Abstract
Brugada syndrome is an inherited channelopathy with an increased risk of sudden cardiac death (SCD) due to ventricular arrhythmias (VA) and an increased incidence of supraventricular arrhythmias, as compared with the general population. For the prevention of SCD, the guidelines recommend the implantable cardioverter-defibrillator (ICD); however, ICD does not prevent VA. In this article, we provide a brief review of the literature on the Brugada syndrome pharmacological therapy, mainly focusing on quinidine treatment. The efficacy of quinidine therapy in the prevention of VA in Brugada syndrome has been demonstrated by several small studies in patients with ICD and recurrent shocks or in asymptomatic patients with inducible ventricular fibrillation (VF) at electrophysiological study. Quinidine has also been tested for the prophylaxis of supraventricular arrhythmias, especially atrial fibrillation/flutter, and in paediatric patients. In these studies, quinidine proved highly effective in preventing re-induction of VF and spontaneous recurrences of both ventricular and supraventricular arrhythmias. Unfortunately, this therapy is burdened by a high incidence of side effects, which may lead to drug discontinuation.
Keywords: Brugada syndrome, Quinidine, Ventricular arrhythmias, Sudden cardiac death, Atrial fibrillation
Brugada syndrome is an inherited channelopathy with an increased risk of sudden cardiac death (SCD) due to ventricular arrhythmias (VA).1 An increased incidence of supraventricular arrhythmias, such as atrial fibrillation (AF), atrio-ventricular (AV) nodal re-entrant tachycardia, and AV reciprocating tachycardia due to an accessory pathway has also been reported.2
The 2022 ESC guidelines on VA3 recommend for all Brugada patients some behavioural rules such as avoidance of drugs that may induce ST-segment elevation in the right precordial leads (www.brugadadrugs.org), cocaine, cannabis, excessive alcohol intake, and prompt treatment of fever with anti-pyretic drugs.4 Moreover, the current guidelines recommend implantable cardioverter-defibrillator (ICD) as the only therapy (class I) in patients who are survivors of an aborted SCD or have documented spontaneous sustained ventricular tachycardia (VT) and in patients with type 1 Brugada pattern and an arrhythmic syncope (class IIa).3
However, ICD does not prevent VA, thus the patient remains exposed to the stress of receiving one or multiple shocks. ICD has an impact on quality of life, especially in young patients, and can be associated with significant complications, such as infections, lead failure, and even risk of death in case the electrode needs to be removed.5 Recently, subcutaneous ICD has partially reduced the problems related to transvenous implantation, but not all patients are eligible. Ablation of the arrhythmic substrate6 and pharmacological treatment with quinidine might be alternative options, especially in asymptomatic patients with spontaneous type 1 ECG pattern, but their long-term efficacy and safety are still unclear.
The 2022 ESC guidelines on VA consider the use of quinidine only to prevent arrhythmic recurrences and multiple ICD shocks or as primary prevention in the case of patients who refuse or are ineligible for ICD.3 The reason is probably that the studies addressing the efficacy of quinidine therapy in preventing VA in Brugada patients are small and mainly non-randomized.
The first observation suggesting the efficacy of quinidine comes from Belhassen et al.,7 who, in 2004, in a prospective study used quinidine bisulphate (mean dose 1483 ± 240 mg) in 25 Brugada patients (24 men, 1 woman; age 19–80 years) with inducible ventricular fibrillation (VF) at baseline electrophysiological study (EPS). That cohort included 15 symptomatic (seven cardiac arrest survivors, seven patients with unexplained syncope, and one with symptomatic VT) and 10 asymptomatic patients. Quinidine prevented VF re-induction in 22 of the 25 patients (88%) and there were no arrhythmic events at a follow-up of 6 months to 22 years. However, 36% of patients showed side effects that resolved after drug discontinuation.
In the same period, Hermida et al.8 reported on 35 subjects (32 men; mean age 48 ± 11 years) who received hydroquinidine (HQ) 600–900 mg/day. Thirty-one had asymptomatic Brugada ECG pattern and inducible VA and four had multiple appropriate ICD shocks. HQ prevented VT/VF inducibility in 76% of asymptomatic patients who repeated EPS on therapy. Syncope occurred in two of the 21 patients who received long-term (17 ± 13 months) HQ therapy (one syncope associated with QT interval prolongation and one unexplained syncope associated with probable non-compliance). HQ prevented VT/VF recurrences in all patients with multiple ICD shocks at a mean follow-up of 14 ± 8 months. Side effects occurred in 14% of patients.
