Abstract
Background
The COVID-19 pandemic strained oncologic care access and delivery, yet little is known about how it impacted hepatocellular carcinoma (HCC) management. Our study sought to evaluate the annual effect of the COVID-19 pandemic on time to treatment initiation (TTI) for HCC.
Methods
The National Cancer Database was queried for patients diagnosed with clinical stages I–IV HCC (2017–2020). Patients were categorized based on their year of diagnosis as “Pre-COVID” (2017–2019) and “COVID” (2020). TTI based on stage and type of treatment first received was compared by the Mann-Whitney U test. A logistic regression model was used to evaluate factors of increased TTI and treatment delay (> 90 days).
Results
In total, 18,673 patients were diagnosed during Pre-COVID, whereas 5249 were diagnosed during COVID. Median TTI for any first-line treatment modality was slightly shorter during the COVID year compared with Pre-COVID (49 vs. 51 days; p < 0.0001), notably in time to ablation (52 vs. 55 days; p = 0.0238), systemic therapy (42 vs. 47 days; p < 0.0001), and radiation (60 vs. 62 days; p = 0.0177), but not surgery (41 vs. 41 days; p = 0.6887). In a multivariate analysis, patients of Black race, Hispanic ethnicity, and uninsured/Medicaid/Other Government insurance status were associated with increased TTI by factors of 1.057 (95% CI: 1.022–1.093; p = 0.0013), 1.045 (95% CI: 1.010–1.081; p = 0.0104), and 1.088 (95% CI: 1.053–1.123; p < 0.0001), respectively. Similarly, these patient populations were associated with delayed treatment times.
Conclusions
For patients diagnosed during COVID, TTI for HCC, while statistically significant, had no clinically significant differences. However, vulnerable patients were more likely to have increased TTI.
The coronavirus disease 2019 (COVID-19) pandemic was responsible for the deaths of over 6 million people worldwide, with over 1 million deaths in the United States (US).1 Since its declaration in March of 2020, the pandemic has inflicted an unprecedented and devastating strain on the healthcare system.2 Non-emergent surgeries for both benign and malignant diseases were canceled or postponed.3–5 Outpatient clinics were suspended, and underwent an expedited transition to the virtual platform of telemedicine.6 Additionally, healthcare providers were plagued by on-going ethical dilemmas in care delivery.7,8 As a result, the effects of the COVID-19 pandemic have had a drastic impact on the delivery of surgical care as well as cancer management.
During the pandemic, there were notable declines in breast and colorectal cancer screenings that suggest the risk of later-stage diagnoses in affected populations with subsequent worsened prognoses.9 In addition, cancer-related encounters have notably declined across multiple cancer types such as breast cancer, prostate cancer, and melanoma by 47.7%, 49.1%, and 51.8%, respectively.9,10 These findings are concordant with patient survey data reporting delays to follow-up clinical appointments, infusion-based treatments, surgeries, as well as surveillance imaging during the pandemic.11,12 Oncologic treatment delays for surgery, infusion-based therapies, and radiation have been shown to have deleterious effects on patient overall survival in a multitude of cancers.13,14 While studies have started to characterize the impact of the pandemic on cancer care delivery in breast, colon, and prostate cancer, no study to our knowledge has yet examined the national impact of the pandemic on the management of hepatocellular carcinoma (HCC) in the US.
Primary liver cancer is recognized as the sixth most diagnosed cancer and third leading cause of cancer-related deaths in the world, with HCC comprising 75–85% of these cancers.15 Yet, little is known about how national efforts in response to the COVID-19 pandemic impacted HCC care delivery and time to treatment. Thus, our study utilized the National Cancer Database (NCDB) to evaluate the annual impact of the COVID-19 pandemic on time to treatment initiation (TTI) in patients with HCC, and to identify patient and hospital factors predictive of increased treatment intervals.
Methods
Data Source
The NCDB is a clinical oncology database jointly established by the Commission on Cancer (CoC) of the American College of Surgeons (ACS) and the American Cancer Society.16 This nationwide database captures cancer outcomes data from more than 1500 CoC-accredited facilities, translating to information collected on approximately 72% of newly diagnosed cancers in the US and Puerto Rico.16,17 Given that all data was de-identified, this study was considered exempt from review by the Institutional Review Board at our institution.
Patient Selection
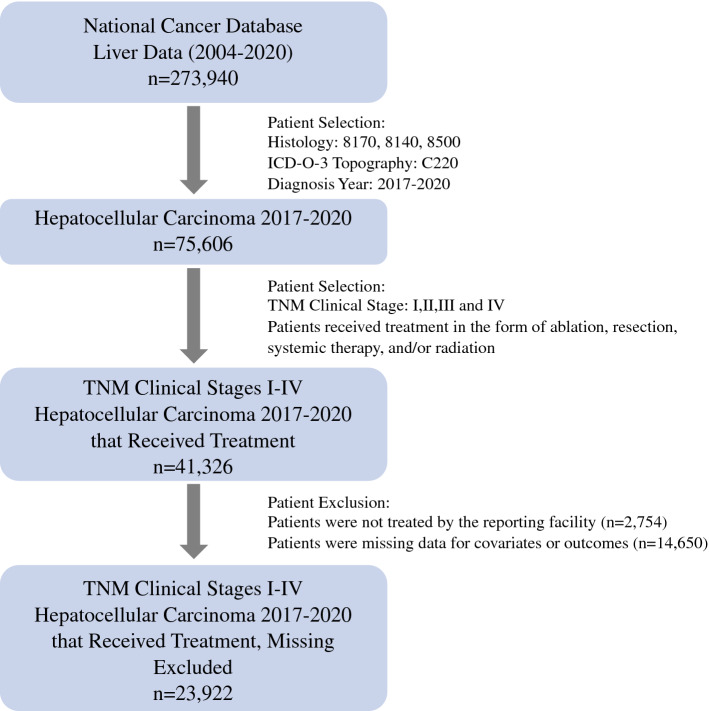
The NCDB was queried for patients diagnosed with hepatocellular carcinoma (HCC) of American Joint Committee on Cancer (AJCC) TNM clinical stages I–IV for the years 2017 to 2020 (Fig. 1). Patients diagnosed in 2017 were staged using the 7th Edition of the AJCC Staging Manual while those diagnosed in 2018–2020 were staged using the 8th Edition.18,19 Patients diagnosed with HCC were identified using the International Classification of Disease for Oncology 3 topography (C220) and histology (8170, 8140, 8500) codes. Patients were categorized into the following cohorts based on their year of diagnosis: “Pre-COVID” (2017–2019) and “COVID” (2020). To allow for comparison of TTI, the cohorts were further restricted to patients who received treatment in the form of ablation, liver resection, systemic therapy, or radiation. Ablation and surgical treatment were assigned using the following CoC Facility Oncology Registry Data Standards (FORDS) codes: 16, 20–26, 30, 36–38, 50–52, 59–60, 66.20 Systemic therapy was defined as either chemotherapy or immunotherapy. Liver transplantation, while a curative therapy, was not included given that the receipt of transplantation is confounded by a system-based allocation driven by patient disease severity and geographic variations amongst cadaveric and living organ availability.21,22
Fig. 1.
