Abstract
Criteria for diagnosis of arrhythmogenic cardiomyopathy (ACM) were first proposed in 1994 and subsequently revised in 2010 and in 2020 by an international task force. According to the last consensus of 2020, ACM is defined as a heart muscle disease affecting right ventricle, left ventricle or both, whose principal pathologic feature is fibrofatty myocardial replacement that impairs systolic ventricular function and predisposes to lethal ventricular arrhythmias. ECG findings not only could help to early recognize affected patients but also could identify the ones with maximum risk of ventricular arrhythmias and sudden cardiac death.
Keywords: Arrhythmogenic ventricular cardiomyopathy, Cardiomyopathy, Death, sudden, cardiac, Prognosis, ECG, Arrhythmias
Introduction
The 2020 international consensus (Padua Criteria) includes three different phenotypic variants:
The dominant right variant; the classic arrhythmogenic right ventricular cardiomyopathy (ARVC) phenotype with right ventricle involvement without abnormalities in the left ventricle
The biventricular variant: a phenotype characterized by the involvement of right and left ventricle
The dominant left variant, also called arrhythmogenic left ventricular cardiomyopathy (ALVC); a phenotype characterized by left ventricular involvement without abnormalities in right ventricle.1,2
The right ventricular phenotype
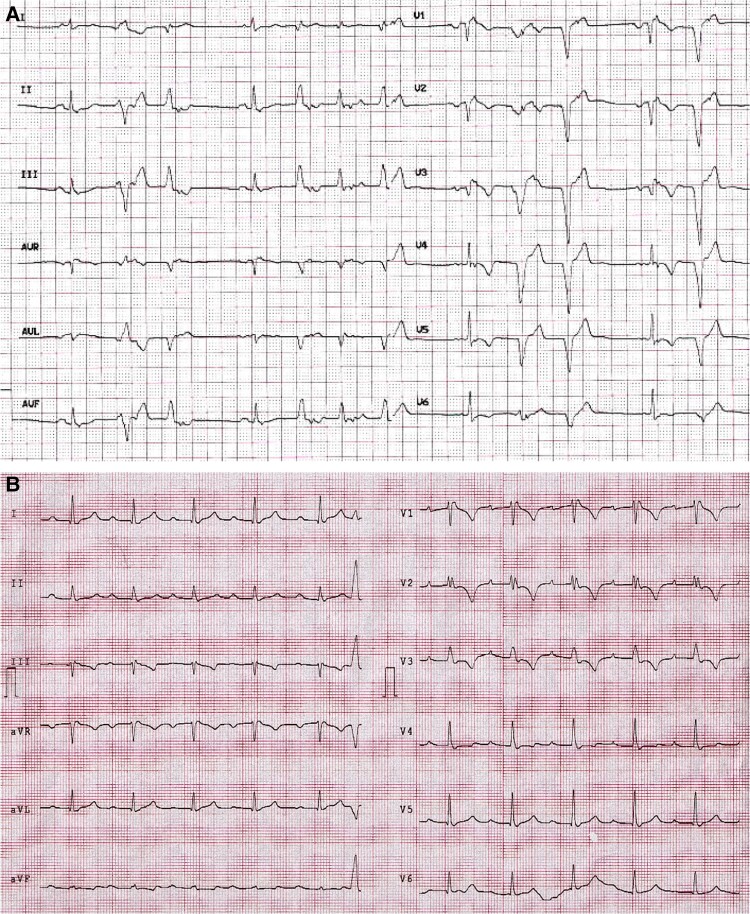
ECG abnormalities: repolarization and depolarization (Figure 1)
Figure 1.
ECG findings of two patients with ARVC. Panel A. ECG performed in a 21-year-old woman shows the classical epsilon wave in lead V1 and T-wave inversion in V1–V6. This patient shows also premature ventricular beats with two LBBB morphologies. Panel B. ECG performed in 43-year-old woman shows epsilon wave in lead V1 and T-wave inversion in V1–V3 leads. LBBB, left bundle branch block.