In 2009, Belhassen et al.9 published on the long-term efficacy of EPS-guided quinidine therapy in a small group of patients. Eight patients had a previous aborted cardiac arrest and one had recurrent syncope, for five patients clinical events were due to Brugada syndrome and for four to idiopathic VF. All patients had inducible sustained VF at baseline EPS that was prevented by quinidine therapy. All underwent another EPS on medication after 1.7–23.6 years (>5 years in eight patients) to ascertain persistent long-term drug efficacy in six patients and to elucidate the reasons for syncopal episodes during therapy in three. No sustained VA could be induced in any patient and no recurrent arrhythmic events were documented at 15 ± 7 years of follow-up.
Márquez et al.10 in 2012 published a retrospective analysis on six men with Brugada syndrome who received ≤600 mg/day of quinidine sulphate or HQ after ICD implantation. Quinidine was initiated after appropriate shocks, including arrhythmic storm in four cases. Quinidine prevented the recurrence of VA in all patients, without side effects, during a median follow-up of 4 years. Three subjects who discontinued the medication experienced VA recurrences afterwards, successfully treated by restarting quinidine.
In 2014, Bouzeman et al.11 evaluated the long-term efficacy and safety of an EPS-guided HQ therapy in 44 asymptomatic Brugada patients with inducible VF. HQ dosage was 600 mg/day, targeting a therapeutic range between 3 and 6 μmol/L. Of these, 34 (77%) were no more inducible and were maintained under HQ alone during a mean follow-up of 6 years. In this group, an ICD was eventually implanted in four patients (12%, two with syncope and two with major HQ intolerance), with the occurrence of appropriate ICD therapy in one out of the four. Among the other 10 patients (22%), who remained inducible and received an ICD, none received appropriate therapy during a mean follow-up of 7.7 + 2 years. The overall annual rate of arrhythmic events was 1.04% (95% CI: 0.00–2.21), without any significant difference according to the result of EPS under HQ. However, this study did not show any event in the patients receiving HQ therapy. The main problems were related to drug intolerance leading to drug discontinuation and to the possibility of low compliance.
In 2016, Anguera et al.12 conducted a nationwide observational survey and demonstrated the beneficial effects of quinidine as secondary prevention in symptomatic Brugada patients, all with an ICD. Twenty-nine patients were prescribed quinidine for recurrent VA, quinidine bisulphate in 10 (mean dose 591 ± 239 mg/day), and HQ in 19 (mean dose 697 ± 318 mg/day). After a mean period of 60 ± 41 months under quinidine treatment, 19 patients (66%) remained free of appropriate ICD discharges. Quinidine administration was associated with a significant reduction of ICD shocks. Predictors of recurrent shocks in the univariate analysis were a temporary discontinuation of quinidine, due to side effects or to a patient decision (HR 4.6; 95% CI 1.28–16.6) and the number of shocks prior to quinidine initiation (HR 1.13; 95% CI 1.01–1.26). The authors hypothesized that some patients may be relatively resistant to quinidine and may require higher doses to control VA.
The QUIDAM study by Probst et al.13 in 2017 was the first prospective randomized double-blind study. Fifty patients were enrolled, all implanted with an ICD. During the 36-month follow-up, one appropriate ICD shock (0.97% event per year) and one self-terminating VF occurred under placebo therapy. No arrhythmic events were reported under HQ therapy. However, a statistically significant difference compared with the placebo was not observed, mainly because of the very low incidence of arrhythmic events in the enrolled population. Moreover, 68% of patients presented HQ-related side effects, mainly gastrointestinal, which led to discontinuation of the therapy in 26%.
