Abstract
Late gadolinium enhancement (LGE) is the most relevant tool of cardiac magnetic resonance for tissue characterization, and it plays a pivotal role for diagnostic and prognostic assessment of cardiomyopathies. The pattern of presentation of LGE allows differential diagnosis between ischaemic and non-ischaemic heart disease with high diagnostic accuracy, and among different cardiomyopathies, specific presentation of LGE may help to make a diagnosis. Late gadolinium enhancement may be caused by conditions that significantly increase the interstitial space or, less frequently, that slow down Gd exit, like myocardial fibrosis. In chronic myocardial infarction, hypertrophic cardiomyopathies (HCM), dilated cardiomyopathy, Fabry disease, and other conditions, LGE is a marker of myocardial fibrosis, but also in patients with acute myocarditis where LGE may be also explained by the increase of interstitial space caused by interstitial oedema or by tissue infiltration of inflammatory cells. In cardiac amyloidosis, LGE represents myocardial fibrosis but the interstitial overload of amyloid proteins should also be considered as a potential cause of LGE. The identification of the pattern of presentation of LGE is also very important. In the ischaemic pattern, LGE always involves the subendocardial layer with more or less transmural extent, it is confluent, and every single scar should be located in the territory of one coronary artery. In the non-ischaemic pattern, LGE does not fulfil the previous criteria, being midwall, subepicardial, or mixed, not necessarily confluent or confined to a territory of one coronary artery. For cardiomyopathies, the exact pattern of non-ischaemic LGE is important. Quantitative analysis of LGE is required in some specific conditions as in HCM. Magnetic resonance imaging with LGE technique should be performed in every patient with suspect of cardiomyopathy. The lack of standardization of pulse sequence and mostly of quantification methods is the main limitation of LGE technique.
Keywords: Late gadolinium enhancement, Cardiomyopathy, Cardiac magnetic resonance, Prognosis
Introduction
Cardiac magnetic resonance (CMR) is probably the best diagnostic tool to evaluate morphological, functional and tissue phenotype of cardiomyopathies. It is considered the gold standard imaging modality for the assessment of cardiac function and morphological parameters as wall thickness, left ventricular (LV) mass and volumes. CMR is able of myocardial tissue characterization by the combination of different pulse sequences. However, among all the different CMR techniques, late gadolinium enhancement (LGE) remains the most relevant tool of CMR for tissue characterization.
LGE plays a pivotal role for diagnostic and prognostic assessment of cardiomyopathies.1 The term LGE is generally used to refer for both the imaging technique and for the presence of myocardial hyper-enhancement in LGE images. In this review, for convenience, LGE is used for the identification of myocardial hyper-enhancement, whereas the terms LGE technique and LGE images are used referring respectively to the technique and images.
The pattern of presentation of LGE allows differential diagnosis between ischaemic and non-ischaemic heart disease with high diagnostic accuracy and, among different cardiomyopathies, specific presentation of LGE may help to make diagnosis.
Similarly, different characteristics of LGE, as the pattern, the intramyocardial distribution and the extent, have different prognostic role in different cardiomyopathies.
Aim of this review is to discuss the diagnostic and prognostic role of LGE in cardiomyopathies.
Mechanism of LGE
LGE technique consists in the acquisition of images starting after 10 min (or less in some conditions) following the injection of Gadolinium-based contrast media.
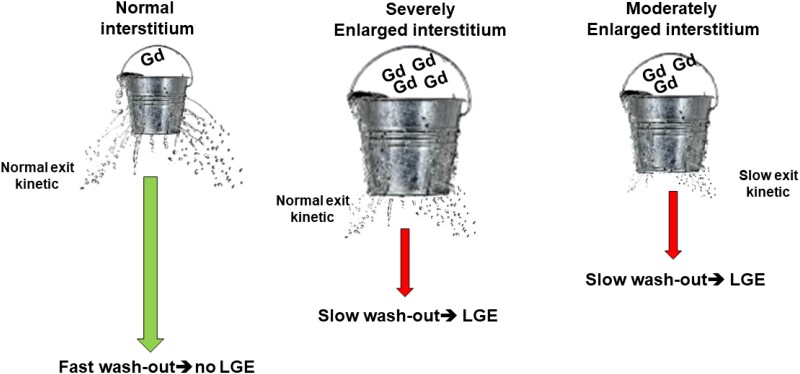
Gadolinium-based media (Gd) are extracellular contrast agents. After injection in peripheral veins, Gd exits from blood vessel spreading in interstitial space. In normal myocardium, interstitium is a relatively small space and Gd stays within it only few minutes since the exit ways through venules or lymphatic vessel are very efficient. LGE may be caused by conditions that significantly increase the interstitial space or less frequently that slow down Gd exit (Figure 1). Then, LGE is not specific for fibrosis as well as Gd is not a specific agent for fibrosis. However, the collagen matrix of scar, replacing dead myocyte, increases interstitial space and the myocardial distribution volume of gadolinium. For this reason, LGE is generally considered a valid marker of myocardial fibrosis in the majority of cardiac conditions. Fibrosis is an irreversible phenomenon because in human heart myocardium is a permanent tissue and myocytes cannot spontaneously regenerate.