An outline of the patient selection process using the National Cancer Database
Covariates
Patient demographics collected for this study were age at diagnosis, sex, race, and ethnicity. Socioeconomic factors collected included insurance status, median annual income, educational attainment, and area of residency. Educational attainment was provided by the NCDB and is defined as an estimate on the proportion of adults who live in the same zip code as the patient who have not graduated from high school, derived from 2020 American Community Survey data files (2016–2020).20 Hospital-specific factors included median travel distance (≤ 14.8 miles, > 14.8 miles), facility type, and facility regional location. Comorbidities were defined based on the Charlson-Deyo comorbidity score and were grouped as the following: 0, 1, and ≥ 2.23 Further details about the covariates are provided in the Appendix. Patients were excluded if missing data for any covariates.
Outcome of Interest
Treatment patterns based on stage at diagnosis were compared between the Pre-COVID and COVID cohorts. Treatment regimens were categorized as procedural intervention only, systemic therapy only, radiation therapy only, and combined treatment. Procedural intervention only comprised patients who received either ablation only or resection only due to small sample sizes in patients with stage IV HCC. The combined treatment regimen was defined as treatment regimens using more than one of the following treatments: ablation, resection, systemic therapy, or radiation.
For TTI, the NCDB provides the number of days between the date of diagnosis and the date of treatment administration for each of the following specified treatments: ablation, liver resection, chemotherapy, immunotherapy, and radiation.20 For ablation or resection, TTI was defined as the number of days from diagnosis to the most definitive surgical intervention and delineated based on the FORDS procedure codes. Since systemic therapy is defined as patients receiving either chemotherapy or immunotherapy, the minimum number of days to either treatment modality was used to define the TTI for systemic therapy. For patients receiving multimodal therapy, TTI was defined as the minimum number of days to treatment amongst all received treatments. Patients were excluded if they received treatment but were missing TTI data and if their TTI for first received therapy was equal to more than one treatment modality (n = 308). To assess for predictors of delayed treatment in the setting of the COVID-19 pandemic, TTI was formatted into a dichotomous variable (≤ 90 days, > 90 days) where delayed treatment was defined as time from diagnosis to treatment of greater than 90 days, based on prior literature.24,25
Statistical Analysis
Baseline characteristics of patients and treatment patterns between the Pre-COVID and COVID patients were compared using the chi-square test. Time to treatment initiation based on clinical stage and on first treatment modality received were compared using the Mann-Whitney U test. A negative binomial regression model was used to evaluate patient and hospital-related factors predictive of increased TTI. A multivariate logistic regression model was used to identify predictors of delayed treatment (> 90 days) with backward selection (significance level of 0.05). Significance for all analyses was defined as p < 0.05. Statistical analyses were performed using version 9.4 SAS software (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of the General Cohort
A total of 23,922 patients with clinical stages I–IV HCC were identified who were diagnosed in either the Pre-COVID (n = 18,673; 78.06%) or COVID (n = 5249; 21.94%) years. The overall cohort had a median age of 65 years [interquartile range (IQR), 60–71]. The majority of patients were male (n = 18,262; 76.34%), White (n = 17,733; 74.13%), and non-Hispanic (n = 20,492; 85.66%). In terms of socioeconomic factors, the majority of patients had Medicare insurance (n = 12,544; 52.44%), an estimated income of ≥ $74,063 (n = 7316; 30.58%) and resided in metropolitan areas (n = 20,608; 86.15%). Patients were mostly managed at academic centers (n = 14,253; 59.58%) and in the Southern region of the US (n = 9542; 39.89%).
Comparing Baseline Characteristics of Patients Diagnosed in Pre-COVID and COVID Periods
There were significantly fewer patients diagnosed during COVID (n = 5249) than the years prior (2017: n = 5969, 2018: n = 6304, and 2019: n = 6400) (p < 0.0001). When comparing patients diagnosed during the COVID year with those in the Pre-COVID years, there was a significant difference in the proportion of patients diagnosed at ages greater than 65 years (52.96% vs. 46.92%, p < 0.0001), respectively (Table 1). There was no significant difference in terms of sex, race, or ethnicity between patients diagnosed in either time period. In terms of socioeconomic status, there was a significant difference in the proportion of patients with Medicare insurance when comparing patients diagnosed during the COVID year with those in Pre-COVID years (55.44% vs. 51.59%, p < 0.0001); no difference was seen in terms of estimated annual income or education status. There was a significant difference in the proportion of patients diagnosed with stage IV disease when comparing the COVID year with Pre-COVID years (16.90% vs. 13.91%, p < 0.0001).
