing head-to-head. Contact snout-rear: when two mice are less than 2cm apart inside the central arena, facing head-to-tail. Additional group social parameters are detailed below ("Characterization and quantification of dominance hierarchy").
Principal component analysis
Principal component analysis (PCA) was employed to explore the sources of variance in our multi-dimensional behavioral dataset. Specifically, we performed PCA using the Z scores of the different individual, pairwise, and group behavioral parameters extracted from our tracking data in lab and wild mice of both sexes (see Table S1 for the full array of behavioral parameters introduced into the PCA). The principal components (PCs) obtained were ranked by the total amount of variance explained. The top 3 PCs, namely PC1, PC2, and PC3, contained most of the variance in our dataset and were thus used to investigate the effects of sex and type (wild vs. lab). The relation between behavioral parameters and each PC was calculated using Spearman's correlation with Bonferroni correction for multiple comparisons.
In addition, Z scores of individual and social behaviors were compared between wild TrpC2-/- mice of both sexes and their respective controls, and the difference ('delta') for each parameter was plotted as bubbles with varying sizes representing the relative change.
Pareto task interface (ParTI)
In order to characterize and distinguish different behavioral archetypes within our datasets of wild mice, the Pareto optimality analysis was employed as previously described.49,50,51,57 Briefly, normalized individual parameters of either WT (control) or TrpC2-/- mice were analyzed using the ParTI software49 on a Matlab platform. The software performs dimensionality reduction using PCA and fits a polytope which best confines the data within the geometric space of the PCA. Each vertex of the polytope constitutes an 'archetype', i.e. distinguished behavioral personality. The Pareto algorithm recommends the number of archetypes and their distribution to produce the most reliable and significant model. The error for each archetype was calculated by repeated shuffling of the dataset and plotted as ellipsoids/ellipses at the respective vertices of the polytopes.
Then the correlation was calculated between each original parameter and the normalized proximity to each archetype (i.e. the additive inverse of the normalized distance) such that parameters with the highest positive correlation are enhanced near the respective archetype (Tables S2A and S2B). For each archetype, the set of parameters which are significantly enhanced by the specific archetype and depleted by the other archetypes were examined by calculating their fold enhancement. This was defined for each mouse for parameter/s X as (Xi-Xminimum)/Xminimum and plotted as a function of the normalized distance from each archetype in bins of 5 mice each. Thus, the significance level for fold enhancement in each archetype was calculated for the first bin. Then for each archetype, the cumulative percentage of mice from each group (males vs. females, dominants vs. submissives) were plotted as a function of the normalized distance from the archetype or by a receiver operating characteristic (ROC) classifier curve.136 This allowed identifying the individuals that are near each behavioral archetype, and therefore show enhanced levels of a unique phenotype.
Characterization and quantification of dominance hierarchy
Formation of hierarchy for each group of mice was calculated using the Glicko rating system.32,52,53 This rating system is a method for calculating the relative skill level of players in competitor-versus-competitor games such as chess52 and it was successfully applied to evaluate dominance relationships within groups of mice living under semi-natural conditions.7,32,53 Glicko rating was calculated based on pairwise chasing/being-chased interactions, continuously updating the ratings following each event. After every event, the winner (the chasing mouse) takes points from the loser (being-chased mouse). The number of points gained depends on the prior social ranking of both interacting mice. For each experimental group of 5 mice, the dominant mouse (alpha), as well as the other ranks (i.e. beta, gamma, delta & epsilon), were determined based on their Glicko rating at the end of the experiment (i.e. day 6). A set of experiments was performed to verify social ranking by the Glicko approach in a separate cohort of control lab male mice in which two female intruders were introduced following termination of the experiment (Figure S4C). Analysis of the mice behavior showed that the dominant male indeed exhibits substantially higher aggression compared to submissives, spends more time with the females, and is generally more active. Similar effects were seen in TrpC2-/- lab male mice. In addition, we validated our hierarchy findings in comparison to the standard laboratory tube test, where we showed that control lab males, like TrpC2-/- lab males, establish clear winner-looser relations in pairs, while lab females do not (Figure S4D).