In a recent study, Mazzanti et al.14 compared the clinical course of 53 Brugada patients treated with HQ to that of 441 matched untreated controls and the annual incidence of arrhythmic events in 123 patients with aborted SCD (27 treated with HQ and 96 not treated). HQ, at a mean dosage of 439 ± 115 mg/day, reduced by 26% the overall risk of experiencing an arrhythmic event at a mean follow-up of 5 years. In the 123 patients with previous VA, the annual rate of recurrences decreased from 14.7% off-quinidine to 3.9% on-quinidine (HR 0.33; 95% CI 0.12–0.92). In this study, a lower mean dosage made the drug better tolerated, with only 6% of drug discontinuation, but at the expense of an incomplete protection from VA recurrences.
Quinidine therapy for supraventricular arrhythmias
The relation between Brugada ECG pattern and supraventricular arrhythmias, especially AF, has been reported with a highly variable prevalence, ranging from 11% to 39%.2,15 The use of class IC anti-arrhythmic drugs (AAD) in the prevention of supraventricular arrhythmias is contraindicated in Brugada patients because they can unmask the coved-type ST-segment elevation and pre-dispose to VA.4
In 2014, our group reported the prevalence of AF or atrial flutter (AFl) among 560 patients with Brugada type 1 ECG and analysed its correlation with prognosis and the efficacy of HQ treatment.16 Forty-eight patients (9%) had AF/AFl: in 23 patients the diagnosis of Brugada ECG pattern preceded that of AF/AFl (group 1), while in 25 patients AF/AFl were diagnosed first and the Brugada ECG pattern was seen only after administration of class IC AADs for cardioversion/prophylaxis (group 2). Nine patients in group 1 and nine in group 2 received HQ for AF/AFl prophylaxis and none had recurrences on therapy. Moreover, patients in group 1 were significantly younger than those in group 2 (mean age 47 ± 15 years vs. 59 ± 11 years) and had a worse prognosis, indeed AF in this group could be a marker of more advanced disease.
All these studies show that quinidine may be a useful alternative anti-arrhythmic therapy for supraventricular arrhythmias in Brugada patients.
Quinidine therapy in the paediatric Brugada population
It is well known that ICD implantation is associated with significant morbidity in children, therefore, quinidine may represent a good alternative in younger Brugada patients at risk for malignant arrhythmias, at least until adult age.
Probst et al.17 described 30 children and adolescents (<16 years, mean age 8 ± 5) with Brugada syndrome. Ten out of 11 patients with symptoms had spontaneous type 1 ECG pattern. VA generally occurred at rest, often during febrile episodes. An ICD was implanted in five children and therapy with quinidine was started in four. No VA occurred in patients treated with quinidine during a mean follow-up of 28 ± 24 months and the treatment was well tolerated. Moreover, 13% of these paediatric patients presented AF/AFl, which is very uncommon in children without structural heart disease, and without recurrences in HQ.
Andorin et al.18 in 2016 evaluated a population of 106 patients younger than 19 years (67 under the age of 15), 22 treated with ICD (21%). Hydroquinidine was started in 11 patients (10%): of these, eight (73%) had experienced an arrhythmic event and nine (82%) had spontaneous type 1 ECG pattern. No side effects were reported. Eight out of 11 (73%) patients receiving HQ remained free from VA during therapy. On the other hand, 41% of patients experienced ICD-related complications.