Table 1.
Comparison of demographic and clinical characteristics of the Pre-COVID and COVID periods
| Pre-COVID n=18,673 |
COVID n=5249 |
P-value | |
|---|---|---|---|
| Age | |||
| ≤ 65 years | 9911 (53.08%) | 2469 (47.04%) | < 0.0001 |
| > 65 years | 8762 (46.92%) | 2780 (52.96%) | |
| Sex | |||
| Male | 14,236 (76.24%) | 4026 (76.70%) | 0.4866 |
| Female | 4437 (23.76%) | 1223 (23.30%) | |
| Race | |||
| White | 13,825 (74.04%) | 3908 (74.45%) | 0.4565 |
| Black | 2702 (14.47%) | 770 (14.67%) | |
| Other | 2146 (11.49%) | 571 (10.88%) | |
| Ethnicity | |||
| Non-Hispanic | 16,028 (85.84%) | 4464 (85.04%) | 0.1489 |
| Hispanic | 2645 (14.16%) | 785 (14.96%) | |
| Insurance status | |||
| Private | 4972 (26.63%) | 1296 (24.69%) | < 0.0001 |
| Medicare | 9634 (51.59%) | 2910 (55.44%) | |
| Uninsured, medicaid, other government | 4067 (21.78%) | 1043 (19.87%) | |
| Incomea | |||
| < $46,277 | 4313 (23.10%) | 1173 (22.35%) | 0.3949 |
| $46,277–$57,856 | 4263 (22.83%) | 1237 (23.57%) | |
| $57,857–$74,062 | 4410 (23.62%) | 1210 (23.05%) | |
| ≥ $74,063 | 5687 (30.46%) | 1629 (31.03%) | |
| Educationb | |||
| ≥ 15.3% | 5861 (31.39%) | 1596 (30.41%) | 0.2779 |
| 9.1%–15.2% | 5599 (29.98%) | 1626 (30.98%) | |
| 5.0%–9.0% | 4546 (24.35%) | 1250 (23.81%) | |
| < 5.0% | 2667 (14.28%) | 777 (14.80%) | |
| Residence | |||
| Metro | 16,102 (86.23%) | 4506 (85.84%) | 0.4739 |
| Non-metro | 2571 (13.77%) | 743 (14.16%) | |
| Travel distance | |||
| ≤ 14.8 miles | 9292 (49.76%) | 2678 (51.02%) | 0.1074 |
| > 14.8 miles | 9381 (50.24%) | 2571 (48.98%) | |
| Facility location | |||
| Northeast | 3453 (18.49%) | 944 (17.98%) | 0.0068 |
| Midwest | 3701 (19.82%) | 1055 (20.10%) | |
| South | 7361 (39.42%) | 2181 (41.55%) | |
| West | 4158 (22.27%) | 1069 (20.37%) | |
| Facility type | |||
| Community | 4696 (25.15%) | 1420 (27.05%) | 0.0003 |
| Integrated cancer network | 2724 (14.59%) | 829 (15.79%) | |
| Academic | 11,253 (60.26%) | 3000 (57.15%) | |
| Clinical stage | |||
| Stage I | 7619 (40.80%) | 2047 (39.00%) | < 0.0001 |
| Stage II | 4570 (24.47%) | 1215 (23.15%) | |
| Stage III | 3887 (20.82%) | 1100 (20.96%) | |
| Stage IV | 2597 (13.91%) | 887 (16.90%) | |
| Comorbidities | |||
| None | 8709 (46.64%) | 2411 (45.93%) | 0.3363 |
| 1 | 4071 (21.80%) | 1194 (22.75%) | |
| 2 or more | 5893 (31.56%) | 1644 (31.32%) | |
aIncome is the median household income for the patient’s area of residence, serving as an estimate for financial status based on 2020 American Community Survey data.
bEducation is the percentage of adults who live in the patient’s area of residence at the time of diagnosis who did not graduate from high school, serving as an estimate of patient education status based on 2020 American Community Survey data
Comparing Treatment Modalities in Pre-COVID and COVID Periods
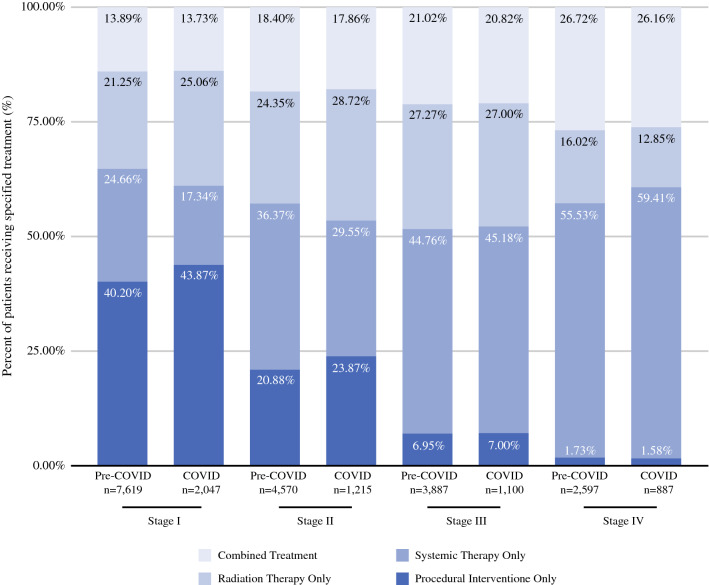
In stage I HCC, a greater proportion of patients that were diagnosed during the COVID year underwent radiation therapy (25.06% vs. 21.25%; p < 0.0001) and a lesser proportion received systemic therapy (17.34% vs. 24.66%; p < 0.0001) when compared with those diagnosed during Pre-COVID years (Fig. 2). Similar trends were seen in stage II HCC when comparing the COVID year with the Pre-COVID years (radiation therapy: 28.72% vs. 24.35%) (systemic therapy: 29.55% vs. 36.37%). Minimal differences were seen in patients undergoing ablation or resection for stage I and II HCC. For later stage patients (stages III and IV), no significant differences were found amongst treatment strategies.