Additional parameters for evaluating the power and type of dominance hierarchy within each group of mice were calculated using functions made available in the R package ‘compete’ as previously described.32 These included the directional consistency (DC) index, phi, despotism, Landau's h, triangle transitivity, steepness, and other parameters as previously described.32 Briefly, the DC index measures how many of the total agonistic interactions between each pair within a group are directed from the more dominant individual to the subordinate individual. Phi indicates the degree by which the dominant male is exclusively aggressive. Despotism is the proportion of wins made by the alpha mouse. Landau's h evaluates the level of linearity within the hierarchy, i.e. the alpha mouse dominates all the others, beta dominates all but alpha, and so on. Triangle transitivity measures the proportion of all mice triads that are transitive (i.e. if mouse A dominates mouse B and mouse B dominates mouse C, then mouse A also dominates mouse C). The steepness of hierarchy represents the magnitude of difference in ratings between each 2 adjacent ranks. We also calculated the difference (i.e. 'delta') between the last day Glicko scores of alpha mice, to the beta, epsilon, and average of submissives as parameters of hierarchy. All hierarchy parameters were employed in the flexible discriminant analysis (FDA) as previously described,54 to further examine the differences in hierarchy characteristics between our experimental groups. In addition, David scores were calculated for each mouse, as previously described.137,138
Quantification and statistical analysis
Analysis of Glicko values per day for each experimental group in lab and wild mice was performed using two-way repeated-measures ANOVA with sex as the independent variable and with rank and day as dependent variables. Analysis of PCA was performed using factorial ANOVA with sex and type as independent variables. Each ANOVA was followed by post-hoc Tukey's test. Analysis of David scores was performed using non-parametric Friedman's ANOVA for dependent samples with post-hoc pairwise signed-ranks tests. Correlations of Glicko scores with proximity/distance from Pareto archetypes were analyzed using non-parametric Spearman's correlation. Analysis of group parameters such as no. of chasing events, delta between dominant and submissives, and stability, as well as hierarchy parameters in the ‘compete’ package, was performed using the non-parametric Mann-Whitney test between males and females or between TrpC2-/- mice and their respective wild control groups. Comparisons of fold enhancement between different archetypes were performed using the Mann-Whitney test followed by Benjamini-Hochberg correction for multiple comparisons. The cumulative distances from archetypes were analyzed using the Kolmogorov-Smirnov test for analysis of distributions. The difference between male and female TrpC2-/- and control mice in the set of social and individual parameters (i.e. bubble plots) was calculated using the Wilcoxon signed-rank test. The classifier ROC curves comparing accumulation of different groups of mice with respect to each archetype were analyzed using repeated randomized shuffling. Full information with all statistical values is detailed in Table S3. A p-value ≤ 0.05 was considered statistically significant. Analyses were performed using Statistica_14 software (TIBCO Software Inc., Palo-Alto, USA), MATLAB (R2020b, MathWorks, Natick, MA, USA), or R version 4.2.
Acknowledgments
We thank Genia Brodsky for her assistance with the graphics and Dr. Noa Stettner, Osnat Amram, Sharon Ovadia, Omri Meir, and Beni Siani from the Weizmann Veterinary department for their great assistance. We also wish to thank all members of the Kimchi lab, as well as Prof. Nir Gov and Amir Haluts, for their helpful comments on the manuscript. This work was supported by the Israeli Science Foundation (grant no. 2141/21) and the European Research Council (grant agreement no. 856487).
Author contributions
Conceptualization, N.Z., Y.S., and T.K.; methodology, N.Z., Y.S., S.G.C., A.M., U.A., and T.K.; software, S.G.C. and A.M.; formal analysis, N.Z. and S.G.C.; investigation, N.Z., Y.S., Y.P., and M.C.; data curation, S.G.C.; writing – original draft, N.Z. and T.K.; writing – review & editing, N.Z., Y.S., S.G.C., A.M., U.A., and T.K.; funding acquisition, U.A. and T.K.; supervision, T.K.
Declaration of interests
The authors declare no competing interests.
Published: March 13, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2023.02.039.
Supplemental information
Total behavioral parameters employed for PCA, with their correlation to PC1-3. For each PC, the behavioral parameters significantly correlated (p < 0.05, Spearman correlation with Bonferroni correction) are presented in BOLD. Parameters were classified as either individual behaviors, dyadic social behaviors, or group organization behaviors. Parameters that were not significantly correlated are presented as plain text.
Full array of behavioral parameters and their correlations with each archetype, in control (A) and TrpC2-/- (B) wild mice. Parameters with the highest positive correlation are the ones that are most enriched near each archetype. Significant correlations are marked in BOLD.
Data and code availability