Quinidine mechanism of action
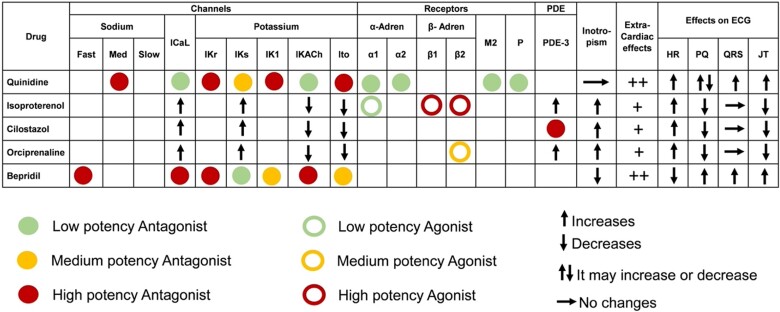
Quinidine, as well as its derivative HQ, has multi-channel blocking properties leading to very complex and still not completely unravelled electrophysiological effects, also depending on baseline conditions and cardiac ionic channel balance. Quinidine has been historically classified among the class I (membrane stabilizing), sub-group B (intermediate offset kinetics) AAD within the Vaughan Williams classification, because it blocks the rapid sodium channel (INa) in the activated state with a high relative blocking potency and an intermediate (200–1500 ms) time constant for recovery from block. Yet, this kind of classification can be misleading, since numerous other cardiac channels and receptors are affected by quinidine, well explaining its potential usage in different clinical settings, but also its potential for pro-arrhythmic effects (Figure 1).19 Quinidine is an at least moderate potency blocker of the transient outward potassium (K+) current (Ito), of the inward rectifier K+ current (IK1), and of both the component of the delayed rectifier K+ current (IKr and IKs). The drug also reduces the L-type calcium (ICa) and the late inward sodium (INa) currents, as well as being a low potency competitive and reversible antagonist of alfa-1 and alfa-2 adrenergic receptors. Finally, quinidine exerts prominent peripheral post-synaptic anti-cholinergic effects most likely due to the combination of a direct and competitive anti-muscarinic (M1 and M2) receptors blockage, and a direct inhibition of the muscarine receptor-activated K+ channel current (IKAch).20 Altogether, these complex molecular effects affect all phases of cardiac action potential (AP) and translate into a prominent change of AP duration and shape.21 Quinidine decreases the phase zero of rapid depolarization, prolongs early repolarization (phase 1), shortens the plateau (phase 2), and prolongs the late repolarization (phase 3), thereby leading to AP duration prolongation and a more triangular shape (an effect better known as AP triangulation). Notably, although quinidine prolongs AP duration, due to the relatively long kinetics of dissociation of the drug from INa, sodium channels are still largely blocked even after the restoration of resting membrane potential. This leads to a prolongation of the effective refractory period beyond AP duration (post-repolarization refractoriness).
Figure 1.
Main electrophysiological, clinical, and electrocardiographic effects of the anti-arrhythmic drugs most used (quinidine and isoproterenol) and tested (cilostazol, orciprenaline, bepridil) for Brugada syndrome, described in the text. Full and empty circles refer to the direct effect of the drug on the channel, receptor, or enzyme, while arrows refer to functional/indirect effects (e.g. as part of the intracellular cascade response to the drug). HR, heart rate; PDE, phosphodiesterase.
The most generally accepted pathophysiological interpretation of the Brugada syndrome is that the main arrhythmogenic mechanism is related to the local disruption of phase 1 of ventricular AP at the right ventricular epicardial level, due to a combination of reduced inward currents (INa and ICa), and increased outward currents (mostly Ito, but also IKATP, IKr, IKs, IKAch, and IK1), leading to the accentuation of the AP notch and eventual loss of AP dome. Loss of the AP dome typically occurs at some epicardial sites, but not others, resulting in a marked increase of ventricular repolarization and refractoriness dispersion within the epicardium as well as between epicardium and endocardium (transmural dispersion of repolarization, TDR) and finally, giving rise to phase-2 related short-coupled pre-mature ventricular beats and re-entrant polymorphic VA. Accordingly, pre-clinical data clearly showed that Ito block restores epicardial AP dome, normalizes the ST segment, and prevents VA in experimental models of Brugada syndrome, and the same was demonstrated for quinidine, although direct Ito blockade is not the only mechanism responsible for quinidine anti-arrhythmic effects. Quinidine may directly counteract the pro-arrhythmic consequences of cholinergic activation in the Brugada syndrome that have been related to a combination of bradycardia (that intrinsically increases Ito current due to its slow recovery from inactivation and its higher availability at slower heart rate), increased heart rate variability (leading to increased AP instability and temporal dispersion), IKAch augmentation and ICaL suppression.21
Quinidine side effects
Side effects are common during quinidine therapy, often leading to drug discontinuation. The most common side effects are gastrointestinal, usually diarrhoea. The electrolyte imbalances may be harmful by facilitating the onset of VA, therefore, in the case of diarrhoea, the therapy with quinidine should be reduced or possibly interrupted.