Fig. 2.
Stage at diagnosis and treatment received compared between the Pre-COVID and COVID periods
Time to Treatment Initiation Based on Clinical Stage at Diagnosis
In the overall cohort, the median interval to any treatment was slightly shorter for patients diagnosed during the COVID year compared with those during Pre-COVID years (49 vs. 51 days; p < 0.0001) (Table 2). For TTI for any treatment modality based on clinical stage at diagnosis, the median time interval was shorter for patients diagnosed with stage I HCC during the COVID year than for those during Pre-COVID years (53 vs. 56 days; p = 0.0235). For patients diagnosed with stage II, III, and IV HCC, the median TTI did not significantly differ between those diagnosed during the COVID and Pre-COVID years.
Table 2.
Time to treatment initiation of any treatment modality based on clinical stage at diagnosis comparing Pre-COVID and COVID periods
| Pre-COVID (Median, IQR) | COVID (Median, IQR) | P-value | |
|---|---|---|---|
| TTI for all patients (days) n=23,922 | 51 (30–83) | 49 (29–78) | < 0.0001 |
| TTI for stage I (days) n=9666 | 56 (33–88) | 53 (33–82) | 0.0235 |
| TTI for stage II (days) n=5785 | 58 (35–92) | 57 (34–91) | 0.1538 |
| TTI for stage III (days) n=4987 | 48 (28–77) | 45 (28–76) | 0.1186 |
| TTI for stage IV (days) n=3484 | 36 (20–61) | 34 (21–56) | 0.1027 |
TTI: Time to treatment initiation.
Time to Treatment Initiation Based on Treatment Modality
In the overall cohort, TTI for any of the included treatment modalities was slightly shorter for patients diagnosed during the COVID year compared with those during Pre-COVID (49 vs. 51 days; p < 0.0001) (Table 3). For TTI based on the treatment modality first received, the median time from diagnosis to treatment was shorter for patients diagnosed during the COVID year compared with those during Pre-COVID for the following treatments: ablation (52 vs. 55 days; p = 0.0238), systemic therapy (42 vs. 47 days; p < 0.0001), and radiation (60 vs. 62 days; p = 0.0177). For patients who first received liver resection as part of their treatment course, there was no difference in TTI between the COVID and Pre-COVID years (41 vs. 41 days; p = 0.6887).
Table 3.
Time to treatment initiation based on the first received treatment modality, comparing Pre-COVID and COVID periods
| Pre-COVID (Median, IQR) | COVID (Median, IQR) | P-value | |
|---|---|---|---|
| TTI for any first received treatment modalitya (days) n=23,922 | 51 (30–83) | 49 (29–78) | < 0.0001 |
| TTI for ablation as first-line treatment (days) n=3462 | 55 (34–89) | 52 (31–80) | 0.0238 |
| TTI for liver resection as first-line treatment (days) n=2796 | 41 (17–66) | 41 (19–67) | 0.6887 |
| TTI for systemic therapy as first-line treatmentb (days) n=10,850 | 47 (27–78) | 42 (25–70) | < 0.0001 |
| TTI for radiation therapy as first–line treatment (days) n=7078 | 62 (41–92) | 60 (38–90) | 0.0177 |
TTI: Time to treatment initiation
aAny first received treatment modality refers to any of the following treatments: ablation, liver resection, systemic therapy, and radiation therapy
bSystemic therapy consists of either or both chemotherapy and immunotherapy treatments
Sub-Analysis of Patients Who Received Liver Transplantation
The study conducted a sub-analysis including patients who received liver transplantation and compared the findings when these patients were excluded. In the total cohort of 25,182 patients, 414 (1.64%) patients received liver transplantation as their first-line therapy. In terms of TTI for patients who first received liver transplantation, patients diagnosed in the COVID year had shorter median time intervals compared with those diagnosed in Pre-COVID years (64 vs. 121 days; p = 0.001). No difference was seen amongst the baseline characteristics and TTI of the general cohort.
Factors Associated with Time to Treatment Initiation
In the multivariate analysis, several patient and hospital related factors were found to be associated with increased TTI (Table 4). The incidence rate ratio (IRR) of increased TTI was higher for patients of Black race [vs. White: 1.057; 95% confidence interval (CI): 1.022–1.093; p = 0.0013] and Hispanic ethnicity (vs. non-Hispanic: 1.045; 95% CI: 1.010–1.081; p = 0.0104). Patients that were non-privately insured were also found to have increased TTI (Medicare vs. Private: 1.047; 95% CI: 1.017–1.078; p = 0.0023; and uninsured/Medicaid/Other Government insurance vs. Private: 1.088; 95% CI: 1.053–1.123, p < 0.0001). Furthermore, patients of lower income at ranges of $57,857–$74,062, $46,277–$57,856, and < $46,277 were found to be at a steadily increasing risk of increased TTI (vs. ≥ $74,063: 1.034; 95% CI, 1.001–1.068, p = 0.0413), (vs. ≥ $74,063: 1.067; 95% CI, 1.029–1.105, p = 0.0004), and (vs. ≥ $74,063: 1.085; 95% CI, 1.042–1.130, p < 0.0001, respectively).
Table 4.