-
•
All tracking data has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
All original code has been deposited at Github and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Silk J.B. The adaptive value of sociality in mammalian groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2007;362:539–559. doi: 10.1098/rstb.2006.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holekamp K.E., Strauss E.D. Aggression and dominance: an interdisciplinary overview. Curr. Opin. Behav. Sci. 2016;12:44–51. [Google Scholar]
- 3.Abbott D.H., Keverne E.B., Bercovitch F.B., Shively C.A., Mendoza S.P., Saltzman W., Snowdon C.T., Ziegler T.E., Banjevic M., Garland T., et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm. Behav. 2003;43:67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 4.Tibbetts E.A., Pardo-Sanchez J., Weise C. The establishment and maintenance of dominance hierarchies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022;377:20200450. doi: 10.1098/rstb.2020.0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murlanova K., Kirby M., Libergod L., Pletnikov M., Pinhasov A. Multidimensional nature of dominant behavior: insights from behavioral neuroscience. Neurosci. Biobehav. Rev. 2022;132:603–620. doi: 10.1016/j.neubiorev.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Milewski T.M., Lee W., Champagne F.A., Curley J.P. Behavioural and physiological plasticity in social hierarchies. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022;377:20200443. doi: 10.1098/rstb.2020.0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williamson C.M., Lee W., DeCasien A.R., Lanham A., Romeo R.D., Curley J.P. Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. Sci. Rep. 2019;9:7324. doi: 10.1038/s41598-019-43747-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapolsky R.M. The influence of social hierarchy on primate health. Science. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 9.Lewin N., Treidel L.A., Holekamp K.E., Place N.J., Haussmann M.F. Socioecological variables predict telomere length in wild spotted hyenas. Biol. Lett. 2015;11:20140991. doi: 10.1098/rsbl.2014.0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebbesen P., Villadsen J.A., Villadsen H.D., Heller K.E. Effect of subordinance, lack of social hierarchy, and restricted feeding on murine survival and virus leukemia. Exp. Gerontol. 1991;26:479–486. doi: 10.1016/0531-5565(91)90036-l. [DOI] [PubMed] [Google Scholar]
- 11.Archie E.A., Altmann J., Alberts S.C. Social status predicts wound healing in wild baboons. Proc. Natl. Acad. Sci. USA. 2012;109:9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razzoli M., Nyuyki-Dufe K., Gurney A., Erickson C., McCallum J., Spielman N., Marzullo M., Patricelli J., Kurata M., Pope E.A., et al. Social stress shortens lifespan in mice. Aging Cell. 2018;17:e12778. doi: 10.1111/acel.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snyder-Mackler N., Burger J.R., Gaydosh L., Belsky D.W., Noppert G.A., Campos F.A., Bartolomucci A., Yang Y.C., Aiello A.E., O’Rand A., et al. Social determinants of health and survival in humans and other animals. Science. 2020;368:eaax9553. doi: 10.1126/science.aax9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forkman B., Haskell M.J. The maintenance of stable dominance hierarchies and the pattern of aggression: support for the suppression hypothesis. Ethology. 2004;110:737–744. [Google Scholar]
- 15.Hyde J.S., Ebert P.D. Correlated response in selection for aggressiveness in female mice. I. Male aggresiveness. Behav. Genet. 1976;6:421–427. doi: 10.1007/BF01065699. [DOI] [PubMed] [Google Scholar]
- 16.Chalfin L., Dayan M., Levy D.R., Austad S.N., Miller R.A., Iraqi F.A., Dulac C., Kimchi T. Mapping ecologically relevant social behaviours by gene knockout in wild mice. Nat. Commun. 2014;5:4569. doi: 10.1038/ncomms5569. [DOI] [PubMed] [Google Scholar]
- 17.Ebert P.D., Hyde J.S. Selection for agonistic behavior in wild female Mus musculus. Behav. Genet. 1976;6:291–304. doi: 10.1007/BF01065725. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari P.F., Palanza P., Rodgers R.J., Mainardi M., Parmigiani S. Comparing different forms of male and female aggression in wild and laboratory mice: an ethopharmacological study. Physiol. Behav. 1996;60:549–553. doi: 10.1016/s0031-9384(96)80030-8. [DOI] [PubMed] [Google Scholar]
- 19.Palanza P., Parmigiani S., vom Saal F.S. Male urinary cues stimulate intra-sexual aggression and urine-marking in wild female mice, Mus musculus domesticus. Anim. Behav. 1994;48:245–247. [Google Scholar]
- 20.Stockley P., Campbell A. Female competition and aggression: interdisciplinary perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20130073. doi: 10.1098/rstb.2013.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zilkha N., Sofer Y., Beny Y., Kimchi T. From classic ethology to modern neuroethology: overcoming the three biases in social behavior research. Curr. Opin. Neurobiol. 2016;38:96–108. doi: 10.1016/j.conb.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 22.Palanza P., Re L., Mainardi D., Brain P.F., Parmigiani S. Male and female competitive strategies of wild house mice pairs (Mus musculus domesticus) confronted with intruders of different sex and age in artificial territories. Behaviour. 1996;133:863–882. [Google Scholar]