Another important adverse effect of quinidine is the ventricular pro-arrhythmic effect (torsade de pointes with recurrent syncope and even SCD), due to QT prolongation, occurring generally within 1–3 h after the last quinidine dose and more frequently in the first 48–72 h from the beginning of therapy. Therefore, it is recommended to start quinidine administration during a short hospitalization or at least to record an ECG after the first administration. Monitoring quinidine plasma level can be useful to maintain a therapeutic blood level range, as suggested by a few studies.8,11,13
Digoxin can have an increased arrhythmic risk when associated with quinidine, because quinidine reduces its renal tubular secretion, increasing digoxin blood levels. The association with other QT-prolonging drugs should be avoided, for the increased risk of torsade de pointes.
Other dose-related and reversible side effects include tinnitus, headache, dizziness, visual disturbances, nausea, and decreased hearing, usually defined as ‘cinchonism’. Hypersensitivity reaction, as haemolytic anaemia, agranulocytosis and thrombocytopenia, rashes, lupus, and hepatitis can rarely occur.22
Other anti-arrhythmic drugs
Other drugs with possible anti-arrhythmic effect in Brugada syndrome have been described.4 In 1996, the group of Miyazaki et al.23 explored the effect of different AAD on ECG Brugada pattern in four patients, and described how the Brugada ECG pattern was mitigated during beta-adrenoceptor stimulation by isoproterenol. The efficacy of isoproterenol infusion in the suppression of electrical storms in Brugada patients was later reported24,25 and its use in this context is supported by the most recent ESC guidelines with a class IIa recommendation.3
As a possible alternative to quinidine, ESC guidelines suggest the phosphodiesterase-3 inhibitor cilostazol3 that acts by increasing the inward calcium current (ICa-L) and inhibiting the transient outward potassium current (Ito).25,26
Several small reports described the use of other drugs such as the beta-stimulator orciprenaline (10–40 times less potent than isoprenaline) in electrical storms27 or bepridil as a chronic anti-arrhythmic treatment, a drug originally proposed as a calcium antagonist, also inhibiting outward K+ currents and Ito.25,28 These drugs are not available in all countries.
Despite many authors reporting on the possible mechanisms and efficacy of diverse anti-arrhythmic treatments in Brugada patients, the evidence for their use, with the exception of quinidine and isoproterenol, remains insufficient to draw certain conclusions and recommendations.
Discussion
The main clinical reasons that can lead to the prescription of quinidine therapy in Brugada patients are as follows:
recurrent VA and multiple ICD shocks3
EPS-guided therapy in asymptomatic Brugada patients with VA induced at the basal EPS8,9,11,13
supraventricular arrhythmias, mainly AF16
The efficacy of quinidine therapy in the prevention of VA in Brugada syndrome has been demonstrated by several small studies. Those studies which considered patients with previous cardiac arrest, already implanted with an ICD, have clearly proved the ability of the drug to prevent or significantly reduce arrhythmic recurrences.8,10,12 In several studies, VA recurrences were observed in patients who discontinued the medication, and successfully treated by restarting therapy.10
The asymptomatic patients with spontaneous type 1 ECG pattern have a low but not negligible risk of SCD. The 2022 ESC guidelines consider EPS for risk stratification and ICD implantation in the case of inducible VF (class IIb).3 The possibility of long-term device-related complications (e.g. lead fracture, infections, psychological distress, and so on) in this population, however, can outweigh the expected benefits. In the asymptomatic patients with inducible VF at EPS, who represent indeed the majority of subjects enrolled in the pharmacological studies, quinidine could be an alternative. Indirect evidence of its value is given by the prevention of VF inducibility; however, the low incidence of spontaneous arrhythmic events in this group does not allow to definitely prove its efficacy.
Supraventricular arrhythmias, especially AF, have a higher incidence in Brugada patients, as compared with the general population, and often occur at a younger age. Quinidine is the only pharmacological option in the prevention of recurrences, the alternative being represented by catheter ablation.16
Quinidine is particularly indicated in the paediatric population, also as a bridge to ICD, considering the problems associated with device implantation in young patients.17,18
Unfortunately, quinidine therapy presents a relatively high incidence of side effects that can lead to drug discontinuation. An option in these cases may be dose reduction: low-dose quinidine (<600 mg daily) has been associated with greater tolerability, which however might imply a reduced efficacy.14
Quinidine mechanism of action is not completely understood, but it seems to be at least partially due its Ito blocking effect and its anti-cholinergic activity.22
In conclusion, to date quinidine is the only drug with sufficient evidence of efficacy and safety to be considered among the therapeutic tools for the prevention of both ventricular and supraventricular arrhythmias in Brugada patients.