Factors associated with time to treatment initiation for all clinical stages of hepatocellular carcinoma
| Incidence rate ratio (95% CI) | P-value | |
|---|---|---|
| Age | ||
| ≤ 65 years | Reference | |
| > 65 years | 0.948 (0.923–0.973) | < 0.0001 |
| Sex | ||
| Male | Reference | |
| Female | 0.989 (0.964–1.015) | 0.4112 |
| Race | ||
| White | Reference | |
| Black | 1.057 (1.022–1.093) | 0.0013 |
| Other | 0.946 (0.912–0.980) | 0.0023 |
| Ethnicity | ||
| Non-Hispanic | Reference | |
| Hispanic | 1.045 (1.010–1.081) | 0.0104 |
| Insurance status | ||
| Private | Reference | |
| Medicare | 1.047 (1.017–1.078) | 0.0023 |
| Uninsured, medicaid, other government | 1.088 (1.053–1.123) | < 0.0001 |
| Incomea | ||
| ≥ $74,063 | Reference | |
| $57,857–$74,062 | 1.034 (1.001–1.068) | 0.0413 |
| $46,277–$57,856 | 1.067 (1.029–1.105) | 0.0004 |
| < $46,277 | 1.085 (1.042–1.130) | < 0.0001 |
| Educationb | ||
| < 5.0% without HSD | Reference | |
| 5.0%–9.0% without HSD | 1.015 (0.978–1.054) | 0.4308 |
| 9.1%–15.2% without HSD | 1.044 (1.004–1.086) | 0.0293 |
| ≥ 15.3% without HSD | 1.039 (0.994–1.085) | 0.0886 |
| Residence | ||
| Metro | Reference | |
| Non-metro | 0.981 (0.947–1.016) | 0.2891 |
| Travel distance | ||
| ≤ 14.8 miles | Reference | |
| > 14.8 miles | 1.041 (1.015–1.067) | 0.0015 |
| Facility location | ||
| Northeast | Reference | |
| Midwest | 0.991 (0.956–1.028) | 0.6255 |
| South | 1.031 (0.999–1.065) | 0.0582 |
| West | 1.218 (1.175–1.262) | < 0.0001 |
| Facility type | ||
| Community | Reference | |
| Integrated cancer network | 1.034 (0.998–1.072) | 0.0677 |
| Academic | 1.119 (1.089–1.150) | < 0.0001 |
| Clinical stage at diagnosis | ||
| Stage I | Reference | |
| Stage II | 1.043 (1.015–1.073) | 0.0028 |
| Stage III | 0.863 (0.838–0.889) | < 0.0001 |
| Stage IV | 0.691 (0.669–0.715) | < 0.0001 |
| Comorbidities | ||
| None | Reference | |
| 1 | 0.999 (0.972–1.028) | 0.9655 |
| 2 or more | 1.006 (0.981–1.032) | 0.6255 |
| Year of diagnosis | ||
| Pre-COVID | Reference | |
| COVID | 0.944 (0.919–0.969) | < 0.0001 |
aIncome is the median household income for the patient’s area of residence, serving as an estimate for financial status based on 2020 American Community Survey data
bEducation is the percentage of adults who live in the patient’s area of residence at time of diagnosis who did not graduate from high school, serving as an estimate of patient education status based on 2020 American Community Survey data
For hospital-related factors, patients managed at academic centers (vs. community: 1.119, 95% CI, 1.089–1.150, p < 0.0001) and in hospitals located in the Western regions of the US (vs. Northeast: 1.218, 95% CI, 1.175–1.262, p < 0.0001) were likely to have increased TTI. When comparing patients and year of diagnosis, TTI was decreased for patients diagnosed during the COVID year (vs. Pre-COVID years: 0.944; 95% CI, 0.919–0.969, p < 0.0001).
Factors Associated with Delayed Treatment
Several patient and hospital related factors were found to be associated with delayed treatment (> 90 days) (Table 5). The odds ratio (OR) of delayed treatment was higher for patients of Black race (vs. White: 1.172; 95% CI: 1.065–1.290; p = 0.0012) and Hispanic ethnicity (vs. non-Hispanic: 1.121; 95% CI: 1.023–1.228; p = 0.0141). Non-privately insured patients were more likely to have delayed treatment (Medicare vs. Private: 1.127; 95% CI: 1.032–1.230; p = 0.0079) (uninsured/Medicaid/Other Government insurance vs. Private: 1.356; 95% CI: 1.236–1.487, p < 0.0001). Furthermore, patients of lower income were found to be at a steadily increasing risk of delayed treatment.
Table 5.