- 23.Fitzgerald B.M., Karl B.J., Moller H. Spatial organization and ecology of a sparse population of house mice (Mus musculus) in a New Zealand forest. J. Anim. Ecol. 1981;50:489–518. [Google Scholar]
- 24.Berry R.J. The natural history of the house mouse. Fld. Stud. 1970;3:219–262. [Google Scholar]
- 25.Berry R.J., Jakobson M.E. Vagility in an island population of the house mouse. J. Zool. 1974;173:341–354. [Google Scholar]
- 26.Chambers L.K., Singleton G.R., Krebs C.J. Movements and social organization of wild house mice (Mus domesticus) in the wheatlands of Northwestern Victoria, Australia. J. Mammal. 2000;81:59–69. [Google Scholar]
- 27.Singleton G.R., Krebs C.J. In: The Mouse in Biomedical Research. Second Edition. Fox J.G., Davisson M.T., Quimby F.W., Barthold S.W., Newcomer C.E., Smith A.L., editors. Academic Press; 2007. The secret world of wild mice; pp. 25–51. [Google Scholar]
- 28.Latham N., Mason G. From house mouse to mouse house: the behavioural biology of free-living Mus musculus and its implications in the laboratory. Appl. Anim. Behav. Sci. 2004;86:261–289. [Google Scholar]
- 29.Rosvall K.A. Intrasexual competition in females: evidence for sexual selection? Behav. Ecol. 2011;22:1131–1140. doi: 10.1093/beheco/arr106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stockley P., Bro-Jørgensen J. Female competition and its evolutionary consequences in mammals. Biol. Rev. Camb. Philos. Soc. 2011;86:341–366. doi: 10.1111/j.1469-185X.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- 31.Forkosh O., Karamihalev S., Roeh S., Alon U., Anpilov S., Touma C., Nussbaumer M., Flachskamm C., Kaplick P.M., Shemesh Y., et al. Identity domains capture individual differences from across the behavioral repertoire. Nat. Neurosci. 2019;22:2023–2028. doi: 10.1038/s41593-019-0516-y. [DOI] [PubMed] [Google Scholar]
- 32.Williamson C.M., Lee W., Curley J.P. Temporal dynamics of social hierarchy formation and maintenance in male mice. Anim. Behav. 2016;115:259–272. [Google Scholar]
- 33.Fan Z., Zhu H., Zhou T., Wang S., Wu Y., Hu H. Using the tube test to measure social hierarchy in mice. Nat. Protoc. 2019;14:819–831. doi: 10.1038/s41596-018-0116-4. [DOI] [PubMed] [Google Scholar]
- 34.Shemesh Y., Forkosh O., Mahn M., Anpilov S., Sztainberg Y., Manashirov S., Shlapobersky T., Elliott E., Tabouy L., Ezra G., et al. Ucn3 and CRF-R2 in the medial amygdala regulate complex social dynamics. Nat. Neurosci. 2016;19:1489–1496. doi: 10.1038/nn.4346. [DOI] [PubMed] [Google Scholar]
- 35.Shemesh Y., Sztainberg Y., Forkosh O., Shlapobersky T., Chen A., Schneidman E. High-order social interactions in groups of mice. eLife. 2013;2:e00759. doi: 10.7554/eLife.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varholick J.A., Bailoo J.D., Palme R., Würbel H. Phenotypic variability between social dominance ranks in laboratory mice. Sci. Rep. 2018;8:6593. doi: 10.1038/s41598-018-24624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou T., Sandi C., Hu H. Advances in understanding neural mechanisms of social dominance. Curr. Opin. Neurobiol. 2018;49:99–107. doi: 10.1016/j.conb.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 38.van den Berg W.E., Lamballais S., Kushner S.A. Sex-specific mechanism of social hierarchy in mice. Neuropsychopharmacology. 2015;40:1364–1372. doi: 10.1038/npp.2014.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thoß M., Luzynski K.C., Enk V.M., Razzazi-Fazeli E., Kwak J., Ortner I., Penn D.J. Regulation of volatile and non-volatile pheromone attractants depends upon male social status. Sci. Rep. 2019;9:489. doi: 10.1038/s41598-018-36887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.König B., Lindholm A.K., Lopes P.C., Dobay A., Steinert S., Buschmann F.J.-U. A system for automatic recording of social behavior in a free-living wild house mouse population. Anim. Biotelem. 2015;3:39. [Google Scholar]
- 41.Karamihalev S., Brivio E., Flachskamm C., Stoffel R., Schmidt M.V., Chen A. Social dominance mediates behavioral adaptation to chronic stress in a sex-specific manner. eLife. 2020;9:e58723. doi: 10.7554/eLife.58723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ribeiro A.C., Musatov S., Shteyler A., Simanduyev S., Arrieta-Cruz I., Ogawa S., Pfaff D.W. siRNA silencing of estrogen receptor-α expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc. Natl. Acad. Sci. USA. 2012;109:16324–16329. doi: 10.1073/pnas.1214094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ragnauth A.K., Devidze N., Moy V., Finley K., Goodwillie A., Kow L.M., Muglia L.J., Pfaff D.W. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes Brain Behav. 2005;4:229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 44.Wolf M., van Doorn G.S., Leimar O., Weissing F.J. Life-history trade-offs favour the evolution of animal personalities. Nature. 2007;447:581–584. doi: 10.1038/nature05835. [DOI] [PubMed] [Google Scholar]
- 45.Harrison L.M., Noble D.W.A., Jennions M.D. A meta-analysis of sex differences in animal personality: no evidence for the greater male variability hypothesis. Biol. Rev. Camb. Philos. Soc. 2022;97:679–707. doi: 10.1111/brv.12818. [DOI] [PubMed] [Google Scholar]
- 46.Cabrera D., Nilsson J.R., Griffen B.D. The development of animal personality across ontogeny: a cross-species review. Anim. Behav. 2021;173:137–144. [Google Scholar]