Contributor Information
Carla Giustetto, Division of Cardiology, Cardiovascular and Thoracic Department, “Città della Salute e della Scienza” Hospital, C.so Bramante, 88. 10126, Turin, Italy; Department of Medical Sciences, University of Turin, C. so Dogliotti, 14, 10126, Turin, Italy.
Natascia Cerrato, Division of Cardiology, Cardinal G. Massaia Hospital, 14100 Asti, Italy.
Veronica Dusi, Division of Cardiology, Cardiovascular and Thoracic Department, “Città della Salute e della Scienza” Hospital, C.so Bramante, 88. 10126, Turin, Italy; Department of Medical Sciences, University of Turin, C. so Dogliotti, 14, 10126, Turin, Italy.
Filippo Angelini, Division of Cardiology, Cardiovascular and Thoracic Department, “Città della Salute e della Scienza” Hospital, C.so Bramante, 88. 10126, Turin, Italy.
Gaetano De Ferrari, Division of Cardiology, Cardiovascular and Thoracic Department, “Città della Salute e della Scienza” Hospital, C.so Bramante, 88. 10126, Turin, Italy; Department of Medical Sciences, University of Turin, C. so Dogliotti, 14, 10126, Turin, Italy.
Fiorenzo Gaita, Department of Medical Sciences, University of Turin, C. so Dogliotti, 14, 10126, Turin, Italy; Maria Pia Hospital, GVM Care & Research, 10132 Torino, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado Det al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005;111:659–670. [DOI] [PubMed] [Google Scholar]
- 2. Schimpf R, Giustetto C, Eckardt L, Veltmann C, Wolpert C, Gaita Fet al. Prevalence of supraventricular tachyarrhythmias in a cohort of 115 patients with Brugada syndrome. Ann Noninvasive Electrocardiol 2008;13:266–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 4. Postema PG, Wolpert C, Amin AS, Probst V, Borggrefe M, Roden DMet al. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website (www.brugadadrugs.org). Heart Rhythm 2009;6:1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sacher F, Probst V, Iesaka Y, Jacon P, Laborderie J, Mizon-Gérard Fet al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study. Circulation 2006;114:2317–2324. [DOI] [PubMed] [Google Scholar]
- 6. Pappone C, Brugada J, Vicedomini G, Ciconte G, Manguso F, Saviano Met al. Electrical substrate elimination in 135 consecutive patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2017;10:e005053. [DOI] [PubMed] [Google Scholar]
- 7. Belhassen B, Glick A, Viskin S. Efficacy of quinidine in high-risk patients with Brugada syndrome. Circulation 2004;110:1731–1737. [DOI] [PubMed] [Google Scholar]
- 8. Hermida JS, Denjoy I, Clerc J, Extramiana F, Jarry G, Milliez Pet al. Hydroquinidine therapy in Brugada syndrome. J Am Coll Cardiol 2004;43:1853–1860. [DOI] [PubMed] [Google Scholar]
- 9. Belhassen B, Glick A, Viskin S. Excellent long-term reproducibility of the electrophysiologic efficacy of quinidine in patients with idiopathic ventricular fibrillation and Brugada syndrome. Pacing Clin Electrophysiol 2009;32:294–301. [DOI] [PubMed] [Google Scholar]
- 10. Márquez MF, Bonny A, Hernández-Castillo E, De Sisti A, Gómez-Flores J, Nava Set al. Long-term efficacy of low doses of quinidine on malignant arrhythmias in Brugada syndrome with an implantable cardioverter-defibrillator: a case series and literature review. Heart Rhythm 2012;9:1995–2000. [DOI] [PubMed] [Google Scholar]
- 11. Bouzeman A, Traulle S, Messali A, Extramiana F, Denjoy I, Narayanan Ket al. Long-term follow-up of asymptomatic Brugada patients with inducible ventricular fibrillation under hydroquinidine. Europace 2014;16:572–577. [DOI] [PubMed] [Google Scholar]