Figure 1.
Mechanism of late gadolinium enhancement (LGE). A small concentration of Gadolinium-based (Gd) contrast agent in normal interstitium and the exit kinetic is effective producing a fast wash out. In case di LGE, interstitial space may be moderately or severely increased and exit kinetic preserved or impaired, producing a slower wash out of Gd.
In chronic myocardial infarction, in hypertrophic cardiomyopathies (HCM), in dilated cardiomyopathy (DCM), in Fabry disease and other conditions, LGE is a marker of myocardial fibrosis. On contrast, in 10–15% of patients with myocarditis, LGE, detected within the first week from symptoms onset, may completely disappear after 6 months.1,2 In such cases of myocarditis, LGE may be explained by the increase of interstitial space caused by interstitial oedema or by tissue infiltration of giant inflammatory cells as macrophages and fibroblasts. Macrophages may also incorporate interstitial Gd in phagosomes during the process of phagocytosis of dead myocytes. LGE could also be explained by slow down Gd exit through a congestion or obstruction of lymphatic or venous vessel caused by the inflammatory cells. All these phenomena are potentially reversible, it is always advisable to repeat the MRI after 6 months in case of myocarditis to confirm the presence of definite fibrosis.
LGE is already seen since the first hours from acute myocardial infarction event if there is no sufficient time for a complete replacement of dead myocytes by collagen matrix. Indeed, in the first hours/days of acute myocardial infarction, LGE may be secondary to the passage of Gd within the sarcoplasm of irreversibly damaged myocytes with ruptured sarcolemma. Although this explanation is widely accepted, actually it was never demonstrated and other phenomena as the slow down of Gd reabsorption due to different mechanisms could have a role.
In cardiac amyloidosis, LGE represents myocardial fibrosis but the interstitial overload of amyloid proteins (with their osmotic effect attracting water in the interstitium) should also be considered as potential cause of LGE.3
Finally, in conventional pulse sequence used for LGE technique, fat is hyperintense as LGE. Then, in case of myocardial fat infiltration may be difficult to distinguish between intramyocardial fibrosis and fat by the analysis of only the LGE images. In such cases, the ‘India ink’ sign of cine steady-state free precession (SSFP) images or the hyperintensity/hypointensity, respectively, in non-fat-saturated and fat-saturated fast spin echo (FSE) can make this differential diagnosis.
The term ‘delayed enhancement’ is sometimes used as a synonymous of LGE. A necessary condition for LGE is the patency of micro-vessel permitting to Gd to arrive in the interstitium. On contrast, differently from intracellular agents of nuclear medicine as technetium, the presence of viable myocytes is not necessary for LGE. Then, in majority of conditions with LGE, the enhancement is usually present since the first pass of Gd in myocardium and persists for long time after. Then, the term ‘delayed’ is not correct: enhancement is seen also in ‘late’ phases but is not ‘delayed’ compared with normal myocardium. Actually, the wash out is ‘delayed’, not the enhancement.
LGE technique
Different pulse sequences are currently available for LGE technique but despite some vendor-dependent differences, major characteristics are common for all them.