- 47.Weissbrod A., Shapiro A., Vasserman G., Edry L., Dayan M., Yitzhaky A., Hertzberg L., Feinerman O., Kimchi T. Automated long-term tracking and social behavioural phenotyping of animal colonies within a semi-natural environment. Nat. Commun. 2013;4:2018. doi: 10.1038/ncomms3018. [DOI] [PubMed] [Google Scholar]
- 48.Beny Y., Kimchi T. Innate and learned aspects of pheromone-mediated social behaviours. Anim. Behav. 2014;97:301–311. [Google Scholar]
- 49.Hart Y., Sheftel H., Hausser J., Szekely P., Ben-Moshe N.B., Korem Y., Tendler A., Mayo A.E., Alon U. Inferring biological tasks using Pareto analysis of high-dimensional data. Nat. Methods. 2015;12:233. doi: 10.1038/nmeth.3254. [DOI] [PubMed] [Google Scholar]
- 50.Shoval O., Sheftel H., Shinar G., Hart Y., Ramote O., Mayo A., Dekel E., Kavanagh K., Alon U. Evolutionary trade-offs, Pareto optimality, and the geometry of phenotype space. Science. 2012;336:1157–1160. doi: 10.1126/science.1217405. [DOI] [PubMed] [Google Scholar]
- 51.Szekely P., Korem Y., Moran U., Mayo A., Alon U. The mass-longevity triangle: Pareto optimality and the geometry of life-history trait space. PLoS Comput. Biol. 2015;11:e1004524. doi: 10.1371/journal.pcbi.1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glickman M.E. Parameter estimation in large dynamic paired comparison experiments. J. R. Stat. Soc. C. 1999;48:377–394. [Google Scholar]
- 53.So N., Franks B., Lim S., Curley J.P. A social network approach reveals associations between mouse social dominance and brain gene expression. PLoS One. 2015;10:e0134509. doi: 10.1371/journal.pone.0134509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee W., Dowd H.N., Nikain C., Dwortz M.F., Yang E.D., Curley J.P. Effect of relative social rank within a social hierarchy on neural activation in response to familiar or unfamiliar social signals. Sci. Rep. 2021;11:2864. doi: 10.1038/s41598-021-82255-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adler M., Tendler A., Hausser J., Korem Y., Szekely P., Bossel N., Hart Y., Karin O., Mayo A., Alon U. Controls for phylogeny and robust analysis in Pareto task inference. Mol. Biol. Evol. 2022;39:msab297. doi: 10.1093/molbev/msab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hausser J., Szekely P., Bar N., Zimmer A., Sheftel H., Caldas C., Alon U. Tumor diversity and the trade-off between universal cancer tasks. Nat. Commun. 2019;10:5423. doi: 10.1038/s41467-019-13195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheftel H., Szekely P., Mayo A., Sella G., Alon U. Evolutionary trade-offs and the structure of polymorphisms. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20170105. doi: 10.1098/rstb.2017.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adler M., Szekely P., Mayo A., Alon U. Optimal regulatory circuit topologies for fold-change detection. Cell Syst. 2017;4:171–181.e8. doi: 10.1016/j.cels.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 59.Korem Y., Szekely P., Hart Y., Sheftel H., Hausser J., Mayo A., Rothenberg M.E., Kalisky T., Alon U. Geometry of the gene expression space of individual cells. PLoS Comput. Biol. 2015;11:e1004224. doi: 10.1371/journal.pcbi.1004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stowers L., Holy T.E., Meister M., Dulac C., Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 61.Kimchi T., Xu J., Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 62.Ben-Shaul Y., Katz L.C., Mooney R., Dulac C. In vivo vomeronasal stimulation reveals sensory encoding of conspecific and allospecific cues by the mouse accessory olfactory bulb. Proc. Natl. Acad. Sci. USA. 2010;107:5172–5177. doi: 10.1073/pnas.0915147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bergan J.F., Ben-Shaul Y., Dulac C. Sex-specific processing of social cues in the medial amygdala. eLife. 2014;3:e02743. doi: 10.7554/eLife.02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beny-Shefer Y., Zilkha N., Lavi-Avnon Y., Bezalel N., Rogachev I., Brandis A., Dayan M., Kimchi T. Nucleus accumbens dopamine signaling regulates sexual preference for females in male mice. Cell Rep. 2017;21:3079–3088. doi: 10.1016/j.celrep.2017.11.062. [DOI] [PubMed] [Google Scholar]
- 65.Gray S.J., Hurst J.L. Competitive behaviour in an island population of house mice, Mus domesticus. Anim. Behav. 1998;56:1291–1299. doi: 10.1006/anbe.1998.0890. [DOI] [PubMed] [Google Scholar]
- 66.Bronson F.H. The reproductive ecology of the house mouse. Q. Rev. Biol. 1979;54:265–299. doi: 10.1086/411295. [DOI] [PubMed] [Google Scholar]
- 67.Phifer-Rixey M., Nachman M.W. Insights into mammalian biology from the wild house mouse Mus musculus. eLife. 2015;4:e05959. doi: 10.7554/eLife.05959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stagkourakis S., Spigolon G., Williams P., Protzmann J., Fisone G., Broberger C. A neural network for intermale aggression to establish social hierarchy. Nat. Neurosci. 2018;21:834–842. doi: 10.1038/s41593-018-0153-x. [DOI] [PubMed] [Google Scholar]
- 69.Wang F., Kessels H.W., Hu H. The mouse that roared: neural mechanisms of social hierarchy. Trends Neurosci. 2014;37:674–682. doi: 10.1016/j.tins.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Wang F., Zhu J., Zhu H., Zhang Q., Lin Z., Hu H. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334:693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- 71.Blanchard D.C., Fukunaga-Stinson C., Takahashi L.K., Flannelly K.J., Blanchard R.J. Dominance and aggression in social groups of male and female rats. Behav. Processes. 1984;9:31–48. doi: 10.1016/0376-6357(84)90006-8. [DOI] [PubMed] [Google Scholar]