Factors associated with delayed treatment time for all clinical stages of hepatocellular carcinoma
| Odds ratio (95% CI) | P-value | |
|---|---|---|
| Age | ||
| ≤ 65 | Reference | |
| > 65 | 0.874 (0.810–0.943) | 0.0005 |
| Race | ||
| White | Reference | |
| Black | 1.172 (1.065–1.290) | 0.0012 |
| Other | 0.911 (0.820–1.013) | 0.0863 |
| Ethnicity | ||
| Non-Hispanic | Reference | |
| Hispanic | 1.121 (1.023–1.228) | 0.0141 |
| Insurance status | ||
| Private | Reference | |
| Medicare | 1.127 (1.032–1.230) | 0.0079 |
| Uninsured, Medicaid, Other Government | 1.356 (1.236–1.487) | < 0.0001 |
| Incomea | ||
| ≥ $74,063 | Reference | |
| $57,857–$74,062 | 1.144 (1.045–1.252) | < 0.0001 |
| $46,277–$57,856 | 1.320 (1.204–1.447) | < 0.0001 |
| < $46,277 | 1.385 (1.260–1.522) | 0.0037 |
| Travel distance | ||
| ≤ 14.8 miles | Reference | |
| > 14.8 miles | 1.077 (1.006–1.153) | 0.0334 |
| Facility location | ||
| Northeast | Reference | |
| Midwest | 0.920 (0.823–1.028) | 0.1406 |
| South | 1.115 (1.012–1.229) | 0.0282 |
| West | 1.771 (1.595–1.965) | < 0.0001 |
| Facility type | ||
| Community | Reference | |
| Integrated cancer network | 1.093 (0.978–1.221) | 0.1182 |
| Academic | 1.371 (1.263–1.488) | < 0.0001 |
| Stage | ||
| Stage I | Reference | |
| Stage II | 1.148 (1.064–1.239) | 0.0004 |
| Stage III | 0.700 (0.641–0.765) | < 0.0001 |
| Stage IV | 0.414 (0.367–0.466) | < 0.0001 |
| Year of diagnosis | ||
| Pre-COVID | Reference | |
| COVID | 0.896 (0.828–0.970) | 0.0065 |
aIncome is the median household income for the patient’s area of residence, serving as an estimate for financial status based on 2020 American Community Survey data
Discussion
At the onset of the COVID-19 pandemic, access to cancer care was abruptly interrupted for many patients with both new and current diagnoses. This study is one of the first to conduct an annual nation-wide analysis on the impact of the pandemic and TTI in patients with clinical stages I–IV HCC as well as to evaluate patient and hospital factors predictive of increased treatment time intervals. We found a 12.2% drop in the overall number of patients diagnosed with HCC in 2020 compared with prior years. However, of those patients who did present with HCC, we observed no clinically significant differences in TTI for first-line therapies during the COVID year compared with pre-COVID years, although the median TTI was statistically significantly less during the COVID year. These findings suggest that amidst a national—and global—emergency, the US healthcare system was able to maintain the initialization of cancer care delivery in patients with HCC.
While this study is unable to identify the exact mechanisms that buffered the impact of the COVID-19 pandemic on delivery times of HCC therapies in the US, there are several possibilities. At the onset of the pandemic, national societies released recommendations that urged providers to continue cancer treatment efforts while accounting for hospital resource availability. The American College of Surgeons, while recommending curtailing “non-emergent” procedures, provided specific guidelines for the triage of cancer surgery patients and emphasized the importance of balancing the risk of delayed treatment with the availability of institutional resources.26,27
For HCC specific recommendations, the American Association for the Study of Liver Diseases (AASLD) recommended providers to avoid treatment delays in patients diagnosed with HCC if possible.28 In terms of new referrals for HCC evaluation and diagnosis, providers were encouraged to review imaging at virtual multidisciplinary tumor board conferences prior to scheduling in-patient visits to limit potential exposures. The Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) in collaboration with the Americas Hepato-Pancreato-Biliary Association (AHPBA) offered alternative or “next-best” HCC treatment strategies using a phase-based approach that consisted of three phases and accounted for both the number of COVID-19 patients in the hospital and the availability of ventilators and beds in the intensive care unit (ICU).29 For hospitals in the “emergent”—or severest—phase, where most ICU resources were directed to COVID-19 patients, providers were recommended to offer patients transarterial chemoembolization, ablation, or observation if unable to provide definitive treatment for early stage HCC. The Society of Surgical Oncology (SSO) echoed similar recommendations.30 The judicious efforts of national leadership to limit delay to HCC care may have contributed to the minimal differences seen in time to treatment between the Pre-COVID and COVID cohorts, especially in terms of liver resection.
Our findings are similarly reflected in institutional studies of other cancers that have investigated the impact of the COVID-19 pandemic on timing of cancer care delivery. A single-institution study revealed non-statistically significant differences in TTI for patients diagnosed with colorectal cancer prior to and during the pandemic.31 The authors attributed the lack of difference in TTI to the fact that their hospital was an academic referral center that continued treatment services during the pandemic. A multicenter study echoed similar findings when comparing times to surgery for breast cancer but, on further analysis, revealed significant variation amongst each center included in the hospital system.32 While our study did not evaluate hospitals individually, we did find that patients treated at academic facilities were at risk for increased TTI as well as more likely to have delayed treatment. This may be due to academic centers being utilized as centers for severe COVID-19 infections and consequentially re-directing many resources to addressing the urgent and emergent needs of the patient population.
Interestingly, our study demonstrated a slightly shorter TTI for patients who first received systemic therapy—as well as radiation—and were diagnosed during COVID, compared with those diagnosed during Pre-COVID. This may be due to the fact that these services are offered in an outpatient setting and do not require hospital admission for treatment administration. In addition, national guidelines recommended alternative administration approaches for systemic therapies to allow for the continuance of these therapies while reducing the risk of COVID-19 exposure. For example, immunotherapy—common in the management of intermediate and later-stage HCC—could be administered at higher doses and reduced frequencies to maintain similar pharmacokinetics as standard dosing.33 Patients receiving chemotherapy could be transitioned from intravenous to oral agents as well as be scheduled for a 2-week break or arranged for infusion at a satellite facility.34 These alternative approaches to how treatment was administered may have contributed to the slightly expedited treatment times seen in our study. In addition, our study did not investigate the impact of the pandemic on screening practices and whether delayed screening translated to later-stage diagnoses, which could have played a role in expedited systemic treatments. Future studies must investigate the effects of the pandemic on HCC screening to further contextualize the slightly shorter TTI for systemic therapy that we saw in our study.