As explained in the previous paragraph, LGE is caused by the persistence of Gd in myocardial interstitial space. Gd in an indirect contrast agent, acting by a decrease of T1 of surrounding hydrogen protons. Then, all the pulse sequence for LGE technique need to be T1-weighted to detect Gd deposit. In all the pulse sequence normal myocardium is nulled to amplify the contrast with diseased myocardium with Gd uptake. The dosage of Gd is 0.1–0.2 mmol/Kg, with a fast Gd removal for lower dose and a slower wash out for higher dose. As indicated by current position papers, LGE image should be acquired after 10 min following injection, however in case of low dose of Gd, earlier acquisition is suggested. For the evaluation of LGE in cardiomyopathies, where often small areas of fibrosis are found and multiple acquisition section are often needed, we suggest to use the greater dosage of Gd. Nephrogenic systemic fibrosis (NSF) is a rare complication of Gd, but its occurrence almost disappeared in the last 5 years because of the use of polycyclic contrast agents. However, caution should be taken in patients with chronic kidney disease and an estimated glomerular filtration rate <30 mL/min/1.73 m2). If no alternative is available, in patients in dialytic therapy, dialysis should be programmed within 12 h from Gd injection.
Despite black blood LGE techniques were recently proposed, all the conventional LGE techniques use a bright blood pulse sequence based on gradient-echo (GRE) or SSFP with different strategy to null normal myocardium: inversion recovery (IR) or phase-sensitive inversion recovery (PSIR).
In 2D IR fast-GRE sequences a non-selective 180° IR preparation pulse inverting the polarization from up to down of hydrogen spins.4 After the cessation of the IR pulse, spins come back to the up direction. This process takes different time for different tissue and during this, there is a time when spins of normal myocardium are at the ‘null’ point, meaning absence of polarization and absence of signal. The time from cessation of IR pulse and null point is the inversion time (TI). Because Gd decreases the TI of normal myocardium depending on its concentration, the challenge is to choice the more appropriate TI for LGE images acquisition.5 TI increases with the time after the injection of Gd. Look-Locker or TI-Scout sequences are used to identify the correct TI in order to suppress signal from normal myocardium. This choice is crucial to emphasize the signal intensity differences between normal and pathological tissue and to avoid false positive hyperintensity of myocardium.
PSIR sequences are characterized by the acquisition of IR and proton density (PD)-weighted images at the same cardiac and respiratory phase.4 PSIR technique requires less adjustment of TI due to his intrinsic capability to provide reference for background phase and IR data (correcting the polarity of the signal).6 This technique is less accurate than 2D IR GE sequence for detection of small and focal areas of LGE, but it is less operator dependent and it is recommended in MRI laboratory with small expertise.
Three-dimensional LGE technique is a good option in order to characterized, delineate and quantify myocardial fibrosis with high spatial resolution and utilizing isotropic voxel. This technique is extremally useful for the study of myocardial scar tissue prior to ablation therapy.7 Three-dimensional LGE imaging demonstrated an excellent agreement with 2D LGE, in term of image quality,8 but the presence of cardiac arrhythmias and poor patients compliance may deteriorate the image quality, respect to the 2D approach. Three-dimensional LGE imaging may be superior than conventional 2D acquisition for the detection of papillary muscle scar.9
Three-dimensional LGE sequences are able to acquire the whole heart volume in a single breath-hold (or using a respiratory gated approach), with a single longer apnoea, than 2D method (shorter but repetitive apnoea approach). So the overall acquisition time is shorter than 2D acquisition, but it requires more compliant patients, capable to maintain longer apnoeas, without frequent arrhythmias.
For patients with limitations in breath-hold capability, 3D LGE may be acquired using a respiratory gated approach,10 with reduction of SNR.4
The consideration for choice of appropriate TI of 3D LGE technique are the same of 2D IR GRE.
Single-shot 2D imaging with SSFP readout are particularly useful for a faster acquisition in patients with breath holding difficulties and/or arrhythmias in order to minimize respiratory and motion artefacts.4,6
Qualitative and quantitative assessment of LGE
The visual qualitative assessment of LGE is often sufficient in the majority of cardiomyopathies.
The determination of the presence of LGE is in many cases one of the most important prognostic marker. However, in case of small areas of LGE, image artefacts (inappropriate TI, movement artefacts, trigger artefacts), or when hyperintensity are found in specific myocardial regions (RV insertion points, very basal anterior septum, thinned apex, RV wall), LGE detection could be more challenging. A semiquantitative approach for LGE may be obtained by the count of number of myocardial segments involved, but its capacity to depict the true extent of LGE is less effective in cardiomyopathies than in myocardial infarction.
The identification of the pattern of presentation of LGE is also very important. In the ischaemic pattern, LGE always involves the subendocardial layer with more or less transmural extent, it is confluent and should be located in the territory of one coronary artery.