- 72.Miczek K.A., Barry H. What does the tube test measure? Behav. Biol. 1975;13:537–539. doi: 10.1016/s0091-6773(75)91249-3. [DOI] [PubMed] [Google Scholar]
- 73.Lindzey G., Manosevitz M., Winston H. Social dominance in the mouse. Psychon. Sci. 1966;5:451–452. [Google Scholar]
- 74.Li S.W., Zeliger O., Strahs L., Báez-Mendoza R., Johnson L.M., McDonald Wojciechowski A., Williams Z.M. Frontal neurons driving competitive behaviour and ecology of social groups. Nature. 2022;603:661–666. doi: 10.1038/s41586-021-04000-5. [DOI] [PubMed] [Google Scholar]
- 75.Padilla-Coreano N., Batra K., Patarino M., Chen Z., Rock R.R., Zhang R., Hausmann S.B., Weddington J.C., Patel R., Zhang Y.E., et al. Cortical ensembles orchestrate social competition through hypothalamic outputs. Nature. 2022;603:667–671. doi: 10.1038/s41586-022-04507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou X.H., Hyun M., Taranda J., Huang K.W., Todd E., Feng D., Atwater E., Croney D., Zeidel M.L., Osten P., et al. Central control circuit for context-dependent micturition. Cell. 2016;167:73–86.e12. doi: 10.1016/j.cell.2016.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou T., Zhu H., Fan Z., Wang F., Chen Y., Liang H., Yang Z., Zhang L., Lin L., Zhan Y., et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science. 2017;357:162–168. doi: 10.1126/science.aak9726. [DOI] [PubMed] [Google Scholar]
- 78.Dwortz M.F., Curley J.P., Tye K.M., Padilla-Coreano N. Neural systems that facilitate the representation of social rank. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2022;377:20200444. doi: 10.1098/rstb.2020.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kingsbury L., Huang S., Wang J., Gu K., Golshani P., Wu Y.E., Hong W. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell. 2019;178:429–446.e16. doi: 10.1016/j.cell.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Peters S.M., Pothuizen H.H.J., Spruijt B.M. Ethological concepts enhance the translational value of animal models. Eur. J. Pharmacol. 2015;759:42–50. doi: 10.1016/j.ejphar.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 81.Palanza P., Della Seta D., Ferrari P.F., Parmigiani S. Female competition in wild house mice depends upon timing of female/male settlement and kinship between females. Anim. Behav. 2005;69:1259–1271. [Google Scholar]
- 82.Parmigiani S., Palanza P., Rogers J., Ferrari P.F. Selection, evolution of behavior and animal models in behavioral neuroscience. Neurosci. Biobehav. Rev. 1999;23:957–969. doi: 10.1016/s0149-7634(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 83.Parmigiani S. Rank order in pairs of communally nursing female mice (Mus musculus domesticus) and maternal aggression towards conspecific intruders of differing sex. Aggress. Behav. 1986;12:377–386. [Google Scholar]
- 84.Vom Saal F.S., Franks P., Boechler M., Palanza P., Parmigiani S. Nest defense and survival of offspring in highly aggressive wild Canadian female house mice. Physiol. Behav. 1995;58:669–678. doi: 10.1016/0031-9384(95)00121-x. [DOI] [PubMed] [Google Scholar]
- 85.Blanchard R.J., Hebert M.A., Ferrari P.F., Palanza P., Figueira R., Blanchard D.C., Parmigiani S. Defensive behaviors in wild and laboratory (Swiss) mice: the mouse defense test battery. Physiol. Behav. 1998;65:201–209. doi: 10.1016/s0031-9384(98)00012-2. [DOI] [PubMed] [Google Scholar]
- 86.McCarthy M.M., Vom Saal F.S. The influence of reproductive state on infanticide by wild female house mice (Mus musculus) Physiol. Behav. 1985;35:843–849. doi: 10.1016/0031-9384(85)90248-3. [DOI] [PubMed] [Google Scholar]
- 87.Holekamp K.E., Smale L. Dominance acquisition during mammalian social development: the “inheritance” of maternal rank. Am. Zool. 1991;31:306–317. [Google Scholar]
- 88.Matthews G.A., Tye K.M. Neural mechanisms of social homeostasis. Ann. N. Y. Acad. Sci. 2019;1457:5–25. doi: 10.1111/nyas.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Douglas L.A., Varlinskaya E.I., Spear L.P. Rewarding properties of social interactions in adolescent and adult male and female rats: impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. 2004;45:153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- 90.Zilkha N., Sofer Y., Kashash Y., Kimchi T. The social network: neural control of sex differences in reproductive behaviors, motivation, and response to social isolation. Curr. Opin. Neurobiol. 2021;68:137–151. doi: 10.1016/j.conb.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang Y., Benusiglio D., Lefevre A., Hilfiger L., Althammer F., Bludau A., Hagiwara D., Baudon A., Darbon P., Schimmer J., et al. Social touch promotes interfemale communication via activation of parvocellular oxytocin neurons. Nat. Neurosci. 2020;23:1125–1137. doi: 10.1038/s41593-020-0674-y. [DOI] [PubMed] [Google Scholar]
- 92.Frank L.G. Social organization of the spotted hyaena Crocuta crocuta. II. Dominance and reproduction. Anim. Behav. 1986;34:1510–1527. [Google Scholar]