Twelve-lead ECG is a pivotal diagnostic test in ARVC and shows in up to 90% of patients with ARVC, repolarization and/or depolarization abnormalities.3,4
Negative T waves in the right precordial leads (V1–V2–V3) after the pubertal age are the most significant and common electrocardiographic finding in affected patients.5
Their presence, in the absence of complete right bundle branch block (RBBB), are a major repolarization abnormality criterion.
The presence of epsilon waves in right precordial leads were classified as a major ECG criterion in both the 1994 and the 2010 task force criteria; however, its diagnostic value has been questioned because its identification and interpretation are largely influenced by ECG filtering and sampling rate, determining a large interobserver variability.5,6
Hence, in the 2020 international diagnostic criteria (Padua criteria) this ECG marker has been downgraded to a minor ECG depolarization criterion.2
According to the 2010 TF criteria and to the 2020 TF criteria, the presence of terminal activation duration of QRS >55 msec measured from the nadir of the S-wave to the end of the QRS, including R’ in V1, V2 or V3 without complete RBBB is another minor ECG depolarization criterion.
The presence of late potentials on signal-averaged ECG was a minor criterion in the 2010 TF criteria, is no longer included among the 2020 Padua criteria because the use of the signal-averaged ECG technique has been abandoned worldwide because of its limited diagnostic accuracy.7
Other ECG abnormalities
Beyond the scope of using ECG criteria to the identification of ARVC patients, several authors find other ECG abnormalities that are recurrent in this population.
Peters et al., in 2003, identified the phenomenon of localized right precordial QRS prolongation, defined as QRS duration in V1 + V2 + V3/V4 + V5 + V6 ≥ 1.2, with a sensitivity of 98% and a specificity of 100% in a cohort of 265 patients as the most important ECG finding.8
In 2013, Zusterzeel R et al.9, found in a cohort of 42 ARVC patients that not only T-wave inversion in right precordial leads but also the presence of diminished QRS amplitude in extreme and precordial leads are associated with right ventricular dilatation.
Other variable ECG findings in ARVC patients are:
QRS fragmentation: a slurs or notches on the R- or S-wave. In ARVD fQRS is common and often seen in multiple leads; it is caused by regional delay in propagation of ventricular depolarization caused by fibro/fibrofatty tissue replacement.
Poor R-wave progression: especially in patients with severe right ventricular dilatation.10
ECG abnormalities and risk prediction model
In the last years, several studies have identified multiple predictors of sustained ventricular arrhythmia (VA) and sudden cardiac death (SCD) in patients with ARVC without a prior arrhythmic history; subsequently these predictors were integrated in different decision algorithm for ICD implant.11–13 In 2019 a risk prediction model was developed by a multinational group guided by Cadrin-Tourigny J and including several referral centres for ARVC.14
This model includes different demographical, clinical and electrocardiographic variable as sex, age, history of cardiac syncope, non-sustained ventricular tachycardia, number of premature ventricular complexes (PVCs) on 24 h Holter monitoring, extent of T-wave inversion (TWI) on anterior and inferior leads and right ventricular function.14
In 2022, using a large cohort of patients from 29 centres in six countries in North America and Europe, our group validated the published ARVC risk calculator; the study, furthermore, shows that the risk model provides good prognostic information and outperforms other published decision algorithms for ICD use. Our findings support the use of the model to facilitate the decision-making process about ICD implantation in the primary prevention of SCD in ARVC.15
Left ventricular phenotype
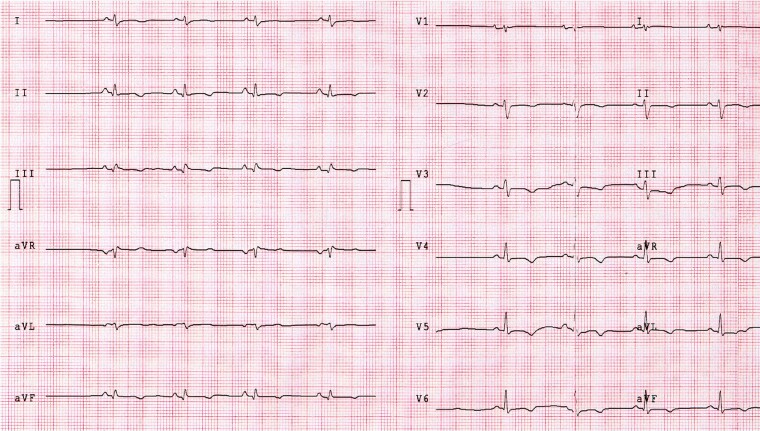
ECG abnormalities: repolarization and depolarization (Figure 2)
Figure 2.