In non-ischaemic pattern, LGE does not fulfil the previous criteria, being midwall, subepicardial or mixed, not necessarily confluent or confined to a territory of one coronary artery.
For cardiomyopathies, the exact pattern of non-ischaemic LGE is important. As described later, midwall pattern involving the interventricular septum or the ‘annular’ pattern are associated with greater risk of malignant arrythmias than other pattern.
Quantitative analysis of LGE is required in some specific condition as in HCM. Several techniques are available for the assessment of LGE extent: (i) manual contouring of scar, (ii) manual thresholding of signal intensity; (iii) multiple standard deviation approach (SD); (iv) full width half maximum (FWHM); (iv) Otzu-auto-threshold (OAT); (v) Rayleigh-curve method.11 A deep description of all these techniques is beyond the scope of this review. However, the absence of a standardization of the measurement is a limitation of LGE technique and in most of MRI laboratories, LGE is only evaluated qualitatively or semi-quantitatively by visual analysis.
All these quantification techniques generate different results, due to intrinsic limitation: ROI positioning, the wrong assumption of a Gaussian distribution of the signal intensity in the nulled myocardium, the fixed cut-off value used to identify the enhanced areas.12 Started by the assumption that normal myocardium is perfected nulled, its intensity is due only to signal noise.12 Nulled myocardium as well as background noise does not follow a gaussian distribution but a Rician distribution. Then, the threshold cut-off of signal intensity to distinguish between normal myocardium and LGE cannot be fixed as mean + 2SDs. The approaches using multiple mean + multiple SD (usually 5 and 6 SDs) as well as the FWHM and the OAT method are empiric methods, working enough well when compared with visual assessment but without a specific physic rationale. The Rayleigh-curve method is based on the reconstruction of the Rician distribution of the noise (normal myocardium) and finding the more appropriate cut-off for LGE.12 However, Rayleigh-curve method requires a complex modelling based of the configuration of the acquisition coils and channels and of magnetic field and it is not generally used in clinical setting.
Other characteristics of LGE can be evaluated. Radiomics application of LGE allows extrapolation of novel parameters. LGE dispersion mapping with the global dispersion score was recently proposed to evaluated the dispersion of LGE within normal myocardium. This technique was able to define the heterogeneous distribution of LGE and was demonstrated to have and incremental prognostic role over LGE extent.13
LGE in hypertrophic cardiomyopathy
In sarcomeric HCM, MRI has an important diagnostic role being the more accurate technique to assess end-diastolic wall thickness and the pattern of hypertrophy. MRI is able to quantify LV mass and to detect secondary phenotype, as crypts, apical aneurysms, papillary muscle abnormalities, elongation of anterior mitral leaflet and RV hypertrophy, that may support the diagnosis.
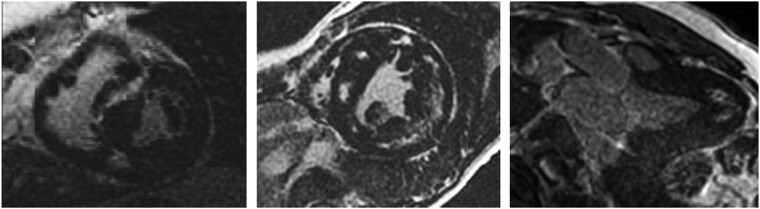
Together with asymmetric hypertrophy and myocardial disarray, the presence of fibrosis is a hallmark of HCM. LGE in HCM is usually located in the midwall layer of the hypertrophied segments but it can be transmural in case of apical aneurisms or for large scar (Figure 2).
Figure 2.
Examples of pattern of LGE in hypertrophic cardiomyopathy (HCM): the pattern of LGE follows the distribution of hypertrophy: in the left panel, hypertrophy is asymmetrical septal and LGE is in the septal midwall; in the middle panel a concentric hypertrophy and diffuse midwall LGE is found; in the right panel, an example of apical HCM with LGE is shown.
In adult patients with HCM, the prevalence of LGE is between 40% and 95% depending of the average age of the studied population with a prevalence that increase with the age. European society of cardiology (ESC) guidelines14 recommend to use the HCM risk score to evaluate the 5-year risk of sudden cardiac death. The indication for implantable cardioverter defibrillator (ICD) is suggested for an estimated 5-year risk ≥6%. However, the overwhelming majority of patients with HCM has an estimated risk <6%, and, in absolute terms, the majority of sudden death occurs in this low-estimated-risk patients. LGE was not included in HCM risk score. As demonstrated, the presence of LGE is associated with worse prognosis. The identification of an early phenotype with greater wall thickness and LGE in young patients is associated with greater risk of events. In studies with an average age greater than 50 years the prevalence of LGE is >80%, then the presence of LGE is not sufficient to stratify the risk of patients.