Most concerning, our logistic regression analyses revealed that patients who were Black, Hispanic, of lower income status, and of non-private insurance were at risk of increased TTI and delayed treatment of greater than 90 days. Our results are concordant with those of prior studies that have demonstrated racial and socioeconomic disparities in the time to treatment of HCC, where Black and low-income patients with HCC were more likely to experience treatment delays.35 In addition, Black, Hispanic, and underinsured/uninsured patients were less likely to undergo curative therapy for HCC.36,37 Our data suggest that the pandemic further exacerbated these inequalities. When evaluating COVID-19 outcomes, these same vulnerable populations were found to have decreased access to clinical care and increased COVID-19 positivity risk, specifically for Black and Hispanic patients.38 Given that the pandemic disproportionately affected patients of minority groups and lower socioeconomic status, future studies must investigate how cancer care delivery—from screening to receipt of treatment—was impacted by the pandemic in underserved populations.
Our study is not without limitations. The NCDB solely collects patient information from CoC-accredited hospitals in the US, limiting the generalizability of our results and introducing the risk of hospital-based sampling bias. In addition, the NCDB provides diagnosis time in terms of years rather than months, leading to our annual-based analysis. Given that the COVID-19 pandemic was announced in March of 2020, our “COVID” cohort captures patients diagnosed with HCC prior to the onset of the pandemic.2 However, the first case of COVID-19 in the US was documented in January of 2020, and many social-distancing practices that would impede access to cancer care were already implemented earlier in the year of 2020.39 Furthermore, the NCDB does not capture information regarding transarterial chemoembolization, a treatment that was recommended as an alternative regimen for hospitals with limited resources. Lastly, while our study offers insight into TTI during and prior to the pandemic, it does not provide information on how HCC screening practices were impacted during the pandemic. Despite these limitations, we have been able to conduct a national annual assessment of time to treatment in HCC for therapies during Pre-COVID and COVID time periods and offer insight into vulnerable populations that may have incurred the negative effects of the pandemic on their cancer treatment delivery.
Conclusion
While the COVID-19 pandemic impeded the cancer care delivery efforts for many other cancers, we found no clinically significant differences in TTI for patients with HCC who were diagnosed during the first COVID year of 2020. However, vulnerable populations were identified to be at risk of increased TTI during this time. Given the disproportionate impact of the pandemic on underserved populations, we will need to identify how to limit the impact of future states of emergency on cancer care delivery, especially in vulnerable populations.
Appendix
The following patient demographics were collected for this study: median age (≤ 65 years, > 65 years), sex (male, female), race (Black, White, other), and ethnicity (non-Hispanic, Hispanic). In terms of race, the NCDB provided a variable named “Race” that details the primary race of each collected patient. The “other” category includes the following racial identities: Aleutian, American Indian, Asian Indian, Cambodian, Chamorran, Chinese, Eskimo, Fiji Islander, Filipino, Guamanian, Hawaiian, Hmong, Japanese, Kampuchean, Khmer, Korean, Laotian, Melanesian, Micronesian, New Guinean, Pakistani, Polynesian, Samoan, Tahitian, Thai, Tongan, and Vietnamese. The “other” category also includes patients identified as “Other Asian, including Asian not otherwise specified and Oriental not otherwise specified,” “Pacific Islander not otherwise specified,” and “Other.” The variable “ethnicity” was dichotomized as “Non-Hispanic” and “Hispanic.” Patients of Hispanic origin were defined as patients who had the following origins: Central American, Chicano, Cuban, Dominican Republican, Hispanic not otherwise specified, Latino not otherwise specified, Mexican, Puerto Rican, South American (except Brazil), Spanish not otherwise specified, and other specified Spanish/Hispanic origin (includes European). Patients were also considered to have Hispanic origin if they had a Spanish surname or maiden name with no contrary evidence that the patient is not Hispanic. Comorbidities were defined based on the Charlson-Deyo comorbidity score and were grouped as no comorbidities, one comorbidity, and greater than or equal to 2 comorbidities.20
Socioeconomic factors collected included insurance status (Private, Medicare, Medicaid/uninsured/other government), median annual income (categorized into quartiles as provided by the NCDB), educational attainment (categorized into quartiles as provided by the NCDB), and area of residency (metro, non-metro). Area of residency is an NCDB-provided variable that estimates the rurality and urban influence of a patient’s residence by matching patient-reported residence zip code with typology files published in 2013 by the United States Department of Agriculture Economic Research Service.20 Hospital-specific factors included median travel distance (≤ 14.8 miles, > 14.8 miles), facility type (academic, community, integrated cancer network), and facility regional location (Northeast, Midwest, South, West). Median travel distance was derived from the “crowfly” variable, where distance is calculated between the patient’s reported zip code at time of diagnosis and hospital location.20 Estimated annual income is a variable by provided by the NCDB and determined by matching the patient’s reported zip code at time of diagnosis with 2020 American Community Survey data files (2016-2020).20 Educational attainment was provided by the NCDB and is defined as an estimate of the proportion of adults who live in the same zip code as the patient who have not graduated from high school. These estimates are made by cross-referencing the patient’s zip code with 2020 American Community Survey data files (2016–2020).20
Author Contribution
Each author meets authorship criteria per the guidelines of the International Committee of Medical Journal Editors (ICMJE) and has participated sufficiently in the manuscript to take public responsibility for appropriate portions of the content.
Funding
Sophie H. Chung and Kelsey S. Romatoski are supported by the Health Resources Services Administration’s (HRSA) National Research Service Award, grant number T32HP10028. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the supporting institution.
Disclosure
The authors have no conflicts of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization Coronavirus (COVID-19) Dashboard. 2022. https://covid19.who.int/. Accessed 11 Nov 2022.
- 2.Centers for Disease Control and Prevention. 2022. https://www.cdc.gov/museum/timeline/covid19.html. Accessed 8 Nov 2022.