ECG findings in a patient with ALVC. This 34-year-old man with history of ventricular arrhythmias had a CMR revealing biventricular dilation and a stria LGE pattern with subepicardial distribution, mainly involving the LV lateral wall. This patient showed the ‘classical’ low QRS voltages in limb leads and T-wave inversion in V2–V6 and inferior leads. CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricular.
Left ventricular involvement can be predicted on standard ECG by the presence of:
Low QRS voltage in the limb leads (peak to peak QRS amplitude <0.5 mV limbs in all leads, in the absence of obesity, emphysema or pericardial effusion
T-wave inversion in the leads exploring the lateral or the infero-lateral leads (V5–V6).
Both ECG abnormalities have been classified as minor criteria, because of the low estimated disease specificity.14–16
The ECG pattern of low QRS voltages is probably caused by the decrease of LV myocardial mass, which mostly accounts for the generation of the electrical activity causing the depolarization current responsible for the QRS complex. At this moment is not clear why it mainly affects the limb leads.17
T-wave inversion limited to the lateral precordial leads (V5–V6) is an ECG marker of left ventricular involvement; it is important to remember than anterolateral T-wave inversion from right precordial leads (V1–V3) to left ones (V4–V6) may be the result of severe RV dilation and dysfunction rather than involvement of left ventricle.17
Novel ECG findings
Recently, our group have found that left posterior fascicular block (LPFB) was 100-fold higher in young patients affected by sudden cardiac death or aborted cardiac arrest than healthy controls. All patients with LPFB, who underwent CMR, showed LV late gadolinium enhancement. Histopathological analysis showed the presence of fibrous/fibro-adipose myocardial replacement in the inferior and infero-lateral LV. We thus hypothesize that LPFB could be an ECG marker of left arrhythmogenic cardiomyopathy.18
Starting with this observation, we hypothesized that specific ECG parameters might represent biomarkers of ALVC and may help in identifying individual at high risk of sudden cardiac death (SCD) as occurred in ARVC patients. In our experience, we observed both the know ECG signs of ALVC (TWI and LQRSV) and the new ones. Specifically, LPFB, pathological Q-waves in inferior and/or lateral leads and a prominent R-wave in V1 with a R/S ratio ≥0.5 represents frequent findings in these patients. Furthermore, we observed that R-wave voltages were markedly lower in infero-lateral leads in comparison with controls and that the sum of the R-wave in I–II ≤8 mm and S-wave in V1 plus R-wave in V6 ≤12 mm were highly specific for ALVC.