In the multicentre study by Chan,15 the extent of LGE ≥ 15% is associated with a greater risk for malignant arrhythmic events in an additive role over conventional arrhythmic risk factors. HCM is the only cardiomyopathy in which the quantitative extent of LGE has a prognostic role.
This is probably secondary to the fact that fibrosis in HCM is caused by a micro-vessel disease that is dependent by the degree of hypertrophy: the greater the hypertrophy, the higher risk of fibrosis. Two recent studies16,17 have evaluated the impact of LGE extent for prognostic stratification of patients with HCM risk score <6%. In the study by Mentias,16 majority of patients had HCM with outflow tract obstruction, whereas in the multicentre study by Todiere et al.,17 majority of patients had non-obstructive HCM. Both the studies demonstrated that, in patients with HCM risk score <6%, a LGE extent >10–15% was associated with higher risk of major arrhythmic events as sudden cardiac death, appropriate ICD intervention or cardiac arrest.
As consequence of such findings, in a recent document of ESC heart failure18 the presence of LGE extent ≥15% should be considered for indication of ICD even in patients with HCM risk score <6%. Similarly, in the more recent AHA/ACC guidelines for HCM,19 the presence of ‘extensive’ LGE has to be considered for the indication of ICD even in absence of conventional arrhythmic risk factors.
Interestingly, in both those cited documents,18,19 apical aneurysm were included as arrhythmic risk factor. Indeed, in HCM apical aneurysms are caused by transmural LGE and it is difficult to ascertain whether the greater risk is secondary of the apical aneurysm itself or by the extensive LGE the causes the aneurysm.
HCM is an evolutive disease, and it was demonstrated that the prevalence of LGE increased from 80% to 95% by repeating MRI after 3 years and the extent of LGE doubled in the same time interval.
By these, ESC guidelines prescribe to repeat every 2–3 years MRI in HCM to assess the evolution of the cardiomyopathy.
As reported above, other features of LGE may have a prognostic role. Previous studies, demonstrated that the ‘grey zone’ of fibrosis, variably named as ‘intermediate-signal LGE’ or ‘mild-enhancement’ was more accurate to predict the risk of ventricular tachycardia than the gross scar, identified as hyper-enhanced LGE. This may be explained by the presence of viable, suffering, myocytes interspersed between small island of scar or plexiform fibrosis.
The problem with the assessment of mild-enhancement LGE is that the threshold of signal intensity to distinguish between mild- and hyper-enhancement is absolutely arbitrary.
More recently, a new technique, named LGE dispersion mapping was proposed. Briefly, with this technique, for every voxel with LGE, the signal of the surrounding voxels is measured and a score from 0 to 8 is assigned to the voxel, where 0 is when all the surrounding voxels are similar to the central one and 8 when all the voxels are different. By these automatic measurement, the global dispersion score (GDS) is obtained. GDS is a measure of the dispersion of LGE in myocardium.
Patients with high GDS had worse prognosis than those without, and GDS demonstrated an additive role over LGE extent for predicting major arrhythmic events.
LGE in cardiac amyloidosis
In cardiac amyloidosis LGE has an important diagnostic role. The pattern of LGE is highly specific for cardiac amyloidosis, consisting in a diffuse subendocardial enhancement (without respecting the irroration territory of the coronary artery), with an early darkening of the signal of LV cavity and with a nulling defect of remaining myocardium. Mechanism of this pattern of LGE is not fully understood but the complex interactions between Gd and amyloid proteins (Gd decreasing and amyloid increasing T1) play probably an important role. This pattern of LGE is unique for cardiac amyloidosis having 100% specificity and 85% of sensitivity because it may be absent in early stage of amyloid deposition. The presence of LGE is associated with a worsening of survival in patients with systemic amyloidosis. Furthermore, in more advanced stage, LGE becomes transmural. Fontana et al., demonstrated that transmural pattern of LGE was associated with a worse prognosis than the subendocardial pattern or than the absence of LGE.