- 3.Bhangu A. Elective surgery cancellations due to the COVID-19 pandemic: global predictive modelling to inform surgical recovery plans: elective surgery during the SARS-CoV-2 pandemic. Br J Surg. 2020 doi: 10.1002/bjs.11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spinelli A, Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020 doi: 10.1002/bjs.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American College of Surgeons. COVID-19: recommendations for management of elective surgical procedures. 2020. https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-surgery/. Accessed 11 Nov 2022.
- 6.Smith WR, Atala AJ, Terlecki RP, Kelly EE, Matthews CA. Implementation guide for rapid integration of an outpatient telemedicine program during the COVID-19 pandemic. J Am Coll Surg. 2020 doi: 10.1016/j.jamcollsurg.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiang YT, Yang Y, Li W, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leo CG, Sabina S, Tumolo MR, et al. Burnout among healthcare workers in the COVID 19 era: a review of the existing literature. Front Public Health. 2021 doi: 10.3389/fpubh.2021.750529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clin Cancer Inform. 2020 doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020 doi: 10.1200/CCI.20.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Cancer Society Cancer Action Network. COVID-19 pandemic impact on cancer patients and survivors survey findings summary. 2020. https://www.fightcancer.org/sites/default/files/National%20Documents/Survivor%20Views.COVID19%20Polling%20Memo.Final. Accessed 12 Nov 2022.
- 12.Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat. 2020 doi: 10.1007/s10549-020-05828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson BA, Waddimba AC, Ogola GO, Fleshman JW, Jr, Preskitt JT. A systematic review and meta-analysis of surgery delays and survival in breast, lung, and colon cancers: implication for surgical triage during the COVID-19 pandemic. Am J Surg. 2021 doi: 10.1016/j.amjsurg.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ. 2020 doi: 10.1136/bmj.m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021 doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 16.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008 doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Surgeons. About the national cancer database. https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/about/. Accessed 8 Nov 2022.
- 18.Edge SB, Compton CC. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 19.Amin MB, Gress DM. AJCC cancer staging manual. 8. New York: Springer; 2017. [Google Scholar]
- 20.American College of Surgeons. National cancer database participant user file. 2022. https://www.facs.org/media/brilfbgu/puf-2020-data-dictionary.pdf Accessed 8 Nov 2022.
- 21.Trieu JA, Bilal M, Hmoud B. Factors associated with waiting time on the liver transplant list: an analysis of the united network for organ sharing (UNOS) database. Ann Gastroenterol. 2017 doi: 10.20524/aog.2017.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman RB, Wiesner RH, Edwards E, et al. Results of the first year of the new liver allocation plan. Liver Transplant. 2004 doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992 doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Singal AG, Waljee AK, Patel N, et al. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J Natl Compr Cancer Netw. 2013;11(9):1101–1108. doi: 10.6004/jnccn.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao A, Rich NE, Marrero JA, Yopp AC, Singal AG. Diagnostic and therapeutic delays in patients with hepatocellular carcinoma. J Natl Compr Cancer Netw. 2021;19(9):1063–1071. doi: 10.6004/jnccn.2020.7689. [DOI] [PubMed] [Google Scholar]
- 26.American College of Surgeons. COVID-19: elective case triage guidelines for surgical care. 2020. https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case/. Accessed 4 Dec 2022.
- 27.American College of Surgeons. COVID-19 guidelines for triage of cancer surgery patients. 2020. https://www.facs.org/for-medical-professionals/covid-19/clinical-guidance/elective-case/cancer-surgery/.Accessed 4 Dec 2022.
- 28.Fix OK, Hameed B, Fontana RJ, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020 doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Society of American Gastrointestinal and Endoscopic Surgeons. SAGES–AHPBA recommendations regarding surgical management of HPB cancer patients during the response to the COVID-19 crisis. 2020. https://www.sages.org/sages-ahpba-recommendations-surgical-management-of-hpb-cancer-COVID-19/ Accessed 12 Nov 2022.
- 30.Society of Surgical Oncology. Resource for management options of GI and HPB cancers during COVID-19. 2020. https://www.surgonc.org/wp-content/uploads/2020/04/GI-and-HPB-Resource-during-COVID-19-4.6.20. Accessed 12 Nov 2022.
- 31.Elamin D, Ozgur I, Steele SR, et al. Impact of COVID-19 pandemic on treatment of colorectal cancer patients. Am J Surg. 2023 doi: 10.1016/j.amjsurg.2023.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escobar N, DiMaggio C, Pocock B, et al. Effects of COVID-19 on surgical delays in patients with breast cancer in NYC public hospitals: a multicenter study. Ann Surg Oncol. 2023;30(1):23–30. doi: 10.1245/s10434-022-12491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segelov E, Underhill C, Prenen H, et al. Practical considerations for treating patients with cancer in the COVID-19 pandemic. JCO Oncol Pract. 2020 doi: 10.1200/OP.20.00229. [DOI] [PubMed] [Google Scholar]
- 34.American Society of Clinical Oncology. Cancer treatment & supportive care. (2020). https://old-prod.asco.org/covid-resources/patient-care-info/cancer-treatment-supportive-care. Accessed 8 Dec 2022.
- 35.Wagle NS, Park S, Washburn D, et al. Racial, ethnic, and socioeconomic disparities in treatment delay among patients with hepatocellular carcinoma in the United States. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobotka LA, Hinton A, Conteh LF. Insurance status impacts treatment for hepatocellular carcinoma. Ann Hepatol. 2019 doi: 10.1016/j.aohep.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Sonnenday CJ, Dimick JB, Schulick RD, Choti MA. Racial and geographic disparities in the utilization of surgical therapy for hepatocellular carcinoma. J Gastrointest Surg. 2007 doi: 10.1007/s11605-007-0315-8. [DOI] [PubMed] [Google Scholar]
- 38.Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic review and meta-analysis. JAMA Netw Open. 2021 doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel Coronavirus in the United States. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]