- 93.Dorning J., Harris S. Individual and seasonal variation in contact rate, connectivity and centrality in red fox (Vulpes vulpes) social groups. Sci. Rep. 2019;9:20095. doi: 10.1038/s41598-019-56713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Angermeier W.F., Phelps J.B., Murray S., Howanstine J. Dominance in monkeys: sex differences. Psychon. Sci. 1968;12:344. [Google Scholar]
- 95.Hausfater G., Altmann J., Altmann S. Long-term consistency of dominance relations among female baboons Papio cynocephalus. Science. 1982;217:752–755. doi: 10.1126/science.217.4561.752. [DOI] [PubMed] [Google Scholar]
- 96.Lea A.J., Akinyi M.Y., Nyakundi R., Mareri P., Nyundo F., Kariuki T., Alberts S.C., Archie E.A., Tung J. Dominance rank-associated gene expression is widespread, sex-specific, and a precursor to high social status in wild male baboons. Proc. Natl. Acad. Sci. USA. 2018;115:E12163–E12171. doi: 10.1073/pnas.1811967115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mast M.S. Gender differences and similarities in dominance hierarchies in same-gender groups based on speaking time. Sex Roles. 2001;44:537–556. [Google Scholar]
- 98.Blanchard R.J., Dulloog L., Markham C., Nishimura O., Nikulina Compton J., Jun A., Han C., Blanchard D.C. Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol. Behav. 2001;72:245–254. doi: 10.1016/s0031-9384(00)00403-0. [DOI] [PubMed] [Google Scholar]
- 99.Plusquellec B., Bouissou G.L., Pape G.L. Early predictors of dominance ability in heifers (Bos taurus, L.) of the hérens breed. Behaviour. 2001;138:1009–1031. [Google Scholar]
- 100.Saltzman W., Schultz-Darken N.J., Abbott D.H. Behavioural and endocrine predictors of dominance and tolerance in female common marmosets, Callithrix jacchus. Anim. Behav. 1996;51:657–674. [Google Scholar]
- 101.Cooney R. Colony defense in Damaraland mole-rats, Cryptomys damarensis. Behav. Ecol. 2002;13:160–162. [Google Scholar]
- 102.Thomas R.M., Hotsenpiller G., Peterson D.A. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J. Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huck U.W., Lisk R.D., McKay M.V. Social dominance and reproductive success in pregnant and lactating golden hamsters (Mesocricetus auratus) under seminatural conditions. Physiol. Behav. 1988;44:313–319. doi: 10.1016/0031-9384(88)90031-5. [DOI] [PubMed] [Google Scholar]
- 104.Dewsbury D.A. Fathers and sons: genetic factors and social dominance in deer mice, Peromyscus maniculatus. Anim. Behav. 1990;39:284–289. [Google Scholar]
- 105.Klass K., Cords M. Agonism and dominance in female blue monkeys. Am. J. Primatol. 2015;77:1299–1315. doi: 10.1002/ajp.22481. [DOI] [PubMed] [Google Scholar]
- 106.Arakawa H. Interaction between isolation rearing and social development on exploratory behavior in male rats. Behav. Processes. 2005;70:223–234. doi: 10.1016/j.beproc.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 107.Blanchard D.C., Blanchard R.J. Behavioral correlates of chronic dominance-subordination relationships of male rats in a seminatural situation. Neurosci. Biobehav. Rev. 1990;14:455–462. doi: 10.1016/s0149-7634(05)80068-5. [DOI] [PubMed] [Google Scholar]
- 108.Meerlo P., Overkamp G.J.F., Koolhaas J.M. Behavioural and physiological consequences of a single social defeat in Roman high- and low-avoidance rats. Psychoneuroendocrinology. 1997;22:155–168. doi: 10.1016/s0306-4530(96)00047-9. [DOI] [PubMed] [Google Scholar]
- 109.Chichinadze K., Chichinadze N., Gachechiladze L., Lazarashvili A., Nikolaishvili M. Physical predictors, behavioural/emotional attributes and neurochemical determinants of dominant behaviour. Biol. Rev. Camb. Philos. Soc. 2014;89:1005–1020. doi: 10.1111/brv.12091. [DOI] [PubMed] [Google Scholar]
- 110.Crowcroft P., Rowe F.P. Social organization and territorial behaviour in the wild. Proc. Zool. Soc. Lond. 1963;140:517–531. [Google Scholar]
- 111.Snyder-Mackler N., Sanz J., Kohn J.N., Voyles T., Pique-Regi R., Wilson M.E., Barreiro L.B., Tung J. Social status alters chromatin accessibility and the gene regulatory response to glucocorticoid stimulation in rhesus macaques. Proc. Natl. Acad. Sci. USA. 2019;116:1219–1228. doi: 10.1073/pnas.1811758115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Magariños A.M.a., McEwen B.S., Flügge G., Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mohrhardt J., Nagel M., Fleck D., Ben-Shaul Y., Spehr M. Signal detection and coding in the accessory olfactory system. Chem. Senses. 2018;43:667–695. doi: 10.1093/chemse/bjy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Y., Dulac C. Neural coding of sex-specific social information in the mouse brain. Curr. Opin. Neurobiol. 2018;53:120–130. doi: 10.1016/j.conb.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 115.Veyrac A., Wang G., Baum M.J., Bakker J. The main and accessory olfactory systems of female mice are activated differentially by dominant versus subordinate male urinary odors. Brain Res. 2011;1402:20–29. doi: 10.1016/j.brainres.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schröder H., Moser N., Huggenberger S. Neuroanatomy of the Mouse: An Introduction. Springer International Publishing; 2020. The mouse olfactory system; pp. 319–331. [Google Scholar]
- 117.Liberles S.D. Mammalian pheromones. Annu. Rev. Physiol. 2014;76:151–175. doi: 10.1146/annurev-physiol-021113-170334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hurst J.L., Beynon R.J. Scent wars: the chemobiology of competitive signalling in mice. BioEssays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- 119.Hurst J.L., Payne C.E., Nevison C.M., Marie A.D., Humphries R.E., Robertson D.H.L., Cavaggioni A., Beynon R.J. Individual recognition in mice mediated by major urinary proteins. Nature. 2001;414:631–634. doi: 10.1038/414631a. [DOI] [PubMed] [Google Scholar]