- 12. Anguera I, García-Alberola A, Dallaglio P, Toquero J, Pérez L, Martínez JGet al. Shock reduction with long-term quinidine in patients with Brugada syndrome and malignant ventricular arrhythmia episodes. J Am Coll Cardiol 2016;67:1653–1654. [DOI] [PubMed] [Google Scholar]
- 13. Andorin A, Gourraud JB, Mansourati J, Fouchard S, le Marec H, Maury Pet al. The QUIDAM study: hydroquinidine therapy for the management of Brugada syndrome patients at high arrhythmic risk. Heart Rhythm 2017;14:1147–1154. [DOI] [PubMed] [Google Scholar]
- 14. Mazzanti A, Tenuta E, Marino M, Pagan E, Morini M, Memmi Met al. Efficacy and limitations of quinidine in patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2019;12:e007143. [Google Scholar]
- 15. Bordachar P, Reuter S, Garrigue S, Caï X, Hocini M, Jaïs Pet al. Incidence, clinical implications and prognosis of atrial arrhythmias in Brugada syndrome. Eur Heart J 2004;25:879–884. [DOI] [PubMed] [Google Scholar]
- 16. Giustetto C, Cerrato N, Gribaudo E, Scrocco C, Castagno D, Richiardi Eet al. Atrial fibrillation in a large population with Brugada electrocardiographic pattern: prevalence, management, and correlation with prognosis. Heart Rhythm 2014;11:259–265. [DOI] [PubMed] [Google Scholar]
- 17. Probst V, Denjoy I, Meregalli PG, Amirault JC, Sacher F, Mansourati Jet al. Clinical aspects and prognosis of Brugada syndrome in children. Circulation 20077;115:2042–2048. [DOI] [PubMed] [Google Scholar]
- 18. Andorin A, Behr ER, Denjoy I, Crotti L, Dagradi F, Jesel Let al. Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm 2016;13:1274–1282. [DOI] [PubMed] [Google Scholar]
- 19. Task Force of the Working Group on Arrhythmias of the European Society of Cardiology. The Sicilian Gambit. A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. Circulation 1991;84:1831–1851. [DOI] [PubMed] [Google Scholar]
- 20. Kurachi Y, Nakajima T, Sugimoto T. Quinidine inhibition of the muscarine receptor-activated K+ channel current in atrial cells of Guinea pig. Naunyn Schmiedebergs Arch Pharmacol 1987;335:216–218. [DOI] [PubMed] [Google Scholar]
- 21. Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation 1999;100:1660–1666. [DOI] [PubMed] [Google Scholar]
- 22. Serdoz L V, Rittger H, Furlanello F, Bastian D. Quinidine-A legacy within the modern era of antiarrhythmic therapy. Pharmacol Res 2019;144:257–263. [DOI] [PubMed] [Google Scholar]
- 23. Miyazaki T, Mitamura H, Miyoshi S, Soejima K, Aizawa Y, Ogawa S. Autonomic and antiarrhythmic drug modulation of ST segment elevation in patients with Brugada syndrome. J Am Coll Cardiol 1996;27:1061–1070. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe A, Fukushima Kusano K, Morita H, Miura D, Sumida W, Hiramatsu Set al. Low-dose isoproterenol for repetitive ventricular arrhythmia in patients with Brugada syndrome. Eur Heart J 2006;27:1579–1583. [DOI] [PubMed] [Google Scholar]
- 25. Ohgo T, Okamura H, Noda T, Satomi K, Suyama K, Kurita Tet al. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm 2007;4:695–700. [DOI] [PubMed] [Google Scholar]
- 26. Tsuchiya T, Ashikaga K, Honda T, Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J Cardiovasc Electrophysiol 2002;13:698–701. [DOI] [PubMed] [Google Scholar]
- 27. Kyriazis K, Bahlmann E, van der Schalk H, Kuck KH. Electrical storm in Brugada syndrome successfully treated with orciprenaline; effect of low-dose quinidine on the electrocardiogram. Europace 2009;11:665–666. [DOI] [PubMed] [Google Scholar]
- 28. Murakami M, Nakamura K, Kusano KF, Morita H, Nakagawa K, Tanaka Met al. Efficacy of low-dose bepridil for prevention of ventricular fibrillation in patients with Brugada syndrome with and without SCN5A mutation. J Cardiovasc Pharmacol 2010;56:389–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.