LGE in Fabry disease
Fabry disease is caused by deficiency of alpha-galactosidase-A enzyme, producing an intracellular overload of sphingolipids. Fabry disease has an aetiological enzyme-replacement therapy which is unique among the cardiomyopathies. In involved hearts, lysosomal sphingolipids increases myocyte dimensions causing hypertrophy which is concentric in most of cases, although in 9% cases of cases asymmetrical hypertrophy was described.20 Myocytes hypertrophy decreases interstitial space. Then, LGE is only seen in more advanced stage, when there is a sufficient amount of necrotic myocytes and macroscopic fibrosis that increases the interstitial space.7 When present, LGE is usually located in the inferolateral basal wall of LV, with a non-ischaemic midwall distribution.11 However, in 7% of cases LGE may be found in other wall as in the interventricular septum or in the LV apex.20
The MRI diagnosis of Fabry disease is made by the identification of a low native T1 of myocardium at T1 mapping, caused by the sphingolipids overload. However, extensive fibrosis may increase the T1 compensating the decrease caused by sphingolipids. This phenomenon is called ‘pseudo-normalization of myocardial T1’. The identification of extensive LGE coexisting with apparently ‘normal’ values of T1 in subjects with LV hypertrophy should raise the suspicion of Fabry disease.
LGE has also an important prognostic role in this cardiomyopathy. Weidemann et al.21 and Beer et al.22 demonstrated that in patients treated with the enzyme-replacement therapy, the presence of LGE was associated with a low likelihood of regression of LV hypertrophy, and with a scarce improvement of exercise capacity. LGE was also associated with an increased risk for malignant arrhythmic events, including sudden cardiac death.23
LGE in dilated cardiomyopathy
Non-ischaemic DCM is a heterogeneous group of conditions, mostly caused by a genetic predisposition, consisting in LV dilatation and dysfunction. DCM is associated with a greater risk of heart failure and of sudden cardiac death. ICD therapy, is suggested in presence of arrhythmic events or in primary prevention in patients with ejection fraction <35%. The Danish trial24 demonstrated that ICD did not improve the overall mortality in DCM because the majority patients die for heart failure. ICD improves only the risk for arrhythmic sudden death. Then, a more accurate selection of patients for ICD implantation is necessary and LGE may have an important role for this selection.
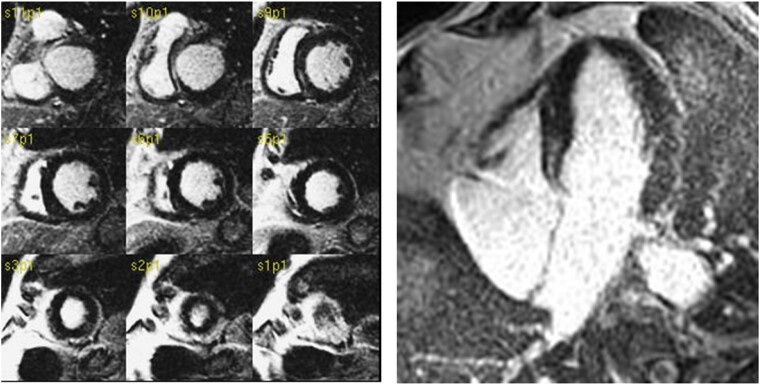
LGE is completely absent in 75% of patients with DCM, in 20–25% of cases LGE is detected with a non-ischaemic pattern (midwall or subepicardial) and in 0–5% of cases it presents an ischaemic pattern (Figure 3). The presence of ischaemic pattern of LGE may be explained as the occurrence ischaemic heart disease during the course of non-ischaemic DCM. In those cases, the extent of ischaemic LGE should not explain the degree of LV dysfunction. The presence of LGE may be considered a sign of a more aggressive phenotype of DCM. Previous studies demonstrated that, in patients with first diagnosis of DCM, the presence of LGE was associated with a low likelihood of reverse remodelling after the instauration of drug therapy for heart failure.25 Furthermore, the presence of LGE is associated with worse prognosis in DCM.25 However, all the studies evaluating the role of quantification of LGE extent in DCM failed to demonstrate a prognostic role of LGE extent.
Figure 3.
LGE in a patient with dilated cardiomyopathy. LGE is seen in midwall of anterior and inferior basal septum.
On contrast, the pattern and the site of distribution may be relevant for prognostic stratification.