- 120.Leypold B.G., Yu C.R., Leinders-Zufall T., Kim M.M., Zufall F., Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim S., Ma L., Jensen K.L., Kim M.M., Bond C.T., Adelman J.P., Yu C.R. Paradoxical contribution of SK3 and GIRK channels to the activation of mouse vomeronasal organ. Nat. Neurosci. 2012;15:1236–1244. doi: 10.1038/nn.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelliher K.R., Spehr M., Li X.H., Zufall F., Leinders-Zufall T. Pheromonal recognition memory induced by TRPC2-independent vomeronasal sensing. Eur. J. Neurosci. 2006;23:3385–3390. doi: 10.1111/j.1460-9568.2006.04866.x. [DOI] [PubMed] [Google Scholar]
- 123.Beny Y., Kimchi T. Conditioned odor aversion induces social anxiety towards females in wild-type and TrpC2 knockout male mice. Genes Brain Behav. 2016;15:722–732. doi: 10.1111/gbb.12320. [DOI] [PubMed] [Google Scholar]
- 124.Cherry J.A., Baum M.J. Sex differences in main olfactory system pathways involved in psychosexual function. Genes Brain Behav. 2020;19:e12618. doi: 10.1111/gbb.12618. [DOI] [PubMed] [Google Scholar]
- 125.Hurst J.L. Behavioral variation in wild house mice Mus domesticus Rutty – a quantitative assessment of female social organization. Anim. Behav. 1987;35:1846–1857. [Google Scholar]
- 126.Berry R.J. Population dynamics of the house mouse. Symp. Zool. Soc. Lond. 1981;47:395–425. [Google Scholar]
- 127.Berry R.J., Bronson F.H. Life history and bioeconomy of the house mouse. Biol. Rev. Camb. Philos. Soc. 1992;67:519–550. doi: 10.1111/j.1469-185x.1992.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 128.Palanza P., Parmigiani S. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci. Biobehav. Rev. 2017;76:134–143. doi: 10.1016/j.neubiorev.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 129.Clipperton Allen A.E., Cragg C.L., Wood A.J., Pfaff D.W., Choleris E. Agonistic behavior in males and females: effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 2010;35:1008–1022. doi: 10.1016/j.psyneuen.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de la Zerda S.H., Netser S., Magalnik H., Briller M., Marzan D., Glatt S., Abergel Y., Wagner S. Social recognition in rats and mice requires integration of olfactory, somatosensory and auditory cues. Psychoneuroendocrinology. 2022;143:105859. doi: 10.1016/j.psyneuen.2022.105859. [DOI] [PubMed] [Google Scholar]
- 131.Zufall F. The TRPC2 ion channel and pheromone sensing in the accessory olfactory system. Naunyn. Schmiedebergs. Arch. Pharmacol. 2005;371:245–250. doi: 10.1007/s00210-005-1028-8. [DOI] [PubMed] [Google Scholar]
- 132.Wu Z., Autry A.E., Bergan J.F., Watabe-Uchida M., Dulac C.G. Galanin neurons in the medial preoptic area govern parental behaviour. Nature. 2014;509:325–330. doi: 10.1038/nature13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller R.A., Dysko R., Chrisp C., Seguin R., Linsalata L., Buehner G., Harper J.M., Austad S. Mouse (Mus musculus) stocks derived from tropical islands: new models for genetic analysis of life-history traits. J. Zool. (1987) 2000;250:95–104. doi: 10.1111/j.1469-7998.2000.tb00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Munkres J. Algorithms for the assignment and transportation problems. J. Soc. Ind. Appl. Math. 1957;5:32–38. [Google Scholar]
- 135.Anpilov S., Shemesh Y., Eren N., Harony-Nicolas H., Benjamin A., Dine J., Oliveira V.E.M., Forkosh O., Karamihalev S., Hüttl R.E., et al. Wireless optogenetic stimulation of oxytocin neurons in a semi-natural setup dynamically elevates both pro-social and agonistic behaviors. Neuron. 2020;107:644–655.e7. doi: 10.1016/j.neuron.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J. Intern. Med. 2013;4:627–635. [PMC free article] [PubMed] [Google Scholar]
- 137.David H.A. Ranking from unbalanced paired-comparison data. Biometrika. 1987;74:432–436. [Google Scholar]
- 138.Gammell M.P., de Vries H., Jennings D.J., Carlin C.M., Hayden T.J. David's score: a more appropriate dominance ranking method than Clutton-Brock et al.'s index. Anim. Behav. 2003;66:601–605. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total behavioral parameters employed for PCA, with their correlation to PC1-3. For each PC, the behavioral parameters significantly correlated (p < 0.05, Spearman correlation with Bonferroni correction) are presented in BOLD. Parameters were classified as either individual behaviors, dyadic social behaviors, or group organization behaviors. Parameters that were not significantly correlated are presented as plain text.
Full array of behavioral parameters and their correlations with each archetype, in control (A) and TrpC2-/- (B) wild mice. Parameters with the highest positive correlation are the ones that are most enriched near each archetype. Significant correlations are marked in BOLD.
Data Availability Statement
-
•
All tracking data has been deposited at Zenodo and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
All original code has been deposited at Github and is publicly available as of the date of publication. DOIs are listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.