Contributor Information
Leonardo Calò, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Giada Oliviero, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Cinzia Crescenzi, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Fabiana Romeo, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Annamaria Martino, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Edoardo Bressi, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Matteo Stefanini, Division of Radiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Elisa Silvetti, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Ludovica Danza, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Marco Rebecchi, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Stefano Canestrelli, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Elisa Fedele, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Chiara Lanzillo, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Armando Fusco, Division of Radiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Ermenegildo De Ruvo, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169 Rome, Italy.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
- 1. Corrado D, Zorzi A, Cipriani A, Bauce B, Bariani R, Beffagna Get al. . Evolving diagnostic criteria for arrhythmogenic cardiomyopathy. J Am Heart Assoc 2021;10:e021987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, De Lazzari Met al. . Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–114. [DOI] [PubMed] [Google Scholar]
- 3. Nasir K, Bomma C, Tandri H, Roguin A, Dalal D, Prakasa Ket al. . Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation 2004;110:1527–1534. [DOI] [PubMed] [Google Scholar]
- 4. Steriotis AK, Bauce B, Daliento L, Rigato I, Mazzotti E, Folino AFet al. . Electrocardiographic pattern in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2009;103:1302–1308. [DOI] [PubMed] [Google Scholar]
- 5. Marcus FL, Mckenna JW, Sherril D, Basso C, Bauce B, Bluemke ADet al. . Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mc Kenna WJ, Thiene G, Nava A, Fontaliran F, Blomstron-Lundqvist C, Fontaine Get al. . Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task force of the working group myocardial and pericardial disease of the European Society of Cardiology and of the scientific council on cardiomyopathies of the international society and federation of cardiology. Br Heart J 1994;71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corrado D, van Tilten PJ, Mckenna JW, Hauer RNW, Anastastakis A, Asimaki Aet al. . Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J 2020;41:1414–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peters S, Trummel M. Diagnosis of arrhythmogenic right ventricular dysplasia-cardiomyopathy: value of standard Ecg revisited. Ann Noninvasive Electrocardiol 2003;8:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zusterezeel R, Ter Bekke MAR, Volders GAP, Leijten MMF, Van der Wijngaard A, Serroyen Jet al. . Right ventricular enlargement in arrhythmogenic right ventricular cardiomyopathy is associate with decrease QRS amplitude and T wave negativity. Ann Noninvasive Electrocardiol 2013;18:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang L, Liu L, Kowey RP, Fontaine GH. The electrocardiographic manifestation of arrhythmogenic right ventricular dysplasia. Curr Cardiol Rev 2014;10:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corrado D, Wichter T, Link MS, Hauer RN, Marchlinski FE, Anastasakis Aet al. . Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation 2015;132:441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. . 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. [DOI] [PubMed] [Google Scholar]
- 13. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCCet al. . 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019;16:e301–e372. [DOI] [PubMed] [Google Scholar]
- 14. Cadrin-Tourigny J, Bosman PL, Nozza A, Wang W, Tadros R, Bhonsale Aet al. . A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2019;40:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jordà P, Bosman LP, Gasperetti A, Mazzanti A, Gourraud JB, Davies Bet al. . Arrhythmic risk prediction in arrhythmogenic right ventricular cardiomyopathy: external validation of the arrhythmogenic right ventricular cardiomyopathy risk calculator. Eur Heart J 2022;43:3041–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Augusto JB, Eiros R, Nakou E, Moura-Ferreira S, Treibel TA, Captur Get al. . Dilated cardiomyopathy and arrhythmogenic left ventricular cardiomyopathy: a comprehensive genotype-imaging phenotype study. Eur Heart J Cardiovasc Imaging 2020;21:326–336. [DOI] [PubMed] [Google Scholar]
- 17. Cipriani A, Bauce B, De Lazzari M, Rigato I, Bariani R, Meneghin Set al. . Arryhthmogenic ventricular cardiomyopathy: characterization of left ventricular phenotype and differential diagnosis with dilated cardiomyopathy. J Am Heart Assoc 2020;9:e014628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calò L, Della Bona R, Martino A, Crescenzi C, Panattoni G, d’Amati Get al. . Left posterior fascicular block and increased risk of sudden cardiac death in young people. J Am Coll Cardiol 2021;77:1143–1145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analysed in support of this research.