In the study by Halliday et al.,26 midwall septal LGE was associated with greater arrhythmic risk than other patterns, particularly when it is combined with LGE at the lateral wall (annular pattern).
The reasons for a major risk of midwall septal pattern are not understood. Interestingly, also in myocarditis the midwall septal pattern was associated to greater risk of events than subepicardial pattern of LGE.
LGE in arrhythmogenic cardiomyopathy
Arrhythmogenic cardiomyopathy (ARC) includes different phenotypic presentation at MRI. The most frequent presentation is with lone RV involvement with dilatation and/or dysfunction, however biventricular or LV dominant presentations are very often detected by MRI. The findings of non-ischaemic LGE and/or of intramyocardial signs of fat infiltration are often the only features permitting to suspect LV dominant phenotype of ARC because LV function is preserved in most of cases. However, the genetic analysis with the identification of a pathogen mutation is mandatory to complete the diagnosis of LV dominant ARC.
A recent multicentre study27 demonstrated that the identification of LV involvement in ARC by LGE and/or fat infiltration is associated to greater risk of major arrhythmic events. Moreover, LV dominant presentation was associated with specific desmosome mutation (desmoplakin and desmoglein) whereas plakophilin mutation was associated with lone or biventricular RV with higher risk for evolution to heart failure but lower risk of arrhythmic events.
Conclusions
LGE remains the most important feature of cardiac MRI and particularly in cardiomyopathies.
LGE is an important diagnostic and prognostic marker for the evaluation of cardiomyopathies, but different aspects of LGE have a role in different conditions as reassumed in Table 1.
Table 1.
Features of LGE and the impact on specific cardiomyopathies
| Features of LGE | Cardiomyopathies with prognostic role |
|---|---|
| Presence/absence | DCM, Fabry, ARVC, amyloidosis, HCM |
| Pattern of distribution (midwall septal/annular) | DCM, myocarditis |
| Pattern of distribution (subendocardial vs. transmural) | Cardiac amyloidosis |
| Extent of LGE | HCM (LGE ≥15%) |
| LGE dispersion | HCM |
MRI with LGE technique should be performed in every patient with suspect of cardiomyopathy.
The lack of standardization of pulse sequence and mostly of quantification methods are the main limitations of LGE technique.
Contributor Information
Giovanni Donato Aquaro, Academic Radiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy.
Carmelo De Gori, Academic Radiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy.
Lorenzo Faggioni, Academic Radiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy.
Maria Luisa Parisella, Academic Radiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy.
Dania Cioni, Academic Radiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy.
Riccardo Lencioni, Academic Radiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy.
Emanuele Neri, Academic Radiology, University of Pisa, Via Savi 10, 56126 Pisa, Italy.
Funding
None declared.
Data availability
All reported data are available in references.
References
- 1. Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro Cet al. Cardiac MR with late gadolinium enhancement in acute myocarditis with preserved systolic function: ITAMY study. J Am Coll Cardiol 2017;70:1977–1987. [DOI] [PubMed] [Google Scholar]
- 2. Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt Get al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation 2006;114:1581–1590. [DOI] [PubMed] [Google Scholar]
- 3. Rapezzi C, Aimo A, Serenelli M, Barison A, Vergaro G, Passino Cet al. Critical comparison of documents from scientific societies on cardiac amyloidosis: JACC state-of-the-art review. J Am Coll Cardiol 2022;79:1288–1303. [DOI] [PubMed] [Google Scholar]
- 4. Kellman P, Arai AE. Cardiac imaging techniques for physicians: late enhancement. J Magn Reson Imaging 2012;36:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim RJ, Shah DJ, Judd RM. How we perform delayed enhancement imaging. J Cardiovasc Magn Reson 2003;5:505–514. [DOI] [PubMed] [Google Scholar]
- 6. Huber A, Hayes C, Spannagl B, Rieber J, Klauss V, Schoenberg SOet al. Phase-sensitive inversion recovery single-shot balanced steady-state free precession for detection of myocardial infarction during a single breathhold. Acad Radiol 2007;14:1500–1508. [DOI] [PubMed] [Google Scholar]
- 7. Andreu D, Ortiz-Pérez JT, Fernández-Armenta J, Guiu E, Acosta J, Prat-González Set al. 3D delayed-enhanced magnetic resonance sequences improve conducting channel delineation prior to ventricular tachycardia ablation. Europace 2015;17:938–945. [DOI] [PubMed] [Google Scholar]
- 8. Goetti R, Kozerke S, Donati OF, Sürder D, Stolzmann P, Kaufmann PAet al. Acute, subacute, and chronic myocardial infarction: quantitative comparison of 2D and 3D late gadolinium enhancement MR imaging. Radiology 2011;259:704–711. [DOI] [PubMed] [Google Scholar]
- 9. Peters DC, Appelbaum EA, Nezafat R, Dokhan B, Han Y, Kissinger KVet al. Left ventricular infarct size, peri-infarct zone, and papillary scar measurements: a comparison of high-resolution 3D and conventional 2D late gadolinium enhancement cardiac MR. J Magn Reson Imaging 2009;30:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foo TK, Stanley DW, Castillo E, Rochitte CE, Wang Y, Lima JAet al. Myocardial viability: breath-hold 3D MR imaging of delayed hyperenhancement with variable sampling in time. Radiology 2004;230:845–851. [DOI] [PubMed] [Google Scholar]
- 11. Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti Cet al. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging 2011;4:150–156. [DOI] [PubMed] [Google Scholar]
- 12. Aquaro GD, Positano V, Pingitore A, Strata E, Di Bella G, Formisano Fet al. Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson 2010;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aquaro GD, Grigoratos C, Bracco A, Proclemer A, Todiere G, Martini Net al. Late gadolinium enhancement-dispersion mapping: a new magnetic resonance imaging technique to assess prognosis in patients with hypertrophic cardiomyopathy and low-intermediate 5-year risk of sudden death. Circ Cardiovasc Imaging 2020;13:e010489. [DOI] [PubMed] [Google Scholar]
- 14. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron Pet al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European society of cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 15. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas Tet al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 2014;130:484–495. [DOI] [PubMed] [Google Scholar]
- 16. Mentias A, Raeisi-Giglou P, Smedira NG, Feng K, Sato K, Wazni Oet al. Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J Am Coll Cardiol 2018;72:857–870. [DOI] [PubMed] [Google Scholar]
- 17. Todiere G, Nugara C, Gentile G, Negri F, Bianco F, Falletta Cet al. Prognostic role of late gadolinium enhancement in patients with hypertrophic cardiomyopathy and low-to-intermediate sudden cardiac death risk score. Am J Cardiol 2019;124:1286–1292. [DOI] [PubMed] [Google Scholar]
- 18. Seferović PM, Polovina M, Bauersachs J, Arad M, Ben Gal T, Lund LHet al. Heart failure in cardiomyopathies: a position paper from the heart failure association of the European society of cardiology. Eur J Heart Fail 2019;21:553–576. [DOI] [PubMed] [Google Scholar]
- 19. Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott Pet al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: executive summary: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 2020;142:e533–e557. [DOI] [PubMed] [Google Scholar]
- 20. Aquaro GD, De Gori C, Faggioni L, Parisella ML, Aringhieri G, Cioni Det al. Cardiac magnetic resonance in fabry disease: morphological, functional, and tissue features. Diagnostics (Basel) 2022;12:2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weidmann F, Niemann M, Breunig F, Herrmann S, Beer M, Störk Set al. Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: evidence for a better outcome with early treatment. Circulation 2009;119:524–529. [DOI] [PubMed] [Google Scholar]
- 22. Beer M, Weidemann F, Breunig F, Knoll A, Koeppe S, Machann Wet al. Impact of enzyme replacement therapy on cardiac morphology and function and late enhancement in fabry’s cardiomyopathy. Am J Cardiol 2006;97:1515–1518. [DOI] [PubMed] [Google Scholar]
- 23. Nojiri A, Anan I, Morimoto S, Kawai M, Sakuma T, Kobayashi Met al. Clinical findings of gadolinium-enhanced cardiac magnetic resonance in fabry patients. J Cardiol. 2020;75:27–33. [DOI] [PubMed] [Google Scholar]
- 24. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup Eet al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221–1230. [DOI] [PubMed] [Google Scholar]
- 25. Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: A review and meta-analysis. JACC Cardiovasc Imaging 2018;11:1274–1284. [DOI] [PubMed] [Google Scholar]
- 26. Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi Cet al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc Imaging 2019;12:1645–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aquaro GD, De Luca A, Cappelletto C, Raimondi F, Bianco F, Botto Net al. Prognostic value of magnetic resonance phenotype in patients with arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2020;75:2753–2765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All reported data are available in references.